Abstract
Heart failure (HF) has been a known complication of HIV/AIDS for three decades. As the treatment of HIV has changed, so has the epidemiology and pathophysiology of HF in people with HIV (PWH). Initial manifestations of HF in uncontrolled HIV primarily included a rapidly evolving cardiomyopathy with pericardial involvement. With the widespread uptake of effective antiretroviral therapy (ART), HF in PWH has become a chronic disease reflective of the aging population and associated comorbidities, albeit with a contribution from HIV-associated chronic immune dysregulation and inflammation. Despite viral suppression, PWH remain at elevated risk for both HF with reduced ejection fraction and HF with preserved ejection fraction. In this review, we discuss the changing epidemiology and mechanisms of HF in PWH and how that may inform HF prevention in this vulnerable population.
Keywords: Heart Failure, HIV, Immune Dysfunction
Introduction
Prior to widespread antiretroviral therapy (ART) uptake, several case reports and series noted a high incidence of heart failure (HF) in people with HIV/AIDS (PWH), marked by aggressive cardiomyopathy secondary to opportunistic infections, idiopathic lymphocytic myocarditis, and noninflammatory myocardial necrosis.1,2 Early control of HIV with ART reduced the risk of AIDS-associated cardiomyopathy but potential cardiotoxic effects of these therapies began to emerge. Initial reports demonstrated zidovudine-induced myocarditis characterized by CD8 T-cell infiltration on endomyocardial biopsy.3 Subsequently, the development of highly effective and less toxic ART over the years has allowed for early initiation and continuous uninterrupted treatment, with associated significant reductions in morbidity and mortality among PWH.4,5
Thus, leading causes of mortality for treated PWH have shifted from AIDS-related deaths to diseases of aging, including cardiovascular diseases (CVD), such as HF.6 However, it is becoming clear that not only is the prevalence of HF rising in PWH, but the risk of HF for PWH is higher than the general population.7–9 This increased risk is not fully explained by: (1) increasing prevalence of traditional CVD risk factors or high-risk behaviors, (2) disparities in treatment of CVD risk factors, or (3) potential harmful effects of current generation ART. Instead, there is mounting evidence that persistent immune dysregulation, even in those started on early suppressive ART, plays an important role in the development of myocardial dysfunction (either directly or through enhancing the effects of traditional CVD risk factors) and incident HF. In this review, we will discuss the changing epidemiology of HF in PWH and attempt to understand the underlying mechanisms that may contribute to HF risk and propose strategies to prevent HF in this vulnerable population.
Epidemiology of HIV-Associated HF
The global epidemiology of HF in PWH varies greatly with geographical location depending on access to, and uptake of, effective ART. Specifically, in resource-limited settings where access to ART is limited, AIDS-associated cardiomyopathy along with pericardial disease remains a common subtype of HF. The prevalence of AIDS-associated cardiomyopathy ranges from 9% to 57% depending on the study population, with one prospective study reporting an incidence of nearly 17% over 18 months.10 AIDS-associated cardiomyopathy is strongly associated with advanced immunosuppression, specifically CD4 count <100/mm3, and has a dismal prognosis.10 Prior to ART, the prevalence of AIDS-associated cardiomyopathy was likewise 30%−40% even in high income countries.11 Additionally, HIV accounted for up to 4% of all unexplained cardiomyopathies in the US during the 1980s and 1990s.12 These data from low-and-middle income countries as well as high-income countries pre-ART clearly suggest that untreated, advanced HIV leads to direct myocardial injury.
While AIDS-associated cardiomyopathy is less of a concern in settings with high rates of continuous ART use, PWH on ART continue to be at increased risk of HF with reduced ejection fraction ( EF; HFrEF) and HF with preserved EF ( HFpEF) compared to the general population. Analysis of HIV-infected and uninfected veterans enrolled in the Veterans Aging Cohort Study (VACS) from 2000 to 2007 demonstrated a nearly 2-fold increased risk of HF in HIV-infected veterans.13 This association persisted in those with and without coronary artery disease (CAD). In subgroup analysis, the association strengthened in those with elevated viral load at baseline while those with sustained suppression of viral load did not have an increased risk of HF. Increased risk was also seen in those with CD4 count below or above 200/mm3 and regardless of ART. In a subsequent analysis from VACS, HF was further categorized into HFrEF (EF<40%), HFpEF (EF≥50%), and borderline HFpEF (EF 40–49%).9 This analysis used administrative codes to define HF (with echocardiographic data to sub-categorize HF types) and included predominantly men enrolled after 2003 and followed up through 2012. There were roughly equal numbers of HFpEF and HFrEF cases in PWH. PWH had an increased risk of all three subtypes of HF, but the association with HFrEF was strongest (hazard ratio 1.61) and persisted after adjustment for incident myocardial infarction (MI) during the follow-up period. The increased risk of overall HF remained in sensitivity analyses that excluded those with hypertension (HTN), alcohol or cocaine abuse, and ever smokers. In those with baseline suppressed viral load, the risk of HFrEF attenuated while the risk of HFpEF and borderline HFpEF remained elevated. In contrast, lower CD4 count, especially <200/mm3 was associated with all three subtypes of HF. The risk attenuated with a rising CD4 count for all three HF subtypes with no increased risk of HFpEF was found in those with CD4 count >500/mm3.
