Summary
Cells interact with their surrounding environment through surface proteins. However, knowledge gaps remain in understanding how these important types of proteins are transported and anchored on the cell surface. In the Gram-negative social bacterium, Myxococcus xanthus, a putative C-terminal sorting tag (MYXO-CTERM) is predicted to help direct 34 different proteins onto the cell surface. Here we investigate the sorting pathway for MYXO-CTERM proteins by using the TraA cell surface receptor as a model. Deleting this motif from TraA abolishes cell surface anchoring and results in extracellular secretion. Our findings indicate that conserved cysteines within the MYXO-CTERM are post-translationally modified and are required for TraA cell surface localization and function. A region immediately upstream of these residues is predicted to be disordered and removing this motif caused a secretion defect and blocked cell surface anchoring. We further show that the type II secretion system is required for translocation across the outer membrane and that a cysteine-rich region directs TraA to the T2SS. Similar results were found with another MYXO-CTERM protein indicating our findings can be generalized. Further, we show the universal distribution of MXYO-CTERM motif across the Myxococcales order and provide a working model for sorting of these proteins.
Keywords: type II secretion system, protein sorting, cell surface receptor, outer membrane, myxobacteria, outer membrane exchange
Introduction
Cells interact with their neighbors and local environment with surface proteins. These proteins therefore play key roles in a variety of cellular processes including cell-cell recognition. However, our current knowledge of bacterial cell surface proteins is limited to a few types. Textbook descriptions of surface proteins from Gram-negative bacteria are largely derived from Escherichia coli, where their surface is composed of β-barrel proteins, pili, flagella and occasionally lipoproteins. Although these are important proteins, relatively recent genomic and proteomic studies show that many pathogenic and environmental species contain other types of cell surface proteins. In particular, comparative genomics found that Gram-negatives and archaea contain open reading frames (ORFs) with C-terminal tags that are similar to prototypic LPXTG sorting tags from Gram-positive bacteria (Schneewind et al., 1993, Haft et al., 2006, Haft & Varghese, 2011, Haft et al., 2012). Here, the sortase enzyme cleaves between the threonine and glycine residues with transient attachment of the threonine to the active site cysteine of sortase, followed by a transpeptidation reaction that covalently attaches the tagged protein to the cell wall (Navarre & Schneewind, 1994, Mazmanian et al., 1999, Ton-That et al., 1999). The resulting cell surface proteins act as enzymes, adhesins, among other functions. In contrast, Gram-negative bacteria have an outer membrane (OM) that encases the cell wall, and therefore proteins with C-terminal sorting tags must be anchored to the cell surface by a mechanism(s) different from sortase. In addition, for proteins to reach the cell surface, they must pass through the cell envelope; a complex structure composed of a peptidoglycan layer sandwiched between the inner membrane (IM), composed of a phospholipid bilayer, and the OM, composed of a phospholipid inner leaflet and a lipopolysaccharide (LPS) outer leaflet (Silhavy et al., 2010).
The PEP-CTERM motif (TIGR02595) is one of several known C-terminal sorting tags found in Gram-negatives thought to be processed by exosortase, an enzyme with analogous functions to sortase. Like LPXTG, PEP-CTERM has a similar tripartite architecture, which consists of a short region of conserved residues (PEP) followed by a hydrophobic transmembrane helix (TMH) and a short stretch of positively charged residues (Haft et al., 2006). The GlyGly-CTERM motif (TIGR03501) is another C-terminal sorting tag found in several genera of Proteobacteria (Haft & Varghese, 2011). A recent study in Vibrio cholerae with VesB, a serine protease, describes the cleavage of the GlyGly-CTERM motif by an intramembrane protease, rhombosortase (Gadwal et al., 2018). Periplasmic processing of VesB is followed by translocation across the OM by the type II secretion system (T2SS) and cell surface localization. In the absence of rhombosortase, VesB is degraded apparently due to the lack of posttranslational modification (PTM). Analogous to GlyGly-CTERM proteins, some well-studied lipoproteins, such as pullulanase (PulA) from Klebsiella pneumoniae and SslE from enteropathogenic Escherichia coli, are extracted from the IM and transported across the OM by the T2SS and anchored, although transiently, to the cell surface by their acyl chains (Pugsley et al., 1986, Baldi et al., 2012, East et al., 2016). Here, the invariant cysteine within the N-terminal lipobox is post-translationally acylated followed by signal peptide (SP) removal (Zuckert, 2014). The PulA lipoprotein SP contains a Lol avoidance sequence that initially targets the protein to the IM (Pugsley, 1993, Hara et al., 2003), which is required for subsequent transport across the OM by T2SS (East et al., 2016). Interestingly, these examples represent two topologies, N-terminal or C-terminal, by which proteins are first anchored in the IM, before translocation across the OM where they are anchored to the cell surface in the same respective orientations. Other T2SS substrates are secreted across the OM from the periplasm as soluble proteins (d’Enfert et al., 1987, Poquet et al., 1993, Baldi et al., 2012).
Similarly, in archaea a C-terminal motif, PGF-CTERM (TIGR04126), found in cell surface glycoproteins, is processed by archaeosortase, an enzyme homologous to exosortase. Other C-terminal motifs with cognate archaeosortase or exosortase enzymes have been identified in archaea and Proteobacteria, respectively (Haft et al., 2012). Many of the archaeosortase/exosortase targeted proteins are also likely to be glycosylated (Haft et al., 2012). The first experimental evidence of archaeosortase mediated C-terminal processing of a S-layer glycoprotein came from a haloarchaea, Haloferax volcanii (Abdul Halim et al., 2013). To date, two co-occurring types of PTM of C-terminal motifs have been demonstrated in cell surface glycoproteins; lipid attachment (Kikuchi et al., 1999, Konrad & Eichler, 2002, Kandiba et al., 2012) and proteolytic cleavage (Abdul Halim et al., 2013).
Many members of the phylum Bacteroidetes secrete proteins across the OM via the type IX secretion system (T9SS) (McBride & Zhu, 2013, Veith et al., 2013). Proteins secreted through this system have N-terminal SP and C-terminal domains (CTDs) that direct them to cross the IM and OM, respectively (Nguyen et al., 2007, Sato et al., 2013, Veith et al., 2013, Kharade & McBride, 2015). For example, the oral pathogen Porphyromonas gingivalis (Sato et al., 2010, Shoji et al., 2011) contains Gingipain, a cell surface-associated virulence protein, where its transport across the OM requires the T9SS (Seers et al., 2006) and deletion of the CTD traps the protein in the periplasm (Nguyen et al., 2007). Interestingly, PorU, an important component of T9SS, acts as the sorting protease (Gorasia et al., 2015) that cleaves the CTD and then covalently attaches it to anionic-LPS, resulting in cell surface anchoring (Paramonov et al., 2005).
Myxococcus xanthus is a Gram-negative soil bacterium well known for complex social behaviors involving direct physical interactions with neighboring cells. We previously showed that cell surface proteins, TraA (MXAN_6895; WP_011556817.1) and TraB (MXAN_6898; WP_011556818.1), are involved in self-recognition (Pathak et al., 2012). These genes overlap in an operon and together function as a cell surface adhesin that discriminate friends from foe based on a specificity region, the variable domain (VD), contained within TraA (Pathak et al., 2013, Cao & Wall, 2017, Cao et al., 2019). Following recognition, cells transfer lipids and proteins in a process called OM exchange (OME) that is thought to be mediated by transient OM fusion (Cao et al., 2015, Cao & Wall, 2019). Analogous to the mentioned sorting tags, TraA contains a C-terminal tripartite motif called MYXO-CTERM (TIGR03901) (Pathak et al., 2012). As implied in the other systems, we hypothesize that the MYXO-CTERM undergoes PTM to allow protein cell surface localization. The M. xanthus genome contains 34 ORFs with MYXO-CTERM suggesting this motif plays a major role in directing proteins to the cell surface, which in turn governs how cells perceive and interact with their external world (Pathak et al., 2012).
Here we investigated the role of MXYO-CTERM in protein sorting with a focus on TraA. As background, TraB resembles other OM proteins in that it contains a SP, a β-barrel domain and a cell wall binding OmpA domain. In prior work we showed that in the absence of TraB, TraA still localizes to the cell surface, thus TraA/B does not act as a two-partner secretion system (Jacob-Dubuisson et al., 2001, Cao & Wall, 2017). For this work we undertook a structure-function studies of TraA to elucidate the roles of its various domains: Cysteine-rich repeats (CRR), intrinsically disordered region (IDR) and the MYXO-CTERM motif in secretion and sorting. Prior work showed that the VD was not required for TraA sorting because in its absence TraA still localizes to the cell surface (Cao & Wall, 2019). In this work we identify a minimal region of TraA responsible for its secretion and cell surface localization. Additionally, we show that an invariant cysteine residue at the beginning of MYXO-CTERM is critical for TraA function, localization and apparent PTM. We further show that two different proteins with MYXO-CTERM motifs are translocated across the OM by the T2SS in a process that does not depend on MYXO-CTERM. In wild-type proteins, the MYXO-CTERM is cleaved and further PTM that allows cell surface anchoring. Finally, we propose a working model for how MYXO-CTERM proteins are processed and sorted to the cell surface.
Results
MYXO-CTERM motif is widely distributed in the order Myxococcales
To understand the distribution of the MYXO-CTERM motif we searched the Integrated Microbial Genomes (IMG) database from the Joint Genome Institute (Nordberg et al., 2014). Since MYXO-CTERM (TIGR03901) is a small degenerate sequence, this motif was frequently found; i.e. one to a few times in thousands of different genomes. However, the vast majority of these hits were false positives because they were not at the C-terminus of bona fide ORFs. According to the founding definition of TIGR03901, MYXO-CTERM is restricted to the Myxococcales order and our analysis supported this conclusion. Therefore, our further analysis only included genomes from this order. According to IMG, Nannocystis exedens DSM 71 contains the most TIGR03901 hits (>60), while Vulgatibacter incomptus DSM 27710, which has a relatively small myxobacterial genome, only contains a few. However, we note that the TIGR03901 hidden Markov model is a general identifier that has not been customized for specific linages, and therefore undercounts the number of MYXO-CTERM ORFs per genome. For example, according to IMG there are seven MYXO-CTERM ORFs in the M. xanthus DK1622 genome, however our prior analysis, which included manual curation, identified 34 ORFs (Pathak et al., 2012).
