Abstract
Nonalcoholic steatohepatitis (NASH) is the progressive subtype of non-alcoholic fatty liver disease and potentiates risks for both hepatic and metabolic diseases. Although the pathophysiology of NASH is not completely understood, recent studies have revealed that macrophage activation is a major contributing factor for the disease progression. Macrophages integrate the immune response and metabolic process and have become promising targets for NASH therapy. Natural products are potential candidates for NASH treatment and have multifactorial underlying mechanisms. Macrophage involvement in the development of steatosis and inflammation in NASH has been widely investigated. In this review, we assess the evidence for natural products or their active ingredients in the modulation of macrophage activation, recruitment, and polarization, as well as the metabolic status of macrophages. Our work may highlight the possible natural products that target macrophages as potential treatment options for NASH.
Keywords: Nonalcoholic steatohepatitis, Macrophages, Natural products, Inflammation, Metabolism
Core tip: Macrophages play a pivotal role in the pathogenesis of nonalcoholic steatohepatitis. Here we discuss the evidence for natural products or their active ingredients in the modulation of macrophage activation, recruitment, and polarization, as well as the metabolic status of macrophages. Our work may highlight the possible natural products that target macrophages as potential treatment options for nonalcoholic steatohepatitis.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is a common liver disease worldwide[1]. Approximately one-quarter of the population suffer from NAFLD[1], and approximately 30% of patients with NAFLD progress to the inflammatory subtype-nonalcoholic steatohepatitis (NASH)[2]. NASH is characterized by steatosis, inflammation, and fibrosis, and serves as a potential risk factor for hepatocellular carcinoma[3]. Lifestyle interventions, such as dieting and exercise, are the general recommendation for NAFLD[4]. Weight control is of great importance, and a weight loss of 7% to 10% can histologically attenuate NASH in patients[5]. Even without weight loss, patients with NAFLD benefit from exercise by improving insulin sensitivity and reducing hepatic lipid content[3,6,7]. However, not all patients are willing or suitable for such interventions, thus making pharmacological agents urgently needed. Although the pathophysiology and treatment of NASH have been extensively investigated, authorized pharmacological agents that are specific for NASH are not yet available.
Macrophages are versatile innate immune cells. As scavengers, they engulf worn-out cells and debris. As secretory cells, they produce a wide array of powerful chemical substances, such as enzymes and complement proteins. In addition, macrophages can present antigens and, along with dendritic cells, initiate adaptive immune response. Tissue macrophages are mainly derived from embryonic progenitors and blood monocytes[8]. Since macrophages obtain phagocytosis and immunoregulating properties, they are involved in tissue development and homeostasis with high plasticity[9]. According to their functions, macrophages are generally divided into two subpopulations, namely, classically activated (M1-type) macrophages and alternatively activated (M2-type) macrophages. The microenvironment determines the phenotype, and the dynamic self-metabolism state inversely regulates its response to the microenvironment. For instance, high levels of lipopolysaccharide (LPS) and interferon γ (IFN-γ) promote M1-type macrophage polarization, whereas interleukin (IL)-4, IL-10, and IL-13 promote M2-type macrophage polarization[9-11]. The predominant phenotype may change at different periods of disease. M1-type macrophages become dominant during inflammation and injury, whereas M2-type macrophages are abundant in the tissue repair and recovery periods.
Macrophages are the main source of inflammatory mediators in the liver, and the activation of macrophages also induces insulin resistance and metabolic dysfunctions. In addition, high titers of immunoglobulin G exist in 40% of adult NAFLD/NASH patients, 60% of pediatric NASH patients, and diet-induced NASH animals, suggesting that adaptive immune responses also take an active part in NASH development[12-14]. The versatile macrophages integrate metabolic and inflammatory responses, as well as adaptive immunity, thus serving as critical targets for the treatment of NASH[15]. Natural products are potential candidates in NASH therapy owing to their safety and multitarget properties, and a series of natural products are reported to modulate macrophages, which may contribute to their effects in preventing or treating NASH.
ROLE OF LIVER MACROPHAGES IN NASH
NASH is characterized by infiltration of inflammatory cells in the liver, and liver macrophages play a central part in the process[16]. Liver macrophages consist of resident Kupffer cells (KCs) and recruited macrophages derived from circulating monocytes. KCs and recruited macrophages have different features in the progression of NASH[17]. KCs are the first line of defense in the liver, and endogenous and exogenous pathogens induce KC activation. The activated KCs clear pathogens depending on their phagocytic activity. Simultaneously, KCs secrete pro-inflammatory cytokines and chemokines, promote the inflammatory response, and recruit peripheral blood monocytes to the liver. With the progression of disease, monocyte-derived macrophages become the dominant macrophages in the liver[17]. Generally, macrophage activation serves as a protector by engulfing pathogens and secreting cytokines or mediators in the early stage of host immunity[18]. However, continuous stimulation induces cell death, liver injury, and related diseases[19].
NATURAL PRODUCTS THAT TARGET MACROPHAGES IN NASH TREATMENT
Natural products regulating macrophage activation
In NASH, pathogen-associated molecular patterns or damage associated molecular models such as gut-derived endotoxins, adipose tissue-derived adipokines, and debris from injured or dead hepatocytes induce KC activation, activated KCs secrete chemokines to recruit monocytes to the liver, and the expanding liver macrophage pool may further promote liver injury[20,21]. KC depletion has been reported to protect mice from hepatic steatosis and insulin resistance upon high fat diet (HFD) feeding, suggesting that KCs play an important role in NAFLD development[22]. Several natural products are reported to inhibit KC activation. Sparstolonin B is an ingredient of Sparganium stoloniferum, and administration of Sparstolonin B to HFD-fed mice decreases the expression of cluster of differentiation 68 (CD68) and chemokine 2 (CCL2) in KCs and reverses NASH features accordingly[23]. Curcuminoids, extracted from the plant Curcuma domestica Val., are found to inhibit KC activation in LPS-treated BALB/C mice[24]. In carbon tetrachloride-induced acute liver injury rats, S. miltiorrhiza extract administration obviously suppresses p38 and nuclear factor-kappa B (NF-κB) signaling in KCs[25]. A six-week supplementation of methanolic extract of Graptopetalum paraguayense was reported to reduce nitric oxide, tumor necrosis factor (TNF)-α, and IL-6 generation and improve liver inflammation and fibrosis in dimethylnitrosamine- or carbon tetrachloride-induced NASH rats[26].
