Clostridioides (formerly Clostridium) difficile is the most common cause of hospital-acquired infection, and advanced age is a risk factor for C. difficile infection. Disruption of the intestinal microbiota and immune responses contribute to host susceptibility and severity of C. difficile infection. However, the specific impact of aging on immune responses during C. difficile infection remains to be well described.
KEYWORDS: Clostridioides difficile, aging, eosinophils, gastrointestinal infection, innate immunity, intestinal colonization
ABSTRACT
Clostridioides (formerly Clostridium) difficile is the most common cause of hospital-acquired infection, and advanced age is a risk factor for C. difficile infection. Disruption of the intestinal microbiota and immune responses contribute to host susceptibility and severity of C. difficile infection. However, the specific impact of aging on immune responses during C. difficile infection remains to be well described. This study explores the effect of age on cellular and cytokine immune responses during C. difficile infection. Young mice (2 to 3 months old) and aged mice (22 to 28 months old) were rendered susceptible to C. difficile infection with the antibiotic cefoperazone and then infected with C. difficile strains with varied disease-causing potentials. We observe that the host age and the infecting C. difficile strain influenced the severity of disease associated with infection. Tissue-specific CD45+ immune cell responses occurred at the time of peak disease severity in the ceca and colons of all mice infected with a high-virulence strain of C. difficile; however, significant deficits in intestinal neutrophils and eosinophils were detected in aged mice, with a corresponding decrease in circulating CXCL1, an important neutrophil recruiter and activator. Interestingly, this lack of intestinal granulocyte response in aged mice during severe C. difficile infection was accompanied by a simultaneous increase in circulating white blood cells, granulocytes, and interleukin 17A (IL-17A). These findings demonstrate that age-related alterations in neutrophils and eosinophils and systemic cytokine and chemokine responses are associated with severe C. difficile infection and support a key role for intestinal eosinophils in mitigating C. difficile-mediated disease severity.
INTRODUCTION
In the last 2 decades, the frequency of Clostridioides difficile infection (CDI) among hospitalized patients has steadily increased, particularly among those 65 years of age and older (1). Several studies have demonstrated that as an individual’s age increases, so does their risk of C. difficile infection and the severity of CDI-associated disease (2, 3). While the connection between advanced age and severe CDI disease outcomes has been well established, the contributions of the aging host’s immune responses during acute CDI disease development and the pathogenicity of the C. difficile strain remain to be clarified.
Eosinophils are innate immune cells that predominantly reside in close proximity to microbes that colonize mucosal surfaces under noninflammatory homeostasis (4). The biological function of eosinophils in health and disease is most well studied and described in the protection against helminth infections (5) and in the pathogenesis of allergy (6). There is now growing evidence supporting a previously underappreciated role for eosinophils as important mediators of intestinal immune responses (7), and the expression of a broad range of pattern recognition receptors in eosinophils suggests a potential role in bacterial infection (8). Recent efforts by multiple research groups have indicated a role for eosinophils in CDI disease (9–11). However, the specific role for eosinophils in CDI disease severity has yet to be completely elucidated, and few studies characterize the innate immune responses to C. difficile strains with a range of virulence potentials in animals of advanced age.
CDI disease severity is influenced by host factors and the virulence of the C. difficile strain (12). Aging is known to cause immune dysfunction and negatively impacts patients in the setting of infectious diseases in the intestine (13). While immunosenescence likely plays a role in modulating CDI outcomes (14, 15), dysregulation of particular cytokine responses and immune cell subsets associated with advanced age may differentially contribute to CDI disease severity. In the present study, we characterize the effect of C. difficile strain virulence and host age on the cellular immune response using a murine model of CDI utilizing C. difficile strain 630 (low virulence) and strain VPI 10463 (high virulence), as well as a young cohort and an aged cohort of adult mice reared in the same animal facility.
RESULTS
Severity of clinical disease and degree of intestinal histopathology associated with C. difficile infection are influenced by host age and infecting strain.
Young mice (2 to 3 months old) and aged mice (22 to 28 months old) were rendered susceptible to C. difficile infection (CDI) by treatment with the antibiotic cefoperazone prior to oral challenge with spores derived from C. difficile strain 630 (low virulence) or strain VPI 10463 (high virulence) (Fig. 1A). Samples utilized in this study were obtained from animals at the times of peak clinical disease severity: day 2 postinfection with C. difficile strain VPI 10463 and day 4 postinfection with C. difficile strain 630 (12). Day 2 postinfection was chosen for infection with VPI 10463, as infected mice succumbed to disease shortly afterwards, hindering the collection of viable tissues for subsequent cellular analyses. There was no age-associated difference in C. difficile colonization in mice infected with strain VPI 10463 or strain 630 (Fig. 1B).
FIG 1.
Host age does not significantly impact C. difficile colonization. (A) Mouse model of C. difficile infection with a low-virulence strain (630) or a high-virulence strain (VPI 10463) in young and aged animals. (B) Cecal contents were collected from young mice and aged mice at the time of peak disease severity (day 2 postinfection with strain VPI 10463 or day 4 postinfection with strain 630) or after mock infection with vehicle and plated anaerobically on selective agar plates to quantify C. difficile burden. Dotted line indicates limit of detection for C. difficile quantification (103 CFU).
