Abstract
Transthyretin amyloid cardiomyopathy (ATTR-CM) continues to be an easily overlooked, life-threatening, yet treatable cause of heart failure. Furthermore, its elusive diagnosis leads to late or misdiagnosis. As therapeutic advancements such as tafamidis usher in a promising new era in the management of ATTR-CM, the need for disease awareness and efficient diagnostic evaluation is crucial. With newer inexpensive imaging modalities and techniques, such as longitudinal strain imaging, T1 mapping on cardiac magnetic resonance imaging, and cardiac scintigraphy, the diagnosis of ATTR-CM no longer requires invasive evaluation with tissue biopsy. Here, the authors review current diagnostic tools to help clinicians diagnose ATTR-CM.
Keywords: Amyloidosis, Cardiac scintigraphy, Cardiomyopathy, Diastolic dysfunction, Transthyretin amyloid
Key Summary Points
| ATTR-CM remains a highly elusive and life-threatening diagnosis. |
| Numerous clinical scenarios with both cardiac and extra-cardiac manifestations can serve as screening tools for clinicians. |
| Definitive diagnosis of ATTR-CM can now be made noninvasively with newer imaging techniques. |
| Cardiac scintigraphy remains an indispensable imaging modality in the diagnosis of ATTR-CM. |
| A positive cardiac scintigraphy scan does not differentiate ATTR-CM from AL-amyloid CM, therefore a thorough multidisciplinary evaluation is still required. |
Introduction
Transthyretin (TTR) amyloid cardiomyopathy (ATTR-CM) remains an elusive diagnosis despite recent advances in both clinical diagnostic tools and treatment. Untreated amyloidosis can progress to end-stage heart failure with a poor prognosis. ATTR-CM results from myocardial deposition of a misfolded protein called transthyretin (previously known as pre-albumin). This misfolded protein forms amyloid fibrils (cross-beta-sheet-rich) that are cytotoxic to several tissue types. This is in contrast to immunoglobulin light chain (AL) amyloidosis, which forms when plasma cells secrete misfolded light chains [1].
The diagnosis of ATTR-CM remains a dilemma as the clinical phenotype of ATTR-CM can be seen in many other cardiac disease states. Additionally, most clinical cardiologists are unclear of the diagnostic evaluation required for AL amyloid cardiomyopathy and often confuse the two entities. It is not surprising therefore that an alarming amount of patients diagnosed with ATTR-CM initially not only received a misdiagnosis, but were treated for the misdiagnosed condition [2]. Common clinical cues can be used to help clinicians raise ATTR-CM on their differential. Therefore, early and optimal use of diagnostic tools remains indispensable for the clinician in making the correct diagnosis. This review discusses the main diagnostic tools that aid in the diagnosis of ATTR-CM. This article does not contain any studies with human participants or animals performed by any of the authors.
Prevalence
The true prevalence of ATTR-CM remains unknown as most patients remain undiagnosed. ATTR-CMwt is the most common type, with autopsy studies showing that its incidence increases with age [3]. Among patients hospitalized with heart failure with preserved ejection fraction, 13% of older adults were found to have ATTR-CM on bone scintigraphy. All of these patients were diagnosed with ATTR-CMwt by age 86 years [4]. ATTR-CMh has a phenotype similar to that of ATTR-CMwt with respect to the late-onset restrictive cardiomyopathy. The average age at presentation is reported to be 69 years [5].
Subtypes and Pathogenesis
ATTR-CM is classified genetically by the TTR gene into wild-type (ATTR-CMwt) or hereditary (ATTR-CMh). The TTR gene is located on chromosome 18 and consists of a 127 amino acid sequence. ATTR-CMwt does not have any identifiable mutation, while ATTR-CMh has an identifiable single amino acid mutation. Recent data suggest that ATTR-CMh is carried by 3.5% of African Americans. In African Americans older than 65 years with congestive heart failure the allele has been found in 10% [6]. Table 1 lists the salient differences in ATTR-CMwt and ATTR-CMh.
Table 1.
Similarities and differences between wild-type and hereditary amyloid transthyretin cardiomyopathy
| ATTR-CMwt | ATTR-CMh | |
|---|---|---|
| Age of onset | Typically > 60 years | Variable depending on mutation (30–80 years) |
| Genotype | Normal | Abnormal, nucleotide mutations present |
| Survival | Approximately 3.5 years | Variable depending on genetic mutation |
| Patient demographics |
Male African American Increased prevalence with age |
Mutations are endemic to certain locations Ireland Japan Sub-Saharan Africa |
TTR is secreted from the liver, choroid plexus, and retinal epithelial cells. In its native form, it is composed of four beta-sheet-rich monomers that circulates as a tetramer. TTR functions as a carrier protein for thyroxine and holo-retinol binding protein [7]. Genetic studies have shown that a single amino acid mutation on the 127 amino acid sequence that codes for TTR is what leads to misfolding and aggregation [8]. Misfolded TTR infiltrates into tissues and causes clinical ATTR amyloidosis [9]. In ATTR-CM, aggregated and misfolded TTR produces a stiff and space-occupying infiltrate that causes myocardium restriction and dysfunction [10].
