Abstract
Many areas intended for crop production suffer from the concomitant occurrence of heavy metal pollution and elevated salinity; therefore, halophytes seem to represent a promising perspective for the bioremediation of contaminated soils. In this study, the influence of Cd treatment (0.01–10.0 mM) and salinity stress (0.4 M NaCl) on the expression of genes involved in heavy metal uptake (irt2–iron-regulated protein 2, zip4–zinc-induced protein 4), vacuolar sequestration (abcc2–ATP-binding cassette 2, cax4–cation exchanger 2 pcs1–phytochelatin synthase 1) and translocation into aerial organs (hma4–heavy metal ATPase 4) were analyzed in a soil-grown semi-halophyte Mesembryanthemum crystallinum. The upregulation of irt2 expression induced by salinity was additionally enhanced by Cd treatment. Such changes were not observed for zip4. Stressor-induced alterations in abcc2, cax4, hma4 and pcs1 expression were most pronounced in the root tissue, and the expression of cax4, hma4 and pcs1 was upregulated in response to salinity and Cd. However, the cumulative effect of both stressors, similar to the one described for irt2, was observed only in the case of pcs1. The importance of salt stress in the irt2 expression regulation mechanism is proposed. To the best of our knowledge, this study is the first to report the combined effect of salinity and heavy metal stress on genes involved in heavy metal trafficking.
Keywords: heavy metal stress, heavy metals transporters, Mesembryanthemum crystallinum, phytoremediation, semi-halophyte
1. Introduction
Among abiotic stresses, salinity is one of the most important factors affecting crop growth. According to the FAO Land and Plant Nutrition Management Service, elevated salinity concerns more than 6% of the land area (approximately 400 million hectares) and increases by approximately 1–2% a year. This phenomenon is predominantly associated with human activities, such as excessive irrigation, deforestation, fertilization and irrigation with salt-contaminated water, resulting in poor drainage, elevated water table, waterlogging and salt accumulation in the root zone [1,2,3,4]. Even good quality water used for irrigation may contain 200–500 mg of soluble salts per litre. According to [2], a hectare of arable land may receive 3–5 tons of salts per year in this way. Salinity stress can be recognized by the occurrence of three symptoms: first, increased osmotic pressure, which results in water deficit conditions; second, accumulation of toxic ions in plant organs [4]; and third, nutritional imbalance, which influences the growth and development of challenged plants [5]. These symptoms are primarily responsible for adverse alterations in morphological, physiological and biochemical processes that disrupt the agricultural production and ecological balance of the area [3].
Many areas, including arable lands, suffer from concomitant contamination with heavy metals (HMs) and salts. Recently, the accumulation of HMs in the environment has increased as a consequence of intensified human activities, such as mining, smelting and agricultural practices, including the long-term application of fertilizers, fungicides or pesticides [6]. Since HMs are non-degradable, they may persist in a contaminated substrate for decades [7]. The threat posed by heavy metals to humans has an origin in plants, which are the first link in the food chain. Plants can take up HMs from various sources, such as air, water or soil; the latter process predominates and is dependent on many factors, such as temperature, soil pH, aeration and, clearly, on the particular species involved in the accumulation process.
Cadmium is one of the HMs that is particularly widely spread in the environment and has gained prominence as an important issue not only in urbanized lands, but also in less explored, rural, agricultural or wildlife areas. Cd toxicity results from altered uptake and transport of nutrient elements (Ca, Mg, P), disruption of water balance, inhibition of enzymes and reactive oxygen species (ROS) overproduction. As a consequence, sensitive plants growing in the Cd-polluted environment display such symptoms as chlorosis, growth inhibition and root tip browning [8]. Since plant responses to salinity and HM stresses rely on common physiological mechanisms, halophytes have been proposed as potentially useful candidates for the phytoremediation of Cd-contaminated saline soils [7,9,10,11]. Halophytes are the plants capable of growth and able to complete their life cycle in environments polluted with high salt concentrations [2]. Although these plants represent only 2% of the total plant species on Earth, they are a highly diverse group of organisms [12]. The high tolerance of halophytes towards salinity is a result of specific properties at the molecular, cellular and whole-plant levels, and is at least partially related to enhanced resistance to oxidative stress [2,5]. In addition to effective scavenging of salt stress-derived ROS, halophytes display a whole range of various mechanisms, including the development of special tubers at leaves or stems that may contain large amounts of salt, the production of enzymes that limit ion transfer through membranes, the generation of stress proteins and osmolytes and active metal detoxification in vacuoles, which enable homeostasis maintenance under stressful conditions [11].
Halophytic plants employ many strategies enabling the undisturbed execution of the developmental program under concurrent salinity and HM stresses. These strategies involve restricted metal uptake, exclusion of toxic ions from roots, efficient neutralization of metal ions in the protoplast and sequestration or translocation to remote organs [13]. The above mechanisms are regulated by a large number of genes and provide higher tolerance to both salt and Cd [5]. The members of the zinc iron permease (ZIP) family, including iron-regulated transporters (IRT), are responsible for zinc (Zn), manganese (Mn), iron (Fe) and Cd uptake [14]. Physiological studies confirmed the overexpression of ZIP family genes in plants exposed to Cd and nickel (Ni) [15]. Among the transporters involved in Cd trafficking, phytochelatins (PCs) are the best-known protoplast chelating agents required for the maintenance of Cd tolerance. Sequestration of Cd in vacuoles is a well-described process directing plant tolerance towards this pollutant, which involves at least a few known transporters. Cation exchangers (CAX) participate in Cd2+/Na+ antiport activity in tonoplasts exploiting a V-H+-ATPase-derived proton motive force [15]. In addition to CAXs, the C subfamily of the ATPase binding cassette (ABCC) has been recognized as an additional vacuolar metal-PC transporter. Although it has been reported that vacuolar sequestration of Cd by ABCC is essential for its complete detoxification, transporters from this subfamily are also involved in the transport of such ions as Zn, Cu, Mn and Fe [16]. Apart from these strategies, the translocation of Cd to remote organs, combined with sequestration in tissues not involved in vital metabolic processes, seems to play a crucial role in Cd tolerance. Effective translocation of Cd to the shoots requires active transport during loading to the xylem. The P-type heavy metal ATPases (HMA) are transporters involved both in Cd loading to xylem from vascular tissues (HMA4) [17]. Regulation of the expression of genes associated with the presented transporters may be necessary for the appropriate distribution of toxic metal ions at the cellular and whole-plant levels, which substantially contributes to the resultant Cd tolerance in plants [15].
Many halophytes were reported to be involved in HM remediation processes [10,18,19,20]. One of them is Mesembryanthemum crystallinum (the common ice plant), the model C3-CAM intermediate semi-halophyte, which is widely studied in physiological and biochemical research. This plant has been found to reveal a high tolerance to excess light, drought and salinity stresses. The dry weight of the plant may contain even 40% NaCl without showing visible symptoms of toxicity [9]. In addition to salinity tolerance, high resistance towards HMs has recently been confirmed. This halophyte is able to accomplish its life cycle at high concentrations of Cu and Zn present in the soil substrate. It was also confirmed that high doses of Ni did not induce any growth or developmental disturbances [9,21,22]. In our studies, we described high tolerance of soil-grown ice plants towards elevated Cd levels, regardless of the photosynthetic metabolism [23]. The obtained data strongly suggest the potential use of M. crystallinum in the phytoremediation of HM-polluted areas. The aim of the present study was to determine expression alterations of transporters involved Cd uptake (zip4, irt2), protoplast detoxification (pcs1), vacuolar sequestration (abbc2, cax4) and xylem loading/shoot translocation (hma4) upon salinity and heavy metal stress in a model semi-halophyte known for enhanced HM resistance. To the best of our knowledge, this study is the first to describe the impact of combined stresses, i.e., salinity and HM, on the expression of genes involved in heavy metals trafficking.
