Abstract
Stevens-Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) are potentially fatal mucocutaneous diseases that can involve many organ systems. Manifestations of SJS/TEN outside of the skin, eyes, and oral mucosa are not well defined or well recognized, and, therefore, are often not addressed clinically. As supportive care improves and mortality from SJS/TEN decreases, chronic complications in affected organ systems are becoming more prevalent. Recognition of the manifestations of SJS/TEN in the acute phase is critical to optimal care. In this review, we review the organ systems that may be involved in SJS/TEN, provide an overview of their management, and propose a list of items that should be communicated to the patient and family upon discharge. The organ systems discussed include the pulmonary, gastrointestinal/hepatic, oral, otorhinolaryngologic, gynecologic, genitourinary, and renal systems. In addition, the significant psychosocial, nutritional, and pain consequences and management of SJS/TEN are discussed.
Keywords: burns, complications, gastrointestinal, gynecologic, ocular, pulmonary, Stevens-Johnson syndrome, toxic epidermal necrolysis
Introduction
Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN) lie on a spectrum of immune-mediated mucocutaneous diseases that cause widespread sloughing of the skin and mucosal surfaces. These ‘immunologic burns’ can be fatal, with a reported mortality rate of up to 35%.1 Unknown to many physicians, SJS/TEN is not a disease limited to the skin and oral mucosa, and survivors are often left with debilitating multi-organ complications.
SJS/TEN is defined as a widespread vesiculobullous rash with epidermal sloughing and necrosis, and mucous membrane involvement, usually of the eyes, oral cavity, and skin. The degree of total body surface area (TBSA) involved determines where on the SJS/TEN spectrum a patient lies (SJS: <10% TBSA; SJS-TEN overlap: 10–30% TBSA; TEN: >30% TBSA).1 Both conditions, SJS and TEN, are also currently known together as a single entity called Epidermal Necrolysis (EN).2 SJS/TEN occurs most commonly as an idiosyncratic reaction to systemic medications, such as antibiotics, anti-epileptic medications and nonsteroidal anti-inflammatory drugs.3 However, no drug origin can be identified for 15% of cases4; SJS/TEN can also occur secondary to viral infections, vaccinations, and other nonpharmacological triggers.5 The ALDEN (algorithm of drug causality for EN) algorithm facilitates identification of the culprit drug in cases of SJS/TEN.4 The ALDEN algorithm assigns a final score based on six criteria to determine as being ‘very probable’, ‘probable’, ‘possible’, ‘unlikely’, and ‘very unlikely’ as having caused SJS/TEN. These criteria also help to rule out unlikely drugs from being labeled as a cause for SJS/TEN (see Table 1).
Table 1.
ALDEN algorithm criteria and scoring for drug causality.
| Criteria | Possible score |
|---|---|
| Time lag between initial drug intake to onset of reaction (index day) | −3 to +3 |
| Presence of drug in the body on index day | 0 to −3 |
| Prechallenge/rechallenge outcome with the suspect drug | −2 to +4 |
| Outcome of rechallenge | 0 to −2 |
| Drug notoriety for causing SJS/TEN | −1 to +3 |
| Other possible etiologic alternatives | −1, if applicable |
The total ALDEN is based on the six criteria listed. A total score of ⩾6 is categorized as very probable, 4–5 as probable, 2–3 as possible, 0–1 as unlikely, and <0 as very unlikely. Specifics of the scoring system for each criterion is not described here but can be found in Sassolas and colleagues.4
ALDEN, algorithm of drug causality for epidermal necrolysis; SJS/TEN, Stevens-Johnson syndrome/toxic epidermal necrolysis.
Many internal organ systems outside of the eyes and skin are affected in SJS/TEN, including the pulmonary, gastrointestinal/hepatic, oral, otorhinolaryngologic, gynecologic, genitourinary, and renal systems.6 There are also significant psychosocial consequences, and nursing and social work services play a critical role in the rehabilitation of these patients. The aim of this review is to give the reader an overview of the organ systems involved in SJS/TEN, and how a multidisciplinary team can provide optimal care for these patients.
Methods
A PubMed database search was conducted for publications until August 2018. The search strategy was performed using the keywords ‘Stevens-Johnson syndrome’ and ‘toxic epidermal necrolysis’. The title and abstracts of all publications were screened manually for content referring to specific organ system involvement. The full texts of all relevant articles were reviewed. Articles in the English language were used. We categorized every article according to the organ system involved.
This study does not aim to conduct a systematic review of the data.
Supportive care
Identification of the culprit drug
The most crucial measure in the acute phase is immediate withdrawal of the culprit drug when there is suspicion of SJS/TEN. Prompt withdrawal of the suspected medication when blisters or erosions appear during the course of the disease may decrease mortality.7 Culprit drugs are identified in 85% cases of SJS/TEN4; however, identification of the causative agent is not straightforward in all cases, especially in patients who are taking multiple drugs concurrently. Although helpful in these scenarios, the ALDEN algorithm is generally used for assessment of drug causality retrospectively and not in the acute phase. Pharmacovigilance data plays a large role here, and identifies a list of drugs that have a very strong association with SJS/TEN and are responsible for 50% of all cases of SJS/TEN. This data has been established with the help of large case-control studies.3,8 From these studies, for example, paracetamol, aspirin, ibuprofen (medications generally given to treat the prodromal symptoms of SJS/TEN), which are common confounders, have not yet been found to have an established association as causative factors of SJS/TEN.
A variety of methods has been used to identify the causal drug using various diagnostic tests. It is universally agreed that oral provocation studies in SJS/TEN patients may be dangerous since they can precipitate a similar episode again. Patch testing has been attempted, but has been found to be positive in only 9–63% of SJS/TEN patients in different studies, and has been noted to be of low diagnostic value.9–13 In addition, patch testing has different levels of sensitivity and specificity for different drugs. Culprit drugs may possibly be identified using in vitro assays, such as drug-induced T-cell proliferation [lymphocyte transformation tests (LTT) and drug-induced lymphocyte cytokine production (cytokine assays). LTT were positive in 21–56% of patients with SJS/TEN14–17 and in 0–37% of control cases.15,17,18 Hence, LTT has not yet been used routinely for identification of the culprit drug in cases of SJS/TEN. Recently, there have been reports of using tests that measure levels of cytokines or other mediators produced by lymphocytes secondary to a reaction a drug. Recent studies have shown IFN-γ drug assays to identify the causal drug in 78% of cases of SJS/TEN, and the IL-4 assay to detect drug causality in 50% of cases.16 Another study showed that the culprit drug could be identified in 55% of cases of SJS/TEN with IFN-γ assays, in 43% of cases with Interleukin-5 assay, in 38% of cases with Interleukin-2 assay, and in 33% of cases with granzyme-B assay.17 This latter study also suggested that that combining different assays may be a more feasible approach to identifying the causative drug in patients with SJS/TEN. However, none of these tests have yet been used in routine hypersensitivity testing, and all warrant further research in larger groups of patients.
Supportive medical care
Supportive care encompasses protecting and restoring the barrier function of the skin, maintaining fluid balance, protecting the airway, and treating infection.19,20 However, several complications of SJS/TEN can result in death, including metabolic imbalance, sepsis, pulmonary embolus, renal failure, hematologic abnormalities, and gastrointestinal hemorrhage.21,22 During admission, the SCORTEN (SCORe of Toxic Epidermal Necrosis) is used to evaluate the risk of death on the basis of seven clinical and biological parameters.23 The seven risk factors considered are age above 40 years, malignancy, tachycardia above 120 beats per minute, initial percentage of epidermal detachment greater than 10% TBSA, serum urea above 10 mmol per liter, serum glucose above 14 mmol per liter, and serum bicarbonate below 20 mmol per liter.23 The primary team usually provides the supportive care needed to prevent mortality from this potentially fatal disease, usually on an inpatient floor, intensive care unit, or burn unit.
Systemic treatment for SJS/TEN varies widely as little evidence-based recommendations exist and there is no clear consensus in acute or chronic systemic management. The use of systemic corticosteroids, intravenous immunoglobulins, and tumor necrosis factor (TNF)-alpha inhibitors have all been described in the acute phase, with mortality results varying from improved mortality, no benefit, to increased mortality.5,20,24 Recent data on cyclosporine, however, includes a report that compared cyclosporine to other systemic therapies, and found that cyclosporine reduced mortality in SJS/TEN patients.25 A recently published systematic review of treatment of SJS/TEN in the acute phase also reported a significant benefit of cyclosporine.26 However, the recently published United Kingdom guidelines for SJS/TEN, which scrutinized all studies with at least eight SJS/TEN patients in the treatment group, did not consider any of the available published data on SJS treatment of sufficient quality or consistency to give any specific recommendations for or against the use of these drugs.27 They highlighted a dire need for more research in this direction, and establishment of case registries to study the effect of these medications systematically.
