Abstract
The coronavirus disease 2019 (COVID-19) pandemic spread globally in the beginning of 2020. At present, predictors of severe disease and the efficacy of different treatments are not well understood. We conducted a systematic review and meta-analysis of all published studies up to 15 March 2020, which reported COVID-19 clinical features and/or treatment outcomes. Forty-five studies reporting 4203 patients were included. Pooled rates of intensive care unit (ICU) admission, mortality, and acute respiratory distress syndrome (ARDS) were 10.9%, 4.3%, and 18.4%, respectively. On meta-regression, ICU admission was predicted by increased leukocyte count (P < .0001), alanine aminotransferase (P = .024), and aspartate transaminase (P = .0040); elevated lactate dehydrogenase (LDH) (P < .0001); and increased procalcitonin (P < .0001). ARDS was predicted by elevated LDH (P < .0001), while mortality was predicted by increased leukocyte count (P = .0005) and elevated LDH (P < .0001). Treatment with lopinavir-ritonavir showed no significant benefit in mortality and ARDS rates. Corticosteroids were associated with a higher rate of ARDS (P = .0003).
Keywords: coronavirus, COVID-19, severe, risk factor, treatment
Predictors of intensive care unit admission, mortality, and acute respiratory distress syndrome in patients with COVID-19 were identified. Lopinavir-ritonavir treatment did not show significant benefit, whereas corticosteroid use was associated with poorer outcome.
A pandemic of coronavirus disease 2019 (COVID-19) caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spread from Asia to the rest of the world in the first 3 months of 2020. The consequences for human health, the global economy, and normal functioning of society have been unprecedented.
COVID-19 causes infection in any age group, although severe disease is more common in older adults [1]. The clinical spectrum of disease ranges from asymptomatic or subclinical infections to organ dysfunction—shock, acute respiratory distress syndrome (ARDS), acute cardiac injury, and acute kidney injury (AKI)—and death [2]. As of 2 May 2020, there was a total of 3 421 226 confirmed cases globally. Of the 1 333 313 cases that reached an outcome, 240 222 resulted in mortality [3].
The growth curve of the COVID-19 academic literature since the first report of this outbreak from Wuhan, Hubei Province, China, in December 2019 has been exponential (13 131 publications found on the National Institutes of Health COVID-19 Portfolio and 8502 publications found on PubMed on 2 May 2020 using the search string “coronavirus disease 2019 OR SARS-CoV-2”). However, systematic reviews that consolidate these findings remain scarce, with none focused on understanding the predictors for severe disease, including the effects of different experimental antiviral and immunomodulatory treatments [4].
To address this gap in the literature, we conducted a systematic review, meta-analysis, and meta-regression to (1) investigate the predictive value of laboratory investigations for severe disease and adverse outcomes and (2) evaluate the efficacy of antivirals and corticosteroids for COVID-19.
METHODS
This review was conducted in accordance to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [5].
Search Strategy
A search string was developed to identify original research studies reporting clinical features and treatment outcomes of patients with COVID-19 (see Supplementary Table 1). The search was applied to the following databases: Ovid MEDLINE, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and PubMed. Searches were performed for each database on 15 March 2020. Limits were applied to the search to identify studies published after 1 November 2019, as the first case of novel coronavirus was only reported in December 2019.
Study Selection
All titles and abstracts were screened independently by 2 reviewers (J. J. Y. Z. and K. S. L.) against a set of predefined eligibility criteria. Potentially eligible studies were selected for full-text analysis. Disagreements were resolved by consensus or appeal to a third senior reviewer (B. E. Y.). Agreement among the reviewers on study inclusion was evaluated using Cohen’s κ [6].
All original studies reporting the clinical characteristics (symptoms and signs, laboratory investigations, and radiological findings) and treatment outcomes of patients with COVID-19 were included in our meta-analysis. Case reports and series with a sample size of fewer than 5 were excluded per recommendations by the Cochrane Statistical Methods Group and in accordance with methodologies of previously published meta-analyses [7–9]. Other exclusion criteria included non-English articles, nonoriginal research papers, laboratory-based and epidemiological studies with no clinical characteristics reported, as well as nonhuman research subjects (see Supplementary Table 2).
Risk-of-Bias Assessment
The quality of included studies was assessed using the Joanna Briggs Institute (JBI) checklist for prevalence studies and the JBI checklist for case series [10]. Full details are shown in Supplementary Tables 3 and 4. In summary, these tools rated the quality of selection, measurement, and comparability for all studies and gave a score for prevalence studies (maximum of 9) and case series (maximum of 10). Two researchers (J. J. Y. Z. and K. S. L.) assessed the quality of all included studies and discussed discrepancies until consensus was reached.
