Supplemental Digital Content is available in the text.
Keywords: administrative claims, healthcare; antibiotic; economics; infections; quality of life
Abstract
Background:
Current understanding of the impact of cardiac implantable electronic device (CIED) infection is based on retrospective analyses from medical records or administrative claims data. The WRAP-IT (Worldwide Randomized Antibiotic Envelope Infection Prevention Trial) offers an opportunity to evaluate the clinical and economic impacts of CIED infection from the hospital, payer, and patient perspectives in the US healthcare system.
Methods:
This was a prespecified, as-treated analysis evaluating outcomes related to major CIED infections: mortality, quality of life, disruption of CIED therapy, healthcare utilization, and costs. Payer costs were assigned using medicare fee for service national payments, while medicare advantage, hospital, and patient costs were derived from similar hospital admissions in administrative datasets.
Results:
Major CIED infection was associated with increased all-cause mortality (12-month risk-adjusted hazard ratio, 3.41 [95% CI, 1.81–6.41]; P<0.001), an effect that sustained beyond 12 months (hazard ratio through all follow-up, 2.30 [95% CI, 1.29–4.07]; P=0.004). Quality of life was reduced (P=0.004) and did not normalize for 6 months. Disruptions in CIED therapy were experienced in 36% of infections for a median duration of 184 days. Mean costs were $55 547±$45 802 for the hospital, $26 867±$14 893, for medicare fee for service and $57 978±$29 431 for Medicare Advantage (mean hospital margin of −$30 828±$39 757 for medicare fee for service and −$6055±$45 033 for medicare advantage). Mean out-of-pocket costs for patients were $2156±$1999 for medicare fee for service, and $1658±$1250 for medicare advantage.
Conclusions:
This large, prospective analysis corroborates and extends understanding of the impact of CIED infections as seen in real-world datasets. CIED infections severely impact mortality, quality of life, healthcare utilization, and cost in the US healthcare system.
Registration:
URL: https://www.clinicaltrials.gov Unique Identifier: NCT02277990
What Is Known?
While cardiac implantable electronic device (CIED) therapies have vastly improved over the decades, patients still experience serious complications such as infection.
Importantly, the impact of CIED infection is substantial for the individual patient, as infection management typically requires hospitalization, prolonged antibiotic therapy, and complete device and lead removal until the infection is resolved.
What the Study Adds?
The prospective, multicenter design of the WRAP-IT (Worldwide Randomized Antibiotic Envelope Infection Prevention Trial) offers a unique opportunity to evaluate the clinical and economic impact of CIED infection in the US healthcare system from the patient, payer, and hospital perspectives, as current understanding is based on retrospective analyses of medical records or administrative claims data.
In this prospectively collected data set, CIED infections were associated with a >3-fold increase in all-cause mortality, a reduction in quality of life for 6 months, and a disruption of CIED therapy in 36% of patients. Mean costs were $55K to the hospital, and $26K to the payer and $2.1K to the patient assuming Medicare fee for service or $57K to the payer and $1.5K to the patient assuming Medicare Advantage.
Cardiac implantable electronic devices (CIEDs) are lifesaving and life-improving technologies for an estimated 1.5 million patients who suffer from electrical disturbances and heart failure disorders every year.1 While these technologies have evolved over the decades, patients still experience serious complications, such as infection. Approximately 1% to 4% of CIED procedures are associated with an infection.2–4
Importantly, the impact of CIED infection is substantial for an individual patient, as infection management typically requires hospitalization, prolonged antibiotic therapy, and often times complete device and lead removal.5 CIED infections carry high short- and long-term mortality risk with an estimated 1 in 5 patient deaths occurring within 1 year, and a 50% risk of mortality at 3 years.6,7 These consequences have a significant financial impact on the healthcare system with prior retrospective estimates of the average cost of treating CIED infections ranging from $45K to $49K from the payer perspective8,9 and ≈$55K in hospital costs.10
Previous efforts to characterize the impact of infection are derived from either single-center experiences or retrospective large claims-based analyses and do not fully explore the perspectives of mortality in infected versus noninfected patients, quality of life (QOL), disruptions in CIED therapy, payer and hospital economics, and patient out-of-pocket costs. The WRAP-IT trial randomized the use of the absorbable antibacterial envelope (TYRX, Medtronic, Minneapolis, MN) envelope among 6983 patients at increased risk of infection, and envelope use resulted in a 40% reduction in major CIED infections.11 The data from the WRAP-IT trial offer a unique opportunity to evaluate the clinical and economic impact of CIED infection.
Methods
This was a prespecified, as-treated analysis of the WRAP-IT trial patients, to evaluate clinical and economic consequences related to major CIED infections. Clinical outcomes were defined as mortality, QOL, and disruption in CIED therapy. Economic outcomes were healthcare utilization (HCU) and costs from the US payer, provider, and patient perspectives. Because of the proprietary nature of the data collected for this trial, data will not be made publicly available.
Trial Design
WRAP-IT was a multicenter, randomized, controlled, prospective, single-blinded, interventional clinical trial comparing standard-of-care antibiotic prophylaxis with the adjunctive use of the TYRX envelope. It included patients undergoing CIED generator replacement or a system upgrade with or without new leads, those undergoing CIED pocket or lead revision, and those undergoing an initial cardiac resynchronization therapy defibrillator procedure. Further details of the trial design have been described previously.12 The protocol was approved by the ethics committee at each participating institution and associated national and local regulatory agencies. All patients provided written informed consent.
