Abstract
Hypothalamic neurons show sex differences in neuritogenesis, female neurons have longer axons and higher levels of the neuritogenic factor neurogenin 3 (Ngn3) than male neurons in vitro. Moreover, the effect of 17-β-estradiol (E2) on axonal growth and Ngn3 expression is only found in male-derived neurons. To investigate whether sex chromosomes regulate these early sex differences in neuritogenesis by regulating the E2 effect on Ngn3, we evaluated the growth and differentiation of hypothalamic neurons derived from the “four core genotypes” mouse model, in which the factors of “gonadal sex” and “sex chromosome complement” are dissociated. We showed that sex differences in neurite outgrowth are determined by sex chromosome complement (XX > XY). Moreover, E2 increased the mRNA expression of Ngn3 and axonal length only in XY neurons. ERα/β expressions are regulated by sex chromosome complement; however, E2-effect on Ngn3 expression in XY neurons was only fully reproduced by PPT, a specific ligand of ERα, and prevented by MPP, a specific antagonist of ERα. Together our data indicate that sex chromosomes regulate early development of hypothalamic neurons by orchestrating not only sex differences in neuritogenesis, but also regulating the effect of E2 on Ngn3 expression through activation of ERα in hypothalamic neurons.
Subject terms: Cell biology, Neuroscience
Introduction
Many of the known sex differences found in mammalian brain networks and behavior are organized during early development and a compelling body of evidence has effectively linked many of these sex differences to the effect of a developmental exposure to gonadal testosterone or its metabolite 17-β-estradiol (E2)1. E2 has been shown to stimulate neuritogenesis at the time when neural circuits become functional in the brain2–5. More recently, the effect of sex chromosome-dependent factors during development on the sexual differentiation of the brain has been studied using the mouse model known as Four Core Genotypes (FCG)6. This animal model allows the evaluation of any sexually differentiated endpoint between XY and XX mice with testes or ovaries, i.e. XY male (XYM) or female (XYF) and XX male (XXM) or female (XXF) mice7,8. Using the FCG model, the early developmental effect of sex chromosome complement on sex differences in the brain has been demonstrated in the number of mesencephalic neurons immunoreactive for tyrosine hydroxylase9, as well as in the expression of the enzyme aromatase in the anterior amygdala10,11 and the neuritogenic factor neurogenin 3 (Ngn3) in hypothalamic neurons12. These sex differences were found in primary neuronal cultures or tissue obtained from FCG mice of embryonic age (E) 14–16 meaning that they cannot be attributed to the prenatal peak in gonadal testosterone occurring at E17–1813,14.
Ngn3, an autosomal gene involved in the signaling pathway of Notch15, is required for neuronal subtype specification in the ventromedial hypothalamus16, and it has been implicated in the degree of dendritic arborization in hippocampal neurons17,18 and in the rate of hypothalamic neuronal development12. Typically, hypothalamic neuronal cultures prepared with E14–16 female embryos develop faster and with longer axons than male neurons and these sex differences depend on a higher expression of Ngn3 in female neurons than in male cultures12. The sex difference in Ngn3 is dependent on the sex chromosome complement since XX male or female hypothalamic neurons express higher levels of Ngn3 than XY cultures12. In addition, E2 increases Ngn3 expression12 and axonal growth12,19,20 only in male neurons, counterbalancing the sex differences in neuritogenesis during early hypothalamic development.
To further investigate the effect of sex chromosomes on sex differences in the E2-effect and neuritogenesis, we evaluated the growth and differentiation of hypothalamic neurons derived from FCG in cultures with or without the hormone. In addition, we tested the hypothesis that sex chromosomes regulate early sex differences in neuritogenesis by regulating the E2 effect on Ngn3.
Results
Female hypothalamic cultures from MF1 and CD1 mice express higher levels of Ngn3 than male cultures
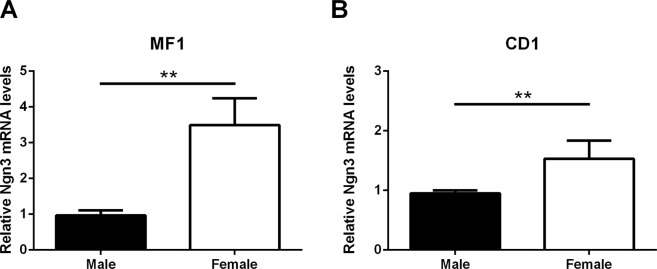
In agreement with previous studies in CD1 mice12, we found that female hypothalamic neurons from MF1 mice expressed higher levels of Ngn3 than male cultures at E14 (Fig. 1; two- tailed T test: t20 = 3.331, P = 0.003 for MF1 cultures; two- tailed T test: t10 = 4.316, P = 0.002 for CD1 cultures).
Figure 1.
Female neuronal cultures express higher levels of Ngn3 than male cultures at embryonic day 14. Hypothalamic neuronal cultures of (A) MF1 and (B) CD1 mice. Bars indicate mean + SEM. n = 11 independent cultures for MF1 cultures and n = 6 for CD1 cultures. **P ≤ 0.01.
