Abstract
Mixed corticomedullary tumor is an adrenal tumor intermixed with cortical and medullary cells. It is extremely rare with unclear tumorigenesis. We reported a 32-year-old female, manifested with typical Cushing’s syndrome and hypertension, to be diagnosed with right huge adrenal mixed corticomedullary tumor (8.8 cm). Right adrenalectomy was done to document the tumor intimately admixed with adrenal cortical adenoma and pheochromocytoma by biochemistry and immunohistochemistry. A case-control study was designed to explore the tumorigenesis of mixed corticomedullary tumor by whole exome sequencing. Expression of the stemness markers was controlled by a tissue array of 80 adrenal tumors. Overall, 1559 identical variants coexisted in parts of adrenal cortical adenoma and pheochromocytoma, which mainly (85.8%) originated from germline mutations. These enriched mutations were engaged in stemness control, coherent with substantial expression of the stemness markers (SOX2, CD44 and OCT4) in both parts. The differential stemness expressions were demonstrated in other adrenal tumors as well. The germline mutations were also enriched in signaling involving cancer proliferation, hypoxia inducible factor-1, focal adhesion and extracellular matrix receptor interaction. Somatic mutations affecting mitogen-activated protein kinase signaling, glycolysis and the citrate cycle were found in some tumor elements. This is the first study to verify the rare mixed corticomedullary tumor by molecular and genetic evidence to link with its phenotype. Germline mutations involving the stemness regulation and cancer proliferative signaling may drive intermixed tumor formation. Somatic mutations related to glycolysis and the citrate cycle may contribute to greater tumor outgrowth.
Abbreviations: MCT, adrenal mixed corticomedullary tumor; WES, whole exome sequencing; ACA, adrenal cortical adenoma; PHEO, pheochromocytoma; NGS, next-generation sequencing; INDEL, insertion/deletion; SNP, single nucleotide polymorphism; ROH, runs of heterozygosity; CNV, copy number variations; SOX2, sex determining region Y-box 2; CD44, cluster of differentiation 44; OCT4, octamer-binding transcription factor 4; POU5F1B, a pseudogene of OCT4, POU domain, class 5, transcription factor 1B; KEGG, Kyoto Encyclopedia of Genes and Genomes; ECM, extracellular matrix; HIF-1, hypoxia-inducible factors-1; cAMP, 3′,5′ cyclic adenosine monophosphate; Wnt, mammalian wingless-type integration signaling; ZEB1, zinc finger E-box binding homeobox 1 protein; Ngn3, Neurogenin 3; LRP5, low-density lipoprotein receptor-related protein 5; TCF, β-catenin/T-cell factor; GPR39, G-protein coupled receptor 39; GLI1, glioma-associated oncogene homolog 1; EMR2, mucin-like hormone receptor-like 2; IGF, insulin-like growth factor; PKA, protein kinase A; TCGA, The Cancer Genome Atlas; TCA, tricarboxylic acid; PNMT, phenylethanolamine N-methyltransferase
Keywords: Mixed corticomedullary tumor (MCT), Stemness, Whole exome sequencing (WES), Adrenocortical adenoma, Pheochromocytoma
Introduction
Adrenal mixed corticomedullary tumor (MCT) is defined histopathologically as a single mass intimately admixed cortical cells and chromaffin cells. It is definitely different from adrenal collision tumors, where two adjacent and histologically distinct neoplasms exist without admixture at the interface. Adrenal MCT was first reported by Mathison and Waterhouse in 1969. It remains extremely rare and only 30 cases have been reported so far [1]. Since case numbers are so limited, the clinical course, prognosis and true etiology are still unclear.
The adrenal tumors are classified into tumors originating from the adrenal cortex and medulla without mentioning the rare MCT category [2]. Based on the concept of precision medicine, a molecular biology-based taxonomy has been proposed for pheochromocytoma to define its distinct molecular-biochemical-imaging and its metastatic features [3]. MCT, usually demonstrated with Cushing’s syndrome and pheochromocytoma, is regarded as a new disease entity because of its quite different manifestation to other adrenal tumors. Here we diagnosed a female patient with a hug MCT. To explore the tumorigenesis by molecular approach, whole exome sequencing (WES) was conducted and analyzed.
Material and methods
Whole exome sequencing for MCT
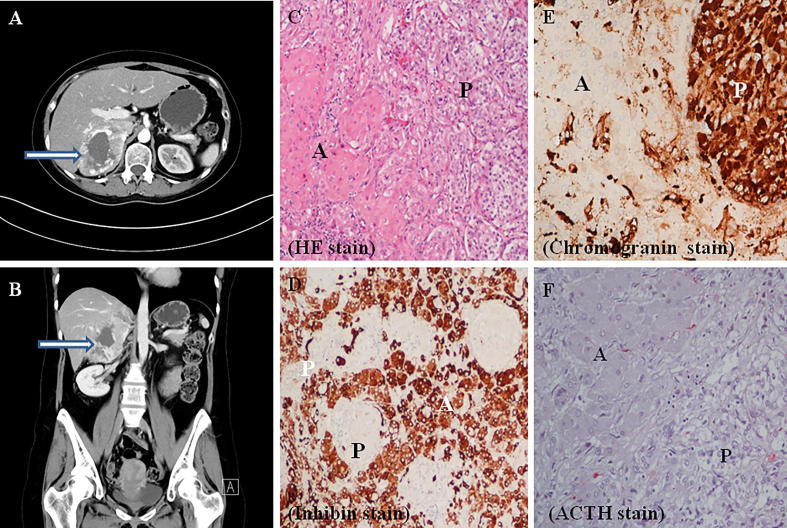
A 32-year-old female suffered from paroxysmal attack of hypertension, headache and palpitation for years followed with rapid weight gain and appearance of Cushing’s manifestations. She was diagnosed with adrenal Cushing’ syndrome (cortisol 38.08 μg/dL at 8 am, 41.62 μg/dL at 4 pm; ACTH 14.51 pg/ml (normal, 10–50) without pituitary tumor in magnetic resonance image. Her hypertension was secondary to hypercortisolism and pheochromocytoma (urine vanillylmandelic acid (VMA) 54.06, 74.03 mg/d (normal 1–7.5). Computerized tomography revealed a huge hypervascular tumor (8.8 cm) with central necrosis over her right adrenal gland. Right adrenalectomy was done to document adrenal MCT (Fig. 1). The Ki-67 index was 2% and 1% in part of adrenal cortical adenoma (ACA) and pheochromocytoma (PHEO), respectively (https://ppt.cc/fH7DEx, Supplementary Fig. 1). She is still in remission without recurrence for 7 years.
