Highlights
-
•
We compared ICNs between PTSD, dissociative subtype PTSD, and healthy controls.
-
•
Results revealed unique group connectivity to brain areas associated with PTSD symptoms.
-
•
Classification machine learning algorithms predicted diagnosis with high accuracy.
-
•
Alterations within ICNs may underlie unique psychopathology among PTSD subtypes.
Abstract
Intrinsic connectivity networks (ICNs), including the default mode network (DMN), the central executive network (CEN), and the salience network (SN) have been shown to be aberrant in patients with posttraumatic stress disorder (PTSD). The purpose of the current study was to a) compare ICN functional connectivity between PTSD, dissociative subtype PTSD (PTSD+DS) and healthy individuals; and b) to examine the use of multivariate machine learning algorithms in classifying PTSD, PTSD+DS, and healthy individuals based on ICN functional activation. Our neuroimaging dataset consisted of resting-state fMRI scans from 186 participants [PTSD (n = 81); PTSD + DS (n = 49); and healthy controls (n = 56)]. We performed group-level independent component analyses to evaluate functional connectivity differences within each ICN. Multiclass Gaussian Process Classification algorithms within PRoNTo software were then used to predict the diagnosis of PTSD, PTSD+DS, and healthy individuals based on ICN functional activation. When comparing the functional connectivity of ICNs between PTSD, PTSD+DS and healthy controls, we found differential patterns of connectivity to brain regions involved in emotion regulation, in addition to limbic structures and areas involved in self-referential processing, interoception, bodily self-consciousness, and depersonalization/derealization. Machine learning algorithms were able to predict with high accuracy the classification of PTSD, PTSD+DS, and healthy individuals based on ICN functional activation. Our results suggest that alterations within intrinsic connectivity networks may underlie unique psychopathology and symptom presentation among PTSD subtypes. Furthermore, the current findings substantiate the use of machine learning algorithms for classifying subtypes of PTSD illness based on ICNs.
1. Introduction
1.1. PTSD heterogeneity
Modern medicine has demonstrated a substantial interest in exploring heterogenous subtypes of psychiatric illness, corresponding to the characterization of unique psychopathology and associated aberrant neural circuitry (Etkin et al., 2019; Fenster et al., 2018). Critically, a dissociative subtype of posttraumatic stress disorder (PTSD+DS) has been formalized recently, which corresponds to a specific group of individuals that exhibit additional symptoms of depersonalization/derealization and related emotion overmodulation (APA, 2013). In contrast to the typical presentation of PTSD, PTSD+DS is often associated with more severe PTSD symptoms, childhood trauma, increased comorbidity, and increased suicidality, pointing towards a patient group with heightened psychopathology and additional treatment needs (Lanius et al., 2012; Stein et al., 2013; Mergler et al., 2017). It has been shown repeatedly that posttraumatic stress disorder (PTSD) is associated with decreased regulation and inhibition from the medial prefrontal cortex (mPFC), where increased activation of the amygdala/limbic system and midbrain is thought to precipitate associated symptoms of hyperarousal, vivid re-experiencing, and emotion undermodulation (Etkin and Wager 2007; Pitman et al., 2012; Yehuda et al., 2015; Nicholson et al., 2017; Nicholson et al., 2018a; Fenster et al., 2018; Fitzgerald et al., 2018; Henigsberg et al., 2018). By contrast, PTSD+DS is uniquely characterized by increased regulatory activation of the mPFC, resulting in hypoactivation of the amygdala/limbic system, with associated symptoms of depersonalization, derealization, and emotion overmodulation (Hopper et al., 2007a; Lanius et al., 2010; Mickleborough et al., 2011; Nicholson et al., 2017; AA 2018a; Fenster et al., 2018; Melara et al., 2018). Indeed, PTSD and PTSD+DS show directed connectivity patterns that are consistent with their emotion under- and over-modulation symptom profiles, respectively. For example, Nicholson et al. (2017) showed that PTSD patients demonstrate bottom-up directed connectivity from the amygdala and periaqueductal gray, brain regions involved in emotion generation and threat processing, to the vmPFC. By contrast, PTSD+DS patients displayed top-down directed connectivity from the vmPFC, an area involved in emotion regulation, to the amygdala and periaqueductal gray, which may facilitate emotion overmodulation symptoms observed in these patients. Interestingly, unique neural correlates have also been documented within central hubs of intrinsic connectivity networks (ICNs) when comparing PTSD, PTSD+DS, and healthy individuals during both symptom provocation and the resting state (Hopper et al., 2007b; Nicholson et al., 2015, 2016b; Nicholson et al., 2017; Harricharan et al., 2016; Rabellino et al., 2017, 2018; Terpou et al., 2018).
1.2. Intrinsic connectivity networks
Neuroscientists have increasingly begun to emphasize the characterization of psychiatric disorders as conditions reflecting altered distributed neural networks (Hulshoff and Bullmore 2013; Lanius et al., 2015). The default mode network (DMN), which consists of the posterior cingulate cortex (PCC), ventromedial prefrontal cortex (vmPFC), hippocampus, and cortical midline/parietal structures, is active predominantly at rest and is critical to autobiographical self-referential processing, future-oriented thinking, and continuous experience of the self across time and into the future (Greicius et al., 2003; Buckner et al., 2008; Spreng et al., 2008; Qin and Northoff 2011; Frewen et al., 2020). The central executive network (CEN) is a frontoparietal and cerebellar network centered around the dorsolateral prefrontal cortex (dlPFC) and is involved in higher order executive functioning, including the cognitive control of thought, emotion regulation, and working memory (Miller and Cohen 2001; Petrides 2005; Koechlin and Summerfield 2007; Seeley et al., 2007; Habas et al., 2009; Akiki et al., 2017). The salience network (SN) encompasses the insula, the dorsal anterior cingulate cortex (ACC), and the amygdala, where this network is involved in interoceptive processing, environmental monitoring and the subsequent apprehension of personally salient stimuli (Dosenbach et al., 2007; Seeley et al., 2007; Sridharan et al., 2008). Indeed, central hubs within the DMN, CEN, and SN display aberrant activation and functional connectivity patterns in PTSD and its dissociative subtype during both symptom provocation and during the resting-state (Hopper et al., 2007b; Nicholson et al., 2015, 2016b, 2018a, 2017; Rabellino et al., 2017, 2018; Olivé et al., 2018; Terpou et al., 2018).
1.3. Intrinsic connectivity networks in PTSD
Neuropathological disruptions within ICNs have been shown to be related to specific symptoms of PTSD and PTSD+DS, which are closely tied to the unique function of each network (Lanius et al., 2015; Fenster et al., 2018). DMN functional disruptions in PTSD patients are hypothesized to be related to negative self-referential thoughts as well as alterations in social cognition and autobiographical memory, where PTSD+DS patients additionally experience alterations in somatic self-referential processing, including symptoms of depersonalization (i.e., out-of-body experiences) (Bluhm et al., 2009; Daniels et al., 2010; Tursich et al., 2015b; Fenster et al., 2018; Frewen et al., 2020). CEN functional disruptions are associated with PTSD symptoms of decreased cognitive functioning across multiple domains, as well as emotion undermodulation associated with impaired regulation of limbic structures (Aupperle et al., 2012; Polak et al., 2012; St. Jacques et al. 2013; Cisler et al., 2014; Lanius et al., 2015; Block et al., 2017). Interestingly, PTSD+DS is uniquely characterized by emotion overmodulation from executive functioning areas, and dissociative symptoms are transdiagnostically related to exacerbated CEN impairments of attention, executive functioning, memory, and social cognition (McKinnon et al., 2016; Boyd et al., 2018; Fenster et al., 2018; Lanius et al., 2018). Finally, alterations within the SN have been linked to PTSD symptoms of hyperarousal and bodily reactivity, as well as PTSD+DS symptoms of emotion overmodulation associated with emotional detachment and lack of interoceptive awareness related to symptoms of depersonalization (Sripada et al., 2012; Tursich et al., 2015a; Yehuda et al., 2015; Nicholson et al., 2016b; Akiki et al., 2017; Harricharan et al., 2019).
Neuroimaging studies examining functional connectivity within the DMN among subtype non-differentiated PTSD patients at rest reveal decreased coupling between the PCC, vmPFC and other DMN structures (Daniels et al., 2010; Qin et al., 2012; Sripada et al., 2012a; Chen and Etkin 2013; Shang et al., 2014; Kennis et al., 2016; Koch et al., 2016; Miller et al., 2017). Here, recent studies have found decreased DMN functional connectivity to be associated with increased PTSD symptom severity, with both decreased functional integration (less efficiency of overall communication across the network) (Akiki et al., 2018; Holmes et al., 2018), and increased segregation (the capacity of specialized processing) in patients with PTSD (Akiki et al., 2018). With regard to PTSD+DS, depersonalization/derealization symptoms have been associated with reduced connectivity of regions linked to self-referential processing within the DMN (Tursich et al., 2015a). Moreover, during tasks that require executive functioning, previous studies report decreased functional connectivity within the CEN in PTSD and increased connectivity within the DMN (Daniels et al., 2010). This suggests aberrant ICN functioning in PTSD, as DMN recruitment would typically be decreased during tasks that require executive functioning (Daniels et al., 2010). Indeed, this may underlie aforementioned disruptions in cognition functioning among PTSD patients (McKinnon et al., 2016). Additionally, during the resting-state, subtype non-differentiated PTSD patients demonstrate reduced functional integration of the CEN, which is associated with reduced orbitofrontal-amygdala connectivity, indicative of reduced regulation of the limbic system as compared to healthy controls (Barredo et al., 2018). Supporting models of PTSD emotion undermodulation, a recent study has also shown weaker connectivity within nodes of the CEN (right and left dlPFC) in patients with PTSD (Holmes et al., 2018). By contrast, it is hypothesized that PTSD+DS patients exhibit over-recruitment of CEN areas relating to emotion overmodulation (Yehuda et al., 2015; Fenster et al., 2018). Finally, it is well documented that subtype non-differentiated PTSD patients often display elevated SN connectivity during the resting-state indicative of hyperarousal, hypervigilance and threat processing symptoms (Rabinak et al., 2011; Sripada et al., 2012; Koch et al., 2016).
Although disruptions in these aforementioned neural networks have already been associated with subtype nonspecific PTSD populations (Akiki et al., 2017; Krause et al., 2017; Lanius et al., 2015; Menon, 2011; Rabellino et al., 2015; Shalev et al., 2017; Shang et al., 2014; Yehuda et al., 2015), functional connectivity of the DMN, CEN and SN has not been compared between PTSD, PTSD+DS and healthy controls, nor has activation within these networks been used to classify diagnoses via machine learning. Hence, it is unclear how alterations within the DMN, CEN and SN may contribute to heterogeneous symptom presentation in PTSD versus PTSD+DS patients, an effort which may guide/inform treatment interventions aimed at restoring these brain networks in trauma-related illness.
1.4. Multivariate pattern analysis: machine learning applications in neuroimaging
In contrast to univariate neuroimaging analyses, multivariate machine learning applications for fMRI constitute a more powerful method by which to evaluate subtle and spatially distributed signal patterns within the brain (Schrouff et al., 2013). Multivariate machine learning algorithms for neuroimaging data provide a means by which to classify patients, identify illness subtypes, and predict response to treatment based on highly complex sources of neural information, and due to their multivariate properties, these machine learning methods can achieve relatively greater sensitivity (Schrouff et al., 2013). Of particular relevance to psychiatry, these analyses are sensitive enough to facilitate inference/classification at the single-subject level (Orrù et al., 2012; Fu and Costafreda 2013; Wolfers et al., 2015), hence offering the capacity to predict individual diagnoses. Indeed, identifying objective neural network classifiers that can categorize PTSD heterogeneity may offer valuable clinical insight into guiding treatments for PTSD versus PTSD+DS by matching specific individuals to a personalized treatment. Recently, a growing number of studies have applied multivariate machine learning methods to neuroimaging datasets to predict and characterize psychiatric disease (Bleich-cohen et al., 2014; Mikolas et al., 2016; Rive et al., 2016; Ranlund et al., 2018), including PTSD (Gong et al., 2014; Karstoft et al., 2015; Liu et al., 2015; Omurca and Ekinci 2015; Galatzer-Levy et al., 2017; Gradus et al., 2017; Jin et al., 2017; Saxe et al., 2017). Indeed, we have shown recently that machine learning algorithms were able to accurately classify PTSD, PTSD+DS, and healthy controls based on neural activation (91.63% accuracy), amygdala complex functional connectivity (85.00% accuracy) (Nicholson et al., 2018a) and insula subregion functional connectivity (80.40% accuracy) (Harricharan et al., 2019). To date, however, no studies have utilized fMRI machine learning to classify PTSD and its dissociative subtype from healthy individuals based on ICN activation with the DMN, CEN, and SN.
1.5. Study objective
The purpose of the current study was to a) compare DMN, CEN, and SN functional connectivity between PTSD, PTSD+DS and healthy individuals; and b) to examine the predictive validity of machine learning algorithms in classifying PTSD, PTSD+DS, and healthy individuals based on DMN, CEN and SN activation. Given the distinct neurobiological underpinnings of PTSD and PTSD+DS with respect to both activation and functional connectivity within central hubs of ICNs, we hypothesized unique group differences between the two PTSD groups and healthy controls in terms of functional connectivity within the DMN (PCC and mPFC), CEN (dlPFC), and the SN (insula and amygdala). Specifically, with regard to the CEN and SN, we predicted increased integration of prefrontal cortex emotion regulation regions in PTSD+DS as compared to PTSD, indicative of emotion overmodulation. In addition, we predicted increased DMN connectivity in PTSD+DS as compared to PTSD and healthy controls, a pattern reflective of the altered self-referential processing and bodily self-consciousness associated with depersonalization (i.e., out-of-body experiences, emotional detachment). By contrast, in the PTSD group as compared to PTSD+DS and healthy controls, we predicted decreased CEN and SN connectivity to prefrontal cortex emotion regulation regions, consistent with emotion undermodulation. Moreover, in the PTSD group as compared to PTSD+DS and healthy controls, we predicted increased SN connectivity to the insula, consistent with hyper-monitoring of salient stimuli and hyperarousal at rest seen in this group. Finally, we hypothesized that these distinct ICN dynamics may contribute to high predictive accuracy when classifying individuals using machine learning computations based on whole-network activation.
