Abstract
Obesity has reached epidemic proportions in the United States and in much of the westernized world, contributing to considerable morbidity. Several of these obesity-related morbidities are associated with greater risk for death with coronavirus disease 2019 (COVID-19). Severe acute respiratory syndrome coronavirus 2 penetrates human cells through direct binding with angiotensin-converting enzyme 2 receptors on the cell surface. Angiotensin-converting enzyme 2 expression in adipose tissue is higher than that in lung tissue, which means that adipose tissue may be vulnerable to COVID-19 infection. Obese patients also have worse outcomes with COVID-19 infection, including respiratory failure, need for mechanical ventilation, and higher mortality. Clinicians need to be more aggressive when treating obese, especially severely obese, patients with COVID-19 infection.
Abbreviations and Acronyms: ACE, angiotensin-converting enzyme; ACEi, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; Ang II, angiotensin II; ARB, angiotensin receptor blocker; BMI, body mass index; CHD, coronary heart disease; CKD, chronic kidney disease; COVID-19, coronavirus disease 2019; CVD, cardiovascular disease; ET, exercise training; HF, heart failure; HTN, hypertension or hypertensive; MetS, metabolic syndrome; OR, odds ratio; PA, physical activity; RAAS, renin-angiotensin-aldosterone system; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SNS, sympathetic nervous; T2DM, type 2 diabetes mellitus; VTE, venous thromboembolism
Introduction
Obesity is at epidemic levels in the United States and most of the westernized world.1 Nearly three-fourths of adults older than 20 years in the United States meet criteria for being overweight or obese, and 42% are obese (body mass index [BMI; calculated as the weight in kilograms divided by the height in meters squared] ≥30 kg/m2). More than 9% of the US adult population meet criteria for morbid or severe (class III) obesity, with BMI of 40 kg/m2 or greater. Other westernized countries have also noted an increasing prevalence of obesity.2
Obesity is known to influence most major cardiovascular disease (CVD) risk factors, including dysglycemia, metabolic syndrome (MetS) and type 2 diabetes mellitus (T2DM), elevated blood pressure and hypertension (HTN), with adverse effects on cardiovascular structure and function.3 , 4 Additional morbidity includes chronic lung disease, asthma, and systemic inflammation.4 , 5 Not surprisingly, almost all forms of CVD are increased in the setting of increased adiposity,6 especially heart failure (HF), especially HF with preserved ejection fraction, as well as HTN, coronary heart disease (CHD), atrial fibrillation (AF), and peripheral arterial disease.4 , 5 , 7 , 8
Many reports identified obesity and severe obesity as risk factors for hospitalization, mechanical ventilation, and mortality from H1N1 influenza.9 Obesity increases the duration of virus shedding of influenza A virus.10 Based on currently available information and clinical expertise, the Centers for Disease Control and Prevention has identified severe obesity (ie, BMI ≥40 kg/m2) as a common clinical risk factor for worse prognosis and higher mortality in patients with coronavirus disease 2019 (COVID-19) infection.11 Furthermore, any degree of obesity (BMI ≥30 kg/m2) has been associated with poor prognosis in patients with COVID-19.12
Obesity Paradox vs Antiparadox in COVID-19 Infection
We and others proposed the “obesity paradox,” which suggests that overweight and those obese with established CVD appear to have a better short- and medium-term prognosis compared with their lean counterparts. We believe that this obesity paradox is “heavily” influenced by physical activity (PA) and fitness because generally those with high PA and fitness have a good prognosis regardless of weight, whereas the leaner patients with CVD and low PA/fitness have a particularly poor prognosis.4 , 5 , 7 , 13 , 14 Nevertheless, many obesity and CVD prognosis reports do not include data for PA/fitness. We have demonstrated this obesity paradox in patients with lung diseases, including chronic obstructive pulmonary disease and pulmonary embolism,15 , 16 as well as in patients with advanced renal disease.17 However, evidence suggests that obesity may be detrimental in patients with COVID-19 infection,18 with the potential for more development of disease; worse outcomes, including respiratory and multiple organ failure; and higher mortality (Figure 1 ).1 , 12 , 19 , 20
Figure 1.
Potential obesity implications and mechanisms in coronavirus disease 2019 (COVID-19) infection. AF = atrial fibrillation = eGFR = estimated glomerular filtration rate; ERPF = effective renal plasma flow; ERV = expiratory reserve volume; FC = functional capacity; FF = filtration fraction; HDL = high-density lipoprotein; HFpEF = heart failure with preserved ejection fraction; IL-6 = interleukin 6; LDL = low-density lipoprotein; RSC = respiratory system compliance; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; TNF-α = tumor necrosis factor α.
