Abstract
Background
The treatment of upper respiratory tract infections (URTIs) accounts for the majority of antibiotic prescriptions in primary care, although an antibiotic therapy is rarely indicated. Non-clinical factors, such as time pressure and the perceived patient expectations are considered to be reasons for prescribing antibiotics in cases where they are not indicated. The improper use of antibiotics, however, can promote resistance and cause serious side effects. The aim of the study was to clarify whether the antibiotic prescription rate for infections of the upper respiratory tract can be lowered by means of a short (2 x 2.25h) communication training based on the MAAS-Global-D for primary care physicians.
Methods
In total, 1554 primary care physicians were invited to participate in the study. The control group was formed from observational data. To estimate intervention effects we applied a combination of difference-in-difference (DiD) and statistical matching based on entropy balancing. We estimated a corresponding multi-level logistic regression model for the antibiotic prescribing decision of German primary care physicians for URTIs.
Results
Univariate estimates detected an 11-percentage-point reduction of prescriptions for the intervention group after the training. For the control group, a reduction of 4.7% was detected. The difference between both groups in the difference between the periods was -6.5% and statistically significant. The estimated effects were nearly identical to the effects estimated for the multi-level logistic regression model with applied matching. Furthermore, for the treatment of young women, the impact of the training on the reduction of antibiotic prescription was significantly stronger.
Conclusions
Our results suggest that communication skills, implemented through a short communication training with the MAAS-Global-D-training, lead to a more prudent prescribing behavior of antibiotics for URTIs. Thereby, the MAAS-Global-D-training could not only avoid unnecessary side effects but could also help reducing the emergence of drug resistant bacteria. As a consequence of our study we suggest that communication training based on the MAAS-Global-D should be applied in the postgraduate training scheme of primary care physicians.
Introduction
The widespread use of antibiotics and the lack of new drug development serve as the main causes for the emergence of drug resistant bacteria [1], limiting the effectiveness of antimicrobial therapy [2]. The rapid increase of resistant bacteria is regarded as one of the greatest threats to global health [3]. Infections with antibiotic-resistant bacteria may cause higher severity of illness, mortality rates, risk of complications, admissions to hospital, hospital length of stay and health care costs [2, 4–7].
Especially, not indicated antibiotic use is considered to be a primary cause of increasing risk of bacterial resistance [8]. Therefore, several initiatives address the improvement of prescribing practices of antibiotics worldwide [9–11]. A prominent example for an irrational use of antibiotics can be found in primary care, where primary care physicians (PCPs) often treat upper respiratory tract infections (URTIs) with antibiotics [12]. URTIs are one of the most common reasons for encounter in primary care and are mostly caused by viral infections, making antibiotic-therapy appropriate for only a small number of high risk patients [13]. However, the treatment of URTIs accounts for the majority of antibiotic prescriptions in primary care [12, 14, 15], although there is very limited evidence for their benefits [16–18]. Besides characteristics of the physicians (e.g., specialty, training, experience), patients (e.g., sex, age, insurance status, comorbidities) and environmental factors (e.g. access to and quality of care), patient knowledge and expectations, as well as the physicians’ assumptions regarding these expectations play a crucial role in the prescribing process [19–21]. Furthermore, evidence strongly suggests that antibiotic prescriptions are associated with a communication problem. Most patients seem to possess insufficient knowledge about the difference between viral and bacterial infections [22]. Due to the patients’ belief that a previously received antibiotic drug cured their infection, their expectations to receive antibiotic therapy when next presenting with URTI symptoms will increase [23]. Additionally, physicians may wrongly assume that the patient will demand antibiotics and preemptively prescribe the medicine [24–26]. Moreover, due to an overload of patients, physicians might not take the time to change the patient’s expectations by explaining the differences between viruses and bacteria in an understandable and effective way [27–29]. Therefore, patient expectations could strongly influence physicians, who are willing to prescribe an antibiotic to maintain a good relationship and to save time [20, 30, 31].
Communication trainings have been found to be effective in decreasing the antibiotic prescription rate [32–37]. Although the benefits of adequate communication skills are well known, they are not part of the postgraduate training scheme of any medical specialty in Germany [38]. In the Netherlands, a mandatory instrument for training and measuring physicians’ communication and medical skills is widely used in under- and postgraduate training [39]. This instrument, named Maastricht history taking and advice scoring list (MAAS-Global), has been recently translated and adapted for use in Germany (MAAS-Global-D) [40].
The aim of this study was to investigate whether a communication training based on the MAAS-Global-D can reduce the rate of antibiotic prescribing for URTIs. Since the expectations of the patients and their perceptions of the physicians are subjective and might differ between patients, we additionally evaluate the intervention effect by the patient’s age and sex to increase the insights of the communication effect.
Materials and methods
Data source
This study was based on the analysis of routine data of the years 2013 to 2016 from the Association of Statutory Health Insurance Physicians (ASHIP) of the federal state Schleswig-Holstein, located in Northern Germany. The ASHIP is in charge for the reimbursement of services that are provided to patients within the statutory health insurance system. The dataset covers 85% of the population and 83% of the PCPs of Schleswig-Holstein [41, 42]. The URTI cases were identified by the target-diagnoses of acute bronchitis, sinusitis and pharyngitis (classified by the International Classification of Diseases, version 10 (ICD-10) codes: J01.-; J02.-; J20.- [43]). We concentrate the analysis to these diagnoses, since only in some cases the use of antibiotics is suggested by respective guidelines within these diagnoses. For cases of acute bronchitis (J20) an antibiotic prescription is indicated for elderly patients as well as for those with a severe cardiac or respiratory disease or a congenital or acquired immunodeficiency [44]. In the case of acute pharyngitis (J02), the indications for an antibiotic therapy are: pharyngitis due to group A streptococcus bacterial infections (GAS pharyngitis), scarlet fever, peritonsillar abscess, a suspected serious illness or clinical worsening as well as consumptive diseases, immunosuppression and acute rheumatic fever in the personal or family history [45]. For acute sinusitis (J01), an antibiotic therapy should be considered for patients with specific risk factors, as well as complications such as severe headache, facial swelling, lethargy and acute exacerbation of recurrent sinusitis. Moreover, severe pain and an increased inflammation score complaints in the course of the disease and with fever above 38.5°C [46].
Since the antibiotic prescriptions have been inferred based on the visit diagnoses, we excluded cases with additional diagnoses. This includes the presence of diagnoses regarding puerperium/pregnancy (O00-O99), further (bacterial) infections (A00 to A37, A39 to A79, J15, J17, J18) or chronic diseases (I50, J44, J45, C00 to C75). If the diagnosis had been made several times or more than one diagnosis had been made from the three groups (J01, J02, J20), the corresponding cases were also excluded. To increase the comparability of the included cases and, thus, minimize a potential estimation bias of the communication training effect, only cases of patients that were older than 18 years are included in the analysis.
Recruitment and inclusion criteria
All primary care physicians in private practices, working in a contract with statutory public health insurance and with a work experience of at least five years, who have patients with at least one of the target-diagnoses between 2013 and 2015 were considered for the intervention. In total, 1554 (76%) primary care physicians of Schleswig-Holstein have been invited by letter to participate in a study named “Effects of communication training with the MAAS-Global-D on the prescription of antibiotics for respiratory infections”.