Clinical Manifestations
Cardiac Involvement and Differential Diagnoses
The common clinical scenario for ATTR-CM is an elderly patient who has been diagnosed with heart failure with preserved ejection fraction. They often have a challenging history of worsening heart failure that has been refractory to multiple therapies. These patients exhibit typical echocardiographic findings of left ventricular thickness as covered below, and have a progressive decline despite therapy. Their hemodynamic profile often demonstrates a restrictive physiology. It is not uncommon that these patients do not tolerate beta blockade and have self-improving hypertension even without any therapy or lifestyle changes [10]. The average age at diagnosis of ATTR-CMwt is reported to be 74 years, with a male predominance [11]. Thus, the name “senile” amyloidosis can be used to refer to this population.
ATTR-CM can be an obscure diagnosis as its presentation can mimic other well-established cardiac problems, most commonly aortic stenosis, hypertrophic cardiomyopathy, and AL amyloidosis. Other potentially confounding diagnoses include, but are not limited to, dilated cardiomyopathies, toxic cardiomyopathies, peripartum cardiomyopathy, cardiac sarcoid, or even stress-induced cardiomyopathy. Therefore, it is not uncommon that patients with these diagnoses are underdiagnosed for ATTR-CM.
Retrospective studies have shown a relatively high prevalence of ATTR-CM in patients with severe aortic stenosis undergoing surgical valve replacement [12]. Furthermore, for patients found to have a low-flow, low-gradient severe aortic stenosis, the restrictive physiology and minimal improvement post valvular replacement is thought to be because of coexistent ATTR-CM. These patients have been shown to have a worse prognosis despite valve replacement and improvement in hemodynamics [13]. As more long-term data emerge for patients post transcatheter aortic valve replacement, further understanding of ATTR-CM in these patients will be possible.
Previous studies have shown that asymmetric septal hypertrophy occurs in up to 25% of patients with ATTR-CMwt [2]. These patients are often diagnosed with hypertrophic cardiomyopathy. Incidental ATTR-CM has been shown to be diagnosed histologically in patients undergoing septal myectomy for left ventricular outflow tract (LVOT) obstruction [14]. Clinicians who manage patients with diagnoses of hypertrophic cardiomyopathy should consider the possibility of ATTR-CM. Furthermore, in patients who undergo septal myectomies for LVOT obstruction, histopathology should be sought to determine whether ATTR-CM is present.
Extracardiac Involvement
Extracardiac manifestations of ATTR serve as potential screening tools to allow clinical cardiologists to diagnose ATTR-CM before cardiac manifestations begin. Extracardiac manifestations include central nervous system involvement, nephrotic syndrome, ocular manifestations, gastrointestinal illness, autonomic neuropathy, and peripheral neuropathy. However, the most common and consistent extracardiac findings in ATTR are bilateral carpal tunnel syndrome, spinal stenosis, and spontaneous bicep tendon rupture [15, 16]. In general, ATTR-CMwt and ATTR-CMh have similar extracardiac manifestations, however genetic studies show that extracardiac manifestations of ATTR-CMh may vary slightly depending on the exact genotype. For example, the VAL122lle (pV1421) mutation does not normally cause polyneuropathy [17]. Conversely, the Thr60AIa (pT80A) mutation, seen in Ireland, causes a phenotype with a high rate of carpal tunnel syndrome [18].
ATTR amyloid fibrils can infiltrate soft tissue causing nerve entrapment, the most common being median nerve entrapment or carpal tunnel syndrome. Interesting to clinicians, studies have shown that the symptoms of carpal tunnel syndrome existed up to 10 years prior to the clinical ATTR-CM [19]. Among patients with ATTR-CMwt, carpal tunnel syndrome presents in approximately 50% [20]. These studies, among many others, show that existence of carpal tunnel syndrome should remain an important clinical tool to screen patients who have heart failure symptoms and a history of symptoms related to median nerve entrapment. This could aid in earlier and more efficient diagnosis. Furthermore, if patients have undergone surgery for carpal tunnel syndrome, sending a sample of tenosynovium to pathology can be helpful if amyloid deposits are identified, as shown in a previous study [21].