General supportive care is the mainstay of treatment. While it is vital to improving survival, a lack of multidisciplinary care can leave survivors with long-term sequelae in various organ systems. We have found that, even for organ systems for which there is significant evidence of chronic sequelae, specialists are infrequently involved early on in the care of these patients. For example, despite well-documented significant acute and chronic ocular involvement, only 66% of burn centers across the US routinely consult ophthalmology for SJS/TEN patients.28
As mortality from SJS/TEN decreases with increasingly expedient supportive care, assessing for, and addressing, the acute pathology that can give rise to chronic complications becomes even more critical, as are social services support and patient education. Given that SJS/TEN is a rare disease, there are few prospective studies, and even fewer randomized clinical trials from which evidence-based recommendations can be made. Based on our experience seeing some of the highest volumes of SJS/TEN patients in the United States, however, we bring attention in this review to the need for multidisciplinary care in the management of these patients. We provide a comprehensive systems-based approach to the review and management of SJS/TEN.
We begin by reviewing the most commonly involved systems in SJS/TEN, current practices in care, and addressing how multidisciplinary teams can be integrated into the acute care phase as early as possible. We would like to emphasize that most of the recommendations below are based on Level IV and V evidence from case series and the experiences of experts in the field, and therefore represent Grade C and D recommendations.
Integument
Skin involvement in SJS/TEN in the acute phase is well described, and occurs in 100% of SJS/TEN patients in the form of confluent purpuric macules, or atypical flat target lesions, with blisters and erosions.20,29 Cutaneous lesions begin symmetrically on the face and the upper part of the body, and extend rapidly across the entire body, predominantly on the trunk and proximal limbs, with maximal extension of lesions occurring over 2–3 days (Figure 1a). A sheet-like loss of epidermis in regions involved by confluent erythema is characteristic of TEN.29 The degree of skin involvement, as categorized by percent of TBSA, is an indication of the severity of the disease and should be assessed daily, especially during the first week after disease onset.
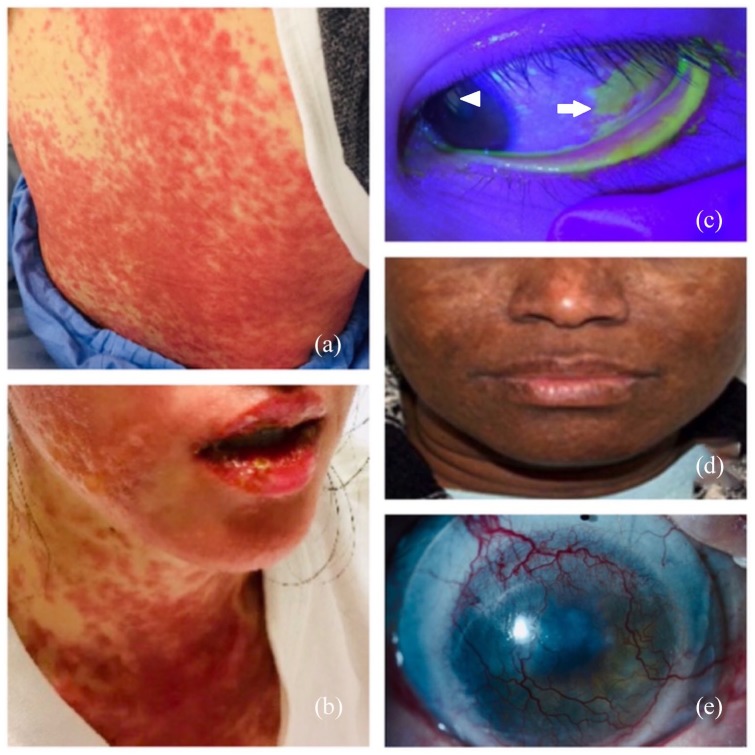
Figure 1.
Skin and ocular complications of SJS/TEN in the acute and chronic phases. (a) Acute SJS/TEN with maculopapular rash of skin. (b) Mucositis of the oral mucosa can also be seen. (c) Fluorescein dye illuminated with cobalt blue light showing lower eyelid margin skin sloughing and conjunctival (arrow) and corneal (arrowhead) epithelial defect in the acute phase. (d) Hypertrophic facial scarring in the chronic phase. (e) Corneal neovascularization in the chronic phase (a scleral contact lens can be also be seen in the image). (Informed consent has been obtained for publication of images).
SJS/TEN, Stevens-Johnson syndrome/toxic epidermal necrolysis.
As in burn injuries, when the barrier function of the skin is lost, homeostatic mechanisms cannot be maintained. A host of consequences can occur as a result, including fluid and electrolyte loss, hypovolemia, susceptibility to infection, impaired thermoregulation, altered immunologic functions, increased energy expenditure, and impaired substrate utilization.30 The occurrence of these consequences is proportional to the degree of epidermal loss.
There are different approaches to skin care in the acute phase, with little evidence to support one over the other.27 A conservative approach involves aspiration of blister fluid while leaving the detached epidermis intact to protect the underlying dermis. If the dermis is exposed, an appropriate dressing is used to reduce fluid loss, prevent infection secondary to microbial colonization, and provide a moist wound environment to optimize re-epithelialization. A surgical approach involves debridement of the detached epidermis, followed by usage of synthetic dressings, allograft, or xenograft for wound closure. This approach removes any infected material at the skin site. The recently published UK guidelines for the management of SJS/TEN shed some light on the indications for conservative and surgical approaches.27 However, there is still no evidence that one approach is more successful than the other in terms of long-term morbidity.
Most skin involvement resolves over time without chronic sequelae, but pigmentation abnormalities and scarring can occur. Involvement of the paronychium and nail plate in the acute phase can also lead to long-term deformities of the fingernails.31 Hair loss during the first 6 months after hospital discharge has also been reported, with the areas most commonly affected being the scalp, eyebrows, and eyelashes.32 Long-term sequelae of the integumentary system are common, but are generally not severe and include pigmentary changes, scarring, and nail dystrophy (Figure 1b).33,34 In a recently published survey of 17 patients at a mean follow-up period of 51.6 ± 74.7 months after SJS/TEN, 15 (88%) patients had long-term cutaneous complications such as postinflammatory skin changes, scars, milia, and urticaria.32 These sequelae should be managed by a dermatologist or burn/plastic surgeon. Upon discharge, clear recommendations on sun protection and the use of sunscreens should be provided to prevent postinflammatory hyperpigmentation.32
Ocular involvement
Acute ocular involvement occurs in 60–100% of SJS/TEN patients,35–37 and can range from conjunctival hyperemia to near total sloughing of the ocular surface, including the tarsal conjunctiva and eyelid margins (Figure 1c). Ophthalmologic consultation upon admission is critical as there are windows of opportunity during which vision-saving treatments can be employed. Once these windows of opportunity pass, there are irreversible changes to the eye that can eventually lead to blindness. Specific management is beyond the scope of this review, but aggressive lubrication, judicious use of topical corticosteroid eye drops, prevention of infection with antibiotic eye drops, as well as amniotic membrane transplantation (a noninvasive and low-risk ocular surface procedure) have significantly improved the outcomes of ocular disease in SJS/TEN patients.38,39
Long-term ocular sequelae occur in 20–79% of SJS/TEN survivors, and include dry eye, keratinization of eyelid margins, loss of corneal epithelial stem cell function, and opacification and xerosis of the ocular surface leading to blindness (Figure 1d).6,31,34–37,40,41 SJS/TEN is a cause of bilateral corneal blindness.42 In a recent study, almost 66% children with SJS/TEN who did not receive appropriate care in the acute phase were blind 1 year after the acute episode of SJS/TEN.43 Of the organ systems discussed in this review, complications of the ocular surface are thought to be the most severe, and have a significant impact on quality of life and well being.44,45
All SJS/TEN patients should be seen by an ophthalmologist in the acute phase, and be followed closely during the chronic phase. Patients with any acute ocular involvement in SJS/TEN, regardless of severity, should be seen by an ophthalmologist for life, as severe and irreversible complications can occur at any time, even decades after acute disease.