Interestingly, aging and obesity share numerous causative mechanisms largely linked to dysfunctional adipose tissue, such as metabolic dysfunction, multiorgan damage, endocrine disruption, impaired immune function, and chronic inflammation.21
Potential Obesity Implications and Mechanisms in COVID-19 Infection
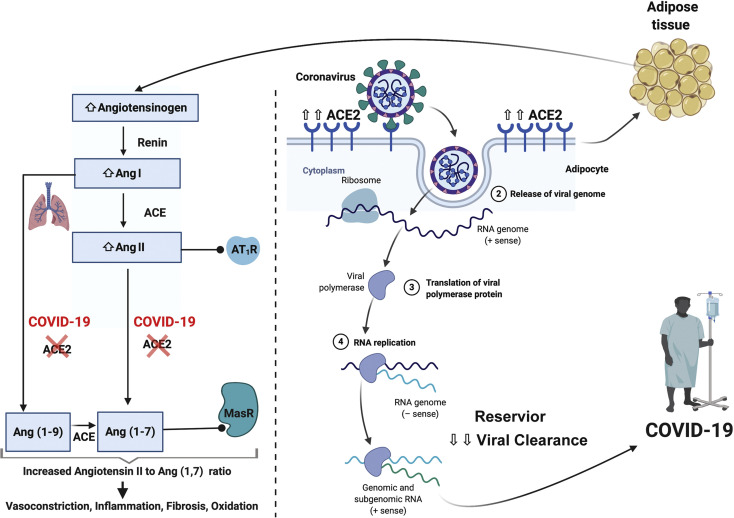
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) penetrates human cells through direct binding with angiotensin-converting enzyme 2 (ACE2) receptors on the cell surface. The obese demonstrate insulin resistance and overactivity of the renin-angiotensin-aldosterone system (RAAS), which is implicated with worse outcomes in COVID-19 infection (Table )22.23 The ACE2 expression in adipose tissue is higher than that in the lung, a major target organ affected by COVID-19,24 suggesting that adipose tissue may be more vulnerable to COVID-19 infection. The obese population have more adipose tissue and consequently higher ACE2 levels (Figure 2 , right panel).24
Table.
Obesity-Associated Comorbid Conditions Potentially Implicated in Worsening Prognosis in Patients With Coronavirus Disease 2019
| Endocrine and metabolic | - Insulin resistance, prediabetes, and type 2 diabetes mellitus - Dyslipidemia including hypertriglyceridemia |
| Autonomic nervous system | - Chronic and excessive activation of the sympathetic nervous and renin-angiotensin-aldosterone systems - Systemic arterial HTN, CVD, and chronic kidney disease |
| Immune system | - Chronic inflammatory reaction |
| Cardiovascular | - Atherosclerotic and nonatherosclerotic CVD and cerebrovascular disease - HTN, including treatment-resistant HTN |
| Pulmonary and sleep apnea | - Sleep-disordered breathing, obesity hypoventilation syndrome, and obstructive sleep apnea - Secondary chronic hypoxia, pulmonary arterial hypertension, and cor pulmonale - Systemic arterial HTN, which may be treatment resistant |
| Hematologic | - Hypercoagulability and a tendency to thromboembolic disease |
| Cancer | - Increased risk for cancer: colon, gastric, esophageal, hepatic, biliary duct, pancreatic, and renal cell |
CVD = cardiovascular disease; HTN = hypertension.
Adapted from Prog Cardiovasc Dis22 with permission.
Figure 2.
Scheme of the effects of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in obese individuals. Increased production of angiotensinogen by adipose tissue leads to elevated angiotensin (Ang) II levels in obesity. SARS-CoV-2 attenuates Ang II metabolism by binding to angiotensin-converting enzyme 2 (ACE2), promoting a system imbalance. High Ang II levels lead to pulmonary vasoconstriction and inflammation that contributes to acute lung injury (left side of figure). ACE2 expression in adipose tissue is higher than that in the lung, a major target organ affected by coronavirus disease 2019 (COVID-19). Increased ACE2 expression in adipocytes may make them more vulnerable to SARS-CoV-2 infection and a potential viral reservoir leading to prolonged viral clearance (right side of the figure). AT1R = angiotensin II type I receptor; MasR = G-protein coupled Mas receptor.