Similar to carpal tunnel syndrome, ATTR deposition into musculoskeletal tissue has been shown to cause compression and narrowing of the spinal canal through the ligamentum flavum. Prior studies have shown that over 45% of older patients undergoing spinal stenosis surgery were found to have ATTR deposition [22]. Additionally, the prevalence of ATTR deposits was 33% in patients who suffered from spontaneous rupture of the distal biceps tendon [15]. In orthopedic studies, amyloid deposits have been found in tissue samples of patients who underwent rotator cuff repairs [23] and total knee and hip arthroplasties [24]. These musculoskeletal manifestations of ATTR may serve as important clues in screening for patients at risk for ATTR-CM.
Diagnostic Tools
Serology
Unlike AL amyloidosis in which there are circulating biomarkers (light chains), ATTR-CM has not been shown to have specific biomarkers for which to test. There are newer serologic tests for endogenous TTR ligand retinol binding protein 4, which may serve as a testable biomarker in the future [25]. Classic cardiac biomarkers have been shown to be persistently elevated in ATTR-CM and have been incorporated into its staging and prognosis [26]. Serum troponin levels have been found to be persistently elevated in the absence of any apparent cardiomyopathy. Similarly, pro-B-type natriuretic peptide can be seen as elevated out of proportion to the patient’s clinical heart failure [27].
Electrocardiogram
ATTR-CM can be suspected based on a simple electrocardiographic (ECG) pattern. Patients with increased left ventricular wall thickness found to have a low-voltage ECG pattern should be further evaluated for ATTR-CM. Normally, hypertrophic cardiomyopathy and hypertensive heart disease patients would demonstrate increased left ventricular wall thickness, but with left ventricular hypertrophy criteria on their ECG. Low voltage would not be expected for these conditions. Unfortunately, however, studies have shown that only up to 40% of patients with ATTR-CM meet the low-voltage criteria [28].
Another important ECG finding is atrioventricular block seen in patients with ATTR-CM. Amyloid infiltration of the sinus and atrioventricular nodes may often necessitate pacemaker implantation. Atrioventricular block has been seen in up to 22% of patients with cardiac amyloidosis [29].
Imaging
Imaging remains at the heart of a noninvasive diagnosis of ATTR-CM. The three modalities that have been shown to be useful and, at times, diagnostic for ATTR-CM are transthoracic echocardiography, cardiovascular magnetic resonance (CMR), and cardiac scintigraphy.
Transthoracic Echocardiography
Although limited, there are a number of findings on transthoracic echocardiography that should alert the clinician of possible cardiac amyloid. Notably, symmetrical increase in left ventricular (LV) thickness is seen that is often mislabeled as LV hypertrophy. LV hypertrophy is due to myocyte hypertrophy as opposed to cardiac amyloidosis in which there is increased extracellular deposition of amyloid protein with normal myocytes. Due to the thick and dense myocardium, a classic sparking or “speckled appearance” term is often used to describe the myocardium. It is not uncommon to have pleural and pericardial effusions, although they are often dismissed as trivial with respect to hemodynamic significance. Diastolic parameters demonstrated a restrictive filling pattern with severe bi-atrial dilatation. However, these diastolic changes are often noted to be in later stages of the disease process [30]. As alluded to, these findings are often severely limited. For example, increased LV wall thickness and a speckled pattern can often be seen with other conditions, such as end-stage renal disease and glycogen storage diseases.
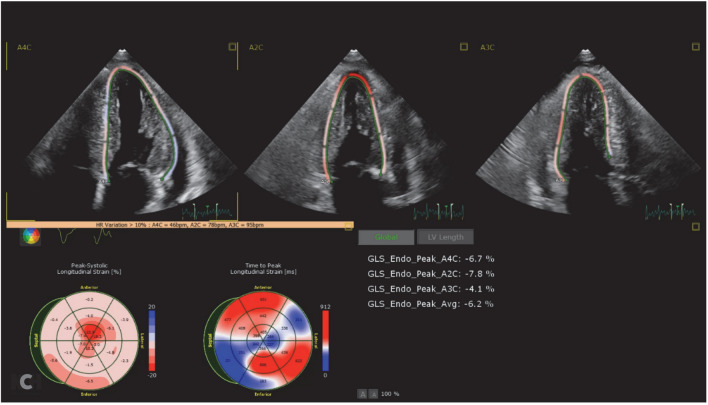
Newer echocardiographic techniques have allowed for more precise analysis of cardiac deformation. Longitudinal systolic strain imaging has been shown to be reduced in ATTR-CM. Specifically, there is preserved longitudinal systolic strain in the apical region of the left ventricle compared with reduced strain in the mid and basal regions. This produces an easily recognizable longitudinal stain image map (Fig. 1) that resembles a “cherry on top.” An abnormal apical to mid or basal strain ratio has been shown to have a strong diagnostic accuracy for cardiac amyloidosis and helps to differentiate cardiac amyloidosis from other etiologies [31]. This is extremely useful when conflicting diagnoses, such as hypertensive heart disease or aortic stenosis, can also explain the particular basic echocardiographic findings. However, longitudinal strain image cannot necessarily differentiate the type of cardiac amyloidosis, as AL amyloidosis can produce a similar pattern.