2. Methods
2.1. Participants
Our sample consisted of 186 participants [PTSD (n = 81); PTSD+DS (n = 49); healthy controls (n = 56); Table 1]. Although there were no statistically significant associations between group and biological sex, the majority of the sample consisted of female participants. There were also no statistically significant differences in terms of age between groups (see Supplemental Material for details). Machine learning analyses on a portion of this sample have been reported in previous manuscripts (Nicholson et al., 2018a; Harricharan et al., 2019). Participants were recruited from 2009–2018 through referrals from family physicians, mental health professionals, psychology/psychiatric clinics, community programs for traumatic stress, and posters/advertisements within the London, Ontario community. The inclusion criteria for either PTSD group included a primary diagnosis of PTSD as determined using the Clinician-Administered PTSD Scale [CAPS; versions IV (for 85 participants) and 5 (for 45 participants] (Blake et al., 1995; Weathers et al., 2013) and the DSM-IV Structured Clinical Interview (SCID) (First et al., 2002). Additionally, PTSD+DS patients were identified by scoring ≥ 2 for both frequency and intensity on either depersonalization or derealization CAPS-IV symptoms, or at least two in symptom severity on the CAPS-5 scale for depersonalization or derealization symptoms, as per standard methods (S Harricharan et al., 2017; Nicholson et al., 2018a). Exclusion criteria for patients included alcohol or substance abuse/dependence not in sustained full remission and diagnosis of bipolar disorder or schizophrenia. Exclusion criteria for the control group included lifetime Axis-I psychiatric disorders, evaluated using the SCID and CAPS. Ideally, all patients would be evaluated with the same version of the CAPS; however, this large dataset of almost 200 participants has been collected since 2010, and participants were evaluated with the best diagnostic criteria at the time. Exclusion criteria for all participants included non-compliance with 3 Tesla fMRI safety standards, significant untreated medical illness, pregnancy, a history of neurological or pervasive developmental disorders, and previous head injury with loss of consciousness. The study was approved by the Research Ethics Board at Western University, Canada, and written informed consent was obtained from all participants.
Table 1.
Demographic and Clinical Information.
| PTSD | PTSD+DS | Healthy Controls | ||||
|---|---|---|---|---|---|---|
| N | 81 | 49 | 56 | |||
| Sex | 46 female | 38 female | 36 female | |||
| Measure | Value | SD | Value | SD | Value | SD |
| Age | 39 | 11.79 | 40 | 13.52 | 34 | 11.98 |
| CAPS-IV Total* | 66.60 a | 14.91 | 81.60 a,b | 12.89 | 0.60 | 2.59 |
| CAPS-5 Total | 36.58 | 9.21 | 41.37 | 7.76 | n/a | n/a |
| CTQ-Total* | 56.06 a | 23.00 | 69.74 a,b | 19.41 | 32.10 | 8.80 |
| BDI* | 23.21 a | 8.33 | 35.13 a,b | 11.70 | 0.96 | 1.91 |
| MDI-Total* | 53.64 a | 14.83 | 80.89 a,b | 22.20 | 33.96 | 3.82 |
| MDI-Dep/Dereal* | 7.72 a | 2.73 | 12.97 a,b | 4.59 | 5.20 | 0.51 |
| STAI | 5.6 a | 2.1 | 6.2 a | 2.5 | 3.3 | 0.6 |
| RSDI-Dissociation* | 3.6 a | 1.4 | 4.9 a,b | 2.0 | 2.7 | 0.4 |
| RSDI-Reliving Experiences | 3.0 a | 1.3 | 3.3 a | 1.5 | 2.1 | 0.3 |
| n | Past | n | Past | n | Past | |
| MDD* | 12 a | 24 | 23 a,b | 9 | – | – |
| Panic Disorder/Agoraphobia | 10 | 6 | 9 | 6 | – | – |
| Social Phobia | 2 | 2 | 6 | 0 | – | – |
| OCD | 3 | 2 | 0 | 2 | – | – |
| GAD | 1 | 0 | 0 | 0 | – | – |
| Medication | 29 | 19 | – | – | ||
Abbreviations: PTSD= Posttraumatic Stress Disorder, PTSD+DS= Dissociative Subtype Posttraumatic Stress Disorder Patients, CAPS = Clinician-Administered PTSD Scale, CTQ = Childhood Trauma Questionnaire (none or minimal childhood trauma = 25–36, moderate = 56–68, extreme trauma > 72), BDI = Beck's Depression Inventory, MDI = Multiscale Dissociation Inventory, Dep/Dereal = Depersonalization and Derealization Average, MDD = Major Depressive Disorder, OCD = Obsessive Compulsive Disorder, GAD = Generalized Anxiety Disorder. State clinical measures taken during the scan: STAI = State Trait Anxiety Inventory, RSDI = Responses to Script-Driven Imagery Scale.* indicates the clinical variables on which all groups differed significantly from one another (p < .05). a. indicates significantly higher clinical measures within a group as compared to the control group, b. indicates significantly higher clinical measures as compared to the PTSD group.
A battery of questionnaires was administered consisting of the Childhood Trauma Questionnaire (CTQ) (Bernstein et al., 2003), Beck's Depression Inventory (BDI) (Beck et al., 1997), and the Multiscale Dissociation Inventory (MDI) (Briere 2002). Additionally, scores on the Responses to Script-Driven Imagery Scale (RSDI; Hopper et al., 2007), and the State-Trait Anxiety Inventory (STAI; Spielberger, 2010) were collected during the fMRI scan to evaluate state clinical PTSD symptoms. Ninety percent of PTSD patients had early aversive experiences (confirmed if the patient scored above the ‘none/minimal’ threshold for any trauma category according to the CTQ scoring manual). Among PTSD participants, 48 (PTSD, n = 29; PTSD+DS, n = 19) were receiving psychotropic treatment at the time of the study. Medications included antidepressants, atypical antipsychotics, sedatives, and anticonvulsants (see Supplemental Material for details). Although the healthy control group was free of Axis-I disorders, a small portion of this group reported elevated levels on the CTQ. Critically, medication use was not found to significantly affect results when used as a covariate.
2.2. fMRI image acquisition, protocol and preprocessing
We acquired and preprocessed the resting-state neuroimaging data according to standard procedures in several of our manuscripts (Nicholson et al., 2015, Nicholson et al., 2017, Nicholson et al., 2018). We utilized a 3 Tesla MRI Scanner (Trio, Siemens Medical Solutions, Erlangen, Germany) with a 32-channel phased array head coil for brain imaging. During the resting-state scan, 120 volumes of whole brain BOLD (blood oxygen level dependent) images were acquired with the manufacturer's standard T2* gradient-echo planar imaging (EPI) pulse sequence (single-shot, blipped-EPI, interleaved slice acquisition order and tridimensional prospective acquisition correction) with the following parameters: TR = 3000 ms, TE = 20 ms, isotropic resolution 2 mm, FOV = 192 × 192 × 128 mm3 (94 × 94 matrix, 64 slices), flip angle = 90° High-resolution T1-weighted anatomical images were acquired with a Magnetization-Prepared Rapid Acquisition Gradient Echo sequence (192 slices, 1 mm isotropic resolution). For the resting-state procedure, participants were instructed to close their eyes and let their minds wander while trying not to focus on anything in particular for 6 minutes (Bluhm et al., 2009; Fransson, 2005; Harricharan et al., 2016), after which we assessed state-based clinical symptoms experienced during the scans (see below).
Preprocessing of the functional images was performed with SPM12 (Wellcome Department of Cognitive Neurology, London, UK) within Matlab 2017a. After discarding the 4 initial volumes, the standard preprocessing routine included spatial alignment to the mean image using a rigid body transformation, reslicing, and co-registration of the functional mean image to the anatomical image. The co-registered images were segmented using the “New Segment” method implemented in SPM12. The functional images were normalized to MNI space (Montréal Neurological Institute) and were subsequently smoothed with a FWHM (full-width at half-maximum) Gaussian kernel of 6 mm. Additional correction for motion was implemented using the ART software package (Gabrieli Lab, McGovern Institute for Brain Research, Cambridge, MA), which computes regressors that account for outlier volumes, in addition to the six movement regressors computed during standard realignment in general linear modeling. The smoothed functional images were subsequently bandpass filtered (high-pass 0.012 Hz, low-pass 0.1 Hz) (software by co-author Jean Théberge).
3. Data analyses
3.1. Part A) analysis of intrinsic connectivity networks
This section of the methods describes the analysis pertaining to objective one of the study, which was to compare DMN, CEN, and SN functional connectivity between PTSD, PTSD+DS and healthy individuals.
3.1.1. Independent component analysis
Group spatial independent component analysis (ICA) was performed on the resting-state fMRI data with all subjects in order to identify spatially independent networks using the Group ICA of fMRI Toolbox (GIFT v4.0b) (Calhoun et al., 2001, 2009; Allen et al., 2011). The Infomax algorithm was used to identify 20 independent components (ICs) within the data set, following minimum description length (MDL) criteria (Allen et al., 2011; Rosazza et al., 2012; St. Jacques et al. 2013; Kluetsch et al., 2014). In order to ensure reliability of the components, the ICA estimation was repeated 20 times through ICASSO (Himberg et al., 2004). This procedure resulted in a set of group aggregate spatial maps (which included brain regions that represent a network/component) and corresponding time courses of the BOLD signal change across time. For each component, single-subject spatial maps and time courses were then back-reconstructed to individual subject space and converted to Z-scores, which denote the strength of each voxel's correlation (i.e., connectivity) with the aggregate component's time course. This data-driven approach was used to identify ICNs instead of masking the data with standard ICN templates, as previous studies suggest that ICNs within patients with PTSD networks may be aberrant (Daniels et al., 2010; Shang et al., 2014; Rabellino et al., 2015b; Tursich et al. 2015; Nicholson et al., 2018b). Of importance, ICA also identifies motion-related sources, along with vascular, ventricular, and susceptibility artifacts, which are incorporated into “noise” components.
3.1.2. Component identification: spatial sorting analysis
We first visually inspected the obtained components for the presence of artifacts (ensuring peak activations in gray matter, low spatial overlap with known vascular, ventricular, motion, and susceptibility artifacts, and investigated signal time course frequency fluctuations) (Allen et al., 2011). Subsequently, the spatial sorting function within the GIFT toolbox was used to identify components that shared features with reference network templates in the literature. Here, we utilized reference ICN masks derived from the GIFT toolbox (GIFT v4.0b), and from https://findlab.stanford.edu/functional_ROIs.html (Shirer et al., 2012; Rabellino et al., 2015a; Nicholson et al., 2018b). Rationale for examining the left and right CENs separately was derived in part from our recent ICA study, which suggests differential hemisphere recruitment within the CEN among PTSD patients during emotion regulation tasks (Nicholson et al., 2018b). Furthermore, recent findings suggest greater involvement of the left CEN in explicit cognitive emotion regulation and language paradigms, while the right CEN is associated with implicit perceptual, somesthetic, and nociceptive processing (Smith et al., 2009; Laird et al., 2011; Heine et al., 2012). We also examined the anterior and posterior SN separately, as we have recently shown differential connectivity patterns of the anterior salience network (anterior insula) and posterior salience network (posterior insula) among PTSD, PTSD+DS, and healthy controls (Nicholson et al., 2016b; Harricharan et al., 2019). Furthermore, it has been shown that the anterior insula is associated more with arousal/interoceptive awareness, cognitive emotional processing (Craig 2010; Menon and Uddin 2010), heightened alertness, and autobiographical memory (Kurth et al., 2010), representing a major hub within the anterior SN along with the dorsal ACC (Shirer et al., 2012). By contrast, the posterior insula has been identified as a multimodal convergence zone for sensory information, including pain processing (Craig et al., 2011; Deen et al., 2011), and is the major hub of the posterior SN (Shirer et al., 2012). Interestingly, the CEN and SN naturally separated into left/right and anterior/posterior networks, respectively, when conducting our ICA protocol. In summary, we carried forward 1 DMN component, 2 CEN components (left/right), and 2 SN components (anterior/posterior).
3.1.3. Group spatial comparisons for intrinsic networks
The resulting component spatial maps of networks of interest were entered into second-level analyses within SPM12, with the central aim to examine network differences in the strength of regional functional connectivity between participant groups. For each network, a mask was created by entering the single-subject spatial maps into a voxel-wise one-sample t-test. This was thresholded at q < 0.05 with false discovery rate (FDR) correction and saved as a mask for use in subsequent paired t-tests on the single-subject spatial maps. This ensured that all findings would be restricted to brain regions actually contributing to the respective component. We first conducted a 3 (group) × 5 (network) full-factorial ANOVA that principally focused on the interaction between participant group (PTSD, PTSD+DS, healthy controls) and network (DMN, left/right CEN, anterior/posterior SN). Next, we evaluated between group differences in functional connectivity for each network, with separate two-sample t-tests. All analyses were evaluated at the conservative threshold of p-FDR < 0.05 k = 10 observed at the cluster-corrected level in order to control for multiple comparisons (see Eklund et al., 2016) where we set the initial uncorrected cluster-forming threshold in SPM at p < .001, k = 20.
3.2. Part B) multivariate machine learning classification analysis on network activation
This section of the methods describes the analysis pertaining to the second objective of the study, which was to observe the predictive validity of machine learning algorithms with regard to classifying PTSD, PTSD+DS, and healthy individuals based neural activation within ICNs.
3.2.1. Extraction of resting-state data for the classification machine learning analysis
We first extracted individual subject activation maps that would serve as inputs for the multivariate classification machine learning analyses after being masked with network maps. Specifically, we computed the mean amplitude of low-frequency fluctuations (mALFF) of the BOLD signal, denoting spontaneous resting-state brain activation across the whole-brain. The mALFF has been used previously as an input for machine learning analyses to predict accurately PTSD symptoms (Gong et al., 2014; Liu et al., 2015; Nicholson et al., 2018a), which was found to be more accurate than functional connectivity features (Nicholson et al., 2018). Here, we used the REST toolbox (http:// www.restfmri.net/forum) within Matlab2012a and SPM8 in order to de-trend and extract individual ALFF maps from the preprocessed fMRI data for each participant across the frequency band 0.01 Hz to 0.08 Hz. Individual subject mALFF maps were then obtained by normalizing ALFF spatial maps (where each voxel was divided by the whole-brain ALFF mean).