The affinity between ACE2 and SARS-CoV-2 is several-fold higher than the affinity between ACE2 and the former SARS-CoV.25 The presence of ACE2 may enable the entry of SARS-CoV-2 into adipocytes, which makes adipose tissue an important viral reservoir.20 Accordingly, adipose tissue might also be infected by SARS-CoV-2 and allow spread to other organs.26 Moreover, obesity, along with low PA/fitness, is the leading cause of T2DM, and T2DM is also causally linked with elevated ACE2 expression.27 Cell`s expressing ACE2 are also connected to progression of idiopathic pulmonary fibrosis.20
Obesity may induce alterations in the RAAS that promote further derangement in COVID-19 infection. Adipocytes may substantially contribute to the production of circulating angiotensinogen, which after metabolism by renin and ACE, produces angiotensin II (Ang II).28 As such, obesity may lead to an overactive RAAS (Figure 2, left panel). In a small study by Liu et al,29 patients with COVID-19 infection were shown to have elevated Ang II levels that correlated with severity of lung injury measured by PaO2 to fraction of inspired oxygen ratio. High Ang II levels in the lung can induce pulmonary vasoconstriction leading to ventilation/perfusion mismatch and hypoxemia, as well as inflammation and oxidative damage, promoting acute lung injury.30 , 31 In obese individuals with T2DM, Ang II levels were shown to positively correlate with body weight.32 Thus, the elevated baseline Ang II levels in the obese may exacerbate COVID-19–induced Ang II level increase, leading to more severe lung injury. Interestingly, Ang II levels decreased in response to dietary weight loss.32 As such, dietary modification and PA may be beneficial at reducing this potential mechanism of enhanced COVID-19 infection in obesity.
CVD is significantly increased with obesity,33 which represents an important risk factor for CHD and more so for HF. Obesity is also linked to other numerous risk factors for CHD; that is, T2DM, high low-density lipoprotein cholesterol levels, triglyceride levels, HTN, and MetS.34 Importantly, T2DM and HTN themselves also represent a frequent comorbid condition in patients with COVID-19 infection. The risk for CHD is higher in people with “central” or “visceral” obesity (concentrated in the abdomen).
Likewise, obesity is one of the leading risk factors for AF; in many cases, AF is present in the obese with HF, especially HF with preserved ejection fraction.33 Accordingly, the pathways among obesity, HF, and AF are all closely related.33 In effect, AF is a common comorbid condition present in severe forms of COVID-19 infection,35 which may lead to a worse prognosis or even higher mortality. In a report published by the Italian National Institute of Health, approximately one-quarter of patients who died of COVID-19 infection had AF, which was hence the fourth most common condition in these patients.36 Moreover, data provided by the New York State Department of Health also reported that AF is among the top 10 COVID-19 comorbid conditions, specifically occupying the seventh position.37
Additionally, obesity is associated with impaired pulmonary function, with decreased expiratory reserve volume, functional capacity, and respiratory system compliance.9 Increased abdominal obesity compromises pulmonary function in supine patients by decreased diaphragmatic excursion, while the base of the lung ventilation is also impaired, resulting in reduced oxygen-saturated blood levels.9 Furthermore, chronic low-grade inflammation and increased levels of circulating proinflammatory cytokines associated with obesity, such as leptin, tumor necrosis factor α, and interleukin 6, may impair immune response and affect the lung parenchyma and bronchi, thus contributing to the increased morbidity associated with obesity in COVID-19 infection.
There is evidence of endothelial dysfunction in obesity,5 as well as renal disease,22 and this may lead to more potential for endothelial cell infection in obesity, as discussed next. Obesity-related endothelial dysfunction is caused by several mechanisms, such as low-grade inflammation produced by either perivascular adipose tissue or the vasculature itself.38 Endothelial dysfunction results from an imbalance in the production of vasodilatory and vasoconstricting agents.39 These alterations prompt the vascular endothelium toward prothrombotic and proatherogenic states, reflected by platelet hyperactivation, enhanced leukocyte adhesiveness, vasoconstriction, pro-oxidation, mitogenesis, vascular inflammation, impaired hemostasis, atherosclerosis, and thrombosis, with subsequent increased CVD.39 These factors all provoke the development of and the further progression to vascular endothelial dysfunction, with subsequent damage to some vital organs.