Gynecologic involvement
Although not widely reported, acute gynecologic involvement occurs in up to 77% of female SJS/TEN patients, and long-term complications are neither insignificant nor infrequent.46 We believe a gynecological exam should be a part of every examination protocol in female patients suspected of having SJS/TEN, and that a gynecologist should be involved early in the care of these patients. A recent survey of national burn intensive care units (ICUs) showed that only 13% of units routinely consult gynecology for female SJS/TEN patients.28 Acute involvement includes vaginal erosions and ulcerations, dysuria, urinary retention, and vaginal discharge, pain, and bleeding.47 There are limited data on effective interventions, but acute gynecologic care may help mitigate the chronic sequelae of vulvar adhesions and vaginal stenosis. These sequelae can occur in up to 25% of survivors and cause dyspareunia, chronic pain and bleeding, and difficulty conceiving.46
Similar to the ocular surface, interventions to decrease inflammation in the acute phase can prevent the development of adhesions.48 Aggressive lubrication of vulvar skin with ointments, and judicious use of high potency steroid ointments on the vulva and in the vagina (+/– fungal prophylaxis) should be undertaken. Vaginal molds/dilators should be used to separate the vaginal walls and prevent vaginal adhesions.46 Patients who feel uncomfortable with the use of a dilator or mold can apply medication with a vaginal applicator. Finally, all female patients with SJS/TEN should be seen by a gynecologist within 3 months of hospital discharge.
Genito-urinary involvement
The exact incidence of genitourinary involvement in patients during the acute phase of SJS/ TEN is not known; however, a recent study by Van Batavia and colleagues reported that 71% (22/31) of children with SJS/TEN had genitourinary involvement in the acute phase.49 Penile erosions were the most common manifestations, followed by meatal involvement, leading to dysuria and hematuria in the acute phase. Management consists primarily of local wound care with petroleum jelly, and symptomatic relief of dysuria with oral phenazopyridine. Importantly, the study found that urethral catheterization was safe in the acute phase, with no short-term or long-term complications. A genitourinary exam should be performed during, or after, the height of cutaneous involvement, and should include an assessment of preputial retractability in uncircumcised male patients.27
Long-term genitourinary sequelae are rare, but may manifest as penile adhesions, urethral strictures, and phimosis.50–52 There may be a greater incidence of urethral complications in TEN compared with SJS.51 There is overlap between the genitourinary and gynecologic systems. Gynecologic sequelae are discussed in the section above.
Oral involvement
Oral mucosal involvement occurs in up to 100% of SJS/TEN patients in the acute stage, manifesting as mucositis and ulceration.52 A large subset of patients will require nasogastric tube feeding in the acute phase because of oral mucosa pain and difficulty swallowing. Acute care of the oral cavity is generally performed by the primary team, and often includes mouth washes for both infectious prophylaxis and prevention of desiccation. Among the chronic oral sequelae, dry mouth has been reported in 40% of survivors.53 Also, survivors of SJS/TEN have saliva that is acidic and is reduced in quantity with abnormal viscosity. These changes may promote dental caries, gingival inflammation, and periodontitis.51 There is also a risk of dental root and growth abnormalities, tooth decay, and strictures that can lead to infection and speech impediments.54,55 We do not believe sufficient data exists to recommend dental/oral surgery consultation in the acute phase, but patients should establish care with such providers upon discharge from the hospital.
Otorhinolaryngologic complications
The otorhinolaryngologic complications of SJS/TEN are an extension of the oral mucosal involvement. In the acute stage, this manifests as dysphagia, dysphonia, dyspnea, odynophagia, otalgia, and nasal obstruction.56 Otitis externa due to epidermolysis of the ear canal has also been reported in the acute phase. Laryngeal lesions in the form of enanthemas, edema, erosions, and pseudomembranes were found in 29% (14/49) of patients in the acute phase of SJS/TEN in a recent study.56 Furthermore, if a patient in the acute phase complains of dysphonia or dyspnea, evaluation with nasal fiberoptic endoscopy is indicated to rule out laryngeal lesions and airway obstruction.56 Although Bequignon and colleagues reported that most affected mucosal surfaces completely healed within a year of disease onset, chronic otorhinolaryngologic complications have also been reported. Hypopharyngeal stenosis causing impaired swallowing, external auditory canal stenosis, and synechiae between the pinna of the ear and scalp, have been reported in isolated case reports.57–59 Insufficient data exist to recommend otolaryngology consultation in the acute phase, but any related symptoms should prompt care from a specialist.
Respiratory complications
Epidermal sloughing of the bronchial epithelium has been reported in the acute phase of SJS/TEN, as diagnosed by fiberoptic bronchoscopy60; this is a frequently fatal complication. Up to 38% patients with SJS/TEN may require mechanical ventilation in the acute phase.60–62 The need for mechanical ventilation in adult patients with TEN is associated with higher mortality. Delayed pulmonary complications in the form of pulmonary edema, atelectasis, and bacterial pneumonia have been reported in 25% of patients who showed no evidence for pulmonary involvement early in their disease course (Figure 2).60
Figure 2.
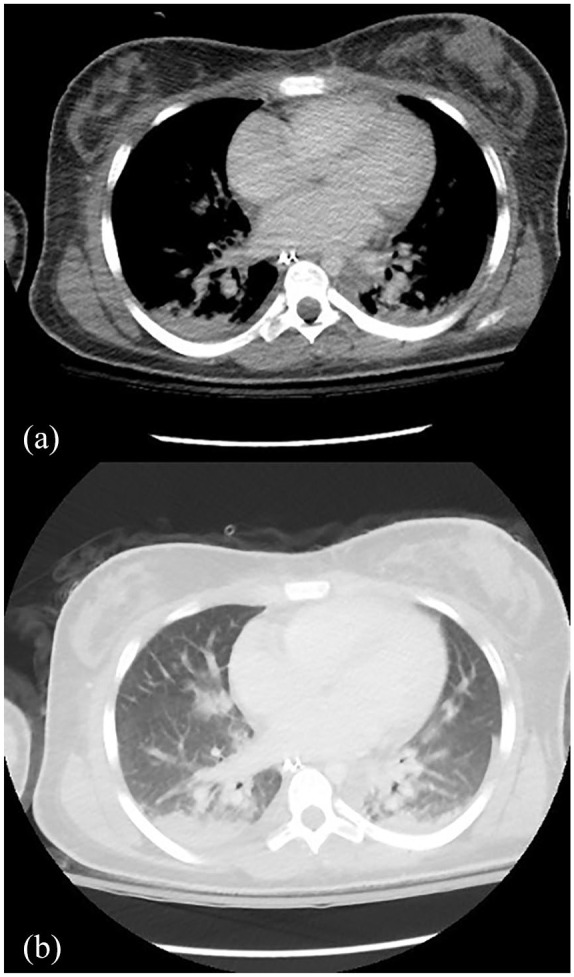
Pulmonary complications in acute SJS/TEN. Axial views of computed tomography chest scan showing (a) patchy consolidation in the lung bases bilaterally with bilateral pleural effusions, and (b) ground-glass opacities.
SJS/TEN, Stevens-Johnson syndrome/toxic epidermal necrolysis.
Acute phase care is not specifically tailored to prevent SJS/TEN-related chronic complications, as little is known about the pathogenesis and extent of respiratory involvement in the disease; however, it is essential to transfer the patient to a burn center/ICU as soon as respiratory symptoms and hypoxemia in the acute phase are encountered. Management includes the use of supplemental oxygen, bronchodilators, bronchial aspiration, and physical therapy. In patients with respiratory involvement in the acute phase, fiberoptic bronchoscopy should be undertaken to identify bronchial involvement, and all patients with respiratory symptoms should be monitored to identify delayed complications.
Sequelae in the chronic phase include bronchiolitis obliterans, respiratory tract obstruction, and bronchiectasis.63 Duong and colleagues noted that 56% of patients in their study on the chronic phase of SJS/TEN had abnormalities in pulmonary functions tests, mainly in the form of diffusion impairment, although the mechanism for this was unknown.64 Thus, patients should be monitored closely after discharge for the development of obstructive lung diseases.27,65,66
Renal failure
In the acute phase, acute renal failure requiring dialysis, and the development of hypokalemia have been reported (Figure 3).67,68 Renal complications are more common in older patients who have pre-existing comorbidities.69 Proteinuria, microscopic hematuria, uremia, and azotemia have also been described in the acute phase, and a serum urea level >10 mmol/l is an independent risk factor for mortality in the acute phase of SJS/TEN.23,70 The incidence of acute kidney injury has been found to be significantly higher in patients who developed SJS/TEN secondary to allopurinol.69,71
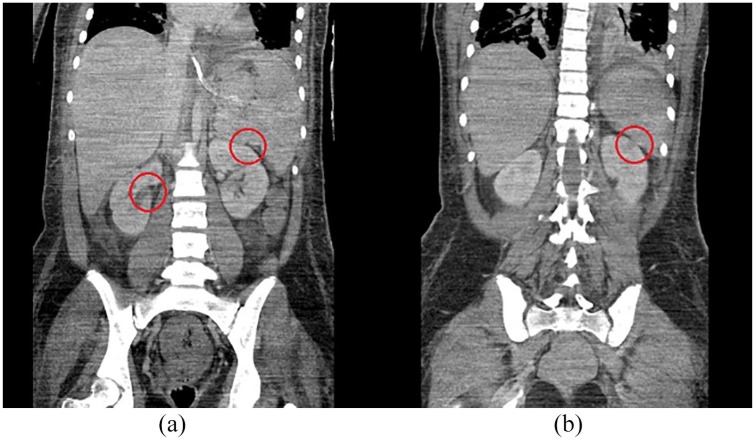
Figure 3.
