Abstract
Background:
The search for an accurate, gene-based test to identify heritable risk factors for Reward Deficiency Syndrome (RDS) was conducted based on hundreds of published studies about the role of dopamine in addictive behaviors, including risk for drug dependence and compulsive/impulsive behavior disorders. The term RDS was first coined by Blum’s group in 1995 to identify a group of behaviors with a common neurobiological mechanism associated with a polymorphic allelic propensity for hypodopaminergia.
Objectives:
To outline the process used to select risk alleles of reward genes for the Genetic Addiction Risk Score (GARS) test. Consequently, to address the limitations caused by inconsistent results that occur in many case-control behavioral association studies. These limitations are perhaps due to the failure of investigators to adequately screen controls for drug and alcohol use disorder, and any of the many RDS behaviors, including nicotine dependence, obesity, pathological gambling, and internet gaming addiction.
Methods:
Review of the literature related to the function of risk alleles of reward genes associated with hypodopaminergia relevant case-control association studies for the selection of alleles to be measured by the Genetic Addiction Risk Score (GARS) test.
Results:
The prevalence of the DRD2 A1 allele in unscreened controls (33.3%), compared to “Super-Controls” [highly screened RDS controls (3.3%) in proband and family] is used to exemplify a possible solution.
Conclusion:
Unlike one gene-one disease (OGOD), RDS is polygenetic, and very complex. In addition, any RDS-related behaviors must be eliminated from the control group in order to obtain the best possible statistical analysis instead of comparing the phenotype with disease-ridden controls.
Keywords: Behavioral genetic research, case controlled studies, genetic addiction association studies, genetic prevalence, hypodopaminergia, Reward Deficiency Syndrome (RDS), Single Nucleotide Polymorphisms (SNPs), study controls, super controls
1. INTRODUCTION
The primary basis for an accurate test to identify heritable risk resides in the identification of risk alleles linked to genes in the brain’s reward circuitry, that contribute to reward deficiency. The test recently developed by Blum’s group is known as the Genetic Addiction Risk Score (GARS), for which the United States Patent and Trademark Office (USPTO) issued a patent on 9/11/18 [1]. Reward Deficiency Syndrome (RDS) was first coined by Blum’s group [2] to identify a group of behaviors that includes both drug addictive, compulsive and impulsive behaviors, and provides the rationale for suggesting a common genetic rubric.
1.1. Reward Deficiency Syndrome (RDS)
Reward Deficiency Syndrome (RDS) involves dopamine resistance, a form of sensory deprivation of the brain’s reward or pleasure mechanisms. The syndrome occurs because of an individual’s inability to derive reward from ordinary, everyday activities. Reward deficiency can be relatively mild or severe, and addiction is a manifestation of RDS. The subject of extensive peer review RDS is a disorder of the neurochemistry of the brain and affects over one-third of the US population. Dopamine is a principal component of brain function and RDS. The healthy function of molecular neuroanatomy ultimately results in the release of the neurotransmitter dopamine which is the key to feelings of well-being, motivation and happiness.
Dopamine induces “pleasure” and reduces “stress.” This phenomenon, the neuronal release of dopamine at the reward site of the brain, the Nucleus Accumbens (NAc), involves a complicated cascade of neurotransmission called the “Brain Reward Cascade” (BRC). Dopamine released into the synapse results in feelings of well-being and reduced stress.
1.1.1. The Brain Reward Cascade (BRC)
The activation of the dopamine post-receptor site in the brain reward center is facilitated by the interaction of many other brain chemicals. The amount of dopamine release relies on the upstream neurotransmitter serotonin, to stimulate endorphins and enkephalin. Subsequently, endorphins regulate the activity of GABA then GABA regulates the actual release of dopamine in the reward site of the brain [3].
The BRC of the mesocorticolimbic dopaminergic pathway plays an especially important role in mediating natural reward like sexual drive and hunger, as well as unnatural rewards, like substance-seeking. Natural rewards include the satisfaction of physiological drives, while unnatural rewards are learned and involve satisfaction of acquired pleasures, such as hedonic sensations. Alcohol and other drugs, as well as most positive reinforcers like sex, food, gambling and aggressive thrills, cause activation and neuronal release of brain dopamine into the synapses. The dopamine release can decrease negative feelings and satisfy abnormal cravings for alcohol, cocaine, heroin, nicotine, and with chronic use, exacerbate low dopamine function.
Following extensive research, a new understanding of how these substances influence the neurology of dopamine release and addictive behaviors arose. The research determined that alcoholism is like dependence on opiates, cocaine, nicotine, food, and some repetitive behaviors, like gaming, sex addiction. Both psychoactive drugs and certain behaviors produce a surge of dopamine in the midbrain (mesolimbic reward center), the biological substrate for addictive behavior. Individuals, genetically predisposed to crave (“want”) dopamine release, are at higher risk for addiction due to environmental and genetic factors that can, especially, in combination, reduce dopamine release and cause craving.
There are multitudes of genetic studies that associate specific behaviors with identified reward gene alleles, Single Nucleotide Polymorphisms (specific SNPs) within the mesolimbic pathway [4, 5]. These studies are descriptions of the contribution made by polymorphisms (sequence variations) that affect the healthy function of reward genes and association studies that illustrate why the presence of these alleles in a gene panel indicates genetic risk for associated behaviors. Notably, the suggestion is that the actual phenotype is RDS, and deficiencies in the brain’s reward cascade, either hereditary or environmental (epigenetically produced), are responsible for impulsive, compulsive, and addictive behaviors both substance and non-substance [2, 6] (Table 1).
Table 1.
The basis of the RDS behaviors listed here is a shared hypodopaminergia.
| Addictive Behaviors | Impulsive Behaviors | Obsessive Compulsive Behaviors | Personality Disorders | ||
|---|---|---|---|---|---|
| Substance-related | Non-substance Related | Spectrum Disorders | Disruptive Impulsive | ||
| Alcohol | Thrill-seeking (novelty) | Attention-deficit Hyperactivity | Anti-social | Body Dysmorphic | Paranoid |
| Cannabis | Sexual Sadism | Tourette and Tic Syndrome | Conduct | Hoarding | Schizoid |
| Opioids | Sexual Masochism | Autism | Intermittent Explosive | Trichotillomania-mania (hair pulling) | Borderline |
| Sedatives Hypnotics | Hypersexual | - | Oppositional Defiant | Excoriation (skin picking) | Schizotypal |
| Stimulants | Gambling | - | Exhibitionistic | Non-suicidal Self-injury | Histrionic |
| Tobacco | Internet Gaming | - | - | - | Narcissistic |
| Glucose | - | - | - | - | Avoidant |
| Food | - | - | - | - | Dependent |
RDS behaviors include substance use disorders. Dependence on alcohol, psychostimulants, marijuana, nicotine (smoking) and opioid misuse with altered opiate receptor function, carbohydrates,; sugar-binging and obesity are substance-related RDS. Pathological gambling, sex addiction, reactive aggression, pathological aggression, and some personality disorders are non-substance RDS behaviors. RDS personality disorders include novelty seeking and non-suicidal self-mutilation. Polygenes are involved, and these RDS behaviors induce pre-synaptic dopamine release in the NAc. Spectrum disorders, such as Attention Deficit Hyperactivity Disorder (ADHD), Tourette’s Syndrome, and Autism, involve dopamine deficiency due to genetic, dopamine dysregulation. There are many hundreds of study results that support the theory that polymorphisms of the reward genes identified in the brain reward system are significantly associated with these reward-dependent traits.
