Abstract
Background
Vaccine wastage is one of quality indicators of immunization program and high vaccine wastage will increase overall costs and impede efforts towards a more efficient and sustainable program. We aimed at estimating of the wastage rates of Measles-Mumps-Rubella (MMR) and pentavalent (diphtheria-tetanus-pertussis-hepatitis B -Haemophilus influenza type b) vaccines in different vaccine vial sizes.
Study design
Multicentre descriptive study using existing data.
Methods
This study was in three provinces (Hamadan, Kermanshah and Kordestan) of Iran including 131,135 populations with 2,548 under-1years children. Twenty-seven health facilities were selected randomly from nine districts in three provinces of western part of Iran. Six-months data including vaccination and vaccine stock records collected from April to September 2017. Finally, number of opened vials and number of target population vaccinated were collected and data were analysed to estimate the wastage rates in both unopened and opened vials of both antigens.
Results
The wastage rate for combined MMR 2-dose and 5-dose opened vials for three provinces was 29%(Hamadan 18%, Kermanshah 14% and Kordestan 52%). The wastage rate for combined pentavalent single-dose and 10-dose vials for three provinces was 17% (in Kordestan33%, 11% Kermanshah 11% and Hamedan 3%). The total average of pentavalent single-dose and 10-dose vials wastage rate was 5% and varied 13% for urban and 3% for rural areas. The average of discarded unopened vials wastage rate in all facilities for MMR was 3.9% (3.2% for MMR 2-dose vial and 10.2% for MMR 5-dose vial). This rate was 1.7% for pentavalent total (1.9% for single dose vial and 0.4% for 10 dose vial).
Conclusion
The vaccine wastage rates in Iran are in line with other countries and lower than the suggested rate based on WHO policies for multi-dose vials. The wastage rates were different for in provinces, districts and health facilities. The MMR total wastage rate in rural is higher than those in urban areas. However, the pentavalent total wastage rate was higher in urban area.
Keywords: Public health, Wastage rate, Pentavalent, Vaccine, MMR, Iran, Vaccination
Public Health; Wastage rate; Pentavalent; Vaccine; MMR; Iran; Vaccination.
1. Introduction
The vaccine wastage rate is one of the critical quality indicators of an immunization program since vaccines represent a significant proportion of the program costs and defined as the proportion of doses of opened or unopened vaccine vials are not used for vaccination [1, 2, 3]. Based on the World Health Organization (WHO) reports the vaccine wastage around the world is over 50% [4]. The introduction of new and more expensive vaccines brings additional complexity to vaccine wastage control. In one hand, a high vaccine wastage will increase overall costs and impede efforts towards a more efficient and sustainable program [5, 6]. In the other hand, the fear of increasing wastage by opening multi-dose vials potentially limits access to vaccination services and contributes to low coverage and/or late protection of children by national immunization programs [7].
There are two types of vaccine wastage including opened and unopened or closed vial wastages [1, 7]. The opened vial wastage is associated with the use of multi-dose vials for vaccination. Unopened or closed vial wastage is related to gaps in stock inventory control and poor vaccine management practices [8, 9]. Every effort must be made to avoid wastage in unopened vials. The successful implementation of effective vaccine management practices will maintain low wastage rates of unopened vials [1, 2]. In contrast, opened vial wastage is unavoidable. Doses discarded with multi-dose vials may be high depending on the vial size, the compliance with multi-dose vial policy (MDVP), and the size and frequency of vaccination sessions [8]. Undue pressure on health workers to reduce wastage may push them to abstain from opening multi-dose vials when the number of children is insufficient. This can lead to a low coverage and must be avoided [3, 8].
The Government of Islamic Republic of Iran is investing great efforts for sustaining the high performance of the national immunization program [10, 11] and administrating new vaccines such as pentavalent (including diphtheria-tetanus-pertussis-hepatitis B-Haemophilus influenza type b) [12]. pentavalent vaccine (adsorbed) as a part of the national paediatric vaccination schedule in Iran started from 2014 and administrated for three time in under 1 years old children at 2, 4 and 6 months after birth [12, 13]. Measles-Mumps-Rubella (MMR) vaccination in Iran started at 2004 and MMR vaccine scheduled two times in children. The first dose administrating at 12th and second dose at 18th month after birth [14].
In Iran where all costs for vaccination are covered by the government alone, that high wastage increases vaccine demand and inflates vaccine procurement and supply costs [10, 15]. Reduction of vaccine wastage is a priority of the Ministry of Health and Medical Education. A special focus is made on MMR vaccine, as the country is moving towards disease elimination [16, 17]. Currently, by using a mix of 2 and 5-dose vials of MMR vaccines, a high and on-time immunization coverage is acquired in Iran [11, 18]. However, the decision to open the relevant vaccine vial size at each service delivery point for each session remains at the intuitive decision of individual healthcare workers [5, 6, 9]. Overall reported wastage rates remain high globally. In 1992, WHO estimated that the amount of vaccine wasted (60%) accounted for more vaccine than was administered. In 1994, after switching to smaller multi-dose vials, vaccine wastage rates in some areas were reduced to 45% of vaccine demand [19]. Selection of the appropriate vial size is a complex decision that should be supported by an evidence-based rational by a cost-effective approach to minimize the cost of vaccination [20].
To now, we not found an estimate from vaccine wastage in Iran based a national survey. The current study aimed to provide an estimation of vaccine wastage rate, type and place of occurrence for Measles-Mumps-Rubella (MMR) and pentavalent vaccines.
