Abstract
Monosodium glutamate is a sodium salt of a nonessential amino acid, L-glutamic acid, which is widely used in food industry. Glutamate plays an important role in principal brain functions including formation and stabilization of synapses, memory, cognition, learning, as well as cellular metabolism. However, ingestion of foodstuffs rich in monosodium glutamate can result in the outbreak of several health disorders such as neurotoxicity, hepatotoxicity, obesity and diabetes. The usage of medicinal plants and their natural products as a therapy against MSG used in food industry has been suggested to be protective. Calendula officinalis, Curcuma longa, Green Tea, Ginkgo biloba and vitamins are some of the main natural products with protective effect against mentioned monosodium glutamate toxicity through different mechanisms. This review provides a summary on the toxicity of monosodium glutamate and the protective effects of natural products against monosodium glutamate -induced toxicity.
Key Words: Food industry, Herbal medicine, Monosodium glutamate, Neurotoxicity, Phytochemical
Introduction
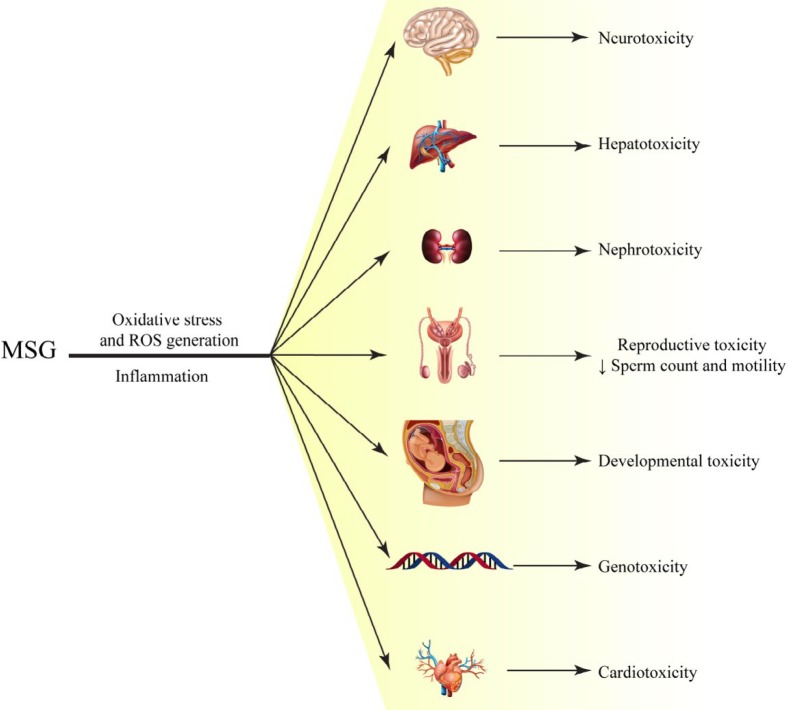
Food additives are extensively used in food industry in small quantities to improve the flavor, color, taste, appearance and texture of food (1). In food industry, the food flavoring agents are categorized to 25 classes, which contain about 230 different compounds (2). Among them, flavor enhancers are responsible for giving the food an umami, brothy and savory taste (3). Monosodium glutamate (MSG) is one of the most extensively used flavor enhancers and stabilizers in processed foods (4). The daily intake of glutamate depends on the region. The average intake of MSG has been estimated to be 0.3-1.0 g/day in developed countries (5). The average intake of MSG in UK was 0.58 g/day (6), 10.0 g in Germany (7) while it has recently been reported that in other European countries, the average daily intakes of glutamate is about 1.0 g/day. In Nigeria, an average intake of 0.56–1.00 g/day has been reported whereas in Asia it is higher with an intake of 1.1–1.6 g/day in Japan, 1.5–3.0 g/day in Taiwan and 1.6–2.3 g/day in South Korea (4, 8). Although the Food and Drug Administration (FDA) stated that MSG is a safe substance, several studies in animals have indicated negative effects after chronic consumption of MSG. These adverse effects have been shown in different organs such as thymus (9), brain (10), pancreas (11), testis (12), liver and kidney (13), and have been linked with several diseases including obesity, hypertension, headaches, asthma exacerbation, neurotoxic effects and detrimental effects on the reproductive organs (14). Figure 1 is a schematic representation of different organs that may be affected by MSG toxicity. Several natural products can exert protective effect against toxicity induced by MSG. In this review, the protective effects of medicinal plants and natural products against MSG-induced toxicities are discussed.
Figure 1.
A schematic representation of Monosodium glutamate (MSG) toxicity and the organs may be affected by MSG
Search strategy
This comprehensive review was performed by searching in Scopus, Web of Science, PubMed and Google Scholar to identify all published articles about the chemistry, toxicity of MSG, and protective effect of natural products against MSG from their inception up to August 2019. The search terms included “monosodium glutamate”, “toxicity”, “nephrotoxicity”, “hepatotoxicity”, “neurotoxicity”, “reproductive toxicity”, “oxidative stress”, “genotoxicity” and “natural products” in titles and abstracts.
Chemistry of MSG
MSG (Figure 2) was first extracted from the seaweed Laminaria japonica and identified by the Japanese chemist Kikunae Ikeda in 1908 (15). It is a sodium salt of a nonessential amino acid known as L-glutamic acid, with chemical formula of C5H8NO4.Na, IUPAC-ID name of sodium 2-aminopentanedioate that specified by name of E621 in food industry (16). It is a white, odorless, and crystalline powder with a molecular mass of 169.11 g/mol and melting point of 232 °C. It has a unique taste known as umami which is a savory, broth-like or meaty taste and once dissolves in aqueous solution; it will dissociate to form sodium and free glutamate. It is sparingly soluble in alcohol but the solubility in water is 385,000 mg/l at 25 °C. MSG is also soluble in oil or organic solvents (17). It is a common glutamic acid salt which contains 78% glutamic acid, 22% sodium salt and also water (18). The major reason of using such an additive is that MSG has higher and more rapid dissolution performance against glutamic acid (19).
Figure 2.
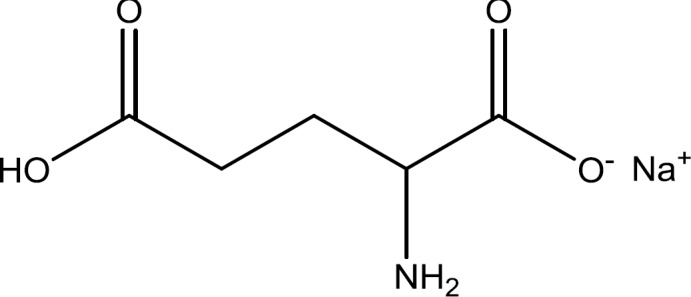
Chemical structure of Monosodium glutamate (MSG)
Pharmacokinetics
Once ingested, MSG dissociates in the body into glutamate and sodium ions. Glutamate is one of the most common amino acid and abundant in the central nervous system (18). It is found in high concentrations in the regions of brain with essential role in mediating cognition incluan increase ding striatum, dentate gyrus of hippocampus and cerebral cortex (20). Since MSG does not require any enzyme activity to break it down, it is absorbed very quickly in our body which can spike the blood plasma levels of glutamate (21). Then, the absorbed glutamate is transported in the intestine lumen via sodium carboxylate transporter (NaDC-1) and excitatory amino acid transporter (EAAT-1), and circulated in the body through the bloodstream (22). There is a consensus that nearly whole ingested glutamate is metabolized in the intestine and most of its carbon skeleton is converted to CO2 or consumed for the intestinal production of amino acids such as glutathione, alanine, arginine, lactate and so others. Furthermore, most of its nitrogen is participated in the synthesis of proline, glutamine, amino acids of the urea cycle, and branched-chain amino acids. Remained little glutamate is absorbed into the portal vein which may be the reason for the low systemic concentration of glutamate (23). The plasma glutamate level peaked within 80 min, when it administered orally at dosage of 30 mg/kg every 20 min for 220 min, without any side effects (24). It was reported that excessive ingestion of MSG increased the concentration of glutamate and aspartate in plasma for 1–2 hr (25). Excessive daily intake of MSG causes an elevated plasma level of glutamate. The plasma levels of glutamate are depending on some factors including dose, concentration and age. For example, an increase in the concentration of MSG in neonatal rats (from 2 to 10%) leads to five-fold increase in plasma (26). It was found that doses of up to 1 g/kg of MSG do not significantly cross the blood–brain barrier (27). Furthermore, the glutamate levels in the brain are far higher than those in plasma of mice, rats, guinea pigs and rabbits (26). Actually, plasma glutamate concentrations are 50–100 μM; while, they are 10,000–12,000 μM in whole brain and just 0.5–2 μM in extracellular fluids (28).
Toxicity and side effects of MSG
Obesity and diabetes
During recent decades, obesity has become a serious global health issue (29, 30). It plays an essential role in developing a wide range of human diseases such as dyslipidemia, diabetes, coronary heart disease, hypertension and cancer (30). Diet and lifestyle modification are suggested as two important aspects associated with reduced diabetes risk (31). It was suggested that uncontrolled use of food additives such as MSG can cause obesity (32). MSG has been used in several experimental models for inducing obesity which causes diabetes. Dietary MSG consumption is associated with obesity and overweight in healthy adults (33). The possible mechanisms involved in MSG-induced obesity may be the influence of MSG on energy balance by enhancing palatability through disrupting the hypothalamic signaling network of leptin action (33, 34). Administration of MSG enhances body weight, triacylglycerol, cholesterol, glucose, insulin, leptin and reduces high-density lipoprotein (HDL) levels in male Wistar rats (35). Injection of MSG (2 mg/g body weight) to the newborn male and female mice lead to increase blood glucose and diabetes (36). At the same dosage, MSG causes obesity and diabetes and moderates severe microvesicular fatty changes throughout the liver parenchyma in newborn mice (37).
Oxidative stress and hepatotoxicity
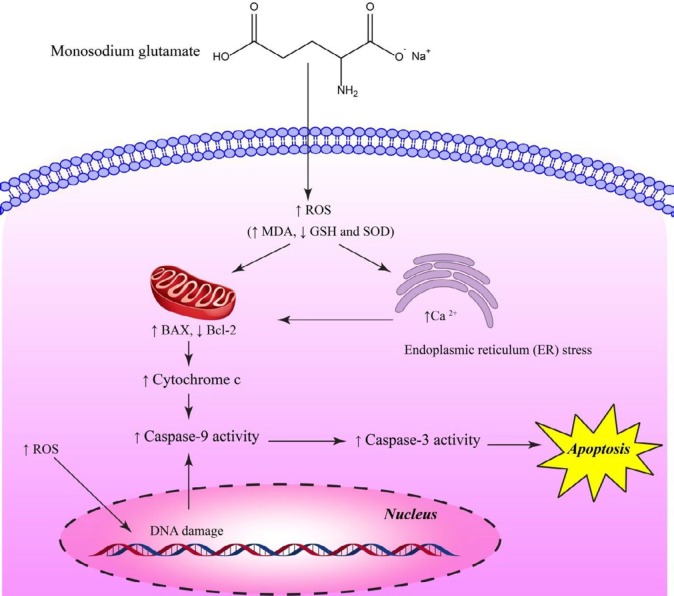
The mechanism of action of MSG-induced damage to different organs such as the liver, brain, testis, and kidney is related to the induction of oxidative stress (38). Oxidative stress is a situation when intracellular levels of reactive oxygen species (ROS) are enhanced, which leads to the disruption of cellular metabolism and damage to lipids, carbohydrates, proteins and nucleic acids. Oxidative stress is associated with many human disorders including neurodegenerative diseases, diabetes, cardiovascular diseases, atherosclerosis, inflammatory bowel disease, osteoporosis and carcinogenesis (31, 39). Pavlovic et al. found that MSG increased rat thymocytes apoptosis via reducing Bcl-2 expression (40). The same authors also showed that prolonged administration of MSG to animals led to increased thymocytes apoptosis through oxidative stress (9). Molecular mechanism of MSG-induced cell death is shown in Figure 3. In another study on rats, dependent to the age, time of administration, and susceptibility of brain and spinal cord regions, MSG induced nociception and oxidative stress (41). Administration of MSG at doses more than 4 mg/g of body weight (bw) for adult male mice encouraged oxidative stress in erythrocytes by increasing lipid peroxidation (LPO, as a marker of oxidative stress) (42). Elshafey et al. (43) have demonstrated that MSG-induced hepatic toxicity through oxidative stress evidenced by increased lipid peroxidation, reduced antioxidant enzymes and fibrosis. Similarly, administration of MSG also has shown to induce oxidative stress through induction of lipid peroxidation, reduction of glutathione (GSH) and enhancement of the activities of superoxide dismutase (SOD), glutathione-s-transferase (GST), and catalase in the liver of the experimental animals which in turn, result in hepatotoxicity at a dose of 0.6 mg/g bw (44). Furthermore, administration of MSG induced oxidative stress in adult Wistar rats and causes some undesirable effects on the liver at higher doses (45).
Figure 3.
