ABSTRACT
Study Objective:
We evaluated the early hemodynamic profile of patients presenting with acute circulatory failure to the Emergency Department (ED) using focused echocardiography performed by emergency physicians after a dedicated training program.
Methods:
Patients presenting to the ED with an acute circulatory failure of any origin were successively examined by a recently trained emergency physician and by an expert in critical care echocardiography. Operators independently performed and interpreted online echocardiographic examinations to determine the leading mechanism of acute circulatory failure.
Results:
Focused echocardiography could be performed in 100 of 114 screened patients (55 with sepsis/septic shock and 45 with shock of other origin) after a median fluid loading of 500 mL (interquartile range: 187–1,500 mL). A hypovolemic profile was predominantly observed whether the acute circulatory failure was of septic origin or not (33/55 [60%] vs. 23/45 [51%]: P = 0.37). Although a vasoplegic profile associated with a hyperkinetic left ventricle was most frequently identified in septic patients when compared with their counterparts (17/55 [31%] vs. 5/45 [11%]: P = 0.02), early left or right ventricular failure was observed in 31% of them. Hemodynamic profiles were adequately appraised by recently trained emergency physicians, as reflected by a good-to-excellent agreement with the expert's assessment (Κ: 0.61–0.85).
Conclusions:
Hypovolemia was predominantly identified in patients presenting to the ED with acute circulatory failure. Although vasoplegia was more frequently associated with sepsis, early ventricular dysfunction was also depicted in septic patients. Focused echocardiography seemed reliable when performed by recently trained emergency physicians without previous experience in ultrasound.
Keywords: Echocardiography, Emergency Department, hemodynamic, sepsis, shock
INTRODUCTION
Background
Acute circulatory failure, associated or not with hypotension, is commonly encountered in the Emergency Department (ED) and recognized as a strong predictor of inhospital mortality (1), sepsis remaining a leading cause (2). The recent Surviving Sepsis Campaign (SSC) update has elicited a bundle which should be initiated within the first hour after the identification of sepsis, especially to urge the frontline physician to perform prompt fluid resuscitation in septic patients presenting to the ED acute circulatory failure (3). This bundle includes blood sampling for lactate measurement and blood cultures, administration of 30 mL/kg of intravenous crystalloids and antibiotics, and initiation of vasopressor therapy in the presence of sustained hypotension (3). Nevertheless, such standardized fluid resuscitation may not be adapted in certain patients with early sepsis-induced cardiac dysfunction. In addition, excessive fluid administration in the ED has been shown to be deleterious (4).
Point-of-care echocardiography is a focused examination performed at the bedside to guide patient's management (5). It is ideally suited to assess patients with acute circulatory failure in the ED (6). After a short training program, emergency physicians can perform and interpret focused echocardiography to accurately identify the leading mechanism of acute circulatory failure (7). Information on the hemodynamic status of patients admitted to the ED with acute circulatory failure when evaluated using echocardiography is scarce.
Goals of the investigation
We sought to describe the early hemodynamic profile assessed using focused echocardiography in patients who present to the ED with acute circulatory failure of any origin. The secondary objective of the study was to validate a training program dedicated to emergency physicians who are novice in ultrasound to reach competence in focused echocardiography for the early hemodynamic assessment of patients who present to the ED with acute circulatory failure.
PATIENTS AND METHODS
Patients
We prospectively enrolled adult patients from January 2017 to July 2018 in the ED of our Teaching Hospital when presenting with acute circulatory failure defined as a combination of clinical, hemodynamic, and biochemical signs (8): arterial hypotension (systolic blood pressure <90 mmHg or mean blood pressure <65 mmHg), altered tissue perfusion (skin: cold, clammy, mottling; kidneys: urine output <0.5 mL/kg/h; brain: altered mental status), and cellular dysoxia (lactate >2 mmol/L). Importantly, the presence of low blood pressure was not required due to compensatory mechanisms that may preserve blood pressure through vasoconstriction despite the development of tissue dysoxia (8). Patients were classified into two groups according to the presence of underlying sepsis or septic shock according to the Sepsis-3 definition (9), or not. Patients were not studied in the presence of a moribund status or pregnancy. The study protocol was reviewed and approved by the local Ethics committee (no. 210-2016-24) and the study was registered (NCT02974790).
Focused echocardiography
Each eligible patient was successively examined by a recently trained emergency physician and by an expert in critical care echocardiography. Echocardiographic examinations were performed at the earliest convenience of investigators after the identification of the acute circulatory failure and in random order, depending on the availability of investigators, but within a 1-h time frame. All examinations were performed using a portable full-feature system (CX 50, Philips Healthcare, France).
