Abstract
The first bacterial α2−6-sialyltransferase cloned from Photobacterium damselae (Pd2,6ST) has been widely applied for the synthesis of various α2−6-linked sialosides. However, the extreme substrate flexibility of Pd2,6ST makes it unsuitable for site-specific α2−6-sialylation of complex substrates containing multiple galactose and/or N-acetylgalactosamine units. To tackle this problem, a general redox-controlled site-specific sialylation strategy using Pd2,6ST is described. This approach features site-specific enzymatic oxidation of galactose units to mask the unwanted sialylation sites and precisely controlling the site-specific α2−6-sialylation at intact galactose or N-acetylgalactosamine units.
Graphical Abstract

Sialic acids (Sias) are the most common termini and among the most abundant monosaccharides of mammalian glycans.1 As ubiquitous components of glycoproteins and glycolipids, three common sialic acids of N-acetylneuraminic acid (Neu5Ac, 1), N-glycolylneuraminic acid (Neu5Gc, 2) and 2-keto-3-deoxy-nonulosonic acid (Kdn, 3) are found α2−6-linked to galactose (Gal) or N-acetylgalactosamine (GalNAc) residues (Scheme 1a). Owing to their remarkable structural diversity, sialic acid-containing glycans play important roles in many physiological and pathological processes, and most sialic acid-related biological processes require specific sialic acid forms, glycosidic linkage and defined underlying glycan chains.2 For example, avian influenza viruses primarily bind to α2−3-linked sialic acid, whereas human influenza viruses preferentially recognize α2−6-linked sialic acid.3 Recent studies also demonstrated that α2−6-sialylation is required for the anti-inflammatory activities of intravenous immunoglobulin (IVIG), and in vitro glycoengineered IVIG with uniform α2−6-sialylated N-glycans showed 10-fold enhancement in anti-inflammatory activities compared to unfractionated IVIG.4 It is also well known that the high expression levels of α2−6-sialylated glycans on a number of carcinomas are correlated with cancer progression and poor prognosis.2b, 5
Scheme 1.

a) Common sialic acid forms and α2−6-linkages; b) The known, and c) proposed enzymatic α2−6-sialylation approaches.
The past few decades have witnessed increasing attention on using glycosyltransferases for the synthesis of various glycans and glycoconjugates.6 However, only very few recombinant sialyltransferases (SiaTs) from both mammals and bacteria have been widely applied for the construction of both α2−6-sialyl linkages 4 and 5, (Scheme 1a). ST6Gal I is a mammalian α2−6-SiaT which has been widely used for the α2−6-sialylation of terminal Gal residue, but it has very strict substrate specificity that can only use terminal type-2 glycan (Galβ1−4GlcNAc) as acceptor substrate (Scheme 1b, i).7 ST6GalNAc I is another recombinant mammalian α2−6-SiaT which has been used for the α2−6-sialylation of GalNAc residue, but it can only use Tn antigen or T antigen as acceptor substrates (Scheme 1b, i).8 The α2−6-sialylation of terminal Gal residues of type-1 glycan (Galβ1−3GlcNAc) and LacdiNAc (GalNAcβ1−4GlcNAc) have also been identified in a number of naturally occurring glycans9, however, the SiaTs responsible for the α2−6-sialylation modification are still unknown. Moreover, α-series cholinergic neuron-specific gangliosides, a subgroup of gangliosides with α2−6-sialylation on the GalNAc residue of extended type 1 glycan chain, play important roles in the development and regeneration of nervous system.10 However, due to the substrate restriction and unavailability of siaT for modification of GalNAc residue, none of these complex sialoglycans have been enzymatically synthesized yet.11
In contrast to mammalian α2−6-SiaTs, the first recombinant bacterial α2−6-SiaT cloned from Photobacterium damselae (Pd2,6ST) can be overexpressed in a conventional E. coli strain.12 Owing to its remarkable activity and extreme substrate specificities, the Pd2,6ST has been extensively used for the construction of both Siaα2−6Gal and Siaα2−6GalNAc sequences for the synthesis of various O-glycans, N-glycans and human milk oligosaccharides.7d, 12a, 13 Unfortunately, previous studies shown that the Pd2,6ST can recognize both internal and terminal Gal and GalNAc moieties of complex substrates, resulting a mixture of sialylated products (Scheme 1b, ii).7d, 13e–g To overcome the limitations of both mammalian and bacterial α2−6-SiaTs, we describe herein a novel redox-controlled site-specific α2−6-sialylation approach to precisely control the reaction sites of Pd2,6ST for the synthesis of complex α2−6-linked sialosides (Scheme 1c).