Renal complications in acute SJS/TEN. Coronal view of computed tomography scan of the abdomen of an SJS/TEN patient with acute renal failure showing (a) wedge-shaped cortical perfusion defect in the right and left kidney (highlighted by red circles), and (b) wedge-shaped cortical perfusion defect in the left kidney (highlighted by a red circle).
SJS/TEN, Stevens-Johnson syndrome/toxic epidermal necrolysis.
Renal histopathology examinations in the chronic phase have revealed membranoproliferative glomerulonephritis.70 Although there are reports of developing chronic renal insufficiency years after the acute episode of SJS/TEN, it is not clear if this is due to drug nephrotoxicity or other comorbidities. Insufficient data exist to recommend nephrology consultation in the acute phase, but any related symptoms should prompt care from a specialist.
Gastrointestinal/hepatic involvement
Although SJS/TEN is known to affect mucosal surfaces, reports on gastrointestinal involvement are rare. There have been isolated reports of inflammatory necrosis of the stomach, jejunum, ileum, and colon in the acute phase of SJS/TEN, leading to intestinal infarcts and mesenteric ischemia, which manifest as severe abdominal pain, hematemesis, and diarrhea.72–74 This can be treated by resection of the ischemic portion of the gastrointestinal tract. These symptoms, however, are also frequently attributed to sepsis, often delaying treatment for this subset of patients. In the chronic phase, there have been several reports of esophageal strictures occurring months-to-years after the acute phase (Figure 4).75–77 These are treated successfully with endoscopic dilation, and supportive targeted care in the acute phase may mitigate chronic complications, or may alert patients and providers of their potential development. Gastrointestinal consultation is warranted if a patient complains of dysphagia in the acute phase of SJS/TEN.
Figure 4.
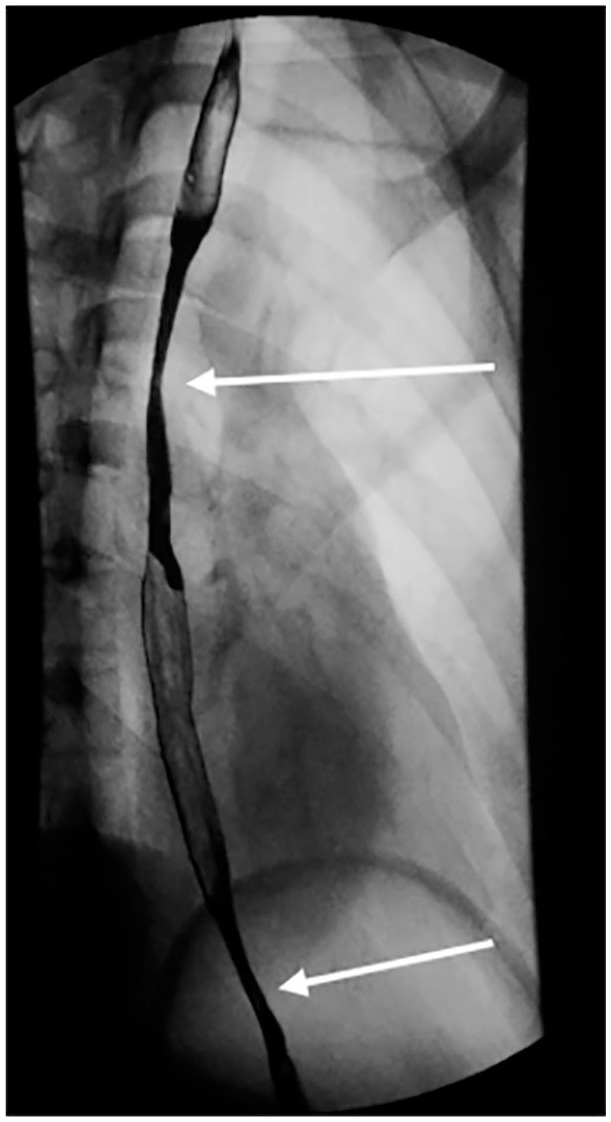
Gastro-intestinal complication in acute SJS/TEN. Barium swallow demonstrating two areas of luminal narrowing and mucosal irregularity in the thoracic esophagus, suggestive of esophageal strictures (highlighted by white arrows). The first stricture is at the level of the aortic arch (3–4 cm long, approximately 67% narrowed), and the second stricture is at the level of the pulmonary artery (1 cm long, approximately 50% narrowed).
SJS/TEN, Stevens-Johnson syndrome/toxic epidermal necrolysis.
Liver involvement in the form of hepatitis has also been reported in the acute phase of SJS/TEN. This has been attributed to sloughing of the epithelial lining of the bile ducts, causing obstruction.78 Hence, in the acute phase, monitoring of liver function tests and coagulation parameters is needed to diagnose and assess the progression of liver involvement. Vanishing bile duct syndrome (VBDS) is a rare complication reported in the acute phase, characterized by rapidly progressive destruction of the intrahepatic bile ducts.79,80 If not treated aggressively with immunosuppressive agents, VBDS can result in severe cholestasis and biliary cirrhosis, necessitating liver transplantation.81,82 Chronic hepatic involvement, in the form of persistent cholestasis, has been reported.83 However, in comparison with other drug hypersensitivity diseases, such as drug reaction with eosinophilia and systemic symptoms (DRESS), the severity and duration of liver involvement in patients with SJS/TEN is lower.84
Psychiatric impacts
The psychological impact of SJS/TEN on survivors and families represents another area typically underaddressed by providers. SJS/TEN is both a physiologically and psychologically stressful experience and survivors may have long-term psychological sequelae, including post-traumatic stress disorder (PTSD). In one study, questionnaires answered by 17 SJS/TEN survivors revealed that 65% of participants showed symptoms of PTSD an average of 51.6 months after hospital discharge.85 An additional number of participants demonstrated significant psychological distress. This distress may also result in an intense fear of medications and hospitalization. Small studies have shown that many SJS/TEN survivors are fearful of taking any new medications, and, thus, may avoid seeking medical attention even when ill.86 Interestingly, the severity of SJS/TEN does not seem to correlate with severity of psychiatric disease. Finally, despite the high preponderance of survivors developing psychological distress, limited data show that only 33% of survivors were assessed by a mental health professional during the period following SJS/TEN.85
Several psychiatric drugs are implicated in the development of SJS/TEN, making the psychological impact of the disease particularly concerning. These drugs must be stopped to prevent worsening of disease, but withdrawing the offending agent can further exacerbate a patient’s mental illness. This could also mean that a patient may no longer be able to take a first-line drug for their mental illness. Thus, patients may relapse and have suboptimal control of their existing psychiatric disease. Furthermore, mental illness can become more severe when coupled with a life-altering disease such as SJS/TEN. We believe a psychiatric consultation should be offered to all patients with SJS/TEN. This is particularly important for patients who develop SJS/TEN secondary to a psychiatric drug or if the patient has a pre-existing psychiatric illness.87
Nutritional support
SJS/TEN is characterized by metabolic disturbances in the acute phase, and a personalized nutritional regimen is essential. Nitrogen and energy requirements are high because of wound exudate losses, impaired substrate utilization, and the subsequent hypermetabolic response. Similar to burn injuries, nutritional requirements are dependent on the percentage of skin involvement.88 There is a high amount of protein loss via wound exudates and blister fluid, and protein intake must be increased for optimum wound healing and to maintain immune function.89 Nutritional intake through the oral route may be difficult secondary to mucosal ulceration and odynophagia, and there should be a low threshold for placing a nasogastric tube in these patients.90 If neither oral nor enteral nutrition is possible, total parenteral nutrition should be initiated to ensure adequate nutrition.90
Physical and occupational therapy
In the acute phase of SJS/TEN, patients are often critically ill, with limited mobility, and pharmacologic prophylaxis for deep vein thrombosis is essential. The prevention of pressure ulcers is also necessary, and can be achieved with the use of air-fluidized patient beds. Once the patient is stable and conscious, a physical therapist should evaluate the patient daily for range of motion, mobility, strength, and endurance impairments.91 Daily physical therapy should be initiated as early as possible to preserve limb mobility and improve strength of endurance, while limiting joint contractures.
After discharge from a burn center, patients with SJS/TEN are often transferred to a rehabilitation facility. This ensures that the patient receives physical therapy until they gain enough strength to be ambulatory. An occupational therapist may ensure that the patient can conduct tasks of daily living independently before discharge. After discharge from the rehabilitation center, patients may also require rehabilitation support through periodic outpatient physical therapy. We recommend comprehensive evaluation by a physical therapist when a patient is admitted to a burn center/ICU, and continued oversight by a physical therapist in the long-term setting.