To compare genomes within the Myxococcales order, MYXO-CTERM ORFs identified in IMG were curated and genes that did not contain an apparent SP and were shorter than 100 amino acid residues were excluded. The ORFs were then grouped into the three well established phylogenetic suborders and a catchall unclassified suborder (Fig. 1). The resulting 704 sequences were manually aligned with Jalview (Waterhouse et al., 2009) and binned into the four respective suborders to create WebLogos (Crooks et al., 2004). The average length of MYXO-CTERM sequences were 33 amino acids. This motif has a characteristic tripartite organization; a periplasmic N-terminal GC sequence, a hydrophobic TMH and a cytoplasmic C-terminal tail with positively charged arginine residues. While the first position residue was often, but not always, a glycine the second position was an invariant cysteine (Fig. 1). From our analysis the MYXO-CTERM motif is universally found within the diverse and ancient Myxococcales order and, to our knowledge, is currently the only motif with these characteristics within this group of bacteria.
Fig. 1.
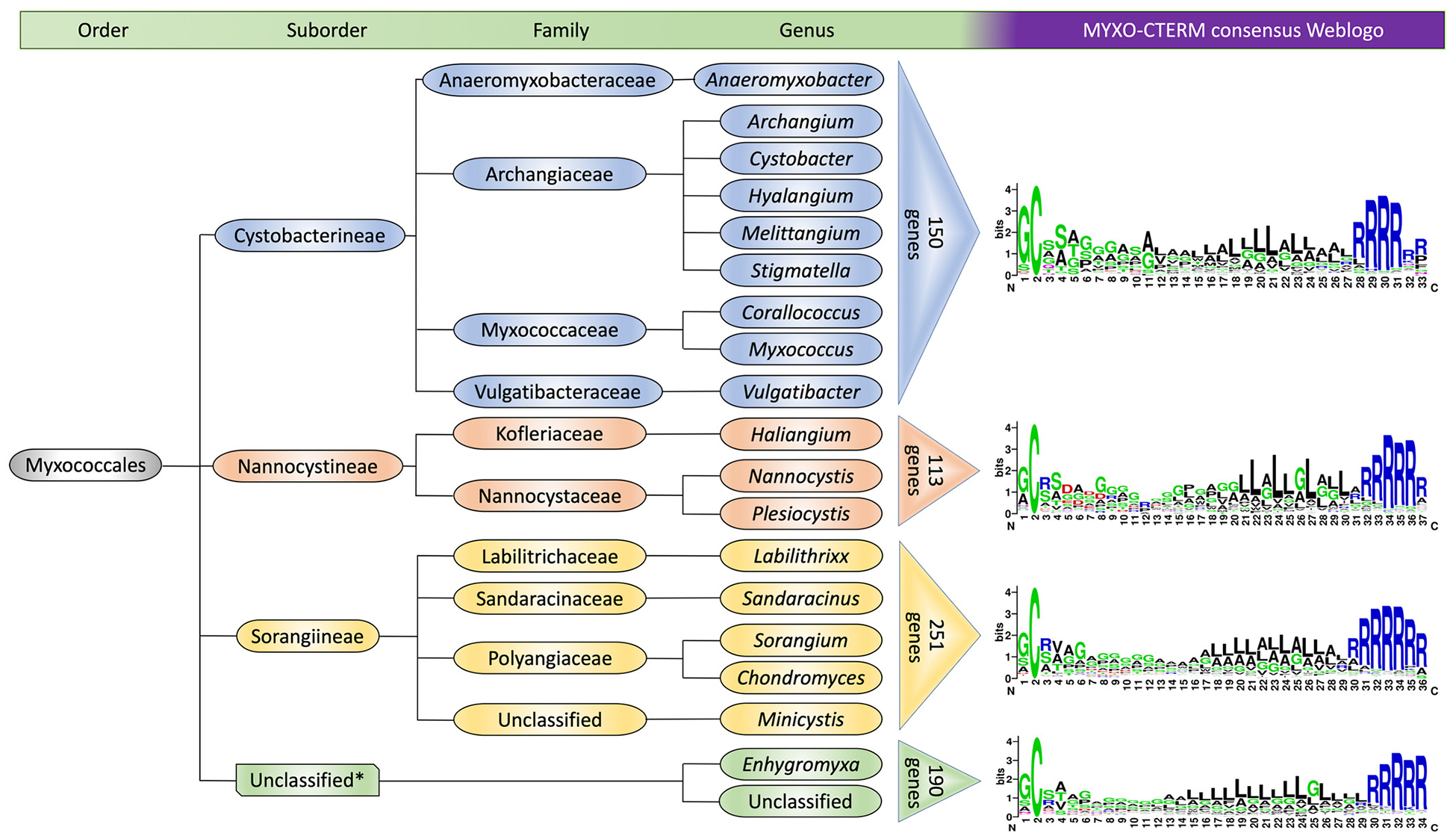
MYXO-CTERM is a universal motif in the order Myxococcales. Four different WebLogos show the MYXO-CTERM motif from three defined suborders and an unclassified (catchall) suborder. Colored triangles indicate the number of proteins binned into each suborder.
MYXO-CTERM motif is essential for maturation and PTM of TraA
We hypothesized that the MYXO-CTERM allows proteins, such as TraA, to be transiently anchored in the IM. Further, we predicted a proteolytic cleavage site, in the case of TraA, after the second cysteine residue in MYXO-CTERM (note: TraA contains a CC dipeptide instead of the more prevalent GC, Figs. 1 and 2A) (Pathak et al., 2012). To test this, we created two tagged variants of TraA; N-terminal (TraA-nFLAG) and C-terminal (TraA-cFLAG) constructs (Fig. 2A) and tested for C-terminal processing by western analysis. Since TraA contains an N-terminal SP, the FLAG-tag was inserted downstream of that processing site. Equal amounts of whole cell lysates were probed with anti-FLAG antibodies and, as a control, anti-VD antibodies were also used. As shown in Figure 2B, the VD antibodies detected both variants of TraA and the levels were equal. However, anti-FLAG antibodies only detected abundant amounts of TraA-nFLAG, while TraA-cFLAG was marginally detected. These results suggest that the sequence upstream of the FLAG-tag, namely MYXO-CTERM, in TraA-cFLAG was cleaved. Next we created a truncated variant of TraA where the MYXO-CTERM was deleted (TraA_ΔMXCT) and tested its localization and activity. Importantly, deleting MYXO-CTERM resulted in TraA secretion to the extracellular medium and abolished cell association (Fig. 2C) and rendered cells nonfunctional for OME (Fig. S1A). Here we report that wild-type (WT) TraA localizes to both the cell and supernatant fractions and the migration of TraA_ΔMXCT was identical to the supernatant fraction of WT TraA (Fig. 2C). For WT TraA, the migration differences suggest that it was cleaved and resulted in a processed version that lacked the MYXO-CTERM, like TraA_ΔMXCT, and the presumed PTM therein. Additionally, ultracentrifugation experiments found the secreted version of WT TraA was not associated with OM vesicles and instead was a soluble extracellular protein (Fig. S1B).
Fig. 2.