Fig. 1.
Transthoracic echocardiography demonstrating a classic cardiac amyloid longitudinal strain pattern of the “cherry on top”
CMR Imaging
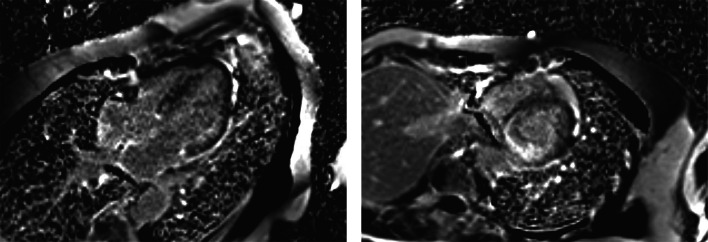
CMR has been increasingly utilized in the evaluation of numerous cardiac pathologies. The addition of gadolinium enhancement helps detect infiltrative cardiomyopathies. An inability to suppress the myocardial signal (inability for the gadolinium to leave the myocardium) or presence of diffuse subendocardial or transmural enhancement is suggestive of amyloidosis with impressive sensitivity and specificity [32]. This late enhancement is not seen in other cardiac diseases such as hypertensive heart disease because the gadolinium washes out of the myocardium (Fig. 2).
Fig. 2.
a Four-chamber phase-sensitive inversion recovery sequences (inversion time 240 ms) 10-min post gadolinium demonstrated late gadolinium enhancement in all four chambers, inability to null the myocardium and transmural involvement. b Short-axis phase-sensitive inversion recovery sequence (inversion time 240 ms) 10-min post gadolinium demonstrated late gadolinium enhancement in all four chambers, inability to null the myocardium and transmural involvement.
Figure images courtesy of Dr Mohammad Al-Ani, Division of Cardiovascular Medicine, University of Florida, Gainesville, Florida
Further CMR techniques such as T1 mapping provide further sensitivity and a quantitative measure of ATTR infiltration into the myocardium. For example, quantitative measures such as extracellular volume fraction are higher in cardiac amyloidosis than in other disease states affecting the myocardium [33].
Despite the utility of CMR in diagnosing an infiltrative process such as cardiac amyloidosis, it does not provide the ability to differentiate the type of amyloid infiltration, therefore it cannot reliably differentiate between ATTR-CM and AL amyloid cardiomyopathy [34].
Cardiac Scintigraphy with 99m Technetium Pyrophosphate (TC-99m PYP)
Currently, the only imaging modality that allows accurate diagnosis of the exact type of cardiac amyloidosis is nuclear scintigraphy using bone-avid radiotracers.
Briefly, three technetium-labeled radiotracers have been used to identify ATTR-CM: TC-99m-PYP; TC-99m-3,3-diphosphono-1,2-propanodicarboxylic acid; or TC-99m-hydroxymethylene diphosphonate [10]. Myocardial uptake of these radiotracers is compared with adjacent bone uptake of the rib and graded to indicate that cardiac uptake is greater than rib uptake. The exact mechanism of radiotracer uptake by the myocardium is not fully elucidated at this time, although several hypotheses appear in the literature [35]. It has been demonstrated that these radiotracers confer 100% specificity for ATTR-CM when grade 2 or 3 uptake is seen in heart failure patients without monoclonal protein (which would indicate AL amyloidosis) and echocardiographic or CMR findings of amyloidosis [36]. Therefore, cardiac scintigraphy allows a noninvasive approach to a diagnosis of ATTR-CM.
Despite the remarkable advantage of cardiac scintigraphy, the approach to diagnosis of ATTR-CM rests greatly on the ability to rule out AL amyloidosis. Therefore, a multidisciplinary approach remains indispensable as a thorough hematologic and nephrologic evaluation is needed to confidently rule out AL amyloidosis. AL amyloid cardiomyopathy can itself cause increased uptake on cardiac scintigraphy. Common serology used for AL amyloidosis, such as serum and urine protein electrophoresis, is unreliable and challenging to interpret for non-specialists. Furthermore, it is not uncommon to have concomitant monoclonal gammopathy found in patients with ATTR-CM [37]. Thus, a multidisciplinary approach along with genetic testing and tissue biopsy should still be highly considered in challenging cases. Table 2 summarizes diagnostic clues for suspecting ATTR-CM noninvasively.
Table 2.
