Abstract
Melatonin (MT) is an important electroactive hormone that regulates different physiological actions in the brain, ranging from circadian clock to neurodegeneration. An impressive number of publications have highlighted the effectiveness of MT treatments in different types of sleep and neurological disorders, including Alzheimer’s and Parkinson’s disease. The ability to detect MT in different regions of the brain would provide further insights into the physiological roles and therapeutic effects of MT. While multiple electrochemical methods have been used to detect MT in biological samples, monitoring MT in the brain of live animals has not been demonstrated. Here, we optimized a square wave voltammetry (SWV) electro-analytical method to evaluate the MT detection performance at CFEs in vitro and in vivo. SWV was able to sensitively detect the MT oxidation peak at 0.7 V, and discriminate MT from most common interferents in vitro. More importantly, using the optimized SWV, CFEs successfully detected and reliably quantified MT concentrations in the visual cortex of anesthetized mice after intraperitoneal injections of different MT doses, offering stable MT signals for up to 40 minutes. To the best of our knowledge, this is the first electrochemical measurement of exogenously administered MT in vivo. This electrochemical MT sensing technique will provide a powerful tool for further understanding MT's action in the brain.
Introduction
Melatonin (MT) is an electroactive hormone primarily synthesized in the pineal gland in dark conditions, but it is also produced in glial cells, some neurons, and in the meninges.1-3 Commonly known as the sleep hormone,4-6 MT also presents antioxidant,7-11 anti-inflammatory,10-13 antiapoptotic,14 and neuroprotective2-8,9 effects, and regulates different physiological actions in the brain. Further, MT can easily diffuse through the blood–brain-barrier and be effective in treating brain injuries.7,10,15 Within the last decade, hundreds of studies have provided scientific evidence of tissue recovery after MT treatment in several oxidative stress-related diseases7,10,11,13 and demonstrated promising MT therapeutic effects in different types of sleep4,5,16 and neurological disorders,2,15,17 including Parkinson’s disease,8,9,17 Alzheimer’s disease,2,7,18 epilepsy,19,20 alcoholism21,22 and depression.21,23 A recent study from our lab12 provides evidence of MT’s potent effect in reducing inflammation and oxidative stress around implanted neural electrode arrays and improving neuronal health and recording performance in the visual cortex of mice.
The ability to detect MT concentrations – endogenously produced or exogenously administered – in different regions of the brain could provide useful insight on MT’s mechanisms of action in diverse models of sleep disorder, neural injuries, and neurodegenerative conditions. In vivo microdialysis has been successfully used to monitor pineal MT secretion in living animals.24,25 Implantation of microdialysis probes, which typically have diameters of approximately 300 μm, creates substantial tissue damage that greatly diminishes extraction efficiency and can influence the experimental outcome.26-28 To overcome these limitations, Si-based microfabricated probes with a cross-sectional area 79% smaller than the smallest conventional probes have been recently introduced.29 However, microdialysis presents limited temporal resolution in comparison to direct electrochemical techniques, such as fast scan cyclic voltammetry (FSCV) and square wave voltammetry (SWV), and does not provide real time information regarding changes in the neurochemical environment.30,31 Electrochemical methods have been used to detect MT from plasma,32 human urine,33 pharmaceutical formulations,34 food supplements35 and the intestinal epithelium.36 However, monitoring the concentration of MT in vivo has not been demonstrated.
FSCV has been recently optimized at carbon-fiber microelectrodes (CFEs) to selectively detect MT in intact lymph node tissue.37 FSCV at CFEs has enabled monitoring of various electroactive neurotransmitter releases in the brain.38-40 The small footprint of the 7 μm carbon fiber elicits minimum tissue injury and inflammatory response,41 while the fast scan rate of FSCV enables the detection of concentration change of electroactive species in a sub-second scale.38-40 Although FSCV offers unparalleled temporal resolution to capture fast phasic neurotransmitter release,42-44 the necessity for background subtraction limits its ability to monitor slow changes of concentration.42,44
Another electrochemical sensing method is SWV, which uses a combined symmetrical square wave superimposed on a staircase potential sweep, enabling the isolation of faradaic current with improved rate of collection and increased peak current amplitude as compared to differential pulse voltammetry.45-47 SWV has been widely used in biosensing applications spanning from diagnostic, environmental and food monitoring and enzyme kinetics.45,47 Particularly for MT detection, SWV has been shown to successfully measure MT concentration in urine samples,33,48 and pharmaceutical tablet.33,45,49 Furthermore, SWV has been capable of simultaneously detecting MT and DA using graphene decorated with Fe3O4 magnetic nanoparticles on a carbon paste electrode.50 Unlike FSCV, SWV has a lower temporal resolution (~seconds) but can be used for direct measurement of analyte concentration without background subtraction. Recently, we demonstrated that SWV at PEDOT/functionalized carbon nanotube (PEDOT/CNT) microelectrodes allows the detection of basal DA levels in rat brains with high sensitivity and selectivity.51
Here, we optimized a SWV waveform to detect MT from CFEs in vitro and in vivo. We demonstrated that MT detection can be accomplished in vitro with high sensitivity and selectivity among the most common interferents and, more importantly, in mice brain after administration of different MT doses through intraperitoneal (i.p.) injections.
To the best of our knowledge, this is the first reproducible electrochemical measurement of exogenously administered MT in vivo. This provides a powerful tool for further understanding MT’s action in the brain.
Materials and methods
Electrode preparation and characterization
CFEs were fabricated as previously described in ref. 51 and 52. Briefly, borosilicate capillaries (0.4 mm ID, 0.6 mm OD; A-M systems Inc., Sequim, WA, USA), each containing a single carbon fiber (7 μm diameter, T650; Cytec Carbon Fibers LLC., Piedmont, SC, USA), were pulled to a fine tip using a vertical puller (Narishige, Los Angeles, CA, USA). The tip was sealed with epoxy (Spurr Epoxy, Polysciences Inc., Warrington, PA, USA) and the exposed fiber was cut 400 μm from the glass seal using a scalpel under an optical microscope (Szx12, Olympus). A mercury drop was placed in the barrel for electrical contact to a hookup wire (Nichrome; Goodfellow, Oakdale, PA, USA). CFEs were soaked in isopropyl alcohol53 (Fisher Chemical, USA) for 20 minutes prior to use.