3.2.2. Multivariate machine learning classification analysis
To assess the predictive value of neural activation within the DMN, CEN, and SN as a means to classify PTSD, PTSD+DS, and healthy controls, we implemented Multiclass Gaussian Process Classification algorithms within PRoNTo toolbox (http:// www.mlnl.cs.ucl.ac.uk/pronto/) (Schrouff et al., 2013) running under Matlab2017a . Using standard template ICN masks from the GIFT toolbox (GIFT v4.0b), mALFF maps denoting activation within either the DMN, left CEN, right CEN, anterior SN or posterior SN were inputted into separate machine learning analyses. For each network, a resting-state design was modelled with no conditions. A feature set was prepared on mALFF data for voxels within each network of interest. Features were mean-centered, and a Multiclass Gaussian Process Classifier (MGPC) (Rasmussen et al., 2006; Schrouff et al., 2013) was used to test if network activation could accurately predict the three groups (PTSD, PTSD+DS, and healthy controls). Critically, PRoNTo software implements kernel methods as a result of the high dimensionality of pattern vectors in neuroimaging data relative to the number of subjects (for more information see Schrouff et al., 2013). We implemented a supervised machine learning approach such that MGPC could inform multiclass membership with predictive probabilities (i.e., PTSD, PTSD+DS and healthy control group classification) (Rasmussen et al., 2006; Schrouff et al., 2013; Wegrzyn et al., 2015). We then used a leave-one-subject-out (LOSO) cross-validation procedure to estimate the generalizability of our classifiers. Consistent with our previous machine learning publication, we also computed a leave-one-subject-out-per-group (LOSOPG) cross-validation procedure in order to provide an exhaustive approach to this analysis (see Supplemental Results) (Nicholson et al., 2018a). The MGPC analysis provided probabilistic predictions for each diagnostic category in which balanced accuracy measures were computed to account for unequal group sizes. Statistical significance of these accuracy measures was determined by permutation testing (1000 permutations). For visualization purposes, anatomical atlas weights were computed to illustrate the regional pattern of weights used by the decision function of the machine to classify each group (Schrouff et al., 2018). Finally, we evaluated the predictive accuracy of activation features from all networks (DMN, left/right CEN and anterior/posterior SN) within the same MGPC analysis.
Of importance, utilizing reference template network masks of the DMN, CEN, and SN in Part B (as opposed to corresponding network components/masks identified in Part A) ensured that the features inputted into the classification machine learning analysis were not biased by the previous independent component analysis. In Part A, the independent component analysis has indeed 'learnt' from the BOLD signal in all of the subjects, where the cross-validation procedure used in the machine learning analysis for Part B assumes that the hold-out set has not informed the decision function of the machine. As PRoNTo software does not allow for a feature extraction in which an ICA can be run on the inner cross validation loop of the MGPC (i.e., generating the ICA based on the training data by leaving-one-subject-out, then training the MGPC on these features, and finally, classifying the hold-out subject), we elected to utilize non-bias template ICN masks and not components identified in Part A.
3.3. Clinical data and motion statistical analyses
We computed Kruskal-Wallis H tests and Games-Howell post-hoc analyses in order to test for potential group differences with regard to the following clinical measures: CAPS-total, CTQ, BDI, MDI-total, and MDI depersonalization/derealization average scores. Additionally, scores on the RSDI scale (used to assess dissociation and reliving experiences subscales at the time of the scan) and the STAI (assessing state anxiety at the time of the scan) were compared among groups to assess for differences on state-based clinical symptoms. Finally, we computed separate chi-squared statistics on the number of motion outlier parameters generated by ART across each group, MDD diagnosis between patient groups, and frequency of medication use between patient groups.
4. Results
4.1. Part A) analysis of intrinsic connectivity networks
4.1.1. Spatial sorting analysis: component identification
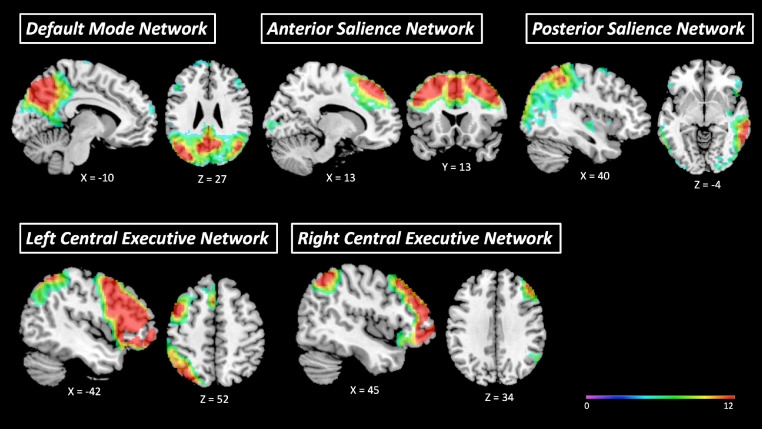
We identified five artifact-free components corresponding to the DMN, left CEN, right CEN, anterior SN, and posterior SN (see Fig. 1). The DMN component consisted mainly of a large bilateral cluster in the posterior cingulate cortex (PCC), precuneus, and superior parietal lobe (SPL), as well bilateral clusters in medial and lateral PFC regions, the temporal pole and ACC. The left CEN component primarily covered a large prefrontal area in the left hemisphere, including the left dlPFC, the dmPFC, and the orbitofrontal cortex, as well as clusters in the left SPL. The right CEN component primarily covered a large prefrontal area in the right hemisphere, including the vmPFC, the dlPFC, the dmPFC, and the orbitofrontal cortex, as well as clusters in the right SPL. The anterior SN component comprised mainly a large bilateral anterior cluster spanning the dorsal ACC, as well as the bilateral anterior insula. The posterior SN mainly comprised clusters in the bilateral posterior insula, supramarginal gyrus, precuneus and cerebellum (lobules V, VI).
Fig. 1.
Mean components generated by the group-level independent component analysis (ICA), pertaining to the default mode network, left and right central executive networks, and the anterior and posterior salience networks.
4.1.2. Group spatial comparison for intrinsic networks: Summary
In summary, when comparing functional connectivity within ICNs between PTSD, PTSD+DS and healthy controls, we found differential patterns of connectivity to brain regions involved in emotion regulation, in addition to limbic structures and areas involved in self-referential processing, interoception, consciousness, attention, orienting responses, and depersonalization/derealization. Specifically, the omnibus full-factorial ANOVA revealed a significant interaction effect between group and network involving the cuneus/precuneus and the middle dorsal PFC (see Table 2). Below we present in detail follow-up comparison results for each ICN.
Table 2.
Intrinsic Network Spatial Comparison: Full-Factorial ANOVA.
| Intrinsic Network | Brain Region | H | BA | Cluster Size | MNI Coordinate | F Stat. | Z score | p FDR Cluster | ||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||||
| Network by Group Interaction | Cuneus/precuneus | 17 | 94 | −2 | −92 | 20 | 5.63 | 4.88 | 0.036 | |
| Middle dorsal PFC | R | 9 | 93 | 28 | 36 | 40 | 4.41 | 4.01 | 0.037 | |
Results from the full-factorial 3 (group) by 5 (network) ANOVA (p-FDR cluster-corrected < 0.05, k = 10). Abbreviations: PFC = Prefrontal cortex, H = Hemisphere, BA = Brodmann area, p FDR = False discovery rate correction for multiple comparisons.
4.1.3. Default mode network
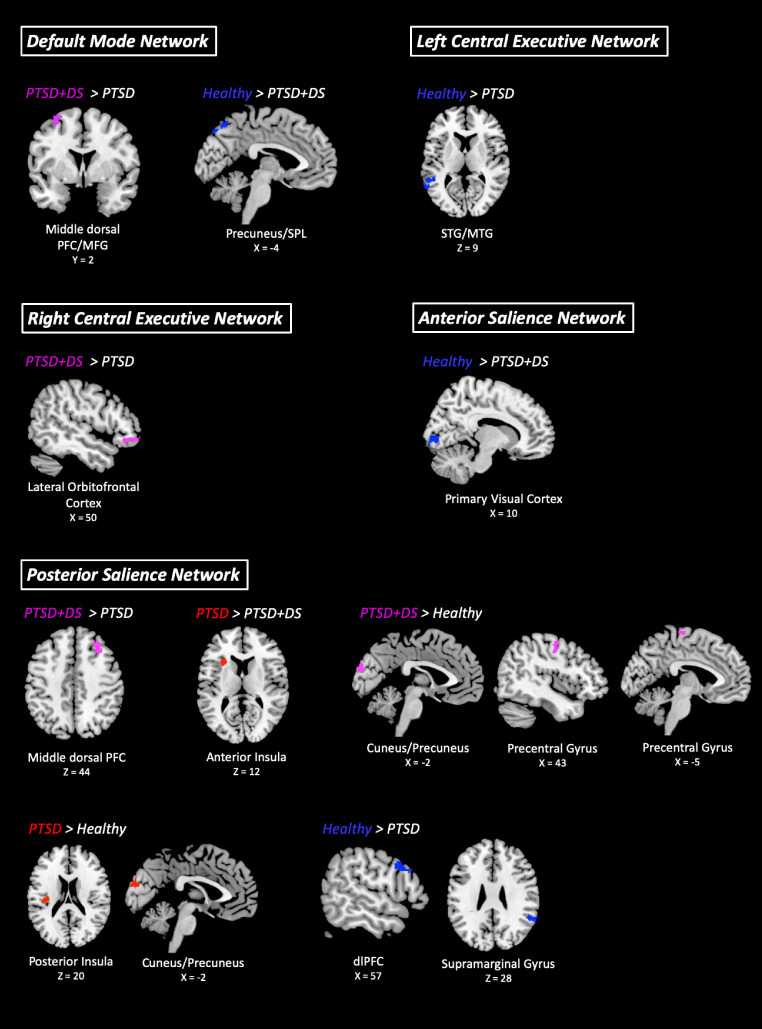
PTSD+DS patients showed more DMN functional connectivity to the left middle dorsal PFC/middle frontal gyrus as compared to PTSD patients (see Table 3 and Fig. 2). Additionally, the healthy control group displayed increased DMN functional connectivity to the left precuneus/SPL, as compared to PTSD+DS patients.
Table 3.
Follow-up Between Group Spatial Comparisons for Intrinsic Networks.
| Intrinsic Network | Group Comparison | Brain Region | H | BA | Cluster Size | MNI Coordinate | t Stat. | Z score | p FDR Cluster | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||||
| DMN | PTSD+DS > PTSD | Middle dorsal PFC/MFG | L | 8/9 | 85 | −32 | 0 | 64 | 4.77 | 4.74 | 0.016 |
| Healthy > PTSD+DS | Precuneus/ superior parietal lobe | L | 7 | 75 | −6 | −68 | 54 | 4.71 | 4.68 | 0.032 | |
| Left CEN | Healthy > PTSD | Superior/middle temporal gyrus | L | 21 | 129 | −64 | −54 | 10 | 4.33 | 4.31 | 0.008 |
| Right CEN | PTSD+DS > PTSD | Lateral orbitofrontal cortex | R | 11 | 91 | 48 | 30 | −10 | 3.92 | 3.90 | 0.012 |
| Anterior SN | Healthy > PTSD+DS | Primary visual cortex | R | 17 | 113 | 9 | −84 | 1 | 5.08 | 5.04 | 0.005 |
| Posterior SN | PTSD+DS > PTSD | Middle dorsal PFC | R | 8/9 | 201 | 28 | 30 | 46 | 4.50 | 4.48 | 0.003 |
| PTSD > PTSD+DS | Anterior insula | L | 79 | −28 | 18 | 10 | 4.58 | 4.55 | 0.029 | ||
| PTSD+DS > Healthy | Cuneus/precuneus | 17 | 86 | 0 | −92 | 20 | 4.95 | 4.92 | 0.020 | ||
| Precentral gyrus | L | 4 | 55 | −3 | −22 | 70 | 4.64 | 4.61 | 0.039 | ||
| R | 4 | 54 | 42 | −8 | 54 | 4.09 | 4.07 | 0.039 | |||
| PTSD > Healthy | Posterior insula | L | 88 | −36 | −20 | 17 | 5.15 | 5.11 | 0.007 | ||
| Cuneus/precuneus | 17 | 124 | −2 | −90 | 20 | 4.92 | 4.89 | 0.002 | |||
| Healthy > PTSD | Supramarginal gyrus | R | 40 | 205 | 64 | −46 | 34 | 5.11 | 5.08 | 0.001 | |
| dlPFC | R | 9 | 68 | 60 | 14 | 27 | 5.11 | 5.07 | 0.040 | ||
Follow-up group comparisons of network functional connectivity evaluated via 2-sample t-tests (p-FDR cluster-corrected < 0.05, k = 10). Data represents between group differences in terms of network functional connectivityfor the DMN, CEN and SN. Comparisons were computed between PTSD patients, PTSD+DS patients, and healthy controls, as indicated by contrast notation (< or >). Abbreviations: DMN = Default mode network, CEN = central executive network, SN = salience network, dlPFC = Dorsolateral prefrontal cortex, PFC = Prefrontal cortex, MFG = Middle frontal gyrus, PTSD = Posttraumatic stress disorder patient group, PTSD+DS = Dissociative subtype posttraumatic stress disorder group, Healthy= age-matched healthy control group, BA = Brodmann Area, MNI = Montreal Neurological Institute, FDR = False discovery rate cluster corrected, H = Hemisphere.
Fig. 2.
Between group comparisons of default mode, central executive, and salience network connectivity (FDR-cluster level p < .05, k = 10) comparing PTSD patients, PTSD+DS patients, and healthy controls, as indicated by contrast notation (< or >). Abbreviations: PTSD = Posttraumatic stress disorder group, PTSD+DS = Dissociative subtype posttraumatic stress disorder group, FDR = False discovery rate cluster corrected, dlPFC = Dorsolateral prefrontal cortex, SPL = Superior parietal lobe, MFG = Middle frontal gyrus, STG = Superior temporal gyrus, MTG = Middle temporal gyrus. Coordinates are given in MNI space and images were produced using MRIcron.