Pain management
Pain management in SJS/TEN is critical. SJS/TEN is characterized by often severe cutaneous pain, and patients may need long courses of pain medication in the acute and subacute phases of disease.30 Multimodal therapy is often instituted in the acute phase of the disease. Oral nonsteroidal anti-inflammatory drugs and opioid-based regimens are used when necessary. Opioid-based regimens are frequently used in high doses and for long duration, necessitating respiratory monitoring.92 Dressing changes and other procedures require further supplementation or sedation. One study on pain management in SJS/TEN patients assessed pain on a 10-point visual analog scale (VAS) every 4 h, and, based on the VAS score, the pain control regimen was modified. If the VAS score was higher than 4, morphine was initiated by patient-controlled analgesia (PCA) mechanisms.92 However, patients in the acute phase of SJS/TEN are often incapable of using PCA mechanisms for delivery of pain medications, and may need infusions of opioid drugs, or their derivatives, although PCA devices are useful in the recovery period.93
Pain control is essential, but the high doses administered for long inpatient stays can lead to addiction and dependence once discharged. Patients need their pain to be well controlled to maintain a sense of mental and physical well being, and in order to be productive members of society; however, they often cannot be discharged from the hospital on narcotic medication above a threshold level and risk addiction. Any patient unable to be weaned off pain medication as discharge planning approaches should be seen by the pain management team and be followed closely upon discharge. Long-acting opiates like methadone may be used in the subacute and the chronic phases for pain control, and gabapentin may be employed for neuropathic pain in the chronic phase of SJS/TEN.
Social work
SJS/TEN can have significant economic and societal impact. In the acute and subacute stage of the disease, which can last weeks to months, patients are unable to return to work, and inpatient care can become extremely costly. Even after recovery from the acute illness, a patient’s general health-related quality of life may be adversely affected. Up to 71% of patients may remain unemployed following SJS/TEN.85 Following hospital discharge, patients sometimes require weeks, months, and even years of recovery, during which time they may be unable to work. Furthermore, they often require caregiver support, which can keep at least two people out of the workforce. The blindness and other disabilities that can result abruptly from SJS/TEN limit the jobs these patients can perform, even when they are ready to re-enter the workforce. Because of these limitations, patients may find it difficult to comply with the often frequent visits to healthcare professionals. We believe it is imperative for the social work team to work with the patient and family as early in hospitalization as possible.
In addition to addressing the above, the social worker may be able to connect the patient and family to a support group of SJS/TEN survivors, both on and offline. Because of the rarity of the condition, SJS/TEN survivors may not find any other survivors in their vicinity, and internet support groups become an invaluable tool. Patients can be made aware of national support groups such as the Stevens-Johnson Syndrome Foundation in the United States, CAST (Canadians Against Stevens-Johnson Syndrome and toxic epidermal necrolysis), and Amalyste in France. The website http://www.sjsupport.org/sjsupport_group_facilitators.shtml provides information on support groups in different countries. Studies on SJS/TEN support groups have noted that the motivation for both survivors and their families to partake in these groups stems from a desire to connect on shared experiences and to seek advice from others.92 These support groups can also help survivors and their families regain their trust in healthcare professionals. SJS/TEN survivors may develop mistrust due to the trigger of their disease by a medication prescribed by a healthcare professional, and the often initial misdiagnosis, which complicates access to and delivery of necessary medical care.94
Genetic assessment of susceptibility to SJS/TEN
Identification of genetic risk factors for the development of SJS/TEN is an area of active research. While no polymorphism has been identified as a universal risk factor, genetic variables that are population-and drug-specific have been discovered. At present, the most widely accepted associations are those between HLA-B*15:02 and carbamazepine-induced SJS/TEN in the Han Chinese population,95 and between HLA-B*58:01 and allopurinol-induced SJS/TEN in various races.96–99 HLA-B12, HLA-DQB1*0601, HLA-A*0206, and HLA-B*44:03 have also been associated with an increased risk of SJS/TEN to varying degrees.5,100–103 Polymorphisms in other genes have also been associated with SJS/TEN and are an area of active research. Other than for HLA-B*1502, HLA testing is not performed routinely prior to starting new medications. In Taiwan and Hong Kong, genetic testing for human leukocyte antigen (HLA)-B*1502 is available and recommended before administering carbamazepine, and has successfully reduced carbamazepine-induced SJS/TEN in both countries.104,105 Such screening programs have also been implemented in Thailand and Singapore.106,107 The United States Food and Drug Administration (FDA) also recommends this genetic testing before administering carbamazepine in at-risk Asian patients.
Support and risk communication
Although some SJS/TEN survivors may develop mistrust of healthcare professionals, others may have positive views if they perceive that they have been given clear and honest information at the time of their illness.84 This includes appropriate discharge planning and communication about the risks and complications associated with SJS/TEN.
Many patients have multiple inaccurate drug allergies listed after developing SJS/TEN out of abundance of caution. However, this is unnecessary and promotes an unfounded fear of medications, even of those that are unrelated to the implicated drug. It is important to remember, and to articulate to the patient, that SJS/TEN due to a medication is a drug-/drug-class-specific disease.
Communication about what to expect is necessary, and we recommend the following be done prior to discharge:
(1) Clear communication with the patient about the nature of the disease, the need for continued medical care, and the different organ systems that might be affected in the future. Furthermore, the patient’s medical records should accurately reflect the diagnoses for which the patient was hospitalized.
(2) Ensure that the patient and family know that the culprit drug, and drugs in the same chemical class, should be avoided at all costs. They should be educated that there is no increased risk of SJS/TEN from unrelated drugs. Patients should also be informed about the availability of in vitro and in vivo tests to identify drug causality.
(a) In addition to communicating the future risk of SJS/TEN to the patient, the treating physician should report the occurrence of an adverse drug reaction like SJS/TEN to specific regulatory agencies and to the manufacturer of the culprit drug. This is the only way adverse reactions gain recognition, particularly for a rare occurrence such as SJS/TEN. The FDA’s Adverse Event Reporting System is a primary tool used in pharmacovigilance, especially for adverse drug reactions like SJS.108
(3) Ensure that the patient establishes care with a primary care physician, if one does not already exist.
(4) Relay information regarding wound care, dressings, medications, and future appointments for all organ systems.
(5) Specific follow-up and discharge instructions for the most commonly affected organ systems including the eye, skin, and genitourinary system.
(6) Follow up with specialists for any additional organ system involved in the acute phase (e.g. pulmonary if the patient with has a history of ventilator use).
(7) Schedule an appointment with a mental health professional.
(8) Set up for transfer of social services.
Conclusion
SJS/TEN is a devastating and potentially fatal mucocutaneous disease. As access to expedient care improves, and supportive care is initiated earlier in the disease, mortality in SJS/TEN will continue to decrease. As more and more patients survive SJS/TEN, the number of those with chronic complications will also increase, necessitating the development of a protocol initiated at the time of admission to address every sphere of involvement from physical to mental to social. Although few high-level evidence-based recommendations exist, establishing a protocol for care will allow healthcare professionals and researchers to develop a deeper understanding of the natural history and pathogenesis of this disease, and, in turn, allow for evidence-based recommendations in the future.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and publication of this article: This article was supported by the NIH/NEI (grant number K23 EY028230-01).
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Hajirah N. Saeed  https://orcid.org/0000-0002-7385-6534
https://orcid.org/0000-0002-7385-6534
Contributor Information
Swapna S. Shanbhag, Department of Ophthalmology, Massachusetts Eye and Ear Infirmary, Boston, MA, USA LV Prasad Eye Institute, Tej Kohli Cornea Institute, Hyderabad, Telangana, India.
James Chodosh, Department of Ophthalmology, Massachusetts Eye and Ear Infirmary, Boston, MA, USA.
Cherie Fathy, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, MA, USA.
Jeremy Goverman, Department of Surgery, Massachusetts General Hospital, Sumner Redstone Burn Center, Boston, MA, USA.
Caroline Mitchell, Department of Obstetrics and Gynecology, Massachusetts General Hospital, Boston, MA, USA.
Hajirah N. Saeed, Department of Ophthalmology, Massachusetts Eye and Ear Infirmary, 243 Charles St, Boston, MA 02114, USA.