The electrochemical properties of the CFE were evaluated in vitro in artificial cerebral spinal fluid (aCSF, 142 mM NaCl, 1.2 mM CaCl2, 2.7 mM KCl, 1.0 mM MgCl2, 2.0 mM NaH2PO4, pH 7.4) by electrochemical impedance spectroscopy (EIS). During the EIS measurements, a sine wave (10 mV RMS amplitude) was superimposed onto the open circuit potential while varying the frequency from 1 to 105 Hz. EIS were carried out using a potentiostat/galvanostat (Autolab, Metrohm, USA) connected to a three-electrode electrochemical cell with a platinum counter electrode and an Ag/AgCl reference electrode.
SWV in vitro calibration
Electrochemical detection of MT was performed via SWV. SWV experiments were carried out using a potentiostat/galvanostat (Autolab, Metrohm, USA) connected to a three-electrode electrochemical cell with a platinum counter electrode and an Ag/AgCl reference electrode. SWV is a pulse voltammetry technique that consists of a square wave superimposed on a staircase that allows for the isolation of faradaic current with improved rate of collection and increased peak current amplitude and high sensitivity screening pulse voltammetry.45-47 The SWV waveform was repeatedly applied from 0.4 V to 0.9 V with a 10 Hz step frequency, a 50 mV pulse amplitude and a 5 mV step height every 30 seconds. Potential was held at 0.4 V between scans. In vitro MT calibrations were performed using freshly prepared MT solutions dissolved in aCSF (142 mM NaCl, 1.2 mM CaCl2, 2.7 mM KCl, 1.0 mM MgCl2, 2.0 mM NaH2PO4, pH 7.4) in a 0.1–5 μM concentration range. Electrode sensitivity was determined by the slope of the linear range of the calibration plot relating MT peak current at 0.7 V to MT concentration.
The MT selectivity was determined among different possible interferents, i.e. dopamine (DA), serotonin (5-HT), histamine (HA), homovanillic acid (HVA), adenosine (AD), hydrogen peroxide (H2O2), uric acid (UA) and ascorbic acid (AA).
In vivo procedures
The in vivo performance of CFEs using SWV was determined through acute surgical experiments conducted in the visual cortex of 22 male mice (C57BL/6J, 8–12 weeks, 22–35 g; Jackson Laboratory, Bar Harbor, ME). We chose C57BL.6J mice for the low endogenous MT background.54 All animal care and procedures were performed under approval of the University of Pittsburgh Institutional Animal Care and Use Committee and in accordance with regulations specified by the Division of Laboratory Animal Resources.
All animals were induced with 1.5–2% isoflurane mixed with oxygen flow at 1 L min−1, then maintained at 1.25–1.5%. The body temperature was maintained at 37 °C with a thermostatically controlled heating pad (Harvard Apparatus, Holliston, MA, USA) and lacrigel (Dechra Puralube Vet Ointment) was placed on eyes to avoid dryness.
After the animal head was fixed in a stereotaxic frame (Narishige International USA, Inc), the skin and connective tissue on the surface of the skull was removed. A small pinhole craniotomy was made over visual cortex (1.0 mm anterior to lambda, and 1.5 mm lateral from midline) with a high-speed dental drill (0.007 drill bit, Fine Science Tools, Inc., Foster City, CA) and bone fragments were carefully removed with forceps and saline. Saline was applied continuously onto the skull to dissipate heat from the high-speed drill. We chose to measure MT in the left monocular visual cortex, to be consistent with a recent study from our lab12 that provides evidence of MT’s potent effect in reducing inflammation and oxidative stress around neural electrode arrays implanted in the same area of C57BL.6J mice.
The 400 μm CFEs were lowered 0.8–1 mm deep into the left monocular visual cortex, spanning approximately 900 μm, using a hand-driven micromanipulator. The MT response to an intraperitoneal (i.p.) injection of different MT concentrations (30–180 mg kg−1) was measured using SWV. In particular, during SWV experiments, n = 6 mice received 30 mg kg−1 MT, n = 6 mice received 90 mg kg−1 MT, n = 6 mice received 180 mg kg−1 MT and n = 4 receive saline vehicle. 2 to 10 μl dimethylsulfoxide (DMSO), depending by the MT doses, were added in order to improve the solubility of the MT in 100 μl saline vehicle. 10 μl of DMSO were also added to the saline vehicIe for the control group.
Two additional small pinhole craniotomies were performed for the introduction of the reference electrode (1.0 mm anterior to bregma, and 1.5 mm right from midline) and for the insertion of a bone screw (1.0 mm anterior to lambda, and 2.5 mm right from midline) on the skull, used as counter electrode for SWV detection. SWV experiments were acquired using a potentiostat/galvanostat (Autolab, Metrohm, USA) connected to the three-electrode configuration: CFE working electrode, bone screw (counter electrode), and Ag/AgCl reference electrode via a salt bridge. In vivo MT concentration was determined for all in vivo experiments by converting SWV peak current to MT concentration using the pre-calibration electrode sensitivity (see section Calibration in ESI†).
Data analysis
MT peaks were isolated from the nonfaradaic background current for each SWV scan by subtracting a modelled polynomial baseline, using a previously described methodology.51 Statistical analyses were conducted using IBM SPSS software (v22, IBM Corp, Armonk, NY, USA). SWV data was analyzed using MATLAB (MathWorks Inc., Natick, MA, USA) and Origin Pro 8.5.
Results and discussion
MT detection using SWV
SWV has been widely used for trace analyses, especially in pharmacology, being considered one of the most sensitive technique for the direct evaluation of analyte concentrations.45 The goal of this study was to optimize a SWV protocol to achieve sensitive and selective MT detection at CFEs in vitro and in vivo.