Excess lipids in the liver may cause lipotoxicity, and lipid intermediate metabolites such as palmitate, ceramides, and free cholesterol are crucial contributors to macrophage activation, oxidative stress, and apoptosis[27-29]. Natural products with the function of reducing lipotoxicity are candidates for NASH treatment. The tuber of Alisma orientalis (Sam.) Juzep. is a commonly used herbal medicinal material, and administration of its extract prevents endoplasmic reticulum stress and lipogenic gene expression in palmitate-stimulated HepG2 cells as well as in diet-induced NAFLD mice[30,31]. Serine palmitoyltransferase is a key enzyme in ceramide metabolism, and the fungal compound myriocin inactivates serine palmitoyltransferase by forming a C18 aldehyde and prevents sphingolipid biosynthesis in hepatocytes[32].
In addition to endogenous liver stress, liver macrophage activation can also be mediated by extrahepatic stimuli, such as gut-derived endotoxins, translocated bacteria and microbiota metabolites, and adipose-derived cytokines. Blocking or alleviating these stimuli is expected to suppress macrophage activation and improve the NASH phenotype[27,33-36]. Certain natural products may affect the structure of the gut microbiome, and the related metabolites are reported in NASH treatment. Gallic acid is a naturally abundant plant phenolic compound in vegetables and fruits; it partially reshapes gut dysbiosis, reduces the choline metabolites dimethylamine and trimethylamine, and prevents NAFLD development in HFD-fed mice[37]. The natural plant alkaloid berberine can be found in plants, such as Coptis chinensis Franch. and Phellodendron chinense Schneid. Short-term berberine exposure in mice reshapes gut microbiota by reducing Clostridium clusters XIVa and IV, and shows beneficial effects on NASH mice[38]. In db/db mice, administration of dendrobium extract increases the Bacteroidetes to Firmicutes ratio and the relative abundance of Prevotella and Akkermansia, and reduces the relative abundance of S24-7, Rikenella, and Escherichia coli., thus alleviating hepatic steatosis in mice[39]. Certain natural products are found to improve NAFLD/NASH through modulation of adipokines. Dihydromyricetin is the main ingredient of the edible medicinal plant Ampelopsis grossedentata. In a double-blind clinical trial, dihydromyricetin treatment reduces resistin levels and improves insulin intolerance in patients with NAFLD[40]. Korean Red Ginseng is found to increase adiponectin and reduce pro-inflammatory TNF-α levels in patients with NAFLD[41]. Total alkaloids of Rubus alceaefolius Poir have beneficial effects on NAFLD by reducing serum leptin and resistin and increasing adiponectin levels in HFD-induced rats[42]. Additionally, the edible plants Opuntia ficus indica (nopal), umbelliferone, and piperine have been reported to improve insulin resistance and oxidative stress by upregulating serum adiponectin and downregulating leptin levels in obese animals[43-45].
Natural products regulating liver macrophage recruitment
In NASH, classical LY6Chigh (mice) and CD14+ (human) monocytes are recruited to the inflamed area in the liver through chemokines[46]. CCL2 is present at a very low level in the physiological state but is significantly increased in NASH. The interaction of CCL2 with its receptor C-C motif receptor 2 (CCR2) is required for monocyte migration to the liver, and knockout of CCL2 or CCR2 significantly reduces macrophage accumulation and mitigates NASH severity in mice[21]. Therefore, inhibiting macrophage recruitment to the liver is considered an effective strategy for NASH treatment. Chemokine antagonists have been found in natural products, suggesting that natural products play a positive role in this process[47]. Flavonoids derived from modified apple reduce the transcription of CCR2, chemokine ligand 10, and CCR10 in mice[48]. Dietary broccoli can reverse dextran sulfate sodium-evoked CCR2 upregulation in mice[49]. Berberine reduces CCL2 levels and inhibits macrophage recruitment in HFD-fed rats[50]. In high refined carbohydrate-containing diet-fed BALB/c mice, supplementation with crude extract of Rudgea viburnoides (Cham.) benth. (Rubiaceae) leaves lowers hepatic CCL2, reduces macrophage recruitment, and improves the inflammatory response in NASH animals[51]. In HFD-induced NASH mice and ApoE-/- mice, administration of Long ya Aralia chinensis L-derived total saponins of Aralia elata (Miq) Seem for 12 wk decreases CCL2, blocks the inosital-requiring enzyme-1α (IRE1α)-mediated c-Jun N-terminal kinase pathway and significantly improves hepatic steatosis[52].
Natural products regulating macrophage polarization
Polarization of macrophages is determined by the local environment[53]. The inflammatory microenvironment with LPS and IFN-γ induces macrophage polarization to the pro-inflammatory M1-type, characterized by increased pro-inflammatory cytokines, chemokines, and reactive nitrogen and oxygen intermediates[54]. IL-4, IL-10, and IL-13 induce polarization towards the anti-inflammatory M2-type (e.g., M2a, M2b, and M2c) characterized by increased scavenger receptors and enhanced phagocytosis activity[11,55]. In addition, PPAR-γ regulates M2-type polarization, and low levels of IFN-γ or high levels of CSF-1 induce recruited monocytes to differentiate into M2-type macrophages[56,57]. Certain stimuli may switch macrophages from M1-type to M2-type, or vice versa[53,58-60]. Failure to appropriately control this switch may cause progression of the disease[61]. In NASH, rapid and abundant pro-inflammatory macrophages are required and of benefit in the early stage; however, the constant existence of pro-inflammatory macrophages results in aggravated inflammation and fibrogenesis[62,63]. A series of natural products have been proven to regulate macrophage polarization and thus alleviate NASH and related complications. Celastrol is found to attenuate lipid accumulation and improve insulin sensitivity in NAFLD mice and regulate macrophage polarization through mitogen-activated protein kinase-NF-κB pathways in mice[64]. Smiglaside A is a phenylpropanoid glycoside isolated from Smilax riparia, and it has been found to upregulate M2-type and downregulate M1-type macrophage biomarkers in LPS-stimulated RAW264.7 cells and mouse peritoneal macrophages[65]. Asperlin isolated from marine Aspergillus versicolor LZD4403 fungus significantly reduces the expression of pro-inflammatory mediators such as inducible nitric oxide synthase, IL-1β, and TNF-α, and increases expression of IL-4 and IL-10 in LPS-stimulated RAW264.7 cells[66]. The pentacyclic triterpene lupeol regulates macrophage polarization by reducing pro-inflammatory and increasing anti-inflammatory cytokines in intestinal epithelial cells[67]. Baicalin upregulates IL-10, arginase 1, and IFN regulatory factor 4 (IRF4), downregulates TNF-α, IFN regulatory factor 5, IL-6, and IL-23, and enhances the phagocytosis and efferocytosis of macrophages, thus promoting macrophage polarization to the M2-type in mice with inflammatory bowel disease[68,69]. The Salvia miltiorrhiza ingredient tanshinone IIA and Tabebuia avellanedae Lorentz ex Griseb extract were found to promote M2-type macrophage polarization in colitis mice[70,71]. Emodin can be found in Chinese herbs such as Rheum palmatum and Polygonum multiflorum; it bidirectionally modulates the polarization of primary mouse macrophages, inhibits pro-inflammatory genes when challenged with LPS/IFN-γ, but increases pro-inflammatory genes under IL-4 stimulation in macrophages[10]. Inactivation of the Notch signaling pathway contributes to M2-type polarization[72]. Natural products such as Trichosanthes kirilowii lectin and oridonin are reported to deactivate Notch signaling, induce M2-type macrophage polarization, and inhibit the inflammatory response in rodents[73,74].
