Abstract
Social robots are increasingly demonstrating effectiveness as low-intensity behavior change agents. Key targets for these behavioral interventions include daily lifestyle behaviors with significant health consequences, such as the consumption of high-calorie foods and drinks (‘snacks’). A pilot randomized controlled trial using a stepped-wedge design was conducted to determine the efficacy of a motivational intervention by an autonomous robot, to help reduce high-calorie snacks. Twenty-six adults were randomized to receive Immediate or 4-week Delayed treatment, with assessments at Baseline and Weeks 4 and 8. The treatment comprised motivation enhancement and self-management training using mental imagery (Functional Imagery Training). A significant condition by time effect for snack episode reduction was obtained, F(2, 32.06) = 4.30, p = .022. The Immediate condition significantly reduced snacking between Baseline and Week 4 (d = −1.06), while the Delayed condition did not (d = −0.08). Immediate participants maintained their improvement between Weeks 4 and 8 (d = −0.18), and Delayed participants then showed a significant fall (d = −1.42). Overall, ‘Immediate’ participants decreased their snack episodes by 54% and ‘Delayed’ decreased by 62% from Baseline to Week 8, and an average weight reduction of 4.4 kg was seen across over the first 2 weeks of treatment. Four weeks after starting the intervention, both conditions had significant increases in perceived confidence to control snack intake for time duration, specific scenarios and emotional states (d = 0.61 to 1.42). Working alliance was significantly correlated with reduced snack episodes. The pilot's results appear to suggest that the robot-delivered intervention may be as effective as a human clinician delivering a similar intervention. The robot-delivered pilot achieved similar snack episode reduction in the first four weeks (FIT-R, 55%) when compared with the human-delivered version by a trained clinician (FIT-H, 49%).
Overall, the results provide preliminary evidence for an autonomous social robot to deliver a low-intensity treatment on dietary intake without the need for human intervention. Future trials should extend the deployment of the robot-delivered intervention protocol to other low-intensity behavioral outcomes.
Keywords: Social robot, Healthcare, Motivation, Intervention, Imagery
Highlights
-
•
Social robots can autonomously deliver a behavior change treatment.
-
•
Results found >50% snack episode reduction and 4.4 kg average weight reduction.
-
•
A robot-delivered intervention may be as effective as a human clinician delivering a similar intervention.
1. Introduction
1.1. The use case for social robots in healthcare
Social robots are gaining traction to deliver healthcare information, assessment, and treatment as a supplement to other digital health services (Robinson et al., 2019; Lal and Adair, 2014; Drigas et al., 2011). Social robots that can interact with people hold some unique advantages for healthcare treatment delivery compared to other digital modes, such as smartphones, computers, or screen-based avatars e.g. Li (2015); Saunderson and Nejat (2019). Embodied social robots tend to elicit more favourable responses from people compared to telepresence or virtual agents, including higher scores on dimensions such as overall impression, preference, engagement, helpfulness, appeal and enjoyment (Bainbridge, Hart, Kim, & Scassellati, 2011; Jost, Le Pévédic, & Duhaut, 2012; Lee, Jung, Kim, & Kim, 2006; Pereira, Martinho, Leite, & Paiva, 2008; Wainer, Feil-Seifer, Shell, & Mataric, 2007). Interpersonal benefits linked to embodied robots include higher levels of credibility, trust, attention, perceived empathy and being able to elicit more descriptive conversational language from people (Fischer, Lohan, & Foth, 2012; Kwak, Kim, Kim, Shin, & Cho, 2013; Looije, Neerincx, & Cnossen, 2010; Looije, Zalm, Neerincx, & Beun, 2012; Reeves et al., 2003; Seo, Geiskkovitch, Nakane, King, & Young, 2015; Wang & Rau, 2019). In relation to characteristics linked to health activities, embodied robots often receive higher ratings on persuasion, individual likelihood to accept a recommendation, and better task-related outcomes, including people being more likely to choose a health bar over a candy bar when an embodied robot was present with them compared to a virtual agent (Li, 2015; Bainbridge et al., 2011; Kiesler et al., 2008; Shinozawa et al., 2005). Collectively, these attributes represent an important set of characteristics for a digital treatment method in healthcare, if social robots will begin to take on treatment roles that require strong clinical expertise and talk-based interpersonal connection, such as robot-delivered psychotherapy.
1.2. Social robots, healthcare and robot-delivered psychotherapy
Tests of the health benefits that can be obtained from social robots have shown some positive effects, but have been restricted to a narrow range of contexts (Robinson et al., 2019). One line of research has used animal-like robots primarily in aged care to replicate beneficial effects of animal-assisted therapy (Abbott et al., 2019). Meta-analysis outcomes of feasibility and phase 2 RCTs include reduced agitation together with qualitative evidence reporting a perceived reduction of loneliness and increased pleasure described by residents, staff and family members (Abbott et al., 2019; Jøranson et al., 2015; Moyle et al., 2017; Moyle et al., 2018). Other studies have used social robots to teach social skills to children with autism spectrum disorder (Pennisi et al., 2016; Ismail et al., 2019; Scassellati et al., 2012). These trials have shown improved social behaviors from robot-delivered training sessions, such as increased gesture recognition, question-asking and participation in social interactions (So et al., 2017; Pop et al., 2013; Huskens et al., 2013). A third application of social robots has been to health-enhancing behaviors (Robinson et al., 2019; Moerman et al., 2018), including giving advice about drinking water, assisting the tracking of calorie intake, or coaching physical exercise sessions (Fasola, 2014; Fasola and Mataric, 2012; Kidd and Breazeal, 2008; Powers and Kiesler, 2006; Schrum et al., 2019). However, these trials have not yet tracked health outcomes across multiple sessions or timepoints.
Social robots also have emergent potential to facilitate and deliver low-intensity behavioral interventions that simulate evidence-based treatments used in routine clinical practice (Robinson et al., 2019; Moerman et al., 2018). They have been used less often than their digital counterparts (i.e. virtual avatars and conversational agents), which have delivered clinical interviews or psychotherapeutic treatment in depression, anxiety, post-traumatic stress disorder, suicidal behavior and substance abuse (Provoost et al., 2017; Martínez-Miranda, 2017; Laranjo et al., 2018; Vaidyam et al., 2019; Gaffney et al., 2019). Social robot-delivered interventions for children show some positive effects when used as part of a medical intervention (Moerman et al., 2018; Trost et al., 2019). This includes a humanoid social robot using distraction to reduce children's perception of pain and distress during a vaccination session (Beran et al., 2013), and a multi-session, robot-delivered psychotherapy for child oncology patients reduced their anxiety, depression and anger scores (Alemi et al., 2016). Social robots have been used less often with healthy adult populations. Trials include a robot-assisted program providing cognitive training sessions to increase memory, attention, and executive function improved the functioning of older adults (Kim et al., 2015), and a social robot delivering a low-intensity behavioral intervention via a 15-min Motivational Interview to encourage healthy behavior changes, which included increased physical activity (Galvão Gomes da Silva et al., 2018). This emerging body of work shows that embodied social robots can assist and lead health interventions, including ones that provide support, coaching and guidance for behavior change.
1.3. Clinician-led behavior change interventions
Motivation for behavior change is essential to successful sustained adoption of functional behaviors (ones that give valued outcomes to people). Daily selection of actions that promote or undermine health is driven by the incentives for attaining a behavioral goal, and the person's confidence in reaching it (Bandura, 1986). Desires that underpin a target behavior such as healthy eating (e.g. increased fitness, reduced weight) are typically in competition with desires for less healthy targets such as high-calorie foods. At certain times (e.g. when trying on clothes), the healthy goal seems most attractive; at others (e.g. when presented with an attractive cake) the less healthy one swamps our attention. An important reason for this is that motivational targets that are proximal in time (such as a high-calorie food) tend to have greater emotional impact than ones that are more delayed or distal (such as being more healthy; (Loewenstein and Thaler, 1989).
The intervention strategy that currently has the strongest supportive evidence in building motivation is Motivational Interviewing MI, (Miller and Rollnick, 2013; Britt et al., 2004). MI involves establishing a collaborative and empathic context, where people can feel comfortable talking about the advantages and possibility of change. Participants explore their ambivalence around behavior change, with a particular focus on whether their functional goals are linked to their most highly valued outcomes. They reflect on functional achievements in the past, and their implications for their confidence in achieving future success. If they commit to a functional goal, they are assisted in making plans to achieve it. Practitioners use reflective listening and ask open questions, drawing attention to statements that support functional change, summarizing frequently and only providing information or advice with permission (Miller and Rollnick, 2013).