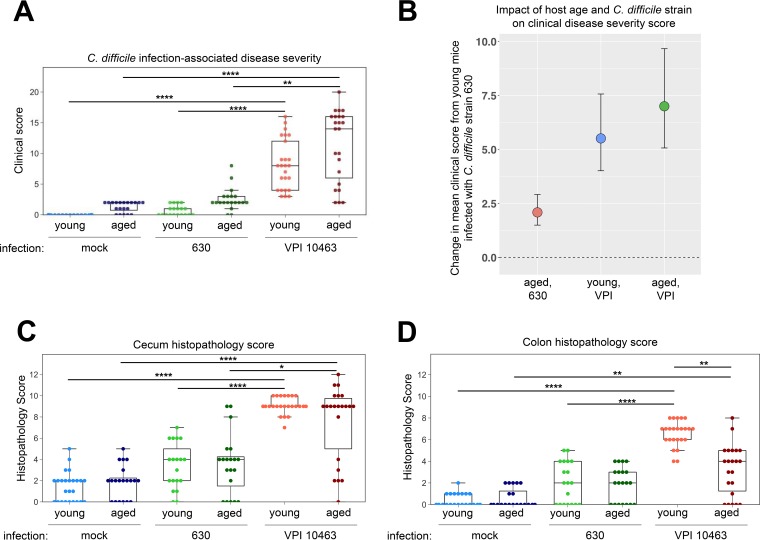
We confirmed that C. difficile strain VPI 10463 causes significantly worse disease than strain 630, regardless of age (Fig. 2A) (P values were <0.0001 for young mice and <0.01 for aged mice). At the time of peak clinical disease, two factors significantly contributed to developing severe disease: the infecting strain of C. difficile and age. Linear regression modeling demonstrated that both advanced age and infection with C. difficile strain VPI 10463 interacted to increase the clinical score. Therefore, the largest difference in infected mice was seen between young mice infected with strain 630 and aged mice infected with strain VPI 10463 (mean change in clinical score of 7, 95% confidence interval [CI], 5 to 10) (Fig. 2B) (P values for regression modeling of individual effects are reported in Table S1 in the supplemental material).
FIG 2.
C. difficile strain VPI 10463 causes more severe CDI than strain 630, regardless of age. (A) Clinical scores of young and aged mice infected with C. difficile strain VPI 10463 or strain 630 at peak clinical disease severity (day 2 and 4 postinfection, respectively). (B) Mean changes in clinical score with the 95% confidence interval compared to that in young mice infected with strain 630. Data are arranged by magnitude of clinical score change, and modeling coefficients are shown in Table S1 in the supplemental material. Histopathology scores for cecum (C) and colon (D) tissues collected from young and aged mice. Epithelial destruction, immune cell infiltration, and edema were scored on a 4-point scale for each category, and the sum of these scores determined the histological score in each tissue. Kruskal-Wallis one-way ANONA with Dunn’s post hoc test: *, P < 0.05; **, P < 0.01; ****, P < 0.0001.
In addition to assessing overt clinical disease, we explored whether aging modifies the degree of intestinal tissue pathology during C. difficile infection. Histopathology in the ceca (Fig. 2C) and colons (Fig. 2D) of young and aged mice was quantified by a veterinary pathologist that scored tissues for the amount of edema, infiltration of leukocytes, and epithelial damage. Cecum and colon histopathology scores were similar between young and old mice at baseline as well as during CDI with strain 630 (Fig. 2C and D), corresponding to the minimal clinical disease observed with this strain of C. difficile (Fig. 2A). In contrast, infection with C. difficile strain VPI 10463 was associated with a significantly higher degree of cecum (P < 0.0001) and colon (P < 0.0001 for young mice and P < 0.01 for aged mice) histopathology, regardless of age (Fig. 2C and D). Interestingly, mice of advanced age responded with significantly less colonic histopathology during infection with C. difficile strain VPI 10463 than young mice (Fig. 2D) (P < 0.01).
Age and infecting C. difficile strain influence the cellular immune response in the intestines of mice.