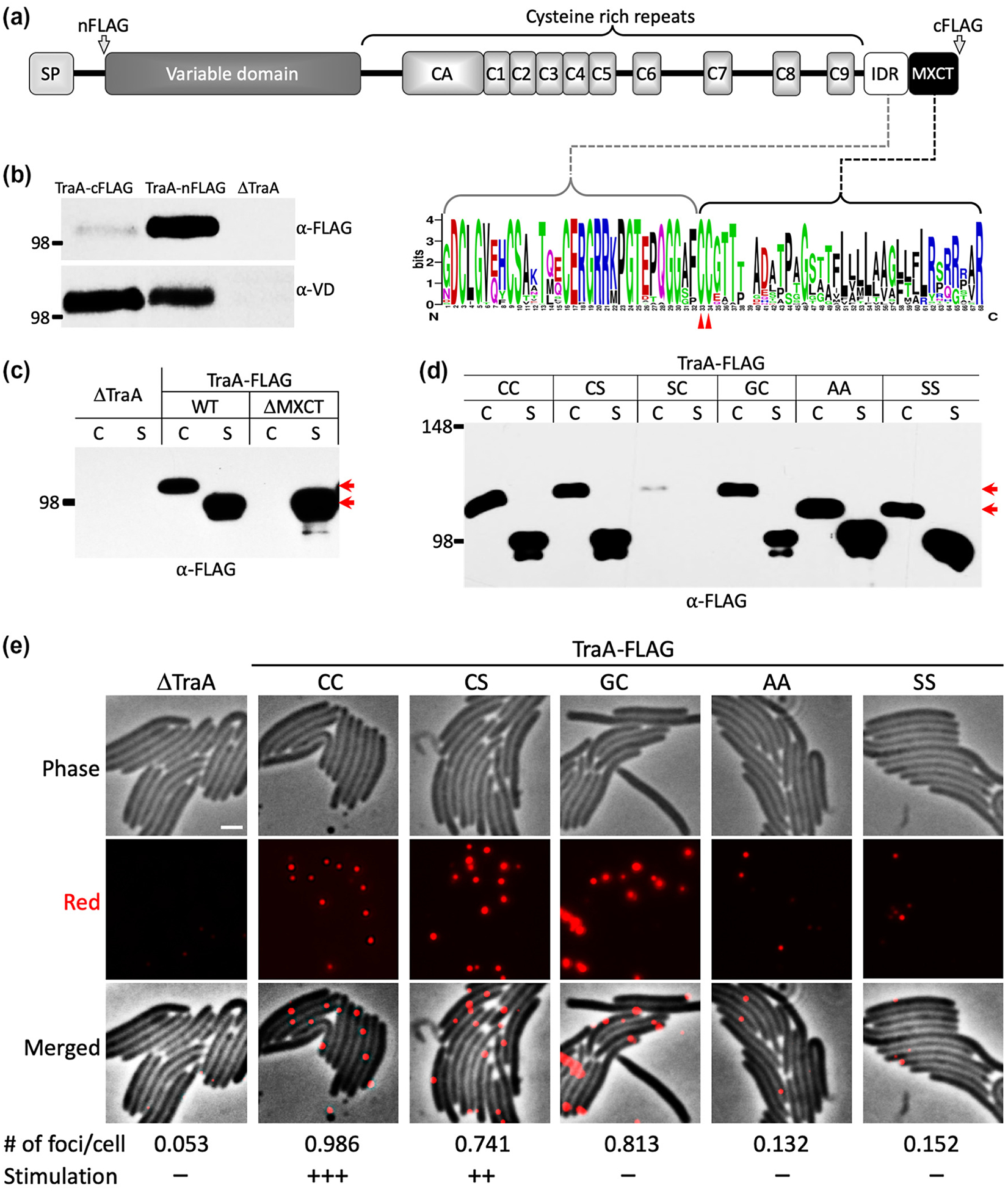
MYXO-CTERM is essential for TraA cell surface localization and conserved cysteines therein are the sites for PTM. (A) Domain architecture of TraA (MXAN_6895) and location of FLAG-tags (arrows). WebLogo (below) derived from 59 TraA sequences illustrates the IDR is conserved and is enriched with polar and charged residues. Red triangles mark the location of two invariant cysteines within the MYXO-CTERM. (B) TraA is processed at the C-terminus. Cell lysates with FLAG-tags engineered at the N- or C-termini were loaded with equal amounts in replicates and each half of the blot was probed with either anti-FLAG or anti-VD antibody. A ΔtraA mutant served as a negative control. Left, molecular weight marker, kDa. (C) Western blot of indicated constructs found in the cell ‘C’ and/or supernatant ‘S’ fractions. Red arrows mark size difference (~20 kDa) between C and S fractions of WT TraA. (D) Western blot of substitution mutants in C and S fractions. For better separation, an 8% SDS-PAGE was instead used and the relative migration of MW markers and TraA differed from other 10% gels. Top red arrow marks migration of WT TraA (CC) and bottom arrow marks migration of AA and SS mutants. The size difference corresponds to ~8 kDa. The SC mutant was not stable. (E) Live-cell immunofluorescence evaluating cell surface localization of wild type (CC) and mutant TraA proteins tagged with FLAG. As previously described (Cao & Wall, 2019), red foci indicate cell surface localized TraA detected with anti-VD primary antibodies (1:700) followed by Alexa Fluor 594-conjugated donkey anti-rabbit IgG secondary antibodies. Bottom, the average number of foci per cell, 150 cells counted for each strain. Plus (+) indicates activity while minus (–) indicates no activity of TraA variants for OME. See Figure S1A for details. Scale bar = 1 μm.
All 59 of the TraA proteins from the Cystobacterineae suborder contain tandem cysteines within their MYXO-CTERM, while other proteins typically contain GC at those positions (Figs. 1 and 2A). To test the idea that the invariant cysteine within the MYXO-CTERM was the site of PTM, we made TraA variants where either one or both of the cysteines were substituted with alanine, serine or glycine. Interestingly, by western analysis a migration difference, and hence molecular weight difference, was found between the WT (CC) and the double mutants (CC→SS or CC→AA) (Fig. 2D). This relatively large difference (~8 kDa) was unlikely caused by molecular weight differences from amino acid substitutions. Instead, we propose that the size differences represent a lack of PTM when both cysteines were replaced with alternative amino acids. However, consistent with TraA_ΔMXCT, there was no defect in protein secretion for any of these TraA substitution mutants, as indicated by equal levels of protein in supernatant fractions. Next, we tested whether the cell associated proteins were on the surface and functional. Live-cell immunofluorescence found that at least one cysteine was critical for surface localization because substitution of both cysteines diminished cell surface abundance (Fig. 2E). Curiously, the mutant that convert CC to the canonical GC MYXO-CTERM motif (Fig. 1) was not functional for OME, although it was localized on the cell surface. In contrast, the CC→CS mutant was functional and properly localized (Figs. 2E and S1A). Taken together these results suggest that either of these cysteines was sufficient for surface localization and PTM. However, the first cysteine was required for TraA function (Fig. S1A). Having shown that the CC residues play crucial roles in TraA localization/function, we asked whether the spacing (proximal or distal) around these residues was important. Here FLAG-tags were inserted immediately upstream or downstream of CC. Inserting an eight amino acid charged FLAG sequence immediately upstream of CC abolished TraA function, whereas inserting the same sequence immediately downstream resulted in partial activity (Fig. S2A). Interestingly, the constructs were found in both the cell and supernatant fractions like WT TraA (Fig. S2B)
IDR plays a role in periplasmic processing and sorting of TraA
A short region in TraA (32 aa; IDR in Fig. 2A) found immediately upstream of MYXO-CTERM is predicted to be disordered (Fig. S3) (Kozlowski & Bujnicki, 2012). Sequence alignments of 59 TraA proteins showed the IDR was well-conserved, implying it was important (Fig. 2A). To test the role of IDR in TraA maturation, an in-frame deletion (TraA_ΔIDR) was made. Western analysis showed a uniform distribution of TraA_ΔIDR in the cell and the supernatant fractions like WT TraA (Fig. 3A), however the mutant was nonfunctional for OME (Fig. S1A). Protease accessibility and live-cell immunofluorescence found that TraA_ΔIDR associated with the cell fraction was defective in surface localization (Figs. 3B–C). We conclude that TraA_ΔIDR has a processing defect and that a subpopulation that crossed the OM was poorly anchored to the cell surface, while the remaining fraction was apparently trapped in the periplasm.
Fig. 3.
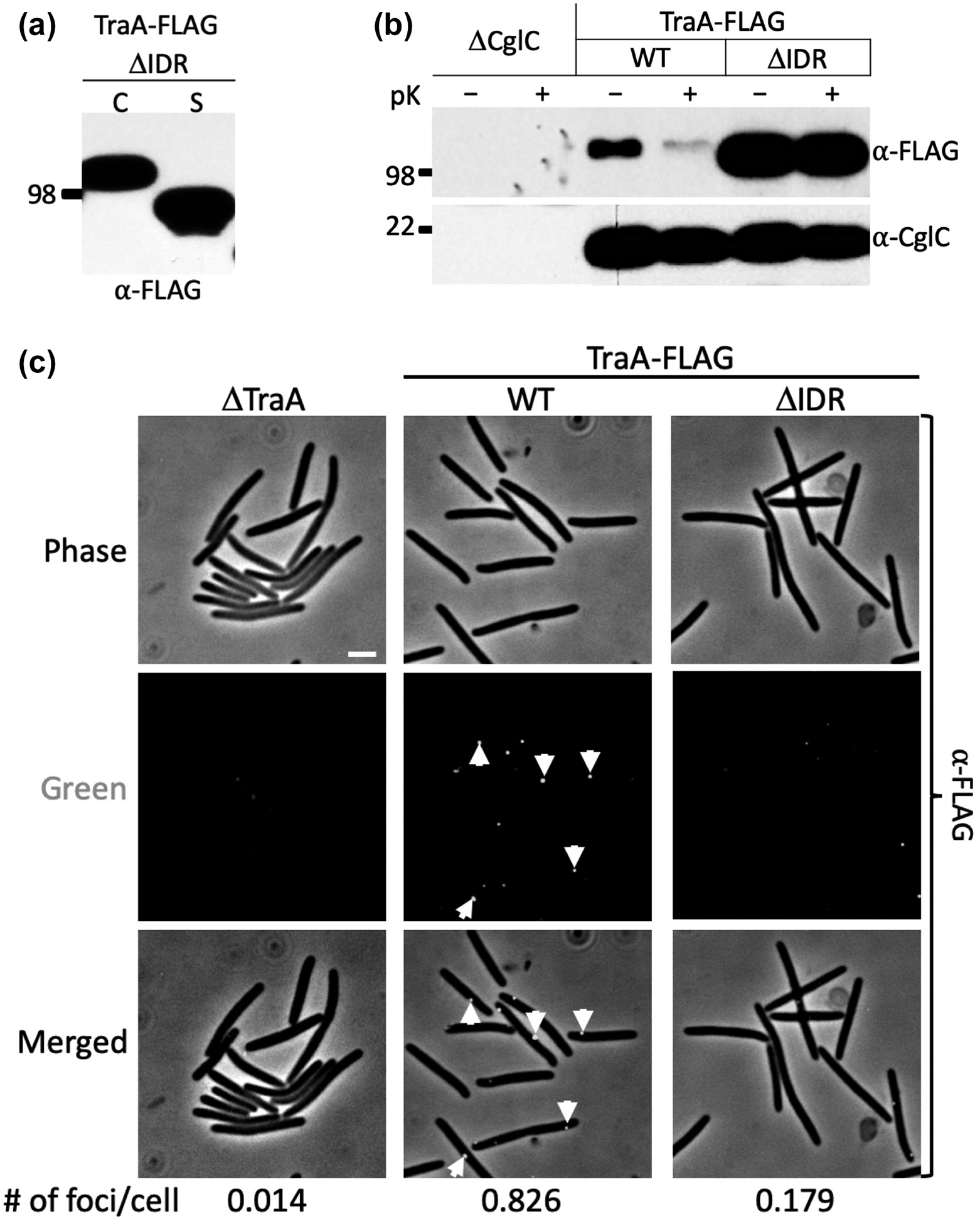
The role of IDR in cell surface localization of TraA. (A) Western blot shows TraA_ΔIDR is found in C and S fractions. (B) Protease accessibility assay reveals TraA_ΔIDR is blocked from the cell surface localization. Proteinase K (pK) treated (+) and untreated (–) cell samples. CglC, an inner leaflet OM lipoprotein (Cao & Wall, 2017), was used as a loading control for cell integrity during pK treatment. Blots were probed with indicated antibodies. (C) Live-cell immunofluorescence shows a reduction of TraA_ΔIDR from the cell surface. Cells were treated with anti-FLAG antibody followed by Alexa fluor 488-conjugated donkey anti-rabbit IgG. Green foci indicate cell surface localized TraA. Foci were quantified as in Figure 2E. Scale bar = 1 μm.