Sex differences in neurite outgrowth are determined by sex chromosome complement
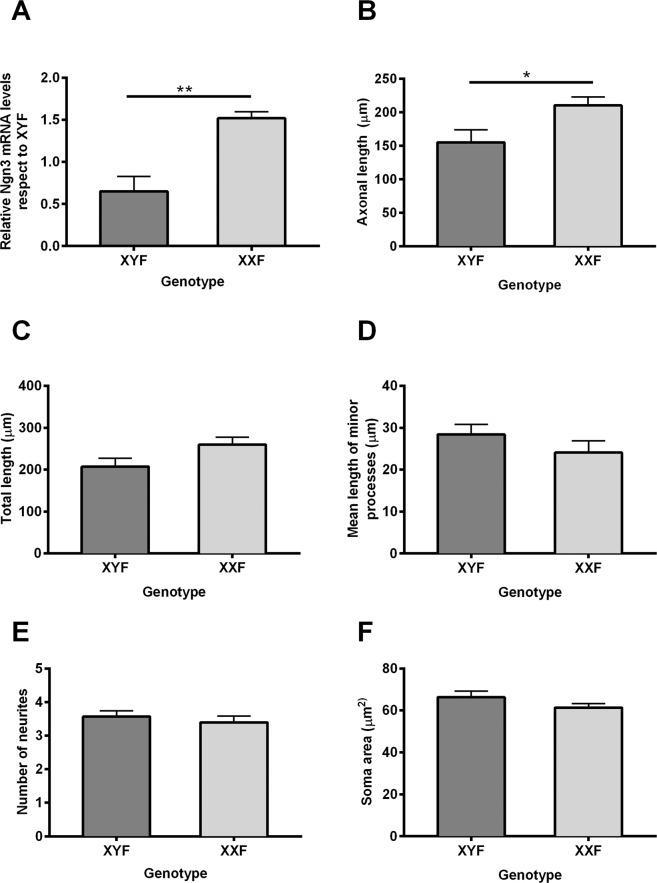
Our previous results indicate that male and female hypothalamic neurons from CD1 mouse cultures present morphologic sex differences in neurite outgrowth starting at 2 days in vitro (DIV) and these sex differences are maintained through 7 DIV12. Female neurons present longer axons and higher expression of Ngn3 than male neurons12. In order to evaluate the direct role of sex chromosome complement in the described sex differences in neuronal maturation, we analyzed different morphological parameters of cellular shape and Ngn3 expression in hypothalamic neurons of XYF and XXF cultures of MF1 mice after 2 DIV (Fig. 2). The comparison of XY and XX female neurons allowed us to exclude any effects of gonadal testosterone produced before E14. As expected, XXF neurons expressed higher levels of Ngn3 than XYF neurons (two- tailed T test: t11 = 3.681, P = 0.004; Fig. 2A). Regarding to morphological parameters of neuronal development, XXF neurons presented longer axons than XYF neurons (two- tailed T test: t8 = 2.487, P = 0.04; Figs. 2B and 3A). There were no effects of sex chromosomes on total neuritic length, mean length of minor processes, number of neurites, or soma area (Fig. 2C–F).
Figure 2.
Sex chromosome complement determines sex differences in neuritogenesis. (A) Relative Ngn3 mRNA expression, (B) axonal length, (C) total length, (D) length of minor processes, (E) number of neurites and (F) soma area of XY female (XYF) and XX female (XXF) hypothalamic neurons. XXF neurons present higher levels of the neuritogenic factor Ngn3 as well as longer axonal length. Data are mean ± SEM. n = 5–8 independent cultures for each genotype. *P < 0.05; **P < 0.01.
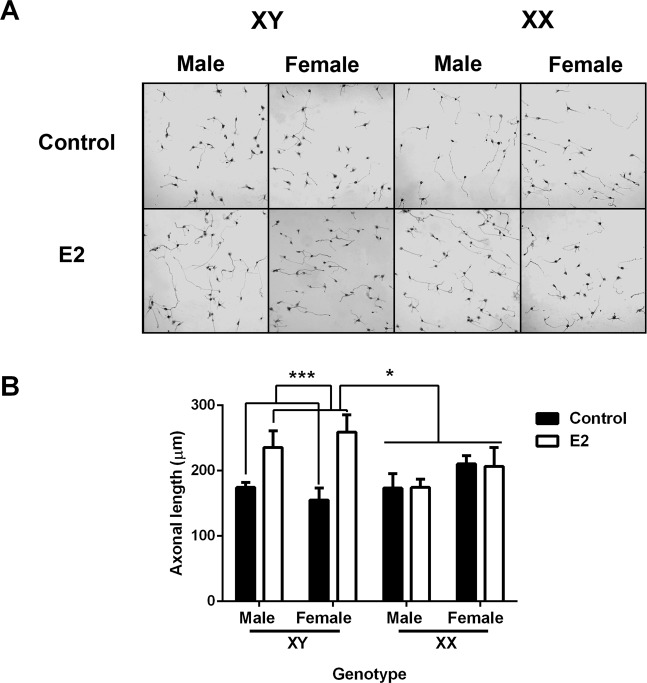
Figure 3.
Sex chromosome complement determines the neuritogenic effects of estradiol in hypothalamic neurons. (A) Representative images of 2 DIV hypothalamic neurons derived from the “four core genotype” mouse model treated with or without 17-β-estradiol (E2), (B) E2 significantly increased the axonal length only in XY male and female (XYM and XYF) neuronal cultures. Data are mean ± SEM. n = 5–7 independent cultures for each genotype. *P < 0.05; ***P < 0.001.
Estradiol increases axonal length and Ngn3 mRNA expression only in XY neurons
We next asked whether sex chromosome complement determines the effect of E2 on axonal growth and Ngn3 expression in male and female cultures of FCG MF1 mice. Three-way ANOVA indicated a significant effect of treatment (F1,29 = 6.98; P = 0.013) and genotype by treatment interaction (F1,29 = 7.466; P = 0.01). E2 significantly increased axonal length in XY neurons, irrespectively if they were male or female (P < 0.001, Fig. 3). In contrast, E2 did not affect this parameter in XX neurons.
Regarding the effect of E2 on Ngn3 expression, a three-way ANOVA indicated a significant effect of gonadal sex (F1,45 = 10.90; P = 0.0019) as well as genotype by treatment interaction (F1,45 = 90.20; P = 0.004). E2 treatment significantly increased the relative expression of Ngn3 mRNA in XYM and XYF neurons (P = 0.001, Fig. 4). Importantly, the effect of E2 on XY neurons abolished the sex chromosome-dependent difference with XX control cultures (XY + E2 vs. XX control cultures: P = 0.71).
Figure 4.
