Abstract
Purpose:
To document the mammographic breast density (MBD) distribution of Jordanian women and the relationship with MBD with age. Correlation between breast cancer diagnosis and density was also explored.
Methods:
A retrospective review of 660 screening mammograms from King Abdullah University Hospital was conducted. Mammograms were classified into 2 groups: normal (return to routine screening) and breast cancer and rated using the American College of Radiology (ACR) Breast Imaging-Reporting and Data System (BI-RADS) 5th edition for MBD. The association between MBD and age was assessed by descriptive analyses and Kruskal-Wallis test. To compare between normal and breast cancer groups, chi-square post hoc tests with Bonferroni adjustment was used.
Results:
Groups consisted of 73.9% (n = 488) normal group and 26.1% (n = 172) breast cancer group. A significant inverse relationship was demonstrated between age and MBD among the normal (r = −.319, P < .01) and breast cancer group (r = −.569, P < .01). In total, 69% (n = 336) of women in the normal group and 71% (n = 122) in the breast cancer group and 79.1% (n = 159) of the normal group and 100% (n = 48) of the breast cancer group aged 40 to 49 years reported high MBD (ACR BI-RADS c or d).
Conclusions:
Most of women in both the normal and breast cancer groups evidenced increased MBD. Increased MBD was inversely proportional to age. As MBD has a known link to increased breast cancer risk and the decreased sensitivity of mammography and it is vital that future screening guidelines for Jordanian women consider the unique breast density distribution of this population.
Keywords: Breast cancer, mammographic breast density, mammography, breast cancer screening, population health
Introduction
Breast cancer is the most frequently diagnosed cancer in women worldwide, accounting for 25.4% of all newly diagnosed cases in 2018.1 In Jordan, breast cancer is the most common cancer and represents 36.4% of all cancers among women in 2018.2 The age-standardised incidence rate of breast cancer has increased from 50.4/100 000 in 2008 to 57.4/100 000 in 20182,3 and the age-standardised mortality rate was 18.5 per 100 000 females.2 In Jordan, the highest incidence of breast cancer is found in women aged 40 to 49 years (30%) and 50 to 59 years (25%).4 However, 15% of breast cancer was diagnosed in women aged less than 40 years.4 In 2012, the Jordan National Cancer Registry reported that 70% of breast cancers were diagnosed at late stages (III-IV), possibly due to breakdown in breast cancer screening implementation process.5
Mammography is an effective modality for the early detection of breast cancer.6 However, mammographic sensitivity and specificity decreases when imaging women with high mammographic breast density (MBD) due to the potential obscuring of breast lesions by areas of fibroglandular tissue.7 Mammographic breast density describes the proportion of fibroglandular and adipose tissue present in the breast.8 Mammographic breast density is strongly and reproducibly associated with a 4- to 6-fold increase in breast cancer risk.9 The use of adjunct modalities for women with high MBD such as ultrasound, magnetic resonance imaging (MRI), and digital breast tomosynthesis (DBT) has been shown to improve cancer detection rates by 4.2,10 5,11 and 4.212 per 1000 women, respectively.
Different classification systems have been developed to standardise MBD assessment and mammographic reporting including those by Wolfe,13 Boyd et al,8 Gramme et al,14 and the American College of Radiology Breast Imaging-Reporting and Data System (ACR BI-RADS).15 The BI-RADS 5th edition describes MBD as a reflection of breast composition as follows: (1) the breasts are almost entirely fatty; (2) there are scattered areas of fibroglandular density; (3) the breasts are heterogeneously dense, which may obscure small masses; and (4) the breasts are extremely dense, which lowers the sensitivity of mammography. In several states in the United States, it is mandatory to incorporate MBD notification in mammographic reports using the ACR BI-RADS system to alert patients’ and their physicians of mammographic sensitivity and the risk of developing breast cancer.16
Breast density varies in association with age, ethnicity, and geographical location.17,18 Therefore, knowledge of an MBD profile of a particular population is important for planning population-based screening programmes. The Jordan Breast Cancer Program (JBCP) first initiated a screening service for the early detection of breast cancer in 2007. The JBCP initially adapted the US National Cancer Comprehensive Network (NCCN) guidelines for annual mammography screening of women aged 40 years and older.19 However, although typical Western screening programmes are effective according to the data derived from their populations, these guidelines were not deemed to be suitable for Jordanian women. The JBCP edited the NCCN guidelines to allow women aged 20 years and older with a high risk of breast cancer to begin annual mammography screening 5 to 10 years prior to the age of the youngest family member diagnosed with breast cancer.19 This change acknowledges that 15% of breast cancer cases are in Jordanian women aged less than 40 years4 compared with 4% of cancer cases in the same age group in Western countries.20,21 In addition, the adapted NCCN guidelines do not take into consideration Jordanian woman’s MBD profile, which has not previously been reported. This study documents for the first time the MBD distribution of Jordanian women and the relationship of breast density and age. Relationships between breast cancer diagnosis and density will also be explored. Results of this study may inform age-specific imaging protocols based on MBD to enhance the early detection of breast cancer in Jordanian women.