Study design and estimation strategy
The intervention and the previously planned randomized controlled trial (RCT) has been described by Hammersen et al. [47] (Trial registration: DRKS00009566). The study was originally designed to consist of two interventional study arms. In addition to the communication training, the second intervention group received an educational introduction into the use of and online-access to EbMG online (Evidence–based Medicine Guidelines) [48]. This point-of-care online tool provides further information material on the prescribing of antibiotics for uncomplicated respiratory infections. Since the inclusion rate was lower than initially expected, both intervention groups have been consolidated. Furthermore, a comparison between the pooled intervention group and the control group did not yield significant results due to a lack of power because of the small sample size. Instead, we formed a control group from observational data and applied a combination of difference-in-difference (DiD) estimation and matching approach that is considered to reproduce the results of RCTs very well under certain assumptions [49]. For instance, under the assumption that the average outcomes for the intervention and control group would have followed parallel trends over time before intervention, the DiD estimator identifies causal effects by contrasting the change for the intervention and control groups in pre- and post-intervention outcomes [50]. However, the assumption of parallel trends might be implausible in our setting. For instance, if physicians recognized a too high antibiotic prescription rate for URTIs, they presumably tried reducing it. Therefore, they might have been more likely to respond to the training offer that advertised a reduction of the prescription rate through improved communication skills. Consequently, the evolutions of the prescription rates were suspected to differ between the intervention and control group if the control group, as in this case, had not been built upon a controlled randomization. An alternative identifying assumption is that the potential outcomes are independent of intervention status, conditional on past outcomes and covariates [51]. By means of balancing the intervention and control group according to pre-intervention outcomes and covariates all potential outcome trends are perfectly aligned and the DiD estimates can be interpreted as causal effects [49, 52]. However, recent studies showed that the combination of DiD and matching might also deliver biased estimates [53]. In order to enhance the robustness of our findings and minimize the risk of estimation bias we compared DiD estimates from both unmatched and matched (on pre- intervention outcomes) data [54].
In the search for relevant variables determining the decision to prescribe an antibiotic for a specific URTI case (our dependent variable) we first estimated a multi-level random effects logistic regression model based on case-, patient- and physician-level data of the pre-intervention period. A logistic regression model was chosen to account for the binary nature of the dependent variable. Moreover, the logit model showed computational merits and, unlike the probit model, it did not suffer from any convergence failures. Random effects were specified on the physician level to account for intra-physician variability [55]. In a second step, we aggregated the data on the physician level and matched the intervention and control groups according to aggregated pre- intervention outcomes and covariates by means of entropy balancing [56]. Based on the balanced data, in the third step, we estimated a multi-level random effects logistic DiD regression model using the weights of the physician-level from entropy balancing. Alternatively, we also specified fixed physician effects in the pre-intervention analysis and the DiD regression models. For all models the results between fixed and random effects models are very similar and we conclude there is no correlation between the explanatory variables and the individual effects. The physicians, who had previously been selected to the control group were excluded from the third step of the analysis, since we could not rule out that their prescribing behavior might have been affected by the cancellation of participation in the communication training.
The study was approved by the ethics committee of Luebeck University before the recruitment of participants on 9 June 2015 (number of approval: 15–139). Statistical analyses were performed with STATA 15 (StataCorp LLC, College Station, TX, USA).
Intervention
The intervention group received a communication training with an interactive workshop character (two times 2.25 hours), which was held at the Institute of Family Medicine in February and March of 2016. It was delivered face-to-face by members of the research team, including an expert in physician-patient communication. The curriculum of the training was derived from the German version (MAAS-Global-D [40]) of the Dutch instrument MAAS-Global [39]. After establishing the relevance and success of physician-patient communication, the participant were provided with information concerning the associated evidence base regarding treatment of URTIs. Furthermore, they learned about the different communicative phases of a consultation, corresponding communication skills as well as general communication skills for the whole consultation (e.g. adequate provision of information, structuring and empathy, shared decision-making).
Measurements
As the outcome variable we considered the binary choice whether an antibiotic was prescribed for a URTI case. The selection of potential determinants serving as control variables in both the pre- intervention and the DiD regression analyses was based on related previous literature [25, 57]. They can be classified into three categories: (i) case related (year, quarter, diagnosis and its certainty, emergency service), (ii) patient specific (insurance status, age, sex) and (iii) physician characteristics (age, sex, number of URTI-patients in that quarter). Seasonal effects and a general trend in the prescribing pattern were considered by respective dummy variables identifying the quarter and the year of the consultation, respectively. As the prescription rate might differ between the considered diagnoses, we introduced dummy variables for sinusitis and pharyngitis with bronchitis serving as reference. According to the German coding policy, primary care physicians are required to designate their diagnoses as validated (certain) or suspected (cases without an established definite diagnosis). We controlled for the cases with a certain diagnosis by including a respective dummy variable. Further, we distinguish whether the patient visited an emergency care center during the out-of-hours care (emergency service). Demographic variables of the patient were comprised of the sex (sex = 1: female), the age and the insurance status (normal, family or retired). The age was grouped by respective dummy variables for patients aged <35, 35–65, 65+ to allow for nonlinear age effects. In Germany, the insurance status signifies whether the patient is ordinary insured, retired or coinsured. Children and grandchildren aged below 25 as well as spouses that are unemployed, not self-employed and are not exceeding an income of EUR 450 per month are coinsured with an ordinary insurance member. The considered age and insurance status based clusters reflect different stages of life that might go along with different expectations about the treatment. At the physician level, we controlled for the specialty, since primary care physician workforce in Germany consists of general practitioners, physicians in general internal medicine and a declining number of practitioners without special training in primary care (12%). Previous studies have shown substantial differences in prescribing behavior between general internists and general practitioners [58]. Further, we considered the age and the sex of the physician. Finally, to approximate the workload of the physician’s practice we included the number of total URTI patients in the respective quarter. The logarithmic function to this variable accounts for unequal variation.
In the intervention analysis, the DiD dummy variable identifies observations of the intervention group for the post-intervention period. To control for any other time-invariant differences between both groups a dummy variable trained is additionally included.
Results
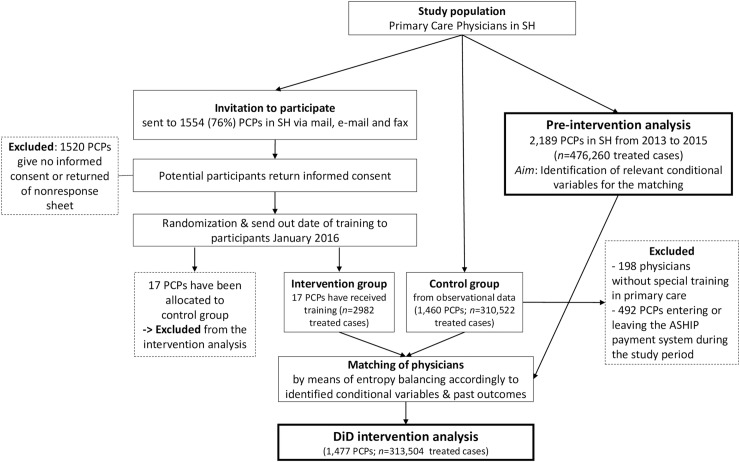
In the first part of the analysis (pre-intervention), the sample of the pre-intervention analysis (2013 to 2015) consisted of 315,752 adult patients with 476,260 cases from 2,189 PCPs. For the second part of the study, we invited 1,554 PCPs in SH to participate in the training. The group of interested participants has been divided randomly in a control and an intervention group with each 17 PCPs. Due to a lack of power, we alternatively form the control group from observational data. Do to so, we excluded the prior control group physicians (n = 17) and practitioners without special training in primary care, since they are lacking in the intervention group (n = 198). Moreover, 492 PCPs are not considered because they are not treating URTIs in each of the considered years, for instance since they are entering or leaving the ASHIP payment system during the study period. Finally, the intervention/control group in the intervention analysis consisted of 17/1,460 PCPs with 1,807/170,683 patients with 2,284/235,355 cases in the pre-intervention period (2013:q1 to 2015:q4) and 585/61,755 patients with 698/75,167 cases after the intervention (2016:q2 to 2016:q4) (Fig 1).
Fig 1. Flow chart.