2. Materials and methods
This multicentre descriptive study using existing data was conducted in 27 health facilities that selected randomly in nine districts of three western provinces that including 131,135 populations (2,548 were under-1 age, 1.94%). The setting provinces were Hamedan including 40,319 people (830 were under-1 age, 2.06%), Kermanshah including 49,724 people (926 were under-1 age, 1.86%) and Kordestan including 41,092 people (792 were under-1 age, 1.93%). Three studied provinces have same socioeconomic status at west of Iran. The maximum, average and minimum of the target population (under-1 age group) in all 27 facilities included in the study were 202, 47 and 2, respectively.
There are 9 regional vaccine stores in Iran. One of the nine was selected randomly which includes 3 provinces named Kordestan, Kermanshah and Hamadan in western part of the country. Then three districts were selected from each of the provinces randomly. Two rural health facilities and one urban health post were again randomly selected from the list of all the existing health facilities in the selected districts. Therefore, the total of selected 27 health facilities in three provinces are shown in Table 1 and the target population of each facility considered per annum.
Table 1.
The catchment area of the selected facilities and codes in Hamadan, Kermanshah and Kordestan provinces and codes of each districts.
| Province | district | Type of facility | Name of Health Facility | Health Facility code | |
|---|---|---|---|---|---|
| 1 | Kordestan | Divandarreh | Health Post | Qods1 | 331 |
| 2 | Kordestan | Divandarreh | Health house 2 | Ghaleh kohne | 332 |
| 3 | Kordestan | Divandarreh | Health house 1 | Papaleh | 333 |
| 4 | Kordestan | Sanandaj | Health Post | Asaveleh | 312 |
| 5 | Kordestan | Sanandaj | Health house 1 | Barghru | 311 |
| 6 | Kordestan | Sanandaj | Health house 2 | Avihang | 313 |
| 7 | Kordestan | Baneh | Health Post | Health Facility No 4 | 321 |
| 8 | Kordestan | Baneh | Health house 1 | Aloot | 322 |
| 9 | Kordestan | Baneh | Health house 2 | Bardeh rash | 323 |
| 10 | Kermanshah | Eslamabad gharb | Health house 2 | Bagher abad | 232 |
| 11 | Kermanshah | Eslamabad gharb | Health house 1 | Shiyan | 231 |
| 12 | Kermanshah | Eslamabad gharb | Health Post | Health Facility No 2 | 233 |
| 13 | Kermanshah | Salas Babajani | Health Post | Nazeh abad | 221 |
| 14 | Kermanshah | Salas Babajani | Health house 1 | Ziarat Tamrkhan | 222 |
| 15 | Kermanshah | Salas Babajani | Health house 2 | Pookehabbas | 223 |
| 16 | Kermanshah | Kermanshah | Health house 1 | Hojoom abad | 211 |
| 17 | Kermanshah | Kermanshah | Health house 2 | Zameleh | 212 |
| 18 | Kermanshah | Kermanshah | Health Post | Zeynabiyeh | 213 |
| 19 | Hamedan | Hamedan | Health house 1 | Varkaneh | 111 |
| 20 | Hamedan | Hamedan | Health house 2 | Khakoo | 113 |
| 21 | Hamedan | Hamedan | Health Post | Chamran | 112 |
| 22 | Hamedan | Toyserkan | Health house 2 | Hoosh | 132 |
| 23 | Hamedan | Toyserkan | Health house 1 | Ghelghel | 131 |
| 24 | Hamedan | Toyserkan | Health Post | Ghaem | 133 |
| 25 | Hamedan | Bahar | Health house 2 | Mihmaleh Olaya | 123 |
| 26 | Hamedan | Bahar | Health house 1 | Dahangerd | 121 |
| 27 | Hamedan | Bahar | Health Post | Bahar 2 | 122 |
The lasted 6 months and consisted of retrospective data collection conducted and analysis of vaccine utilization and wastage data was done at selected sites. This study covers data from April to September 2017. Data on number and frequency of vaccination sessions collected and data were analysed to map the number of doses administered per session.
2.1. Data collection
The vaccine stock and vaccination records were collected for each district and used for analysis. Vaccine stock records was the source in all vaccine stores and health facilities included in the assessment. The wide implementation of Web-based Vaccination Supplies Stock Management (wVSSM) facilitated the collection of stock data. This tool approved by WHO and UNICEEF for vaccine stock management. Different data were collected and used to estimate wastage occurring in unopened vials during the storage including stock at the beginning and the end of month, number of vaccine doses received per month throughout the project period, number of vaccine doses discarded due to expiry during the month, number of vaccine doses discarded due to reaching stages 3 and 4 of vaccine vial monitor (VVM) during the month, number of vaccine doses discarded due to exposure to freezing temperatures (only for pentavalent) during the month and number of vaccine doses discarded due to any other reason different from those listed above during the month.
Vaccination records were collected data at the service delivery level. At the health facilities the vaccination records were collected using documents/forms available (daily vaccination tally sheets, vaccination registers and any other document available with the relevant information). Therefore, data of vaccination records including date and type of vaccination session conducted (fixed, outreach, mobile), number of children vaccinated per vaccination session and number of vaccine vials opened for use, were collected.