Materials and Methods
Ethics approval was obtained from the Jordan University of Science and Technology (Project Number 20170023). A retrospective review of all mammograms from King Abdullah University Hospital imaged between January 2016, when picture archiving and communication system (PACS) was introduced, and August 2018 was performed. These dates were chosen to maximise sample size as women typically undergo screening every 2 years and to ensure all images were produced by the same technology. A total of 660 mammograms were included. Bilateral standard 2-view mammographic images (cranio-caudal and medio-lateral oblique projections) were obtained using a Mammorex Peruru MGU-1000A digital mammography unit (Toshiba Medical Systems Corporation, Tokyo, Japan). Exclusion criteria were based on incomplete examination of both breasts, bilateral breast cancer, and breast implants. Women were categorised into normal or breast cancer groups. The normal group included women who were returned to routine screening. Breast cancer cases were assigned based on histopathology reports.
Mammographic breast density was classified according to ACR BI-RADS version 5.15 Mammographic breast density was assessed by 3 radiologists with more than 10 years of experience in breast imaging. The majority classification of MBD (2 of 3 readers) was used to manage discordance. In the breast cancer group, the cancer-free side was used to assess MBD so as to assess normal MBD.
The quadratic weighted Cohen kappa statistics and 95% confidence intervals (CIs) were used to calculate the interobserver agreement between pairs of radiologists for MBD classification, and then the average kappa for all 3 readers was computed. The kappa value was interpreted as follows: slight agreement for a kappa value of 0.0 to 0.2, fair agreement for a kappa value of 0.21 to 0.41, moderate agreement for a kappa value of 0.41 to 0.60, substantial agreement for a kappa value of 0.61 to 0.80, and almost perfect agreement for a kappa value of 0.81 to 0.99.
Age was elicited from the data embedded in the images and was recorded in years. Women were categorised into 5 age groups:<40, 40 to 49, 50 to 59, 60 to 69, and ⩾70 and descriptive analyses were used to examine the association between age groups and MBD. Correlations between MBD and age as a continuous variable were analysed using Kruskal-Wallis and Spearman tests. To compare between normal and breast cancer groups, chi-square post hoc tests with Bonferroni adjustment were used. All analyses were performed using SPSS software (version 20.0; SPSS). Statistical significance was determined where P < .05.
Results
MBD classification inter-observer agreement
Table 1 shows almost perfect agreement between the 3 radiologists for MBD classification. The overall kappa value was calculated to be 0.93 (95% CI: 0.90-0.96).
Table 1.
Inter-observer agreement for MBD classification.
| Comparisons | Kappa value (95% confidence intervals) |
|---|---|
| Radiologists 1 and 2 | 0.94 (0.89-0.98) |
| Radiologists 1 and 3 | 0.91 (0.86-0.96) |
| Radiologists 2 and 3 | 0.93 (0.88-0.95) |
Abbreviation: MBD, mammographic breast density.
Age and MBD
A total of 73.9% (n = 488) mammograms were reported as normal with a median age of 49 years (25th percentile, 44th and 75th percentile, 54 years); 26.1% (n = 172) as having breast cancer with a median age of 51.5 years (25th percentile, 43rd and 75th percentile, 58 years) (see Table 2). Mammographic breast density distribution showed that, based on ACR BI-RADS system, 68.9% of women in the normal group and 70.9% of women in the cancer group reported high density – ACR BI-RADS c and d type density.
Table 2.