Varga et al40 recently demonstrated the involvement of endothelial cells of different organs in a series of patients with COVID-19 infection. They found endothelial cells infected with SARS-CoV-2 and diffuse endothelial inflammation, suggesting that because ACE2 receptors are widely expressed on endothelial cells of multiple organs, SARS-CoV-2 infection may result in extensive endothelial dysfunction related with apoptosis, which ultimately leads to induction of endotheliitis in several organs. For that reason, the authors hypothesized that anti-inflammatory anticytokine drugs, ACE inhibitors (ACEis), and statins may represent relevant strategies in COVID-19 treatment, particularly for more vulnerable patients with worse prognosis (ie, patients with preexisting endothelial dysfunction associated with male sex, chronic respiratory diseases, HTN, T2DM, obesity, and established CVD).40
Chronic kidney disease (CKD) is also among the top 10 COVID-19 comorbid conditions.37 , 41 Obesity provokes structural and functional changes at the nephron level, including podocyte dysfunction with decreased podocyte density and number, podocyte hypertrophy, glomerular capillary hypertension and glomerular hypertrophy, glomerulomegaly, and a secondary adaptive form of focal and segmental glomerulosclerosis in the long term.22 Also, central adiposity decreases estimated glomerular filtration rate and effective renal plasma flow, as well as increases filtration fraction, leading to subnephrotic proteinuria.22
Overall, obesity increases the odds of developing 2 of the major risk factors with direct impact on the progress to CKD, that is, T2DM and HTN, and end-stage renal disease.42 Again, therapy with ACEis and angiotensin receptor blockers (ARBs) has been shown to protect the kidneys and delay progression of CKD in patients with T2DM, being the preferred drugs in patients with CKD.43 For that reason, and in agreement with Varga et al,40 ACEis/ARBs may be recommended as part of the treatment armamentarium for COVID-19 infection.44
Finally, immune dysfunction is also linked to obesity, with increased susceptibility to infection or bacteremia.45 , 46 Functions of T lymphocytes and their subpopulations are impaired in obese individuals.47
Obesity–SARS-CoV-2–Hypercoagulability/Thrombosis: The Lethal Triad of COVID-19
Patients with COVID-19 infection are at risk for venous thromboembolism (VTE), even when using systemic anticoagulation therapy, and/or are at risk for disseminated intravascular coagulation.48 The severity of COVID-19 infection is intimately connected with a hypercoagulable status and thrombosis,49 , 50 the so called “COVID-19–associated coagulopathy” or “sepsis-induced coagulopathy.” Fulminant activation of coagulation and consumption of clotting factors reflected by moderate to severe thrombocytopenia, prolongation of the prothrombin time and activated partial thromboplastin time, elevation of d-dimer levels, and decreased fibrinogen values has been observed in severe COVID-19 infection.51 Abnormal coagulation parameters are associated with poor prognosis in all patients with severe coronavirus pneumonia.52 Many patients with COVID-19 infection who die during hospitalization also show criteria for disseminated intravascular coagulation.52 Notably, d-dimer has been associated with the severity of COVID-19 infection and thus represents a useful index for identifying patients at higher risk for developing VTE.53 An increased incidence of VTE among patients with COVID-19 with severe pneumonia has also been reported, which may be related to poor prognosis.54 Importantly, obesity and overweight have been consistently associated with increased risk for developing VTE.55, 56, 57 Hypercoagulability has been found in overweight patients without MetS and increases with the severity of obesity.58 Numerous mechanisms are implicated in obesity-related hypercoagulability and/or thrombosis, including action of adipocytokines (eg, leptin and adiponectin), coagulation factor hyperactivity (fibrinogen, factor VII, factor VIII, and von Willebrand factor), hypofunctional fibrinolysis (plasminogen activator inhibitor), increased inflammation (tumor necrosis factor and interleukin 6); elevated Ang II levels (endothelial dysfunction and elevated plasminogen activator inhibitor 1), increased oxidative stress and endothelial dysfunction, and lipid and glucose tolerance disorders together with MetS, as well as venous stasis and impaired venous return.59, 60, 61 Therefore, increased hypercoagulability and thrombosis in patients with COVID-19 infection may be the result of additive effects of obesity and SARS-CoV-2 infection.
Clinical Evidence Linking Obesity to Worse Outcomes in Patients With COVID-19
Qingxian et al19 reported for the first time that obesity increased the risk for developing severe pneumonia in patients with COVID-19 infection. These authors examined the association of obesity with severity COVID-19 infection in 383 patients with COVID-19 admitted to the Third People’s Hospital of Shenzhen, China, from January 11 to February 16, 2020. Compared with normal weight, the obesity group showed 2.42-fold higher odds of developing severe pneumonia after adjusting for potential confounders. The odds ratios (ORs) for severe pneumonia in overweight and obese men were 1.96 (95% CI, 0.78-4.98) and 5.70 (95% CI, 1.83-17.76), respectively.
Simonnet et al62 described that obesity represented a risk factor for severity in patients with COVID-19 infection. These authors retrospectively analyzed BMI and other outcomes among 124 consecutive patients with COVID-19 infection consecutively admitted to the intensive care unit. Patients with COVID-19 infection with BMI greater than 35 kg/m2 had more than 7-fold greater risk for invasive mechanical ventilation than patients with BMI less than 25 kg/m2 (OR, 7.36; 95% CI, 1.63-33.14; P=.02). Overall, 47.5% of patients with COVID-19 infection admitted to the ICU had BMI of 30 kg/m2 or greater, 13.7% had BMI between 35 and 39 kg/m2, and 14.5% had BMI of 40 kg/m2 or greater, respectively.