4.1.4. Left central executive network
As compared to patients with PTSD, the healthy control group showed increased left CEN functional connectivity to the left superior/middle temporal gyrus (see Table 3 and Fig. 2).
4.1.5. Right central executive network
PTSD+DS patients displayed increased right CEN functional connectivity to the right lateral orbitofrontal cortex, as compared to PTSD patients (see Table 3 and Fig. 2).
4.1.6. Anterior salience network
The healthy control group showed more anterior SN functional connectivity to the right primary visual cortex as compared to PTSD+DS patients (see Table 3 and Fig. 2).
4.1.7. Posterior salience network
PTSD+DS patients displayed more posterior SN functional connectivity to the right middle dorsal PFC as compared to PTSD patients (see Table 3 and Fig. 2). By contrast, we found that the PTSD group displayed increased posterior SN connectivity to the left anterior insula, as compared to the PTSD+DS group. The PTSD+DS group also showed more posterior SN functional connectivity to the cuneus/precuneus, and the bilateral precentral gyrus, as compared to the healthy control group. Additionally, PTSD patients displayed increased posterior SN functional connectivity to the left posterior insula and the cuneus/precuneus, as compared to the healthy control group. Finally, healthy controls showed more posterior SN functional connectivity to the right dlPFC and the right supramarginal gyrus as compared to PTSD patients.
4.2. Part B) multivariate machine learning classification analysis on network activation
4.2.1. Default mode network
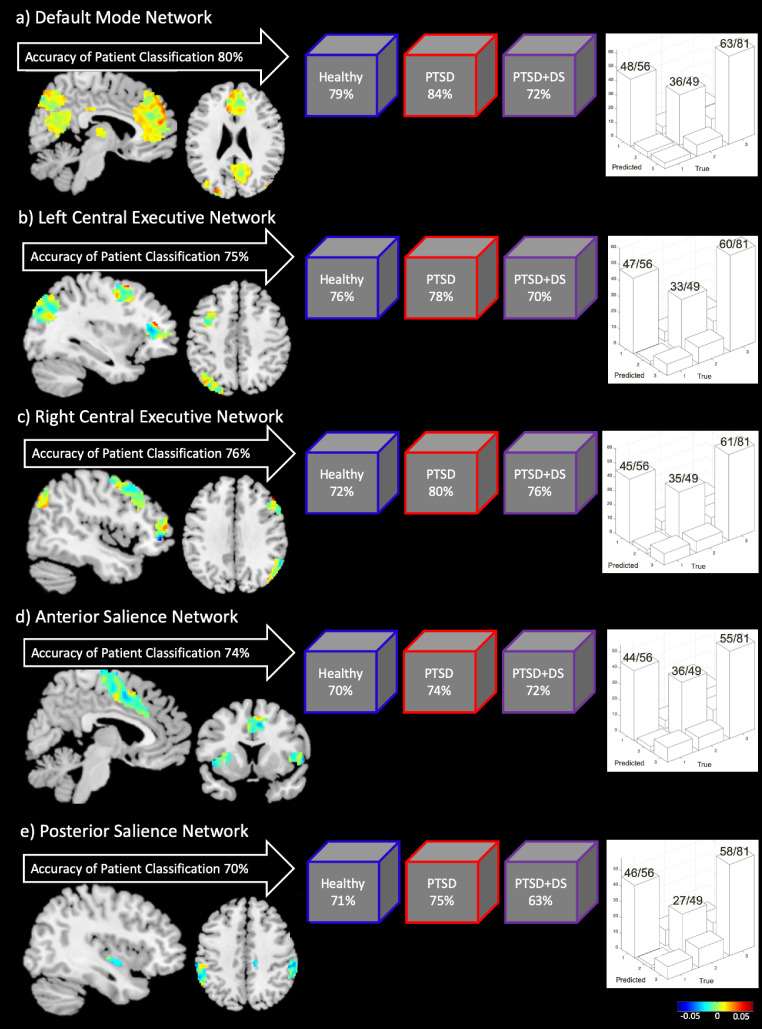
Activation within the DMN resulted in the highest accuracy measures across all ICNs with regard to classifying PTSD diagnoses with machine learning computations. Indeed, we found that activation within the DMN was able to predict group classification of PTSD, PTSD+DS, and healthy individuals with 80% balanced accuracy (p < .005 during permutation testing) (Fig. 3). Here, the class accuracy was 86% for controls, 78% for PTSD, and for 74% for PTSD+DS. Moreover, the class predictive value was 79% for controls, 84% for PTSD, and 72% for PTSD+DS. Network regions with the highest decision function weights included multiple clusters in the mPFC, as well as midline parietal areas in the SPL and the PCC.
Fig. 3.
Results for the Multiclass Gaussian Process Classification machine learning analysis, examining the predictive validity of activation within intrinsic connectivity networks: a) default mode network, b) left central executive network, c) right central executive network, d) anterior salience network, e) posterior salience network. On the left side of each section is the balanced accuracy of group classification for each network, with regional decision function weight vectors displayed below (visual representation of the weights that the machine learning algorithm uses to classify each group, based on features inputted from each network). Displayed in the middle are the individual class predictive values for each group. Finally, the graphs on the right side of each section displays the respective confusion matrix, where an ideal confusion matrix is diagonal and all predicted class labels correspond to the truth. Here, no classes are sacrificed in order to gain accuracy in other classes. For the confusion matrix, Group 1 = healthy control group, Group 2 = PTSD+DS patients, Group 3 = PTSD patients. Numbers on top of the bars in each graph correspond to the number of correctly classified individuals in each group. Abbreviations: PTSD = posttraumatic stress disorder group, PTSD+DS = dissociative subtype posttraumatic stress disorder group.
4.2.2. Left central executive network
Activation within the left CEN was able to predict group classification of PTSD, PTSD+DS, and healthy individuals with 75% balanced accuracy (p < .005 during permutation testing) (Fig. 3). The class accuracy was 84% for controls, 74% for PTSD, and 68% for PTSD+DS. Moreover, the class predictive value was 76% for controls, 78% for PTSD, and 70% for PTSD+DS. Network regions with the highest weights included areas in the left hemisphere pertaining to the dlPFC, the SPL, and the cerebellum crus 1.
4.2.3. Right central executive network
We found that activation within the right CEN was able to predict group classification of PTSD, PTSD+DS, and healthy individuals with 76% balanced accuracy (p < .005 during permutation testing) (Fig. 3). The class accuracy was 81% for controls, 75% for PTSD, and 72% for PTSD+DS. Moreover, the class predictive value was 72% for controls, 80% for PTSD, and 76% for PTSD+DS. Network regions with the highest weights included areas in the right hemisphere pertaining to the inferior orbitofrontal cortex, the dlPFC, the SPL, the supramarginal gyrus, and the cerebellum crus 1.
4.2.4. Anterior salience network
Activation within the anterior SN was able to predict group classification of PTSD, PTSD+DS, and healthy individuals with 74% balanced accuracy (p < .005 during permutation testing) (Fig. 3). The class accuracy was 79% for controls, 68% for PTSD, and 74% for PTSD+DS. Moreover, the class predictive value was 70% for controls, 74% for PTSD, and 72% for PTSD+DS. Network regions with the highest decision function weights included the anterior dorsal ACC, the bilateral middle dorsal PFC, and the right anterior insula.
4.2.5. Posterior salience network
Activation within the posterior SN was able to predict group classification of PTSD, PTSD+DS, and healthy individuals with 70% balanced accuracy (p < .005 during permutation testing) (Fig. 3). The class accuracy was 82% for controls, 72% for PTSD, and 56% for PTSD+DS. Moreover, the class predictive value was 71% for healthy individuals, 75% for PTSD, and 63% for PTSD+DS. Network regions with the highest weights included the right posterior insula, the left superior temporal gyrus, the right postcentral gyrus, and the bilateral supramarginal gyrus.
A noteworthy concept is that all voxels within each of the specified ICN activation maps will contribute to the decision function of the machine. Indeed, the weight maps produced by the machine learning analysis are a spatial representation of the predictive function and show the relative contribution of all voxels for the model. Hence, we present regional contributions above and in Fig. 3 for illustrative purposes only. This is in contrast to many common neuroimaging analyses which utilize a univariate approach. In this multivariate analysis, as all voxels inputted into the algorithm will contribute to the machine's prediction, it is not possible to arbitrarily threshold or to single out whether any one region is predictive in isolation. Finally, similar results were obtained for all machine learning analyses when using a LOSOPG cross-validation procedure. Here, accuracy measures were within +/- 1% of original balanced accuracy percent values reported via the LOSO cross-validation (see Supplemental Results). Moreover, all anatomical weights retained expected ranking positions, indicating stable ranking of the regions across folds. Of importance, confusion matrices in Fig. 3 show an optimal and diagonal pattern of classification, where no classes are sacrificed in order to gain accuracy in other classes. Finally, including activation features from all networks in the same MGPC analysis yielded a balanced accuracy measure of 88.99% (p < .005 during permutation testing) (class accuracy 98.21% for healthy individuals, 81.63% for PTSD+DS, and 90.12% for PTSD; class predictive value 88.71% for healthy individuals, 90.91% for PTSD+DS, and 91.25% for PTSD). Collectively, these results highlight the importance of examining ICNs in PTSD; below we discuss each network in turn, an approach consistent with these findings demonstrating the unique contributions of each network to the accuracy of classification.
4.3. Clinical data and motion artefacts
We found that all three groups (PTSD, PTSD+DS and controls) differed significantly in terms of CAPS-IV, CTQ, BDI, MDI-total, and MDI depersonalization/derealization average scores. The PTSD+DS group exhibited the highest scores among these clinical variables as compared to the PTSD and control groups (see Supplemental Material Table s1 and Table 1), where the PTSD group had higher scores on these clinical variables as compared to the control group only.
With regard to state-based clinical measures assessed at the time of the fMRI scan, the PTSD+DS group scored significantly higher on state dissociation scores as measured by the RSDI-dissociation subscale as compared to the PTSD group (see Supplemental Material Table s1 and Table 1). By contrast, scores for state anxiety (STAI) and reliving symptoms (RSDI scale) did not differ significantly between the PTSD and PTSD+DS groups. However, all state-based clinical measures were significantly higher in the PTSD and PTSD+DS groups as compared to the healthy control group, which is a common finding in the PTSD literature and suggests active psychopathology even at rest among PTSD patients (also see Harricharan et al., 2016b; Nicholson et al., 2017a; Lanius et al., 2018). Dissociative symptoms collected prior to the scan (MDI depersonalization/derealization averages) and during the scan (RSDI depersonalization/derealization averages) were highly correlated (r = 0.70, p <0.001).
The relation between medication use frequency and group was non-significant. MDD diagnosis was significantly more frequent in the PTSD+DS group as compared to the PTSD group (p < .001), where it has been suggested that higher scores of depression and PTSD symptom severity are associated with the dissociative subtype of PTSD (Stein et al., 2013; Hansen et al., 2017). Finally, when MDD diagnosis was used as a covariate in Part A, regional clusters did not change, albeit the magnitude of statistical significance decreased marginally. Similarly, medication use was not found to significantly affect results when used as a covariate. Our analysis regarding motion outliers yielded non-significant results when comparing observed outlier frequencies across groups X2 (2, N = 186) = 0.90, ns.
5. Discussion
In comparison to patients with PTSD, PTSD+DS is characterized by additional psychopathology, more severe PTSD symptoms, increased comorbidity, and suicidality, which together translates into unique treatment needs (Lanius et al., 2012; Stein et al., 2013; Mergler et al., 2017). Critically, the functional connectivity of intrinsic networks has not been compared among heterogeneous PTSD subtypes (PTSD versus PTSD+DS) and healthy controls, nor has activation within these networks been used to predict PTSD subtype diagnosis using machine learning. Indeed, it was previously unclear how alterations within the DMN, CEN and SN may contribute to heterogeneous symptom presentation in PTSD versus PTSD+DS patients, which may inform treatment interventions aimed at restoring large scale brain networks. Given the distinct neurobiological correlates of PTSD and PTSD+DS at the activation and functional connectivity level within central hubs of ICNs, we hypothesized unique group differences in terms of functional connectivity within the DMN (PCC and mPFC), CEN (dlPFC), and the SN (insula and amygdala). In Part A, we observed differential group connectivity of the DMN, CEN, and SN among PTSD, PTSD+DS and healthy individuals to brain regions involved in emotion regulation, in addition to limbic structures and areas involved in self-referential processing, interoception, bodily self-consciousness, attention, orienting responses, and depersonalization/derealization. In support of our hypotheses, our results generally support increased connectivity with PFC emotion regulation areas among all intrinsic networks in patients with PTSD+DS as compared to decreased PFC emotion regulation connectivity among patients with PTSD. Importantly, unique ICN functional connectivity within each PTSD group may underlie differential symptom presentations among PTSD subtypes. In Part B, using machine learning classification algorithms, we were also able to show that activation within each of the aforementioned networks accurately predicts the classification of PTSD, PTSD+DS, and healthy individuals. Here, the DMN was the most accurate in classifying PTSD patient diagnoses — an expected finding given that feature selection was performed on resting-state data and that the DMN has been shown to be highly implicated in PTSD psychopathology (Lanius et al., 2015; Yehuda et al., 2015; Akiki et al., 2017). The second-best predictor of PTSD patient diagnoses using machine learning classification algorithms was activation within the CEN (the ICN most implicated in emotion regulation and executive functioning), which is a finding consistent with emotion-modulation models of PTSD (see below; Lanius et al., 2010; Fenster et al., 2018).