Pre-intervention analysis
The mean values of the considered variables in the pre-intervention analysis and the regression results are shown in Table 1. An antibiotic was prescribed in half of the considered cases (49%).
Table 1. Multilevel logistic regression analysis of the pre-intervention period of prescribing an antibiotic.
| Variable | means | (1) | (2) | |
|---|---|---|---|---|
| Dependent variable | ||||
| antibiotic prescription (= 1) | 0.49 | |||
| Case characteristics | ||||
| quarter | ||||
| 2nd quarter | 0.21 | -0.01 | -0.01 | |
| 3rd quarter | 0.17 | -0.07** | -0.07** | |
| 4th quarter | 0.26 | -0.08** | -0.08** | |
| (Reference: 1st quarter) | ||||
| year | ||||
| 2014 | 0.32 | -0.03** | -0.03** | |
| 2015 | 0.33 | -0.14** | -0.14** | |
| (Reference: 2013) | ||||
| diagnosis | ||||
| sinusitis (J01) | 0.21 | -0.19** | -0.19** | |
| pharyngitis (J02) | 0.29 | -0.18** | -0.18** | |
| (Reference: bronchitis (J20)) | ||||
| certainty | ||||
| certain diagnosis | 0.99 | 0.38** | 0.38** | |
| type of service | ||||
| emergency service | 0.03 | 0.40** | 0.40** | |
| Patient characteristics | ||||
| Insurance status | ||||
| Family insured | 0.12 | 0.11** | 0.11** | |
| Pensioners insured | 0.16 | 0.16** | 0.17** | |
| (Reference: ordinary insured) | ||||
| Patient demographics | ||||
| Patient aged 35–65 | 0.52 | 0.33** | 0.45** | |
| Patient aged 65+ | 0.14 | 0.28** | 0.39** | |
| (Reference: < 35) | ||||
| Female patient | 0.59 | 0.09** | ||
| (Reference: male) | ||||
| sex-age interactions | ||||
| Female patient aged <35 | 0.19 | 0.22** | ||
| (Reference: male<35) | ||||
| Female patient aged 35–65 | 0.31 | 0.02 | ||
| (Reference: male aged 35–65) | ||||
| Female patient aged 65+ | 0.09 | 0.04 | ||
| (Reference: male aged 65+) | ||||
| Physician characteristics | ||||
| PCP specialty | ||||
| PCP without special training | 0.13 | 0.16 | 0.16 | |
| General Internist | 0.18 | 0.00 | 0.00 | |
| (Reference: GP) | ||||
| PCP demographics | ||||
| Physician age | 55.11 | 0.00 | 0.00 | |
| Female physician | 0.31 | -0.04 | -0.04 | |
| (Reference: male) | ||||
| PCP workload | ||||
| log(#URTI-patients) | 3.60 | 0.15** | 0.15** | |
| intercept | -1.19** | -1.27** | ||
| (Variance of random effects on physician level) | 0.90** | 0.91** | ||
| Intra-class correlation (in %) | 21.57 | 21.58 | ||
| Log-Like | -288,587 | -288,480 | ||
| Akaike Info Criterion (AIC) | 577,216 | 577,006 | ||
| R2-MacFadden (in %) | 12.56 | 12.60 | ||
The first column presents sample means. The other columns display the estimated regression coefficients. Based on 476,260 observations (315,752 patients from 2,189 primary care physicians). Estimated by means of Maximum Likelihood. Significance levels: * 5%, ** 1%.
The results of two logistic regression models with specified random effects on the physician level are shown in the third and fourth column of Table 1. In both models, the estimated intra-class coefficients (21.6%) suggest that conditional on the covariates, almost one quarter of total variation in antibiotic prescription could be explained by the individual physician’s practice style. The estimated regression coefficients indicated that patients aged over 35 years were significantly more likely to receive an antibiotic prescription than younger patients. The strongest effect was achieved for patients aged between 35–65 years. Female patients were also more likely to receive an antibiotic. The interaction effects between the patients’ gender and age groups in Model (2) signified that the gender difference only exists for patients younger than 35 years. As indicated by the smaller Akaike Information Criterion (AIC), the fit of the model was significantly improved, leading to our final model that was considered for the intervention analysis.
Matching
The entropy balancing was applied to match physicians of the intervention group with physicians of the control group in the pre-intervention period. In addition to the control variables, the pre-intervention prescription rates served as conditional variables used in the matching. Case- and patient-level variables were aggregated on the physician level. Table 2 shows the means of the variables for the intervention as well as the matched and non-matched control group. Further, the differences between intervention group and unmatched controls as well as the share of missing observations are shown for each variable.
Table 2. Means of aggregated variables before intervention.
| variables | Intervention group | Control group | Difference between (a) and (b) | Share of missing observations (in %) | ||
|---|---|---|---|---|---|---|
| Un matched | matched | |||||
| (a) | (b) | (c) | ||||
| Outcome: Prescription rate (in %) | ||||||
| 2013 | 51.5 | 46.5 | 51.5 | 5.0 | 3.74 | |
| 2014 | 48.3 | 44.7 | 48.3 | 3.6 | 2.01 | |
| 2015 | 47.6 | 43.2 | 47.6 | 4.4 | 2.64 | |
| Number of URTI-patients | ||||||
| 2013 | 66.5 | 88.4 | 66.4 | -21.9 | 3.74 | |
| 2014 | 65.1 | 79.0 | 65.0 | -13.9 | 2.01 | |
| 2015 | 69.3 | 82.3 | 69.3 | -13.0 | 2.64 | |
| Share of cases (in %) | ||||||
| quarter | ||||||
| 2nd quarter | 22.3 | 21.8 | 22.3 | 0.5 | 0 | |
| 3rd quarter | 18.6 | 18.1 | 18.6 | 0.5 | 0 | |
| 4th quarter | 27.6 | 26.2 | 27.6 | 1.4 | 0 | |
| (Reference: 1st quarter) | ||||||
| diagnosis | ||||||
| sinusitis (J01) | 14.8 | 19.7 | 14.8 | -4.9 | 0 | |
| pharyngitis (J02) | 47.2 | 41.3 | 47.2 | 5.9 | 0 | |
| (Reference: bronchitis (J20)) | ||||||
| certainty | ||||||
| certain diagnosis | 99.3 | 97.9 | 99.3 | 1.4** | 0 | |
| service-type | ||||||
| Emergency services | 6.6 | 5.5 | 6.6 | 1.1 | 0 | |
| Patient demographics | ||||||
| Patients aged 35–65 | 55.0 | 51.5 | 55.0 | 3.5 | 0 | |
| Patients aged >65 | 13.6 | 14.0 | 13.6 | -0.4 | 0 | |
| Female patients | 59.4 | 60.6 | 59.4 | -1.2 | 0.02 | |
| sex-age interactions | ||||||
| Female patients aged 35–65 | 32.9 | 31.3 | 32.9 | 1.6 | 0.02 | |
| Female patients aged >65 | 7.9 | 8.5 | 7.9 | -0.6 | 0.02 | |
| Insurance status | ||||||
| Patients family insurance | 8.7 | 12.4 | 8.7 | -3.7* | 0 | |
| Patients pensioners insurance | 15.6 | 16.1 | 15.6 | -0.5 | 0 | |
| Physician characteristics | ||||||
| PCP specialty | ||||||
| General Internists (in %) | 23.5 | 26.4 | 23.5 | -2.9 | 0 | |
| (Reference: GP) | ||||||
| Female physician (in %) | 23.5 | 37.9 | 23.5 | -14.4 | 0 | |
| (Reference: male) | ||||||
| Physician age | 54.3 | 53.6 | 54.3 | 0.7 | 0.00 | |
| Number of PCPs | 17 | 1,460 | 17 | |||
The first three columns present means of selected variables used for the matching before intervention for trained controls and matched controls, respectively. The last column displays the differences between intervention and control group before matching. Significance levels: * 5%, ** 1%. Patient variables are aggregated on physician level by summing up (Number of URTI-patients) or computing as shares of cases.