2.2. Data analysis
The vaccine utilization and wastage indicators were calculated including 1. Cumulative wastage rates in opened vials that were calculated for each service point using the total number of doses of vials opened and the total vaccinations given. 2. Wastage rates in unopened vials that was calculated for each storage point as the proportion of total doses discarded in closed vials over the total doses handled. 3. The weighted average wastage for urban versus health facilities.
Data collected were analysed to provide an estimation of frequency and number of children vaccinated in vaccination sessions conducted per service point, number of vaccine doses used versus vaccine doses administered, number of vaccine doses received and wasted in unopened vials during storage and handling, and vaccine wastage rates, both in unopened vials and in opened vials. Vaccine wastage was defined as 1 minus vaccine usage and the vaccine usage can be defined as the proportion of vaccine issued (including doses used for immunization and all doses discarded or lost for any reason such as expiry, VVM indication, cold chain failure, freezing, missing inventory or routine discard of open vials of vaccine at the end of a session) which is administered [4]. Therefore, descriptive statistics including mean and percent were used. Moreover, to compare between provinces, districts and facilities, chi square test was used. The formula of vaccine usage and vaccine wastage rate is as bellow [4].
| Vaccine wastage rate = 100 – vaccine usage rate |
3. Results
3.1. MMR vaccine wastage
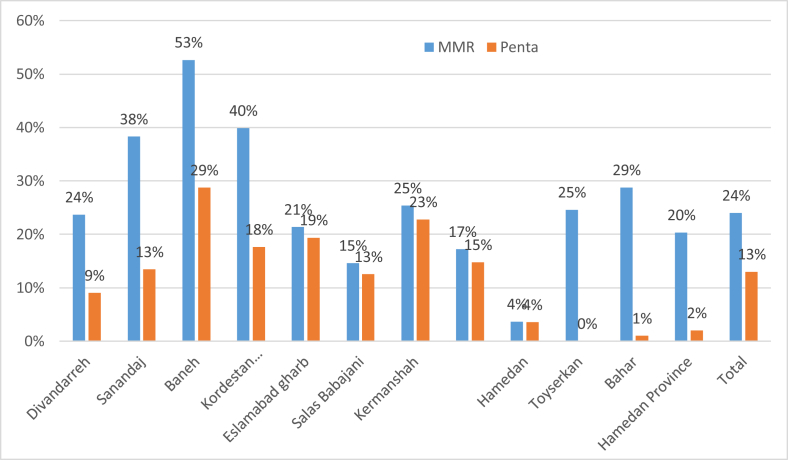
The six months' wastage rate for combined MMR 2-dose and 5-dose opened vials for three studied provinces was 29% (CI 95%; 28%,30%), Hamadan 18%(CI 95%; 17.9%,18.1%), Kermanshah 14%(CI 95%; 13.9%,14.1%), and Kordestan 52% (CI 95%; 51.9%,52.1%). MMR is used in 2-dose and 5-dose vials. Figure 1 shows the opened vial wastage rates in urban and rural facilities. and Table 2 shows the total wastage rate of opened MMR 2-dose and 5-dose vials for three provinces and nine districts included in the study for the period of six months. The more detail about the MMR wastage rate by facilities is provided in Table 2.
Figure 1.
The Opened vial wastage rates of MMR and Pentavalent in nine districts and based on each providence.
Table 2.
Opened vial wastage rates of MMR and Pentavalent in comparison between rural and urban selected facilities.
| Provinces | Districts | Facility codes | Total wastage |
Pentavalent |
MMR |
|||
|---|---|---|---|---|---|---|---|---|
| Pentavalent | MMR | Urban | Rural | Urban | Rural | |||
| Kordestan | Divandarreh | 331 | 10% | 22% | 10% | 22% | ||
| Kordestan | Divandarreh | 332 | 0% | 71% | 0% | 71% | ||
| Kordestan | Divandarreh | 333 | 0% | 18% | 0% | 18% | ||
| Divandarreh District | 9% | 24% | 10% | 13 | 22% | 41% | ||
| Kordestan | Sanandaj | 311 | 7% | 43% | 7% | 43% | ||
| Kordestan | Sanandaj | 313 | 0% | 37% | 0% | 37% | ||
| Kordestan | Sanandaj | 312 | 15% | 38% | 15% | 38% | ||
| Sanandaj District | 13% | 38% | 15% | 18 | 38% | 40% | ||
| Kordestan | Baneh | 321 | 30% | 53% | 30% | 53% | ||
| Kordestan | Baneh | 322 | 12% | 43% | 12% | 43% | ||
| Kordestan | Baneh | 323 | 0% | 45% | 0% | 45% | ||
| Baneh District | 29% | 53% | 30% | 6% | 53% | 44% | ||
| Kordestan Province | 18% | 40% | 19% | 2% | 40% | 41% | ||
| Kermanshah | Eslamabad gharb | 232 | 2% | 5% | 2% | 5% | ||
| Kermanshah | Eslamabad gharb | 231 | 0% | 7% | 0% | 7% | ||
| Kermanshah | Eslamabad gharb | 233 | 23% | 24% | 23% | 24% | ||
| Eslamabad gharb District | 19% | 21% | 23% | 1% | 24% | 6% | ||
| Kermanshah | Salas Babajani | 221 | 13% | 15% | 13% | 15% | ||
| Kermanshah | Salas Babajani | 222 | 3% | 3% | 3% | 3% | ||
| Kermanshah | Salas Babajani | 223 | 0% | 17% | 0% | 17% | ||
| Salas Babajani District | 13% | 15% | 13% | 2% | 15% | 4% | ||
| Kermanshah | Kermanshah | 211 | 20% | 43% | 20% | 43% | ||
| Kermanshah | Kermanshah | 212 | 0% | 50% | 0% | 50% | ||
| Kermanshah | Kermanshah | 213 | 23% | 24% | 23% | 24% | ||
| Kermanshah District | 23% | 25% | 23% | 15% | 24% | 46% | ||
| Kermanshah Province | 15% | 17% | 15% | 3% | 17% | 13% | ||
| Hamedan | Hamedan | 111 | 0% | 17% | 6% | 17% | ||
| Hamedan | Hamedan | 113 | 0% | 25% | 0% | 25% | ||
| Hamedan | Hamedan | 112 | 4% | 2% | 5% | 2% | ||
| Hamedan District | 4% | 4% | 5% | 4% | 2% | 19% | ||
| Hamedan | Toyserkan | 132 | 0% | 44% | 0% | 44% | ||