In vitro SWV sensitivity and selectivity
MT oxidizes at carbon-based electrodes following a three-step reaction.37,55,56 One step involving the formation of a quinoneimine, which is both highly reactive and susceptible to electropolymerization, may cause undesirable adsorption products to foul the electrode surface.37,55,56 In a recent study, reporting the first FSCV real-time detection of MT in live lymph node slices, A. L. Hensley et al.37 minimized the extent of MT fouling by developing an optimized FSCV waveform that combines a positive holding potential (0.2 V) with a faster scan rate (600 V s−1).37
The performance of CFEs for the electrochemical detection of MT was first determined via in vitro calibration experiments performed in aCSF. The waveform for MT determination was optimized by varying the parameters that can influence the voltammetric responses, i.e. frequency, step potential, pulse amplitudes, holding potential and holding time.48 The optimum waveform was determined to be a square wave with 50 mV pulse amplitude, 5 mV step height and 10 Hz frequency, scanned from 0.4 to 0.9 V. In order to prevent unwanted fouling, we decided to use 0.4 V as lower bound and to hold the potential at 0.4 V for 30 s in between different repetitions. Indeed, using negative potential bounds, a secondary oxidation peak of MT was observed at 0.05 V (Fig. 2a). The quinoneimine produced from this secondary reaction can undergo nucleophilic attack with free MT, causing electropolymerization and unwanted adsorption products that foul the electrode surface. In FSCV, the oxidation peak due to this reaction was observed at 0.6 V, due to the faster scan rate.37
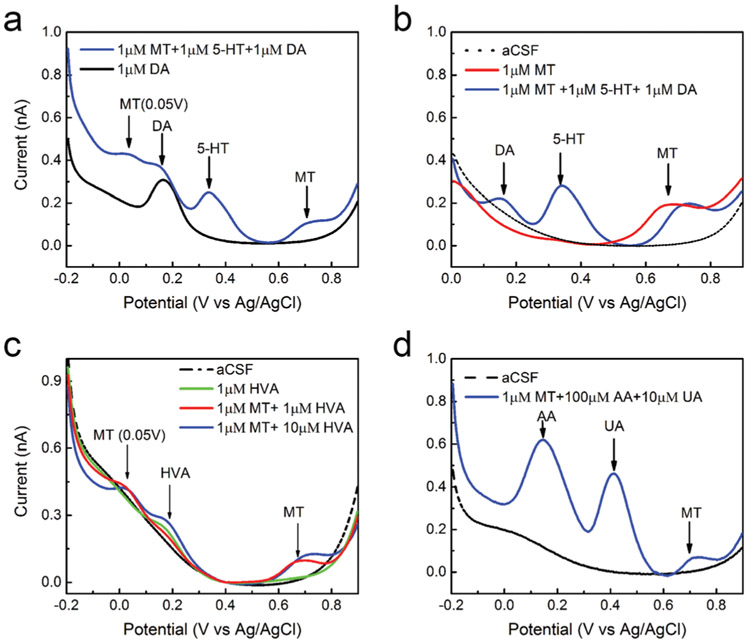
Fig. 2.
MT square wave voltammetry (SWV) selectivity: (a) SWV waveforms performed respectively in 1 μm DA (black) and in a mixture of 1 μm MT, 1 μM 5-HT and 1 μM DA (blue) in aCSF. MT can be distinguished among DA and 5-HT. DA and 5-HT present an oxidation potential peak respectively at 0.15 V and 0.35 V, easily distinguished from the MT one at 0.7 V. However, extending the potential window to negative potentials, we observed that MT presents a secondary oxidation peak at 0.05 V. A previous study demonstrated that the quinoneimine produced from this secondary reaction can undergo nucleophilic attack with free MT, causing electropolymerization and unwanted adsorption products that foul the electrode surface.37 (b) SWV waveforms performed respectively in 1 μM MT (red) and in a mixture of 1 μM MT, 1 μM 5-HT and 1 μM DA (blue) in aCSF (black). (c) SWVs (potential window from −0.2 V to 0.9 V) performed in aCSF (black), 1 μM HVA (green), a mixture of 1 μM MT and 1 μM HVA (red) and a mixture of 1 μM MT and 10 μM HVA (blue) respectively. HVA present an oxidation peak around 0.2 V and do not interfere with MT detection. (d) SWV waveform performed in a mixture of 1 μM MT, 100 μM AA and 10 μM UA (blue) in aCSF (black) shows that MT can be distinguished among AA and UA.
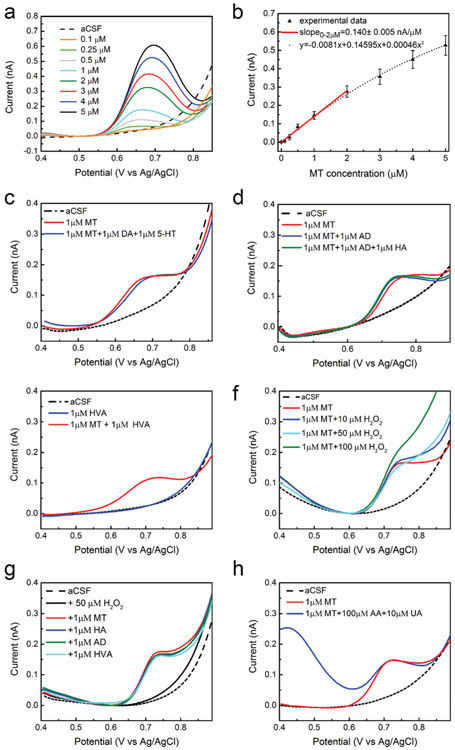
CFEs were subjected to SWV measurement, first in aCSF and then in solutions of increasing MT concentration from 0.1 μM to 5 μM. CFEs exhibit clear detection of MT at each concentration with the average SWV traces (SEM removed for clarity) revealing a single concentration dependent peak located near 0.7 V (Fig. 1a). The CFE response to MT is linear (r2 = 0.99) up to 2 μM (red line, Fig. 1b) with a sensitivity of 0.140 ± 0.005 nA μM−1, defined by the slope. For the concentrations above 2 μM, a non-linear trend is observed and agrees very well (r2 = 0.99) with a second-degree polynomial equation (dot black line, Fig. 1b), suggesting saturation of the electrode at higher concentrations. The average LOD (±SEM, n = 5), defined as 3 times the standard deviation of the noise, was estimated to be 38.1 ± 11.8 nM. The sensitivity is ~60-fold lower than the value reported using the FSCV recently optimized for MT detection at shorter (100 μM) CFEs. However, these results exceed the performance obtained using other SWV waveforms at glassy carbon,33 acetylene black-chitosan modified glassy carbon,57 and boron-doped diamond electrodes48 with millimeter-range sizes.