Fig. 1.
Right adrenal mixed cortiomedullary tumor. Computerized tomography demonstrated a huge hypervascular tumor (8.8 cm) over right adrenal gland with central necrosis (Se/Im: 5/21), indicated by arrow head in (A) horizontal view and (B) coronal view. The approximate Hounsfield unit value at 0, 35, 60 s and 10 min were 42, 94, 110, 64 with the absolute enhancement washout of 65% and relative washout of 42 %. (C) HE stain, (D) Inhibin stain, (E) Chromogranin stain, (F) ACTH stain. (A: cortical adenoma; P: pheochromocytoma).
Tumor tissues were dissected into dominant ACA and PHEO parts by a pathologist. Tumor DNA from each part was extracted and patient’s blood DNA served as a non-tumor control. The library for WES was prepared using the Agilent SureSelect V6 kit (Agilent, Santa Clara USA) for next-generation sequencing (NGS) of the genomic DNA by Illumina HiSeq system. Data analysis was conducted by VARBANK pipeline v.2.15 using the corresponding filter interface as previously described [4]. The primers to amplify the mutated DNA fragments were designed from the Primer 3 web (http://bioinfo.ut.ee/primer3-0.4.0/). The amplified products were purified after being dissolved for agarose electrophoresis and Sanger sequencing using ABI 3730.
Immunohistochemical analysis
Immunohistochemistry was performed for histopathological identification by H&E and chromogranin stain to label PHEO. A purchased tissue array of human adrenal tumors (US Biomax Inc. Derwood, MD, Cat. No. AG801), including adrenal cortical adenoma (ACA, n = 40 from 14 males and 16 females, mean aged 42.6 ± 11.3 years), adrenal cortical carcinoma (ACC, n = 10 from 7 males and 3 females, mean aged 40.2 ± 16.2 years) and pheochromocytoma (PHEO, n = 30 from 15 males and 15 females, mean aged 39.7 ± 12.6 years), was used as control. The stemness marker stained with anti-SOX2 (sex determining region Y-box 2, 1:100, GTX 101507, Genetex Hsinchu, Taiwan), anti-CD44 (cluster of differentiation 44, 1:100, GTX 102111) and anti-OCT4 (octamer-binding transcription factor 4, 1:200, GTX 101497, Genetex Hsinchu, Taiwan) were compared among our MCT and tissue array of adrenal tumors. The staining intensity, using Panoramic MIDI digital scanner, was semi-quantitatively assessed using a HistoQuant module (3DHISTECH, Budapest, Hungary) as “histo” score (H-score). The H-score, ranging from 0 to 300, was calculated from [1× (% cells 1 + 2 × (% cells 2+) + 3 × (% cells 3+)] to give relative greater impact for higher intensity [5]. The expression intensity was defined as High (H-score ≥200), intermediate (H-score, 100–199) and low (H-score, 0–100). Quantitative data were expressed as means ± SEM. Statistical significance was determined as p < 0.05 using Student’s t test between two groups.