Data Extraction and Outcome Measures
Data were extracted on the following variables: study details, sample size of study, method of diagnosis, age, gender, coexisting medical conditions, clinical symptoms, laboratory investigations, radiological findings, treatment details, and patient outcomes.
Primary outcome measures were intensive care unit (ICU) admission rate, mortality rate, and the event rate of ARDS. Intensive care unit admission was used as a surrogate marker for severe infection. Secondary outcome measures included other complications such as respiratory failure, septic shock, coagulopathy, acute cardiac injury, AKI, and secondary infection, as well as length of hospital stay and discharge rate at the point of study completion.
Statistical Analysis
To account for intra- and interstudy variance, random-effects models were used for meta-analyses of variables and endpoints [11]. Pooled proportions were computed with the inverse variance method using the variance-stabilizing Freeman-Tukey double arcsine transformation [12]. Confidence intervals (CIs) for individual studies were calculated using the Wilson score CI method with continuity correction. The I2 statistic was used to present between-study heterogeneity, where I2 ≤30%, between 30% and 50%, between 50% and 75%, and ≥75% were considered to indicate low, moderate, substantial, and considerable heterogeneity, respectively [13]. P values for the I2 statistic were derived from the chi-square distribution of the Cochran Q test. For pooling of means of numerical variables, we computed missing means and standard deviations (SDs) from medians, ranges (minimum to maximum), and interquartile ranges (IQRs) using the methods proposed by Hozo et al [14] and Wan et al [15].
Summary-level meta-regression was performed using the mixed-effects model after computation of the SD of Freeman-Tukey double arcsine transformed proportions. Publication bias of studies was assessed using funnel plots, where an asymmetrical distribution of studies was suggestive of bias [16]. Quantitative analysis of funnel plot asymmetry was done using Egger’s regression test, based on a weighted linear regression of the treatment effect (expressed as a Freeman-Tukey double arcsine transformed proportion) on its standard error [17]. The GRADE (Grading of Recommendations Assessment, Development, and Evaluation) approach was used to evaluate the quality of evidence for each outcome [18].
All statistical analyses were performed using R software version 3.4.3 (R Foundation for Statistical Computing, 2016), with the package meta [19]. P-values less than .05 were considered statistically significant.
RESULTS
Our search yielded 1481 unique publications. After screening of titles and abstracts, 109 publications were reviewed in full text. A total of 45 studies reporting on 4203 patients met eligibility criteria for inclusion in our meta-analysis (see Supplementary Figure 1). Reliability of study selection between observers was substantial at both the title and abstract screening stage (Cohen’s κ = 0.86) and the full-text review stage (Cohen’s κ = 0.85).
All included studies were nonrandomized, retrospective observational studies. Forty-two studies reported data from China, with one each from Singapore, South Korea, and Hong Kong. Details of included studies are reported in Supplementary Table 5. Of the 36 prevalence studies, 33 studies attained a full score of 9 on the JBI checklist for prevalence studies, 2 studies attained a score of 8, and 1 study attained a score of 7 (see Supplementary Table 3). Of the 9 case series, 7 studies attained a full score of 10, 1 study attained a score of 8, and 1 study attained a score of 7 (see Supplementary Table 4).
Of the total 4203 patients, 2797 were male (66.5%) and 1406 were female (33.5%). Supplementary Table 6 summarizes the demographics, medical comorbidities, clinical symptoms, laboratory investigations, radiological findings, treatment details, and outcomes of all included patients. Pooled mean age was 45 years (95% CI, 35.5–54.5 years). The most common medical comorbidities were hypertension, cardiovascular disease, and diabetes (16.4%, 12.1%, and 9.8%, respectively). The most common clinical symptoms were fever, cough, and dyspnea (80.5%, 58.3%, and 23.8%, respectively). Definition of fever was specified in 9 studies (7 studies, ≥37.3°C; 2 studies, ≥37.5°C). The pooled mean incubation period was 6.1 days (95% CI, 5.0–7.3 days). The pooled mean time from symptom onset to hospital admission was 7.2 days (5.5–8.9 days).
The most common blood abnormalities observed were elevated C-reactive protein (CRP) (59.4%), decreased albumin (58.6%), elevated lactate dehydrogenase (LDH) (51.7%), and lymphopenia (47.7%). The most common radiological abnormalities seen on chest computed tomography (CT) scan were bilateral infiltrates (80.8%), ground-glass opacities (73.0%), interlobular septal thickening (46.3%), subpleural lines (45.5%), and consolidation (41.6%). In terms of treatment, the type of antivirals used included combinations of oseltamivir, ganciclovir, lopinavir, ritonavir, ribavirin, and arbidol. The type of antibiotics used comprised moxifloxacin, ceftriaxone, and azithromycin.