Fig. 1.
In vitro SWV MT detection at CFEs: (a) average (n = 5, SEM omitted for clarity) in vitro SWV MT calibrations conducted at CFEs in aCSF reveal clear MT peaks at 0.7 V. (b) In vitro SWV MT calibration curve conducted at CFEs. The average (n = 6) sensitivity (baseline subtracted peak current vs. MT concentration) is linear up to 2 μM (slope: 0.140 ± 0.005 nA μM−1, red line). For the concentrations above 2 μM, a non-linear trend is observed and agrees very well (r2 = 0.99) with a second-degree polynomial equation (y = −0.0081 + 0.14595x + 0.000459872x2, dot black line). (c) MT can be distinguished among DA and 5-HT. SWVs relative to 1 μM MT (red) and a contaminant mixture (1 μM MT plus 1 μM DA plus 1 μM 5-HT, blue) show that DA and 5-HT do not present oxidation peaks in the potential window used. (d) MT can be distinguished among AD and HA. SWVs relative to 1 μM MT (red) and a 1 μM MT plus 1 μM AD (blue) and a contaminant mixture (1 μM MT plus 1 μM AD plus 1 μM 5-HT, green) show that AD and HA do not present oxidation peaks in the potential window used. (e) MT can be detected in presence of HVA. SWVs relative to 1 μM HVA do not show peaks in the potential range used at neutral pH. (f) MT can be detected in presence of H2O2 in the concentration range of 10–50 μM. (g) MT can also be distinguished among AD, HA, HVA, in presence of H2O2. SWVs do not shown peaks in the potential range used and SWV corresponding to 1 μM MT collected in mixtures of these molecules does not differ from the one collected in aCSF. (h) MT can be distinguished among AA and UA. SWV corresponding to 1 μM MT collected in a mixture of 100 μM AA and 10 μM UA (blue) in aCSF shows that MT can be detected in presence of high concentration of AA and UA. In red, the SWV corresponding to 1 μM MT collected in aCSF is reported for comparison.
The optimized SWV waveform allows for MT detection in presence of DA and 5-HT. The simultaneous detection of these different biomolecules is extremely important to give further insight into the interaction between melatoninergic, dopaminergic and serotonergic systems in the brain and their involvement in neurological disorders.50,58,59 Fig. 1c compares representative SWV waveforms performed in 1 μm MT and a mixture of 1 μm MT, 1 μm 5-HT and 1 μm DA in aCSF respectively. DA and 5-HT do not present oxidation peaks in this potential window and we can clearly detect MT in presence of a mixture of DA and 5-HT. Extending the potential window from −0.2 to 0.9 V, we can observe the DA and 5-HT oxidation potential peaks at 0.15 V and 0.35 V respectively, easily distinguished from the MT one at 0.7 V (Fig. 2a and b). This allows for the simultaneous detection of the three biomolecules, as shown in Fig. 2a and b. However, we also observed the secondary oxidation peak of MT at 0.05 V (Fig. 2a). This factor must be considered, in order to prevent unwanted fouling, when a wider potential scan would be required, i.e. for the simultaneous detection of MT, DA or 5-HT.
For direct measurements in the brain, we need to consider the selectivity of detection over other electroactive species, such as 3,4-dihydroxyphenylacetic acid (DOPAC), 5-hydroxyin-doleacetic acid (5-HIAA), epinephrine (EP), norepinephrine (NE), histamine (HA), homovanillic acid (HVA) and adenosine (AD), that might potentially interfere with MT signals, depending on the targeted area.
We first ruled out DOPAC and 5-HIAA, the precursors of DA and 5-HT, as they have been shown to present oxidation peaks at 0.15 V and 0.3 V at the surface of carbon-based electrodes respectively60,61 which should not overlap with the MT peak at 0.7 V. EP and NE can also be ruled out, because these molecules showed oxidation peaks around 0.15–0.20 V and reduction peaks at −0.384 V, −0.39 V and −0.05 V, respectively by cyclic square-wave voltammetry at unmodified carbon electrodes.62 These peaks are outside of the window we used for SWV detection of MT.
We then tested the selectivity of our MT detection against AD, HA, HVA. Fig. 1d compares representative SWV performed in 1 μM MT (red), in a mixture of 1 μM MT and 1 μM AD (blue) and in a mixture of 1 μM MT, 1 μM AD and 1 μM HA (green) in aCSF, respectively. We do not observe HA electrochemical reactions using the optimized SWV (0.4–0.9 V) for MT detection. This result agrees with multiple studies that reports HA oxidation at potentials higher than 1.1 V at carbon electrodes in aqueous solution.63-65 Accordingly, when we extended the SWV potential window from 0.4 to 1.4 V, we observe a HA reaction around 1.3 V, starting from concentration higher than 3 μM (ESI Fig. 1a, red and ESI†). description).
Additionally, no AD oxidation peaks in the 0.4–0.9 V potential window (Fig. 1d) and the extended 0.4–1.4 V potential window (ESI Fig. 1a†) were observed.
Furthermore, using the SWV waveform optimized for MT detection, we do not observe HVA peaks and we were able to detect a clear MT peak in presence of 1 μm HVA (Fig. 1e). Previous differential pulse voltammetry studies showed that HVA presents only one oxidation peak, and the peak current is strongly dependent on the pH of the buffer solution, with the highest reported at pH 3.0–4.0. With the increase of pH, the peak potential of HVA shifts to lower values and the peak current dramatically decreases.66-68 At neutral pH, HVA presents an oxidation peak around 0.25 V both at non-modified GCE67 and l-leucine modified sol–gel-carbon electrode.68 Similarly, extending the SWV waveform at more negative potentials, from −0.2 V to +0.9 V, we can observe an oxidation peak for HVA around 0.2 V, well separated from the MT peak, that can be discriminated in presence of 1–10 μm HVA (Fig. 2c).
