Abstract
Bodily sensations are closely linked to emotional experiences. However, most research assessing the body-emotion link focuses on young adult samples. Inspired by prior work showing age-related declines in autonomic reactivity and interoception, we present two studies investigating age-related differences in the extent to which adults (18–75 years) associate interoceptive or internal bodily sensations with emotions. Study 1 (N=150) used a property association task to assess age effects on adults’ tendencies to associate interoceptive sensations, relative to behaviors or situations, with negative emotion categories (e.g., anger, sadness). Study 2 (N=200) used the Day Reconstruction experience sampling method to assess the effect of age on adults’ tendencies to report interoceptive sensations and emotional experiences in daily life. Consistent with prior literature suggesting that older adults have more muted physiological responses and interoceptive abilities than younger adults, we found that older adults’ mental representations (Study 1) and self-reported experiences (Study 2) of emotion are less associated with interoceptive sensations than are those of younger adults. Across both studies, age effects were most prominent for high arousal emotions (e.g., anger, fear) and sensations (e.g., racing heart) that are often associated with peripheral psychophysiological concomitants in young adults. These findings are consistent with psychological constructionist models and a “maturational dualism” account of emotional aging, suggesting additional pathways by which emotions may differ across adulthood.
Keywords: aging, emotional experience, emotion concepts, development, interoception
Interoceptive sensations—those flutters, pangs, gurgles, flushes, and tightening sensations felt in the body—are closely tied to emotional experiences. In common parlance, people describe these sensations as key to emotions: hearts are said to race with excitement, palms sweat with anxiety, and faces blush with embarrassment (Kövecses, 2000). Similarly, when asked to draw where in the body emotions occur, people across the world associate emotions with an array of bodily sensations (Nummenmaa, Glerean, Hari, & Hietanen, 2014; Nummenmaa, Hari, Hietanen, & Glerean, 2018). Although there are long-standing debates (e.g., Cannon, 1927; James, 1890) about whether emotions cause peripheral changes or vice versa, few researchers would disagree that emotional experiences involve the body in some capacity. Objective peripheral nervous system measures (e.g., shifts in heart rate, respiration, blood pressure, gastric motility) confirm that visceral bodily changes generally accompany emotional experiences (although evidence for autonomic differentiation or links between specific emotions and specific bodily changes are less clear; e.g., Blascovich & Mendes, 2010; Cacioppo, Berntson, Larsen, Poehlmann, & Ito, 2000; Laird & Lacasse, 2013; Siegel et al., 2018).
Neuroimaging meta-analyses of emotion similarly reveal increased activation in brain areas associated with visceromotor control of the peripheral nervous system and motor outputs (e.g., ventromedial prefrontal cortex, amygdala, basal ganglia, periaqueductal gray; for meta-analyses, see Lindquist, Satpute, Wager, Weber, & Barrett, 2016; Lindquist, Wager, Kober, Bliss-Moreau, & Barrett, 2012; Vytal & Hamann, 2010). Other evidence examines how afferent visceral signals can influence emotion (e.g., Craig, 2003; Garfinkel et al., 2014; Kleckner et al., 2017). Indeed, greater interoceptive ability 1 or the perception of on-going visceral changes is associated with more intense and more highly aroused emotions (Barrett, Quigley, Bliss-Moreau, & Aronson, 2004; Critchley, Wiens, Rotshtein, Öhman, & Dolan, 2004; Schulz & Vögele, 2015). Reducing the intensity of peripheral changes through pharmacological blockade can correspondingly reduce the intensity of emotional experiences, particularly the experience of negative, high arousal emotions during stress (MacCormack, Armstrong-Carter, et al., 2019).
Together, these findings are consistent with psychological constructionist and active inference models of emotion, which hypothesize that on-going changes in the peripheral body actively contribute to the creation of emotional experiences (Allen, Levy, Parr, & Friston, 2019; Barrett, 2017; Barrett & Bliss-Moreau, 2009; Critchley & Garfinkel, 2017; MacCormack & Lindquist, 2017; Seth, 2013). In particular, constructionist approaches hypothesize that people experience emotions when the brain uses knowledge accumulated from prior experiences to make a situated prediction that on-going afferent information from the body has emotional meaning; relative shifts in the amount or quality of afferent information received should in turn alter the nature of emotional experience. Yet, to date, most research on the body’s role in emotion investigates young adult samples. The processes that produce emotions are not immutable across the life span (Davidson, 2003); similarly, the body’s role in emotion may differ with age (Mendes, 2010). The present report addresses for the first time whether adults’ associations between emotions and interoceptive sensations (Study 1) and self-reports of emotion-related interoceptive sensations (Study 2) decrease with increasing age.
The Body in Emotion Across Adulthood
Initial studies on peripheral nervous system structure and function hint that the role of the body in emotion may diminish with age. For example, during healthy physical aging, peripheral nerve myelination decreases, ultimately driving age-related declines in nerve conduction velocity, sensory discrimination, and autonomic responding (Verdú, Ceballos, Vilches, & Navarro, 2000). In healthy adults, these peripheral declines begin emerging in midlife (around age 45) and become increasingly pronounced into old age (Palve & Palve, 2018), meaning that the brain can less efficiently transmit and receive information from the periphery during emotions and other states starting around mid-life. Consistent with the declines observed in the structure and function of peripheral nerves, older adults exhibit reduced autonomic reactivity (e.g., heart rate, skin conductance) during emotion inductions compared to younger adults (e.g., Tsai, Levenson, & Carstensen, 2000; see Uchino, Birmingham, & Berg, 2010 for meta-analysis). Similarly, older adults perform worse on tasks assessing interoceptive ability (Khalsa, Rudrauf, & Tranel, 2009; Murphy, Geary, Millgate, Catmur, & Bird, 2018). Neurally, there are further age-related differences in the functional activation of brain regions involved in both marshaling visceromotor changes in the body (e.g., the amygdala) and brain regions involved in representing and detecting those autonomic changes (e.g., the insula; Good et al., 2001; Moriguchi et al., 2011; Raz et al., 2005; for meta-analysis, see MacCormack, Stein, et al., 2019).
These findings occur alongside well-documented shifts in healthy older adults’ self-reported emotional experiences. Healthy older adults (>60 years) generally report experiencing more intense and frequent positive emotions, fewer and less intense negative emotions, greater emotion regulation success, and greater equanimity during interpersonal conflicts compared to younger adults (for reviews, see Isaacowitz & Livingstone, 2015; Mather, 2012; Mather & Carstensen, 2005; Urry & Gross, 2010). To date, these differences in self-reports have been largely attributed to age-related changes in motivation and regulatory expertise (e.g., Carstensen, Isaacowitz, & Charles, 1999; Labouvie-Vief, DeVoe, & Bulka, 1989), with the ideas being that (1) older adults want to experience more positive and fewer negative emotions as the end of life draws near and (2) older adults possess a lifetime’s worth of emotional expertise and skills with which to meet these goals. A complementary explanation is that age-related physical changes to the peripheral nervous system and brain could also cause emotions to involve fewer, less intense internal bodily sensations, resulting in “maturational dualism” (Mendes 2010).
The Present Studies
Although prior work has focused on both age differences in interoceptive ability and peripheral nervous system structure and function in the context of emotion, to our knowledge no studies have yet determined whether there are age differences in adults’ knowledge and reported experiences of interoceptive sensations during emotion. For example, work with younger adults demonstrates that people vary in their tendency to think about and report interoceptive sensations in relation to emotion (Garfinkel et al., 2015; Oosterwijk & Barrett, 2014); there are also both cross-cultural and individual differences in which sensations people tend to associate with specific emotions (e.g., Breugelmans et al., 2005; O’Brien, Oosterwijk, & Barrett, 2016).
We wanted to examine whether increasing age is linked with adults’ decreased tendency to associate emotions with interoceptive sensations and to self-report less intense experiences of interoceptive sensations during episodes of emotion in daily life. If indeed peripheral physiology and interoceptive processes decline with age, one hypothesis is that adults’ conceptual associations for emotions and their self-reported experiences should show similar evidence of maturational dualism effects. Rather than focusing on age as a categorical variable comparing older vs. younger adults. we opted to recruit samples that varied in age from early adulthood throughout midlife into the retirement years (e.g., 60s and 70s), drawing on evidence that aging exists as a continuum (Song & Johnson, 2018; Sun et al., 2016; Wilson et al., 2018), that age-related physiological decrements are observed as early as mid-life (Palve & Palve, 2018), and that prior studies find both linear vs. curvilinear effects of age on emotion across adulthood (e.g., Carstensen et al., 2000; Mroczek & Kolarz, 1998; Schilling, Wahl, & Wiegering, 2013).
Study 1 used a property association task from cognitive psychology to examine the nature of emotion concept associations across adulthood (ages 18–75). In typical property association tasks, participants rate how much a given property (e.g., red) relates to a given concept (e.g., apple), which reveals information about participants’ representations of different categories (e.g., cats, apples; Kosslyn, 1976; Simmons et al., 2007). In emotion research, property association tasks have been used to compare the extent to which internally-focused associations (including, but not limited to interoceptive sensations) vs. externally-focused associations (nonverbal behaviors) are associated with emotion categories (Oosterwijk et al., 2015; Oosterwijk et al., 2012). Building on this past work, participants in Study 1 rated how much a given emotion-relevant interoceptive sensation (e.g., heart racing), came to mind when thinking of a specific negative emotion category (e.g., how much does heart racing come to mind when thinking about anger). In comparison, participants also rated the extent to which emotion-relevant behaviors (e.g., clenched fists) and situations (e.g., being insulted) came to mind. We hypothesized that, given maturational dualism effects, there should be age-related differences in associations between emotion categories and interoceptive sensations, but not necessarily between emotion categories and behaviors or situations.
In Study 2, we investigated whether age-related differences in the association between interoceptive sensations and emotions would extend into self-reported daily experience. Prior work shows that asking participants to make summary judgments about their experiences can lead individuals to rely on mental representations like those we tested in Study 1, rather than actual experience (Barrett, Robin, Pietromonaco, & Eyssell, 1998; Robinson & Clore, 2002). Thus, Study 2 used a method that helps limit reliance on conceptual knowledge, in hopes of better capturing experience. Individuals aged 18–67 completed the Day Reconstruction Method (Kahneman, Krueger, Schkade, Schwarz, & Stone, 2004) in which they reported the emotion-relevant interoceptive sensations, behaviors, and situations they experienced during the prior day. As in Study 1, we included emotional behaviors and situations as comparisons for interoceptive sensations, hypothesizing that older adults would report less intense interoceptive sensations (especially high arousal sensations) relative to younger and middle-aged adults, with no age effect for emotional situations and perhaps less, if any, age effect for behaviors.
Study 1
As a first step, in Study 1, we measured age-related differences in mental representations of emotion by assessing the properties that participants associate with specific negative emotion categories. Emotion representations are acquired in early childhood (e.g., Castro, Halberstadt, & Garrett-Peters, 2016; Pons, Lawson, Harris, & de Rosnay, 2003; Widen & Russell, 2010), but are not static. They change with shifting experiences throughout early childhood and adolescence and even into adulthood (Doyle & Lindquist, 2018; Lebois, Wilson-Mendenhall, Simmons, Barrett, & Barsalou, 2018; Nook et al., 2018, in press). Indeed, encountering novel instances of an emotion category can update and shift category representations in adults (Doyle & Lindquist, 2018). We thus reasoned that age-related shifts in emotional experiences might be reflected as differences in adults’ mental representations for emotion categories.
To measure emotion representations, we assessed the extent to which adults associated different features of emotional experience, including interoceptive sensations (e.g., heart racing), behaviors (e.g., clenched fists), and situations (e.g., insulted), with different emotion categories (e.g., anger). To identify a set of interoceptive sensations, behaviors, and situations that adults across the age-span readily associate with certain emotion categories, we conducted a pilot study (N= 170, aged 18–72; Table 1). We focused on the negative emotions of anger, fear, disgust, sadness and boredom because these emotions are prototypically experienced as high vs. low in arousal (i.e., anger, fear, disgust vs. sadness, boredom; Yik, Russell, & Barrett, 1999). Hereafter we refer to these as “high arousal” and “low arousal” emotions based on their average or prototypical features in Western samples, although we note that there is important within-category variance in how much arousal and valence are associated with an emotion category across instances (Wilson-Mendenhall, Barrett, & Barsalou, 2013).
Table 1.
Full list of original vs. validated emotion category properties by modality.
| Interoceptive Properties | Behavioral Properties | Situational Properties | |||
|---|---|---|---|---|---|
| Aching s | Loose limbs | Aggressive | Loud a | Abandoned s | Incompetent a |
| Agitated | Low | Aloof | Lurch d | Ambiguous b | Inferior s |
| Blood pumping a | Muscle knots f | Antagonist | Mind-wandering | Beaten | Inhibited |
| Breathless f | Nauseous d | Approach f | Moaning s | Blame | Injustice a |
| Cold | Numb s | Argumentative | Mumbling s | Broken | Insulted a |
| Contraction | Pain s | Arrogant | Outward focused | Cheated a | Intolerable s |
| Dazed | Pale f | Avoidant f | Protective f | Comfortable | Lonely s |
| Dizzy f | Red-faced a | Careful f | Push away a | Competitive | Loss s |
| Down | Relaxed | Careless a | Ready to act | Critical | Mistreated a |
| Drained s | Restless | Cautious f | Resistant f | Cruel a | Mistrust |
| Droopy | Scalp prickles f | Clenched fists | Retch | Danger f | Monotonous |
| Drowsy b | Sick d | Confront | Retreat f | Defeated s | Nonchalant |
| Empty | Sleepy b | Contemplative b | Running f | Defensive | Oppressed a |
| Exhausted s | Sluggish b | Cover mouth | Sarcastic a | Defiant | Persecuted a |
| Faint f | Soft | Crying | Screaming a | Deflated | Putrid |
| Fatigued s | Stomach butterflies f | Curled lip | Scrunch nose | Degraded s | Rejection s |
| Flushed a | Sunken | Falling | Seek comfort s | Dependent | Submissive |
| Full | Sweating f | Fidgety | Shout | Despicable d | Superior |
| Goosebumps f | Tense f | Fight | Shrink back | Difficult | Thwarted a |
| Hard | Tightness a | Fixed gaze a | Sighing b | Disengaged | Uncertain f |
| Head rush a | Tingly f | Freeze in place | Silent s | Disgraced a | Unclear |
| Heartbeat f | Tired b | Frowning s | Sitting b | Distant | Uncomfortable |
| Heavy limbs | Turned stomach d | Furrowed brow | Snarl | Dull | Unexpected |
| Hollow | Warm f | Gag | Squint d | Explosive a | Unfriendly |
| Hot a | Weak s | Grimace | Staring a | Failure s | Uninteresting |
| Hungry | Weary s | Growling a | Watchful | Harm a | Unprotected f |
| Ill d | White-faced f | Hunched | Widen eyes | Helpless | Urgent |
| Itchy | White-knuckled | Inward focused | Wiggle | Immorality d | Violence a |
| Jittery f | Wide awake f | Lazy | Willful a | Impotent | Vulnerable s |
| Juiced a | Worn out s | Look away d | Withdraw s | Impure | Wistful s |
Note: This table presents the original 180 properties, with a balance of 60 items per modality. Bolded items were those most reliably classified as belonging to only one modality and as being most clearly associated with only one emotion category. Superscript letters denote which of the five negative emotion categories was uniquely associated with the given property:
anger,
boredom,
fear,
disgust,
sadness.