Additional cohorts have further corroborated these findings. In an electronic health records-derived database of patients receiving care for HIV at a large urban medical center in Chicago from 2000 to 2016, a rigorous, systematic approach was taken to adjudicate HF endpoints with the goal of enhancing diagnostic accuracy and HF sub-phenotyping.14 Similar to prior studies of administrative records, PWH had a 2-fold higher risk of adjudicated HF compared to controls.15 Higher viral load was associated with increased HF risk while higher CD4 count was associated with lower HF risk. Another study from a Taiwanese nationwide cohort using data from 2003 to 2014 also showed an increased risk of incident HF in PWH using a competing risk model.16 In addition, those receiving ART had significantly higher risk of incident HF than those not receiving ART. However, no specific class of ART was associated with a higher risk of HF, and the risk of HF decreased as ART duration increased. Despite a small number of women in the Taiwanese cohort, in subgroup analyses, the HIV-associated risk of HF appeared to be stronger in women (HR 2.5) than in men (HR 1.44). Finally, in an analysis of a large healthcare database that used administrative codes to define HF, risks for HF among PWH were likewise elevated, with an adjusted relative risk of 1.66 for PWH vs. HIV-uninfected persons.7 An analysis of women in VACS from 2003 to 2009 showed that women with HIV had a significantly higher risk of CVD than HIV-uninfected women (HR 2.8).17 After excluding atherosclerotic CVD, the risk of HF remained higher in women with HIV (HR 2.5). The median age of CVD onset was lower in women with HIV. Among those with HF, women with HIV have higher rates of HF hospitalization, longer HF hospital stays, and higher rates of CVD mortality compared to uninfected women.18
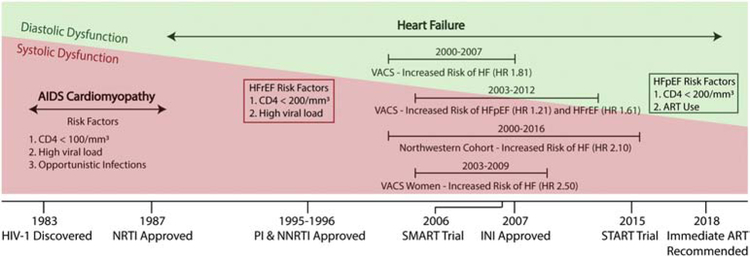
These studies done in the era of modern ART have helped establish HIV as an independent risk factor for incident HF even in the absence of the classic AIDS-associated cardiomyopathy. However, few studies distinguished between HFpEF and HFrEF and none comprehensively phenotyped HF etiologies (e.g. ischemic vs. non-ischemic) – a clear area of need. Several studies have evaluated phenotypes of subclinical myocardial dysfunction. One meta-analysis evaluated the prevalence and type of cardiac dysfunction in HIV.19 This analysis included 18 studies from North America, 15 from Europe, 11 from Africa, and 10 from Asia. The pooled relative risk of HF was 1.7, similar to the studies described above. The pooled prevalence of left ventricular (LV) systolic dysfunction and any diastolic dysfunction were 12.3% and 29.3%, respectively. The prevalence of advanced diastolic dysfunction was 11.7%. The prevalence of LV systolic dysfunction was higher in studies with greater proportion of participants with AIDS and was lower among more recent studies and those with higher proportion of participants on ART. These results, along with the findings from the cohort studies discussed above, suggest that systolic dysfunction and HFrEF are strongly linked to burden of HIV viremia. It is important to note that the 2014 HIV treatment guidelines were the first to recommend ART regardless of CD4 count and only the most recent 2018 guidelines stress the importance of very early ART initiation, including immediately after diagnosis.20,21 These recommendations have led to an increase in the proportion of PWH on ART and a significant decrease in the median time from HIV diagnosis to ART initiation (418 days to 77 days) during this decade.22 Thus, it stands to reason that with the evolution of early to immediate ART initiation, HFpEF may become the dominant subtype of HF in PWH living in the developed world (Figure 1). Future studies more heavily weighted toward the modern ART era are needed to better characterize this changing epidemiology.
Figure 1.
Evolving epidemiology of heart failure and cardiac dysfunction. The pre-ART era was characterized by AIDS-cardiomyopathy. The post-ART era is characterized by less systolic dysfunction and rising diastolic dysfunction. Major cohort studies have demonstrated an increased risk of heart failure in people with HIV. Both HFpEF and HFrEF are associated with immune dysfunction as characterized by nadir CD4+ T cell count. HFrEF is also associated with viral load while HFpEF is associated with certain ART regimens.
HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; VACS, Veterans Aging Cohort Study; ART, antiretroviral therapy; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; INI, integrase inhibitor; SMART Trial4, continuous ART superior to episodic ART guided by CD4+ T cell count; START Trial5, early ART initiation at CD4+ T cell count >500/mm3 was beneficial.
Pathophysiology of HIV-Associated Heart Failure
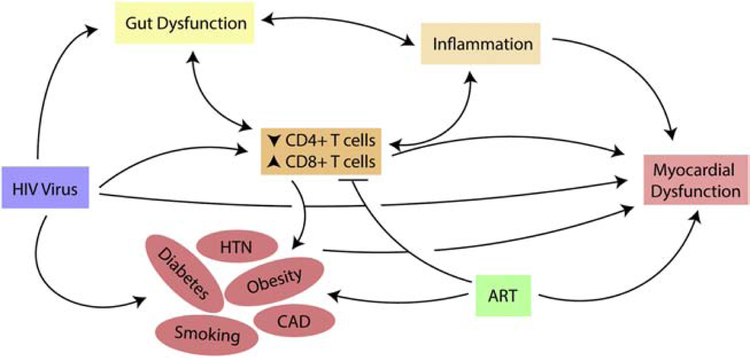
Given the changing epidemiology of HF as well as treatment of HIV in this population, the exact mechanisms underlying the increased risk of HF have not been fully elucidated. However, there are many potential pathways that may be implicated in HIV-associated HF pathogenesis based on emerging data (Figure 2).
Figure 2.
Pathways leading to myocardial dysfunction and development of heart failure in people with HIV.
CAD, coronary artery disease; HTN, hypertension; ART, antiretroviral therapy.
AIDS-Associated Cardiomyopathy and Systolic Dysfunction – Relevance After Immune Recovery
Early studies evaluating AIDS-associated severe, dilated cardiomyopathy described the pathophysiology as related to opportunistic infections, autoimmune myocarditis, and direct HIV-induced myocardial damage. In autopsy studies of PWH, many different organisms including fungi, protozoa, bacteria, and viruses were identified in the myocardium.23 Mechanisms for increased risk of autoimmune myocarditis have also been identified. These include a greater presence of cardiac-specific autoantibodies in PWH, potentially resulting in increased cardiac expression of HLA antigens and subsequent cardiac injury.24,25 An initial landmark study showed that HIV nucleic acid sequences could be detected in the myocytes of patients with AIDS-associated cardiomyopathy but, importantly, this manuscript was later retracted due to concerns for falsification of data.26 Subsequent studies have demonstrated that the site of cardiac infection is more likely to be the macrophages, dendritic cells, and endothelial cells, rather than cardiomyocytes which lack the HIV-1 receptor protein.27 The HIV protein Tat has also been shown to cause dilated cardiomyopathy through mitochondrial damage in a transgenic mouse model.28
In the current era of early and uninterrupted ART, it is also important to understand whether there are any mechanisms that lead to cardiac injury during the acute phase of the infection that can contribute to downstream incident HF. Acute HIV infection is characterized by uncontrolled viral replication and results in a cytokine cascade involving tumor necrosis factor (TNF) α, interferon α, interferon γ, interleukin (IL) 6, IL 8, and IL 15.29 While one purpose of this cytokine release is to activate the immune system to help control viral replication, it may also contribute to early cardiac pathology. In a study of 49 patients with no underlying CVD, levels of N-terminal prohormone of brain natriuretic peptide (NT-proBNP) and troponin T during acute HIV infection were significantly elevated compared to levels after achieving viremic control.30 Both biomarkers had a strong correlation with viral load. Thus, cardiac injury appears to begin early after HIV infection. More studies are needed to understand whether this early cardiac injury may create vulnerability to “second-hit” HF-inducing comorbidities, even after considerable immune recovery following uninterrupted ART.