MBD distribution according to ACR BI-RADS system and age.
| MBD | No. (%) | Median age in years | 25th age percentile in years | 75th age percentile in years | Range age in years |
|---|---|---|---|---|---|
| Normal group | |||||
| ACR BI-RADS (a) | 47 (9.6) | 56.06 | 50.00 | 62.00 | (42-75) |
| ACR BI-RADS (b) | 105 (21.5) | 51.00 | 45.00 | 55.5 | (35-77) |
| ACR BI-RADS (c) | 282 (57.8) | 48.00 | 43.00 | 53.00 | (35-79) |
| ACR BI-RADS (d) | 54 (11.1) | 45.00 | 41.00 | 47.25 | (32-54) |
| Breast cancer group | |||||
| ACR BI-RADS (a) | 11 (6.4) | 69.00 | 58.00 | 79.00 | (54-86) |
| ACR BI-RADS (b) | 39 (22.7) | 58.00 | 53.00 | 65.00 | (50-77) |
| ACR BI-RADS (c) | 94 (54.7) | 49.00 | 42.75 | 53.00 | (32-85) |
| ACR BI-RADS (d) | 28 (16.3) | 42.00 | 39.00 | 48.75 | (34-72) |
Abbreviations: ACR BI-RADS, American College of Radiology Breast Imaging-Reporting and Data System; MBD, mammographic breast density.
Age ranged from 32 to 86 years with an average of 50 years. For both groups, the most frequent age was 50 to 59 years (36.5% and 35.5%, respectively) (see Table 3). All cancers found in women aged below 49 years were classified as high-density ACR BI-RADS c or d (see Table 3).
Table 3.
Group distribution of MBD and age (n = 660).
| Age group | No. (%) of women per ACR BI-RADS category |
Total | |||
|---|---|---|---|---|---|
| a | b | c | d | ||
| Normal group | |||||
| <40 | — | 9 (15.3%) | 39 (66.1%) | 11 (18.6%) | 59 (12.1%) |
| 40-49 | 11 (5.5%) | 31 (15.4%) | 126 (62.7%) | 33 (16.4%) | 201 (41.2%) |
| 50-59 | 22 (12.4%) | 49 (27.5%) | 97 (54.5%) | 10 (5.6%) | 178 (36.5%) |
| 60-69 | 10 (27.0%) | 12 (32.4%) | 15 (40.5%) | — | 37 (7.6%) |
| >70 | 4 (30.8%) | 4 (30.8%) | 5 (38.5%) | — | 13 (2.7%) |
| Total | 47 (9.6%) | 105 (21.5%) | 282 (57.8%) | 54 (11.1%) | 488 (100%) |
| Breast cancer group | |||||
| <40 | — | — | 15 (62.5%) | 9 (37.5%) | 24 (14%) |
| 40-49 | — | — | 34 (70.8%) | 14 (29.2%) | 48 (27.9%) |
| 50-59 | 3 (4.9%) | 22 (36.1%) | 33 (54.1%) | 3 (4.9%) | 61 (35.5%) |
| 60-69 | 3 (13.0%) | 12 (52.2%) | 7 (30.4%) | 1 (4.3%) | 23 (13.4%) |
| >70 | 5 (31.3%) | 5 (31.3%) | 5 (31.3%) | 1 (6.3%) | 16 (9.3%) |
| Total | 11 (6.4%) | 39 (22.7%) | 94 (54.7%) | 28 (16.3%) | 172 (100%) |
Abbreviations: ACR BI-RADS, American College of Radiology Breast Imaging-Reporting and Data System; MBD, mammographic breast density.
A significant inverse relationship was demonstrated between age and MBD overall among normal (r = −.319, P < .01) and breast cancer group (r = −.569, P < .01) (see Figure 1A and B).
Figure 1.