Noninvasive diagnostic tests and indicators of suspected ATTR-CM
| Test | Salient features |
|---|---|
| Electrocardiogram | Discrepancy between left ventricular thickness and QRS voltage |
| Echocardiogram |
Increased left ventricular wall thickness Reduced longitudinal strain with apical sparing on strain imaging |
| Cardiac magnetic resonance imaging |
Marked extracellular volume expansion Abnormal nulling of myocardium on T1 mapping Delayed gadolinium enhancement |
| Cardiac scintigraphy | Increased radiotracer uptake |
| Serology |
Mild chronic cardiac biomarker elevation Lack of biomarkers for amyloid light-chain amyloidosis |
| Salient clinical features |
Heart failure with preserved ejection fraction Intolerance to beta-blockers or ace inhibitors No longer hypertensive Neuropathy Carpal tunnel syndrome Biceps tendon rupture Lumbar spinal stenosis |
Genetic Testing
Genetic testing has been recommended for all patients with ATTR-CM regardless of age. This is because of the potential significant impact on family members. Currently, surveillance for those with a variant genotype is unknown [10].
Endomyocardial Biopsy
Tissue biopsy with histopathology and immunohistochemistry has been used to definitively diagnose amyloidosis. Extracardiac tissue biopsies (such as abdominal fat pad aspirate) have varying and unreliable sensitivity [38]. Tissue samples from orthopedic procedures, such as carpal tunnel syndrome surgery, have unclear diagnostic reliability at this time but may be useful.
Traditionally, endomyocardial biopsy has been the gold standard for diagnosing ATTR-CM as tissue diagnosis provides extremely high sensitivity and specificity, especially if multiple sites on the myocardium are biopsied and histopathologically evaluated for amyloid using Congo Red staining [39]. Further immunologic techniques with tandem mass spectrometry analysis helps with a definitive diagnosis of ATTR-CM.
Given advances in noninvasive imaging modalities, the need for endomyocardial biopsy will likely decrease, especially given the risk of complications with endomyocardial biopsy. However, it is likely endomyocardial biopsy will still be an important part of the definitive diagnosis of ATTR-CM, especially when AL amyloid cardiomyopathy cannot be confidently excluded.
Disease Progression and Prognosis
The clinical course of ATTR-CM varies depending on whether the etiology is ATTR-CMh or ATTR-CMwt. Generally, both types will progress to frank heart failure with conduction system disease [28]. The clinical course of ATTR-CM is a slowly progressive disease. This is in contrast to AL amyloid cardiomyopathy, which progresses rapidly with severe heart failure [28]. Specifically, ATTR-CMh classically presents with either a primary cardiomyopathy or primary neuropathy, however mixed phenotypes can occur in ATTR-CMh, especially if the disease is recognized late [40]. The natural history and clinical course of ATTR-CMh will further vary based on various mutations, fibril type, and the particular genetic makeup. Cardiac involvement is a strong determinant of outcome. The median survival reported in the literature for ATTR-CMh is around 10 years for those with ATTR polyneuropathy as opposed to 3 years in those diagnosed with ATTR-CMh [40]. Conversely, ATTR-CMwt natural history and disease progression are more consistent with median survival from diagnosis of 3.5 years [10]. However, the stage of the disease has shown to also impact prognosis. For example, thresholds of troponin T and N-terminal pro-B type natriuretic peptide levels are used in the Mayo Clinic ATTR-CMwt staging system and influence survival [41]. Other staging systems incorporate both ATTR-CMh and ATTR-CMwt patients with similar and additional biomarkers. Conduction system disease is more prevalent in ATTR-CMwt, manifesting as increased rates of permanent pacemakers and atrial arrhythmias such as atrial fibrillation [41].
Conclusions
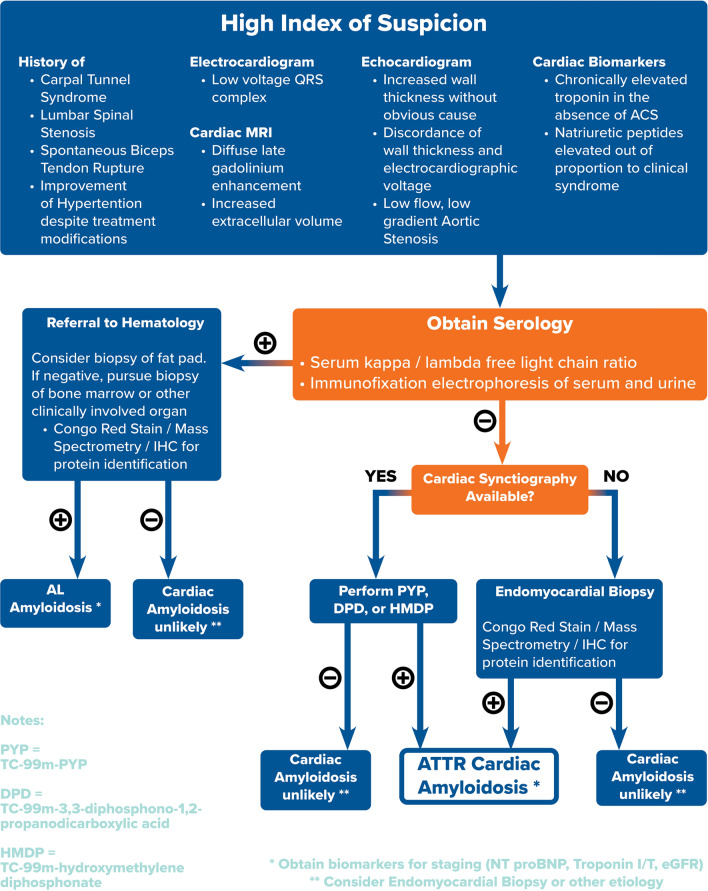
ATTR-CM is no longer an undiagnosable or incurable life-threatening disease. Clinical cardiologists now have noninvasive tools to screen and diagnose patients with ATTR-CM long before deleterious clinical cardiac disease manifestation. Namely, musculoskeletal and neuropathic changes can serve as clinical clues years before ATTR-CM manifests. Baseline ECG and echocardiogram with longitudinal strain imaging can increase clinical suspicion for ATTR-CM. Lastly, newer imaging with cardiac scintigraphy allows further differentiation of types of amyloidosis. Combined with serology to exclude AL amyloidosis and the clinical context, ATTR-CM can be confidently diagnosed noninvasively by clinical cardiologists. Our algorithmic approach to the diagnosis of ATTR-CM is proposed in Fig. 3.