Traditional Heart Failure Risk Factors
The predictors of HFpEF and HFrEF in the general population primarily consist of traditional cardiovascular risk factors such as age, HTN, diabetes mellitus (DM), smoking, along with underlying atherosclerotic CVD.31 Therefore, measuring the prevalence of these risk factors in PWH is necessary to determine if they help explain the increased risk of HF. However, these risk factors are also deeply intertwined with HIV-associated immune dysfunction and ART, thus making it difficult to determine whether they are confounders or mediators of the HIV effect.
A meta-analysis of studies with data collected during 1996–2014 from 63,554 participants across the world showed an overall estimated prevalence of 25.2% for HTN. The prevalence was 34.7% for the ART-experienced group and 12.7% for the ART-naïve group.32 The lower prevalence of HTN in the ART-naïve group has been shown in other studies as well and may be accounted for by factors associated with advanced HIV such as younger age, lower body mass index (BMI), and perhaps increased vasodilation in the setting of opportunistic infections.33 While the prevalence of HTN in PWH on ART might be greater than that of the uninfected population, it is not clear whether HIV increases the risk of developing HTN compared to the general population34–36 or whether this is merely an epidemiological artifact.
The rising rates of obesity and DM have contributed significantly to the increase in prevalence of HF, especially HFpEF, in the general population. These same risk factors are also affecting the HIV population.37 Therefore, it is crucial to understand whether underlying HIV pathophysiology and associated factors, such as ART potentiate this relationship between metabolic dysfunction and HF in PWH. HIV infection was initially a wasting syndrome, although some studies in the pre-ART era showed significantly increased triglyceride levels in patients with AIDS compared to controls.38 Lipodystrophy began to occur as a consequence of initial ART regimens, characterized by peripheral lipoatrophy and excess visceral adiposity eventually leading to dyslipidemia, insulin resistance, and DM. These toxicities were primarily attributed to PIs and mitochondrial toxicity from thymidine-based NRTIs.39–41 While the currently recommended therapies are significantly less toxic, they continue to have some metabolic effects. There has been some concern regarding the association of abacavir with MI based on some cohort studies, although other analyses have not found an association.42–44 Integrase strand inhibitors (INSTIs) are well-tolerated and have minimal effect on the lipid profile; however, some studies suggest increased risk of insulin resistance and there have been case reports of DM.45 Both INSTIs and the current generation of PIs, which are considered second line, still lead to increase in peripheral and central fat depots after initiation.46
While the current generation of ART may contribute to metabolic dysfunction, their potential effects are modest; thus, other HIV-related factors are likely implicated in the increased risk of metabolic disease such as DM. A recent survey from a nationally representative US sample of PWH showed a higher prevalence of DM than in uninfected adults, potentially at younger ages and lower BMI.47 Potential mechanisms involve accessory proteins produced by HIV that are present in the blood even in the absence of viremia and contribute to adipocyte dysfunction. These proteins have been shown to inhibit pre-adipocyte differentiation as well as peroxisome proliferator-activated receptor gamma (PPAR-γ) in hepatocytes, while stimulating glucocorticoid receptors in adipocytes leading to hyperglycemia, dyslipidemia, and hepatic steatosis.48 They may also regulate gene transcription by inhibiting Dicer, an RNAse that plays a role in maintaining the functionality of subcutaneous adipose tissue. Immune dysfunction in adipose tissue is thought to play a role as well. T-cells within the white adipose tissue of lean PWH are enriched for CD8+ T-cells, which is characteristic of obesity. These CD8+ T cells lead to increased adipocyte inflammation which promotes insulin resistance and DM through dysregulation of adipokines.48 Furthermore, a study among PWH in Uganda demonstrated that pericardial fat was associated with subclinical myocardial dysfunction, suggesting that metabolic abnormalities in PWH may directly affect the heart.49
Cigarette smoking is another important risk factor for HF that disproportionately affects the HIV population. Rates of current smoking in PWH are 2- to 3-fold higher than in the general population. Prior data have shown that the risk of MI associated with being a previous or current smoker compared with a never smoker was significantly higher in PWH than in uninfected controls.50 Similarly, exposure to smoking is associated with a population attributable fraction of 72% in PWH compared to 24% in uninfected controls.50 This augmented effect of smoking on CVD in HIV may be explained, in part, by the increased levels of immune activation seen in smokers with HIV.51
The risk of HF is not only affected by a direct effect of risk factors discussed above on the myocardium but also through pathways involving coronary artery disease (CAD). While studies investigating risk of HF in PWH excluded baseline CVD and adjusted for interval MI in their analyses, CAD remains the most common cause of HF and thus is integral for HF prevention. The association between HIV and increased risk of MI has been demonstrated in several cohorts. In a large healthcare system-based cohort of PWH followed between 1996 and 2004, PWH had 1.75-fold higher adjusted relative risk for MI compared with HIV-uninfected persons.52 Similarly, in a subsequent analysis of VACS data, PWH were at increased risk of MI compared with HIV-uninfected controls across 3 decades, from ages 40–70.53 This increased risk of nearly 50% persisted despite adjusting for traditional CVD risk factors including cigarette smoking and substance use. Additional analyses with physician adjudication and phenotyping of MI in the Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) cohort showed approximately half of all MIs in PWH were type 2 MIs caused by clinical conditions such as infection and illicit drug use.54 While a type 2 MI does not reflect an atherothrombotic event, it usually occurs in the setting of underlying CAD and leads to myocardial injury. In the general population, incident type 2 MI has been shown to predict CVD death as well as HF.55 Whether it also increases the risk of HF in PWH is not known, although a recent analysis demonstrated very high rates of mortality after type 2 MI for PWH.56
Myocardial Dysfunction from Persistent Immune Dysregulation
While traditional risk factors are certain to play a big role in the development of HF, chronic immune dysfunction in PWH likely contributes to myocardial dysfunction directly and perhaps by enhancing the effects of those risk factors on the myocardium. We have only recently started to appreciate the role of the immune system in cardiac homeostasis and disease in the general population. The response of immune cells to different cardiac insults including MI, infections, and CVD risk factors, such as HTN and DM, is important in understanding the subsequent development of HFpEF or HFrEF. Therefore, chronic immune dysfunction in HIV should be viewed as a key enhancing risk factor for HF in this population.