Following pilot testing, we identified 100 properties that were strongly associated with each emotion category across the adult age-span and used these validated properties as part of a property association task (adapted from Kan et al., 2003; Pecher et al., 2004) in a separate sample of participants (aged 18–75). Across 100 trials, participants explicitly rated how much a given property came to mind when thinking about a specific emotion category (e.g., how much does hot come to mind when thinking about anger?). In line with maturational dualism and psychological constructionist models of emotion, we predicted that as age increased, adults would rate interoceptive properties in general as less central to emotion categories, but that this relationship with age would not exist for behavioral or situational properties. Because high arousal emotions may be particularly linked to peripheral reactivity and interoception (e.g, Barrett et al., 2004), we also predicted that these age differences in interoceptive properties would especially hold for sensations that are high arousal (e.g., blood pumping).
Study 1 Method
Participants.
Sample size was determined ahead of time based on a priori power analysis. Data were not analyzed until all data collection ended. We used our pilot data to establish an empirically-derived estimate of age effects on ratings of interoceptive items (r= −.23, p= .009) and behavioral vs. situational items (rs= −.16, −.05, ps= .077, .572 respectively). We used multilevel modeling to address the hierarchical, partially within-subject nature of the data and thus relied on power simulations. With the small effect size observed from the pilot sample, power simulations suggested that a Level 1 sample (trials) greater than 30 nested within a Level 2 sample (individuals) greater than 40, would give us 90% power to observe an effect (ps< .05; see simulations in Scherbaum & Ferreter, 2009). Thus, we aimed to have around 30 trials for each modality of interoception, behavior, and situation in our design. We recruited 150 participants as our target sample to ensure that we recruited a wide enough age-range of participants and to account for potential data loss on the online platform.
One hundred fifty participants completed the study via MTurk. Participants ranged in age from 18–75, with 32.8% falling between 18–30, 41.9% between 31–49, and 25.3% between 50–75. In the sample, 78.7% were European American, 5.3% were African American, 6.0% were Asian American, and 6.0% were Latin American. Self-reported annual income ranged from $0.00 to $167,000 per year (Mincome= $48,292, SDincome= $35,384). No individuals were from the same IP address and seven participants were removed from analysis due to failed attention checks. All participants reported their age. The final sample was N= 143 (Mage= 39.87 years, SDage=12.93 years; 57.3% female).
Materials.
The final list of 100 emotion properties derived from our pilot study included 40 interoceptive properties, 29 behavioral properties, and 31 situational properties that were most strongly associated with each of five emotion categories.2 Based on the pilot ratings, each property and its most strongly associated emotion category were paired to create a category-property item (for example, “ANGER-hot” where “anger” is the emotion category and “hot” is the interoceptive property). See the Supplementary Materials for pilot study details.
Procedure.
This study was approved by the primary university’s institutional review board and conducted in accordance with APA ethical conduct of research with human subjects (IRB# 14–2319). Participants read that this was an “emotion knowledge survey” and were directed to Qualtrics via Mechanical Turk. The task began after individuals granted informed consent. Next, participants read the following instructions: “In this task, you will rate how much a word comes to mind when thinking about a specific emotion. This task is timed, so please work as quickly and accurately as you can.” Participants were given this instruction to ensure that they relied on semantic associations and to reduce effortful deliberation. We also encouraged participants to only complete the task if they were able to work uninterpreted on it in a quiet space with few distractions.
On each trial, participants saw a randomly-selected single category-property item (e.g., ANGER-hot) on the screen after a fixation cross and were asked to indicate how much the property came to mind when they thought about that category (e.g., “How much does HOT come to mind when you think about ANGER?”). Participants answered using a 10-point Likert scale (0 = Did not come to mind at all to 9 = Immediately came to mind). Participants rated all 100 category-property items, with no item shown more than once. Two attention checks (false items with instructed answers) were randomly presented throughout the task to identify whether individuals were maintaining attention. As this was a property association task, we only included false items as attention checks. Additionally, false foils as used in property verification tasks are harder to determine with emotions (e.g., ANGER-cold could be similarly or more valid across some people and cultures than ANGER-hot) given that there is much more conceptual variability in people’s associations for emotion categories. This is different from classic property verification studies (see Kan et al., 2003; Pecher et al., 2004) which focused on exteroceptive modalities where there are much clearer, consistent false item foils that can be reliably verified across people (e.g., GRASS-red is typically false whereas GRASS-green is more reliably true).
Stimuli were presented in Qualtrics using the QRTEngine (Barnhoorn, Haasnoot, Bocanegra, & van Steenbergen, 2015), which provided a platform for conducting reaction time-based trials in Qualtrics. To measure reaction time, we used Qualtrics’ built-in tool, computed from the time that the page fully loaded on the user’s internet browser to when they rendered their responses. Although we were interested in participants’ explicit ratings of category-property items, we collected reaction time data to exclude overly long trials that might indicate that participants were overly deliberating on items or not paying attention. Reaction times were also included as a covariate in all models to control for any potential cognitive differences between older and younger adults. However, there was no effect of modality on reaction time or a modality x age interaction.
Analyses.
All analyses were conducted in R using the lme4 and simpleboot packages (Bates, Mächler, Bolker, & Walker, 2015; Peng, 2008). Data and R code for both Studies 1 and 2 can be accessed at https://dataverse.unc.edu/dataverse/agingemotions. We first cleaned the data by removing the eight individuals who failed the attention checks. Additionally, based on common practice in cleaning reaction time data (Whelan, 2008) we excluded any ratings under 200 milliseconds (ms). Based on the data’s distribution, we also removed any responses that were over one minute long as these were outliers (99.4% observed responses occurred within the first 30 seconds). To confirm that older adults were not more likely to be reaction time outliers relative to younger adults, we used generalized mixed effect modeling with a logit link predicting outlier rates with age and age2, nested within participant. This analysis showed no relation between age and age2 with outlier rates (odds ratios= −.18, −.002; ps= .905, .996), suggesting that older adults in the study did not have more extreme outlier reaction times than younger adults.
To test our prediction that older adults would be less likely than younger adults to rate interoceptive properties as associated with emotion categories, we ran a multilevel linear model (as per Raudenbush & Bryk, 2001) using a cross-classified multi-level design, with modality items nested within person at Level 1 and age as a Level 2 between-subject predictor. Our two clustering groups were subject and item. We treated age as a continuous variable and centered it at the lowest age in the sample (18 years old) so that positive beta values would indicate an increase in ratings compared to ratings at age 18 (the intercept). Consequently, negative beta values would indicate a decrease in ratings compared to ratings at age 18. Age was also scaled by dividing by the standard deviation which assists with estimation by ensuring the units of age are not vastly different from the units of other variables. Additionally, we computed an age2 term to address potential curvilinear (quadratic) effects of age, given that prior emotion literature finds a mix of linear vs. curvilinear age effects (e.g., Carstensen, Pasupathi, Mayr, & Nesselroade, 2000; Mroczek & Kolarz, 1998; Schilling, Wahl, & Wiegering, 2013); this age2 term was computed by squaring the age-centered and scaled term.
Each modality was dummy-coded (0–1) and situational items served as the primary reference group. Therefore, the model intercept represents the mean rating of situational items for individuals at 18 years of age, while the fixed effect of age represents the impact of age on the rating of situational items for individuals at 18 years of age. The interaction effects represent the relative difference in the effect of age and age2 between situations and interoceptive items and situations and behavioral items, respectively. Reaction time for each item was included in the model to control for potential effects of cognitive aging on task performance. The random intercept and random slope effects of item modality were allowed to correlate freely with one another. As our observations are clustered both within subjects and items, we used a cross-classified multilevel model with random intercepts for both subjects and items, as well as a subject-specific random slope for modalities. Furthermore, we analyzed the relation of age and the quadratic effect of age (age2) using both main effects and cross-level interactions with modality. This sort of idiographic approach allows us to model both individual-specific modality effects and item-specific effects. Standardized betas (β) are presented in the tables (calculated as per Cohen, Cohen, West, & Aiken, 2003) as these allow for effect size comparison. Random effects for all models are presented in the Supplementary Materials.
Study 1 Results
Modality effects.
For our primary model, we examined the main effects of age, age2, modality, and the interactions of age x modality and age2 x modality on participants’ likert ratings for each category-property item (Table 2). We found several significant fixed effects. There was a significant main effect of reaction time on likert ratings, b=−.03, S.E.=.01, p<.0001, 95% CIs [−.042, −.023], suggesting that the more an emotion category was associated with a property, the quicker participants were to rate that item in general. Additionally, the main effect (i.e., intercept) for interoceptive properties was marginally lower than that of situational properties (b=−.66, S.E.=.39, p=.085, 95% CIs [−1.42, .09]), suggesting that on average, participants rated interoceptive items as less related to emotions than situational items. This finding might be related to known individual differences in both interoception and the association of interoceptive sensations and emotions (e.g., O’Brien et al., 2016; Schulz & Vögele, 2015). Moreover, situations are external, easily observable, and often explicitly linked to the definitions of emotion categories across the age-span (Nook et al., in press). There was no main effect of behavioral properties compared to situational properties, b=−.56, S.E.=.39, p=.15, 95% CIs [−.1.33, .21], suggesting that individuals did not rate behavioral properties as coming to mind for emotions less than situational properties.
Table 2.
Study 1 fixed effects for age x modality on category-property ratings
| Fixed Effects | b | β | S.E. | t | 95% CIs |
|---|---|---|---|---|---|
| Intercept | 6.09 | .00 | .44 | 13.81*** | 5.22, 6.95 |
| Age | −.61 | −.19 | .53 | −1.16 | −1.66, .43 |
| Age2 | .10 | .10 | .15 | .62 | −.21, .40 |
| Behavior | −.56 | −.09 | .39 | −1.42 | −1.33, .21 |
| Interoception | −.66 | −.12 | .39 | −1.72† | −1.42, .09 |
| Age x Behavior | .13 | .04 | .32 | .40 | −.49, .75 |
| Age x Interoception | .90 | .29 | .34 | 2.63** | .23, 1.57 |
| Age2 x Behavior | −.02 | −.02 | .09 | −.25 | −.20, .16 |
| Age2 x Interoception | −.23 | −.19 | .10 | −2.30* | −.42, −.03 |
| Reaction Time | −.03 | −.05 | .01 | −6.88*** | −.04, −.02 |
Note. Standard errors are for the unstandardized betas. Situational property ratings serve as the reference category.
p < .10,
p < .05,
p < .01,
p < .001.
As predicted, neither the main effects of age nor age2 were significant (ps>.25), meaning that there were not age-related differences in ratings of situational property. Moreover, there were no interactions between age, age2, and behavioral properties (ps>.250), meaning that there were not age-related differences in ratings of behavioral properties. However, as predicted, there were significant interactions between age x interoceptive properties (b=.90, S.E.=.34, p=.008, 95% CIs [.23, 1.57]) and age2 x interoceptive properties (b=−.23, S.E.=.10, p=.021, 95% CIs [−.42, −.03]). Together, the significant linear and curvilinear age effects suggest that the association between interoceptive properties and age increases until middle age but decreases thereafter into late adulthood. Probing the curvilinear effect revealed that, on average, the age around which interoceptive sensations were most associated with emotion categories occurred around age 45 with declines in associations between emotions and interoceptive sensations occurring from mid into late adulthood. Note that although the situational modality was the primary reference category here, effects were not a product of analysis choice. When we re-ran the model with behavior as the reference category, results fully replicated, again showing age and age2 effects on interoceptive, but not other properties.
High vs. low arousal effects.
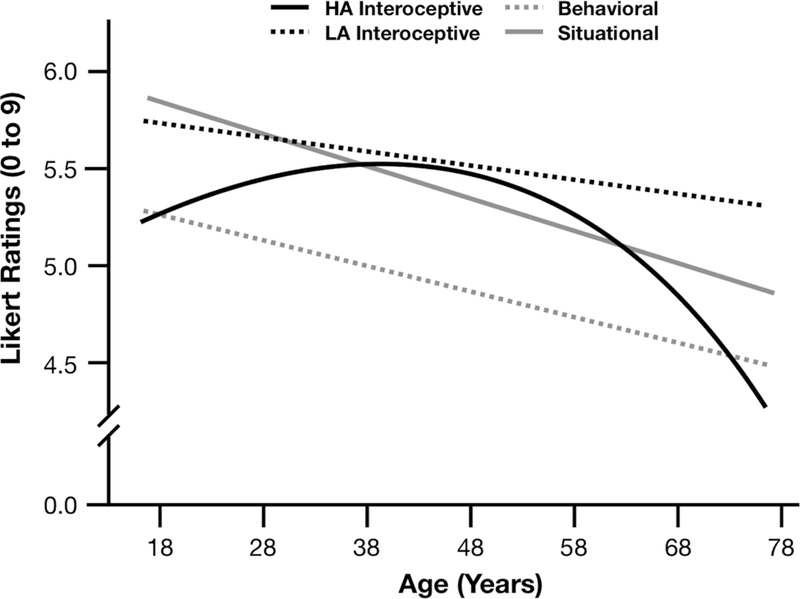
Given our specific predictions for high arousal sensations, we separated the interoceptive ratings into high vs. low arousal items (Table 3, Figure 1). Interoceptive items were treated as high arousal vs. low arousal based on pilot study ratings of which sensations were most associated with prototypically high arousal (anger, disgust, fear) vs. low arousal (sadness, boredom) emotions. We examined the effects of age, age2, modality, and the interactions of age x modality and age2 x modality on participants’ likert ratings for each category-property item, but now compared high arousal interoceptive items to behavioral and situational items, and again with low arousal interoceptive items to behavioral and situational items.