References
- 1. Bastuji-Garin S, Rzany B, Stern RS, et al. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol 1993; 129: 92–96. [PubMed] [Google Scholar]
- 2. Ingen-Housz-Oro S, Duong TA, Bensaid B, et al. Epidermal necrolysis French national diagnosis and care protocol (PNDS; protocole national de diagnostic et de soins). Orphanet J Rare Dis 2018; 13: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roujeau JC, Kelly JP, Naldi L, et al. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Eng J Med 1995; 333: 1600–1607. [DOI] [PubMed] [Google Scholar]
- 4. Sassolas B, Haddad C, Mockenhaupt M, et al. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson syndrome and toxic epidermal necrolysis: comparison with case-control analysis. Clin Pharmacol Ther 2010; 88: 60–68. [DOI] [PubMed] [Google Scholar]
- 5. Kohanim S, Palioura S, Saeed HN, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis–a comprehensive review and guide to therapy. I. systemic disease. Ocul Surf 2016; 14: 2–19. [DOI] [PubMed] [Google Scholar]
- 6. Lee HY, Walsh SA, Creamer D. Long-term complications of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN): the spectrum of chronic problems in patients who survive an episode of SJS/TEN necessitates multidisciplinary follow-up. Br J Dermatol 2017; 177: 924–935. [DOI] [PubMed] [Google Scholar]
- 7. Garcia-Doval I, LeCleach L, Bocquet H, et al. Toxic epidermal necrolysis and Stevens-Johnson syndrome: does early withdrawal of causative drugs decrease the risk of death? Arch Dermatol 2000; 136: 323–327. [DOI] [PubMed] [Google Scholar]
- 8. Mockenhaupt M, Viboud C, Dunant A, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol 2008; 128: 35–44. [DOI] [PubMed] [Google Scholar]
- 9. Wolkenstein P, Chosidow O, Flechet ML, et al. Patch testing in severe cutaneous adverse drug reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Contact Dermatitis 1996; 35: 234–236. [DOI] [PubMed] [Google Scholar]
- 10. Lin YT, Chang YC, Hui RC, et al. A patch testing and cross-sensitivity study of carbamazepine-induced severe cutaneous adverse drug reactions. J Eur Acad Dermatol Venereol. 2013; 27: 356–364. [DOI] [PubMed] [Google Scholar]
- 11. Barbaud A, Collet E, Milpied B, et al. A multicentre study to determine the value and safety of drug patch tests for the three main classes of severe cutaneous adverse drug reactions. Br J Dermatol 2013; 168: 555–562. [DOI] [PubMed] [Google Scholar]
- 12. Hassoun-Kheir N, Bergman R, Weltfriend S. The use of patch tests in the diagnosis of delayed hypersensitivity drug eruptions. Int J Dermatol 2016; 55: 1219–1224. [DOI] [PubMed] [Google Scholar]
- 13. Pinho A, Coutinho I, Gameiro A, et al. Patch testing - a valuable tool for investigating non-immediate cutaneous adverse drug reactions to antibiotics. J Eur Acad Dermatol Venereol 2017; 31: 280–287. [DOI] [PubMed] [Google Scholar]
- 14. Tang YH, Mockenhaupt M, Henry A, et al. Poor relevance of a lymphocyte proliferation assay in lamotrigine-induced Stevens-Johnson syndrome or toxic epidermal necrolysis. Clin Exp Allergy 2012; 42: 248–254. [DOI] [PubMed] [Google Scholar]
- 15. Roujeau JC, Albengres E, Moritz S, et al. Lymphocyte transformation test in drug-induced toxic epidermal necrolysis. Int Arch Allergy Appl Immunol 1985; 78: 22–24. [DOI] [PubMed] [Google Scholar]
- 16. Polak ME, Belgi G, McGuire C, et al. In vitro diagnostic assays are effective during the acute phase of delayed-type drug hypersensitivity reactions. Br J Dermatol 2013; 168: 539–549. [DOI] [PubMed] [Google Scholar]
- 17. Porebski G, Pecaric-Petkovic T, Groux-Keller M, et al. In vitro drug causality assessment in Stevens-Johnson syndrome - alternatives for lymphocyte transformation test. Clin Exp Allergy 2013; 43: 1027–1037. [DOI] [PubMed] [Google Scholar]
- 18. Nyfeler B, Pichler WJ. The lymphocyte transformation test for the diagnosis of drug allergy: sensitivity and specificity. Clin Exp Allergy 1997; 27: 175–181. [PubMed] [Google Scholar]
- 19. Saeed H, Mantagos IS, Chodosh J. Complications of Stevens-Johnson syndrome beyond the eye and skin. Burns 2016; 42: 20–27. [DOI] [PubMed] [Google Scholar]
- 20. Dodiuk-Gad RP, Chung WH, Valeyrie-Allanore L, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: an update. Am J Clin Dermatol 2015; 16: 475–493. [DOI] [PubMed] [Google Scholar]
- 21. Mahar PD, Wasiak J, Paul E, et al. Comparing mortality outcomes of major burns and toxic epidermal necrolysis in a tertiary burns centre. Burns 2014; 40: 1743–1747. [DOI] [PubMed] [Google Scholar]
- 22. Weinand C, Xu W, Perbix W, et al. 27 years of a single burn centre experience with Stevens-Johnson syndrome and toxic epidermal necrolysis: analysis of mortality risk for causative agents. Burns 2013; 39: 1449–1455. [DOI] [PubMed] [Google Scholar]
- 23. Bastuji-Garin S, Fouchard N, Bertocchi M, et al. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol 2000; 115: 149–153. [DOI] [PubMed] [Google Scholar]
- 24. Saeed HN, Chodosh J. Immunologic mediators in Stevens-Johnson syndrome and toxic epidermal necrolysis. Semin Ophthalmol 2016; 31: 85–90. [DOI] [PubMed] [Google Scholar]
- 25. González-Herrada C, Rodríguez-Martín S, Cachafeiro L, et al. PIELenRed therapeutic management working group. Cyclosporine use in epidermal necrolysis is associated with an important mortality reduction: evidence from three different approaches. J Invest Dermatol 2017; 137: 2092–2100. [DOI] [PubMed] [Google Scholar]
- 26. Zimmermann S, Sekula P, Venhoff M, et al. Systemic immunomodulating therapies for Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol 2017; 153: 514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Creamer D, Walsh SA, Dziewulski P, et al. U.K. guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults 2016. Br J Dermatol 2016; 174: 1194–1227. [DOI] [PubMed] [Google Scholar]
- 28. Le HG, Saeed H, Mantagos IS, et al. Burn unit care of Stevens Johnson syndrome/toxic epidermal necrolysis: a survey. Burns 2016; 42: 830–835. [DOI] [PubMed] [Google Scholar]
- 29. Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: part I. Introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol 2013; 69: 173.e1–e13; quiz 85–86. [DOI] [PubMed] [Google Scholar]
- 30. Roujeau JC, Chosidow O, Saiag P, et al. Toxic epidermal necrolysis (Lyell syndrome). J Am Acad Dermatol 1990; 23: 1039–1058. [DOI] [PubMed] [Google Scholar]
- 31. Haber J, Hopman W, Gomez M, et al. Late outcomes in adult survivors of toxic epidermal necrolysis after treatment in a burn center. J Burn Care Rehabil 2005; 26: 33–41. [DOI] [PubMed] [Google Scholar]
- 32. Olteanu C, Shear NH, Chew HF, et al. Severe physical complications among survivors of Stevens-Johnson syndrome and toxic epidermal necrolysis. Drug Saf 2018; 41: 277–284. [DOI] [PubMed] [Google Scholar]
- 33. Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: part II. Prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol 2013; 69: 187.e1–e16; quiz 203–204. [DOI] [PubMed] [Google Scholar]
- 34. Yang CW, Cho YT, Chen KL, et al. Long-term sequelae of Stevens-Johnson syndrome/toxic epidermal necrolysis. Acta Derm Venereol 2016; 96: 525–529. [DOI] [PubMed] [Google Scholar]
- 35. Morales ME, Purdue GF, Verity SM, et al. Ophthalmic manifestations of Stevens-Johnson syndrome and toxic epidermal necrolysis and relation to SCORTEN. Am J Ophthal 2010; 150: 505–510.e1. [DOI] [PubMed] [Google Scholar]
- 36. Yip LW, Thong BY, Lim J, et al. Ocular manifestations and complications of Stevens-Johnson syndrome and toxic epidermal necrolysis: an Asian series. Allergy 2007; 62: 527–531. [DOI] [PubMed] [Google Scholar]