Results
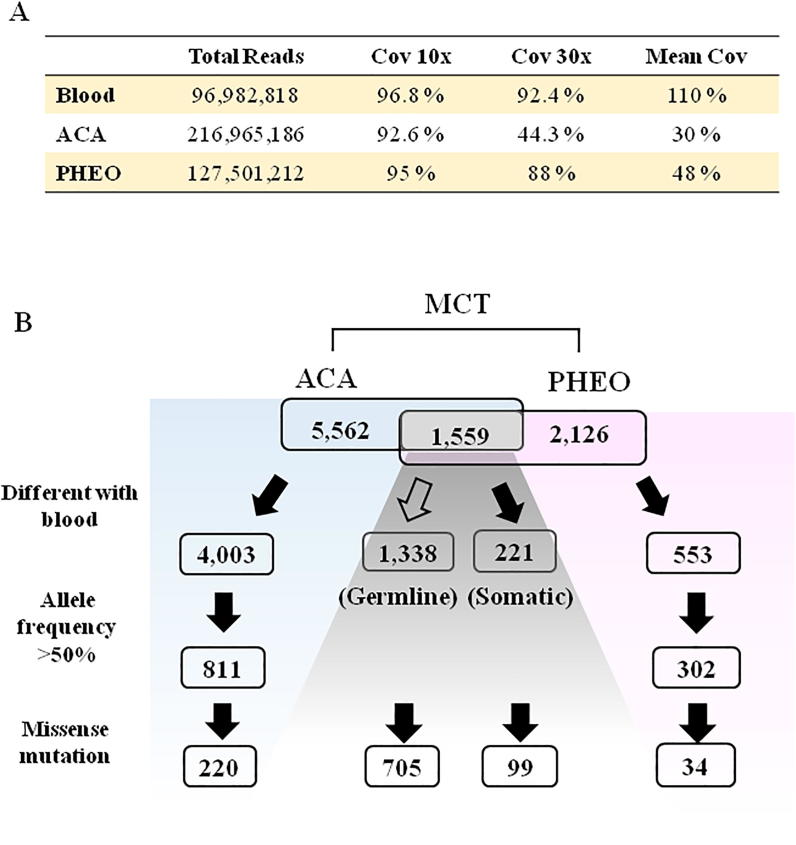
WES identified 5562 variants (5233 SNP, 170 INDEL, and 159 ROH) from ACA, and 2126 variants (1767 SNP, 131 INDEL, and 228 ROH) from PHEO. The previously well-known mutations (GNAS, CTNNB1, PRKAR1A, PRKACA, PDE11A, PDE8B, KCNJ5, CACNA1D) for adrenocortical adenoma [6] and mutations (RET, VHL, NF1, SDHA, SDHB, SDHC, SDHD, SDHAF2, TMEM127, MAX [7], EGLN1(PHD2), EPAS1(HIF2A), KIF1B, MET, FH, and H-RAS [8]) for pheochromocytoma were not detected in this MCT. A total of 1559 identical variants appeared in both parts, and 1338 variants (85.8%) of these were recognized as germline mutations because of their co-occurrence in blood (Fig. 2). Overall, there were 804 missense mutations (nonsynonymous substitution) encoding 758 unique genes. The further pathway enrichment analysis, illustrated by the Kyoto Encyclopedia of Genes and Genomes (KEGG) using DAVID web (https://david.ncifcrf.gov/), demonstrated the top 6 enriched pathways sequentially linked to cancer pathway (hsa05200; 3.2%), endocytosis (hsa04144; 2.1%), focal adhesion (hsa04510; 1.8%), protein digestion and absorption (hsa04974; 1.7%), extracellular matrix (ECM)-receptor interaction (hsa04512; 1.3%), and hypoxia-inducible factors-1 (HIF-1) signaling pathway (hsa04066; 1.3%). Furthermore, the 32 genes enriched in “pathways in cancer” were clarified by the KEGG system to demonstrate signaling involving PI3K-Akt (map04151, 34.85%), 3′,5′ cyclic adenosine monophosphate (cAMP) (map04024, 17.4%), Rap1 (map04015,17.4%), Hedgehog (map04340, 13%), apoptosis (map04210, 13%), HIFs (map04066, 13%), and pathways regulating pluripotency of stem cells (Wnt, mammalian wingless-type integration) signaling (map04550, 13%) (https://ppt.cc/fH7DEx, supplementary Table 1). The KEGG maps labeled with the mutants are shown in supplementary Fig. S1-4 (https://ppt.cc/fH7DEx).
Fig. 2.
Filter algorithm of whole exome sequencing to analyze gene mutations. The numbers of mutant variants by filtering were compared in parts of adrenocortical adenoma (ACA), pheochromocytoma (PHEO) and blood. Blank arrow indicates to filter out the variant versus blood DNA. Black arrow indicates the variants to pass the filtering criteria.
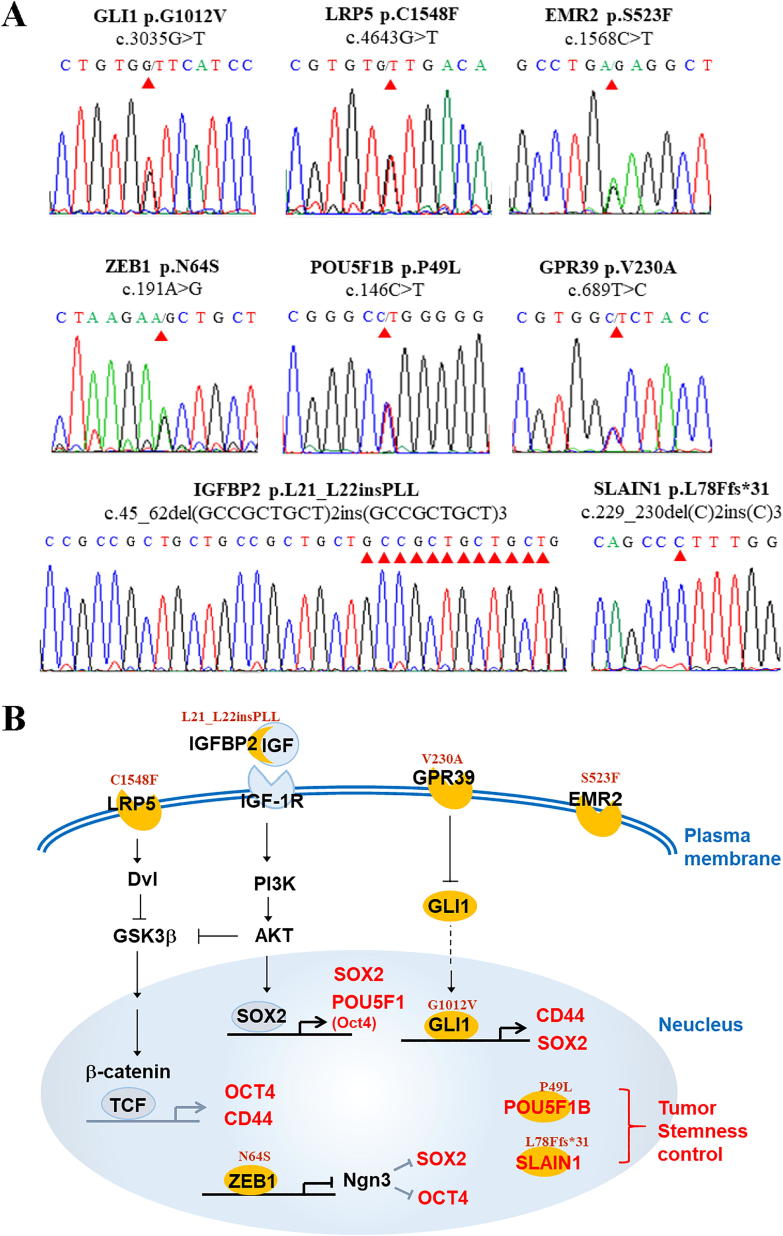
The potential impact on protein function and damage score of these genes were predicted by polyphe-2 module (http://genetics.bwh.harvard.edu/pph2/) (https://ppt.cc/fH7DEx, Supplementary Tables 1–3). It revealed 35 missense mutations encoding 29 genes (29/758, 3.8%) closely involved stemness control (https://ppt.cc/fH7DEx, Supplementary Table1). These mutations, selected by polyphen-2 score >0.15, were validated by Sanger sequencing and all the validated SNPs were heterozygous variants. The INDELs affecting IGFBP2 and SLAIN1 were also confirmed by Sanger sequencing (Fig. 3A). These mutants were previously found to regulate expression of the stemness markers, SOX2, OCT4, and CD44, in a direct or indirect manner [8], [9], [10], [11], [12]. Based on these evidences, Fig. 3B summarizes the potential stemness regulation linked to our mutated genes.