The intervention group is characterized by higher average prescriptions per physician in comparison with the control group. This hints for a selection of the participants in the intervention group due to their pre-intervention outcome. Furthermore, the change over time differed between both groups, underlining that the assumption of parallel trends might not hold. The average number of patients was higher in the control group. However, none of the differences were significant, except for the fraction of patients with a certain diagnosis and family insurance. This might have been due to the low number of observations at the physician level in the intervention group (n = 17). Nevertheless, after applying the reweighting approach based on entropy balancing the means in the control group equaled the means in the intervention group.
Univariate DiD analysis
To assess the sensitivity of the DiD analysis due to model specifications and the balancing we started presenting univariate DiD estimates (simple mean comparison) based on unmatched and matched sample data in Table 3. Neglecting physician-specific effects and other covariates, the reduction in the overall prescription rate of the intervention group between the pre-intervention and post-intervention period was 11.2%. For the control group a reduction of 4.7% could be detected. The difference between both groups in the difference between the periods is the DiD estimator, which is -6.5% [95% CI: (-10.7%; -2.3%)], and significant. Reweighting the observations of the case-level by the entropy weights on the physician-level increased the prescription rate of the matched control group to 52.9%. This was also slightly smaller than the rate of the intervention group (55.4%), which might be, because the matching was done at the physician level and not at the case level. However, the DiD estimate for the matched sample was rather similar (-6.1% [95% CI: (-12.0%; -0.2%)],) and also significant. Concluding, both univariate DiD estimates suggest a significant reduction of antibiotic prescriptions after the communication training.
Table 3. Univariate difference-in-difference analysis of the communication training on the antibiotic prescribing behavior.
| Prescribing rate (in %) | |||||
|---|---|---|---|---|---|
| Intervention Group | Control Group | Difference-in-Difference | |||
| unmatched | matched | unmatched | matched | ||
| Before | 55.43 | 47.27 | 52.86 | ||
| (2014–2015) | (n = 2284) | (n = 235355) | (∑wi≈2282) | ||
| After | 44.27 | 42.61 | 47.80 | ||
| (2016) | (n = 698) | (n = 75167) | (∑wi≈736) | ||
| Difference | -11.16** | -4.65** | -5.07* | -6.51** | -6.10* |
| p-value | <0.001 | <0.001 | 0.017 | 0.003 | 0.043 |
313,504 observations (234,723 patients from 1,477 general practitioners). Significance levels: * 5%, ** 1%. Matching is based on Entropy balancing using the variables listed in Table 2 and wi denotes the Entropy balancing weights.
Multilevel DiD regression analysis
To take into account the control variables and the random effects on the physician-level, we estimated the specification of Model (2) based on the extended data set, as well as the DiD and training dummy variable. Table 4 shows the estimated DiD effects and the moderation effects. To ease the interpretation of the estimated DiD coefficient, the marginal effect on the prescription rate was also shown for the direct effects.
Table 4. Multilevel logistic regression analysis of the difference-in-difference effect of the communication training on prescribing an antibiotic.
| (3) | (4) | (5) | (6) | |
|---|---|---|---|---|
| matching | no | yes | no | yes |
| trained | 0.15 | -0.08 | 0.14 | -0.08 |
| DiD | -0.31** | -0.28* | 0.19 | 0.14 |
| 95%-CIa of DiD | [-0.50, -0.12] | [-0.50, -0.05] | ||
| MEb of DiD (in %) | -6.34** | -6.44* | ||
| 95%-CIa of ME (in %) | [-10.31–2.37] | [-11.67, -1.22] | ||
| Odds Ratio DiD | 0.73** | 0.76* | ||
| 95%-CI of OR | [0.61, 0.89] | [0.60, 0.95] | ||
| Interaction effects | ||||
| DiD*Pat age (35–65) | -0.52 | -0.49* | ||
| DiD*Pat age (65+) | -0.33 | -0.26 | ||
| DiD*Fem pat (<35) | -0.73* | -0.65** | ||
| DiD*Fem pat (35–65) | -0.13 | -0.03 | ||
| DiD*Fem pat (65+) | -0.00 | 0.07 |
313,504 observations (234,723 patients from 1,477 general practitioners). Significance levels: ** 5%, *** 1%.
a Confidence interval
b Marginal Effect. Estimated coefficients of the control variables and the variance of the random effects are not shown. Matching is based on Entropy balancing using the variables listed in Table 2.
All specifications obtained a significant reduction of the prescription rate due to the intervention. There were no substantial differences between the estimates of the matched and unmatched sample. The marginal effects were close to the estimated univariate DiD effects.
The results of a moderation effect of the DiD effect by the age and sex of the patients are also shown in Table 4. They suggest that the intervention had a significantly stronger effect on the treatment of female patients aged below 35.
Discussion
In this study, we estimated the effect and its moderations of a communication training based on the MAAS-Global-D instrument on the antibiotic prescription rate of primary care physicians for the treatment of upper respiratory tract infections. Since the control group was formed from observational data, we applied a combination of difference-in-difference estimation and statistical matching based on entropy balancing to estimate the intervention effect. Relevant variables for the matching were selected after estimating a multi-level logistic regression model for the antibiotic prescribing decision, based on case-, patient- and physician-level data of the pre-intervention period in the first stage. In the second stage, the same model was estimated, based on matched data and extended by the intervention period and DiD specification.
Pre-intervention analysis
During the pre-intervention period, an antibiotic was prescribed in almost half of the considered cases. This relatively high number of antibiotic prescriptions is also observed in related studies [57, 59]. In both groups (intervention and control), the prescription rate slightly decreased over time. This is similar to the declining trend of general antibiotic use in other countries [60] and might be explained by an increased awareness of antimicrobial resistance [61], e.g. due to successful antibiotic stewardship programs as the German Strategy against Antibiotics Resistance [62]. The estimated intra-class coefficient of the multilevel regression model shows that the individual physician’s practice style explains about 22% of the total variance in antibiotic prescription and is similar to the results of related studies [25, 35, 63]. It suggests the prospect of a successful reduction in the prescription rate by changing the individual physician’s prescribing behavior. Most of the observable characteristics of the physician do not explain the variance in prescribing behavior. Only the number of URTI patients (serving as a proxy of the physician’s workload) increases the probability of antibiotic prescription. This effect underlines the hypothesis that insufficient communication determines the antibiotic prescription. It is more complicated for physicians lacking sufficient time for the consultation due to an overload of patients, to change the patients’ expectations [28, 64]. A similar mechanism might explain the positive association of emergency service and the antibiotic prescription probability. In Germany, PCPs face an overload of patients, especially when providing out-of-hours care in emergency service [65]. Patients visiting the emergency service for respiratory complaints might be more severe and therefore might have a strong expectation of receiving an antibiotic [66]. The expectations might also differ between patients, as suggested by the estimated effects of the patient characteristics.
Patients above the age of 35 years receive a significantly higher number of antibiotic prescriptions than younger patients do. For patients belonging to a higher-risk group (e.g., elderly patients) respective guidelines suggest the use of antibiotics in some cases [44–46]. Therefore, the application of guidelines cannot explain the lower prescription rates for patients aged above 65 in comparison with patients aged between 35 and 65 (Model (1): 0.28 vs. 0.33). Differences between the patients’ expectations in the age-groups below and above 35 respectively, might be more likely to serve as an explanation. Work pressure and other related stress cause patients to desire rapid relieve from symptoms and cure of their sickness [67]. The perceived importance of the patient’s job promotes the decision to prescribe an antibiotic [30, 68]. This might explain that the antibiotic prescription rate considerably exceeds the clinically justified amount for young and middle-aged adults with respiratory infections in the UK [69].