Molecular mechanism of monosodium glutamate (MSG)–induced cell death. As shown in the picture, MSG can activate intrinsic apoptosis pathway, leading cell death
Neurotoxicity
MSG induces neurotoxicity through increasing LPO, oxidative stress and subsequent apoptosis and cholinergic dysfunction (46, 47). Although it is neurotoxic, manufacturers are using glutamic acid because it is cheap and do not want the public to know that (48). Its neurotoxicity associates with over-activation of excitatory amino acid receptors which causes enhanced intracellular calcium that triggers a cascade of enzymatic activities which resulting in cell death (49). Several studies also have demonstrated the neurotoxic effects of neonatal MSG administration (50, 51). MSG administration destroys neurons of the hypothalamus in rats which causes many metabolic abnormalities such as growth disturbances, self-mutilation, pseudo-obesity and hypogonadism (52-54). In addition, it is documented that MSG induces a neuroendocrine abnormalities leading to hypophagic which causes anxiogenic-like and depressive-like behaviors, metabolic dysfunctions, obesity, insulin resistance, changes in analgesic responses, glucose intolerance, and chronic inflammation (55-57). It is also found that MSG could be associated with other neurodegenerative illnesses such as amyotrophic lateral sclerosis, Alzheimer’s and Parkinson’s disease (58).
Hippocampus, as one of the most vulnerable brain regions to glutamate-mediated excitotoxicity, has an important role in spatial learning and memory (59). Recently, it was found that rats exposed to low dose of MSG showed reduction in learning capabilities and short memory on forebrain in the hippocampus (48). Ali et al. have documented that low doses of MSG affects cognitive functions and has toxic effect during early childhood in which the blood-brain-barrier is highly permeable to large and small molecules (60). Such behavioral tests of animals exposed to MSG are also obtained by other studies (61, 62). In another study, exposed to MSG caused impairment of memory in rats associated to the inhibition of Na+, K+-ATPase activity in the hippocampus of rats (63).
Genotoxicity
Several reports have been indicated the genotoxicicty of several flavour enhancers including MSG (64). It was also reported that MSG develops genotoxicity and cytotoxicity in the root tip cells of the plant Allium cepa (65). MSG presented both genotoxicity and mutagenicity in Allium cepa assay, that was manifested by chromosomal abnormalities and affected through mitotic index in root tips (66). In vitro studies have shown that MSG is genotoxic to the human peripheral blood lymphocytes (67). Furthermore, some other authors have demonstrated that MSG has genotoxic effects (66, 68, 69). The oral median lethal dose (LD50) of MSG in rats and mice was reported as 15 and 18 g/kg bw (70). Administration of MSG at doses of 20 and 40 mg/kg bw (equivalent to 1 and 2 g/person) for 2 months caused genotoxicity in rats’ palatal mucosa (71). ROS and oxidative stress are involved in MSG-induced cytotoxicity and genotoxicity, as indicated by increasing LPO in three major organs; liver, kidney and brain of rats (11). In contrast, some other researchers believed that MSG is not genotoxic (72, 73).
Reproductive toxicity
MSG induced testicular lesions in rats through oxidative stress. Administration of MSG at low and high doses caused alterations in the testis tissue of rats which may related to the male infertility in rats (74). Uses of MSG resulted in damage to the testes due to its effect in inducing oligospermia and glycogen accumulation in male Wistar rats (75). In another study, oral administration of MSG caused reduced levels of serum testosterone and decrease in the cauda epididymal sperm reserves in young male rats as well as adult ones (76). Several observed abnormalities in both microarchitecture of testis and semen characteristics has associated with using MSG administration in Wistar rats which could affect male fertility (77). A number of studied have mentioned that the ingestion of MSG could affect male fertility (78-80). It was reported that half lethal dose (LD50) of MSG in rats and mice is 15 and 18 g/kg BW respectively (70). Recently, European Food Safety Authority (EFSA) suggested the acceptable daily intake of MSG as 30 mg/kg BW.
Protective effect of plants or plant-derived natural products
Calendula officinalis
Shivasharan et al. (81) demonstrated the protective influence of the flower extract of C. officinalis against MSG-induced oxidative stress in addition to excitotoxic brain damage in rats. Orally administered of MSG to adult Wistar rats for 7 days resulted in increasing of oxidative and nitrative stress which evidenced by the reduced GSH, GST, total thiols, catalase activity and increased the MDA and nitrite levels in the brain tissues. It was previously reported that induction of apoptosis in the brain tissue resulted in neurotoxicity by MSG. When, animals were treated with the dosage of 100 and 200 mg/kg of the extract after one hour of MSG administration, it significantly reduced the oxidative stress through increased levels of CAT, GSH, TT, GST and reduced levels of LPO and nitrite. Since previous studies have demonstrated the antioxidant (82, 83) and anti-flammatory (84, 85) activities of C. officinalis flawers, they have suggested that the observed protective activity may relate to the antioxidant and anti-inflammatory activities of C. officinalis (81). Lima et al. (86) have also reported that 90 mg/kg extract of C. officinalis significantly reduced oxidative stress (increased GSH, SOD, CAT and reduced MDA) in rats. There are so many identified bioactive compounds in C. officinalis extract, which may responsible for aforementioned activities. Among them flavonoids such as quercetine (87) and the triterpenoids especially, the faradiol monoester (88), seem to play an important role as antioxidant and anti-inflammatory activities of C. officinalis extract, respectively.
Cucurbita ficifolia
Numerous experimental models have demonstrated that MSG in high doses can cause the increased body weight and fat mass (89). The increased body weight by MSG may be due to an increase in energy intake that subsequently lead to obesity (90), or interference with signaling systems regulating appetite centers, also upscaling food consumption led to weight gain (91). It is accepted that increased weight is associated with several diseases such as type 2 diabetes (T2D), reduced life expectancy, cardiovascular disease, psychological dysfunction and hypertension (92). Recently natural medicine or plant extracts with lowering body weight activity has attracted much attention due to its fewer side effects than chemical pharmaceuticals. Previous studies in T2D patients have confirmed that C. ficifolia (cucurbitaceae) has hypoglycemic activity (93). In other hand, a recent study showed that the aqueous extract of the endocarp of fruits from C. ficifolia (pumpkin) decreased the body weight and inflammation in an obesity model induced by MSG in an obesity model mice (MSG-obese mice) through inhibiting the expression of tumor necrosis factor type alpha (TNF-α), interleukin-6 (IL-6), tumor necrosis factor receptor 2 (TNFR2), while it increased the protein levels of interferon-gamma (IFN-γ) and IL-10. Moreover, the extract enhanced the protein levels of IL-10 in lean mice in addition to IFN-c in both lean and MSG-obese mice (94). Several studies have suggested that plants belong to cucurbitaceae family have anti-adipogenic property (95).
Curcuma longa
It was well-documented that C. longa, especially its bioactive component curcumin have numerous health effects including neuroprotective and anticancer properties (96-102). Khalil and Khedr (103) showed that curcumin has a protective role against MSG-induced neurotoxicity in rats. Curcumin treatment considerably attenuated both AChE activity and TNFα in MSG-treated rats. They suggested that anti-inflammatory activities of curcumin may explain this neuroprotective action. In another study, Vucic and colleagues reported that treatment of rat thymocytes with curcumin decreased MSG-induced apoptosis and ROS production, restored MMP and upregulated the Bcl-2/Bax protein ratio (104). In addition, they proposed that inhibition of PI3K/Akt signaling pathway in MSG-induced apoptosis was the main mechanism of anti-apoptotic effects of curcumin. Finally, the protective effects of curcumin on MSG-induced reproductive toxicity was shown by restoring testis weight and sperm count and decreasing the incidence of abnormal sperm in male rats (105).
Hibiscus sabdariffa
Hibiscus sabdariffa belongs to the Malvaceae family, rich in several bioactive compounds such as flavonoids, anthocyanins, proanthocyanidins, polysaccharides and organic acids (106). Olaleye et al. identified also a verity of other compounds such as cardiac glycosides, saponins, alkaloids, and flavonoids in aqueous extract of H. sabdariffa (107). It traditionally used to treat many diseases including liver disease, colds, hypertension, urinary tract infections, cholesterol-lowering and mutagenicity (106). In study of Gheller et al. aqueous extract of H. sabdariffa (at dose of 400 mg/kg/ day) exhibited considerable anti-mutagenic effects against MSG-induced DNA damage in male Wistar rats (108). The methanolic flower extract of another Hibiscus species; H. tiliaceus also has reported to have anti-mutagenic effects in vivo (109). Observed protection against mutagenic processes may be due to the presence of anthocyanins in the plants which act as potent antioxidants (110). Furtheremore, the anthocyanins extracted from H. sabdariffa have been reported as anti-carcinogenic and anti-mutagenic (111). Pre-clinical data have shown the anti-obesity effects of H. sabdariffa (112, 113). An in vivo study by Alarcon-Aguilar et al. (114) showed that orally administration of the aqueous extract of H. sabdariffa (120 mg/kg/day) for 60 days significantly reduced body weight gain and glycemia in MSG-obese mice.
Green tea
Obesity is known as an important risk factor for chronic morbidities such as cardiovascular diseases, some cancers (e.g. breast, colon and prostate), pulmonary and metabolic diseases (115). Both experimental and clinical studies have reported the anti-obesity effects of green tea (115, 116). MSG-induced obesity is widely used as an experimental model for investigation of obesity and its complications. Toxic effects of MSG on neurons of hypothalamus areas that control body mass and energy metabolism was reported as major mechanism of MSG-induced obesity (16). Study of Bártíková et al. showed significant anti-obesity effects of green tea. Oral administration of green tea extract (GTE) to obese mice resulted in reduction of food intake as well as level of insulin and leptin but did not significantly change the body weight (117). In addition, in another study, also it has reported that GTE could improve MSG-induced obesity and reduce insulin and leptin concentrations (118). Numerous studies have confirmed that MSG could cause damage to the ovaries of female rats, resulting in infertility (119, 120). MSG administration could cause oxidative stress in different organs which is associated with infertility in animal and in vitro models (121). Ali et al. have reported that GTE due to its potent antioxidant activity can protect ovarian against damages induced by MSG. They have concluded that the protection role of GTE against MSG is contribute to the ability of the green tea to trapping of ROS (122). Similar results were obtained by Yulianti et al. who reported that GTE (with 1.4 mg/day as an optimum dose) significantly increased the serum 17β-estradiol levels and Graafian follicles numbers in white rats exposed to MSG (123). In another study, an increased level of plasma total cholesterol, LDL-cholesterol and triglycerides was observed by oral administration MSG for 60 days. However, reduced gain body weight was observed in the groups treated with GTE at dose 1.5 ml/rat/day for 60 days (124). This observation is in accordance with other study finding that green tea catechins are associated to body weight regulation via inhibition of catechol O-methyl-transferase and phosphodiesterase which lead to thermogenesis, fat oxidation, and sparing fat free mass (125). Their results have also shown that GTE supplementation has the ability to normalize glucose levels and improve and ameliorate liver and kidney toxicity induced by MSG, restores the activity of antioxidant enzymes, and reduces the generation of ROS and lipid peroxidation (126). These results are in agreement with the findings reported in a previous study carried out by Hamza et al. (127), who noted that GTE conjugated with zinc oxide nanoparticles (ZnO/NPs) considerably recovers the hepatotoxicity developed by MSG through improving the liver enzyme activity (significantly reduced activities of LDH, ALT, AST, ALP as well as γ-GT in the serum) and the lipid profile (significantly decreased activities of LDH, ALT, AST, TC, γ-GT, TG, LDL-C, and VLDL-C).
Mangifera indica L (Mango)
Anthony and his colleague confirmed that MSG induced oxidative stress in brain of rats which evidenced by hypothalamic neuronal necrosis and degeneration of the brain histology. Therefore, they have studied the protective effect of mango (Mangifera indica L.) seed kernel against MSG toxicity in rats. Their study showed that high antioxidant potential of mango (increased the DPPH, total antioxidant capacity (TAC), and ferric reducing antioxidant power (FRAP) in vitro, besides increased catalase (CAT) and superoxide dismutase (SOD) activity and reduced MDA, glutathione peroxidase (GPx), glutathione (GSH) and uric acid (UA) in vivo) has a significant role in improving and regulating of the brain histology and serum antioxidant capacity of normal and MSG-intoxicated rats (128). The same author has already recorded similar results that demonstrated the modulation of MSG-induced toxicity in rats by mango seed kernel extract. Egbuonu and his coleagues have suggested that mentioned protective effect of the extract could be associated with the high vitamin C content of mango seed kernel which may enhance its antioxidant activity (129, 130).
Solanum lycopersicum (Tomato)
As mentioned earlier, reproductive toxicity and infertility is one of the concerns of MSG usage in food industry. Given tomato to animals that exposed to MSG improved motility and morphology of spermatozoa in mice. Previously, the antioxidant effects of tomato content especially lycopene was suggested as the main mechanism (131). Lycopene, a naturally occurring bioactive compound in tomato is the best scavenger of the free radicals among carotenoids (132).
It was shown that lycopene had remarkable neuroprotective effects against MSG-induced cholinergic dysfunction, Bcl-2/Bax imbalance and neurotoxicity (47). Recently, Badawi has studied the protective effects of lycopene against MSG-induced nephrotoxicity in vivo. Orally administration of lycopene in a dose of 4 mg/kg/ day for 14 days in adult male albino rats showed that lycopene has protective effects against MSG-induced nephrotoxicity, through reduction of serum creatinine and blood urea nitrogen level, inhibition of apoptosis (decrease Bax, increase Bcl2), and prevention of kidney damage (133). Similar findings were obtained by Kadry (47) who proved that lycopene has protective effects against MSG-neurotoxicity through ameliorating oxidative stress (decrease LPO levels, SOD, GST, and catalase activities in addition to downregulating CAT and GST gene expression; increase GSH in the brain of rats) and apoptosis (decrease Bax, increase Bcl2), inducing improvement of the acetylcholinesterase activity, modulating antioxidant enzymes gene transcripts, and decreasing body weight. Numerous clinical trials have also confirmed that lycopene inhibited oxidative stress through preventing LDL oxidation (134, 135). In 2018, Lu et al. (136) have suggested that the inhibition of glutamate release is one of the potentially mechanism for neuroprotective actions of lycopene. They have confirmed that lycopene prevents glutamate release from rat cortical synaptosomes through inhibiting of presynaptic Ca2+ entry and PKC activity.