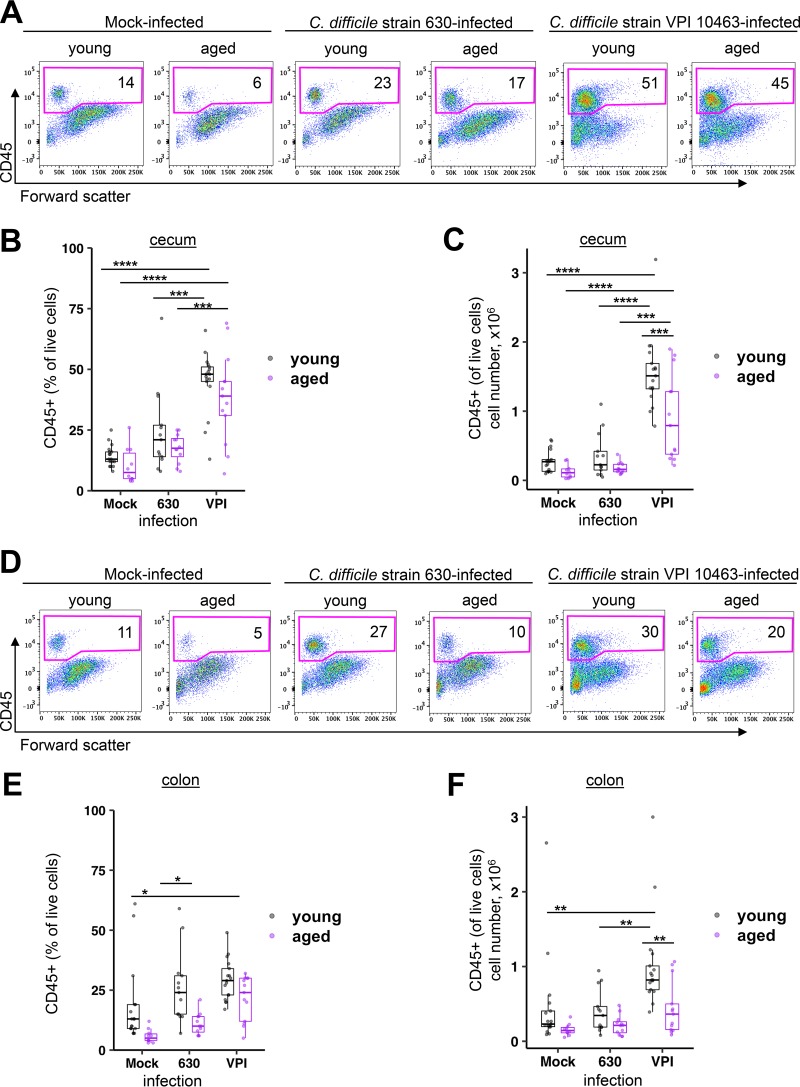
Total immune cells and myeloid cell subsets in the laminae propriae of ceca and colons from young and aged mice were analyzed by flow cytometry at the time of peak disease severity (representative plots, Fig. 3A and D). C. difficile strain 630 did not elicit an early cellular intestinal immune response in the cecum (Fig. 3B and C) or colon (Fig. 3E and F), independent of age. Due to the absence of a local intestinal cellular immune response during infection with the low-virulence C. difficile strain 630, we focused on further characterizing the nature of the immune cells infiltrating the distal intestinal tract during infection with the more virulent C. difficile strain VPI 10463.
FIG 3.
CD45+ leukocytes are preferentially increased in the cecum and colon laminae propriae of young and aged mice infected with C. difficile strain VPI 10463 compared to those infected with C. difficile strain 630. Mice were mock infected with vehicle or infected with C. difficile strain VPI 10463 or strain 630, and total immune cells were quantified in the cecum and colon at the time of peak disease severity. (A) Representative flow cytometry plots indicating the percentage of CD45+ leukocytes in cecum lamina propria. Percentages (B) and absolute numbers (C) of CD45+ leukocytes in total live lamina propria cells harvested from cecum. (D) Representative flow cytometry plots indicating the percentages of CD45+ leukocytes in colon lamina propria. Percentages (E) and absolute numbers (F) of CD45+ leukocytes in total live lamina propria cells harvested from colon. ANOVA and Tukey’s test: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Of the CD45+ immune cells in the ceca and colons of young and aged mice infected with C. difficile strain VPI 10463 at the time of peak disease severity, the majority were CD11b+ cells (Fig. 4A and B). While there was a significant increase in CD11b+ cell numbers in the ceca of young (P < 0.0001) and aged (P < 0.01) mice infected with C. difficile VPI 10463, this response was significantly blunted in aged mice (Fig. 4C and D) (P < 0.001). Interestingly, we observed local differences in intestinal CD11b+ cell responses in aged mice. There was a lack of response by CD11b+ cells in the colons of aged mice infected with C. difficile VPI 10463 in contrast to a significant increase in colonic CD11b+ cells in young counterparts (Fig. 4E and F) (P < 0.0001). Similarly, subsets of CD11b+ myeloid cells, including CD11b+ Ly6G+ Siglec-F− neutrophils and CD11b+ Ly6G− Siglec-F+ eosinophils, showed an age-dependent difference in intestinal response to C. difficile infection (Fig. 5). While aged mice indeed mounted a cecal neutrophil response during severe CDI (P < 0.01), it was significantly dampened compared to that of their young counterparts (Fig. 5C) (P < 0.01). Furthermore, colonic neutrophil infiltration during CDI was not observed in aged mice during severe CDI (Fig. 5C).
FIG 4.
CD11b+ cells are the dominant immune cell type in the cecum and colon laminae propriae of young and aged mice with severe CDI. Immune cell subsets from mock-infected or C. difficile strain VPI 10463-infected young and aged mice 2 days postinfection were analyzed by flow cytometry. The ratios of CD11b+ cells, CD3+ lymphocytes, and “other” CD11b− CD3− cells of the total CD45+ immune cells in ceca (A) and colons (B) of mock- or C. difficile strain VPI 10463-infected young and aged mice 2 days postinfection. (C) Representative flow cytometry plots indicating the percentages of CD11b+ myeloid cells in cecum lamina propria. (D) Absolute numbers of CD11b+ cells determined by flow cytometry in cecum. (E) Representative flow cytometry plots indicating the percentages of CD11b+ cells in colon lamina propria. (F) Absolute numbers of CD11b+ cells determined by flow cytometry in colon. ANOVA and Tukey’s test: **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
FIG 5.