Cysteine-rich repeats (CRR) facilitate secretion across the OM and cell surface attachment
Following the VD in TraA, a conserved CRR contains 71 cysteine residues that includes nine imperfect repeats (TIGR04201; ~25 amino acid motif found in myxobacteria), that we previously postulated acts as a stalk for displaying the specificity domain (VD; aka PA14-like domain) on the cell surface (Pathak et al., 2012). In support of this, we recently showed that parts of the CRR were essential for TraA function (Cao et al., 2019). Here we hypothesized that CRR plays an additional role in the translocation of TraA across the OM. To test this, we made a series of in-frame deletions in TraA and tested their sub-cellular localization (Fig. 4A). From this analysis, constructs with a minimum of the CA-C2 region (i.e., ΔC3-C9 construct) crossed the OM but did not associate with the cell (Fig. 4B). We also found that all constructs lacking CA-C2 did not cross the OM (Figs. 4B–C and S4).
Fig. 4.
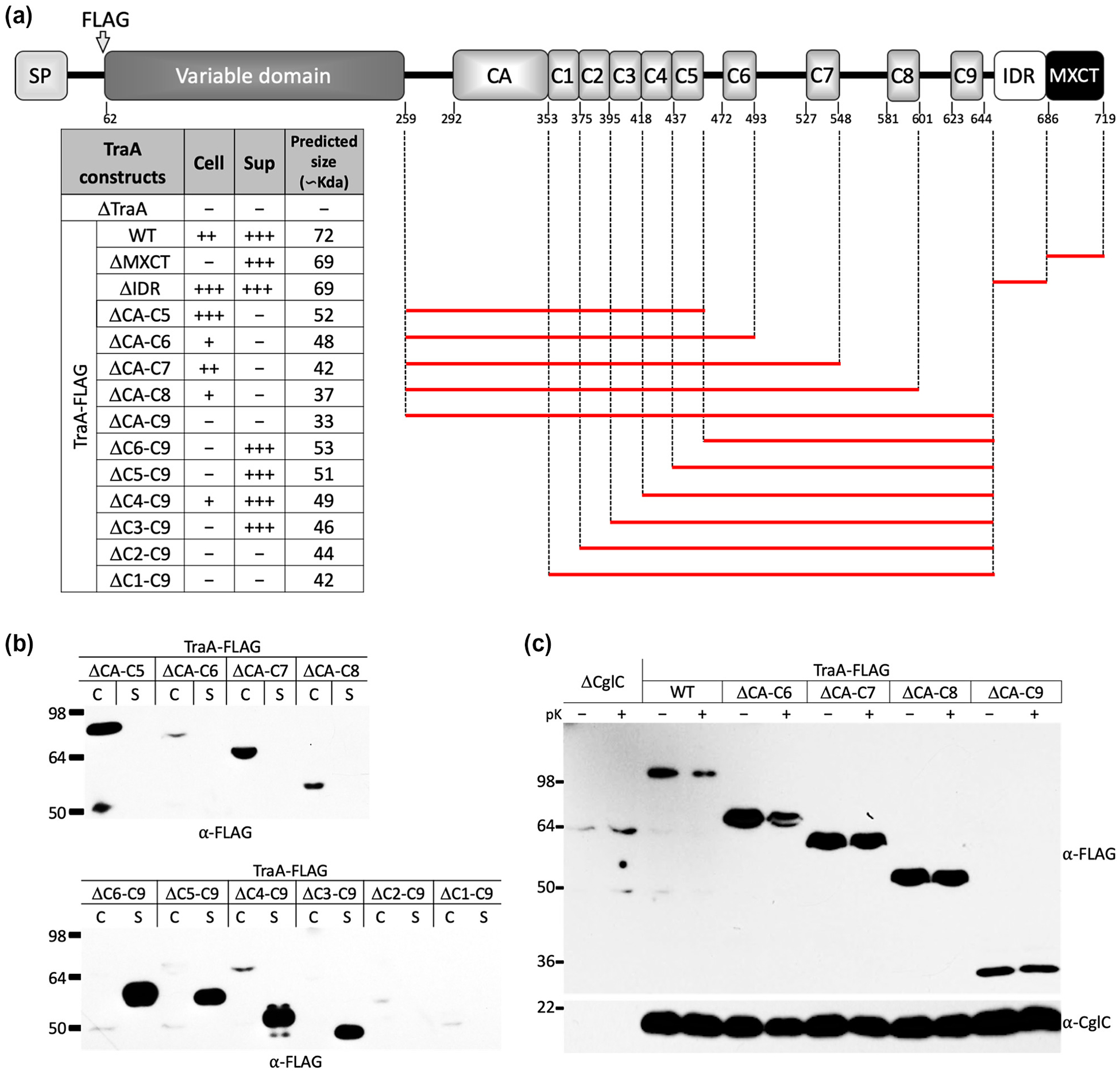
Structure-function analysis of TraA domains and their roles in sorting. (A) Graphical representation of TraA deletion constructs. Deleted regions are represented by red bars. All constructs contain a FLAG-tag. Left, table summarizes stability, localization and predicted molecular weight of constructs. Plus (+) indicates the presence and minus (–) indicates the absence of proteins from the corresponding fractions relative to WT TraA abundance. (B) Western blot of C and S fractions for TraA in-frame deletion constructs. Equivalent amount of cell material was loaded between samples. (C) Protease accessibility assay indicates a block in secretion of the mutant constructs.
Next, we sought to find a minimal region within CRR that facilitated TraA secretion across the OM. For this, we created two truncated versions of TraA fused with monomeric super-folder green fluorescent protein (msfGFP) (Fig. S5A). In the first chimera, msfGFP was fused with a ΔVD-C5 variant (TraAΔVD-C5-msfGFP), while the second chimera only had the VD removed (TraAΔVD-msfGFP). Western analysis found that both chimeras were expressed in the cell fraction at their predicted sizes (Fig. S5B), however only the TraAΔVD-msfGFP chimera was anchored to the cell surface as confirmed by live-cell immunofluorescence (Fig. S5C) and protease accessibility assays (Cao & Wall, 2019). Together these results indicate that the complete CRR is essential for secretion and cell surface attachment/stability of TraA.
MYXO-CTERM functions as a general sorting tag for cell surface localization
From proteomics studies, nine of the 34 MXYO-CTERM proteins from the M. xanthus laboratory strain (DK1622) were found to reside on the cell surface (Kahnt et al., 2010, Pathak et al., 2012), which excluded TraA, likely because of its low expression level. Since MYXO-CTERM is universally distributed across the Myxococcales order and every ORFs encoding this motif contains a SP, we predicted they all reside on the cell surface and share a common sorting pathway. To test this, we selected another uncharacterized MYXO-CTERM protein, MXAN_4924 (WP_011554902.1) for analysis, which shares sequence homology to TraA (Fig. 5A) and it was not detected in the mentioned proteomic study. Here we engineered a FLAG-tag in 4924 and did protease shaving and live-cell immunofluorescence experiments. In both assays, 4924 was found to reside on the cell surface (Fig. 5C–D). Next, we deleted the MYXO-CTERM (4924_ΔMXCT) and, as expected, 4924_ΔMXCT was not on the cell surface (Fig. 5D) and instead was exclusively found in the supernatant (Fig. 5B). Similar to TraA, a large size difference (~20 kDa) was found between the WT (4924) and the truncated version (4924_ΔMXCT) (Fig. 5B), again supporting the MYXO-CTERM was PTM and that TraA and 4924 share a common sorting pathway.
Fig. 5.