Each patient was systematically screened for the long- and short-axis parasternal views, the apical four-chamber view, the subcostal four-chamber view, and the inferior vena cava (IVC) view. The emergency physicians and the expert answered a limited number of binary clinical questions (10): global left ventricular (LV) size and systolic function, homogeneous or heterogeneous LV contraction pattern, global right ventricular (RV) size and systolic function, identification of pericardial fluid and tamponade, assessment of both the size and respiratory variations of the IVC, and presence of a severe (acute) left-sided valvular regurgitation. In case of undetermined interpretation due to suboptimal imaging quality, the corresponding clinical question was considered not addressed.
Echocardiography diagnostic criteria were standardized (11). Moderately and severely depressed LV systolic dysfunction corresponded to a visually estimated ejection fraction of 30% to 50% and less than 30%, respectively. LV dilatation was defined by an increased end-diastolic diameter of LV cavity (men >59 mm, women >53 mm) when measured in the parasternal long axis view. LV failure corresponded to the identification of an LV systolic dysfunction, irrespective of its severity and LV cavity size, in the absence of hypovolemia. RV dilation corresponded to a RV/LV end-diastolic diameter ratio more than 0.6 when measured in the apical four-chamber view. RV failure corresponded to the conjunction of a dilatation of the RV cavity and a systemic venous congestion, as reflected by a dilated IVC (12). A dilated RV associated with a paradoxical septal motion identified in the parasternal short-axis view of the heart was consistent with the presence of a cor pulmonale, the most severe presentation of RV failure (13). IVC dilatation and collapse corresponded to an end-expiratory diameter at least 23 mm and at most 15 mm (14) respectively, when measured proximally in the subcostal view between the right atrial junction and the superior hepatic vein. The presence of normal respiratory variations of IVC size corresponded to a visually assessed diameter reduction during inspiration more than 50% in spontaneously breathing patients. Pericardial effusion was identified by the presence of fluid (i.e., echo free space) within the pericardium and tamponade was defined as a compressive pericardial effusion, typically on right cardiac cavities. Severe left-sided valvular regurgitation was identified using color Doppler flow mapping with appropriate setting of the Nyquist limit (10).
After the completion of echocardiography, investigators interpreted the onlineexamination and determined the leading mechanism of acute circulatory failure as being hypovolemia, LV failure, RV failure with acute cor pulmonale being its most severe presentation, vasoplegia with hyperdynamic state, tamponade, or severe (and usually acute) mitral or aortic regurgitation (Supplementary Table 1, Supplemental Digital Content 1). Only expert's interpretation was used to address the primary objective of the study.
Training and curriculum
Five emergency physicians without previous experience in ultrasound participated in the study. A previously validated curriculum (15) was fully conducted and supervised by two experienced intensivists who were board-certified in echocardiography, had a level 3 competence according to the American Society of Echocardiography standards (16), and were experts in critical care echocardiography (10). The training program included 6 h of didactics, 3 h of interactive clinical cases, and 3 h of hands-on tutorial (Supplementary Table 2, Supplemental Digital Content 1) (15). Hands-on sessions were initially performed on normal volunteers and mannequin to master technical skills, including probe handling, spatial orientation, imaging planes acquisition, and anatomical structure identification (17). Subsequently, hands-on sessions were organized in patients with hemodynamic failure and an abnormal echocardiogram. Care was taken to systematically answer all clinical questions covered by focused echocardiography in each examined patient.
Statistical analysis
Results are expressed as means and standard deviations, or percentages. Quantitative variables were compared using Student t test and qualitative variables using χ2 test, or Fisher exact test when necessary. The proportion of addressed clinical questions was compared between emergency physicians and expert intensivists using the McNemar test. An inter-rater reliability analysis using the Cohen's κ statistics with 95% confidence intervals (CIs) was performed to determine the degree of agreement between the five emergency physicians and the expert for each clinical question (18). The concordance of the two-dimensional measurements (IVC diameter, LV diameter, and RV/LV end-diastolic diameter) was evaluated using the κ intra-class coefficient with 95% CIs (19). Expert's interpretation was used as reference (15). A P <0.05 was considered statistically significant.