Neutrophil and eosinophil infiltration in the distal intestine is significantly decreased in aged mice with severe CDI compared to that in young counterparts. CD11b+ Ly6G+ Siglec-F− neutrophils and CD11b+ Ly6G− Siglec-F+ eosinophils from mock-infected or C. difficile strain VPI 10463-infected young and aged mice 2 days postinfection analyzed by flow cytometry. Representative flow cytometry plots indicating the percentages of CD11b+ Ly6G+ Siglec-F− neutrophils and CD11b+ Ly6G− Siglec-F+ eosinophils in cecum (A) and colon (B) laminae propriae. (C) Absolute numbers of neutrophils in the cecum and colon laminae propriae. (D) Absolute eosinophil numbers in cecum and colon laminae propriae. ANOVA and Tukey’s test: **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Differential intestinal and systemic neutrophil and eosinophil cellular responses during severe C. difficile infection in aged mice.
Granulocytes, including neutrophils and eosinophils, are important cellular mediators of innate immunity. Since it has been suggested that eosinophils play a protective role in CDI using a mouse model of C. difficile infection (9), we hypothesized that eosinophil responses in older animals during C. difficile infection would differ significantly from those of their relatively young counterparts. Young mice at the time of peak CDI disease severity mounted a robust cecal and colonic eosinophil cellular response; however, intestinal eosinophil infiltration during peak CDI disease severity was absent in aged mice infected with C. difficile strain VPI 10463 (Fig. 5D). We sought to determine if there was an age-related difference in peripheral white blood cells, with a focus on neutrophil and eosinophil responses during C. difficile infection (Fig. 6). We found that aged mice infected with the high-virulence C. difficile strain VPI 10463 responded with an increased eosinophil-to-total leukocyte ratio (Fig. 6C) (P < 0.01) compared to that of young mice infected with the same strain of C. difficile. Although there was a complete lack of local eosinophil infiltration in the distal intestines of aged mice with severe CDI, there was a concomitant significant increase in the absolute number of peripheral eosinophils in the blood of aged mice infected with C. difficile strain VPI 10463 compared to that in their young counterparts (Fig. 6C) (P < 0.01). In contrast, young mice did not demonstrate a change in peripheral blood eosinophil levels at the peak of CDI severity, regardless of infecting C. difficile strain (Fig. 6C).
FIG 6.
Severe CDI results in differential systemic neutrophil and eosinophil responses in aged mice and young mice. (A) Numbers of white blood cells in mock-infected or C. difficile strain VPI 10463-infected young and aged mice 2 days postinfection. Frequencies and absolute numbers of neutrophils (B) and eosinophils (C) in young and aged mice at baseline or during peak CDI disease severity. ANOVA and Sidak’s test: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Age alters systemic cytokine and chemokine levels during severe C. difficile infection.
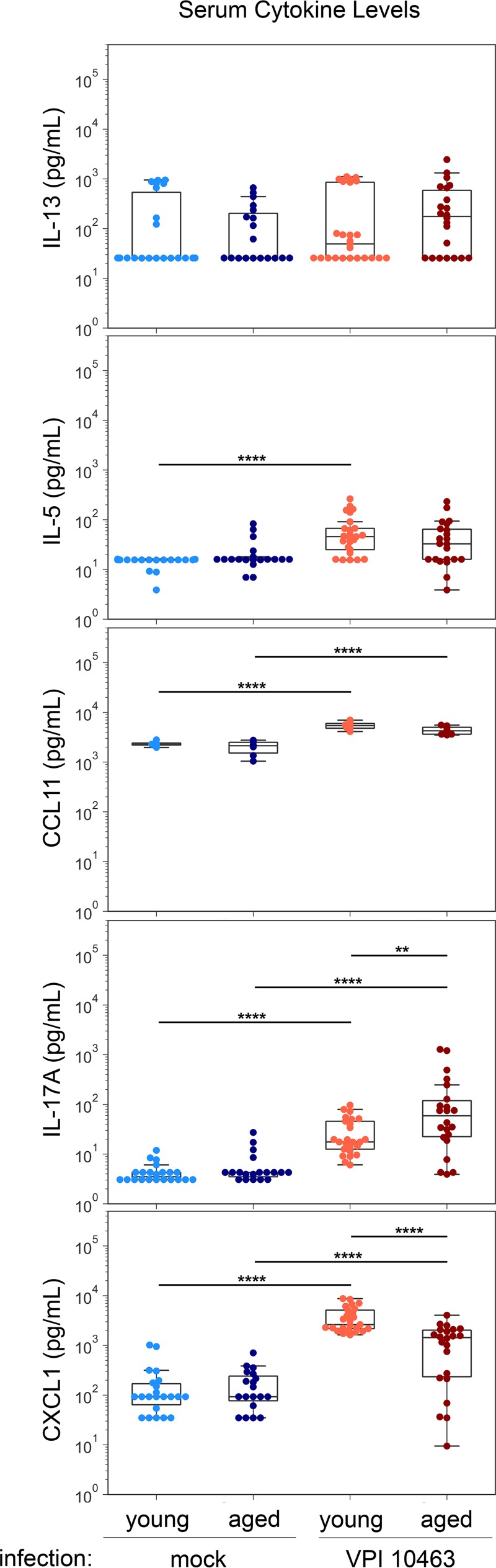
We sought to determine if age affected particular circulating cytokine and chemokine responses associated with granulocytes during severe C. difficile infection (Fig. 7). Severe CDI induced significant increases of serum C-C motif chemokine 11 (CCL11; P < 0.0001), interleukin 17A (IL-17A; P < 0.0001), and CXCL1 (P < 0.0001) in both young and aged mice. Interestingly, in the setting of severe CDI, IL-17A levels were significantly elevated (P < 0.01) and CXCL1 levels were significantly reduced (P < 0.0001) in aged mice compared to levels in young mice. In addition, while serum levels of IL-5 were significantly elevated in young mice during severe CDI (P < 0.0001), the amount of IL-5 in aged mice with severe CDI was not statistically different from that in age-matched controls (Fig. 7). We found no measurable age-related difference in CDI-induced IL-13 in the serum of young or aged mice.