| Hamedan | Toyserkan | 131 | 0% | 21% | 0% | 21% | ||
| Hamedan | Toyserkan | 133 | 0% | 24% | 0% | 24% | ||
| Toyserkan District | 0% | 25% | 0% | 0% | 24% | 28% | ||
| Hamedan | Bahar | 123 | 0% | 50% | 0% | 50% | ||
| Hamedan | Bahar | 121 | 0% | 21% | 0% | 21% | ||
| Hamedan | Bahar | 122 | 1% | 26% | 1% | 26% | ||
| Bahar District | 1% | 29% | 1% | 0% | 26% | 44% | ||
| Hamedan Province | 2% | 20% | 2% | 1% | 18% | 34% | ||
| Total | 13% | 24% | 14% | 2% | 25% | 31% | ||
Table 2 showed that the maximum wastage rate for total MMR (2-dose and 5-dose vials) is 53% and the minimum rate is 0% for the facilities in the study the study facilities regardless of type and whether they are in urban or rural areas. Table 2 showed that the wastage rate of Pentavalent and MMR was 24% and 13% respectively. It is also shows the difference between MMR wastage and pentavalent wastage. It can be seen that only in two facilities pentavalent wastage is slightly more than MMR wastage. In only two facilities (codes 111 and 112 and district 11) the wastage rates of MMR are slightly less than wastage rates of pentavalent.
The average of MMR 2-dose and 5-dose vials wastage rates by urban and rural areas in this study was 26% (23% in urban and 29% in rural areas). The MMR total wastage rate in rural facilities is higher than those in urban areas (28.6% versus 21.2%).
The average of discarded unopened vials wastage rate in all facilities for MMR total was 3.9% (3.2% for MR 2-dose vial and 10.2% for MMR 5-dose vial). The total MMR (2-dose and 5-dose vials) wastage rate in relation to the size of the target population showed that there is no clear or systematic correlation between the target population and MMR total wastage rates (Table 3). Table 3 showed the detail of all data collected for MMR 2-dose and 5-dose vials in terms of wastage rate, areas (urban versus rural), session size for each individual facility, separated by district and province. These results, only two facilities had only MMR 5-dose vials whereas 17 facilities had only MMR 2-dose vial and the rest (eight facilities) had both MMR 2-dose and 5-dose vials. However, the MOH recommends to seize any opportunity for vaccination of children in order to maintain a high coverage and little attention is made to vaccine wastage rate.
Table 3.
Summary of MMR wastage by facility (opened vial), vial size, number of sessions per month and based on urban versus rural areas.
| Districts | Facility codes | Target Population(children) | MMR wastage rate (%) | Number of MMR Vaccination sessions per month | Average number of MMR Vaccination per sessions | Combination of 2-dose and 5-dose vials∗ |
|---|---|---|---|---|---|---|
| Divandarreh | 331 | 174 | 22 | 24 | 3.19 | 1.42 |
| Divandarreh | 332 | 6 | 50 | 24 | 0.03 | 0.42 |
| Divandarreh | 333 | 11 | 18 | 2 | 1.50 | Only 2 dose vials |
| Sanandaj | 311 | 7 | 43 | 4 | 1.04 | Only 2 dose vials |
| Sanandaj | 313 | 10 | 37 | 4 | 1.09 | Only 2 dose vials |
| Sanandaj | 312 | 57 | 23 | 4 | 5.42 | 0.76 |
| Baneh | 321 | 140 | 53 | 8 | 0.00 | Only 5 dose vials |
| Baneh | 322 | 6 | 33 | 4 | 0.36 | Only 2 dose vials |
| Baneh | 323 | 6 | 45 | 4 | 0.46 | Only 2 dose vials |
| Eslamabad gharb | 232 | 13 | 5 | 4 | 0.88 | Only 2 dose vials |
| Eslamabad gharb | 231 | 14 | 7 | 4 | 1.63 | Only 2 dose vials |
| Eslamabad gharb | 233 | 123 | 24 | 4 | 11 | 18.33 |
| Salas Babajani | 221 | 202 | 0 | 8 | 9.81 | 2.1 |
| Salas Babajani | 222 | 15 | 3 | 24 | 0.27 | Only 2 dose vials |
| Salas Babajani | 223 | 3 | 17 | 24 | 0.03 | Only 2 dose vials |
| Kermanshah | 211 | 4 | 33 | 24 | 0.06 | Only 2 dose vials |
| Kermanshah | 212 | 2 | 50 | 24 | 0.03 | Only 2 dose vials |
| Kermanshah | 213 | 90 | 24 | 8 | 0.0 | Only 5 dose vials |
| Hamedan | 111 | 6 | 17 | 24 | .010 | Only 2 dose vials |
| Hamedan | 113 | 4 | 25 | 24 | 0.04 | Only 2 dose vials |
| Hamedan | 112 | 124 | 2 | 24 | 1.87 | 0.96 |
| Toyserkan | 132 | 5 | 44 | 24 | 0.06 | Only 2 dose vials |
| Toyserkan | 131 | 11 | 21 | 24 | 0.23 | Only 2 dose vials |
| Toyserkan | 133 | 82 | 15 | 12 | 2.57 | 5.39 |
| Bahar | 123 | 20 | 50 | 24 | 0.19 | Only 2 dose vials |
| Bahar | 121 | 5 | 21 | 24 | 0.08 | Only 2 dose vials |
| Bahar | 122 | 142 | 26 | 24 | 1.94 | 1.48 |
∗Digits in last column indicate the ratio of the total number of doses in 5-dose vials to total number of doses in 2-dose vials. For instance, on the first row 1.42 means that there were 230 MMR doses in 5-dose vials and 162 MMR doses in 2-dose vials.
