Abstract
Characterization of decision-making in cells in response to received signals is of importance for understanding how cell fate is determined. The problem becomes multi-faceted and complex when we consider cellular heterogeneity and dynamics of biochemical processes. In this paper, we present a unified set of decision-theoretic, machine learning and statistical signal processing methods and metrics to model the precision of signaling decisions, in the presence of uncertainty, using single cell data. First, we introduce erroneous decisions that may result from signaling processes and identify false alarms and miss events associated with such decisions. Then, we present an optimal decision strategy which minimizes the total decision error probability. Additionally, we demonstrate how graphing receiver operating characteristic curves conveniently reveals the trade-off between false alarm and miss probabilities associated with different cell responses. Furthermore, we extend the introduced framework to incorporate the dynamics of biochemical processes and reactions in a cell, using multi-time point measurements and multi-dimensional outcome analysis and decision-making algorithms. The introduced multivariate signaling outcome modeling framework can be used to analyze several molecular species measured at the same or different time instants. We also show how the developed binary outcome analysis and decision-making approach can be extended to more than two possible outcomes. As an example and to show how the introduced methods can be used in practice, we apply them to single cell data of PTEN, an important intracellular regulatory molecule in a p53 system, in wild-type and abnormal cells. The unified signaling outcome modeling framework presented here can be applied to various organisms ranging from viruses, bacteria, yeast and lower metazoans to more complex organisms such as mammalian cells. Ultimately, this signaling outcome modeling approach can be utilized to better understand the transition from physiological to pathological conditions such as inflammation, various cancers and autoimmune diseases.
Keywords: Cell decision making, noise, decision theory, machine learning, signaling errors, p53 system
INSIGHT BOX
Cells are supposed to make correct decisions, i.e. respond properly to various signals and initiate certain cellular functions, based on the signals they receive from the surrounding environment. Due to signal transduction noise, signaling malfunctions or other factors, cells may respond differently to the same input signals, which may result in incorrect cell decisions. Modeling and quantification of decision-making processes and signaling outcomes in cells have emerged as important research areas in recent years. Here we present univariate and multivariate data-driven statistical models and machine learning methods for analyzing dynamic decision-making processes and signaling outcomes. Furthermore, we exemplify the methods using single cell data generated by a p53 system, in wild-type and abnormal cells.
INTRODUCTION
Understanding how cells make decisions in response to input signals is an important challenge in molecular and cell biology. Depending on the signals they receive, cells can adopt different fates. Emergence of single cell data and methods [1–3] has made it possible to study and model the behavior of each cell individually. An important factor that affects cell decisions is biological noise in various organisms [4], which can cause cells to exhibit different behaviors, when receiving the same input signal. For example, under the same stimuli, some cells may decide to survive, whereas others may undergo apoptosis. Signaling outcomes can be affected by genetic and epigenetic regulation and misregulation, leading to errors in signaling outcomes and ensuing cell decisions.
Given the probabilistic nature of cellular decisions [1, 3], it is of interest to have a unified set of statistical metrics and methods to systematically study and characterize the signaling outcomes that may inform them and determine probabilities associated with different outcomes. Using statistical signal processing and decision theory concepts, recently a framework was introduced by Habibi et al. [1], to compute optimal decision thresholds and probabilities for incorrect cell decisions using single cell data. More specifically, in the transcription factor nuclear factor κB (NF-κB) pathway regulated by the tumor necrosis factor (TNF) [3], the optimal decision threshold which minimized the decision probability to distinguish between two different TNF levels was computed from data [1]. Probabilities of incorrect cell decisions were computed from data as well.
One goal of this paper is to show how the statistical decision theoretic framework [1] can be used to study other molecular systems and signaling outcomes. The other goal is to extend the decision modeling framework such that one can model and analyze multi-dimensional signaling outcome processes using multi-time point measurements. This allows to incorporate signaling dynamics into decision-making analysis. Application of receiver operating characteristic (ROC) curve as a graphical tool to visualize decisions and outcomes under normal and abnormal conditions is introduced here as well. In this paper, we use the tumor suppressor p53 system, as an example, to present the concepts, metrics and algorithms related to decision-making and outcome analysis.