RESULTS
Characteristics of study patients
Of 114 eligible patients, 14 patients were excluded (10 patients with sepsis and 4 with other shock) because investigators were not timely available for the performance of the two hemodynamic assessments within 1-h time frame (n = 11) or no acoustic windows were available (Fig. 1). With the exception of oxygen saturation, no significant difference was observed between excluded and studied patients (Supplementary Table 3, Supplemental Digital Content 1). Focused echocardiography was performed after the administration of a median volume of 500 mL of crystalloids (interquartile range [IQR]: 187–1,500 mL). Indications for performing focused echocardiography was an acute circulatory failure from sepsis origin (n = 55), or not including hypovolemia (n = 27), cardiac failure (n = 13), hemodynamic obstruction (n = 3), and undetermined cause (n = 2). Mean SOFA score was 5.0 ± 3.1, mean lactate reached 5.2 ± 4.9 mmol/L, and overall 28 days mortality reached 36% (Table 1). All septic patients had a qSOFA score at least 2 points on ED admission (systolic blood pressure <100 mmHg: 89%; respiratory rate ≥22/min: 91%; altered mental status: 53%). Dysfunction of the central nervous system was more frequently observed, and heart rate and hemoglobin level were significantly higher in septic patients than in patients with circulatory failure of other origin, whereas mean SOFA score, lactate, and mortality were similar between groups (Table 1).
Fig. 1.
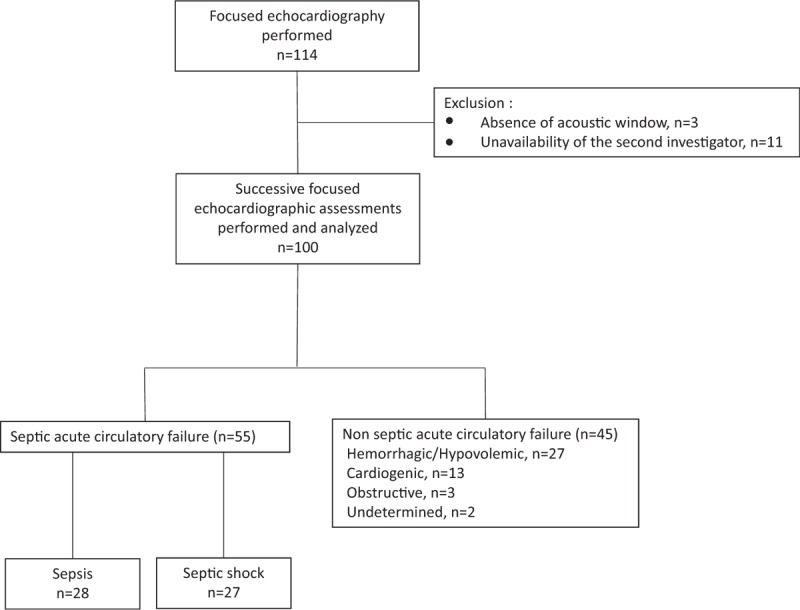
Flow chart of the study.
Table 1.
Characteristics of the study population, according to the septic or nonseptic origin of acute circulatory failure (n = 100)
| Study population | Acute circulatory failure of septic origin | Other acute circulatory failure | ||
| n = 100 | n = 55 | n = 45 | P* | |
| Age, y | 70 ± 15 | 70 ± 15 | 70 ± 16 | 0.92 |
| Male | 62 (62) | 33 (60) | 29 (65) | 0.64 |
| Comorbidities | ||||
| Chronic heart disease | 13 (13) | 5 (9) | 8 (18) | 0.24 |
| Arrhythmia | 28 (28) | 13 (24) | 15 (33) | 0.37 |
| Hemodynamic parameters: | ||||
| Heart rate, bpm | 100 ± 25 | 107 ± 24 | 91 ± 27 | 0.003 |
| Mean blood pressure, mmHg | 66 ± 19 | 67 ± 19 | 65 ± 20 | 0.59 |
| Systolic blood pressure, mmHg | 90 ± 24 | 91 ± 23 | 90 ± 26 | 0.86 |
| O2 saturation | 96 ± 4 | 95 ± 5 | 96 ± 3 | 0.11 |
| Fluid resuscitation†, median [interquartile range], mL | 500 [187–1,500] | 500 [250–1,500] | 500 [0–1,500] | 0.76 |
| Vasopresseur support | 9 (9) | 4 (7) | 5 (11) | 0.72 |
| Mean SOFA score | 5.0 ± 3.1 | 5.3 ± 3.0 | 4.6 ± 3.1 | 0.29 |
| Organ dysfunction: | ||||
| Renal | 57 (57) | 32 (58) | 25 (56) | 0.79 |
| Central nervous system | 41 (41) | 28 (51) | 13 (29) | 0.02 |
| Cardiovascular | 86 (86) | 48 (87) | 38 (84) | 0.68 |