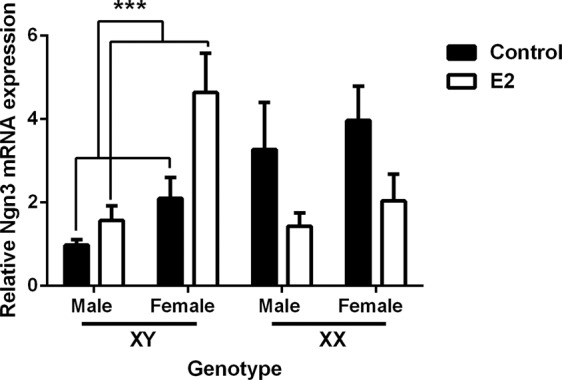
Estradiol increases the mRNA expression of Ngn3 only in XY chromosome complement. 17-β-estradiol (E2) significantly increased Ngn3 expression only in XY male and female (XYM and XYF) neuronal cultures. Data are mean ± SEM. n = 4–7 independent cultures for each genotype. ***P < 0.001.
XY regulates estrogen receptor expression in hypothalamic neurons
To determine whether neuritogenic effects seen in hypothalamic neurons depend on a differential availability of estrogen receptors (ER), we assessed the mRNA levels of ERα (Esr1), ERβ (Esr2) and G protein-coupled estrogen receptor 1 (GPER, Gpr30) in cultures of FCG MF1 mice. As shown in Fig. 5, two-way ANOVA found a significant interaction of sex chromosomes and gonadal sex for Esr1 expression (F1,18 = 6.02; P = 0.024). XYM neurons express higher levels of Esr1 than XYF or XX (male or female) neurons (P < 0.05). In addition, Esr2 expression was determined by sex chromosome complement (F 1, 17 = 6.06; P = 0.024; Fig. 5). XY male or female neurons express higher levels of Esr2 mRNA than XX male or female neurons (P < 0.05). The expression of Gpr30 did not differ among groups.
Figure 5.
Sex chromosome complement determines the mRNA expression of ERα and ERβ in hypothalamic neurons. mRNA expression of ERα (Esr1), ERβ (Esr2) and G protein-coupled estrogen receptor 1 (GPER, Gpr30) in hypothalamic neurons of MF1 FCG cultures. Data are mean ± SEM. n = 4–6 independent cultures. *P < 0.05.
Neuritogenic effect of estradiol on Ngn3 expression depends on ERα
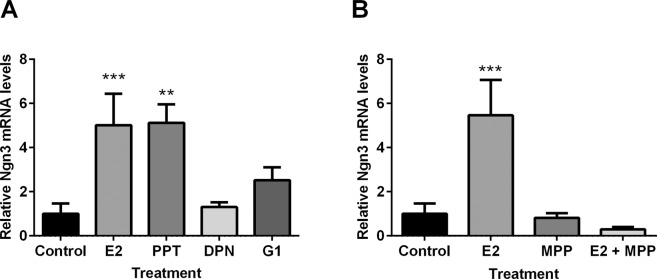
In the analyses above, ERα mRNA expression was higher in XYM neurons, while ERβ mRNA expression was higher in XY male and female neurons. These results suggest that E2 dependent Ngn3 expression was mediated by either one of these ERs. In order to further identify the subtype of ER involved in the effect of E2 on Ngn3 expression, we used wild type CD1 male hypothalamic cultures. Male neuronal cultures were incubated with either E2 or specific agonists for ERα, ERβ, or GPER. One-way ANOVA found a significant effect of treatment (F4,29 = 5.35; P = 0.002; Fig. 6A). As expected, E2 significantly increased Ngn3 expression (P = 0.0006) and this effect was mimicked by the ERα agonist PPT (control cultures compared to PPT-treated cultures: P = 0.0028). Treatment with the ERβ agonist DPN or with the GPER agonist G1 did not significantly affect Ngn3 expression (P > 0.05). To further demonstrate that ERα is involved in the E2-induced upregulation of Ngn3 in male cultures, hypothalamic cultures were co-treated with E2 and the ERα antagonist MPP (Fig. 6B). One-way ANOVA found a significant effect of treatment (F3,21 = 6.19; P = 0.003): as expected, E2 upregulated Ngn3 expression (P = 0.0007) and this effect was blocked when MPP was added in combination with E2 (P = 0.64). MPP alone did not affect the expression of the neuritogenic gene (P > 0.05).
Figure 6.
ERα mediates the neuritogenic effect of estradiol on Ngn3 expression. (A) The ERα agonist PPT mimicked the effect of estradiol (E2) on Ngn3 expression while (B) the selective ERα antagonist MPP abolished the E2-induced increase in Ngn3 expression in male neuronal cultures from CD1 mice. Data are mean ± SEM. N = 3–7 independent cultures. **P < 0.01; ***P < 0.001.
Discussion
Previously, we have demonstrated a sex difference in neuritogenesis and Ngn3 expression in hypothalamic cultures of E14 CD1 mouse12. In order to corroborate this sex difference in the FCG mouse model, we used MF1 WT mice to prepare primary neuronal cultures since this strain is the genetic background of our FCG mouse colony. In agreement with our previous data in CD1 mice, female neuronal cultures from MF1 embryos expressed higher levels of Ngn3 mRNA. Therefore, although the use in the present study of two different mice strains, MF1 and CD1, is not exempt of some limitations, our findings suggest that both strains have similar characteristics concerning Ngn3 expression in the embryonic hypothalamus, and that the FCG MF1 mouse is a valid model to study the mechanism of hormonal regulation of neuritogenesis in vitro.
Here, we found that sex chromosomes orchestrate sex differences in neuritogenesis in hypothalamic neurons. In concordance with our previous results12, we have shown that XXF neurons present longer axons than XYM; furthermore, our present results demonstrate that XY hypothalamic neurons respond to E2 increasing axonal length regardless of their gonadal status. This estrogenic effect was concomitant with enhanced Ngn3 expression in XY cultures, thereby enhanced neuritogenesis. We also found that E2 and the ERα agonist PPT increased Ngn3 expression in male hypothalamic neurons. In addition, the ERα antagonist MPP completely blocked the effect of E2 on Ngn3 expression. Remarkably, our present results are in agreement with previous findings21,22, indicating that the axogenic effect of E2 in male hypothalamic neurons rely on an ERα-dependent mechanism.