3.2. Pentavalent vaccine wastage
The six months' retrospective total wastage rate for combined pentavalent single-dose and 10-dose vials was 17% (CI 95%; 16.5%,17.5%) for three provinces. This rate was 33%(CI 95%; 32.9%,33.1%) in Kordestan, 11%(CI 95%; 10.9%,11.1%) in Kermanshah and 3%(CI 95%; 2.9%,3.1%) in Hamedan. The maximum wastage was in Baneh 29% and Kermanshah 22% and the minimum in Salas e babajani 0%. In addition, the total average of pentavalent single-dose and 10-dose vials wastage rate was 5% and varied 13% for urban and 3% for rural areas. The average of discarded unopened vials wastage rate in all facilities for pentavalent total was 1.7% including 1.9% for single dose vial and 0.4% for 10 dose vial.
Table 4 shows the detail of all data collected for pentavalent single-dose and 10-dose vials in terms of wastage rate, areas (urban versus rural), session size for each individual facility, combined for district and province. As it can be seen from Table 4 that two facilities had both pentavalent single-dose vials and 10-dose vials whereas 17 facilities had only pentavalent single-dose vials and the rest (six facilities) had both only pentavalent 10-dose vials.
Table 4.
Summary of pentavalent wastage by facility, vial size, number of sessions per month and based on urban versus rural areas– opened vial.
| Districts | Facility codes | Target Population | Penta wastage rate (%) | Number of Penta Vaccination sessions per month | Average number of Penta Vaccination per sessions | Combination of single-dose and 10-dose vials∗ |
|---|---|---|---|---|---|---|
| Divandarreh | 331 | 174 | 10 | 24 | 3.85 | 5.68 |
| Divandarreh | 332 | 6 | 0 | 24 | 0.20 | 1.67 |
| Divandarreh | 333 | 11 | 0 | 24 | 0.28 | Only single dose vials |
| Sanandaj | 311 | 7 | 0 | 24 | 0.09 | Only single dose vials |
| Sanandaj | 313 | 10 | 0 | 24 | 0.17 | Only single dose vials |
| Sanandaj | 312 | 57 | 12 | 24 | 1.53 | 3.05 |
| Baneh | 321 | 140 | 30 | 24 | 0 | Only 10 dose vials |
| Baneh | 322 | 6 | 0 | 24 | 0.10 | Only single dose vials |
| Baneh | 323 | 6 | 0 | 24 | 0.12 | Only single dose vials |
| Eslamabad gharb | 232 | 13 | 2 | 24 | 0.35 | Only single dose vials |
| Eslamabad gharb | 231 | 14 | 0 | 24 | 0.29 | Only single dose vials |
| Eslamabad gharb | 233 | 123 | 2.3 | 24 | 2.80 | 73.33 |
| Salas Babajani | 221 | 202 | 0 | 24 | 5.38 | 8.42 |
| Salas Babajani | 222 | 15 | 0 | 24 | 0.27 | Only single dose vials |
| Salas Babajani | 223 | 3 | 0 | 24 | 0.06 | Only single dose vials |
| Kermanshah | 211 | 4 | 0 | 24 | 0.06 | Only single dose vials |
| Kermanshah | 212 | 2 | 0 | 24 | 0.02 | Only single dose vials |
| Kermanshah | 213 | 90 | 23 | 24 | 0 | Only 10 dose vials |
| Hamedan | 111 | 6 | 23 | 24 | 0.21 | Only single dose vials |
| Hamedan | 113 | 4 | 0 | 24 | 0.15 | Only single dose vials |
| Hamedan | 112 | 124 | 5 | 24 | 2.65 | 3.86 |
| Toyserkan | 132 | 5 | 0 | 24 | 0.15 | Only single dose vials |
| Toyserkan | 131 | 11 | 0 | 24 | 0.22 | Only single dose vials |
| Toyserkan | 133 | 82 | 3 | 24 | 1.49 | 21.58 |
| Bahar | 123 | 20 | 0 | 24 | 0.33 | Only single dose vials |
| Bahar | 121 | 5 | 0 | 24 | 0.10 | Only single dose vials |
| Bahar | 122 | 142 | 2 | 24 | 2.52 | 5.92 |
∗Digits in this column indicate the ratio of the total number of doses in 10-dose vials to total number of doses in single-dose vials. For instance, on the first row 2.14 means that there were 420 pentavalent doses in 10-dose vials and 162 pentavalent doses in single-dose vials. The digits below one, means that number of doses in single-dose vial was more than number of doses in 10-dose vials. These figures are one of the important parameters used in the WHO model.