The primary trial end point for WRAP-IT was major CIED infection within 12 months of the index procedure, where major infection was defined as infection resulting in CIED system removal, an invasive CIED procedure (eg, pocket revision without removal), treatment with long-term suppressive antibiotic therapy (if the patient was not a candidate for system removal) with infection recurrence after discontinuation of antibiotic therapy or resulting in death. Major CIED infection is a subset of all CIED infections, defined as either superficial cellulitis in the region of the CIED pocket with wound dehiscence, erosion, or purulent drainage; deep incisional or space (pocket) surgical-site infection that met the Centers for Disease Control and Prevention criteria, independent of time from surgery; persistent bacteremia; or endocarditis.
Cohort Selection
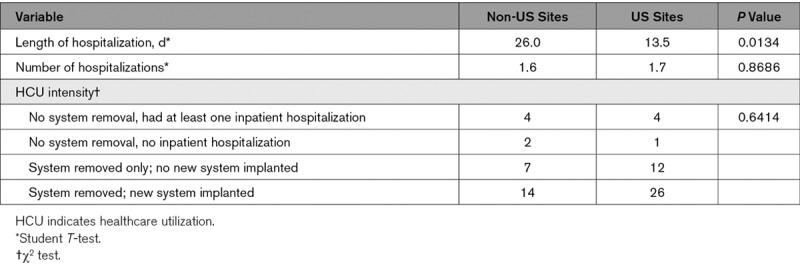
For this analysis, we evaluated the consequences of infections independently from the use of the envelope and considered all major infections for analysis. Poolability of data between the envelope and no envelope arms was confirmed by comparing hospitalization length of stay, total number of hospitalizations per infection, and healthcare utilization intensity (Tables I and II and Figure I in the Data Supplement). Healthcare utilization intensity was defined similar to a previous analysis by categorizing the consequence of the infection as no hospitalization, no system removal, and at least one inpatient hospitalization, CIED system removed without replacement or CIED system removed with replacement.8
Poolability tests revealed significant differences in HCU between infections treated at US and non-US sites (Table 1), thus infections were stratified into 2 cohorts: Cohort with major infections inclusive of all infections at all sites, and US cohort with major infections inclusive of only infections that occurred at US sites. Economic outcomes were analyzed only in the US cohort with major infections. All HCU and costs associated with trial end points were analyzed, even if they occurred >12 months after the index procedure.
Table 1.
Geography Poolability Analysis
Mortality and Quality of Life
Clinical outcomes were analyzed in the full global dataset for primary trial end points. The mortality analysis compared the risk of death at 12 months and throughout all follow-up in patients experiencing a primary trial end point (major infection within 12 months of index procedure) to those with no primary trial end point, and observationally for all follow-up postinfection diagnosis. QOL was collected using EuroQOL-5D (EQ-5D) at baseline, infection diagnosis, 1, 3, and 6 months after diagnosis, and at 12 months after the index procedure. EQ-5D health states were converted to utilities (single cardinal values between 0 and 1 reflecting the health-related QOL of an individual at a point in time)13 using weights reflecting US societal preferences.14
Cost Assignment
Costs reflect inpatient and outpatient hospital visits, clinic visits, long-term stays, home healthcare, and associated professional services. All currency reflects 2017 US dollars.
Payer Perspective
For the payer perspective, costs were assigned as if all HCUs occurred for members of Medicare fee for service (FFS), and also as if all were members of a Medicare Advantage plan. HCU reimbursement codes were retrospectively assigned by a certified medical coding professional and validated by a second. Both coding professionals were blinded to the therapy randomization but had full access to the trial adverse event and HCU data. Current Procedural Terminology,15 International Classification of Diseases, Tenth Revision, Procedure Coding System,16 and International Classification of Diseases, 10th Revision, Clinical Modification17 codes were imputed based on HCU type, diagnosis, and healthcare description. For Medicare FFS payments, codes were mapped to a Medicare Severity-Diagnosis Related Group and used to determine local reimbursement rates,18 accounting for outlier payments based on Centers for Medicare & Medicaid Services reimbursement rules and regulations.19 For Medicare Advantage payments, codes were used to determine average standardized costs for claims in the de-identified Optum Clinformatics Data Mart, an administrative health plan database associated with a large US healthcare organization.
Hospital Perspective
Hospital cost assignments were determined by matching each hospital visit with average costs from comparable events in the Premier Healthcare Database, with data on hospital costs and coding histories for >970 healthcare facilities in the United States.20 For inpatient admissions, inpatient hospitalizations with an infection diagnosis code (Table III in the Data Supplement) in any position were selected, and the match was based on year, Medicare Severity-Diagnosis Related Group and procedure type, and costs were scaled by length of stay. For hospital outpatient and observation stays, same day surgery procedures or observation hospitalizations were selected, and the match was based on year, Current Procedural Terminology and diagnosis codes, and type of encounter. Emergency department visits were matched by year, diagnosis code, and physician specialty. A sensitivity analysis was performed to determine the impact of excluding infections that were not resolved before trial exit.
Hospital margin was calculated for each infection by subtracting total hospital revenue assuming physicians were not employees of the hospital (ie, inpatient and outpatient hospital facility reimbursement only) from total hospital costs. A sensitivity analysis was performed to determine the impact of physician employment status by including professional services in the margin calculation.