Walnut
Walnut kernel mostly used as food but it also has been recorded as medicinal plant in traditional medicine (137). Study of Liang et al. showed that walnut meal extracts are rich in polyphenols such as glansreginin A, gallic acid and ellagic acid, significantly improved adverse effects of MSG on metabolic disorders including change in blood glucose, TG, TC, LDL-C, and insulin and liver dysfunction, as well as reduced the weight gain and fat accumulation (138). Previously, Rock et al. (139) have evaluated a study including one-hundred obese adults with two dietary strategies; a walnut-enriched diet group and a standard diet group for six months which resulted in reduced systolic blood pressure, triglycerides, total cholesterol, and low-density lipoprotein cholesterol (LDL-C), whereas their HDL-C, α-linolenic acid and linoleic acid were increased in the walnut-enriched diet group. They have suggested that the presence of γ-tocopherol and polyunsaturated fatty acids, such as α-linolenic and linoleic fatty acids are associated with the reduction in energy intake and weight loss. However, in another study, it was reported that gallic acid, as one of the main component of walnut, is able to reduce the body weight in rats, so it might be responsible for its weight-loss potential (140). The mechanism of action of the regulation of the body weight by gallic acid may explain in part via activating the AMP-activated protein kinase (AMPK) and improving mitochondrial function via the activation of peroxisome proliferator-activated receptor-γ coactivator1α (PGC1α) (141).
Zingiber officinale (Ginger)
Ginger is traditionally used as spice since 2000 years ago. In addition, it is well known for its anti-emetic properties due to anti-5HT3-receptor effect, mostly used during pregnancy (142). Study of Hussein et al. showed that ginger had protective effects on MSG-induced neurotoxicity. Ginger reduced oxidative stress represented by decreasing MDA level as lipid peroxidation marker as well as increasing antioxidant enzymes activity. Moreover, treatment of rats with ginger (500 mg/kg orally) decreased MSG-induced elevated level of Na+ and Ca+2 in the brain while enhanced K+ concentration (143). Waggas (144) has studied the neuroprotective potential of the aqueous extract of ginger in MSG-induced neurotoxicity in male albino rats. He recorded that the chronic administration of 100 mg/kg of ginger extract ameliorated the toxic effects of MSG, evidenced by significant increase in epinephrine, norepinephrine, dopamine and serotonin (5-HT) content. In previous study made by Gomar et al. (145), the antioxidant and neuroprotective effect of ginger has confirmed. These beneficial effect of ginger may due to the presence of 6-gingerol and its derivatives such as 6-shogaol and 6-paradol, as well as zingerone which have potent antioxidant and anti-inflammatory activities (146-148).
Ginkgo biloba
Ginkgo biloba is well-known as neurodegenerative agent without side effects. Earliest Chinese medical record back to 2800 BC (149). G. biloba has been reported to have palliative effects on neuropathologic effects of MSG in male rats (150). G. biloba also prevented most of the damage caused by MSG in retinal pigmented cells. In addition, Elatrash et al. reported that G. biloba (80 mg/kg) had renoprotective and hepatoprotective activity exhibited by reduced serum level of urea, creatinine and uric acid (kidney function), alanine aminotransferase (ALT) and serum aspartate aminotransferase (AST) activity (liver function), and lipid peroxidation level of liver and kidney associated with MSG administration (151). Similar findings were obtained by Arafa (152), who stated that the administration of G. biloba extract in rats exposed to MSG, reduced serum activities of liver enzymes, kidney function, MDA and metallothionein (MT), while it increased glucose-6-phosphate dehydrogenase (G- 6-PD), GSH, and SOD activities. Furthermore, G. biloba has also shown protective effect on spinal cord neurons from MSG excitotoxicity and oxidative stress. This protection is via suppression of cytosolic phospholipase A2 (cPLA2) activation through ERK1/2 signaling pathway, which evidenced by significantly decreased in the expression of phosphorylated cPLA2 (p-cPLA2) and prostaglandin E2 (PGE2) release (153). It was found that the above mentioned beneficial effect of G. biloba may be associated with the presence of a sesquiterpene lactone compound named bilobalide (154). In fact, bilobalide was found as a potent neuroprotective in vivo through the reduction of ischemia-induced glutamate release, and reducing excitotoxicity (155). The same authors have demonstrated that bilobalide protected rats against glutamate-induced excitotoxicity neuronal death about 20–fold (EC50 = 5 μg/ml) better than G. biloba extract (EC50 = 100 μg/ml) through several mechanisms including anti-excitotoxicity, inhibition of ROS generation, scavenging of ROS, and regulation of mitochondrial gene expression (156).
Algae
Ovarian dysfunction that subsequently may results in female infertility rise some concern during last decades. Experimental studies reported adverse effects of MSG on female reproductive system showed by fetal growth retardation (157), neuroendocrine disorders in neonatal (158), hypothalamus and pituitary disorders (159), and changes in rat’s uterine tube (160). Algae such as Spirulina platensis and Chlorella vulgaris, as functional foods, has remarkable amount of components including carotenoids, micronutrient and essential amino acids that are necessary in human health (161). Regarding this, Abdel-Aziem and the colleagues showed that oral administration of female mice with C. vulgaris or S. platensis aqueous extracts, for 28 days, had protective effects against MSG-induced ovarian dysfunction represented by alleviate ovarian tissue histological change, sex hormones content and increased the ovarian enzymatic antioxidants (162). The protective effect of the aqueous extracts of both S. platensis and C. vulgaris against MSG toxicity including oxidative stress, genotoxicity and cell death pathway in male mice have studied (163). Administration of the aqueous extracts at dosage of 500 mg/kg bw showed hepatoprotective activity against MSG through enhancement of liver functions (MDA improvement, antioxidant activities, histochemical and histological alterations and DNA fragmentation), genotoxicity prevention, and apoptosis inhibition by means of advancing the Bcl-2 mRNA expression. They have suggested that the protective effect of both extracts against MSG-induced toxicities may be through the prevention of ROS because of the presence of natural antioxidants including minerals such as selenium and manganese, vitamins such as E and C, as well as β-carotene, tocopherol, C-phycocyanins and phenolic compounds (164).
Vitamins
Vitamins C
As vitamins and MSG may be present in human diet, it is, therefore, necessary to evaluate their interactions, in order to establish whether vitamins would exacerbate or ameliorate the adverse effects of MSG. Vitamin C (500 mg/kg) co-administered along with MSG for 45 days, showed hepatoprotective effect on the parenchymal architecture of the liver against MSG in rats through reducing cellular proliferation which evidenced by the low expression of ki-67 and tumor suppressor genes mutation (165). This anti-proliferative activity of vitamin C are mainly based on its extracellular action and induction of apoptosis, inducing cell cycle arrest and inhibition of expression of genes involved in protein synthesis (166, 167). Ashraf Waiz et al. (168) confirmed that administration of MSG at dose of 6 mg/g bw for 10 days induced hepatotoxicity and oxidative stress. Vitamin C (500 mg/kg) co-administered with MSG, significantly reduced the oxidative stress and hepatic toxicity with decreased LPO, ALT and AST activity and liver weight and reduced the hepatic activity of catalase. In another study, orally administration of vitamin C (100 mg/kg) attenuate the effect of MSG at doses of 2 and 4 mg/kg induced toxicity on the weight of testes and epididymis, sperm motility, sperm count as well as sperm head abnormality in rats (169). Vitamin C also inhibited the MSG-induced cytotoxicity in rat thymocytes through up-regulation of Bcl-2 protein expression (170). These results are in accordance with those concluded recently that MSG has cytotoxic effect on the germ cells in testicular tissue of adult rats, but vitamin C at dosage of 150 mg/kg due to its antioxidant properties has beneficial effect on reducing the cytotoxicity mediated by administration of MSG (171). Vitamin C has been demonstrated as protective agent against some other MSG-induced toxicities including histological changes in oviduct of rats (172), hepatotoxicity (173), sperm toxicity (169), Obesity (174), and neurobehavioral changes in periadolescent rats (175).
Vitamin D
It was found that vitamin D has protective effect against MSG-induced obese rats. Rats administrated with MSG showed the increase in the body weight, food and water intake. However, co-administration of vitamin D with MSG significantly suppresses body weight gain (176). Similar results were obtained by Elbassuoni et al. who reported that vitamin D and L-arginine have protective role in liver and kidney damage induced by MSG via inhibiting oxidative stress and decreasing food intake and body weight. The MSG-induced oxidative liver evidenced by increasing of renal MDA and decreasing of liver TAC and also kidney damage proved by increasing in the level of renal function markers, urea, and creatinine in rats. Concomitant vitamin D or L-Arginine administration with MSG protected liver and kidney against hepatic and renal injurious induced by MSG which is proved by decreasing in serum ALT and AST levels, and reducing serum urea and creatinine (the renal injury markers). Its molecular mechanisms may be the reduction of oxidative stress that was indicated by considerable decrease in MDA and remarkable increase in TAC in both hepatic and renal tissues (177). Furthermore, Nandan et al. (178) have reported that vitamin D might be helpful in the inhibition of the pre-and postnatal exposed MSG-induced steatohepatitis. They have also suggested that the diet rich in vitamin D might be beneficial in reducing the hepatic toxicity in the pregnant women consuming food containing MSG.
Vitamin E
Vitamin E is one of the most important antioxidants present in daily diet, which has protective effects against several human diseases (179). MSG at a dose of 0.6 mg/g bw made oxidative stress and hepatotoxicity in rats by induction of LPO, decreased the level of GSH, and increased the activities of GST, SOD and catalase in the liver of rats. Vitamin E (0.2 mg/g bw) co-administered with MSG (0.6 mg/g bw), ameliorated the LPO, increased the GSH level and decreased the hepatic SOD activities of GST, catalase, and reduced the ALT, AST and GGT activities in the serum (44). The administration of combined vitamin C and E prevented MSG-induced ovarian toxicity as shown by a significant decrease in the MDA levels and the quantity of atresia follicle and increased FSH level and quantity of primary follicles (180). Herbal oils such as sesame oil contain considerable amounts of vitamin E, polyunsaturated fatty acids and lignans, which are responsible for its high antioxidant capacity (181). Oral administration of sesame oil to rats reduced MSG-induced liver damages, showed by decreased in AST and ALT as well as oxidative stress indicators, and improved lipid profile (182). Furthermore, α-tocopherol, main constituent of vitamin E, at dosage of 200 mg/kg for 180 days protected nephrotoxicity caused by MSG which revealed by a significantly decreased in lipid peroxidation and oxidative stress (reduced MDA and conjugated dienes), improved renal function (decreased urea, uric acid, and creatinine) and increased antioxidant defense systems (SOD, CAT, GPx, GST, and GSH) (183). The same authors also have reported the protective role of α-tocopherol against MSG-induced cardiotoxicity in rats. Administration of MSG led to the oxidative stress which indicated by significant increasing in MDA and CD and decreasing in the activities of antioxidant defense systems; SOD, catalase, GSH, GSHpx and glutathione S-transferase, and increased activities of biochemical markers of cardiac dysfunction; creatine phosphokinase, aspartate transaminase, and lactate dehydrogenase. However, intake of α-tocopherol (200 mg/kg) was effective in reducing the cardiotoxicity of MSG (4 g/kg). In the other words, administration of α-tocopherol at dosage of 200 mg/kg for 180 days significantly reduced the MSG-induced oxidative stress and cardiac toxicities (184). α-Tocopherol as a potent scavenger of free radicals (185) may prevent development of oxidative stress related diseases possibly through antioxidant status mechanism via increasing the reduced glutathione and decrease the lipid peroxidation in the body (186). Therefore, the presence of vitamins especially C, D and E in foods containing MSG could be beneficial against MSG-induced toxicity.
Other natural compounds
In addition to the mentioned compounds above, there are other natural compounds that have been shown to possess protective effects against various MSG toxicities. These compounds are summarized in Table 1.
Table 1.