NATURAL PRODUCTS THAT MODULATE METABOLIC STATUS OF MACROPHAGES
The liver is an important metabolic organ and provides a favorable environment for macrophages[19,75,76]. As immune cells have high plastic functions, macrophages autonomously change their self-metabolism state to adapt to the micro-environment[77-79]. Alterations in the metabolic state influence the energy supply as well as the function and phenotype of macrophages[80]. Metabolic pathways in macrophages include amino acid metabolism, glycolysis, mitochondrial oxidative phosphorylation (OXPHOS), pentose phosphate pathway, fatty acid synthesis, and fatty acid oxidation[81]. Activated macrophages are characterized by abnormal amino acid metabolism, upregulated glycolytic metabolism, and damaged OXPHOS[80,82-84]. M1-type macrophages display activated pentose phosphate pathway and a broken tricarboxylic acid (TCA) cycle[85]. M2-type macrophages show enhanced OXPHOS and normal TCA cycle function[86-88]. The damaged TCA cycle promotes the accumulation of succinate and citrate, followed by the generation of IL-1β, and thus contributes to the M1-type macrophage response[89].
Macrophages acquire energy to support their functions; M2-type macrophages obtain energy mainly from OXPHOS, whereas M1-type macrophages obtain energy through glycolysis. Glycolysis is inefficient at ATP generation, so the process is enhanced, and substrate production is accelerated to guarantee the functions of M1 macrophages in the inflammatory state[90]. Accumulated substrates act as stimulants that strengthen the macrophage response and activate other signaling pathways. Pyruvate is one of the end products of glycolysis, and an increase in pyruvate dehydrogenase kinase-2 (PDK2) and pyruvate dehydrogenase phosphorylation decreases pyruvate/acetyl-CoA conversion, reactive oxygen species secretion, and IL-1β production[91-93]. Several natural products are reported to affect the metabolic status of macrophages in NASH treatment. Ampelopsis brevipedunculata (Vitaceae) berries are a medicinal plant for treating liver disease, and its ethanol extract decreases pyruvate, superoxide dismutase, and dimethyl sulfoxide levels in ferrous iron-stimulated liver injury rats[94]. Aim Scutellariae Radix and Coptidis Rhizoma are found to upregulate pyruvate kinase activity in the liver, and thus improve the dysfunctional lipid metabolism in diabetic rats[95]. Hyacinth bean (Dolichos lablab L) ameliorates pyruvate-derived amino acid metabolism and prevents obesity in HFD-fed mice[96]. PDK1 is associated with the M1-type response and aerobic glycolysis, and inhibition of PDK1 promotes M2-type polarization[97]. It has been reported that methanol extracts of Mycetia cauliflora Reinw. (Rubiaceae) and Dipterocarpus tuberculatus Roxb. (Dipterocarpaceae) target PDK1 and suppress the NF-κB signaling pathway in LPS-stimulated RAW264.7 cells[98,99]. Pyruvate kinase M2 inhibits LPS-induced M1-type polarization while evoking M2-type polarization by inhibiting IL-1β and increasing IL-10 generation. Natural products that regulate pyruvate kinase M2 may also benefit NASH therapy, and further studies are needed to explore such agents[100].
SUMMARY AND PERSPECTIVES
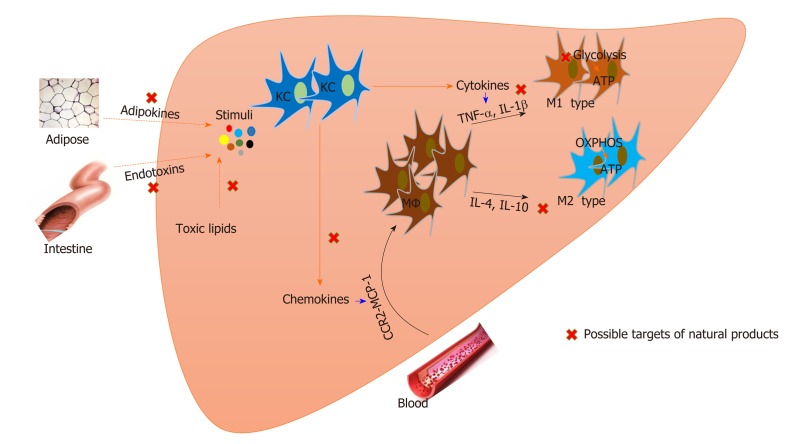
Macrophages play a pivotal role in NASH development. Macrophages in the liver integrally regulate immune and metabolic responses and have become attractive targets for NASH treatment. Natural products are important candidates for NASH and are involved in regulating macrophage activation, recruitment, and polarization. Inversely, metabolic status affects the function of macrophages, and enzymes that modulate metabolic processes can also be regulated by natural products (Figure 1, Table 1).
Figure 1.
Natural products that target macrophages for nonalcoholic steatohepatitis treatment. Both resident Kupffer cells and recruited macrophages are involved in the pathogenesis of nonalcoholic steatohepatitis. Modulation of macrophage activation, polarization, and recruitment by natural products contributes to nonalcoholic steatohepatitis improvement. Metabolic status affects the function of macrophages, and natural products also regulate macrophage metabolism. KC: Kupffer cell; MΦ: Macrophage; OXPHOS: Mitochondrial oxidative phosphorylation; IL: Interleukin; TNF: Tumor necrosis factor; CCR2: C-C motif receptor 2; MCP: Monocyte chemotactic protein.
Table 1.