- 37. Gueudry J, Roujeau JC, Binaghi M, et al. Risk factors for the development of ocular complications of Stevens-Johnson syndrome and toxic epidermal necrolysis. Arch Dermatol 2009; 145: 157–162. [DOI] [PubMed] [Google Scholar]
- 38. Gregory DG. Treatment of acute Stevens-Johnson syndrome and toxic epidermal necrolysis using amniotic membrane: a review of 10 consecutive cases. Ophthalmology 2011; 118: 908–914. [DOI] [PubMed] [Google Scholar]
- 39. Ma KN, Thanos A, Chodosh J, et al. A Novel Technique for amniotic membrane transplantation in patients with acute Stevens-Johnson syndrome. Ocul Surf 2016; 14: 31–36. [DOI] [PubMed] [Google Scholar]
- 40. Lopez-Garcia JS, Rivas Jara L, Garcia-Lozano CI, et al. Ocular features and histopathologic changes during follow-up of toxic epidermal necrolysis. Ophthalmology 2011; 118: 265–271. [DOI] [PubMed] [Google Scholar]
- 41. Sheridan RL, Schulz JT, Ryan CM, et al. Long-term consequences of toxic epidermal necrolysis in children. Pediatrics 2002; 109: 74–78. [DOI] [PubMed] [Google Scholar]
- 42. Vazirani J, Nair D, Shanbhag S, et al. Limbal stem cell deficiency-demography and underlying causes. Am J Ophthalmol 2018; 188: 99–103. [DOI] [PubMed] [Google Scholar]
- 43. Basu S, Shanbhag SS, Gokani A, et al. Chronic ocular sequelae of Stevens-Johnson syndrome in children: long-term impact of appropriate therapy on natural history of disease. Am J Ophthalmol 2018; 189: 17–28. [DOI] [PubMed] [Google Scholar]
- 44. Papakostas TD, Le HG, Chodosh J, et al. Prosthetic replacement of the ocular surface ecosystem as treatment for ocular surface disease in patients with a history of Stevens-Johnson syndrome/toxic epidermal necrolysis. Ophthalmology 2015; 122: 248–253. [DOI] [PubMed] [Google Scholar]
- 45. Kohanim S, Palioura S, Saeed HN, et al. Acute and chronic ophthalmic involvement in Stevens-Johnson syndrome/toxic epidermal necrolysis - a comprehensive review and guide to therapy. II. Ophthalmic disease. Ocul Surf 2016; 14: 168–188. [DOI] [PubMed] [Google Scholar]
- 46. Meneux E, Wolkenstein P, Haddad B, et al. Vulvovaginal involvement in toxic epidermal necrolysis: a retrospective study of 40 cases. Obstet Gynecol 1998; 91: 283–287. [DOI] [PubMed] [Google Scholar]
- 47. Petukhova TA, Maverakis E, Ho B, et al. Urogynecologic complications in Stevens-Johnson syndrome and toxic epidermal necrolysis: presentation of a case and recommendations for management. JAAD Case Rep 2016; 2: 202–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kaser DJ, Reichman DE, Laufer MR. Prevention of vulvovaginal sequelae in Stevens-Johnson syndrome and toxic epidermal necrolysis. Rev Obstet Gynecol 2011; 4: 81–85. [PMC free article] [PubMed] [Google Scholar]
- 49. Van Batavia JP, Chu DI, Long CJ, et al. Genitourinary involvement and management in children with Stevens-Johnson syndrome and toxic epidermal necrolysis. J Pediatr Urol 2017; 13: 490.e1–e7. [DOI] [PubMed] [Google Scholar]
- 50. Dore J, Salisbury RE. Morbidity and mortality of mucocutaneous diseases in the pediatric population at a tertiary care center. J Burn Care Res 2007; 28: 865–870. [DOI] [PubMed] [Google Scholar]
- 51. Yang MS, Lee JY, Kim J, et al. Incidence of Stevens-Johnson syndrome and toxic epidermal necrolysis: a nationwide population-based study using national health insurance database in Korea. PLoS One 2016; 11: e0165933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Revuz J, Penso D, Roujeau JC, et al. Toxic epidermal necrolysis. Clinical findings and prognosis factors in 87 patients. Arch Dermatol 1987; 123: 1160–1165. [DOI] [PubMed] [Google Scholar]
- 53. Roujeau JC, Phlippoteau C, Koso M, et al. Sjogren-like syndrome after drug-induced toxic epidermal necrolysis. Lancet 1985; 1: 609–611. [DOI] [PubMed] [Google Scholar]
- 54. Gaultier F, Rochefort J, Landru MM, et al. Severe and unrecognized dental abnormalities after drug-induced epidermal necrolysis. Arch Dermatol 2009; 145: 1332–1333. [DOI] [PubMed] [Google Scholar]
- 55. Bajaj N, Madan N, Rathnam A. Cessation in root development: ramifications of ‘Stevens-Johnson’ syndrome. J Indian Soc Pedod Prev Dent 2012; 30: 267–270. [DOI] [PubMed] [Google Scholar]
- 56. Bequignon E, Duong TA, Sbidian E, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: ear, nose, and throat description at acute stage and after remission. JAMA Dermatol 2015; 151: 302–307. [DOI] [PubMed] [Google Scholar]
- 57. Barrera JE, Meyers AD, Hartford EC. Hypopharyngeal stenosis and dysphagia complicating toxic epidermal necrolysis. Arch Otolaryngol Head Neck Surg 1998; 124: 1375–1376. [DOI] [PubMed] [Google Scholar]
- 58. Hotaling JM, Hotaling AJ. Otologic complications of Stevens-Johnson syndrome and toxic epidermal necrolysis. Int J Pediatr Otorhinolaryngol 2014; 78: 1408–1409. [DOI] [PubMed] [Google Scholar]
- 59. Heimbach DM, Engrav LH, Marvin JA, et al. Toxic epidermal necrolysis. A step forward in treatment. JAMA 1987; 257: 2171–2175. [PubMed] [Google Scholar]
- 60. Lebargy F, Wolkenstein P, Gisselbrecht M, et al. Pulmonary complications in toxic epidermal necrolysis: a prospective clinical study. Intensive Care Med 1997; 23: 1237–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. de Prost N, Mekontso-Dessap A, Valeyrie-Allanore L, et al. Acute respiratory failure in patients with toxic epidermal necrolysis: clinical features and factors associated with mechanical ventilation. Crit Care Med 2014; 42: 118–128. [DOI] [PubMed] [Google Scholar]
- 62. Beck A, Cooney R, Gamelli RL, et al. Predicting mechanical ventilation and mortality: early and late indicators in Steven-Johnson syndrome and toxic epidermal necrolysis. J Burn Care Res 2016; 37: e47–e55. [DOI] [PubMed] [Google Scholar]
- 63. Kamada N, Kinoshita K, Togawa Y, et al. Chronic pulmonary complications associated with toxic epidermal necrolysis: report of a severe case with anti-Ro/SS-A and a review of the published work. J Dermatol 2006; 33: 616–622. [DOI] [PubMed] [Google Scholar]
- 64. Duong TA, de Prost N, Ingen-Housz-Oro S, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: follow-up of pulmonary function after remission. Br J Dermatol 2015; 172: 400–405. [DOI] [PubMed] [Google Scholar]
- 65. McIvor RA, Zaidi J, Peters WJ, et al. Acute and chronic respiratory complications of toxic epidermal necrolysis. J Burn Care Rehabil 1996; 17: 237–240. [DOI] [PubMed] [Google Scholar]
- 66. Kim MJ, Lee KY. Bronchiolitis obliterans in children with Stevens-Johnson syndrome: follow-up with high resolution CT. Pediatr Radiol 1996; 26: 22–25. [DOI] [PubMed] [Google Scholar]
- 67. Hung CC, Liu WC, Kuo MC, et al. Acute renal failure and its risk factors in Stevens-Johnson syndrome and toxic epidermal necrolysis. Am J Nephrol 2009; 29: 633–638. [DOI] [PubMed] [Google Scholar]
- 68. Blum L, Chosidow O, Rostoker G, et al. Renal involvement in toxic epidermal necrolysis. J Am Acad Dermatol 1996; 34: 1088–1090. [DOI] [PubMed] [Google Scholar]
- 69. Cooney R, Beck A, Gonzalez B, et al. Not all drugs are created equal: the importance of causative agent in toxic epidermal necrolysis. J Burn Care Res 2016; 37: e69–e78. [DOI] [PubMed] [Google Scholar]
- 70. Krumlovsky FA, Del Greco F, Herdson PB, et al. Renal disease associated with toxic epidermal necrolysis (Lyell’s disease). Am J Med 1974; 57: 817–825. [DOI] [PubMed] [Google Scholar]
- 71. Chung WH, Chang WC, Stocker SL, et al. Insights into the poor prognosis of allopurinol-induced severe cutaneous adverse reactions: the impact of renal insufficiency, high plasma levels of oxypurinol and granulysin. Ann Rheum Dis 2015; 74: 2157–2164. [DOI] [PubMed] [Google Scholar]
- 72. Fava P, Astrua C, Cavaliere G, et al. Intestinal involvement in toxic epidermal necrolysis. A case report and review of literature. J Eur Acad Dermatol Venereol 2015; 29: 1843–1845. [DOI] [PubMed] [Google Scholar]