FIG 7.
Aged mice mount altered systemic cytokine responses during severe CDI compared to those of young mice. Select cytokine and chemokine levels in the sera of young and aged mice mock infected or 2 days postinfection with C. difficile strain VPI 10463 were quantified using a Luminex multiplex system. ANOVA and Sidak’s test: **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
DISCUSSION
Although advanced age is a risk factor for C. difficile infection (CDI) (1), the relationship between the aging immune system and C. difficile infection is not well known. This study demonstrates an overall decrease in intestinal innate immune responses during acute C. difficile infection in mice with advanced age and identifies specific aging-related alterations in neutrophil and eosinophil responses during CDI. We show that mice of advanced age (22 to 24 months old) and infected with a highly virulent strain of C. difficile (VPI 10463) develop more severe CDI disease and mount a significantly blunted intestinal cellular immune response compared to that in young mice (2 to 3 months old), with a notable absence of an intestinal eosinophil response. Young animals have a robust cellular intestinal immune response during acute CDI, whereas aged mice have a blunted intestinal neutrophil and eosinophil cellular response to C. difficile infection with concomitant peripheral neutrophilia and eosinophilia. Recently, peripheral eosinophil counts were found to be predictive of CDI disease severity and mortality in patients (11), and eosinophils were shown to potentially be protective in mouse models of CDI (9, 10). Interestingly, aged mice had a significant peripheral eosinophil response, whereas young mice lacked a detectable increase in circulating eosinophils during severe CDI. Our results also suggest that eosinophils may play differential roles, whether that be protective or pathogenic. Additionally, eosinophil counts may predict different disease outcomes during C. difficile infection depending on their location in intestinal tissue or circulation in the periphery. In the present study, we characterized the intestinal cellular immune response to C. difficile infection during peak disease severity in young and aged mice, with a focus on myeloid cell subsets mobilized during the innate immune response.
While the aging immune system is known to increase the risk for CDI, the protective or pathogenic contributions of particular immune responses to disease outcomes are currently being explored in more detail. Peniche et al. showed that middle-aged mice (12 to 14 months old) have increased susceptibility to C. difficile infection and worse disease than young controls (16). They report that this observation was driven by impaired innate immune responses; however, eosinophils were not evaluated in this study. Our data showing a decreased intestinal neutrophil response in aged mice infected with C. difficile agree with a recent study that examined the effect of age on C. difficile infection in a mouse model (15). Type 2 immune responses include IL-5, IL-13, and C-C motif chemokine 11 (CCL11), also known as eosinophil chemotactic protein and eotaxin-1, while CXCL1 and IL-17A are associated with type 3 immune responses. Our results demonstrate an age-related defect in IL-5 and CCL11, which are responses associated with eosinophil recruitment. Although IL-17 is known to induce CXCL1, we measured significantly lower levels of CXCL1 in the serum of aged mice with severe CDI than in young mice. The contributions of type 2 and type 3 immune responses to C. difficile pathogenesis and infection are an active area of research (17, 18).
Our results add to the growing evidence that eosinophils play a critical role in the pathogenesis of CDI. One study demonstrated that C. difficile binary toxin suppresses host-protective colonic eosinophil responses in a Toll-like receptor 2 (TLR2)-dependent manner (10). However, our study explores responses to infection with C. difficile strain VPI 10463, which does not express binary toxin produced by C. difficile strains typically associated with severe CDI (19). Our data indicate that severe disease associated with C. difficile infection also occurs via binary toxin-independent modulation of eosinophils. Recently, Buonomo et al. reported that an increase in intestinal eosinophils was associated with reduced host mortality during C. difficile infection (9). In the aforementioned study, cytokine, IgA, IgG, and muc2 were assessed in the cecum while eosinophils were enumerated in the colon. While we detected robust eosinophil infiltration in the ceca of young mice, we did not observe this in the colons of young or aged animals. Another group found that peripheral loss of eosinophils in patients with C. difficile infection was predictive of severe disease (11). We report that young mice had significantly increased numbers of intestinal eosinophils during C. difficile infection compared to that in mock-infected young controls, while aged mice did not have intestinal eosinophil infiltration and had significantly worse CDI disease than young mice. However, eosinophils in the blood of aged mice infected with the more virulent strain of C. difficile were significantly increased at the time of peak CDI disease severity compared to those in young counterparts. It is possible that eosinopenia at the time of symptom onset is predictive of increased CDI disease severity and mortality, but eosinophil levels may increase in the blood as CDI disease progresses over time. Additionally, eosinophil responses may be altered in older patient populations and may not be predictive of CDI outcomes, an important consideration for studies utilizing immune response biomarkers for CDI outcome prediction (20, 21). Our results also suggest tissue-specific eosinophil responses in the distal intestinal tract during C. difficile infection, warranting further examination of local tissue responses associated with CDI.