Petrilli et al12 carried out a cross-sectional analysis of all patients with laboratory-confirmed COVID-19 infection in New York City between March 1, 2020, and April 2, 2020. Among 4103 patients with COVID-19 infection, 445 required mechanical ventilation, 162 (36.4%) of whom finally died. A total number of 1100 patients with COVID-19 infection (26.8%) had obesity, whereas hospitalized patients were also more likely to be obese (39.8% vs 14.5%). Moreover, BMI greater than 40 kg/m2 was found to be the strongest risk factor for hospitalization (OR, 6.2; 95% CI, 4.2-9.3).
Lighter et al63 performed a retrospective analysis of BMI stratified by age in symptomatic patients with COVID-19 infection who tested positive and who presented to a large academic hospital system in New York City between March 4 and April 4, 2020. Of the 3615 symptomatic patients who tested positive, 775 (21%) had BMI between 30 and 34 kg/m2 and 595 (16%) had BMI greater than 35 kg/m2. Patients younger than 60 years with BMI between 30 and 34 kg/m2 were 2-fold (95% CI, 1.6-2.6; P<.0001) and 1.8-fold (95% CI, 1.2-2.7; P=.006) more likely to be admitted to acute and critical care, respectively, compared with individuals with BMI less than 30 kg/m2. Moreover, patients with COVID-19 infection with BMI greater than 35 kg/m2 and younger than 60 years were 2.2-fold (95% CI, 1.7-2.9; P<.0001) and 3.6-fold (95% CI, 2.5-5.3; P≤.0001) more likely to be admitted to acute and critical care compared with patients in the same age category and with BMI less than 30 kg/m2.
Additionally, Goyal et al64 performed a retrospective case series study that included confirmed COVID-19–positive cases consecutively admitted at a referral hospital in New York City (Manhattan) between March 5 and March 27, 2020. Among the patients finally included in the study, 136 of 380 (35.8%) were obese and 43.4% needed invasive mechanical ventilation. Interestingly, these authors underlined that the number of patients who received invasive mechanical ventilation was more than 10 times higher compared with China.
Improving PA, Exercise, and Fitness for the Next Pandemic
There is a large amount of evidence supporting the role of PA or exercise training (ET) in decreasing inflammation in overweight or obese individuals.14 PA/ET prevents CVD and improves the prognosis of patients with CVD, particularly CHD and HF. In obese patients with CHD and/or HF, survival rates are excellent regardless of adiposity when fitness is preserved. Thus, improving fitness through PA/ET is needed for primary and secondary prevention of CVD and all-cause mortality in obese populations.14 Maintaining body weight and remaining fit represent a crucial goal for possible future pandemics.
Conclusion
Obesity represents a risk factor for higher severity and worse prognosis in patients with COVID-19 infection. Likewise, obesity-induced adipose tissue inflammation and its effects on the immune system play a crucial role in the pathogenesis of COVID-19 infection. Moreover, it also results in metabolic dysfunction, which may lead to dyslipidemia, insulin resistance, CVD, MetS/T2DM, and HTN. Older age also represents a risk factor for poor prognosis in patients with COVID-19 infection. Clearly, prevention of obesity in the first place and especially its progression to more severe forms is desperately needed throughout the health care system and society. These efforts are also needed to help improve prognosis in the next pandemic, as well as for primary and secondary prevention of CVD and diabetes mellitus. In the ongoing COVID-19 pandemic, clinicians should recognize that the obese, and more so the more severely obese, are at higher risk for clinical deterioration with COVID-19. As such, these patients need to be carefully monitored and treated more aggressively to reduce morbidity and mortality.
Footnotes
Grant Support: F.S.-G. is supported by a postdoctoral contract granted by “Subprograma Atracció de Talent - Contractes Postdoctorals de la Universitat de València.”
Potential Competing Interests: Dr Mehra reports consultancy with Abbott, Janssen, Roivant, Baim Institute for Clinical Research, Leviticus, FineHeart, NupulseCV, Portola, Bayer, Triple Gene, and Mesoblast. The other authors report no competing interests.