Persistent immune dysfunction in HIV primarily results from maintenance of viral reservoirs. HIV-1 preferentially infects activated memory CD4+ T-cells, most of which die, but a small proportion become part of a latent reservoir.57 All antiretroviral medications work by preventing the virus from replicating but they do not target HIV-1 genomic material that is already integrated into the host cell DNA and they cannot eliminate the cells which harbor the integrated DNA. Interestingly, these memory CD4+ T cells and HIV-1 help promote each other. HIV-1 genes are often integrated with host genes associated with cell growth, thus promoting expansion of these T cells. At the same time, T cell activation creates chronic inflammation which stimulates immunoregulatory responses and down regulates effector cells that could help decrease HIV replication.57 While memory CD4+ T cells are the primary reservoir cell for HIV-1 and predominantly reside in secondary lymph nodes, spleen, and gut mucosa, there is evidence that other T cell subsets and macrophages may also serve as an important reservoir.58 Given that recruited monocytes from the periphery contribute to cardiac fibrosis and dysfunction in the general population, this could be a potential mechanism through which chronic HIV infection contributes to myocardial damage.59 A study using the simian immunodeficiency virus model of AIDS in Rhesus macaques illustrated that blocking monocyte/macrophage trafficking to the heart decreases cardiac fibrosis and dysfunction.60 There is also evidence in humans that there is persistent monocyte/macrophage activation despite suppressive ART.61
Cardiovascular imaging studies have allowed us to better quantify the relationship between myocardial dysfunction and persistent immune dysregulation in PWH. In a cross-sectional study, treated PWH without underlying CVD had increased levels of intramyocardial lipid and fibrosis compared to matched uninfected controls on cardiac magnetic resonance imaging (CMR). These findings strongly correlated with increased levels of markers associated with inflammation and monocyte activation.62 Similarly, women with HIV on ART had increased myocardial fibrosis and diastolic dysfunction compared to matched uninfected controls. Both myocardial fibrosis and diastolic dysfunction significantly correlated with markers of monocyte activation.63 Markers of monocyte and macrophage activation are also significantly associated with increased levels of arterial wall inflammation in the aorta of PWH.64 As aortic stiffness is known to contribute to HFpEF pathophysiology in the general population, these findings might represent another mechanism through which risk of HFpEF is increased in this population.65
Other studies have not evaluated myocardial dysfunction in the context of immune dysfunction in HIV. However, they have illustrated that subclinical myocardial dysfunction is significantly increased in PWH without other comorbidities, suggesting pathways that bypass traditional cardiovascular risk factors. In a study comparing normotensive PWH on ART with normotensive uninfected controls with similar fasting glucose and cholesterol levels, PWH had increased LV mass and worse diastolic function.66 In fact their LV mass and diastolic function was similar to hypertensive uninfected controls. In a study of nonsmokers without history of CVD or DM and no difference between groups with respect to blood pressure or anthropometric measures, PWH on ART had higher myocardial lipid levels and myocardial fibrosis, along with a lower LV systolic longitudinal strain compared to uninfected controls.67 Additional CMR studies have confirmed these findings of increased myocardial mass, myocardial lipid, diastolic dysfunction, localized scar, and diffuse myocardial fibrosis in PWH on ART without prior CVD.62,68–70 While these studies have focused on PWH without prior CVD, there is evidence that HIV-infected adults develop more extensive myocardial scar than uninfected persons in the post-MI setting.71
In totality, these results lend credence to the hypothesis that immune dysfunction contributes significantly to the increased risk of incident HF in PWH. However, there is a strong need for further delineating the pathways that lead from immune dysregulation to myocardial dysfunction. This will need a combination of additional studies using both animal models such as SIV-infected macaques and well-curated cohorts of PWH on current generation ART with linked biorepositories, myocardial imaging, and adjudicated HF events.
Heart Failure Prevention
The first step in preventing incident HF in this population is to effectively treat the underlying traditional cardiovascular risk factors. The rates of appropriate guideline-recommended cardiovascular care are lower in PWH compared to the general population.72 PWH are also at high risk for mental health disorders such as depression, which is associated with an increased risk of incident HF.73 This disparity is further compounded by factors such as sex and race. Compared to whites, blacks are less likely to receive ART, experience viral control, HTN control, DM control or lipid monitoring.74 Further, recent data suggest that racial disparities in appropriate statin prescription may be exacerbated for PWH compared with HIV-uninfected persons.75 Removing these barriers to primary prevention through public policy and promoting awareness of the increased risk of CVD in this population are key initial steps. Furthermore, comprehensive scientific statements reviewing the best available data and determining practices most likely to prevent CVD among PWH are essential; the recent American Heart Association scientific statement on HIV and CVD provides general guidance regarding CVD prevention for PWH,76 albeit in the context of still-limited data.
Risk prediction is another important tool in prevention. Yet, unlike atherosclerotic CVD, there is no well-accepted HF risk prediction tool for the general population. Nonetheless, a risk prediction score can aid in risk stratification and provide physicians the impetus to intensify available therapies; however, quantification of HF risk attributable to HIV is not exact. As described earlier, studies have demonstrated a 1.5–2 fold greater risk for HF in PWH, especially in those who had a delay in ART initiation and were exposed to a prolonged period of viremia with evidence of immune dysfunction (current low CD4 count, low CD4/CD8 ratio, or low nadir CD4). In the AHA scientific statement discussed above, the writing group offers a general guide to assessing CVD risk for PWH.76
Early identification of subclinical myocardial damage through serum and imaging-based markers may also be important in preventing incident HF for PWH. This will be especially important as the epidemiology shifts to HFpEF, which can be more difficult to diagnose. Markers such as NT-proBNP have been associated with CVD events, including HF, and mortality in the HIV population.77,78 However, using NT-proBNP as a screening tool in PWH to reduce incident HF has not been evaluated. Other biomarkers expressed by cardiac tissue in the setting of stress, such as soluble ST2 and growth differentiation factor-15, have also been associated with adverse cardiac mechanics and overall mortality in PWH.79,80 Routine use of speckle tracking to quantify left ventricular longitudinal strain might also help identify early myocardial dysfunction in PWH. Decrease in left ventricular global longitudinal strain has been associated with immune dysfunction as measured by nadir CD4 <250 cells/mm3 or current CD4 <500 cells/mm3.81
In PWH with risk factors such as DM and HTN, data are sparse with respect to which treatment options might be most effective in decreasing long-term CVD risk. Small studies with Telmisartan, which acts as both an angiotensin receptor blocker and a PPAR-γ agonist, have illustrated dramatic effects on blood pressure as well improvement in lipid metabolism, renal function, and a potential endothelial protective effect in PWH.82 Given that HIV-1 promotes production of renin by CD4 T cells and, in return, renin is important for HIV replication within T cells, inhibition of RAAS is appealing as the first line therapy for HTN.83
Similarly, there are no HIV-specific guidelines for first-line therapy for DM. The rising prevalence of metabolic dysfunction, obesity, and DM should make sodium-glucose cotransporter 2 (SGLT2) inhibitors an attractive agent in PWH as a HF prevention therapy. The cardio-protective effects of SGLT2 inhibitors work through mechanisms that are enhanced in HIV. These effects include amelioration of cardiac fibrosis, reduction in sympathetic activity and epicardial fat, improved metabolism leading to weight loss and decreased insulin resistance, and potential improvement in systemic inflammation and endothelial dysfunction.84 However, the potential benefits of metformin in HIV are worth considering as well. Metformin appears to improve metabolic indices and even reduce atherosclerosis progression in PWH.85 However, it might have unique benefits in PWH by reducing the HIV reservoir through immunomodulatory effects, as metformin may actually help improve CD4+ T cell counts in diabetic PWH.86 The effects of metformin in PWH could be due to multiple pathways including overcoming the inhibition of adenosine monophosphate-activated protein kinase (energy sensor for cells including T cells) from HIV Tat protein, anti-inflammatory effect on the gut by suppressing nuclear factor κB activation, reduction of anti-inflammatory cytokines such as TNF-α, and changing the gut microbiota composition.87 The hypothesis that metformin can reduce HIV reservoir in the gut and decrease immune activation is currently being evaluated in the Lilac study.87
Statins have also been shown to have immunomodulatory and anti-inflammatory effects. Therefore, statin therapy could play a role in HF prevention in PWH beyond effects related to prevention of MI. Studies have shown a decrease in markers of fibrosis in PWH with statin therapy, an effect that correlates closely with decrease in immune activation.88 Pitavastatin has also been shown to significantly reduce markers of immune activation and arterial inflammation.89 The effects of Pitavastatin on CVD in PWH will be comprehensively evaluated in the ongoing REPRIEVE trial.90
While these therapies have been translated from work done in the general population, it will be important to develop therapies that address HIV-specific pathophysiology, such as the underlying immune dysfunction. These therapies have been broadly focused on eradicating latent reservoirs of HIV. One strategy has been to induce latently infected cells to produce virus and reverse latency with subsequent interventions to augment the ability of the host to clear these cells.91 These interventions could include vaccines, neutralizing monoclonal antibodies, and immune checkpoint blockade. Both vaccines and immune checkpoint inhibitors allow for a more robust HIV-1 specific CD8+ T cell response, which has also been observed in elite controllers (individuals who can control viral replication without ART). Development of targeted therapies that eradicate the latent reservoirs and restore immune function may help treat chronic diseases such as HF in PWH.