Multiple studies have investigated the neuritogenic effect of E2, which is differentially exerted depending on the brain region23–25, sex19,24, and age of the donor neural tissue19,20,24. However, few studies have taken into account neuritogenesis prior to the organizational actions of gonadal hormones26. Previous work from our laboratory has demonstrated sex differences in neuritogenesis in primary neuronal hypothalamic cultures prepared before the peak of testosterone production by fetal testis12. Female neurons show increased axogenesis than male neurons suggesting cell-autonomous actions of sex chromosomes. In the present study, we found that female neurons bearing XX sex chromosome complement show higher axonal length than female neurons with XY complement (XX > XY). Hormonal treatment increased axon length only in XY neurons (irrespectively of gonadal sex), resulting in the reversion of sex differences in neuritogenesis (XX < XY) by a mechanism that also depends on genetic factors.
Sex differences in neuritogenesis have been related to sex differences in the expression of the proneural gene Ngn3 (located in autosome 10), not only in the developing hypothalamus12 but also in the hippocampus27. Indeed, a down-regulation of Ngn3 expression in hypothalamic cultures decreased neuronal maturation, axonal length, and the proportion of cells with branched neurites in female cultures, but not in male cultures12. Moreover, Ngn3 has also been involved in the neuritogenic actions of E2 in developing hippocampal neurons28. Previously, we showed that sex chromosomes generate the sex difference in Ngn3 expression in hypothalamic neurons12. Our present results corroborate these findings and extend the role of sex chromosomes to the E2 effect on Ngn3 expression in hypothalamic neurons. E2 enhanced Ngn3 levels only in neurons from XY embryos whether the embryos had ovaries or testes. In contrast, no increased level of Ngn3 by E2 was found in cultures from embryos with XX sex chromosome complement. One possible explanation for the different response to E2 is a varied enrichment of classical and non-classical ERs in hypothalamic neurons according to the sex chromosome complement. For example, our previous results using FCG mice indicate that the amygdala in XY males and females express higher levels of ERβ (Esr2) than XX mice, both in vivo10 and in vitro11, whereas ERα (Ers1) is regulated by both sex chromosome complement and gonadal phenotype11. Here we found that in the developing hypothalamus, Esr1 and Esr2 expression was also dependent on XY sex chromosome complement. XYM neurons express higher levels of Esr1 and Esr2 than XX (male or female) neurons. Moreover, Esr2 was also increased in XYF neurons. The expression of the non-classical ER, Gpr30, was not subjected to sex chromosome influence. These findings open up the question of ERα/β involvement on E2-dependent regulation of Ngn3 expression. To unravel this possibility, we evaluated several compounds that act as agonists or antagonists of classical steroid action. PPT, an ERα agonist, imitates the effect of E2 on Ngn3 expression. By contrast, DPN and G1, selective agonists of ERβ and GP30‐dependent mechanisms, respectively, were not able to imitate the effect of E2 on Ngn3. Furthermore, MPP, a selective antagonist of ERα‐mediated transcription, was able to antagonize the effect of E2. Given that ER expression was dependent on XY sex chromosome complement; all these findings suggest that sex chromosome genes might primarily orchestrate the effectiveness of E2 effects by regulating the expression of ERα.
The difference in X- and Y- gene dosage resulting from the inheritance of XX vs. XY sex chromosome complement is one of the mechanisms producing sex differences in non-gonadal tissues8. Moreover, sex chromosomes may also counteract the effects of each other, reducing rather than producing sex differences in phenotype29. In contrast to the small and gene-poor Y chromosome (containing mostly genes related to testis determination and fertility), the X chromosome is much larger and encodes genes with a much broader range of functions30,31. Interestingly, many of these genes are involved in brain development32 and their mutations produce X-linked neurological diseases that are more harmful in males than in females33. Among the X-linked genes associated with neuronal growth and differentiation, there are some that escape X-inactivation34 and encode proteins related to chromatin remodeling, such as the histone demethylases Kdm6a and Kdm5c35,36. In the developing hypothalamus, these genes could potentially mediate the regulation of autosomal genes such as Ngn3, Esr1 and Esr2 in a sex dependent manner. Particularly, Kdm6a and Kdm5c are expressed in the brain37 and show higher levels of expression in XX vs. XY mice37,38, both before and after gonadal differentiation39. Moreover, the inhibition of Kdm5c in primary mammalian neurons impairs dendritic morphogenesis35, suggesting that it may regulate the autosomal gene expression involved in neuritogenesis. Unfortunately, the use of unsexed cultures in this study35 limits the interpretation of sex differences. Other gene that escapes X-inactivation in mice is Mid140, a microtubule-associated protein that influences microtubule dynamics41. In polarized neurons, the endogenous Mid1 expression is mainly located in the soma and axon. Moreover, silencing Mid1 in developing neurons promotes axon growth and branch formation, resulting in a disruption of callosal axon projections in the contralateral cortex42. The male-specific effect of E2 on axonal growth and Ngn3 expression could be mediated through a downregulation of Mid1 expression. Based on this evidence, the most likely interpretation is that one or more X-linked genes may be contributing to a sex-specific effect of E2 in male neurons by regulating the expression of ERs, and thus influencing the sensitivity of the developing hypothalamus to sex steroid hormones.