MI has been applied to a wide variety of health maintenance behaviors (Martins and McNeil, 2009). A meta-analysis of 119 MI trials investigating a variety of behavior change targets found significant health behavior benefits compared to control conditions (i.e. wait-list groups or no treatment controls, treatment as usual, or provision of written materials) (Lundahl et al., 2010). A separate meta-analysis of 11 randomized controlled trials on MI for weight management, healthy eating and physical activity also showed greater reductions in body mass compared with control conditions (Armstrong et al., 2011). However, a systematic review by Morton, et al. (Morton et al., 2015) on MI for health behavior change with non-clinical populations in primary care, which included studies on dietary behaviors, showed only 50% of trials had positive effects on behavior change. Furthermore, average relative effect sizes in these reviews were small, especially when MI was compared with active controls.
Several limitations to MI may be associated with its limited impact. Functional targets often have higher personal value when considered outside a consumption context, such as a clinic room. However, when presented with temptations, the more proximal nature of the related incentives gives them stronger motivational impact when making a consumption decision. Challenges for maintaining healthy eating involve making the related incentives more salient and emotionally powerful, and reducing the power of temptations at times when we are making consumption decisions (Hofmann and Nordgren, 2015). MI does not attempt to teach participants how to do that. It also relies heavily on verbal discussion, which is less strongly linked with emotions than is more sensory experiences, including mental imagery (Pearson et al., 2015).
Mental imagery involves creating a mental representation of an object, activity or experience, which simulates actual experience, and carries a similar emotional charge (Pearson and Kosslyn, 2013). Multisensory imagery has an important influence on desires for a wide range of motivational targets, including foods, alcohol, cigarettes, and engaging in sport (Kavanagh et al., 2009; May et al., 2008; Kemps and Tiggemann, 2007; May et al., 2010). Individuals who want to moderate their desires or cravings can focus on interfering with the imagery that gives them emotional power, using cognitive tasks that compete for the same working memory capacity, including other mental imagery (May et al., 2008; Skorka-Brown et al., 2015; Baddeley and Andrade, 2000; Kavanagh et al., 2005). One important type of competing mental imagery involves images about a more beneficial goal, such as maintaining a healthy diet. Rehearsing that imagery may not only build motivation for healthy food consumption, but also blunt the power of other food cravings, allowing individuals to overcome momentary temptations and maintain their progress towards long-term health goals.
1.4. A new behavior change intervention
Functional Imagery Training (FIT, (May et al., 2015a)) was created to address these limitations. This new motivational change approach was based on extensive theoretical and empirical work, which demonstrated the power of mental imagery in desires and motivation (Kavanagh et al., 2005; May et al., 2015b).
FIT incorporates the spirit of MI in its initial stages, but conducts it using mental imagery (Kavanagh et al., 2005). This imagery brings important distal outcomes from functional behaviors into immediate experience, augmenting their motivational impact. If the person is committed to making a change, they are then shown how to use motivational imagery in situations where they are confronted with a consumption-related decision, allowing that decision to be more rationally based and less influenced by proximal temptations. Thus, FIT teaches individuals how to use sensory-rich imagery to build motivation towards a beneficial goal and deal with problematic craving.
An initial trial of FIT for the reduction of high-energy snacking used a stepped wedge 2-week delay design and a single treatment session. It found that participants who received FIT immediately had greater reductions in both the number of snacks and snacking occasions over 2 weeks than did those who received it after that assessment. The delayed group then achieved similar reductions over the next 2 weeks (Andrade et al., 2016). Over the 4 weeks, the sample obtained a 33% reduction for the number of occasions. More recently, a larger randomized controlled trial on weight control that compared 4 h of FIT or MI over 3 months demonstrated sustained benefits from FIT (Solbrig et al., 2019). While MI only gave a reduction of 0.67 kg over a 12-month period, FIT resulted in a 6.44 kg reduction in weight. These trials indicate that FIT is an emerging but competitive psychotherapeutic intervention with significant impact.
1.5. Behavioral target: high-calorie consumption
High-calorie food consumption is a key health risk factor underpinned by behavioral lifestyle choices, which is receptive to motivational intervention. It is also a key modifiable behavioral determinant of obesity, high blood glucose, and poor dental and bone health (Hsiao and Wang, 2013; Malik et al., 2010; Woodward-Lopez et al., 2011; World Health Organization, 2015). Routine adherence to a healthy diet aids in preventing and managing serious health conditions, including cardiovascular disorders, diabetes and cancers (World Health Organization, 2017). Reduction of high-calorie food intake is therefore a key goal for interventions that attempt to improve both immediate and long-term health outcomes.
1.6. Preliminary pilot: human-delivery (FIT-H)
We developed a protocol-based version of the intervention for reducing high-calorie snack intake. This script was tested using a human clinician to deliver it (FIT-H). This preliminary pilot was to ensure the creation and delivery of an intervention script using FIT could achieve behavioral change on its own, prior to the implementation of the robot. A case series study was conducted with adults (aged 18+) who had daily access to a smartphone, were snacking at least twice a day on high-fat or high-sugar foods or drinks, wanted to reduce that type of snack intake, and had never been diagnosed with an eating disorder. The intervention was delivered in three 60-min face-to-face treatment sessions and five 15-min phone sessions, giving a total of approximately 4.25 h of treatment over 12 weeks. Over a 2-month recruitment period, 14 participants began the trial. They were between 20 and 62 years (M = 38.5, SD = 13.03), and 13 (93%) were female. Eight participants (57%) provided data at 4 weeks, and seven (50% of those entering the study) completed assessments at 12 weeks. All those completing post-baseline assessments showed reductions in snack episodes: Baseline (M = 14.6, SD = 3.8), 4 Weeks (M = 7.4, SD = 4.4), and Week 12 (M = 5.9, SD = 3.1), giving a 49% reduction between the first 4 weeks followed by an additional 20% reduction between 4 and 12 weeks. Using standard deviation units across the whole baseline sample, this corresponded to a Cohen's d of 1.65 for completers to Week 4, and 1.93 for completers to Week 12. Even though this intervention used a highly structured script, the results compared very favourably with the previous results from a manualised but less constrained intervention (Andrade et al., 2016), even though the earlier study only had 2 rather than 4-week phases and 3 rather than 14 days of recalled snacking. These data provided a strong foundation for using a social robot to deliver a similar script, after some modifications to allow for a social robot to deliver an intervention. Further details on the design, implementation, treatment scripts and results of this study are at https://github.com/nrbsn/robofit.
1.7. Aims and hypotheses
The primary study was a pilot randomized controlled trial on the delivery of FIT by a social robot (FIT-R). It had a stepped-wedge design, comparing the effects of immediate delivery of FIT-R on enrolment to the trial (‘Immediate’), with FIT-R that was delayed until 4 weeks after enrolment (‘Delayed’). The ‘Delayed’ group received an instruction to self-monitor their snack intake for the initial 4 weeks - a common strategy seen in control groups in behavioral weight loss trials (Burke et al., 2011). Outcomes were measured at Baseline, Week 4 and Week 8. This design was chosen to investigate differential changes over time, and control for the effects of signing up to a behavior change study and self-monitoring snack intake.
We hypothesized that:
-
•
From 0 to 4 weeks, the Immediate condition would show a greater reduction in snack episodes (primary outcome), a greater increase in motivational thoughts, and a greater reduction in craving after FIT-R than the Delayed condition.
-
•
From 4 to 8 weeks, the Immediate condition would maintain their treatment gains, while the Delayed condition (who now received FIT-R) would display a reduction in snack episodes, an increase in motivational thoughts, and a reduction in craving after treatment.
The study comprised an initial test of feasibility for a social robot to deliver a verbal intervention on its own with a clear focus on one behavioral target. Accordingly, additional medical assessments were not included.
2. Methodology
2.1. Participants
Participants were invited to participate using email recruitment to university staff and students, social media networks, media releases, networking sites, electronic and physical noticeboard flyers over a period of 11 months. Participants had to be aged 18 or over, consuming at least one high-calorie food or drink per day that was not part of their daily meals. They also must have wanted to reduce that type of snack intake. They could not screen positive for a disorder on the Eating Attitudes Test-26 (Garner et al., 1982) or have ever been diagnosed with an eating disorder. Participants also needed to complete baseline assessments and provide contact details. No other exclusion criteria were applied, and individuals were able to concurrently take part in other programs or services at their own discretion, since the study had a clear focus on one specific behavioral component related to the more multifaceted condition of healthy eating and weight management.
2.2. Materials
2.2.1. NAO Humanoid Robot
The NAO Humanoid Robot (See Fig. 1) from Softbank Robotics (SoftBank Robotics, 2019) is 58 cm tall. It has 25 degrees of body movement freedom, tactile sensors, two cameras, directional microphones and a linear inverse pendulum for omnidirectional movement. It also has an intel ATOM 1.6 ghz CPU, sonar rangefinder, an inertial board, voice synthesiser, and 48.6 watt-hour battery with 1.5+ battery life (SoftBank Robotics, 2019). The robot was programmed and run using the Choregraphe Software Development Kit by Softbank Robotics. The NAO was selected for this research study because it had a friendly humanoid and child-like appearance, a simple sensor system for participants to use, and had been used in several previous health-related trials e.g. (Robinson et al., 2019).
