Abstract
Objective
To determine whether right ventral stream and limbic structures (including posterior superior temporal gyrus [STG], STG, temporal pole, inferior frontal gyrus pars orbitalis, orbitofrontal cortex, amygdala, anterior cingulate, gyrus, and the sagittal stratum) are implicated in emotional prosody identification.
Methods
Patients with MRI scans within 48 hours of unilateral right hemisphere ischemic stroke were enrolled. Participants were presented with 24 sentences with neutral semantic content spoken with happy, sad, angry, afraid, surprised, or bored prosody and chose which emotion the speaker was feeling based on tone of voice. Multivariable linear regression was used to identify individual predictors of emotional prosody identification accuracy from a model, including percent damage to proposed right hemisphere structures, age, education, and lesion volume across all emotions (overall emotion identification) and 6 individual emotions. Patterns of recovery were also examined at the chronic stage.
Results
The overall emotion identification model was significant (adjusted r2 = 0.52; p = 0.043); greater damage to right posterior STG (p = 0.038) and older age (p = 0.009) were individual predictors of impairment. The model for recognition of fear was also significant (adjusted r2 = 0.77; p = 0.002), with greater damage to right amygdala (p = 0.047), older age (p < 0.001), and less education (p = 0.005) as individual predictors. Over half of patients with chronic stroke had residual impairments.
Conclusions
Right posterior STG in the right hemisphere ventral stream is critical for emotion identification in speech. Patients with stroke with damage to this area should be assessed for emotion identification impairment.
Comprehending the intended meaning of a speaker requires understanding both the content of what is said and the emotional prosody (expression of emotion in speech). The same words shouted in anger have a very different meaning when exclaimed with joy (e.g., “It's you!”). Damage to right hemisphere (RH) regions is associated with difficulty understanding emotional prosody,1–5 termed emotional or affective receptive aprosodia. Poststroke aprosodia is associated with decreased marital satisfaction6 and increased caregiver burden.7
Schirmer and Kotz8 proposed RH ventral (sound-to-meaning) and dorsal (sound-to-articulation) streams for processing emotional prosody, analogous to left hemisphere streams for language processing.9,10 The proposed RH ventral stream projects from the superior temporal gyrus (STG) to the anterior superior temporal sulcus (STS) and subsequently to frontal regions where evaluative judgments are made; subcortical structures also subserve emotional prosody identification.
We propose that identification of emotional prosody relies on a right ventral stream and limbic structures, including posterior STG, STG, temporal pole, inferior frontal gyrus (IFG) pars orbitalis, orbitofrontal cortex (OFC), amygdala, anterior cingulate gyrus, and the sagittal stratum. We evaluated this hypothesis by examining relationships between damage to these structures and performance on an emotional prosody recognition task in a group of 23 patients with acute stroke. Studying the acute stage offers the advantage of examining lesion effects before any functional reorganization occurs during recovery.11,12 We also hypothesized that not all patients recover from acute deficits in emotional prosody identification by the time of reevaluation at 6-12 months, suggesting that interventions are needed to address this deficit.
Methods
Participants
We enrolled 23 patients (mean age 55.09 years, SD 17.22) with acute RH ischemic stroke who provided informed consent to participate in the study. The patients included 11 women and 12 men; 69.6% were African American and 30.4% were white. The majority of strokes were within the middle cerebral artery territory (78%), but also included were strokes within the posterior cerebral artery territory (13%), as well as 1 (4%) lenticulostriate stroke, and 1 (4%) stroke involving multiple territories. Stroke etiology was determined to be embolic in 43% of patients. At the acute stage of stroke 4 of the 23 patients were determined to have visual neglect, and 4 patients experienced cognitive and communication deficits (other than prosodic deficits) including impaired empathy and dysarthria. Many patients had a history of hypertension prior to stroke (15/23), several patients were tobacco users at time of stroke (8/23), and a small number of patients (3/23) had diabetes.