Taken together, our results suggest a direct role of sex chromosomes in the development of sex differences in neuronal differentiation and maturation via an ERα-dependent mechanism. The scenario is likely to be strengthened in a region such as the ventromedial hypothalamus which contains a highly heterogeneous population of ERα-expressing neurons with different projections and sex-related functions43–45. The sex differences in neuritic development may establish the organization of hypothalamic neuronal projections to ensure precise structural and functional patterning, which is crucial for proper circuit formation of sex-related behaviors. The developing hypothalamus selectively expresses the steroidogenic factor 1 (SF-1) around E1446; this event is paramount for the proper organization of the VMH47,48. Interestingly, Ngn3 promotes the development of ventromedial SF1 neurons16, and given the role of sex chromosome complement in regulating hormonal and non-hormonal expression of Ngn3, these results suggest that sex chromosomes might participate in the generation of neuronal populations controlling sexual development and reproduction in mice. Since the E2 effect on Ngn3 expression in XY neurons abolished basal differences with control XX neurons, the final result might be the compensation of sex differences in Ngn3 expression which would ensure equivalent specification of SF1 neurons in males and females during brain development.
Sexual differentiation of the brain has long been considered a hormonally driven process, in great part, due to multiple evidence coming from the field of neuroendocrinology. Indeed, the classical hypothesis of hormonal sexual differentiation has effectively explained many of the known sex differences in the brain and other non-gonadal tissues. The hypothalamus, a major brain region controlling sexual and reproductive functions is a main example of a brain region considered predominantly, or exclusively, under hormonal control during development. However, there is evidence of sex chromosome complement playing a role in the organization of sex differences in the hypothalamus49–51. We recently demonstrated gonadal hormone-independent sex differences in GABAA receptors in hypothalamic cultures of rat embryos. Specifically, these effects were observed before the in utero surge in gonadal hormones and thus were independent of hormonal treatment of cultures52. Taken together, all these studies provide evidence to rethink the classic model and to incorporate the role of sex chromosome complement, not only as a primary orchestrator of early sex differences in the development of hypothalamic neurons, but also as gating the action of sex steroids at this early stage of development. This action might be relevant for the sexual differentiation hypothesis as it contributes to the integration of multiple, separate sex-biasing factors.
Methods
Animals
The embryos used in this study were obtained from CD1 mice raised at the Instituto Cajal (Madrid, Spain) or at the Instituto Ferreyra (Córdoba, Argentina). The researcher who did the experiments in Madrid and Córdoba was always the same (C.D.C.). Some experiments also involved transgenic mice obtained from MF1 mice of the Four Core Genotypes (FCG) mouse model, bred in our colony at the Instituto Ferreyra. This MF1 outbred colony was a gracious donation from Dr. Paul Burgoyne (National Institute for Medical Research, London UK). The embryos used in FCG cultures were produced by breeding transgenic MF1 XYM mice with wild-type MF1 females (Harlan Laboratories Inc. US). Mice were maintained on a 12:12 light dark cycle with ad libitum access to food and water. All procedures involving the animals used in this study were approved by the animal care and use committees at our institutions (Animal Care and Use Committee, CICUAL-IMMF, INIMEC-CONICET-UNC; Comité de Ética de Experimentación Animal, Instituto Cajal) and by the Consejeria del Medio Ambiente y Territorio (Comunidad de Madrid, Ref. PROEX 200/14) and followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Genotyping was performed as previously described in10. Timed pregnancies were established by pairing breeders within an hour of lights off and removing males the following morning after lights on. E1 was defined as the day of vaginal plug presence.
Cell cultures
Embryos of E14 were used to prepare hypothalamic neuronal cultures as previously described in12. The embryos were classified according to the sex (by observation of the spermatic artery of the developing testes) and/or genotype (by PCR). The ventromedial hypothalamic region was dissected out and stripped off the meninges and the blocks of tissue were then mechanically dissociated to single cells after digestion for 15 min at 37 ◦C with 0.5% trypsin (Worthington Biochemicals, Freehold, NJ, USA). The medium was phenol red-free Neurobasal (Invitrogen, United Kingdom) to avoid “estrogen-like effects”53 and was supplemented with B-27, N-2, 0.043% L-alanyl-L-glutamine (GlutaMAX I), 0.15% glucose, 100 U/ml penicillin and 100 mg/ml streptomycin (Invitrogen). The cells were seeded on the surfaces of glass coverslips or plates that were coated with poly-L-lysine (1 µg/µl; Sigma-Aldrich, USA).
For the analyses of gene expression, cells were plated onto 6-well plates and after 3 days in vitro (DIV) the medium was replaced by Neurobasal without supplements for 2 hours. The neuronal cultures were then treated for 2 h with the following compounds alone or in combination: E2 (10−10 M; Sigma-Aldrich), the selective ERα agonist propylpyrazoletriol (PPT, 10−7 M; Tocris Bioscience, United Kingdom), the selective ERβ agonist dyarilpropionitrile (DPN, 10−9 M; Tocris), the GPER agonist G1 (10−6 M, Calbiochem), the selective ERα antagonist 1,3-Bis(4-hydroxyphenyl)−4-methyl-5-[4-(2-piperidinylethoxy)phenol]−1H-pyrazole dihydrochloride (MPP, 10−7 M; Tocris). The concentrations used were based on our previous results11,22,28. For morphometric analyses, neurons were seeded onto coverslips of 12 mm diameter (Assistant, Germany) and treated with of E2 (10−9 M, Sigma-Aldrich) for 2 DIV.
RNA purification and qRT-PCR
Total RNA was isolated from neuronal cultures with illustra RNAspin Mini RNA isolation kit from GE Healthcare (Buckinghamshire, UK). Single strand cDNA was prepared from total RNA using the M-MLV reverse transcriptase (Promega Corp., Madison, Wisconsin) following the manufacturer’s instructions. Quantitative PCR (qRT-PCR) reactions were performed on an ABI Prism 7000 Sequence Detector (Applied Biosystems, Weiterstadt, Germany) using the TaqMan or Sybr Green Universal PCR Master Mix. TaqMan probes and primers for Ngn3 were Assay-on-Demand gene expression products (Applied Biosystems). Primer sequences for the control housekeeping gene 18S rRNA and ERs are described in Table 1. All primers were verified to amplify with an efficiency of 95–100% using 4-point calibration curves. Relative quantification of mRNA expression was determined with the ΔΔCt method using the average of XY-Sry male as calibrator group for FCG cultures or, the average of male cultures as a calibrator group for wild type cultures.