Statistical Analysis
Kaplan-Meier (KM) methods were used to construct event-rate plots. Time-to-event for the major CIED infection end point was set to the interval from the time of the index procedure to the time of the first major CIED infection within 365 days. Patients with no major CIED infections in the first 365 days were censored at 365 days or the last follow-up visit, if it occurred before 365 days. Time-to-event for the all-cause mortality end point was calculated in a similar fashion. Hazard ratios and P values for time-to-event analyses were derived with the use of a Cox proportional-hazards regression model, and all-cause mortality was adjusted for CIED device type (pacemaker, defibrillator, CRT), age, sex, history of cardiomyopathy, coronary artery disease, myocardial infarction, chronic obstructive pulmonary disease, diabetes mellitus, and renal disease (renal failure requiring dialysis, or renal insufficiency not requiring dialysis [glomerular filtration rate <60 mL/min per 1.73 m2]). Change in QOL from baseline to the time of major infection, 1, 3, and 6 months post-infection was assessed through repeated measures modeling, assuming compound symmetry within patient. Poolability between the envelope group and the no envelope group was assessed by Student t-test for continuous variables and χ2 test for the categories of HCU intensity. Descriptive and summary statistics are reported. All analyses were performed with the use of the R statistical package (R Project for Statistical Computing) or SAS software, version 9.4 (SAS Institute).
Results
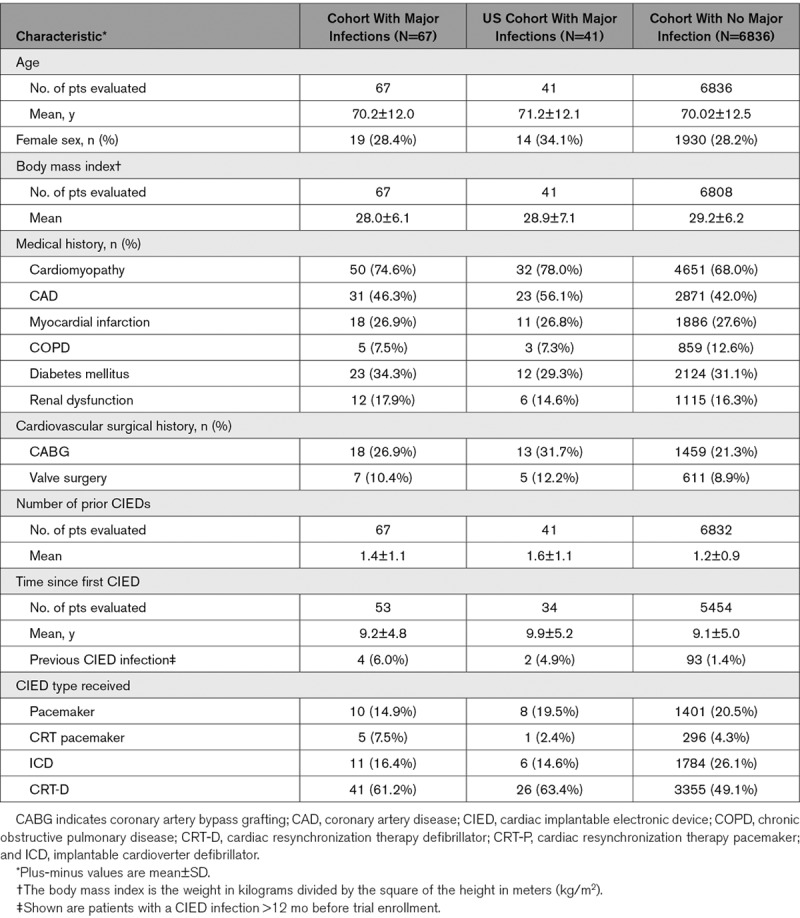
There were 6903 patients from 181 centers in 25 countries within North America, Europe, Asia, and South America included in the analysis. A total of 70 major infections occurred in 67 patients (3 patients had 2 major infections each) through 12 months follow-up (Cohort with Major Infections). Infections occurred at 49 centers in 14 different countries, with no more than 3 infections at any 1 center. There were 43 major infections among 41 patients seen in the US healthcare system (US Cohort with Major Infections; Table 2).
Table 2.
Baseline Demographics and Clinical Characteristics of Patients With and Without Major Infection Within 12 mo
Mortality
In the WRAP-IT trial, 355 patients died within 12 months of the index procedure: 10 of the 67 patients in the infection group (12-month Kaplan-Meier estimate: 16%) and 345 of the 6836 patients in the no infection group (12-month Kaplan-Meier estimate: 5%). As compared with the no infection group, infections were associated with an increased risk of death (risk-adjusted hazard ratio, 3.41 [95% CI, 1.81–6.41]; P<0.001). The effect on mortality was sustained beyond 12 months (hazard ratio through all follow-up: 2.30 [95% CI, 1.29–4.07]; P=0.004; Figure 1A). Of the patients in the cohort with major infections, the Kaplan-Meier estimates of mortality after major infection onset were 16% at 12 months and 23% at 24 months (Figure 1B).
Figure 1.