All of the patients met the following criteria: (1) never had a previous symptomatic stroke (2 patients had experienced a single TIA previously; 1 patient was found to have small cerebellar infarcts, which were asymptomatic, as the patient was unaware that she had experienced any previous stroke); (2) were premorbid fluent speakers of English; (3) were right-handed; (4) did not have a neurologic disease other than stroke; (5) did not have reduced level of consciousness or receive ongoing sedation; and (6) had normal or corrected-to-normal auditory and visual acuity based on neurologic examination. At the acute time point, 23 patients were tested within 5 days of stroke. We conducted follow-up testing on 9 of these patients (mean age 47 years, SD 17.65; 2 women and 7 men) at the chronic time point, which was defined as at least 6 months after stroke. Performance on recognizing emotional prosody was compared to a group of 10 age-matched healthy control participants (mean age 53.33 years, SD 18.43; 8 women and 2 men).
Standard protocol approvals, registrations, and patient consents
All of the participants in this study, or their legally authorized representative, provided informed consent using methods approved by the Johns Hopkins Institutional Review Board (protocol NA_00042097).
All patients were evaluated with MRI diffusion-weighted imaging (DWI) and fluid-attenuated inversion recovery to rule out old lesions, susceptibility-weighted imaging to rule out hemorrhage, and T2-weighted imaging to check for other structural lesions. Scans were acquired clinically within 48 hours of admission on a 3.0T Siemens (Munich, Germany) Trio scanner. Whole-brain DWI had 40 slices with a 2.0 mm3 voxel size. Technicians blinded to the prosody evaluation results identified the presence or absence of tissue dysfunction, which was defined as dense ischemia/infarct (bright on DWI maps). These areas of tissue dysfunction were traced using MRIcron software13 and were coregistered to the Johns Hopkins University atlas using SPM12 (nitrc.org/projects/clinicaltbx/). In this way, comparisons can be made across the participants in the study while preserving individual local features of each brain.14
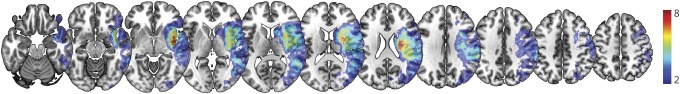
Lesion overlap maps were created for all participants included at the acute time point using normalized DWI trace images and the corresponding lesion tracings (see lesion overlap map in figure 1). The percent damage to each of the areas in the proposed RH ventral stream and underlying white matter structures (posterior STG, STG, temporal pole, IFG pars orbitalis, lateral OFC, amygdala, anterior cingulate gyrus, and the sagittal stratum) was calculated for each participant using NiiStat software (github.com/neurolabusc/NiiStat).
Figure 1. Lesion overlay map.
Map shows the number of patients with damage to each area. The color scale indicates the number of patients with damage to each area, ranging from 2 to 8 patients.
As a control, we also evaluated the extent to which damage to dorsal stream structures could account for impaired identification of emotion in speech, independently of age, education, and lesion volume. Dorsal stream and underlying white matter structures include angular gyrus, supramarginal gyrus, posterior STG, premotor cortex, IFG opercularis, arcuate fasciculus, caudate nucleus, and putamen.
Emotional prosody identification testing
Participants listened to 24 prerecorded semantically neutral sentences (e.g., “We walked through the park to get here.”) spoken by a female speaker with happy, sad, angry, afraid, surprised, or bored emotional prosody. After each sentence, the participants were presented with a screen on a laptop that displayed each of the 6 possible emotion words and were asked to select the emotion they believed the speaker was feeling based on the tone of voice. The 6 emotion words were presented in 2 vertical columns presented in the center of the screen. Emotion words were presented in the same order for each item. Prior to the emotional prosody task, patients were tested for presence of visual neglect, and in those cases the emotion words were presented on the patients’ non-neglect side. Patients were not given a time limit for a response. In cases where they were not sure about their answer, they were encouraged to select 1 emotion from the 6 emotions before moving to the next item. This task was given at the acute (n = 23) and chronic (n = 9) time points. Impaired performance was defined as performance 1 SD below the mean of the control group’s performance.15,16
Statistical analysis
Multivariable linear regression was used to identify the independent predictors of overall emotional prosody identification impairment (percent accuracy on overall emotion recognition task). The following independent variables were entered into the initial model: percent damage to each of 7 regions of interest (posterior STG, STG, temporal pole, IFG pars orbitalis, lateral OFC, amygdala, sagittal stratum), overall lesion volume, age, and education. Only regions from the proposed ventral stream where at least 2 participants had damage were included in the analysis; thus, the model did not include the anterior cingulate gyrus. Next, we evaluated differences between patients with and without damage to any brain regions that were revealed as individual predictors in the model of overall emotion identification in terms of age, education, and accuracy on recognizing 6 emotions using unpaired t tests. Multivariable linear regression was then repeated using the same model (percent accuracy on individual emotion recognition, age, education, lesion volume, percent damage to posterior STG, STG, temporal pole, IFG pars orbitalis, lateral OFC, amygdala, and sagittal stratum) for each of the 6 emotions individually.