Table 3.
Study 1 fixed effects for age x high arousal vs. low arousal on category-property ratings
| Fixed Effects | b | β | S.E. | t | 95% CIs |
|---|---|---|---|---|---|
| High arousal (HA) interoceptive model | |||||
| Intercept | 6.08 | .00 | .44 | 13.76*** | 5.22, 6.95 |
| Age | −.61 | −.19 | .53 | −1.16 | −1.64, .43 |
| Age2 | .10 | .10 | .15 | .62 | −.21, .40 |
| Behavior | −.56 | −.10 | .40 | −1.41 | −1.33, .22 |
| HA Interoception | −.76 | −.13 | .43 | −1.76† | −1.61, .09 |
| Age x Behavior | .13 | .04 | .32 | .40 | −.49, .74 |
| Age x HA Interoception | 1.00 | .30 | .39 | 2.58** | .24, 1.76 |
| Age2 x Behavior | −.02 | −.02 | .09 | −.25 | −.20, .16 |
| Age2 x HA Interoception | −.27 | −.20 | .11 | −2.37* | −.49, −.05 |
| Reaction Time | −.03 | −.04 | .01 | −6.23*** | −.04, −.02 |
| Low arousal (LA) interoceptive model | |||||
| Intercept | 6.07 | .00 | .44 | 13.84*** | 5.21, 6.93 |
| Age | −.61 | −.19 | .53 | −1.15 | −1.65, .43 |
| Age2 | .09 | .10 | .15 | .61 | −.21, .40 |
| Behavior | −.56 | −.10 | .39 | −1.44 | −1.32, .20 |
| LA Interoceptive | −.46 | −.07 | .52 | −.90 | −1.48, .55 |
| Age x Behavior | .13 | .04 | .32 | .34 | −.49, .75 |
| Age x LA Interoception | .69 | .17 | .45 | 1.53 | −.19, 1.58 |
| Age2 x Behavior | −.02 | −.02 | .09 | −.25 | −.20, .16 |
| Age2 x LA Interoception | −.15 | −.09 | .13 | −1.14 | −.20, .16 |
| Reaction Time | −.03 | −.04 | .01 | −5.20*** | −.04, −.02 |
Note. Standard errors are for the unstandardized betas. Situational property ratings serve as the reference category.
p < .10,
p < .05,
p < .01,
p < .001.
Figure 1.
Study 1 self-reported high vs. low arousal interoceptive sensations relative to behavioral and situational reports across the age span.
As predicted, age-related differences in associations between interoceptive sensations and emotions occurred only for high arousal interoceptive items. There were significant interactions for age x high arousal interoceptive properties (b=1.00, S.E.=.39, p=.009, 95% CIs [.24, 1.76]) and age2 x high arousal interoceptive properties (b=−.27, S.E.=.11, p=.018, 95% CIs [−.49, −.05]). Together, the significant linear and curvilinear age effects suggest that the association between high arousal interoceptive properties and age increases into midlife (around age 45) before decreasing thereafter into late adulthood. However, there were no significant age or age2 interactions for low arousal interoceptive items in comparison to behavioral or situational items. This suggests that older age predicts decreasing associations between emotions and high arousal interoceptive sensations (e.g., ANGER-blood pumping), but that associations for low arousal interoceptive sensations (e.g., SADNESS-drained) remain unrelated to age between individuals.
Study 1 Discussion
Study 1 used a property association task to assess the extent to which individuals associated interoceptive, behavioral, and situational properties with different emotion categories across adulthood. We found a curvilinear effect, whereby adults increasingly associated interoceptive properties with emotion categories until around middle adulthood, after which adults were less likely to associate interoceptive sensations with emotion categories in later life. As predicted, age effects were interoceptive-specific; age was not associated with differences in participants’ behavioral or situational emotion ratings. We further showed that these interoceptive age effects were primarily driven by high arousal (e.g., “blood pumping”) but not low arousal (e.g., “drained”) interoceptive sensations, although low arousal items were limited due to fewer items populating this space relative to high arousal items. These findings serve as initial evidence for age-related variation in adults’ mental representations of the physiological concomitants of emotion.
Of course, there are alternate interpretations of our findings. First, we used a cross-sectional sample, so we cannot rule out cohort effects. It is possible that, due to generational differences in emotion representations, older adults are less likely to think of emotions as involving bodily changes. We know of no relevant research that would imply historical differences in the understanding of emotions as embodied phenomena, although this finding would be interesting unto itself. An alternate interpretation for Study 1 is that interoceptive properties become less strongly associated with emotion categories in later life because adults have accumulated sufficient knowledge of the more externally-focused properties of emotions (behavioral, situational cues) and no longer need to focus on interoceptive cues when representing their own or other’s emotions. However, this interpretation is less plausible for a few reasons. First, we found no significant age differences in behavioral and situational properties: older adults do not appear to associate these properties with emotion categories to a greater extent than younger and middle-aged adults do. Second, even young children understand the situational properties of emotion and these presumably reflect a more basic understanding of emotion concepts (Nook et al., in press). It is thus unlikely that a shift away from interoceptive sensations with increasing age reflects emotional expertise per se. Instead, the interoceptive-specific decrement we observed is more consistent with maturational dualism. As these bodily signals become less clear, intense, or reliable with age, adults’ representations of emotion categories may become correspondingly less linked to interoceptive representations.
Study 1 also only assesses adults’ conceptual representations of emotion. Although emotion concepts are updated based on experience (Doyle & Lindquist, 2018), it is still in principle possible that emotion concepts reflect culturally learned symbols and do not reflect participants’ daily experiences. We thus conducted Study 2 to examine whether age-related differences in the association between interoceptive sensations and emotions would extend to self-reported emotional experience. Asking participants to make summary judgments or to generally report their emotional experiences can elicit retrospective memory biases or reliance on culturally-proscribed concepts (e.g., stereotypes, norms) rather than idiographic experiences (Barrett et al., 1998; Robinson & Clore, 2002). Therefore, we used an experience sampling method that is designed to help limit participants’ exclusive reliance on conceptual representations while better assessing actual subjective experiences (Kahneman et al., 2004).
Study 2
Study 2 built on Study 1 in at least three ways. First, Study 1 only assessed participants’ associations with a small set of negative emotion categories, many of which are typically experienced as highly arousing (e.g., anger, disgust, fear; e.g., Bradley & Lang, 1994; Russell, 1980). Given that Study 1 revealed arousal-driven effects for interoceptive sensations, we sought to more clearly examine the effect of age on high vs. low arousal emotions and their interoceptive concomitants in Study 2. Second, given that old age tends to bring relative increases in wellbeing and positive emotional experience (e.g., Carstensen et al., 2011), we also assessed adults’ experiences of positive emotion categories in Study 2.
Third and most importantly, Study 2 built on Study 1 by assessing participants’ self-reported experiences rather than their mental representations of emotion categories. A constructionist approach suggests that conceptual knowledge about emotions and experience are linked, insofar as participants are drawing on said knowledge when making predictions about the meaning of their physiologically-driven affective states (Barrett, 2017, 2018; Lindquist, 2013). Nonetheless, we wanted to more directly assess participants’ experiences, given that Study 1 could represent what participants thought of emotions in general (i.e., cultural norms for emotion categories) rather than their own personal experiences of emotions in daily life. We used the Day Reconstruction Method (DRM; Kahneman et al., 2004) to collect participants’ self-reported emotional experiences, given that the DRM has been shown to somewhat ameliorate effects of retrospective memory bias on self-reports. The DRM has also been validated against more traditional types of ecological momentary assessments, with the added benefit of lower participant burden than ecological momentary assessment items that require weeks of ratings (Diener & Tay, 2014; Dockray et al., 2010). In our case, it was ideal for use on an online platform.
Using the DRM, we assessed the intensity of individuals’ self-reported interoceptive sensations, behaviors, and situations (“modalities”) from the prior day across multiple episodes, as well as their emotional, physical, and cognitive states (“states”). We assessed age differences in both the intensity and co-occurrence of adults’ modality and state reports, again using the behavior and situation items as comparisons for the interoceptive items. In Study 2, we also used physical and cognitive state items as comparisons for the emotion items, given prior work showing that in folk theories of the mind, individuals often categorize their experiences as physical vs. emotional vs. cognitive states (Weisman, Dweck, & Markman, 2017).
Intensity hypotheses.
As in Study 1, we predicted that with increasing age, adults would report experiencing less intense emotion-related interoceptive sensations throughout the previous day; we did not predict age-related differences for the behaviors or situations typically associated with emotions. Additionally, given the predictions of maturational dualism and the findings of Study 1, we predicted that as age increased into late adulthood, older adults would report less intense high arousal interoceptive sensations and emotion categories, but would report either equal or more intense low arousal interoceptive sensations and emotion categories relative to younger and middle-aged adults. We also expected to replicate prior work demonstrating that older adults experience more positive and fewer negative emotions (e.g., Carstensen et al., 2011; Charles et al., 2001).
Co-occurrence hypotheses.
Age may also bring with it differences in the structure of emotion experience—that is, how the different components of emotion co-occur across time. Given our interoceptive-specific predictions for aging, we took a network-based analytic approach to explore age differences in the co-occurrence between interoceptive sensations, behaviors, and situations during emotions. Specifically, consistent with maturational dualism, we expected that with increasing age, adults would show greater distancing of interoceptive sensations from emotional behaviors and situations, but relatively stable co-occurrences between emotional behaviors and situations.
Study 2 Method
Participants.
Two-hundred participants completed the study via MTurk. The final sample was N= 198 (Mage= 34.27 years, SDage=12.15 years, 18–67 years; 65.2% female), with 47.5% falling between ages 18–30, 40.9% between ages 31–49, and 11.6% between ages 50–67, after checking for any duplicate IP addresses and removing two individuals who failed attention checks. In the sample, 76.3% were European American, 11.1% were African American, 7.1% were Asian American, and 6.6% were Latin American. Self-reported total annual income ranged from $0 to $460,000 per year (Mincome = $56,657.16, SDincome= $52,982.45).
As in Study 1, sample size was determined based on a priori power analysis and data were not analyzed until all data collection was completed. Prior studies have used the DRM to assess older adult’s emotional experiences and wellbeing (reviewed in Steptoe, Deaton, & Stone, 2015). Based on this prior work, we expected a small effect size (Cohen’s ds= .03-.10) for the interaction of age and self-reported emotions. As in Study 1, we used multilevel modeling to analyze the hierarchical, partially within-subject data and thus relied on power simulations. Power simulations suggest that with the observed small effect size, a Level 1 sample (trials) greater than 30 nested within a Level 2 sample (individuals) greater than 40, would give us 90% power to observe an effect (p< .05-.01; Scherbaum & Ferreter, 2009). Thus, for each episode of the day reported (9–15 episodes per person), participants rated their experiences for n>30 modality properties and n>30 states. We recruited 200 participants as our target sample size to ensure that we included a wide enough age-range of participants and to account for data loss on the online platform.
Materials.
Interoceptive, behavioral, and situational items in Study 1 had been validated for negative emotions only. For Study 2, we also wanted to investigate positive emotions. Therefore, to account for the addition of positive emotions, we expanded our stimuli set of interoceptive, behavioral, and situational items to include items associated with positive affective states, such as the interoceptive experience of “muscles relaxed,” behaviors like “smiling,” or situations like “success”. Emotion items were drawn from previous studies that use the DRM for ratings of emotional experiences and expanded to include a balanced number of items across the dimensions of valence and arousal (e.g., Fredrickson, Tugade, Waugh, & Larkin, 2003; Gruber, Kogan, Quoidbach, & Mauss, 2013). Specifically, we added in 10 positive emotions that spanned the higher and lower arousal affective spaces (amused, awe, excitement, and pride vs. content, grateful, love, happy, serene, and pleased). Physical state and cognitive state items were created for this study to serve as comparisons for emotional state items.
For each episode, participants rated the extent to which they had experienced 20 interoceptive items (e.g., “heart racing”), 24 behavioral items (e.g., “laughter”), 24 situational items (e.g., “failure”), 19 physical states (e.g., “hunger”), 23 emotional states (e.g., “embarrassed”), and 18 cognitive states (e.g., “lost in thought”), with a total of 128 items per episode. Each item was rated on a 7-point likert scale, with 0=Did not experience this at all, 3= Experienced this moderately, and 6= Extremely experienced this. See Table 4 for full list of items used in Study 2.
Table 4.
Day Reconstruction Method items used in Study 2.
| Interoceptive Modality | Behavioral Modality | Situational Modality |
|---|---|---|
| Blood pumping | Bite fingernails | All is right with the world |
| Body or limbs heavy | Clenched jaw | Almost had an accident |
| Butterflies in stomach | Closed eyes | Bad news |
| Cold or clammy | Fidgety | Being alone |
| Dizzy or lightheaded | Frowning | Being with someone you dislike |
| Easy breathing | Grind teeth | Being with someone you love |
| Feeling warm | Help someone else | Failure |
| Goosebumps | Hugging | Good news / compliment |
| Heart calm | Laughter | Heard about a disaster |
| Heart racing | Look away | Made a mistake |
| Hot or flushed | Lower eyebrows | Received kindness |
| Lump in throat | Pace back and forth | See someone get hurt |
| Muscle tension | Savor something | Someone offends you |
| Muscles relaxed | Seek comfort | Something is certain |
| Nausea | Sighing | Something is distasteful |
| Heavier breathing | Slouch | Something is putrid |
| Shakiness | Smile | Something is uncertain |
| Stomach full | Speak faintly | Something special happened |
| Stomach growling | Speak loudly | Something is unexpected |
| Sweating | Stare into space | Something is unfair |
| Tap foot | Something uplifts you | |
| Weeping | Success | |
| Wide eyes | Uneventful | |
| Wrinkled nose | Urgent | |
| Physical States | Emotional States | Cognitive States |
| Activated | Amusement | Clear thinking |
| Awake | Anger | Confusion |
| Deactivated | Anxious | Creative |
| Energized | Awe | Daydreams |
| Exhaustion | Bittersweet | Decisions |
| Feeling healthy | Boredom | Doubt |
| Hunger | Contentment | Focused |
| Illness | Disgust | Fuzzy thinking |
| Inflammation | Dissatisfied | Lost in thought |
| Jittery | Downhearted | Making plans |
| Juiced | Embarrassed | Mind racing |
| Lazy | Excitement | Mind wandering |
| Restless | Fear | Puzzled |
| Satiated | Gratitude | Reflecting |
| Sluggish | Guilt | Remembering |
| Stressed | Happiness | Speculations |
| Thirst | Irritable | Thoughts rushing |
| Tired | Jealousy | Wondering |
| Well-rested | Love | |
| Pleased | ||
| Proud | ||
| Sadness | ||
| Serenity | ||
Procedure.
