Abstract
Although the clinical high risk for psychosis (CHR) paradigm has become well-established over the past two decades, one key component has received surprisingly little investigative attention: the predictive validity of the criteria for conversion or transition to frank psychosis. The current study evaluates the predictive validity of the transition to psychosis as measured by the Structured Interview for Psychosis-Risk Syndromes (SIPS) in CHR individuals. Participants included 33 SIPS converters and 399 CHR non-converters both from the North American Prodromal Longitudinal Study (NAPLS-2), as well as a sample of 67 separately ascertained first-episode psychosis (FEP) patients from the STEP program. Comparisons were made at baseline and one-year follow-up on demographic, diagnostic stability (SCID), and available measurement domains relating to severity of illness (psychotropic medication, psychosocial treatment, and resource utilization). Principal findings are: 1) a large majority of cases in both SIPS converters (n=27/33, 81.8%) and FEP (n=57/67, 85.1%) samples met criteria for continued psychosis at one-year follow-up; 2) follow-up prescription rates for current antipsychotic medication were higher in SIPS converters (n=17/32, 53.1%) compared to SIPS non-converters (n=81/397, 20.4%), and similar as compared to FEP cases (n=39/65, 60%); and 3) at follow-up, SIPS converters had higher rates of resource utilization (psychiatric hospitalizations, day hospital admissions, and ER visits) than SIPS non-converters and were similar to FEP in most categories. The results suggest that the SIPS definition of psychosis onset carries substantial predictive validity. Limitations and future directions are discussed.
Keywords: SIPS, transition, predictive validity
1. Introduction
The clinical high risk syndrome for psychosis (CHR), also referred to as ultra-high risk or at-risk mental state, has been extensively researched over the past two decades (Fusar-Poli et al., 2012; Fusar-Poli et al., 2013b; van Os and Guloksuz, 2017). The advent of the CHR diagnosis has allowed for prospective identification of a pre-psychotic syndrome characterized by attenuated positive symptoms, negative symptoms, and functional impairment(Woods et al., 2001). This CHR syndrome often lasts 1 to 3 years (Powers III et al., under revision) and carries a substantial risk of progression to frank psychosis (Miller et al., 2002; Yung, 1998; Yung et al., 1996; Yung et al., 2004b). A recent meta-analysis concluded that 22% of at-risk patients convert to psychosis within one year, 29% within two years, and 36% within three years (Fusar-Poli et al., 2012).
Structured interviews to diagnose CHR (Fusar-Poli et al., 2015; McGlashan et al., 2010; Yung et al., 2004a) have been widely employed, as both research and clinical tools, (Allen et al., 2018; Bossong et al., 2018; Cannon et al., 2015; Cao et al., 2018; Corcoran et al., 2018; Davies et al., 2018a; Davies et al., 2018b; Dutt et al., 2015; Egerton et al., 2014; Fusar-Poli et al., 2013b; Ho et al., 2017; Howes et al., 2011; Jagannath et al., 2017; Jeffries et al., 2016; Jeffries et al., 2018; Koutsouleris et al., 2018; Lepock et al., 2018; McGorry, 2008; Miller et al., 2002; Modinos et al., 2018; NHS England, 2016; Schmidt et al., 2017; Schneider et al., 2016; Seidman et al., 2016; Selvaraj et al., 2018; US Substance Abuse and Mental Health Services Administration, 2018; Yung, 2017; Yung et al., 2005). The reliability and validity of one such tool, the Structured Interview for Psychosis-risk Syndromes (SIPS) (McGlashan et al., 2010), have each been reported in more than 20 samples (Addington et al., 2011; Carrión et al., 2018; Koike et al., 2013; Liu et al., 2011; McFarlane et al., 2015; Miller et al., 2002; Raballo et al., 2018; Simon et al., 2012; Tso et al., 2017; Waltz et al., 2015), and the large majority of reported data strongly support the accuracy of the instrument (Powers III et al., in press; Woods et al., 2019).
One key component of the CHR paradigm has received relatively little direct investigative attention: the criteria for conversion or transition to frank psychosis. While there is substantial evidence for the validity of the CHR converter designation as compared to non-converters (Sun, 2009; Takahashi et al., 2009; Walterfang et al., 2008; Wood, 2011; Yung et al., 2010; Yung et al., 2003), there has been relatively little attention to the predictive validity of the conversion assessment for clinical outcome over time (Hengartner et al., 2017) and in comparison to separate first episode samples. It is not sufficient that no CHR subjects receive psychosis diagnoses on Structured Interview for DSM (SCID) or that all CHR structured interview-defined psychosis subjects do (Woods et al., 2009), because the SCID and CHR judgments are generally not made independently in CHR research clinics. We are aware of only five samples where psychotic patients identified by the SIPS were compared to CHR (Koike et al., 2013; Liu et al., 2011; Raballo et al., 2018; Simon et al., 2012; Tso et al., 2017; Waltz et al., 2015). Although these data do support the validity of the SIPS CHR versus psychosis distinction, they are limited: analyses are restricted to baseline construct validity (concurrent rating scale) comparisons; and there is relatively little overlap in validity measures across studies (Powers III et al., in press; Woods et al., in press). Neurobiological studies of predictors of conversion and of longitudinal changes in converter versus non-converter samples can support validity (McGorry et al., 2018; Sun, 2009; Takahashi et al., 2009; Walterfang et al., 2008; Wood, 2011).
This paucity of evidence for validity of the conversion assessment is unfortunate because many CHR treatment and prediction studies rely on the conversion measure as an outcome. Further, many studies are limited due to lack of follow-up data after conversion to psychosis, as the point of conversion tends to be the stopping point or lead to exclusion of further study participation. One existent longitudinal study of converters is limited in that it did not identify a specific point of conversion and compare it to an outcome point, instead the clinical measures were averaged over the entire observation period, including both before and after conversion (Hengartner et al., 2017). Unsurprisingly several observers have questioned the validity of the conversion determination (Fusar-Poli et al., 2014a; Fusar-Poli and Van Os, 2013; Fusar-Poli et al., 2014b; Hengartner et al., 2017; Lin et al., 2012; van Os and Guloksuz, 2017; Yung et al., 2010) and some have raised the possibility that some conversions from CHR may even be “trivial”, for instance, if people convert and continue to function well and/or experience remission or attenuated symptoms (Fusar-Poli et al., 2014b; Hengartner et al., 2017; Lin et al., 2012; Lin et al., 2011; Yung et al., 2010).
Arguably the most important needed information for the CHR structured interview identification of psychosis is on its predictive validity. If the CHR interview identification of psychosis is valid, individuals who make the transition to psychosis should be expected to have a less favorable subsequent course of illness than those who do not make the transition (Hengartner et al., 2017; Yung et al., 2010), a measure of discriminant predictive validity. Moreover, individuals who make the transition should generally have a similar course of illness in comparison to other first episode psychosis (FEP) samples, a measure of convergent predictive validity. However, specialized treatment in the prodromal phase may shorten DUP which may have a favorable effect on long term outcomes (Valmaggia et al., 2015). There is a need for studies evaluating CHR patients at the point of transition and then at 1 year post-conversion follow-up, in comparison to a separate first episode sample.
The purpose of this paper is to evaluate the predictive validity of the SIPS-defined transition to psychosis in CHR individuals, by comparing SIPS-converters (CV) both to SIPS non-converters (NCV) and to a sample of separately ascertained FEP patients on diagnostic stability and available measures of illness severity at one-year follow-up evaluation. It is hypothesized that SIPS CV will show similar diagnostic stability and severity of illness compared to the FEP sample, and will differ significantly from SIPS NCV on clinical severity.