2. Results
2.1. Cadmium Treatment up to 10 mM Does Not Affect the Growth and Development of Soil-Grown Common Ice Plants
In our previous paper, we showed that Cd concentrations up to 1 mM had no detrimental effects on soil-grown ice plants [23]. In this study, with the treatment extended to a Cd concentration of 10 mM, no visible morphological symptoms of heavy metal stress in either NaCl-untreated (−NaCl) or salt-stressed (+NaCl) plants were observed. In particular, none of the applied treatments significantly modified the dry weight of roots and shoots of NaCl-untreated and salt-stressed ice plants (Table 1). The water status of the roots and shoots of the −NaCl and +NaCl ice plants was not altered by any of the applied Cd concentrations (Table 2). Moreover, the unaffected plant growth was also confirmed by maintaining a constant shoot-to-root (DW) ratio, which demonstrated that neither root nor shoot growth was disrupted by Cd presence (Table 3). Taken together, the results obtained through biometric analysis have demonstrated high tolerance of both plant groups towards Cd treatments of up to 10 mM.
Table 1.
Weight of roots and shoots of soil-grown NaCl-untreated (−NaCl) and salt-stressed (+NaCl) Mesembryanthemum crystallinum plants subjected to the elevated concentrations of Cd and control.
| Cadmium Concentration [mM] | Root DW [g/Plant] | Shoot DW [g/Plant] | ||
|---|---|---|---|---|
| −NaCl | +NaCl | −NaCl | +NaCl | |
| 0 | 0.29 ± 0.06a | 0.18 ± 0.06abc | 1.93 ± 0.13a | 2.03 ± 0.58a |
| 0.01 | 0.27 ± 0.05a | 0.13 ± 0.01bc | 1.90 ± 0.18a | 2.20 ± 0.41a |
| 0.1 | 0.20 ± 0.05abc | 0.21 ± 0.09abc | 1.97 ± 0.22a | 2.29 ± 0.57a |
| 1.0 | 0.26 ± 0.07a | 0.25 ± 0.04ab | 2.09 ± 0.33a | 2.06 ± 0.33a |
| 10.0 | 0.21 ± 0.03abc | 0.12 ± 0.04c | 1.80 ± 0.35a | 1.97 ± 0.37a |
Means within root/shoot columns followed by the same letters are not significantly different at p < 0.05 according to Duncan’s test (N = 4, mean value ± SD).
Table 2.
Root and shoot water content of soil-grown NaCl-untreated (−NaCl) and salt-stressed (+NaCl) M. crystallinum plants subjected to the elevated concentrations of Cd and control.
| Cadmium Concentration [mM] | Root Water Content [cm3 g−1 DW] | Shoot Water Content [cm3 g−1 DW] | ||
|---|---|---|---|---|
| −NaCl | +NaCl | −NaCl | +NaCl | |
| 0 | 11.78 ± 4.27ab | 8.77 ± 2.41abc | 22.27 ± 1.43a | 14.54 ± 1.48b |
| 0.01 | 8.97 ± 0.53abc | 6.47 ± 1.03bc | 23.10 ± 2.22a | 15.10 ± 1.37b |
| 0.1 | 6.79 ± 0.89bc | 5.34 ± 0.28bc | 22.49 ± 2.84a | 15.37 ± 1.48b |
| 1.0 | 6.94 ± 0.32bc | 7.99 ± 0.94abc | 22.70 ± 2.75a | 15.46 ± 1.53b |
| 10.0 | 7.23 ± 1.15abc | 10.04 ± 2.55ab | 23.24 ± 2.61a | 14.98 ± 2.81b |
Means within root/shoot columns followed by the same letters are not significantly different at p < 0.05 according to Duncan’s test (N = 4, mean value ± SD).
Table 3.
Shoot-to-root dry weight (DW) ratio in soil-grown NaCl-untreated (−NaCl) and salt-stressed (+NaCl) M. crystallinum plants subjected to elevated Cd concentrations and control.
| Cadmium Concentration [mM] | Shoot-to-Root (DW) Ratio | |
|---|---|---|
| −NaCl | +NaCl | |
| 0 | 7.01 ± 1.54c | 10.22 ± 1.74bc |
| 0.01 | 7.11 ± 0.84c | 15.51 ± 3.75ab |
| 0.1 | 9.57 ± 1.75bc | 11.13 ± 3.19b |
| 1.0 | 7.58 ± 2.33c | 8.57 ± 0.78c |
| 10.0 | 7.44 ± 0.54c | 16.88 ± 3.46ab |
Means within columns followed by the same letters are not significantly different at p < 0.05 according to Duncan’s test (N = 4, mean value ± SD).
2.2. NaCl-Stressed Plants Accumulated More Cd than NaCl-Untreated Plants; Cd Was Deposited mostly in the Roots
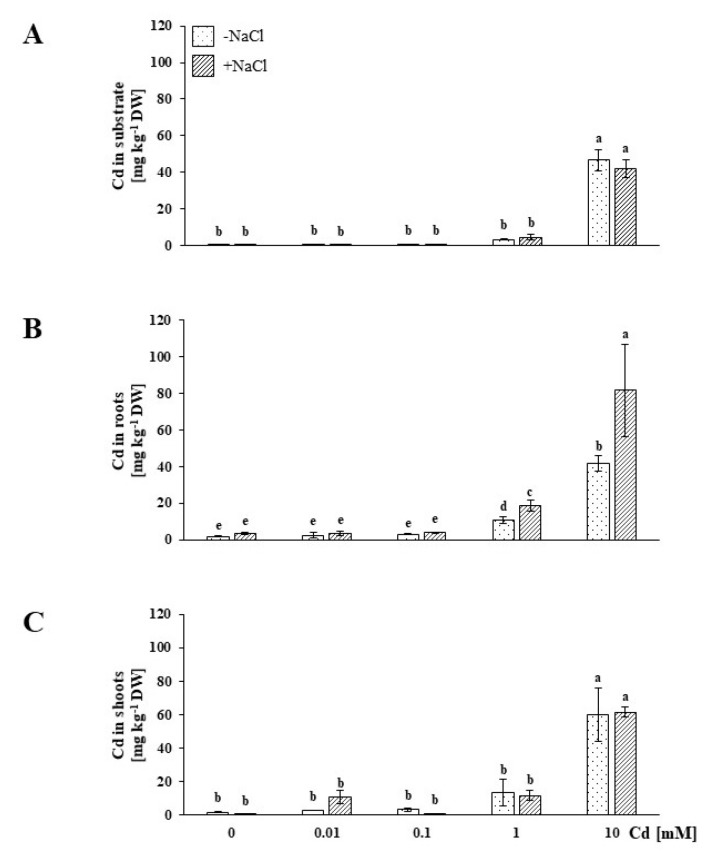
To determine the concentration of Cd fraction available for the plants during the experiment, the substrate samples were analyzed with atomic absorption spectrometry (AAS). Detailed results regarding changes in bioavailable Cd concentration during subsequent days of treatment are presented in the supplementary data set (Figure S1). It is worth mentioning that, upon administration of the highest Cd treatment (10 mM), the salt-stressed (+NaCl) plants, beginning on the first day of the experiment, were exposed to a significantly higher concentration of bioavailable Cd in comparison to the NaCl-untreated plants. According to our preceding study concerning the M. crystallinum-Cd interaction [23], we focused on the results of the eighth day of treatment. In this study, we confirmed our previous observations by showing that with up to 0.1 mM treatment, the amount of bioavailable Cd was very low (Figure 1A). The low heavy metal bioavailability in the substrate was reflected by low Cd concentrations measured in corresponding root and shoot samples (Figure 1B,C). On the other hand, we found that under 1 and 10 mM Cd treatment, both −NaCl and +NaCl plants were exposed to similar concentrations of bioavailable Cd. As in our earlier work, the salt-stressed plants subjected to a 1 mM concentration accumulated more Cd compared to the NaCl-untreated plants, and the heavy metal was stored mainly in the roots (Figure 1B). Interestingly, this relationship was also maintained under 10 mM Cd treatment, where the salt-stressed plant roots accumulated almost two-fold more Cd in comparison to the roots of NaCl-untreated plants. The elevated Cd amounts were deposited in shoots of NaCl-untreated and salt-stressed plants only under 10 mM concentration, without distinctive differences being observed between both plant groups.