Table 1.
Primer sequences used for gene expression assays.
| Primer name | Forward sequence 5′−3′ | Reverse sequence 5′−3′ |
|---|---|---|
| Esr1 | ATGAAAGGCGGCATACGGAAAG | CACCCATTTCATTTCGGCCTTC |
| Esr2 | CCTGGTCTGGGTGATTTCGA | ACTGATGTGCCTGACATGAGAAAG |
| Gper1 | TGCTGCCATCCAGATTCAAG | GGGAACGTAGGCTATGGAAAGAA |
| 18S rRNA | CGCCGCTAGAGGTGAAATTCT | CATTCTTGGCAAATGCTTTCG |
Immunocytochemistry and morphometric analyses
After 2 DIV, the hypothalamic cultures were fixed by immersion in 4% paraformaldehyde with 0.12 M sucrose at 37 °C. After extensive rinses in phosphate-buffered saline solution (PBS), cultures were stained against β-tubulin class III (SDL.3D10). The protocol is described in detail elsewhere54. Briefly, on the first day cells were permeabilized with 0.2% Triton X-100 at room temperature followed by incubation in bovine serum albumin (BSA) and incubation in primary antibody against β-tubulin class III (1:400, Sigma-Aldrich, St. Louis, MO, USA) at 4 °C. On the second day, cells were rinsed in PBS and then incubated with anti-mouse biotinylated secondary antibody for one hour at room temperature. Reaction was magnified by incubation in Vectastain ABC immunoperoxidase reagent (Vector Laboratories, Burlingame, CA, USA), and visualized by reaction with 1.4 mM 3,3′-diaminobenzidine in phosphate buffer with H2O2. Cells were then dehydrated with serial dilutions of ethanol and mounted on glass slides for morphometric analysis.
Morphometric analyses were done using an image processor (Jandel Inc., Richmond, CA, USA) through an optical microscope (Carl Zeiss, Germany). The operator doing all morphometric analyses was blind to the genotype and treatment of the cell cultures. At 40X magnification, the operator evaluated all labeled neurons identified as a single cell from all other surrounding cells. Morphometric parameters of hypothalamic neurons in vitro have been previously described24 and follow those described for hippocampal neurons in culture23,55. According to these morphologic criteria, after 2 DIV most cells have a single long “major” process, thin and uniform in diameter, while several shorter “minor” processes also emerge from the soma55. Neurons with a major process 3 to 5 times longer than the rest of other processes were considered to have developed an axon (stage III of development). We recorded the total axonal length, soma area, length of minor processes, and the number of neurites per cell of 60 neurons for every experimental condition in each culture.
Statistical analyses
Data are expressed as mean + SEM. The sex chromosome effect on the expression of Ngn3 in FCG MF1 or CD1 cultures, as well as the neurite outgrowth were evaluated using two-tailed independent t-tests. The neuritogenic effect of E2, sex chromosomes and gonadal sex in FCG cultures was evaluated using three-way ANOVA. Two-way ANOVA was used to evaluate the effect of sex chromosomes and gonadal sex on the expression of ERs. One-way ANOVA was used to evaluate the mechanism of E2 effect on Ngn3 expression in male cultures. ANOVA was followed by Fisher’s least significance difference (LSD) post hoc test when appropriate. A level of p < 0.05 was considered as statistically significant. The n for statistical analysis was the number of independent cultures that corresponds to the number of pregnant mothers in CD1 cultures. For cell cultures prepared from FCG mice, the number of independent neuronal cultures corresponds to the number of embryos of each genotype and treatment. The FCG embryos were obtained from at least 5 pregnant mothers.
Acknowledgements
This research was supported by grants from Argentina: ANPCyT (PICT 2015 No. 1333) and SECyT-UNC (2018–2021) to MJC, and from Spain: Agencia Estatal de Investigación (BFU2017-82754-R) and CIBERFES to LMGS and MAA, and Programa CSIC de Cooperación Científica para el Desarrollo Programa EMHE-CSIC 2017 to LMGS and MJC. MJC would like to thank Dr. Paul Burgoyne (MRC National Institute for Medical Research, London, UK) for providing the MF1 transgenic mice.
Author contributions
C.D.C., L.M.G.S. and M.J.C. conceived and designed the research. C.D.C., L.E.C.Z., M.J.S., and F.R.M. performed all experiments and analyzed data. C.D.C., M.A.A., L.M.G.S. and M.J.C. interpreted results of experiments. C.D.C. prepared figures. C.D.C. and M.J.C. wrote manuscript. M.A.A. and L.M.G.S. edited and revised manuscript. M.J.C. drafted manuscript.