Additionally, hydrogen peroxide (H2O2) fluctuations, usually indicating the presence of tissue dysfunctions,69 may potentially affect the determination of MT in the brain. MT detection in presence of different concentration of H2O2 was investigated. Fig. 1f compares representative SWVs corresponding to 1 μm MT collected in aCSF and after the addition of 10 to 100 μm H2O2 respectively. We can clearly discriminate MT in presence of 10 and 50 μm H2O2, while the further addition of 50 μm H2O2 (100 μm H2O2 in total) starts to affect the baseline, showing a less clear MT peak. We do not think this should affect the MT discrimination in vivo because the concentrations of stimulus-evoked endogenous H2O2 measured in the extracellular space of the brain are on the order of a few μm.70 Moreover, the optimized SWVs corresponding to 1 μm MT collected in 50 μm H2O2 in aCSF with subsequent additions of 1 μm HA, 1 μm AD, and 1 μm HVA, show a clear MT detection, not affected by the presence of H2O2 or the mixture of other contaminants (Fig. 1g).
Finally, the selective detection of MT in the presence of ascorbic acid (AA, 100-fold higher concentration) and uric acid (UA, 10-fold higher concentration) was investigated, both of which are typically present in the tissue at high concentrations. Fig. 1h compares representative SWV waveforms performed respectively in 1 μm MT and in a mixture of 1 μm MT, 100 μm AA and 10 μm UA in aCSF using the optimized waveform. A clear MT (1 μm) peak was observed in presence of 100 μm AA and 10 μm UA. When extending the potential window from −0.2 to 0.9 V, we can detect AA and UA oxidation potential peaks respectively at 0.16 V and 0.4 V, easily distinguished from the MT peak at 0.7 V (Fig. 2d). Since we observed MT peak shift due to a change of the aCSF pH48,50 following the AA addition (ESI Fig. 1b†), the aCSF was pH adjusted to 7.4. We do not expect that this pH dependency should affect the SWV detection of MT in vivo since the local pH changes follow the rapid dynamics of metabolism and carbon dioxide clearance, usually requiring a sub-second temporal resolution to be captured.71 Also, the aCSF we used in vitro is not an ideal buffer while, in physiological conditions, brain homeostasis maintains a relatively stable pH.72
In summary, we demonstrated that, using CFE microelectrodes, the optimized SWV waveform detects a single concentration dependent MT peak located near 0.7 V and provides an excellent selectivity over a wide miscellaneous of possible interferents.
In vivo MT detection using SWV
Finally, the optimized SWV waveform was used in vivo on CFEs implanted in the visual cortex of anesthetized mice to detect MT i.p. injected at different doses.
The SWV waveform was applied immediately upon finalizing electrode placement in the brain and repeated every 30 seconds over a period of 50 minutes. Following 10 minutes of baseline SWV collection, n = 6 mice received a single 30 mg kg−1, i.p. MT injection, n = 6 mice received a single 90 mg kg−1, i.p. MT injection and n = 6 mice received a single 180 mg kg−1, i.p. MT injection. Furthermore, n = 4 mice have been used as control group and received a saline vehicle injection. 30 mg kg−1 is a pharmacologically meaningful dose and have been used to treat amyotrophic lateral sclerosis,73 Huntington’s disease74 and improve the quality of neural recordings in chronic implants.12 90 mg kg−1 dose has been shown to have analgesic actions to lower pain in animals affected by chronic pain, fibromyalgia, irritable bowel syndrome, migraine.75 We also tested 180 mg kg−1 dose, considered a high dosage,76 to show that this SWV can detect different concentration in the brain, without saturation at high concentrations. Similar high MT dosages showed substantial anticonvulsant effects in mice and rat models of epilepsy.76,77
MT peaks were isolated from the non-faradaic background current for each SWV scan by subtracting a modeled polynomial baseline, using a methodology we previously reported.51 In vivo MT concentrations were determined for all in vivo experiments by converting SWV peak current to MT concentration using the pre-calibration curve of electrode sensitivity (see section Calibration in ESI†). Specifically, the linear regression model has been used in the 0–2 μM range and the second-degree polynomial approximation for concentration >2 μM.
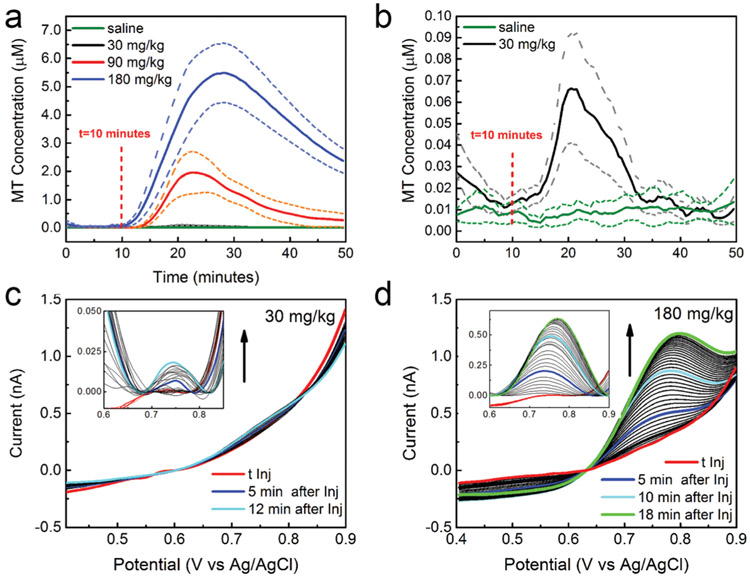
A clear peak was observed following the i.p. MT injections, with the size proportional to the MT concentrations (Fig. 3). Average in vivo MT concentrations (±SEM) were plotted over time for both experimental and control groups (Fig. 3a and b). For all the concentrations, the CFEs can detect the MT dynamics along the entire recording periods. No change has been observed in the SWV baseline after injection of saline vehicle in the control group (Fig. 3a and b). Fig. 3c and d report examples of SWVs collected every 30 seconds starting from the MT injection until the time at which the peak concentrations have been reached, in response to 30 mg kg−1 (c) and 180 mg kg−1 (d) MT respectively.
Fig. 3.