Figure 1.
Contents measured in the substrate (A), roots (B) and shoots (C) of soil-grown NaCl-untreated (−NaCl) and salt-stressed (+NaCl) Mesembryanthemum crystallinum plants subjected to concentrations of 0.01, 0.1, 1, 10 mM Cd and the control (0.0 mM). Different letters above the bars indicate statistically significant differences at p ≤ 0.05 by Duncan’s post hoc test (N = 4, mean value ± SD).
2.3. Genes Involved in Divalent Cation Uptake, Vacuolar Sequestration and Translocation Are Upregulated in the Roots of Plants Exposed to Salinity Stress
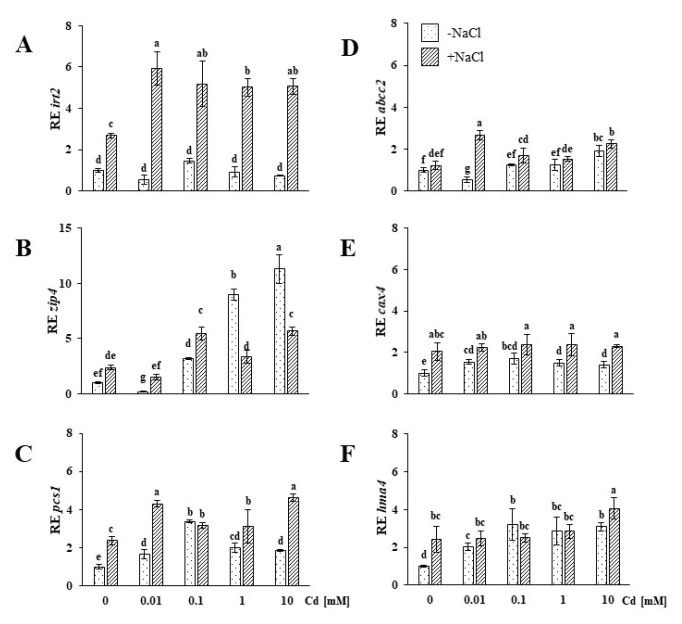
To determine how salinity stress occurrence and/or Cd exposure affect the expression of the genes involved in uptake, vacuolar sequestration and aerial organ translocation, corresponding transcript levels were analysed with a qPCR technique in roots and shoots of the NaCl-untreated and salt-stressed plants exposed to elevated Cd concentrations. Expression of irt2 and zip4, i.e., plasma membrane transporters involved in divalent cation uptake, were measured exclusively in the roots of both plant groups. The expression of irt2 found in the Cd-untreated +NaCl plants was over two-fold higher in comparison to the control (Cd-, NaCl-untreated plants, Figure 2A). Cd treatment caused a greater than two-fold increase in irt2 expression in the roots of salt-stressed plants, contrary to −NaCl roots, where none of the applied Cd treatments altered the gene expression. In the case of zip4, no significant difference was found between the control and salt-stressed plants (Figure 2B). The lowest Cd concentration (0.01 mM) caused zip4 downregulation in the NaCl-untreated plants, while in the salt-stressed plants, the expression of this gene remained unaltered. Upon treatment with 0.1 mM Cd, zip4 was upregulated in both groups; however, the concentration-dependent tendency to increase expression was maintained only in the −NaCl plants. In the roots of the salt-stressed (+NaCl) plants, treatment with 1 and 10 mM Cd resulted in similar zip4 upregulation.
Figure 2.
Expression of iron-regulated protein 2, irt2 (A), zinc-induced protein 4, zip4 (B), phytochelatin synthase 1, pcs1 (C), ATP-binding cassette type c 2, abcc2 (D), cation exchanger 4, cax4 (E), and heavy metal ATPase 4, hma4 (F) genes in the roots of soil-grown NaCl-untreated (−NaCl) and salt-stressed (+NaCl) Mesembryanthemum crystallinum plants subjected to concentrations of 0.01, 0.1, 1, 10 mM Cd and control (0.0 mM). Different letters above the bars indicate statistically significant differences at p ≤ 0.05 by Duncan’s post hoc test (N = 4, mean value ± SD).
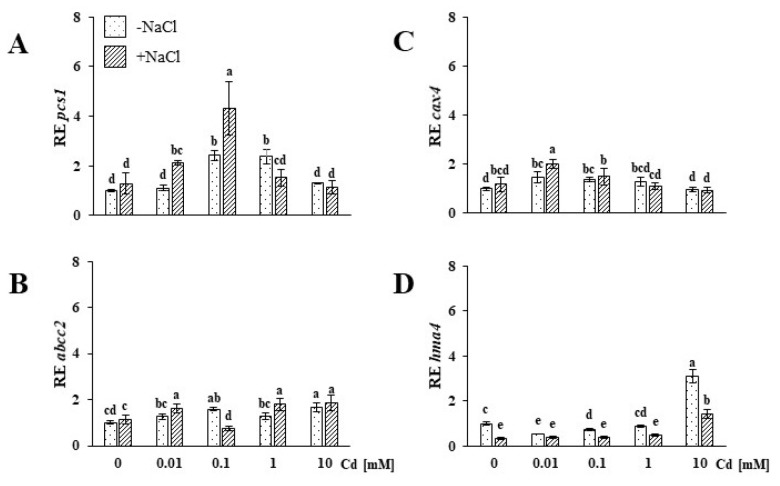
Phytochelatin synthase 1 (pcs1) expression found in the roots of the control (Cd-, NaCl-untreated) plants was lower compared to the salt-stressed plants (Figure 2C). Upregulation of pcs1 in roots of NaCl-untreated (−NaCl) plants was observed for all Cd treatments. In the case of the salt-stressed plants, Cd-induced pcs1 expression in all tested treatments. One can note that a similar cumulative effect of both stressors was observed for irt2, as described above. In both tested plant groups, the upregulation effect was not concentration-dependent, and the most pronounced induction was found at 0.1 mM Cd in −NaCl plants and 0.01 and 10 mM Cd in +NaCl plants. The regulation of pcs1 expression in shoots was affected differently by the applied stressors. No significant differences in the pcs1 expression between shoots of untreated −NaCl and +NaCl plants, were found (Figure 3A). Upon Cd treatment of NaCl-untreated plants, the gene expression was upregulated only in the cases of 0.1 and 1 mM Cd. Concentration-dependent upregulation of pcs1 expression was observed in shoots of salt-stressed plants subjected to up to 0.1 mM Cd.
Figure 3.
Expression of phytochelatin synthase 1, pcs1 (A), ATP-binding cassette type c 2, abcc2 (B), cation exchanger 4, cax4 (C), and heavy metal ATPase 4, hma4 (D) genes in the shoots of soil-grown NaCl-untreated (−NaCl) and salt-stressed (+NaCl) Mesembryanthemum crystallinum plants subjected to concentrations of 0.01, 0.1, 1, 10 mM Cd and control (0.0 mM). Different letters above the bars indicate statistically significant differences at p ≤ 0.05 by Duncan’s post hoc test (N = 4, mean value ± SD).