2. Method
2.1. Subjects
The CHR sample was obtained through the North American Prodrome Longitudinal Study (NAPLS-2). NAPLS-2 recruited 764 CHR subjects ages 12–35 who met Structured Interview for Psychosis-risk Syndrome (McGlashan et al., 2010) (SIPS, n=743) or young schizotypal personality disorder (Addington et al., 2012; Woods et al., 2009) criteria (n=21) at eight sites from 2008 to 2013. Participants were referred from health care providers, educators, social service agencies or were self-referred in response to community education efforts (Addington et al., 2012). Of the 764 subjects, 94 converted to psychosis and 670 did not convert. Among those who converted, twelve did not have in-person conversion assessments and eight did not have time for 12-month post-conversion assessment before data collection ended. Of the remaining 74, 33 (44.6%) completed one-year follow-up. Among non-converters, 399/670 (59.6%) had clinical visits at one-year follow-up.
The FEP sample was obtained from a coordinated specialty care (CSC) clinic within the Program for Specialized Treatment Early in Psychosis (STEP) (Srihari et al., 2014) in New Haven, CT. STEP recruited individuals aged 16–35 who had their first episode of a non-affective psychotic disorder within three years. The majority of referrals came from inpatient units and other acute facilities. Multiple sources of information contributed to determine eligibility for STEP, including a SCID interview, collateral from medical records and families, as well as a retrospective SIPS to estimate duration of psychosis. STEP excluded referrals with established diagnoses of affective psychosis or psychosis secondary to substance-use or medical illness, but there was no exclusion for comorbid nonpsychotic diagnoses. Eligible participants were offered CSC together with the option of STEP research. A total of 90 FEP patients were enrolled in the research program between February 2014 and September 2016 who were potentially eligible for a one-year follow-up assessment. Of the 90 research participants, five were found ineligible shortly after the baseline assessment due to psychosis duration greater than 3 years. By September 2017, 67 of the eligible 85 had a completed baseline and follow-up assessments (78.8%) and were included in the current study. Eighteen subjects either never completed baseline or were lost to follow-up.
2.2. Assessments
For SIPS CV, baseline is date at which the participants received their “conversion” assessment. Follow-up assessments were conducted one year after the baseline/conversion visit. For SIPS NCV, baseline assessments were the initial assessment in the NAPLS study, with follow-up assessment occurring one year later. FEP baseline assessments were conducted at admission and one year follow-up. For FEP, days since onset of psychosis is the time from psychosis onset, according to SIPS POPS criteria (see supplementary), to admission to STEP (see Table 1).
Table 1.
Baseline Demographic Statistics for the Sample
| Measure | SIPS NCV n=399 | SIPS CV n=33 | FEP n=67 |
|---|---|---|---|
| Age, years | 19.1±4.4 | 19.6±3.7b | 22.1±3.4b |
| Gender, Male, n (%) | 233 (58.4%) | 18 (54.5%) | 49 (73.1%) |
| Race, White, n (%) | 224 (56.1%) | 18 (54.5%) | 27 (40.3%) |
| Days Since Onset of Psychosis | n/a | M = 62 ± 47.7a | M = 370 ±339a |
| Median = 44 days | Median = 258 days |
Note: SIPS NCV=clinical high risk non-converter; SIPS CV=clinical high risk converter;
FEP=first episode psychosis.
groups with this label differ p<0.001
groups with this label differ p<0.01
Data related to severity of illness for both samples included: 1) DSM-IV psychotic disorder diagnoses, 2) psychotropic medication use, 3) psychosocial treatment, 4) resource utilization. Unfortunately, no direct measures of functional status were collected in the SIPS-CV at follow-up.
2.3. DSM-IV TR psychotic disorder diagnoses
Comparisons on psychotic disorder diagnoses were limited to the SIPS CV versus the FEP group, since the NCV did not have a psychotic disorder. For the SIPS CV sample, current DSM-IV psychotic disorder diagnoses were assessed prospectively at conversion and follow-up using the Structured Clinical Interview for DSM-IV Axis-I Disorders, Clinician Version (SCIDI), incorporating ratings on whether lifetime psychotic symptoms were present in the past month. Affective psychoses in remission were coded as no current psychosis. For FEP sample, the SCID-I was used prospectively to determine lifetime baseline and lifetime follow-up psychotic disorder diagnoses. The determination of current psychosis diagnoses at follow-up was generated by SCID-certified raters incorporating all available research study and clinical chart information.
Follow-up psychotic diagnoses for both samples were further categorized as: 1) continued psychosis if current affective or non-affective psychosis was present or if antipsychotic medication was prescribed for psychosis or 2) no continued psychosis.
2.4. Psychotropic medication prescription
For NAPLS-2 at baseline and follow-up and for STEP at baseline, an inventory of psychotropic medication prescribed to the patient was collected based primarily on patient and informant interview using the NAPLS Prescription Med Log (Woods et al., 2013). For each course of medication, data included start/stop date, and total prescribed daily dose. “Accounted-for” medication time constituted all time between birth and the time point of interest for which courses of medication or no medication were recorded (see supplementary for additional methods).
2.5. Psychosocial treatment
For collection of the CHR data, at each assessment participants and/or family members were queried about psychosocial treatments received currently and over the past 6 months during the time between the current and previous assessment. For each treatment received, participants were asked to provide start/stop dates, number of sessions, and the nature of the therapy to best determine type. For comparison with the FEP sample, treatment types were collapsed into an “Any Therapy” category.
Information on FEP psychosocial treatment was collected through the use of multiple sources, including an adapted version of the Service Utilization and Resources Form (SURF) (Srihari et al., 2014), medical records, and a review of the clinic’s weekly clinical activity log. A best estimate method was utilized to aggregate all available information on FEP to create a comprehensive frequency of psychosocial services defined as “Any Therapy.”
2.6. Resource utilization
Resource use for CHR sample was coded at each visit using a running log with codes for various types of utilization and start/stop dates. For FEP, resources were coded at baseline and follow-up using the SURF (Rosenheck and Lieberman, 2007; Srihari et al., 2014). The SURF is a multi-item form that uses participants’ or caregivers’ report to document comprehensively the number, type, and duration of health services and consumption of non-health resources. This form has been adapted for use with the younger population in this study (Srihari et al., 2014).
Resource use considered most relevant for the present analyses were those that reflect need for intensive psychiatric treatment related to high severity of illness: inpatient unit stays, Emergency Room (ER) visits, and day hospital use for psychiatric reasons. Based upon data availability in the FEP sample, resource use in CHR at baseline was restricted to the preceding six months.
2.7. Statistical Analyses
Analyses were conducted using the Statistical Package for Social Sciences (SPSS), Version 22. Univariate group comparisons were conducted with independent t tests for continuous variables and Fisher’s exact test for categorical variables. Analyses compared variables related to severity of illness across groups, within time point.
3. Results
3.1. Demographics
The mean age at baseline evaluation of SIPS converters was significantly lower than that in the FEP sample (see Table 1). As expected, the duration of psychosis was substantially longer for FEP enrollees than for SIPS CV. Other differences between the SIPS CV and comparator groups were nonsignificant. The majority of participants across all three samples was male. The majority of participants in both SIPS CV and NCV samples identified as white, whereas only 40.3% of FEP sample identified as white. The SIPS CV with follow-up did not differ significantly on any measure in Table 1 from converters who could not be included.