In vivo MT detection: (a) average in vivo MT concentrations, plotted over time, for 30 mg kg−1, 90 180 mg kg−1 i.p. MT injections (n = 6) and saline control (n = 4) in vivo; (b) higher magnification for 30 mg kg−1 MT dose (n = 6) and saline control (n = 4). A clear peak was observed following the i.p. MT injections, with the size proportional to the MT concentrations. No change has been observed in the SWV baseline after injection of saline vehicle in the control group. The peak concentrations were 65.9 nM (41.0 nM min, 91.8 nM max) after 10.9 minutes, 1.95 μM (1.2 μM min, 2.7 μM max) after 12.4 minutes and 5.5 μM (4.4 μM min, 6.5 μM max) after 17.9 minutes following the MT administration of 30, 90, 180 mg kg−1 doses, respectively. (c, d) SWVs in response to 30 mg kg−1 (c) and 180 mg kg−1 (d) i.p. MT injections respectively, collected every 30 seconds starting from the MT injection until the time at which the maximum concentration has been reached. (12 and 18 minutes after injection for 30 and 180 mg kg−1 respectively.) In inset, the baseline subtracted SWV reveal the MT peaks at 0.75 V, proportional to the corresponding concentrations.
The maximum detected concentrations were 65.9 nM (41.0 nM min, 91.8 nM max) after 10.9 minutes, 1.95 μM (1.2 μM min, 2.7 μM max) after 12.4 minutes and 5.5 μM (4.4 μM min, 6.5 μM max) after 17.9 minutes following the MT administration of 30, 90, 180 mg kg−1 doses, respectively. To the best of our knowledge, the level of MT in the brain following i.p. administration has not been measured. The closest study we were able to find is the MT detection in the rat jugular vein following i.p. administration, using in vivo microdialysis coupled with liquid chromatography/tandem mass spectrometry.78 The measured MT concentration after 30 minutes from the i.p. injection of ~60 mg kg−1 was approximately 1.72 μm, which is on the same order of magnitude as our measurement. Clearly, the two studies differ in animal models (mouse vs. rat), temporal resolution (microdialysis measures an averaged concentration over 30 min), and region of detection (jugular vein vs. brain), and this comparison should be taken as a mere indication.
I.p. administration is widely used in rodents as a route of drug administration. The drug transport may occur by the mesenterical–portal vasculature, the mesenterical–extraportal and the extra-mesenterical vasculature, as well as the lymph vessels. Although the compounds are partially subjected to hepatic first-pass elimination, the absorption into the blood flow occurs more rapidly than with subcutaneous injection, more similar to intravenous administration.79-81 Furthermore, the absorption depends on the dimension of the molecules and their water-solubility. Hence, smaller and water-soluble compounds will reach the bloodstream more quickly than larger molecules. For example, tribromoethanol and ethyl carbamate can reach the brain within few minutes, inducing rapidly anesthetic effects in rodents.82 Thus, it is not surprising that MT, a relatively small molecule well known to easily diffuse through the blood–brain-barrier, rapidly reached the brain.
We found that the uptake and elimination kinetics are different for the different doses, with the lower dose being adsorbed faster. The half-lives are respectively 19.4, 22.9 and 36.6 minutes for the 30, 90 and 180 mg kg−1 doses. This result is in agreements with other studies, documenting MT elimination half-lives for oral and intravenous ranging from 20 to 60 minutes,83,84 showing a fast distribution and elimination kinetics.85
This dose study suggests that CFEs can detect different concentrations of MT following the i.p. administration of different MT doses, with uptake and elimination kinetics proportional to the amount of MT administrated.
Conclusions
In this study we optimized a SWV waveform to evaluate the MT detection performance of CFEs in vitro and in vivo. SWV was able to sensitively and selectively detect MT oxidation peak – at 0.7 V vs. Ag/AgCl – in vitro in aCSF. MT can be discriminated among the most common electroactive biomolecules that can potentially interfere with MT in the brain, such as DA, 5-HT, NE, HA, HVA, AD, AA and UA. More importantly, the optimized SWV at CFEs can successfully detect different MT concentration administered via i.p. injections in the visual cortex of anesthetized mice. For all the concentrations, the CFEs can detect the MT dynamics along the entire recording periods, with uptake and elimination kinetics proportional to the amount of MT administrated.
These results suggest that the optimized SWV technique could be extremely useful for the monitoring of MT over time in the brain. To the best of our knowledge, this is the first electrochemical measurement of MT in vivo. This electrochemical MT sensing technique will provide a powerful tool for further understanding MT’s action in the brain.
Supplementary Material
Acknowledgements
Thank you to Dr Adrian Michael for the use of his laboratory and FSCV instrumentation.
This work was supported by NIH (Grants R01NS062019, R01NS089688, R21DA043817, and R21DA049592).
Footnotes
Conflicts of interest
There are no conflicts to declare.