To validate the feasibility of our proposed redox-controlled site-specific α2−6-sialylation strategy, lacto-N-neohexaoside (LNnH) 11 containing three Gal residues (Scheme 2) was investigated first as a model substrate. LNnH potentially has seven α2−6-sialylated products, including three mono-sialylated heptasaccharides 17, 19 and 29, and three di-sialylated octasaccharides 22, 26 and 32. Galactose oxidase (GOase), a commercially available copper metalloenzyme, has been widely used in biosensor for detecting Gal or terminal Gal-containing glycans by selective oxidation of the C6-hydroxyl group of free galactose or terminal Gal residue into C6-aldehyde galactose (Gal6-Ald).14 The resulting C6-aldehyde group has also been extensively used as a chemical handle for further labelling and derivatization purposes.14–15 We envisioned that the Gal6-Ald would act as a protected Gal residue and would not be sialylated by Pd2,6ST. Therefore, the site-specifically introduction of α2−6-linked sialic acid to the intact Gal residues could be achieved. To obtain mono-sialylated heptasaccharide 17 with an α2−6-linked sialic acid at non-reducing end of hexasaccharide 11, both internal Gal residues of 11 need to be protected by oxidation, while the terminal Gal was left intact for site-specific α2−6-sialylation. As shown in Scheme 2, lactoside 12 was treated with oxidation module in the presence of GOase and peroxidase to convert the terminal Gal residue into Gal6-Ald. The resulting disaccharide was then extended by a one-pot three-enzyme β1−3-N-acetylglucosaminylation module (EM1)13b, 13d to give trisaccharide 13 in 73% yields for 2 steps. Trisaccharide 13 was elongated by a β1−4-linked Gal using enzyme module 2 (EM2)13b, 13d, and the nascent terminal Gal was also converted into Gal6-Ald by oxidation module, and then extended by sequential glycosylation with EM1 and EM2 to afford the hexasaccharide intermediate 15. Although, all C6-aldehyde groups of oxidized Gal moieties exist as hydrated germinal diols, none of them could be utilized by Pd2,6ST in EM313b, 13d as sialylation site. The mono-sialylated 16 was isolated as only product in 94% yield. Two Gal6-Ald residues of 16 can be reduced back to the Gal moieties by simply treating with NaBH4 in aqueous solution to provide mono-sialylated heptasaccharide 17 in 98% yield. The same redox-controlled site-specific α2−6-sialylation strategy was also successfully applied in the synthesis of mono-sialylated heptasaccharide 19, di-sialylated octasaccharides 22 and 26 using corresponding Gal6-Ald-containing glycans as intermediates. The syntheses of mono-sialylated heptasaccharide 29 and di-sialylated octasaccharide 32 can be realized by simply changing the glycosylation sequence of three same enzyme modules (EM1-EM3) without the need of oxidation module (Scheme 2).
Scheme 2.
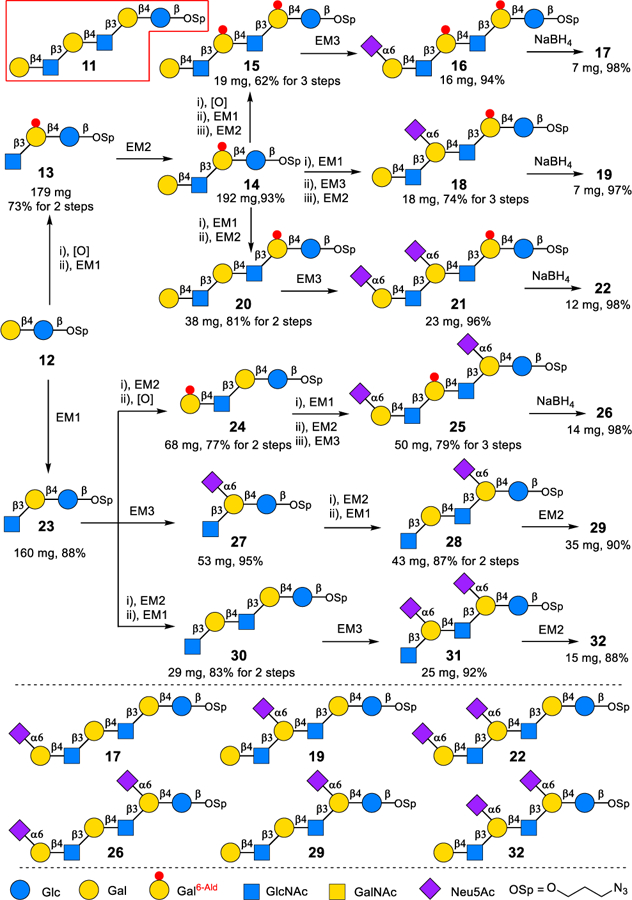
Redox-controlled site specific α2–6-sialylation of lacto-N-neohexaoside (LNnH) 11a
aReagents and conditions: [O], one-pot two enzyme oxidation module with commercial galactose oxidase and peroxidase; EM1, one-pot three-enzyme β1–3-N-acetylglucosaminylation module with BlNahK, EcGlmU and HpLgtA; EM2, one-pot three-enzyme β1–4-galactosylation module with EcGalK, BLUSP and NmLgtB; EM3, one-pot two-enzyme α2–6-sialylation module with NmCSS and Pd2,6ST, see Supporting Information for details.