Protective effects of some other plants or natural products against various Monosodium glutamate (MSG) toxicities
| Compound | Dose or Concentration | Experimental model | Target MSG-induced toxicity | Effects | References |
|---|---|---|---|---|---|
| Tanshinone IIA | 2.5-10 μM | Human neuroblastoma cell line SH-SY5Y | Neurotoxicity | ↑SOD, CAT, Bcl-2, ↓ROS, MDA, Protein carbonyl content, Bax, cleaved caspase-3 apoptosis, JNK1/2, p38 MAPK |
(187) |
| Sida acuta Leaf Extract | 400 mg/kg/day, p.o., 14 days | Female rats | Neurotoxicity | ↑ GSH,SOD and CAT activity↓ MDA level | (188) |
| Ethanolic extract of Pongamia pinnata | 200 and 400 mg/kg/day, p.o., 7 days | Wistar albino rats | Neurotoxicity | ↑ GSH,SOD and CAT activity↓ MDA level↓ Ca+2 and Na+ levels↑ level of K+ | (189) |
| Naringenin | 25, 50, 75 and 100 mg/L | Cultured hippocampal cells | Neurotoxicity | ↑ Erk1/2 and Akt phosphorylation↓ Caspase-3 | (190) |
| Methanolic and hydroalcoholic extract of Solanum torvum | 100 and 300 mg/kg/day, p.o, 14 days | Swiss albino mice | Neurotoxicity | ↑ GSH,SOD and CAT activity↓ MDA level | (191) |
| tetramethylpyrazine | 10, 20 and 40 mg/kg/day, i.p, 10 days | Kunming mice | Neurotoxicity | ↓ Expression NMDA receptor type 1 blocking Ca2+ influx |
(192) |
| Aqueous extract of Rosemary | 10 ml/kg/day, p.o., 42 days | Male albino rats | Neurotoxicity | ↑ CAT activity, HDL level↓ MDA level, Cholesterol and LDL | (193) |
| Piperine | 10 mg/kg/day, p.o., 14 days | Male Wistar rats | Neurotoxicity | ↑ GSH content ↓ MDA level, glial fibrillary acidic protein (GFAP) and caspase-3 | (194) |
| Butanolic extract of Tinospora cordifolia | 10 and 20 μg/mL | Primary cerebellar cells | Neurotoxicity | ↑ BCL-XL, MAP-2,GAP-43 and NF200↓ NF-κB, AP-1,iNOS, Cyclin D1 and IL-6 | (195) |
| Ethanolic extract of garlic (Allium sativum) | 12.5, 25, and 50 mg/kg/day, p.o., 10 days | Male Wistar rats | Neuronal excitotoxicity | Improved working memory performances | (196) |
| Moringa oleifera leaves extract | 200 mg/kg/day, p.o., 28 days | Male albino rats | Hepatotoxicity and Genotoxicity | ↑ GSH,SOD, GST and CAT activity↓ MDA level and hepatic enzymes (AST, ALT, ALP, and GGT) | (197) |
| Aqueous extract of Trigonella foenum-graecum | 0.5 and 1 g/kg/day, p.o., 28 days | Neonatal Wistar rats | Dyslipidemia | ↑ HDL, GSH,SOD, GST and CAT activity↓ MDA level, total cholesterol, triglyceridesand and hepatic enzymes (AST, ALT) | (198) |
| Aqueous extract of Qing brick tea | 75, 150, and 300 mg/kg/day, p.o., 140 days | CD1 mice | Metabolic syndrome | ↑ CAT, SOD and GPx activity↑ HO-1, Nrf2 and p-Akt expression ↓ MDA level, ROS and protein carbonylation↓ Cholesterol, triglyceride and FBS | (199) |
| Quercetin | 75 mg/kg/day, i.p., 42 days | Male Wistar rats | Metabolic syndrome | ↑ HDL, Total protein ↓ AST, ALT, ALP, LDH and Amylase↓ Cholesterol, triglyceride , LDL and VLDL, Creatinine | (35) |
| Quince (Cydonia Oblonga) leaf extract | 500 mg/kg/day, p.o., 56 days | Male Wistar rats | Reproductive toxicity | ↑ FSH and testosterone ↑ Epididymal sperm population and motility | (200) |
| Centella asciatica | 100 and 200 mg/kg/day, 7 days | Female SpragueDawley rats | excitotoxicity | ↑CAT, SOD ↓GSH, ROS, TBAR |
(201) |
| Piper longum | 300 mg/kg, 21 days | Male Wistar rats | Oxidative stress | ↑ALT, AST ↓Lipid peroxides, GSH, Cholesterol, triacylglycerol |
(202) |
| Garlic (Allium sativum) | 100 mg/kg, 60 days | Wistar rats | Fibroid | ↓ Cholesterol, estrogen, serum protein | (203) |
| Flaxseed Oil | 1.2 ml/kg , 6 weeks | Male Wistar albino rats | Brain Injury | ↑ Norepinephrine, Dopamine, Serotonin ↓ ALT, AST, Urea, Creatinine, MDA, |
(204) |
| Syzygium cumini | 0.5 or 1.0 g/kg/day, 8 weeks | Newborn male Wistar rats | Obesity | ↑ Liver function ↓ Body weight, Fasting glucose, cholesterol, Triglycerides, Free fatty acids, ER Stress, Hepatic XBP-1s/PDI/MTP Axis |
(205) |
| Syzygium cumini | 500 mg/kg, 30 days | Newborn male Wistar rats | Metabolic syndrome | ↓white adipose tissue, weight gain, Lee Index, triglyceride, cholesterol | (206) |
| Qing brick tea | 75, 150 and 300 mg/kg, 20 weeks | Breeding CD1 mice | Metabolic syndrome | ↑ SOD, GPx, CAT, GR, Nrf2/ HO-1, expression of p-AKT and GLUT4 ↓ MDA, ROS, protein carbonylation |
(207) |
| coconut water | 10 mL/kg b.w, 15 days | Male mice | Male infertility | ↑ Sperm concentration, sperm motility, viable sperm | (208) |
|
Trigonella foenum-graecum
seeds |
0.5 and 1.0 g/ kg b.w. |
Neonatal male Wistar rats | Fat deposition and dyslipidemia | ↓ Body weight, Lee’s index, white adipose tissue weights, adiposity index, glucose, insulin, leptin, LDL-C, VLDL-C, atherogenic index, coronary risk index, and homeostatic model assessment index | (209) |
| Sapindus emariganatus | 200 mg/kg and 400 mg/kg, 28 days | wistar albino rats | Obesity | ↓ Body weight, glucose, cholesterol, LDL-C, HDL-C, triglycerides, SGOT, SGPT, ALP | (210) |
| Cedrus deodara | 100 and 200 mg/kg, p.o./day, 60 days. | Female Albino Wistar rats | Hyperlipidemic | ↑ HDL ↓ body weight, serum glucose, cholesterol, triglyceride, LDL, VLDL |
(174) |
| Mimusops elengi | 100 and 200 mg/kg, 7 days | Adult female Wistar rats | Oxidative stress and excitotoxicity | ↑ Locomotor activity, GSH, total thiols, GST, CAT ↓ LPO, brain nitrite |
(211) |
| Tinospora cordifolia | 20 μg/ml, 24 h | Primary cerebellar neurons | Excitotoxicity | ↑ NF-κB, AP-1, HSP70, Mortalin ↓ MAP-2, GAP-43, NF200,Bcl-xL, |
(195) |
Conclusion
MSG as a flavor enhancer is still being widely used in a variety of food preparations. Although this substance it is generally recognized as safe for limited use by FDA, numerous studies have recently indicated unwanted side effects of long-term consumption of MSG, making its safety and toxicity a controversial issue. However, a number of in vitro and in vivo animal models and even clinical trials have shown several potential health hazards of MSG particularly at high doses. There has been a consensus by many researchers that unusual effect of MSG extends to other tissues in the body. As discussed above, MSG can increase the risk of hypercholesterolemia, hypertriglyceridemia, obesity and diabetes. Furthermore, it can induce oxidative stress, hepatotoxicity and neurotoxicity. The aforementioned undesirable effects of MSG can be minimized by some medicinal plants and their constituents. This review provides some information on the protective role of medicinal plants and their active compounds against MSG-induced toxicity. Such natural products include curcumin from Curcuma longa, gingerols and shogaols from ginger (Zingiber officinale), lycopene from tomato (Solanum lycopersicum), rosmarinic acid from rosemary (Rosmarinus officinalis), piperine from pepper (Piper nigrum), and several vitamins which are suitable compounds to be added to food. These products have been shown to ameliorate health hazards of MSG via several mechanisms including enhancement of antioxidant status, inhibition of oxidative stress and reduction of apoptosis. For example, foods rich in lycopene, curcumin, quercetin and naringenin could inhibit oxidative stress-associated neuronal and liver damage in several related diseases. Based on the reviewed studies, it suggests that MSG should be eliminated from diet until its safety is re-examined. Moreover, it is imperative to do additional studies with more details to further understand the mechanisms underlying the serious health risks of MSG.
Acknowledgment
The results described in this paper were part of student thesis.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Moldes AB, Vecino X, Cruz JM. 6-Nutraceuticals and food additives. In: Pandey A, Sanromán MÁ, Du G, Soccol CR, Dussap C-G, editors , editors. Current Developments in Biotechnology and Bioengineering. 2017. pp. 143–164. [Google Scholar]
- 2.Martins F, Sentanin MA, De Souza D. Analytical methods in food additives determination: Compounds with functional applications. Food Chem. 2019;272:732–750. doi: 10.1016/j.foodchem.2018.08.060. [DOI] [PubMed] [Google Scholar]
- 3.Silva HLA, Balthazar CF, Esmerino EA, Vieira AH, Cappato LP, Neto RPC, et al. Effect of sodium reduction and flavor enhancer addition on probiotic Prato cheese processing. Food Res Int. 2017;99:247–255. doi: 10.1016/j.foodres.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 4.Beyreuther K, Biesalski HK, Fernstrom JD, Grimm P, Hammes WP, Heinemann U, et al. Consensus meeting: monosodium glutamate - an update. Eur J Clin Nutr. 2007;61:304–313. doi: 10.1038/sj.ejcn.1602526. [DOI] [PubMed] [Google Scholar]
- 5.Husarova V, Ostatnikova D. Monosodium glutamate toxic effects and their implications for human intake: a review. JMED Research. 2013:1–12. [Google Scholar]
- 6.Rhodes J, Titherley AC, Norman JA, Wood R, Lord DW. A survey of the monosodium glutamate content of foods and an estimation of the dietary intake of monosodium glutamate. Food Addit Contam. 1991;8:663–672. doi: 10.1080/02652039109374021. [DOI] [PubMed] [Google Scholar]
- 7.Park E, Yu KH, Kim DK, Kim S, Sapkota K, Kim S-J, et al. Protective effects of N-acetylcysteine against monosodium glutamate-induced astrocytic cell death. Food Chem Toxicol. 2014;67:1–9. doi: 10.1016/j.fct.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Tome D. The roles of dietary glutamate in the intestine. Ann Nutr Metab. 2018;73 Suppl 5:15–20. doi: 10.1159/000494777. [DOI] [PubMed] [Google Scholar]
- 9.Pavlovic V, Pavlovic D, Kocic G, Sokolovic D, Jevtovic-Stoimenov T, Cekic S, et al. Effect of monosodium glutamate on oxidative stress and apoptosis in rat thymus. Mol Cell Biochem. 2007;303:161–166. doi: 10.1007/s11010-007-9469-7. [DOI] [PubMed] [Google Scholar]
- 10.Ugur Calis I, Turgut Cosan D, Saydam F, Kerem Kolac U, Soyocak A, Kurt H, et al. The effects of monosodium glutamate and tannic acid on adult rats. Iran Red Crescent Med J. 2016;18:e37912. [Google Scholar]
- 11.Farombi EO, Onyema OO. Monosodium glutamate-induced oxidative damage and genotoxicity in the rat: modulatory role of vitamin C, vitamin E and quercetin. Hum Exp Toxicol. 2006;25:251–259. doi: 10.1191/0960327106ht621oa. [DOI] [PubMed] [Google Scholar]
- 12.Onakewhor JU, Oforofuo IA, Singh SP. Chronic administration of monosodium glutamate induces oligozoospermia and glycoen accumulation in Wistar rat testes. Afr J Reprod Health. 2017;2:37–43. [Google Scholar]
- 13.Ortiz GG, Bitzer-Quintero OK, Zarate CB, Rodriguez-Reynoso S, Larios-Arceo F, Velazquez-Brizuela IE, et al. Monosodium glutamate-induced damage in liver and kidney: a morphological and biochemical approach. Biomed Pharmacother. 2006;60:86–91. doi: 10.1016/j.biopha.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Niaz K, Zaplatic E, Spoor J. Extensive use of monosodium glutamate: A threat to public health? EXCLI J. 2018;17:273–278. doi: 10.17179/excli2018-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurihara K. Glutamate: from discovery as a food flavor to role as a basic taste (umami) Am J Clin Nutr. 2009;90:719S–722S. doi: 10.3945/ajcn.2009.27462D. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez Bautista RJ, Mahmoud AM, Konigsberg M, Lopez Diaz Guerrero NE. Obesity: Pathophysiology, monosodium glutamate-induced model and anti-obesity medicinal plants. Biomed Pharmacother. 2019;111:503–516. doi: 10.1016/j.biopha.2018.12.108. [DOI] [PubMed] [Google Scholar]
- 17.Rim K-T. Toxicological evaluation of MSG for the manufacturing workers’ health: A literature review. J Toxicol Environ Health Sci. 2017;9:1–11. [Google Scholar]
- 18.Samuels A. The toxicity/safety of processed free glutamic acid (MSG): a study in suppression of information. Account Res. 1999;6:259–310. doi: 10.1080/08989629908573933. [DOI] [PubMed] [Google Scholar]
- 19.Cetin Kardesler A, Baskale E. Investigation of the behavioral and neurochemical effects of monosodium glutamate on neonatal rats. Turk J Med Sci. 2017;47:1002–1011. doi: 10.3906/sag-1511-92. [DOI] [PubMed] [Google Scholar]