Fig. 1.

NAO Humanoid Robot.
2.2.2. Assessment outcomes
All assessments listed below (excluding Body Mass Index) were self-report measures that were completed online in response to emails that included links to the surveys. This approach allowed participants to complete these assessments in their own time to reduce research burden, and allowed the assessments to both be independent of the research team and separate from the robot intervention.
2.3. Number of snack episodes (primary outcome)
The number of high-calorie snack episodes was calculated using a diary-based recall over the previous 2 weeks, which was taken at each assessment time-point. A high-calorie snack was defined as any high-sugar or high-fat food or drink that was consumed between regular meals. High-calorie snack intake is identified as a key risk factor towards obesity and high-blood glucose levels, with promotion to reduce consumption to improve overall health outcomes well-established in national health guidelines (World Health Organization, 2015). They are often consumed instead of more nutritious foods, contain low levels of essential nutrients, and not considered essential for a healthy diet (World Health Organization, 2015). Provided examples included, but were not limited to, sugary soft drinks, doughnuts or buns, confectionery, sweet or savoury pies/crumbles, fruit juice and sugary muesli bars, but excluding alcoholic drinks (National Health and Medical Research Council, 2013). For each day, the number and type of snacks or drinks were recorded for three periods: morning (12:00 am to 12:00 pm), afternoon (12:00 pm to 6:00 pm) and evening (6:00 pm to 12:00 am). Participants were asked to report any snacks they were unsure about for review. A snack episode was defined as any snacks consumed in any morning, afternoon or evening period for that time-frame, regardless of the total number of snacks or drinks. Total possible snack episodes ranged from 0 to 42 (i.e. 3 time periods by 14 days) with the final reported outcome listed as an average across the 2-week period to find a weekly average (i.e. total/2). Total snack episodes represented the primary outcome because it is a metric that is identifiable, easy to understand, measurable across multiple individuals, and a clear behavioral outcome from an intervention designed to initiate behavior change. This also avoided potential issues with item counts or quantity assessments conducted by different participants, or with decisions about whether a series of snacks or drinks might constitute different episodes. Other more technical methods would have provided more details about dietary quality, but require significantly more time to train participants to use them correctly before they would have produced meaningful results, such as calorie counting total energy intake ( Johnson, 2002; Shim, 2014).
2.3.1. Motivational and craving cognition
The 13-item Motivation Thought Frequency MTF, (Kavanagh et al., 2018; Parham et al., 2017; Robinson et al., 2016) scale was used to measure the frequency of motivational thoughts over the previous week about their motivation to want to change their high-calorie snack intake. Motivation is expected to have a strong influence on health behavior change with motivation towards the routine selection of health foods over high-calorie snacks leading to better health practices and benefits, both in short and long-term scenarios (Hofmann and Nordgren, 2015). Confirmatory factor analyses show the MTF as having four subscales that underpin a variety of motivational cognitions (intensity, incentives imagery, self-efficacy imagery, availability).
The 10-item Craving Experience Questionnaire Frequency CEQ-F, (May et al., 2014) measured the frequency of cravings or desires towards high-calorie snack intake over the previous week, which can interfere with motivational cognition for wanting to change high-calorie snack episodes (Kavanagh et al., 2005). The CEQ-F has three subscales (intensity, imagery, intrusiveness). Both the MTF and CEQ have items rated from 0 (‘never’) to 10 (‘constantly’). The State Motivation and CEQ Strength (Kavanagh et al., 2018; Robinson et al., 2016) was also used at each assessment point. Those results are available at https://github.com/nrbsn/robofit. These scores were presented in the results as mean items scores for each subscale (i.e. 0–10).
2.3.2. Confidence to Control Snacking (CCS)
The CCS was created for the current study as a measure of self-efficacy to control snack intake across different time periods, scenarios and emotional states. It has two subscales, each of 10 items: reported confidence to control over each of 1–6 days, 1, 2, 3 and 4 weeks (CCS-Days) and reported confidence to control during emotional states or specific scenarios, such as feeling angry, bored or if others were having one (CCS-Situations). All items that are each rated from 0 (‘Not at all confident’) to 10 (‘Extremely confident’, and subscale scores are reported the average confidence across items within the subscale. The scale has not yet been tested in other research, but its construction closely apparels other self-efficacy measures that have established internal consistency (Kavanagh et al., 1996).
2.3.3. Working Alliance
The Working Alliance Inventory (WAI) by Horvath and Greenberg (Horvath and Greenberg, 1989) is a self-report measure to assess the therapeutic alliance between a professional and a patient, a theoretical approach which has often been investigated to explore its relationship to psychotherapy and behavior change outcomes (Ardito and Rebellino, 2011). Items are rated on a 7-point Likert scale from 1 (‘never’) to 7 (‘always’) and scores on the therapeutic bond, roles and tasks. ‘Goal’ refers to what the individual wants to achieve from the intervention with both parties selecting the target of the intervention, ‘Task’ involves what the individual and treatment agent (in this case, the robot) propose should be undertaken to reach the goal, and ‘Bond’ refers to attributes of the treatment agent such as trust, acceptance and confidence (Horvath and Greenberg, 1989). The inventory was completed after the first robot session. Permission to use the inventory was granted.
2.3.4. Weight and Body Mass Index (BMI, secondary outcome)
Weight and Body Mass Index (BMI, [kg/m2]) were assessed in each robot treatment session using the same set of scales. Participants were asked to remove their shoes and other items of heavy clothing, such as coats or jackets to increase the likelihood of taking an accurate between-session reading. Since the time-frame between the two robot sessions was very short (2 weeks) and weight measurements were not mandatory, BMI was a secondary outcome measure only.
2.4. Robot-delivered intervention
2.4.1. Design
The timeline for delivery of FIT-R is shown in Table 1. In recognition that much of the effect in the human pilot study appeared to be from the initial sessions, the number of sessions was reduced from eight to three and the total contact time from 4.25 to 2.25 h, FIT-R had two face-to-face sessions with the robot at Week 1 and Week 3 post-baseline (2 × 60 min), and a pre-recorded video session delivered online at Week 2 (1 × 15 min). Instead of additional booster sessions, participants in the Immediate group, received a weekly text-message reminder (<160 standard characters) from Week 4, to encourage them to continue imagery practice until their final assessment point in Week 8.
Table 1.
Pilot RCT timeline.
| Month 1 | Baseline | Week 1 | Week 2 | Week 3 | Week 4 |
| Immediate | Assessment | Session 1 | Video Session | Session 2 | Assessment |
| Delayed | Assessment | – | – | – | Assessment |
| Month 2 | Week 5 | Week 6 | Week 7 | Week 8 | |
| Immediate | Reminder | Reminder | Reminder | Assessment | |
| Delayed | Session 1 | Video Session | Session 2 | Assessment |
2.5. Intervention design and content
2.5.1. Functional imagery training delivered by a social robot (FIT-R)
Delivery of FIT by the robot was similar to the human-therapist version (FIT-H). Both scripts used an encouraging and collaborative approach, supporting whichever snack reduction goal the individual chose, and there was substantial re-use of wording. However, based on observations in the human-therapist study, the robot intervention script gave an additional explanation of mental imagery. Adaptation to the robot delivery also required that some previously open-ended questions be delivered in a branched format, to ensure the accuracy of the robot's response. The robot intervention content was written, reviewed and approved by trained clinical psychologists with extensive experience in large-scale behavior change interventions.
FIT-R had a largely linear script with a series of nodes that participants progressed through using a sensor touch or basic oral branching points (i.e. Yes/No). All segments were custom-animated and aural pronunciation was selected to closely represent a natural, realistic and collaborative conversation with the robot. Individual face-to-face sessions with the robot were conducted in a private room without others present, to maximise the likelihood of disclosure and participation in treatment. Sessions were monitored using a wireless router and observed by a researcher to allow expeditious responses to any error or accident. However, the robot was not designed to wait for any commands or prompts by the researcher (i.e. no Wizard-of-Oz control was used). The full human and robot therapist intervention scripts, including content differences, can be seen at https://github.com/nrbsn/robofit.