| Liver | 25 (25) | 15 (27) | 10 (22) | 0.56 |
| Coagulation | 34 (34) | 21 (38) | 13 (29) | 0.33 |
| Respiratory | 61 (61) | 36 (65) | 25 (56) | 0.31 |
| Biology | ||||
| pH | 7.31 ± 0.15 | 7.31 ± 0.14 | 7.30 ± 0.17 | 0.88 |
| Lactates, mmol/L | 5.2 ± 4.9 | 5.5 ± 5.0 | 4.8 ± 4.7 | 0.55 |
| Creatinin, μmol/L | 175 ± 240 | 135 ± 78 | 227 ± 346 | 0.11 |
| Platelets, 109 G/L | 210 ± 123 | 193 ± 127 | 232 ± 116 | 0.11 |
| Hemoglobin, g/dL | 12.2 ± 2.7 | 12.7 ± 2.5 | 11.4 ± 2.8 | 0.015 |
| ICU admission | 32 (32) | 22 (40) | 10 (22) | 0.06 |
| 28-d mortality | 36 (36) | 22 (40) | 13 (31) | 0.36 |
Results are expressed as numbers or mean ± standard deviations. Numbers in parentheses are percentages.
*P value was calculated after the comparison of the variables between the two groups (septic and other).
†Volume of fluid administered at the time of focused echocardiography.
Main results
Hypovolemia was predominantly identified in both septic and nonseptic patients (33/55 [60%] vs. 23/45 [51%]: P = 0.37). LV hyperkinesia associated with profound vasoplegia was more frequently observed in septic patients than in their counterparts (17/55 [31%] vs. 5/45 [11%]: P = 0.02). This hyperkinetic state was associated with hypovolemia in 11 septic patients (20%) and in 5 nonseptic patients (11%). In contrast, LV failure was more frequently observed in patients with nonseptic acute circulatory failure than in septic patients (16/45 [36%] vs. 10/55 [18%]: P = 0.04), with severely depressed LV systolic function more often identified in the nonseptic group (Table 2). Among septic patients with LV failure (n = 10), five patients had a worsened pre-existing chronic LV systolic dysfunction, whereas the remaining patients exhibited an acute sepsis-related cardiomyopathy. IVC size was more frequently reduced (26/55 [47%] vs. 12/45 [27%], P = 0.03) and inspiratory collapse more frequently observed in septic patients (35/55 [64%] vs. 15/45 [33%], P <0.01), whereas a dilated non-collapsible vessel was more frequently identified in patients sustaining acute circulatory failure of other origin (14/45 [31%] vs. 8/55 [15%], P = 0.046) (Table 2). The proportion of RV failure was not statistically different between groups, as reflected by the absence of intergroup difference of RV cavity size (Table 2), and only three patients exhibited acute cor pulmonale (all patients without sepsis). Of note, 17 septic patients (31%) exhibited either an LV or a RV failure after a median fluid resuscitation of only 1,000 mL (IQR: 500–1,500 mL), as compared with a median volume loading of 500 mL (IQR: 500–1,500 mL) in the remaining 38 septic patients. The two cases of tamponade and the acute massive mitral regurgitation were all responsible for shock in patients without sepsis (Table 2).
Table 2.