Natural products that target macrophage for nonalcoholic steatohepatitis therapy
| Section | Drugs | Model | Functions | Ref. |
| Macrophage activation | Sparstolonin B | HFD-fed mice | ↓CD68, MCP-1 | [23] |
| Curcuminoids | LPS-treated BALB/C mice | ↓Phagocytic activity of KCs | [24] | |
| S. miltiorrhiza extract | CCl4-induced liver injury rats | ↓p38, NF-κB signaling | [25] | |
| Extract of Graptopetalum paraguayense | Liver fibrosis rats, primary HSCs and KCs | ↓KC activation, nitric oxide, TNF-α, IL-6 | [26] | |
| Alisma orientale extract | PA-stimulated HepG2 cells, NAFLD mice | ↓ER stress, lipogenic gene expression | [30,31] | |
| Myriocin | Co-culture SPT with myriocin | ↓SPT activation | [32] | |
| Gallic acid | HFD-induced NAFLD mice | ↓TMA, DMA | [37] | |
| Berberine | NAFLD mice | ↓Clostridium cluster XIVa and IV; | [38] | |
| Dendrobium extract | db/db mice | ↑The bacteroidetes to firmicutes ratio, Prevotella, Akkermansia; ↓S24-7, Rikenella, Escherichia coli. | [39] | |
| Dihydromyricetin | Adult NAFLD patients | ↓Resistin, IR | [40] | |
| Korean Red Ginseng | NAFLD patients | ↑Adiponectin, ↓TNF-α | [41] | |
| Total alkaloids of Rubus alceaefolius Poir | HFD-fed NAFLD rats | ↑Adiponectin; ↓Leptin, resistin | [42] | |
| Opuntia ficus indica | Obese Zucker (fa/fa) rats | ↑Adiponectin; ↓leptin, IR | [43] | |
| Umbelliferone | HFD- and STZ-induced type 2 diabetic rats | ↑Adiponectin; ↓leptin, IR | [44] | |
| Piperine | HFD-induced obese rats | ↑Adiponectin; ↓leptin, IR | [45] | |
| Macrophage recruitment | Flavonoids | Mice | ↓CCR2, CXCL10, CCR10 | [48] |
| Broccoli | DSS-induced colitis mice | ↓CCR2 | [49] | |
| Berberine | HFD-fed rats | ↓CCL2 | [50] | |
| Rudgea viburnoides (Cham.) Benth. (Rubiaceae) leaves | HC-diet fed BALB/c mice | ↓CCL2 | [51] | |
| Total aralosides of Aralia elata (Miq) seem | HFD-induced NASH mice, ApoE-/- mice | ↓CCL2, JNK signaling pathway | [52] | |
| Macrophage polarization | Celastrol | RAW264.7 cells and diet-induced obese mice | ↓TNF-α, IL-6, IL-1β, iNOS, MAPK activation, NF-κB nuclear translocation; ↑Nrf2 and HO-1 | [64] |
| Smiglaside A | LPS-stimulated RAW264.7 cells, mouse peritoneal macrophages | ↑AMPK-PPARγ, M2-type macrophages; ↓M1-type macrophages | [65] | |
| Asperlin | LPS-stimulated RAW264.7 cells | ↓TNF-α, IL-1β, iNOS; ↑IL-4, IL-10 | [66] | |
| The pentacyclic triterpene lupeol | DSS-induced colitis mice | ↓TNF-α, IL-6, IL-1β, IL-12, p38, MAPK, CD86, IRF5; ↑IL-10, CD206 | [67] | |
| Baicalin | BMDMs, PMs, colitis mice | ↓TNF-α, IL-6, IL-23, IRF5; ↑IL-10, Arg-1, IRF4 | [68,69] | |
| Tanshinone IIA | HFD fed ApoE-/- mice | ↑M2-type macrophage, ↓miR-375 | [70] | |
| Tabebuia avellanedae Lorentz ex Griseb extract | Mesenteric lymph nodes of DSS-induced colitis mice | ↑M2-type macrophage | [71] | |
| Emodin | Primary mouse macrophages | ↓NF-κB/IRF5/STAT1 and IRF4/STAT6 signaling, H3K27 acetylation; ↑H3K27 trimethylation | [10] | |
| Trichosanthes kirilowii lectin | STZ-induced diabetic DN rats | ↓Notch signaling | [73] | |
| Oridonin | LPS-stimulated RAW264.7 cells | ↓Notch signaling | [74] | |
| Macrophage metabolism | Ampelopsis brevipedunculata (Vitaceae) berries | Ferrous iron-stimulated rat hepatocyte | ↓Pyruvate, superoxide dismutase, dimethyl sulfoxide | [94] |
| Aim Scutellariae Radix and Coptidis Rhizoma | HFD-induced diabetic rats | ↑Pyruvate kinase activities | [95] | |
| Hyacinth bean | HFD-fed mice | ↓Pyruvate-derived amino acids metabolism | [96] | |
| Mycetia cauliflora Reinw. | LPS-activated RAW264.7 cells | ↓PDK1, NF-κB signaling pathway | [98] | |
| Dipterocarpus tuberculatus Roxb. | LPS-activated RAW264.7 cells | ↓PDK1, NF-κB signaling pathway | [99] |
HFD: High fat diet; LPS: lipopolysaccharide; KCs: Kupffer cells; CCl4: Carbon tetrachloride; HSCs: Hepatic stellate cells; NAFLD: Non-alcoholic fatty liver disease; SPT: Serine palmitoyltransferase; DMA: Dimethylamine; TMA: Trimethylamine; CCR2: C-C motif receptor; CXCL10: Chemokine ligand 10; DSS: Dextran sulfate sodium; HC: High refined carbohydrate; JNK: c-Jun N-terminal kinase; MAPK: Mitogen-activated protein kinase; iNOS: Inducible nitric oxide synthase; IRF: Interferon regulatory factor; PDK: Pyruvate dehydrogenase kinase; IL: Interleukin; TNF: Tumor necrosis factor; CCR2: C-C motif receptor 2; MCP: Monocyte chemotactic protein; NF-κB: Nuclear factor-kappa B.
There are plenty of reports about natural products for treating liver-related diseases, and on the basis of the available experimental results, curcumin, berberine, flavonoids, sparstolonin B, baicalin, and emodin are among the most promising agents in NASH treatment. Actually, several natural products are already under clinical investigation. Curcumin is currently in phase II/III clinical trials, expecting to improve liver steatosis, fibrosis, and liver inflammatory mediators in NAFLD patients[101,102]. Administration of berberine plus lifestyle intervention has been proven to reduce body weight, hepatic fat content, and serum lipid profiles, improve insulin sensitivity, and increase brown adipose tissue mass in NAFLD patients[103,104].
Although the effects of natural products on NASH are confirmed, available studies lack consensus standards and specifications, leading to the evaluation system being in an immature state and the potential mechanisms remaining unclear. The variance of patient choice and adherence, dosing methods, as well as test cycle may cause inconclusive results, and large-scale, multicenter random control trials are needed. In addition, many natural products show low bioavailability, thus strategies in promoting drug utilization or improving dosage form (nanoparticle and biological vector) need to develop. Considering the complex pathology of NASH, natural products are quite feasible to solve the problems. However, more work should be done to connect and integrate the two complex systems.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: The authors declare no conflict of interests for this article.
Peer-review started: January 10, 2020
First decision: February 28, 2020
Article in press: April 24, 2020
P-Reviewer: Sutti S S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Zhang YL
Contributor Information
Chun-Lin Li, Institute of Digestive Diseases, Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai 200032, China.
Wen-Jun Zhou, Institute of Digestive Diseases, Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai 200032, China.