ATPase-binding cassette 2 (abcc2) gene expression levels measured in the roots of the control (Cd-, NaCl-untreated plants) and salt-stressed plants differed insignificantly (Figure 2D). In −NaCl plants, treatment with 0.01 mM Cd resulted in the downregulation of abcc2. Application of 0.1 and 1 mM did not affect the expression of the gene. At the highest Cd concentration (10 mM), abcc2 was upregulated. On the other hand, in the roots of the salt-stressed plants, Cd application induced random changes that were not correlated with increasing heavy metal concentrations: 0.01 and 10 mM Cd treatment led to abcc2 upregulation, while 0.1 and 1 mM did not affect the expression of this gene. We found no significant differences in the shoot abcc2 expression levels of the −NaCl (control) and salt-stressed (+NaCl) plants (Figure 3B). In the case of the NaCl-untreated plants, the gene expression was slightly modified only in response to 0.1 and 10 mM Cd concentrations. In turn, in the shoots of salt-stressed plants, all the treatments, except 0.1 mM Cd, significantly upregulated abcc2 expression.
As in the case of the hma4 and pcs1 genes, the cax4 (cation exchanger 4) expression found in roots of the Cd-untreated, salt-stressed plants was higher in comparison to controls (Figure 2E). None of the applied Cd concentrations enhanced the cax4 expression, as established in the salt-stressed roots. On the other hand, in the roots of the −NaCl plants, all Cd treatments upregulated cax4. We found no difference in the shoot cax4 expression of control (Cd-untreated, NaCl-untreated plants) and salt-stressed plants (Figure 3C). Cd treatments caused concentration-independent alterations of cax4 expression in the shoots of both plant groups. The gene was upregulated by 0.01 and 0.1 mM Cd in NaCl-untreated plants and by 0.01 mM in salt-stressed plants.
In the roots of the salt-stressed ice plants, we found a significantly higher hma4 (heavy metal ATPase 4) expression compared to the control (Cd-untreated, NaCl-untreated plants) (Figure 2F). Cd treatments did not modify the hma4 expression established in plant roots after salt stress, except for the highest concentration, which resulted in a significant gene upregulation. In the NaCl-untreated plants, the Cd application starting with the lowest applied concentration induced hma4 to the level found for the salt-stressed plants. The hma4 expression determined in the shoots of salt-stressed plants was lower than that observed for the control (Figure 3D). Cd treatments caused downregulation of the gene in most of the examined variants. hma4 upregulation was observed in both tested groups only in response to the highest Cd treatment (10 mM), and the effect was more pronounced for the NaCl-untreated plants.
3. Discussion
Essential heavy metals, e.g., Zn or Fe, are vital for plants to achieve intact growth and development, while the involvement of their non-essential relatives, i.e., Hg and Cd, in these processes is doubtful at best. Cadmium uptake from the soil is performed predominantly in the form of divalent cations and to some extent as chelates [15]. IRT1 and IRT2, as well as their respective orthologues, are ZIP family members. As divalent transporters, these proteins are responsible mostly for the uptake of Fe; however, their involvement in the trafficking of non-essential metals, such as Cd, was confirmed [24,25,26]. On the other hand, insufficient Fe levels promote Cd influx and accumulation in plants, while Fe supply initiates IRT1-mediated Cd uptake [27,28]. Based on the soil substrate analysis, for concentrations up to 1 mM, the amount of bioavailable Cd was very low. Despite this finding, the salt-stressed plants somehow sensed the introduced heavy metal which, in our opinion, was reflected by the induced irt2 expression already observed at the lowest Cd treatment. It was recently discovered that irt1 and irt2 expression was amplified in glycophytic green grams (Vigna radiata) under Cd treatment in Fe-sufficient conditions [29]. In this study, we observed that salt stress upregulated irt2 expression, and further Cd application enhanced this effect. The lack of Cd-triggered upregulation of irt2 found in the NaCl-untreated plants could suggest that for halophytes, salt stress is required for Cd-induced modulation of irt2 expression. Moreover, the observed interplay between salinity stress and irt2 transcription regulation suggests an upstream position of salt stress in the irt2 expression regulation pathway. The rate of Cd and Fe uptake via IRT transporters is orchestrated with a complex regulatory system, in which bioavailability seems to play an important role. This property is a result of antagonistic interactions recognized between both metals. For example, Cd presence can interfere with Fe assimilation, and thus contribute to Fe deficiency, despite its availability in the soil [30]. It was earlier proposed that the Cd-induced enhanced expression of irt2 found in green grams is a part of the mechanism that allows coping with elevated Cd concentrations through improved Fe uptake [29]. However, at high bioavailable Cd concentrations, the effectiveness of such a mechanism could be negligible. In our opinion, more Cd accumulated in the roots of plants exposed to salinity (+NaCl), in comparison to NaCl-untreated plants (−NaCl) found under 10 mM treatment, despite similar Cd bioavailabilities, which could result from enhanced irt2 expression. On the other hand, unaltered irt2 transcript levels observed in the NaCl-untreated (−NaCl) plants could be responsible for restricted Cd uptake and significantly lower root accumulation compared to salt-stressed (+NaCl) plants. In conclusion, in a model of semi-halophyte M. crystallinum, salt stress plays an important role in the regulation of irt2 expression during the plant response to elevated Cd concentrations. Moreover, both the salt and Cd stress effects on irt2 expression were cumulative. The involvement of other ZIP family members, e.g., ZIP4, in essential (Mn) and non-essential (Cd) metal trafficking was confirmed [31]. Transcriptional regulation of ZIP transporters under Cd or salinity stress has not been thoroughly elucidated. Recently, enhanced expression of zip4 in response to elevated Cd concentrations was described for non-hyperaccumulating ecotypes of Sedum alfredii [32]. A parallel result was observed in our experiment for both the NaCl-untreated and salt-stressed plants. Interestingly, our findings suggest that for the examined model semi-halophyte, the scheme of zip4 expression, contrary to irt2, was not affected to such an extent by salt stress. The enhanced zip4 expression found in response to elevated Cd concentration in the NaCl-untreated plants implies that plant salinity stress was not required for Cd-induced modification of the zip4 expression scheme. Moreover, contrary to irt2 regulation, no cumulative effect was observed, and both stressors caused zip4 expression upregulation independently. Although the influence of the two stress factors on zip4 expression was confirmed in this study, the accumulated Cd amounts, especially in salt-stressed roots, suggest the existence of a more complex mechanism orchestrating heavy metal uptake.