Fig. 3.
Algorithmic approach to the diagnosis of ATTR-CM
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Anthony A. Bavry is an Editorial Board member of the journal; Current affiliation is Department of Medicine, University of Texas Southwestern, Dallas, Texas. Adam S. Hafeez has nothing to disclose.
Compliance with Ethics Guidelines
This article does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Footnotes
Digital Features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12032166.
References
- 1.Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016;387(10038):2641–2654. doi: 10.1016/S0140-6736(15)01274-X. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Lopez E, Gagliardi C, Dominguez F, Quarta CC, de Haro-Del Moral FJ, Milandri A, et al. Clinical characteristics of wild-type transthyretin cardiac amyloidosis: disproving myths. Eur Heart J. 2017;38(24):1895–1904. doi: 10.1093/eurheartj/ehx043. [DOI] [PubMed] [Google Scholar]
- 3.Cornwell GG, 3rd, Murdoch WL, Kyle RA, Westermark P, Pitkanen P. Frequency and distribution of senile cardiovascular amyloid. A clinicopathologic correlation. Am J Med. 1983;75(4):618–623. doi: 10.1016/0002-9343(83)90443-6. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez-Lopez E, Gallego-Delgado M, Guzzo-Merello G, de Haro-Del Moral FJ, Cobo-Marcos M, Robles C, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36(38):2585–2594. doi: 10.1093/eurheartj/ehv338. [DOI] [PubMed] [Google Scholar]
- 5.Dungu JN, Papadopoulou SA, Wykes K, Mahmood I, Marshall J, Valencia O, et al. Afro-Caribbean heart failure in the United Kingdom: cause, outcomes, and ATTR V122I cardiac amyloidosis. Circ Heart Fail. 2016;9(9):e003352. doi: 10.1161/CIRCHEARTFAILURE.116.003352. [DOI] [PubMed] [Google Scholar]
- 6.Buxbaum JN, Ruberg FL. Transthyretin V122I (pV142I)* cardiac amyloidosis: an age-dependent autosomal dominant cardiomyopathy too common to be overlooked as a cause of significant heart disease in elderly African Americans. Genet Med. 2017;19(7):733–742. doi: 10.1038/gim.2016.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly JW, Colon W, Lai Z, Lashuel HA, McCulloch J, McCutchen SL, et al. Transthyretin quaternary and tertiary structural changes facilitate misassembly into amyloid. Adv Protein Chem. 1997;50:161–181. doi: 10.1016/S0065-3233(08)60321-6. [DOI] [PubMed] [Google Scholar]
- 8.Purkey HE, Dorrell MI, Kelly JW. Evaluating the binding selectivity of transthyretin amyloid fibril inhibitors in blood plasma. Proc Natl Acad Sci USA. 2001;98(10):5566–5571. doi: 10.1073/pnas.091431798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westermark P, Sletten K, Johansson B, Cornwell GG., 3rd Fibril in senile systemic amyloidosis is derived from normal transthyretin. Proc Natl Acad Sci USA. 1990;87(7):2843–2845. doi: 10.1073/pnas.87.7.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin amyloid cardiomyopathy: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73(22):2872–2891. doi: 10.1016/j.jacc.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connors LH, Sam F, Skinner M, Salinaro F, Sun F, Ruberg FL, et al. Heart failure resulting from age-related cardiac amyloid disease associated with wild-type transthyretin: a prospective, observational cohort study. Circulation. 2016;133(3):282–290. doi: 10.1161/CIRCULATIONAHA.115.018852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Treibel TA, Fontana M, Gilbertson JA, Castelletti S, White SK, Scully PR, et al. Occult transthyretin cardiac amyloid in severe calcific aortic stenosis: prevalence and prognosis in patients undergoing surgical aortic valve replacement. Circ Cardiovasc Imaging. 2016;9(8):e005066. doi: 10.1161/CIRCIMAGING.116.005066. [DOI] [PubMed] [Google Scholar]