Reward deficiency [7] is a type of flawed dopamine metabolism and function, linked to gene variants that cause hypodopaminergia. These polymorphisms affect the function of the genes involved in the Brain Reward Cascade, for example, the dopamine D2 receptor gene makes D2 receptors, and the polymorphism (variation) A1 causes a reduction in receptor numbers (30–40% fewer receptors at birth) [8]. The established concept of RDS helps to identify a complex array of behaviors, associated with molecular dysfunctions in the mesolimbic system of the brain. Essentially, high-risk individuals seek behaviors and substances including alcohol, opiates, cocaine, nicotine, and glucose known to cause the preferential release of dopamine at the NAc. Activation of the dopaminergic pathways offset low dopaminergic function, caused by gene variants in the BRC.
Comprehension of this shared mechanism will eventually lead to improved diagnosis, treatment, and relapse prevention. We cannot as yet claim that we have “hatched the behavioral addiction egg,” We are, however, starting to make the right inquiries. Based on numerous independent studies from around the world, it is becoming increasingly clear that risk analysis of reward gene polymorphisms can provide vital information for addiction clinicians. Meanwhile, many studies are investigating high and low drug metabolism, in particular, for opiates like buprenorphine/naloxone with polymorphisms of the P450 system [9]. Many firmly believe that pharmacogenetic testing is relevant to clinical practice. However, RDS risk for drug and non-drug addictive behaviors cannot be identified by using pharmacogenetics alone [10] and requires a detailed understanding of the BRC and related genetic variants.
2. MATERIALS AND METHODS: DEVELOPMENT OF GARS
To develop the Genetic Addiction Risk Score (GARS), we first selected ten candidate genes from the plethora of chemical messengers involved in the neurotransmission of dopamine. The neurotransmission of dopamine follows a systematic interaction of many neurotransmitters and secondary messengers involved in signal transmission across the brain circuitry. Indeed, it is the net release, regulated catabolism, and receptor function of dopamine that is responsible for brain health and impulse control. The neurotransmitter dopamine is responsible for feelings of well-being and stress reduction [11]. Genes selected based on their influence on the net release of dopamine at the brain reward site were the Dopamine Receptors (DRD1, 2, 3, 4), Dopamine Transporter (DAT1), Serotonin Transporter (5-HTTLPR), COMT, MAO, GABA, Mu Opiate Receptor (OPRM1). The sequence variants or SNPs, including point-mutations of those genes, were chosen to reflect a hypodopaminergic trait. The basis of the selection was association studies; experimental vs. controls provided strong evidence that specific alleles support a hypodopaminergic trait (Table 2).
Table 2.
The reward gene studies found in PUBMED on November 12th, 2017.
| GENE | STUDIES |
|---|---|
| SEROTONIN RECEPTOR 2a/c | 108 |
| SEROTONIN TRANSPORTER | 391 |
| COMT | 579 |
| MONAMINE OXIDASE-A | 253 |
| DOPAMINE D1 RECEPTOR | 468 |
| DOPAMINE D2 RECEPTOR | 1493 |
| DOPAMINE D3 RECEPTOR | 322 |
| DOPAMINE D4 RECEPTOR | 521 |
| DOPAMINE TRANSPORTER | 237 |
| DOPAMINE-BETA-HYDROXYLASE | 142 |
| OPIOID RECEPTOR | 739 |
| GABA RECEPTOR | 183 |
After an exhaustive review of the genetic literature related to all RDS behaviors followed by initial testing, only those alleles that lead to hypodopaminergia as the overall risk were selected (except for dopamine D3 gene). In the review process, we sought to reduce the number of possible genes and alleles and to eliminate spurious results and, as such, by trial and error, following adding and subtracting genes and alleles, decided on the proposed 11 allele panel from ten genes. For example, in place of using serotoninergic receptors, the serotonin transport was chosen as a way to track serotonin in the synapse [12] which resulted in an accurate and significant prediction of drug and alcohol severity, linked to a clinical outcome referred to as the Addiction Severity. Index (ASI-Media version V). This work was a substantial undertaking involving many alleles, genes, kinases, and second messengers. The use of the Brain Reward Cascade (BRC), the result of many years of work done by Blum et al. and others globally [1], Fig. (1) helped guide our search. In support, Li and associates [6] found over 800 haplotypes but tracked them to two major pathways glutaminergic and dopaminergic; this provided further rationale for the GARS selection criteria.
Fig. 1.
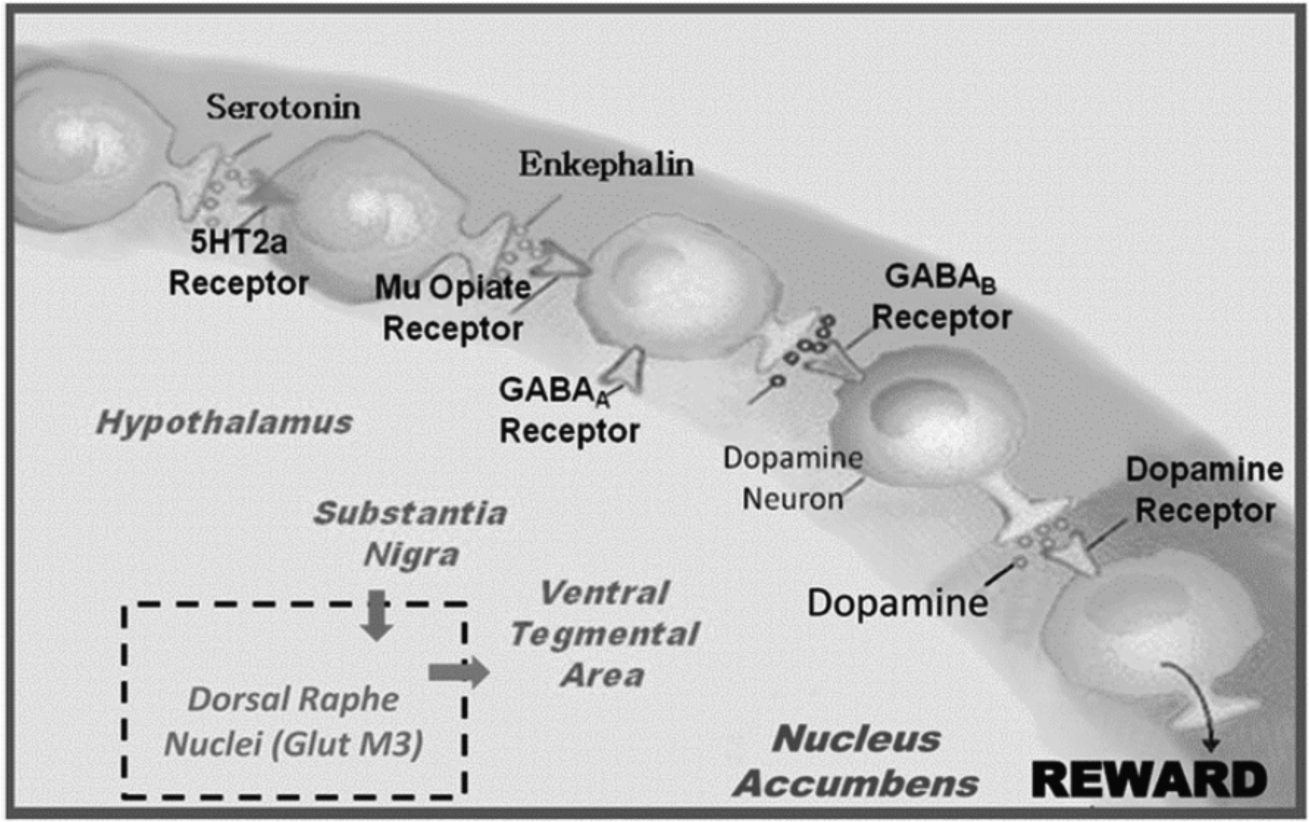
The interaction of the primary neurotransmitters of the Brain Reward Cascade (BRC). Schematic of the interaction of neurotransmitters within the mesolimbic reward system. Modified from [4].