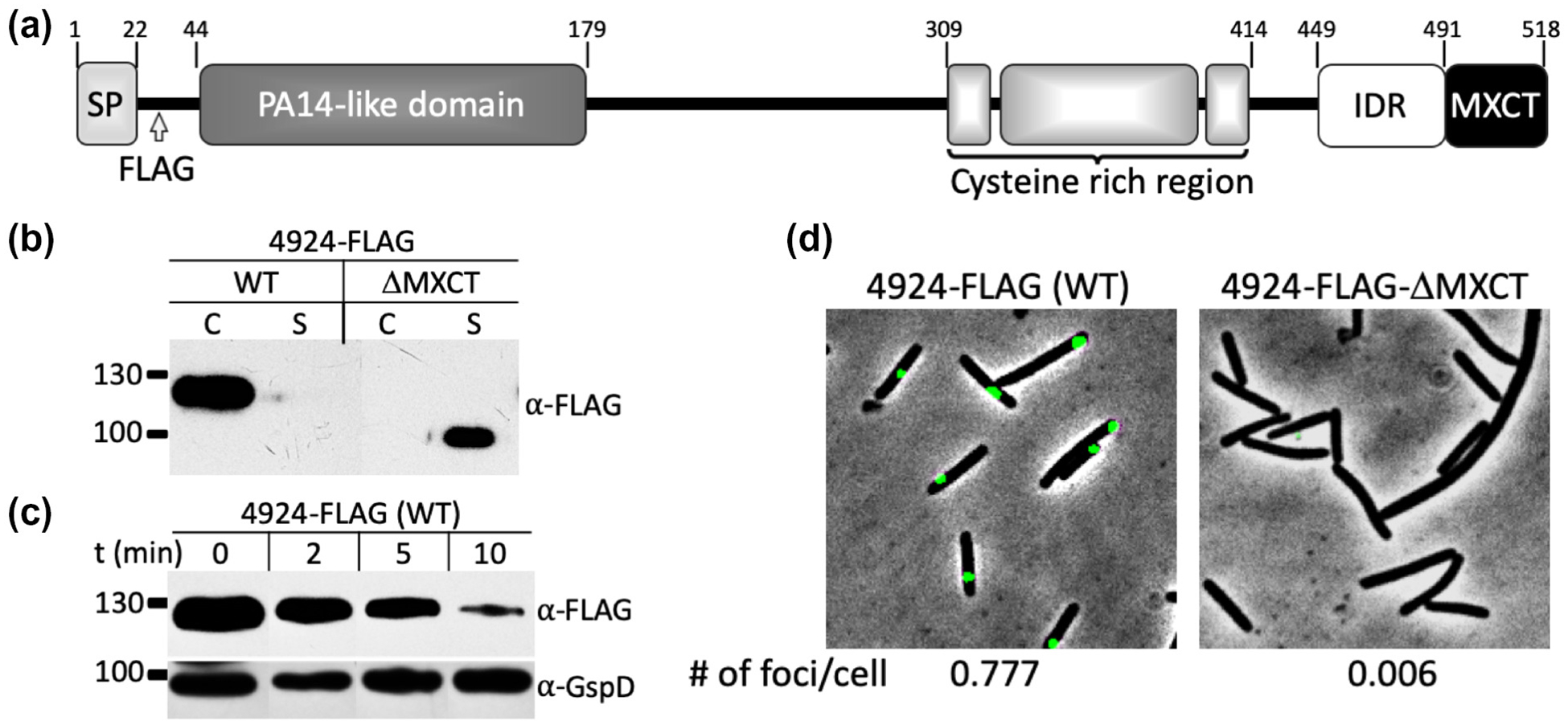
MYXO-CTERM in MXAN_4924 functions as a sorting tag. (A) Domain architecture of 4924. Numbers indicate domain boundaries. The PA14-like domain is a distant homolog of the VD from TraA and a cysteine rich region is different than the one in TraA. Arrow marks engineered FLAG-tag. (B) Western blot shows MYXO-CTERM is required for cell surface localization. Equal volumes of C and S fractions probed with anti-FLAG followed by HRP conjugated anti-rabbit-IgG secondary antibody. 4924_ΔMXCT is exclusively in the S fraction. (C) Protease accessibility assay indicates 4924-FLAG is cell surface localized. Cells were incubated with 5 μg/ml of pK for indicated times. GspD, an OM integral protein, was used as a loading and cell integrity control. Blot was treated with two primary antibodies: anti-FLAG antibody and affinity purified anti-GspD antibody followed by HRP conjugated anti-rabbit-IgG secondary antibody. (D) Live-cell immunofluorescence demonstrating cell surface localization of 4924 (WT). Cells were treated and foci were quantified as in Figure 2E. Scale bar = 1 μm.
MYXO-CTERM proteins are translocated across the OM by the T2SS
The majority of secreted proteins in Gram-negative bacteria follow the Sec pathway to cross the IM (Chatzi et al., 2014). Subsequent periplasmic proteins that are destined to cross the OM typically do so via the T2SS (Tsirigotaki et al., 2017). In addition, a recent study showed that GlyGly-CTERM proteins also use the T2SS (Gadwal et al., 2018). For these reasons we postulated that MYXO-CTERM proteins employ the T2SS to cross the OM. To test this, we used an M. xanthus gspD conditional knockdown mutant expressed under the control of the copper inducible promoter, PcuoA (Gomez-Santos et al., 2012) . GspD is a secretin that forms the OM embedded T2SS channel that is essential for M. xanthus viability (E. Hoiczyk & D. Zuckerman, personal communication). We transformed this strain with vectors that express TraA_ΔMXCT or 4924_ΔMXCT from the chromosome. The resulting strains were then tested for T2SS-dependent secretion of the mentioned proteins using the experimental design outlined in Figure 6A. Cells grown in the presence of copper maintained a constant level of GspD and of the secreted proteins (TraA_ΔMXCT and 4924_ΔMXCT) (Fig. 6B–C). In contrast, cells grown in the absence of copper showed a decrease in GspD levels and a corresponding block in TraA_ΔMXCT and 4924_ΔMXCT secretion. Furthermore, with TraA_ΔMXCT there was a corresponding build-up of the protein in the whole cell fraction indicating a secretion block (Fig. 6B). In the absence of copper, 4924_ΔMXCT was unstable, apparently because it was trapped in the periplasm (Fig. 6C). We conclude that MYXO-CTERM proteins cross the OM by the T2SS.
Fig. 6.
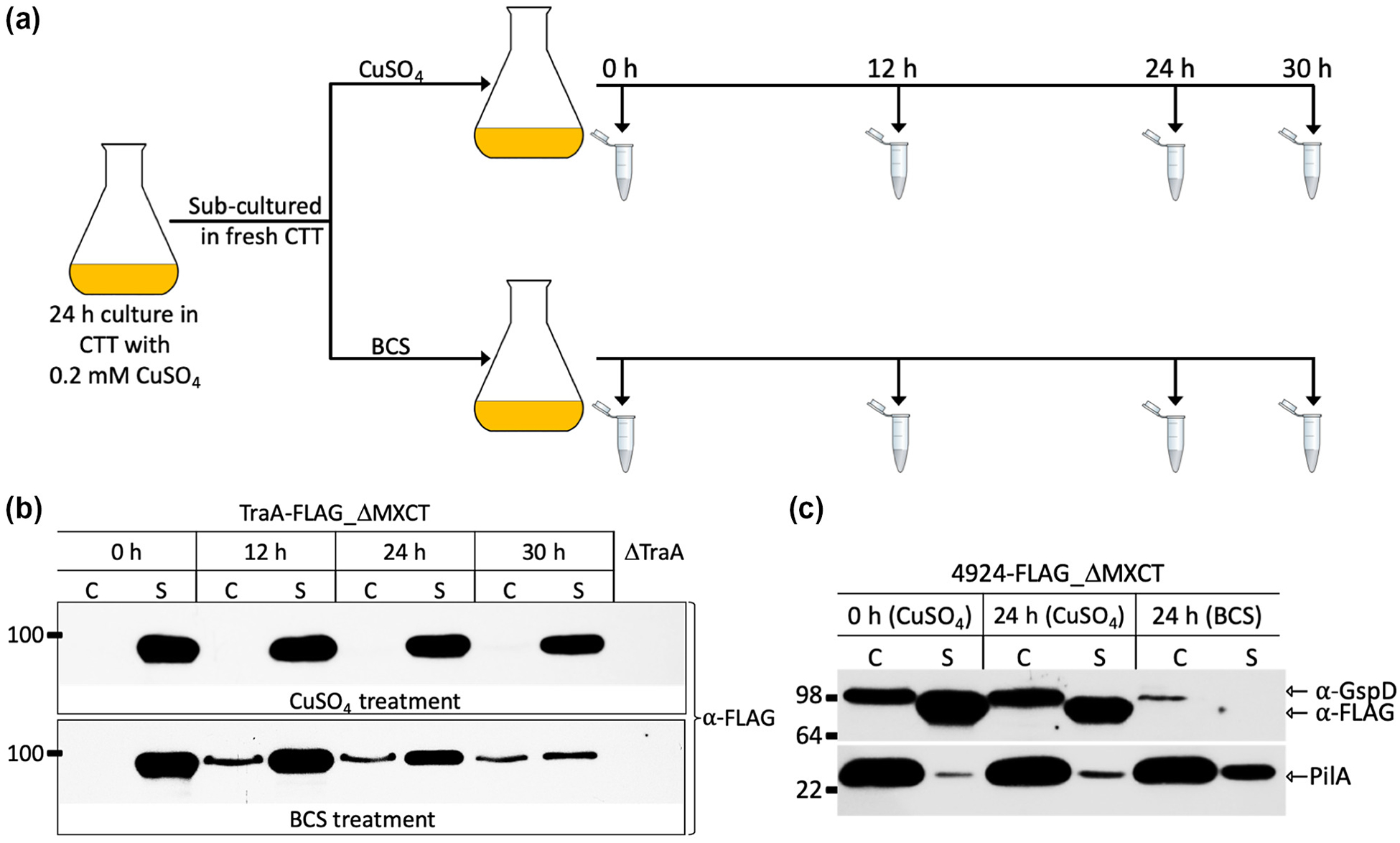
TraA and MXAN_4924 are secreted by the T2SS. (A) Schematic of GspD depletion assay. CuSO4 induces GspD expression and BCS (copper chelator) blocks GspD expression. (B) Western blot shows GspD dependent secretion of TraA_ΔMXCT. BCS culture accumulates TraA_ΔMXCT in C fraction indicating a secretion block. (C) Western blot shows 4924_ΔMXCT secretion depends on GspD expression. Cells grown in BCS have reduced levels of GspD and 4924_ ΔMXCT is absent from S fraction, suggesting a secretion block made it unstable. Equal amounts of cell materials were loaded, and blots were probed with anti-FLAG, anti-GspD and anti-PilA antibodies. PilA encodes the cell surface pilus that is secreted independently of T2SS, i.e. the type IV pilus system, and serves as a negative control for the GspD depletion assay.
Discussion
Key findings from this study are summarized in Figure 7A. These findings include that the MYXO-CTERM is cleaved from their respective proteins and that deleting this motif prevents proteins from being anchored to the cell surface, which instead are secreted into the extracellular milieu. Site directed mutagenesis suggested that at least one of the two tandem cysteines in the MYXO-CTERM is required for PTM and the first cysteine is required for TraA function. Removal of the IDR partly blocks TraA secretion and cell surface anchoring, perhaps again by interfering with PTM. We defined a region in TraA, CA-C2, which plays a crucial role in secretion across the OM. C6-C9 is required for stable attachment to the cell surface because removal of this region results in TraA being extracellular. Although C6-C9 is not found in other MYXO-CTERM proteins, our results indicate this region is required for PTM, perhaps by providing a structurally stable context for processing. Finally, we show that MYXO-CTERM proteins are transported across the OM by the T2SS.
Fig. 7.