Echocardiographic findings obtained by the expert intensivists (n = 100)
| Septic group | Other acute circulatory failure | P | |
| n = 55 | n = 45 | ||
| Echocardiographic patterns | |||
| Left ventricular characteristics | |||
| Global systolic function | |||
| Normal or increased | 43 (78) | 28 (62) | 0.08 |
| Depressed | 7 (13) | 6 (13) | 0.93 |
| Severely depressed | 3 (5) | 10 (22) | 0.01 |
| Non addressed | 2 (4) | 1 (2) | 1.0 |
| Cavity size | |||
| Enlarged | 5 (9) | 5 (11) | 0.75 |
| Normal | 41 (75) | 32 (71) | 0.70 |
| Reduced | 6 (11) | 7 (16) | 0.49 |
| Non addressed | 3 (5) | 1 (2) | 0.62 |
| Right ventricular characteristics | |||
| Global function | |||
| Normal | 45 (82) | 33 (73) | 0.31 |
| Failure | 7 (13) | 11 (24) | 0.30 |
| Non addressed | 3 (5) | 1 (2) | 0.62 |
| Cavity size | |||
| Normal | 25 (45) | 24 (53) | 0.43 |
| Dilated | 17 (30) | 15 (33) | 0.79 |
| Non addressed | 13 (23) | 6 (13) | 0.19 |
| End-diastolic RV/LV diameter (mean) | 0.72 ± 0.21 | 0.80 ± 0.36 | 0.43 |
| Inferior vena cava characteristics | |||
| Size | |||
| Small (≤15 mm) | 26 (47) | 12 (27) | 0.03 |
| Normal | 18 (33) | 12 (27) | 0.51 |
| Dilated (≥23 mm) | 8 (15) | 14 (31) | 0.046 |
| Non addressed | 3 (5) | 7 (15) | 0.11 |
| Respiratory variations | |||
| Inspiratory collapse | 35 (64) | 15 (33) | <0.01 |
| Non addressed | 3 (5) | 7 (15) | 0.11 |
| Pericardial effusion | 0 (0) | 4 (9) | — |
| Non addressed | 3 (5) | 1 (2) | 0.62 |
| Severe valvular regurgitation | 0 (0) | 1 (2) | — |
| Non addressed | 3 (5) | 3 (7) | 1.0 |
| Hemodynamic profile | |||
| Hypovolemia | 33 (60) | 23 (51) | 0.37 |
| Left ventricular failure | 10 (18) | 16 (36) | 0.04 |
| Hyperdynamic left ventricle associated with vasoplegia* | 17 (31) | 5 (11) | 0.02 |
| Right ventricular failure | 7 (13) | 11 (24) | 0.30 |
| Tamponade | 0 (0) | 2 (4) | — |
| Acute massive left-sided valvular regurgitation | 0 (0) | 1 (2) | — |
| No relevant hemodynamic abnormality | 8† (14) | 2 (4) | 0.18 |
Numbers between parentheses indicate percentages.
*Associated with hypovolemia, explaining that percentages exceed 100%.
†All in patients with sepsis.
During the study period, recently trained emergency physicians performed a median of 22 (IQR = 19–23) echocardiographic examinations. When compared with trainees, experts performed shorter examinations (7 ± 4 min vs. 11 ± 4 min: P < 0.01). When addressed by the two investigators, clinical questions were adequately appraised by recently trained emergency physicians with a good-to-excellent agreement (range: 0.61–1.0) (Table 3). Accuracy of two-dimensional measurements performed by emergency physicians was good for LV end-diastolic diameter (κ = 0.71; 95% CI, 0.55–0.81) and for RV/LV end-diastolic diameter (κ = 0.64; 95% CI, 0.42–0.77), and excellent for end-expiration IVC diameter (κ = 0.90, 95% CI, 0.85–0.94).
Table 3.
Agreement of hemodynamic profiles identified independently by trainees and experts in critical care echocardiography
| Clinical questions (n = 100) | Concordant agreement | Discordant positive results by the emergency physician | Discordant negative results by the emergency physician | Questions nonaddressed by the EP/expert | Kappa value for clinical questions addressed by the two investigators* |
| Severe hypovolemia | 81 | 14 | 2 | 2/1 | 0.65 (0.50–0.80) |
| Left ventricular failure | 87 | 6 | 4 | 3/0 | 0.74 (0.59–0.89) |
| Hyperdynamic left ventricle associated with vasoplegia | 82 | 11 | 4 | 2/1 | 0.61 (0.43–0.78) |
| Right ventricular failure | 85 | 10 | 2 | 2/1 | 0.63 (0.44–0.81) |
| Pericardial effusion | 96 | 1 | 0 | 3/0 | 0.85 (0.57–1.0) |
| Severe valvular regurgitation | 94 | 0 | 0 | 5/1 | 1.0 (1–1) |
*Numbers between brackets indicate 95% confidence interval.
DISCUSSION
Our study confirmed the high prevalence of sepsis in patients admitted to the ED with acute circulatory failure and the predominance of hypovolemia and vasoplegia in these patients. Interestingly, echocardiography early depicted LV or RV systolic failure in more than 30% of septic patients who were not eligible for empiric fluid administration which could have been deleterious. In addition, we showed that focused echocardiography allowed identifying accurately the mechanisms of circulatory failure when performed by recently trained emergency physicians without previous knowledge in ultrasound.