Conclusions
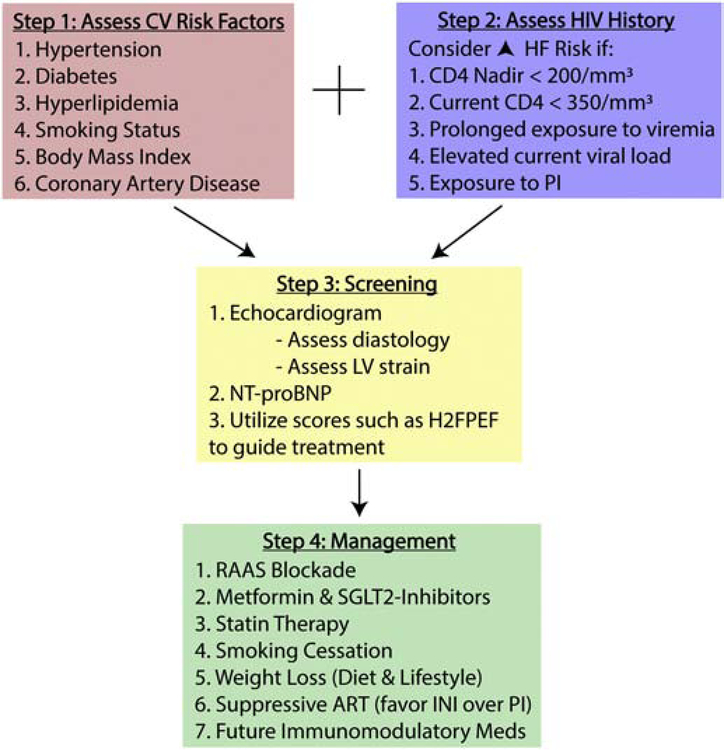
There are numerous pathways that require further investigation using methods spanning the translational spectrum to lower the elevated risk of incident HF in PWH. This involves improving systems of care to allow for more equitable delivery of healthcare and more trials in the HIV population, as findings in the general population may not always apply. Developing tools and drugs that selectively reverse immune dysfunction may help prevent and treat HF in this population. In the meantime, it will be important for clinicians taking care of PWH to identify individuals at the highest risk (presence of immune dysfunction and CVD risk factors) and consider early screening for further risk stratification (sample pathway for HF prevention in PWH provided Figure 3). For those at highest risk, clinicians should consider aggressive risk factor modification with RAAS inhibition, statin therapy, and perhaps SGLT2 inhibitor or metformin when applicable.
Figure 3.
Proposed algorithm to prevent heart failure in people with HIV.
CV, cardiovascular; HF, heart failure; LV, left ventricular; NT-proBNP, N-terminal-pro B-type Natriuretic Peptide; H2FPEF, echocardiogram and clinical characteristics score to determine risk of heart failure with preserved ejection fraction92; RAAS, renin-angiotensin-aldosterone system; SGLT2, sodium-glucose cotransporter 2; ART, antiretroviral therapy; PI, protease inhibitor; INSTI, integrase strand transfer inhibitor.
As the HIV population ages, the morbidity and mortality attributable to HF will continue to rise. The focus of HIV treatment has switched from preventing opportunistic infections to preventing chronic diseases such as HF. While important advances have been made in identifying the increased risk of HF in HIV, many gaps remain in our understanding. These include the changing epidemiology of HF in the setting of current ART guidelines, underlying immune mechanisms that contribute to myocardial dysfunction and exacerbate the myocardial effects of traditional CVD risk factors, and optimal methods for HF risk assessment and prevention. These gaps need to be filled to address the growing burden of HF in this population.
Acknowledgments
Funding:
American Heart Association Fellow-to-Faculty Award (16FTF31200010; PI: Feinstein) National Institutes of Health (P30AI117943)
Abbreviations:
- ART
antiretroviral therapy
- BMI
body mass index
- BP
blood pressure
- CAD
coronary artery disease
- CMR
cardiac magnetic resonance imaging
- CNICS
Centers for AIDS Research Network of Integrated Clinical Systems
- CVD
cardiovascular disease
- DM
diabetes mellitus
- EF
ejection fraction
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- HR
hazard ratio
- HTN
hypertension
- IL
interleukin
- INI
integrase inhibitors
- LV
left ventricular
- MI
myocardial infarction
- NNRTI
non-nucleoside reverse transcriptase inhibitors
- NRTI
nucleoside reverse transcriptase inhibitor
- NT-proBNP
N-terminal prohormone of brain natriuretic peptide
- PWH
people living with HIV
- PI
protease inhibitors
- PPAR-γ
peroxisome proliferator-activated receptor gamma
- RAAS
renin-angiotensin-aldosterone system
- SGLT2
sodium-glucose cotransporter 2
- SIV
simian immunodeficiency virus
- TNF
tumor necrosis factor
- VACS
Veterans Aging Cohort Study
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None.