Fig. 3.
Mutation genes validated by Sanger sequencing. Sanger sequencing chromatograms confirmed 8 missense mutations (A) and their potential mechanisms related to stemness regulation, expressed by stemness markers (SOX2, CD44, OCT4) (B). Red arrowheads indicated the mutation sites. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The allele frequency of mutation less than 50% were excluded to identify the tissue-specific somatic mutations in MCT. Eventually, 220 missense mutations in ACA and 34 missense mutations in PHEO part were found, which encoded 142 and 31 genes, respectively (Fig. 2). Our results displayed a greater mutation burden in the ACA than in the PHEO part, which were linked to three function processes: kinase signaling, energy metabolism involving glycolysis and the citrate cycle, and focal adhesion/ECM-receptor interaction. Analysis of the enriched KEGG pathways mapped the ACA-specific somatic mutations to the PI3K-Akt signaling pathway (hsa04151; 5.7%), Carbon metabolism (hsa01200; 4.4%), Focal adhesion (hsa04510; 4.4%), Biosynthesis of amino acids (hsa01230; 3.8%), and ECM-receptor interaction (hsa04512; 3.8%) (https://ppt.cc/fH7DEx, Supplementary Table 2). Seven of these somatic mutation genes enriched in Carbon metabolism and Biosynthesis of amino acids pathways were precisely mapped to the Glycolysis module (hsa_M00002, including GAPDH, PGK1, ENO1, and PKM1) and Citrate cycle (has_M00010, including GOT2, ACO2, and GLUD2) in pathways (https://ppt.cc/fH7DEx, Supplementary Fig. S5). Five genes enriched in the pathway of ECM-receptor interaction were identically mapped to Focal adhesion, both of which were reclassified to the same cluster. Only one PHEO-specific missense mutation (MAPKAPK2, NM_032960.3, c.46G>C, p.A16P) was detected involving mitogen-activated protein kinase (MAPK) signaling (https://ppt.cc/fH7DEx, Supplementary Table 3). In ACA part, some ATPase gene variants were also identified (https://pptcc/fH7Dfx, Supplementary Table 4).
Generally, all the stemness markers, SOX2, CD44, and OCT4, were expressed in MCT and a tissue array of human adrenal tumors. They were highly expressed in MCT with a greater density in part of ACA than PHEO (Fig. 4A). The tissue array of human adrenal tumors also expressed differential intensities of stemness markers (Fig. 4B, C). The CD44-membanous expression was consistently distributed in high intensity in our MCT and all adrenal tumors, while the CD44-cytosol expression was highly expressed in our MCT compared with a wide distribution in intensity of other adrenal tumors. These results revealed that stemness control was critically involved in the tumorigenesis of adrenal tumors and MCT.
Fig. 4.