This study was approved by the institutional review board and conducted in accordance with APA ethical conduct of research with human subjects (IRB# 14–2213). Individuals read that this was a “daily experiences study” and were directed to Qualtrics via Mechanical Turk. After informed consent, participants completed a demographic questionnaire in which they reported their age, gender, ethnicity, level of education, and annual household income. A computerized version of the DRM was then administered. Individuals began the task by selecting which day the previous day of the week was. To avoid weekend effects on emotion reports (Stone, Schneider, & Harter, 2012), we only collected data on Wednesdays-Fridays (so that reports were about Tuesdays-Thursdays).
Per standard DRM instructions, participants first broke the day down into episodes. The goal of this portion of the DRM is to help participants focus on finite episodes of the day rather than reflect in general on especially memorable moments. To facilitate their subsequent reporting, participants named each episode and made notes about it so that they could better remember it later. Participants broke down the previous day’s morning (from waking up until lunchtime) into a minimum of three and maximum of five episodes. They then did the same for the previous day’s afternoon (from lunch until dinner) and evening (from dinner until bed). We required a minimum of three episodes per time of day to ensure we had enough data to reliably estimate each part of the day. Thus, each participant had a minimum of nine episodes or a maximum of fifteen episodes from the previous day. Due to the many items in the study, we limited participants to fifteen episodes total to ensure participants were not overburdened with reporting.
After sub-dividing their day into episodes, participants used a 1–7 likert scale to rate the extent to which each episode was described by modalities (interoceptive sensations, behaviors, situations replicated from Study 1) and subjective states (emotional, physical, and cognitive); see Table 4. Each episode’s name and the participant’s notes about that episode were fed back to the participant to serve as memory cues when rating. Participants read that they should “Take a moment to recall this moment from yesterday to mind. Remember what you were doing, thinking, and feeling in that situation. Below, you will rate what behaviors you did, what sorts of situations you were in, and what sorts of internal experiences you had during this episode. Please do your best to recall that time / episode as accurately as you can.”
All items were randomly presented within each episode to avoid order effects. Participants completed these likert ratings for every episode they identified throughout the morning, afternoon, and evening of the previous day. Three attention checks (false items with instructed answers) were included at random to assess participant engagement and fatigue. Participants were offered a three-minute break between the times of day (e.g., after rating the morning episodes, before rating the afternoon episodes). After a participant finished rating all episodes, they completed debriefing.
Analyses.
All analyses were conducted in R using the lme4 and simpleboot packages (Bates et al., 2015; Peng, 2008). Age was a continuous variable centered at the lowest age in the sample (18 years old) and scaled via dividing by the standard deviation, thus assisting with model estimation. As we found curvilinear effects of age in Study 1, we again included an age2 term in all models. Standardized betas (β) are presented in the main tables to allow for effect size comparison. Model random effects are presented in the Supplementary Materials.
Multi-level models of intensity.
We hypothesized that as age increases, the intensity of experienced interoceptive sensations (especially high arousal sensations), negative emotions, and high arousal emotions would decrease. On the other hand, we predicted that as age increases, the intensity of positive emotions, and low arousal emotions would either increase or remain stable. To test these predictions, we ran multilevel linear models with age and age2 as the key predictors. As in Study 1, these models used a cross-classified multi-level design with the exception that in Study 2, each model had three levels: self-report items nested within episodes at Level 1, episodes nested within person at Level 2, and age as the Level 3 between-subject characteristic. As in Study 1, we first compared interoceptive vs. behavioral vs. situational items across episodes on all items, and then ran our planned analyses parsing apart age effects on high vs. low arousal interoceptive items. Additionally, given the valence-based age effects in the literature (e.g., Carstensen et al., 2011), we examined age differences in high vs. low arousal emotions and negative vs. positive emotions. All emotions models used reports of physical and cognitive states as comparisons for the reported emotional states, paralleling how behaviors and situations are compared with interoceptive sensations.
Network analysis of co-occurrence.
We used a network approach to test age differences in the structure of emotional experience where, as age increased, we expected to observe a lower co-occurrence of interoceptive sensations with emotional behaviors and situations, but stable co-occurrence between emotional behaviors and situations. This analysis proceeded as follows: First, similarity networks of individuals’ emotional modalities were constructed by calculating the Euclidean distance between each emotion property vector across every event. For example, the distance between “blood pumping” and “nausea” was calculated for an individual by collecting their responses for each of those properties into vectors B and N, each with a number of entries equal to the number of events the individual responded about, E, and using the following equation for Euclidean distance
Following the calculation of the distance networks, we normalized them into similarity networks with
where D is the distance network for a given individual. This transformation accomplishes two things: first, the similarity between two properties are scaled between 0 and 1, with increasing similarity indicating that a subject expressed similar levels of two properties for all events and second, the similarity measure is comparable across all subjects. The distance measure is not comparable across all subjects, as subjects could differ in their number of events. By examining the similarity network, we can analyze differences in modality co-occurrence rather than simply analyzing differences in the level of each modality, as was done in the multi-level models. From the property-wise similarity network, we calculated network statistics of between-modality co-occurrence as the mean of the similarities between modalities. For example, to calculate between-modality co-occurrence for interoceptive vs. situational properties, we averaged all similarity values between properties in one modality relative to the other modality.
The relation between age and between-modality co-occurrence was calculated using simple linear regression with robust bootstrapped standard errors and 95% confidence intervals. Bootstrap standard errors (using 1000 bootstrap samples) were used as the distributional properties of our co-occurrence measure are unknown. Results are presented with unstandardized betas and confidence intervals for significance testing. Confidence intervals that do not contain zero are indicative of statistical significance. Our co-occurrence metrics assess coupling between modalities. For example, we expected that with increasing age, there would be less co-occurrence amongst all emotion modalities, such that interoceptive, behavioral, and situational reports within episodes are less well-integrated and experienced as a tightly cohering unit. In contrast, for younger individuals, we expected stronger co-occurrence between modalities, with self-reported interoceptive sensations, behaviors and situations, being strongly associated across episodes.
Study 2 Results
Intensity analyses: Self-reported modalities.
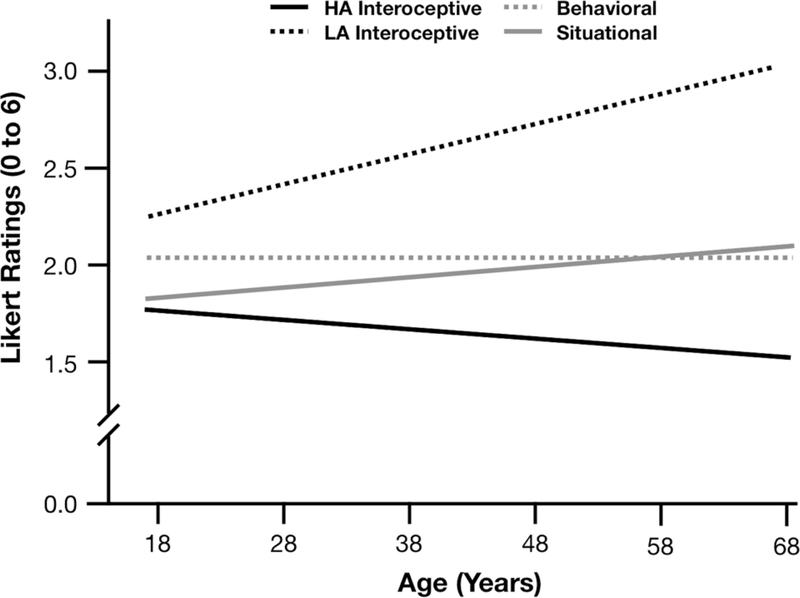
All results are multilevel models with cross-level interactions between age x modality or age x state as well as curvilinear effects of age2. As in Study 1, we found no significant effect of age nor age2 on how much individuals reported experiencing emotion-relevant behaviors and situations (ps> .25). Unlike Study 1, age and age2 did not predict interoceptive sensations overall. However, like Study 1 and as predicted, we found that age predicted high arousal interoceptive experiences (b= −.14, p< .001, 95% CIs [−.21, −.06]), such that adults reported experiencing fewer high arousal interoceptive experiences with increasing age. There was no effect of age2 (p=.16) on interoceptive sensations nor an effect of age or age2 on low arousal sensations (ps> .10). Thus, as age increased, adults were less likely to report experiencing high arousal interoceptive sensations in daily life. See Table 5, Figure 2 for full high arousal fixed effects.
Table 5.
Study 2 fixed effects for age x high arousal vs. low arousal on modality self-reports
| Fixed Effects | b | β | S.E. | t | 95% CIs |
|---|---|---|---|---|---|
| High arousal (HA) interoceptive model | |||||
| Intercept | 2.02 | .00 | .16 | 12.64*** | 1.70, 2.33 |
| Age | −.13 | −.08 | .16 | −.83 | −.44, .18 |
| Age2 | .04 | .09 | .04 | .89 | −.04, .12 |
| Behavior | .15 | .04 | .16 | .93 | −.16, .46 |
| HA Interoception | −.01 | −.01 | .18 | −.06 | −.37, .34 |
| Age x Behavior | −.01 | −.01 | .03 | −.43 | −.08, .05 |
| Age x HA Interoception | −.14 | −.07 | .04 | −3.64*** | −.21, −.06 |
| Age2 x Behavior | −.01 | −.02 | .01 | −1.39 | −.03, .01 |
| Age2 x HA Interoception | .01 | .02 | .01 | 1.40 | −.01, .03 |
| Low arousal (LA) interoceptive model | |||||
| Intercept | 2.01 | .00 | .18 | 11.02*** | 1.65, 2.36 |
| Age | −.11 | −.07 | .15 | −.74 | −.41, .18 |
| Age2 | .03 | .07 | .04 | .79 | −.05, .11 |
| Behavior | .15 | .04 | .20 | .72 | −.25, .55 |
| LA Interoception | .51 | .11 | .26 | 1.94† | −.01, 1.03 |
| Age x Behavior | −.01 | −.01 | .04 | −.39 | −.08, .06 |
| Age x LA Interoception | .02 | .01 | .05 | .35 | −.07, .10 |
| Age2 x Behavior | −.01 | −.02 | .01 | −1.33 | −.03, .01 |
| Age2 x LA Interoception | .02 | .02 | .01 | 1.32 | −.01, .04 |
Note. Standard errors are for the unstandardized betas. Situational modality ratings serve as the reference category.
p< .10,
p < .001.
Figure 2.
Study 2 self-reported high vs. low arousal interoceptive sensations relative to behavioral and situational reports across the age span.
Intensity analyses: Self-reported states.
Next, we examined age as a predictor of emotional vs. physical vs. cognitive states. There was no significant effect of age or age2 on self-reports of emotion categories, physical state categories or cognitive state categories (ps> .10), suggesting that on the whole across adulthood, people do not differ in the extent to which they experience these daily states.
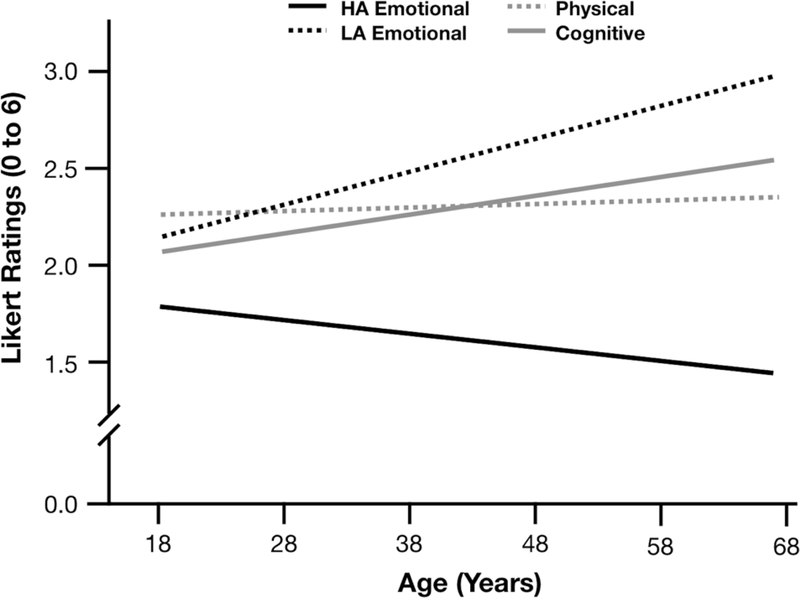
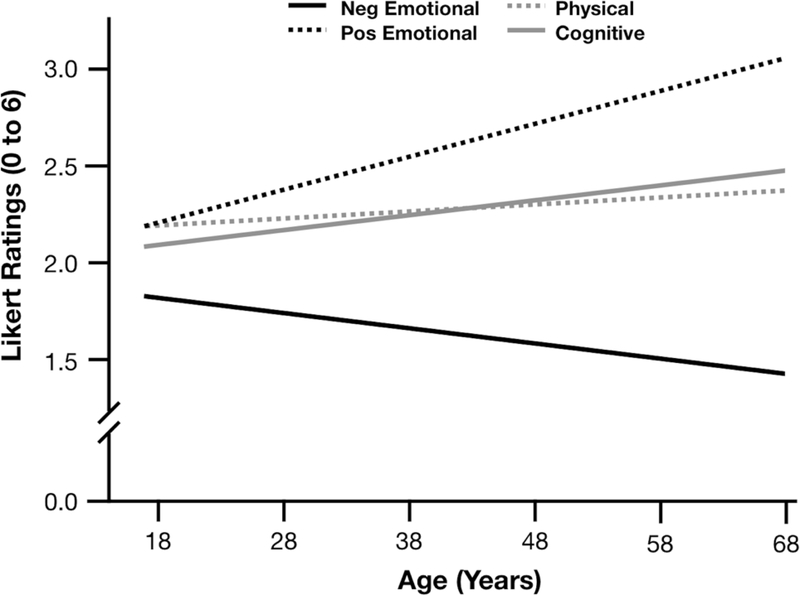
However, our planned arousal vs. valence analyses did reveal that age predicted differences in the quality of emotions experienced (Tables 6 and 7). Age significantly predicted less intense high arousal emotions (b=−.09, S.E.=.42, p=.036, 95% CIs [−.17, −.01]), and more intense low arousal emotions (b=.15, S.E.=.04, p<.001, 95% CIs [.07, .24]), Figure 3. Consistent with prior literature, we also replicated the well-known positivity effect, such that age significantly predicted more intense positive emotions (b=.23, S.E.=.05, p<.001, 95% CIs [.14, .32]), and less intense negative emotions (b=−.12, S.E.=.04, p=.003, 95% CIs [−.19, −.04]), Figure 4. There were no significant curvilinear effects of age in these models (all ps> .10).