3.2. DSM-IV psychotic disorder diagnoses
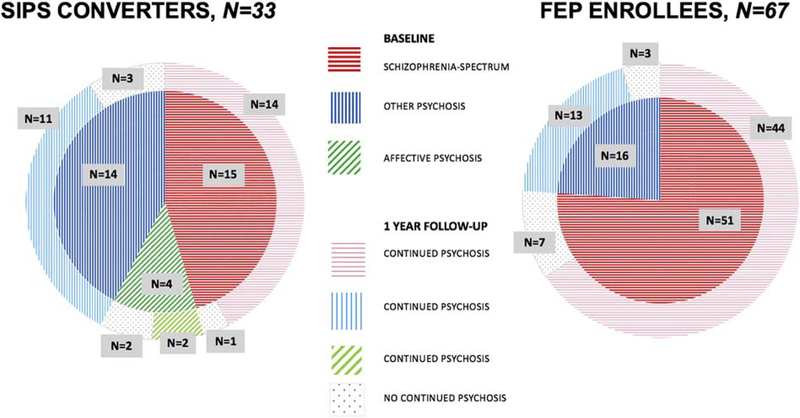
As shown in Figure 1 and as detailed in Table S1, the proportion of schizophrenic psychosis diagnosis among SIPS converters at baseline was lower than in FEP enrollees but did not differ significantly (p=.051). The proportion of the sample meeting criteria for other psychoses was higher among SIPS converters but also did not differ significantly (p=.062), whereas a diagnosis of affective psychosis was present in SIPS converters but not in FEP participants, consistent with STEP exclusion criteria.
Fig, 1.
Diagnostic Stability at Baseline and One-year Follow-up for SIPS Clinical High Risk Converters and First Episode Psychosis (FEP) Enrollees.
At one-year follow-up, the large majority of subjects in both SIPS CV (n=27/33, 81.8%) and FEP (n=57/67, 85.1%) qualified for a continued psychosis diagnosis. The group comparison was not statistically significant (p=.674). Similar findings were observed among the subgroup diagnosed with schizophrenic psychosis at baseline (SIPS CV vs FEP p=.469) as well among the subgroup diagnosed with other psychosis at baseline (SIPS CV vs FEP p=.86). The small number of SIPS CV with baseline affective psychosis qualified for a psychosis diagnosis at follow-up in half of the cases.
3.3. Psychotropic medication
At baseline, accounted-for medication time was similar in SIPS CVs versus SIPS NCVs but 28 months shorter in SIPS CVs than in FEPs (Table 2), consistent with the younger age of the SIPS CV sample (Table 1). SIPS CVs differed from SIPS NCVs in having significantly higher rates of current AP, AT, MS, and any psychotropic. Duration of use at baseline was significantly longer in SIPS CVs for AP, AT, and AD, as compared to SIPS NCV. Compared to FEPs, SIPS CVs were less frequently prescribed AP and any psychotropic at baseline but had longer duration of psychotropic use at baseline in several categories (AP, AT, AD, ST, BZ). CPZ equivalent doses in SIPS CVs were significantly higher than in the NCV group, and similar to those in FEP group.
Table 2.
Psychotropic Medication at Baseline and One Year Follow-up.
| Baseline | One Year | |||||
|---|---|---|---|---|---|---|
| Variable | SIPS NCV n=398 | SIPS CV n=33 | FEP n=64 | SIPS NCV n=397 | SIPS CV n=32 | FEP n=65 |
| Current AP | 67 (16.8%)a | 21 (63.6%)a,c | 54 (84.4%)c | 81 (20.4%)a | 17 (53.1%)a | 39 (60.0%) |
| Current AT | 66 (16.6%)a | 21 (63.6%)a | 45 (70.3%) | 79 (19.9%)a | 16 (50.0%)a | 31 (47.7%) |
| Current CV | 1 (0.3%) | 1 (3.0%) | 9 (14.1%) | 1 (0.3%) | 1 (3.1%) | 9 (13.8%) |
| Current AD | 119 (29.9%) | 9 (27.3%) | 8 (12.5%) | 119 (30.0%) | 6 (18.8%) | 6 (9.2%) |
| Current MS | 11 (2.8%)c | 4 (12.1%)c | 3 (4.7%) | 23 (5.8%) | 2 (6.3%) | 7 (10.8%) |
| Current ST | 32 (8.0%) | 1 (3.0%) | 0 (0.0%) | 39 (9.8%) | 1 (3.1%) | 0 (0.0%) |
| Current BZ | 31 (7.8%) | 4 (12.1%) | 2 (3.1%) | 32 (8.1%) | 5 (15.6%)c | 2 (3.1%)c |
| Current NA | 4 (1.0%) | 2 (6.1%) | 0 (0.0%) | 3 (0.8%) | 0 (0.0%) | 2 (3.1%) |
| Current Any | 173 (43.5%)c | 22 (66.7%)c | 54 (84.4%)a | 187 (47.1%) | 17 (53.1%) | 42 (64.6%) |
| FU Time Months | na | na | na | 12.9±1.79 | 12.8±3.82 | 12.3±0.87 |
| Meds Time Months1 | 230±59 | 238±46b | 266±44b | 12.8±1.78 | 12.6±3.87 | 11.7±2.49 |
| Months AP1 | 1.24±5.37b | 6.68±9.96b,c | 2.84±5.25c | 2.45±4.37a | 5.87±4.96a | 7.52±4.67 |
| Months AT1 | 1.23±5.36b | 6.66±9.91b,a | 2.11±4.04a | 2.42±4.35a | 5.84±4.95a | 5.89±4.81 |
| Months CV1 | 0.01±0.13 | 0.02±0.13 | 0.89±3.63 | 0.03±0.35 | 0.17±0.80 | 1.52±3.33 |
| Months AD1 | 7.15±19.7 | 10.0±15.8a | 0.42±1.77a | 3.80±5.09c | 2.22±3.67c | 1.56±3.42 |
| Months MS1 | 1.00±6.66 | 2.23±7.40 | 0.73±3.75 | 0.68±2.52 | 0.65±2.37 | 1.02±3.12 |
| Months ST1 | 9.51±29.6 | 8.14±21.1b | 0.00±0.01b | 1.01±3.09a | 0.12±0.48a | 0.00±0.00 |
| Months BZ1 | 0.97±5.09 | 2.07±6.23c | 0.21±1.31c | 0.97±3.07 | 1.12±2.97 | 0.48±1.44 |
| Months NA1 | 0.08±1.25 | 0.35±1.90 | 0.04±0.31 | 0.07±0.73 | 0.06±0.24 | 0.27±1.28 |
| Current CPZ eq2 | 224±170c | 395±348c | 272±285 | 298±282 | 380±189 | 405±217 |
Note: SIPS=Structured Interview for Psychosis Risk Syndromes; SIPS CV=clinical high risk converters; SIPS NCV=clinical high risk non converters; FEP=first episode psychosis; AP=antipsychotic; AT=atypical; CV=conventional; AD=antidepressant; MS=mood stabilizer; ST=stimulant; BZ=benzodiazepine/hypnotic; NA=nonbenzodiazepine anxiolytic; CPZ eq=chlorpromazine equivalent.
groups with this label differ p<0.001, Fisher’s exact test or t-test
groups with this label differ p<0.01, Fisher’s exact test or t-test
groups with this label differ p<0.05, Fisher’s exact test or t-test na -- not available
Lifetime for baseline (n=397 for SIPS NCV for some measures, n=61–67 for FEP across measures), since baseline for one year (n=396 for SIPS NCV for some measures, n=60–67 for FEP across measures). If start or stop dates were missing for one or more specific medication type courses, the case was counted as missing for duration analysis.
Among those with current AP, at baseline and at one year. Dose missing for one CV and two FEP at one year. One extreme dose outlier(Tukey, 1977) for CV at one year excluded. Dose for FEP at baseline n=35.
At follow-up, accounted-for medication time in SIPS CVs was similar to both SIPS NCVs and FEPs. SIPS CVs differed from SIPS NCVs in having significantly higher rates of current AP and AT. Duration of use over follow-up was significantly longer in SIPS CVs as compared to NCV for AP, AT, and AD and was significantly shorter for ST. Compared to FEPs, frequency of current prescription and duration of psychotropic use for SIPS CVs did not differ in any category, except for higher rates of BZ prescription. CPZ equivalent doses in SIPS CVs did not differ from either comparator group.