Substantial expressions of the stemness marker in MCT. (A) The histopathological identification was stained by H&E (a, b) and Chromogranin (c, d) for pheochromocytoma. The stemness markers, SOX2 (e, f), CD44 (g, h) and OCT4 (i, j), were highly expressed in MCT by immunohistochemistry. (B, C) Expression intensity of the SOX2 and CD44 was semi-quantitatively assessed H-score with Panoramic MIDI digital scanner among MCT and tissue array of adrenal tumors (adrenal cortical adenoma (ACA), adrenal cortical carcinoma (ACC) and pheochromocytoma (PHEO). (CD44-M: membranous CD44; CD44-C: cytosol CD44; Blue: tissue array; Red: cortical adenoma (A) and pheochromocytoma (P) of MCT). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
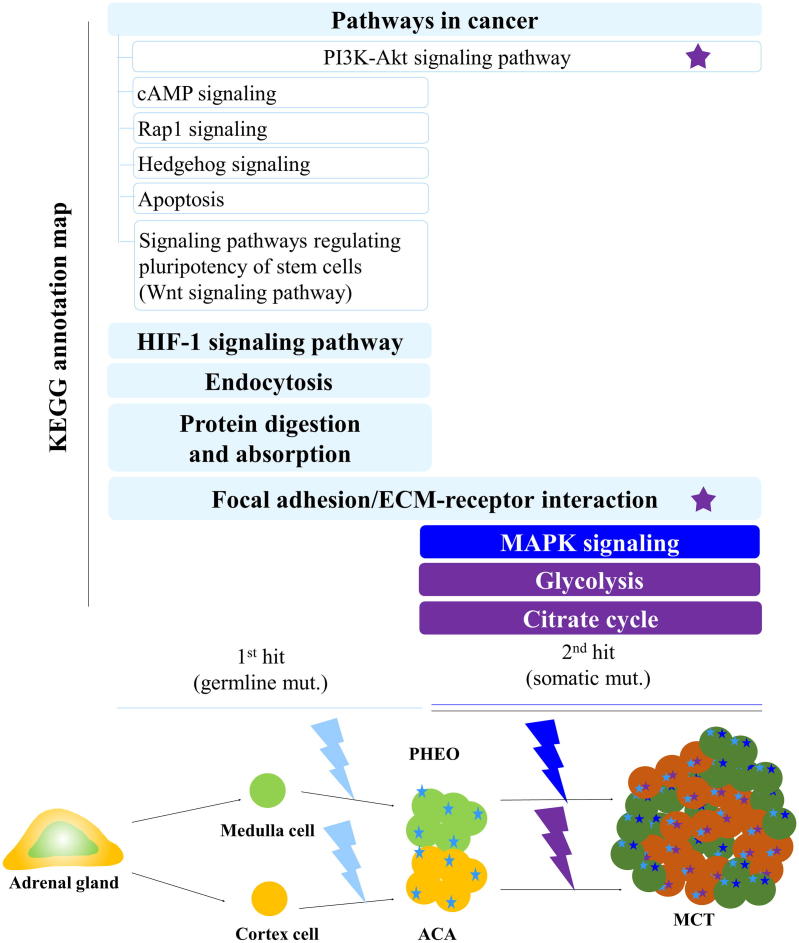
Fig. 5 illustrates the mechanism of MCT tumorigenesis. Most (∼86%) of the identical mutants in ACA and PHEO originated from germline mutations. Overall, 35 proteins from 29 germline mutations were involved in stemness regulation, which may be the “first hit” driving ACA and PHEO tumor formation. Additional mutations affecting kinase signaling, focal adhesion/ECM-receptor interaction, and HIF-1 signaling pathway may accelerate tumor growth and intimately admix ACA and PHEO together. The somatic mutation enriched pathways in PI3K-Akt signaling, MAPK signaling, glycolysis and citrate cycle may be a “second hit” triggering greater tumor burden and endocrine hyperfunction.
Fig. 5.
Speculated tumorigenesis of MCT. Upper panel: to demonstrate the enriched KEGG annotation map for 1st and 2nd hits. Lower panel: to illustrate the speculated intermixed phenotype for tumor formation. Two-hits hypothesis was suggested for MCT tumorigenesis. Germline mutations involving stemness activation may be the “first hit” to drive the tumor formation of ACA and PHEO, respectively. And gene mutations involved in signaling of cancer pathway, HIF-1, focal adhesion and ECM-receptor interaction may accelerate the tumor to grow intimately admixed together. The somatic mutations enriched pathways in the PI3K-Akt signaling, MAPK, glycolysis and citrate cycle may be as a “second hit” to generate a greater tumor burden and facilitate endocrine hyperfunction. (Light blue star: identical mutations between ACA and PHEO; Dark blue star: PHEO-specific mutations; Purple star: ACA-specific mutations).
Discussion and conclusion
Existing evidence shows that the maintenance of adrenal volume and function are presumably replenished from a pool of somatic stem and progenitor cells. Adrenal stem/progenitor cells play essential roles in adrenocortical development, maintenance, tumor formation and steroidogenesis [13]. Previous reports have emphasized the adrenal tumorigenesis is directly regulated by stem cells/progenitor cells. Mouse experiments have confirmed that SOX2 and OCT4 could induce pluripotent stem cells and maintain their function. The SOX2 overexpression, as an oncogene, may induce the cancer formation of lung and colon. OCT4 is a master regulator of pluripotency controlling the lineage commitment and tumorigenesis of adult germ cells, adult somatic stem cells and cancer stem cells. The CD44 antigen is a membranous glycoprotein involved in cell proliferation, differentiation, migration, adhesion, angiogenesis, and tumor metastasis [14]. The transmembrane domain of CD44 interacts with co-factors and adaptor proteins, while intracellular domain functions in nuclear localization and transcription mediation. It has been evidenced the intracellular domain of CD44 linked with the progression and metastatic potential of breast cancers. The alternative spliced variant of CD44 (CD44 isoform) may cause both conformational changes and modulate the intracellular signaling, which may regulate epithelial to mesenchymal transition, and adaptive plasticity of cancer cells [14], [15]. The differential intensity of the CD44 expression in membrane and cytosol may implicate the functional variety of the tumor progression and metastatic potential for the MCT and adrenal tumors. This study revealed a total of 35 missense mutations engaged in the stemness control in MCT. The stemness markers were substantially expressed both in MCT and other adrenal tumors. Therefore, stemness regulation plays a critical role in the adrenal tumor formation, including MCT.