The clinical value of echocardiography to guide the diagnostic work-up in patients admitted to the ED with acute circulatory failure has long been documented (2, 20). Not surprisingly, persisting hypovolemia was the leading mechanism in our patients when they were hemodynamically assessed using early focused echocardiography in the ED after a median fluid loading of only 500 mL (IQR: 187–1,500 mL), irrespective of its origin. This result is in keeping with that reported in previous studies, especially in septic patients (2, 20). In the present study, a vasoplegic profile was more frequently observed in septic patients than in patients with circulatory failure of other origin, and frequently associated with hypovolemia. Profound alteration of vasomotor tone results in markedly decreased LV afterload, hence associated ventricular hyperkinesia with frequent end-systolic cavity obliteration, especially in septic patients (21). Of note, only 9 of our patients had a vasopressor support at the time of echocardiography because hemodynamic assessment was performed very early during initial therapeutic management. In patients admitted to the ED with symptomatic nontraumatic undifferentiated hypotension, Jones et al. (20) showed that the presence of a hyperdynamic LV was suggestive of a septic origin. Similarly, Volpicelli et al. (2) showed that a majority of septic patients were diagnosed with hypovolemia and vasoplegia when hemodynamically assessed using echocardiography in the ED setting. This legitimates the need for early fluid resuscitation in these patients, as currently recommended by the SSC (3, 21).
In the present study, early hemodynamic assessment using echocardiography allowed identifying LV or RV failure in up to 44 of patients presenting to the ED with acute circulatory failure. This hemodynamic profile involved predominantly patients with nonseptic cardiovascular compromise, but was also depicted in 31% of septic patients. The reported prevalence of LV systolic dysfunction in patients with septic shock varies widely, depending on the timing of echocardiographic assessment and the definition used (22). As the diagnosis of LV failure commonly relies on the presence of a decreased ejection fraction, this hemodynamic profile is more frequently described when the hemodynamic assessment is performed later during the sepsis course, in a fluid-filled patient under vasopressor support with restored loading conditions which unmask the underlying septic cardiomyopathy (23). LV systolic dysfunction is the most frequent hemodynamic disturbance ascribed to septic cardiomyopathy (24). Sepsis-induced LV systolic dysfunction is typically identified during the initial hemodynamic assessment performed usually within the first hours after admission, but it may also develop only on day 2 or even 3 (25). Nevertheless, nearly one-third of our septic patients early exhibited either an LV or a RV failure after only a median fluid resuscitation of 1,000 mL (IQR: 500–1,500 mL). This underlines the clinical value of performing early hemodynamic assessment using echocardiography in the ED setting and challenges the potential benefit of a standardized 30-mL fluid resuscitation initiated within the first hour after sepsis recognition, as currently recommended by the last SSC bundles (26, 27). Echocardiographic assessment performed during the initial management of acute circulatory failure in the ED promises to best guide personalized therapy, whether the cardiovascular compromise is of septic origin (26) or not. Such implementation of early noninvasive hemodynamic assessment may reduce unnecessary fluid loading and resulting positive fluid balance which has been shown to be associated with poor outcome (28, 29). Although it has been recently suggested that point-of-care ultrasonography fails to alter survival in the ED setting (30), its impact on intermediate patient-centered outcomes such as organ failures when guiding tailored acute therapy remains to determine, especially in patients presenting with sepsis.
In the present study, emergency physicians without previous knowledge in ultrasound obtained a good-to-excellent agreement with expert intensivists when interpretating the hemodynamic profile of acute circulatory failure after a limited training to perform focused echocardiography. Such dedicated curriculum has previously been validated both in the intensive care unit and ED settings (7, 11). The herein obtained agreement between trainees and experts is similar to that reported in previous studies involving recently trained emergency physicians (7, 31). This reinforces the external validity of our results because echocardiography is currently recommended as a first-line imaging technique for the hemodynamic assessment of shock (8). When focused, the echocardiographic examination can be rapidly performed and therefore appears ideally suited for its routine use in the specific workflow of the ED.
Our study knows substantial limitations. First, we did not evaluate the potential therapeutic impact of the systematic focused echocardiographic assessment because our objective was primarily to describe early hemodynamic profiles in patients presenting to the ED with acute circulatory failure of any origin, and secondarily to validate our dedicated training program. Second, this single-center study involved only a few emergency physicians without previous experience in ultrasound and certain patients could not be evaluated because of unavailability of operators within a restrained time frame. Nevertheless, this failed introducing a recruitment bias as shown by the absence of relevant statistically significant differences between excluded and enrolled patients.