Supplemental Online Material
References
- 1.Hales C.M., Carroll M.D., Fryar C.D., Ogden C.L. National Center for Health Statistics; Hyattsville, MD: 2020. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. [Google Scholar]
- 2.Ng M., Fleming T., Robinson M. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013 [erratum in Lancet. 2014;384(9945):746] Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lavie C.J., Sanchis-Gomar F., Henry B.M., Lippi G. COVID-19 and obesity: links and risks. Expert Rev Endocrinol Metab. 2020 doi: 10.1080/17446651.2020.1767589. in press. [DOI] [PubMed] [Google Scholar]
- 4.Lavie C.J., Laddu D., Arena R., Ortega F.B., Alpert M.A., Kushner R.F. Healthy weight and obesity prevention: JACC health promotion series. J Am Coll Cardiol. 2018;72(13):1506–1531. doi: 10.1016/j.jacc.2018.08.1037. [DOI] [PubMed] [Google Scholar]
- 5.Elagizi A., Kachur S., Lavie C.J. An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog Cardiovasc Dis. 2018;61(2):142–150. doi: 10.1016/j.pcad.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Neeland I.J., Yokoo T., Leinhard O.D., Lavie C.J. Twenty-first century advances in multimodality imaging of obesity for care of the cardiovascular patient [published online ahead of print April 10, 2020] https://doi.org/10.1016/j.jcmg.2020.02.031 JACC Cardiovasc Imaging. [DOI] [PMC free article] [PubMed]
- 7.Lavie C.J., Pandey A., Lau D.H., Alpert M.A., Sanders P. Obesity and atrial fibrillation prevalence, pathogenesis, and prognosis: effects of weight loss and exercise. J Am Coll Cardiol. 2017;70(16):2022–2035. doi: 10.1016/j.jacc.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Carbone S., Lavie C.J., Elagizi A., Arena R., Ventura H.O. The impact of obesity in heart failure. Heart Fail Clin. 2020;16(1):71–80. doi: 10.1016/j.hfc.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Dietz W., Santos-Burgoa C. Obesity and its implications for COVID-19 mortality [published online ahead of print April 1, 2020] https://doi.org/10.1002/oby.22818 Obesity (Silver Spring) [DOI] [PubMed]
- 10.Milner J.J., Rebeles J., Dhungana S. Obesity increases mortality and modulates the lung metabolome during pandemic H1N1 influenza virus infection in mice. J Immunol. 2015;194(10):4846–4859. doi: 10.4049/jimmunol.1402295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention Coronavirus disease 2019 (COVID-19). Groups at higher risk for severe illness. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/groups-at-higher-risk.html [PubMed]
- 12.Petrilli C.M., Jones S.A., Yang J. Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in New York City [preprint April 11, 2020] medRxiv. [DOI]
- 13.Moholdt T., Lavie C.J., Nauman J. Sustained physical activity, not weight loss, associated with improved survival in coronary heart disease. J Am Coll Cardiol. 2018;71(10):1094–1101. doi: 10.1016/j.jacc.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Lavie C.J., Carbone S., Kachur S., O'Keefe E.L., Elagizi A. Effects of physical activity, exercise, and fitness on obesity-related morbidity and mortality. Curr Sports Med Rep. 2019;18(8):292–298. doi: 10.1249/JSR.0000000000000623. [DOI] [PubMed] [Google Scholar]
- 15.Keller K., Hobohm L., Munzel T. Survival benefit of obese patients with pulmonary embolism. Mayo Clin Proc. 2019;94(10):1960–1973. doi: 10.1016/j.mayocp.2019.04.035. [DOI] [PubMed] [Google Scholar]
- 16.Chittal P., Babu A.S., Lavie C.J. Obesity paradox: does fat alter outcomes in chronic obstructive pulmonary disease? COPD. 2015;12(1):14–18. doi: 10.3109/15412555.2014.915934. [DOI] [PubMed] [Google Scholar]
- 17.Naderi N., Kleine C.E., Park C. Obesity paradox in advanced kidney disease: from bedside to the bench. Prog Cardiovasc Dis. 2018;61(2):168–181. doi: 10.1016/j.pcad.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kassir R. Risk of COVID-19 for patients with obesity. Obes Rev. 2020;21(6):e13034. doi: 10.1111/obr.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qingxian C., Fengjuan C., Fang L. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China (3/13/2020) 2020. https://ssrn.com/abstract=3556658 Lancet. Accessed June 18, 2020. [DOI] [PubMed]
- 20.Kruglikov I.L., Schere P.E. The role of adipocytes and adipocyte-like cells in the severity of COVID-19 infections [published online ahead of print April 27, 2020] https://doi.org/10.1002/oby.22856 Obesity (Silver Spring) [DOI] [PMC free article] [PubMed]