Electronic supplementary information (ESI) available. See DOI: 10.1039/d0an00051e
References
- 1.Pandi-Perumal SR, Trakht I, Srinivasan V, Spence DW, Maestroni GJ, Zisapel N and Cardinali DP, Prog. Neurobiol, 2008, 85, 335–353. [DOI] [PubMed] [Google Scholar]
- 2.Tan D-X, Curr. Neuropharmacol, 2010, 8, 161–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vanecek J, Physiol. Rev, 1998, 78, 687–721. [DOI] [PubMed] [Google Scholar]
- 4.Doghramji K, J. Clin. Sleep Med, 2007, 3, S17. [PMC free article] [PubMed] [Google Scholar]
- 5.Zhdanova IV, Lynch HJ and Wurtman RJ, Sleep, 1997, 20, 899–907. [PubMed] [Google Scholar]
- 6.Prater WT, Swamy M, Beane MD and Lester DB, J. Behav. Brain Sci, 2018, 8, 117–125. [Google Scholar]
- 7.Pappolla M, Chyan Y-J, Poeggeler B, Frangione B, Wilson G, Ghiso J and Reiter RJ, J. Neural Transm, 2000, 107, 203–231. [DOI] [PubMed] [Google Scholar]
- 8.Sharma R, McMillan CR and Niles LP, J. Pineal Res, 2007, 43, 245–254. [DOI] [PubMed] [Google Scholar]
- 9.Sharma R, McMillan CR, Tenn CC and Niles LP, Brain Res, 2006, 1068, 230–236. [DOI] [PubMed] [Google Scholar]
- 10.Barlow KM, Esser MJ, Veidt M and Boyd R, J. Neurotrauma, 2019, 36, 523–537. [DOI] [PubMed] [Google Scholar]
- 11.Marra A, McGrane TJ, Henson CP and Pandharipande PP, Crit. Care Clin, 2019, 35, 329–340. [DOI] [PubMed] [Google Scholar]
- 12.Golabchi A, Wu B, Li X, Carlisle DL, Kozai TD, Friedlander RM and Cui XT, Biomaterials, 2018, 180, 225–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esposito E and Cuzzocrea S, Curr. Neuropharmacol, 2010, 8, 228–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu S, Madu CO and Lu Y, Oncomedicine, 2018, 3, 37–47. [Google Scholar]
- 15.Srinivasan V, Pandi-Perumal S, Maestroni G, Esquifino A, Hardeland R and Cardinali D, Neurotoxic. Res, 2005, 7, 293–318. [DOI] [PubMed] [Google Scholar]
- 16.Jan JE and O’Donnell ME, J. Pineal Res, 1996, 21, 193–199. [DOI] [PubMed] [Google Scholar]
- 17.Mayo JC, Sainz RM, Tan D-X, Antolín I, Rodríguez C and Reiter RJ, Endocrine, 2005, 27, 169–178. [DOI] [PubMed] [Google Scholar]
- 18.Reiter RJ, Manchester LC and Tan D-X, Curr. Neuropharmacol, 2010, 8, 194–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldberg-Stern H, Oren H, Peled N and Garty B-Z, J. Child Neurol, 2012, 27, 1524–1528. [DOI] [PubMed] [Google Scholar]
- 20.Fauteck J-D, Schmidt H, Lerchl A, Kurlemann G and Wittkowski W, Neurosignals, 1999, 8,105–110. [DOI] [PubMed] [Google Scholar]
- 21.Emet M, Ozcan H, Ozel L, Yayla M, Halici Z and Hacimuftuoglu A, Eurasian J. Med, 2016, 48,135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baydas G, Yasar A and Tuzcu M, J. Pineal Res, 2005, 39, 346–352. [DOI] [PubMed] [Google Scholar]
- 23.Weil ZM, Hotchkiss AK, Gatien ML, Pieke-Dahl S and Nelson RJ, Brain Res. Bull, 2006, 68, 425–429. [DOI] [PubMed] [Google Scholar]
- 24.Drijfhout W, Brons H, Oakley N, Hagan R, Grol C and Westerink B, Neuroscience, 1997, 80, 233–239. [DOI] [PubMed] [Google Scholar]
- 25.Nakahara D, Nakamura M, Iigo M and Okamura H, Proc. Natl. Acad. Sci. U. S. A, 2003, 100, 9584–9589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan ME, Singhal D and Anderson BD, J. Pharmacol. Exp. Ther, 1996, 277, 1167–1176. [PubMed] [Google Scholar]
- 27.Di GC, Tanda G and Carboni E, Behav. Pharmacol, 1996, 7, 640–657. [PubMed] [Google Scholar]
- 28.Jaquins-Gerstl A and Michael AC, Analyst, 2015, 140, 3696–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee WH, Ngernsutivorakul T, Mabrouk OS, Wong J-MT, Dugan CE, Pappas SS, Yoon HJ and Kennedy RT, Anal. Chem, 2016, 88, 1230–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chefer VI, Thompson AC, Zapata A and Shippenberg TS, Curr. Protoc. Neurosci, 2009, 47(1), 7.1.1–7.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beyene AG, Yang SJ and Landry MP, J. Vac. Sci. Technol., A, 2019, 37, 040802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vieira R, Míguez J, Lema M and Aldegunde M, Anal. Biochem, 1992, 205, 300–305. [DOI] [PubMed] [Google Scholar]
- 33.Kumar N and Goyal RN, Curr. Pharm. Anal, 2017, 13, 85–90. [Google Scholar]
- 34.Apetrei IM and Apetrei C, Int. J. Nanomed, 2016, 11, 1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alpar N, Pınar PT, Yardım Y and Şentürk Z, Electroanalysis, 2017, 29, 1691–1699. [Google Scholar]
- 36.Bertrand PP, Polglaze KE, Bertrand RL, Sandow SL and Pozo MJ, Curr. Pharm. Des, 2014, 20, 4802–4806. [DOI] [PubMed] [Google Scholar]
- 37.Hensley AL, Colley AR and Ross AE, Anal. Chem, 2018, 90, 8642–8650. [DOI] [PubMed] [Google Scholar]
- 38.Wood KM and Hashemi P, ACS Chem. Neurosci, 2013, 4, 715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacobs CB, Ivanov IN, Nguyen MD, Zestos AG and Venton BJ, Anal. Chem, 2014, 86, 5721–5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keithley RB, Takmakov P, Bucher ES, Belle AM, Owesson-White CA, Park J and Wightman RM, Anal. Chem, 2011, 83, 3563–3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kozai TDY, Langhals NB, Patel PR, Deng X, Zhang H, Smith KL, Lahann J, Kotov NA and Kipke DR, Nat. Mater, 2012, 11, 1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeWaele M, Oh Y, Park C, Kang YM, Shin H, Blaha CD, Bennet KE, Kim IY, Lee KH and Jang DP, Analyst, 2017, 142, 4317–4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oh Y, Park C, Kim DH, Shin H, Kang YM, DeWaele M, Lee J, Min H-K, Blaha CD and Bennet KE, Anal. Chem, 2016, 88, 10962–10970. [DOI] [PubMed] [Google Scholar]