Phytochelatins (PC) are a well-described group of metal-complexing glutathione (GSH) derivatives, whose synthesis is rapidly induced in response to heavy metals [33]. PC synthase (PCS), whose gene pcs1 has been recognized in A. thaliana and yeast, is responsible for phytochelatin synthesis [34,35]. The roles of PC and, indirectly, PCS in non-essential heavy metal detoxification have been unequivocally indicated with A. thaliana PC-deficient cad1 mutants (AtPCS1 mutation) hypersensitive to As, Cd and Hg [36,37]. The GSH pool and PC synthesis remain interconnected, which revealed enhanced PCS activity, along with increasing concentrations of exogenously applied GSH and Cd in the tobacco BY-2 cell line [38]. The alteration of the GSH pool in response to different abiotic stresses is a well-described phenomenon, related mostly to antioxidative protection [39]. Salinity stress-induced CAM is accompanied by a significant increase in the GSH pool in Mesembryanthemum crystallinum [40]. The results of our study suggest that salt stress could fortify PC synthesis, not only by elevating the GSH pool, but also through an enhanced expression of pcs1. The mentioned expression alteration, however, was organ-specific and pronounced, especially in the roots. Interestingly, Cd treatment affected the root pcs1 expression in both the NaCl-untreated and salt-stressed plants, which resulted in even stronger gene expression in the latter group, and further confirmed the cumulative effect of both stressors on the pcs1 regulatory pathway. In earlier studies, it was documented that overexpression of the A. thaliana pcs1 gene enhanced Cd accumulation and tolerance in tobacco seedlings as a result of increased PC synthesis [41]. High levels of PC identified in the Brassica napus phloem sap [42], as well as highly expressed pcs1 in the A. thaliana phloem-loading cells, support the involvement of the PC-PCS system in long-distance metal distribution [43]. According to the above-mentioned studies, one cannot exclude the involvement of upregulated pcs1 expression in phloem-mediated Cd-PC complex translocation, since significant concentrations of Cd were measured in shoots of both the NaCl-untreated and salt-stressed plants at the highest applied Cd concentration. In summary, the results of this study suggest that applied abiotic stressors, namely, salt and Cd stress, can synergistically affect the regulatory pathway of pcs1, contributing to the enhanced detoxifying potential of salt-stressed halophytes.
The next stage of heavy metal management involves ATP-dependent sequestration of metal-PC complexes within the vacuoles. Translocation of metal complexes via the tonoplast is carried out mainly by ATP-binding cassette (ABC)-type membrane transporters. The role of two best-described A. thaliana ABCC-type transporters, namely, AtABCC1 and AtABCC2, in Cd and Hg detoxification through vacuolar sequestration was demonstrated earlier [44]. As in the case of previously described genes involved in heavy metal homeostasis, the influence of salt stress on abcc2 expression regulation has not been fully elucidated. The results obtained in this study suggest that, in contrast to pcs1 and irt2, salt stress did not participate in abcc2 expression regulation of roots and shoots. The response of ABCC-type transporter genes to heavy metal stress is considerably better known. It was demonstrated that Cd treatment did not affect A. thaliana abcc1 and abbc2 gene expression [45]; however, it enhanced the abcc3 transcript level. Recently, similar abcc1 and abcc2 responses to Cd were also confirmed for rapeseed and wheat [46,47]. Our findings agree with the above-mentioned findings and support the idea of the lack of heavy metal involvement in halophyte abcc2 expression regulation. Taken together, the experimental data indicate that both salt stress and Cd treatment have a rather slight effect on the abcc2 gene regulatory pathway.
In addition to ATP-dependent transporters, such as ABCC-type transporters, the vacuolar sequestration of cations relies on antiporters energized by V-H+-ATPases and V-PPases. Cation exchangers (CAX) are a family of such antiporters predominantly responsible for Ca2+ homeostasis, but are also involved in other divalent cation trafficking [48]. Among the six CAXs identified in Arabidopsis thaliana, CAX2 and CAX4 are strongly involved in vacuolar sequestration of Cd, thereby conferring heavy metal tolerance [49,50]. The response of monovalent cation exchangers to salt stress is a well-described phenomenon [51,52]. However, the behavior of divalent transporters, such as CAXs, during salinity stress has not been determined. This subject requires elucidation, since the strong induction of activity and increased content of V-H+-ATPase, the enzyme responsible for the accumulation of an electrochemical gradient exploited during cation transport, has been revealed in M. crystallinum during salinity stress [53,54]. According to [55], the cax4 steady-state expression level of the shoot is very low compared to the root. In this study, we confirmed a small share of both salinity and Cd treatment in the regulation of shoot cax4 expression. On the other hand, cax4 expression in roots was enhanced by salt stress and Cd treatment independently.
Heavy metals that became neutralized inside the protoplast, either as free cations or chelates, can be applied to the metal circulation system of xylem and phloem, respectively [43]. While the mechanism regarding phloem loading and circulation still requires elucidation, the involvement of heavy metal ATPases (HMAs) in Cd xylem loading leaves no doubt. Most HMAs are influx pumps involved in the trafficking of both essential and toxic heavy metals. These ATPases may confer heavy metal resistance, such as AtHMA4, for which dysfunctional mutants were shown to be sensitive to elevated Cd and Zn levels [56]. It was proved that AtHMA2 and AtHMA4 were extraordinary ATPases [57]. Contrary to other ATPases, these proteins act as efflux pumps responsible for Cd and Zn xylem loading, thereby establishing the root-to-shoot translocation of both heavy metals. Although AtHMA4 is expressed predominantly in the roots, some environmental factors can alter its expression in other plant organs. It was shown that in the shoots of the A. thaliana seedlings, hma4 expression was upregulated in response to sodium chloride [58]. In contrast to the aforementioned study, salt stress downregulated and upregulated hma4 in shoots and roots, respectively. On the other hand, Cd treatment applied in our experiment to the NaCl-untreated plants upregulated not only hma4 but also cax4, pcs1 and zip4 in the roots. Interestingly, hma4 upregulation, as observed either for NaCl- or Cd-treatments, was not reflected by a significant rate of Cd translocation, with the only exception being the 10 mM Cd supplementation. Taken together, the above data, analogous to the cases of cax4 and pcs1, show that both salt and Cd stress enhanced hma4 expression in an organ-specific manner. However, similar to cax4, but contrary to irt2 and pcs1 regulation, the effect of both stressors was not cumulative; hence, NaCl and Cd could independently establish root readiness for heavy metal translocation at the transcriptional level. A different approach, however, was found earlier in glycophytes representative. Namely, [59] reported that AtHMA4 root expression was downregulated upon exposure to Cd stress. A similar response was later observed in the shoots of A. thaliana seedlings [58]. On the other hand, in Cd hyperaccumulators, such as Arabidopsis helleri and Thlaspi caerulescens, the HMA4 constitutive expression in roots and shoots was significantly higher in comparison to their Cd-sensitive counterparts [60,61]. In summary, it is possible that halophytes may represent intermediate features between sensitive and hyperaccumulating plants.
4. Materials and Methods
4.1. Plant Cultivation and Cd Treatment
Mesembryanthemum crystallinum L. seeds were sown on soil substrate in a greenhouse under 300–350 µmol photons m−2 s−1 of photosynthetically active radiation (PAR), a 16/8 h day/night period (25 °C /17 °C temperature) and 60%/65% relative humidity (RH). For plant cultivation, the market-available universal soil substrate “Hawita Fruhstorfer type LD80” (Hawita Gruppe GmbH, Germany) was used. The “LD80” soil is a ready-to-use substrate made with peat (H4–H6 and H6–H8), clay and type dependent ingredients, pH value (CaCl2) of 5.9, and salinity (1.0 in g/L KCl). The substrate contained CaCO3 and a long-term delivery fertilizer, providing N, P, K and Mg in concentrations of 150, 150, 250 and 130 mg/L, respectively. Two weeks after sowing, each seedling with a fully developed second leaf pair was transferred to an individual 1.2-L round pot with dimensions of 100 mm height × 125 mm deep. Six-week-old plants were divided into two groups: the first was irrigated with water (NaCl-untreated, −NaCl), while the second one was irrigated with 0.4 M NaCl (salt-stressed, +NaCl) for 14 days. In the next step, 8-week-old plants of both mentioned groups were irrigated with 10 cm3 solution of 0 (control), 0.01, 0.1, 1 and 10 mM CdCl2 (Sigma Aldrich, Saint Louis, MO, USA) each day of the 8-day-long treatment. No leakage from pots was detected during Cd application. On the day after the last Cd treatment, four plants of each experimental variant were harvested (roots and shoots independently). The fourth pair of leaves of each plant were collected, immediately frozen in liquid nitrogen, ground and then stored at −80 °C for molecular analyses. The remaining shoots (including leaves) and roots were used for biometric and Cd concentration analyses.