Ten genes and 11 common polymorphisms including Single Nucleotide Polymorphisms (SNPs), and Variable Number Tandem Repeats (VNTRs) connected to the promotion of a genetically-induced hypodopaminergia were selected for the GARS test [13]. The presence of hypodopaminergia is a complicated but determining condition of the GARS test results. The search for studies that report low-dopamine function associated with specific SNPs of reward genes formed the cornerstone of the development of the GARS test [14]. While there are many possible addiction-related genes; as pointed out by Li et al. [6], neurotransmitter pathways located in the mesolimbic/pre-frontal cortices including the Serotonergic, Cannabinoidergic, Endorphinergic, GABAergic, Glutaminergic, and Dopaminergic, pathways are related to brain reward functioning and any dysfunction can result in unwanted dopaminergic dysregulation [6]. Polymorphisms of reward genes that have been correlated with chronic dopamine deficiency and reward-seeking behavior were selected for the genetic panel (Table 3).
Table 3.
GARS is based on the following genes linked to hypodopaminergia.
| Gene | Polymorphism | Location | Risk Allele | Function Linked to Hypodopaminergia | Reference |
|---|---|---|---|---|---|
| DRD1 | rs4531 or rs4532 | Chr −5 | A | rs4532 is known to reduce the function of the DRD1 gene, which is needed as a “go” drive to activate D1 receptors causing normal dopamine function. | Batel et al. (2008) [15]. |
| DRD2 | rs1800497 | Chr-11 | A | rs1800497 equates functionally to 30–40% lower density of dopamine D2 receptors resulting hypodopaminergic function | Noble et al (1991) [8]. |
| DRD3 | rs6280 | Chr3 | C | Rs6280 in D3 causes an imbalance of the DRD3 function and has assiciated with Heroin Dependence. | Kuo et al. (2014) [16]. |
| DRD4 | 48 base repeat VNTR | Chr 11 exon 3 | Above 7 R | Similar to the D2, the VNTR 7 R and above results in a hypodopaminergia due to lower receptor function. | Van Tol (1998) [17] |
| COMT | rs4680 | Chr 22 | G | Carrying the 9 R allele leads to a high activity of catabolism of dopamine in synapse inducing a hypodopaminergia in the synapse. | Isir et al. (2008) [18] and Wichers et al. [19] |
| OPRM1 | rs1799971 | Chr 6 | G | The G allele is the risk variant of the MOR 118A>G (p.Asn40Asp; SNP rs1799971) promotes a low dopamine function because there will be a lack of inhibition via the GABA inhibitory control of dopamine release at the reward site Nucleus Accumbens (NAc) inducing hypodopaminergia. | Ray et al. (2011) [20] |
| DAT1 | 40 base repeat VNTR | Chr 5 exon 15 | 9R | Carrying the 9 R allele leads to a high activity inducing a hypodopaminergia in the synapse. The DAT1 clears excess dopamine released from the pre-neuron into the synapse and prevents uptake into the receptors on the next neuron. | Byerley et al. (1993) [21]. |
| MOA-A | 30 base repeat VNTR | Chr X Promotor | 3.5 R, 4R | The 3.5 R and 4 R variants are more active than 3 R or 5 R. Excessive amounts of dopamine are broken down in the presynaptic neuron which may result is less dopamine availability for release into the synaptic cleft arriers of the 3.5 and 4 R may display hypodopaminergia (low dopamine function). | Contini et al. (2006) [22]. |
| 5HTTLPR | 43 base repeat INDEL/VNTR plus rs 25531 | Chr 17 | LG, S | The risk variant has 43 base -pair 5″ insertion/deletion, S′ at SNP rs25531. The long allele results in higher serotonin transporter mRNA transcription in human cell lines., The result is that Serotonin is highly reabsorbed from the synapse into the pre-nerve cell causing low serotonin content in the synapse leading to reduced function. | Merenakk et al. (2011) [23] and van der Zwaluw et al. (2010) [24]. |
| GABRB3 | CA repeat DNR | Chr 15 (downstream) | 181 | GABRA3 gene and the risk variant is CA-repeat, whereby allele 181 results in higher activity. This risk allele if overexpressed will cause low dopamine function hypodopaminergia leading to SUD because of inhibition of dopamine release at reward site | Namkoong et al. (2008) [25]. |
In terms of selection, one example of the detailed and careful selection relates to the dopamine transporter (DAT1) gene. The authors theorized that carriers of the 9 allele of the DAT1 gene would present an improved treatment reaction with buprenorphine, because its transport function; which results in the hypodopaminergic attribute, is four times faster than the 10 allele.
The development of a polygenic polymorphic test to evaluate the risk for all addictive behaviors is a worthwhile endeavor, and some studies have addressed this possibility for future clinical practice. Gerra et al. [10] provided clear evidence that buprenorphine treatment response in humans with heroin use disorder is related to the dopaminergic system. In the incidence of kappa opioid receptor (OPRK1) 36G>T SNP, they were surprised to have found no difference between responders and non-responders to buprenorphine. Nevertheless, the incidence of DAT gene polymorphism allele 10 (SLC6A3/DAT1), was significantly increased in “non-responder,” above “responder” persons (64.9% vs. 55.9%). The incidence of the class of additional alleles was increased in the responder group, rather than in non-responder persons (11.0% vs. 2.1% respectively). These outcomes dovetail with the effort of others, presenting improved treatment results and agreement also based on dopaminergic polymorphisms, where hypodopaminergic qualities facilitate an enhanced reaction throughout treatment [26]. While the ten gene panel with associated polymorphisms based on these and many other studies reviewed previously represents the “state of the art,” we encourage others to further the development of a risk stratification test for RDS behaviors.
Pearson-Fuhrhop et al. [26] produced a genetic risk score by merging the functional polymorphisms from five genes involved in synaptic dopamine availability, the DAT and COMT, and genes involved in dopamine receptor binding DRD1, DRD2, DRD3. They drew data from three separate groups: 1) a discovery group of healthy adult subjects (n = 273); 2) a duplication group of adults suffering from depression, (n = 1,267); and 3) a group of healthy adult subjects (n = 382). Their genetic risk score associated with depressive symptomatology with poor dopamine function and specified decreased dopaminergic neurotransmission that anticipated increased levels of depression. The authors then simulated these results based on genetic data from adults suffering from depression using a comparable genetic risk score. Based on these results, Pearson-Fuhrhop et al. [26] suggested that a sequence variation in multiple dopaminergic genes may influence depressive symptoms in an additive manner. There are, however, negative reports, from others in terms of depression and dopaminergic genetics [27]. While the GARS test is not designed to determine depressive symptoms per se, Blum’s group and others have suggested that a primary symptom of depression is anhedonia, which is, indeed, a subset of RDS behaviors [28].