In summary, when early assessed using focused echocardiography, patients presenting to the ED with an acute circulatory failure of any origin predominantly exhibited hypovolemia. Although a vasoplegic profile with hyperkinetic LV was more frequently observed in septic patients, a substantial proportion of them exhibited early left or right ventricular failure despite low volume of fluid resuscitation at the time of hemodynamic assessment. When performed by recently trained emergency physicians without previous experience in ultrasound, this hemodynamic assessment appears reliable. Taken together, these results suggest that early focused echocardiographic assessment promises to help the frontline physician tailoring the therapeutic management of patients presenting with acute circulatory failure to the ED, especially regarding fluid resuscitation of septic patients.
Supplementary Material
Acknowledgment
The authors thank François Dalmay for his advice in data analysis.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Jones AE, Aborn LS, Kline JA. Severity of emergency department hypotension predicts adverse hospital outcome. Shock 22 (5):410–414, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Volpicelli G, Lamorte A, Tullio M, Cardinale L, Giraudo M, Stefanone V, Boero E, Nazerian P, Pozzi R, Frascisco MF. Point-of-care multiorgan ultrasonography for the evaluation of undifferentiated hypotension in the emergency department. Intensive Care Med 39 (7):1290–1298, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Crit Care Med 46 (6):997–1000, 2018. [DOI] [PubMed] [Google Scholar]
- 4.Andrews B, Semler MW, Muchemwa L, Kelly P, Lakhi S, Heimburger DC, Mabula C, Bwalya M, Bernard GR. Effect of an early resuscitation protocol on in-hospital mortality among adults with sepsis and hypotension: a randomized clinical trial. JAMA 318 (13):1233–1240, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore CL, Copel JA. Point-of-care ultrasonography. N Engl J Med 364 (8):749–757, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Sasmaz MI, Gungor F, Guven R, Akyol KC, Kozaci N, Kesapli M. Effect of focused bedside ultrasonography in hypotensive patients on the clinical decision of emergency physicians. Emerg Med Int 2017:6248687, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bustam A, Noor Azhar M, Singh Veriah R, Arumugam K, Loch A. Performance of emergency physicians in point-of-care echocardiography following limited training. Emerg Med J 31 (5):369–373, 2014. [DOI] [PubMed] [Google Scholar]
- 8.Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, Jaeschke R, Mebazaa A, Pinsky MR, Teboul JL, et al. Consensus on circulatory shock and haemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med 40 (12):1795–1815, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315 (8):801–810, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayo PH, Beaulieu Y, Doelken P, Feller-Kopman D, Harrod C, Kaplan A, Oropello J, Vieillard-Baron A, Axler O, Lichtenstein D, et al. American College of Chest Physicians/La Société de Réanimation de Langue Française statement on competence in critical care ultrasonography. Chest 135 (4):1050–1060, 2009. [DOI] [PubMed] [Google Scholar]
- 11.Labovitz AJ, Noble VE, Bierig M, Goldstein SA, Jones R, Kort S, Porter TR, Spencer KT, Tayal VS, Wei K. Focused cardiac ultrasound in the emergent setting: a consensus statement of the American Society of Echocardiography and American College of Emergency Physicians. J Am Soc Echocardiogr 23 (12):1225–1230, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Vieillard-Baron A, Naeije R, Haddad F, Bogaard HJ, Bull TM, Fletcher N, Lahm T, Magder S, Orde S, Schmidt G, et al. Diagnostic workup, etiologies and management of acute right ventricle failure: a state-of-the-art paper. Intensive Care Med 44 (6):774–790, 2018. [DOI] [PubMed] [Google Scholar]
- 13.Jardin F, Dubourg O, Bourdarias JP. Echocardiographic pattern of acute cor pulmonale. Chest 111 (1):209–217, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Vieillard-Baron A, Evrard B, Repessé X, Maizel J, Jacob C, Goudelin M, Charron C, Prat G, Slama M, Geri G, et al. Limited value of end-expiratory inferior vena cava diameter to predict fluid responsiveness impact of intra-abdominal pressure. Intensive Care Med 44 (2):197–203, 2018. [DOI] [PubMed] [Google Scholar]