Our results further suggest that women receive more frequent an antibiotic prescription. Women are more likely to visit a physician for URTI than men [57] and are found to be more skeptical towards the physician’s suggestions [70]. This patient group might combine higher expectations and wariness that might lead to additional communication requirements. This hypothesis is in line with the higher antibiotic prescription rate that is observed for female patients in our data. However, the underlying mechanisms were not aim of our research focus and is, therefore, subjected to future research. Similar to other studies, the gender gap vanishes with the increasing patient-age [71]. Only female patients below the age of 35 receive a significantly higher number of antibiotic prescriptions. This result might indicate that the communication problem is mostly pronounced for the treatment of this group of patients. In the following, we discuss the effects of the communication training on the antibiotic prescription probability.
Intervention analysis
We applied different approaches and specifications to robustly estimate the effect of the communication training on the antibiotic prescribing behavior. The univariate approach estimates an 11-percentage-point reduction of prescriptions for the intervention group after the training. This result is very similar to a related study [72]. All our approaches (univariate and multivariate) estimate a decrease of around 6.5 percentage-points in the prescription probability of the trained physicians. These robust estimates are in line with the findings of other related studies applying RCT methodology [32, 35, 73]. The moderation analysis confirms that the effect of the communication training is stronger for the treatment of patients marked by a larger communication problem. The impact of the training on the reduction of antibiotic prescription is significantly stronger for the treatment of young women. Thus, physicians with improved communication skills might be able to better address the potentially higher expectations of young female patients to receive an antibiotic therapy and their wariness towards the physician’s suggestions [70].
As argued by Fritz and Holton [74], the lack of trust in the patient-doctor relationship enhances the likelihood of overprescribing. A patient trusting in the physician’s clinical judgment, can be reassured to accept non-prescribing [75]. Furthermore, secured trust between the patient and physician could reduce the probability of the physician to misperceive the patient’s expectation to receive antibiotic treatment. To establish a trustful relationship it is important for the patient to recognize the physician’s trust in them and believe that the physician acts in their best interest [76]. Signals of trustworthiness are given by verbal and nonverbal communication and serve to establish patients’ trust, and, thus, influence the doctor-patient relationship [77]. For this purpose, the MAAS-Global-D might be a promising tool to improve effective communication since both verbal and nonverbal communication skills are part of the training. To comprehend emotions as well as feelings and to react adequately, the MAAS-global-D-manual proposes the physician to render the feelings expressed by the patient during the consultation either in words or nonverbally [39]. Trust is considered for most patients to be an integral part of an ongoing relationship with a physician [78]. An increased continuity of care enables, on the one hand, physicians to better evaluate the patient’s expectations of receiving an antibiotic by the more intimate knowledge of their living conditions. On the other hand, patients can build up a deep understanding of appropriate antibiotic use and will change their expectations permanently. The findings of Robert et al. [79] suggest that receiving information about antibiotics from family physicians is usually not associated with an increased knowledge of the patients. A trustful and continued relationship might be helpful for physicians to provide information about the use of antibiotics, and to improve knowledge about antibiotics especially among target groups [79, 80]. As we found in our pre-intervention data analysis, one specific target group consists of young female patients.
Limitations and strengths
The study estimated the effects of a communication training for primary care physicians on the antibiotic prescription rate for infections of the upper respiratory tract and its moderation by age and gender of the patients.
The study has strengths as well as limitations. In contrast to the previously planned randomized controlled trial [47], in this study we formed a control group from observational data. In contrast to the control group physicians the members of the intervention group did know that their prescription data would be analyzed for the periods before and after the training. Therefore, we cannot exclude that behavior change in the intervention group is due to the physicians’ awareness of being under observation rather than solely due to the intervention (Hawthorne effect) [81]. However, since we considered the data of the physicians up to one year after the training, we do not believe that this effect is responsible for persistent behavior changes. Further, the approach that has been applied to estimate the intervention effect is more sophisticated and is, thus, more susceptible to misspecification than an RCT [82]. To minimize the risk of biased estimates we applied several alternative approaches (univariate, multivariate, matching, no-matching) and specifications (fixed and random effects) as robustness checks. All estimated effects of the intervention are very similar. Therefore, we believe that misspecification is not a big issue here.
A strength of this study is that it relies on routine data collected from all primary care physicians in a specific region of Germany. The relatively large number of physicians of the (matched) control group (n = 1,460) might ensure a higher external validity of our findings than the rather small sample sizes of other related studies applying an RCT [32, 33, 35, 36]. However, the small number of the intervention group highlights the problem to convince PCPs to participate in intervention studies [83, 84]. Another reason for the low response rate might have been rooted in the PCPs’ (who already faced an overload of patients) concerns that improved communication skills would prolong the consultation, although so far, there is no evidence to support this claim [85].
While on the one hand, the focus on the federal state of Schleswig-Holstein constrained the representativeness of the findings, it on the other hand also reduced practice variations based on regional differences and state-specific regulations [86]. The analyzed moderation of the patient’s age and gender on the communication training effect further increased the insights of antibiotic prescribing behavior. In line with the findings of our pre-intervention data analysis, our results suggest that improved communication skills are mostly effective in cases where the underlying communication problem is particularly pronounced due to high expectations of the patient to receive an antibiotic or due to the physicians’ perceptions. To clarify the moderating role of expectation and its perception for the communication training effect on antibiotic usage future research should include direct measures of these variables [25].
Conclusion
In this study, we estimated the effect and its moderations of a communication training on the antibiotic prescription rate of primary care physicians for the treatment of upper respiratory tract infections, i.e. acute bronchitis, sinusitis and pharyngitis. The short communication training has been based on the MAAS-Global-D [40], the German version of the Dutch instrument MAAS-Global [39]. Since the control group has been formed from observational data, a combination of difference-in-difference (DiD) and matching has been applied to estimate the intervention effect. To minimize the risk of biased estimates we applied several alternative approaches and specifications as robustness checks that all reveal similar intervention effects. The results show that the communication training decreases the prescribing probability by around 6.5-percentage-points for the physicians of the intervention group. For the treatment of female patients aged below 35, the intervention has a stronger impact.
Our results suggest that communication skills implemented via MAAS-Global-D-training lead to more prudent prescribing of antibiotics for URTIs. Therefore, the MAAS-Global-D-training could not only avoid unnecessary side effects but could also help to reduce the emergence of drug resistant bacteria. The instrument MAAS-Global-D has been proven to provide a valid tool for a training of physicians that encourages an effective communication with the patient. In the Netherlands, communication training is an integral part in the postgraduate-training program of general practitioners. A similar communication training based on the MAAS-Global-D could also be applied in Germany, as well as in other countries, where postgraduate training schemes of PCPs lack in training of communication skills. The instrument and the explanatory manual in German language are available for free download [87].
Trial registration
The intervention and the previously planned randomized controlled trial (RCT) has been registered in the German Clinical Trial Register (DRKS00009566).
Supporting information
(DOCX)
(ZIP)
Data Availability
The dataset used and analyzed during the current study is extracted from administrative data and provided by the Association of Statutory Health Insurance Physicians of the Federal State of Schleswig-Holstein. The data are secured by strict German data protection regulation and we are not allowed to share the data with other researchers. However, a request to ac-cess the data for scientific purposes and to replicate the findings can be made to the Associa-tion of Statutory Health Insurance Physicians (ASHIP) in official writing at Dr. Monika Schliffke, service@kvsh.de, ASHIP, Bismarckallee 1-6, 23795 Bad Segeberg. The relevant software codes and technical support to replicate the findings are stored in the Supporting Information files.