Guang Ji, Institute of Digestive Diseases, Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai 200032, China.
Li Zhang, Institute of Digestive Diseases, Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai 200032, China. zhangli.hl@163.com.
References
- 1.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 2.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 3.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–2273. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 4.Rinella ME, Sanyal AJ. Management of NAFLD: a stage-based approach. Nat Rev Gastroenterol Hepatol. 2016;13:196–205. doi: 10.1038/nrgastro.2016.3. [DOI] [PubMed] [Google Scholar]
- 5.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, Romero-Gomez M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149:367–78.e5; quiz e14-5. doi: 10.1053/j.gastro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Oh S, Shida T, Yamagishi K, Tanaka K, So R, Tsujimoto T, Shoda J. Moderate to vigorous physical activity volume is an important factor for managing nonalcoholic fatty liver disease: a retrospective study. Hepatology. 2015;61:1205–1215. doi: 10.1002/hep.27544. [DOI] [PubMed] [Google Scholar]
- 7.St George A, Bauman A, Johnston A, Farrell G, Chey T, George J. Independent effects of physical activity in patients with nonalcoholic fatty liver disease. Hepatology. 2009;50:68–76. doi: 10.1002/hep.22940. [DOI] [PubMed] [Google Scholar]
- 8.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ginhoux F, Schultze JL, Murray PJ, Ochando J, Biswas SK. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat Immunol. 2016;17:34–40. doi: 10.1038/ni.3324. [DOI] [PubMed] [Google Scholar]
- 10.Iwanowycz S, Wang J, Altomare D, Hui Y, Fan D. Emodin Bidirectionally Modulates Macrophage Polarization and Epigenetically Regulates Macrophage Memory. J Biol Chem. 2016;291:11491–11503. doi: 10.1074/jbc.M115.702092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Nobili V, Parola M, Alisi A, Marra F, Piemonte F, Mombello C, Sutti S, Povero D, Maina V, Novo E, Albano E. Oxidative stress parameters in paediatric non-alcoholic fatty liver disease. Int J Mol Med. 2010;26:471–476. doi: 10.3892/ijmm_00000487. [DOI] [PubMed] [Google Scholar]
- 13.Albano E, Mottaran E, Vidali M, Reale E, Saksena S, Occhino G, Burt AD, Day CP. Immune response towards lipid peroxidation products as a predictor of progression of non-alcoholic fatty liver disease to advanced fibrosis. Gut. 2005;54:987–993. doi: 10.1136/gut.2004.057968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baumgardner JN, Shankar K, Hennings L, Albano E, Badger TM, Ronis MJ. N-acetylcysteine attenuates progression of liver pathology in a rat model of nonalcoholic steatohepatitis. J Nutr. 2008;138:1872–1879. doi: 10.1093/jn/138.10.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krenkel O, Puengel T, Govaere O, Abdallah AT, Mossanen JC, Kohlhepp M, Liepelt A, Lefebvre E, Luedde T, Hellerbrand C, Weiskirchen R, Longerich T, Costa IG, Anstee QM, Trautwein C, Tacke F. Therapeutic inhibition of inflammatory monocyte recruitment reduces steatohepatitis and liver fibrosis. Hepatology. 2018;67:1270–1283. doi: 10.1002/hep.29544. [DOI] [PubMed] [Google Scholar]
- 17.Tosello-Trampont AC, Landes SG, Nguyen V, Novobrantseva TI, Hahn YS. Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-α production. J Biol Chem. 2012;287:40161–40172. doi: 10.1074/jbc.M112.417014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baeck C, Wehr A, Karlmark KR, Heymann F, Vucur M, Gassler N, Huss S, Klussmann S, Eulberg D, Luedde T, Trautwein C, Tacke F. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut. 2012;61:416–426. doi: 10.1136/gutjnl-2011-300304. [DOI] [PubMed] [Google Scholar]
- 21.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 22.Huang W, Metlakunta A, Dedousis N, Zhang P, Sipula I, Dube JJ, Scott DK, O'Doherty RM. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes. 2010;59:347–357. doi: 10.2337/db09-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dattaroy D, Seth RK, Das S, Alhasson F, Chandrashekaran V, Michelotti G, Fan D, Nagarkatti M, Nagarkatti P, Diehl AM, Chatterjee S. Sparstolonin B attenuates early liver inflammation in experimental NASH by modulating TLR4 trafficking in lipid rafts via NADPH oxidase activation. Am J Physiol Gastrointest Liver Physiol. 2016;310:G510–G525. doi: 10.1152/ajpgi.00259.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lukita-Atmadja W, Ito Y, Baker GL, McCuskey RS. Effect of curcuminoids as anti-inflammatory agents on the hepatic microvascular response to endotoxin. Shock. 2002;17:399–403. doi: 10.1097/00024382-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Yue S, Hu B, Wang Z, Yue Z, Wang F, Zhao Y, Yang Z, Shen M. Salvia miltiorrhiza compounds protect the liver from acute injury by regulation of p38 and NFκB signaling in Kupffer cells. Pharm Biol. 2014;52:1278–1285. doi: 10.3109/13880209.2014.889720. [DOI] [PubMed] [Google Scholar]
- 26.Su LJ, Chang CC, Yang CH, Hsieh SJ, Wu YC, Lai JM, Tseng TL, Huang CY, Hsu SL. Graptopetalum paraguayense ameliorates chemical-induced rat hepatic fibrosis in vivo and inactivates stellate cells and Kupffer cells in vitro. PLoS One. 2013;8:e53988. doi: 10.1371/journal.pone.0053988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marra F, Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol. 2018;68:280–295. doi: 10.1016/j.jhep.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 28.Ertunc ME, Hotamisligil GS. Lipid signaling and lipotoxicity in metaflammation: indications for metabolic disease pathogenesis and treatment. J Lipid Res. 2016;57:2099–2114. doi: 10.1194/jlr.R066514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jang MK, Han YR, Nam JS, Han CW, Kim BJ, Jeong HS, Ha KT, Jung MH. Protective Effects of Alisma orientale Extract against Hepatic Steatosis via Inhibition of Endoplasmic Reticulum Stress. Int J Mol Sci. 2015;16:26151–26165. doi: 10.3390/ijms161125944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han CW, Kang ES, Ham SA, Woo HJ, Lee JH, Seo HG. Antioxidative effects of Alisma orientale extract in palmitate-induced cellular injury. Pharm Biol. 2012;50:1281–1288. doi: 10.3109/13880209.2012.673629. [DOI] [PubMed] [Google Scholar]