- 15.Vignon P, Mücke F, Bellec F, Marin B, Croce J, Brouqui T, Palobart C, Senges P, Truffy C, Wachmann A, et al. Basic critical care echocardiography: validation of a curriculum dedicated to noncardiologist residents. Crit Care Med 39 (4):636–642, 2011. [DOI] [PubMed] [Google Scholar]
- 16.Quiñones MA, Douglas PS, Foster E, Gorcsan J, 3rd, Lewis JF, Pearlman AS, Rychik J, Salcedo EE, Seward JB, Stevenson JG, et al. ACC/AHA clinical competence statement on echocardiography: a report of the American College of Cardiology/American Heart Association/American College of Physicians-American Society of Internal Medicine Task Force on clinical competence. J Am Soc Echocardiogr 16 (4):379–402, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Vignon P, Pegot B, Dalmay F, Jean-Michel V, Bocher S, L’her E, Cros J, Prat G. EchoSimu Group: acceleration of the learning curve for mastering basic critical care echocardiography using computerized simulation. Intensive Care Med 44 (7):1097–1105, 2018. [DOI] [PubMed] [Google Scholar]
- 18.Fermanian J. [Measurement of agreement between 2 judges. Qualitative cases]. Rev Epidemiol Sante Publique 32 (2):140–147, 1984. [PubMed] [Google Scholar]
- 19.Bloch DA, Kraemer HC. 2 x 2 kappa coefficients: measures of agreement or association. Biometrics 45 (1):269–287, 1989. [PubMed] [Google Scholar]
- 20.Jones AE, Craddock PA, Tayal VS, Kline JA. Diagnostic accuracy of left ventricular function for identifying sepsis among emergency department patients with nontraumatic symptomatic undifferentiated hypotension. Shock 24 (6):513–517, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med 45 (3):486–552, 2017. [DOI] [PubMed] [Google Scholar]
- 22.Aneman A, Vieillard-Baron A. Cardiac dysfunction in sepsis. Intensive Care Med 42 (12):2073–2076, 2016. [DOI] [PubMed] [Google Scholar]
- 23.Zaky A, Deem S, Bendjelid K, Treggiari MM. Characterization of cardiac dysfunction in sepsis: an ongoing challenge. Shock 41 (1):12–24, 2014. [DOI] [PubMed] [Google Scholar]
- 24.Vieillard-Baron A, Cecconi M. Understanding cardiac failure in sepsis. Intensive Care Med 40 (10):1560–1563, 2014. [DOI] [PubMed] [Google Scholar]
- 25.Boissier F, Razazi K, Seemann A, Bedet A, Thille AW, de Prost N, Lim P, Brun-Buisson C, Mekontso Dessap A. Left ventricular systolic dysfunction during septic shock: the role of loading conditions. Intensive Care Med 43 (5):633–642, 2017. [DOI] [PubMed] [Google Scholar]
- 26.Coopersmith CM, De Backer D, Deutschman CS, Ferrer R, Lat I, Machado FR, Martin GS, Martin-Loeches I, Nunnally ME, Antonelli M, et al. Surviving sepsis campaign: research priorities for sepsis and septic shock. Crit Care Med 46 (8):1334–1356, 2018. [DOI] [PubMed] [Google Scholar]
- 27.Malbrain MLNG, Van Regenmortel N, Saugel B, De Tavernier B, Van Gaal PJ, Joannes-Boyau O, Teboul JL, Rice TW, Mythen M, Monnet X. Principles of fluid management and stewardship in septic shock: it is time to consider the four D's and the four phases of fluid therapy. Ann Intensive Care 8 (1):66, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakr Y, Rubatto Birri PN, Kotfis K, Nanchal R, Shah B, Kluge S, Schroeder ME, Marshall JC, Vincent JL. Intensive care over nations investigators: higher fluid balance increases the risk of death from sepsis: results from a large international audit. Crit Care Med 45 (3):386–394, 2017. [DOI] [PubMed] [Google Scholar]
- 29.Kalil AC, Johnson DW, Lisco SJ, Sun J. Early goal-directed therapy for sepsis: a novel solution for discordant survival outcomes in clinical trials. Crit Care Med 45 (4):607–614, 2017. [DOI] [PubMed] [Google Scholar]
- 30.Atkinson PR, Milne J, Diegelmann L, Lamprecht H, Stander M, Lussier D, Pham C, Henneberry R, Fraser JM, Howlett MK, et al. Does point-of-care ultrasonography improve clinical outcomes in Emergency Department patients with undifferentiated hypotension? An International Randomized Controlled Trial from the SHoC-ED Investigators. Ann Emerg Med 72 (4):478–489, 2018. [DOI] [PubMed] [Google Scholar]
- 31.Moore CL, Rose GA, Tayal VS, Sullivan DM, Arrowood JA, Kline JA. Determination of left ventricular function by emergency physician echocardiography of hypotensive patients. Acad Emerg Med 9 (3):186–193, 2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