Overall Outcomes
Table 1 summarizes the overall outcome findings. At the point of study completion, pooled ICU admission rate was 10.9% (95% CI, 4.5–19.3%) (see Supplementary Figure 2). The pooled mortality rate was 4.3% (95% CI, 1.0–9.1%) (see Supplementary Figure 3). Acute respiratory distress syndrome was the most common complication, with a pooled event rate of 18.4% (95% CI, 7.4–32.4%) (see Supplementary Figure 2). Study heterogeneity was considerable for ICU admission, mortality, and ARDS rates (Table 1). In 16.2% of all patients (95% CI, .4–43.3%), ARDS progressed to respiratory failure. The second and third most common complications were secondary infections (8.7%; 95% CI, 4.9–13.3%), such as hospital-acquired or ventilator-associated pneumonia and acute cardiac injury (7.8%; 95% CI, 1.2–18.2%), respectively. Among the studies that reported secondary infections, 4 specified the method of diagnosis. In all 4 of these studies, diagnosis required a positive bacterial culture, either from a lower respiratory tract specimen (including qualified sputum, endotracheal aspirate, or bronchoalveolar lavage fluid) or from blood samples. Acute cardiac injury was defined consistently across studies as an elevated level of serum cardiac biomarkers (eg, troponin I) above the 99th percentile upper reference limit, or new abnormalities demonstrated in electrocardiography and echocardiography.
Table 1.
Pooled Outcomes of Included Patients
| Outcome | No. of Studies Reporting Variable | No. of Patients Analyzed | Pooled Mean Value or Percentage of Patients (95% CI) | I 2, % | P Value of χ2 | Quality of Evidence (GRADE) |
|---|---|---|---|---|---|---|
| ICU admission | 14 | 2153 | 10.9 (4.5–19.3) | 94.9 | <.0001 | Low |
| Mortality | 23 | 2921 | 4.3 (1.0–9.1) | 94.1 | <.0001 | Low |
| Complications | ||||||
| ARDS | 13 | 2221 | 18.4 (7.4–32.4) | 97.7 | <.0001 | Low |
| Respiratory failure | 8 | 552 | 16.2 (.4–43.3) | 97.2 | <.0001 | Very low |
| Shock | 8 | 1738 | 4.3 (.3–11.3) | 93.6 | <.0001 | Low |
| Coagulopathy | 3 | 1299 | 3.3 (.0–27.7) | 98.3 | <.0001 | Low |
| Acute cardiac injury | 7 | 592 | 7.8 (1.2–18.2) | 90.6 | <.0001 | Low |
| Acute kidney injury | 11 | 1958 | 5.5 (1.1–12.2) | 93.8 | <.0001 | Low |
| Secondary infection | 8 | 588 | 8.7 (4.9–13.3) | 55.7 | .0270 | Low |
| Length of hospital stay, days | 6 | 1531 | 12.2 (10.9–13.4) | 94.3 | <.0001 | Low |
| Duration of viral shedding, days | 5 | 261 | 14.8 (11.5–18.2) | 96.0 | <.0001 | Low |
| Discharged | 22 | 2922 | 37.4 (23.6–52.3) | 98.1 | <.0001 | Low |
Abbreviations: ARDS, acute respiratory distress syndrome; CI, confidence interval; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; ICU, intensive care unit.
Publication Bias
Funnel plots and an Egger’s regression test were done to assess for publication bias for ICU admission, mortality, and ARDS rates. There was no evidence of publication bias for ICU admission (P = .42), mortality (P = .41), and ARDS (P = .14) (see Supplementary Figure 4).
GRADE Assessment
At baseline, the quality of evidence derived from a review of COVID-19 studies was assessed as low, due to their observational nature. The quality of evidence for respiratory failure was rated down to very low for imprecision, due to the large CI range and the relatively small sample size analyzed. Despite considerable study heterogeneity demonstrated by the I2 values for most outcome measures, there was no rating down due to inconsistency, as the heterogeneity could likely be explained by differences in patient demographics, diagnostic criteria, treatment methods, and management protocols given that COVID-19 is a newly emergent disease.