Table 6.
Study 2 fixed effects for age x state on self-reports, with high and low arousal emotions.
| Fixed Effects | b | β | S.E. | t | 95% CIs |
|---|---|---|---|---|---|
| High arousal (HA) emotion model | |||||
| Intercept | 2.20 | .00 | .18 | 12.50*** | 1.85, 2.54 |
| Age | −.10 | −.06 | .17 | −.57 | −.42, .23 |
| Age2 | .04 | .09 | .04 | .87 | −.05, .12 |
| HA Emotions | −.26 | −.07 | .20 | −1.34 | −.65, .12 |
| Physical States | .10 | .03 | .18 | .57 | −.25, .45 |
| Age x HA Emotions | −.09 | −.04 | .04 | −2.10* | −.17, −.01 |
| Age x Physical States | −.01 | −.01 | .04 | −.15 | −.81, .07 |
| Age2 x HA Emotions | −.01 | −.01 | .01 | −.91 | −.03, .01 |
| Age2 x Physical States | −.01 | −.01 | .01 | −.34 | −.02, .02 |
| Low arousal (LA) emotion model | |||||
| Intercept | 2.19 | .00 | .19 | 11.60*** | 1.82, 2.57 |
| Age | −.09 | −.05 | .17 | −.52 | −.42, .24 |
| Age2 | .03 | .08 | .04 | .78 | −.05, .12 |
| LA Emotions | −.03 | −.01 | .22 | −.12 | −.46, .40 |
| Physical States | .10 | .03 | .20 | .50 | −.30, .50 |
| Age x LA Emotions | .15 | .07 | .04 | 3.46*** | .07, .24 |
| Age x Physical States | −.01 | −.01 | .04 | .78 | −.05, .12 |
| Age2 x LA Emotions | −.02 | −.02 | .01 | −1.49 | −.04, .01 |
| Age2 x Physical States | −.01 | −.01 | .01 | −.32 | −.02, .02 |
Note. Standard errors are for the unstandardized betas. Cognitive state ratings serve as the reference category.
p < .05,
p < .001.
Table 7.
Study 2 fixed effects for age x state on self-reports, with negative and positive emotions.
| Fixed Effects | b | β | S.E. | t | 95% CIs |
|---|---|---|---|---|---|
| Negative emotion model | |||||
| Intercept | 2.20 | .00 | .17 | 12.72*** | 1.86, 2.54 |
| Age | −.10 | −.06 | .17 | −.57 | −.43, .24 |
| Age2 | .04 | .09 | .04 | .86 | −.05, .13 |
| Neg Emotions | −.30 | −.09 | .18 | −1.70† | −.66, .05 |
| Physical States | .10 | .03 | .17 | .61 | −.23, .44 |
| Age x Neg Emotions | −.12 | −.06 | .04 | 2.95** | −.19, −.04 |
| Age x Physical States | −.01 | −.01 | .04 | −.15 | −.08, .07 |
| Age2 x Neg Emotions | −.01 | −.02 | .01 | −1.02 | −.03, .01 |
| Age2 x Physical States | −.01 | −.01 | .01 | −.35 | −.02, .02 |
| Positive emotion model | |||||
| Intercept | 2.19 | .00 | .19 | 11.77*** | 1.83, 2.56 |
| Age | −.09 | −.05 | .17 | −.51 | −.42, .25 |
| Age2 | .03 | .08 | .04 | .76 | −.05, .12 |
| Pos Emotions | .07 | .02 | .22 | .31 | −.37, .50 |
| Physical States | .10 | .03 | .20 | .52 | −.28, .49 |
| Age x Pos Emotions | .23 | .10 | .05 | 4.97*** | .14, .32 |
| Age x Physical States | −.01 | −.01 | .04 | −.14 | −.09, .08 |
| Age2 x Pos Emotions | −.02 | −.02 | .01 | −.31 | −.04, .01 |
| Age2 x Physical States | −.01 | −.01 | .01 | −.31 | −.02, .02 |
Note. Standard errors are for the unstandardized betas. Cognitive state ratings serve as the reference category.
p< .10,
p < .01,
p < .001.
Figure 3.
Study 2 reports of high vs. low arousal emotional states relative to physical and cognitive states across the age span.
Figure 4.
Study 2 reports of negative vs. positive emotional states relative to physical and cognitive states across the age span.
Co-occurrence analyses.
Next, using a network approach, we assessed age effects on the co-occurrence between the three emotion modalities of interoception, behaviors, and situations. First, overall between modalities, we found that as age increased, there was less co-occurrence between interoceptive sensations and emotional situations (b=−.001, SD=.0004, 95% CIs [−.002, −.001]). In contrast, and replicating our previous findings, there were no significant age effects in co-occurrence between emotional behaviors and situations. However, we did not observe a decrease in co-occurrence between interoception and behaviors with age (b=−.001, SD=.001, 95% CIs [−.001, .001]), suggesting that with increasing age, interoceptive sensations are still experienced as co-occurring with overt nonverbal behaviors.
Study 2 Discussion
Study 2 extended the interoceptive-specific age effects for emotion categories found in Study 1 into the realm of self-reported experience. In line with hypotheses, in the multilevel models, we found evidence that as age increased, adults reported experiencing less intense high arousal interoceptive sensations. For emotion, as age increased, adults also reported experiencing less intense negative and high arousal emotions, but more intense positive and low arousal emotions.
Of note, the curvilinear age effect found for interoceptive properties in Study 1 did not replicate in Study 2. This may reflect differences in the structure of emotion category representations vs. reports of emotional experience or may be due to differences between samples unrelated to the stimuli used. For instance, the age range of Study 2 was slightly smaller, with fewer late age older adults, perhaps limiting our ability to observe curvilinear effects at the upper limits of the age distribution. However, the literature on aging routinely finds conflicting results, with some studies finding linear vs. curvilinear effects of age (e.g., Carstensen et al., 2000). More work in larger samples with relatively equal sampling across each decade of adulthood would help clarify the extent to which linear vs. quadratic patterns consistently characterize age effects on interoceptive sensations and emotions while also identifying which factors might be contributing to linear vs. quadratic effects across different studies. However, despite fewer older adults in Study 2, we believe findings still reflect broad age-related decrements in the subjective link between the body and emotion in self-reported experience, given that age was a continuous variable (thus allowing us to better estimate age differences) and given that physiological declines begin appearing as early as midlife (e.g., around age 45, Palve & Palve, 2018), not just old age (e.g., ages 60–80) or very old age (e.g., age 80 and above).
The network co-occurrence analyses further intimate that there may be differences in the structure of emotional experience across adulthood, although the sizes of effects were small and should be replicated in future samples. Here we found that, as age increased, self-reported experiences of interoceptive items across multiple episodes became increasingly decoupled from situational items. Given that situational items in our studies likely approximate appraisals of a given situation (e.g., “X” situation is threatening, demanding, safe, etc.; Siemer, Mauss, & Gross, 2007; Smith & Kirby, 2009), this finding suggests that adults in later life likely still rely on situational meanings when experiencing emotions. This finding is predicted by maturational dualism, which suggests that older adults may rely relatively more on external vs. internal cues when experiencing emotions (Mendes, 2010).
In contrast, we did not find an age-related decoupling of interoceptive sensations and behaviors, meaning that as age increased, interoceptive sensations continued to co-occur on average with overt emotional behaviors (e.g., frowning, clenching fits, laughing). Given that physiological changes help enact behavior (Tomaka, Blascovich, Kelsey, & Leitten, 1993), this finding may even suggest that adults in later life do not tend to experience physiological changes in emotion in the absence of overt behaviors. Younger adults, in contrast, may experience interoceptive sensations (e.g., increased heart rate) regardless of whether they are engaging in overt behavior (e.g., running away from a threat) or not (e.g., thinking about an upcoming deadline). The neuroimaging literature is consistent with this hypothesis, insofar as younger vs. older adults experience more activity within brain regions involved in visceromotor control during emotions, even when lying inert in the fMRI scanner (MacCormack, Stein, et al., 2019).
Study 2, like Study 1, was limited by its cross-sectional design. Also compared to Study 1, there were fewer adults over the age of 50 in Study 2; accessing equal numbers of adults across the age-span is a limitation of the online platform that we used, although it affords other benefits (we were able to collect data from a group of individuals who ranged in age, had the computer skills and facility with technology to complete our task, and spanned the United States across an array of incomes, ethnicities, and genders). The fact that both studies’ findings conceptually replicate each other with slightly different samples and completely different methods is thus promising.
Study 2‘s use of the DRM is not without limitations. The DRM helps people more accurately recall the events of the prior day and is shown to reduce biases related to recall (Dockray et al., 2010). However, participants only reported a single day’s experiences, which might not be representative of a typical day. We cannot speak to day-to-day variability or stability over time. Future research could address these concerns by using longer experience sampling procedures across multiple days. There may be differences in how older adults perform on and use the DRM compared to younger adults, although there is prior work published that uses the DRM across a range of adult ages, even into 80+ years old (Ayuso-Mateos et al., 2013; Freedman et al., 2014). Nonetheless, age-related declines in episodic memory could perhaps impact older adults’ performance on the DRM, but this would suggest an overall difference in how older adults remember and recall the previous day’s emotion episodes and does not account for why we might observe age differences that are specific to high arousal interoceptive sensations and emotions.
General Discussion
Prior literature shows that emotional life differs across adulthood. Our findings add to this literature by demonstrating that individuals’ characterizations and reported experiences of the body during emotion can differ across adulthood. In Study 1, we found a curvilinear effect of age whereby younger adults reported stronger associations for interoceptive sensations like “heart racing” and “blood pumping” with negative emotion categories. However, from mid-life onward, and especially in later life, adults associated these sensations with emotions to a lesser degree than do younger individuals. These effects occurred especially for high arousal interoceptive items that are more likely to involve activation of the autonomic nervous system (e.g., heart racing).
In Study 2, we found a linear effect of age whereby older adults reported less intense high arousal interoceptive sensations relative to younger and middle-aged adults with the Day Reconstruction Method. Older adults’ interoceptive reports were also more decoupled from situational features than in younger adults. Critically, alongside these interoception-specific changes, Study 2 replicated prior experience sampling and longitudinal work in which older adults report less negative and more positive emotions (e.g., Carstensen et al., 2000; Charles et al., 2001). We also found that older adults reported fewer high arousal and greater low arousal emotional states. These findings are consistent with maturational dualism, which suggests that with increasing age, emotions might become less arousing (Mendes, 2010).
Implications
The present data add to other well-documented findings that healthy older adults appear emotionally better off than healthy young adults. Healthy older adults report experiencing fewer negative emotions and more positive emotions, report being more skilled at and prefer using effective emotion regulation techniques, and are less likely to use ineffective strategies to resolve interpersonal conflict3 (Birditt & Fingerman, 2005; Birditt, Fingerman, & Almeida, 2005; Carstensen et al., 2000; Charles et al., 2001; Cheng, 2004; Coats & Blanchard-Fields, 2008; Gross et al., 1997; Livingstone, Castro, & Isaacowitz, 2018; Neupert, Almeida, & Charles, 2007; Shallcross, Ford, Floerke, & Mauss, 2013). To date, these differences were primarily attributed to motivational changes or increased expertise in later life. For instance, socioemotional selectivity theory proposes that older adults are motivated to avoid negativity and achievement-orientations and pursue positivity and relationship-orientations as the end of life looms closer (e.g., Carstensen et al., 1999; Carstensen & Mikels, 2005; Mather & Carstensen, 2005). On the other hand, expertise theories suggest that older adults are more competent and comfortable across diverse emotional situations after a lifetime of accumulated experiences and are thus better at avoiding situations that are unpleasant and selecting effective regulation strategies (e.g., Diehl & Hay, 2011; Labouvie-Vief et al., 1989; Livingstone et al., 2018; Lockenhoff, Costa, & Lane, 2008).
However, there are also conflicting age-related differences in emotion that are less consistent with socioemotional selectivity theory or expertise accounts. Although older adults exhibit greater subjective well-being, they also perform worse on tasks that rely on emotional information to guide decisions (e.g., affective “gut”-based signals). For instance, older adults are more likely to trust scam artists or make sub-optimal financial and health decisions (e.g., Castle et al., 2012; Kircanski et al., 2018; Zebrowitz et al., 2017). Presumably, the same affective motivations and expertise that allow older adults to better manage their emotions and interpersonal interactions should also prevent older adults from wrongfully trusting others or making poorer decisions. To the extent that body-based representations contribute to both emotional experiences and affect-based decisions (e.g., Clithero & Rangel, 2014), maturational dualism and a constructionist account of emotional aging could parsimoniously describe both sets of results. If older adults experience fewer peripheral sensations during emotions, then this may make unpleasant and highly activated emotions both less frequent and easier to regulate when they do occur. The same effects would make it harder for adults to make decisions that rely on afferent interoceptive signals (e.g., Damasio, 1994).