References
- 1.Acierno LJ. Cardiac complications in acquired immunodeficiency syndrome (AIDS): a review. Journal of the American College of Cardiology. 1989;13(5):1144–1154. [DOI] [PubMed] [Google Scholar]
- 2.Cohen IS, Anderson DW, Virmani R, et al. Congestive cardiomyopathy in association with the acquired immunodeficiency syndrome. N Engl J Med 1986;315(10):628–630. [DOI] [PubMed] [Google Scholar]
- 3.Herskowitz A, Willoughby SB, Baughman KL, Schulman SP, Bartlett JD. Cardiomyopathy associated with antiretroviral therapy in patients with HIV infection: a report of six cases. Ann Intern Med 1992;116(4):311–313. [DOI] [PubMed] [Google Scholar]
- 4.Strategies for Management of Antiretroviral Therapy Study G, El-Sadr WM, Lundgren J, et al. CD4+ count-guided interruption of antiretroviral treatment. The New England journal of medicine. 2006;355(22):2283–2296. [DOI] [PubMed] [Google Scholar]
- 5.Lundgren JD, Babiker AG, Gordin F, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med 2015;373(9):795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith CJ, Ryom L, Weber R, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet 2014;384(9939):241–248. [DOI] [PubMed] [Google Scholar]
- 7.Al-Kindi SG, ElAmm C, Ginwalla M, et al. Heart failure in patients with human immunodeficiency virus infection: Epidemiology and management disparities. International journal of cardiology. 2016;218:43–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feinstein MJ, Steverson AB, Ning H, Pawlowski AE, Schneider D, Ahmad FS, Sanders JM, Sinha A, Nance RM, Achenbach CJ, Delaney JAC, Heckbert SR, Shah SJ, Hanna DB, Hsue PY, Bloomfield GS, Longenecker CT, Crane HM, Lloyd-Jones DM Adjudicated Heart Failure in HIV-Infected and Uninfected Men and Women. Journal of the American Heart Association. 2018;7(21):e009985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freiberg MS, Chang CH, Skanderson M, et al. Association Between HIV Infection and the Risk of Heart Failure With Reduced Ejection Fraction and Preserved Ejection Fraction in the Antiretroviral Therapy Era: Results From the Veterans Aging Cohort Study. JAMA Cardiol 2017;2(5):536–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ntsekhe M, Mayosi BM. Cardiac manifestations of HIV infection: an African perspective. Nat Clin Pract Cardiovasc Med 2009;6(2):120–127. [DOI] [PubMed] [Google Scholar]
- 11.Barbarinia G, Barbaro G. Incidence of the involvement of the cardiovascular system in HIV infection. Aids 2003;17 Suppl 1:S46–50. [PubMed] [Google Scholar]
- 12.Felker GM, Thompson RE, Hare JM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med 2000;342(15):1077–1084. [DOI] [PubMed] [Google Scholar]
- 13.Butt AA, Chang CC, Kuller L, et al. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Archives of internal medicine. 2011;171(8):737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steverson AB, Pawlowski AE, Schneider D, et al. Clinical characteristics of HIV-infected patients with adjudicated heart failure. European journal of preventive cardiology. 2017;24(16):1746–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feinstein MJ, Steverson AB, Ning H, et al. Adjudicated Heart Failure in HIV-Infected and Uninfected Men and Women. J Am Heart Assoc 2018;7(21):e009985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yen YF, Ko MC, Yen MY, et al. Human Immunodeficiency Virus Increases the Risk of Incident Heart Failure. Journal of acquired immune deficiency syndromes. 2019;80(3):255–263. [DOI] [PubMed] [Google Scholar]
- 17.Womack JA, Chang CC, So-Armah KA, et al. HIV infection and cardiovascular disease in women. Journal of the American Heart Association. 2014;3(5):e001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janjua SA, Triant VA, Addison D, et al. HIV Infection and Heart Failure Outcomes in Women. Journal of the American College of Cardiology. 2017;69(1):107–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erqou S, Lodebo BT, Masri A, et al. Cardiac Dysfunction Among People Living With HIV: A Systematic Review and Meta-Analysis. JACC Heart failure. 2019;7(2):98–108. [DOI] [PubMed] [Google Scholar]
- 20.Gunthard HF, Aberg JA, Eron JJ, et al. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society-USA Panel. Jama 2014;312(4):410–425. [DOI] [PubMed] [Google Scholar]
- 21.Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2018 Recommendations of the International Antiviral Society-USA Panel. JAMA : the journal of the American Medical Association. 2018;320(4):379–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medland NA, Chow EPF, McMahon JH, Elliott JH, Hoy JF, Fairley CK. Time from HIV diagnosis to commencement of antiretroviral therapy as an indicator to supplement the HIV cascade: Dramatic fall from 2011 to 2015. PLoS One. 2017;12(5):e0177634–e0177634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Currie PF, Boon NA. Immunopathogenesis of HIV-related heart muscle disease: current perspectives. Aids 2003;17 Suppl 1:S21–28. [DOI] [PubMed] [Google Scholar]
- 24.Currie PF, Goldman JH, Caforio AL, et al. Cardiac autoimmunity in HIV related heart muscle disease. Heart. 1998;79(6):599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herskowitz A, Wu TC, Willoughby SB, et al. Myocarditis and cardiotropic viral infection associated with severe left ventricular dysfunction in late-stage infection with human immunodeficiency virus. J Am Coll Cardiol. 1994;24(4):1025–1032. [DOI] [PubMed] [Google Scholar]
- 26.Drazen JM, Curfman GD. Retraction: Barbaro et Al. Incidence of dilated cardiomyopathy and detection of HIV in myocardial cells of HIV-positive patients. N Engl J Med 1998;339:1093–9. [DOI] [PubMed] [Google Scholar]; N Engl J Med 2002;347(2):140.12110745 [Google Scholar]
- 27.Shannon RP, Simon MA, Mathier MA, Geng YJ, Mankad S, Lackner AA. Dilated cardiomyopathy associated with simian AIDS in nonhuman primates. Circulation. 2000;101(2):185–193. [DOI] [PubMed] [Google Scholar]
- 28.Raidel SM, Haase C, Jansen NR, et al. Targeted myocardial transgenic expression of HIV Tat causes cardiomyopathy and mitochondrial damage. Am J Physiol Heart Circ Physiol 2002;282(5):H1672–1678. [DOI] [PubMed] [Google Scholar]
- 29.Stacey AR, Norris PJ, Qin L, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol 2009;83(8):3719–3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuster C, Mayer FJ, Wohlfahrt C, et al. Acute HIV Infection Results in Subclinical Inflammatory Cardiomyopathy. J Infect Dis 2018;218(3):466–470. [DOI] [PubMed] [Google Scholar]
- 31.Ho JE, Lyass A, Lee DS, et al. Predictors of new-onset heart failure: differences in preserved versus reduced ejection fraction. Circ Heart Fail 2013;6(2):279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Y, Chen X, Wang K. Global prevalence of hypertension among people living with HIV: a systematic review and meta-analysis. J Am Soc Hypertens 2017;11(8):530–540. [DOI] [PubMed] [Google Scholar]