Our results showed that the total pentavalent wastage (single-dose and 10-dose vials) versus target population. It showed that rural health facilities have had the least wastage rate because they were received just single dose vials (Table 4). This pattern is not observed for MMR (Table 3).
Reasons for discarding unopened vials in all three provinces were 44 doses VVM at stages 3 and 4 (22 vials) 2 doses (1 vials) lost and 2 (1 vials) doses brooked for MMR 2-dose vial. Only 5 doses (1 Vial) lost in MMR 5-dose vial and 10 doses (1 vial) of pentavalent 10-dose vial lost. In pentavalent single dose vial also 3 doses (3 vials) discarded due to VVM at stages 3 and 4, 1 dose (1 vial) due to lost and 2 doses (2 vials) brooked.
4. Discussion
The average wastage rate for MMR opened vials in this study estimated to be 29%. The highest rate was observed in Kordestan (52%), and was 14% in Kermanshah, while this rate estimated 18% for Hamadan in 2-dose and 5-dose vials. The Global Alliance for Vaccines and Immunizations (GAVI), recommended that the maximum wastage rate of vaccine should reduce to 25% at the first year and reduce it to 15% at the third year. Although, the maximum wastage allowance for single-dose or two-dose vaccines is 5% [4]. Based on our results, the vaccine wastage in Iran is over the GAVI guidelines. The mean wastage rates in facility-level of Cambodia varied from 4% for single-dose of pentavalent vaccine to 60% for 10-dose MCV [1]. Another study showed that the mean of vaccine wastage rates in Nigeria varied from 18% to 35% based on vaccine type [7]. The wastage rate for combined pentavalent single-dose and 10-dose vials was 17% for three studied provinces including 33% in Kordestan, 11% in Kermanshah and 3% in Hamedan for single and 10-doses vials of pentavalent. In Addition, the total average of pentavalent single-dose and 10-dose vials wastage rate was 5% and varied 13% for urban and 3% for rural areas. The GAVI/WHO recommended wastage rate for pentavalent is 5% [8] equal to our study. The average of discarded unopened vials wastage rate in all facilities for pentavalent total was 1.7% including 1.9% for single dose vial and 0.4% for 10 dose vial. However, the average of wastage rate in Iran for single dose and multi dose of Pentavalent is 2% and 10% respectively [21] but we did not find data about MMR wastage rate. Another study in Nigeria showed that the monthly wastage rate for all vaccines were estimated 25% [7]. The wastage vaccine rate was 35% for MMR and 31 for DPT vaccines in Nigeria according to the facility stock-based records wastage [7]. The wastage rate for measles and DPT also estimated in Surat (India) 28% and 16% respectively [22].
This study shows that the average wastage rate of MMR was 28.6% mainly due to very small target population. The MMR total wastage rate in rural facilities is higher than those in urban areas. In rural areas of Iran, only 2 dose MMR vials distributed in order to decrease wastage. However, the vaccine wastage is related to population size of districts. In the other hand higher wastage rate in urban areas is due to a reflection of more dosages of the trivalent vaccine than pentavalent vaccine being administered. Wallace et al study showed that the wastage rates are lower in large facilities than small facilities in some vaccines [1]. Another studies showed that similar results [22]. However, using single doses in rural area caused lower vaccine wastage and as our results the vaccine wastage is related to population size. The average number of target population in rural health facilities was 15 (277 children in 18 health facilities). Based on EPI schedule in Iran, every child receives 2 dose of MMR at 12 and 18 months of age. This means that on average less than three children were inoculated with MMR per month. Therefore, it would be unavoidable to have MMR wastage rate above 25% by use of 2-dose vials in rural areas. On the other hand, every urban health facility covers 251 of target population (2,264 children in 9 health facilities). Based on EPI schedule every urban health facility vaccinate 42 children per month. Therefore, by use of 5-dose vial MMR vaccine, it would be possible for them to have lower wastage rate if they managed their MMR vaccination sessions. In addition, some urban health facilities used both 2 and 5-dose vials of MMR e.g. Health facilities 221 and 112. These two facilities had the lowest wastage rate, 0% and 3%, respectively. The main reason is the difference between content of MMR and pentavalent vaccines. The reconstituted MMR vials (no preservative) should be discarded at the end of vaccination session while pentavalent multi-dose vials can be used for 28 days after the opening of the vial in compliance with multi-dose vial policy. Furthermore, distribution of single-dose vials of pentavalent versus 2-dose vials of MMR vaccine has been influenced in obtaining lower wastage rates of pentavalent in rural health facilities.
Based on the WHO about MDVP, the maximum recommended wastage rates for multi-dose vials vaccines with preserved lyophilized varied between 30 to 50% [8]. The estimated wastage of MMR and pentavalent were lower than the MDVP. However, the wastage rate of MMR should be reduced in Kermanshah and pentavalent in Kordestan to reached to WHO recommendations. The higher rate of vaccine wastage could be related to higher rural and nomads' population. In addition, as Karami et al study, the reporting of administrative vaccination data is adequate at all levels of the healthcare provider centers [23].