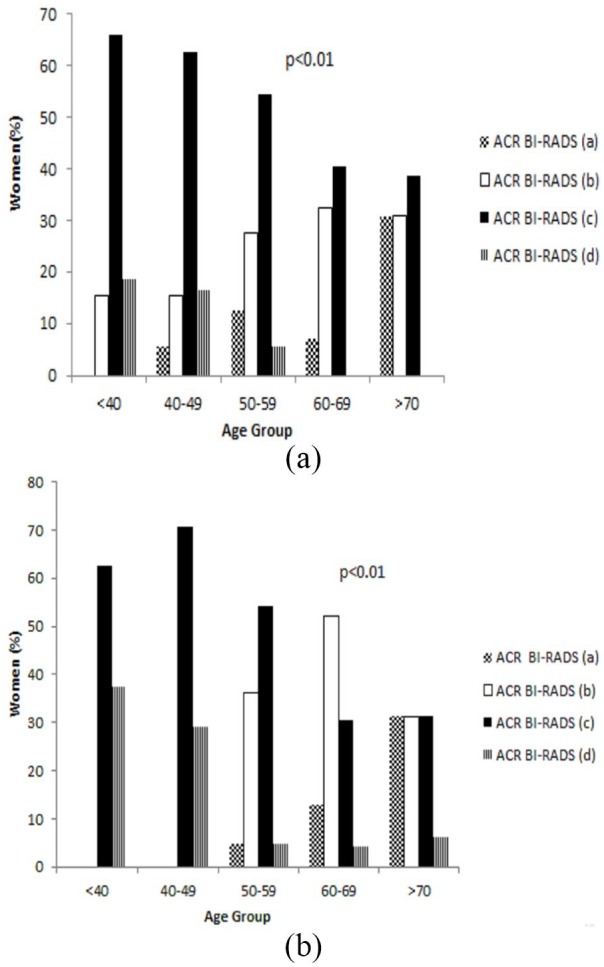
Women age and MBD as defined by ACR BI-RADS among (A) normal group and (B) breast cancer group. ACR BI-RADS indicates American College of Radiology Breast Imaging-Reporting and Data System; MBD, mammographic breast density.
The ratio of high to low MBD was demonstrated to reverse among the study population reflective of reproductive life (see Figure 2). There was a significant difference between MBD and age (P < .001).
Figure 2.
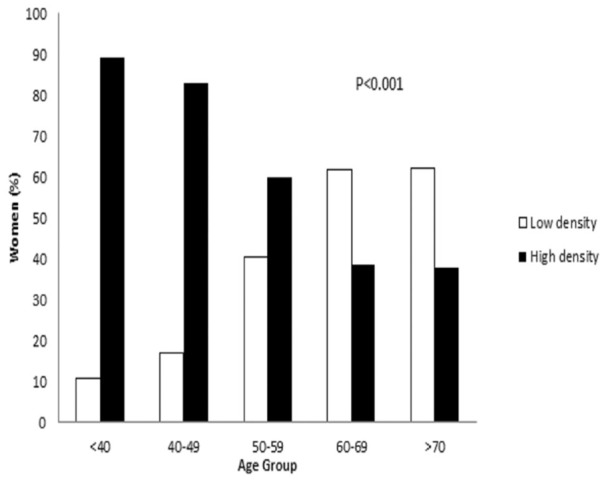
Age and MBD as defined by ACR BI-RADS. ACR BI-RADS indicates American College of Radiology Breast Imaging-Reporting and Data System; MBD, mammographic breast density.
Using chi-square post hoc tests with Bonferroni adjustment based on adjusted standardised residuals between 2 groups, normal and breast cancer (Table 4), breast density in the breast cancer group was significantly denser (ACR-BI-RADS c and d) compared with normal group in women aged less than 40 and 40 to 49 years.
Table 4.
MBD comparison between normal and breast cancer groups.
| MBD | Number (%) of women per ACR BI-RADS category |
||||
|---|---|---|---|---|---|
| <40 y | 40-49 y | 50-59 y | 60-69 y | >70 y | |
| Normal group | |||||
| a + b | 9 (15.3%) | 42 (20.8%) | 71 (39.8%) | 22 (59.4%) | 8 (61.5%) |
| c + d | 50 (84.7%) | 159 (79.1%) | 107 (60.1%) | 15 (40.5%) | 5 (38.4%) |
| Total | 59 (12.1%) | 201 (41.2%) | 178 (36.5%) | 37 (7.5%) | 13 (2.7%) |
| Breast cancer group | |||||
| a + b | — | — | 25 (40.9%) | 15 (65.2%) | 10 (62.5%) |
| c + d | 24 (100%) | 48 (100%) | 36 (59.0%) | 8 (34.8%) | 6 (37.5%) |
| Total | 24 (14%) | 48 (27.9%) | 61 (35.5%) | 23 (13.4%) | 16 (9.3%) |
| Z | −2.0 | −3.5 | −0.2 | −0.2 | −0.1 |
| P | .05 | >.01 | .84 | .84 | .92 |
Abbreviations: ACR BI-RADS, American College of Radiology Breast Imaging-Reporting and Data System; MBD, mammographic breast density.