The Siaα2−6Gal sequence is not only a common terminal component of various N-, O-glycans and glycolipids, but also has been identified as internal moieties in a number of naturally occurring glycans.9d, 16 However, the SiaT from either mammalian or bacteria source that can catalyze site-specific α2−6-sialylation at the internal Gal is still unknown. The redox-controlled α2−6-sialylation strategy provides a practical approach to harness the extreme substrate flexibility offered by readily available bacterial sialyltransferase Pd2,6ST, thus provides the first synthetic approach for on demand site-specific α2−6-sialylation of poly-LacNAc glycans.
This redox-controlled site-specific α2−6-sialylation strategy also provides an easy access for the synthesis of complex sialosides bearing different α2−6-linked sialic acid forms. As shown in Scheme 3a, the tetrasaccharide 24 with a terminal Gal6-Ald was extended using EM1, then the innermost intact Gal was modified with an α2−6-linked Neu5Gc using EM3 in the presence of Neu5Gc as the donor precursor to give hexasaccharide 34. The 34 was treated with EM2 to form a LacNAc termini which was further modified with an α2−6-linked Neu5Ac by EM3 in the presence of Neu5Ac as the donor precursor to afford intermediate 35. The Gal6-Ald moiety of 35 was reduced back to Gal by NaBH4 to give di-sialyl octasaccharide 36 containing hybrid sialic acid forms in excellent overall yields. Taking advantage of this redox-controlled site-specific sialylation strategy, octasaccharide 38 with two different sialic acid forms, and nonasaccharide 39 with three different sialic acid forms at designated positions were also achieved from 24 (Scheme 3a). Moreover, a one-pot two-enzyme α2−8-sialylation enzyme module (EM4) comprising a recombinant α2−8-sialyltransferase from Campylobacter jejuni (CjCstII)17 was applied for the modification of innermost α2−6-linked Neu5Ac to produce nonasaccharide 42 and 45 in good overall yields, respectively (Scheme 3a, see Supporting Information for details).
Scheme 3.

Redox-controlled site specific α2–6-sialylation with different sialic acid forms and substrate scope and application of redox-controlled site-specific α2–6-sialylation strategy.a
aReagents and conditions: EM4, one-pot two-enzyme α2–8-sialylation module with NmCSS and CjCstII; EM5, one-pot three-enzyme β1–3-galactosylation module with EcGalK, BLUSP and EcWbgO; EM6, one-pot three-enzyme β1–4-N-acetylgalactosaminylation module with BlNahK, EcGlmU and GalT1 Y289L; EM7, one-pot two-enzyme α2–3-sialylation module with NmCSS and PmST1, see Supporting Information for details.
Having established the redox-controlled site-specific α2−6-sialylation strategy for poly-LacNAc glycan receptors, the substrate scope and general applicability of the strategy was explored next. As shown in Scheme 3b, the terminal GlcNAc moiety of trisaccharide 13 was parallelly elaborated to type 2 chain by EM2 to give 14, type 1 chain by EM5 to give 48, and LacdiNAc by EM6 to give 51, respectively. In addition to bacterial β1−4GalT NmLgtB in EM2, the recombinant β1−3GalT from E. coli (EcWbgO)18 in EM5, and recombinant bovine GalT 1 mutant (GalT1 Y289L)19 in EM6 all utilize trisaccharide 13 as receptor efficiently. As anticipated, the extreme substrate flexibility of Pd2,6ST in EM3 ensured the α2−6-sialylation at the terminal Gal of 14 and 48, and terminal GalNAc of 51 furnishing pentasaccharides 46, 49 and 52, which were then treated with NaBH4 to give the sialyl LNnT (LSTc) 47, sialyl LNT 50 and sialyl LacdiNAc (sialyl LDNT) 53 in excellent yields, respectively (Scheme 3b). The GOase can also selectively oxidize the terminal GalNAc unit. Therefore, the selective oxidation of terminal GalNAc unit of LDNT 54 could achieve site-specific α2−6-sialylation at the internal Gal. After reduction, the mono-sialylated LDNT 57 was obtained in 69% overall yields for 3 steps (Scheme 3c).