- 20.Onaolapo OJ, Onaolapo AY, Akanmu MA, Gbola O. Evidence of alterations in brain structure and antioxidant status following ‘low-dose’ monosodium glutamate ingestion. Pathophysiology. 2016;23:147–156. doi: 10.1016/j.pathophys.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Stegink LD, Filer LJ Jr, Baker GL. Plasma glutamate concentrations in adult subjects ingesting monosodium L-glutamate in consomme. Am J Clin Nutr. 1985;42:220–225. doi: 10.1093/ajcn/42.2.220. [DOI] [PubMed] [Google Scholar]
- 22.Shannon M, Wilson J, Xie Y, Connolly L. In vitro bioassay investigations of suspected obesogen monosodium glutamate at the level of nuclear receptor binding and steroidogenesis. Toxicol Lett. 2019;301:11–16. doi: 10.1016/j.toxlet.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura H, Kawamata Y, Kuwahara T, Torii K, Sakai R. Nitrogen in dietary glutamate is utilized exclusively for the synthesis of amino acids in the rat intestine. Am J Physiol Endocrinol Metab. 2013;304:E100–108. doi: 10.1152/ajpendo.00331.2012. [DOI] [PubMed] [Google Scholar]
- 24.Rutten EP, Engelen MP, Wouters EF, Deutz NE, Schols AM. Effect of glutamate ingestion on whole-body glutamate turnover in healthy elderly and patients with chronic obstructive pulmonary disease. Nutrition. 2006;22:496–503. doi: 10.1016/j.nut.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Graham TE, Sgro V, Friars D, Gibala MJ. Glutamate ingestion: the plasma and muscle free amino acid pools of resting humans. Am J Physiol Endocrinol Metab. 2000;278:E83–89. doi: 10.1152/ajpendo.2000.278.1.E83. [DOI] [PubMed] [Google Scholar]
- 26.Boisrobert C OS, Stjepanovic A, Lelieveld H. Ensuring global food safety: Exploring global harmonization. Academic Press; 2009. [Google Scholar]
- 27.Caccia S, Garattini S, Ghezzi P, Zanini MG. Plasma and brain levels of glutamate and pyroglutamate after oral monosodium glutamate to rats. Toxicol lett. 1982;10:169–175. doi: 10.1016/0378-4274(82)90070-4. [DOI] [PubMed] [Google Scholar]
- 28.Hawkins RA. The blood-brain barrier and glutamate. Am J Clin Nutr. 2009;90:867S–874S. doi: 10.3945/ajcn.2009.27462BB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dehghani S, Mehri S, Hosseinzadeh H. The effects of crataegus pinnatifida (Chinese hawthorn) on metabolic syndrome: A review. Iran J Basic Med Sci. 2019;22:460–468. doi: 10.22038/IJBMS.2019.31964.7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hajian-Tilaki K, Heidari B. Variations in the pattern and distribution of non-obese components of metabolic syndrome across different obesity phenotypes among Iranian adults’ population. Diabetes Metab Syndr. 2019;13:2419–2424. doi: 10.1016/j.dsx.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Dow C, Balkau B, Bonnet F, Mancini F, Rajaobelina K, Shaw J, et al. Strong adherence to dietary and lifestyle recommendations is associated with decreased type 2 diabetes risk in the AusDiab cohort study. Prev Med. 2019;123:208–216. doi: 10.1016/j.ypmed.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Leshchenko IV, Shevchuk VH, Falalieieva TM, Beregova TV. [The influence of long-term monosodium glutamate feeding on the structure of rats pancreas] Fiziol Zh. 2012;58:59–65. [PubMed] [Google Scholar]
- 33.He K, Zhao L, Daviglus ML, Dyer AR, Van Horn L, Garside D, et al. Association of monosodium glutamate intake with overweight in Chinese adults: the INTERMAP Study. Obesity. 2008;16:1875–1880. doi: 10.1038/oby.2008.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hermanussen M, Garcia AP, Sunder M, Voigt M, Salazar V, Tresguerres JA. Obesity, voracity, and short stature: the impact of glutamate on the regulation of appetite. Eur J Clin Nutr. 2006;60:25–31. doi: 10.1038/sj.ejcn.1602263. [DOI] [PubMed] [Google Scholar]
- 35.Seiva FR, Chuffa LG, Braga CP, Amorim JP, Fernandes AA. Quercetin ameliorates glucose and lipid metabolism and improves antioxidant status in postnatally monosodium glutamate-induced metabolic alterations. Food Chem Toxicol. 2012;50:3556–3561. doi: 10.1016/j.fct.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Nagata M, Suzuki W, Iizuka S, Tabuchi M, Maruyama H, Takeda S, et al. Type 2 diabetes mellitus in obese mouse model induced by monosodium glutamate. Exp Anim. 2006;55:109–115. doi: 10.1538/expanim.55.109. [DOI] [PubMed] [Google Scholar]
- 37.Nakanishi Y, Tsuneyama K, Fujimoto M, Salunga TL, Nomoto K, An JL, et al. Monosodium glutamate (MSG): a villain and promoter of liver inflammation and dysplasia. J Autoimmun. 2008;30:42–50. doi: 10.1016/j.jaut.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 38.Umukoro S, Oluwole GO, Olamijowon HE, Omogbiya AI, Eduviere AT. Effect of monosodium glutamate on behavioral phenotypes, biomarkers of oxidative stress in brain tissues and liver enzymes in mice. World J Neurol. 2015;5:339–349. [Google Scholar]
- 39.Saeidnia S, Abdollahi M. Toxicological and pharmacological concerns on oxidative stress and related diseases. Toxicol Appl Pharmacol. 2013;273:442–455. doi: 10.1016/j.taap.2013.09.031. [DOI] [PubMed] [Google Scholar]
- 40.Pavlovic V, Cekic S, Sokolovic D, Djindjic B. Modulatory effect of monosodium glutamate on rat thymocyte proliferation and apoptosis. Bratisl Lek Listy. 2006;107:185–191. [PubMed] [Google Scholar]
- 41.Rosa SG, Chagas PM, Pesarico AP, Nogueira CW. Monosodium glutamate induced nociception and oxidative stress dependent on time of administration, age of rats and susceptibility of spinal cord and brain regions. Toxicol Appl Pharmacol. 2018;351:64–73. doi: 10.1016/j.taap.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 42.Ahluwalia P, Tewari K, Choudhary P. Studies on the effects of monosodium glutamate (MSG) on oxidative stress in erythrocytes of adult male mice. Toxicol Lett. 1996;84:161–165. doi: 10.1016/0378-4274(95)03612-1. [DOI] [PubMed] [Google Scholar]
- 43.Elshafey M, Eladl MA, El-Sherbiny M, Atef H, El Morsi DA. Hepatotoxicity of monoglutamate sodium: Oxidative stress and histopathlogical study. FASEB J. 2017;31:lb31–lb. [Google Scholar]
- 44.Onyema OO, Farombi EO, Emerole GO, Ukoha AI, Onyeze GO. Effect of vitamin E on monosodium glutamate induced hepatotoxicity and oxidative stress in rats. Indian J Biochem Biophys. 2006;43:20–24. [PubMed] [Google Scholar]
- 45.Eweka A, Om’iniabohs F. Histological studies of the effects of monosodium glutamate on the ovaries of adult Wistar rats. Ann Med Health Sci Res. 2011;1:37–43. [PMC free article] [PubMed] [Google Scholar]
- 46.Babu GN, Bawari M, Ali MM. Lipid peroxidation potential and antioxidant status of circumventricular organs of rat brain following neonatal monosodium glutamate. Neurotoxicology. 1994;15:773–777. [PubMed] [Google Scholar]
- 47.Sadek K, Abouzed T, Nasr S. Lycopene modulates cholinergic dysfunction, Bcl-2/Bax balance, and antioxidant enzymes gene transcripts in monosodium glutamate (E621) induced neurotoxicity in a rat model. Can J Physiol Pharmacol. 2016;94:394–401. doi: 10.1139/cjpp-2015-0388. [DOI] [PubMed] [Google Scholar]
- 48.Abdel Moneim WM, Yassa HA, Makboul RA, Mohamed NA. Monosodium glutamate affects cognitive functions in male albino rats. Egypt J Forensic Sci. 2018;8:9. [Google Scholar]
- 49.Lucas DR, Newhouse JP. The toxic effect of sodium L-glutamate on the inner layers of the retina. Arch Ophthalmol. 1957;58:193–201. doi: 10.1001/archopht.1957.00940010205006. [DOI] [PubMed] [Google Scholar]
- 50.Olney JW, Sharpe LG. Brain lesions in an infant rhesus monkey treated with monsodium glutamate. Science. 1969;166(3903):386–388. doi: 10.1126/science.166.3903.386. [DOI] [PubMed] [Google Scholar]
- 51.Burde RM, Schainker B, Kayes J. Acute effect of oral and subcutaneous administration of monosodium glutamate on the arcuate nucleus of the hypothalamus in mice and rats. Nature. 1971;233:58–60. doi: 10.1038/233058a0. [DOI] [PubMed] [Google Scholar]
- 52.Bodnár I, Göõz P, Okamura H, Tóth BE, Vecsernyé M, Halász B, et al. Effect of neonatal treatment with monosodium glutamate on dopaminergic and L-DOPA-ergic neurons of the medial basal hypothalamus and on prolactin and MSH secretion of rats. Brain Res Bull. 2001;55:767–774. doi: 10.1016/s0361-9230(01)00584-6. [DOI] [PubMed] [Google Scholar]
- 53.Moreno G, Perello M, Gaillard RC, Spinedi E. Orexin a stimulates hypothalamic-pituitary-adrenal (HPA) axis function, but not food intake, in the absence of full hypothalamic NPY-ergic activity. Endocrine. 2005;26:99–106. doi: 10.1385/ENDO:26:2:099. [DOI] [PubMed] [Google Scholar]
- 54.Perello M, Gaillard RC, Chisari A, Spinedi E. Adrenal enucleation in MSG-damaged hyperleptinemic male rats transiently restores adrenal sensitivity to leptin. Neuroendocrinology. 2003;78:176–184. doi: 10.1159/000072799. [DOI] [PubMed] [Google Scholar]
- 55.Gobel CH, Tronnier VM, Munte TF. Brain stimulation in obesity. Int J Obes. 2017;41:1721–1727. doi: 10.1038/ijo.2017.150. [DOI] [PubMed] [Google Scholar]
- 56.Miranda RA, Torrezan R, de Oliveira JC, Barella LF, da Silva Franco CC, Lisboa PC, et al. HPA axis and vagus nervous function are involved in impaired insulin secretion of MSG-obese rats. J Endocrinol. 2016;230:27–38. doi: 10.1530/JOE-15-0467. [DOI] [PubMed] [Google Scholar]
- 57.Rosa SG, Quines CB, da Rocha JT, Bortolatto CF, Duarte T, Nogueira CW. Antinociceptive action of diphenyl diselenide in the nociception induced by neonatal administration of monosodium glutamate in rats. Eur J Pharmacol. 2015;758:64–71. doi: 10.1016/j.ejphar.2015.03.060. [DOI] [PubMed] [Google Scholar]
- 58.Appaiah KM. Chapter 13 - Monosodium Glutamate in Foods and its Biological Effects. In: Boisrobert CE, Stjepanovic A, Oh S, Lelieveld HLM, editors. Ensuring Global Food Safety. San Diego: Academic Press; 2010. pp. 217–26. [Google Scholar]
- 59.Schmidt-Kastner R, Freund TF. Selective vulnerability of the hippocampus in brain ischemia. Neuroscience. 1991;40:599–636. doi: 10.1016/0306-4522(91)90001-5. [DOI] [PubMed] [Google Scholar]
- 60.Ali MM, Bawari M, Misra UK, Babu GN. Locomotor and learning deficits in adult rats exposed to monosodium-l-glutamate during early life. Neurosci Lett. 2000;284:57–60. doi: 10.1016/s0304-3940(00)00958-7. [DOI] [PubMed] [Google Scholar]
- 61.Olvera-Cortés E, López-Vázquez MA, Beas-Zárate C, González-Burgos I. Neonatal exposure to monosodium glutamate disrupts place learning ability in adult rats. Pharmacol Biochem Behav. 2005;82:247–251. doi: 10.1016/j.pbb.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 62.Beas-Zarate C, Flores-Soto ME, Armendariz-Borunda J. NMDAR-2C and 2D subunits gene expression is induced in brain by neonatal exposure of monosodium L-glutamate to adult rats. Neurosci Lett. 2002;321:9–12. doi: 10.1016/s0304-3940(01)02388-6. [DOI] [PubMed] [Google Scholar]
- 63.Ramalho JB, Izaguirry AP, Soares MB, Spiazzi CC, Pavin NF, Affeldt RF, et al. Selenofuranoside improves long-term memory deficits in rats after exposure to monosodium glutamate: Involvement of Na+, K+-ATPase activity. Physiol Behav. 2018;184:27–33. doi: 10.1016/j.physbeh.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 64.Lestari B, Novitasari D, Putri H, Haryanti S, Sasmito E, Meiyanto E. Evaluation of the genotoxicity of three food additives using CHO-K1 Cells under in vitro micronucleus flow cytometry assay. IJCC. 2017;8:74–80. [Google Scholar]
- 65.Adeyemo OA, Farinmade AE. Genotoxic and cytotoxic effects of food flavor enhancer, monosodium glutamate (MSG) using Allium cepa assay. Afr J Biotechnol. 2013;12:1459–1466. [Google Scholar]
- 66.Khatab HA, Elhaddad NS. Evaluation of mutagenic effects of monosodium glutamate using Allium cepa and antimutagenic action of Origanum majorana L and Ruta chalepensis medical plants. Biotechnol J. 2015;8:1–11. [Google Scholar]
- 67.Ataseven N, Yuzbasioglu D, Keskin AC, Unal F. Genotoxicity of monosodium glutamate. Food Chem Toxicol. 2016;91:8–18. doi: 10.1016/j.fct.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 68.Turkoglu S. Evaluation of genotoxic effects of five flavour enhancers (glutamates) on the root meristem cells of Allium cepa. Toxicol Ind Health. 2015;31:792–801. doi: 10.1177/0748233713475509. [DOI] [PubMed] [Google Scholar]