Clinical heterogeneity of disease associated with C. difficile infection is a major medical challenge in the management of CDI, particularly in vulnerable patients at risk for increased morbidity and mortality. Focus on precision medicine-based therapeutic approaches adapted to fit the specific characteristics of a particular patient population with CDI should be explored to advance current CDI treatment methods. Understanding the age-related and site-specific differences in immune responses during CDI is critical for the appropriate care of inherently diverse adult patient populations. The general blunted cellular immune response observed in the intestines of aged mice with CDI appears to lead to worse clinical disease, yet intestine-specific changes in immune responses would not be accurately captured by routine blood testing in patients. Our data support further exploration of innate immune mediators in age-associated CDI outcomes, including neutrophils and eosinophils and accompanying cytokine/chemokine responses. Knowledge of the relationship between local and systemic immune responses and CDI-associated disease in high-risk patient populations is required to effectively monitor disease progression and to tailor patient-specific treatment.
MATERIALS AND METHODS
Mice.
Male and female specific-pathogen-free (SPF) C57BL/6 wild-type adult mice that were young (2 to 3 months old) or aged (22 to 28 months old) were used in these studies. These mice were from a breeding colony at the University of Michigan that was originally founded with breeding stock obtained from the Jackson Laboratories in 2002. Euthanasia was carried out via CO2 inhalation at the conclusion of the experiment. Animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Michigan, and animal husbandry was performed in an AAALAC-accredited facility.
C. difficile strains and experimental murine infection.
The C. difficile strains used in this study include reference strain VPI 10463 (ATCC 43255) and strain 630 (ATCC BAA-1382). The differential virulence of these two strains, with VPI 10463 causing rapidly fatal infection and strain 630 causing a more limited disease, was previously described in a murine model of CDI by Theriot et al. (12). Mice were rendered susceptible to C. difficile infection by placing them on 0.5 mg/ml cefoperazone (MP Pharmaceuticals) in sterile distilled drinking water (Gibco) ad libitum. The antibiotic-supplemented water was provided for 10 days, followed by 2 days of drinking water without antibiotics. Animals were then inoculated by oral gavage with 103 to 104 CFU of C. difficile spores suspended in 20 to 100 μl of distilled water (Gibco) or were mock infected with vehicle alone. Viable spores in each inoculum were enumerated by plating for CFU per milliliter on prereduced taurocholate-cycloserine-cefoxitin fructose agar (TCCFA). TCCFA was prepared as originally described (22) with modifications. Briefly, the agar base consisted of 40 g of proteose peptone no. 3 (BD Biosciences), 5 g of Na2HPO4 (Sigma-Aldrich), 1 g of KH2PO4 (Fisher), 2 g NaCl (J.T. Baker), 0.1 g MgSO4 (Sigma), 6 g fructose (Fisher), and 20 g of agar (Life Technologies) dissolved in 1 liter of Milli-Q water. The prepared medium was autoclaved and supplemented with a final concentration of 250 μg/ml d-cycloserine (Sigma-Aldrich), 16 μg/ml cefoxitin (Sigma-Aldrich), and 0.1% taurocholate (Sigma). Over the course of the experiment, mice were regularly weighed and cecal contents were collected for quantitative culture.
C. difficile quantification.
Cecal contents were collected in a preweighed sterile tube from each mouse at the time of euthanasia. Immediately following collection, the tubes were reweighed to determine fecal weight and passed into an anaerobic chamber (Coy Laboratories). Each sample was then diluted 10% (wt/vol) with prereduced sterile phosphate-buffered saline (PBS) and serially diluted onto prereduced TCCFA plates with or without erythromycin supplementation. C. difficile strain 630 is erythromycin resistant, whereas C. difficile strain VPI 10463 is sensitive to erythromycin. The plates were incubated anaerobically at 37°C, and colonies were enumerated after 18 to 24 h of incubation.
Clinical disease severity scoring.
Mice were monitored for clinical signs of disease. Disease scores were averaged based on scoring of the following features for signs of disease: weight loss, activity, posture, coat, diarrhea, eyes/nose. A 4-point scale was applied for weight loss and activity, and a 3-point score was applied for each of the other features; the sum of these scores (with 0 representing no signs of disease and 20 representing signs of the most severe disease) determined the clinical disease severity score (23). Formalin-fixed tissue sections prepared from cecum and colon were hematoxylin and eosin (H&E) stained and evaluated by an animal pathologist blinded to the experimental conditions. Histopathologic damage in each tissue was scored for epithelial destruction, immune cell infiltration, and edema on a 4-point scale for each category, and the sum of these scores determined the histological score (12, 24).
Lamina propria cell isolation.