The algorithm governing GARS identifies the gene in which the polymorphism is found and counts the risk alleles for each subject. Each allelic gene polymorphism was selected based on an understanding of the physiology and many case-controlled associations found in the literature. The exponential growth of the Behavioral Genetics field notwithstanding, there is confidence that we can rely on the case controls used in the GARS research, and that, based on results from the literature, each allele selected is associated with high risk for RDS behaviors (Table 3). However, it is noteworthy that highly-screened controls (eliminating any addictive, compulsive and impulsive behaviors in both proband and family), have importance in all genetically based research in the field of behavioral addictions especially in the study of vulnerable, addiction-prone populations. While the selection of the ten gene panel relies on the reward cascade (BRC) which mirrors the mesolimbic reward circuit (Fig. 1), the selection also included consideration of hundreds of case-control studies of those genes and their polymorphic variants. Additional GARS research involved the relationship of dopaminergic genes and the prediction of risk severity for substance misuse [1, 2] without needed RDS free controls. While as yet positive GARS results have not been compared to RDS free controls, the goal is to do so.
3. SOME LIMITATIONS AND STRATEGIES OF GENETIC ASSOCIATION STUDIES
3.1. Adequately Screened Controls
Blum’s laboratory is currently developing RDS-free controls which will provide additional adjustments to GARS. The development of RDS free controls will enable the calculateion of Odds Ratios (ORs) for each experimental risk allele. The ORs may provide the actual contribution to the variance for any risk allele and enable the “true” weighting of each polymorphism.
The goal is to develop RDS-free controls by eliminating all known RDS behaviors in, not only the probands but their family as well. These subjects were referred to as “Super Controls” in a previously published study [29]. “Super Controls” were identified for the DRD2 A1 allele and were also used to determine the role of percent body fat as a function of the DRD2 A1 allele [30].
Much of the current literature involving genetic studies in the field of drug and non-drug behavioral addictions is flawed because of disease-laden controls displaying many RDS behaviors. Reward Deficiency Syndrome, now a featured disorder in the SAGE Encyclopedia of Abnormal & Clinical Psychology [31], includes a remarkable list of behaviors (Table 1). While exploring the dopamine D2 receptor gene (DRD2) variants and percent body fat, a known subset of RDS, in an earlier study [29] Blum’s group genotyped 122 obese/overweight (O/OW) Caucasian subjects and compared them with 30 non-obese Caucasian controls, screened to exclude substance abuse. These subjects were assessed for weight, body mass index (BMI; kg m-2) and percent body fat using Dual-Energy X-Ray Absorptiometry (DEXA).
It is noteworthy that this previous research controlling for any known RDS behaviors, in, for example, overweight probands and their families resulted in a very significant reduction of DRD2 A1allelic prevalence “Super Controls” (Fig. 2) [29].
Fig. 2.
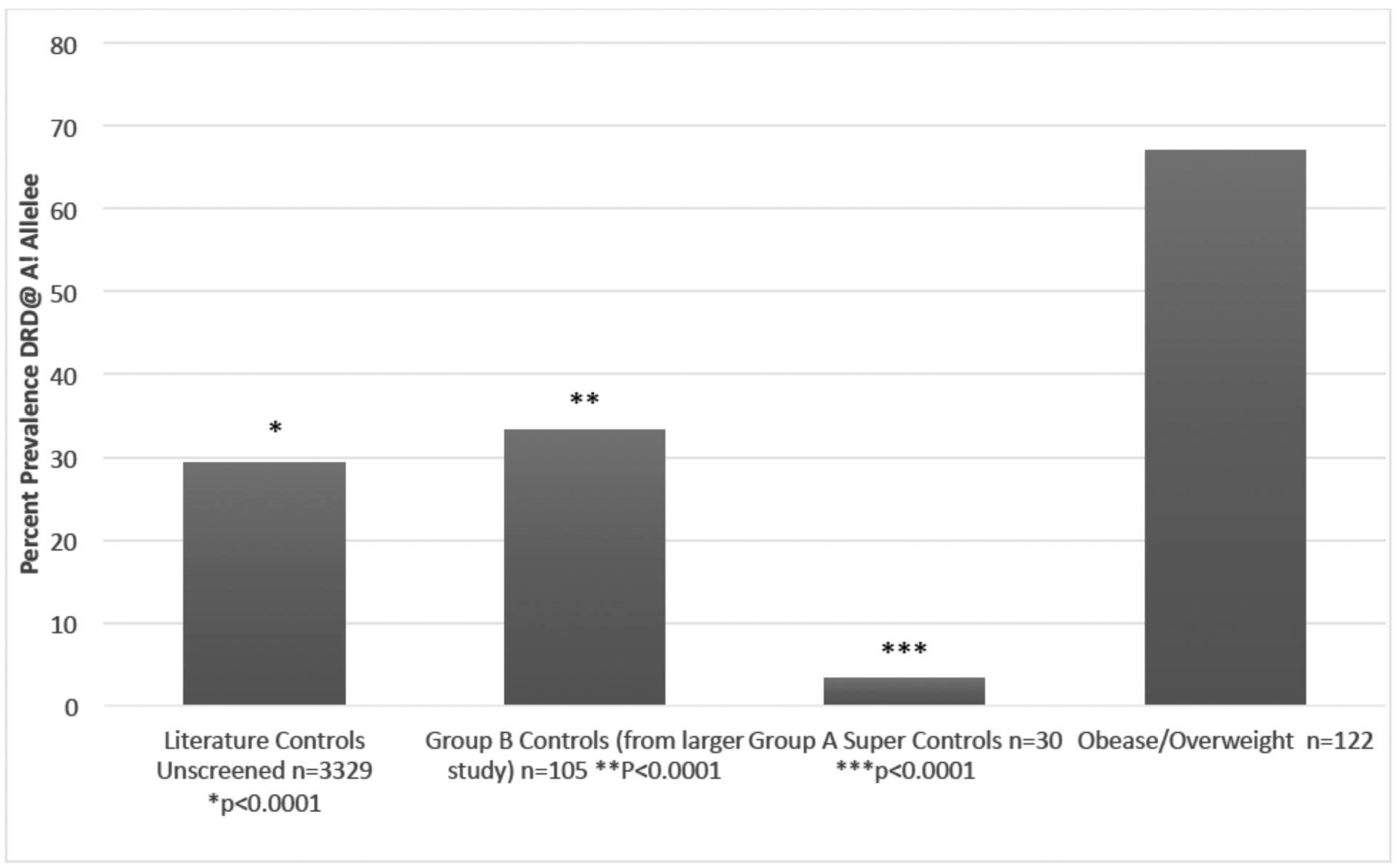
The percentage prevalence of the A1 allele in variously screened controls.
Fig. (2) compares the percentages of the DRD2 Taq1 A1 allele in an unscreened literature controls 29.4% (P≤0.001) [30] to Group B Controls from an earlier larger study screened (for drug abuse and obesity) 33.3% [29], Super controls 3.3%, and an Overweight and Obese cohort 67% [30].
The point here is to illustrate that after carefully eliminating every RDS behavior in the proband and family of 183 people attending a family practice clinic, we found a total of 30 people that were considered RDS-free. When this RDS free cohort was genotyped for the DRDA1 allele, one individual out of 30 carried the risk allele - a 3.3% prevalence [29]. This finding strongly suggests that the completion of an on-going study to obtain a larger RDS-free control cohort will facilitate the most accurate results possible for the GARS test and other genetic associations.