- 13.Cavalcante JL, Rijal S, Abdelkarim I, Althouse AD, Sharbaugh MS, Fridman Y, et al. Cardiac amyloidosis is prevalent in older patients with aortic stenosis and carries worse prognosis. J Cardiovasc Magn Reson. 2017;19(1):98. doi: 10.1186/s12968-017-0415-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helder MR, Schaff HV, Nishimura RA, Gersh BJ, Dearani JA, Ommen SR, et al. Impact of incidental amyloidosis on the prognosis of patients with hypertrophic cardiomyopathy undergoing septal myectomy for left ventricular outflow tract obstruction. Am J Cardiol. 2014;114(9):1396–1399. doi: 10.1016/j.amjcard.2014.07.058. [DOI] [PubMed] [Google Scholar]
- 15.Geller HI, Singh A, Alexander KM, Mirto TM, Falk RH. Association between ruptured distal biceps tendon and wild-type transthyretin cardiac amyloidosis. JAMA. 2017;318(10):962–963. doi: 10.1001/jama.2017.9236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Westermark P, Westermark GT, Suhr OB, Berg S. Transthyretin-derived amyloidosis: probably a common cause of lumbar spinal stenosis. Ups J Med Sci. 2014;119(3):223–228. doi: 10.3109/03009734.2014.895786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Connors LH, Prokaeva T, Lim A, Theberge R, Falk RH, Doros G, et al. Cardiac amyloidosis in African Americans: comparison of clinical and laboratory features of transthyretin V122I amyloidosis and immunoglobulin light chain amyloidosis. Am Heart J. 2009;158(4):607–614. doi: 10.1016/j.ahj.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Reilly MM, Staunton H, Harding AE. Familial amyloid polyneuropathy (TTR ala 60) in north west Ireland: a clinical, genetic, and epidemiological study. J Neurol Neurosurg Psychiatry. 1995;59(1):45–49. doi: 10.1136/jnnp.59.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakagawa M, Sekijima Y, Yazaki M, Tojo K, Yoshinaga T, Doden T, et al. Carpal tunnel syndrome: a common initial symptom of systemic wild-type ATTR (ATTRwt) amyloidosis. Amyloid. 2016;23(1):58–63. doi: 10.3109/13506129.2015.1135792. [DOI] [PubMed] [Google Scholar]
- 20.Pinney JH, Whelan CJ, Petrie A, Dungu J, Banypersad SM, Sattianayagam P, et al. Senile systemic amyloidosis: clinical features at presentation and outcome. J Am Heart Assoc. 2013;2(2):e000098. doi: 10.1161/JAHA.113.000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sperry BW, Reyes BA, Ikram A, Donnelly JP, Phelan D, Jaber WA, et al. Tenosynovial and cardiac amyloidosis in patients undergoing carpal tunnel release. J Am Coll Cardiol. 2018;72(17):2040–2050. doi: 10.1016/j.jacc.2018.07.092. [DOI] [PubMed] [Google Scholar]
- 22.Yanagisawa A, Ueda M, Sueyoshi T, Okada T, Fujimoto T, Ogi Y, et al. Amyloid deposits derived from transthyretin in the ligamentum flavum as related to lumbar spinal canal stenosis. Mod Pathol. 2015;28(2):201–207. doi: 10.1038/modpathol.2014.102. [DOI] [PubMed] [Google Scholar]
- 23.Sueyoshi T, Ueda M, Jono H, Irie H, Sei A, Ide J, et al. Wild-type transthyretin-derived amyloidosis in various ligaments and tendons. Hum Pathol. 2011;42(9):1259–1264. doi: 10.1016/j.humpath.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 24.Rubin J, Alvarez J, Teruya S, Castano A, Lehman RA, Weidenbaum M, et al. Hip and knee arthroplasty are common among patients with transthyretin cardiac amyloidosis, occurring years before cardiac amyloid diagnosis: can we identify affected patients earlier? Amyloid. 2017;24(4):226–230. doi: 10.1080/13506129.2017.1375908. [DOI] [PubMed] [Google Scholar]
- 25.Arvanitis M, Simon S, Chan G, Fine D, Beardsley P, LaValley M, et al. Retinol binding protein 4 (RBP4) concentration identifies V122I transthyretin cardiac amyloidosis. Amyloid. 2017;24(sup1):120–121. doi: 10.1080/13506129.2017.1295371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gillmore JD, Damy T, Fontana M, Hutchinson M, Lachmann HJ, Martinez-Naharro A, et al. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J. 2018;39(30):2799–2806. doi: 10.1093/eurheartj/ehx589. [DOI] [PubMed] [Google Scholar]