Risk Factors of Intensive Care Unit Admission, Mortality, and Acute Respiratory Distress Syndrome
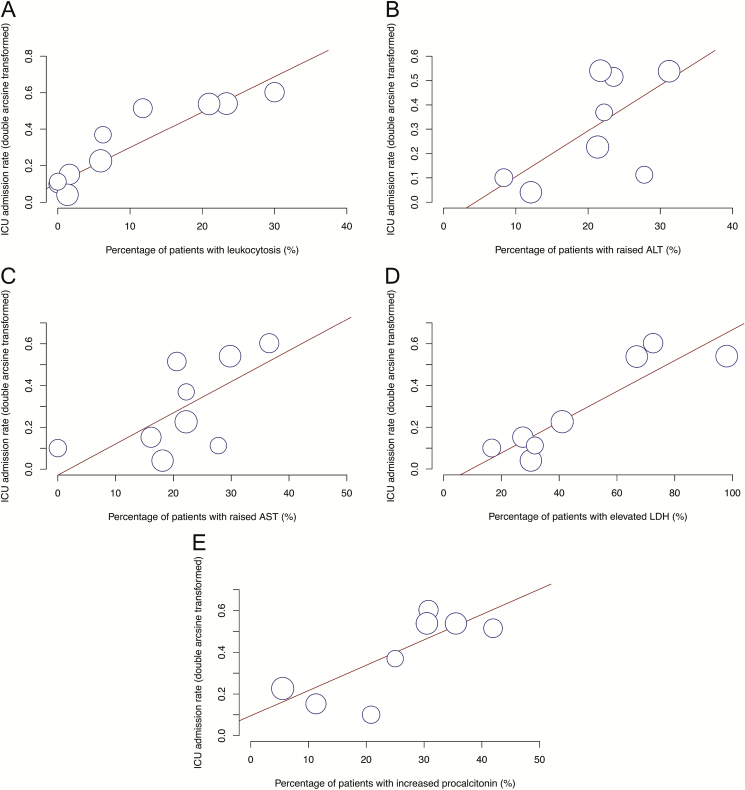
Meta-regression was performed to identify risk factors of ICU admission, ARDS, and mortality (Table 2). Fourteen studies with a total of 2153 patients reported ICU admission rates. Intensive care unit admission was predicted by increased leukocyte count (P < .0001), alanine aminotransferase (ALT) (P = .024), and aspartate transaminase (AST) (P = .0040); elevated LDH (P < .0001); and increased procalcitonin (P < .0001) (Figure 1). Intensive care unit admission was not significantly associated with elevated creatine kinase (P = .053), decreased leukocyte count (P = .29), lymphopenia (P = .44), thrombocytopenia (P = .80), elevated D-dimer (P = .41), elevated creatinine (P = .63), and elevated CRP (P = .88).
Table 2.
Summary of Laboratory Predictors of Intensive Care Unit Admission, Acute Respiratory Distress Syndrome, and Mortality Identified on Meta-Regression
| Outcome | No. of Studies Reporting Outcome | Risk Factor | P |
|---|---|---|---|
| ICU admission | 14 | Leukocytosis Increased ALT Increased AST Elevated LDH Increased procalcitonin | <.0001 .0235 .0040 <.0001 <.0001 |
| ARDS | 13 | Elevated LDH | <.0001 |
| Mortality | 23 | Leukocytosis Elevated LDH | .0005 <.0001 |
Abbreviations: ALT, alanine aminotransferase; ARDS, acute respiratory distress syndrome; AST, aspartate aminotransferase; ICU, intensive care unit; LDH, lactate dehydrogenase.
Figure 1.
Bubble plot for meta-regression of transformed ICU admission rate against percentages of patients with leukocytosis (A), increased ALT (B), increased AST (C), elevated LDH (D), and increased procalcitonin (E) in each study. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; ICU, intensive care unit; LDH, lactate dehydrogenase.