Initially, all participants completed a brief practice session to become familiar with the robot, including use of the sensors and responses to basic verbal prompts. This segment provided a validation check on their ability to navigate through the session on their own. Each participant then received an identical intervention with the only minor customization, such as their name and pre-scripted feedback at designated branching points (e.g. their reported confidence number for making a change). Participants were asked to answer open-ended questions aloud (i.e. how did that imagery exercise make you feel?), although their verbal responses were not recorded or analysed. The omission of changes to the script based on answers to open-ended questions was because these questions can elicit an infinite number of possible responses, and because natural language processing by social robots remains an emergent field of work and is not yet sufficiently error-free for clinical applications. The decision not to directly respond to each individual answer reduced the likelihood of errors, the complexity of the session, or other inaccurate responses that could have undermined perceived trust or confidence in the robot session, or the treatment content. The fixed script intervention design also increased the ability to systematically draw conclusions about the impact of its content, and was similar to other forms of digital programs (Oosterveen et al., 2017). Given the static nature of the program, the robot-delivered intervention could not fully implement FIT, but it did approximate and simulate the content as well as a linear, fixed dialogue is able to do.
2.5.2. Session 1-Robo-FIT (60 min)
Session 1 commenced with the robot introducing itself as ‘Andy’, provided information about the session agenda, and general mental imagery psychoeducation. The robot elicited practice of positive imagery, and provided a rationale for the intervention. Then, participants described the positives and downsides about their current snack intake, and created mental imagery about a time that was worse than usual, followed by that problem disappearing, and others praising them for making a change. If they wanted to work on their snack intake, they were asked to identify a potential goal. Otherwise, if they declined, the session would conclude. They imagined reaching that goal for a week, the outcomes that would give them, rated their confidence in reaching that goal, and imagined how they would do it. They added imagery about how they would address challenges, and a specific occasion in the next week when they could use that idea, and reported whether their confidence had risen. The session concluded with them summarizing out loud what they wanted to do, why, how they would do it, and asked to practise the imagery at home.
2.5.3. Session 2-Robo-FIT online video (15 min)
A 15-min pre-recorded video was provided as a short booster session. It involved the robot delivering a piece to camera monologue with further information and guided technique rehearsal to support the intervention content. The robot asked participants to reflect on their progress over the week, and elicited imagery about an occasion in the week when they resisted having a high-calorie snack. They refined imagery about their plans for the next week, recalled a time in the past when they had yielded to temptation, and imagined choosing an alternative snack or doing something else. Participants were only able to access the video session once to avoid receipt of multiple top-up sessions, and the video session did not contain any customised content or personalisation, instead using a neutral greeting to open and close the session.
2.5.4. Session 3-Robo-FIT (60 min and 30-min semi-structured interview)
Session 3 commenced with a session overview and proposal of agenda. Participants again reflected on any changes in their snacking and re-rated their confidence. They practised imagery about further actions they could take when tempted to have a high-energy snack or drink in the next week, and if a recent challenge to their control were to recur. They re-rated their confidence in maintaining control and reflected on whether it had changed. They practised imagery about preparing for a future challenge, maintaining control, and how good they would then feel. They were encouraged to practise similar imagery at home, and set reminders to practise using their phone.
A 30-min semi-structured interview was conducted at the end of their final robot session by a member of the research team, which was their first substantial interaction with the researcher since commencing the program. Detailed results of the qualitative interview will be analysed and reported in depth in a separate publication.
2.6. Procedure
Human research ethical approval was obtained (HREC #1500000934). Participants provided informed consent online, and whether they met eligibility criteria. They completed the EAT-26 and baseline assessments, including demographic characteristics. Ineligible participants were given feedback, offered healthy eating and eating disorder information, and contact details for any questions. Eligible participants were randomized into the Immediate or Delayed condition through an automated computer program using random permutations of the digits 1–2 and 1–4 with no stratification. This random permutation set was undertaken to minimise differences in numbers between conditions and avoid guessing of likely future allocations by the researcher who determined eligibility. Immediate commenced treatment from Baseline to Week 4, with text reminder follow-up until Week 8, and the Delayed group commenced treatment from Week 4 to Week 8.
2.7. Statistical analyses
Outcomes were analysed using the full randomized sample (i.e. intention- to-treat). Linear mixed models (IBM™ SPSS Version 23) assessed changes over three time points: Baseline, Week 4 and Week 8. An autoregressive relationship between an individual's repeated data with a single-time lag provided best fit. Condition and time were fixed effects with participants as a random effect. Restricted maximum likelihood was used for estimation. Effect sizes of contrasts between conditions used Cohen's d, reported in standard deviation units across conditions at Baseline. Preliminary analyses checked for outliers and assumptions of normality and distribution, and all assumptions were met. Any missing data from snack episode reports were substituted by prorating. For example, a participant who reported data for 10 out of 14 days and had 5 snacking episodes, prorating gave a score of 7 (for the 14 days). No significant differences were found when conducting all statistical analyses using the raw or prorated data, leading to the prorated data reported in the final analysis below due to its likelihood to more closely represent their behavior over the last two weeks. Correlations with changes in snack episodes used baseline snack episodes minus episodes immediately post-intervention (i.e. Immediate at 4 weeks and Delayed at 8 weeks).
3. Results
3.1. Baseline data
A total of 122 participants commenced the survey: 104 provided consent, and 83 fulfilled initial eligibility criteria. Only 33 (42%) completed the baseline measures, and 26 submitted their contact details and therefore randomized into conditions. In the final sample, most participants were female (n = 18, 69%) and were aged from 19 to 69 years (M = 37, SD = 13.47). The sample had high levels of education: 10 (38%) had an undergraduate degree, and 7 (27%) had a postgraduate degree. The rest of the sample had completed Grade 12 (n = 8, 31%) or a trade (n = 1, 4%). Most were employed (part-time: n = 12, 46%; full-time: n = 13, 50%), had partners (n = 16, 61%), or were single (n = 8, 31%). Only two (8%) were separated or divorced. Most were born in Oceania (n = 23, 89%), two from the United States and one from Europe. The conditions received an equal number of allocations across Immediate (n = 13) and Delayed (n = 13) treatment. There were no significant differences between conditions on any demographic characteristics or other baseline assessments. At Session 1, BMI average was high (M = 30.66, SD = 10.50, Range = 17.38–53.11).
3.2. Intervention involvement and retention
In the treatment sessions, all participants gave descriptive answers out loud to each question, and appeared to attempt the imagery segments. None declined to work on snack intake as their main goal or withdrew involvement during treatment sessions. All live sessions were completed within the recommended timeframes of 60 min. Components within sessions were completed at a different pace between individual participants, depending on the extent of discussion required to identify their individual goal, discuss their reasons for change, or talk through ideas for their treatment plan.
The 4-week assessment was completed by 19 of the 26 participants who were randomly allocated to conditions (73%), and the 8-week assessment was completed by 16 (62%). Everyone who completed the 8-week assessment had completed all previous assessments. Completion rates for the Immediate group (n = 13, Session 1 = 100%, Video 1 = 84.6%, Session 2 = 76.9%, All Sessions = 76.9%). Completion rates for the Delayed group (n = 13, Session 1 = 76.9%, Video 1 61.5%, Session 2 = 76.9%, All Sessions = 61.5%).
3.3. Snack episodes
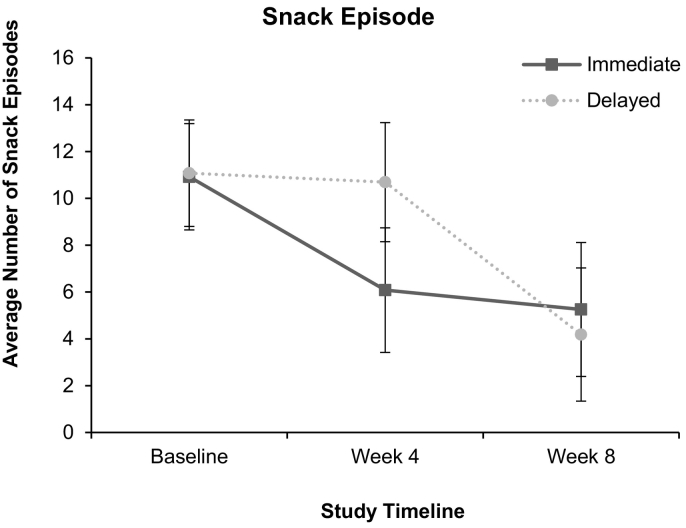
A significant condition by time effect for snack episode reduction was found, F(2, 32.06) = 4.30, p = .022 (See Fig. 2). Pairwise comparisons revealed no significant differences between allocated conditions at Baseline, F(1, 45.09) = 0.009, p = .924, or Week 8, F(1, 50.24) = 0.284, p = .596, but found significant differences at the key assessment in Week 4, F(1, 51.35) = 6.31, p = .015. The Immediate condition had a significant fall in snack episodes from Baseline to Week 4 (F(1, 30.24) = 11.18, p = .002, d = −1.06), and this was maintained between Weeks 4 and 8 (F(1, 25.92) = 0.273, p = .605, d = −0.18). In contrast, the Delayed condition had no significant change between Baseline and Week 4 (F(1, 28.93) = 0.08, p = .785, d = −0.08), but fell significantly between Weeks 4 and 8 (F(1, 27.58) = 17.46, p < .001, d = −1.42). Reductions in snack episodes in the robot-delivered version: Immediate Baseline (M = 10.92, SD = 4.68) with 4 Weeks (M = 5.39, SD = 3.02) and Week 8 (M = 5.06, SD = 3.42) and Delayed Baseline (M = 11.08, SD = 4.65) with 4 Weeks (M = 10.85, SD = 4.25) and Week 8 (M = 4.25, SD = 2.64). In the first four weeks of treatment, the sample reduced by 55%. The Immediate group reduced by 51% in the first 4 weeks, followed by a 6% reduction between 4 and 8 weeks (overall reduction = 54%). The Delayed group reduced by 2% in the first 4 weeks, followed by 61% decrease between 4 and 8 weeks (overall reduction = 62%).