Another implication of our findings relates to grounded models of cognition (Borghi & Pecher, 2011; Lindquist, MacCormack, & Shablack, 2015; Niedenthal et al., 2005; Wilson-Mendenhall, Barrett, & Barsalou, 2013). Psychological constructionist models emphasize that emotions are abstract categories comprised of “populations” of modality-specific prior experiences: that is, emotion categories include associated information about the thoughts, behaviors, and experiences (including interoceptive sensations) that characterized prior emotional experiences accumulated across an individual’s life (Lindquist & Barrett, 2008; Wilson-Mendenhall, Barrett, Simmons, & Barsalou, 2011). Although some research assesses how these representations are developed in childhood (MacCormack, Castro, Halberstadt, & Rogers, 2019; Pons et al., 2003; Widen & Russell, 2010), very little research examines how they might change and differ across adulthood. One recent study demonstrated that young adults can update the visual information associated with the categories “fear” and “anger” to include new representations of facial expressions within a single experimental session (Doyle & Lindquist, 2018). Thus, it stands to reason that throughout adulthood even into late life, mental representations of emotion categories may also change as the nature of emotional experiences change. More research is clearly needed to model this process longitudinally.
Limitations and Future Directions
Our findings are consistent with constructionist accounts to emotion and the theory of maturational dualism insofar as age increasingly tracks with reduced associations between emotions and interoceptive sensations (Study 1) and reduced reports of interoceptive sensations (Study 2) and high arousal emotions in daily adult life. However, they should be viewed as provisional on the basis of our sample characteristics and methods used. The data are cross-sectional, correlational, and drawn from an online sample. Future studies should thus replicate these findings using longitudinal measures, experimental methods, and a stratified sampling technique that equally samples younger, middle, and older adults.
For instance, due to our cross-sectional methods, it remains unclear whether the process of aging itself causes these differences in interoceptive, high arousal associations and self-reports or whether this pattern is due to cohort effects. Given the potential for cohort effects, we are cautious to emphasize age differences (rather than age changes) in our interpretations throughout the paper. If cohort effects are at play, then findings in Study 1 could be due to differences in semantic knowledge as a byproduct of how older vs. younger generations learned about emotion categories. American adults born in the earlier versus latter half of the 20th century may think about emotions differently due to historical events and cultural differences across time. This is an interesting question unto itself. To address causality more precisely, future research might use longitudinal methods to examine whether the interoceptive qualities of emotion shift within individuals across the adult age-span.
Another means of addressing causality is to use experimental methods. Although the present data demonstrate that older adults think about and report their emotions as involving less high arousal and interoceptive features, they are an important descriptive first step and do not address deeper mechanisms. The inspiration for these studies stemmed from prior evidence in psychophysiology, interoceptive science, and neuroimaging suggesting that the peripheral body and how the brain represents the periphery during emotion both change with age. As such, biological aging may be one explanation for the present studies’ findings. However, no published work to our knowledge yet tests whether these biological changes are causally contributing to effects observed in the present study or in the emotional aging literature more generally (e.g., the positivity effect). One means of addressing this question would be to block afferent feedback during emotion with drugs such as with a peripheral-specific beta-adrenergic blockade (e.g., nadolol) and compare effects across adults of different ages.
Despite the cross-sectional and correlational nature of the present work, a relative strength of our studies is that we sample across adulthood, rather than focusing exclusively on separate cohorts of older and younger adults. By sampling across middle adulthood, we are able to more accurately assess the age distribution of our effects and better model continuous age effects for emotion representations and self-reported experience. As such, although we cannot rule out cohort effects with a cross-sectional study design, our findings across the adult age-span are at least suggestive of a continuous process across life rather than a categorical cohort effect. It is harder to justify why semantic knowledge about emotions, specifically in relation to interoceptive sensations and arousal, would have shifted linearly or curvilinearly in modern American history. Nonetheless, future studies should use a stratified sampling technique to ensure equal representation of younger, middle-aged and older adults when modelling age as a continuous variable. It would also be beneficial to test these hypotheses in a representative sample rather than a sample of convenience recruited online, as online workers may differ from the population in important ways (e.g., education, socio-economic status, etc.).
Finally, a limitation of the present studies is that we do not fully account for the role of other cognitive and motivational variables that also characterize aging. For example, older adults experience well-documented declines in cognitive abilities such as episodic memory (Morcom & Friston, 2012; Tromp et al., 2015) that could have contributed to our effects. Although it is possible that older adults have a harder time recalling certain types of information than younger and middle-aged adults, this does not explain why the observed findings are specific to interoceptive, arousal-based emotion representations and experiences—unless somehow episodic memory declines impact interoceptively-relevant information more than other domains of episodic memory, such as recalling emotional behaviors or situations.
Young children rely heavily on situations to understand and describe emotion categories, an effect which levels off around age 15 and remains stable into early adulthood. Thus, our finding that older adults maintain their situational and behavioral representations relative to interoceptive sensations could be consistent with “first in, last out” models of cognitive aging, where information learned first is the last to show the effects of cognitive aging (Rogers, Ivanoiu, Patterson, & Hodges, 2006). Even if this were the case, it would be an interesting finding unto itself. Future research should investigate how episodic memory, as well as other aspects of executive function such as working memory, might impact older adults’ ability to access and report on their interoceptive representations and experiences. Additionally, although both category associations and self-reports generally rely on retrospective memory and are susceptible to retrospective memory biases (Robinson & Clore, 2002), the experience sampling method we used in Study 2 is designed to help minimize retrospective memory confounds. As such, Study 1 most clearly speaks to how adults cognitively represent emotion categories across the age-span, but Study 2 may also suggest that adults’ actual experiences in daily life may shift in interoceptive-focus as well.
Beyond cognitive aging effects, it is likely that maturational dualism occurs alongside shifting motivations and expertise, working together to additively contribute to late life emotional differences. Examining how these different aspects of emotional aging may work together to exacerbate vs. buffer against different emotional outcomes (e.g., geriatric depression, affect-based decisions) is, we believe, an important future direction. For instance, we cannot rule out that the maturational differences in emotion that we observed are associated with age-related differences in motivation to experience certain states or age-related differences in emotion regulation skills, more generally. Older adults are known to avoid situations that cause negative and high arousal emotions (Isaacowitz & Ossenfort, 2017; Livingstone & Isaacowitz, 2015; Sands & Isaacowitz, 2017); it is thus possible that the findings in Study 2 could be explained by older adults’ motivation to avoid situations that lead to more intense, highly arousing interoceptive sensations. We cannot fully rule out this alternate explanation, as Study 2 did not ask participants to report on motivation for situation selection or the specifics of the situations they were experiencing (but see Supplementary Materials for exploratory analyses examining the link between situation endorsement and the differential experience of positive and negative emotions across adulthood). Similarly, we did not assess whether participants actively sought to regulate their emotions. Although older adults often report greater emotion regulation success than younger adults (see reviews and discussions in Carstensen, Fung, & Charles, 2003; Coats & Blanchard-Fields, 2008), older adults do not necessarily perform better than younger adults on momentary emotion regulation tests in the lab (Livingstone & Isaacowitz, 2019; Tuck, Mauss, & Consedine, 2014). As such, it remains an open question how much age-related differences in emotion regulation motivation and ability are driving the present effects.
Other future directions should continue to examine how individual differences interact with aging to predict differences in the quality of emotional experiences. For example, individual differences in cognitive aging predict the well-known positivity effect in late life (Carstensen & DeLiema, 2018). Similarly, older adults who exercise, maintain a healthy diet, or who have experienced less “wear and tear” on their biological systems (e.g., due to lower chronic life stressors; more resilient genetic predispositions, etc.) may exhibit different autonomic reactivity and interoception compared to peers, in turn affecting the quality of their emotions relative to other older adults. Health behaviors such as exercise and diet could perhaps help buffer against demyelination of peripheral nerves and promote the maintenance and efficiency of nervous system structures and functions. Indeed, exercise and diet can promote peripheral myelin repair (Zhou & Notterpek, 2016). As such, future studies could collect larger samples of older adults and consider the role that healthy lifestyle factors play in physiological contributions to aging emotions. These sorts of studies would also be invaluable for public health, given that exercise, diet, and other lifestyle factors are feasible targets for behavior change.
Conclusion
In sum, our findings demonstrate that individuals across different phases of adulthood think about and experience their emotions in qualitatively different ways. As age increases, adults are less likely to associate interoceptive sensations with emotions and this effect was specific to the interoceptive aspects of emotions; it did not occur for situations or behaviors associated with emotions. Even if they are the product of cohort effects, our findings suggest that emotion, health, and aging researchers should think carefully about how they measure and assess emotion as well as somatic symptoms and other interoceptive-based self-reports in older adults. On the other hand, if our effects are related to biological differences in the contribution of the peripheral nervous system to emotion (or even how the brain is processing peripheral signals during emotion), then researchers need to consider the many impacts that these findings might have for affect-based processes beyond emotion such as the role of peripheral and autonomic aging in the context of affective learning (e.g., aversion), person perception (e.g., trustworthiness), and affect-based decisions (e.g., in finances, politics, health). If the peripheral nervous system contributes less to affect-based phenomena with age, then behaviors and decisions that typically incorporate more of this interoceptive input may be adversely impacted.
We believe the most exciting work ahead lies at the intersection of different facets of aging, testing how end-of-life motivations, expertise in emotion knowledge and regulation, psychophysiology, and interoception may interact to shape emotional processes across the life span. Such work across perspectives, methodologies, and disciplines would not only help unravel interactions between the aging body and other domains of aging (e.g., social skills, motivation, memory, executive function), but could also provide a richer, more holistic picture of how and why emotional processes differ and change across the life span.
Supplementary Material
Acknowledgments
This work was supported by a Ruth L. Kirschstein National Research Service Award predoctoral fellowship to JKM from the National Institute on Aging (1F31AG055265-01A1).
Footnotes
The term “interoception” encompasses several different constructs which are actively under investigation in the emerging field of interoceptive science (Khalsa et al., 2018; Pollatos & Herbert, 2018). For example, behavioral measures of interoceptive ability such as the heartbeat detection and heartbeat counting tasks seek to assess objective sensitivity or accuracy in identifying one’s heartbeat. Other work identifies constructs such as interoceptive sensibility (beliefs about how interoceptive one is; Garfinkel et al., 2015) and interoceptive knowledge (what an individual knows about the bodily sensations associated with emotions and other states as drawn from idiographic experience and cultural transmission; MacCormack, Castro, Halberstadt, & Rogers, 2019), of relevance to the present paper.
In the pilot study, we began with an even number of items across modalities (Table 1). However, for the purposes of Study 1‘s association task, we excluded any pilot items that were ambiguously classified as belonging to more than one modality or as being strongly associated with more than one emotion category. For example, participants were more likely to classify “retch” as belonging to both the interoceptive and behavioral modalities or to strongly associate “fight” with both anger and fear. Although unequal numbers of modality items in Study 1 lend greater power to find an effect for interoceptive items relative to behavioral or situational items, this concern is partially mitigated by the use of multi-level modeling. Specifically, multilevel modeling helps account for different numbers of observations, providing greater power to detect effects across all modalities.
Importantly, these general age differences in emotion are not universal (Isaacowitz, Livingstone, & Castro, 2017). For example, although older adults in general appear to be biased towards positive stimuli, this is not always the case and does not always link to positive outcomes (Isaacowitz & Blanchard-Fields, 2012). Cross-cultural differences in these age effects also remain understudied (Grossmann, Karasawa, Kan, & Kitayama, 2014). See also Tuck, Mauss, and Consedine (2014) for a discussion of the ways in which emotion regulation does not always improve with age.