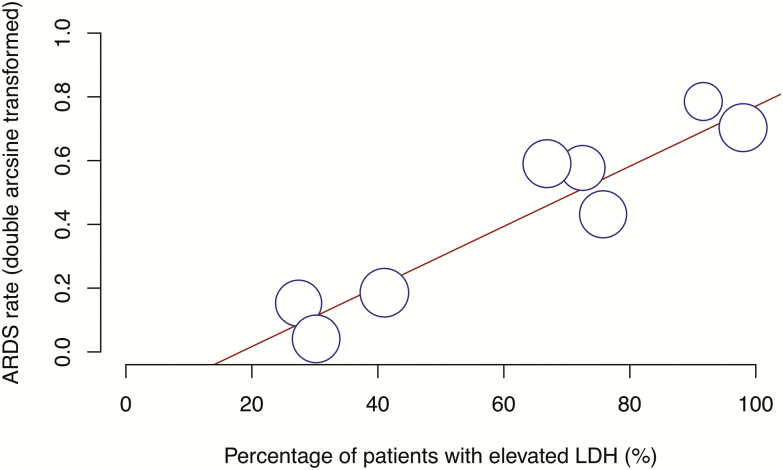
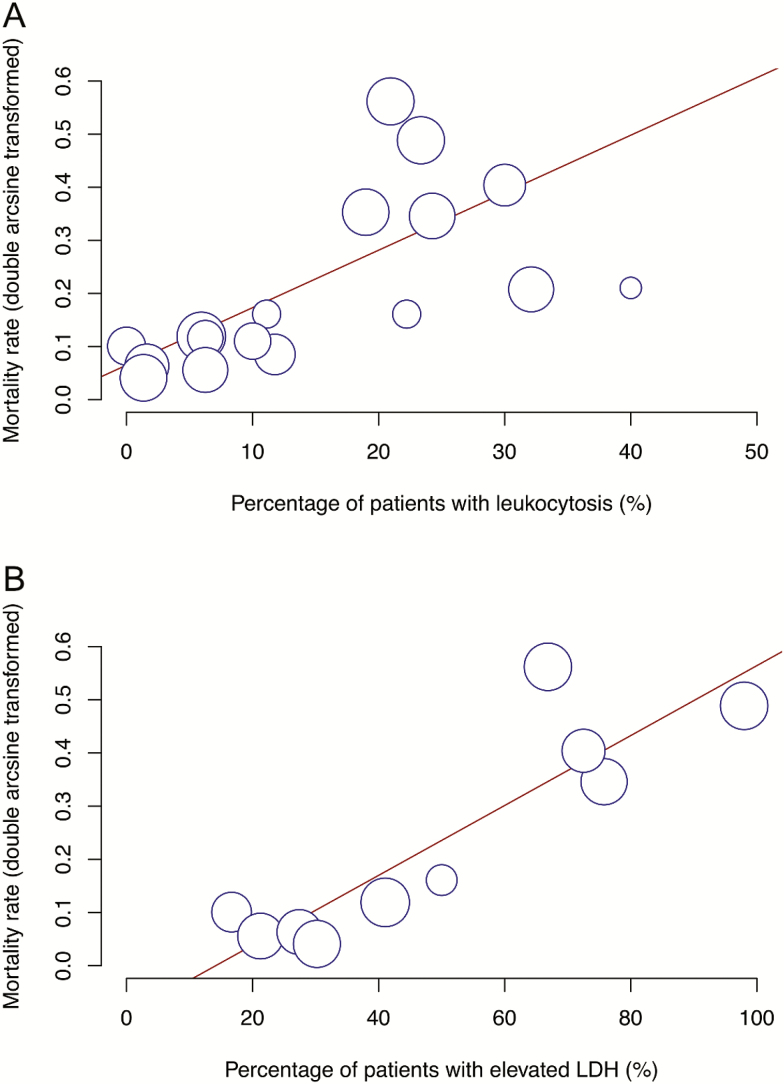
Acute respiratory distress syndrome was significantly predicted by elevated LDH (P < .0001) (Figure 2), while mortality was predicted by increased leukocyte count (P = .0005) and elevated LDH (P < .0001) (Figure 3).
Figure 2.
Bubble plot for meta-regression of transformed proportion of ARDS against percentage of patients with elevated LDH in each study. Abbreviations: ARDS, acute respiratory distress syndrome; LDH, lactate dehydrogenase.
Figure 3.
Bubble plot for meta-regression of transformed mortality rate against percentages of patients with leukocytosis (A) and elevated LDH (B) in each study. Abbreviation: LDH, lactate dehydrogenase.
Supplementary Table 7 summarizes all risk factors for ARDS, mortality, and severe COVID-19 infection reported in the literature to date.
Efficacy of Lopinavir-Ritonavir Treatment
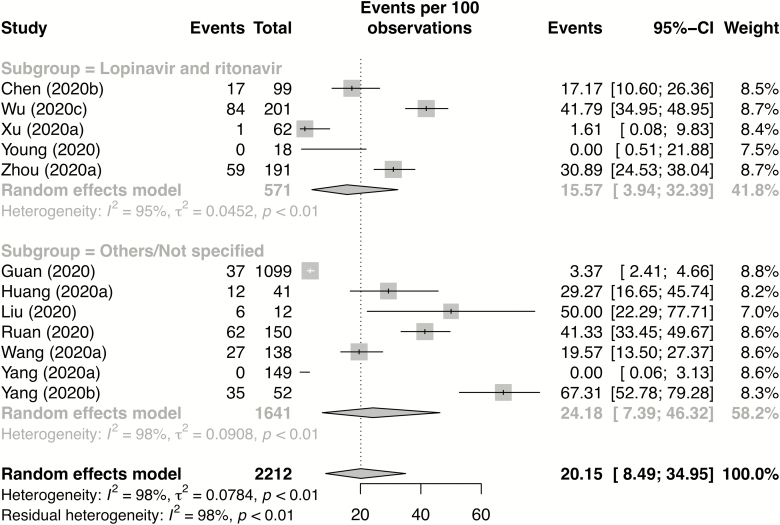
Subgroup analysis was performed according to reported antiviral treatment. Studies reporting the use of lopinavir and ritonavir were grouped together and compared with studies that reported other antiviral combinations or did not specify the type of antivirals used. Eight studies reporting on 633 patients used the combination of lopinavir and ritonavir (lopinavir-ritonavir group) and 13 studies reporting on 2079 patients used other combinations of antivirals or did not specify the type of antiviral (“Others/Not specified” group). Other combinations included oseltamivir, ganciclovir, ribavirin, and arbidol. Of these, 18 studies reported mortality rate and 12 studies reported the percentage of patients with ARDS.