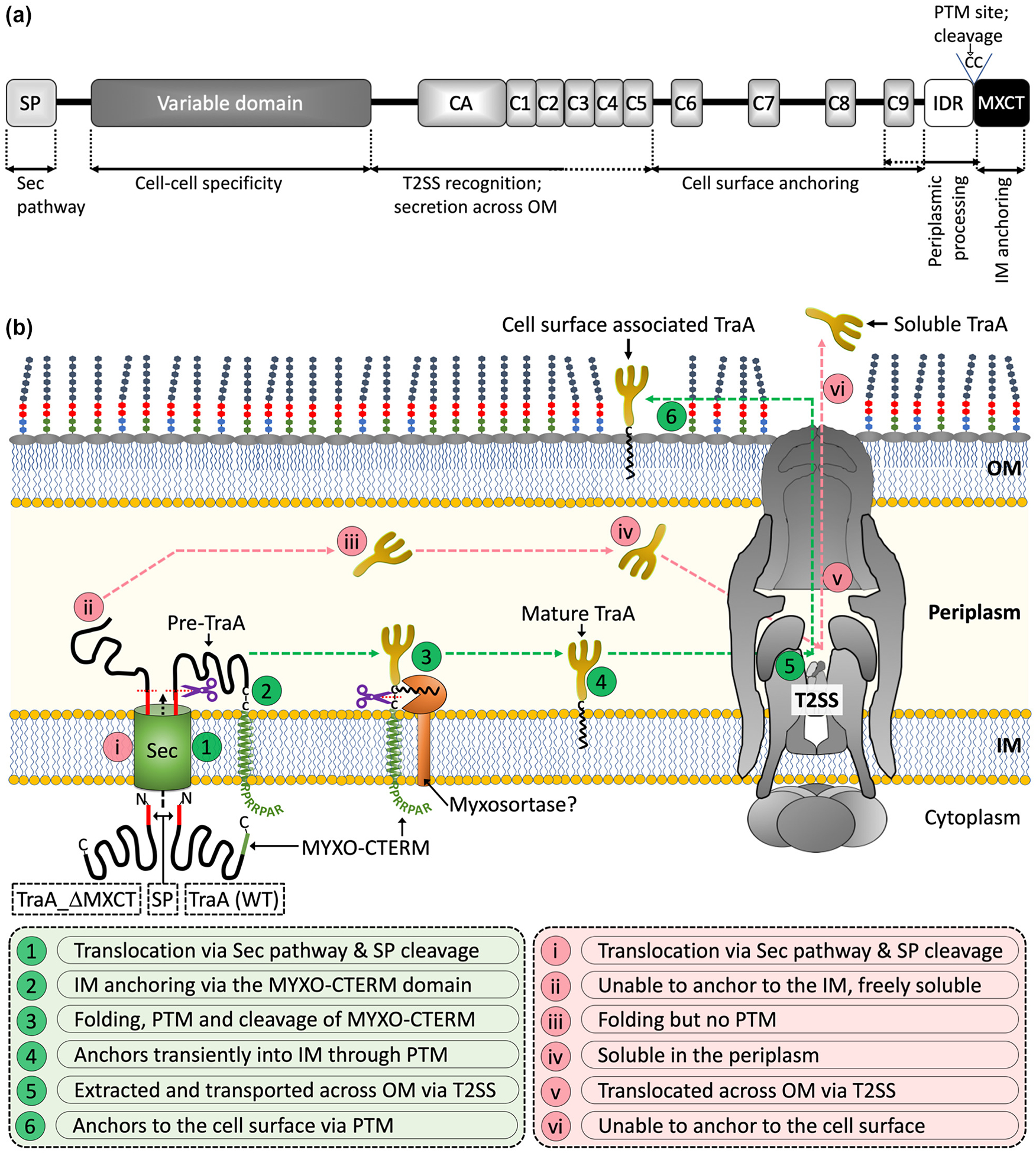
TraA and the MYXO-CTERM sorting pathway. (A) Domain architecture of TraA with assigned roles for function and sorting. (B) Working model of the MYXO-CTERM sorting pathway. Two different versions of TraA are shown. The steps for secretion and sorting are indicated and defined by numbers in green circles for WT TraA and roman numerals in light pink circles for TraA_ΔMXCT. See text for details.
Based on these results, we propose a working model for the sorting pathway of TraA and other MYXO-CTERM proteins (Fig. 7B). First, nascent TraA crosses the IM via its N-terminal SP and the Sec pathway followed by IM anchoring via the TMH within MYXO-CTERM. This intermediate anchoring provides a temporal and spatial step for protein folding and PTM. Based on analogous CTERM systems (Haft et al., 2006, Haft & Varghese, 2011, Haft et al., 2012), we propose an IM-associated (and still unidentified) myxosortase enzyme adds a lipid moiety(s) at one or both cysteines and then cleaves off the distal MYXO-CTERM sequence. The theorized myxosortase could be a single transpeptidase, like sortase from Gram-positives, which cleaves and transfers the bound cysteine to a lipid molecule. Alternatively, processing could involve two or more enzymes analogous to lipoprotein processing (Zuckert, 2014). In the model, once processed TraA remains associated with the IM via a proposed C-terminal lipid moiety until it is recognized by the T2SS. If TraA does not contain MYXO-CTERM, it is neither anchored in the IM nor modified and instead is a soluble periplasmic protein. Next, the T2SS loads IM bound as well as soluble versions of TraA for transport across the OM. Our working model is also analogous to lipoprotein transport (Pugsley et al., 1986, Baldi et al., 2012, East et al., 2016). Following secretion, the fate of TraA, surface attachment or extracellular milieu, is determined by its PTM or lack thereof.
Within the suborder Cystobacterineae, every TraA contains tandem cysteines (CC) within their MYXO-CTERM, as opposed to the GC dipeptide found in many members of TIGR03901. In the case of GC, a single cysteine suggests that PTM occurs on the sole cysteine residue and that is sufficient to anchor those proteins, e.g. enzymes and adhesins, on the cell surface. In contrast, TraA has tandem cysteines and consequently may be modified on both residues. In turn, this may provide a more robust anchoring mechanism that facilitates TraA function as a fusogen to catalyze OME (Pathak et al., 2012, Cao & Wall, 2019).
The IDR in TraA is located immediately upstream of MYXO-CTERM and, according to the SPOT-Disorder2 server (Hanson et al., 2018), nearly all of the 34 M. xanthus proteins contain divergent IDR sequences immediately preceding their respective MYXO-CTERM motifs. This suggests that IDRs play a general and important role in PTM and sorting. In this regard IDRs could serve as a flexible handle for PTM within the MYXO-CTERM. Similarly, the PulA lipoprotein contains an IDR immediately after its lipobox that promotes PulA binding to the IM and translocation across the OM by the T2SS (East et al., 2016). By analogy, the IDR in TraA could play a similar role.
MYXO-CTERM tripartite structure and protein sorting function is analogous to other C-terminal sorting tags such as PEP-CTERM and GlyGly-CTERM from Gram-negative bacteria. Among these, MYXO-CTERM and GlyGly-CTERM are both secreted across the OM through the T2SS and PTM involves apparent lipidation and motif cleavage (Gadwal et al., 2018). However, the shorter GlyGly-CTERM motif (~22 residues-long) is processed by an intramembrane rhombosortase, while the longer MYXO-CTERM motif (33 residues) extends the GC/CC dipeptide out into the periplasm where myxosortase is thought to process it. Consistent with this view, we showed that the sequence context around CC residues is important. For instance, inserting a short linker immediate downstream of CC diminished TraA OME activity significantly, while addition of the same linker immediately upstream abolished function. In the first case, the inserted linker is predicted to move the processing site farther from the membrane, which may reduce the efficiency of PTM. In the second case, by inserting an upstream linker, the IDR is no longer adjacent to CC and TraA OME function was abolished.
The first five repeats within the CRR are in tandem (Fig. 7A) and based on I-TASSER (Zhang, 2008), are predicted to form a compact 3D fold. Recently we showed that deleting any of the CRR from C5-C9 rendered TraA nonfunctional for OME (Cao et al., 2019). Here we show that C6-C9 are essential for stable cell surface attachment and therefore provides an explanation for why these repeats are essential for TraA function. Upstream of the IDR in TraA is C9, which is essential for cell surface anchoring because all constructs lacking C9 were secreted. For this reason, C9 may contribute to PTM and anchoring TraA to the cell surface. We also note that the ΔCA-C9 construct migrated at its predicted molecular size (~35 kDa) while all other cell associated TraA variants that retained C9 migrated at much higher mobilities than their predicted molecular sizes (Fig. 4C). This suggests that C9 is critical for the high mobility behavior of TraA, which could be caused by intrinsic properties of its sequence and/or that C9 plays a key role in processing and PTM.
Sorting of cell surface proteins in Gram-negative bacteria is an emerging field and in a significant number of cases depends on a variety of C-terminal sorting tags. Although these tags vary in their specific sequences there are common themes in their architecture and processing. Like other C-terminal sorting tags, we describe a sorting tag that is only found in one group of bacteria. Here, MYXO-CTERM is universally distributed in Myxococcales, where most genomes have dozens of proteins with this motif. Since myxobacteria have complex intercellular interactions with siblings, non-siblings and prey cells, understanding the sorting of their surface proteins will provide important insights into their social behaviors. Future studies are needed to address the chemical nature of the PTM and to identify the myxosortase enzyme(s) responsible for processing.
Experimental procedures
Bacterial strains and growth conditions
Bacterial strains and plasmids used in this study are listed in Table S1. M. xanthus was routinely cultured in the dark at 33°C with continuous shaking in CTT medium (1% Casitone, 1 mM KH2PO4, 8 mM MgSO4, 10 mM Tris-HCl [pH 7.6]). For stimulation assays CTT agar with 0.5% Casitone (½ CTT) and 2 mM CaCl2 was used. TPM buffer (10 mM Tris [pH 7.6], 1 mM KH2PO4 and 8 mM MgSO4) was used to wash cells. E. coli was cultured at 37°C with shaking in LB medium. For plates, 1.5% agar (w/v) was added to the respective media. Media were supplemented with 50 μg/ml of kanamycin (Km) for both M. xanthus and E. coli when necessary. For GspD depletion assay, cells were grown in CTT medium with Km and supplemented with 0.2 mM CuSO4 or 0.2 mM bathocuproine sulfonate (BCS) as needed.