References
- Allen M, Levy A, Parr T, & Friston KJ (2019). In the body’s eye: The computational anatomy of interoceptive inference. BioRxiv Pre-Print. 10.1101/603928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayuso-Mateos JL, Miret M, Caballero FF, Olaya B, Haro JM, Kowal P, & Chatterji S (2013). Multi-country evaluation of affective experience: Validation of an abbreviated version of the day reconstruction method in seven countries. PLoS ONE, 8, e61534. 10.1371/journal.pone.0061534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhoorn JS, Haasnoot E, Bocanegra BR, & van Steenbergen H (2015). QRTEngine: An easy solution for running online reaction time experiments using Qualtrics. Behavior Research Methods, 47, 918–929. 10.3758/s13428-014-0530-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF (2017). The theory of constructed emotion: An active inference account of interoception and categorization. Social Cognitive and Affective Neuroscience, 12, 1–23. 10.1093/scan/nsw154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF (2018). Emotions are constructed with interoception and concepts within a predicting brain. In Fox AS, Lapate RC, Shackman AJ, & Davidson RJ (Eds.), The nature of emotion: Fundamental questions (2nd ed., pp. 33–38). New York: Oxford University Press. [Google Scholar]
- Barrett LF, & Bliss-Moreau E (2009). Affect as a psychological primitive. In Zanna MP (Ed.), Advances in Experimental Social Psychology (Vol. 41, pp. 167–218). Burlington: Academic Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Quigley KS, Bliss-Moreau E, & Aronson KR (2004). Interoceptive sensitivity and self-reports of emotional experience. Journal of Personality and Social Psychology, 87(5), 684–697. 10.1037/0022-3514.87.5.684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Robin L, Pietromonaco PR, & Eyssell KM (1998). Are women the “more emotional” sex? Evidence from emotional experiences in social context. Cognition & Emotion, 12, 555–578. 10.1080/026999398379565 [DOI] [Google Scholar]
- Bates D, Mächler M, Bolker B, & Walker S (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67, 1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Birditt KS, & Fingerman KL (2005). Do we get better at picking our battles? Age group differences in descriptions of behavioral reactions to interpersonal tensions. Journals of Gerontology - Series B Psychological Sciences and Social Sciences, 60(121–128). 10.1093/geronb/60.3.P121 [DOI] [PubMed] [Google Scholar]
- Birditt KS, Fingerman KL, & Almeida DM (2005). Age differences in exposure and reactions to interpersonal tensions: A daily diary study. Psychology and Aging, 20, 330–340. 10.1037/0882-7974.20.2.330 [DOI] [PubMed] [Google Scholar]
- Blascovich J, & Mendes WB (2010). Social psychophysiology and embodiment. In Fiske ST, Gilbert DT, & Lindzey G (Eds.), The Handbook of Social Psychology (5th ed., pp. 194–227). New York, NY: John Wiley & Sons, Inc. [Google Scholar]
- Borghi AM, & Pecher D (2011). Introduction to the special topic embodied and grounded cognition. Frontiers in Psychology, 2, 187. 10.3389/fpsyg.2011.00187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, & Lang PJ (1994). Measuring emotion: The self-assessment manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry, 25, 49–59. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7962581 [DOI] [PubMed] [Google Scholar]
- Breugelmans SM, Poortinga YH, Ambadar Z, Setiadi B, Vaca JB, Widiyanto P, & Philippot P (2005). Body sensations associated with emotions in Rarámuri Indians, rural Javanese, and three student samples. Emotion, 5, 166–174. 10.1037/1528-3542.5.2.166 [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Larsen JT, Poehlmann KM, & Ito TA (2000). The psychophysiology of emotion. In Lewis M & Haviland-Jones JM (Eds.), The Handbook of Emotion (pp. 173–191). New York: Guilford Press. [Google Scholar]
- Cannon WB (1927). The James-Lange theory of emotions: A critical examination and an alternative theory. The American Journal of Psychology, 39, 106. 10.2307/1415404 [DOI] [PubMed] [Google Scholar]
- Carstensen LL, & DeLiema M (2018). The positivity effect: A negativity bias in youth fades with age. Current Opinion in Behavioral Sciences, 19, 7–12. 10.1016/j.cobeha.2017.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL, Fung H, & Charles ST (2003). Socioemotional selectivity theory and the regulation of emotion in the second half of life. Motivation and Emotion, 27(2), 103–123. Retrieved from http://link.springer.com/article/10.1023/A:1024569803230 [Google Scholar]
- Carstensen LL, Isaacowitz DM, & Charles ST (1999). Taking time seriously: A theory of socioemotional selectivity. American Psychologist, 54, 165–181. 10.1037/0003-066X.54.3.165 [DOI] [PubMed] [Google Scholar]
- Carstensen LL, & Mikels JA (2005). At the intersection of emotion and cognition: Aging and the positivity effect. Current Directions in Psychological Science, 14, 117–121. 10.1111/j.0963-7214.2005.00348.x [DOI] [Google Scholar]
- Carstensen LL, Pasupathi M, Mayr U, & Nesselroade JR (2000). Emotional experience in everyday life across the adult life span. Journal of Personality and Social Psychology, 79, 644–655. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11045744 [PubMed] [Google Scholar]
- Carstensen LL, Turan B, Scheibe S, Ram N, Ersner-Hershfield H, Samanez-Larkin GR, … Nesselroade JR (2011). Emotional experience improves with age: Evidence based on over 10 years of experience sampling. Psychology and Aging, 26, 21–33. 10.1037/a0021285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle E, Eisenberger NI, Seeman TE, Moons WG, Boggero IA, Grinblatt MS, & Taylor SE (2012). Neural and behavioral bases of age differences in perceptions of trust. Proceedings of the National Academy of Sciences, 109, 20848–20852. 10.1073/pnas.1218518109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro VL, Halberstadt AG, & Garrett-Peters P (2016). A three-factor structure of emotion understanding in third-grade children. Social Development, 25, 602–622. 10.1111/sode.12162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles ST, Reynolds CA, & Gatz M (2001). Age-related differences and change in positive and negative affect over 23 years. Journal of Personality and Social Psychology, 80, 136–151. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11195886 [PubMed] [Google Scholar]
- Cheng S-T (2004). Age and subjective well-being revisited: A discrepancy perspective. Psychology and Aging, 19, 409–415. 10.1037/0882-7974.19.3.409 [DOI] [PubMed] [Google Scholar]
- Clithero JA, & Rangel A (2014). Informatic parcellation of the network involved in the computation of subjective value. Social Cognitive and Affective Neuroscience, 9, 1289–1302. 10.1093/scan/nst106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coats AH, & Blanchard-Fields F (2008). Emotion regulation in interpersonal problems: The role of cognitive-emotional complexity, emotion regulation goals, and expressivity. Psychology and Aging, 23, 39–51. 10.1037/0882-7974.23.1.39 [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, & Aiken LS (2003). Applied multiple regression/correlation analysis in the behavioral sciences (3rd ed.). Mahwah, NJ: Erlbaum. [Google Scholar]
- Craig AD (2003). Interoception: the sense of the physiological condition of the body. Current Opinion in Neurobiology, 13, 500–505. 10.1016/S0959-4388(03)00090-4 [DOI] [PubMed] [Google Scholar]
- Critchley HD, & Garfinkel SN (2017). Interoception and emotion. Current Opinion in Psychology, 17, 7–14. 10.1016/j.copsyc.2017.04.020 [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Öhman A, & Dolan RJ (2004). Neural systems supporting interoceptive awareness. Nature Neuroscience, 7, 189–195. 10.1038/nn1176 [DOI] [PubMed] [Google Scholar]
- Damasio AR (1994). Descartes’ error: Emotion, reason, and the human brain. New York: Putnam. Retrieved from https://scholar.google.nl/scholar?start=0&q=AR+Damasio&hl=en&as_sdt=0,5#0%0Ahttps://scholar.google.nl/scholar?q=AR+Damasio&btnG=&hl=en&as_sdt=0%2C5#0 [Google Scholar]
- Davidson RJ (2003). Seven sins in the study of emotion: Correctives from affective neuroscience. Brain and Cognition, 52, 129–132. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12812811 [DOI] [PubMed] [Google Scholar]
- Diehl M, & Hay EL (2011). Self-concept differentiation and self-concept clarity across adulthood: Associations with age and psychological well-being. International Journal of Aging & Human Development, 73, 125–152. 10.2190/AG.73.2.b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener E, & Tay L (2014). Review of the day reconstruction method (DRM). Social Indicators Research, 116, 255–267. 10.1007/s11205-013-0279-x [DOI] [Google Scholar]
- Dockray S, Grant N, Stone AA, Kahneman D, Wardle J, & Steptoe A (2010). A comparison of affect ratings obtained with ecological momentary assessment and the day reconstruction method. Social Indicators Research, 99, 269–283. 10.1007/s11205-010-9578-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle CM, & Lindquist KA (2018). When a word is worth a thousand pictures: Language shapes perceptual memory for emotion. Journal of Experimental Psychology: General, 147, 62–73. 10.1037/xge0000361 [DOI] [PubMed] [Google Scholar]
- Fredrickson BL, Tugade MM, Waugh CE, & Larkin GR (2003). What good are positive emotions in crises? A prospective study of resilience and emotions following the terrorist attacks on the United States on September 11th, 2001. Journal of Personality and Social Psychology, 84, 365–376. 10.1037/0022-3514.84.2.365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman VA, Conrad F, Cornman J, Schwarz N, & Stafford F (2014). Does time fly when you are having fun? A day reconstruction method analysis. Journal of Happiness Studies, 15, 639–655. 10.1007/s10902-013-9440-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel SN, Minati L, Gray M. a, Seth AK, Dolan RJ, & Critchley HD (2014). Fear from the heart: sensitivity to fear stimuli depends on individual heartbeats. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 34(19), 6573–6582. 10.1523/JNEUROSCI.3507-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel SN, Seth AK, Barrett AB, Suzuki K, & Critchley HD (2015). Knowing your own heart: Distinguishing interoceptive accuracy from interoceptive awareness. Biological Psychology, 104, 65–74. 10.1016/j.biopsycho.2014.11.004 [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, & Frackowiak RS (2001). A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage, 14, 21–36. 10.1006/nimg.2001.0786 [DOI] [PubMed] [Google Scholar]
- Gross JJ, Carstensen LL, Pasupathi M, Tsai J, Skorpen CG, & Hsu AY (1997). Emotion and aging: Experience, expression, and control. Psychology and Aging, 12, 590–599. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9416628 [DOI] [PubMed] [Google Scholar]
- Grossmann I, Karasawa M, Kan C, & Kitayama S (2014). A cultural perspective on emotional experiences across the life span. Emotion, 14(4), 679–692. 10.1037/a0036041 [DOI] [PubMed] [Google Scholar]
- Gruber J, Kogan A, Quoidbach J, & Mauss IB (2013). Happiness is best kept stable: Positive emotion variability is associated with poorer psychological health. Emotion, 13, 1–6. 10.1037/a0030262 [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM, & Blanchard-Fields F (2012). Linking process and outcome in the study of emotion and aging. Perspectives on Psychological Science, 7, 3–17. 10.1177/1745691611424750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacowitz DM, & Livingstone KM (2015). Emotion in adulthood: What changes and why? In Branscombe NR & Reynolds K (Eds.), Psychology of Change (pp. 116–132). New York: Psychology Press. [Google Scholar]
- Isaacowitz DM, Livingstone KM, & Castro VL (2017). Aging and emotions: Experience, regulation, and perception. Current Opinion in Psychology, 17, 79–83. 10.1016/j.copsyc.2017.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacowitz DM, & Ossenfort KL (2017). Aging, attention and situation selection: Older adults create mixed emotional environments. Current Opinion in Behavioral Sciences, 15, 6–9. 10.1016/j.cobeha.2017.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W (1890). The principles of psychology. New York: Henry Holt and Company. [Google Scholar]
- Kahneman D, Krueger AB, Schkade DA, Schwarz N, & Stone AA (2004). A survey method for characterizing daily life experience: The day reconstruction method. Science, 306, 1776–1780. 10.1126/science.1103572 [DOI] [PubMed] [Google Scholar]
- Kan IP, Barsalou LW, Solomon KO, Minor JK, & Thompson-Schill SL (2003). Role of mental imagery in a property verification task: fMRI evidence for perceptual representations of conceptual knowledge. Cognitive Neuropsychology, 20, 525–540. 10.1080/02643290244000257 [DOI] [PubMed] [Google Scholar]
- Khalsa SS, Adolphs R, Cameron OG, Critchley HD, Davenport PW, Feinstein JS, … Zucker N (2018). Interoception and mental health: A roadmap. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3, 501–513. 10.1016/j.bpsc.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, & Tranel D (2009). Interoceptive awareness declines with age. Psychophysiology, 46(6), 1130–1136. 10.1111/j.1469-8986.2009.00859.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircanski K, Notthoff N, DeLiema M, Samanez-Larkin GR, Shadel D, Mottola G, … Gotlib IH (2018). Emotional arousal may increase susceptibility to fraud in older and younger adults. Psychology and Aging, 33, 325–337. 10.1037/pag0000228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner IR, Zhang J, Touroutoglou A, Chanes L, Xia C, Simmons WK, … Barrett LF (2017). Evidence for a large-scale brain system supporting allostasis and interoception in humans. Nature Human Behaviour, 1. 10.1038/s41562-017-0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslyn SM (1976). Can imagery be distinguished from other forms of internal representation? Evidence from studies of information retrieval times. Memory & Cognition, 4, 291–297. 10.3758/BF03213178 [DOI] [PubMed] [Google Scholar]
- Kövecses Z (2000). Metaphor and Emotion: Language, Culture, and Body in Human Feeling. Studies in Emotion and Social Interaction (2nd Editio, Vol. Studies in). Cambridge, UK: Cambridge University Press. 10.1207/S15327868MS1703_5 [DOI] [Google Scholar]
- Labouvie-Vief G, DeVoe M, & Bulka D (1989). Speaking about feelings: Conceptions of emotion across the life span. Psychology and Aging, 4, 425–437. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2619949 [DOI] [PubMed] [Google Scholar]
- Laird JD, & Lacasse K (2013). Bodily Influences on Emotional Feelings: Accumulating Evidence and Extensions of William James’s Theory of Emotion. Emotion Review, 6(1), 27–34. 10.1177/1754073913494899 [DOI] [Google Scholar]
- Lebois LAM, Wilson-Mendenhall CD, Simmons WK, Barrett LF, & Barsalou LW (2018). Learning situated emotions. Neuropsychologia 10.1016/j.neuropsychologia.2018.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA (2013). Emotions emerge from more basic psychological ingredients: A modern psychological constructionist model. Emotion Review, 5, 356–368. 10.1177/1754073913489750 [DOI] [Google Scholar]
- Lindquist KA, & Barrett LF (2008). Emotional complexity. In Lewis M, Haviland-Jones JM, & Barrett LF (Eds.), Handbook of emotions (3rd ed., pp. 513–530). New York: The Guilford Press. [Google Scholar]