5.1. Default mode network
The DMN is thought to mediate altered self-referential processing, autobiographical memory, and social cognition (Lanius et al., 2015), where trauma has been shown to have lasting effects on the sense of self manifested at both the cognitive and somatic level (Frewen et al., 2020). When comparing PTSD patient groups, we hypothesized unique connectivity differences as PTSD+DS patients experience additional alterations in self-referential processing and bodily self-consciousness associated with depersonalization (i.e., out-of-body experiences, emotional numbing) (Lanius et al., 2018; Frewen et al., 2020). Studies investigating DMN intrinsic functional connectivity among subtype non-differentiated PTSD at rest generally report decreased coupling between the PCC, vmPFC, and other DMN structures where aberrant DMN functioning has been associated with PTSD symptoms (St. Jacques et al. 2013; Lanius et al., 2015; Yehuda et al., 2015; Akiki et al., 2017; Fenster et al., 2018). Importantly, during tasks that require executive functioning, previous studies report increased connectivity within the DMN and decreased connectivity within the CEN among patients with PTSD as compared to healthy individuals (Daniels et al., 2010).
While previous studies typically examine undifferentiated PTSD patient samples as compared to healthy controls, results from the current study demonstrate unique DMN connectivity between patients with PTSD and its dissociative subtype. Here, increased DMN connectivity to the middle dorsal PFC in the PTSD+DS as compared to the PTSD group likely represents emotion overmodulation (over regulation of the hyperactive limbic system in PTSD) related to increased symptoms of depersonalization/derealization in this group (Lanius et al., 2010, 2012; Rabellino et al., 2015a; Nicholson et al., 2017; Nicholson et al., 2018a; Fenster et al., 2018). Interestingly, and in direct support of our results, the frequency of dissociative experiences has been positively correlated to DMN connectivity with the dorsal PFC, a region involved in the CEN and emotion overmodulation (Bluhm et al., 2009). It is noteworthy that dissociative responses have also been shown to involve key areas of the DMN, including the medial PFC, medial parietal lobe, and the temporoparietal junction (Frewen and Lanius (2015)), further suggesting that alterations within the DMN may be a potential mechanism underlying depersonalization-related disturbances in self-referential processing (Rabellino et al., 2015a; Frewen et al., 2020). Critically, hyperconnectivity of the DMN with prefrontal CEN areas in PTSD+DS may also reduce the availability of the CEN for use in other cognitively demanding tasks, hence underlying symptoms of poor cognitive performance in this group (McKinnon et al., 2016). Moreover, PTSD+DS patients may be uniquely characterized by alterations in connectivity between the DMN and CEN regions, which may also relate to aforementioned difficulties in switching between DMN and CEN modalities (Lanius et al., 2015; Lanius et al., 2018; McKinnon et al., 2016). Mirroring our results and further supporting models of emotion undermodulation in PTSD patients without the dissociative subtype, a recent study found that PFC dysconnectivity in the DMN was linked to PTSD symptom severity (Akiki et al., 2018).
The precuneus is an additional node within the DMN and is critical for relating the self to socially relevant emotional stimuli, bodily self-consciousness, first-person perspective taking, visuo-spatial imagery and episodic memory retrieval (Greicius et al., 2003; Cavanna and Trimble 2006; Cabanis et al., 2013). Here, decreased precuneus connectivity in PTSD+DS as compared to healthy controls may point to alterations in these functions related to depersonalization (i.e., out-of-body experiences) and dissociation driven changes in social-cognition (disruption in the ability to use, encode, and store information about others that we gain from social interactions) (McKinnon et al., 2016). The PTSD+DS group also displayed decreased connectivity to the SPL involved in attention (Behrmann et al., 2004; Wang et al., 2015), as compared to healthy controls. This may be a mechanism underlying dissociation driven changes in attention and cognitive dysfunction that occur in PTSD patients and in other psychiatric disorders transdiagnostically (De Bellis et al., 2013; McKinnon et al., 2016; Lanius et al., 2018).
Additionally, results from the multivariate machine learning analysis in Part B suggest that aberrant DMN activation may be a useful predictor of PTSD group classification, where the DMN yielded the most accurate classification results when predicting individual diagnoses among PTSD, PTSD+DS and healthy controls. Taken together, this provides further evidence that the DMN may support unique clinical presentations of self-referential processing, including depersonalization and related identity disturbance.
5.2. Central executive network
Alterations within the CEN are thought to underlie both emotion regulatory and cognitive dysfunctions observed in PTSD (Daniels et al., 2010; Frewen et al., 2015; Lanius et al., 2015; McKinnon et al., 2016; Akiki et al., 2017; Shalev et al., 2017). A recent study found that during the resting-state, subtype non-differentiated PTSD patients demonstrated reduced CEN convergence which was associated with decreased orbitofrontal-amygdala connectivity in PTSD, indicative of reduced prefrontal regulation on the resting limbic system (Barredo et al., 2018). This is consistent with previous literature that suggests decreased recruitment and functional connectivity within the CEN among subtype non-differentiated PTSD patients (Cisler et al., 2013; St. Jacques et al. 2013; Lanius et al., 2015; Holmes et al., 2018). Critically, these studies suggest that altered CEN functional connectivity may have cascading negative effects on emotion regulation, and thus may be critical to the neural underpinnings of emotion undermodulation symptoms observed in PTSD. By contrast, PTSD+DS patients are known to exhibit emotion overmodulation patterns of neural connectivity, paralleling extreme top-down regulation from CEN areas, including the prefrontal cortex, which may promote emotional detachment symptoms including depersonalization and derealization (Lanius et al., 2010; Nicholson et al., 2017; Fenster et al., 2018). Furthermore, PTSD is associated with disrupted cognitive functioning across multiple domains (declarative memory, short-term memory, attention, and executive functioning) (Aupperle et al., 2012; Polak et al., 2012; McKinnon et al., 2016).
In support of our hypotheses, we found increased right CEN connectivity with the right lateral orbitofrontal cortex in PTSD+DS as compared to PTSD. Increased CEN connectivity with the orbitofrontal cortex may contribute to the neurobiological basis of emotion overmodulation among PTSD+DS patients. Indeed, exacerbated recruitment of emotion regulation areas, including the orbitofrontal cortex among PTSD+DS patients has repeatedly been shown to be related to over-regulation of the limbic system with associated depersonalization, derealization and emotional detachment (Hopper et al., 2007b; Felmingham et al., 2008; Lanius et al., 2010; Weston 2014; Nicholson et al., 2017; Nicholson et al., 2018a; Fenster et al., 2018; Melara et al., 2018; D Rabellino et al., 2018). Furthermore, dissociative symptoms have been shown to impair CEN functions related to attention, executive functioning, memory, and social cognition (Aupperle et al., 2012; Polak et al., 2012; Cisler et al., 2013; St. Jacques et al. 2013; McKinnon et al., 2016; Block et al., 2017).
Additionally, the PTSD group displayed decreased left CEN connectivity to the left superior/middle temporal gyri as compared to the healthy control group. Interestingly, alterations in superior/middle temporal gyri processing have been associated with trauma-related symptoms, such as reexperiencing and avoidance, where decreased CEN connectivity to this area my correspond to decreased control over these symptoms (Lanius et al., 2002; Hopper et al., 2007b; Sierra et al., 2014; Schiavone et al., 2018). Interestingly, the temporoparietal junction, which includes the posterior superior temporal gyrus and the supramarginal gyrus, is critical for multisensory integration, bodily self-consciousness and embodiment (which is the sense of being localized within one's physical body and therefore constitutes a fundamental aspect of the self) (Arzy et al., 2006; Blanke 2012; Igelström et al., 2015). Furthermore, the left superior/middle temporal gyrus is also part of the dorsal attention network critical for multisensory integration and executive functioning (Dixon et al., 2018). Critically, these functions related to bodily self-consciousness, multisensory integration, embodiment, and executive functioning are hypothesised to be disrupted in patients with PTSD (Lanius et al., 2015; S Harricharan et al., 2017).
Finally, results from the multivariate machine learning analysis in Part B suggest that activation within the left and right CEN may be useful biomarkers for predicting PTSD subtype classification. Indeed, our CEN results from Part A & B strongly support the hypothesis that unique symptom presentation among PTSD subtypes may emanate from emotion under- and over- modulation within the CEN in PTSD and PTSD+DS patients, respectively.
5.3. Salience network
Alterations in SN functioning in patients with PTSD may contribute to the hypervigilance and hyperarousal symptoms commonly observed in patients with PTSD (Lanius et al., 2015; Yehuda et al., 2015; Fenster et al., 2018), where subtype non-differentiated PTSD patients have been shown to display elevated SN connectivity during the resting state (Rabinak et al., 2011; Sripada et al., 2012; Koch et al., 2016). Additionally, the SN and anterior insula are thought to mediate “dynamic switching” between DMN and the CEN, in order to bring online higher-order cognitive processing (Seeley et al., 2007; Sridharan et al., 2008; Menon and Uddin 2010).
The anterior insula is associated with arousal/interoceptive awareness, cognitive emotional processing (Craig 2010; Menon and Uddin 2010), heightened alertness, and auto- biographical memory (Kurth et al., 2010) and represents a major hub within the anterior SN along with the dorsal ACC (Shirer et al., 2012). In the current study, our results showed decreased anterior SN connectivity to the primary visual cortex in PTSD+DS as compared to healthy controls. Critically, the anterior SN facilitates orienting responses to salient stimuli via connections with dorsal visual stream regions, which includes the primary visual cortex (Menon and Uddin 2010; Uddin 2015). Here, we hypothesize that PTSD+DS patients may exhibit disrupted SN processing with regard to orienting to salient visual stimuli in the environment, which may be mediated by depersonalization and derealization symptoms (Lanius et al., 2015; Harricharan et al., 2019).
By contrast, the posterior insula region has been identified as a multimodal convergence zone for sensory information, including pain and body condition (Craig et al., 2011; Deen et al., 2011) and is the major hub of the posterior SN (Shirer et al., 2012). In the current study, PTSD patients displayed increased posterior SN connectivity to the anterior insula as compared to PTSD+DS, and additionally displayed increased posterior SN connectivity with the posterior insula as compared to healthy controls. In the PTSD group, increased posterior SN connectivity with the anterior insula may represent exacerbated sensory processing related to arousal/interoceptive awareness, emotional state processing and heightened alertness (Craig 2010; Menon and Uddin 2010; Lanius et al., 2015; Nicholson et al., 2016b), as compared to PTSD+DS. Indeed, PTSD+DS and accompanying emotional detachment, depersonalization and derealization, has been associated with anterior insula under-engagement (Hopper et al., 2007b; Frewen et al., 2008; Lanius et al., 2010; Fenster et al., 2018). These findings support exacerbated interoceptive/arousal processing in the anterior insula among PTSD patients as compared to PTSD+DS (Nicholson et al., 2016a), which may precipitate hypervigilance and avoidance symptoms in PTSD patients (Paulus and Stein 2006; Yehuda et al., 2015). Notably, hyperactivation in the anterior insula has been correlated positively with state re-experiencing scores and negatively with state dissociation scores (Hopper et al., 2007b). By contrast, increased posterior SN connectivity to the posterior insula in PTSD as compared to healthy controls may reflect exacerbated multisensory processing related to salient threat detection, pain and body condition, hypervigilance and hyperarousal PTSD symptoms (Hopper et al., 2007b; Lanius et al., 2010; Pitman et al., 2012; Weston 2014; Meier et al., 2015; Fenster et al., 2018).
Finally, both the PTSD and PTSD+DS groups showed increased posterior SN connectivity to areas within attention/memory networks and areas related to episodic memory processing (precuneus, cuneus, precentral gyrus), as well as areas within the posterior insula network related to consciousness (precuneus) and motor processing (precentral gyrus) (Cavanna and Trimble 2006; Cauda et al., 2011; Burianová et al., 2012) as compared to healthy controls. Additionally, when compared to healthy individuals, the PTSD group evidenced decreased posterior SN connectivity with the supramarginal gyrus, a temporoparietal junction area heavily implicated in self-referential processing, bodily self-consciousness and embodiment, where these results collectively support disruptions among these neural processes within patients with PTSD (Blanke 2012; Serino et al., 2013; Igelström et al., 2015; Harricharan et al., 2017; Terpou et al., 2018).
The emotion modulation model also appears to be a relevant framework for characterizing PTSD subtypes with regard to SN connectivity. In support of our hypotheses, we found increased posterior SN connectivity among PTSD+DS patients to the middle dorsal PFC as compared to PTSD patients. This finding suggests that the middle dorsal PFC may be attenuating/ overmodulating salient threat processing and multisensory integration of the posterior SN within PTSD+DS patients (Etkin et al., 2015; Nicholson et al., 2017). Similarly, PTSD patients also displayed decreased posterior SN connectivity to the dlPFC as compared to healthy controls. This further supports models of emotion undermodulation in PTSD patients, consistent with attenuated connectivity between emotion regulation areas and limbic structures (Lanius et al., 2010; Yehuda et al., 2015; Nicholson et al., 2017; Fenster et al., 2018). Finally, we were able to predict the diagnosis PTSD, PTSD+DS and healthy controls based on SN activation using machine learning computations. The anterior salience network had greater classification accuracy as compared to the posterior SN during our resting-state scans. Notably, SN functioning related to threat detection may predict classification with higher accuracy during symptom provocation/emotion induction as opposed to resting-state paradigms. Indeed, functional connectivity patterns of ICNs may further polarize during emotion induction paradigms when comparing PTSD versus PTSD+DS patients, where these patient groups are known to exhibit unique symptoms in response to such paradigms (Hopper et al., 2007b; Lanius et al., 2010).
5.4. Future directions and limitations
It will be critical for future studies to investigate additional subtypes of PTSD illness, as well as investigate accuracy of prediction during emotion induction paradigms. Future studies may benefit from a longitudinal experimental design that would allow for prospective examination of the effects of treatment response and medication type/dosage on neural network integrity in PTSD. Additional studies are also required to elucidate further the effects of different classes of medication on patterns of neural response, and to determine how specific biomarkers of psychiatric illness may predict response to specific treatments. Future studies should also examine the predictive accuracy of data driven networks from PTSD populations and explore influences of sex on network classification in PTSD. It will also be important to examine changes in network connectivity in relation to patterns of patient comorbidity. Additionally, PTSD and PTSD+DS differed significantly on CAPS-IV total severity scores; no such finding emerged for the CAPS-5. The absence of an effect in participants assessed by the CAPS-5 may stem, in part, from the smaller sample size of these participants, thus limiting power to detect a difference. Future studies that include a larger sample size will be needed to confirm this hypothesis and to additionally compare the sensitivity of such diagnostic measures. Finally, we implemented a very conservative data driven approach, where additional group differences were found at a more liberal threshold for the DMN, SN, and CEN; hence, replication and increased power are critical to fully elucidating these dynamics.