- 32.Wadsworth JM, Clarke DJ, McMahon SA, Lowther JP, Beattie AE, Langridge-Smith PR, Broughton HB, Dunn TM, Naismith JH, Campopiano DJ. The chemical basis of serine palmitoyltransferase inhibition by myriocin. J Am Chem Soc. 2013;135:14276–14285. doi: 10.1021/ja4059876. [DOI] [PubMed] [Google Scholar]
- 33.Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G, Vecchio FM, Rapaccini G, Gasbarrini G, Day CP, Grieco A. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 34.Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, Gill SR. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 35.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 36.Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206–211. doi: 10.1136/gut.48.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chao J, Huo TI, Cheng HY, Tsai JC, Liao JW, Lee MS, Qin XM, Hsieh MT, Pao LH, Peng WH. Gallic acid ameliorated impaired glucose and lipid homeostasis in high fat diet-induced NAFLD mice. PLoS One. 2014;9:e96969. doi: 10.1371/journal.pone.0096969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian Y, Cai J, Gui W, Nichols RG, Koo I, Zhang J, Anitha M, Patterson AD. Berberine Directly Affects the Gut Microbiota to Promote Intestinal Farnesoid X Receptor Activation. Drug Metab Dispos. 2019;47:86–93. doi: 10.1124/dmd.118.083691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li XW, Chen HP, He YY, Chen WL, Chen JW, Gao L, Hu HY, Wang J. Effects of Rich-Polyphenols Extract of Dendrobium loddigesii on Anti-Diabetic, Anti-Inflammatory, Anti-Oxidant, and Gut Microbiota Modulation in db/db Mice. Molecules. 2018:23. doi: 10.3390/molecules23123245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen S, Zhao X, Wan J, Ran L, Qin Y, Wang X, Gao Y, Shu F, Zhang Y, Liu P, Zhang Q, Zhu J, Mi M. Dihydromyricetin improves glucose and lipid metabolism and exerts anti-inflammatory effects in nonalcoholic fatty liver disease: A randomized controlled trial. Pharmacol Res. 2015;99:74–81. doi: 10.1016/j.phrs.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Hong M, Lee YH, Kim S, Suk KT, Bang CS, Yoon JH, Baik GH, Kim DJ, Kim MJ. Anti-inflammatory and antifatigue effect of Korean Red Ginseng in patients with nonalcoholic fatty liver disease. J Ginseng Res. 2016;40:203–210. doi: 10.1016/j.jgr.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng H, Zhao J, Zheng Y, Wu J, Liu Y, Peng J, Hong Z. Protective effects and mechanisms of total alkaloids of Rubus alceaefolius Poir on non‑alcoholic fatty liver disease in rats. Mol Med Rep. 2014;10:1758–1764. doi: 10.3892/mmr.2014.2403. [DOI] [PubMed] [Google Scholar]
- 43.Morán-Ramos S, Avila-Nava A, Tovar AR, Pedraza-Chaverri J, López-Romero P, Torres N. Opuntia ficus indica (nopal) attenuates hepatic steatosis and oxidative stress in obese Zucker (fa/fa) rats. J Nutr. 2012;142:1956–1963. doi: 10.3945/jn.112.165563. [DOI] [PubMed] [Google Scholar]
- 44.Naowaboot J, Somparn N, Saentaweesuk S, Pannangpetch P. Umbelliferone Improves an Impaired Glucose and Lipid Metabolism in High-Fat Diet/Streptozotocin-Induced Type 2 Diabetic Rats. Phytother Res. 2015;29:1388–1395. doi: 10.1002/ptr.5392. [DOI] [PubMed] [Google Scholar]
- 45.BrahmaNaidu P, Nemani H, Meriga B, Mehar SK, Potana S, Ramgopalrao S. Mitigating efficacy of piperine in the physiological derangements of high fat diet induced obesity in Sprague Dawley rats. Chem Biol Interact. 2014;221:42–51. doi: 10.1016/j.cbi.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 46.Ziegler-Heitbrock L, Hofer TP. Toward a refined definition of monocyte subsets. Front Immunol. 2013;4:23. doi: 10.3389/fimmu.2013.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yao W, Ba Q, Li X, Li H, Zhang S, Yuan Y, Wang F, Duan X, Li J, Zhang W, Wang H. A Natural CCR2 Antagonist Relieves Tumor-associated Macrophage-mediated Immunosuppression to Produce a Therapeutic Effect for Liver Cancer. EBioMedicine. 2017;22:58–67. doi: 10.1016/j.ebiom.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Espley RV, Butts CA, Laing WA, Martell S, Smith H, McGhie TK, Zhang J, Paturi G, Hedderley D, Bovy A, Schouten HJ, Putterill J, Allan AC, Hellens RP. Dietary flavonoids from modified apple reduce inflammation markers and modulate gut microbiota in mice. J Nutr. 2014;144:146–154. doi: 10.3945/jn.113.182659. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Jeffery EH, Miller MJ, Wallig MA, Wu Y. Lightly Cooked Broccoli Is as Effective as Raw Broccoli in Mitigating Dextran Sulfate Sodium-Induced Colitis in Mice. Nutrients. 2018:10. doi: 10.3390/nu10060748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang X, Zhao Y, Zhang M, Pang X, Xu J, Kang C, Li M, Zhang C, Zhang Z, Zhang Y, Li X, Ning G, Zhao L. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PLoS One. 2012;7:e42529. doi: 10.1371/journal.pone.0042529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Almeida JMA, Ferreira AVM, Oliveira VB, Oliveira MC, Teixeira MM, Brandão MGL. Effects of Rudgea viburnoides (Cham.) Benth. (Rubiaceae) Leaves on Metabolic and Inflammatory Dysfunction Induced by High Refined Carbohydrate-Containing Diet in Mice. J Med Food. 2018;21:1266–1275. doi: 10.1089/jmf.2018.0016. [DOI] [PubMed] [Google Scholar]
- 52.Luo Y, Dong X, Yu Y, Sun G, Sun X. Total aralosides of aralia elata (Miq) seem (TASAES) ameliorate nonalcoholic steatohepatitis by modulating IRE1α-mediated JNK and NF-κB pathways in ApoE-/- mice. J Ethnopharmacol. 2015;163:241–250. doi: 10.1016/j.jep.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 53.Murray PJ. Macrophage Polarization. Annu Rev Physiol. 2017;79:541–566. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 54.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soudja SM, Ruiz AL, Marie JC, Lauvau G. Inflammatory monocytes activate memory CD8(+) T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion. Immunity. 2012;37:549–562. doi: 10.1016/j.immuni.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van den Bossche J, Baardman J, Otto NA, van der Velden S, Neele AE, van den Berg SM, Luque-Martin R, Chen HJ, Boshuizen MC, Ahmed M, Hoeksema MA, de Vos AF, de Winther MP. Mitochondrial Dysfunction Prevents Repolarization of Inflammatory Macrophages. Cell Rep. 2016;17:684–696. doi: 10.1016/j.celrep.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 59.Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005;65:3437–3446. doi: 10.1158/0008-5472.CAN-04-4262. [DOI] [PubMed] [Google Scholar]