Data availability
No datasets were generated or analyzed during the current study. RRID:AB_2314198, Sigma-Aldrich Cat# SDL.3D10. RRID:AB_2338504, Jackson ImmunoResearch Labs Cat# 115-035-062.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lenz KM, McCarthy MM. Organized for sex - steroid hormones and the developing hypothalamus. Eur. J. Neurosci. 2010;32:2096–104. doi: 10.1111/j.1460-9568.2010.07511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Annu. Rev. Neurosci. 1984;7:413–42. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- 3.McEwen BS, et al. Steroid hormones as mediators of neural plasticity. J. Steroid Biochem. Mol. Biol. 1991;39:223–32. doi: 10.1016/0960-0760(91)90067-F. [DOI] [PubMed] [Google Scholar]
- 4.Carrer HF, Cambiasso MJ. Sexual differentiation of the brain: genes, estrogen, and neurotrophic factors. Cell. Mol. Neurobiol. 2002;22:479–500. doi: 10.1023/A:1021825317546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCarthy MM. Estradiol and the developing brain. Physiol. Rev. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm. Behav. 2009;55:570–578. doi: 10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold AP. A general theory of sexual differentiation. J. Neurosci. Res. 2017;95:291–300. doi: 10.1002/jnr.23884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold AP. Rethinking sex determination of non-gonadal tissues. Curr. Top. Dev. Biol. 2019;134:289–315. doi: 10.1016/bs.ctdb.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carruth LL, Reisert I, Arnold AP. Sex chromosome genes directly affect brain sexual differentiation. Nat. Neurosci. 2002;5:933–934. doi: 10.1038/nn922. [DOI] [PubMed] [Google Scholar]
- 10.Cisternas C, et al. Sex chromosome complement determines sex differences in aromatase expression and regulation in the stria terminalis and anterior amygdala of the developing mouse brain. Mol. Cell. Endocrinol. 2015;414:99–110. doi: 10.1016/j.mce.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 11.Cisternas C, Cabrera Zapata L, Arevalo M, Garcia-Segura L, Cambiasso M. Regulation of aromatase expression in the anterior amygdala of the developing mouse brain depends on ERβ and sex chromosome complement. Sci. Rep. 2017;7:5320. doi: 10.1038/s41598-017-05658-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scerbo MJ, et al. Neurogenin 3 mediates sex chromosome effects on the generation of sex differences in hypothalamic neuronal development. Front. Cell. Neurosci. 2014;8:1–13. doi: 10.3389/fncel.2014.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Shaughnessy PJ, et al. Fetal development of Leydig cell activity in the mouse is independent of pituitary gonadotroph function. Endocrinology. 1998;139:1141–1146. doi: 10.1210/endo.139.3.5788. [DOI] [PubMed] [Google Scholar]
- 14.O’Shaughnessy, P. J., Baker, P. J. & Johnston, H. The foetal Leydig cell–differentiation, function and regulation. Int. J. Androl. 29, 90–95; discussion 105-108 (2006). [DOI] [PubMed]
- 15.Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat. Rev. Neurosci. 2002;3:517–30. doi: 10.1038/nrn874. [DOI] [PubMed] [Google Scholar]
- 16.Pelling M, et al. Differential requirements for neurogenin 3 in the development of POMC and NPY neurons in the hypothalamus. Dev. Biol. 2011;349:406–16. doi: 10.1016/j.ydbio.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Salama-Cohen P, Arévalo M-A, Grantyn R, Rodríguez-Tébar A. Notch and NGF/p75NTR control dendrite morphology and the balance of excitatory/inhibitory synaptic input to hippocampal neurones through Neurogenin 3. J. Neurochem. 2006;97:1269–78. doi: 10.1111/j.1471-4159.2006.03783.x. [DOI] [PubMed] [Google Scholar]
- 18.Simon-Areces J, Membrive G, Garcia-Fernandez C, Garcia-Segura LM, Arevalo M-A. Neurogenin 3 cellular and subcellular localization in the developing and adult hippocampus. J. Comp. Neurol. 2010;518:1814–24. doi: 10.1002/cne.22304. [DOI] [PubMed] [Google Scholar]
- 19.Cambiasso MJ, Díaz H, Cáceres A, Carrer HF. Neuritogenic effect of estradiol on rat ventromedial hypothalamic neurons co-cultured with homotopic or heterotopic glia. J. Neurosci. Res. 1995;42:700–9. doi: 10.1002/jnr.490420513. [DOI] [PubMed] [Google Scholar]
- 20.Cambiasso, M. J., Colombo, J.A. & Carrer, H. F. Differential effect of oestradiol and astroglia-conditioned media on the growth of hypothalamic neurons from male and female rat brains. Eur. J. Neurosci. 12, 2291–2298 (2000). [DOI] [PubMed]
- 21.Gorosito SV, Cambiasso MJ. Axogenic effect of estrogen in male rat hypothalamic neurons involves Ca(2+), protein kinase C, and extracellular signal-regulated kinase signaling. J. Neurosci. Res. 2008;86:145–57. doi: 10.1002/jnr.21466. [DOI] [PubMed] [Google Scholar]
- 22.Gorosito SV, Lorenzo AG, Cambiasso MJ. Estrogen receptor alpha is expressed on the cell-surface of embryonic hypothalamic neurons. Neuroscience. 2008;154:1173–1177. doi: 10.1016/j.neuroscience.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Blanco G, Diaz H, Carrer HF, Beaugé L. Differentiation of rat hippocampal neurons induced by estrogen in vitro: effects on neuritogenesis and Na, K-ATPase activity. J. Neurosci. Res. 1990;27:47–54. doi: 10.1002/jnr.490270108. [DOI] [PubMed] [Google Scholar]
- 24.Díaz H, Lorenzo A, Carrer HF, Cáceres A. Time lapse study of neurite growth in hypothalamic dissociated neurons in culture: sex differences and estrogen effects. J. Neurosci. Res. 1992;33:266–281. doi: 10.1002/jnr.490330210. [DOI] [PubMed] [Google Scholar]
- 25.Lorenzo A, Díaz H, Carrer H, Cáceres A. Amygdala neurons in vitro: neurite growth and effects of estradiol. J. Neurosci. Res. 1992;33:418–435. doi: 10.1002/jnr.490330308. [DOI] [PubMed] [Google Scholar]
- 26.Reisert I, Pilgrim C. Sexual differentiation of monoaminergic neurons–genetic or epigenetic? Trends Neurosci. 1991;14:468–473. doi: 10.1016/0166-2236(91)90047-X. [DOI] [PubMed] [Google Scholar]