Ceca and colons were excised and separated, and the lumens were flushed. Residual fat was removed and tissues were opened longitudinally. Tissue was placed in prewarmed RPMI medium containing 0.5 M EDTA, dithiothreitol, and fetal bovine serum (FBS) and incubated at 37°C on an orbital shaker at 150 rpm for 15 min. After incubation, a steel strainer was used to separate tissue pieces from the epithelium-containing supernatant. Tissue was minced in RPMI medium containing dispase, collagenase II, DNase I, and FBS and incubated at 37°C on an orbital shaker at 150 rpm for 30 min. Digested tissue was filtered through a 100-μm cell strainer followed by a 40-μm cell strainer. The resultant single cell suspensions were counted on a hemocytometer by using the trypan blue exclusion test.
Flow cytometry.
Lamina propria single-cell suspensions from colon or cecum were incubated with anti-CD16/32 antibody to reduce nonspecific binding. Cells were incubated on ice for 30 min in the dark, with a cocktail of fluorescent antibodies consisting of anti-CD45.2 peridinin chlorophyll protein (PerCP)-Cy5 (clone 104), CD3 phycoerythrin (PE; clone 145-2C11), CD11b PE-eFluor 610 (clone M1/70), CD11c Alexa Fluor 700 (clone N418), Ly6G PE-Cy7 (clone 1A8), and Siglec-F Alexa Fluor 647 (clone E50-2440) antibodies. All antibodies were purchased from eBioscience, BioLegend, or BD Biosciences. Stained cells were incubated with an eFluor 450 fixable viability dye (eBioscience) and fixed with 0.5% paraformaldehyde. Cells were analyzed using a BD LSRFortessa X-20 flow cytometer (BD Biosciences, San Jose, CA) and analyzed with FlowJo v10 software (Tree Star Inc., Ashland, OR).
White blood cell enumeration in blood.
Blood was collected via cardiac puncture in microtainer tubes with K2 EDTA (Sarstedt, Nümbrecht, Germany) at the experimental endpoint. Blood samples were taken immediately to the Unit for Laboratory Animal Medicine In-Vivo Animal Core and processed for a complete blood count on an automated hematology analyzer (Hemavet 950; Drew Scientific, Miami Lakes, FL).
Serum preparation and cytokine analysis.
Blood was collected via cardiac puncture utilizing a polymer gel-based separator tube (BD Microtainer SST) at the experimental endpoint. Tubes were centrifuged according to the manufacturer’s instructions, and serum was collected and stored at −80°C until use. Cytokine levels in the serum were measured using a Luminex multiplex system (Invitrogen).
Statistics.
One-way analysis of variance (ANOVA) with Tukey’s or Sidak’s post hoc test for C. difficile burden, cell population, and serum cytokine analyses was performed using R or GraphPad Prism 8. Clinical, cecum, and colon summary scores were analyzed with a Kruskal-Wallis test and Dunn’s post hoc test using R or GraphPad Prism 8. Clinical score as an outcome was modeled using multivariable linear regression modeling to minimize the sum of squares of the residuals using the R function lm. Clinical scores were log transformed, and a significant interaction term was found between age and infecting strain as input variables. Standard errors for the mean clinical change for each condition were calculated using the deltamethod in the r package msm. A P value of less than 0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank Naomi Perlman and James M. George for their assistance in the laboratory and Anna Colvig and Florin Timpau of the ULAM In-Vivo Animal Core for hematology assessment. We also thank Patrick Schloss for providing aged mice for the University of Michigan colony for experimentation.
This study was funded by grant U01AI124255 awarded to V.B.Y. by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health. In addition, M.G.D. was supported by NIH grants T32GM007863 and T32DK094775. The funding agencies had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Leffler DA, Lamont JT. 2015. Clostridium difficile infection. N Engl J Med 372:1539–1548. doi: 10.1056/NEJMra1403772. [DOI] [PubMed] [Google Scholar]
- 2.Carignan A, Allard C, Pépin J, Cossette B, Nault V, Valiquette L. 2008. Risk of Clostridium difficile infection after perioperative antibacterial prophylaxis before and during an outbreak of infection due to a hypervirulent strain. Clin Infect Dis 46:1838–1843. doi: 10.1086/588291. [DOI] [PubMed] [Google Scholar]
- 3.Lessa FC, Gould CV, McDonald LC. 2012. Current status of Clostridium difficile infection epidemiology. Clin Infect Dis 55(Suppl 2):S65–S70. doi: 10.1093/cid/cis319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Travers J, Rothenberg ME. 2015. Eosinophils in mucosal immune responses. Mucosal Immunol 8:464–475. doi: 10.1038/mi.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang L, Appleton JA. 2016. Eosinophils in helminth infection: defenders and dupes. Trends Parasitol 32:798–807. doi: 10.1016/j.pt.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin LB, Kita H, Leiferman KM, Gleich GJ. 1996. Eosinophils in allergy: role in disease, degranulation, and cytokines. Int Arch Allergy Immunol 109:207–215. doi: 10.1159/000237239. [DOI] [PubMed] [Google Scholar]