As a control, we also repeated the same analyses, including percent accuracy on the emotion recognition task, age, education, and lesion volume, along with percent damage to dorsal stream structures in place of ventral stream structures (angular gyrus, supramarginal gyrus, posterior STG, premotor cortex, IFG opercularis, arcuate fasciculus, caudate nucleus, and putamen).
Data availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
Healthy controls
Overall emotional prosody identification and individual emotions
The controls attained a mean accuracy (SD) of 82.50% (5.20%) for overall emotional prosody identification. The accuracy for each individual emotion was 95.00% (10.54%) for happy, 95.00% (15.81%) for sad, 97.22% (8.33%) for angry, 27.78% (31.73%) for afraid, 100% (0%) for surprised, and 80.56% (16.67%) for bored sentences. The controls were best able to identify surprised prosody and had the most difficulty with identifying fearful prosody.
Patients with RH stroke
Acute time point
Overall emotional prosody identification
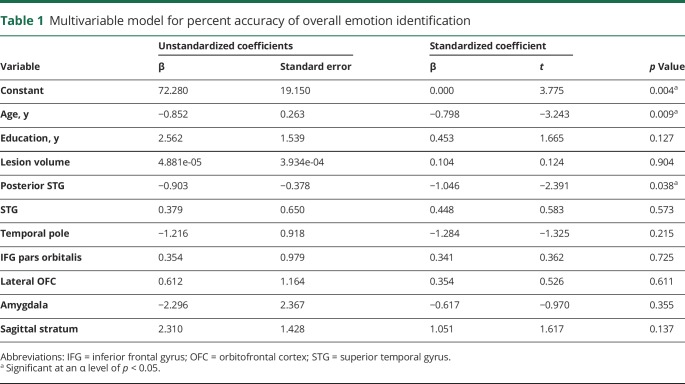
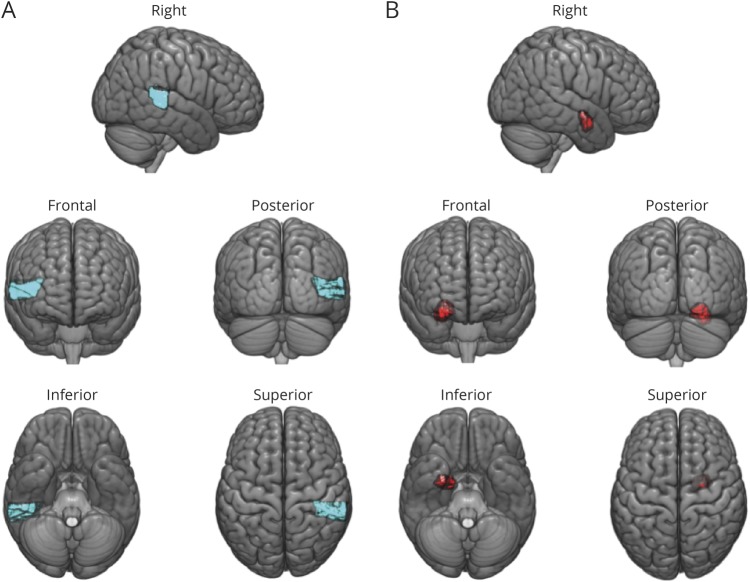
Overall emotional prosody identification was impaired relative to controls in 16/23 patients with acute RH stroke. The group of 23 patients achieved a mean accuracy (SD) of 57.25% (19.67%) vs 82.50% (5.20%) in age-matched controls. The model for overall emotion identification was significant, F10,10 = 3.15, p = 0.043, and accounted for approximately 52% of the variance (r2 = 0.76, adjusted r2 = 0.52) (table 1). Individual predictors of overall emotion identification impairment were older age (p = 0.009) and greater damage to posterior STG (p = 0.038) (figure 2A). We compared patients with and without damage to posterior STG on age, education, lesion volume, and percent accuracy identifying happy, sad, angry, afraid, surprised, and bored emotions in speech. Patients with right posterior STG lesions had significantly larger lesion volume (t = 3.07, p = 0.006) and were significantly worse at recognizing happy (t = 4.32, p < 0.001), sad (t = 3.48, p = 0.001), angry (t = 2.90, p = 0.004), and bored (t = 2.12, p = 0.023) emotional prosody (table 2). In addition, the multivariable model evaluating dorsal stream structures in place of ventral stream structures, along with age, education, and lesion volume, was not significant.