3.4. Psychosocial treatment
As detailed in Table 3, at baseline, the three groups had comparable proportions of subjects who received “Any Therapy” within the prior 6 months. However, the number of sessions received varied, as the sample of SIPS CV had a significantly greater number than FEP enrollees.
Table 3.
Psychosocial Treatment at Baseline and One-year Follow-up
| Six Months before Baseline | One Year after Baseline | |||||
|---|---|---|---|---|---|---|
| Proportion Receiving Treatment N (%) | Proportion Receiving Treatment N (%) | |||||
| Variable | SIPS NCV n=397 | SIPS CV n=33 | FEP n=67 | SIPS NCV n=394 | SIPS CV n=33 | FEP n=67 |
| Any Therapy | 207 (52.1%) | 21 (63.6%) | 34 (50.8%) | 273(69.3%)ac | 17(51.5%)ac | 66 (98.5%)a |
| # of Sessions M ± SD | # of Sessions M ± SD | |||||
| Any Therapy | 6.47 ± 11.07 | 9.73 ± 16.91c | 4.61± 8.60c | 17.30± 26.00c | 12.03± 21.84b | 24.06 ± 15.18bc |
Note: SIPS CV= clinical high risk converters; SIPS NCV= clinical high risk non converters; FEP=first episode psychosis.
groups with this label differ p<0.001
groups with this label differ p<0.01
groups with this label differ p <.05
At one-year follow-up, the proportion of SIPS CV receiving “Any Therapy” was significantly lower than that in SIPS NCV and that in FEP enrollees. The number of sessions received also varied, as the sample of SIPS CV had a significantly lower number than those of the FEP enrollees.
3.5. Resource utilization
As detailed in Table 4, at baseline, the proportion of patients who reported an inpatient psychiatric hospitalization in the prior six months was similar across SIPS CV and NCV; however, a significantly lower proportion of SIPS CV had inpatient hospitalizations compared to FEP enrollees. Also, when hospitalized, SIPS CV averaged significantly fewer hospital nights compared to FEP enrollees.
Table 4.
Resources Utilization at Baseline and One-year Follow-up.
| Six Months before Baseline | One Year after Baseline | |||||
|---|---|---|---|---|---|---|
| Variable | SIPS NCV n=397 | SIPS CV n=33 | FEP* n=65 | SIPS NCV n=396 | SIPS CV n=33 | FEP N=67 |
| Any Psychiatric Hospitalization1 | 43 (10.8%) | 7 (21.2%)a | 51 (78.5%)a | 35 (8.8%)a | 11 (33.3%)a | 25 (37.3%) |
| # of Psychiatric Hospitalization | 0.13±0.38 | 0.18±0.39a | 1.14±1.26a | 0.12±0.43c | 0.73±1.38c | 0.66±1.02 |
| Psychiatric Hospital nights | 1.42±6.49 | 1.67±3.95a | 11.7±12.8a | 1.61±7.16c | 8.52±17.9c | 11.2±26.2 |
| Any Day Hospital1 | 8 (2.0%) | 2 (6.1%) | 8 (12.3%) | 11 (2.8%)c | 3 (9.1%)c | 3 (4.5%) |
| # of Day Hospital admission | 0.02±0.12 | 0.06±0.24 | 0.09±0.29 | 0.03±0.16 | 0.12±0.42 | 0.04±0.20 |
| Day Hospital visits | 0.34±3.14 | 2.55±10.7 | 1.31±4.94 | 1.15±9.82 | 3.27±11.0 | 0.63±3.44 |
| Any Emergency Room visit1 | 42 (10.5%) | 3 (9.1%) | 6 (9.2%) | 25 (6.3%)c | 6 (18.2%)b,c | 3 (4.5%)b |
| # of Emergency Room visits** | 0.13±0.41 | 0.09±0.29 | 0.11±0.35 | 0.07±0.21 | 0.21±0.48 | 0.06±0.29 |
Note: SIPS CV= clinical high risk converters; SIPS NCV= clinical high risk non converters; FEP=first episode psychosis.
groups with this label differ p<0.001, Fisher’s exact test or t-test
groups with this label differ p<0.01, Fisher’s exact test or t-test
groups with this label differ p<0.05, Fisher’s exact test or t-test
FEP: calculated on 65 (2 are missing).
One subject had one Emergency Room visit the lasted two nights. Five subjects had one Emergency Room visit. One subject had two visits in the Emergency Room.
At follow-up, SIPS CV and FEP groups showed a similar proportion of subjects who were admitted to inpatient units, with a non-statistically different length of hospital stay. By contrast, when compared to SIPS NCV, SIPS CV were more likely to be admitted to the hospital and for a significantly longer number of nights. Emergency room utilization occurred significantly more frequently in the SIPS CV group, when compared to SIPS NCV and also to the FEP sample. Number of day hospital treatment days was similar across groups.
4. Discussion
Although other aspects of validity have been previously studied (Sun, 2009; Takahashi et al., 2009; Walterfang et al., 2008; Wood, 2011; Yung et al., 2010; Yung et al., 2003), this study specifically investigates the predictive validity of the conversion assessment in the context of one year converter follow-up data, in comparison to a separately ascertained FEP sample. The principal findings are: 1) a large and similar majority of cases in both SIPS CV and FEP samples met criteria for continued psychosis at one-year follow-up; 2) SIPS CV had similar follow-up prescription rates of antipsychotic medication as compared to FEP cases and higher compared to SIPS NCVs; and 3) SIPS CV had similar rates of follow-up resource utilization as FEP in most categories and higher than SIPS NCV. These findings provide both convergent and discriminant support for the predictive validity of the SIPS conversion determination as an indicator of the onset of psychosis.
The findings are strengthened by evidence that our samples appear fairly typical of those reported in the literature. In our current FEP sample, 37% were hospitalized in the year after baseline, similar to 44% in our previous FEP sample (Srihari, 2015) and within the range reported by four other FEP samples focusing on one-year follow-up (12–59%) (Birchwood, 1992; Grawe, 1991; Gupta, 1997; Ucok, 2006). Three FEP studies report one-year rates of follow-up lifetime diagnoses whose presence at follow-up generally indicates that the original condition was transient and no longer present (brief, schizophreniform, or substance-induced psychosis) (Addington et al., 2006; Amini et al., 2005; Pope et al., 2013). Our FEP rate of no continued psychosis (15%) is within the range reported in these studies (2–29%). Our CHR converter sample received affective psychosis diagnoses at baseline in 12%, comparable to the 11% reported in a 2013 meta-analysis of CHR conversion diagnoses in 23 studies that did not include the present sample (Fusar-Poli et al., 2013a). Our CHR converter rate of schizophrenia spectrum conversion diagnoses (45%) was somewhat lower, however, and our rate of other psychoses (42%) was somewhat higher than the meta-analytic estimates (73% and 16%, respectively). Since other psychoses may show lower rates of continued psychosis (Figure 1), these differences if anything suggest that CHR converters on the whole may have somewhat higher rates of continued psychosis at follow-up than shown in our sample.