Our results showed that only two facilities had only MMR 5-dose vials whereas 17 facilities had only MMR 2-dose vial and the rest (eight facilities) had both MMR 2-dose and 5-dose vials. However, the MOH recommends to seize any opportunity for vaccination of children in order to maintain a high coverage and little attention is made to vaccine wastage rates [2, 24]. Our results showed that the wastage rate was higher in opened vials than closed vials and it is different among studied facilities. Other showed same results and this difference due to some factors including immersion of opened vials in water, uncertainty about the sterility of prior withdrawals, thermal handling, and poor vaccine administration practices [25, 26, 27].
The total MMR (2-dose and 5-dose vials) wastage rate in relation to the size of the target population showed that there is no clear or systematic correlation between the target population and MMR total wastage rates. It could be caused by the recommendations from the higher levels to seize any opportunity to vaccinate children and giving priority to vaccination coverage rather than to the wastage rate. Moreover, pentavalent vaccine in liquid form are used in single dose and 10-dose vials. Our results showed that rural health facilities have had the least wastage rate because they were received just single dose vials. This pattern is not observed for MMR. However, it is understanding that usage of single dose of pentavalent or 2 doses of MMR could yield the best results and lower vaccine wastage. But the cost effectiveness analysis should be considered. The cost of each dose of vaccine in MMR vials for single, 2 and 5 doses is near 2, 1.5 and 0.6 US $. This costs for one dose of pentavalent in single and 10 doses in Iran is estimated 2 and 1.2 US $. Based on a cost analysis, it is suggested that 2 doses vial of MMR and 10 doses of pentavalent are more effectiveness after considering the population size of each districts. These activities could reduce the cost of vaccinations [6, 7, 27, 28].
However, our finding is helpful as guide in implementation of future improvement activities including contribute to development of policies, strategies and standard operating procedures regarding vaccine procurement, organization and implementation of services and vaccine wastage control in MOH. Nevertheless, future studies suggested to estimate the national vaccine wastages for all vaccines in routine immunization program in Iran.
5. Conclusion
The vaccine wastage rate in Iran are equivalent to the other countries and lower than of maximum rate based on WHO multi-dose vial policy. The wastage rates varied in different provinces, districts and health facilities. The MMR total wastage rate in rural is higher than those in urban areas, but, the pentavalent total wastage rate was higher in urban area. The relation between the wastage rates for both vaccines is related to average number of vaccinations given during each session, number of vaccination sessions per month and the size of the target population plus some other factors looked at in this report.
Declarations
Author contribution statement
M. Zahraei: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
G. Zamani and A. Mohammadbeigi: Analyzed and interpreted the data.
A. Asgarian and S. Afrashteh: Analyzed and interpreted the data; Wrote the paper.
H. Gharibnavaz, S. Kone and M. Haghgou: Conceived and designed the experiments; Analyzed and interpreted the data.
Funding statement
This work was supported by the WHO (2017/758863-0 cod).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Wallace A.S., Krey K., Hustedt J., Burnett E., Choun N., Daniels D. Assessment of vaccine wastage rates, missed opportunities, and related knowledge, attitudes and practices during introduction of a second dose of measles-containing vaccine into Cambodia's national immunization program. Vaccine. 2018;36(30):4517–4524. doi: 10.1016/j.vaccine.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duttagupta C., Bhattacharyya D., Narayanan P., Pattanshetty S.M. Vaccine wastage at the level of service delivery: a cross-sectional study. Publ. Health. 2017;148:63–65. doi: 10.1016/j.puhe.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 3.Usuf E., Mackenzie G., Ceesay L., Sowe D., Kampmann B., Roca A. Vaccine wastage in the Gambia: a prospective observational study. BMC Publ. Health. 2018;18(1):864. doi: 10.1186/s12889-018-5762-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Organization W.H. World Health Organization; Geneva: 2005. Monitoring Vaccine Wastage at Country Level: Guidelines for Programme Managers. [Google Scholar]
- 5.Ebong C.E., Levy P. Impact of the introduction of new vaccines and vaccine wastage rate on the cost-effectiveness of routine EPI: lessons from a descriptive study in a Cameroonian health district. Cost effectiveness and resource allocation. C/E. 2011;9(1):9. doi: 10.1186/1478-7547-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee B.Y., Norman B.A., Assi T.M., Chen S.I., Bailey R.R., Rajgopal J. Single versus multi-dose vaccine vials: an economic computational model. Vaccine. 2010;28(32):5292–5300. doi: 10.1016/j.vaccine.2010.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallace A.S., Willis F., Nwaze E., Dieng B., Sipilanyambe N., Daniels D. Vaccine wastage in Nigeria: an assessment of wastage rates and related vaccinator knowledge, attitudes and practices. Vaccine. 2017;35(48 Pt B):6751–6758. doi: 10.1016/j.vaccine.2017.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization . 2000. WHO Policy Statement: the Use of Opened Multi-Dose Vials of Vaccine in Subsequent Immunization Sessions. [Google Scholar]