Of all the patients in whom antiviral use was reported, the overall rate of mortality and ARDS was 5.7% and 20.2%, respectively. On subgroup analysis, the lopinavir-ritonavir treatment group had a lower rate of ARDS, although this difference was not statistically significant (15.6% vs 24.2%, P = .49) (Figure 4). The mortality rate was comparable between the lopinavir-ritonavir group and the “Others/Not specified” group (6.2% vs 5.5%, respectively; P = .93) (see Supplementary Figure 5).
Figure 4.
Forest plot for subgroup analysis of ARDS by type of antiviral used. The event rate is the incidence of ARDS in lopinavir and ritonavir recipients (top of figure) versus recipients of other/nonspecified antivirals (bottom of figure). Abbreviations: CI, confidence interval; ARDS, acute respiratory distress syndrome.
Efficacy of Corticosteroids
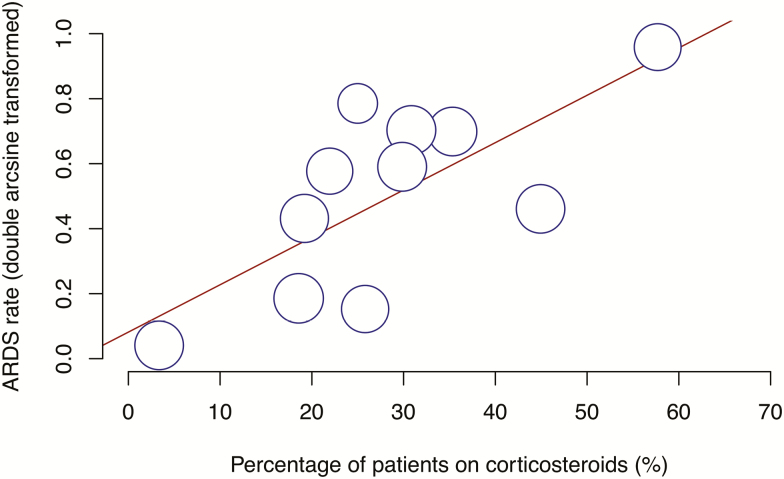
Subgroup analysis was performed for studies with the use of corticosteroids reported. Sixteen studies with a total of 2407 patients reported the use of corticosteroids. The pooled mortality rate in these patients was 7.2% (95% CI, 1.7–15.4%) and the pooled ARDS rate was 22.7% (95% CI, 9.9–38.6%). Meta-regression demonstrated a significant association between corticosteroid use and higher rate of ARDS (P = .0003) (Figure 5).
Figure 5.
Bubble plot for meta-regression of transformed ARDS rate against percentage of patients on corticosteroids in each study. Abbreviation: ARDS, acute respiratory distress syndrome.
DISCUSSION
Our meta-analysis provides an in-depth analysis of the key epidemiological features, clinical characteristics, laboratory investigations, radiological findings, treatment details, and outcomes of COVID-19 from the published literature. We identified elevated LDH as a significant predictive marker of ARDS and found that both elevated leukocyte count and elevated LDH predict mortality. Treatment with the antiretroviral drug lopinavir-ritonavir was not associated with significant benefit, while corticosteroids were associated with possible harm.