Other reports have previously addressed the post-conversion course of CHR converters, but methodologic issues relating to control groups or to the timing of conversion and follow-up assessments limit their comparison to our findings. One group reported that of 59 transitioned CHR patients, 38 (64%) met criteria for SCID current or lifetime psychotic disorder at follow-up(Lin et al., 2011). The precise duration of follow-up after conversion was not reported but appears to have been five years or longer on average. No FEP control group was included to evaluate the expected degree of diagnostic change. Another paper reported that the average course over three years of 24 CHR patients who converted at some time during the three-year follow-up was one of symptomatic and functional improvement rather than worsening (Hengartner et al., 2017). Ratings were timed relative to the CHR baseline rather than relative to time of conversion, however, so that the meaning of the average decline was unclear. The symptom and functioning means in the converter group at each follow-up time-point averaged a varying mixture of patient statuses: not yet converted, recently converted, and converted months or years earlier. Again the study did not provide for a FEP control group.
4.1. “Trivial” Conversions?
Despite the present evidence of the validity of the SIPS conversion determination in the sample as a group, could any of the individual SIPS conversions be considered “trivial?” Of the 33 SIPS converters followed for a year, only six subjects did not have continued psychosis at follow-up. Of these six, two had baseline diagnoses of affective psychosis. Of the remaining four (14% of 29), three (10%) were prescribed antipsychotics for four months or less during the one year follow-up period. These cases could be considered “trivial” (Yung et al., 2010), however, we note that if so, a similar proportion of our FEP cases would also need to be considered “trivial.” Eight or 12% of 67 of our FEP cases similarly did not show continued psychosis at follow-up and were prescribed antipsychotics for four months or less during the one year follow-up period. It is important to emphasize that these outcomes were assessed at one year, and that some of these “trivial” cases could experience distressing and disabling relapses over the longer term. Additionally, it is possible some patients may have become fully psychotic in between assessment points and this conversion was not detected. If these unobserved conversions did occur, then they could be considered “trivial.”
4.2. Additional Findings
The current analyses also revealed other findings of interest. SIPS CV were less likely to receive psychotherapy, and for fewer sessions, than NCV or FEP patients. While reported receipt of treatment can be considered a proxy for need for treatment, and thus for severity of illness, other factors should be considered. The SIPS CV versus FEP difference can be interpreted in the context of the assertive CSC treatment model available to everyone in the FEP comparator sample. By contrast, the research programs that followed CHR subjects through conversion to psychosis were not funded to provide treatment after conversion. Converters to psychosis were always referred for treatment in the community but may have faced particular challenges with access. Supporting this interpretation is the related finding that ER utilization over follow-up was higher in SIPS CV compared to FEP. In addition, SIPS CV were less likely to receive stimulant prescriptions relative to SIPS NCV, before and after the point of conversion to frank psychosis. Given reported risks of exacerbating psychotic symptoms with these medications, avoiding them may reflect appropriate pharmacologic management, although empirical data on the contribution of stimulants to psychosis specifically in CHR appear to be sparse.
4.3. Limitations
Limitations of the current study should be considered when evaluating the findings. First, the current study focused on SIPS-defined conversion to psychosis, whereas other instruments that capture psychosis risk and subsequent conversion, such as the CAARMS, might have yielded different results. However, a previous comparison has shown fairly good inter-instrument agreement on psychotic conversion rates despite some criterion variance (Fusar-Poli et al., 2016).
Second, the methodology used for determination of continued psychosis may have under-or over-estimated rates at which psychotic disorders were no longer present at follow-up in both samples. This concern stems from the SCID-I instrument querying about current psychotic symptoms but not directly specifying criteria for presence of current psychotic disorder. Future longitudinal work should more carefully document whether psychotic disorder is currently present at each assessment. The new SCID-5-RV does specify whether psychotic diagnoses are current or not, and also whether psychotic disorders are currently in full or partial remission (Addington et al., 2017).
Third, our analyses integrated two protocols that were designed for different purposes (i.e., NAPLS – longitudinal monitoring and prediction of conversion; STEP-FEP early intervention outcomes). Consequent limitations included the unequal sample sizes and different inclusion criteria, variability of power across pairwise comparisons, and the absence of social/role functioning data for the SIPS CV. Additionally, given treatment was not a part of the NAPLS study, the circumstances under which SIPS CV and NCV received antipsychotic medications were unknown.
Fourth, interpreting psychosocial treatment utilization as a proxy for illness severity carries limitations. Potential variability in access to post-conversion care for the SIPS CV could clearly impact utilization independent from clinical severity, as discussed above. Engagement in psychosocial treatment could also be a marker of those who had more insight and/or were higher functioning.
Lastly, the focus of the current paper on the validity of the conversion outcome should not be taken as endorsement of a notion that conversion is the only outcome of interest for CHR. In our view many other clinical outcomes of CHR are of interest, including functioning, remission, negative symptoms, cognition, depression, and anxiety.
SIPS-defined conversion to psychosis signaled a non-trivial change, as evidenced by diagnostic stability and severity of illness outcomes that are comparable to a FEP sample. Additional prospective comparisons of CHR converter and FEP samples are needed across domains of diagnosis, treatment access, resource utilization, and functioning.
Supplementary Material
ACKNOWLEDGEMENTS
The co-authors would like to acknowledge Philip Markovich and Shadie Burke for their efforts in data collection for this manuscript.
Funding
This study was supported by the NIMH (U01MH081984 to JA; U01 MH081928; P50 MH080272; Commonwealth of Massachusetts SCDMH82101008006 to LJS; R01 MH60720, U01 MH082022, and K24 MH76191 to KSC; U01MH081902 to TDC; P50 MH066286 (Prodromal Core) to CEB; U01MH082004 to DOP; grant U01MH081988 to EFW; U01MH082022 to SWW; UO1 MH081857-05 to BAC; and R01 MH103831 to VHS.) The NIMH had no further role in study design; collection, analysis, and interpretation of data; writing of the report; and the decision to submit the paper for publication.