- 33.Peck RN, Shedafa R, Kalluvya S, et al. Hypertension, kidney disease, HIV and antiretroviral therapy among Tanzanian adults: a cross-sectional study. BMC Med 2014;12:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mills KT, Bundy JD, Kelly TN, et al. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation. 2016;134(6):441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryscavage P, Still W, Nyemba V, Stafford K. Prevalence of Systemic Hypertension Among HIV-Infected and HIV-Uninfected Young Adults. Open Forum Infectious Diseases. 2017;4(Suppl 1):S59–S59. [DOI] [PubMed] [Google Scholar]
- 36.Gelpi M, Afzal S, Lundgren J, et al. Higher Risk of Abdominal Obesity, Elevated Low-Density Lipoprotein Cholesterol, and Hypertriglyceridemia, but not of Hypertension, in People Living With Human Immunodeficiency Virus (HIV): Results From the Copenhagen Comorbidity in HIV Infection Study. Clin Infect Dis 2018;67(4):579–586. [DOI] [PubMed] [Google Scholar]
- 37.Tate T, Willig AL, Willig JH, et al. HIV infection and obesity: where did all the wasting go? Antivir Ther 2012;17(7):1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grunfeld C, Kotler DP, Hamadeh R, Tierney A, Wang J, Pierson RN. Hypertriglyceridemia in the acquired immunodeficiency syndrome. Am J Med 1989;86(1):27–31. [DOI] [PubMed] [Google Scholar]
- 39.Tsiodras S, Mantzoros C, Hammer S, Samore M. Effects of protease inhibitors on hyperglycemia, hyperlipidemia, and lipodystrophy: a 5-year cohort study. Archives of internal medicine. 2000;160(13):2050–2056. [DOI] [PubMed] [Google Scholar]
- 40.Carr A, Samaras K, Thorisdottir A, Kaufmann GR, Chisholm DJ, Cooper DA. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet 1999;353(9170):2093–2099. [DOI] [PubMed] [Google Scholar]
- 41.Caron M, Auclairt M, Vissian A, Vigouroux C, Capeau J. Contribution of mitochondrial dysfunction and oxidative stress to cellular premature senescence induced by antiretroviral thymidine analogues. Antivir Ther 2008;13(1):27–38. [PubMed] [Google Scholar]
- 42.Sabin CA, Worm SW, Weber R, et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet 2008;371(9622):1417–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elion RA, Althoff KN, Zhang J, et al. Recent Abacavir Use Increases Risk of Type 1 and Type 2 Myocardial Infarctions Among Adults With HIV. J Acquir Immune Defic Syndr 2018;78(1):62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ding X, Andraca-Carrera E, Cooper C, et al. No association of abacavir use with myocardial infarction: findings of an FDA meta-analysis. J Acquir Immune Defic Syndr 2012;61(4):441–447. [DOI] [PubMed] [Google Scholar]
- 45.Hulgan T Factors Associated With Insulin Resistance in Adults With HIV Receiving Contemporary Antiretroviral Therapy: a Brief Update. Curr HIV/AIDS Rep 2018;15(3):223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McComsey GA, Moser C, Currier J, et al. Body Composition Changes After Initiation of Raltegravir or Protease Inhibitors: ACTG A5260s. Clin Infect Dis 2016;62(7):853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hernandez-Romieu AC, Garg S, Rosenberg ES, Thompson-Paul AM, Skarbinski J. Is diabetes prevalence higher among HIV-infected individuals compared with the general population? Evidence from MMP and NHANES 2009–2010. BMJ Open Diabetes Res Care. 2017;5(1):e000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Godfrey C, Bremer A, Alba D, et al. Obesity and Fat Metabolism in Human Immunodeficiency Virus–Infected Individuals: Immunopathogenic Mechanisms and Clinical Implications. The Journal of Infectious Diseases. 2019;220(3):420–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buggey J, Yun L, Hung CL, et al. HIV and pericardial fat are associated with abnormal cardiac structure and function among Ugandans. Heart. 2020;106(2):147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rasmussen LD, Helleberg M, May MT, et al. Myocardial Infarction Among Danish HIV-Infected Individuals: Population-Attributable Fractions Associated With Smoking. Clinical Infectious Diseases. 2015;60(9):1415–1423. [DOI] [PubMed] [Google Scholar]
- 51.Valiathan R, Miguez MJ, Patel B, Arheart KL, Asthana D. Tobacco smoking increases immune activation and impairs T-cell function in HIV infected patients on antiretrovirals: a cross-sectional pilot study. PLoS One. 2014;9(5):e97698–e97698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. The Journal of clinical endocrinology and metabolism. 2007;92(7):2506–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freiberg MS, Chang C-CH, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA internal medicine. 2013;173(8):614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crane HM, Paramsothy P, Drozd DR, et al. Types of Myocardial Infarction Among Human Immunodeficiency Virus-Infected Individuals in the United States. JAMA cardiology. 2017;2(3):260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gaggin HK, Liu Y, Lyass A, et al. Incident Type 2 Myocardial Infarction in a Cohort of Patients Undergoing Coronary or Peripheral Arterial Angiography. Circulation. 2017;135(2):116–127. [DOI] [PubMed] [Google Scholar]
- 56.Feinstein MJ, Nance RM, Delaney JAC, et al. Mortality following myocardial infarction among HIV-infected persons: the Center for AIDS Research Network Of Integrated Clinical Systems (CNICS). BMC Med 2019;17(1):149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barouch DH, Deeks SG. Immunologic strategies for HIV-1 remission and eradication. Science. 2014;345(6193):169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wong ME, Jaworowski A, Hearps AC. The HIV Reservoir in Monocytes and Macrophages. Front Immunol 2019;10:1435–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swirski FK, Nahrendorf M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nature reviews Immunology. 2018;18(12):733–744. [DOI] [PubMed] [Google Scholar]
- 60.Walker JA, Beck GA, Campbell JH, Miller AD, Burdo TH, Williams KC. Anti-α4 Integrin Antibody Blocks Monocyte/Macrophage Traffic to the Heart and Decreases Cardiac Pathology in a SIV Infection Model of AIDS. Journal of the American Heart Association. 2015;4(7):e001932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wada NI, Jacobson LP, Margolick JB, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS (London, England). 2015;29(4):463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thiara DK, Liu CY, Raman F, et al. Abnormal Myocardial Function Is Related to Myocardial Steatosis and Diffuse Myocardial Fibrosis in HIV-Infected Adults. The Journal of infectious diseases. 2015;212(10):1544–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zanni MV, Awadalla M, Toribio M, et al. Immune Correlates of Diffuse Myocardial Fibrosis and Diastolic Dysfunction Among Aging Women With Human Immunodeficiency Virus. The Journal of infectious diseases. 2019:jiz184. [DOI] [PMC free article] [PubMed]