Early recognition of severe infection may allow early intervention with supportive measures and therapeutics and improve outcomes [20]. Our meta-regression identified 5 significant markers of ICU admission: increased leukocyte count, ALT, and AST, in addition to elevated LDH and finally increased procalcitonin. While 10.7% of patients had an increased leukocyte count in our meta-analysis, the degree of leukocytosis was modest (pooled mean leukocyte count was 6.0 × 109/L). Increased ALT and AST in severe COVID-19 may be a result of liver damage caused by the direct binding of SARS-CoV-2 to angiotensin-converting enzyme 2–positive cholangiocytes [21]. In our analysis, LDH was the only marker that significantly predicted all 3 measured outcomes: ICU admission, ARDS, and mortality. LDH is released from cells upon damage to their cytoplasmic membrane and is not only a metabolic but also an immune surveillance prognostic biomarker [22, 23]. LDH increases the production of lactate, which leads to enhancement of immune-suppressive cells and inhibition of cytolytic cells [24]. These changes could weaken the immune response mounted against the viral infection, resulting in more severe disease in patients with elevated LDH. Increased procalcitonin may have been a marker of bacterial coinfection, thereby resulting in complications of COVID-19 and hence a higher rate of ICU admission in these patients [25]. Interestingly, lymphopenia was not found to be a significant predictor of ICU admission, mortality, and ARDS in our meta-analysis. A possible explanation may be that we analyzed lymphopenia as a dichotomous variable without taking into account the degree of lymphopenia (ie, the numerical value of lymphocyte count), which lies on a spectrum and could affect disease severity among patients with lymphopenia.
The results of randomized clinical trials of COVID-19 interventions are of critical importance as only weak evidence supports the currently available repurposed and novel antivirals [26]. Among the patients with antiviral use reported in our meta-analysis, overall rates of mortality, ICU admission, and ARDS were 5.7%, 11.8%, and 20.2%, respectively. We found no overall benefit from treatment with lopinavir-ritonavir, in line with a recent randomized controlled trial (RCT) of 199 patients by Cao et al [27] (published after our search was conducted). However, this trial demonstrated that lopinavir-ritonavir treatment granted a significant reduction in ICU length of stay in survivors. Further trials (NCT04252885 and NCT04307693) are in progress to assess the efficacy of both lopinavir and ritonavir in reducing the COVID-19 viral load, and we look forward to future developments to provide recommendations on the use of antiviral therapy [28, 29].
Severe COVID-19 is associated with a dysregulated host inflammatory response, suggesting immune modulators as an attractive treatment modality [30]. Corticosteroids were used during the SARS-CoV outbreak; however, in a meta-analysis, only 4 studies provided conclusive data, and all 4 indicated possible harm [31, 32]. These harms included risks of prolonged viremia, corticosteroid-induced diabetes, avascular necrosis, and psychosis [31, 33, 34]. Our meta-analysis suggested that the use of corticosteroids is associated with disease severity (ICU admission) and higher ARDS rates. It is not clear if this effect is a consequence of corticosteroid treatment or confounding by indication bias where sicker patients are more likely to receive corticosteroids. An RCT of corticosteroids in severe respiratory viral infections has long been called for, and at least 1 clinical trial in COVID-19 (NCT04244591) is ongoing [35].
SARS-CoV-2–induced pneumonia is marked by a cytokine storm—hyperactivation of effector T cells and excessive production of inflammatory cytokines, particularly interleukin-6 (IL-6) [36]. Blockade of IL-6 function using tocilizumab, a specific monoclonal antibody against its receptor, appears to be useful in alleviating hyperinflammation symptoms in severe cases [37, 38]. Selective Janus kinase-signal transducer and activator of transcription (JAK-STAT) inhibitors such as baricitinib may also be beneficial, although clinical trials are required and any benefit is likely to be greatest in combination with an effective antiviral [39].
There are several limitations to our study. First, only studies published in English were included, which may have introduced selection bias. Unpublished materials (such as recently completed studies) were also excluded, which might have affected the conclusions drawn. Second, the results may be not be generalizable to healthcare systems outside Asia as no studies from Europe or the United States were available for inclusion in our review at the time of the literature search. Additionally, all included studies were observational. Clinical parameters were often not clearly defined and clinical follow-up time varied. Finally, there was heterogeneity in the range of symptoms and comorbidities recorded by different studies due to the lack of objective measurements.