The redox-controlled sialylation strategy could also applied for the site-specific α2−6-sialylation of internal GalNAc residue for the synthesis of sialyl GNB 61, di-sialyl GNB 62 and α–series ganglioside glycan GM1α 65. It was shown that the Pd2,6ST could modify both Gal and GalNAc residues of GNB 58α or 58β, resulting a mixture of two mono-sialylated and one di-sialylated products for each of them (Scheme 1b, ii).13f For site-specific α2−6-sialylation at internal GalNAc residue, the terminal Gal of 58α or 58β was converted into Gal6-Ald by oxidation enzyme module to give 59α or 59β. Both 59α or 59β were treated with α2−6-sialylation module EM3, however, only 59β could be utilized by Pd2,6ST to afford the sialoside 60. These results were consistent with previous report that the α-linked GalNAc is not a good substrate for Pd2,6ST.12a Sialoside 60 was reduced with NaBH4 to afford sialyl GNB 61 in 73% yields for 3 steps. The 61 could be further elaborated to di-sialyl GNB 62 by an α2−3-sialylation enzyme module (EM7)13b, 20 (Scheme 3d).
As aforementioned, owing to the substrate restriction and unavailability of siaT for modification of GalNAc residue, none of α-series cholinergic neuron-specific gangliosides has been enzymatically synthesized yet. The first enzymatic synthesis of α-series ganglioside glycan GM1α 65 using redox-controlled sialylation strategy was also explored. Starting from known asialo-GM1 (GA1) 6321, a similar sequence involving enzymatic oxidation, selective α2−6-sialylation of internal GalNAc, and subsequent reduction provided ganglioside GM1α 65 in 74% yields for 3 steps (Scheme 3e). Interestingly, the Pd2,6ST in EM3 can only introduce α2−6-linked Neu5Ac at GalNAc residue to give pentasaccharide 64, while sialylation of innermost Gal residue was not observed. The redox-controlled sialylation strategy utilized for the synthesis of GM1α 65 provides a novel approach for the synthesis of other α-series gangliosides. Besides, by replacing NaBH4 with NaBD4 in the reduction step, the Gal6-Ald-containing intermediates in this study, such as 64, can be transformed into deuterium labeled products (e.g. GM1α 66), which could be used as probe in elucidating multiple biochemical processes (Scheme 3d, see Supporting Information for details).
The binding profiles of synthesized sialosides with sialic acid-recognition proteins were also examined using printed sialoglycan slides. The plant lectin Sambucus nigra agglutinin (SNA) is known to be able to specifically recognize α2−6-linked sialosides.22 SNA exhibited very strong binding exclusively to all sialosides with a terminal α2−6-sialylated LacNAc moiety, while no significant binding was observed for internal α2−6-sialylated glycans (Figure S7). Chicken polyclonal anti-Neu5Gc antibody IgY (pChGc) bound to all Neu5Gc-containg glycans with a preference toward terminal α2−6-linked Neu5Gc (Figure S8). In contrast, human anti-Neu5Gc antibody rich serum only bound tightly with terminal α2−6-linked Neu5Gc (Figure S9). The His-tagged typhoid toxin (PltB-His) recognized all Neu5Ac modified glycans (Figure S10), while human sialic acid-binding lectin Siglec-9 (hSiglec-9-Fc) only bound tightly to terminal Neu5Ac-modified glycans and Neu5Acα2−8Neu5Acα2−6-linked glycan (Figure S11). Plant lectins Maackia amurensis lectin I and II (MAL-I-II) were also examined. As expected only α2−3-linked sialoside 62 exhibited strong affinity to MAL-II (Figure S12–S13).22
In summary, a novel substrate engineering strategy23 was developed to harness the extreme substrate flexibility of Pd2,6ST for site-specific α2−6-sialylation of both Gal and GalNAc residues of various substrates. This strategy overcomes the limitation of availability and substrate specificities of known mammalian and bacterial α2−6-sialyltransferases, thereby providing a general and concise approach for the synthesis of complex α2−6-sialylated glycans with a single bacterial α2−6-sialyltransferase Pd2,6ST.