Fig. 2.
Snack episode reduction across 8 weeks.
3.4. Frequency of motivational cognitions
No significant effects were found on the MTF subscales for either condition by time (intensity: F(2, 37.29) = 0.14, p = .871, self-efficacy imagery: F(2, 35.06) = 0.90, p = .417, incentives imagery: F(2, 35.52) = 0.92, p = .407, availability: F(2, 34.25) = 0.83, p = .446) or time (intensity: F(2, 37.29) = 0.15, p = .858, self-efficacy imagery: F(2, 35.06) = 0.65, p = .531, incentives imagery: F(2, 35.52) = 0.49, p = .616, availability: F(2, 34.25) = 1.31, p = .282). The average scores at baseline were moderately high even before commencing treatment (Intensity M = 7.8, SD = 1.8; Self-efficacy Imagery M = 6.2, SD = 2.78; Incentives Imagery M = 6.8, SD = 2.27; Availability M = 6.7, SD = 2.46). This would have left little room for further increase, since the cognitions were not either expected or intended to be constantly experienced throughout the day and may have already been at a peak for the individual which prompted their self-referral into the study. There were no significant correlations found between changes in pre-to-post treatment snack episodes and changes in the frequency of motivational cognitions.
3.5. Frequency of craving cognitions
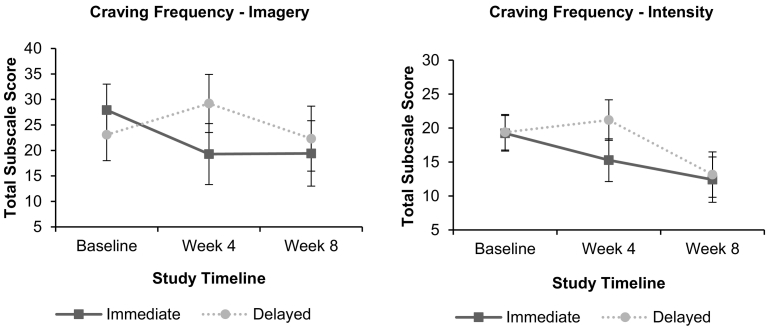
The condition by time effect for craving imagery was statistically significant (F(2, 35.81) = 5.25, p = .010). The Immediate condition had a moderately large reduction in craving imagery from Baseline to 4 weeks (F(1, 33.77) = 6.86, p = .013, d = −0.89), while absolute values appeared to rise for the Delayed condition (F(1, 32.47) = 3.75, p = .062, d = 0.63). From 4 to 8 weeks, the Immediate condition remained stable, (F(1, 29.43) = 0.001, p = .972, d = 0.01), but craving imagery for the Delayed participants had an apparent but non-significant fall (F(1, 31.11) = 3.77, p = .061, d = −0.71). While the condition by time effect for craving intensity fell short of the 0.05 level of significance (F(2, 36.93) = 2.56, p = .091), a similar pattern of mean scores was found to those of craving imagery (See Fig. 3). Over the first 4 weeks, the fall in the Immediate condition gave Cohen's d = −0.76, while the Delayed condition had d = 0.34. From Weeks 4 to 8, the Immediate condition had a further apparent fall of d = 0.55, while the Delayed condition fell by d = 1.53. No significant effects for time by condition (F(2, 38.03) = 1.76, p = .186) or time (F(2, 38.03) = 0.92, p = .407) were seen for the intrusiveness of craving cognitions.
Fig. 3.
Craving frequency reduction across 8 weeks.
3.6. Confidence to control snacking
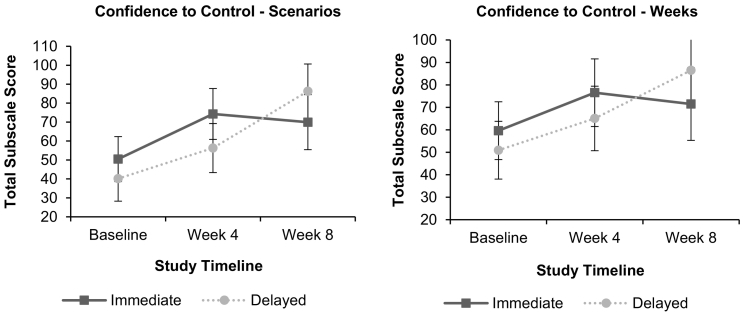
There was a significant condition by time effect for CCS-Situations (F(2, 32.54) = 6.66, p = .004, See Fig. 4). The Immediate condition had a significant increase in average confidence over the first 4 weeks (F(1, 32.39) = 14.65, p < .001, d = 1.13), as did the Delayed condition to some extent (F(1, 31.47) = 7.33, p = .011, d = 0.76). From Weeks 4 to 8, the Immediate condition maintained their improvement (F(1, 29.75) = 0.42, p = .522, d = −0.20), while the Delayed condition had a further substantial increase (F(1, 30.86) = 20.46, p < .001, d = 1.42).
Fig. 4.
Confidence to control snack intake across 8 weeks.
On CCS-Days, there was no condition by time effect (F(2, 34.59) = 2.40, p = .106), but there was a significant increase in average confidence over time (F(2, 34.59) = 6.67, p = .004). However, only the Immediate condition showed a statistically significant improvement from Baseline to 4 weeks (F(1, 32.97) = 4.35, p = .045, d = 0.73, vs F(1, 31.71) = 3.29, p = .079, d = 0.61). As in CCS-Situations, from 4 to 8 weeks the Immediate group maintained its improvement (F(1, 32.97) = 0.33, p = .572, d = −0.22), while the Delayed condition had a significant increase (F(1, 30.41) = 6.04, p = .020, d = 0.93).
3.7. Weight and Body Mass Index (BMI)
Participants were weighed during the two face-to-face sessions only, so no comparisons between conditions could be tested or follow-up data obtained. Despite the brevity of the 2-week interval between these sessions, participants achieved an average weight reduction of 4.4 k (Session 1: N = 21, M = 85.24, SD = 28.26, Range = 48.20 to 153.50; Session 2: N = 20, M = 80.84, SD = 25.48, Range = 47.25 to 151.25). A reduction in mean BMI was also seen, from 30.66 (SD = 10.50, Range = 17.38 to 53.11) to 28.77 (SD = 9.34, Range = 17.17 to 52.34).
3.8. Working Alliance Inventory
The WAI subscales correlated positively with snack episode reduction from pre-to-post treatment (Task: r = 0.71, p = .001; N = 17; Bond: r = 0.67, p = .003; N = 17; Goal: r = 0.75, p < .001; N = 17).
4. Discussion
This pilot randomized controlled trial revealed that a pre-programed social robot can autonomously deliver a behavior change intervention translated from an evidence-based psychotherapeutic program. Individuals reduced the total average number of snack episodes after completing the social robot intervention without any human involvement. The robot-delivered pilot achieved similar snack episode reduction in the first four weeks (FIT-R, 55%) when compared with the human-delivered version by a trained clinician (FIT-H, 49%). Overall reductions were seen in the frequency of craving imagery, showing that treatment techniques did help to some extent to blunt the impact of craving episodes. Significant increases in motivational cognition frequency were not found, although motivations were already moderately high at the start of the intervention. A significant treatment effect on perceived confidence to control snack intake across specific situations and emotional states was seen, and while the condition by time effect was not significant for confidence in the number of days of adherence, a rise in confidence across conditions was observed, and there was a similar pattern of results within treatment groups to those for CCS-Situations. Participants rated the robot highly on working alliance subscales after the first session, and before they started experiencing personal benefits from treatment, and significant positive correlations were found between snack episode reductions and working alliance, including the personal ‘bond’ between the social robot and the individual. Overall, despite its small sample size, this pilot study provided strong initial support for use of a social robot to conduct a motivational interview using an imagery-based intervention.