Table 1.
Multivariable model for percent accuracy of overall emotion identification
Table 2.
Comparing participants with and without posterior superior temporal gyrus (STG) lesions
Identification of individual emotions in speech
The mean accuracy (SD) for identifying each individual emotion was 66.25% (30.65%) for happy, 73.75% (29.77%) for sad, 53.75% (36.52%) for angry, 11.25% (15.12%) for afraid, 78.75% (23.33%) for surprised, and 43.75% (29.10%) for bored. The patients displayed the same pattern as controls, with highest accuracy identifying surprised prosody and lowest accuracy identifying fearful sentences. Their accuracy was lower for each individual emotion when compared to controls.
Of the multivariable linear regression models evaluating emotion identification in each of the 6 emotions, only the fear identification model was significant, F10,10 = 7.61, p = 0.002. This model accounted for approximately 77% of the variance (r2 = 0.88, adjusted r2 = 0.77). Individual independent predictors of lower accuracy of fearful prosody identification were percent damage to the right amygdala (p = 0.047) (figure 2B), older age (p < 0.001), and less education (p = 0.005). No other predictors were independently associated with the error rate of emotion identification in fearful speech. Furthermore, no models evaluating emotion identification in each of the 6 emotions using dorsal stream structures were significant.
Figure 2. Brain regions associated with emotional prosody identification.
(A) Right posterior STG (in blue), where greater damage was significantly associated with impaired overall emotional prosody identification. The brain template is partially transparent to allow viewing of this area from different orientations. (B) Right amygdala (in red), where greater damage was significantly associated with impaired identification of fearful prosody. The brain template is partially transparent to allow viewing of this area from different orientations.
Chronic time point
Nine patients were also tested at the chronic time point (at least 6 months poststroke) to test the hypothesis that only a subset of people with RH ischemic stroke recover emotional prosody identification without specific intervention directed toward this deficit. Five of these patients had impaired overall emotional prosody identification acutely and 4 patients were impaired at the chronic time point. Two patients with impaired overall emotional prosody identification acutely recovered to normal at the chronic stage. However, one patient who performed within normal limits acutely experienced a decline in emotional prosody identification and was impaired at chronic follow-up testing. Imaging obtained at the chronic stage confirmed that she did not have an extension of her infarct or a new infarct that could explain her decline. Three of the 5 patients with unimpaired emotion identification at the chronic stage were also unimpaired at the acute time point, meaning only 2 patients with impairment at baseline recovered to normal performance. One additional patient improved but still had impaired overall emotion identification.
Discussion
We confirmed that identification of emotional prosody is often impaired in patients with acute RH strokes. This result is in line with previous studies showing that RH damage often results in difficulty recognizing emotional tones of voice.3,5,17 More importantly, we tested the hypothesis that damage to the RH ventral stream would explain impairment in emotional prosody identification, independently of demographics and overall lesion volume. We confirmed that our model including RH ventral stream structures, as well as age, education, and lesion volume, predicted performance on an emotion recognition task. Older age and greater damage to the right posterior STG were independent predictors of impairment in overall emotion identification. The analyses of individual emotions only revealed a significant model for the identification of fearful speech. Significant individual predictors of impaired identification of fear in speech included greater percent damage to the amygdala, older age, and less education.
Schirmer and Kotz8 proposed a 3-stage model for the identification of emotion in speech, of which STG and amygdala play essential roles. Stage 1 (sensory processing) consists of processing speech in bilateral auditory processing areas. Stage 2 (integration) consists of integrating emotionally meaningful acoustic cues along the auditory “what” processing stream from STG to anterior STS. During this stage, the emotional significance of vocalizations is encoded. Finally, in stage 3 (cognition), emotionally significant information is made available for higher-order cognitive processing, including evaluative judgments, in the right IFG and OFC. The integration of emotional prosody with language also engages the left IFG. Schirmer and Kotz8 also proposed that subcortical structures, including the amygdala, are also important to emotional prosody identification. For example, they suggested that the amygdala mediates the processing of specific emotional expressions that are either unexpected or important, such as changes in prosody signaling a threat.