- 7.Jung Y, Rothenberg ME. 2014. Roles and regulation of gastrointestinal eosinophils in immunity and disease. J Immunol 193:999–1005. doi: 10.4049/jimmunol.1400413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kvarnhammar AM, Cardell LO. 2012. Pattern-recognition receptors in human eosinophils. Immunology 136:11–20. doi: 10.1111/j.1365-2567.2012.03556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buonomo EL, Cowardin CA, Wilson MG, Saleh MM, Pramoonjago P, Petri WA. 2016. Microbiota-regulated IL-25 increases eosinophil number to provide protection during Clostridium difficile infection. Cell Rep 16:432–443. doi: 10.1016/j.celrep.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cowardin CA, Buonomo EL, Saleh MM, Wilson MG, Burgess SL, Kuehne SA, Schwan C, Eichhoff AM, Koch-Nolte F, Lyras D, Aktories K, Minton NP, Petri WA. 2016. The binary toxin CDT enhances Clostridium difficile virulence by suppressing protective colonic eosinophilia. Nat Microbiol 1:16108. doi: 10.1038/nmicrobiol.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kulaylat AS, Buonomo EL, Scully KW, Hollenbeak CS, Cook H, Petri WA, Stewart DB. 2018. Development and validation of a prediction model for mortality and adverse outcomes among patients with peripheral eosinopenia on admission for Clostridium difficile infection. JAMA Surg 153:1127. doi: 10.1001/jamasurg.2018.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Theriot CM, Koumpouras CC, Carlson PE, Bergin II, Aronoff DM, Young VB. 2011. Cefoperazone-treated mice as an experimental platform to assess differential virulence of Clostridium difficile strains. Gut Microbes 2:326–334. doi: 10.4161/gmic.19142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mabbott NA, Kobayashi A, Sehgal A, Bradford BM, Pattison M, Donaldson DS. 2015. Aging and the mucosal immune system in the intestine. Biogerontology 16:133–145. doi: 10.1007/s10522-014-9498-z. [DOI] [PubMed] [Google Scholar]
- 14.van Opstal E, Kolling GL, Moore JH, Coquery CM, Wade NS, Loo WM, Bolick DT, Shin JH, Erickson LD, Warren CA. 2016. Vancomycin treatment alters humoral immunity and intestinal microbiota in an aged mouse model of Clostridium difficile infection. J Infect Dis 214:130–139. doi: 10.1093/infdis/jiw071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin JH, Gao Y, Moore JH, Bolick DT, Kolling GL, Wu M, Warren CA. 2018. Innate immune response and outcome of Clostridium difficile infection are dependent on fecal bacterial composition in the aged host. J Infect Dis 217:188–197. doi: 10.1093/infdis/jix414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peniche AG, Spinler JK, Boonma P, Savidge TC, Dann SM. 2018. Aging impairs protective host defenses against Clostridioides (Clostridium) difficile infection in mice by suppressing neutrophil and IL-22 mediated immunity. Anaerobe 54:83–91. doi: 10.1016/j.anaerobe.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donlan A, Petri WA. 2020. The inflammasome and type-2 immunity in Clostridium difficile infection. Clin Colon Rectal Surg 33:67–72. doi: 10.1055/s-0040-1701231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saleh MM, Petri WA. 2019. Type 3 immunity during Clostridioides difficile infection: too much of a good thing? Infect Immun 88:e00306-19. doi: 10.1128/IAI.00306-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammond GA, Johnson JL. 1995. The toxigenic element of Clostridium difficile strain VPI 10463. Microb Pathog 19:203–213. doi: 10.1016/S0882-4010(95)90263-5. [DOI] [PubMed] [Google Scholar]
- 20.Walker AS, Eyre DW, Wyllie DH, Dingle KE, Griffiths D, Shine B, Oakley S, O'Connor L, Finney J, Vaughan A, Crook DW, Wilcox MH, Peto TEA, Infections in Oxfordshire Research Database. 2013. Relationship between bacterial strain type, host biomarkers, and mortality in Clostridium difficile infection. Clin Infect Dis 56:1589–1600. doi: 10.1093/cid/cit127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu H, Chen K, Sun Y, Carter M, Garey KW, Savidge TC, Devaraj S, Tessier ME, von Rosenvinge EC, Kelly CP, Pasetti MF, Feng H. 2017. Cytokines are markers of the Clostridium difficile-induced inflammatory response and predict disease severity. Clin Vaccine Immunol 24:e00037-17. doi: 10.1128/CVI.00037-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.George WL, Sutter VL, Citron D, Finegold SM. 1979. Selective and differential medium for isolation of Clostridium difficile. J Clin Microbiol 9:214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warren CA, van Opstal E, Ballard TE, Kennedy A, Wang X, Riggins M, Olekhnovich I, Warthan M, Kolling GL, Guerrant RL, Macdonald TL, Hoffman PS. 2012. Amixicile, a novel inhibitor of pyruvate: ferredoxin oxidoreductase, shows efficacy against Clostridium difficile in a mouse infection model. Antimicrob Agents Chemother 56:4103–4111. doi: 10.1128/AAC.00360-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reeves AE, Theriot CM, Bergin IL, Huffnagle GB, Schloss PD, Young VB. 2011. The interplay between microbiome dynamics and pathogen dynamics in a murine model of Clostridium difficile infection. Gut Microbes 2:145–158. doi: 10.4161/gmic.2.3.16333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.