The adrenocortical stem cells were regulated by some paracrine signaling pathways. The Hedgehog pathway regulates mitogenic signaling for capsular stem and progenitor cells to differentiate into cortical cells. Wnt/β-catenin signaling, residing in the outer subcapsular region of cortex, is an essential regulator of stem cell development, maintenance and cortical zonation, which has been proposed to control the steroidogenesis [16]. In humans, excessive expression of β-catenin has been identified in most adrenocortical tumors. Since β-catenin mutation is found in both aldosterone- and cortisol-producing adenoma, Wnt/β-catenin signaling is critical for adrenal tumorigenesis [17]. Fibroblast growth factor (FGF) signaling exhibits a mitogenic effect by stimulating downstream pro-proliferative and angiogenic signaling, including PI3K and MAPK, to convey proper development and maintenance of adrenal gland. Over-expression of the FGF receptor has been detected in up to 65% of human adrenocortical tumors and also predicts a poor outcome [13], [18]. The insulin-like growth factor (IGF) signaling, expressed throughout all cortical zones with enriched IGF-1R in the subcapsular region, is implicated in the growth and differentiation of the human adrenal cortex. IGF signaling is regarded as a potent mitogen for postnatal growth maintenance of the adrenal cortex [13], [19]. The adrenal steroidogenesis and cell proliferation of the zona fasciculata critically depend on PI3K-Akt and cAMP signaling [16]. The cAMP could act directly on protein kinase A (PKA) to mediate intracellular signaling transduction. Aberrant cAMP-PKA signaling correlates with many proliferative adrenocortical diseases both in human and mouse models [13]. Both ACA and PHEO tissues in MCT had enriched gene mutations involving the above pathways, suggesting that abnormal paracrine regulation in adrenocortical stem cells may cause greater tumor outgrowth and hypercortisolism.
The Cancer Genome Atlas (TCGA) project recognized a driver mutation in 73% of pheochromocytoma, 27% harboring a germline mutation with autosomal dominant inheritance, and 46% as a somatic mutation in sporadic cases. Currently, pheochromocytoma tumorigenesis has been categorized into three clusters involving pseudohypoxia, Wnt-, and kinase signaling [3]. Tumor hypoxia/pseudohypoxia is supposed to be an oncogenic signaling to regulate tricarboxylic acid (TCA) cycle related enzymes, succinate dehydrogenase, fumarate hydratase and malate dehydrogenase. Truncating gene mutations encoding these enzymes could cause accumulation of the intermediates to alter cell signaling and chromatin maintenance. Pseudohypoxia signaling also involves activation of HIFs proteins, which may modify the target genes to increase angiogenesis, cellular proliferation and reduced apoptosis [3]. Wnt- and kinase signaling in pheochromocytoma could enhance neuronal differentiation to highly express phenylethanolamine N-methyltransferase (PNMT) and facilitate synthesis of catecholamines [20], [21]. The cancer stem cell theory holds that tumors originate from the cells exhibiting stem cell properties in various tumors, but evidence reported for pheochromocytoma is scarce. One European study, assembling a tissue array of 166 pheochromocytomas and 42 paragangliomas, documented expression of stem cell markers (SOX2, LIN28, NGFR, THY1) in more than 10% of tumors, significantly associated with SDHx mutation [22]. The PHEO in MCT was not identified with any of the previously reported common mutations. However, PHEO tumorigenesis could be coherently attributable to the germline mutations involving HIF-1, Wnt- and PI3k-Akt kinase signaling as well as stemness activation.
Physiologically, the adult adrenal outer cortex maintains a relatively proliferative activity with a centripedal migration for turnover. Homeostasis of adrenocortical cells is sustained by a balance of subcapsular cell proliferation, migration, and apoptosis at the cortical-medullary boundary [16], [19]. Extracellular matrix (ECM), including type IV collagen, laminin and fibronectin, links with integrin receptors to keep cell behavior specific within zone. The laminin or collagen not only contributes to maintaining cell morphology, but also increases cell proliferation, while fibronectin is involved in regulating apoptosis. Type IV collagen significantly amplifies the ACTH-stimulated cortisol secretion [19]. Adrenal glucocorticoids stimulate the hyperfunction of chromaffin cells by upregulating PNMT to produce epinephrine in adrenal medulla [19]. Rap1, a small GTPase, is a down-stream effector of Epac proteins regulating cell polarity, cell adhesion, cell–cell junction, cell-matrix interaction by modulating the integrins. The cAMP-Epac-Rap1 pathway is engaged in cell–cell junction, cell adhesion, exocytosis, cell differentiation, proliferation, and apoptosis [23]. To elucidate the mechanism of the closely intermixed ACA and PHEO tissues in MCT, we assumed coordinating all these mutations involving cAMP, Rap 1, apoptosis, and focal adhesion/ECM-receptor interaction may cause aberrant cellular proliferation, adhesion and cell junction behavior to develop MCT.
Most tumor cells prefer glycolysis instead to convert a majority of glucose into lactate even in the presence of oxygen, known as Warburg’s effect (aerobic glycolysis). Aerobic glycolysis produces less adenosine triphosphate, but the altered energy metabolism and the generation of abnormal metabolite may benefit tumor formation and growth. Intratumoral hypoxia significantly enhances expression of HIF-1 to reprogram the tumor metabolism. HIF-1 involves the regulation of glucose transporters, glycolysis and the TCA cycle. Glycolysis is the organized center to generate energy, which is tightly coordinated with gluconeogenesis and the TCA cycle. Glycolysis is closely controlled by three key enzymes: hexokinase, pyruvate kinase and 6-phosphofructo-1-kinase. These enzymes are highly regulated by several oncogenes and tumor suppressors, and the imbalanced function of these enzymes is seen in tumor cells [24]. The Warbug effect in cancer cells reduces citrate biosynthesis and diminishes intracellular acidity, both of which promote glycolysis to sustain tumor growth. Citrate and isocitrate, as intermediates in the TCA cycle, negatively regulate the enzyme pyruvate dehydrogenase in glycolysis. Citrate functions as an essential donor for protein acetylation. Reduced citrate concentration causes deacetylation of proteins, which contributes to epigenetic changes and resistance to apoptosis, both processes favoring tumor aggressiveness. Therefore, the reduced concentration of citrate in cancer cells significantly indicates cancer aggressiveness [25]. Evidence has demonstrated Na/K-ATPase could interact with protooncogene Src kinase to increase aerobic glycolysis and tumor growth [26]. Somatic mutations in this MCT included several enzymes involved in the glycolysis and citrate cycle pathways. These missense mutations may alter the tumor metabolism and accelerate greater tumor burden.