6. Conclusion
Alterations within intrinsic connectivity networks have long been shown to be associated with psychiatric illnesses, including PTSD. Of importance, we present the first study to compare directly intrinsic connectivity network (DMN, CEN, and SN) functional connectivity among PTSD, dissociative subtype PTSD and healthy individuals. Specifically, we show unique group differences in terms of ICN functional connectivity with emotion regulation areas, limbic structures, somatosensory and interoception brain areas, as well as regions involved in self-referential processing, consciousness, and depersonalization/derealization. Using multivariate machine learning classification algorithms, we were also able to show that activation within these networks predicts accurately the diagnosis of PTSD, its dissociative subtype, and healthy individuals with high accuracy. Critically, identifying objective neural network classifiers that can categorize PTSD heterogeneity may prove valuable by pointing towards targeted treatments for PTSD and its dissociative subtype that address specific alterations in the neural networks associated with each and thus match specific individuals to personalized treatment approaches. The present results also suggest strongly that the specific patterns of alterations within intrinsic connectivity networks associated uniquely with PTSD and with PTSD+DS may contribute, in part, to the contrasting clinical presentation observed across these subtypes.
CRediT authorship contribution statement
Andrew A. Nicholson: Conceptualization, Data curation, Funding acquisition, Formal analysis, Writing - original draft. Sherain Harricharan: Conceptualization, Data curation, Writing - review & editing, Visualization. Maria Densmore: Formal analysis, Methodology. Richard W.J. Neufeld: Conceptualization, Writing - review & editing. Tomas Ros: Conceptualization, Writing - review & editing. Margaret C. McKinnon: Conceptualization, Writing - review & editing. Paul A. Frewen: Conceptualization, Writing - review & editing. Jean Théberge: Conceptualization, Writing - review & editing. Rakesh Jetly: Conceptualization, Writing - review & editing. David Pedlar: Conceptualization, Writing - review & editing. Ruth A. Lanius: Conceptualization, Supervision, Data curation, Funding acquisition, Writing - original draft.
Declaration of Competing Interest
All authors declare no financial interests or potential conflicts of interest with regard to the current study.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgements
Dr. Andrew A. Nicholson has received funding support from the European Union's Horizon 2020 research and innovation program under the Marie Sklodowska-Curie Individual Fellowship (grant agreement No. 897709). Our research group would also like to acknowledge the following funding sources in which we are extremely grateful for their support: the Canadian Institutes of Health Research (CIHR), General Dynamics Land Systems, Mitacs, the Canadian Institute for Veteran Health Research (CIMVHR), IBM and the Homewood Research Institute. We also thank Suzy Southwell and Stephanie Nevill for their contributions with data collection, and Dr. David Steyrl for providing a high level of machine learning expertise. Open access funding provided by the University of Vienna.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2020.102262.
Appendix. Supplementary materials
References
- Akiki T.J., Averill C.L., Abdallah C.G. A Network-Based Neurobiological Model of PTSD : evidence From Structural and Functional Neuroimaging Studies. Curr Psychiatry Rep. 2017 doi: 10.1007/s11920-017-0840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiki T.J., Averill C.L., Wrocklage K.M., Scott J.C., Averill L.A., Schweinsburg B., Alexander-Bloch A., Martini B., Southwick S.M., Krystal J.H., Abdallah C.G. Default mode network abnormalities in posttraumatic stress disorder: a novel network-restricted topology approach. Elsevier Ltd NeuroImage. 2018;176:489–498. doi: 10.1016/j.neuroimage.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen E.A., Erhardt E.B., Damaraju E., Gruner W., Segall J.M., Silva R.F., Havlicek M., Rachakonda S., Fries J., Kalyanam R., Michael A.M., Caprihan A., Turner J.A., Eichele T., Adelsheim S., Bryan A.D., Bustillo J., Clark V.P., Feldstein Ewing S.W., Filbey F., Ford C.C., Hutchison K., Jung R.E., Kiehl K.A., Kodituwakku P., Komesu Y.M., Mayer A.R., Pearlson G.D., Phillips J.P., Sadek J.R., Stevens M., Teuscher U., Thoma R.J., Calhoun V.D. A Baseline for the Multivariate Comparison of Resting-State Networks. Front Syst Neurosci. 2011;5:1–23. doi: 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA . 5th edn Ed. American Psychiatric Publishing; Washington DC: Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental disorders. American Journal of Psychiatry. VAP Publishing. [Google Scholar]
- Arzy S., Thut G., Mohr C., Michel C.M., Blanke O. Neural Basis of Embodiment: distinct Contributions of Temporoparietal Junction and Extrastriate Body Area. Journal of Neuroscience. 2006;26:8074–8081. doi: 10.1523/JNEUROSCI.0745-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperle R.L., Melrose A.J., Stein M.B., Paulus M.P. Executive function and PTSD: disengaging from trauma. Elsevier Ltd Neuropharmacology. 2012;62:686–694. doi: 10.1016/j.neuropharm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barredo J., Aiken E., Van T Wout-Frank M., Greenberg B.D., Carpenter L.L., Philip N.S. Network functional architecture and aberrant functional connectivity in post-traumatic stress disorder: A clinical application of network convergence. Brain Connectivity. 2018;8(9) doi: 10.1089/brain.2018.0634. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Guth D., Steer R.A., Ball R. Screening for major depression disorders in medical inpatients with the beck depression inventory for primary care. Behav Res Ther. 1997;35:785–791. doi: 10.1016/s0005-7967(97)00025-9. [DOI] [PubMed] [Google Scholar]
- Behrmann M., Geng J.J., Shomstein S. Parietal cortex and attention. Curr. Opin. Neurobiol. 2004;14:212–217. doi: 10.1016/j.conb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- De Bellis M.D., Woolley D.P., Hooper S.R. Neuropsychological findings in pediatric maltreatment: relationship of PTSD, dissociative symptoms, and abuse/neglect indices to neurocognitive outcomes. Child Maltreat. 2013;18:171–183. doi: 10.1177/1077559513497420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein D.P., Stein J.A., Newcomb M.D., Walker E., Pogge D., Ahluvalia T., Stokes J., Handelsman L., Medrano M., Desmond D., Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse and Neglect. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Blake D.D., Weathers F.W., Nagy L.M., Kaloupek D.G., Gusman F.D., Charney D.S., Keane T.M. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Blanke O. Vol. 13. Nature Publishing Group; 2012. (Multisensory Brain Mechanisms of Bodily Self-Consciousness). [DOI] [PubMed] [Google Scholar]
- Bleich-cohen M., Jamshy S., Sharon H., Weizman R., Intrator N., Poyurovsky M., Hendler T. Machine learning fMRI classi fi er delineates subgroups of schizophrenia patients. Elsevier B.V. Schizophrenia Research. 2014;160:196–200. doi: 10.1016/j.schres.2014.10.033. [DOI] [PubMed] [Google Scholar]
- Block S.R., King, Sripada A.P., Weissman R.K., Welsh D.H., Liberzon I R. Behavioral and neural correlates of disrupted orienting attention in posttraumatic stress disorder. Cognitive, Affective, & Behavioral Neuroscience. 2017:422–436. doi: 10.3758/s13415-016-0488-2. [DOI] [PubMed] [Google Scholar]
- Bluhm R.L., Williamson P.C., Osuch E.A., Frewen P.A., Stevens T.K., Boksman K., Neufeld R.W.J., Théberge J.…Lanius R.A. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. Journal of Psychiatry and Neuroscience. 2009;34:187–194. [PMC free article] [PubMed] [Google Scholar]
- Boyd J.E., Protopopescu A., O’Connor C., Neufeld R.W.J., Jetly R., Hood H.K., Lanius R.A., McKinnon M.C. Dissociative symptoms mediate the relation between PTSD symptoms and functional impairment in a sample of military members, veterans, and first responders with PTSD. Eur J Psychotraumatol. 2018;9 doi: 10.1080/20008198.2018.1463794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briere J. Multiscale Dissociation Inventory Professional Manual. Odessa, Florida: Psychological Assessment Resources. 2002 [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The Brain’s Default Network Anatomy. Function, and Relevance to Disease. 2008;38:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Burianová H., Ciaramelli E., Grady C.L., Moscovitch M. Top-down and bottom-up attention-to-memory: mapping functional connectivity in two distinct networks that underlie cued and uncued recognition memory. Elsevier Inc. NeuroImage. 2012;63:1343–1352. doi: 10.1016/j.neuroimage.2012.07.057. [DOI] [PubMed] [Google Scholar]
- Cabanis M., Pyka M., Mehl S., Müller B.W., Loos-Jankowiak S., Winterer G., Wölwer W., Musso F., Klingberg S., Rapp A.M., Langohr K., Wiedemann G., Herrlich J., Walter H., Wagner M., Schnell K., Vogeley K., Kockler H., Shah N.J., Stöcker T., Thienel R., Pauly K., Krug A., Kircher T. The precuneus and the insula in self-attributional processes. Cognitive, Affective and Behavioral Neuroscience. 2013;13:330–345. doi: 10.3758/s13415-012-0143-5. [DOI] [PubMed] [Google Scholar]
- Calhoun V.D., Adali T., Pearlson G.D., Pekar J.J., 2001. A Method for Making Group Inferences from Functional MRI Data Using Independent Component Analysis. 151, 140–151. [DOI] [PMC free article] [PubMed]
- Calhoun V.D., Liu J., Adal T., 2009. NeuroImage A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. 45, 163–172. [DOI] [PMC free article] [PubMed]
- Cauda F., D'Agata F., Sacco K., Duca S., Geminiani G., Vercelli A. Functional connectivity of the insula in the resting brain. Elsevier Inc. NeuroImage. 2011;55:8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chen A.C., Etkin A. Hippocampal Network Connectivity and Activation Differentiates Post-Traumatic Stress Disorder From Generalized Anxiety Disorder. Nature Publishing Group Neuropsychopharmacology. 2013;38:1889–1898. doi: 10.1038/npp.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler J.M., Steele J.S., Lenow J.K., Smitherman S., Everett B., Messias E., Kilts C.D. Functional reorganization of neural networks during repeated exposure to the traumatic memory in posttraumatic stress disorder: an exploratory fMRI study. Elsevier Ltd Journal of Psychiatric Research. 2014;48:47–55. doi: 10.1016/j.jpsychires.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler J.M., Steele J.S., Smitherman S., Lenow J.K., Kilts C.D. Psychiatry Research : neuroimaging Neural processing correlates of assaultive violence exposure and PTSD symptoms during implicit threat processing : a network-level analysis among adolescent girls. Elsevier Psychiatry Research: Neuroimaging. 2013;214:238–246. doi: 10.1016/j.pscychresns.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A.D. The sentient self. Brain Structure and Function. 2010:1–15. doi: 10.1007/s00429-010-0248-y. [DOI] [PubMed] [Google Scholar]
- Craig, A.D.B., 2011. Significance of the insula for the evolution of human awareness of feelings from the body. Ann. N. Y. Acad. Sci. 1225, 7282. [DOI] [PubMed]
- Daniels J.K., Mcfarlane A.C., Bluhm R.L., Moores K a., Richard Clark C., Shaw M.E., Williamson P.C., Densmore M., Lanius R a. Switching between executive and default mode networks in posttraumatic stress disorder: alterations in functional connectivity. Journal of Psychiatry and Neuroscience. 2010;35:258–266. doi: 10.1503/jpn.090175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen, B., Pitskel, N.B., Pelphrey, K.A., 2011. Three systems of insular functional connectivity identified with cluster analysis. Cereb. Cortex 21, 14981506. [DOI] [PMC free article] [PubMed]
- Dixon M.L., De La Vega, Mills A., Andrews-Hanna C., Spreng J., Cole R.N., Christoff K M.W. Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. Proc. Natl. Acad. Sci. U.S.A. 2018;115:E1598–E1607. doi: 10.1073/pnas.1715766115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U.F., Fair D a, Miezin F.M., Cohen A.L., Wenger K.K., Dosenbach R a T, Fox M.D., Snyder A.Z., Vincent J.L., Raichle M.E., Schlaggar B.L., Petersen S.E. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. U.S.A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences. 2016 doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Büchel C., Gross J.J. The neural bases of emotion regulation. Nature Publishing Group Nature Reviews Neuroscience. 2015;16:693–700. doi: 10.1038/nrn4044. [DOI] [PubMed] [Google Scholar]
- Etkin A., Maron-Katz A., Wu A., Fonzo G.A., Huemer J., Vértes P.E.…O’Hara R. Using FMRI connectivity to define a treatment-resistant form of post-traumatic stress disorder. Sci. Transl. Med. 2019;11(486) doi: 10.1126/scitranslmed.aal3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Wager T.D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham K., Kemp a H, Williams L., Falconer E., Olivieri G., Peduto A., Bryant R. Dissociative responses to conscious and non-conscious fear impact underlying brain function in post-traumatic stress disorder. Psychol Med. 2008;38:1771–1780. doi: 10.1017/S0033291708002742. [DOI] [PubMed] [Google Scholar]
- Fenster R.J., Lebois L.A.M., Ressler K.J., Suh J. Springer US Nature Reviews Neuroscience; 2018. Brain Circuit Dysfunction in Post-Traumatic Stress disorder: from Mouse to Man. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. Biometrics Research, New York State Psychiatric Institute; New York: 2002. Structured Clinical Interview For DSM-IV-TR Axis I Disorders, Patient Edition (SCID-I/P, 11/2002 revision). For DSMIV. [Google Scholar]
- Fitzgerald J.M., Digangi J.A., Phan K.L. Functional Neuroanatomy of Emotion and Its Regulation in PTSD. Harv Rev Psychiatry. 2018;26:116–128. doi: 10.1097/HRP.0000000000000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen and Lanius, 2015. Healing the Traumatized Self: Consciousness, Neuroscience, Treatment. New York, W.W. Norton and Company.