4.2. Biometric Analysis
For growth parameter and tissue water content determination, plant organs were prepared according to the procedures described in [9], with a slight modification regarding desiccation temperature. Specifically, the collected root parts were gently rinsed with cold distilled water until the soil substrate was removed; the shoots were rinsed briefly, and the roots were blotted with filter papers. For biometric analyses, the fresh weight was measured immediately, and the dry weight was assessed after 48 h of desiccation in an oven at 105 °C. The tissue water content (TWC) was calculated on a dry weight basis as follows:
| TWC (cm3 g−1 DW) = (FW − DW)/DW |
where FW and DW are the fresh and dry weights, respectively.
4.3. Cadmium Concentration Analysis
The soil substrate remaining after plant harvest was collected and stored at 4 °C. Prior to the analysis, the substrate was dried for 48 h in an oven at 105 °C and sieved (2 mm). For determination of the substrate, the Cd content extraction method involving the use of 0.01 M CaCl2, as described in [62], was employed. Each extraction was performed on 270 mg of soil substrate. Determination of M. crystallinum root and shoot Cd concentrations was carried out according to the extraction method previously described by [63]. The Cd content within the substrate and plant organs was measured with the method employing Graphite Furnace-Atomic Absorption Spectrometry (GF-AAS, Thermo iCE3000, Waltham, MA, USA). All standards were purchased from Sigma Aldrich (Saint Louis, MO, USA). All the reagents used in the experiment were trace element grade.
4.4. RNA Preparation
Total RNA was extracted from ground M. crystallinum roots and shoots frozen in liquid nitrogen (three plants per sample) using a Total RNA Mini Kit (Bio-Rad, Hercules, CA, USA). RNA purity and quantity were determined using a Biospec-Nano (Shimadzu, Japan).
4.5. qPCR
An iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA) was used for reverse transcription carried out on 1000 ng of total RNA after digestion with DNase I (DNA I Amplification Grade, Merck, US). During qPCR, the samples were labelled with SYBR Green (iQ™ SYBR® Green Supermix, Bio-Rad, Hercules, CA, USA) fluorescent dye. For a single reaction, 10–15 ng of cDNA and 150 nM of gene-specific primers were used (Supplementary Table S1). To test amplification specificity, a dissociation curve was acquired by heating samples from 60 °C to 95 °C. As a housekeeping reference, ubiquitin was used. The reaction efficiency was tested by serial dilutions of cDNAs with gene-specific primers (Supplementary Figure S1). The expression was calculated from at least three reactions according to [64]. Plants not treated with NaCl (−NaCl) and irrigated with 0 mM Cd served as calibrators.
4.6. Statistical Analysis
The results were analysed with Statistica 13.3 (StatSoft, Kraków, Poland) statistical software. One-way ANOVA followed by a post hoc test was used to evaluate individual treatment effects at p ≤ 0.05.
5. Conclusions
Salinity and Cd stress-enhanced expression of the root genes irt2, cax4, hma4, pcs1 and zip4 was verified using the ice plant model. For irt2 and pcs1, a cumulative effect of both stressors on gene expression was found. Moreover, the role of salinity stress as an upstream regulator in the halophyte irt2 expression scheme was suggested.
Abbreviations
ABCC: ATPase-binding cassette type c; CAM, Crassulacean acid metabolism; CAX, cation exchanger; GF-AAS, Graphite Furnace-Atomic Absorption Spectrometry; HM, heavy metal; HMA, heavy metal ATPase; IRT, iron-regulated transporter; PCS, phytochelatin synthase; TWC, tissue water content; ZIP, ZRT IRT-like Protein; ZRT, zinc-regulated protein.
Supplementary Materials
The following are available online at https://www.mdpi.com/2223-7747/9/4/475/s1, Figure S1. Cadmium concentrations measured on the 1st, 3rd and 8th day of the experiment in the substrate of soil-grown NaCl-untreated (−NaCl) and salt-stressed (+NaCl) Mesembryanthemum crystallinum plants; Table S1. Sequences of primers employed for quantitative analysis of gene expression.
Author Contributions
M.N.—formal analysis, investigation, visualization, writing—original draft; A.K.—investigation, resources, writing—original draft; R.J.J.—formal analysis, investigation; P.S.—investigation, resources; P.K.—writing—review & editing; Z.M.—supervision, writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Science Centre (Poland), OPUS Project 2016/21/B/NZ9/00813.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Machado R., Serralheiro R. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae. 2017;3:30. doi: 10.3390/horticulturae3020030. [DOI] [Google Scholar]
- 2.Parihar P., Singh S., Singh R., Singh V.P., Prasad S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015;22:4056–4075. doi: 10.1007/s11356-014-3739-1. [DOI] [PubMed] [Google Scholar]
- 3.Shrivastava P., Kumar R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015;22:123–131. doi: 10.1016/j.sjbs.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zanetti F., Zegada-Lizarazu W., Lambertini C., Monti A. Salinity effects on germination, seedlings and full-grown plants of upland and lowland switchgrass cultivars. Biomass Bioenerg. 2019;120:273–280. doi: 10.1016/j.biombioe.2018.11.031. [DOI] [Google Scholar]
- 5.Hasanuzzaman M., Hakeem K., Nahar K., Alharby H. Plant Abiotic Stress Tolerance Agronomic, Molecular and Biotechnological Approaches, Plant Abiotic Stress Tolerance. Springer Nature; Cham, Switzerland: 2019. [Google Scholar]
- 6.Asrari E. Heavy Metal Contamination of Water and Soil: Analysis, Assessment, and Remediation Strategies. Apple Academic Press; New York, NY, USA: 2014. [Google Scholar]
- 7.Lutts S., Lefèvre I. How can we take advantage of halophyte properties to cope with heavy metal toxicity in salt-affected areas? Ann. Bot. 2015;115:509–528. doi: 10.1093/aob/mcu264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagajyoti P.C., Lee K.D., Sreekanth T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010;8:199–216. doi: 10.1007/s10311-010-0297-8. [DOI] [Google Scholar]
- 9.Amari T., Ghnaya T., Debez A., Taamali M., Youssef N.B., Lucchini G., Sacchi G.A., Abdelly C. Comparative Ni tolerance and accumulation potentials between Mesembryanthemum crystallinum (halophyte) and Brassica juncea: Metal accumulation, nutrient status and photosynthetic activity. J. Plant Physiol. 2014;171:1634–1644. doi: 10.1016/j.jplph.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 10.Wang H.L., Tian C.Y., Jiang L., Wang L. Remediation of heavy metals contaminated saline soils: A halophyte choice? Environ. Sci. Technol. 2014;48:21–22. doi: 10.1021/es405052j. [DOI] [PubMed] [Google Scholar]
- 11.Zhu J.K. Plant salt tolerance. Trends Plant Sci. 2001;6:66–71. doi: 10.1016/S1360-1385(00)01838-0. [DOI] [PubMed] [Google Scholar]