- 60.Saccani A, Schioppa T, Porta C, Biswas SK, Nebuloni M, Vago L, Bottazzi B, Colombo MP, Mantovani A, Sica A. p50 nuclear factor-kappaB overexpression in tumor-associated macrophages inhibits M1 inflammatory responses and antitumor resistance. Cancer Res. 2006;66:11432–11440. doi: 10.1158/0008-5472.CAN-06-1867. [DOI] [PubMed] [Google Scholar]
- 61.Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, Hainzl A, Schatz S, Qi Y, Schlecht A, Weiss JM, Wlaschek M, Sunderkötter C, Scharffetter-Kochanek K. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121:985–997. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol. 2014;60:1090–1096. doi: 10.1016/j.jhep.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 63.Karlmark KR, Weiskirchen R, Zimmermann HW, Gassler N, Ginhoux F, Weber C, Merad M, Luedde T, Trautwein C, Tacke F. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50:261–274. doi: 10.1002/hep.22950. [DOI] [PubMed] [Google Scholar]
- 64.Luo D, Guo Y, Cheng Y, Zhao J, Wang Y, Rong J. Natural product celastrol suppressed macrophage M1 polarization against inflammation in diet-induced obese mice via regulating Nrf2/HO-1, MAP kinase and NF-κB pathways. Aging (Albany NY) 2017;9:2069–2082. doi: 10.18632/aging.101302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y, Xu Y, Zhang P, Ruan W, Zhang L, Yuan S, Pang T, Jia AQ. Smiglaside A ameliorates LPS-induced acute lung injury by modulating macrophage polarization via AMPK-PPARγ pathway. Biochem Pharmacol. 2018;156:385–395. doi: 10.1016/j.bcp.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 66.Zhou Y, Chen R, Liu D, Wu C, Guo P, Lin W. Asperlin Inhibits LPS-Evoked Foam Cell Formation and Prevents Atherosclerosis in ApoE-/- Mice. Mar Drugs. 2017:15. doi: 10.3390/md15110358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu Y, Li X, Chen J, Chen T, Shi Z, Lei M, Zhang Y, Bai P, Li Y, Fei X. The pentacyclic triterpene Lupeol switches M1 macrophages to M2 and ameliorates experimental inflammatory bowel disease. Int Immunopharmacol. 2016;30:74–84. doi: 10.1016/j.intimp.2015.11.031. [DOI] [PubMed] [Google Scholar]
- 68.Lai YS, Putra RBDS, Aui SP, Chang KT. M2C Polarization by Baicalin Enhances Efferocytosis via Upregulation of MERTK Receptor. Am J Chin Med. 2018;46:1899–1914. doi: 10.1142/S0192415X18500957. [DOI] [PubMed] [Google Scholar]
- 69.Zhu W, Jin Z, Yu J, Liang J, Yang Q, Li F, Shi X, Zhu X, Zhang X. Baicalin ameliorates experimental inflammatory bowel disease through polarization of macrophages to an M2 phenotype. Int Immunopharmacol. 2016;35:119–126. doi: 10.1016/j.intimp.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 70.Chen W, Li X, Guo S, Song N, Wang J, Jia L, Zhu A. Tanshinone IIA harmonizes the crosstalk of autophagy and polarization in macrophages via miR-375/KLF4 pathway to attenuate atherosclerosis. Int Immunopharmacol. 2019;70:486–497. doi: 10.1016/j.intimp.2019.02.054. [DOI] [PubMed] [Google Scholar]
- 71.Park HJ, Lee SW, Kwon DJ, Heo SI, Park SH, Kim SY, Hong S. Oral administration of taheebo (Tabebuia avellanedae Lorentz ex Griseb.) water extract prevents DSS-induced colitis in mice by up-regulating type II T helper immune responses. BMC Complement Altern Med. 2017;17:448. doi: 10.1186/s12906-017-1952-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang YC, He F, Feng F, Liu XW, Dong GY, Qin HY, Hu XB, Zheng MH, Liang L, Feng L, Liang YM, Han H. Notch signaling determines the M1 versus M2 polarization of macrophages in antitumor immune responses. Cancer Res. 2010;70:4840–4849. doi: 10.1158/0008-5472.CAN-10-0269. [DOI] [PubMed] [Google Scholar]
- 73.Jiandong L, Yang Y, Peng J, Xiang M, Wang D, Xiong G, Li S. Trichosanthes kirilowii lectin ameliorates streptozocin-induced kidney injury via modulation of the balance between M1/M2 phenotype macrophage. Biomed Pharmacother. 2019;109:93–102. doi: 10.1016/j.biopha.2018.10.060. [DOI] [PubMed] [Google Scholar]
- 74.Xu L, Li L, Zhang CY, Schluesener H, Zhang ZY. Natural Diterpenoid Oridonin Ameliorates Experimental Autoimmune Neuritis by Promoting Anti-inflammatory Macrophages Through Blocking Notch Pathway. Front Neurosci. 2019;13:272. doi: 10.3389/fnins.2019.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11:738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 2013;339:172–177. doi: 10.1126/science.1230721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Loftus RM, Finlay DK. Immunometabolism: Cellular Metabolism Turns Immune Regulator. J Biol Chem. 2016;291:1–10. doi: 10.1074/jbc.R115.693903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lampropoulou V, Sergushichev A, Bambouskova M, Nair S, Vincent EE, Loginicheva E, Cervantes-Barragan L, Ma X, Huang SC, Griss T, Weinheimer CJ, Khader S, Randolph GJ, Pearce EJ, Jones RG, Diwan A, Diamond MS, Artyomov MN. Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation. Cell Metab. 2016;24:158–166. doi: 10.1016/j.cmet.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.O'Neill LA, Pearce EJ. Immunometabolism governs dendritic cell and macrophage function. J Exp Med. 2016;213:15–23. doi: 10.1084/jem.20151570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Munder M, Eichmann K, Modolell M. Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. J Immunol. 1998;160:5347–5354. [PubMed] [Google Scholar]
- 81.Van den Bossche J, O'Neill LA, Menon D. Macrophage Immunometabolism: Where Are We (Going)? Trends Immunol. 2017;38:395–406. doi: 10.1016/j.it.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 82.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.O'Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16:553–565. doi: 10.1038/nri.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pearce EL, Poffenberger MC, Chang CH, Jones RG. Fueling immunity: insights into metabolism and lymphocyte function. Science. 2013;342:1242454. [Google Scholar]