An enormous amount of research, funding and diligent work has accrued since1990 in the area under investigation; Psychiatric Genetics, however, without carefully controlling for RDS-free controls, we are mixing diseased controls with experimental subjects. The results, as observed, for example, in the Bolos et al. [32] study, failed to show the now well-known association of the DRD2 A1 and alcoholism. The flaw in their study was the use of non-alcoholic controls from a French population which was later found to have Tourette’s Syndrome, a subset disorder in RDS. Many other studies and meta-analyses which have at least controlled for smoking behavior along with SUD, have resulted in a clear, worldwide acceptance of the association between DRD2-A1 and alcohol use disorder (AUD). In a recent study, involving almost 275,000 subjects, Penn State investigators found that at least two polymorphisms of the DRD2 gene are required to identify risk for heavy drinking and AUD [33] as stated originally in the 1990 JAMA study Blum and Noble et al. [8, 34] albiet a different allele.
3.2. Strategy to Obtain RDS-free Controls
The initial plan to obtain RDS free controls involves the validation of a 29-item questionnaire related to a Reward Deficiency Syndrome Index (RDSI). The work is in progress in conjunction with Dr. Zsolt Demetrovics and staff at the Eotvos Loránd University, Institute of Psychology, Budapest, Hungary. The RDSI data has been collected from over 1500 students attending the University, and 850 of these students have been GARS tested. The goal is to match low RDSI behavior scores with GARS genotypes to determine RDS-free controls. Another goal is a collaboration with a Texas-based hospital to develop a computer-based program to identify subjects without any RDS behaviors using interview histories (both proband and family) from the hospital population.
Also, we intend to utilize the RDSI in a local collection of the general population to obtain and genotype RDS free individuals. Determination of RDS free controls would require that the subjects have from 0 to 3 of any alleles measured with GARS test and a low RDS Index. The final criteria to find RDS free controls is to determine an odds ratio (OR) to measure the strength of the association between each allele with the experimental group and the RDS-free control group and then to develop a weighted analysis providing multiplier power for each gene in GARS.
3.3. Allelic Prevalence
As stated previously, earlier work from Blum’s group [29, 30] compared the results of DRD2 A1 association studies with a tested RDS-free control and found that the percent prevalence of just one gene the DRD2 A1 allele was different across unscreened, screened and RDS free controls. The study involved 183 probands attending a generalized family practice clinic in Princeton, NJ. Through a computerized algorithm involving many RDS behavioral search words related to the probands and their families, using behaviors based on intake questionnaires and structured interviews, a total of 30 RDS free controls were obtained. When genotyped the range of prevalence of the DRD2 A1 allele was from 49.3% to 29.4% in non-alcoholic screened controls. RDS free controls showed only one out of 30 and, as such, only a 3.3% prevalence in these non-disease super controls (Fig. 3).
Fig. 3.
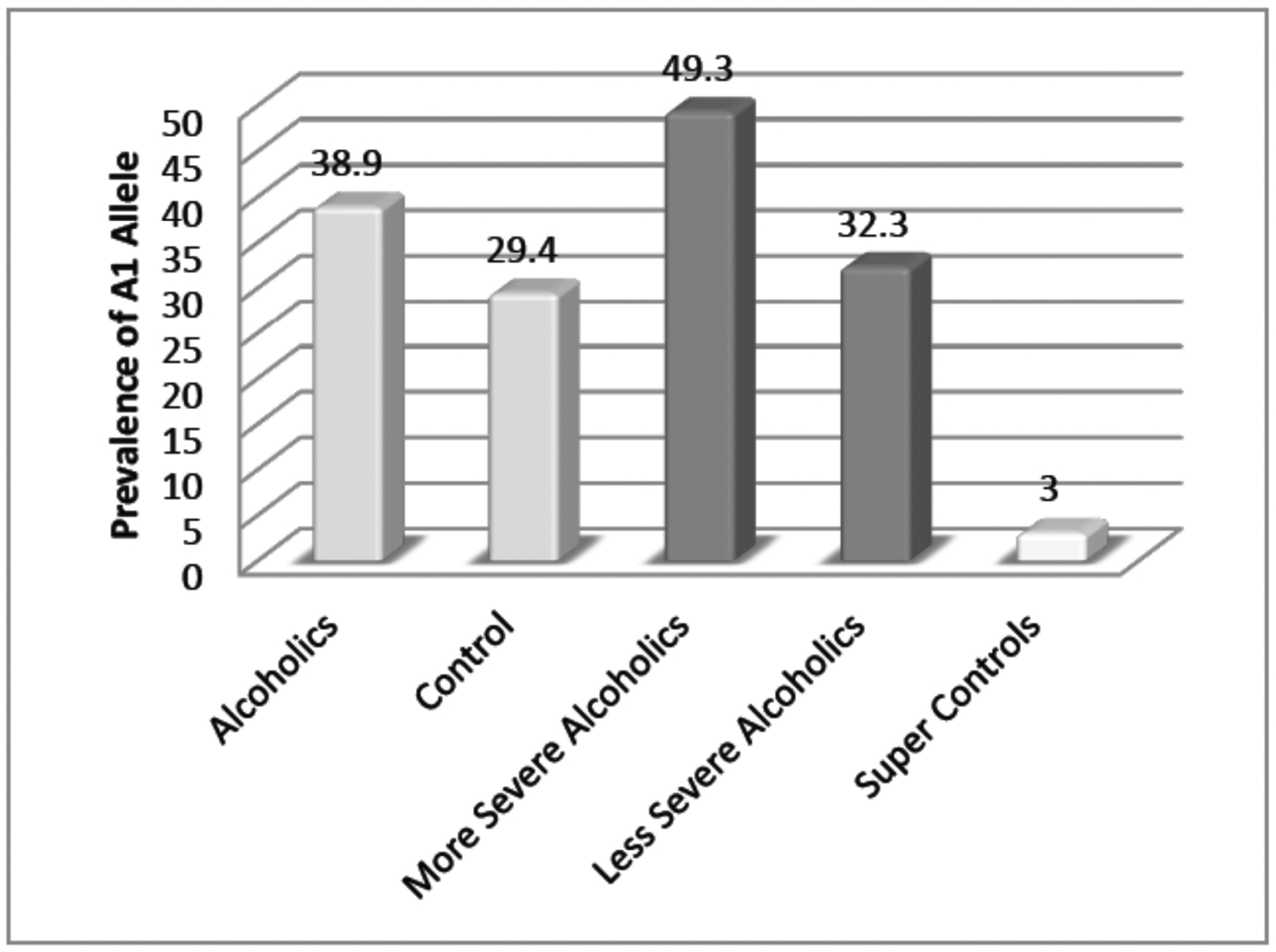
Prevalence of DRD2 A1 allele in severe alcoholics and less severe alcoholics compared to super controls.
Blum along with Marjorie Gondre Lewis of Howard University, in unpublished research, has already shown that 100 percent of 35 methadone or Suboxone dependent patients carry at least 4 or more alleles found in the GARS panel of ten genes and eleven polymorphisms. Sixty percent of these patients tested showed 7 or more alleles and as such revealing high risk for alcoholism.
Table 4 represents a very small sample of percent prevalence of each SNP measured in GARS in a mixed population with poorly screened controls (Table 5). It is provided so that the interested reader can observe that the prevalence for each SNP seems relatively high and therefore RDS -free controls are needed. However, the magnitude of many (in some cases thousands of studies) known functions of each SNP leading to physiologically low dopamine function provides strong confidence that our risk allele gene selection across the reward circuitry is prudent. However, the goal is to develop these controls not because it is easy, but because it is hard. NIDA scientists, including Jean Lud Cadet, Chief of the Molecular Neuropsychiatry Research Branch and his team agree that the development of RDS free controls is essential. They pointed out in their study of genetic and environmental risk factors for Cannabis use: “Exclusion criteria included serious mental health disorders and severe somatic disorders, use of other drugs and alcohol abuse; control subjects were not screened to remove Reward Deficiency Syndrome (RDS) behaviors” [46].