Supplementary Material
ACKNOWLEDGMENT
We thank Prof. Xi Chen at the University of California at Davis for sharing plasmids and providing helpful discussions, and Sandra Diaz at UCSD for technical help. This project was financially supported by National Natural Science Foundation of China (Grant Nos. 21672128, 21877072, 21807064), State Key Laboratory of Microbial Technology (M2016–06), Department of Science and Technology of Shandong Province (2016GGH4502, 2016GSF121002, ZR201709190252), Shandong University (2018JC053) and NIH grant R01GM32373.
Footnotes
ASSOCIATED CONTENT
Supporting Information. Detailed experimental procedures and product characterization. This material is available free of charge via the Internet at http://pubs.acs.org
The authors declare no competing financial interests.
REFERENCES
- (1).Werz DB; Ranzinger R; Herget S; Adibekian A; von der Lieth C-W; Seeberger PH, Exploring the structural diversity of mammalian carbohydrates (“glycospace”) by statistical databank analysis. ACS Chem. Biol 2007, 2, 685–691. [DOI] [PubMed] [Google Scholar]
- (2).a) Chen X; Varki A, Advances in the biology and chemistry of sialic acids. ACS Chem. Biol 2010, 5, 163–176; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Varki A, Sialic acids in human health and disease. Trends Mol. Med 2008, 14, 351–360; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Angata T; Varki A, Chemical diversity in the sialic acids and related α-keto acids: an evolutionary perspective. Chem. Rev 2002, 102, 439–470; [DOI] [PubMed] [Google Scholar]; d) Schauer R, Achievements and challenges of sialic acid research. Glycoconj. J 2000, 17, 485–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Rogers G; Paulson J; Daniels R; Skehel J; Wilson I; Wiley D, Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature 1983, 304, 76. [DOI] [PubMed] [Google Scholar]
- (4).Anthony RM; Nimmerjahn F; Ashline DJ; Reinhold VN; Paulson JC; Ravetch JV, Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science 2008, 320, 373–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).a) Rodrigues E; Macauley M, Hypersialylation in cancer: modulation of inflammation and therapeutic opportunities. Cancers 2018, 10, 207; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Büll C; Stoel MA; den Brok MH; Adema GJ, Sialic acids sweeten a tumor’s life. Cancer Res 2014, 74, 3199–3204. [DOI] [PubMed] [Google Scholar]
- (6).a) For recent reviews, see: Wen L; Edmunds G; Gibbons C; Zhang J; Gadi MR; Zhu H; Fang J; Liu X; Kong Y; Wang PG, Toward automated enzymatic synthesis of oligosaccharides. Chem. Rev 2018, 118, 8151–8187; [DOI] [PubMed] [Google Scholar]; b) Yu CC; Withers SG, Recent developments in enzymatic synthesis of modified sialic acid derivatives. Adv. Syn. Catal 2015, 357, 1633–1654; [Google Scholar]; c) Schmaltz RM; Hanson SR; Wong C-H, Enzymes in the synthesis of glycoconjugates. Chem. Rev 2011, 111, 4259–4307; [DOI] [PubMed] [Google Scholar]; d) Palcic MM, Glycosyltransferases as biocatalysts. Curr. Opin. Chem. Biol 2011, 15, 226–233. [DOI] [PubMed] [Google Scholar]
- (7).a) Moremen KW; Ramiah A; Stuart M; Steel J; Meng L; Forouhar F; Moniz HA; Gahlay G; Gao Z; Chapla D, Expression system for structural and functional studies of human glycosylation enzymes. Nature Chem. Biol 2018, 14, 156–162; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Prudden AR; Liu L; Capicciotti CJ; Wolfert MA; Wang S; Gao Z; Meng L; Moremen KW; Boons G-J, Synthesis of asymmetrical multiantennary human milk oligosaccharides. Proc. Natl. Acad. Sci. USA 2017, 114, 6954–6959; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Wang Z; Chinoy ZS; Ambre SG; Peng W; McBride R; de Vries