- 64.Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. JAMA. 2012;308(4):379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reddy YNV, Andersen MJ, Obokata M, et al. Arterial Stiffening With Exercise in Patients With Heart Failure and Preserved Ejection Fraction. Journal of the American College of Cardiology. 2017;70(2):136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grandi AM, Nicolini E, Giola M, et al. Left ventricular remodelling in asymptomatic HIV infection on chronic HAART: comparison between hypertensive and normotensive subjects with and without HIV infection. J Hum Hypertens 2012;26(10):570–576. [DOI] [PubMed] [Google Scholar]
- 67.Holloway CJ, Ntusi N, Suttie J, et al. Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation. 2013;128(8):814–822. [DOI] [PubMed] [Google Scholar]
- 68.Ntusi N, O’Dwyer E, Dorrell L, et al. HIV-1-Related Cardiovascular Disease Is Associated With Chronic Inflammation, Frequent Pericardial Effusions, and Probable Myocardial Edema. Circ Cardiovasc Imaging. 2016;9(3):e004430–e004430. [DOI] [PubMed] [Google Scholar]
- 69.Luetkens JA, Doerner J, Schwarze-Zander C, et al. Cardiac Magnetic Resonance Reveals Signs of Subclinical Myocardial Inflammation in Asymptomatic HIV-Infected Patients. Circ Cardiovasc Imaging. 2016;9(3):e004091–e004091. [DOI] [PubMed] [Google Scholar]
- 70.Nelson MD, Szczepaniak LS, LaBounty TM, et al. Cardiac steatosis and left ventricular dysfunction in HIV-infected patients treated with highly active antiretroviral therapy. JACC Cardiovasc Imaging. 2014;7(11):1175–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Feinstein MJ, Mitter SS, Yadlapati A, et al. HIV-Related Myocardial Vulnerability to Infarction and Coronary Artery Disease. Journal of the American College of Cardiology. 2016;68(18):2026–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ladapo JA, Richards AK, DeWitt CM, et al. Disparities in the Quality of Cardiovascular Care Between HIV-Infected Versus HIV-Uninfected Adults in the United States: A Cross-Sectional Study. Journal of the American Heart Association. 2017;6(11):e007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.White JR, Chang C-CH, So-Armah KA, et al. Depression and human immunodeficiency virus infection are risk factors for incident heart failure among veterans: Veterans Aging Cohort Study. Circulation. 2015;132(17):1630–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Richardson KK, Bokhour B, McInnes DK, et al. Racial Disparities in HIV Care Extend to Common Comorbidities: Implications for Implementation of Interventions to Reduce Disparities in HIV Care. J Natl Med Assoc 2016;108(4):201–210.e203. [DOI] [PubMed] [Google Scholar]
- 75.Riestenberg RA, Furman A, Cowen A, et al. Differences in statin utilization and lipid lowering by race, ethnicity, and HIV status in a real-world cohort of persons with human immunodeficiency virus and uninfected persons. Am Heart J 2018;209:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Feinstein MJ, Hsue PY, Benjamin LA, et al. Characteristics, Prevention, and Management of Cardiovascular Disease in People Living With HIV: A Scientific Statement From the American Heart Association. Circulation. 2019;140(2):e98–e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gingo MR, Zhang Y, Ghebrehawariat KB, et al. Elevated NT-pro-brain natriuretic peptide level is independently associated with all-cause mortality in HIV-infected women in the early and recent HAART eras in the Women’s Interagency HIV Study cohort. PLoS One. 2015;10(3):e0123389–e0123389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Duprez DA, Neuhaus J, Tracy R, et al. N-terminal-proB-type natriuretic peptide predicts cardiovascular disease events in HIV-infected patients. AIDS (London, England). 2011;25(5):651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scherzer R, Shah SJ, Secemsky E, et al. Association of Biomarker Clusters With Cardiac Phenotypes and Mortality in Patients With HIV Infection. Circulation Heart failure. 2018;11(4):e004312–e004312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Secemsky EA, Scherzer R, Nitta E, et al. Novel Biomarkers of Cardiac Stress, Cardiovascular Dysfunction, and Outcomes in HIV-Infected Individuals. JACC Heart failure. 2015;3(8):591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alenezi F, Bloomfield GS, Okeke NL, et al. Global Longitudinal Strain and Immune Status in Patients Living With Human Immunodeficiency Virus. The American journal of cardiology. 2019;124(6):966–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vecchiet J, Ucciferri C, Falasca K, Mancino P, Di Iorio A, De Caterina R. Antihypertensive and metabolic effects of telmisartan in hypertensive HIV-positive patients. Antiviral therapy. 2011;16(5):639–645. [DOI] [PubMed] [Google Scholar]
- 83.Chandel N, Ayasolla K, Lan X, et al. Renin modulates HIV replication in T cells. J Leukoc Biol 2014;96(4):601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wojcik C, Warden BA. Mechanisms and Evidence for Heart Failure Benefits from SGLT2 Inhibitors. Current Cardiology Reports. 2019;21(10):130. [DOI] [PubMed] [Google Scholar]
- 85.Fitch K, Abbara S, Lee H, et al. Effects of lifestyle modification and metformin on atherosclerotic indices among HIV-infected patients with the metabolic syndrome. AIDS (London, England). 2012;26(5):587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moyo D, Tanthuma G, Cary MS, et al. Cohort study of diabetes in HIV-infected adult patients: evaluating the effect of diabetes mellitus on immune reconstitution. Diabetes Res Clin Pract 2014;103(3):e34–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Routy J-P, Isnard S, Mehraj V, et al. Effect of metformin on the size of the HIV reservoir in non-diabetic ART-treated individuals: single-arm non-randomised Lilac pilot study protocol. BMJ Open. 2019;9(4):e028444–e028444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.deFilippi C, Christenson R, Joyce J, et al. Brief Report: Statin Effects on Myocardial Fibrosis Markers in People Living With HIV. Journal of acquired immune deficiency syndromes (1999). 2018;78(1):105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Toribio M, Fitch KV, Sanchez L, et al. Effects of pitavastatin and pravastatin on markers of immune activation and arterial inflammation in HIV. AIDS (London, England). 2017;31(6):797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grinspoon SK, Fitch KV, Overton ET, et al. Rationale and design of the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE). Am Heart J 2019;212:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barouch DH, Deeks SG. Immunologic strategies for HIV-1 remission and eradication. Science. 2014;345(6193):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure With Preserved Ejection Fraction. Circulation. 2018;138(9):861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]