Discussion
To the best of our knowledge, this is the first study reporting the frequency and age distribution of MBD among women in Jordan and the first to examine MBD in women diagnosed with breast cancer.
The study demonstrated a significant inverse relationship between age and MBD among normal and breast cancer groups of Jordanian women. The results revealed that 68.9% of women in the normal and 70.9% of breast cancer group reported high MBD – ACR BI-RADS c or d. This is higher than the recorded percentage for women in the United States (55.4%),17 China (52.8%),22 Lebanon (52.9%),23 Uganda (39%),24 United Arab Emirates (23.6%),25 and India (16%).26 This density difference emphasises both the increased risk of breast cancer in this population and the importance of local data informing screening policy rather than that of the US or other international data.
In Jordan, the age-standardised incident rate of breast cancer is 57.4 per 100 000 persons which is lower than Western countries such as United Kingdom (93.6 per 100 000), United States (84.9 per 100 000), and Canada (83.8 per 100 000).2 However, it is higher than the surrounding countries including Saudi Arabia (27.3 per 100 000), Turkey (45.6 per 100 000), Qatar (42.1 per 100 000), Oman (34.7 per 100 000), United Arab Emirates (52.9 per 100 000), Iran (31.0 per 100 000), Egypt (52.4 per 100 000), and Iraq (38.4 per 100 000).2 Importantly, Jordanian women are known to develop breast cancer at least 15 years earlier than women in the United States or United Kingdom, with a median age of 46, 62, 67 years, respectively.27,28 In Jordan, the highest incidence of breast cancer occurs among women aged 40 to 49 years (30%) in contrast to United Kingdom and Canada where the highest incidence of all new breast cancer cases is among women aged 60 to 69 years (24.2% and 27%, respectively).21,29
This study documented that in the normal group, 79.1% of women aged 40 to 49 years had dense breasts (ACR BI-RADS c and d), whereas in the breast cancer group, 100% of women had dense breasts. In addition, the MBD of the patients with breast cancer in the subgroup of <40 and 40 to 49 years old was significantly higher compared with that of women in the normal group. In the age groups 50 years old and older, MBD of the normal group was similar to that of the breast cancer groups. It is well established that mammographic sensitivity decreases with increased MBD7,30 and a study by Buist et al31 found that for women less than 50 years of age, interval cancer rates were higher in high MBD (90%) compared with low MBD (0%) breasts.
It can be hypothesised that in this snapshot of Jordanian women, where the majority 69.4% (n = 458) have high MBD breasts, commencing breast screening with mammography only may not afford full benefit from the programme as many lesions might be masked by dense tissue. To minimise the potential for false-negative diagnosis and interval cancers, Jordanian women may benefit from an individualised breast cancer screening programme that takes into account individual risk factors such as density and incorporates additional imaging such as DBT, ultrasound, and MRI. This is supported by the literature which has shown that such combinations increase breast cancer screening programme sensitivity.32,33 The addition of ultrasound to mammography improved sensitivity by 27.5%.32 Compared with mammography alone, the addition of MRI and DBT improved sensitivity from 78.3% to 97.8% and 88.2%, respectively.33
Consideration should also be given to further tailoring of the screening programme to take into account the decreased density of women aged 60 years and above where breast cancer risk and incidence are reduced; cancer detection is better facilitated and where mammography alone may be deemed sufficient.
There is a worldwide movement to tailor breast cancer screening according to age, as well as MBD; however, no clear guidelines have yet been established. In United States, women are notified about their MBD after mammography and additional imaging may be requested by a referring physician.34 In France, Austria, and Germany, women with high MBD have an additional ultrasound imaging and women are also given this option in Greece.34 In United Kingdom, Canada, and Australia, MBD is promoted by many advocacy groups to be incorporated into national screening guidelines.