Our findings specifically implicate the right posterior STG in overall emotional prosody identification. The right posterior STG is part of the proposed ventral stream for identifying emotional prosody and has been associated with emotional prosody identification in several previous studies. For example, Frühholz et al.18 investigated white matter fiber connectivity between regions responding to emotional prosody in neurologically healthy participants, and found that right posterior STG was sensitive to variations in pitch and intensity in emotional prosody. The finding that older age was an individual predictor of impaired emotion identification was not surprising given that both pitch identification19 and emotion identification20,21 skills decline as individuals age even in neurologically unimpaired adults, and that older age is associated with poorer functional and cognitive outcomes following stroke.22–25 However, our results are novel in showing that right posterior STG damage is independent of age and total lesion volume.
Our findings also suggest that the right amygdala is essential for the recognition of fearful speech. This is intriguing, since functional neuroimaging studies in neurologically healthy participants26,27 and lesion studies28,29 have implicated the amygdala in recognizing fear in facial expressions. However, in a study of patients with acute stroke, Tippett et al.30 found a strong association between lesions in right amygdala and impaired recognition of happy and angry facial expressions, but not fearful expressions. They proposed that the role of the amygdala is more complex than simply the recognition of fear in facial expression alone. This conclusion corresponds with the findings of Phillips et al.,31 who found that the amygdala is involved in more than just the identification of fearful facial expressions, and was also activated by fearful speech in an fMRI study. Furthermore, Leitman et al.,32 using MRI in neurologically unimpaired participants, found an RH ventral temporo-frontal network activated in emotional prosody recognition. In addition, increased activation in the right amygdala and temporal cortex was found when the stimuli contained rich emotional prosodic cues, whereas increased activation in IFG was observed when the emotional prosody was more ambiguous. Our results lend further support to the finding that the right amygdala is essential for identifying fear in speech. Furthermore, our results indicated that education was also an independent predictor of recognizing fear. Both patients and controls had the most difficulty recognizing fearful speech. Perhaps a higher education level was an independent predictor of fearful speech recognition, and not overall emotion recognition, because it helped patients overcome this more difficult task. Several studies have found that education may be neuroprotective against cognitive decline or may promote neuroplasticity following stroke, making people with higher education more resilient to damage.33,34
Testing of 9 patients at the chronic time point revealed that 2 patients improved to normal emotional prosody identification from the acute to chronic stages, 3 patients remained impaired, 3 patients remained unimpaired, and 1 patient had weaker emotional prosody recognition skills at follow-up testing. These results are comparable to studies of change in language35 and cognition22,36,37 after stroke, which show that up to 25% show decline in performance after the acute stage of stroke (possibly due to vascular dementia triggered by the stroke). While there were a small number of patients at the chronic time point, these data suggest that a large portion of patients with acute deficits will experience chronic receptive emotional aprosodic deficits, indicating that intervention targeting emotional recognition from prosody might be warranted.
It should be noted that our study had several limitations. There were a relatively small number of participants with longitudinal data at both the acute and chronic stages, which prevented us from conducting brain-behavior mapping in chronic patients. Moreover, while posterior STG was implicated at the acute stage, only 1 of the 9 patients at the chronic stage had a lesion in posterior STG. We therefore were not able to determine whether a posterior STG lesion would also be implicated in chronic deficits. Future research will need to examine longitudinal changes in emotional prosody identification in a larger group of participants to gain a better understanding of whether and how these deficits evolve over time. Furthermore, we do not have information about the effect of emotional prosody identification deficits on patients and their families. Future studies will examine the functional nature of emotional prosody impairments.