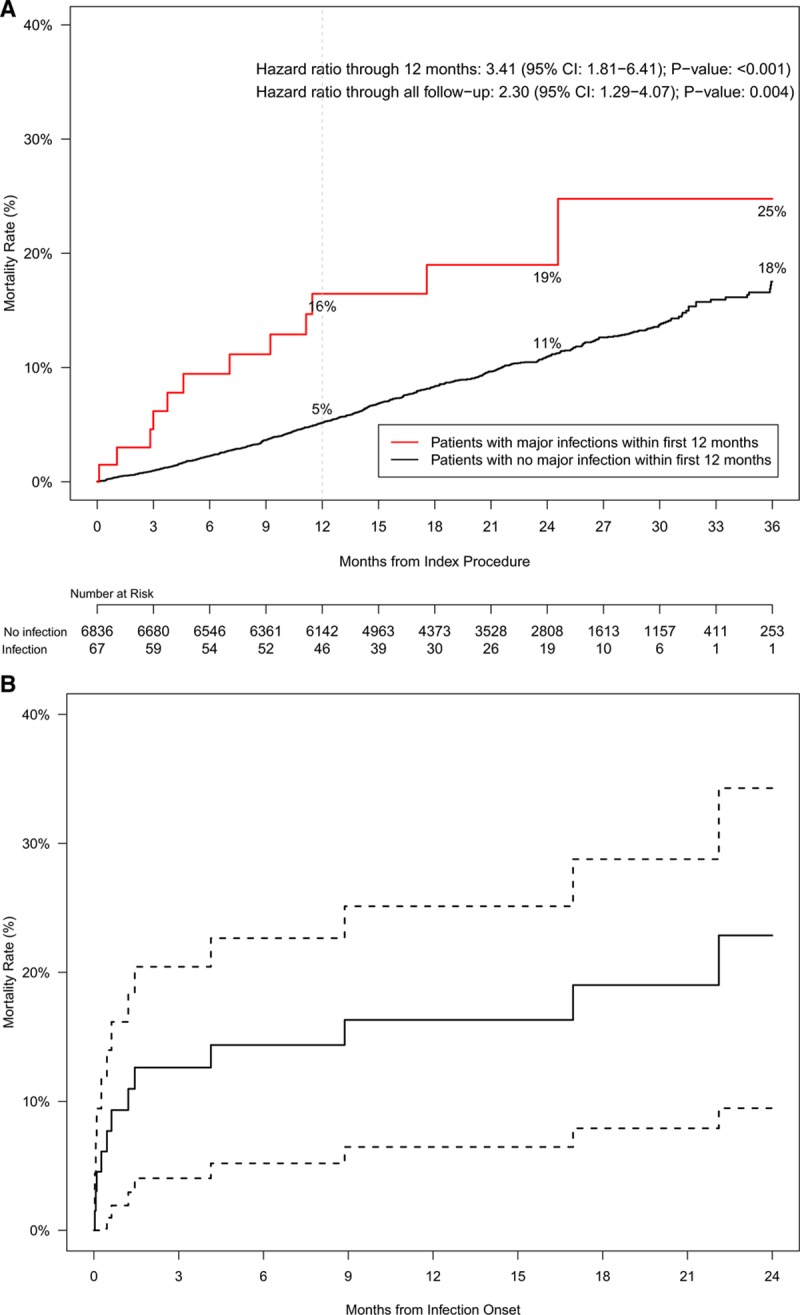
Kaplan-Meier all-cause mortality curves.
A, Patients with (cohort with major infections) and without infections (cohort with no major infections). Hazard ratios and P values are calculated using Cox proportional regression modeling. As compared with the no infection group, infections were associated with an increased risk of death. The effect on mortality was sustained beyond 12 mo. B, Kaplan-Meier (KM) curve and 95% CI for patients with major infection from infection onset. Of the patients in the cohort with major infections, the KM estimates of mortality after major infection onset were 16% at 12 mo and 23% at 24 mo.
Quality of Life
EQ-5D-based utilities at baseline and 12-month follow-up (presumed well states) were similar for patients in the cohort with major infections across devices types. Utilities were significantly reduced at time of infection diagnosis versus baseline (adjusted mean difference 0.09, P=0.004) and did not normalize until 6 months post-diagnosis (Figure 2, Tables IV and V in the Data Supplement).
Figure 2.
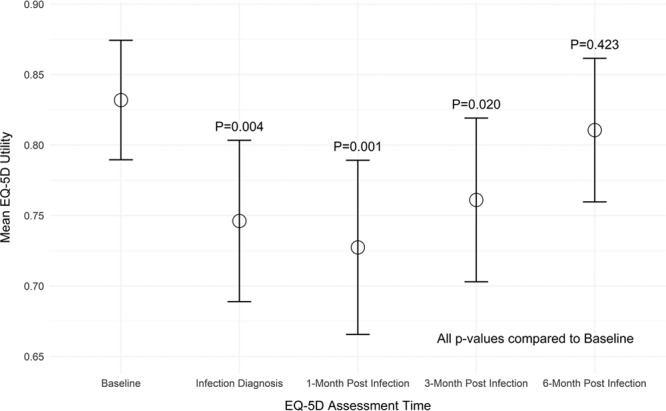
Quality of life.
Impact of infections on quality of life. Data were analyzed using linear mixed-effects modeling. EuroQOL-5D utilities were significantly reduced at time of infection diagnosis vs baseline and did not normalize until 6 mo post-diagnosis. Dots represent the mean and the whiskers represent the 95% CI.
CIED Therapy Disruption
Of the 70 major infections that occurred within 12 months of the index procedure, 11 did not lead to removal of the CIED and 59 did lead to removal of the CIED. Of those with CIED removal, 5 deaths occurred before infection resolution and CIED re-implantation (median of 14 [range, 6–55] days from system removal to death), 14 did not have the CIED re-implanted during the course of the trial (median of 164 [range, 0–749] days from system removal to trial exit), 11 had the CIED explanted in one hospitalization and re-implanted in another (median of 74 [range, 27–288] days from system removal to re-implant), and 29 had the CIED re-implanted during the same hospitalization (median of 6.5 [range, 0–22] days from system removal to re-implant). Disruptions in CIED therapy were experienced in 36% of infections for a median duration of 8474 days (range, 0–749 days; mean duration, 184 days; Figure 3).