Table 4.
Percent prevalence GARS measured SNPs in non-RDS free controls and mixed ethnicity.*
| GENE | SNP | Percent Prevalence | Comment | Reference |
|---|---|---|---|---|
| DRD1 | rs4532 | AG = 49* | Drug & alcohol-free but not nicotine and other RDS behaviors Indian population | Prasad et al. [35]. |
| DRD2 | rs1800497 | DRD2-ANKK1 2137G>A (Taq1A) = 22.7 | Controls Never smokers but not well screened for other RDS behaviors | Verde et al. [36]. |
| DRD3 | rs6280 | Ser9Gly variant = 41 | Western Region of Minas Gerais, MG, Brazil, unscreened | Pinto et al. [37]. |
| DRD4 | 48 base repeat VNTR (Chr11 exon3) 7 R & 8 R | Greater than 7R = 19.2 | Worldwide general population | Ding et al. [38]. |
| DAT1 | 40 bases repeat VNTR (hr5,exon15) 9R | VNTR Polymorphism of DAT Gene (SLC6A3) = 9/10 = 38 | Controls healthy European Americans, however, a smoking status not established nor many other RDS behaviors excluding substance abuse. | van Dyck et al. [39]. |
| COMT | Val158/108Met-A allele | GA = 29.4 | Without any history of systematic diseases, psychiatric disorders, asthma, cancer, cataracts, or psychotic disorder with major depression, schizophrenia, and bipolar disorder were selected. But not specific to RDS behaviors. | Saravani et al. [40]. |
| MOA-A | 30 bases repeat VNTR Chr X Promoter 3.5 R, 4 R | 3.5 R =58; 4.5R = 41 | Korean children only ADHD screened but no RDS behaviors too young to determine | Hwang et al. [41] |
| 5HTTLPR: (SLC6A4) | 43 bases repeat INDEL/VNTR Chr 17 risk alleles LG. S | Heterozygous short allele = 41 | General population white non-Hispanic | Haberstick et al. [42]. |
| 5HTTLPR: (SLC6A4) | rs25531 and risk alleles | Rs2531= 68 | Indian control no alcohol dependence and other drugs and no psychosis or bipolar depression but nicotine not excluded or any other RDS behavior | Malhotra et al. [43]. |
| Rs4532 Mu-opioid Receptor (OPRM1) | rs1799971 Chr6 risk allele G | rs1799971 AG = 30 | Treatment-Emergent Suicidal Ideation (TESI) subjects following anti-depressant medication divided into TESI (yes) or TESI (No). No RDS screened. | Nobile et al. [44]. |
| GABRB3 | Alpha 3 Chr15 (DNR) risk allele 181 (non G1) | 181= 35 | ADHD compared to non-ADHD controls not screened for RDS behaviors | Gade et al. [45]. |
Table 5.
Global heterozygous prevalence.
| SNP | Global Heterozygous Prevalence |
|---|---|
| rs4532 | 32% |
| rs1800497 | 46% |
| rs 6280 | 41% |
| rs1800955 | Frequency of C allele =.42* |
| rs4680 | 42% |
| rs1799971 | 29% |
Prevalence not available
Note resources used to build Table 5:
CEU-180 samples of Utah residents with Northern and Western European ancestry from the CEPH collection (originally 30 mother-father-child trios); CHB-90 samples of Han Chinese in Beijing, China (previously called HCB, originally 45 unrelated samples); JPT - 91 samples of Japanese in Tokyo, Japan (originally 44 unrelated samples);
YRI-180 samples of Yoruba in Ibadan, Nigeria (originally 30 Yoruba mother-father-child trios); In Phase III of the study, seven additional populations were added to the study;
ASW-90 samples of African ancestry in Southwest USA;
CHD-100 samples of Chinese in Metropolitan Denver, Colorado;
GIH-100 samples of Gujarati Indians in Houston, Texas;
LWK-100 samples of Luhya in Webuye, Kenya;
MEX-90 samples of Mexican ancestry in Los Angeles, California;
MKK-180 samples of Maasai in Kinyawa, Kenya;
TSI-100 samples of Toscani in Italia.
4. POPULATION GARS PREVALENCE
Table 6 represents the GARS results involving 419 samples just stratified by ancestry in terms of allelic frequency. While there are prevalence differences between ethnic groups, the number is too small to make any definitive statements. However, ethnicity is an essential caveat in trying to understand actual genetic risk and must be factored into all association and microarray studies.
Table 6.
Frequency of risk alleles in GARS results.
| GENE | COMT | DRD1 | DRD2 | DR3 | DRD4 | OPRM1 | DAT1-repeats | MAOA | GABRB3 | DRD4-repeats | HTTLPR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Asian n=9 | 56% | 100% | 33% | 56% | 44% | 56% | 0% | 33% | 33% | 11% | 100% |
| African American n=42 | 43% | 95% | 55% | 93% | 57% | 2% | 19% | 38% | 19% | 31% | 71% |
| Hispanic or Latino n=29 | 69% | 86% | 59% | 52% | 55% | 28% | 0% | 69% | 62% | 66% | 76% |
| Mixed Race n=1 | 0% | 100% | 100% | 100% | 100% | 0% | 0% | 0% | 100% | 0% | 100% |
| Other n=9 | 67% | 100% | 22% | 67% | 78% | 0% | 0% | 56% | 44% | 44% | 56% |
| Unknown n=36 | 72% | 83% | 31% | 56% | 58% | 39% | 0% | 67% | 56% | 25% | 81% |
| Caucasian n=293 | 76% | 84% | 34% | 54% | 70% | 21% | 1% | 77% | 65% | 30% | 76% |
Shields et al. [47] performed a thoughtprovo-king association study. In an attempt to find a genetic basis for smoking, they compared polymorphisms of the DRD4 gene and risk for smoking between African Americans and Caucasians. Although the number of African Americans is small in this study, they found that African American smokers who at least have one L allele of the serotonin transporter gene have an increased risk of smoking. The increased risk caused by polymorphic loci in African Americans, but not Caucasians, implied that the VNTR here is a marker for another polymorphic site in African Americans, but not in Caucasians.
CONCLUSION
This article elucidates the basis for the Genetic Addiction Risk Score (GARS), a comprehensive test that identifies alleles linked to genes in the brain reward circuitry. There are many possible addiction-related genes as indicated by the work of Li and others [48]. At least it is agreed that neurotransmitter pathways and respective candidate genes that constitute the meso-limbic/ pre-frontal cortical pathways, including Serotonergic, Cannabinoidergic, Endorphinergic, GABAergic, Glutaminergic, and Dopaminergic systems are linked to healthy brain reward functioning. Any deviation can result in unwanted reward dysregulation.
A review of the literature related to the function of many reward genes led to the identification of allelic gene polymorphism based on an understanding of the physiology, as well as, many case-controlled associations and meta-analyses found in the literature, that provided the means to identify risk alleles. These polymorphisms selected to be counted in the GARS test associated with low dopamine function (hypodopaminergia). We have confidence that we can rely on these case controls used in the GARS test. However, it is noteworthy that highly-screened controls (eliminating any addictive, compulsive and impulsive behaviors in both proband and family), have importance in all genetically based research in the field of behavioral addictions. Also, in the current case-control literature, spurious results occur in many of the association studies because the investigators failed to appropriately screen controls for drugs and alcohol use disorder and many of the RDS behaviors listed in Table 1. The authors of this perspective and mini-review show evidence for the prevalence of the DRD2 A1 allele in unscreened controls (33.3%) compared to “Super-Controls” highly RDS-screened controls (3.3%) in proband and family. The message is that unlike one gene-one disease (OGOD), RDS is very complex and any associated RDS behaviors must be eliminated from controls in order to obtain an acceptable statistical analysis, instead of analyzing disease with disease-ridden controls.