Plasmids and strains construction
Plasmids and primers used in this study are listed in Table S2 respectively. TraA in-frame truncation were made by deleting internal regions and tagging them with FLAG at the N-terminus. FLAG-tag was added between SP and VD (after the 60th residue from the N-terminus). Briefly, the strong pilA promoter (PpilA), traA fragments with FLAG-tag were PCR amplified. A vector with the Mx8 attachment site (pDP22) was linearized with EcoRI and XbaI and the fragments containing ~25 bp overlaps were ligated using the Gibson Assembly Master Mix (New England Biolabs). To create msfGFP-TraA fusions, msfGFP was fused with two separate version of traA: traAΔVD-C5 and traAΔVD, using Gibson assembly. To create CC point mutations in TraA, primers with substituted bases were used for PCR amplification. MXAN_4924 was FLAG-tagged (4924-FLAG) by inserting the FLAG sequence after the SP (22nd residue) and cloning it between XbaI and HindIII site in pDP22. A similar approach was used to create a MYXO-CTERM truncated version of 4924 (4924_ΔMXCT). M. xanthus GspD conditional mutants for secreted version of TraA and 4924 were created by transforming pDP22 based plasmid with either traA_ΔMXCT or 4924_ΔMXCT into EH098 (ΔgspD, PcuoA-gspD). All plasmids were verified by PCR, restriction digestion and DNA sequencing before transforming into M. xanthus and then selected on CTT-Km plates. All the constructs were integrated into the Mx8 attachment site and were stably expressed.
MYXO-CTERM bioinformatics analysis
A complete list of TIGR03901 was populated using the IMG database (Nordberg et al., 2014). For this study, we only included the genomes from the order Myxococcales since our analysis found other hits were false positives or belonged to other TIGRfams. Within the gene cart the list was filtered by peptide length (>100 amino acids) and the presence of a SP. This generated 955 genes. Genes that contained other (similar) CTERM TIGRfams were removed leaving 747 genes that were used for alignments with Jalview (Waterhouse et al., 2009). After a manual curation of the genes, we binned a total of 704 into three suborders and a catchall unclassified Myxococcales suborder. Weblogos (Crooks et al., 2004) were generated to highlight sequence conservation.
Stimulation assay
Stimulation (extracellular complementation) assays were performed as described (Cao & Wall, 2017). Briefly, cells were grown to mid-log phase in CTT, harvested by centrifugation and resuspended in TPM buffer to a calculated density of ~2.5×109 cells per ml. Nonmotile donor strains, which themselves cannot be stimulated, contained different TraA constructs (DW1467, DW2288, DW2289, DW2500, DW2513, DW2514, DW2515, DW2517, DW2520 and DW2521) were mixed 1:1 with a nonmotile recipient strain (DW1466) that could be simulated and 5 μL were spotted on ½ CTT agar with 2 mM CaCl2. See Table S1 for strain details. Stimulation was confirmed by flares of cells moving out of the colony edges. Following overnight incubation at 33°C, colony edges were imaged with a Nikon E800 phase contrast 10× objective lens.
Protease Accessibility Assay
The assay was performed as described (Cao & Wall, 2017) with changes. Briefly, exponentially grown cells in CTT were directly incubated with 100 μg/ml of pK (New England Biolabs) in an Eppendorf tube at 33°C with gentle shaking for 15 min. Complete EDTA-free protease inhibitor cocktail (Roche) was added to a final concentration of 1× to stop the reaction and centrifuged at 8000 × g for 2 min to pellet cells. Next, cells were washed with TPM containing 1× protease inhibitor and centrifuged. Finally, the cell pellet was resuspended in SDS lysis buffer containing 1× protease inhibitor cocktail and boiled for 15 min to inactivate pK. For each sample, a negative control was included where TPM was added in lieu of pK. Cell lysate were analyzed by immunoblots.
Live-cell immunofluorescence assay
Live-cell immunofluorescence was done as described (Pathak et al., 2013) with modifications. Briefly, cells were grown in CTT overnight to mid-log phase and harvested at 8000 × g for 2 min. Cell pellets were washed once in TPM and then ~ 9×108 cells were incubated in 1 ml of TPM with 2% BSA for 30 min. Following incubation, primary antibody (1:500 dilution) was added and further incubated for 30 min. Cell pellets were then resuspended in TPM containing 2% BSA with a fluorophore conjugated secondary antibody (1:300; Alexa Fluor 488-conjugated donkey anti-rabbit IgG or Alexa Fluor 594-conjugated donkey anti-rabbit IgG; Jackson ImmunoResearch) and incubated for 30 min in the dark. All the incubations were done at room temperature (RT) with gentle end-to-end rocking. Finally, cells were washed four times in TPM and spotted on 1% agarose pads (w/v) in TPM and imaged using a phase contrast 100× objective lens on a Nikon E800 fluorescence microscope.
Isolation of outer membrane vesicles (OMVs)
OMVs were isolated using the protocol as described earlier (Kahnt et al., 2010). Briefly, cells were harvested using an overnight culture in CTT to a density of 5×108 cells per ml. Culture supernatant was used for the isolation of vesicles. Culture supernatants were passed through a 0.2 μm syringe filter to separate cell debris from the supernatant. The resulting filtrate (S) was centrifuged at 150,000 × g for 2 h at 4 °C to recover OMVs. The supernatant (UC-S) was carefully removed, and the vesicle pellet was resuspended in 50 mM Tris-HCl, pH 8.0. Proteins in S and UC-S fractions were recovered by TCA precipitation method as described earlier. All the three fractions were resuspended in 1× SDS sample buffer to a comparable density and boiled for 10 min before loading into SDS gel.
Western blotting
Cells grown overnight in CTT to mid-log phase were harvested and washed twice in TPM. Pellets were resuspended in 1× SDS sample buffer to a density of 4.5×109 cells/ml. Proteins in the spent media were precipitated as described (Koontz, 2014). In brief, 1 ml supernatant was incubated with 250 μl of 100% TCA on ice for 30 min. Following centrifugation at 16,000 × g, supernatant was carefully aspirated, and pellets were washed twice with 500 μl of ice-cold acetone and dried at RT. Finally, pellets were resuspended in 1× SDS sample buffer to a density equivalent to their respective cell samples. Samples were boiled for 10 min before loading into SDS gel. Western blot assay was performed using standard protocols (Sambrook et al., 2006). Protein samples were separated by SDS-PAGE and then transferred onto PVDF membranes. 10% acrylamide gels were used unless stated otherwise. A 5% skimmed milk solution in TBST was used for blocking overnight at 4°C with gentle shaking. Primary rabbit antibodies were used at their respective concentrations: α-FLAG (1:5,000 dilution; Sigma-Aldrich), α-GspD (1:5,000 dilution), α-GFP (1:30,000 dilution; Invitrogen) and α-VD (1:30,000 dilution; (Pathak et al., 2013)) and α-CglC (1:15,000 dilution; (Cao & Wall, 2017)). For detection, HRP conjugated goat anti-rabbit secondary antibody was used (1:20,000; Pierce). Blots were washed three times for 15 min each with TBST following each antibody treatment. Finally, blots were developed using SuperSignal West Pico Plus chemiluminescent substrate (Thermo Scientific).