Figure 3.
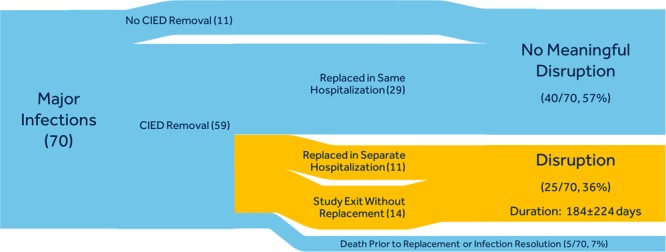
Cardiac implantable electronic device (CIED) therapy disruption.
Time spent without previously indicated CIED therapy. Widths of the paths are proportional to the number of patients in the Sankey Diagram indicating treatment pathway for infections with (blue) and without (orange) CIED therapy disruption. Approximately 36% of infections involved disruption of CIED therapy.
HCU and Costs in the US Healthcare System
The US cohort with major infections experienced a mean of 3.6±5.6 clinic visits and 1.7±0.9 hospital admissions (total hospital days 13.5±11.2 days) per infection. Mean payer costs per infection were $26 867±$14 893 for Medicare FFS and $57 978±$29 431 for Medicare Advantage (Figure 4A).
Figure 4.
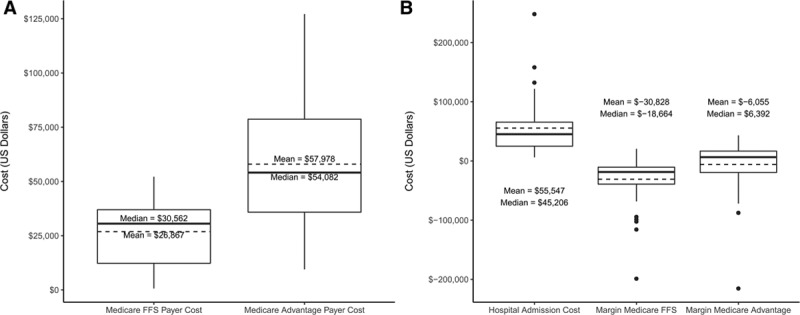
Payer and hospital costs and margins.
A, Total payer costs per infection in the US Cohort with Major Infections for Medicare fee for service (FFS) and Medicare Advantage. B, Total hospital costs and margins per infection in the US Cohort with major infections based on Medicare FFS and Medicare Advantage payments. Box-and-whisker plots represent distribution of data as follows: solid line=median; dashed line=mean; box=interquartile range; whiskers=minimum and maximum within 1.5× interquartile range; dots=outliers (outside of 1.5× interquartile range). All currency reflects 2017 US dollars.
Mean hospital costs were $55 547±$45 802 per infection (Figure 4B). Costs varied by treatment intensity ($16 592±11 293 for 5 infections treated without extraction, $45 694±34 936 for 12 infections treated with extraction and no replacement, $67 586±49 660 for 26 infections treated with extraction and replacement). Mean hospital margins (hospital reimbursement minus cost) per infection were negative: −$30 828±$39 757 assuming Medicare FFS reimbursement and −$6055±$45 033 for Medicare Advantage (Figure 4B).
Mean patient costs per infection were $2156±$1999 assuming Medicare FFS membership and $1658±1250 assuming Medicare Advantage (Figure II in the Data Supplement).
Sensitivity Analyses
Among the infections in the US Cohort with Major Infections, there were 5 infections that did not involve a full course of treatment (3 died and 2 exited the trial before resolution). When these infections were excluded, mean payer costs were $28 229±$15 066 for Medicare FFS and $58 797±$30 587 for Medicare Advantage; mean hospital costs were $56 159±$46 698.
Among the infections in the US Cohort with Major Infections, when including reimbursement payments for professional services, mean hospital margins were negative at −$29 393±$39 399 assuming Medicare FFS reimbursement and slightly positive at $31±$45 999 for Medicare Advantage.
Discussion
In this prospective evaluation of the WRAP-IT trial data, CIED infection was associated with a greater than 3-fold risk of mortality at 12 months after the index procedure, with mortality after major infection onset of 16% at 12 months and 23% at 24 months. QOL was significantly reduced at time of infection diagnosis as compared with baseline and did not return to normal levels before 6 months after diagnosis. Disruptions in CIED therapy were experienced in 36% of the infections with a mean duration of ≈6 months. Average costs in the US healthcare system for an infection were $26 867±$14 893 and $57 978±$29 431 for Medicare FFS and Medicare Advantage payers, respectively; $55 547±$45 802 for the hospital, $2156±$1999 for the Medicare FFS member, and $1658±$1250 for the Medicare Advantage member.
Historically, there is an expectation that CIED infection rates range from 1% to 4%.2–4 The recent WRAP-IT and PADIT (Prevention of Arrhythmia Device Infection Trial) reported overall control infection rates of 1.2% and 1.03%, respectively, which fall within the expected range.11,21 For perspective, it is important to note that both trials had different inclusion/exclusion criteria, with WRAP-IT excluding de novo implants of implantable pulse generator and ICD devices, as well as, patients on hemodialysis and immunosuppressive therapy, and PADIT excluding patients with prior infection and limiting some centers to high-risk patients only.11,21 The rates observed in these trials may also deviate from real-world expectations, both in selection of implant sites with a high implant volume and best practice infection prevention techniques, and the potential of a Hawthorne effect because of a clinical trial focusing on infections as an end point.