- 44.Meunier CJ, McCarty GS and Sombers LA, Anal. Chem, 2019, 91, 7319–7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dogan-Topal B, Ozkan SA and Uslu B, Open Chem. Biomed. Methods J, 2010, 3, 56–73. [Google Scholar]
- 46.Dauphin-Ducharme P, Arroyo-Currás N, Kurnik M, Ortega G, Li H and Plaxco KW, Langmuir, 2017, 33, 4407–4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen A and Shah B, Anal. Methods, 2013, 5, 2158–2173. [Google Scholar]
- 48.Levent A, Diamond Relat. Mater, 2012, 21, 114–119. [Google Scholar]
- 49.Levent A, Diamond Relat. Mater, 2012, 21, 114–119. [Google Scholar]
- 50.Bagheri H, Afkhami A, Hashemi P and Ghanei M, RSC Adv, 2015, 5, 21659–21669. [Google Scholar]
- 51.Taylor IM, Patel NA, Freedman NC, Castagnola E and Cui XT, Anal. Chem, 2019, 91, 12917–12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taylor IM, Nesbitt KM, Walters SH, Varner EL, Shu Z, Bartlow KM, Jaquins-Gerstl AS and Michael AC, J. Neurochem, 2015, 133, 522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bath BD, Michael DJ, Trafton BJ, Joseph JD, Runnels PL and Wightman RM, Anal. Chem, 2000, 72, 5994–6002. [DOI] [PubMed] [Google Scholar]
- 54.Roseboom PH, Namboodiri MA, Zimonjic DB, Popescu NC, Rodriguez IR, Gastel JA and Klein DC, Mol. Brain Res, 1998, 63, 189–197. [DOI] [PubMed] [Google Scholar]
- 55.Vasantha V and Chen S-M, J. Electrochem. Soc, 2005, 152, D151–D159. [Google Scholar]
- 56.Xiao-Ping W, Lan Z, Wen-Rong L, Jian-Ping D, Hong-Qing C and Guo-Nan C, Electroanalysis, 2002, 14,1654–1660. [Google Scholar]
- 57.Thomas A and Kumar KG, Ionics, 2019, 25, 2337–2349. [Google Scholar]
- 58.Zisapel N, Cell. Mol. Neurobiol, 2001, 21, 605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cardinali D, Nagle C, Freire F and Rosner J, Neuroendocrinology, 1975, 18, 72–85. [DOI] [PubMed] [Google Scholar]
- 60.Raj M and Goyal RN, J. Electrochem. Soc, 2017, 164, B695–B703. [Google Scholar]
- 61.Liu A, Honma I and Zhou H, Biosens. Bioelectron, 2005, 21, 809–816. [DOI] [PubMed] [Google Scholar]
- 62.Cho T and Wang J, Electroanalysis, 2018, 30, 1028–1032. [Google Scholar]
- 63.Sarada B, Rao TN, Tryk D and Fujishima A, Anal. Chem, 2000, 72, 1632–1638. [DOI] [PubMed] [Google Scholar]
- 64.Hashemi P, Dankoski EC, Wood KM, Ambrose RE and Wightman RM, J. Neurochem, 2011, 118, 749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Puthongkham P, Lee ST and Venton BJ, Anal. Chem, 2019, 91(13), 8366–8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Q, Batchelor-McAuley C and Compton RG, J. Phys. Chem. B, 2010, 114, 9713–9719. [DOI] [PubMed] [Google Scholar]
- 67.Baluchová S, Barek J, Tomé LI, Brett CM and Schwarzová-Pecková K, J. Electroanal. Chem, 2018, 821, 22–32. [Google Scholar]
- 68.El Khamlichi R, Bouchta D, Atia MB, Attar A, Choukairi M, Tazi S, Ihssane R, Faiza C, Khalid D and Khalid RT, Mater. Sci. Eng., C, 2017, 71, 870–878. [DOI] [PubMed] [Google Scholar]
- 69.Spanos M, Gras-Najjar J, Letchworth JM, Sanford AL, Toups JV and Sombers LA, ACS Chem. Neurosci, 2013, 4, 782–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kulagina NV and Michael AC, Anal. Chem, 2003, 75, 4875–4881. [DOI] [PubMed] [Google Scholar]
- 71.Takmakov P, Zachek MK, Keithley RB, Bucher ES, McCarty GS and Wightman RM, Anal. Chem, 2010, 82, 9892–9900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tyrtyshnaia AA, Lysenko LV, Madamba F, Manzhulo IV, Khotimchenko MY and Kleschevnikov AM, J. Neuroinflammation, 2016, 13, 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Y, Cook A, Kim J, Baranov SV, Jiang J, Smith K, Cormier K, Bennett E, Browser RP and Day AL, Neurobiol. Dis, 2013, 55, 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pandi-Perumal S, Melatonin: biological basis of its function in health and disease, CRC Press, 2005. [Google Scholar]
- 75.Wilhelmsen M, Amirian I, Reiter RJ, Rosenberg J and Gögenur I, J. Pineal Res, 2011, 51, 270–277. [DOI] [PubMed] [Google Scholar]
- 76.Sugden D, J. Pharmacol. Exp. Ther, 1983, 227, 587–591. [PubMed] [Google Scholar]
- 77.Albertson TE, Peterson S, Stark L, Lakin M and Winters W, Neuropharmacology, 1981, 20, 61–66. [DOI] [PubMed] [Google Scholar]
- 78.Wong PS, Yoshioka K, Xie F and Kissinger P, Rapid Commun. Mass Spectrom, 1999, 13, 407–411. [DOI] [PubMed] [Google Scholar]
- 79.Boudreau E, Chen G, Li X, Buck K, Hitzemann R and Hickman D, Lab. Anim, 2010, 39, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fox JG, Barthold S, Davisson M, Newcomer CE, Quimby FW and Smith A, The mouse in biomedical research: normative biology, husbandry, and models, Elsevier, 2006. [Google Scholar]
- 81.Claassen V, Neglected factors in pharmacology and neuro-science research, Elsevier, Amsterdam, 1994, pp. 46–56. [Google Scholar]
- 82.Kohn DF, Wixson SK, White WJ and Benson GJ, Anesthesia and analgesia in laboratory animals, Elsevier, 1997. [Google Scholar]
- 83.DeMuro RL, Nafziger AN, Blask DE, Menhinick AM and Bertino JS Jr., J. Clin. Pharmacol, 2000, 40, 781–784. [DOI] [PubMed] [Google Scholar]
- 84.Salehi B, Sharopov F, Fokou PVT, Kobylinska A, Jonge LD, Tadio K, Sharifi-Rad J, Posmyk MM, Martorell M and Martins N, Cells, 2019, 8, 681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Andersen LP, Werner MU, Rosenkilde MM, Harpsøe NG, Fuglsang H, Rosenberg J and Gögenur I, BMC Pharmacol. Toxicol, 2016, 17, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.