Finally, careful scientific exploration, especially, as it relates to an inheritable basis of risk severity, by the elimination RDS behaviors is tantamount to provide disease-free controls. This simple fact is imperative, and any departure will provide untruths instead of objective scientific evidence.
ACKNOWLEDGEMENTS
The authors appreciate the expert edits by Margaret A. Madigan.
FUNDING
Dr. Rajendra D. Badgaiyan is supported by the National Institutes of Health (NIH) grants R01NS073884 and 1R21MH073624, and Dr. Blum along with Marjorie C Gondre Lewis of Howard University are recipients of grant 1R41MD012318-01.
Footnotes
Publisher's Disclaimer: DISCLAIMER: The above article has been published in Epub (ahead of print) on the basis of the materials provided by the author. The Editorial Department reserves the right to make minor modifications for further improvement of the manuscript.
“All truth passes through three stages. First, it is ridiculed. Second, it is violently opposed. Third, it is accepted as being self-evident”. Arthur Schopenhauer, (1788–1860).
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
Dr. Blum is the inventor of GARS and along with Dr. Siwicki own Geneus Health. Lisa Lott and Jessica Ponce are paid by Geneus Health.
REFERENCES
- [1].Blum K, Chen ALC, Thanos PK, et al. Genetic addiction risk score (GARS) ™, a predictor of vulnerability to opioid dependence. Front Biosci (Elite Ed.) 2018; 10: 175–96. 10.2741/e816 [DOI] [PubMed] [Google Scholar]
- [2].Blum K, Sheridan PJ, Wood RC, et al. The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. J R Soc Med 1996; 89(7): 396–400. 10.1177/014107689608900711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Blum K, Kozlowski G. Ethanol and neuromodulator interactions: a cascade model of reward. Alcohol Behav 1990; 131–50. [Google Scholar]
- [4].Erickson C The Science of Addiction. New York: W.W. Norton 2007. [Google Scholar]
- [5].Blum K, Thanos PK, Badgaiyan RD, et al. Neurogenetics and gene therapy for reward deficiency syndrome: Are we going to the Promised Land? Expert Opin Biol Ther 2015; 15(7): 973–85. 10.1517/14712598.2015.1045871 [DOI] [PubMed] [Google Scholar]
- [6].Li CY, Zhou WZ, Zhang PW, Johnson C, Wei L, Uhl GR. Meta-analysis and genome-wide interpretation of genetic susceptibility to drug addiction. BMC Genomics 2011; 12: 508. 10.1186/1471-2164-12-508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 2016; 3(8): 760–73. 10.1016/S2215-0366(16)00104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Noble EP, Blum K, Ritchie T, Montgomery A, Sheridan PJ. Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch Gen Psychiatry 1991; 48(7): 648–54. 10.1001/archpsyc.1991.01810310066012 [DOI] [PubMed] [Google Scholar]
- [9].Kranzler HR, Smith RV, Schnoll R, Moustafa A, Greenstreet-Akman E. Precision medicine and pharmacogenetics: What does oncology have that addiction medicine does not? Addiction 2017; 112(12): 2086–94. 10.1111/add.13818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gerra G, Somaini L, Leonardi C, et al. Association between gene variants and response to buprenorphine maintenance treatment. Psychiatry Res 2014; 215(1): 202–7. 10.1016/j.psychres.2013.11.001 [DOI] [PubMed] [Google Scholar]
- [11].Madrid GA, MacMurray J, Lee JW, Anderson BA, Comings DE. Stress as a mediating factor in the association between the DRD2 TaqI polymorphism and alcoholism. Alcohol 2001; 23(2): 117–22. 10.1016/S0741-8329(00)00138-5 [DOI] [PubMed] [Google Scholar]
- [12].Thompson MD, Kenna GA. Variation in the serotonin transporter gene and alcoholism: risk and response to pharmacotherapy. Alcohol Alcohol 2016; 51(2): 164–71. 10.1093/alcalc/agv090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gold MS, Badgaiyan RD, Blum K. A Shared molecular and genetic basis for food and drug addiction: overcoming hypodopaminergic trait/state by incorporating dopamine agonistic therapy in psychiatry. Psychiatr Clin North Am 2015; 38(3): 419–62. 10.1016/j.psc.2015.05.011 [DOI] [PubMed] [Google Scholar]
- [14].Gold MS, Blum K, Oscar-Berman M, Braverman ER. Low dopamine function in attention deficit/hyperactivity disorder: should genotyping signify early diagnosis in children? Postgrad Med 2014; 126(1): 153–77. 10.3810/pgm.2014.01.2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Batel P, Houchi H, Daoust M, Ramoz N, Naassila M, Gorwood P. A haplotype of the DRD1 gene is associated with alcohol dependence. Alcohol Clin Exp Res 2008; 32(4): 567–72. 10.1111/j.1530-0277.2008.00618.x [DOI] [PubMed] [Google Scholar]
- [16].Kuo SC, Yeh YW, Chen CY, et al. DRD3 variation associates with early-onset heroin dependence, but not specific personality traits. Prog Neuropsychopharmacol Biol Psychiatry 2014; 51: 1–8. 10.1016/j.pnpbp.2013.12.018 [DOI] [PubMed] [Google Scholar]
- [17].Van Tol HH. Structural and functional characteristics of the dopamine D4 receptor. Adv Pharmacol 1998; 42: 486–90. 10.1016/S1054-3589(08)60794-2 [DOI] [PubMed] [Google Scholar]
- [18].Baransel Isir AB, Oguzkan S, Nacak M, Gorucu S, Dulger HE, Arslan A. The catechol-O-methyl transferase Val158Met polymorphism and susceptibility to cannabis dependence. Am J Forensic Med Pathol 2008; 29(4): 320–2. 10.1097/PAF.0b013e3181847e56 [DOI] [PubMed] [Google Scholar]
- [19].Wichers M, Aguilera M, Kenis G, et al. The catechol-O-methyl transferase Val158Met polymorphism and experience of reward in the flow of daily life. Neuropsychopharmacology 2008; 33(13): 3030–6. 10.1038/sj.npp.1301520 [DOI] [PubMed] [Google Scholar]
- [20].Ray R, Ruparel K, Newberg A, et al. Human Mu Opioid Receptor (OPRM1 A118G) polymorphism is associated with brain mu-opioid receptor binding potential in smokers. Proc Natl Acad Sci USA 2011; 108(22): 9268–73. 10.1073/pnas.1018699108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Byerley W, Hoff M, Holik J, Caron MG, Giros B. VNTR polymorphism for the human dopamine transporter gene (DAT1). Hum Mol Genet 1993; 2(3): 335. 10.1093/hmg/2.3.335 [DOI] [PubMed] [Google Scholar]
- [22].Contini V, Marques FZ, Garcia CE, Hutz MH, Bau CH. MAOA-uVNTR polymorphism in a Brazilian sample: further support for the association with impulsive behaviors and alcohol dependence. Am J Med Genet B Neuropsychiatr Genet 2006; 141b(3): 305–8. [DOI] [PubMed] [Google Scholar]
- [23].Merenäkk L, Mäestu J, Nordquist N, et al. Effects of the serotonin transporter (5-HTTLPR) and α2A-adrenoceptor (C-1291G) genotypes on substance use in children and adolescents: a longitudinal study. Psychopharmacology (Berl) 2011; 215(1): 13–22. 10.1007/s00213-010-2109-z [DOI] [PubMed] [Google Scholar]