- 73. Pradka SP, Smith JR, Garrett MT, et al. Mesenteric ischemia secondary to toxic epidermal necrolysis: case report and review of the literature. J Burn Care Res 2014; 35: e346–e352. [DOI] [PubMed] [Google Scholar]
- 74. Chosidow O, Delchier JC, Chaumette MT, et al. Intestinal involvement in drug-induced toxic epidermal necrolysis. Lancet 1991; 337: 928. [DOI] [PubMed] [Google Scholar]
- 75. Tan YM, Goh KL. Esophageal stricture as a late complication of Stevens-Johnson syndrome. Gastrointest Endosc 1999; 50: 566–568. [DOI] [PubMed] [Google Scholar]
- 76. Misra SP, Dwivedi M, Misra V. Esophageal stricture as a late sequel of Stevens-Johnson syndrome in adults: incidental detection because of foreign body impaction. Gastrointest Endosc 2004; 59: 437–440. [DOI] [PubMed] [Google Scholar]
- 77. Agrawal A, Bramble MG, Shehade S, et al. Oesophageal stricturing secondary to adult Stevens-Johnson syndrome: similarities in presentation and management to corrosive injury. Endoscopy 2003; 35: 454–457. [DOI] [PubMed] [Google Scholar]
- 78. Morelli MS, O’Brien FX. Stevens-Johnson syndrome and cholestatic hepatitis. Dig Dis Sci 2001; 46: 2385–2388. [DOI] [PubMed] [Google Scholar]
- 79. Garcia M, Mhanna MJ, Chung-Park MJ, et al. Efficacy of early immunosuppressive therapy in a child with carbamazepine-associated vanishing bile duct and Stevens-Johnson syndromes. Dig Dis Sci 2002; 47: 177–182. [DOI] [PubMed] [Google Scholar]
- 80. Karnsakul W, Arkachaisri T, Atisook K, et al. Vanishing bile duct syndrome in a child with toxic epidermal necrolysis: an interplay of unbalanced immune regulatory mechanisms. Ann Hepatol 2006; 5: 116–119. [PubMed] [Google Scholar]
- 81. Srivastava M, Perez-Atayde A, Jonas MM. Drug-associated acute-onset vanishing bile duct and Stevens-Johnson syndromes in a child. Gastroenterology 1998; 115: 743–746. [DOI] [PubMed] [Google Scholar]
- 82. Harimoto N, Wang H, Ikegami T, et al. Education and imaging. Hepatology: rare Stevens-Johnson syndrome and vanishing bile duct syndrome induced by acetaminophen, requiring liver transplantation. J Gastroenterol Hepatol 2015; 30: 656. [DOI] [PubMed] [Google Scholar]
- 83. Cavanzo FJ, Garcia CF, Botero RC. Chronic cholestasis, paucity of bile ducts, red cell aplasia, and the Stevens-Johnson syndrome. An ampicillin-associated case. Gastroenterology 1990; 99: 854–856. [DOI] [PubMed] [Google Scholar]
- 84. Lee T, Lee YS, Yoon SY, et al. Characteristics of liver injury in drug-induced systemic hypersensitivity reactions. J Am Acad Dermatol 2013; 69: 407–415. [DOI] [PubMed] [Google Scholar]
- 85. Dodiuk-Gad RP, Olteanu C, Feinstein A, et al. Major psychological complications and decreased health-related quality of life among survivors of Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol 2016; 175: 422–424. [DOI] [PubMed] [Google Scholar]
- 86. Butt TF, Cox AR, Lewis H, et al. Patient experiences of serious adverse drug reactions and their attitudes to medicines: a qualitative study of survivors of Stevens-Johnson syndrome and toxic epidermal necrolysis in the UK. Drug Saf 2011; 34: 319–328. [DOI] [PubMed] [Google Scholar]
- 87. Bliss SA, Warnock JK. Psychiatric medications: adverse cutaneous drug reactions. Clin Dermatol 2013; 31: 101–109. [DOI] [PubMed] [Google Scholar]
- 88. Coss-Bu JA, Jefferson LS, Levy ML, et al. Nutrition requirements in patients with toxic epidermal necrolysis. Nutr Clin Pract 1997; 12: 81–84. [DOI] [PubMed] [Google Scholar]
- 89. Cartotto R. Burn center care of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. Clin Plast Surg 2017; 44: 583–595. [DOI] [PubMed] [Google Scholar]
- 90. Clayton NA, Kennedy PJ. Management of dysphagia in toxic epidermal necrolysis (TEN) and Stevens-Johnson syndrome (SJS). Dysphagia 2007; 22: 187–192. [DOI] [PubMed] [Google Scholar]
- 91. McDonald K, Johnson B, Prasad JK, et al. Rehabilitative considerations for patients with severe Stevens-Johnson syndrome or toxic epidermal necrolysis. A case report. J Burn Care Rehabil 1989; 10: 167–171. [DOI] [PubMed] [Google Scholar]
- 92. Valeyrie-Allanore L, Ingen-Housz-Oro S, Colin A, et al. Pain management in Stevens-Johnson syndrome, toxic epidermal necrolysis and other blistering diseases. Ann Dermatol Venereol 2011; 138: 694–697; quiz 2–3, 8. [DOI] [PubMed] [Google Scholar]
- 93. Jennes S, Pierard GE, Paquet P. Deciphering supportive treatment strategies for toxic epidermal necrolysis. Curr Drug Saf 2012; 7: 361–366. [DOI] [PubMed] [Google Scholar]
- 94. Butt TF, Cox AR, Oyebode JR, et al. Internet accounts of serious adverse drug reactions: a study of experiences of Stevens-Johnson syndrome and toxic epidermal necrolysis. Drug Saf 2012; 35: 1159–1170. [DOI] [PubMed] [Google Scholar]
- 95. Hung SI, Chung WH, Jee SH, et al. Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharmacogenet Genomics 2006; 16: 297–306. [DOI] [PubMed] [Google Scholar]
- 96. Hung SI, Chung WH, Liou LB, et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A 2005; 102: 4134–4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lonjou C, Borot N, Sekula P, et al. A European study of HLA-B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenet Genomics 2008; 18: 99–107. [DOI] [PubMed] [Google Scholar]
- 98. Tohkin M, Kaniwa N, Saito Y, et al. A whole-genome association study of major determinants for allopurinol-related Stevens-Johnson syndrome and toxic epidermal necrolysis in Japanese patients. Pharmacogenomics J 2013; 13: 60–69. [DOI] [PubMed] [Google Scholar]
- 99. Tassaneeyakul W, Jantararoungtong T, Chen P, et al. Strong association between HLA-B*5801 and allopurinol-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in a Thai population. Pharmacogenet Genomics 2009; 19: 704–709. [DOI] [PubMed] [Google Scholar]
- 100. Roujeau JC, Huynh TN, Bracq C, et al. Genetic susceptibility to toxic epidermal necrolysis. Arch Dermatol 1987; 123: 1171–1173. [PubMed] [Google Scholar]
- 101. Power WJ, Saidman SL, Zhang DS, et al. HLA typing in patients with ocular manifestations of Stevens-Johnson syndrome. Ophthalmology 1996; 103: 1406–1409. [DOI] [PubMed] [Google Scholar]
- 102. Ueta M, Tokunaga K, Sotozono C, et al. HLA class I and II gene polymorphisms in Stevens-Johnson syndrome with ocular complications in Japanese. Mol Vis 2008; 14: 550–555. [PMC free article] [PubMed] [Google Scholar]
- 103. Ueta M, Tokunaga K, Sotozono C, et al. HLA-A*0206 with TLR3 polymorphisms exerts more than additive effects in Stevens-Johnson syndrome with severe ocular surface complications. PLoS One 2012; 7: e43650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chen P, Lin JJ, Lu CS, et al. Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. N Engl J Med 2011; 364: 1126–1133. [DOI] [PubMed] [Google Scholar]
- 105. Chen Z, Liew D, Kwan P. Effects of a HLA-B*15:02 screening policy on antiepileptic drug use and severe skin reactions. Neurology 2014; 83: 2077–2084. [DOI] [PubMed] [Google Scholar]
- 106. Toh DS, Tan LL, Aw DC, et al. Building pharmacogenetics into a pharmacovigilance program in Singapore: using serious skin rash as a pilot study. Pharmacogenomics J 2014; 14: 316–321. [DOI] [PubMed] [Google Scholar]
- 107. Rattanavipapong W, Koopitakkajorn T, Praditsitthikorn N, et al. Economic evaluation of HLA-B*15:02 screening for carbamazepine-induced severe adverse drug reactions in Thailand. Epilepsia 2013; 54: 1628–1638. [DOI] [PubMed] [Google Scholar]
- 108. Abe J, Mataki K, Umetsu R, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: the food and drug administration adverse event reporting system, 2004-2013. Allergol Int 2015; 64: 277–279. [DOI] [PubMed] [Google Scholar]