The Japan Strategic Anti-cancer Randomized Trial (J-STAT) investigated the efficacy of additional ultrasound imaging in women aged 40 to 49 years in the large nationwide Japanese breast cancer screening programme. The addition of ultrasound resulted in a significantly improved detection rate by 4.2 per 1000 women10 and less interval cancers compared with imaging with mammography alone.35
Another additional screening modality that can be offered to women with high MBD is MRI; however, there are no data available on its role in women with high MBD. In a UK cost-effectiveness study, MRI screening is recommended for women with family history of breast cancer aged 40 to 49 years at a 10-year risk greater than 12% when mammography has demonstrated a dense breast pattern.36 The American Cancer Society guidelines stated that breast MRI may be recommended for women with high MBD (ACR BI-RADS c or d) as an additional modality to mammography37; however, the value of adding MRI is still unclear. According to ACR guidelines, where women aged 25 to 30 years are identified to be at high risk of breast cancer, annual MRI is recommended.37 In addition, ACR guidelines recommend annual MRI for all women with a previous history of breast cancer that was diagnosed before the age of 50 years and for women diagnosed at a later age with high MBD.38 The NCCN guidelines recommend MRI instead of mammography for women aged 25 to 29 years at high risk and thereafter as an additional imaging to mammography.34 However, adding MRI as a screening tool for women with high MBD is known to be associated with increased recall rates with increased sensitivity.34
Digital breast tomosynthesis is a promising modality for screening women with high MBD because it improves cancer detection, increases positive predictive value, and reduces recall rates.39 This imaging tool has the ability to eliminate the overlapping of breast tissue; hence, more lesions are revealed. Rafferty et al12 found that the addition of DBT to mammography for women with high MBD resulted in a significant advantage when comparing area under the receiver operating curve, 0.88, for DBT with mammography alone, 0.79. Adding DBT imaging to mammography demonstrated reduced recall rates for all breast density and age groups, with significant differences in recall rates for ACR BI-RADS c and d, and this reduction was higher for women younger than 50 years old and in women with dense breasts.39
This study has some limitations, most notably its small sample size and that the population is from north and middle areas in Jordan only and not representative of all Jordanian women. To validate this research, a larger and more representative sample of the population is required. Although it is outside of the scope of the study, the current data also lack information on whether all lesions were detected by mammography only as a number of women also went for adjunct imaging modalities. In addition, the study sample was unequally distributed across different groups and ages which may have skewed results. The recorded age distribution was skewed with 379 women in the normal group aged between 40 and 59 years, but only 50 women aged greater than 60 years. In the breast cancer group, the study reviewed 109 women aged 40 to 59 years compared with only 39 women aged 60 years and above. The relatively high proportion of breast cancer cases in younger women reflects this. If more equal numbers of women were recorded, the percentage differences in breast density patterns and the relationship of ACR BI-RADS category and age may change. Another limitation is the use of the subjective visual ACR BI-RADS classifying of MBD; however, it has been used in many studies,17,23,25,26 and in the current study, almost perfect agreement was demonstrated between the 3 radiologists (kappa value of 0.93). It is more accurate to use automated methods for MBD measurement but semi-automated and automated methods are not currently available in Jordan. Other factors which have previously been shown to be associated with increased MBD and breast cancer risk such as body mass index, parity, menopausal status, age at first menarche, age at first birth, and hormonal use were outside the scope of this study; the authors acknowledge that these parameters should be included in future studies.
In conclusion, this study has demonstrated a significant linear inverse relationship between age and MBD among normal and breast cancer groups. A meaningful population of Jordanian women were reported to have high MBD and reflecting reproductive life; the distribution of MBD reversed among the study population at 60 years of age and above. The results of this study may inform a more population-specific approach to breast cancer screening in Jordan. Further research that investigates the impact of offering women aged less than 60 years additional imaging such as ultrasound, MRI, or DBT to increase the sensitivity of the screening process is required to further inform the current screening programme. Findings of this study emphasise the importance of developing national breast cancer screening guideline based on the local data which additionally takes in consideration a woman’s age, MBD, and overall breast cancer risk.
This snapshot of Jordanian women has identified a population with a unique MBD profile that reflects a significant increased risk of breast cancer that is evidenced in breast cancer incidence. Further research is needed to validate the results of this study.