Currently, cancer formation is explained by the cellular plasticity model in which most cancers originate from cells with a flexible nature preserving a tumor-initiating capacity or cancer stemness activity. This dedifferentiation capacity of cancer cells may be either inherited (hierarchical theory) or acquired via mutations that lead to a stem-cell-like permissive epigenome (stochastic theory) [27]. Our MCT results and case series of adrenal tumors match the cancer plasticity model. Stemness activation may drive tumor formation and the greater tumor growth may be accelerated by the complex proliferative signaling caused by germline and somatic mutations. Due to the scarcity of MCT and the multiple mutations involved, it is hard to verify our hypothesis by cell experiments. Initiation of the stemness activation and the asymmetrical tumorigenesis seen in adrenal gland of MCT remain unclear at this time. Patient with pheochromocytoma is suggested to keep regular surveillance forever because of its malignant potential. However, the malignant potential, best follow-up schedule and even prospective therapy for MCT are also uncertain because the identified gene mutations are very different from those previously reported [3].
In conclusion, this very rare MCT is a diverse adrenal tumor with different gene mutation profiles. This study provided a speculative tumorigenesis for MCT that germline mutations involved in stemness activation and cancer proliferative signaling may drive the intermixed tumor formation. And somatic mutations related to glycolysis and the citrate cycle may contribute to greater tumor outgrowth.
Declarations of interest
None.
Acknowledgments
Acknowledgements
We are sincerely grateful to doctor, Daw-Yang Hwang, for technical assistance and consultation for the analysis of whole exome sequencing.
Conflict of interest statement
The experiment was conducted under patient’s agreement and approved by Institutional Review Board of Kaohsiung Medical University Hospital (KMUIRB-G (I)-20180029). The informed consent has been obtained from the patient for publication of the case report and accompanying images. The supplementary datasets generated and/or analyzed during the current study are available in the [https://ppt.cc/fH7DEx] repository. The authors declare that they have no competing interests of this study and confirm agreement with the final statement.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Authors’ contribution
Conception and design: HY C Chiou, KK Kuo, PJ Hsiao.
Analysis and interpretation of data: HY C Chiou, HJ Jiang, SFYang, PC Lin, PJ Hsiao.
Drafting and revising article: HY C Chiou, HJ Jiang, PJ Hsiao.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neo.2020.04.003.
Contributor Information
Sheau-Fang Yang, Email: sfyang@kmu.edu.tw.
Pi-Chen Lin, Email: 890063@kmuh.org.tw.
Pi-Jung Hsiao, Email: pjhsiao@cc.kmu.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Donatini G., Van Slycke S., Aubert S., Carnaille B. Corticomedullary mixed tumor of the adrenal gland-a clinical and pathological chameleon: case report and review of literature. Updates Surg. 2013;65:161–164. doi: 10.1007/s13304-011-0132-1. [DOI] [PubMed] [Google Scholar]
- 2.Lam A.K. Update on adrenal tumours in 2017 World Health Organization (WHO) of endocrine tumours. Endocr Pathol. 2017;28:213–227. doi: 10.1007/s12022-017-9484-5. [DOI] [PubMed] [Google Scholar]
- 3.Crona J., Taieb D., Pacak K. New perspectives on pheochromocytoma and paraganglioma: toward a molecular classification. Endocr Rev. 2017;38:489–515. doi: 10.1210/er.2017-00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altmuller J., Motameny S., Becker C., Thiele H., Chatterjee S., Wollnik B., Nurnberg P. A systematic comparison of two new releases of exome sequencing products: the aim of use determines the choice of product. Biol Chem. 2016;397:791–801. doi: 10.1515/hsz-2015-0300. [DOI] [PubMed] [Google Scholar]
- 5.John T., Liu G., Tsao M.S. Overview of molecular testing in non-small-cell lung cancer: mutational analysis, gene copy number, protein expression and other biomarkers of EGFR for the prediction of response to tyrosine kinase inhibitors. Oncogene. 2009;28(Suppl 1):S14–23. doi: 10.1038/onc.2009.197. [DOI] [PubMed] [Google Scholar]
- 6.Lacroix A., Feelders R.A., Stratakis C.A., Nieman L.K. Cushing's syndrome. Lancet. 2015;386:913–927. doi: 10.1016/S0140-6736(14)61375-1. [DOI] [PubMed] [Google Scholar]
- 7.Galan S.R., Kann P.H. Genetics and molecular pathogenesis of pheochromocytoma and paraganglioma. Clin Endocrinol (Oxf) 2013;78:165–175. doi: 10.1111/cen.12071. [DOI] [PubMed] [Google Scholar]