This work was funded in part by the State of Connecticut, DMHAS, but this publication does not express the views of DMHAS or the State of Connecticut. The views and opinions expressed are those of the authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addington J, Cadenhead KS, Cornblatt BA, Mathalon DH, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Woods SW, Addington JA, Cannon TD, 2012. North American Prodrome Longitudinal Study (NAPLS 2): Overview and recruitment. Schizophrenia Research 142(1–3), 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington J, Chaves A, Addington D, 2006. Diagnostic stability over one year in first-episode psychosis. Schizophr Res 86(1–3), 71–75. [DOI] [PubMed] [Google Scholar]
- Addington J, Epstein I, Liu L, French P, Boydell KM, Zipursky RB, 2011. A randomized controlled trial of cognitive behavioral therapy for individuals at clinical high risk of psychosis. Schizophrenia Research 125(1), 54–61. [DOI] [PubMed] [Google Scholar]
- Addington J, Piskulic D, Liu L, Lockwood J, Cadenhead KS, Cannon TD, Cornblatt BA, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Bearden CE, Mathalon DH, Woods SW, 2017. Comorbid diagnoses for youth at clinical high risk of psychosis. Schizophrenia Research 10.1016/j.schres.2017.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen P, Azis M, Modinos G, Bossong MG, Bonoldi I, Samson C, Quinn B, Kempton MJ, Howes OD, Stone JM, Calem M, Perez J, Bhattacharayya S, Broome MR, Grace AA, Zelaya F, McGuire P, 2018. Increased Resting Hippocampal and Basal Ganglia Perfusion in People at Ultra High Risk for Psychosis: Replication in a Second Cohort. Schizophrenia bulletin 44(6), 1323–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini H, Alaghband-rad J, Omid A, Sharifi V, Davari-Ashtiani R, Momeni F, Aminipour Z, 2005. Diagnostic stability in patients with first-episode psychosis. Australas Psychiatry 13(4), 388–392. [DOI] [PubMed] [Google Scholar]
- Birchwood M, Cochrane R, Macmillan F, Copestake S, Kucharska J, Carriss M, 1992. The influence of ethnicity and family structure on relapse in first-episode schizophrenia. A comparison of Asia, Afro-Caribbean, and white patients. British journal of psychiatry 161, 783–790. [DOI] [PubMed] [Google Scholar]
- Bossong MG, Antoniades M, Azis M, Samson C, Quinn B, Bonoldi I, Modinos G, Perez J, Howes OD, Stone JM, Allen P, McGuire P, 2018. Association of Hippocampal Glutamate Levels with Adverse Outcomes in Individuals at Clinical High Risk for Psychosis. JAMA Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Chung Y, He G, Sun DQ, Jacobson A, van Erp TGM, McEwen S, Addington J, Bearden CE, Cadenhead K, Cornblatt B, Mathalon DH, McGlashan T, Perkins D, Jeffries C, Seidman LJ, Tsuang M, Walker E, Woods SW, Heinssen R, North Amer Prodrome Longitudinal, S., 2015. Progressive Reduction in Cortical Thickness as Psychosis Develops: A Multisite Longitudinal Neuroimaging Study of Youth at Elevated Clinical Risk. Biological Psychiatry 77(2), 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Chen OY, Chung Y, Forsyth JK, McEwen SC, Gee DG, Bearden CE, Addington J, Goodyear B, Cadenhead KS, Mirzakhanian H, Cornblatt BA, Carrion RE, Mathalon DH, McGlashan TH, Perkins DO, Belger A, Seidman LJ, Thermenos H, Tsuang MT, van Erp TGM, Walker EF, Hamann S, Anticevic A, Woods SW, Cannon TD, 2018. Cerebello-thalamo-cortical hyperconnectivity as a state-independent functional neural signature for psychosis prediction and characterization. Nature Communications 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrión RE, Walder DJ, Auther AM, McLaughlin D, Zyla HO, Adelsheim S, Calkins R, Carter CS, McFarland B, Melton R, Niendam T, Ragland JD, Sale TG, Taylor SF, McFarlane WR, Cornblatt BA, 2018. From the psychosis prodrome to the first-episode of psychosis: No evidence of a cognitive decline. Journal of Psychiatric Research 96, 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran CM, Carrillo F, Fernández-Slezak D, Bedi G, Klim C, Javitt DC, Bearden CE, Cecchi GA, 2018. Prediction of psychosis across protocols and risk cohorts using automated language analysis. World Psychiatry 17(1), 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C, Cipriani A, Ioannidis JPA, Radua J, Stahl D, Provenzani U, McGuire P, Fusar-Poli P, 2018a. Lack of evidence to favor specific preventive interventions in psychosis: a network meta-analysis. World Psychiatry 17(2), 196–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C, Radua J, Cipriani A, Stahl D, Provenzani U, McGuire P, Fusar-Poli P, 2018b. Efficacy and acceptability of interventions for attenuated positive psychotic symptoms in individuals at clinical high risk of psychosis: A network meta-analysis. Frontiers in Psychiatry 9(JUN). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutt A, Tseng HH, Fonville L, Drakesmith M, Su L, Evans J, Zammit S, Jones D, Lewis G, David AS, 2015. Exploring neural dysfunction in ‘clinical high risk’ for psychosis: A quantitative review of fMRI studies. Journal of Psychiatric Research 61, 122–134. [DOI] [PubMed] [Google Scholar]
- Egerton A, Stone JM, Chaddock CA, Barker GJ, Bonoldi I, Howard RM, Merritt K, Allen P, Howes OD, Murray RM, McLean MA, Lythgoe DJ, O’Gorman RL, McGuire PK, 2014. Relationship between brain glutamate levels and clinical outcome in individuals at ultra high risk of psychosis. Neuropsychopharmacology 39(12), 2891–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Bechdolf A, Taylor MJ, Bonoldi I, Carpenter WT, Yung AR, McGuire P, 2013a. At risk for schizophrenic or affective psychoses? A meta-analysis of DSM/ICD diagnostic outcomes in individuals at high clinical risk. Schizophrenia Bulletin 39(4), 923–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, Barale F, Caverzasi E, McGuire P, 2012. Predicting psychosis: Meta-analysis of transition outcomes in individuals at high clinical risk. Archives of General Psychiatry 69(3), 220–229. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Borgwardt S, Bechdolf A, Addington J, Riecher-Rössler A, Schultze-Lutter F, Keshavan M, Wood S, Ruhrmann S, Seidman LJ, Valmaggia L, Cannon T, Velthorst E, De Haan L, Cornblatt B, Bonoldi I, Birchwood M, McGlashan T, Carpenter W, McGorry P, Klosterkötter J, McGuire P, Yung A, 2013b. The psychosis high-risk state: A comprehensive state-of-the-art review. Archives of General Psychiatry 70(1), 107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Cappucciati M, Rutigliano G, Lee TY, Beverly Q, Bonoldi I, Lelli J, Kaar SJ, Gago E, Rocchetti M, Patel R, Bhavsar V, Tognin S, Badger S, Calem M, Lim K, Kwon JS, Perez J, McGuire P, 2016. Towards a Standard Psychometric Diagnostic Interview for Subjects at Ultra High Risk of Psychosis: CAARMS versus SIPS. Psychiatry J 2016, 7146341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Cappucciati M, Rutigliano G, Schultze-Lutter F, Bonoldi I, Borgwardt S, Riecher-Rossler A, Addington J, Perkins D, Woods SW, McGlashan TH, Lee J, Klosterkotter J, Yung AR, McGuire P, 2015. At risk or not at risk? Meta-analysis of the prognostic accuracy of psychometric interviews for psychosis prediction. World Psychiatry 14, 322–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Carpenter W, Woods S, McGlashan T, 2014a. Attenuated psychosis syndrome: ready for DSM-5.1? Annual review of clinical psychology 10, 155–192. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Van Os J, 2013. Lost in transition: setting the psychosis threshold in prodromal research. Acta psychiatrica Scandinavica 127(3), 248–252. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Yung AR, McGorry P, Van Os J, 2014b. Lessons learned from the psychosis high-risk state: Towards a general staging model of prodromal intervention. Psychological Medicine 44(1), 17–24. [DOI] [PubMed] [Google Scholar]