4.1. Clinical implications
This study has significant implications for clinical practice. Behavioral interventions using FIT have now been shown to support behavior change for diet and weight reduction in multiple studies, including the trials using human therapists by Andrade et al. (2016) and by Solbrig et al. (2019). In the current paper, we report effects of a closely scripted version of FIT delivered by a human therapist over a longer period of time (FIT-H), and then a robot-delivered adaptation of that intervention (FIT-R). These results appear to suggest that a robot delivering an imagery-based intervention may be as effective as a human clinician delivering a similar intervention. They also suggest that the rigid translation of a psychotherapeutic intervention can achieve significant results, despite the loss of fidelity to the full treatment that this entails. In particular, the provision of detailed psychoeducation, a methodized set of goal-setting steps, and guided imagery training may be sufficient to initiate change for those who are willing and capable of making changes. Furthermore, teaching people to use imagery-based techniques in their own time, and the provision of motivational encouragement to continue its use, may have substantial impact from the first session alone. Continuous follow-up might best be offered to those who are experiencing difficulties with imagery-based techniques, require additional motivational support, have experienced a recent setback, or wish to rehearse specific session techniques again, such as generating ideas for change.
Results of the current study are also relevant to an understanding of therapeutic alliance. While alliance was predictive of a reduction in snacking episodes, this alliance was with a robot that was unable to provide highly personalised and empathetic healthcare treatment. The substantial extent of alliance with the robot that was seen in this study is inconsistent with the view that a therapeutic alliance can only be attained with a human clinician. Participants reported high task alignment for their chosen goal, perceived support for the intervention direction, and a sense of connection, trust and acceptance with their robotic therapist. A social robot may therefore be capable of providing essential common and specific factors for psychotherapeutic interventions, by eliciting a perceived sense of relational connection and providing a personally relevant and effective intervention (Ardito and Rebellino, 2011). Given the high level of control that robotic therapists provide over both the content of the intervention and the nature of the interaction, they offer an exciting and hitherto unparalleled opportunity to tease apart common and specific factors in psychotherapies (Mulder et al., 2017).
In summary, the current study illustrates that a digital intervention program that supports self-guided change and teaches techniques that can be rehearsed beyond the conclusion of the session may have a significant impact, particularly when imagery-based techniques are included. Such an approach could enable psychological interventions to have substantial reach and potential cost-effectiveness if it were taken to scale, given its potential to supplement or substitute for face-to-face sessions with practitioners (Provoost et al., 2017; Laranjo et al., 2018).
4.2. Strengths and limitations
Strengths of the pilot included its use of a stepped wedge design, which allowed a replication of treatment effects in a wait-list condition. While a wait-list control is subject to expectancy confounds, it provides a sound basis for later trials that provide additional control. Limitations include its focus on periods of snack episodes rather than changes in total energy consumption, which would have given a more detailed insight into overall food intake patterns, albeit at the trade-off of increasing effort and time commitment to complete the intervention (Johnson, 2002; Shim et al., 2014). The lack of a differential impact from the intervention on the frequency of motivational cognitions and the lack of a relationship between motivational cognitions and snacking frequency cast doubt on our proposed mechanism of effects for FIT-R, although as noted, these cognitions were already quite frequent at baseline. A further limitation included the pilot's short duration, which precluded a test of maintained effects of an impact on sustained reductions in weight or snack episode frequency, although our study provides a direct pathway into such a trial. The relatively long time required to recruit the sample may reflect on the acceptability of the robot treatment, and additional strategies may have been needed, such as an introductory session with the robot. Some participants did not complete the initial questionnaire, including filling out its high-calorie diary-recall segment. For a subset of this group, volunteering and attempting to complete the diary-recall segment may have allowed them to realistically determine that treatment was not required, but others may have withdrawn at that point because it was seen as onerous. Abbreviation of the assessments together with a rationale for each measure and feedback on progress through the assessments may increase their completion in future trials. Among participants who did completed the initial assessment, all but 3 (12%) attended at least one intervention session, and moderately high retention in treatment and subsequent assessments was obtained, while leaving room for further improvement (e.g. through more assertive follow-up).
4.3. Future directions
The current research extends the range of potential health applications of social robots beyond well-established target areas e.g. dementia, autism spectrum disorder, (Robinson et al., 2019) to encompass health maintenance for a major modifiable risk behavior (World Health Organization, 2017). This study raised the possibility of social robots delivering a range of similar interventions for other health behavior targets, such as blood glucose monitoring for diabetes management. While further research that replicates and extends the current results in larger scale trials is necessary to build greater confidence in its acceptability and impact, the current study provides a solid basis for that research program.
Acknowledgements
Nicole Robinson was supported by the Australian Postgraduate Award on behalf of the Department of Education and Training and by a Research Grant from the State of Queensland acting through the Department of Science, Information Technology and Innovation. Leanne Hides is funded by an NHMRC Senior Research Fellowship and by Lives Lived Well, a not-for-profit charity. Nicole Robinson is a director and shareholder in a robotics company, but that company has no financial interest in, or conflict with, the subject matter or materials discussed in this manuscript.
References
- Abbott R., Orr N., McGill P. How do “robopets” impact the health and well-being of residents in care homes? A systematic review of qualitative and quantitative evidence. Int. J. Older People Nursing. 2019;14(3) doi: 10.1111/opn.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemi M., Ghanbarzadeh A., Meghdari A. Clinical application of a humanoid robot in pediatric cancer interventions. Int. J. Soc. Robot. 2016;8(5):743–759. doi: 10.1007/s12369-015-0294-y. [DOI] [Google Scholar]
- Andrade J., Khalil M., Dickson J. Functional imagery training to reduce snacking: testing a novel motivational intervention based on elaborated intrusion theory. Appetite. 2016;100:256–262. doi: 10.1016/j.appet.2016.02.015. [DOI] [PubMed] [Google Scholar]
- Ardito R., Rebellino D. Therapeutic alliance and outcome of psychotherapy: historical excursus, measurements, and prospects for research. Front. Psychol. 2011;2 doi: 10.3389/fpsyg.2011.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong M.J., Mottershead T.A., Ronksley P.E. Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2011;12:709–723. doi: 10.1111/j.1467-789X.2011.00892.x. [DOI] [PubMed] [Google Scholar]
- Baddeley A., Andrade J. Working memory and the vividness of imagery. J. Exp. Psychol. Gen. 2000;129(1):126–145. doi: 10.1037/0096-3445.129.1.126. [DOI] [PubMed] [Google Scholar]
- Bainbridge W., Hart J., Kim E. The benefits of interactions with physically present robots over video-displayed agents. Int. J. Soc. Robot. 2011;3(1):41–52. doi: 10.1007/s12369-010-0082-7. [DOI] [Google Scholar]
- Bandura A. Prentice-Hall; Englewood Cliffs, N.J: 1986. Social Foundations of Thought and Action: A Social Cognitive Theory. [Google Scholar]
- Beran T.N., Ramirez-Serrano A., Vanderkooi O.G. Reducing children’s pain and distress towards flu vaccinations: a novel and effective application of humanoid robotics. Vaccine. 2013;31(25):2772–2777. doi: 10.1016/j.vaccine.2013.03.056. [DOI] [PubMed] [Google Scholar]