RP; Glushka J; Paulson JC; Boons G-J, A general strategy for the chemoenzymatic synthesis of asymmetrically branched N-glycans. Science 2013, 341, 379–383; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Nycholat CM; Peng W; McBride R; Antonopoulos A; de Vries RP; Polonskaya Z; Finn M; Dell A; Haslam SM; Paulson JC, Synthesis of biologically active N-and O-linked glycans with multisialylated poly-N-acetyllactosamine extensions using P. damsela α2–6 sialyltransferase. J. Am. Chem. Soc 2013, 135, 18280–18283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).a) For selected examples, see: Blixt O; Allin K; Pereira L; Datta A; Paulson JC, Efficient chemoenzymatic synthesis of O-linked sialyl oligosaccharides. J. Am. Chem. Soc 2002, 124, 5739–5746; [DOI] [PubMed] [Google Scholar]; b) George SK; Schwientek T; Holm B; Reis CA; Clausen H; Kihlberg J, Chemoenzymatic synthesis of sialylated glycopeptides derived from mucins and T-cell stimulating peptides. J. Am. Chem. Soc 2001, 123, 11117–11125. [DOI] [PubMed] [Google Scholar]
- (9).a) Anugraham M; Jacob F; Nixdorf S; Everest-Dass AV; Heinzelmann-Schwarz V; Packer NH, Specific glycosylation of membrane proteins in epithelial ovarian cancer cell lines: glycan structures reflect gene expression and DNA methylation status. Mol. Cell. Proteomics 2014, 13, 2213–2232; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Saarinen J; Welgus HG; Flizar CA; Kalkkinen N; Helin J, N-Glycan structures of matrix metalloproteinase-1 derived from human fibroblasts and from HT-1080 fibrosarcoma cells. Eur. J. Biochem 1999, 259, 829–840; [DOI] [PubMed] [Google Scholar]; c) Suzuki Y; Nakao T; Ito T; Watanabe N; Toda Y; Guiyun X; Suzuki T; Kobayashi T; Kimura Y; Yamada A, Structural determination of gangliosides that bind to influenza A, B, and C viruses by an improved binding assay: strain-specific receptor epitopes in sialo-sugar chains. Virology 1992, 189, 121–131; [DOI] [PubMed] [Google Scholar]; d) Podolsky DK, Oligosaccharide structures of isolated human colonic mucin species. J. Biol. Chem 1985, 260, 15510–15515; [PubMed] [Google Scholar]; e) Mizuochi T; Yamashita K; Fujikawa K; Kisiel W; Kobata A, The carbohydrate of bovine prothrombin. Occurrence of Galβ1,3-GlcNAc grouping in asparagine-linked sugar chains. J. Biol. Chem 1979, 254, 6419–6425. [PubMed] [Google Scholar]
- (10).Yang L; Zeller CB; Shaper NL; Kiso M; Hasegawa A; Shapiro RE; Schnaar RL, Gangliosides are neuronal ligands for myelin-associated glycoprotein. Proc. Natl. Acad. Sci. USA 1996, 93, 814–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).a) Hotta K; Komba S; Ishida H; Kiso M; Hasegawa A, Synthetic studies on sialoglycoconjugates 61: synthesis of α-series ganglioside GM1α. J. Carbohydr. Chem 1994, 13, 665–677; [Google Scholar]; b) Prabhanjan H; Aoyama K; Kiso M; Hasegawa A, Synthesis of disialoganglioside GD1α and its positional isomer. Carbohydr. Res 1992, 233, 87–99; [DOI] [PubMed] [Google Scholar]; c) Prabhanjan H; Kiso M; Hasegawa A, Total synthesis of a disialoganglioside GD1α. Carbohydr. Res 1991, 222, C1–C4. [DOI] [PubMed] [Google Scholar]
- (12).a) Yu H; Huang S; Chokhawala H; Sun M; Zheng H; Chen X, Highly efficient chemoenzymatic synthesis of naturally occurring and non-natural α−2,6-linked sialosides: a P. damsela α−2,6-sialyltransferase with extremely flexible donor-substrate specificity. Angew. Chem. Int. Ed 2006, 45, 3938–3944; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Yamamoto T; Nakashizuka M; Terada I, Cloning and expression of a marine bacterial β-galactoside α2,6-sialyltransferase gene from Photobacterium damsela JT0160. J. Biochem 1998, 123, 94–100. [DOI] [PubMed] [Google Scholar]