Funding Statement
The study was funded by the German Federal Ministry of Health (grant-number: ZMVI1-2515NIK002). The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
References
- 1.Ventola CL: The Antibiotic Resistance Crisis: Part 1: Causes and Threats. Pharmacy and Therapeutics 2015, 40(4):277–283. [PMC free article] [PubMed] [Google Scholar]
- 2.Rice LB: The clinical consequences of antimicrobial resistance. Curr Opin Microbiol 2009, 12(5):476–481. 10.1016/j.mib.2009.08.001 [DOI] [PubMed] [Google Scholar]
- 3.WHO: Antibiotic Resistance—Fact Sheet. Available online. February 2018:. http://www.who.int/mediacentre/factsheets/antibiotic-resistance/ (Accessed 9 May 2019).
- 4.Kollef MH: Broad-spectrum antimicrobials and the treatment of serious bacterial infections: getting it right up front. Clin Infect Dis 2008, 47(Supplement_1):S3–S13. [DOI] [PubMed] [Google Scholar]
- 5.Livermore DM: Current epidemiology and growing resistance of gram-negative pathogens. The Korean journal of internal medicine 2012, 27(2):128 10.3904/kjim.2012.27.2.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magira EE, Islam S, Niederman MS: Multi-drug resistant organism infections in a medical ICU: Association to clinical features and impact upon outcome. Medicina Intensiva (English Edition) 2018, 42(4):225–234. [DOI] [PubMed] [Google Scholar]
- 7.Smith R, Coast J: The true cost of antimicrobial resistance. BMJ: British Medical Journal 2013, 346. [DOI] [PubMed] [Google Scholar]
- 8.Levy SB, Marshall B: Antibacterial resistance worldwide: causes, challenges and responses. Nat Med 2004, 10(12):S122–S129. [DOI] [PubMed] [Google Scholar]
- 9.Roca I, Akova M, Baquero F, Carlet J, Cavaleri M, Coenen S, et al. : The global threat of antimicrobial resistance: science for intervention. New microbes and new infections 2015, 6:22–29. 10.1016/j.nmni.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergman M, Huikko S, Pihlajamäki M, Laippala P, Palva E, Huovinen P, et al. Resistance FSGfA: Effect of macrolide consumption on erythromycin resistance in Streptococcus pyogenes in Finland in 1997–2001. Clin Infect Dis 2004, 38(9):1251–1256. 10.1086/383309 [DOI] [PubMed] [Google Scholar]
- 11.Laxminarayan R, Chaudhury RR: Antibiotic Resistance in India: Drivers and Opportunities for Action. PLoS Med 2016, 13(3):e1001974 10.1371/journal.pmed.1001974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goossens H, Ferech M, Vander Stichele R, Elseviers M, Group EP: Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. The Lancet 2005, 365(9459):579–587. [DOI] [PubMed] [Google Scholar]
- 13.van der Velden A, Duerden M, Bell J, Oxford J, Altiner A, Kozlov R, et al. : Prescriber and Patient Responsibilities in Treatment of Acute Respiratory Tract Infections—Essential for Conservation of Antibiotics. Antibiotics 2013, 2(2):316. [Google Scholar]
- 14.Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. : Prevalence of inappropriate antibiotic prescriptions among us ambulatory care visits, 2010–2011. JAMA 2016, 315(17):1864–1873. 10.1001/jama.2016.4151 [DOI] [PubMed] [Google Scholar]
- 15.Swedres-Svarm Reports. Swedres-Svarm 2017. http://www.sva.se/globalassets/redesign2011/pdf/om_sva/publikationer/swedres_svarm2017.pdf (Accessed 9 May 2019).
- 16.Smith SM, Fahey T, Smucny J, Becker LA: Antibiotics for acute bronchitis. Cochrane Database Syst Rev 2017(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenealy T, Arroll B: Antibiotics for the common cold and acute purulent rhinitis. Cochrane Database Syst Rev 2013(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zoorob R, Sidani MA, Fremont RD, Kihlberg C: Antibiotic use in acute upper respiratory tract infections. Am Fam Physician 2012, 86(9). [PubMed] [Google Scholar]
- 19.van der Meer JWM, Grol RPTM: The Process of Antibiotic Prescribing: Can It Be Changed? In: Antibiotic Policies: Fighting Resistance. edn. Edited by Gould IM, van der Meer JWM. Boston, MA: Springer US; 2008: 17–27. [Google Scholar]
- 20.Sirota M, Round T, Samaranayaka S, Kostopoulou O: Expectations for antibiotics increase their prescribing: Causal evidence about localized impact. Health Psychol 2017, 36(4):402–409. 10.1037/hea0000456 [DOI] [PubMed] [Google Scholar]
- 21.Yates TD, Davis ME, Taylor YJ, Davidson L, Connor CD, Buehler K, et al. : Not a magic pill: a qualitative exploration of provider perspectives on antibiotic prescribing in the outpatient setting. BMC Fam Pract 2018, 19(1):96 10.1186/s12875-018-0788-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salm F, Ernsting C, Kuhlmey A, Kanzler M, Gastmeier P, Gellert P: Antibiotic use, knowledge and health literacy among the general population in Berlin, Germany and its surrounding rural areas. PLoS One 2018, 13(2):e0193336 10.1371/journal.pone.0193336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oxford J, Kozlov R: Antibiotic resistance–a call to arms for primary healthcare providers. Int J Clin Pract 2013, 67(s180):1–3. [DOI] [PubMed] [Google Scholar]
- 24.Little P, Dorward M, Warner G, Stephens K, Senior J, Moore M: Importance of patient pressure and perceived pressure and perceived medical need for investigations, referral, and prescribing in primary care: nested observational study. BMJ 2004, 328(7437):444 10.1136/bmj.38013.644086.7C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akkerman AE, Kuyvenhoven MM, van der Wouden JC, Verheij TJM: Determinants of antibiotic overprescribing in respiratory tract infections in general practice. J Antimicrob Chemother 2005, 56(5):930–936. 10.1093/jac/dki283 [DOI] [PubMed] [Google Scholar]
- 26.McKay R, Mah A, Law MR, McGrail K, Patrick DM: Systematic Review of Factors Associated with Antibiotic Prescribing for Respiratory Tract Infections. Antimicrob Agents Chemother 2016, 60(7):4106–4118. 10.1128/AAC.00209-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macfarlane J, Holmes W, Macfarlane R, Britten N: Influence of patients' expectations on antibiotic management of acute lower respiratory tract illness in general practice: questionnaire study. BMJ 1997, 315(7117):1211–1214. 10.1136/bmj.315.7117.1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ackerman SL, Gonzales R, Stahl MS, Metlay JP: One size does not fit all: evaluating an intervention to reduce antibiotic prescribing for acute bronchitis. BMC Health Serv Res 2013, 13(1):462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Connor R, O’Doherty J, O’Regan A, Dunne C: Antibiotic use for acute respiratory tract infections (ARTI) in primary care; what factors affect prescribing and why is it important? A narrative review. Irish Journal of Medical Science (1971 -) 2018, 187(4):969–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Björnsdóttir I, Kristinsson KG, Hansen EH: Diagnosing infections: a qualitative view on prescription decisions in general practice over time. Pharm World Sci 2010, 32(6):805–814. 10.1007/s11096-010-9441-6 [DOI] [PubMed] [Google Scholar]
- 31.Kumar S, Little P, Britten N: Why do general practitioners prescribe antibiotics for sore throat? Grounded theory interview study. BMJ 2003, 326(7381):138 10.1136/bmj.326.7381.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Corvoisier P, Renard V, Roudot-Thoraval F, Cazalens T, Veerabudun K, Canoui-Poitrine F, et al. : Long-term effects of an educational seminar on antibiotic prescribing by GPs: a randomised controlled trial. Br J Gen Pract 2013, 63(612):e455–e464. 10.3399/bjgp13X669176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cals JW, Butler CC, Hopstaken RM, Hood K, Dinant G-J: Effect of point of care testing for C reactive protein and training in communication skills on antibiotic use in lower respiratory tract infections: cluster randomised trial. BMJ 2009, 338:b1374 10.1136/bmj.b1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vervloet M, Meulepas MA, Cals JWL, Eimers M, van der Hoek LS, van Dijk L: Reducing antibiotic prescriptions for respiratory tract infections in family practice: results of a cluster randomized controlled trial evaluating a multifaceted peer-group-based intervention. Npj Primary Care Respiratory Medicine 2016, 26:15083 10.1038/npjpcrm.2015.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Altiner A, Brockmann S, Sielk M, Wilm S, Wegscheider K, Abholz H-H: Reducing antibiotic prescriptions for acute cough by motivating GPs to change their attitudes to communication and empowering patients: a cluster-randomized intervention study. J Antimicrob Chemother 2007, 60(3):638–644. 10.1093/jac/dkm254 [DOI] [PubMed] [Google Scholar]
- 36.Coxeter P, Del Mar CB, McGregor L, Beller EM, Hoffmann TC: Interventions to facilitate shared decision making to address antibiotic use for acute respiratory infections in primary care. Cochrane Database Syst Rev 2015(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei X, Zhang Z, Hicks JP, Walley JD, King R, Newell JN, et al. : Long-term outcomes of an educational intervention to reduce antibiotic prescribing for childhood upper respiratory tract infections in rural China: Follow-up of a cluster-randomised controlled trial. PLoS Med 2019, 16(2):e1002733 10.1371/journal.pmed.1002733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ärztekammer Schleswig-Holstein: Weiterbildungsordnung. https://www.aeksh.de/dokument/gesetze-inhaltebogen/weiterbildungsordnung (abgerufen am 16. September 2019).