- Lindquist KA, MacCormack JK, & Shablack H (2015). The role of language in emotion: Predictions from psychological constructionism. Frontiers in Psychology, 6, 1–15. 10.3389/fpsyg.2015.00444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, Satpute AB, Wager TD, Weber J, & Barrett LF (2016). The brain basis of positive and negative affect: Evidence from a meta-analysis of the human neuroimaging literature. Cerebral Cortex, 26, 1910–1922. 10.1093/cercor/bhv001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, & Barrett LF (2012). The brain basis of emotion: A meta-analytic review. Behavioral and Brain Sciences, 35(3), 121–143. 10.1017/S0140525X11000446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone KM, Castro VL, & Isaacowitz DM (2018). Age differences in beliefs about emotion regulation strategies. The Journals of Gerontology: Series B, gby022. 10.1093/geronb/gby022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone KM, & Isaacowitz DM (2015). Situation selection and modification for emotion regulation in younger and older adults. Social Psychological and Personality Science, 6(8), 904–910. 10.1177/1948550615593148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone KM, & Isaacowitz DM (2019). Age similarities and differences in spontaneous use of emotion regulation tactics across five laboratory tasks. Journal of Experimental Psychology: General, epub ahead of print. 10.1037/xge0000556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockenhoff CE, Costa PT, & Lane RD (2008). Age differences in descriptions of emotional experiences in oneself and others. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 63, 92–99. 10.1093/geronb/63.2.P92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCormack JK, Armstrong-Carter EL, Gaudier-Diaz MM, Meltzer-Brody S, Sloan EK, Lindquist KA, & Muscatell KA (2019). Beta-adrenergic contributions to emotion during acute stress: A pharmacological approach. Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCormack JK, Castro VL, Halberstadt AG, & Rogers ML (2019). Mothers’ interoceptive knowledge predicts children’s emotion regulation and social skills in middle childhood. Under Revision. [Google Scholar]
- MacCormack JK, & Lindquist KA (2017). Bodily contributions to emotion: Schachter’s legacy for a psychological constructionist view on emotion. Emotion Review, 9, 36–45. 10.1177/1754073916639664 [DOI] [Google Scholar]
- MacCormack JK, Stein AG, Giovanello KS, Kang J, Satpute AB, & Lindquist KA (2019). Affect in the aging brain: A neuroimaging meta-analysis of functional activation and connectivity differences in older vs. younger adult affective experience and perception. Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M (2012). The emotion paradox in the aging brain. Annals of the New York Academy of Sciences, 1251, 33–49. 10.1111/j.1749-6632.2012.06471.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, & Carstensen LL (2005). Aging and motivated cognition: The positivity effect in attention and memory. Trends in Cognitive Sciences, 9, 496–502. 10.1016/j.tics.2005.08.005 [DOI] [PubMed] [Google Scholar]
- Mendes WB (2010). Weakened links between mind and body in older age: The case for maturational dualism in the experience of emotion. Emotion Review, 2, 240–244. 10.1177/1754073910364149 [DOI] [Google Scholar]
- Morcom AM, & Friston KJ (2012). Decoding episodic memory in ageing: A Bayesian analysis of activity patterns predicting memory. NeuroImage, 59, 1772–1782. 10.1016/j.neuroimage.2011.08.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi Y, Negreira A, Weierich M, Dautoff R, Dickerson BC, Wright CI, & Barrett LF (2011). Differential hemodynamic response in affective circuitry with aging: an FMRI study of novelty, valence, and arousal. Journal of Cognitive Neuroscience, 23(5), 1027–1041. 10.1162/jocn.2010.21527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mroczek DK, & Kolarz CM (1998). The effect of age on positive and negative affect: A developmental perspective on happiness. Journal of Personality and Social Psychology, 75, 1333–1349. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9866191 [DOI] [PubMed] [Google Scholar]
- Murphy J, Geary H, Millgate E, Catmur C, & Bird G (2018). Direct and indirect effects of age on interoceptive accuracy and awareness across the adult lifespan. Psychonomic Bulletin & Review, 25, 1193–1202. 10.3758/s13423-017-1339-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupert SD, Almeida DM, & Charles ST (2007). Age differences in reactivity to daily stressors: The role of personal control. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 62, 216–225. 10.1093/geronb/62.4.P216 [DOI] [PubMed] [Google Scholar]
- Niedenthal PM, Barsalou LW, Winkielman P, Krauth-Gruber S, & Ric F (2005). Embodiment in attitudes, social perception, and emotion. Personality and Social Psychology Review, 9, 184–211. 10.1207/s15327957pspr0903_1 [DOI] [PubMed] [Google Scholar]
- Nook EC, Sasse SF, Lambert HK, McLaughlin KA, & Somerville LH (2018). The nonlinear development of emotion differentiation: Granular emotional experience is low in adolescence. Psychological Science, 29, 1346–1357. 10.1177/0956797618773357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nook EC, Stavish CM, Sasse SF, Lambert HK, Mair P, McLaughlin KA, & Somerville LH (n.d.). Charting the development of emotion comprehension and abstraction from childhood to adulthood using observer-rated and linguistic measures. Emotion. 10.1037/emo0000609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa L, Glerean E, Hari R, & Hietanen JK (2014). Bodily maps of emotions. Proceedings of the National Academy of Sciences of the United States of America, 111(2), 646–651. 10.1073/pnas.1321664111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa L, Hari R, Hietanen JK, & Glerean E (2018). Maps of subjective feelings. Proceedings of the National Academy of Sciences, 115, 9198–9203. 10.1073/pnas.1807390115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien O, Oosterwijk S, & Barrett LF (2016). Where do people feel emotions in their body? A quantitative implementation of the Emotionally}Vague project. In Proceedings of the 10th International Conference on Design & Emotion (pp. 547–552). [Google Scholar]
- Oosterwijk S, & Barrett LF (2014). Embodiment in the construction of emotion experience and emotion understanding. In Shapiro L (Ed.), Routledge Handbook of Embodied Cognition (pp. 250–260). New York: Routledge. [Google Scholar]
- Oosterwijk S, Mackey S, Wilson-Mendenhall C, Winkielman P, & Paulus MP (2015). Concepts in context: Processing mental state concepts with internal or external focus involves different neural systems. Social Neuroscience, 10, 294–307. 10.1080/17470919.2014.998840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterwijk S, Winkielman P, Pecher D, Zeelenberg R, Rotteveel M, & Fischer AH (2012). Mental states inside out: Switching costs for emotional and nonemotional sentences that differ in internal and external focus. Memory & Cognition, 40, 93–100. 10.3758/s13421-011-0134-8 [DOI] [PubMed] [Google Scholar]
- Palve SS, & Palve SB (2018). Impact of aging on nerve conduction velocities and late responses in healthy individuals. Journal of Neurosciences in Rural Practice, 9, 112–116. 10.4103/jnrp.jnrp_323_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecher D, Zeelenberg R, & Barsalou LW (2004). Sensorimotor simulations underlie conceptual representations: modality-specific effects of prior activation. Psychonomic Bulletin & Review, 11(1), 164–167. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15117003 [DOI] [PubMed] [Google Scholar]
- Peng RD (2008). Simpleboot: Simple bootstrap routines. [Google Scholar]
- Pollatos O, & Herbert BM (2018). Interoception: Definitions, dimensions, neural substrates. In Hauke G & Kritikos A (Eds.), Embodiment in Psychotherapy (pp. 15–27). Cham: Springer. 10.1007/978-3-319-92889-0_2 [DOI] [Google Scholar]
- Pons F, Lawson J, Harris PL, & de Rosnay M (2003). Individual differences in children’s emotion understanding: Effects of age and language. Scandinavian Journal of Psychology, 44, 347–353. 10.1111/1467-9450.00354 [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, & Bryk AS (2001). Hierarchical linear models: Applications and data analysis methods (2nd ed.). Thousand Oaks, CA: Sage. [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, … Acker JD (2005). Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cerebral Cortex, 15, 1676–1689. 10.1093/cercor/bhi044 [DOI] [PubMed] [Google Scholar]
- Robinson MD, & Clore GL (2002). Belief and feeling: Evidence for an accessibility model of emotional self-report. Psychological Bulletin, 128, 934–960. 10.1037//0033-2909.128.6.934 [DOI] [PubMed] [Google Scholar]
- Rogers TT, Ivanoiu A, Patterson K, & Hodges JR (2006). Semantic memory in Alzheimer’s disease and the frontotemporal dementias: A longitudinal study of 236 patients. Neuropsychology, 20, 319–335. 10.1037/0894-4105.20.3.319 [DOI] [PubMed] [Google Scholar]
- Russell JA (1980). A circumplex model of affect. Journal of Personality and Social Psychology, 39(6), 1161–1178. 10.1037/h0077714 [DOI] [Google Scholar]
- Sands M, & Isaacowitz DM (2017). Situation selection across adulthood: The role of arousal. Cognition and Emotion, 31, 791–798. 10.1080/02699931.2016.1152954 [DOI] [PubMed] [Google Scholar]
- Scherbaum CA, & Ferreter JM (2009). Estimating statistical power and required sample sizes for organizational research using multilevel modeling. Organizational Research Methods, 12, 347–367. 10.1177/1094428107308906 [DOI] [Google Scholar]
- Schilling OK, Wahl H-W, & Wiegering S (2013). Affective development in advanced old age: Analyses of terminal change in positive and negative affect. Developmental Psychology, 49, 1011–1020. 10.1037/a0028775 [DOI] [PubMed] [Google Scholar]
- Schulz A, & Vögele C (2015). Interoception and stress. Frontiers in Psychology, 6, 993. 10.3389/fpsyg.2015.00993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth AK (2013). Interoceptive inference, emotion, and the embodied self. Trends in Cognitive Sciences, 17(11), 565–573. 10.1016/j.tics.2013.09.007 [DOI] [PubMed] [Google Scholar]
- Shallcross AJ, Ford BQ, Floerke VA, & Mauss IB (2013). Getting better with age: The relationship between age, acceptance, and negative affect. Journal of Personality and Social Psychology, 104, 734–749. 10.1037/a0031180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel EH, Sands MK, Van den Noortgate W, Condon P, Chang Y, Dy J, … Barrett LF (2018). Emotion fingerprints or emotion populations? A meta-analytic investigation of autonomic features of emotion categories. Psychological Bulletin, 144, 343–393. 10.1037/bul0000128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemer M, Mauss I, & Gross JJ (2007). Same situation--different emotions: How appraisals shape our emotions. Emotion, 7, 592–600. 10.1037/1528-3542.7.3.592 [DOI] [PubMed] [Google Scholar]
- Simmons WK, Ramjee V, Beauchamp MS, McRae K, Martin A, & Barsalou LW (2007). A common neural substrate for perceiving and knowing about color. Neuropsychologia, 45, 2802–2810. 10.1016/j.neuropsychologia.2007.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, & Kirby LD (2009). Putting appraisal in context: Toward a relational model of appraisal and emotion. Cognition & Emotion, 23, 1352–1372. 10.1080/02699930902860386 [DOI] [Google Scholar]
- Song S, & Johnson F (2018). Epigenetic mechanisms impacting aging: A focus on histone levels and telomeres. Genes, 9, 201. 10.3390/genes9040201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Deaton A, & Stone AA (2015). Subjective wellbeing, health, and ageing. Lancet, 385, 640–648. 10.1016/S0140-6736(13)61489-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AA, Schneider S, & Harter JK (2012). Day-of-week mood patterns in the United States: On the existence of ‘Blue Monday’, ‘Thank God it’s Friday’ and weekend effects. The Journal of Positive Psychology, 7, 306–314. 10.1080/17439760.2012.691980 [DOI] [Google Scholar]
- Sun FW, Stepanovic MR, Andreano J, Barrett LF, Touroutoglou A, & Dickerson BC (2016). Youthful brains in older adults: Preserved neuroanatomy in the default mode and salience networks contributes to youthful memory in superaging. Journal of Neuroscience, 36, 9659–9668. 10.1523/JNEUROSCI.1492-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaka J, Blascovich J, Kelsey RM, & Leitten CL (1993). Subjective, physiological, and behavioral effects of threat and challenge appraisal. Journal of Personality and Social Psychology, 65, 248–260. 10.1037/0022-3514.65.2.248 [DOI] [Google Scholar]
- Tromp D, Dufour A, Lithfous S, Pebayle T, & Després O (2015). Episodic memory in normal aging and Alzheimer disease: Insights from imaging and behavioral studies. Ageing Research Reviews, 24, 232–262. 10.1016/j.arr.2015.08.006 [DOI] [PubMed] [Google Scholar]
- Tsai JL, Levenson RW, & Carstensen LL (2000). Autonomic, subjective, and expressive responses to emotional films in older and younger Chinese Americans and European Americans. Psychology and Aging, 15, 684–693. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11144327 [DOI] [PubMed] [Google Scholar]
- Tuck N, Mauss I, & Consedine NS (2014). Are we really getting better? Lifespan differences in emotion regulatory ability from the perspective of developmental functionalism. ISSBD Bulletin, 1(65), 22–26. Retrieved from http://www.issbd.org/resources/files/JBD_38_3S.pdf [Google Scholar]
- Uchino BN, Birmingham W, & Berg CA (2010). Are older adults less or more physiologically reactive? A meta-analysis of age-related differences in cardiovascular reactivity to laboratory tasks. The Journals of Gerontology: Series B, 65B, 154–162. 10.1093/geronb/gbp127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, & Gross JJ (2010). Emotion regulation in older age. Current Directions in Psychological Science, 19, 352–357. 10.1177/0963721410388395 [DOI] [Google Scholar]
- Verdú E, Ceballos D, Vilches JJ, & Navarro X (2000). Influence of aging on peripheral nerve function and regeneration. Journal of the Peripheral Nervous System, 5, 191–208. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11151980 [DOI] [PubMed] [Google Scholar]
- Vytal K, & Hamann S (2010). Neuroimaging support for discrete neural correlates of basic emotions: A voxel-based meta-analysis. Journal of Cognitive Neuroscience, 22, 2864–2885. 10.1162/jocn.2009.21366 [DOI] [PubMed] [Google Scholar]
- Weisman K, Dweck CS, & Markman EM (2017). Rethinking people’s conceptions of mental life. Proceedings of the National Academy of Sciences, 114, 11374–11379. 10.1073/pnas.1704347114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan R (2008). Effective analysis of reaction time data. The Psychological Record, 58, 475–482. 10.1007/BF03395630 [DOI] [Google Scholar]
- Widen SC, & Russell JA (2010). Differentiation in preschooler’s categories of emotion. Emotion, 10, 651–661. 10.1037/a0019005 [DOI] [PubMed] [Google Scholar]
- Wilson-Mendenhall CD, Barrett LF, & Barsalou LW (2013). Situating emotional experience. Frontiers in Human Neuroscience, 7(November), 764. 10.3389/fnhum.2013.00764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Mendenhall CD, Barrett LF, Simmons WK, & Barsalou LW (2011). Grounding emotion in situated conceptualization. Neuropsychologia, 49(5), 1105–1127. 10.1016/j.neuropsychologia.2010.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Woody A, Padin AC, Lin J, Malarkey WB, & Kiecolt-Glaser JK (2018). Loneliness and telomere length: Immune and parasympathetic function in associations with accelerated aging. Annals of Behavioral Medicine, kay064. 10.1093/abm/kay064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yik MSM, Russell JA, & Barrett LF (1999). Structure of self-reported current affect: Integration and beyond. Journal of Personality and Social Psychology, 77, 600–619. 10.1037/0022-3514.77.3.600 [DOI] [Google Scholar]
- Zebrowitz LA, Boshyan J, Ward N, Gutchess A, & Hadjikhani N (2017). The older adult positivity effect in evaluations of trustworthiness: Emotion regulation or cognitive capacity? PLOS ONE, 12, e0169823. 10.1371/journal.pone.0169823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, & Notterpek L (2016). Promoting peripheral myelin repair. Experimental Neurology, 283, 573–580. 10.1016/j.expneurol.2016.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.