Acknowledgments
We would like to acknowledge Jordan University of Sciences and Technology for their research grant (grant number: 20170023). Thanks also go to Mr Salim Abudayeh, Mrs Rasha Al Shayeb, Ms Safaa Gharaibeh, and Ms Maryam Hesham for assisting in the research. We would like to thank Eng. Ahmad Bresh (Fujifilm, Jordan) for his technical support during data collection.
Footnotes
Author Contributions: DS Al-M contributed to the study conception and design. Material preparation, data collection and analysis were performed by DS Al-M, MA, KMS, HA, MA, MR and PCB. The first draft of the manuscript was written by DS Al-M and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Jordan University of Science and Technology (grant number: 20170023).
ORCID iDs: Dana S Al-Mousa  https://orcid.org/0000-0002-1315-7459
https://orcid.org/0000-0002-1315-7459
Kelly M Spuur  https://orcid.org/0000-0003-0285-9962
https://orcid.org/0000-0003-0285-9962
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization, International Agency for Research on Cancer. Population fact sheets; 2018. http://gco.iarc.fr/today/fact-sheets-populations. Published May 2019. Accessed July 25, 2019.
- 3. Jordan Breast Cancer Program. Governance. Issue 1st ed. Amman, Jordan: Annual Newsletter of the Jordan Breast Cancer Program; 2008. http://www.jbcp.jo. Accessed June 15, 2019. [Google Scholar]
- 4. Jordan Ministry of Health. Jordan cancer registry: cancer incidence in Jordan. Amman, Jordan; 2014. http://www.moh.gov.jo/Echobusv3.0/SystemAssets/3465ef25-4ef3-4d46-bd6e-c3fcbca5048f.pdf. Published 2016. Accessed July 1, 2019. [Google Scholar]
- 5. Jordan Ministry of Health. Annual incidence of cancer in Jordan. Amman, Jordan; 2012. https://www.moh.gov.jo/Pages/viewpage.aspx?pageID=185. Published 2014. Accessed June 12, 2019. [Google Scholar]
- 6. Tabár L, Vitak B, Chen HHT, Yen MF, Duffy SW, Smith RA. Beyond randomized controlled trials: organized mammographic screening substantially reduces breast carcinoma mortality. Cancer. 2001;91:1724-1731. [DOI] [PubMed] [Google Scholar]
- 7. Carney PA, Miglioretti DL, Yankaskas BC, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138:168-175. [DOI] [PubMed] [Google Scholar]
- 8. Boyd NF, Lockwood GA, Byng JW, Tritchler DL, Yaffe MJ. Mammographic densities and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1998;7:1133-1144. [PubMed] [Google Scholar]
- 9. McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159-1169. [DOI] [PubMed] [Google Scholar]
- 10. Thigpen D, Kappler A, Brem R. The role of ultrasound in screening dense breasts – a review of the literature and practical solutions for implementation. Diagnostics. 2018;8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Riedl CC, Luft N, Bernhart C, et al. Triple-modality screening trial for familial breast cancer underlines the importance of magnetic resonance imaging and questions the role of mammography and ultrasound regardless of patient mutation status, age, and breast density. J Clin Oncol. 2015;33:1128-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315:1784-1786. [DOI] [PubMed] [Google Scholar]
- 13. Wolfe J. Breast patterns as an index of risk for developing breast cancer. AJR Am J Roentgenol. 1976;126:1130-1137. [DOI] [PubMed] [Google Scholar]
- 14. Gram IT, Funkhouser E, Tabár L. The Tabár classification of mammographic parenchymal patterns. Eur J Radiol. 1997;24:131-136. [DOI] [PubMed] [Google Scholar]
- 15. D’Orsi CJSE, Mendelson EB, Morris EA, et al. ACR BI-RADS® Atlas, Breast Imaging-Reporting and Data System. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 16. Dense Breast-info. Legislation and Regulation: Notification required by state; 2019. https://densebreast-info.org/legislation.aspx. Accessed May 5, 2020. [Google Scholar]
- 17. Checka CM, Chun JE, Schnabel FR, Lee J, Toth H. The relationship of mammographic density and age: implications for breast cancer screening. AJR Am J Roentgenol. 2012;198:W292-W295. [DOI] [PubMed] [Google Scholar]
- 18. McCormack VA, Perry N, Vinnicombe SJ, Silva Idos S. Ethnic variations in mammographic density: a British multiethnic longitudinal study. Am J Epidemiol. 2008;168:412-421. [DOI] [PubMed] [Google Scholar]
- 19. Jordan Breast Cancer Program. Breast cancer screening and diagnostic guidelines; 2011. https://www.iccp-portal.org/system/files/plans/jor_D1_guidlines%2021.4.2011%20breast%20cancer.pdf/. Published 2011. Accessed September 12, 2019.