- [24].van der Zwaluw CS, Engels RC, Vermulst AA, et al. A serotonin transporter polymorphism (5-HTTLPR) predicts the development of adolescent alcohol use. Drug Alcohol Depend 2010; 112(1–2): 134–9. 10.1016/j.drugalcdep.2010.06.001 [DOI] [PubMed] [Google Scholar]
- [25].Namkoong K, Cheon KA, Kim JW, Jun JY, Lee JY. Association study of dopamine D2, D4 receptor gene, GABAA receptor beta subunit gene, serotonin transporter gene polymorphism with children of alcoholics in Korea: a preliminary study. Alcohol 2008; 42(2): 77–81. 10.1016/j.alcohol.2008.01.004 [DOI] [PubMed] [Google Scholar]
- [26].Pearson-Fuhrhop KM, Dunn EC, Mortero S, et al. Dopamine genetic risk score predicts depressive symptoms in healthy adults and adults with depression. PLoS One 2014; 9(5): e93772. 10.1371/journal.pone.0093772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kawada Y, Hattori M, Fukuda R, Arai H, Inoue R, Nanko S. No evidence of linkage or association between tyrosine hydroxylase gene and affective disorder. J Affect Disord 1995; 34(2): 89–94. 10.1016/0165-0327(95)00004-7 [DOI] [PubMed] [Google Scholar]
- [28].Gold MS, Blum K, Febo M, et al. Molecular role of dopamine in anhedonia linked to reward deficiency syndrome (RDS) and anti-reward systems. Front Biosci (Schol Ed) 2018; 10: 309–25. 10.2741/s518 [DOI] [PubMed] [Google Scholar]
- [29].Chen TJ, Blum K, Mathews D, et al. Are dopaminergic genes involved in a predisposition to pathological aggression? Hypothesizing the importance of “super normal controls” in psychiatricgenetic research of complex behavioral disorders. Med Hypotheses 2005; 65(4): 703–7. 10.1016/j.mehy.2005.04.037 [DOI] [PubMed] [Google Scholar]
- [30].Chen AL, Blum K, Chen TJ, et al. Correlation of the Taq1 dopamine D2 receptor gene and percent body fat in obese and screened control subjects: a preliminary report. Food Funct 2012; 3(1): 40–8. 10.1039/C1FO10089K [DOI] [PubMed] [Google Scholar]
- [31].Blum K Reward Deficiency Syndrome. In: Wenzel A, Ed. The Sage Encyclopedia of Abnormal Clinical Psychology. Thousand Oaks, California: SAGE publishers; 2017; pp. 4200. [Google Scholar]
- [32].Bolos AM, Dean M, Lucas-Derse S, Ramsburg M, Brown GL, Goldman D. Population and pedigree studies reveal a lack of association between the dopamine D2 receptor gene and alcoholism. JAMA 1990; 264(24): 3156–60. 10.1001/jama.1990.03450240058040 [DOI] [PubMed] [Google Scholar]
- [33].Kranzler HR, Zhou H, Kember RL, et al. Genome-wide association study of alcohol consumption and use disorder in 274,424 individuals from multiple populations. Nat Commun 2019; 10(1): 1499. 10.1038/s41467-019-09480-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Blum K, Noble EP, Sheridan PJ, et al. Allelic association of human dopamine D2 receptor gene in alcoholism. JAMA 1990; 263(15): 2055–60. 10.1001/jama.1990.03440150063027 [DOI] [PubMed] [Google Scholar]
- [35].Prasad P, Ambekar A, Vaswani M. Case-control association analysis of dopamine receptor polymorphisms in alcohol dependence: a pilot study in Indian males. BMC Res Notes 2013; 6: 418. 10.1186/1756-0500-6-418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Verde Z, Santiago C, Rodríguez González-Moro JM, et al. ‘Smoking genes’: a genetic association study. PLoS One 2011; 6(10): e26668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pinto JAF, Freitas PHB, Nunes FDD, Granjeiro PA, Santos LLD, Machado RM. Prevalence of polymorphisms in the ANKK1, DRD2, DRD3 genes and metabolic syndrome in refractory schizophrenia. Rev Lat Am Enfermagem 2018; 26: e2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ding YC, Chi HC, Grady DL, et al. Evidence of positive selection acting at the human dopamine receptor D4 gene locus. Proc Natl Acad Sci USA 2002; 99(1): 309–14. 10.1073/pnas.012464099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].van Dyck CH, Malison RT, Jacobsen LK, et al. Increased dopamine transporter availability associated with the 9-repeat allele of the SLC6A3 gene. J Nucl Med 2005; 46(5): 745–51. 10.1073/pnas.012464099 [DOI] [PubMed] [Google Scholar]
- [40].Saravani R, Galavi HR, Lotfian Sargazi M. Catechol-O-Methyltransferase (COMT) Gene (Val158Met) and Brain-Derived Neurotropic Factor (BDNF) (Val66Met) genes polymorphism in schizophrenia: a case-control study. Iran J Psychiatry 2017; 12(4): 265–70. [PMC free article] [PubMed] [Google Scholar]
- [41].Hwang IW, Lim MH, Kwon HJ, Jin HJ. Association of Monoamine Oxidase A (MAOA) gene uVNTR and rs6323 polymorphisms with attention deficit and hyperactivity disorder in Korean children. Medicina (Kaunas) 2018; 54(3): E32. 10.3390/medicina54030032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Haberstick BC, Smolen A, Williams RB, et al. Population frequencies of the triallelic 5HTTLPR in six ethnicially diverse samples from North America, Southeast Asia, and Africa. Behav Genet 2015; 45(2): 255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Malhotra S, Basu D, Khullar M, Ghosh A, Chugh N. Candidate genes for alcohol dependence: a genetic association study from India. Indian J Med Res 2016; 144(5): 689–96. 10.4103/ijmr.IJMR_1018_14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Nobile B, Ramoz N, Jaussent I, et al. Polymorphism A118G of opioid receptor mu 1 (OPRM1) is associated with emergence of suicidal ideation at antidepressant onset in a large naturalistic cohort of depressed outpatients. Sci Rep 2019; 9(1): 2569. 10.1038/s41598-019-39622-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gade R, Blake H, MacMurray J, et al. Relationship of the GABRB3 gene to adult ADHD and personality traits in Caucasian and African-American samples. Psychiatr Genet 1996; 6: 164–5. [Google Scholar]
- [46].Gerra MC, Manfredini M, Cortese E, et al. Genetic and environmental risk factors for Cannabis use: preliminary results for the role of parental care perception. Subst Use Misuse 2019; 54(4): 670–80. 10.1097/00041444-199623000-00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Shields PG, Lerman C, Audrain J, et al. Dopamine D4 receptors and the risk of cigarette smoking in African-Americans and Caucasians. Cancer Epidemiol Biomarkers Prev 1998; 7(6): 453–8. [PubMed] [Google Scholar]
- [48].Li CY, Mao X, Wei L. Genes and (common) pathways underlying drug addiction. PLoS Comput Biol 2008; 4(1): e2. 10.1371/journal.pcbi.0040002 [DOI] [PMC free article] [PubMed] [Google Scholar]