Overall, our results suggest that patients with damage to the RH ventral stream and underlying subcortical structures (particularly right STG and amygdala) should be assessed for their ability to perceive emotion in speech. This assessment can be done at bedside by instructing patients to try to judge the emotional tone of voice of sentences (e.g., happy, sad, angry) spoken by a clinician. The clinician could then produce a neutral content sentence (e.g., “He left.”) with happy, angry, and sad tone of voice. Clinically identifying patients with impaired emotional prosody identification would benefit both patients and their families, particularly since research demonstrates that emotional prosody disorders negatively affect quality of life in patients and families.38,39 If patients are found to have a deficit, they can be made aware that they will have difficulty interpreting others’ emotions, and should ask people to explicitly state their emotions. It would be beneficial to educate family members by explaining that the patient may have difficulty with emotional prosody identification and recommend they explicitly state their feelings and emotions whenever possible to avoid confusing and frustrating communication breakdowns. Finally, future studies should develop interventions to improve emotional prosody recognition after RH stroke.
Glossary
- DWI
diffusion-weighted imaging
- IFG
inferior frontal gyrus
- OFC
orbitofrontal cortex
- RH
right hemisphere
- STG
superior temporal gyrus
- STS
superior temporal sulcus
Appendix. Authors
Study funding
This work was supported by the NIH (National Institute of Deafness and Other Communication Disorders) (R01 DC015466).
Disclosure
S. Sheppard, L. Keator, B. Breining, A. Wright, S. Saxena, and D. Tippett report no disclosures relevant to the manuscript. A. Hillis receives compensation from the American Heart Association as associate editor of Stroke and from Elsevier as associate editor of Practice Update Neurology and has received compensation from Axovant as a member of a data safety monitoring board. Go to Neurology.org/N for full disclosures.
References
- 1.Dara C, Bang J, Gottesman RF, Hillis AE. Right hemisphere dysfunction is better predicted by emotional prosody impairments as compared to neglect. J Neurol Transl Neurosci 2014;2:1037. [PMC free article] [PubMed] [Google Scholar]
- 2.Wright AE, Davis C, Gomez Y, et al. Acute ischemic lesions associated with impairments in expression and recognition of affective prosody. Perspect ASHA Spec Interest Groups 2016;1:82–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright A, Saxena S, Sheppard SM, Hillis AE. Selective impairments in components of affective prosody in neurologically impaired individuals. Brain Cogn 2018;124:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross ED, Monnot M. Affective prosody: what do comprehension errors tell us about hemispheric lateralization of emotions, sex and aging effects, and the role of cognitive appraisal? Neuropsychologia 2011;49:866–877. [DOI] [PubMed] [Google Scholar]
- 5.Ross ED, Monnot M. Neurology of affective prosody and its functional–anatomic organization in right hemisphere. Brain Lang 2008;104:51–74. [DOI] [PubMed] [Google Scholar]
- 6.Blonder LX, Pettigrew LC, Kryscio RJ. Emotion recognition and marital satisfaction in stroke. J Clin Exp Neuropsychol 2012;34:634–642. [DOI] [PubMed] [Google Scholar]
- 7.Sheehan O, Hillis A. Impairments in communicating emotions through prosody of speech and facial expression are associated with reduced quality of life and increased caregiver burden after stroke. Submitted.
- 8.Schirmer A, Kotz SA. Beyond the right hemisphere: brain mechanisms mediating vocal emotional processing. Trends Cogn Sci 2006;10:24–30. [DOI] [PubMed] [Google Scholar]
- 9.Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition 2004;92:67–99. [DOI] [PubMed] [Google Scholar]
- 10.Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci 2007;8:393–402. [DOI] [PubMed] [Google Scholar]
- 11.Marsh EB, Hillis AE. Recovery from aphasia following brain injury: the role of reorganization. In: Moller AR, ed. Progress in Brain Research: vol 157: Reprogramming of the Brain. Amsterdam: Elsevier; 2006:143–156. [DOI] [PubMed] [Google Scholar]
- 12.Jarso S, Li M, Faria A, et al. Distinct mechanisms and timing of language recovery after stroke. Cogn Neuropsychol 2013;30:454–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci 2007;19:1081–1088. [DOI] [PubMed] [Google Scholar]
- 14.Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol 2000;12:191–200. [DOI] [PubMed] [Google Scholar]