- 8.Edwards L.A., Li A., Berel D., Madany M., Kim N.H., Liu M., Hymowitz M., Uy B., Jung R., Xu M. ZEB1 regulates glioma stemness through LIF repression. Sci Rep. 2017;7:69. doi: 10.1038/s41598-017-00106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou C., Jiang H., Zhang Z., Zhang G., Wang H., Zhang Q., Sun P., Xiang R., Yang S. ZEB1 confers stem cell-like properties in breast cancer by targeting neurogenin-3. Oncotarget. 2017;8:54388–54401. doi: 10.18632/oncotarget.17077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Youssef A., Aboalola D., Han V.K. The roles of insulin-like growth factors in mesenchymal stem cell niche. Stem Cells Int. 2017;2017:9453108. doi: 10.1155/2017/9453108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi H.Y., Saha S.K., Kim K., Kim S., Yang G.M., Kim B., Kim J.H., Cho S.G. G protein-coupled receptors in stem cell maintenance and somatic reprogramming to pluripotent or cancer stem cells. BMB Rep. 2015;48:68–80. doi: 10.5483/BMBRep.2015.48.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hadjimichael C., Chanoumidou K., Papadopoulou N., Arampatzi P., Papamatheakis J., Kretsovali A. Common stemness regulators of embryonic and cancer stem cells. World J Stem Cells. 2015;7:1150–1184. doi: 10.4252/wjsc.v7.i9.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walczak E.M., Hammer G.D. Regulation of the adrenocortical stem cell niche: implications for disease. Nat Rev Endocrinol. 2015;11:14–28. doi: 10.1038/nrendo.2014.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Senbanjo L.T., Chellaiah M.A. CD44: a multifunctional cell surface adhesion receptor is a regulator of progression and metastasis of cancer cells. Front Cell Dev Biol. 2017;5:18. doi: 10.3389/fcell.2017.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen C., Zhao S., Karnad A., Freeman J.W. The biology and role of CD44 in cancer progression: therapeutic implications. J Hematol Oncol. 2018;11:64. doi: 10.1186/s13045-018-0605-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pignatti E., Leng S., Carlone D.L., Breault D.T. Regulation of zonation and homeostasis in the adrenal cortex. Mol Cell Endocrinol. 2017;441:146–155. doi: 10.1016/j.mce.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zennaro M.C., Boulkroun S., Fernandes-Rosa F. Genetic causes of functional adrenocortical adenomas. Endocr Rev. 2017;38:516–537. doi: 10.1210/er.2017-00189. [DOI] [PubMed] [Google Scholar]
- 18.Brito L.P., Ribeiro T.C., Almeida M.Q., Jorge A.A., Soares I.C., Latronico A.C., Mendonca B.B., Fragoso M.C., Lerario A.M. The role of fibroblast growth factor receptor 4 overexpression and gene amplification as prognostic markers in pediatric and adult adrenocortical tumors. Endocr Relat Cancer. 2012;19:L11–L13. doi: 10.1530/ERC-11-0231. [DOI] [PubMed] [Google Scholar]
- 19.Xing Y., Lerario A.M., Rainey W., Hammer G.D. Development of adrenal cortex zonation. Endocrinol Metab Clin North Am. 2015;44:243–274. doi: 10.1016/j.ecl.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fishbein L., Leshchiner I., Walter V., Danilova L., Robertson A.G., Johnson A.R., Lichtenberg T.M., Murray B.A., Ghayee H.K., Else T. Comprehensive molecular characterization of pheochromocytoma and paraganglioma. Cancer Cell. 2017;31:181–193. doi: 10.1016/j.ccell.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisenhofer G., Huynh T.T., Pacak K., Brouwers F.M., Walther M.M., Linehan W.M., Munson P.J., Mannelli M., Goldstein D.S., Elkahloun A.G. Distinct gene expression profiles in norepinephrine- and epinephrine-producing hereditary and sporadic pheochromocytomas: activation of hypoxia-driven angiogenic pathways in von Hippel-Lindau syndrome. Endocr Relat Cancer. 2004;11:897–911. doi: 10.1677/erc.1.00838. [DOI] [PubMed] [Google Scholar]
- 22.Oudijk L., Neuhofer C.M., Lichtenauer U.D., Papathomas T.G., Korpershoek E., Stoop H., Oosterhuis J.W., Smid M., Restuccia D.F., Robledo M. Immunohistochemical expression of stem cell markers in pheochromocytomas/paragangliomas is associated with SDHx mutations. Eur J Endocrinol. 2015;173:43–52. doi: 10.1530/EJE-14-1164. [DOI] [PubMed] [Google Scholar]
- 23.Cheng X., Ji Z., Tsalkova T., Mei F. Epac and PKA: a tale of two intracellular cAMP receptors. Acta Biochim Biophys Sin (Shanghai) 2008;40:651–662. doi: 10.1111/j.1745-7270.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J.Q., Russo J. Dysregulation of glucose transport, glycolysis, TCA cycle and glutaminolysis by oncogenes and tumor suppressors in cancer cells. Biochim Biophys Acta. 2012;1826:370–384. doi: 10.1016/j.bbcan.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Icard P., Lincet H. The reduced concentration of citrate in cancer cells: an indicator of cancer aggressiveness and a possible therapeutic target. Drug Resist Updat. 2016;29:47–53. doi: 10.1016/j.drup.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Banerjee M., Cui X., Li Z., Yu H., Cai L., Jia X., He D., Wang C., Gao T., Xie Z. Na/K-ATPase Y260 phosphorylation-mediated Src regulation in control of aerobic glycolysis and tumor growth. Sci Rep. 2018;8:12322. doi: 10.1038/s41598-018-29995-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang W., Quan Y., Fu Q., Liu Y., Liang Y., Wu J., Yang G., Luo C., Ouyang Q., Wang Y. Dynamics between cancer cell subpopulations reveals a model coordinating with both hierarchical and stochastic concepts. PLoS One. 2014;9 doi: 10.1371/journal.pone.0084654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.