Supplementary Material
Acknowledgments
We are grateful to Egbert Hoiczyk and David Zuckerman for providing a gspD conditional expression strain and antibodies to GspD. This work was support by NIH grant GM101449 to D.W.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon request.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Abdul Halim MF, Pfeiffer F, Zou J, Frisch A, Haft D, Wu S, Tolic N, Brewer H, Payne SH, Pasa-Tolic L, and Pohlschroder M (2013) Haloferax volcanii archaeosortase is required for motility, mating, and C-terminal processing of the S-layer glycoprotein. Mol Microbiol 88: 1164–1175. [DOI] [PubMed] [Google Scholar]
- Baldi DL, Higginson EE, Hocking DM, Praszkier J, Cavaliere R, James CE, Bennett-Wood V, Azzopardi KI, Turnbull L, Lithgow T, Robins-Browne RM, Whitchurch CB, and Tauschek M (2012) The type II secretion system and its ubiquitous lipoprotein substrate, SslE, are required for biofilm formation and virulence of enteropathogenic Escherichia coli. Infect Immun 80: 2042–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao P, Dey A, Vassallo CN, and Wall D (2015) How Myxobacteria Cooperate. J Mol Biol 427: 3709–3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao P, and Wall D (2017) Self-identity reprogrammed by a single residue switch in a cell surface receptor of a social bacterium. Proc Natl Acad Sci U S A 114: 3732–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao P, Wei X, Awal RP, Muller R, and Wall D (2019) A Highly Polymorphic Receptor Governs Many Distinct Self-Recognition Types within the Myxococcales Order. MBio 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao PB, and Wall D (2019) Direct visualization of a molecular handshake that governs kin recognition and tissue formation in myxobacteria. Nature Communications 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzi KE, Sardis MF, Economou A, and Karamanou S (2014) SecA-mediated targeting and translocation of secretory proteins. Biochim Biophys Acta 1843: 1466–1474. [DOI] [PubMed] [Google Scholar]
- Crooks GE, Hon G, Chandonia JM, and Brenner SE (2004) WebLogo: a sequence logo generator. Genome Res 14: 1188–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Enfert C, Chapon C, and Pugsley AP (1987) Export and secretion of the lipoprotein pullulanase by Klebsiella pneumoniae. Mol Microbiol 1: 107–116. [DOI] [PubMed] [Google Scholar]
- Dey A, Vassallo CN, Conklin AC, Pathak DT, Troselj V, and Wall D (2016) Sibling Rivalry in Myxococcus xanthus Is Mediated by Kin Recognition and a Polyploid Prophage. Journal of Bacteriology 198: 994–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East A, Mechaly AE, Huysmans GHM, Bernarde C, Tello-Manigne D, Nadeau N, Pugsley AP, Buschiazzo A, Alzari PM, Bond PJ, and Francetic O (2016) Structural Basis of Pullulanase Membrane Binding and Secretion Revealed by X-Ray Crystallography, Molecular Dynamics and Biochemical Analysis. Structure 24: 92–104. [DOI] [PubMed] [Google Scholar]
- Gadwal S, Johnson TL, Remmer H, and Sandkvist M (2018) C-terminal processing of GlyGly-CTERM containing proteins by rhombosortase in Vibrio cholerae. PLoS Pathog 14: e1007341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Santos N, Treuner-Lange A, Moraleda-Munoz A, Garcia-Bravo E, Garcia-Hernandez R, Martinez-Cayuela M, Perez J, Sogaard-Andersen L, and Munoz-Dorado J (2012) Comprehensive set of integrative plasmid vectors for copper-inducible gene expression in Myxococcus xanthus. Appl Environ Microbiol 78: 2515–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorasia DG, Veith PD, Chen D, Seers CA, Mitchell HA, Chen YY, Glew MD, Dashper SG, and Reynolds EC (2015) Porphyromonas gingivalis Type IX Secretion Substrates Are Cleaved and Modified by a Sortase-Like Mechanism. PLoS Pathog 11: e1005152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haft DH, Paulsen IT, Ward N, and Selengut JD (2006) Exopolysaccharide-associated protein sorting in environmental organisms: the PEP-CTERM/EpsH system. Application of a novel phylogenetic profiling heuristic. BMC Biol 4: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haft DH, Payne SH, and Selengut JD (2012) Archaeosortases and exosortases are widely distributed systems linking membrane transit with posttranslational modification. J Bacteriol 194: 36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haft DH, and Varghese N (2011) GlyGly-CTERM and rhombosortase: a C-terminal protein processing signal in a many-to-one pairing with a rhomboid family intramembrane serine protease. PLoS One 6: e28886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J, Paliwal K, and Zhou Y (2018) Accurate Single-Sequence Prediction of Protein Intrinsic Disorder by an Ensemble of Deep Recurrent and Convolutional Architectures. J Chem Inf Model 58: 2369–2376. [DOI] [PubMed] [Google Scholar]
- Hara T, Matsuyama S, and Tokuda H (2003) Mechanism underlying the inner membrane retention of Escherichia coli lipoproteins caused by Lol avoidance signals. J Biol Chem 278: 40408–40414. [DOI] [PubMed] [Google Scholar]
- Jacob-Dubuisson F, Locht C, and Antoine R (2001) Two-partner secretion in Gram-negative bacteria: a thrifty, specific pathway for large virulence proteins. Molecular Microbiology 40: 306–313. [DOI] [PubMed] [Google Scholar]
- Kahnt J, Aguiluz K, Koch J, Treuner-Lange A, Konovalova A, Huntley S, Hoppert M, Sogaard-Andersen L, and Hedderich R (2010) Profiling the outer membrane proteome during growth and development of the social bacterium Myxococcus xanthus by selective biotinylation and analyses of outer membrane vesicles. J Proteome Res 9: 5197–5208. [DOI] [PubMed] [Google Scholar]
- Kandiba L, Aitio O, Helin J, Guan Z, Permi P, Bamford DH, Eichler J, and Roine E (2012) Diversity in prokaryotic glycosylation: an archaeal-derived N-linked glycan contains legionaminic acid. Mol Microbiol 84: 578–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharade SS, and McBride MJ (2015) Flavobacterium johnsoniae PorV is required for secretion of a subset of proteins targeted to the type IX secretion system. J Bacteriol 197: 147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi A, Sagami H, and Ogura K (1999) Evidence for covalent attachment of diphytanylglyceryl phosphate to the cell-surface glycoprotein of Halobacterium halobium. J Biol Chem 274: 18011–18016. [DOI] [PubMed] [Google Scholar]
- Konrad Z, and Eichler J (2002) Lipid modification of proteins in Archaea: attachment of a mevalonic acid-based lipid moiety to the surface-layer glycoprotein of Haloferax volcanii follows protein translocation. Biochem J 366: 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koontz L (2014) TCA precipitation. Methods Enzymol 541: 3–10. [DOI] [PubMed] [Google Scholar]
- Kozlowski LP, and Bujnicki JM (2012) MetaDisorder: a meta-server for the prediction of intrinsic disorder in proteins. BMC Bioinformatics 13: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian SK, Liu G, Ton-That H, and Schneewind O (1999) Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science 285: 760–763. [DOI] [PubMed] [Google Scholar]
- McBride MJ, and Zhu Y (2013) Gliding motility and Por secretion system genes are widespread among members of the phylum bacteroidetes. J Bacteriol 195: 270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarre WW, and Schneewind O (1994) Proteolytic cleavage and cell wall anchoring at the LPXTG motif of surface proteins in gram-positive bacteria. Mol Microbiol 14: 115–121. [DOI] [PubMed] [Google Scholar]
- Nguyen KA, Travis J, and Potempa J (2007) Does the importance of the C-terminal residues in the maturation of RgpB from Porphyromonas gingivalis reveal a novel mechanism for protein export in a subgroup of Gram-Negative bacteria? J Bacteriol 189: 833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordberg H, Cantor M, Dusheyko S, Hua S, Poliakov A, Shabalov I, Smirnova T, Grigoriev IV, and Dubchak I (2014) The genome portal of the Department of Energy Joint Genome Institute: 2014 updates. Nucleic Acids Res 42: D26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paramonov N, Rangarajan M, Hashim A, Gallagher A, Aduse-Opoku J, Slaney JM, Hounsell E, and Curtis MA (2005) Structural analysis of a novel anionic polysaccharide from Porphyromonas gingivalis strain W50 related to Arg-gingipain glycans. Mol Microbiol 58: 847–863. [DOI] [PubMed] [Google Scholar]
- Pathak DT, Wei X, Bucuvalas A, Haft DH, Gerloff DL, and Wall D (2012) Cell contact-dependent outer membrane exchange in myxobacteria: genetic determinants and mechanism. PLoS Genet 8: e1002626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak DT, Wei X, Dey A, and Wall D (2013) Molecular recognition by a polymorphic cell surface receptor governs cooperative behaviors in bacteria. PLoS Genet 9: e1003891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poquet I, Faucher D, and Pugsley AP (1993) Stable periplasmic secretion intermediate in the general secretory pathway of Escherichia coli. EMBO J 12: 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley AP (1993) The complete general secretory pathway in gram-negative bacteria. Microbiol Rev 57: 50–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley AP, Chapon C, and Schwartz M (1986) Extracellular pullulanase of Klebsiella pneumoniae is a lipoprotein. J Bacteriol 166: 1083–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Russell DW, and Sambrook J, (2006) The condensed protocols from Molecular cloning : a laboratory manual, p. v, 800 p. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [Google Scholar]
- Sato K, Naito M, Yukitake H, Hirakawa H, Shoji M, McBride MJ, Rhodes RG, and Nakayama K (2010) A protein secretion system linked to bacteroidete gliding motility and pathogenesis. Proc Natl Acad Sci U S A 107: 276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Yukitake H, Narita Y, Shoji M, Naito M, and Nakayama K (2013) Identification of Porphyromonas gingivalis proteins secreted by the Por secretion system. FEMS Microbiol Lett 338: 68–76. [DOI] [PubMed] [Google Scholar]
- Schneewind O, Mihaylova-Petkov D, and Model P (1993) Cell wall sorting signals in surface proteins of gram-positive bacteria. EMBO J 12: 4803–4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seers CA, Slakeski N, Veith PD, Nikolof T, Chen YY, Dashper SG, and Reynolds EC (2006) The RgpB C-terminal domain has a role in attachment of RgpB to the outer membrane and belongs to a novel C-terminal-domain family found in Porphyromonas gingivalis. J Bacteriol 188: 6376–6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji M, Sato K, Yukitake H, Kondo Y, Narita Y, Kadowaki T, Naito M, and Nakayama K (2011) Por secretion system-dependent secretion and glycosylation of Porphyromonas gingivalis hemin-binding protein 35. PLoS One 6: e21372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy TJ, Kahne D, and Walker S (2010) The bacterial cell envelope. Cold Spring Harb Perspect Biol 2: a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton-That H, Liu G, Mazmanian SK, Faull KF, and Schneewind O (1999) Purification and characterization of sortase, the transpeptidase that cleaves surface proteins of Staphylococcus aureus at the LPXTG motif. Proc Natl Acad Sci U S A 96: 12424–12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsirigotaki A, De Geyter J, Sostaric N, Economou A, and Karamanou S (2017) Protein export through the bacterial Sec pathway. Nature Reviews Microbiology 15: 21–36. [DOI] [PubMed] [Google Scholar]
- Veith PD, Nor Muhammad NA, Dashper SG, Likic VA, Gorasia DG, Chen D, Byrne SJ, Catmull DV, and Reynolds EC (2013) Protein substrates of a novel secretion system are numerous in the Bacteroidetes phylum and have in common a cleavable C-terminal secretion signal, extensive post-translational modification, and cell-surface attachment. J Proteome Res 12: 4449–4461. [DOI] [PubMed] [Google Scholar]
- Wall D, and Kaiser D (1998) Alignment enhances the cell-to-cell transfer of pilus phenotype. Proc Natl Acad Sci U S A 95: 3054–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse AM, Procter JB, Martin DM, Clamp M, and Barton GJ (2009) Jalview Version 2--a multiple sequence alignment editor and analysis workbench. Bioinformatics 25: 1189–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y (2008) I-TASSER server for protein 3D structure prediction. BMC Bioinformatics 9: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckert WR (2014) Secretion of bacterial lipoproteins: through the cytoplasmic membrane, the periplasm and beyond. Biochim Biophys Acta 1843: 1509–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