- 15.Mike A, Strammer E, Aradi M, et al. Disconnection mechanism and regional cortical atrophy contribute to impaired processing of facial expressions and theory of mind in multiple sclerosis: a structural MRI study. PLoS One 2013;8:e82422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganguli M, Dodge H, Shen C, DeKosky S. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology 2004;63:115–121. [DOI] [PubMed] [Google Scholar]
- 17.Davis CL, Oishi K, Faria AV, et al. White matter tracts critical for recognition of sarcasm. Neurocase 2016;22:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frühholz S, Gschwind M, Grandjean D. Bilateral dorsal and ventral fiber pathways for the processing of affective prosody identified by probabilistic fiber tracking. NeuroImage 2015;109:27–34. [DOI] [PubMed] [Google Scholar]
- 19.Clinard CG, Tremblay KL, Krishnan AR. Aging alters the perception and physiological representation of frequency: evidence from human frequency-following response recordings. Hearing Res 2010;264:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halberstadt J, Ruffman T, Murray J, Taumoepeau M, Ryan M. Emotion perception explains age-related differences in the perception of social gaffes. Psychol Aging 2011;26:133–136. [DOI] [PubMed] [Google Scholar]
- 21.Ryan M, Murray J, Ruffman T. Aging and the perception of emotion: processing vocal expressions alone and with faces. Exp Aging Res 2009;36:1–22. [DOI] [PubMed] [Google Scholar]
- 22.Gottesman RF, Hillis AE. Predictors and assessment of cognitive dysfunction resulting from ischaemic stroke. Lancet Neurol 2010;9:895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jehkonen M, Ahonen JP, Dastidar P, et al. Visual neglect as a predictor of functional outcome one year after stroke. Acta Neurol Scand 2000;101:195–201. [DOI] [PubMed] [Google Scholar]
- 24.Klimkowicz-Mrowiec A, Dziedzic T, Słowik A, Szczudlik A. Predictors of poststroke dementia: results of a hospital-based study in Poland. Dement Geriatr Cogn Disord 2006;21:328–334. [DOI] [PubMed] [Google Scholar]
- 25.Wade DT, Hewer RL. Stroke: associations with age, sex, and side of weakness. Arch Phys Med Rehabil 1986;67:540–545. [PubMed] [Google Scholar]
- 26.Fusar-Poli P, Placentino A, Carletti F, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- 27.Gur RC, Schroeder L, Turner T, et al. Brain activation during facial emotion processing. NeuroImage 2002;16(3, part A):651–662. [DOI] [PubMed] [Google Scholar]
- 28.Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 1994;372:669–672. [DOI] [PubMed] [Google Scholar]
- 29.Calder AJ. Facial emotion recognition after bilateral amygdala damage: differentially severe impairment of fear. Cogn Neuropsychol 1996;13:699–745. [Google Scholar]
- 30.Tippett DC, Godin BR, Oishi K, et al. Impaired recognition of emotional faces after stroke involving right amygdala or insula. Semin Speech Lang 2018;39:87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phillips ML, Young AW, Scott SK, et al. Neural responses to facial and vocal expressions of fear and disgust. Proc R Soc Lond B: Biol Sci 1998;265:1809–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leitman DI, Wolf DH, Ragland JD, et al. “It's not what you say, but how you say it”: a reciprocal temporo-frontal network for affective prosody. Front Hum Neurosci 2010;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez-Fernandez M, Davis C, Molitoris JJ, Newhart M, Leigh R, Hillis AE. Formal education, socioeconomic status, and the severity of aphasia after stroke. Arch Phys Med Rehabil 2011;92:1809–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hillis AE, Tippett DC. Stroke recovery: surprising influences and residual consequences. Adv Med 2014;2014:378263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sebastian R, Long C, Purcell JJ, et al. Imaging network level language recovery after left PCA stroke. Restorative Neurol Neurosci 2016;34:473–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ojala-Oksala J, Jokinen H, Kopsi V, et al. Educational history is an independent predictor of cognitive deficits and long-term survival in postacute patients with mild to moderate ischemic stroke. Stroke 2012;43:2931–2935. [DOI] [PubMed] [Google Scholar]
- 37.Brainin M, Tuomilehto J, Heiss W-D, et al. Post-stroke cognitive decline: an update and perspectives for clinical research. Eur J Neurol 2015;22:229–238. [DOI] [PubMed] [Google Scholar]
- 38.Heilman K. Matter of Mind: A Neurologist's View of Brain–Behavior Relationships. New York: Oxford University Press; 2002. [Google Scholar]
- 39.Ross E. Right hemisphere syndromes and the neurology of emotions. In: Behavioral Neurology and the Legacy of Norman Geschwind. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.