- 27.Ruiz-Palmero I, et al. Oestradiol synthesized by female neurons generates sex differences in neuritogenesis. Sci. Rep. 2016;6:31891. doi: 10.1038/srep31891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruiz-Palmero I, Simon-Areces J, Garcia-Segura LM, Arevalo MA. Notch/Neurogenin 3 Signalling is Involved in the Neuritogenic Actions of Oestradiol in Developing Hippocampal Neurones. J. Neuroendocrinol. 2011;23:355–364. doi: 10.1111/j.1365-2826.2011.02110.x. [DOI] [PubMed] [Google Scholar]
- 29.Arnold, A. P. Conceptual frameworks and mouse models for studying sex differences in physiology and disease: Why compensation changes the game. Exp. Neurol. 1–8 10.1016/j.expneurol.2014.01.021 (2014). [DOI] [PMC free article] [PubMed]
- 30.Graves JAM. Sex chromosome specialization and degeneration in mammals. Cell. 2006;124:901–14. doi: 10.1016/j.cell.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 31.Wijchers PJ, Festenstein RJ. Epigenetic regulation of autosomal gene expression by sex chromosomes. Trends Genet. 2011;27:132–140. doi: 10.1016/j.tig.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Ropers H-H, Hamel BCJ. X-linked mental retardation. Nat. Rev. Genet. 2005;6:46–57. doi: 10.1038/nrg1501. [DOI] [PubMed] [Google Scholar]
- 33.Chiurazzi P, Schwartz CE, Gecz J, Neri G. XLMR genes: update 2007. Eur. J. Hum. Genet. 2008;16:422–34. doi: 10.1038/sj.ejhg.5201994. [DOI] [PubMed] [Google Scholar]
- 34.Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–4. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- 35.Iwase S, et al. The X-linked mental retardation gene SMCX/JARID1C defines a family of histone H3 lysine 4 demethylases. Cell. 2007;128:1077–88. doi: 10.1016/j.cell.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 36.Welstead GG, et al. X-linked H3K27me3 demethylase Utx is required for embryonic development in a sex-specific manner. Proc. Natl. Acad. Sci. USA. 2012;109:13004–9. doi: 10.1073/pnas.1210787109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu J, Disteche CM. Sex differences in brain expression of X- and Y-linked genes. Brain Res. 2006;1126:50–55. doi: 10.1016/j.brainres.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 38.Xu J, Deng X, Disteche CM. Sex-specific expression of the X-linked histone demethylase gene Jarid1c in brain. PLoS One. 2008;3:1–6. doi: 10.1371/annotation/8b913538-74f4-4560-b700-0936a8e35847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolstenholme JT, Rissman EF, Bekiranov S. Sexual differentiation in the developing mouse brain: Contributions of sex chromosome genes. Genes, Brain Behav. 2013;12:166–180. doi: 10.1111/gbb.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang F, Babak T, Shendure J, Disteche CM. Global survey of escape from X inactivation by RNA-sequencing in mouse. Genome Res. 2010;20:614–22. doi: 10.1101/gr.103200.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schweiger S, et al. The Opitz syndrome gene product, MID1, associates with microtubules. Proc. Natl. Acad. Sci. USA. 1999;96:2794–9. doi: 10.1073/pnas.96.6.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu, T. et al. X-linked Microtubule-Associated Protein, Mid1, Regulates Axon Development. Proc. Natl. Acad. Sci. USA 110, (2013). [DOI] [PMC free article] [PubMed]
- 43.Chen R, Wu X, Jiang L, Zhang Y. Single-cell RNA-seq reveals hypothalamic cell diversity. Cell Rep. 2017;18:3227–3241. doi: 10.1016/j.celrep.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Correa SM, et al. An estrogen-responsive module in the ventromedial hypothalamus selectively drives sex-specific activity in females. Cell Rep. 2015;10:62–74. doi: 10.1016/j.celrep.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim D-W, et al. Multimodal Analysis of Cell Types in a Hypothalamic Node Controlling Social Behavior. Cell. 2019;179:713–728.e17. doi: 10.1016/j.cell.2019.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ikeda Y, Shen WH, Ingraham HA, Parker KL. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol. Endocrinol. 1994;8:654–62. doi: 10.1210/mend.8.5.8058073. [DOI] [PubMed] [Google Scholar]
- 47.Shinoda K, et al. Developmental defects of the ventromedial hypothalamic nucleus and pituitary gonadotroph in the Ftz-F1 disrupted mice. Dev. Dyn. 1995;204:22–29. doi: 10.1002/aja.1002040104. [DOI] [PubMed] [Google Scholar]
- 48.Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77:481–90. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 49.Büdefeld, T., SA, Tobet, S. & Majdič, G. Gonadal hormone independent sex differences in steroidogenic factor 1 knockout mice brain. Slov. Vet. Zb. 47 (2010). [PMC free article] [PubMed]
- 50.Majdic G, Tobet S. Cooperation of sex chromosomal genes and endocrine influences for hypothalamic sexual differentiation. Front. Neuroendocrinol. 2011;32:137–145. doi: 10.1016/j.yfrne.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grgurevic N, Büdefeld T, Spanic T, Tobet SA, Majdic G. Evidence that sex chromosome genes affect sexual differentiation of female sexual behavior. Horm. Behav. 2012;61:719–24. doi: 10.1016/j.yhbeh.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mir, F. R., Wilson, C., Cabrera Zapata, L. E., Aguayo, L. G. & Cambiasso, M. J. Gonadal hormone-independent sex differences in GABAA receptor activation in rat embryonic hypothalamic neurons. Br. J. Pharmacol. 10.1111/bph.15037 (2020). [DOI] [PMC free article] [PubMed]
- 53.Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc. Natl. Acad. Sci. USA. 1986;83:2496–500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cabrera Zapata LE, Bollo M, Cambiasso MJ. Estradiol-Mediated Axogenesis of Hypothalamic Neurons Requires ERK1/2 and Ryanodine Receptors-Dependent Intracellular Ca2+ Rise in Male Rats. Front. Cell. Neurosci. 2019;13:122. doi: 10.3389/fncel.2019.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J. Neurosci. 1988;8:1454–68. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study. RRID:AB_2314198, Sigma-Aldrich Cat# SDL.3D10. RRID:AB_2338504, Jackson ImmunoResearch Labs Cat# 115-035-062.