Prior reports estimate that patients with CIED infection were associated with approximately twice the mortality risk after 1 year compared with patients without infection.22,23 We observed in this prospective evaluation that the risk of mortality associated with CIED infection was substantially higher, suggesting a >3-fold risk of death through 1-year follow-up and that this effect was sustained beyond 1 year. The prospective, longitudinal nature of the data collection in this analysis is likely a more accurate representation of true mortality rates, as compared with prior retrospective claims-based analyses. It may also be reflective of the change in mortality incidence over time, since inpatient mortality was previously estimated to increase by 1% per decade over a 16-year period.3 However, all-cause mortality in patients with major infection accounted for only about 0.3% of total deaths in the WRAP-IT trial. As such, it should be noted that while infections are expensive and increase mortality, they represent a small component of overall mortality risk to this cohort.
To date, estimates of the impact of CIED infections on QOL are based on expert opinions rather than quantifiable data.24 This analysis establishes an understanding of the impact of infection on QOL, quantifying the severity and duration of this impact. At infection diagnosis, QOL is reduced by an adjusted mean difference of 0.09, which is more than twice the US-specific instrument-defined minimally important difference of 0.04.25 The data from WRAP-IT implies that there is a full recovery to normal baseline QOL, and this occurs up to 6 months postinfection diagnosis for surviving patients.
The potential of an infection to disrupt CIED therapy has not been characterized previously. The experience in WRAP-IT indicates that ≈40% of infections involved disruption in CIED therapy. This is a novel understanding of a potentially important consequence; a period (median, 166 days) where the patient not treated in a single hospitalization is living without a previously indicated device therapy. In the absence of an infection, patients would have continued receiving their indicated CIED therapy without interruption. It is important to acknowledge that there might be clinical circumstances where there was no intent to replace the device after removal, or where the patient was at low risk for adverse events without a device.
Greenspon et al8 performed a claims-based analysis of Medicare FFS payer costs for patients with and without CIED infection with a weighted average of $27K directly related to infection treatment, which is concordant with the WRAP-IT observation of $27K for Medicare FFS. Although WRAP-IT only collected HCUs directly related to infections, the prior estimate of Medicare FFS payments by Greenspon et al also found that total incremental expenses (not just infection related) totaled $47K, suggesting that the cost impact of CIED infection extends beyond direct infection-related expenses. Sohail et al9 estimated total incremental expenses for commercial plus Medicare Supplemental patients with infection at $46-48K while our estimate was $58K for Medicare Advantage payers. While it is commonly expected that Medicare Advantage reimbursement is higher than Medicare FFS, the higher observed rate is at least partially impacted by higher payments to out-of-network providers.
Our study estimated the actual cost to the hospital for infection treatment at approximately $56K. This seems to be similar to previous estimates.10 Using our estimates of Medicare FFS, Medicare Advantage, and hospital costs, we estimate that treating an infection results in an average margin of −$31K to −$6K, which signals a higher burden on hospitals than previously understood.
Few prior publications have attempted to quantify the out-of-pocket cost impacts that CIED infections have to the patient. Mean out-of-pocket cost for infection treatment in this study was estimated to be $2156 for the Medicare FFS member (Medicare Advantage members see a slightly lower but still substantial cost, $1658). The Medicare Part A inpatient hospital deductible for 2017 was $1309, and all but two of the infections in this study led to a total cost to the patient of at least this amount or higher. This level represents paying at or above worst-case expectations, which may be a significant economic and emotional burden for the average person. Achieving high value for patients is a central goal of value-based health care and tracking both outcomes and costs longitudinally from the patient perspective is the only way to accurately measure that value.26
The burden of an infection impacts multiple stakeholders, which has not previously been evaluated comprehensively in retrospective data sets. This prospective analysis, which considers payer, hospital, and patient costs provides a comprehensive understanding of the consequences of CIED infection and warrants further evaluation of the clinical and economic benefit of technologies designed to prevent CIED infection.
Limitations
Data from the WRAP-IT trial had specific inclusion/exclusion criteria that may not represent real-world practice; however, a real-world perspective can be gained from prior observational analyses in the literature. There is a possibility of under-reporting of HCU, which was mitigated by diligent data collection by the clinical trial team. We also did not collect noninfection related HCU, yet this represents an accurate view of direct infection-related costs and indirect costs can be estimated from other sources. Although our analysis did not consider other payer perspectives (eg, Medicaid, Veterans, employer, private plans), the majority of CIED recipients are Medicare eligible. Payments from Medicare Advantage plans vary considerably; however, these estimates were drawn from the largest commercial payer in the US. Hospital costs estimated from charges multiplied by cost-to-charge ratios may not accurately represent true hospital costs, but this is a generally accepted method. These results are specific to the US healthcare system and cannot be extrapolated to other geographies. Finally, the clinical judgement leading to the decision to explant or re-implant devices were not always available. The results, however, provide insight into the impact of disruptions in CIED therapy.