- 27.Donnelly JP, Hanna M. Cardiac amyloidosis: an update on diagnosis and treatment. Cleve Clin J Med. 2017;84(12 Suppl 3):12–26. doi: 10.3949/ccjm.84.s3.02. [DOI] [PubMed] [Google Scholar]
- 28.Rapezzi C, Merlini G, Quarta CC, Riva L, Longhi S, Leone O, et al. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation. 2009;120(13):1203–1212. doi: 10.1161/CIRCULATIONAHA.108.843334. [DOI] [PubMed] [Google Scholar]
- 29.Huang J, Zhao S, Chen Z, Zhang S, Lu M. Contribution of electrocardiogram in the differentiation of cardiac amyloidosis and nonobstructive hypertrophic cardiomyopathy. Int Heart J. 2015;56(5):522–526. doi: 10.1536/ihj.15-005. [DOI] [PubMed] [Google Scholar]
- 30.Aljaroudi WA, Desai MY, Tang WH, Phelan D, Cerqueira MD, Jaber WA. Role of imaging in the diagnosis and management of patients with cardiac amyloidosis: state of the art review and focus on emerging nuclear techniques. J Nucl Cardiol. 2014;21(2):271–283. doi: 10.1007/s12350-013-9800-5. [DOI] [PubMed] [Google Scholar]
- 31.Pagourelias ED, Mirea O, Duchenne J, Van Cleemput J, Delforge M, Bogaert J, et al. Echo parameters for differential diagnosis in cardiac amyloidosis: a head-to-head comparison of deformation and nondeformation parameters. Circ Cardiovasc Imaging. 2017;10(3):e005588. doi: 10.1161/CIRCIMAGING.116.005588. [DOI] [PubMed] [Google Scholar]
- 32.Zhao L, Tian Z, Fang Q. Diagnostic accuracy of cardiovascular magnetic resonance for patients with suspected cardiac amyloidosis: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2016;16:129. doi: 10.1186/s12872-016-0311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez-Naharro A, Treibel TA, Abdel-Gadir A, Bulluck H, Zumbo G, Knight DS, et al. Magnetic resonance in transthyretin cardiac amyloidosis. J Am Coll Cardiol. 2017;70(4):466–477. doi: 10.1016/j.jacc.2017.05.053. [DOI] [PubMed] [Google Scholar]
- 34.Dungu JN, Valencia O, Pinney JH, Gibbs SD, Rowczenio D, Gilbertson JA, et al. CMR-based differentiation of AL and ATTR cardiac amyloidosis. JACC Cardiovasc Imaging. 2014;7(2):133–142. doi: 10.1016/j.jcmg.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 35.Pilebro B, Suhr OB, Naslund U, Westermark P, Lindqvist P, Sundstrom T. (99m)Tc-DPD uptake reflects amyloid fibril composition in hereditary transthyretin amyloidosis. Ups J Med Sci. 2016;121(1):17–24. doi: 10.3109/03009734.2015.1122687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133(24):2404–2412. doi: 10.1161/CIRCULATIONAHA.116.021612. [DOI] [PubMed] [Google Scholar]
- 37.Phull P, Sanchorawala V, Connors LH, Doros G, Ruberg FL, Berk JL, et al. Monoclonal gammopathy of undetermined significance in systemic transthyretin amyloidosis (ATTR) Amyloid. 2018;25(1):62–67. doi: 10.1080/13506129.2018.1436048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quarta CC, Gonzalez-Lopez E, Gilbertson JA, Botcher N, Rowczenio D, Petrie A, et al. Diagnostic sensitivity of abdominal fat aspiration in cardiac amyloidosis. Eur Heart J. 2017;38(24):1905–1908. doi: 10.1093/eurheartj/ehx047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pellikka PA, Holmes DR, Jr, Edwards WD, Nishimura RA, Tajik AJ, Kyle RA. Endomyocardial biopsy in 30 patients with primary amyloidosis and suspected cardiac involvement. Arch Intern Med. 1988;148(3):662–666. doi: 10.1001/archinte.1988.00380030168027. [DOI] [PubMed] [Google Scholar]
- 40.Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;126(10):1286–1300. doi: 10.1161/CIRCULATIONAHA.111.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grogan M, Scott CG, Kyle RA, Zeldenrust SR, Gertz MA, Lin G, et al. Natural history of wild-type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol. 2016;68(10):1014–1020. doi: 10.1016/j.jacc.2016.06.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.