- Britt E., Hudson S.M., Blampied N.M. Motivational interviewing in health settings: a review. Patient Educ. Couns. 2004;53(2):147–155. doi: 10.1016/S0738-3991(03)00141-1. [DOI] [PubMed] [Google Scholar]
- Burke L.E., Wang J., Sevick M.A. Self-monitoring in weight loss: a systematic review of the literature. J. Am. Diet. Assoc. 2011;111(1):92–102. doi: 10.1016/j.jada.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drigas A., Koukianakis L., Papagerasimou Y. Towards an ICT-based psychology: E-psychology. Comput. Hum. Behav. 2011;27(4):1416–1423. doi: 10.1016/j.chb.2010.07.045. [DOI] [Google Scholar]
- Fasola J. ProQuest Dissertations Publishing; 2014. Socially Assistive and Service Robotics for Older Adults: Methodologies for Motivating Exercise and Following Spatial Language Instructions in Discourse. [Google Scholar]
- Fasola J., Mataric M.J. Using socially assistive human-robot interaction to motivate physical exercise for older adults. Proc. IEEE. 2012;100(8):2512–2526. doi: 10.1109/JPROC.2012.2200539. [DOI] [Google Scholar]
- Fischer K., Lohan K.S., Foth K. 7th ACM/IEEE International Conference on Human-Robot Interaction (HRI) IEEE; Boston Massachusetts, USA: 2012. Levels of embodiment: Linguistic analyses of factors influencing HRI. [Google Scholar]
- Gaffney H., Mansell W., Tai S. Conversational agents in the treatment of mental health problems: mixed-method systematic review. JMIR Mental Health. 2019;6(10) doi: 10.2196/14166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvão Gomes da Silva J., Kavanagh D.J., Belpaeme T. Experiences of a motivational interview delivered by a robot: qualitative study. J. Med. Internet Res. 2018;20(5):e116. doi: 10.2196/jmir.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner D.M., Olmsted M.P., Bohr Y. The eating attitudes test: psychometric features and clinical correlates. Psychol. Med. 1982;12(4):871–878. doi: 10.1017/S0033291700049163. [DOI] [PubMed] [Google Scholar]
- Hofmann W., Nordgren L.F. Guilford Publications; New York City, NY: 2015. The Psychology of Desire. [Google Scholar]
- Horvath A.O., Greenberg L.S. Development and validation of the working alliance inventory. J. Couns. Psychol. 1989;36(2):223–233. doi: 10.1037/0022-0167.36.2.223. [DOI] [Google Scholar]
- Hsiao A., Wang Y.C. Reducing sugar-sweetened beverage consumption: evidence, policies, and economics. Curr. Obes. Rep. 2013;2(3):191–199. doi: 10.1007/s13679-013-0065-8. [DOI] [Google Scholar]
- Huskens B., Verschuur R., Gillesen J. Promoting question-asking in school-aged children with autism spectrum disorders: effectiveness of a robot intervention compared to a human-trainer intervention. Developmental Neurorehabilitation. 2013;16(5):345–356. doi: 10.3109/17518423.2012.739212. [DOI] [PubMed] [Google Scholar]
- Ismail L.I., Verhoeven T., Dambre J. Leveraging robotics research for children with autism: a review. Int. J. Soc. Robot. 2019;11(3):389–410. doi: 10.1007/s12369-018-0508-1. [DOI] [Google Scholar]
- Johnson R. Dietary intake - how do we measure what people are really eating? Obes. Res. 2002;10(s1):63S–68S. doi: 10.1038/oby.2002.192. [DOI] [PubMed] [Google Scholar]
- Jøranson N., Pedersen I., Rokstad A.M.M. Effects on symptoms of agitation and depression in persons with dementia participating in robot-assisted activity: a cluster-randomized controlled trial. J. Am. Med. Dir. Assoc. 2015;16(10):867–873. doi: 10.1016/j.jamda.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Jost C., Le Pevedic B., Duhaut D. 2012 IEEE RO-MAN: The 21st IEEE International Symposium on Robot and Human Interactive Communication. IEEE; 2012. Robot is best to play with human! [Google Scholar]
- Kavanagh D., Andrade J., May J. Imaginary relish and exquisite torture: the elaborated intrusion theory of desire. Psychol. Rev. 2005;112(2):446–467. doi: 10.1037/0033-295X.112.2.446. [DOI] [PubMed] [Google Scholar]
- Kavanagh D., May J., Andrade J. Tests of the elaborated intrusion theory of craving and desire: features of alcohol craving during treatment for an alcohol disorder. Br. J. Clin. Psychol. 2009;48(3):241–254. doi: 10.1348/014466508X387071. [DOI] [PubMed] [Google Scholar]
- Kavanagh D., Robinson N., Connolly J. The revised four-factor motivational thought frequency and state motivation scales for alcohol control. Addict. Behav. 2018;87:69–73. doi: 10.1016/j.addbeh.2018.05.026. [DOI] [PubMed] [Google Scholar]
- Kavanagh D.J., Sitharthan T., Sayer G.P. Prediction of results from correspondence treatment for controlled drinking. Addiction. 1996;91(10):1539–1545. (published online first: 1996/10/01) [PubMed] [Google Scholar]
- Kemps E., Tiggemann M. Modality-specific imagery reduces cravings for food: an application of the elaborated intrusion theory of desire to food craving. J. Exp. Psychol. Appl. 2007;13(2):95–104. doi: 10.1037/1076-898X.13.2.95. [DOI] [PubMed] [Google Scholar]
- Kidd C., Breazeal C. IEEE/RSJ International Conference. 2008. Robots at home: understanding long-term human-robot interaction. Intelligent robots and systems. (Nice, France) [Google Scholar]
- Kiesler S., Powers A., Fussell Sr. Anthropomorphic interactions with a robot and robot-like agent. Soc. Cogn. 2008;26(2):169–181. doi: 10.1521/soco.2008.26.2.169. [DOI] [Google Scholar]
- Kim G., Jeon S., Im K. Structural brain changes after traditional and robot-assisted multi-domain cognitive training in community-dwelling healthy elderly. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0123251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak S., Kim Y., Kim E., Shin C., Cho K. 2013 IEEE RO-MAN. 2013. What makes people empathize with an emotional robot?: The impact of agency and physical embodiment on human empathy for a robot. (26-29 Aug. 2013; Gyeongju, South Korea) [Google Scholar]
- Lal S., Adair C.E. E-mental health: a rapid review of the literature. Psychiatr. Serv. 2014;65(1):24–32. doi: 10.1176/appi.ps.201300009. [DOI] [PubMed] [Google Scholar]
- Laranjo L., Dunn A.G., Tong H.L. Conversational agents in healthcare: a systematic review. J. Am. Med. Inform. Assoc. 2018;25(9):1248–1258. doi: 10.1093/jamia/ocy072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.M., Jung Y., Kim J. Are physically embodied social agents better than disembodied social agents?: the effects of physical embodiment, tactile interaction, and people’s loneliness in human–robot interaction. International Journal of Human - Computer Studies. 2006;64(10):962–973. doi: 10.1016/j.ijhcs.2006.05.002. [DOI] [Google Scholar]
- Li J. The benefit of being physically present: a survey of experimental works comparing copresent robots, telepresent robots and virtual agents. International Journal of Human-Computer Studies. 2015;77:23–37. doi: 10.1016/j.ijhcs.2015.01.001. [DOI] [Google Scholar]
- Loewenstein G., Thaler R.H. Anomalies: intertemporal choice. J. Econ. Perspect. 1989;3(4):181–193. doi: 10.1257/jep.3.4.181. [DOI] [Google Scholar]
- Looije R., Neerincx M.A., Cnossen F. Persuasive robotic assistant for health self-management of older adults: design and evaluation of social behaviors. International Journal of Human-Computer Studies. 2010;68(6):386–397. doi: 10.1016/j.ijhcs.2009.08.007. [DOI] [Google Scholar]
- Looije R. 2012 IEEE RO-MAN: The 21st IEEE International Symposium on Robot and Human Interactive Communication. 2012. Help, I need some body the effect of embodiment on playful learning. (9-13 Sept. 2012) [Google Scholar]
- Lundahl B.W., Kunz C., Brownell C. A meta-analysis of motivational interviewing: twenty-five years of empirical studies. Res. Soc. Work. Pract. 2010;20(2):137–160. doi: 10.1177/1049731509347850. [DOI] [Google Scholar]
- Malik V.S., Popkin B.M., Bray G.A. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care. 2010;33(11):2477–2483. doi: 10.1016/j.jsat.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Miranda J. Embodied conversational agents for the detection and prevention of suicidal behaviour: current applications and open challenges. J. Med. Syst. 2017;41(9):135. doi: 10.1007/s10916-017-0784-6. [DOI] [PubMed] [Google Scholar]
- Martins R.K., McNeil D.W. Review of motivational interviewing in promoting health behaviors. Clin. Psychol. Rev. 2009;29(4):283–293. doi: 10.1016/j.cpr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- May J., Andrade J., Kavanagh D. Imagery and strength of craving for eating, drinking, and playing sport. Cognit. Emot. 2008;22(4):633–650. doi: 10.1080/02699930701446296. [DOI] [Google Scholar]
- May J., Andrade J., Panabokke N. Visuospatial tasks suppress craving for cigarettes. Behav. Res. Ther. 2010;48(6):476–485. doi: 10.1016/j.brat.2010.02.001. [DOI] [PubMed] [Google Scholar]