Conclusions
This large, prospective analysis corroborates and extends understanding of the impact of CIED infections as seen in real-world datasets. Even in the selected context of a randomized trial involving centers with high volumes of CIED implants, CIED infections result in severe impact on mortality, QOL, disruption of CIED therapy, hospitalization, and cost in the US healthcare system. This comprehensive evaluation of the consequences of infections provides a foundation for understanding the clinical and economic impact of strategies designed to address the problem of CIED infection.
Acknowledgments
We are grateful to Kelli Schuett, RHIA, Denise Griesmann, CPC, CCC, and Lucas Higuera, MA from Medtronic for creating the payer, hospital and patient perspective cost assignments, and to Swathi Seshadri, PhD for assistance in the preparation of this manuscript.
Sources of Funding
This work was supported by Medtronic, Inc, Minneapolis, MN.
Disclosures
Dr Wilkoff received honoraria/consultant fees from Abbott, Medtronic, and Philips. Dr Boriani received honoraria/consultant fees from Biotronik, Boston Scientific, and Medtronic. Dr Mittal received honoraria/consultant fees from Abbott, Boston Scientific, and Medtronic. Dr Poole received honoraria/consultant fees from Boston Scientific, EBR Solutions, Kestra, and Medtronic. Dr Kennergren received honoraria/consultant fees Medtronic. Dr Corey received honoraria/consultant fees from Arsanis, Basilea, Bayer, Contrafect, Medtronic, Melinta, Motif, Paratek, Pfizer, Quintiles, Tetraphase, The Medicines Company, Theravance, Bio2 Medical, Cempra, Meiji Seika Pharm, Co, Novella, Regeneron, and SC Pharma. Dr Augostini received honoraria/consulting fees Medtronic, Philips, and Respicardia. Dr Faerestrand received honoraria/consultant fees from Medtronic. Dr Healey received a research grant from Medtronic and speaker fees from Abbott, Boston Scientific, and Medtronic. R. Holbrook, Drs Lande and Lexcen, and S. Willey are employed at Medtronic. Dr Tarakji received honoraria/consultant fees from AliveCor and Medtronic. The other authors report no conflicts.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- CIED
- cardiac implantable electronic device
- FFS
- fee for service
- HCU
- healthcare utilization
- PADIT
- Prevention of Arrhythmia Device Infection Trial
- QOL
- quality of life
- WRAP-IT
- Worldwide Randomized Antibiotic Envelope Infection Prevention Trial
A list of WRAP-IT Investigators is provided in the Data Supplement.
For Sources of Funding and Disclosures, see page 390.
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCEP.119.008280.
References
- 1.Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009–a World Society of Arrhythmia’s project. Pacing Clin Electrophysiol. 2011;34:1013–1027. doi: 10.1111/j.1540-8159.2011.03150.x. doi: 10.1111/j.1540-8159.2011.03150.x. [DOI] [PubMed] [Google Scholar]
- 2.Tarakji KG, Ellis CR, Defaye P, Kennergren C. Cardiac Implantable Electronic Device Infection in Patients at Risk. Arrhythm Electrophysiol Rev. 2016;5:65–71. doi: 10.15420/aer.2015.27.2. doi: 10.15420/aer.2015.27.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT, Pavri BB, Kurtz SM. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008. J Am Coll Cardiol. 2011;58:1001–1006. doi: 10.1016/j.jacc.2011.04.033. doi: 10.1016/j.jacc.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 4.Prutkin JM, Reynolds MR, Bao H, Curtis JP, Al-Khatib SM, Aggarwal S, Uslan DZ. Rates of and factors associated with infection in 200 909 Medicare implantable cardioverter-defibrillator implants: results from the National Cardiovascular Data Registry. Circulation. 2014;130:1037–1043. doi: 10.1161/CIRCULATIONAHA.114.009081. doi: 10.1161/CIRCULATIONAHA.114.009081. [DOI] [PubMed] [Google Scholar]
- 5.Kusumoto FM, Schoenfeld MH, Wilkoff BL, Berul CI, Birgersdotter-Green UM, Carrillo R, Cha YM, Clancy J, Deharo JC, Ellenbogen KA, et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017;14:e503–e551. doi: 10.1016/j.hrthm.2017.09.001. doi: 10.1016/j.hrthm.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Rizwan Sohail M, Henrikson CA, Jo Braid-Forbes M, Forbes KF, Lerner DJ. Increased long-term mortality in patients with cardiovascular implantable electronic device infections. Pacing Clin Electrophysiol. 2015;38:231–239. doi: 10.1111/pace.12518. doi: 10.1111/pace.12518. [DOI] [PubMed] [Google Scholar]
- 7.Tarakji KG, Wazni OM, Harb S, Hsu A, Saliba W, Wilkoff BL. Risk factors for 1-year mortality among patients with cardiac implantable electronic device infection undergoing transvenous lead extraction: the impact of the infection type and the presence of vegetation on survival. Europace. 2014;16:1490–1495. doi: 10.1093/europace/euu147. doi: 10.1093/europace/euu147. [DOI] [PubMed] [Google Scholar]
- 8.Greenspon AJ, Eby EL, Petrilla AA, Sohail MR. Treatment patterns, costs, and mortality among Medicare beneficiaries with CIED infection. Pacing Clin Electrophysiol. 2018;41:495–503. doi: 10.1111/pace.13300. doi: 10.1111/pace.13300. [DOI] [PubMed] [Google Scholar]