- 39.van Thiel J, Ram P, van Dalen J: MAAS-global manual. Maastricht: Maastricht University; 2000:4–5. [Google Scholar]
- 40.Hammersen F, Böhmer K, von der Bey J, Berger S, Steinhäuser J: MAAS-Global-D: Instrument zur Messung und Schulung kommunikativer sowie medizinischer Kompetenzen. Z Allg Med 2016, 92(1):13–18. [Google Scholar]
- 41.Zentralinstitut für die kassenärztliche Versorgung in Deutschland (ZI): Zahl der Versicherten in der GKV gemäß KM6 ‐ Statistiken des Bundesministeriums für Gesundheit (BMG). https://www.versorgungsatlas.de/fileadmin/pdf/Zi-IF_Publ-D%C3%84_vTabelle1_V5_20160609.pdf (Aufgerufen am 17.01.2020).
- 42.Kassenärztliche Bundesvereinigung: Regionale Verteilung der Ärzte in der vertragsärztlichen Versorgung. https://gesundheitsdaten.kbv.de/cms/html/16402.php (Accessed 4 October 2019).
- 43.World Health Organization. ICD-10 Version: 2016. https://icd.who.int/browse10/2016/en (Accessed 9 May 2019).
- 44.Deutsche Gesellschaft für Allgemeinmedizin und Familienmedizin: Husten-Leitlinie Nr. 11. https://www.degam.de/files/Inhalte/Leitlinien-Inhalte/Dokumente/DEGAM-S3-Leitlinien/Leitlinien-Entwuerfe/053-013_Husten/Langfassung_Leitlinie_Husten_20140323.pdf (Accessed 9 May 2019).
- 45.Deutsche Gesellschaft für Allgemeinmedizin und Familienmedizin: Halsschmerzen-Leitlinie Nr. 14:. https://www.degam.de/files/Inhalte/Leitlinien-Inhalte/Dokumente/DEGAM-S3-Leitlinien/Leitlinien-Entwuerfe/053-010_Halsschmerzen/LL-14_Langfassung_ZD.pdf (Accessed 9 May 2019).
- 46.Deutsche Gesellschaft für Allgemeinmedizin und Familienmedizin: S2k-Leitlinie-Rhinosinusitis. https://www.degam.de/files/Inhalte/Leitlinien-Inhalte/Dokumente/DEGAM-S2-Leitlinien/053-012_Rhinosinusitis%20(S2k)/017-049_053-012l_Rhinosinusitis_18-12-17.pdf (Accessed 9 May 2019).
- 47.Hammersen F, Goetz K, Soennichsen A, Emcke T, Steinhaeuser J: Effects of communication training with the MAAS-Global-D instrument on the antibiotic prescribing for respiratory infections in primary care: study protocol of a randomised controlled trial. Trials 2016, 17(1):180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Evidence–based Medicine Guidelines:. www.ebm-guidelines.com (Accessed 9 May 2019).
- 49.Heckman JJ, Ichimura H, Todd PE: Matching As An Econometric Evaluation Estimator: Evidence from Evaluating a Job Training Programme. The Review of Economic Studies 1997, 64(4):605–654. [Google Scholar]
- 50.Abadie A: Semiparametric Difference-in-Differences Estimators. The Review of Economic Studies 2005, 72(1):1–19. [Google Scholar]
- 51.Angrist JD, Pischke J-S: Mostly harmless econometrics: An empiricist's companion: Princeton university press; 2009. [Google Scholar]
- 52.O’Neill S, Kreif N, Grieve R, Sutton M, Sekhon JS: Estimating causal effects: considering three alternatives to difference-in-differences estimation. Health Services and Outcomes Research Methodology 2016, 16(1):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chabé-Ferret S: Analysis of the bias of Matching and Difference-in-Difference under alternative earnings and selection processes. J Econometrics 2015, 185(1):110–123. [Google Scholar]
- 54.Lindner S, McConnell KJ: Difference-in-differences and matching on outcomes: a tale of two unobservables. Health Services and Outcomes Research Methodology 2018. [Google Scholar]
- 55.Mousquès J, Renaud T, Scemama O: Is the “practice style” hypothesis relevant for general practitioners? An analysis of antibiotics prescription for acute rhinopharyngitis. Soc Sci Med 2010, 70(8):1176–1184. 10.1016/j.socscimed.2009.12.016 [DOI] [PubMed] [Google Scholar]
- 56.Hainmueller J: Entropy balancing for causal effects: A multivariate reweighting method to produce balanced samples in observational studies. Political Analysis 2012, 20(1):25–46. [Google Scholar]
- 57.Barlam TF, Morgan JR, Wetzler LM, Christiansen CL, Drainoni M-L: Antibiotics for Respiratory Tract Infections: A Comparison of Prescribing in an Outpatient Setting. Infection Control & Hospital Epidemiology 2015, 36(2):153–159. [DOI] [PubMed] [Google Scholar]
- 58.Brieler JA, Scherrer JF, Salas J: Differences in prescribing patterns for anxiety and depression between General Internal Medicine and Family Medicine. J Affect Disord 2015, 172:153–158. 10.1016/j.jad.2014.09.056 [DOI] [PubMed] [Google Scholar]
- 59.Little P, Stuart B, Francis N, Douglas E, Tonkin-Crine S, Anthierens S, et al. : Effects of internet-based training on antibiotic prescribing rates for acute respiratory-tract infections: a multinational, cluster, randomised, factorial, controlled trial. The Lancet 2013, 382(9899):1175–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Curtis HJ, Walker AJ, Mahtani KR, Goldacre B: Time trends and geographical variation in prescribing of antibiotics in England 1998–2017. J Antimicrob Chemother 2018, 74(1):242–250. [DOI] [PubMed] [Google Scholar]
- 61.Wu J, Taylor D, Ovchinikova L, Heaney A, Morgan T, Dartnell J, et al. : Relationship between antimicrobial-resistance programs and antibiotic dispensing for upper respiratory tract infection: An analysis of Australian data between 2004 and 2015. J Int Med Res 2018, 46(4):1326–1338. 10.1177/0300060517740813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bundesministerium für Gesundheit: DART 2020—Deutsche Antibiotika-Resistenzstrategie. https://www.bundesgesundheitsministerium.de/themen/praevention/antibiotika-resistenzen/antibiotika-resistenzstrategie.html (Accessed 10 March 2020).