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen P a, Dozois DJ a, Lanius R a. Neuroimaging studies of psychological interventions for mood and anxiety disorders: empirical and methodological review. Clin Psychol Rev. 2008;28:228–246. doi: 10.1016/j.cpr.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Frewen P., Schroeter M.L., Riva G., Cipresso P., Fairfield B., Padulo C., Kemp A.H., Palaniyappan L., Owolabi M., Kusi-Mensah K., Polyakova M., Fehertoi N., D’Andrea W., Lowe L., Northoff G. Neuroimaging the Consciousness of Self: review, and Conceptual-Methodological Framework. Neuroscience & Biobehavioral Reviews. 2020 doi: 10.1016/j.neubiorev.2020.01.023. [DOI] [PubMed] [Google Scholar]
- Frewen P.A., Brown M.F.D., Steuwe C., Lanius R.A., 2015. Latent profile analysis and principal axis factoring of the DSM-5 dissociative subtype. 1, 1–16. [DOI] [PMC free article] [PubMed]
- Fu C.H.Y., Costafreda SG Neuroimaging-based biomarkers in psychiatry: clinical opportunities of a paradigm shift. Canadian Journal of Psychiatry. 2013;58:499–508. doi: 10.1177/070674371305800904. [DOI] [PubMed] [Google Scholar]
- Galatzer-Levy I.R., Ma S., Statnikov A., Yehuda R., Shalev A.Y. Utilization of machine learning for prediction of post-traumatic stress: a re-examination of cortisol in the prediction and pathways to non-remitting PTSD. Transl Psychiatry. 2017;7:e0. doi: 10.1038/tp.2017.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Q., Li L., Du M., Pettersson-Yeo W., Crossley N., Yang X., Li J., Huang X., Mechelli A. Quantitative Prediction of Individual Psychopathology in Trauma Survivors Using Resting-State fMRI. Nature Publishing Group Neuropsychopharmacology. 2014;39:681–687. doi: 10.1038/npp.2013.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradus J.L., King M.W., Galatzer-levy I., Street A.E., 2017. Gender Differences in Machine Learning Models of Trauma and Suicidal Ideation in Veterans of the Iraq and Afghanistan Wars, 362–371. [DOI] [PMC free article] [PubMed]
- Greicius M.D., Krasnow B., Reiss A.L., Menon V., 2003. Functional connectivity in the resting brain : a network analysis of the default mode hypothesis.100. [DOI] [PMC free article] [PubMed]
- Habas C., Kamdar N., Nguyen D., Prater K., Beckmann C.F., Menon V., Greicius M.D., 2009. Distinct Cerebellar Contributions to Intrinsic Connectivity Networks. 29, 8586–8594. [DOI] [PMC free article] [PubMed]
- Hansen M., Ross J., Armour C. Evidence of the dissociative PTSD subtype: a systematic literature review of latent class and profile analytic studies of PTSD. . Elsevier B.V. J Affect Disord. 2017;213:59–69. doi: 10.1016/j.jad.2017.02.004. [DOI] [PubMed] [Google Scholar]
- Harricharan S., Nicholson A.A., Densmore M., Théberge J., McKinnon M.C., Neufeld R.W.J., Lanius R.A. Sensory overload and imbalance: resting-state vestibular connectivity in PTSD and its dissociative subtype. Elsevier Ltd Neuropsychologia. 2017;106:169–178. doi: 10.1016/j.neuropsychologia.2017.09.010. [DOI] [PubMed] [Google Scholar]
- Harricharan S., Nicholson A.A., Thome J., Densmore M., McKinnon M.C., Théberge J., Frewen P.A., Neufeld R.W.J., Lanius R.A. PTSD and its dissociative subtype through the lens of the insula: anterior and posterior insula resting‐state functional connectivity and its predictive validity using machine learning. Psychophysiology. 2019:1–23. doi: 10.1111/psyp.13472. [DOI] [PubMed] [Google Scholar]
- Harricharan S., Rabellino D., Frewen P.A., Densmore M., Théberge J., Mckinnon M.C., Schore A.N.…Lanius R.A. fMRI functional connectivity of the periaqueductal gray in PTSD and its dissociative subtype. Brain Behav. 2016:1–16. doi: 10.1002/brb3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine L., Soddu A., Gómez F., Vanhaudenhuyse A., Tshibanda L., Thonnard M., Charland-verville V., Kirsch M., Laureys S., Demertzi A., 2012. Resting state networks and consciousness Alterations of multiple resting state network connectivity in physiological, pharmacological, and pathological consciousness states. 3, 1–12. [DOI] [PMC free article] [PubMed]
- Henigsberg N., Kalember P., Petrović Z.K., Šečić A., 2018. Neuroimaging research in posttraumatic stress disorder – Focus on amygdala, hippocampus and prefrontal cortex. Elsevier Inc Progress in Neuro-Psychopharmacology and Biological Psychiatry, #pagerange#. [DOI] [PubMed]
- Himberg J., Hyva A., Esposito F., 2004. Validating the independent components of neuroimaging time series via clustering and visualization. 22, 1214–1222. [DOI] [PubMed]
- Holmes S.E., Scheinost D., DellaGioia N., Davis M.T., Matuskey D., Pietrzak R.H., Hampson M., Krystal J.H., Esterlis I. Cerebellar and Prefrontal Cortical Alterations in PTSD: structural and Functional Evidence. Chronic Stress. 2018;2 doi: 10.1177/2470547018786390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper J.W., Frewen P.A., Sack M., Lanius R.A., Van Der Kolk B.A. The responses to script-driven imagery scale (RSDI): assessment of state posttraumatic symptoms for psychobiological and treatment research. J Psychopathol Behav Assess. 2007;29:249–268. doi: 10.1007/s10862-007-9046-0. [DOI] [Google Scholar]
- Hopper J.W., Frewen P.A., van der Kolk B.A., Lanius R.A. Neural Correlates of Reexperiencing, Avoidance, and Dissociation in PTSD: symptom Dimensions and Emotion Dysregulation in Responses to Script-Driven Trauma Imagery. J Trauma Stress. 2007;20:713–725. doi: 10.1002/jts.20284. [DOI] [PubMed] [Google Scholar]
- Hulshoff H., Bullmore E. Neural networks in psychiatry. Elsevier European Neuropsychopharmacology. 2013;23:1–6. doi: 10.1016/j.euroneuro.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Igelström K.M., Webb T.W., Graziano M.S.A. Neural processes in the human temporoparietal cortex separated by localized independent component analysis. Journal of Neuroscience. 2015;35:9432–9445. doi: 10.1523/JNEUROSCI.0551-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Jacques P.L.S., Kragel P.A., Rubin D.C., 2013. Neural networks supporting autobiographical memory retrieval in posttraumatic stress disorder, 554–566. [DOI] [PMC free article] [PubMed]
- Jin C., Jia H., Lanka P., Rangaprakash D., Li L., Liu T., Hu X., Deshpande G. Dynamic brain connectivity is a better predictor of PTSD than static connectivity. Hum Brain Mapp. 2017;38:4479–4496. doi: 10.1002/hbm.23676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karstoft K., Galatzer-levy I.R., Statnikov A., Li Z., Shalev A.Y., 2015. Bridging a translational gap : using machine learning to improve the prediction of PTSD, 1–7. [DOI] [PMC free article] [PubMed]
- Kennis M., Van Rooij S.J.H., Van Den Heuvel M.P., Kahn R.S., Geuze E. Functional network topology associated with posttraumatic stress disorder in veterans. The Authors NeuroImage: Clinical. 2016;10:302–309. doi: 10.1016/j.nicl.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluetsch R.C., Ros T., Théberge J., Frewen P a, Calhoun V.D., Schmahl C., Jetly R., Lanius R a. Plastic modulation of PTSD resting-state networks and subjective wellbeing by EEG neurofeedback. Acta Psychiatr Scand. 2014;130:123–136. doi: 10.1111/acps.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S.B.J., van Zuiden M., Nawijn L., Frijling J.L., Veltman D.J., Olff M. Aberrant Resting-State Brain Activity in Posttraumatic Stress Disorder: a Meta-Analysis and Systematic Review. Depress Anxiety. 2016;14 doi: 10.1002/da.22478. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- Koechlin E., Summerfield C., 2007. An information theoretical approach to prefrontal executive function. 11. [DOI] [PubMed]
- Krause A.J., Simon E., Ben, Mander B.A., Greer S.M. Vol. 18. Nature Publishing Group Nature Publishing Group; 2017. pp. 404–418. (The Sleep-Deprived Human Brain). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth F., Zilles K., Fox P.T., Laird A.R., Eickhoff S.B. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Structure and Function. 2010:1–16. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird A.R., Fox P.M., Eickhoff S.B., Turner J.A., Ray K.L., Mckay D.R., Glahn D.C., Beckmann C.F., Smith S.M., Fox P.T., 2011. Behavioral Interpretations of Intrinsic Connectivity Networks, 4022–4037. [DOI] [PMC free article] [PubMed]
- Lanius R a, Brand B., Vermetten E., Frewen P a., Spiegel D. The dissociative subtype of posttraumatic stress disorder: rationale, clinical and neurobiological evidence, and implications. Depress Anxiety. 2012;29:701–708. doi: 10.1002/da.21889. [DOI] [PubMed] [Google Scholar]
- Lanius R a., Vermetten E., Loewenstein R.J., Brand B., Christian S., Bremner J.D., Spiegel D. Emotion modulation in PTSD: clinical and neurobiological evidence for a dissociative subtype. American Journal of Psychiatry. 2010;167:640–647. doi: 10.1176/appi.ajp.2009.09081168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius R a., Williamson P.C., Boksman K., Densmore M., Gupta M., Neufeld R.W.J., Gati J.S., Menon R.S. Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. Biol. Psychiatry. 2002;52:305–311. doi: 10.1016/s0006-3223(02)01367-7. [DOI] [PubMed] [Google Scholar]
- Lanius R.A., Boyd J.E., McKinnon M.C., Nicholson A.A., Frewen P., Vermetten E., Jetly R., Spiegel D. A Review of the Neurobiological Basis of Trauma-Related Dissociation and Its Relation to Cannabinoid- and Opioid-Mediated Stress Response: a Transdiagnostic. Translational Approach. . Current Psychiatry Reports Current Psychiatry Reports. 2018;20:118. doi: 10.1007/s11920-018-0983-y. [DOI] [PubMed] [Google Scholar]
- Lanius R.A., Frewen P.A., Tursich M., Jetly R., Mckinnon M.C., 2015. Restoring large-scale brain networks in PTSD and related disorders: a proposal for neuroscientifically-informed treatment interventions. 1, 1–12. [DOI] [PMC free article] [PubMed]
- Liu F., Xie B., Wang Y., Guo W., 2015. Characterization of Post-traumatic Stress Disorder Using Resting- State fMRI with a Multi-level Parametric Classification Approach, 221–237. [DOI] [PubMed]
- McKinnon M.C., Boyd, Frewen J.E., Lanius P.A., Jetly U.F., Richardson R., Lanius RA J.D. A review of the relation between dissociation, memory, executive functioning and social cognition in military members and civilians with neuropsychiatric conditions. Elsevier Neuropsychologia. 2016;90:210–234. doi: 10.1016/j.neuropsychologia.2016.07.017. [DOI] [PubMed] [Google Scholar]
- Meier L., Friedrich H., Federspiel A., Jann K., Morishima Y., Landis B.N., Wiest R., Strik W., Dierks T. Rivalry of homeostatic and sensory-evoked emotions: dehydration attenuates olfactory disgust and its neural correlates. Elsevier Inc. NeuroImage. 2015;114:120–127. doi: 10.1016/j.neuroimage.2015.03.048. [DOI] [PubMed] [Google Scholar]
- Melara R.D., Ruglass L.M., Fertuck E.A., Hien D.A. Regulation of Threat in Post-traumatic Stress Disorder: associations between Inhibitory Control and Dissociative Symptoms. Elsevier Biological Psychology. 2018;133:89–98. doi: 10.1016/j.biopsycho.2018.01.017. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Elsevier Ltd Trends in Cognitive Sciences. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function. 2010:1–13. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergler M., Driessen M., Lüdecke C., Ohlmeier M., Chodzinski C., Weirich S., Schläfke D., Wedekind D., Havemann-Reinecke U., Renner W., Schäfer I. Relationships between a Dissociative Subtype of PTSD and Clinical Characteristics in Patients with Substance Use Disorders. J Psychoactive Drugs. 2017;49:225–232. doi: 10.1080/02791072.2017.1296209. [DOI] [PubMed] [Google Scholar]
- Mickleborough M.J.S., Daniels J.K., Coupland N.J., Kao R., Williamson P.C., Lanius U.F., Hegadoren K., Schore A., Densmore M., Stevens T., Lanius R a. Effects of trauma-related cues on pain processing in posttraumatic stress disorder: an fMRI investigation. Journal of psychiatry & neuroscience : JPN. 2011;36:6–14. doi: 10.1503/jpn.080188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolas P., Melicher T., Skoch A., Matejka M., Slovakova A., Bakstein E., Hajek T., Spaniel F. Connectivity of the anterior insula differentiates participants with first-episode schizophrenia spectrum disorders from controls: a machine-learning study. Psychol Med. 2016;46:2695–2704. doi: 10.1017/S0033291716000878. [DOI] [PubMed] [Google Scholar]
- Miller D.R., Ba H.M., Bs A.S., Logue M.W., Robinson M.E., Schichman S., Miller M.W., Wolf E.J., Hayes J.P., Mcglinchey R.E., Milberg W.P., 2017. D O N A L D F . K L E I N AWA R D F I N A L I S T Posttraumatic stress disorder symptom severity is associated with reduced default mode network connectivity in individuals with elevated genetic risk for psychopathology, 632–640. [DOI] [PMC free article] [PubMed]
- Miller E.K., Cohen J.D., 2001. A N I NTEGRATIVE T HEORY OF P REFRONTAL C ORTEX F UNCTION, 167–202.