- Grawe R, Levander S, Kruger M, 1991. Incidence, clinical characteristics, and short-term outcome of first-episode schizophrenia. Nordic Journal of Psychiatry 45, 383–390. [Google Scholar]
- Gupta S, Andreasen NC, Arndt S, Flaum M, Hubbard WC, Ziebell S, 1997. The Iowa Longitudinal Study of Recent Onset Psychosis: one-year follow-up of first episode patients. Schizophrenia Research 23, 1–13. [DOI] [PubMed] [Google Scholar]
- Hengartner MP, Heekeren K, Dvorsky D, Walitza S, Rössler W, Theodoridou A, 2017. Course of psychotic symptoms, depression and global functioning in persons at clinical high risk of psychosis: Results of a longitudinal observation study over three years focusing on both converters and non-converters. Schizophrenia Research. [DOI] [PubMed] [Google Scholar]
- Ho NF, Holt DJ, Cheung M, Iglesias JE, Goh A, Wang M, Lim JK, De Souza J, Poh JS, See YM, Adcock AR, Wood SJ, Chee MW, Lee J, Zhou J, 2017. Progressive Decline in Hippocampal CA1 Volume in Individuals at Ultra-High-Risk for Psychosis Who Do Not Remit: Findings from the Longitudinal Youth at Risk Study. Neuropsychopharmacology 42(6), 1361–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Bose SK, Turkheimer F, Valli I, Egerton A, Valmaggia LR, Murray RM, McGuire P, 2011. Dopamine synthesis capacity before onset of psychosis: A prospective (18)F -DOPA PET imaging study. American Journal of Psychiatry 168(12), 1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagannath V, Theodoridou A, Gerstenberg M, Franscini M, Heekeren K, Correll CU, Rössler W, Grünblatt E, Walitza S, 2017. Prediction analysis for transition to schizophrenia in individuals at clinical high risk for psychosis: The relationship of DAO, DAOA, and NRG1 variants with negative symptoms and cognitive deficits. Frontiers in Psychiatry 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffries CD, Perkins DO, Chandler SD, Stark T, Yeo E, Addington J, Bearden CE, Cadenhead KS, Cannon TD, Cornblatt BA, Mathalon DH, McGlashan TH, Seidman LJ, Walker EF, Woods SW, Glatt SJ, Tsuang M, 2016. Insights into psychosis risk from leukocyte microRNA expression. Translational Psychiatry 6(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffries CD, Perkins DO, Fournier M, Do KQ, Cuenod M, Khadimallah I, Domenici E, Addington J, Bearden CE, Cadenhead KS, Cannon TD, Cornblatt BA, Mathalon DH, McGlashan TH, Seidman LJ, Tsuang M, Walker EF, Woods SW, 2018. Networks of blood proteins in the neuroimmunology of schizophrenia. Translational Psychiatry 8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike S, Takano Y, Iwashiro N, Satomura Y, Suga M, Nagai T, Natsubori T, Tada M, Nishimura Y, Yamasaki S, Takizawa R, Yahata N, Araki T, Yamasue H, Kasai K, 2013. A multimodal approach to investigate biomarkers for psychosis in a clinical setting: The integrative neuroimaging studies in schizophrenia targeting for early intervention and prevention (IN-STEP) project. Schizophrenia Research 143(1), 116–124. [DOI] [PubMed] [Google Scholar]
- Koutsouleris N, Kambeitz-Ilankovic L, Ruhrmann S, Rosen M, Ruef A, Dwyer DB, Paolini M, Chisholm K, Kambeitz J, Haidl T, Schmidt A, Gillam J, Schultze-Lutter F, Falkai P, Reiser M, Riecher-Rössler A, Upthegrove R, Hietala J, Salokangas RKR, Pantelis C, Meisenzahl E, Wood SJ, Beque D, Brambilla P, Borgwardt S, 2018. Prediction Models of Functional Outcomes for Individuals in the Clinical High-Risk State for Psychosis or with Recent-Onset Depression: A Multimodal, Multisite Machine Learning Analysis. JAMA Psychiatry 75(11), 1156–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepock JR, Mizrahi R, Korostil M, Bagby RM, Pang EW, Kiang M, 2018. Event-related potentials in the clinical high-risk (CHR) state for psychosis: A systematic review. Clinical EEG and Neuroscience 49(4), 215–225. [DOI] [PubMed] [Google Scholar]
- Lin A, Nelson B, Yung AR, 2012. ‘At-risk’ for psychosis research: where are we heading? Epidemiology and Psychiatric Sciences 21(4), 329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A, Wood SJ, Nelson B, Brewer WJ, Spiliotacopoulos D, Bruxner A, Broussard C, Pantelis C, Yung AR, 2011. Neurocognitive predictors of functional outcome two to 13years after identification as ultra-high risk for psychosis. Schizophrenia Research 132(1), 1–7. [DOI] [PubMed] [Google Scholar]
- Liu C-C, Lai M-C, Liu C-M, Chiu Y-N, Hsieh MH, Hwang T-J, Chien Y-L, Chen WJ, Hua M-S, Hsiung P-C, Huang Y-C, Hwu H-G, 2011. Follow-up of subjects with suspected pre-psychotic state in Taiwan. Schizophrenia Research 126(1–3), 65–70. [DOI] [PubMed] [Google Scholar]
- McFarlane WR, Levin B, Travis L, Lucas FL, Lynch S, Verdi M, Williams D, Adelsheim S, Calkins R, Carter CS, Cornblatt B, Taylor SF, Auther AM, McFarland B, Melton R, Migliorati M, Niendam T, Ragland JD, Sale T, Salvador M, Spring E, 2015. Clinical and functional outcomes after 2 years in the early detection and intervention for the prevention of psychosis multisite effectiveness trial. Schizophrenia Bulletin 41(1), 30–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan TH, Walsh BC, Woods SW, 2010. The Psychosis-Risk Syndrome: Handbook for Diagnosis and Follow-up. Oxford University Press, New York. [Google Scholar]
- McGorry PD, Hartmann JA, Spooner R, Nelson B, 2018. Beyond the “at risk mental state” concept: transitioning to transdiagnostic psychiatry. World Psychiatry 17(2), 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGorry PD, Killackey E, Yung A, 2008. Early intervention in psychosis: concepts, evidence and future directions. World Psychiatry 7(3), 148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K, Woods SW, 2002. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry 159(5), 863–865. [DOI] [PubMed] [Google Scholar]
- Modinos G, Simsek F, Horder J, Bossong M, Bonoldi I, Azis M, Perez J, Broome M, Lythgoe DJ, Stone JM, Howes OD, Murphy DG, Grace AA, Allen P, McGuire P, 2018. Cortical GABA in subjects at ultra-high risk of psychosis: Relationship to negative prodromal symptoms. International Journal of Neuropsychopharmacology 21(2), 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHS England MH, 2016. Guidance and FAQs for reporting against access and waiting time standards: Children and Young People with an Eating Disorder, Early Intervention in Psychosis.
- Pope MA, Joober R, Malla AK, 2013. Diagnostic stability of first-episode psychotic disorders and persistence of comorbid psychiatric disorders over 1 year. Canadian Journal of Psychiatry 58(10), 588–594. [DOI] [PubMed] [Google Scholar]
- Powers III AR, Addington J, Perkins DO, Bearden CE, Cadenhead KS, Cannon TD, Cornblatt BA, Mathalon DH, Seidman LJ, Tsuang MT, Walker EF, McGlashan TH, Woods SW, under revision. Duration of the psychosis prodrome. Schizophrenia Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers III AR, McGlashan TH, Woods SW, in press. Clinical Phenomenology of the Prodrome for Psychosis, in: Tamminga C, van Os J, Ivieva E, Reininghaus U (Eds.), Psychotic Disorders: Comprehensive Conceptualization and Treatments. Oxford University Press, New York. [Google Scholar]
- Raballo A, Monducci E, Ferrara M, Nastro PF, Dario C, 2018. Developmental vulnerability to psychosis: Selective aggregation of basic self-disturbance in early onset schizophrenia. Schizophrenia research. [DOI] [PubMed] [Google Scholar]