CONCLUSIONS
To the best of our knowledge, this is the first systematic review and meta-analysis of COVID-19 to describe specific laboratory predictors of severe disease and adverse outcomes. Our study is also the first meta-analysis to evaluate the efficacy of antivirals and corticosteroids. Careful attention should be given to the management of patients with increased leukocyte count, ALT, and AST; elevated LDH; and increased procalcitonin as these factors predict ICU admission, mortality, and ARDS. In terms of treatment efficacy, the use of corticosteroids in patients with COVID-19 is significantly associated with higher rates of ARDS. Compared with other antivirals, the use of lopinavir and ritonavir is nonsuperior in terms of lowering mortality rate. Further prospective studies are vital to clarify our findings.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was funded by the Singapore National Medical Research Council COVID-19 Research Fund (COVID19RF-001).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Worldometers.info. Dover, DE, ; 2020. [Google Scholar]
- 4. Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis 2020; 94:91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas 1960; 20:37–47. [Google Scholar]
- 7. Saad AF, Rahman M, Maybauer DM, et al. Extracorporeal membrane oxygenation in pregnant and postpartum women with H1N1-related acute respiratory distress syndrome: a systematic review and meta-analysis. Obstet Gynecol 2016; 127:241–7. [DOI] [PubMed] [Google Scholar]
- 8. Zhang JJY, Ong JA, Syn NL, et al. Extracorporeal membrane oxygenation in pregnant and postpartum women: a systematic review and meta-regression analysis. J Intens Care Med 2019:885066619892826. doi:10.1177/0885066619892826 [DOI] [PubMed] [Google Scholar]
- 9. Grainge M. Excluding small studies from a systematic review or meta-analysis. Presented at: CSG Annual Meeting 2015; March 12–18, 2015; Dresden, Germany: Available at: https://skin.cochrane.org/sites/skin.cochrane.org/files/public/uploads/CSG-COUSIN_March%202015_M%20Grainge.pdf. Accessed 1 February 2020. [Google Scholar]
- 10. Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med 2015; 8:2–10. [DOI] [PubMed] [Google Scholar]
- 11. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 2010; 1:97–111. [DOI] [PubMed] [Google Scholar]
- 12. Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health 2014; 72:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. London, UK: The Cochrane Collaboration; 2011. [Google Scholar]
- 14. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sterne JAC, Sutton AJ, Ioannidis JPA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011; 343:d4002. [DOI] [PubMed] [Google Scholar]
- 17. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment. Health 2019; 22:153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chai X, Hu L, Zhang Y, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV Infection. bioRxiv 2020:2020.02.03. 931766. doi:10.1101/2020.02.03.931766. Available at: https://www.biorxiv.org/content/10.1101/2020.02.03.931766v1.full. Accessed 1 May 2020. [Google Scholar]
- 22. Han Y, Zhang H, Mu S, et al. Lactate dehydrogenase, a risk factor of severe COVID-19 patients. medRxiv 2020:2020.03.24.20040162. doi:10.1101/2020.03.24.20040162. Available at: https://www.medrxiv.org/content/10.1101/2020.03.24.20040162v1. Accessed 1 May 2020. [Google Scholar]
- 23. Kuang Z-S, Yang Y-l, Wei W, et al. Clinical characteristics and prognosis of community-acquired pneumonia in autoimmune disease-induced immunocompromised host: a retrospective observational study. World J Emerg Med 2020; 11:145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ding J, Karp JE, Emadi A. Elevated lactate dehydrogenase (LDH) can be a marker of immune suppression in cancer: Interplay between hematologic and solid neoplastic clones and their microenvironments. Cancer Biomark 2017; 19:353–63. [DOI] [PubMed] [Google Scholar]
- 25. Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta 2020; 505:190–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA 2020; 323:1824–36. [DOI] [PubMed] [Google Scholar]
- 27. Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020; 382:1787–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. LI L. The efficacy of lopinavir plus ritonavir and arbidol against novel coronavirus infection (ELACOI) Available at: https://clinicaltrials.gov/ct2/show/NCT04252885. Accessed 18 March 2020.
- 29. Kim S-H. Comparison of lopinavir/ritonavir or hydroxychloroquine in patients with mild coronavirus disease (COVID-19) Available at: https://clinicaltrials.gov/ct2/show/NCT04307693. Accessed 18 March 2020.
- 30. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 2020. doi:10.1038/s41577-020-0311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med 2006; 3:e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arabi YM, Mandourah Y, Al-Hameed F, et al. ; Saudi Critical Care Trial Group Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med 2018; 197:757–67. [DOI] [PubMed] [Google Scholar]
- 33. Lee DT, Wing YK, Leung HC, et al. Factors associated with psychosis among patients with severe acute respiratory syndrome: a case-control study. Clin Infect Dis 2004; 39:1247–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS-associated coronavirus RNA concentrations in adult patients. J Clin Virol 2004; 31:304–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hospital Z. Peking Union Medical College Hospital, Zhongda Hospital, Zhongnan Hospital and Renmin Hospital of Wuhan University. Glucocorticoid therapy for novel coronavirus critically ill patients with severe acute respiratory failure (Steroids-SARI) Available at: https://clinicaltrials.gov/ct2/show/NCT04244591. Accessed 30 March 2020.
- 36. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020; 46:846–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395:1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol 2020. doi:10.1002/jmv.25801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stebbing J, Phelan A, Griffin I, et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis 2020; 20:400–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.