- 69.Ismail N. Assessment of DNA damage in testes from young Wistar male rat treated with monosodium glutamate. Life Sci J. 2012;9:930–939. [Google Scholar]
- 70.Walker R, Lupien JR. The safety evaluation of monosodium glutamate. J Nutr. 2000;130:1049S–1052S. doi: 10.1093/jn/130.4.1049S. [DOI] [PubMed] [Google Scholar]
- 71.Mohammed S. Monosodium glutamate-induced genotoxicity in rat palatal mucosa. Tanta Dent J. 2017;14:112–119. [Google Scholar]
- 72.Rogers MD. Monosodium glutamate is not likely to be genotoxic. Food Chem Toxicol. 2016;94:260–261. doi: 10.1016/j.fct.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 73.Shibata MA, Tanaka H, Kawabe M, Sano M, Hagiwara A, Shirai T. Lack of carcinogenicity of monosodium L-glutamate in Fischer 344 rats. Food Chem Toxicol. 1995;33:383–391. doi: 10.1016/0278-6915(94)00152-e. [DOI] [PubMed] [Google Scholar]
- 74.Alalwani AD. Monosodium glutamate induced testicular lesions in rats (histological study) Middle East Fertil Soc J. 2014;19:274–280. [Google Scholar]
- 75.Joseph UE, Onakewhor Iaoo, Sarrjit P Singh. Chronic administration of monosodium glutamate induces oligozoospermia and glycoen accumulation in Wistar rat testes. Onakewhor. 1998;2:1–3. [Google Scholar]
- 76.Igwebuike UM OI, Ihedinihu BC, Ikokide JE, Idika IK. The effects of oral administration of monosodium glutamate (MSG) on the testicular morphology and cauda epididymal sperm reserves of young and adult male rats. Veterinarski Arhiv. 2011;81:525–234. [Google Scholar]
- 77.Kadir RE OG, Balogun TJ, Oyewopo AO. Effects of monosodium glutamate on semen quality and the cytoarchitecture of the testis of adult Wistar rats. Int J Biol Sci. 2011;7:39–46. [Google Scholar]
- 78.Oforofuo IAO OJ, Idaewor PE. The effect of chronic administration of MSG on the histology of the Adult Wister rat testes. Bios Resch Comms. 1997;9:12–14. [Google Scholar]
- 79.Giovambattista A, Suescun MO, Nessralla CCDL, França LR, Spinedi E, Calandra RS. Modulatory effects of leptin on leydig cell function of normal and hyperleptinemic rats. Neuroendocrinology. 2003;78:270–279. doi: 10.1159/000074448. [DOI] [PubMed] [Google Scholar]
- 80.NA V, AK N, Damodara Gowda KM, Ahamed B, C Ramaswamy, Shabarinath , et al. Effect of monosodium induced oxidative damage on rat testis. J Chin Clin Med. 2008;3:370–373. [Google Scholar]
- 81.Shivasharan BD, Nagakannan P, Thippeswamy BS, Veerapur VP. Protective effect of Calendula officinalis L flowers against monosodium glutamate induced oxidative stress and excitotoxic brain damage in rats. Indian J Clin Biochem. 2013;28:292–298. doi: 10.1007/s12291-012-0256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Babaee N, Moslemi D, Khalilpour M, Vejdani F, Moghadamnia Y, Bijani A, et al. Antioxidant capacity of Calendula officinalis flowers extract and prevention of radiation induced oropharyngeal mucositis in patients with head and neck cancers: a randomized controlled clinical study. Daru. 2013;21:18–32. doi: 10.1186/2008-2231-21-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Preethi KC, Kuttan G, Kuttan R. Antioxidant potential of an extract of Calendula officinalis flowers in vitro and in vivo. Pharm Biol. 2006;44:691–697. [Google Scholar]
- 84.Preethi KC, Kuttan G, Kuttan R. Anti-inflammatory activity of flower extract of Calendula officinalis Linn and its possible mechanism of action. Indian J Exp Biol. 2009;47:113–120. [PubMed] [Google Scholar]
- 85.Parente LML, Lino Júnior RdS, Tresvenzol LMF, Vinaud MC, de Paula JR, Paulo NM. Wound healing and anti-Inflammatory effect in animal models of Calendula officinalis L growing in Brazil. Evid Based Complement Alternat Med. 2012;2012:1–7. doi: 10.1155/2012/375671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lima MdR, Lopes AP, Martins C, Brito GAC, Carneiro VC, Goes P. The effect of Calendula officinalis on oxidative stress and bone loss in experimental periodontitis. Front physiol. 2017;8:1–9. doi: 10.3389/fphys.2017.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fonseca YM, Catini CD, Vicentini FT, Nomizo A, Gerlach RF, Fonseca MJ. Protective effect of Calendula officinalis extract against UVB-induced oxidative stress in skin: evaluation of reduced glutathione levels and matrix metalloproteinase secretion. J Ethnopharmacol. 2010;127:596–601. doi: 10.1016/j.jep.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 88.Della Loggia R, Tubaro A, Sosa S, Becker H, Saar S, Isaac O. The role of triterpenoids in the topical anti-inflammatory activity of Calendula officinalis flowers. Planta Med. 1994;60:516–520. doi: 10.1055/s-2006-959562. [DOI] [PubMed] [Google Scholar]
- 89.Hernández-Bautista RJ, Alarcón-Aguilar FJ, Del C Escobar-Villanueva M, Almanza-Pérez JC, Merino-Aguilar H, Fainstein MK, et al. Biochemical alterations during the obese-aging process in female and male monosodium glutamate (MSG)-treated mice. Int J Mol Sci. 2014;15:11473–11494. doi: 10.3390/ijms150711473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mozes S, Sefcikova Z, Lenhardt L, Racek L. Obesity and changes of alkaline phosphatase activity in the small intestine of 40- and 80-day-old rats subjected to early postnatal overfeeding or monosodium glutamate. Physiol Res. 2004;53:177–186. [PubMed] [Google Scholar]
- 91.Bhattacharya T, Bhakta A, Ghosh SK. Long term effect of monosodium glutamate in liver of Albino mice after neo-natal exposure. Nepal Med Coll J. 2011;13:11–16. [PubMed] [Google Scholar]
- 92.Court-Brown CM, Duckworth AD, Ralston S, McQueen MM. The relationship between obesity and fractures. Injury. 2019;50:1423–1428. doi: 10.1016/j.injury.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 93.Miranda-Perez ME, Ortega-Camarillo C, Del Carmen Escobar-Villanueva M, Blancas-Flores G, Alarcon-Aguilar FJ. Cucurbita ficifolia Bouche increases insulin secretion in RINm5F cells through an influx of Ca(2+) from the endoplasmic reticulum. J Ethnopharmacol. 2016;188:159–166. doi: 10.1016/j.jep.2016.04.061. [DOI] [PubMed] [Google Scholar]
- 94.Fortis-Barrera A, Garcia-Macedo R, Almanza-Perez JC, Blancas-Flores G, Zamilpa-Alvarez A, Flores-Saenz JL, et al. Cucurbita ficifolia (Cucurbitaceae) modulates inflammatory cytokines and IFN-gamma in obese mice. Can J Physiol Pharmacol. 2017;95:170–177. doi: 10.1139/cjpp-2015-0475. [DOI] [PubMed] [Google Scholar]
- 95.Virdi J, Sivakami S, Shahani S, Suthar AC, Banavalikar MM, Biyani MK. Antihyperglycemic effects of three extracts from Momordica charantia. J Ethnopharmacol. 2003;88:107–111. doi: 10.1016/s0378-8741(03)00184-3. [DOI] [PubMed] [Google Scholar]
- 96.Shakeri A, Sahebkar A. Optimized curcumin formulations for the treatment of Alzheimer’s disease: A patent evaluation. J Neurosci Res. 2016;94:111–113. doi: 10.1002/jnr.23696. [DOI] [PubMed] [Google Scholar]
- 97.Shakeri A, Ward N, Panahi Y, Sahebkar A. Anti-angiogenic activity of curcumin in cancer therapy: A narrative review. Curr Vasc Pharmacol. 2019;17:262–269. doi: 10.2174/1570161116666180209113014. [DOI] [PubMed] [Google Scholar]
- 98.Karimian MS, Pirro M, Majeed M, Sahebkar A. Curcumin as a natural regulator of monocyte chemoattractant protein-1. Cytokine Growth Factor Rev. 2017;33:55–63. doi: 10.1016/j.cytogfr.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 99.Sahebkar A, Cicero AFG, Simental-Mendía LE, Aggarwal BB, Gupta SC. Curcumin downregulates human tumor necrosis factor-α levels: A systematic review and meta-analysis ofrandomized controlled trials. Pharmacol Res. 2016;107:234–242. doi: 10.1016/j.phrs.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 100.Cicero AFG, Colletti A, Bajraktari G, Descamps O, Djuric DM, Ezhov M, et al. Lipid-lowering nutraceuticals in clinical practice: Position paper from an International Lipid Expert Panel. Nutr Rev. 2017;75:731–767. doi: 10.1093/nutrit/nux047. [DOI] [PubMed] [Google Scholar]
- 101.Rezaee R, Momtazi AA, Monemi A, Sahebkar A. Curcumin: A potentially powerful tool to reverse cisplatin-induced toxicity. Pharmacol Res. 2017;117:218–227. doi: 10.1016/j.phrs.2016.12.037. [DOI] [PubMed] [Google Scholar]
- 102.Shakeri A, Sahebkar A. Opinion Paper: Phytosome: A fatty solution for efficient formulation of phytopharmaceuticals. Recent Pat Drug Deliv Formul. 2016;10:7–10. doi: 10.2174/1872211309666150813152305. [DOI] [PubMed] [Google Scholar]
- 103.Khalil RM, Khedr NF. Curcumin protects against monosodium glutamate neurotoxicity and decreasing NMDA2B and mGluR5 expression in rat hippocampus. Neuro-Signals. 2016;24:81–87. doi: 10.1159/000442614. [DOI] [PubMed] [Google Scholar]
- 104.Vucic M, Cojbasic I, Vucic J, Pavlovic V. The effect of curcumin and PI3K/Akt inhibitor on monosodium glutamate-induced rat thymocytes toxicity. Gen Physiol Biophys. 2018;37:329–336. doi: 10.4149/gpb_2017050. [DOI] [PubMed] [Google Scholar]
- 105.Sakr S, Badawy G. Protective effect of curcumin on monosodium glutamate-induced reproductive toxicity in male albino rats. Global J Pharmacol. 2013;7:416–422. [Google Scholar]
- 106.Riaz G, Chopra R. A review on phytochemistry and therapeutic uses of Hibiscus sabdariffa L. Biomed Pharmacother. 2018;102:575–586. doi: 10.1016/j.biopha.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 107.Olaleye MT. Cytotoxicity and antibacterial activity of methanolic extract of Hibiscus sabdariffa. J Med Plant Res. 2007;1:9–13. [Google Scholar]
- 108.Gheller AC, Kerkhoff J, Vieira Junior GM, de Campos KE, Sugui MM. Antimutagenic effect of Hibiscus sabdariffa L aqueous extract on rats treated with monosodium glutamate. Sci World J. 2017;2017:1–8. doi: 10.1155/2017/9392532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rosa RM, Melecchi MIS, Abad FC, Simoni CR, Caramão EB, Henriques JAP, et al. Antioxidant and antimutagenic properties of Hibiscus tiliaceus L methanolic extract. J Agric Food Chem. 2006;54:7324–7330. doi: 10.1021/jf061407b. [DOI] [PubMed] [Google Scholar]
- 110.Rodriguez-Medina IC, Beltran-Debon R, Molina VM, Alonso-Villaverde C, Joven J, Menendez JA, et al. Direct characterization of aqueous extract of Hibiscus sabdariffa using HPLC with diode array detection coupled to ESI and ion trap MS. J Sep Sci. 2009;32:3441–3448. doi: 10.1002/jssc.200900298. [DOI] [PubMed] [Google Scholar]
- 111.Tseng TH, Kao TW, Chu CY, Chou FP, Lin WL, Wang CJ. Induction of apoptosis by hibiscus protocatechuic acid in human leukemia cells via reduction of retinoblastoma (RB) phosphorylation and Bcl-2 expression. Biochem Pharmacol. 2000;60:307–315. doi: 10.1016/s0006-2952(00)00322-1. [DOI] [PubMed] [Google Scholar]
- 112.Dickel ML, Rates SMK, Ritter MR. Plants popularly used for loosing weight purposes in Porto Alegre, South Brazil. J Ethnopharmacol. 2007;109:60–71. doi: 10.1016/j.jep.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 113.Da-Costa-Rocha I, Bonnlaender B, Sievers H, Pischel I, Heinrich M. Hibiscus sabdariffa L - a phytochemical and pharmacological review. Food Chem. 2014;165:424–443. doi: 10.1016/j.foodchem.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 114.Alarcon-Aguilar FJ, Zamilpa A, Perez-Garcia MD, Almanza-Perez JC, Romero-Nunez E, Campos-Sepulveda EA, et al. Effect of Hibiscus sabdariffa on obesity in MSG mice. J Ethnopharmacol. 2007;114:66–71. doi: 10.1016/j.jep.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 115.Huang J, Wang Y, Xie Z, Zhou Y, Zhang Y, Wan X. The anti-obesity effects of green tea in human intervention and basic molecular studies. Eur J Clin Nutr. 2014;68:1075–1087. doi: 10.1038/ejcn.2014.143. [DOI] [PubMed] [Google Scholar]
- 116.Hursel R, Viechtbauer W, Westerterp-Plantenga MS. The effects of green tea on weight loss and weight maintenance: a meta-analysis. Int J Obes. 2009;33:956–961. doi: 10.1038/ijo.2009.135. [DOI] [PubMed] [Google Scholar]