- Rosenheck RA, Lieberman JA, 2007. Cost-effectiveness measures, methods, and policy implications from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) for schizophrenia. J Clin Psychiatry 68(2), e05. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Crossley NA, Harrisberger F, Smieskova R, Lenz C, Riecher-Rössler A, Lang UE, McGuire P, Fusar-Poli P, Borgwardt S, 2017. Structural network disorganization in subjects at clinical high risk for psychosis. Schizophrenia Bulletin 43(3), 583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Armando M, Pontillo M, Vicari S, Debbané M, Schultze-Lutter F, Eliez S, 2016. Ultra high risk status and transition to psychosis in 22q11.2 deletion syndrome. World Psychiatry 15(3), 259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Shapiro DI, Stone WS, Woodberry KA, Ronzio A, Cornblatt BA, Addington J, Bearden CE, Cadenhead KS, Cannon TD, Mathalon DH, McGlashan TH, Perkins DO, Tsuang MT, Walker EF, Woods SW, 2016. Association of neurocognition with transition to psychosis: Baseline functioning in the second phase of the north American prodrome longitudinal study. JAMA Psychiatry 73(12), 1239–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj S, Bloomfield PS, Cao B, Veronese M, Turkheimer F, Howes OD, 2018. Brain TSPO imaging and gray matter volume in schizophrenia patients and in people at ultra high risk of psychosis: An [11C]PBR28 study. Schizophrenia Research 195, 206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AE, Grädel M, Cattapan-Ludewig K, Gruber K, Ballinari P, Roth B, Umbricht D, 2012. Cognitive functioning in at-risk mental states for psychosis and 2-year clinical outcome. Schizophrenia Research 142(1–3), 108–115. [DOI] [PubMed] [Google Scholar]
- Srihari VH, Tek C, Pollard J, Zimmet S, Keat J, Cahill JD, Kucukgoncu S, Walsh BC, Li F, Gueorguieva R, Levine N, Mesholam-Gately RI, Friedman-Yakoobian M, Seidman LJ, Keshavan MS, McGlashan TH, Woods SW, 2014. Reducing the duration of untreated psychosis and its impact in the U.S.: the STEP-ED study. BMC Psychiatry 14, 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srihari VH, Tek C, Kucukgoncu S, Phutane V, Breitborde NJ, Pollared J, Ozhan B, Saksa J, Walsh B, Woods SW, 2015. First-episode services for psychotic disorders in the US Public Sector: A pragmatic randomized controlled trial. Psychiatric Services 66, 705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Phillips L, Velakoulis D, Yung A, McGorry PD, Wood SJ, van Erp TGM, Thompson PM, Toga AW, Cannon TD, & Pantelis C, 2009. Progressive brain structural changes mapped as psychosis develops in ‘at risk’ individuals. Schizophrenia Research 108, 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Wood SJ, Yung AR, Soulsby B, McGorry PD, Suzuki M, Kawasaki Y, Phillips LJ, Velakoulis D, Pantelis C, 2009. Progressive Gray Matter Reduction of the Superior Temporal Gyrus During Transition to Psychosis. Jama Psychiat 66(4), 366–376. [DOI] [PubMed] [Google Scholar]
- Tso IF, Taylor SF, Grove TB, Niendam T, Adelsheim S, Auther A, Cornblatt B, Carter CS, Calkins R, Ragland JD, Sale T, McFarlane WR, 2017. Factor analysis of the Scale of Prodromal Symptoms: data from the Early Detection and Intervention for the Prevention of Psychosis Program. Early Intervention in Psychiatry 11(1), 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukey JW, 1977. Exploratory Data Analysis. Addison-Wesley, Reading, MA. [Google Scholar]
- Ucok A, Polat A, Cakir S, Genc A, 2006. One year outcome in first episode schizophrenia. Predictors of relapse. European Archives of Psychiatry and Clinical Neuroscience 256, 37–43. [DOI] [PubMed] [Google Scholar]
- US Substance Abuse and Mental Health Services Administration, 2018. Community Programs for Outreach and Intervention with Youth and Young Adults at Clinical High Risk for Psychosis, SM-18–012.
- Valmaggia LR, Byrne M, Day F, Broome M, Johns L, Howes O, Power P, Badger S, Fusar-Poli P, McGuire P, 2015. Duration of untreated psychosis and need for admission in patients who engage with mental health services in the prodromal phase. British journal of psychiatry 207(2), 130–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os J, Guloksuz S, 2017. A critique of the “ultra-high risk” and “transition” paradigm. World Psychiatry 16(2), 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walterfang M, McGuire P, Yung A, Phillips LJ, Velakoulis D, Wood SJ, Suckling J, Bullmore ET, Brewer W, Soulsby B, Desmond P, McGorry P, Pantelis C, 2008. White matter volume changes in people who develop psychosis. British journal of psychiatry 193(3), 210–215. [DOI] [PubMed] [Google Scholar]
- Waltz JA, Demro C, Schiffman J, Thompson E, Kline E, Reeves G, Xu Z, Gold J, 2015. Reinforcement Learning Performance and Risk for Psychosis in Youth. Journal of Nervous and Mental Disease 203(12), 919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SJ, Yung AR, McGorry PD, & Pantelis C, 2011. Neuroimaging and treatment evidence for clinical staging in psychotic disorders: From the at-risk mental state to chronic schizophrenia. Biol Psychiatry(70), 619–625. [DOI] [PubMed] [Google Scholar]
- Woods SW, Addington J, Bearden CE, Cadenhead KS, Cannon TD, Cornblatt BA, Mathalon DH, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, McGlashan TH, 2013. Psychotropic medication use in youth at high risk for psychosis: Comparison of baseline data from two research cohorts 1998–2005 and 2008–2011. Schizophrenia Research 148(1–3), 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SW, Addington J, Cadenhead KS, Cannon TD, Cornblatt BA, Heinssen R, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, McGlashan TH, 2009. Validity of the prodromal risk syndrome for first psychosis: Findings from the north american prodrome longitudinal study. Schizophrenia Bulletin 35(5), 894–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SW, Miller TJ, McGlashan TH, 2001. The “prodromal” patient: both symptomatic and at-risk. CNS Spectr 6(3), 223–232. [DOI] [PubMed] [Google Scholar]
- Woods SW, Walsh BC, Powers III AR, McGlashan TH, 2019. Reliability, validity, epidemiology, and cultural variation of the Structured Interview for Psychosis-risk Syndromes (SIPS) and the Scale Of Psychosis-risk Symptoms (SOPS) in: Li H, S. D Seidman LJ (Ed.), Handbook of Attenuated Psychosis Syndrome in Youth and Young Adults: Early Identification and Intervention Across Cultures. . Springer, New York, pp. 85–113. [Google Scholar]
- Woods SW, Walsh BC, Powers III AR, McGlashan TH, in press. Reliability, validity, epidemiology, and cultural variation of the Structured Interview for Psychosis-risk Syndromes (SIPS) and the Scale Of Psychosis-risk Symptoms (SOPS), in: Li H, Shapiro DI, Seidman LJ (Eds.), International Handbook of Attenuated Psychosis Syndrome in Youth and Young Adults: Early Identification and Intervention Across Cultures. Springer, New York. [Google Scholar]
- Yung A, Phillips L, McGorry PD, 2004a. Treating Schizophrenia in the Prodromal Phase. Taylor & Francis, London. [Google Scholar]
- Yung AR, 1998. Prediction of psychosis A step towards indicated prevention of schizophrenia. British journal of psychiatry 172(s33), 14–20. [PubMed] [Google Scholar]
- Yung AR, 2017. Treatment of people at ultra-high risk for psychosis. World Psychiatry 16(2), 207–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung AR, McGorry PD, McFarlane CA, Jackson HJ, Patton GC, Rakkar A, 1996. Monitoring and care of young people at incipient risk of psychosis. Schizophrenia Bulletin 22(2), 283–303. [DOI] [PubMed] [Google Scholar]
- Yung AR, Nelson B, Thompson A, Wood SJ, 2010. The psychosis threshold in Ultra High Risk (prodromal) research: is it valid? Schizophr Res 120(1–3), 1–6. [DOI] [PubMed] [Google Scholar]
- Yung AR, Phillips LJ, Yuen HP, Francey SM, McFarlane CA, Hallgren M, McGorry PD, 2003. Psychosis prediction: 12-month follow up of a high-risk (“prodromal”) group. Schizophr Res 60(1), 21–32. [DOI] [PubMed] [Google Scholar]
- Yung AR, Phillips LJ, Yuen HP, McGorry PD, 2004b. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr Res 67(2–3), 131–142. [DOI] [PubMed] [Google Scholar]
- Yung AR, Yuen HP, McGorry PD, Phillips LJ, Kelly D, Dell’Olio M, Francey SM, Cosgrave EM, Killackey E, Stanford C, Godfrey K, Buckby J, 2005. Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust N Z J Psychiatry 39(11–12), 964–971. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.