- (13).a) For selected examples, see: Yu H; Yan X; Autran CA; Li Y; Etzold S; Latasiewicz J; Robertson BM; Li J; Bode L; Chen X, Enzymatic and chemoenzymatic syntheses of disialyl glycans and their necrotizing enterocolitis preventing effects. J. Org. Chem 2017, 82, 13152–13160; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Yu H; Chen X, One-pot multienzyme (OPME) systems for chemoenzymatic synthesis of carbohydrates. Org. Biomol. Chem 2016, 14, 2809–2818; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Shivatare SS; Chang S-H; Tsai T-I; Tseng SY; Shivatare VS; Lin Y-S; Cheng Y-Y; Ren C-T; Lee C-CD; Pawar S; Tsai C-S; Shih H-W; Zeng Y-F; Liang C-H; Kwong PD; Burton DR; Wu C-Y; Wong C-H, Modular synthesis of N-glycans and arrays for the hetero-ligand binding analysis of HIV antibodies. Nature Chem 2016, 8, 338–346; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Chen C; Zhang Y; Xue M; Liu XW; Li Y; Chen X; Wang PG; Wang F; Cao H, Sequential one-pot multienzyme (OPME) synthesis of lacto-N-neotetraose and its sialyl and fucosyl derivatives. Chem. Commun 2015, 51, 7689–7692; [DOI] [PubMed] [Google Scholar]; e) Yu H; Lau K; Thon V; Autran CA; Jantscher-Krenn E; Xue M; Li Y; Sugiarto G; Qu J; Mu S; Ding L; Bode L; Chen X, Synthetic disialyl hexasaccharides protect neonatal rats from necrotizing enterocolitis. Angew. Chem. Int. Ed 2014, 53, 6687–6691; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Meng X; Yao W; Cheng J; Zhang X; Jin L; Yu H; Chen X; Wang F; Cao H, Regioselective chemoenzymatic synthesis of ganglioside disialyl tetrasaccharide epitopes. J. Am. Chem. Soc 2014, 136, 5205–5208; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Chien WTL, C. F.; Yu CC; Lin CH; Li SP; Primadona I; Chen YJ; Mong KK; Lin CC, Sequential one-pot enzymatic synthesis of oligo-N-acetyllactosamine and its multi-sialylated extensions. Chem. Commun 2014, 50, 5786–5789; [DOI] [PubMed] [Google Scholar]; h) Malekan H; Fung G; Thon V; Khedri Z; Yu H; Qu J; Li Y; Ding L; Lam KS; Chen X, One-pot multi-enzyme (OPME) chemoenzymatic synthesis of sialyl-Tn-MUC1 and sialyl-T-MUC1 glycopeptides containing natural or non-natural sialic acid. Bioorg. Med. Chem 2013, 21, 4778–4785; [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Teo CF; Hwang TS; Chen PH; Hung CH; Gao HS; Chang LS; Lin CH, Synthesis of sialyl TN glycopeptides-enzymatic sialylation by α2,6-sialyltransferase from Photobacterium damsela. Adv. Syn. Catal 2005, 347, 967–972; [Google Scholar]; j) Kajihara Y; Yamamoto T; Nagae H; Nakashizuka M; Sakakibara T; Terada I, A novel α−2,6-sialyltransferase: transfer of sialic acid to fucosyl and sialyl trisaccharides. J. Org. Chem 1996, 61, 8632–8635. [Google Scholar]
- (14).a) Parikka K; Master E; Tenkanen M, Oxidation with galactose oxidase: multifunctional enzymatic catalysis. J. Mol. Catal. B 2015, 120, 47–59; [Google Scholar]; b) Rannes JB; Ioannou A; Willies SC; Grogan G; Behrens C; Flitsch SL; Turner NJ, Glycoprotein labeling using engineered variants of galactose oxidase obtained by directed evolution. J. Am. Chem. Soc 2011, 133, 8436–8439. [DOI] [PubMed] [Google Scholar]
- (15).a) Laaf D; Bojarová P; Pelantova H; Kren V; Elling L, Tailored multivalent neo-glycoproteins: synthesis, evaluation, and application of a library of galectin-3-binding glycan ligands. Bioconj. Chem 2017, 28, 2832–2840; [DOI] [PubMed] [Google Scholar]; b) Kupper CE; Rosencrantz RR; Henßen B; Pelantová H; Thönes S; Drozdová A; Křen V; Elling L, Chemo-enzymatic modification of poly-N-acetyllactosamine (LacNAc) oligomers and N,N-diacetyllactosamine (LacDiNAc) based on galactose oxidase treatment. Beilstein J. Org. Chem 2012, 8, 712–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).a) Naumenko