- 20. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [DOI] [PubMed] [Google Scholar]
- 21. Statista. Registrations of newly diagnosed cases of breast cancer in England in 2017, by age group and gender. https://www.statista.com/statistics/312771/breast-cancer-cases-england-age/. Published May 21, 2019. Accessed December 8, 2019.
- 22. Sung H, Ren J, Li J, et al. Breast cancer risk factors and mammographic density among high-risk women in urban China. NPJ Breast Cancer. 2018;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Salem C, Atallah D, Safi J, et al. Breast density and breast cancer incidence in the Lebanese population: results from a retrospective multicenter study. Biomed Res Int. 2017;2017:7594953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gathara J, Galukande M, Kiguli-Malwadde E. Breast density as a risk factor for breast cancer amongst a cohort of women in Uganda. E Afr J Surg. 2012;17:98-103. [Google Scholar]
- 25. Kang Y-J, Ahn SK, Kim SJ, Oh H, Han J, Ko E. Relationship between mammographic density and age in the United Arab Emirates Population. J Oncol. 2019;2019:7351350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Singh T, Khandelwal N, Singla V, et al. Breast density in screening mammography in Indian population – is it different from western population? Breast J. 2018;24:365-368. [DOI] [PubMed] [Google Scholar]
- 27. Najjar H, Easson A. Age at diagnosis of breast cancer in Arab nations. Int J Surg. 2010;8:448-452. [DOI] [PubMed] [Google Scholar]
- 28. Bowen R, Duffy S, Ryan D, Hart I, Jones J. Early onset of breast cancer in a group of British black women. Br J Cancer. 2008;98:277-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Halls S. Breast Cancer Incidence and Mortality Rates Worldwide: Canada, UK, USA. Camrose, AB, Canada: Moose & Doc Breast Cancer; 2017:1 https://breast-cancer.ca/httpbreast-cancer-camortratings/. [Google Scholar]
- 30. Mandelson MT, Oestreicher N, Porter PL, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 2000;92:1081-1087. [DOI] [PubMed] [Google Scholar]
- 31. Buist DSM, Porter PL, Lehman C, Taplin SH, White E. Factors contributing to mammography failure in women aged 40-49 years. J Natl Cancer Inst. 2004;96:1432-1440. [DOI] [PubMed] [Google Scholar]
- 32. Berg WA, Blume JD, Cormack JB, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA. 2008;299:2151-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim WH, Chang JM, Moon H-G, et al. Comparison of the diagnostic performance of digital breast tomosynthesis and magnetic resonance imaging added to digital mammography in women with known breast cancers. Eur Radiol. 2016;26:1556-1564. [DOI] [PubMed] [Google Scholar]
- 34. Vourtsis A, Berg WA. Breast density implications and supplemental screening. Eur Radiol. 2019;29:1762-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ohuchi N, Suzuki A, Sobue T, et al. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-cancer Randomized Trial (J-START): a randomised controlled trial. Lancet. 2016;387:341-348. [DOI] [PubMed] [Google Scholar]
- 36. Griebsch I, Brown J, Boggis C, et al. Cost-effectiveness of screening with contrast enhanced magnetic resonance imaging vs X-ray mammography of women at a high familial risk of breast cancer. Br J Cancer. 2006;95:801-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89. [DOI] [PubMed] [Google Scholar]
- 38. Monticciolo DL, Newell MS, Moy L, Niell B, Monsees B, Sickles EA. Breast cancer screening in women at higher-than-average risk: recommendations from the ACR. J Am Coll Radiol. 2018;15:408-414. [DOI] [PubMed] [Google Scholar]
- 39. Haas BM, Kalra V, Geisel J, Raghu M, Durand M, Philpotts LE. Comparison of tomosynthesis plus digital mammography and digital mammography alone for breast cancer screening. Radiology. 2013;269:694-700. [DOI] [PubMed] [Google Scholar]