- 12.Aslam R., Bostan N., e-Amen N., Maria M., Safdar W. A critical review on halophytes: Salt tolerant plants. J. Med. Plant Res. 2011;5:7108–7118. [Google Scholar]
- 13.Sruthi P., Shackira A.M., Puthur J.T. Heavy metal detoxification mechanisms in halophytes: An overview. Wetl. Ecol. Manag. 2017;25:129–148. doi: 10.1007/s11273-016-9513-z. [DOI] [Google Scholar]
- 14.Rascio N., Navari-Izzo F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011;180:169–181. doi: 10.1016/j.plantsci.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Verbruggen N., Hermans C., Schat H. Molecular mechanisms of metal hyperaccumulation in plants. New Phytol. 2009;181:759–776. doi: 10.1111/j.1469-8137.2008.02748.x. [DOI] [PubMed] [Google Scholar]
- 16.Hasan M.K., Cheng Y., Kanwar M.K., Chu X.Y., Ahammed G.J., Qi Z.Y. Responses of plant proteins to heavy metal stress—A review. Front. Plant Sci. 2017;8:1–16. doi: 10.3389/fpls.2017.01492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siemianowski O., Barabasz A., Kendziorek M., Ruszczyńska A., Bulska E., Williams L.E., Antosiewicz D.M. HMA4 expression in tobacco reduces Cd accumulation due to the induction of the apoplastic barrier. J. Exp. Bot. 2014;65:1125–1139. doi: 10.1093/jxb/ert471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clemente R., Walker D.J., Pardo T., Martínez-Fernández D., Bernal M.P. The use of a halophytic plant species and organic amendments for the remediation of a trace elements-contaminated soil under semi-arid conditions. J. Hazard. Mater. 2012;223:63–71. doi: 10.1016/j.jhazmat.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 19.Manousaki E., Kalogerakis N. Phytoextraction of Pb and Cd by the Mediterranean saltbush (Atriplex halimus L.): Metal uptake in relation to salinity. Environ. Sci. Pollut. Res. 2019;16:844–854. doi: 10.1007/s11356-009-0224-3. [DOI] [PubMed] [Google Scholar]
- 20.Sharma A., Gontia I., Agarwal P.K., Jha B. Accumulation of heavy metals and its biochemical responses in Salicornia brachiata, an extreme halophyte. Mar. Biol. Res. 2010;6:511–518. doi: 10.1080/17451000903434064. [DOI] [Google Scholar]
- 21.Amari T., Ghnaya T., Sghaier S., Porrini M., Lucchini G., Attilio G., Abdelly C. Evaluation of the Ni2+ phytoextraction potential in Mesembryanthemum crystallinum (halophyte) and Brassica juncea. J. Bioremediat. Biodegrad. 2016:7. doi: 10.4172/2155-6199.1000336. [DOI] [Google Scholar]
- 22.Ghnaya T., Nouairi I., Slama I., Messedi D., Grignon C., Abdelly C., Ghorbel M.H. Cadmium effects on growth and mineral nutrition of two halophytes: Sesuvium portulacastrum and Mesembryanthemum crystallinum. J. Plant Physiol. 2005;162:1133–1140. doi: 10.1016/j.jplph.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Nosek M., Kaczmarczyk A., Śliwa M., Jędrzejczyk R., Kornaś A., Supel P., Kaszycki P., Miszalski Z. The response of a model C3/CAM intermediate semi-halophyte Mesembryanthemum crystallinum L. to elevated cadmium concentrations. J. Plant Physiol. 2019;240:153005. doi: 10.1016/j.jplph.2019.153005. [DOI] [PubMed] [Google Scholar]
- 24.Nakanishi H., Ogawa I., Ishimaru Y., Mori S., Nishizawa N.K. Iron deficiency enhances cadmium uptake and translocation mediated by the Fe2+ transporters OsIRT1 and OsIRT2 in rice. Soil Sci. Plant Nutr. 2006;52:464–469. doi: 10.1111/j.1747-0765.2006.00055.x. [DOI] [Google Scholar]
- 25.Vert G., Grotz N., Dédaldéchamp F., Gaymard F., Guerinot M.L., Briat J.-F., Curie C. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell. 2002;133:1223–1233. doi: 10.1105/tpc.001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vert G., Briat J.F., Curie C. Arabidopsis IRT2 gene encodes a root-periphery iron transporter. Plant J. 2001;26:181–189. doi: 10.1046/j.1365-313x.2001.01018.x. [DOI] [PubMed] [Google Scholar]
- 27.He X.L., Fan S.K., Zhu J., Guan M.Y., Liu X.X., Zhang Y.S., Jin C.W. Iron supply prevents Cd uptake in Arabidopsis by inhibiting IRT1 expression and favoring competition between Fe and Cd uptake. Plant Soil. 2017;416:453–462. doi: 10.1007/s11104-017-3232-y. [DOI] [Google Scholar]
- 28.Qureshi M.I., D’Amici G.M., Fagioni M., Rinalducci S., Zolla L. Iron stabilizes thylakoid protein-pigment complexes in Indian mustard during Cd-phytoremediation as revealed by BN-SDS-PAGE and ESI-MS/MS. J. Plant Physiol. 2010;167:761–770. doi: 10.1016/j.jplph.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 29.Muneer S., Jeong B.R., Kim T.H., Lee J.H., Soundararajan P. Transcriptional and physiological changes in relation to Fe uptake under conditions of Fe-deficiency and Cd-toxicity in roots of Vigna radiata L. J. Plant Res. 2014;127:731–742. doi: 10.1007/s10265-014-0660-0. [DOI] [PubMed] [Google Scholar]
- 30.Siedlecka A., Krupa Z. Cd/Fe interaction in higher plants—Its consequences for the photosynthetic apparatus. Photosynthetica. 1999;36:321–331. doi: 10.1023/A:1007097518297. [DOI] [Google Scholar]
- 31.Gallego S.M., Pena L.B., Barcia R.A., Azpilicueta C.E., Iannone M.F., Rosales E.P., Zawoznik M.S., Groppa M.D., Benavides M.P. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environ. Exp. Bot. 2012;83:33–46. doi: 10.1016/j.envexpbot.2012.04.006. [DOI] [Google Scholar]
- 32.Yang Q., Ma X., Luo S., Gao J., Yang X., Feng Y. SaZIP4, an uptake transporter of Zn/Cd hyperaccumulator Sedum alfredii Hance. Environ. Exp. Bot. 2018;155:107–117. doi: 10.1016/j.envexpbot.2018.06.021. [DOI] [Google Scholar]
- 33.Sharma S.S., Dietz K.J. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 2006;57:711–726. doi: 10.1093/jxb/erj073. [DOI] [PubMed] [Google Scholar]
- 34.Hall J.L. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2000;53:1–11. doi: 10.1093/jexbot/53.366.1. [DOI] [PubMed] [Google Scholar]
- 35.Rea P.A. Phytochelatin synthase: Of a protease a peptide polymerase made. Physiol. Plant. 2012;145:154–164. doi: 10.1111/j.1399-3054.2012.01571.x. [DOI] [PubMed] [Google Scholar]
- 36.Ha S.-B., Smith A.P., Howden R., Dietrich W.M., Bugg S., O’Connell M.J., Goldsbrough P.B., Cobbett C.S. Phytochelatin synthase genes from Arabidopsis and the yeast Schizosaccharomyces pombe. Plant Cell. 1999;11:1153–1163. doi: 10.1105/tpc.11.6.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howden R., Goldsbrough P.B., Andersen C.R., Cobbett C.S. Cadmium-sensitive, cad1 mutants of Arabidopsis thaliana are phytochelatin deficient. Plant Physiol. 1995;107:1059–1066. doi: 10.1104/pp.107.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zitka O., Krystofova O., Sobrova P., Adam V., Zehnalek J., Beklova M., Kizek R. Phytochelatin synthase activity as a marker of metal pollution. J. Hazard. Mater. 2011;192:794–800. doi: 10.1016/j.jhazmat.2011.05.088. [DOI] [PubMed] [Google Scholar]