- 21.Perez L.M., Pareja-Galeano H., Sanchis-Gomar F., Emanuele E., Lucia A., Galvez B.G. 'Adipaging': ageing and obesity share biological hallmarks related to a dysfunctional adipose tissue. J Physiol. 2016;594:3187–3207. doi: 10.1113/JP271691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lakkis J.I., Weir M.R. Obesity and kidney disease. Prog Cardiovasc Dis. 2018;61(2):157–167. doi: 10.1016/j.pcad.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Bornstein S.R., Dalan R., Hopkins D., Mingrone G., Boehm B.O. Endocrine and metabolic link to coronavirus infection. Nat Rev Endocrinol. 2020;16(6):297–298. doi: 10.1038/s41574-020-0353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia X., Yin C., Lu S. Two things about COVID-19 might need attention. Preprints. 2020:2020020315. doi: 10.20944/preprints202002.0315.v1. [DOI] [Google Scholar]
- 25.Wrapp D., Wang N., Corbett K.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bourgeois C., Gorwood J., Barrail-Tran A. Specific biological features of adipose tissue, and their impact on HIV persistence. Front Microbiol. 2019;10:2837. doi: 10.3389/fmicb.2019.02837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao S., Lau A., So H.-C. Exploring diseases/traits and blood proteins causally related to expression of ACE2, the putative receptor of 2019-nCov: a Mendelian randomization analysis highlights tentative relevance of diabetes-related traits [preprint May 12, 2020] medRxiv. [DOI] [PubMed]
- 28.Cabandugama P.K., Gardner M.J., Sowers J.R. The renin angiotensin aldosterone system in obesity and hypertension: roles in the cardiorenal metabolic syndrome. Med Clin North Am. 2017;101(1):129–137. doi: 10.1016/j.mcna.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y., Yang Y., Zhang C. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henry B.M., Vikse J. Clinical characteristics of Covid-19 in China. N Engl J Med. 2020;382(19):1860–1861. doi: 10.1056/NEJMc2005203. [DOI] [PubMed] [Google Scholar]
- 31.Kuba K., Imai Y., Rao S. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saiki A., Ohira M., Endo K. Circulating angiotensin II is associated with body fat accumulation and insulin resistance in obese subjects with type 2 diabetes mellitus. Metabolism. 2009;58(5):708–713. doi: 10.1016/j.metabol.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 33.Jin J. JAMA patient page. Obesity and the heart. JAMA. 2013;310(19):2113. doi: 10.1001/jama.2013.281901. [DOI] [PubMed] [Google Scholar]
- 34.Pandey A., Patel K.V., Lavie C.J. Obesity, central adiposity, and fitness: understanding the obesity paradox in the context of other cardiometabolic parameters. Mayo Clin Proc. 2018;93(6):676–678. doi: 10.1016/j.mayocp.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 35.Sanchis-Gomar F, Perez-Quilis C, Lavie CJ. Should atrial fibrillation be considered a cardiovascular risk factor for a worse prognosis in COVID-19 patients? Eur Heart J. In press. [DOI] [PMC free article] [PubMed]
- 36.COVID-19 Surveillance Group Characteristics of COVID-19 patients dying in Italy Report based on available data on April 6th, 2020. https://www.epicentro.iss.it/en/coronavirus/bollettino/Report-COVID-2019_6_april_2020.pdf
- 37.New York State Department of Health Fatalities. https://covid19tracker.health.ny.gov/views/NYS-COVID19-Tracker/NYSDOHCOVID-19Tracker-Fatalities?%3Aembed=yes&%3Atoolbar=no
- 38.Virdis A. Endothelial dysfunction in obesity: role of inflammation. High Blood Press Cardiovasc Prev. 2016;23(2):83–85. doi: 10.1007/s40292-016-0133-8. [DOI] [PubMed] [Google Scholar]
- 39.Kwaifa I.K., Bahari H., Yong Y.K., Noor S.M. Endothelial dysfunction in obesity-induced inflammation: molecular mechanisms and clinical implications. Biomolecules. 2020;10(2):291. doi: 10.3390/biom10020291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varga Z., Flammer A.J., Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henry B.M., Lippi G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection [published online ahead of print March 28, 2020] https://doi.org/10.1007/s11255-020-02451-9 Int Urol Nephrol. [DOI] [PMC free article] [PubMed]
- 42.Kovesdy C.P., Furth S.L., Zoccali C., World Kidney Day Steering Committee Obesity and kidney disease: hidden consequences of the epidemic. Can J Kidney Health Dis. 2017;4 doi: 10.1177/2054358117698669. 2054358117698669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brenner B.M., Cooper M.E., de Zeeuw D., RENAAL Study Investigators Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 44.Sanchis-Gomar F., Lavie C.J., Perez-Quilis C., Henry B.M., Lippi G. Angiotensin-converting enzyme 2 and anti-hypertensives (angiotensin receptor blockers and angiotensin converting enzyme inhibitors) in coronavirus disease 2019 (COVID-19) [published online ahead of print April 4, 2020] https://doi.org/10.1016/j.mayocp.2020.03.026 Mayo Clin Proc. [DOI] [PMC free article] [PubMed]