- May J., Andrade J., Kavanagh D.J. The craving experience questionnaire: a brief, theory-based measure of consummatory desire and craving. Addiction. 2014;109(5):728–735. doi: 10.1111/add.12472. [DOI] [PubMed] [Google Scholar]
- May J., Andrade J., Kavanagh D.J. An imagery-based road map to tackle maladaptive motivation in clinical disorders. Frontiers in Psychiatry. 2015;6:14. doi: 10.3389/fpsyt.2015.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May J., Kavanagh D.J., Andrade J. The elaborated intrusion theory of desire: a 10-year retrospective and implications for addiction treatments. Addict. Behav. 2015;44(C):29–34. doi: 10.1016/j.addbeh.2014.09.016. [DOI] [PubMed] [Google Scholar]
- Miller W.R., Rollnick S. Guilford Press; New York: 2013. Motivational Interviewing: Helping People Change. [Google Scholar]
- Moerman C.J., van der Heide L., Heerink M. Social robots to support children’s well-being under medical treatment: a systematic state-of-the-art review. Journal of Child Health Care. 2018 doi: 10.1177/1367493518803031. [DOI] [PubMed] [Google Scholar]
- Morton K., Beauchamp M., Prothero A. The effectiveness of motivational interviewing for health behaviour change in primary care settings: a systematic review. Health Psychol. Rev. 2015;9(2):205–223. doi: 10.1080/17437199.2014.882006. [DOI] [PubMed] [Google Scholar]
- Moyle W., Jones C.J., Murfield J.E. Use of a robotic seal as a therapeutic tool to improve dementia symptoms: a cluster-randomized controlled trial. J. Am. Med. Dir. Assoc. 2017;18(9):766–773. doi: 10.1016/j.jamda.2017.03.018. [DOI] [PubMed] [Google Scholar]
- Moyle W., Bramble M., Jones C. Care staff perceptions of a social robot called Paro and a look-alike Plush Toy: a descriptive qualitative approach. Aging Ment. Health. 2018;22(3):330–335. doi: 10.1080/13607863.2016.1262820. [DOI] [PubMed] [Google Scholar]
- Mulder R., Murray G., Rucklidge J. Common versus specific factors in psychotherapy: opening the black box. Lancet Psychiatry. 2017;4(12):953–962. doi: 10.1016/S2215-0366(17)30100-1. [DOI] [PubMed] [Google Scholar]
- National Health and Medical Research Council . 2013. Australian Dietary Guidelines. [Google Scholar]
- Oosterveen E., Tzelepis F., Ashton L. A systematic review of eHealth behavioral interventions targeting smoking, nutrition, alcohol, physical activity and/or obesity for young adults. Prev. Med. 2017;99:197–206. doi: 10.1016/j.ypmed.2017.01.009. [DOI] [PubMed] [Google Scholar]
- Parham S.C., Kavanagh D.J., Gericke C.A. Assessment of motivational cognitions in diabetes self-care: the motivation thought frequency scales for glucose testing, physical activity and healthy eating. International Journal of Behavioral Medicine. 2017;24(3):447. doi: 10.1007/s12529-016-9607-2. [DOI] [PubMed] [Google Scholar]
- Pearson J., Kosslyn S. Mental imagery. Front. Psychol. 2013;4:198. doi: 10.3389/fpsyg.2013.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J., Naselaris T., Holmes E.A. Mental imagery: functional mechanisms and clinical applications. Trends Cogn. Sci. 2015;19(10):590–602. doi: 10.1016/j.tics.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi P., Tonacci A., Tartarisco G. Autism and social robotics: a systematic review. Autism research : official journal of the International Society for Autism Research. 2016;9(2):165–183. doi: 10.1002/aur.1527. (published online first: 2015/10/21) [DOI] [PubMed] [Google Scholar]
- Pereira A., Martinho C., Leite I. 7th International Joint Conference on Autonomous Agents and Multiagent Systems Estoril. International Foundation for Autonomous Agents and Multiagent Systems; Portugal: 2008. iCat, the chess player: the influence of embodiment in the enjoyment of a game; pp. 1253–1256. [Google Scholar]
- Pop C.A., Simut R.E., Pintea S. Social robots vs. computer display: does the way social stories are delivered make a difference for their effectiveness on ASD children? J. Educ. Comput. Res. 2013;49(3):381–401. doi: 10.2190/EC.49.3.f. [DOI] [Google Scholar]
- Powers A., Kiesler S. Proceedings of the 1st ACM SIGCHI/SIGART Conference on Human-Robot Interaction. ACM; Salt Lake City, Utah, United States of America: 2006. The advisor robot: tracing people’s mental model from a robot’s physical attributes; pp. 218–225. [Google Scholar]
- Provoost S., Lau H.M., Ruwaard J. Embodied conversational agents in clinical psychology: a scoping review. J. Med. Internet Res. 2017;19(5):e151. doi: 10.2196/jmir.6553. (published online first: 09.05.2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves B., Wise K., Maldonado H. CHI 2003. 2003. Robots versus on-screen agents: effects on social and emotional responses. [Google Scholar]
- Robinson N., Kavanagh D., Connor J. Assessment of motivation to control alcohol use: the motivational thought frequency and state motivation scales for alcohol control. Addict. Behav. 2016;59:1–6. doi: 10.1016/j.addbeh.2016.02.038. [DOI] [PubMed] [Google Scholar]
- Robinson N., Cottier T., Kavanagh D. Psychosocial health interventions by social robots: systematic review of randomized controlled trials. J. Med. Internet Res. 2019;21(5) doi: 10.2196/13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunderson S., Nejat G. How robots influence humans: a survey of nonverbal communication in social human-robot interaction. Int. J. Soc. Robot. 2019;11(4):575–608. doi: 10.1007/s12369-019-00523-0. [DOI] [Google Scholar]
- Scassellati B., Admoni H., Matari M. Robots for use in autism research. Annu. Rev. Biomed. Eng. 2012;14(1):275–294. doi: 10.1146/annurev-bioeng-071811-150036. [DOI] [PubMed] [Google Scholar]
- Schrum M., Park C.H., Howard A. 2019 14th ACM/IEEE International Conference on Human-Robot Interaction (HRI) 2019. Humanoid therapy robot for encouraging exercise in dementia patients. (11-14 March 2019) [Google Scholar]
- Seo S.H., Geiskkovitch D., Nakane M. Proceedings of the Tenth Annual ACM/IEEE International Conference on Human-Robot Interaction. ACM; Portland, Oregon, USA: 2015. Poor thing! Would you feel sorry for a simulated robot?: A comparison of empathy toward a physical and a simulated robot; pp. 125–132. [Google Scholar]
- Shim J.-S., Oh K., Kim H.C. Dietary assessment methods in epidemiologic studies. Epidemiol Health. 2014;36 doi: 10.4178/epih/e2014009. e2014009-e09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozawa K., Naya F., Yamato J. Differences in effect of robot and screen agent recommendations on human decision-making. International Journal of Human - Computer Studies. 2005;62(2):267–279. doi: 10.1016/j.ijhcs.2004.11.003. [DOI] [Google Scholar]
- Skorka-Brown J., Andrade J., Whalley B. Playing Tetris decreases drug and other cravings in real world settings. Addict. Behav. 2015;51:165–170. doi: 10.1016/j.addbeh.2015.07.020. [DOI] [PubMed] [Google Scholar]
- So W.C., Wong M.K., Lam C.K. Using a social robot to teach gestural recognition and production in children with autism spectrum disorders. Disability and rehabilitation Assistive technology. 2017:1–13. doi: 10.1080/17483107.2017.1344886. (published online first: 2017/07/05) [DOI] [PubMed] [Google Scholar]
- SoftBank Robotics . 2019. Find out More about NAO. [Google Scholar]
- Solbrig L., Whalley B., Kavanagh D.J. Functional imagery training versus motivational interviewing for weight loss: a randomised controlled trial of brief individual interventions for overweight and obesity. Int. J. Obes. 2019;43(4):883. doi: 10.1038/s41366-018-0122-1. (2005) [DOI] [PubMed] [Google Scholar]
- Trost M.J., Ford A.R., Kysh L. Socially assistive robots for helping pediatric distress and pain: a review of current evidence and recommendations for future research and practice. Clin. J. Pain. 2019;35(5):451–458. doi: 10.1097/ajp.0000000000000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyam A.N., Wisniewski H., Halamka J.D. Chatbots and conversational agents in mental health: a review of the psychiatric landscape. Can. J. Psychiatry. 2019;64(7):456–464. doi: 10.1177/0706743719828977. (published online first: 2019/03/23) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainer J., Feil-Seifer D.J., Shell D.A. Embodiment and human-robot interaction: a task-based perspective. IEEE. 2007:872–877. [Google Scholar]
- Wang B., P-LP Rau. Influence of embodiment and substrate of social robots on users’ decision-making and attitude. Int. J. Soc. Robot. 2019;11(3):411–421. doi: 10.1007/s12369-018-0510-7. [DOI] [Google Scholar]
- Woodward-Lopez G., Kao J., Ritchie L. To what extent have sweetened beverages contributed to the obesity epidemic? Public Health Nutr. 2011;14(3):499–509. doi: 10.1017/S1368980010002375. [DOI] [PubMed] [Google Scholar]
- World Health Organization . 2015. Guideline: Sugars Intake for Adults and Children. [PubMed] [Google Scholar]
- World Health Organization . 2017. Noncommunicable diseases. [Google Scholar]