- 117.Bártíková H, Boušová I, Matoušková P, Szotáková B, Skálová L. Effect of green tea extract-enriched diets on insulin and leptin levels, oxidative stress parameters and antioxidant enzymes activities in obese mice. Pol J Food Nutr Sci. 2017;67:233–240. [Google Scholar]
- 118.Bousova I, Matouskova P, Bartikova H, Szotakova B, Hanusova V, Tomankova V, et al. Influence of diet supplementation with green tea extract on drug-metabolizing enzymes in a mouse model of monosodium glutamate-induced obesity. Eur J Nutr. 2016;55:361–371. doi: 10.1007/s00394-015-0856-7. [DOI] [PubMed] [Google Scholar]
- 119.Ibegbulem CO, Chikezie PC, Ukoha AI, Opara CN. Effects of diet containing monosodium glutamate on organ weights, acute blood steroidal sex hormone levels, lipid profile and erythrocyte antioxidant enzymes activities of rats. J Acute Dis. 2016;5:402–407. [Google Scholar]
- 120.Rani J, Savalagimath MP. Effect of dooshivishari agada over MSG-induced reproductive toxicity wsr ovary and follicle count. Ayu. 2017;38:88–92. doi: 10.4103/ayu.AYU_166_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ruder EH, Hartman TJ, Goldman MB. Impact of oxidative stress on female fertility. Curr Opin Obstet Gynecol. 2009;21:219–222. doi: 10.1097/gco.0b013e32832924ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ali A, El-Seify G, El Haroun H, Mohammed Soliman M. Effect of monosodium glutamate on the ovaries of adult female Albino rats and the possible protective role of green tea. Menoufia Med J. 2014;27:793–800. [Google Scholar]
- 123.Yulianti S, Andarini S, Keman K. Effects of green tea extract on graafian follicles and serum 17β-estradiol in monosodium glutamate-exposed rats. Baqai J Health Sci. 2018;21:1–7. [Google Scholar]
- 124.Ahmed M. Effect of some food additives consumption on the body weight and toxicity and the possible ameliorative role of green tea extract. Sciences. 2016;6:716–730. [Google Scholar]
- 125.Westerterp-Plantenga MS. Green tea catechins, caffeine and body-weight regulation. Physiol Behav. 2010;100:42–46. doi: 10.1016/j.physbeh.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 126.Helal EG, El-Sayed RA, Gomaa M-H, El-Gamal MS. Effects of some food additives on some biochemical parameters in young male Albino rats and the ameliorative role of royal jelly. Egypt J Hosp Med. 2017;67:605–613. [Google Scholar]
- 127.Hamza RZ, Al-Salmi FA, El-Shenawy NS. Nanoparticles effects on zinc oxide/green tea complex on the lipid profile and liver functions of rats after monosodium glutamate treatment. J Appl Sci. 2018;18:65–70. [Google Scholar]
- 128.Anthony C, Cemaluk E E, Ejike G. Effect of pulverized Mangifera indica (mango) seed kernel on monosodium glutamate-intoxicated rats’ serum antioxidant capacity, brain function and histology. EC Pharmacol Toxicol. 2007;4:228–243. [Google Scholar]
- 129.Egbuonu A, Ekwuribe G. Pulverized Mangifera indica (mango) seed-kernel modulated serum lipid profile in monosodium glutamate-challenged rats. J APPL Biotech. 2017;5:72–87. [Google Scholar]
- 130.Egbuonu ACC, Oriji SO. Pulverized Mangifera indica (mango) seed kernel mitigated monosodium glutamate-intoxicated rats’ kidney histology and bio-functions. J Nutr Health Food Sci. 2017;5:1–7. [Google Scholar]
- 131.Wirandoko IH, Apriyani C, Apriyanto DR. Effectivity of tomato (Solanum lycopersicum) and zinc combination to sperms of male white rats (Rattus norvegicus) exposed to monosodium glutamate. J Phys. 2019;1146:1–3. [Google Scholar]
- 132.Malekiyan R, Abdanipour A, Sohrabi D, Jafari Anarkooli I. Antioxidant and neuroprotective effects of lycopene and insulin in the hippocampus of streptozotocin-induced diabetic rats. Biomed Rep. 2019;10:47–54. doi: 10.3892/br.2018.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sayed Badawi M. Assessment of the possible protective effect of lycopene on monosodium glutamate-induced nephrotoxicity in adult male albino rat. Eur J Anat. 2019;23:215–221. [Google Scholar]
- 134.Chen J, Song Y, Zhang L. Effect of lycopene supplementation on oxidative stress: an exploratory systematic review and meta-analysis of randomized controlled trials. J Med Food. 2013;16:361–74. doi: 10.1089/jmf.2012.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Basu A, Imrhan V. Tomatoes versus lycopene in oxidative stress and carcinogenesis: conclusions from clinical trials. Eur J Clin Nutr. 2006;61:295–303. doi: 10.1038/sj.ejcn.1602510. [DOI] [PubMed] [Google Scholar]
- 136.Lu CW, Hung CF, Jean WH, Lin TY, Huang SK, Wang SJ. Lycopene depresses glutamate release through inhibition of voltage-dependent Ca(2+) entry and protein kinase C in rat cerebrocortical nerve terminals. Can J Physiol Pharmacol. 2018;96:479–484. doi: 10.1139/cjpp-2017-0520. [DOI] [PubMed] [Google Scholar]
- 137.Delaviz H, Mohammadi J, Ghalamfarsa G, Mohammadi B, Farhadi N. A review study on phytochemistry and pharmacology applications of Juglans regia plant. Pharmacogn Rev. 2017;11:145–152. doi: 10.4103/phrev.phrev_10_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liang X, Chen D, Cao L, Zhao S. Effects of pressed degreased walnut meal extracts on lipid metabolism in postnatally monosodium glutamate-induced mice and 3T3-L1 preadipocytes. J Funct Foods. 2017;31:89–96. [Google Scholar]
- 139.Rock CL, Flatt SW, Barkai H-S, Pakiz B, Heath DD. Walnut consumption in a weight reduction intervention: effects on body weight, biological measures, blood pressure and satiety. Nutr J. 2017;16:76–85. doi: 10.1186/s12937-017-0304-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Totani N, Tateishi S, Takimoto T, Maeda Y, Sasaki H. Gallic acid glycerol ester promotes weight-loss in rats. J Oleo Sci. 2011;60:457–462. doi: 10.5650/jos.60.457. [DOI] [PubMed] [Google Scholar]
- 141.Doan KV, Ko CM, Kinyua AW, Yang DJ, Choi YH, Oh IY, et al. Gallic acid regulates body weight and glucose homeostasis through AMPK activation. Endocrinology. 2015;156:157–168. doi: 10.1210/en.2014-1354. [DOI] [PubMed] [Google Scholar]
- 142.Lete I, Allue J. The effectiveness of ginger in the prevention of nausea and vomiting during pregnancy and chemotherapy. Integr Med Insights. 2016;11:11–17. doi: 10.4137/IMI.S36273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hussein UK, Hassan NEY, Elhalwagy MEA, Zaki AR, Abubakr HO, Nagulapalli Venkata KC, et al. Ginger and propolis exert neuroprotective effects against monosodium glutamate-induced neurotoxicity in rats. Molecules. 2017;22:1928–1952. doi: 10.3390/molecules22111928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Waggas AM. Neuroprotective evaluation of extract of ginger (Zingiber officinale) root in monosodium glutamate-induced toxicity in different brain areas male albino rats. Pak J Biol Sci. 2009;12:201–212. doi: 10.3923/pjbs.2009.201.212. [DOI] [PubMed] [Google Scholar]
- 145.Gomar A, Hosseini A, Mirazi N, Gomar M. Effect of Zingiber Officinale (ginger rhizomes) hydroethanolic extract on hyoscine-induced memory impairment in adult male rats. ICNSJ. 2015;2:105–110. [Google Scholar]
- 146.Stoilova I, Krastanov A, Stoyanova A, Denev P, Gargova S. Antioxidant activity of a ginger extract (Zingiber officinale) Food Chem. 2007;102:764–770. [Google Scholar]
- 147.Soliman AF, Anees LM, Ibrahim DM. Cardioprotective effect of zingerone against oxidative stress, inflammation, and apoptosis induced by cisplatin or gamma radiation in rats. Naunyn Schmiedebergs Arch Pharmacol. 2018;391:819–832. doi: 10.1007/s00210-018-1506-4. [DOI] [PubMed] [Google Scholar]
- 148.Sapkota A, Park SJ, Choi JW. Neuroprotective effects of 6-shogaol and its metabolite, 6-paradol, in a mouse model of multiple sclerosis. Biomol Ther. 2019;27:152–159. doi: 10.4062/biomolther.2018.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kobayashi MS, Han D, Packer L. Antioxidants and herbal extracts protect HT-4 neuronal cells against glutamate-induced cytotoxicity. Free Radic Res. 2000;32:115–124. doi: 10.1080/10715760000300121. [DOI] [PubMed] [Google Scholar]
- 150.Zaghlool SS, Hanaf LK, Afifi NM, Ibrahim ER. Histological and immunohistochemical study on the protective effect of Ginkgo biloba extract against glutamate-induced neurotoxicity in male albino rat retinal cells. Egypt J Histol. 2012;35:176–188. [Google Scholar]
- 151.Elatrash AM, Abd El-Haleim SZ. Protective role of Ginkgo biloba on monosodium glutamate: Induced liver and kidney toxicity in rats. Res J Pharm Biol Chem Sci. 2015;6:1433–1441. [Google Scholar]
- 152.Arafa NMM. A Study on the antioxidative properties of Ginkgo biloba leaf extract in Albino rats exposed to gamma radiation or monosodium glutamate. 2014;48:223–231. [Google Scholar]
- 153.Zhao Z, Liu N, Huang J, Lu P-H, Xu X-M. Inhibition of cPLA2 activation by Ginkgo biloba extract protects spinal cord neurons from glutamate excitotoxicity and oxidative stress-induced cell death. J Neurochem. 2011;116:1057–1065. doi: 10.1111/j.1471-4159.2010.07160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chandrasekaran K, Mehrabian Z, Spinnewyn B, Chinopoulos C, Drieu K, Fiskum G. Neuroprotective effects of bilobalide, a component of Ginkgo biloba extract (EGb 761) in global brain ischemia and in excitotoxicity-induced neuronal death. Pharmacopsychiatry. 2003;36:S89–S94. doi: 10.1055/s-2003-40447. [DOI] [PubMed] [Google Scholar]
- 155.Lang D, Kiewert C, Mdzinarishvili A, Schwarzkopf TM, Sumbria R, Hartmann J, et al. Neuroprotective effects of bilobalide are accompanied by a reduction of ischemia-induced glutamate release. Brain Res. 2011;1425:155–163. doi: 10.1016/j.brainres.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chandrasekaran K, Mehrabian Z, Spinnewyn B, Chinopoulos C, Drieu K, Fiskum G. Bilobalide, a component of the Ginkgo biloba extract (EGb 761), protects against neuronal death in global brain ischemia and in glutamate-induced excitotoxicity. Cell Mol Biol. 2002;48:663–669. [PubMed] [Google Scholar]
- 157.El makawy A, Abdou H a. Modulatory effect of ascorbic acid against food additive monosodium glutamate genotoxicity in rats. J Genetic Eng Biotechnol. 2005;3:229–253. [Google Scholar]
- 158.Kuznetsova EG, Amstislavskaya TG, Bulygina VV, Il’nitskaya SI, Tibeikina MA, Skrinskaya YA. Effects of administration of sodium glutamate during the neonatal period on behavior and blood corticosterone levels in male mice. Neurosci Behav Physiol. 2007;37:827–833. doi: 10.1007/s11055-007-0088-2. [DOI] [PubMed] [Google Scholar]
- 159.Ciric M, Najman S, Bojanic V, Cekic S, Nesic M, Puskas N. Neonatal influence of monosodium glutamate on the somatometric parameters of rats. Gen Physiol Biophys. 2009;28:155–161. [PubMed] [Google Scholar]
- 160.Eweka AO, Eweka A, Om’iniabohs FA. Histological studies of the effects of monosodium glutamate of the fallopian tubes of adult female Wistar rats. N Am J Med Sci. 2010;2:146–149. doi: 10.4297/najms.2010.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bishop W, Zubeck H. Evaluation of microalgae for use as nutraceuticals and nutritional supplements. J Nutr Food Sci. 2012;2:1–6. [Google Scholar]
- 162.Abdel-Aziem SH, Abd El-Kader HAM, Ibrahim FM, Sharaf HA, El makawy AI. Evaluation of the alleviative role of Chlorella vulgaris and Spirulina platensis extract against ovarian dysfunctions induced by monosodium glutamate in mice. Genet Eng Biotechnol J. 2018;16:653–660. doi: 10.1016/j.jgeb.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Abd-Elmoneim OM, Darwish AM. Potential modulator role of Chlorella vulgaris and Spirulina platensis on monosodium glutamate oxidative stress, genotoxicity, apoptotic gene expression and histopathological alterations. Int J Pharmtech Res. 2016;9:161–177. [Google Scholar]
- 164.Upasani CD, Balaraman R. Protective effect of Spirulina on lead induced deleterious changes in the lipid peroxidation and endogenous antioxidants in rats. Phytother Res. 2003;17:330–334. doi: 10.1002/ptr.1135. [DOI] [PubMed] [Google Scholar]