- 45.Matarese G., Moschos S., Mantzoros C.S. Leptin in immunology. J Immunol. 2005;174(6):3137–3142. doi: 10.4049/jimmunol.174.6.3137. [DOI] [PubMed] [Google Scholar]
- 46.Laddu D.R., Lavie C.J., Phillips S.A., Arena R. Physical activity for immunity protection: Inoculating populations with healthy living medicine in preparation for the next pandemic [published online ahead of print April 9, 2020] https://doi.org/10.1016/j.pcad.2020.04.006 Prog Cardiovasc Dis. [DOI] [PMC free article] [PubMed]
- 47.Tanaka S., Isoda F., Ishihara Y., Kimura M., Yamakawa T. T lymphopaenia in relation to body mass index and TNF-alpha in human obesity: adequate weight reduction can be corrective. Clin Endocrinol (Oxf) 2001;54(3):347–354. [PubMed] [Google Scholar]
- 48.Klok F.A., Kruip M., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19 [published online ahead of print April 10, 2020] https://doi.org/10.1016/j.thromres.2020.04.013 Thromb Res. [DOI] [PMC free article] [PubMed]
- 49.Wang T., Chen R., Liu C. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7(5):e362–e363. doi: 10.1016/S2352-3026(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wichmann D, Sperhake JP, Lutgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study [published online ahead of print May 6, 2020]. Ann Intern Med. 10.7326/M20-2003. [DOI] [PMC free article] [PubMed]
- 51.Henry B.M., de Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis [published online ahead of print April 10, 2020] https://doi.org/10.1515/cclm-2020-0369 Clin Chem Lab Med. [DOI] [PubMed]
- 52.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lippi G., Favaloro E.J. D-Dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb Haemost. 2020;120(5):876–878. doi: 10.1055/s-0040-1709650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia [published online ahead of print April 9, 2020] https://doi.org/10.1111/jth.14830 J Thromb Haemost. [DOI] [PMC free article] [PubMed]
- 55.Darvall K.A., Sam R.C., Silverman S.H., Bradbury A.W., Adam D.J. Obesity and thrombosis. Eur J Vasc Endovasc Surg. 2007;33(2):223–233. doi: 10.1016/j.ejvs.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 56.Lorenzet R., Napoleone E., Cutrone A., Donati M.B. Thrombosis and obesity: cellular bases. Thromb Res. 2012;129(3):285–289. doi: 10.1016/j.thromres.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 57.Rosito G.A., D'Agostino R.B., Massaro J. Association between obesity and a prothrombotic state: the Framingham Offspring Study. Thromb Haemost. 2004;91(4):683–689. doi: 10.1160/th03-01-0014. [DOI] [PubMed] [Google Scholar]
- 58.Campello E., Zabeo E., Radu C.M. Hypercoagulability in overweight and obese subjects who are asymptomatic for thrombotic events. Thromb Haemost. 2015;113(1):85–96. doi: 10.1160/TH14-02-0156. [DOI] [PubMed] [Google Scholar]
- 59.Reaven G.M., Scott E.M., Grant P.J. Hemostatic abnormalities associated with obesity and the metabolic syndrome. J Thromb Haemost. 2005;3(5):1074–1085. doi: 10.1111/j.1538-7836.2005.01277.x. [DOI] [PubMed] [Google Scholar]
- 60.Mertens I., Van Gaal L.F. Obesity, haemostasis and the fibrinolytic system. Obes Rev. 2002;3(2):85–101. doi: 10.1046/j.1467-789x.2002.00056.x. [DOI] [PubMed] [Google Scholar]
- 61.Samad F., Ruf W. Inflammation, obesity, and thrombosis. Blood. 2013;122(20):3415–3422. doi: 10.1182/blood-2013-05-427708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simonnet A., Chetboun M., Poissy J. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation [published online ahead of print April 9, 2020] https://doi.org/10.1002/oby.22831 Obesity (Silver Spring) [DOI] [PMC free article] [PubMed]
- 63.Lighter J., Phillips M., Hochman S. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission [published online ahead of print April 9, 2020] https://doi.org/10.1093/cid/ciaa415 Clin Infect Dis. [DOI] [PMC free article] [PubMed]
- 64.Goyal P., Choi J.J., Pinheiro L.C. Clinical characteristics of Covid-19 in New York City [published online ahead of print April 17, 2020] https://doi.org/10.1056/NEJMc2010419 N Engl J Med. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.