- 39.Szarka A., Tomasskovics B., Bánhegyi G. The ascorbate-glutathione-α-tocopherol triad in abiotic stress response. Int. J. Mol. Sci. 2012;13:4458–4483. doi: 10.3390/ijms13044458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuźniak E., Kaźmierczak A., Wielanek M., Głowacki R., Kornas A. Involvement of salicylic acid, glutathione and protein S-thiolation in plant cell death-mediated defence response of Mesembryanthemum crystallinum against Botrytis cinerea. Plant Physiol. Biochem. 2013;63:30–38. doi: 10.1016/j.plaphy.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 41.Pomponi M., Censi V., Di Girolamo V., De Paolis A., Di Toppi L.S., Aromolo R., Costantino P., Cardarelli M. Overexpression of Arabidopsis phytochelatin synthase in tobacco plants enhances Cd2+ tolerance and accumulation but not translocation to the shoot. Planta. 2006;223:180–190. doi: 10.1007/s00425-005-0073-3. [DOI] [PubMed] [Google Scholar]
- 42.Mendoza-Cózatl D.G., Butko E., Springer F., Torpey J.W., Komives E.A., Kehr J., Schroeder J.I. Identification of high levels of phytochelatins, glutathione and cadmium in the phloem sap of Brassica napus. A role for thiol-peptides in the long-distance transport of cadmium and the effect of cadmium on iron translocation. Plant J. 2008;54:249–259. doi: 10.1111/j.1365-313X.2008.03410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mendoza-Cózatl D.G., Jobe T.O., Hauser F., Schroeder J.I. Long-distance transport, vacuolar sequestration, tolerance, and transcriptional responses induced by cadmium and arsenic. Curr. Opin. Plant Biol. 2011;14:554–562. doi: 10.1016/j.pbi.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park J., Song W.Y., Ko D., Eom Y., Hansen T.H., Schiller M., Lee T.G., Martinoia E., Lee Y. The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J. 2012;69:278–288. doi: 10.1111/j.1365-313X.2011.04789.x. [DOI] [PubMed] [Google Scholar]
- 45.Brunetti P., Zanella L., De Paolis A., Di Litta D., Cecchetti V., Falasca G., Barbieri M., Altamura M.M., Costantino P., Cardarelli M. Cadmium-inducible expression of the ABC-type transporter AtABCC3 increases phytochelatin-mediated cadmium tolerance in Arabidopsis. J. Exp. Bot. 2015;66:3815–3829. doi: 10.1093/jxb/erv185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhati K.K., Sharma S., Aggarwal S., Kaur M., Shukla V., Kaur J., Mantri S., Pandey A.K. Genome-wide identification and expression characterization of ABCC-MRP transporters in hexaploid wheat. Front. Plant Sci. 2015;6:488. doi: 10.3389/fpls.2015.00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang X.D., Zhao K.X., Yang Z.M. Identification of genomic ATP binding cassette (ABC) transporter genes and Cd-responsive ABCs in Brassica napus. Gene. 2018;664:139–151. doi: 10.1016/j.gene.2018.04.060. [DOI] [PubMed] [Google Scholar]
- 48.Manohar M., Shigaki T., Hirschi K.D. Plant cation/H+ exchangers (CAXs): Biological functions and genetic manipulations. Plant Biol. 2011;13:561–569. doi: 10.1111/j.1438-8677.2011.00466.x. [DOI] [PubMed] [Google Scholar]
- 49.Koren’kov V., Park S., Cheng N.H., Sreevidya C., Lachmansingh J., Morris J., Hirschi K., Wagner G.J. Enhanced Cd2+-selective root-tonoplast-transport in tobaccos expressing Arabidopsis cation exchangers. Planta. 2007;225:403–411. doi: 10.1007/s00425-006-0352-7. [DOI] [PubMed] [Google Scholar]
- 50.Mei H., Hirschi K.D., Cheng N.H., Zhao J., Park S., Escareno R.A., Pittman J.K. Root development under metal stress in Arabidopsis thaliana requires the H+/cation antiporter CAX4. New Phytol. 2009;183:95–105. doi: 10.1111/j.1469-8137.2009.02831.x. [DOI] [PubMed] [Google Scholar]
- 51.Bassil E., Blumwald E. The ins and outs of intracellular ion homeostasis: NHX-type cation/H+ transporters. Curr. Opin. Plant Biol. 2014;22:1–6. doi: 10.1016/j.pbi.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 52.Jiang X., Leidi E.O., Pardo J.M. How do vacuolar NHX exchangers function in plant salt tolerance? Plant Signal. Behav. 2010;5:792–795. doi: 10.4161/psb.5.7.11767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barkla B.J., Zingarelli L., Blumwald E., Smith J. Tonoplast Na+/H+ antiport activity and its energization by the vacuolar H+-ATPase in the halophytic plant Mesembryanthemum crystallinum L. Plant Physiol. 1995;109:549–556. doi: 10.1104/pp.109.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ratajczak R., Richter J., Luttge U. Adaptation of the tonoplast V-type H+-ATPase of Mesembryanthemum crystallinum to salt stress, C3–CAM transition and plant age. Plant. Cell Environ. 1994;17:1101–1112. doi: 10.1111/j.1365-3040.1994.tb02008.x. [DOI] [Google Scholar]
- 55.Cheng N., Pittman J.K., Shigaki T., Hirschi K.D. Characterization of CAX4, an Arabidopsis H+/cation antiporter. Plant Physiol. 2002;128:1245–1254. doi: 10.1104/pp.010857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mills R.F., Francini A., Ferreira Da Rocha P.S.C., Baccarini P.J., Aylett M., Krijger G.C., Williams L.E. The plant P1B-type ATPase AtHMA4 transports Zn and Cd and plays a role in detoxification of transition metals supplied at elevated levels. FEBS Lett. 2005;579:783–791. doi: 10.1016/j.febslet.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 57.Wong C., Cobbett E. HMA P-type ATPases are the major mechanism for root-to-shoot Cd translocation in Arabidopsis thaliana. New Phytol. 2009:71–78. doi: 10.1111/j.1469-8137.2008.02638.x. [DOI] [PubMed] [Google Scholar]
- 58.Xu J., Yin H., Liu X., Li X. Salt affects plant Cd-stress responses by modulating growth and Cd accumulation. Planta. 2010;231:449–459. doi: 10.1007/s00425-009-1070-8. [DOI] [PubMed] [Google Scholar]
- 59.Mills R.F., Krijgert G.C., Baccarini P.J., Hall J.L., Williams L.E. Functional expression of AtHMA4, a P1B-type ATPase of the Zn/Co/Cd/Pb subclass. Plant J. 2003;35:164–176. doi: 10.1046/j.1365-313X.2003.01790.x. [DOI] [PubMed] [Google Scholar]
- 60.Bernard C., Roosens N., Czernic P., Lebrun M., Verbruggen N. A novel CPx-ATPase from the cadmium hyperaccumulator Thlaspi caerulescens. FEBS Lett. 2004;569:140–148. doi: 10.1016/j.febslet.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 61.Papoyan A. Identification of Thlaspi caerulescens genes that may be involved in heavy metal hyperaccumulation and tolerance. Characterization of a novel heavy metal transporting ATPase. Plant Physiol. 2004;136:3814–3823. doi: 10.1104/pp.104.044503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Houba V.J.G., Temminghoff E.J.M., Gaikhorst G.A., van Vark W. Soil analysis procedures using 0.01 M calcium chloride as extraction reagent. Commun. Soil Sci. Plant Anal. 2000;31:1299–1396. doi: 10.1080/00103620009370514. [DOI] [Google Scholar]
- 63.Rozpądek P., Domka A., Ważny R., Nosek M., Jędrzejczyk R., Tokarz K., Turnau K. How does the endophytic fungus Mucor sp. improve Arabidopsis arenosa vegetation in the degraded environment of a mine dump? Environ. Exp. Bot. 2018;147:31–42. doi: 10.1016/j.envexpbot.2017.11.009. [DOI] [Google Scholar]
- 64.Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acid Res. 2001;29:16–21. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.