- 85.Haschemi A, Kosma P, Gille L, Evans CR, Burant CF, Starkl P, Knapp B, Haas R, Schmid JA, Jandl C, Amir S, Lubec G, Park J, Esterbauer H, Bilban M, Brizuela L, Pospisilik JA, Otterbein LE, Wagner O. The sedoheptulose kinase CARKL directs macrophage polarization through control of glucose metabolism. Cell Metab. 2012;15:813–826. doi: 10.1016/j.cmet.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Van den Bossche J, Baardman J, de Winther MP. Metabolic Characterization of Polarized M1 and M2 Bone Marrow-derived Macrophages Using Real-time Extracellular Flux Analysis. J Vis Exp. 2015 doi: 10.3791/53424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jha AK, Huang SC, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, Chmielewski K, Stewart KM, Ashall J, Everts B, Pearce EJ, Driggers EM, Artyomov MN. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42:419–430. doi: 10.1016/j.immuni.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 88.Everts B, Amiel E, Huang SC, Smith AM, Chang CH, Lam WY, Redmann V, Freitas TC, Blagih J, van der Windt GJ, Artyomov MN, Jones RG, Pearce EL, Pearce EJ. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKɛ supports the anabolic demands of dendritic cell activation. Nat Immunol. 2014;15:323–332. doi: 10.1038/ni.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, Zheng L, Gardet A, Tong Z, Jany SS, Corr SC, Haneklaus M, Caffrey BE, Pierce K, Walmsley S, Beasley FC, Cummins E, Nizet V, Whyte M, Taylor CT, Lin H, Masters SL, Gottlieb E, Kelly VP, Clish C, Auron PE, Xavier RJ, O'Neill LA. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Feingold KR, Shigenaga JK, Kazemi MR, McDonald CM, Patzek SM, Cross AS, Moser A, Grunfeld C. Mechanisms of triglyceride accumulation in activated macrophages. J Leukoc Biol. 2012;92:829–839. doi: 10.1189/jlb.1111537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Koo SJ, Garg NJ. Metabolic programming of macrophage functions and pathogens control. Redox Biol. 2019;24:101198. doi: 10.1016/j.redox.2019.101198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang F, Wang K, Xu W, Zhao S, Ye D, Wang Y, Xu Y, Zhou L, Chu Y, Zhang C, Qin X, Yang P, Yu H. SIRT5 Desuccinylates and Activates Pyruvate Kinase M2 to Block Macrophage IL-1β Production and to Prevent DSS-Induced Colitis in Mice. Cell Rep. 2017;19:2331–2344. doi: 10.1016/j.celrep.2017.05.065. [DOI] [PubMed] [Google Scholar]
- 93.Lin CH, Wu JB, Jian JY, Shih CC. (-)-Epicatechin-3-O-β-D-allopyranoside from Davallia formosana prevents diabetes and dyslipidemia in streptozotocin-induced diabetic mice. PLoS One. 2017;12:e0173984. doi: 10.1371/journal.pone.0173984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yabe N, Tanaka K, Matsui H. An ethanol-extract of Ampelopsis brevipedunculata (Vitaceae) berries decreases ferrous iron-stimulated hepatocyte injury in culture. J Ethnopharmacol. 1998;59:147–159. doi: 10.1016/s0378-8741(97)00121-9. [DOI] [PubMed] [Google Scholar]
- 95.Cui X, Qian DW, Jiang S, Shang EX, Zhu ZH, Duan JA. Scutellariae Radix and Coptidis Rhizoma Improve Glucose and Lipid Metabolism in T2DM Rats via Regulation of the Metabolic Profiling and MAPK/PI3K/Akt Signaling Pathway. Int J Mol Sci. 2018:19. doi: 10.3390/ijms19113634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Suh DH, Lee HW, Jung ES, Singh D, Kim SH, Lee CH. In vivo metabolomic interpretation of the anti-obesity effects of hyacinth bean (Dolichos lablab L.) administration in high-fat diet mice. Mol Nutr Food Res. 2017:61. doi: 10.1002/mnfr.201600895. [DOI] [PubMed] [Google Scholar]
- 97.Tan Z, Xie N, Cui H, Moellering DR, Abraham E, Thannickal VJ, Liu G. Pyruvate dehydrogenase kinase 1 participates in macrophage polarization via regulating glucose metabolism. J Immunol. 2015;194:6082–6089. doi: 10.4049/jimmunol.1402469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jeong SG, Kim S, Kim HG, Kim E, Jeong D, Kim JH, Yang WS, Oh J, Sung GH, Hossain MA, Lee J, Kim JH, Cho JY. Mycetia cauliflora methanol extract exerts anti-inflammatory activity by directly targeting PDK1 in the NF-κB pathway. J Ethnopharmacol. 2019;231:1–9. doi: 10.1016/j.jep.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 99.Yang WS, Lee BH, Kim SH, Kim HG, Yi YS, Htwe KM, Kim YD, Yoon KD, Hong S, Lee WS, Cho JY. Dipterocarpus tuberculatus ethanol extract strongly suppresses in vitro macrophage-mediated inflammatory responses and in vivo acute gastritis. J Ethnopharmacol. 2013;146:873–880. doi: 10.1016/j.jep.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 100.Palsson-McDermott EM, Curtis AM, Goel G, Lauterbach MA, Sheedy FJ, Gleeson LE, van den Bosch MW, Quinn SR, Domingo-Fernandez R, Johnston DG, Jiang JK, Israelsen WJ, Keane J, Thomas C, Clish C, Vander Heiden M, Xavier RJ, O'Neill LA. Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab. 2015;21:65–80. doi: 10.1016/j.cmet.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Saadati S, Sadeghi A, Mansour A, Yari Z, Poustchi H, Hedayati M, Hatami B, Hekmatdoost A. Curcumin and inflammation in non-alcoholic fatty liver disease: a randomized, placebo controlled clinical trial. BMC Gastroenterol. 2019;19:133. doi: 10.1186/s12876-019-1055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Panahi Y, Kianpour P, Mohtashami R, Jafari R, Simental-Mendía LE, Sahebkar A. Efficacy and Safety of Phytosomal Curcumin in Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Trial. Drug Res (Stuttg) 2017;67:244–251. doi: 10.1055/s-0043-100019. [DOI] [PubMed] [Google Scholar]
- 103.Wu L, Xia M, Duan Y, Zhang L, Jiang H, Hu X, Yan H, Zhang Y, Gu Y, Shi H, Li J, Gao X, Li J. Berberine promotes the recruitment and activation of brown adipose tissue in mice and humans. Cell Death Dis. 2019;10:468. doi: 10.1038/s41419-019-1706-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yan HM, Xia MF, Wang Y, Chang XX, Yao XZ, Rao SX, Zeng MS, Tu YF, Feng R, Jia WP, Liu J, Deng W, Jiang JD, Gao X. Efficacy of Berberine in Patients with Non-Alcoholic Fatty Liver Disease. PLoS One. 2015;10:e0134172. doi: 10.1371/journal.pone.0134172. [DOI] [PMC free article] [PubMed] [Google Scholar]