- 9.Assi T.M., Brown S.T., Djibo A., Norman B.A., Rajgopal J., Welling J.S. Impact of changing the measles vaccine vial size on Niger's vaccine supply chain: a computational model. BMC Publ. Health. 2011;11:425. doi: 10.1186/1471-2458-11-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehrdad R. Health system in Iran. JMAJ. 2009;52(1):69–73. [Google Scholar]
- 11.Zahraei S.M., Eshrati B., Gouya M.M. Is there still an immunity gap in high-level national immunization coverage, Iran? Arch. Iran. Med. 2014;17(10):698. [PubMed] [Google Scholar]
- 12.Karami M., Ameri P., Bathaei J., Berangi Z., Pashaei T., Zahiri A. Adverse events following immunization with pentavalent vaccine: experiences of newly introduced vaccine in Iran. BMC Immunol. 2017;18(1):42. doi: 10.1186/s12865-017-0226-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shamsizadeh A., Nikfar R., Makvandi M., Hakimzadeh M., Alisamir M., Ziaei T. Seroprevalence of measles, mumps and rubella Antibodies in 18 months and 6.5 years old children: 6 months after measles-mumps-rubella (MMR) vaccination. Jundishapur J. Microbiol. 2012;5(4):578–581. [Google Scholar]
- 14.Shafyi A., Mohammadi A. Measles vaccines in Iran: a 50-year review of vaccine development, production and effectiveness (1967 - 2017) Jundishapur J. Microbiol. 2018;11(5) [Google Scholar]
- 15.Moradi-Lakeh M., Esteghamati A. National Immunization Program in Iran: whys and why nots. Hum. Vaccines Immunother. 2013;9(1):112–114. doi: 10.4161/hv.22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohammadbeigi A., Zahraei S.M., Asgarian A., Afrashteh S., Mohammadsalehi N., Khazaei S. Estimation of measles risk using the world health organization measles programmatic risk assessment tool, Iran. Heliyon. 2018;4(11) doi: 10.1016/j.heliyon.2018.e00886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zahraei S.M., Mohammadbeigi A., Mohammadsalehi N., Sabouri A., Afrashteh S., Arsang Jang S. Monitoring of surveillance quality indicators of measles in Iranian districts: analysis of measles surveillance system 2014-2016. J. Res. Health Sci. 2018;18(3) [PMC free article] [PubMed] [Google Scholar]
- 18.Rejali M., Mohammadbeigi A., Mokhtari M., Zahraei S.M., Eshrati B. Timing and delay in children vaccination; evaluation of expanded program of immunization in outskirt of Iranian cities. J. Res. Health Sci. 2015;15(1):54–58. [PubMed] [Google Scholar]
- 19.Drain P.K., Nelson C.M., Lloyd J.S. Single-dose versus multi-dose vaccine vials for immunization programmes in developing countries. Bull. World Health Organ. 2003;81:726–731. [PMC free article] [PubMed] [Google Scholar]
- 20.Parmar D., Baruwa E.M., Zuber P., Kone S. Impact of wastage on single and multi-dose vaccine vials: implications for introducing pneumococcal vaccines in developing countries. Hum. Vaccine. 2010;6(3) doi: 10.4161/hv.6.3.10397. [DOI] [PubMed] [Google Scholar]
- 21.Teimouri F., Kebriaeezadeh A., Zahraei S.M., Gheiratian M., Nikfar S. Budget impact analysis of vaccination against Haemophilus influenzae type b as a part of a Pentavalent vaccine in the childhood immunization schedule of Iran. Daru. 2017;25(1):1. doi: 10.1186/s40199-017-0166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta S., Umrigar P., Patel P., Bansal R. Evaluation of vaccine wastage in Surat. Nat. J. Commun. Med. 2013;4(1):15–19. [Google Scholar]
- 23.Karami M., Khazaei S., Babaei A., Yaghini F.A., Gouya M.M., Zahraei S.M. Accuracy and quality of immunization data in Iran: findings from data quality self-assessment survey in 2017. BMC Health Serv. Res. 2019;19(1):371. doi: 10.1186/s12913-019-4188-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hibbs B.F., Miller E., Shi J., Smith K., Lewis P., Shimabukuro T.T. Safety of vaccines that have been kept outside of recommended temperatures: reports to the vaccine adverse event reporting system (VAERS), 2008-2012. Vaccine. 2018;36(4):553–558. doi: 10.1016/j.vaccine.2017.11.083. [DOI] [PubMed] [Google Scholar]
- 25.Yang W., Parisi M., Lahue B.J., Uddin M., Bishai D. The budget impact of controlling wastage with smaller vials: a data driven model of session sizes in Bangladesh, India (Uttar Pradesh),Mozambique, and Uganda. Vaccine. 2014;32(49):6643–6648. doi: 10.1016/j.vaccine.2014.09.057. [DOI] [PubMed] [Google Scholar]
- 26.Guichard S., Hymbaugh K., Burkholder B., Diorditsa S., Navarro C., Ahmed S. Vaccine wastage in Bangladesh. Vaccine. 2010;28(3):858–863. doi: 10.1016/j.vaccine.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 27.Jarrahian C., Rein-Weston A., Saxon G., Creelman B., Kachmarik G., Anand A. Vial usage, device dead space, vaccine wastage, and dose accuracy of intradermal delivery devices for inactivated poliovirus vaccine (IPV) Vaccine. 2017;35(14):1789–1796. doi: 10.1016/j.vaccine.2016.11.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee B.Y., Assi T.M., Rookkapan K., Connor D.L., Rajgopal J., Sornsrivichai V. Replacing the measles ten-dose vaccine presentation with the single-dose presentation in Thailand. Vaccine. 2011;29(21):3811–3817. doi: 10.1016/j.vaccine.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]