OI; Zheng H; Sof’ya NS; Wang H; Li Q; Shashkov AS; Wang J; Knirel YA; Xiong Y, Structures and gene clusters of the O-antigens of Escherichia albertii O3, O4, O6, and O7. Carbohydr. Res 2017, 449, 17–22; [DOI] [PubMed] [Google Scholar]; b) Deutschmann R; Boncheff AG; Daraban L; MacInnes JI; Monteiro MA, Common sialylated glycan in Actinobacillus suis. Glycobiology 2010, 20, 1227–1232; [DOI] [PubMed] [Google Scholar]; c) Eserstam R; Rajaguru TP; Jansson PE; Weintraub A; Albert MJ, The structure of the O-chain of the lipopolysaccharide of a prototypal diarrheagenic strain of Hafnia alvei that has characteristics of a new species under the genus Escherichia. Eur. J. Biochem 2002, 269, 3289–3295; [DOI] [PubMed] [Google Scholar]; d) Slomiany BL; Murty V; Slomiany A, Isolation and characterization of oligosaccharides from rat colonic mucus glycoprotein. J. Biol. Chem 1980, 255, 9719–9723. [PubMed] [Google Scholar]
- (17).a) Yu H; Cheng J; Ding L; Khedri Z; Chen Y; Chin S; Lau K; Tiwari VK; Chen X, Chemoenzymatic synthesis of GD3 oligosaccharides and other disialyl glycans containing natural and non-natural sialic acids. J. Am. Chem. Soc 2009, 131, 18467–18477; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Gilbert M; Brisson J-R; Karwaski M-F; Michniewicz J; Cunningham A-M; Wu Y; Young NM; Wakarchuk WW, Biosynthesis of ganglioside mimics in Campylobacter jejuni OH4384 identification of the glycosyltransferase genes, enzymatic synthesis of model compounds, and characterization of nanomole amounts by 600-MHz 1H and 13C NMR analysis. J. Biol. Chem 2000, 275, 3896–3906. [DOI] [PubMed] [Google Scholar]
- (18).Liu X.-w.; Xia C; Li L; Guan W.-y.; Pettit N; Zhang H.-c.; Chen M; Wang PG, Characterization and synthetic application of a novel β1,3-galactosyltransferase from Escherichia coli O55:H7. Bioorg. Med. Chem 2009, 17, 4910–4915. [DOI] [PubMed] [Google Scholar]
- (19).Ramakrishnan B; Qasba PK, Structure-based design of β1,4-galactosyltransferase I (β4Gal-T1) with equally efficient N-acetylgalactosaminyltransferase activity point mutation broadens β4Gal-T1 donor specificity. J. Biol. Chem 2002, 277, 20833–20839. [DOI] [PubMed] [Google Scholar]
- (20).Yu H; Chokhawala H; Karpel R; Yu H; Wu B; Zhang J; Zhang Y; Jia Q; Chen X, A multifunctional Pasteurella multocida sialyltransferase: a powerful tool for the synthesis of sialoside libraries. J. Am. Chem. Soc 2005, 127, 17618–17619. [DOI] [PubMed] [Google Scholar]
- (21).Fair RJ; Hahm HS; Seeberger PH, Combination of automated solid-phase and enzymatic oligosaccharide synthesis provides access to α(2,3)-sialylated glycans. Chem. Commun 2015, 51, 6183–6185. [DOI] [PubMed] [Google Scholar]
- (22).a) Padler-Karavani V; Song X; Yu H; Hurtado-Ziola N; Huang S; Muthana S; Chokhawala HA; Cheng J; Verhagen A; Langereis MA; Kleene R; Schachner M; de Groot RJ; Lasanajak Y; Matsuda H; Schwab R; Chen X; Smith DF; Cummings RD; Varki A, Cross-comparison of protein recognition of sialic acid diversity on two novel sialoglycan microarrays. J. Biol. Chem 2012, 287, 22593–22608; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Song X; Yu H; Chen X; Lasanajak Y; Tappert MM; Air GM; Tiwari VK; Cao H; Chokhawala HA; Zheng H; Cummings RD; Smith DF, A sialylated glycan microarray reveals novel interactions of modified sialic acids with proteins and viruses. J. Biol. Chem 2011, 286, 31610–31622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Lairson LL; Watts AG; Wakarchuk WW; Withers SG, Using substrate engineering to harness enzymatic promiscuity and expand biological catalysis. Nature Chem. Biol 2006, 2, 724–728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