- 63.Francis NA, Butler CC, Hood K, Simpson S, Wood F, Nuttall J: Effect of using an interactive booklet about childhood respiratory tract infections in primary care consultations on reconsulting and antibiotic prescribing: a cluster randomised controlled trial. BMJ 2009, 339:b2885 10.1136/bmj.b2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lundkvist J, Åkerlind I, Borgquist L, Mölstad S: The more time spent on listening, the less time spent on prescribing antibiotics in general practice. Fam Pract 2002, 19(6):638–640. 10.1093/fampra/19.6.638 [DOI] [PubMed] [Google Scholar]
- 65.Baier N, Geissler A, Bech M, Bernstein D, Cowling TE, Jackson T, et al. : Emergency and urgent care systems in Australia, Denmark, England, France, Germany and the Netherlands–Analyzing organization, payment and reforms. Health Policy 2019, 123(1):1–10. 10.1016/j.healthpol.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 66.Ong S, Nakase J, Moran GJ, Karras DJ, Kuehnert MJ, Talan DA: Antibiotic Use for Emergency Department Patients With Upper Respiratory Infections: Prescribing Practices, Patient Expectations, and Patient Satisfaction. Ann Emerg Med 2007, 50(3):213–220. 10.1016/j.annemergmed.2007.03.026 [DOI] [PubMed] [Google Scholar]
- 67.Altiner A, Knauf A, Moebes J, Sielk M, Wilm S: Acute cough: a qualitative analysis of how GPs manage the consultation when patients explicitly or implicitly expect antibiotic prescriptions. Fam Pract 2004, 21(5):500–506. 10.1093/fampra/cmh505 [DOI] [PubMed] [Google Scholar]
- 68.Björnsdóttir I, Hansen E: Ethical dilemmas in antibiotic prescribing: analysis of everyday practice. J Clin Pharm Ther 2002, 27(6):431–440. 10.1046/j.1365-2710.2002.00442.x [DOI] [PubMed] [Google Scholar]
- 69.Gulliford MC, Dregan A, Moore MV, Ashworth M, Staa Tv, McCann G, et al. : Continued high rates of antibiotic prescribing to adults with respiratory tract infection: survey of 568 UK general practices. BMJ Open 2014, 4(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim AM, Bae J, Kang S, Kim Y-Y, Lee J-S: Patient factors that affect trust in physicians: a cross-sectional study. BMC Fam Pract 2018, 19(1):187 10.1186/s12875-018-0875-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schröder W, Sommer H, Gladstone BP, Foschi F, Hellman J, Evengard B, et al. : Gender differences in antibiotic prescribing in the community: a systematic review and meta-analysis. J Antimicrob Chemother 2016, 71(7):1800–1806. 10.1093/jac/dkw054 [DOI] [PubMed] [Google Scholar]
- 72.van der Velden AW, Pijpers EJ, Kuyvenhoven MM, Tonkin-Crine SK, Little P, Verheij TJ: Effectiveness of physician-targeted interventions to improve antibiotic use for respiratory tract infections. Br J Gen Pract 2012, 62(605):e801–e807. 10.3399/bjgp12X659268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ranji SR, Steinman MA, Shojania KG, Gonzales R: Interventions to Reduce Unnecessary Antibiotic Prescribing: A Systematic Review and Quantitative Analysis. Med Care 2008, 46(8):847–862. 10.1097/MLR.0b013e318178eabd [DOI] [PubMed] [Google Scholar]
- 74.Fritz Z, Holton R: Too much medicine: not enough trust? J Med Ethics 2019, 45(1):31–35. 10.1136/medethics-2018-104866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Petursson P: GPs’ reasons for “non-pharmacological” prescribing of antibiotics A phenomenological study. Scand J Prim Health Care 2005, 23(2):120–125. 10.1080/02813430510018491 [DOI] [PubMed] [Google Scholar]
- 76.Meyerson D, Weick KE, Kramer RM: Swift trust and temporary groups. Trust in organizations: Frontiers of theory and research 1996, 166:195. [Google Scholar]
- 77.Riva S, Monti M, Iannello P, Pravettoni G, Schulz PJ, Antonietti A: A Preliminary Mixed-Method Investigation of Trust and Hidden Signals in Medical Consultations. PLoS One 2014, 9(3):e90941 10.1371/journal.pone.0090941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tarrant C, Dixon-Woods M, Colman AM, Stokes T: Continuity and Trust in Primary Care: A Qualitative Study Informed by Game Theory. The Annals of Family Medicine 2010, 8(5):440–446. 10.1370/afm.1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Robert A, Nguyen Y, Bajolet O, Vuillemin B, Defoin B, Vernet-Garnier V, et al. : Knowledge of antibiotics and antibiotic resistance in patients followed by family physicians. Med Mal Infect 2017, 47(2):142–151. 10.1016/j.medmal.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 80.Sabuncu E, David J, Bernède-Bauduin C, Pépin S, Leroy M, Boëlle P-Y, et al. : Significant Reduction of Antibiotic Use in the Community after a Nationwide Campaign in France, 2002–2007. PLoS Med 2009, 6(6):e1000084 10.1371/journal.pmed.1000084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mangione-Smith R, Elliott MN, McDonald L, McGlynn EA: An Observational Study of Antibiotic Prescribing Behavior and the Hawthorne Effect. Health Serv Res 2002, 37(6):1603–1623. 10.1111/1475-6773.10482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Deaton A, Cartwright N: Understanding and misunderstanding randomized controlled trials. Soc Sci Med 2018, 210:2–21. 10.1016/j.socscimed.2017.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peters-Klimm F, Hermann K, Gágyor I, Haasenritter J, Bleidorn J, für das Netzwerk Klinische Studien in der A: Erfahrungen und Einstellungen zu Klinischen Studien in der Hausarztpraxis: Ergebnisse einer Befragung von deutschen Hausärzten. Gesundheitswesen 2013, 75(05):321–327. [DOI] [PubMed] [Google Scholar]
- 84.Hummers-Pradier E, Martin H, Kochen MM, Heinemann S, Himmel W, Scheidt-Nave C: Simply no time? Barriers to GPs' participation in primary health care research. Fam Pract 2008, 25(2):105–112. 10.1093/fampra/cmn015 [DOI] [PubMed] [Google Scholar]
- 85.Noordman J, van der Weijden T, van Dulmen S: Effects of video-feedback on the communication, clinical competence and motivational interviewing skills of practice nurses: a pre-test posttest control group study. J Adv Nurs 2014, 70(10):2272–2283. 10.1111/jan.12376 [DOI] [PubMed] [Google Scholar]
- 86.Corallo AN, Croxford R, Goodman DC, Bryan EL, Srivastava D, Stukel TA: A systematic review of medical practice variation in OECD countries. Health Policy 2014, 114(1):5–14. 10.1016/j.healthpol.2013.08.002 [DOI] [PubMed] [Google Scholar]
- 87.Steinhäuser J, Berger S, Bey Jvd, Böhmer K, Hammersen F, Fichtner C, et al. : MAAS-Global-D Handbuch Leitfaden zur Bewertung von ärztlichen Kommunikations- und klinischen Fähigkeiten. Lübeck: Universität zu Lübeck; 2015. Aavailable at: http://www.uksh.de/uksh_media/Dateien_Kliniken_Institute+/Lübeck+Campuszentrum+/Allgemeinmedizin_HL/MAAS_Global_D_Manual-p-108298.pdf. [Google Scholar]