- Nicholson A., Densmore M., Frewen P.A., Théberge J., Neufeld R.W.J., McKinnon M.C., Lanius The Dissociative Subtype of Posttraumatic Stress Disorder : unique Resting-State Functional Connectivity of Basolateral and Centromedial Amygdala Complexes. Neuropsychopharmacology. Sep 2015;40(10):2317–2326. doi: 10.1038/npp.2015.79. Published online 2015 Apr 22. Prepublished online 2015 Mar 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A.A., Densmore M., McKinnon M., Neufeld R.W.J., Frewen P., Theberge J., Jetly R., Richardson D., Lanius R.A. Machine learning multivariate pattern analysis predicts classification of posttraumatic stress disorder and its dissociative subtype: a multimodal neuroimaging approach. Psychological Medicine. 2018;49(12):2049–2059. doi: 10.1017/S0033291718002866. [DOI] [PubMed] [Google Scholar]
- Nicholson A.A., Friston K.J., Zeidman P., Harricharan S., Mckinnon M.C., Densmore M., Neufeld R.W.J., Th J., Jetly R., Spiegel D., Lanius R.A., 2017. Dynamic Causal Modeling in PTSD and Its Dissociative Subtype : bottom – Up Versus Top – Down Processing Within Fear and Emotion Regulation Circuitry. Volume 38, Issue 11 November 2017 Pages 5551-5561. [DOI] [PMC free article] [PubMed]
- Nicholson A.A., Rabellino, Densmore D., Frewen M., Paret P.A., Kluetsch C., Schmahl R., Théberge C., Neufeld J., McKinnon R.W.J., Reiss M.C., Jetly J., Lanius RA R. The neurobiology of emotion regulation in posttraumatic stress disorder: amygdala downregulation via real-time fMRI neurofeedback. Hum Brain Mapp. 2016;0 doi: 10.1002/hbm.23402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A.A., Rabellino D., Densmore M., Frewen P.A., Paret C., Kluetsch R., Schmahl C., Théberge J., Ros T., Neufeld R.W.J., Mckinnon M.C., Reiss J.P., Jetly R., Lanius R.A. Intrinsic connectivity network dynamics in PTSD during amygdala downregulation. Hum Brain Mapp. 2018:1–18. doi: 10.1002/hbm.24244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A.A., Sapru I., Densmore M., Frewen P.A., Neufeld R.W.J., Theberge J., McKinnon M.C., Lanius R.A. Elsevier Psychiatry Research: Neuroimaging In Press; 2016. Unique Insula Subregion Resting-State Functional Connectivity With Amygdala Complexes in Posttraumatic Stress Disorder and Its Dissociative Subtype; pp. 61–72. [DOI] [PubMed] [Google Scholar]
- Olivé I., Densmore M., Harricharan S., Théberge J., McKinnon M.C., Lanius R. Superior colliculus resting state networks in post-traumatic stress disorder and its dissociative subtype. Hum Brain Mapp. 2018;39:563–574. doi: 10.1002/hbm.23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omurca S.I., Ekinci E. 2015 International Symposium on Innovations in Intelligent SysTems and Applications (INISTA) 2015. An alternative evaluation of post traumatic stress disorder with machine learning methods; pp. 1–7. [Google Scholar]
- Orrù G., Pettersson-Yeo W., Marquand A.F., Sartori G., Mechelli A. Using Support Vector Machine to identify imaging biomarkers of neurological and psychiatric disease: a critical review. Elsevier Ltd Neuroscience and Biobehavioral Reviews. 2012;36:1140–1152. doi: 10.1016/j.neubiorev.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Paulus M.P., Stein M.B. An Insular View of Anxiety. Biol. Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Petrides M., 2005. Lateral prefrontal cortex : architectonic and functional organization, 781–795. [DOI] [PMC free article] [PubMed]
- Pitman R.K., Rasmusson A.M., Koenen K.C., Shin L.M., Orr S.P., Gilbertson M.W., Milad M.R., Liberzon I Biological studies of post-traumatic stress disorder. Nature Publishing Group Nature Reviews Neuroscience. 2012;13:769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak A.R., Witteveen A.B., Reitsma J.B., Olff M. The role of executive function in posttraumatic stress disorder: a systematic review. . Elsevier B.V. J Affect Disord. 2012;141:11–21. doi: 10.1016/j.jad.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Qin L., Wang Z., Sun Y., Wan J., Su S., Zhou Y., Xu J. A preliminary study of alterations in default network connectivity in post-traumatic stress disorder patients following recent trauma. Elsevier Brain Research. 2012;1484:50–56. doi: 10.1016/j.brainres.2012.09.029. [DOI] [PubMed] [Google Scholar]
- Qin P., Northoff G. NeuroImage How is our self related to midline regions and the default-mode network ? . Elsevier Inc. Neuroimage. 2011;57:1221–1233. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Rabellino D., Densmore M., Harricharan S., Jean T., McKinnon M.C., Lanius R.A. Resting-state functional connectivity of the bed nucleus of the stria terminalis in post-traumatic stress disorder and its dissociative subtype. Hum Brain Mapp. 2017:1–13. doi: 10.1002/hbm.23925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabellino D., Densmore M., Théberge J., McKinnon M.C., Lanius R.A. The cerebellum after trauma: resting-state functional connectivity of the cerebellum in posttraumatic stress disorder and its dissociative subtype. Hum Brain Mapp. 2018;39:3354–3374. doi: 10.1002/hbm.24081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabellino D., Tursich M., Frewen P.A., Daniels J.K., Densmore M., Théberge J., Lanius R.A. Intrinsic Connectivity Networks in post-traumatic stress disorder during sub- and supraliminal processing of threat-related stimuli. Acta Psychiatr Scand. 2015 doi: 10.1111/acps.12418. [DOI] [PubMed] [Google Scholar]
- Rabellino D., Tursich M., Pa F., Jk D., Densmore M., 2015b. Intrinsic Connectivity Networks in post-traumatic stress disorder during sub- and supraliminal processing of threat-related stimuli, 1–14. [DOI] [PubMed]
- Rabinak C.A., Angstadt M., Welsh R.C., Kenndy A.E., Lyubkin M., Martis B., Phan K.L. Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Front Psychiatry. 2011;2:62. doi: 10.3389/fpsyt.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranlund S., Rosa M.J., de Jong S., Cole J.H., Kyriakopoulos M., Fu C.H.Y., Mehta M.A., Dima D. Associations between polygenic risk scores for four psychiatric illnesses and brain structure using multivariate pattern recognition. Elsevier NeuroImage: Clinical. 2018;20:1026–1036. doi: 10.1016/j.nicl.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen C.E., Williams C.K.I. MIT Press; 2006. Gaussian processes for machine learning. [Google Scholar]
- Rive M.M., Redlich R., Schmaal L., Marquand A.F., Dannlowski U., Grotegerd D., Veltman D.J., Schene A.H., Ruhé H.G. Distinguishing medication-free subjects with unipolar disorder from subjects with bipolar disorder: state matters. Bipolar Disord. 2016;18:612–623. doi: 10.1111/bdi.12446. [DOI] [PubMed] [Google Scholar]
- Rosazza C., Minati L., Ghielmetti F., Mandelli M.L., Bruzzone M.G., 2012. Functional Connectivity during Resting-State Functional MR Imaging : study of the Correspondence between Independent Component Analysis and Region-of-Interest Based Methods. 1. [DOI] [PMC free article] [PubMed]
- Saxe G.N., Ma S., Ren J., Aliferis C., 2017. Machine learning methods to predict child posttraumatic stress : a proof of concept study, 1–13. [DOI] [PMC free article] [PubMed]
- Schiavone F.L., Mckinnon M.C., Lanius R.A. Treatment, and Assessment of Potential Malingering; 2018. Psychotic-Like Symptoms and the Temporal Lobe in Trauma-Related Disorders : Diagnosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrouff J., Monteiro J.M., Portugal L., Rosa M.J., Phillips C., Mourão-Miranda J. Embedding Anatomical or Functional Knowledge in Whole-Brain Multiple Kernel Learning Models. Neuroinformatics Neuroinformatics. 2018;16:117–143. doi: 10.1007/s12021-017-9347-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrouff J., Rosa M.J., Rondina J.M., Marquand A.F., Chu C., Ashburner J., Phillips C., Richiardi J., Mourão-Miranda J. PRoNTo: pattern recognition for neuroimaging toolbox. Neuroinformatics. 2013;11:319–337. doi: 10.1007/s12021-013-9178-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serino A., Alsmith A., Costantini M., Mandrigin, Tajadura-jimenez A., Lopez C A. Bodily ownership and self-location : components of bodily. Elsevier Inc. Consciousness and Cognition. 2013;22:1239–1252. doi: 10.1016/j.concog.2013.08.013. [DOI] [PubMed] [Google Scholar]
- Shalev A., Liberzon I., Marmar C. Post-Traumatic Stress Disorder. New England Journal of Medicine. 2017;376:2459–2469. doi: 10.1056/NEJMra1612499. [DOI] [PubMed] [Google Scholar]
- Shang J., Lui S., Meng Y., Zhu H., Qiu C., Gong Q., Liao W., Zhang W. Alterations in Low-Level Perceptual Networks Related to Clinical Severity in PTSD after an Earthquake: a Resting-State fMRI Study. PLoS ONE. 2014;9:e96834. doi: 10.1371/journal.pone.0096834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer W.R., Ryali S., Rykhlevskaia E., Menon V., Greicius M.D., 2012. Decoding Subject-Driven Cognitive States with Whole-Brain Connectivity Patterns. 3, 158–165. [DOI] [PMC free article] [PubMed]
- Sierra M., Nestler S., Jay E.L., Ecker C., Feng Y., David A.S. A structural MRI study of cortical thickness in depersonalisation disorder. Elsevier Psychiatry Research - Neuroimaging. 2014;224:1–7. doi: 10.1016/j.pscychresns.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E., Filippini N., Watkins K.E., Toro R., Laird A.R., Beckmann C.F., 2009. Correspondence of the brain ’ s functional architecture during activation and rest. [DOI] [PMC free article] [PubMed]
- Spielberger C.D. The Corsini Encyclopedia of Psychology. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2010. State-Trait Anxiety Inventory. [Google Scholar]
- Spreng R.N., Mar R.A., Kim A.S.N. The Common Neural Basis of Autobiographical Memory, Prospection, Navigation. Theory of Mind, and the Default Mode : A Quantitative Meta-analysis. 2008:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Sridharan D., Levitin D.J., Menon V., 2008. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. 105, 12569–12574. [DOI] [PMC free article] [PubMed]
- Sripada R.K., King a.P, Welsh R.C., Garfinkel S.N., Wang X., Sripada C.S., Liberzon I. Neural Dysregulation in Posttraumatic Stress Disorder: evidence for Disrupted Equilibrium Between Salience and Default Mode Brain Networks. Psychosom Med. 2012;911 doi: 10.1097/PSY.0b013e318273bf33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D.J., Koenen K.C., Friedman M.J., Hill E., McLaughlin K a., Petukhova M., Ruscio A.M., Shahly V., Spiegel D., Borges G., Bunting B., Caldas-De-Almeida J.M., De Girolamo G., Demyttenaere K., Florescu S., Haro J.M., Karam E.G., Kovess-Masfety V., Lee S., Matschinger H., Mladenova M., Posada-Villa J., Tachimori H., Viana M.C., Kessler R.C. Dissociation in posttraumatic stress disorder: evidence from the world mental health surveys. Elsevier Biological Psychiatry. 2013;73:302–312. doi: 10.1016/j.biopsych.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpou B.A., Densmore M., Théberge J., Frewen P., McKinnon M.C., Lanius R.A. Resting-state pulvinar-posterior parietal decoupling in PTSD and its dissociative subtype. Hum Brain Mapp. 2018:4228–4240. doi: 10.1002/hbm.24242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tursich M., Ros T., Frewen P.A., Kluetsch R.C., Calhoun V.D., Lanius R.A. Distinct intrinsic network connectivity patterns of post-traumatic stress disorder symptom clusters. Acta Psychiatr Scand. 2015;132:29–38. doi: 10.1111/acps.12387. [DOI] [PubMed] [Google Scholar]
- Tursich M., Ros T., Pa F., Rc K., Vd C., Ra L., 2015b. Distinct intrinsic network connectivity patterns of post-traumatic stress disorder symptom clusters, 29–38. [DOI] [PubMed]
- Uddin L.Q. Salience processing and insular cortical function and dysfunction. Nature Publishing Group Nature Reviews Neuroscience. 2015;16:55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- Wang J., Yang Y., Fan L., Xu J., Li C., Liu Y., Fox P.T., Eickhoff S.B., Yu C., Jiang T. Convergent functional architecture of the superior parietal lobule unraveled with multimodal neuroimaging approaches. Hum Brain Mapp. 2015;36:238–257. doi: 10.1002/hbm.22626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers, F. W., Blake, D. D., Schnurr, P. P., Kaloupek, D. G., Marx, B. P., & Keane, T. M., 2013. The clinician‐administered PTSD scale for DSM‐5 (CAPS‐5). Interview available from the National Center for PTSD at www.ptsd.va.gov.
- Wegrzyn M., Riehle M., Labudda K., Woermann F., Baumgartner F., Pollmann S., Bien C.G., Kissler J. Investigating the brain basis of facial expression perception using multi-voxel pattern analysis. Elsevier Ltd Cortex. 2015;69:131–140. doi: 10.1016/j.cortex.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Weston CSE Posttraumatic stress disorder: a theoretical model of the hyperarousal subtype. Front Psychiatry. 2014;5:1–20. doi: 10.3389/fpsyt.2014.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfers T., Buitelaar J.K., Beckmann C.F., Franke B., Marquand A.F. From estimating activation locality to predicting disorder: a review of pattern recognition for neuroimaging-based psychiatric diagnostics. Elsevier Ltd Neuroscience and Biobehavioral Reviews. 2015;57:328–349. doi: 10.1016/j.neubiorev.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Hoge C.W., McFarlane A.C., Vermetten E., Lanius R.A., Nievergelt C.M., Hobfoll S.E., Koenen K.C., Neylan T.C., Hyman S.E. Macmillan Publishers Limited Nature Reviews Disease Primers; 2015. Post-traumatic Stress Disorder; p. 15057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.