- 9.Sohail MR, Eby EL, Ryan MP, Gunnarsson C, Wright LA, Greenspon AJ. Incidence, treatment intensity, and incremental annual expenditures for patients experiencing a cardiac implantable electronic device infection: evidence from a large US payer database 1-year post implantation. Circ Arrhythm Electrophysiol. 2016;9:e003929. doi: 10.1161/CIRCEP.116.003929. [DOI] [PubMed] [Google Scholar]
- 10.Shariff N, Eby E, Adelstein E, Jain S, Shalaby A, Saba S, Wang NC, Schwartzman D. Health and economic outcomes associated with use of an antimicrobial envelope as a standard of care for cardiac implantable electronic device implantation. J Cardiovasc Electrophysiol. 2015;26:783–789. doi: 10.1111/jce.12684. doi: 10.1111/jce.12684. [DOI] [PubMed] [Google Scholar]
- 11.Tarakji KG, Mittal S, Kennergren C, Corey R, Poole JE, Schloss E, Gallastegui J, Pickett RA, Evonich R, Philippon F, et al. WRAP-IT Investigators. Antibacterial envelope to prevent cardiac implantable device infection. N Engl J Med. 2019;380:1895–1905. doi: 10.1056/NEJMoa1901111. doi: 10.1056/NEJMoa1901111. [DOI] [PubMed] [Google Scholar]
- 12.Tarakji KG, Mittal S, Kennergren C, Corey R, Poole J, Stromberg K, Lexcen DR, Wilkoff BL. Worldwide Randomized Antibiotic EnveloPe Infection PrevenTion Trial (WRAP-IT). Am Heart J. 2016;180:12–21. doi: 10.1016/j.ahj.2016.06.010. doi: 10.1016/j.ahj.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Torrance GW. Utility approach to measuring health-related quality of life. J Chronic Dis. 1987;40:593–603. doi: 10.1016/0021-9681(87)90019-1. doi: 10.1016/0021-9681(87)90019-1. [DOI] [PubMed] [Google Scholar]
- 14.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43:203–220. doi: 10.1097/00005650-200503000-00003. doi: 10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 15.CPT copyright 2018. American Medical Association. All rights reserved. CPT is a registered trademark of the American Medical Association. Applicable FARS/DARS restrictions apply to government use. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT, and the AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The AMA assumes no liability for the data contained or not contained herein.
- 16.U.S. Centers for Medicare & Medicaid Services. The ICD-10. https://www.cms.gov/Medicare/Coding/ICD10. Accessed December 20, 2019. [PubMed]
- 17.U.S. Centers for Medicare & Medicaid Services. ICD-10-CM/PCS MS-DRG v35.0 Definitions Manual. https://www.cms.gov/icd10m/version35-fullcode-cms/fullcode_cms/P0001.html. Accessed December 20, 2019.
- 18.World Health Organization. ICD-9-PCS. http://www.who.int/classifications/apps/icd/ClassificationDownload. Accessed December 20, 2019.
- 19.U.S. Centers for Medicare & Medicaid Services. The Official U.S. Government Site for Medicare. https://www.medicare.gov. Accessed December 20, 2019.
- 20.Premier Applied Sciences, the Research Division of Premier Inc. Premier Healthcare Database White Paper: Data that informs and performs, March 2, 2020. https://learn.premierinc.com/white-papers/premier-healthcaredatabase-whitepaper. Accessed December 20, 2019.
- 21.Krahn AD, Longtin Y, Philippon F, Birnie DH, Manlucu J, Angaran P, Rinne C, Coutu B, Low RA, Essebag V, et al. Prevention of arrhythmia device infection trial: the PADIT trial. J Am Coll Cardiol. 2018;72:3098–3109. doi: 10.1016/j.jacc.2018.09.068. doi: 10.1016/j.jacc.2018.09.068. [DOI] [PubMed] [Google Scholar]
- 22.Sohail MR, Henrikson CA, Braid-Forbes MJ, Forbes KF, Lerner DJ. Mortality and cost associated with cardiovascular implantable electronic device infections. Arch Intern Med. 2011;171:1821–1828. doi: 10.1001/archinternmed.2011.441. doi: 10.1001/archinternmed.2011.441. [DOI] [PubMed] [Google Scholar]
- 23.Tarakji KG, Chan EJ, Cantillon DJ, Doonan AL, Hu T, Schmitt S, Fraser TG, Kim A, Gordon SM, Wilkoff BL. Cardiac implantable electronic device infections: presentation, management, and patient outcomes. Heart Rhythm. 2010;7:1043–1047. doi: 10.1016/j.hrthm.2010.05.016. doi: 10.1016/j.hrthm.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 24.Kay G, Eby EL, Brown B, Lyon J, Eggington S, Kumar G, Fenwick E, Sohail MR, Wright DJ. Cost-effectiveness of TYRX absorbable antibacterial envelope for prevention of cardiovascular implantable electronic device infection. J Med Econ. 2018;21:294–300. doi: 10.1080/13696998.2017.1409227. doi: 10.1080/13696998.2017.1409227. [DOI] [PubMed] [Google Scholar]
- 25.Luo N, Johnson J, Coons SJ. Using instrument-defined health state transitions to estimate minimally important differences for four preference-based health-related quality of life instruments. Med Care. 2010;48:365–371. doi: 10.1097/mlr.0b013e3181c162a2. doi: 10.1097/mlr.0b013e3181c162a2. [DOI] [PubMed] [Google Scholar]
- 26.Porter ME. What is value in health care? N Engl J Med. 2010;363:2477–2481. doi: 10.1056/NEJMp1011024. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