- 165.El-Meghawry El-Kenawy A, Osman HE, Daghestani MH. The effect of vitamin C administration on monosodium glutamate induced liver injury. An experimental study. Exp Toxicol Pathol. 2013;65:513–521. doi: 10.1016/j.etp.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 166.Frömberg A, Gutsch D, Schulze D, Vollbracht C, Weiss G, Czubayko F, et al. Ascorbate exerts anti-proliferative effects through cell cycle inhibition and sensitizes tumor cells towards cytostatic drugs. Cancer Chemoth Pharm. 2011;67:1157–1166. doi: 10.1007/s00280-010-1418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Belin S, Kaya F, Duisit G, Giacometti S, Ciccolini J, Fontes M. Antiproliferative effect of ascorbic acid is associated with the inhibition of genes necessary to cell cycle progression. PloS one. 2009;4:e4409. doi: 10.1371/journal.pone.0004409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Waiz SA, Raies-ul-Haq M, Waiz HA, Gupta S, Pathak AK. Preliminary study on the protective effect of vitamin C on monosodium glutamate-induced hepatotoxicity in rats. Comp Clin Path. 2015;24:1063–1068. [Google Scholar]
- 169.Ekaluo U, Ikpeme E, Ibiang Y, Amaechina O. Attenuating role of vitamin C on sperm toxicity Induced by monosodium glutamate in albino rats. J Biol Sci. 2013;13:298–301. [Google Scholar]
- 170.Pavlovic V, Pavlovic D, Kocic G, Sokolovic D, Sarac M, Jovic Z. Ascorbic acid modulates monosodium glutamate induced cytotoxicity in rat thymus. Bratisl Lek Listy. 2009;110:205–209. [PubMed] [Google Scholar]
- 171.Anbarkeh Rahimi F, Baradaran R, Ghandy N, Jalali M, Nikravesh MR, Soukhtanloo M. Effects of monosodium glutamate on apoptosis of germ cells in testicular tissue of adult rat: An experimental study. Int J Reprod Biomed. 2019;17:261–270. doi: 10.18502/ijrm.v17i4.4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mohtashami L, Shakeri A, Javadi B. Neuroprotective natural products against experimental autoimmune encephalomyelitis: A review. Neurochem Int. 2019;129:104516. doi: 10.1016/j.neuint.2019.104516. [DOI] [PubMed] [Google Scholar]
- 173.Ibrahim MA, Buhari GO, Aliyu AB, Yunusa I, Bisalla M. Amelioration of monosodium glutamate-induced hepatotoxicity by vitamin C. Eur J Sci Res. 2011;60:159–165. [Google Scholar]
- 174.Patil S, Prakash T, Kotresha D, Rao NR, Pandy N. Antihyperlipidemic potential of Cedrus deodara extracts in monosodium glutamate induced obesity in neonatal rats. Indian J Pharmacol. 2011;43:644–647. doi: 10.4103/0253-7613.89818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Narayanan SN, Kumar RS, Paval J, Nayak S. Effect of ascorbic acid on the monosodium glutamate-induced neurobehavioral changes in periadolescent rats. Bratisl Lek Listy. 2010;111:247–252. [PubMed] [Google Scholar]
- 176.Nandan P, Nayanatara AK, Poojary R, Bhagyalakshmi K, Nirupama M, Kini RD. Protective role of co-administration of vitamin D in monosodium glutamate induced obesity in female rats. J Natl Med Assoc. 2018;110:98–102. doi: 10.1016/j.jnma.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 177.Elbassuoni EA, Ragy MM, Ahmed SM. Evidence of the protective effect of l-arginine and vitamin D against monosodium glutamate-induced liver and kidney dysfunction in rats. Biomed Pharmacother. 2018;108:799–808. doi: 10.1016/j.biopha.2018.09.093. [DOI] [PubMed] [Google Scholar]
- 178.Nandan P, Nirupama M, Nayanatara Arun K, Bhagyalakshmi K, Jyoti Ramnath K, Roopesh P. Ameliorative role of vitamin D on prenatal and postnatal exposure of monosodium glutamate induced steatohepatitis in rat pups. Pharmacogn Res. 2018;10:371–375. [Google Scholar]
- 179.Rizvi S, Raza ST, Ahmed F, Ahmad A, Abbas S, Mahdi F. The role of vitamin E in human health and some diseases. Sultan Qaboos Univ Med J. 2014;14:e157–e165. [PMC free article] [PubMed] [Google Scholar]
- 180.Rohmawati W, Istiananingsih Y, Nurdiana N, Barlianto W, Dwijayasa PM. Concomitant exposure to vitamin C and E inhibited monosodium glutamate-induced ovarian toxicity in female rats. Cukurova Med J. 2014;39:517–524. [Google Scholar]
- 181.Namiki M. Nutraceutical functions of sesame: a review. Crit Rev Food Sci Nutr. 2007;47:651–673. doi: 10.1080/10408390600919114. [DOI] [PubMed] [Google Scholar]
- 182.Hareeri NA, Alrasheedi AA, Eassaw MM. Effect of sesame on liver enzymes and lipid profile inrats exposed to oxidative stress induced by monosodium glutamate. J Am Sci. 2017;13:71–78. [Google Scholar]
- 183.Paul MV, Abhilash M, Varghese MV, Alex M, Nair RH. Protective effects of alpha-tocopherol against oxidative stress related to nephrotoxicity by monosodium glutamate in rats. Toxicol Mech Methods. 2012;22:625–630. doi: 10.3109/15376516.2012.714008. [DOI] [PubMed] [Google Scholar]
- 184.Paul S, Mohanan A, Varghese MV, Alex M, H N. Ameliorative effect of α-tocopherol on monosodium glutamate-induced cardiac histological alterations and oxidative stress. J Sci Food Agric. 2012;92:3002–3006. doi: 10.1002/jsfa.5714. [DOI] [PubMed] [Google Scholar]
- 185.Xue H, Ren H, Zhang L, Sun X, Wang W, Zhang Sh, et al. Alpha-tocopherol ameliorates experimental autoimmune encephalomyelitis through the regulation of Th1 cells. Iran J Basic Med Sci. 2016;19:561–566. [PMC free article] [PubMed] [Google Scholar]
- 186.Newaz MA, Nawal NN. Effect of alpha-tocopherol on lipid peroxidation and total antioxidant status in spontaneously hypertensive rats. Am J Hypertens. 1998;11:1480–1485. doi: 10.1016/s0895-7061(98)00167-8. [DOI] [PubMed] [Google Scholar]
- 187.Li H, Han W, Wang H, Ding F, Xiao L, Shi R, et al. Tanshinone IIA inhibits glutamate-induced oxidative toxicity through prevention of mitochondrial dysfunction and suppression of MAPK activation in SH-SY5Y human neuroblastoma cells. Oxid Med Cell Longev. 2017;2017:1–13. doi: 10.1155/2017/4517486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Owoeye O, Salami OA. Monosodium glutamate toxicity: Sida acuta leaf extract ameliorated brain histological alterations, biochemical and haematological changes in Wistar rats. Afr J Biomed Res. 2017;20:173–182. [Google Scholar]
- 189.Swamy AH, Patel NL, Gadad PC, Koti BC, Patel UM, Thippeswamy AH, et al. Neuroprotective activity of Pongamia pinnata in monosodium glutamate-induced neurotoxicity in rats. Indian J Pharm Sci. 2013;75:657–663. [PMC free article] [PubMed] [Google Scholar]
- 190.Xu X-H, Ma C-M, Han Y-Z, Li Y, Liu C, Duan Z-H, et al. Protective effect of naringenin on glutamate-induced neurotoxicity in cultured hippocampal cells. Arch Biol Sci. 2015;67:639–646. [Google Scholar]
- 191.Mohan M, Gangurde SK, Kadam V. Protective effect of Solanum torvum on monosodium glutamate-induced neurotoxicity in mice. Indian J Nat Prod Resour. 2018;8:351–359. [Google Scholar]
- 192.Zhang Y, Huang Z, Yu L, Zhang L. Protective effects of tetramethylpyrazine on glutamate-induced neurotoxicity in mice. J Behav Brain Sci. 2012;2:326–332. [Google Scholar]
- 193.Mahran HA, Arisha SM. The ameliorative effects of the aqueous extract of rosemary against monosodium glutamate neurotoxicity in adult male albino rats: Histological, ultrastructural and biochemical studies. Eur J Pharm Med Res. 2018;5:79–90. [Google Scholar]
- 194.Omar AI, Farag EA, Yousry MM. The possible protective effect of piperine versus vitamin C on monosodium glutamate-induced cerebellar toxicity in adult male rats: a histological and immunohistochemical study. Egypt J Histol. 2016;39:362–371. [Google Scholar]
- 195.Sharma A, Kaur G. Tinospora cordifolia as a potential neuroregenerative candidate against glutamate induced excitotoxicity: an in vitro perspective. BMC Complement Altern Med. 2018;18:268. doi: 10.1186/s12906-018-2330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Nurmasitoh T, Sari DCR, Partadiredja G. The effects of black garlic on the working memory and pyramidal cell number of medial prefrontal cortex of rats exposed to monosodium glutamate. Drug Chem Toxicol. 2018;41:324–329. doi: 10.1080/01480545.2017.1414833. [DOI] [PubMed] [Google Scholar]
- 197.Albrahim T, Binobead MA. Roles of Moringa oleifera Leaf extract in improving the impact of high dietary intake of monosodium glutamate-induced liver toxicity, oxidative stress, genotoxicity, DNA damage, and PCNA alterations in male rats. Oxid Med Cell Longev. 2018;2018:1–11. doi: 10.1155/2018/4501097. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 198.Kumar P, Bhandari U. Protective effect of Trigonella foenum-graecum Linn on monosodium glutamate-induced dyslipidemia and oxidative stress in rats. Indian J Pharmacol. 2013;45:136–140. doi: 10.4103/0253-7613.108288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gao W, Xiao C, Hu J, Chen B, Wang C, Cui B, et al. Qing brick tea (QBT) aqueous extract protects monosodium glutamate-induced obese mice against metabolic syndrome and involves up-regulation Transcription Factor Nuclear Factor-Erythroid 2-Related Factor 2 (Nrf2) antioxidant pathway. Biomed Pharmacother. 2018;103:637–644. doi: 10.1016/j.biopha.2018.04.043. [DOI] [PubMed] [Google Scholar]
- 200.Kianifard D, Saiah GV, Rezaee F. Study of the protective effects of quince (Cydonia Oblonga) leaf extract on fertility alterations and gonadal dysfunction induced by monosodium glutamate in adult male Wistar rats. Rom J Diabetes Nutr Metab Dis. 2015;22:375–384. [Google Scholar]
- 201.Ramanathan M, Sivakumar S, Anandvijayakumar PR, Saravanababu C, Pandian PR. Neuroprotective evaluation of standardized extract of Centella asciatica in monosodium glutamate treated rats. Indian J Exp Biol. 2007;45:425–431. [PubMed] [Google Scholar]
- 202.Thomas M, Sujatha KS, George S. Protective effect of Piper longum Linn on monosodium glutamate induced oxidative stress in rats. Indian J Exp Biol. 2009;47:186–192. [PubMed] [Google Scholar]
- 203.Obochi G, Malu S, Obi-Abang M, Alozie Y, Iyam M. Effect of garlic extracts on monosodium glutamate (MSG) induced fibroid in Wistar rats. Pak J Nutr. 2009;8:970–976. [Google Scholar]
- 204.Youness ER, Hussein JS, Ibrahim AM, Agha FE. Flaxseed oil attenuates monosodium glutamate-induced brain injury via improvement of fatty acids contents. Biomed Pharmacol J. 2019;12:527–532. [Google Scholar]
- 205.França LM, Ferreira Coêlho CF, Costa Freitas LN, Santos Souza IL, Chagas VT, et al. Syzygium cumini leaf extract reverts hypertriglyceridemia via downregulation of the hepatic XBP-1s/PDI/MTP axis in monosodium L-glutamate-induced obese rats. Oxid Med Cell Longev. 2019;2019:1–14. doi: 10.1155/2019/9417498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sanches JR, França LM, Chagas VT, Gaspar RS, Dos Santos KA, Gonçalves LM, et al. Polyphenol-rich extract of Syzygium cumini leaf dually improves peripheral insulin sensitivity and pancreatic islet function in monosodium L-glutamate-induced obese rats. Front Pharmacol. 2016;10:48–63. doi: 10.3389/fphar.2016.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Gao W, Xiao C, Hu J, Chen B, Wang C, Cui B, et al. Qing brick tea (QBT) aqueous extract protects monosodium glutamate-induced obese mice against metabolic syndrome and involves up-regulation transcription factor nuclear factor-erythroid 2-related factor 2 (Nrf2) antioxidant pathway. Biomed Pharmacother. 2018;103:637–644. doi: 10.1016/j.biopha.2018.04.043. [DOI] [PubMed] [Google Scholar]
- 208.Aliff MH, Nooraain H, Nurdiana S. Ameliorating effects of coconut water on sperm quality and selected organs histology in monosodium glutamate pre treated male mice (Mus musculus) Malays Appl Biol. 2017;46:27–35. [Google Scholar]
- 209.Kumar P, Bhandari U. Fenugreek seed extract prevents fat deposition in monosodium glutamate (MSG)-obese rats. Drug Res. 2016;66:174–180. doi: 10.1055/s-0035-1555812. [DOI] [PubMed] [Google Scholar]
- 210.Suneetha D, Banda SDT, Ali F. Antiobesity values of methanolic extract of Sapindus emariganatus on monosodium glutamate induced model in rats. IJPPR. 2013;14:267–270. [Google Scholar]
- 211.Nagakannan P, Shivasharan B, Thippeswamy B, Veerapur V. Restoration of brain antioxidant status by hydroalcoholic extract of Mimusops elengi flowers in rats treated with monosodium glutamate. J Environ Pathol Toxicol Oncol. 2012;31:213–221. doi: 10.1615/jenvironpatholtoxicoloncol.v31.i3.30. [DOI] [PubMed] [Google Scholar]