The tumor suppressor p53 is an important transcription factor that is responsible for DNA repair, cell cycle suppression, cell growth control and initiation of apoptosis [5–8]. When a healthy cell is exposed to ionizing radiation (IR), DNA damage occurs [9]. Due to the DNA damage, p53 becomes activated [5, 10, 11], and the cell takes one of two possible actions: it can either survive by repairing the DNA or trigger apoptosis [8, 12, 13]. Our focus here is to demonstrate how such outcomes can be systematically modeled. We accomplish this by introducing metrics and methods to evaluate success and failure rates of the signaling outcomes and actions, in response to the DNA damage caused by different IR doses and under various conditions. In order to do this, we collected data using the simulator of Hat et al. [9], to obtain single cell data of healthy cells, when different IR doses are applied. Moreover, we collected single cell data of abnormal cells exposed to different IR doses, to measure how the decision-making is affected when there is an anomaly in the system, in addition to the DNA damage.
The rest of the paper is organized as follows. First, we briefly explain the p53 system and its response to DNA damage. Then we present decision-making and outcome analysis as a hypothesis testing problem on the IR level, define probabilities associated with various decisions, introduce the optimal decision-maker and describe the single cell data used to determine the decision probabilities in the presence of noise and under normal and abnormal conditions. Methods for computing optimal decision thresholds and the associated decision error rates are presented afterwards, using either single, double or multiple time point measurements in individual cells. The latter is particularly useful to understand the effect of temporal variations and dynamical changes. Additionally, ROC curves are computed and presented as useful tools to visualize the trade-off between decision error rates and how they are affected by decision thresholds and other factors. A comparison between binary and ternary decision-making and outcome analysis and their error rates is provided as well. The paper concludes with a summary of the highlights of the methods and their biological implications for understanding signaling outcomes and decisions in the exemplary p53 system and extensions to other systems.
SIGNALING OUTCOMES AND DECISIONS IN THE P53 SYSTEM WHEN DNA DAMAGE OCCURS: A CASE STUDY
The transcription factor p53 has a significant role in DNA repair, cell cycle suppression, regulation of cell growth and initiation of apoptosis [5–8]. It becomes active in response to DNA damage that may occur when the cell is exposed to IR, ultraviolet radiation, heat shock, etc. [5, 10, 11]. In particular, exposure to IR results in DNA double-strand breaks (DSBs), which are the most serious DNA lesion. When DSB is not repaired, it can cause cell death or DNA mutations which can propagate to new cell generations [12, 14, 15]. When DNA damage occurs, p53 can assume two phosphorylation states: p53Arrester and p53Killer. Afterwards, the p53 system can take two actions: it either suppresses cell cycle until DNA is repaired, if the damage is low and repair is possible, or can trigger apoptosis if the damage is high and repair is not possible [8, 12, 13]. Herein, we intend to compute decision thresholds and incorrect decision rates when the DNA damages caused by various IR doses occur in a cell. With this goal in mind, we conduct stochastic simulations of cells exposed to different IR doses [9], to obtain in silico single cell data.
Consider the p53 system model [9] shown in Fig. 1. The p53 system is activated due to a DNA damage induced by IR. Initially the protein kinase ataxia-telangiectasia mutated (ATM) is activated by the DNA damage [16, 17]. The active ATM phosphorylates Mdm2, which is a p53 inhibitor [18]. The ATM also activates p53 by phosphorylating it to one of its active phosphoforms, p53Arrester, which further phosphorylates p53 to the p53Killer form [19–21]. Moreover, the p53Arrester activates the Mdm2 [22] and wild-type p53-induced phosphatase 1 (Wip1) [23, 24]. The active Wip1 inhibits the ATM [25] and dephosphorylates the p53Killer to the p53Arrester form [26]. The p53Killer regulates another phosphatase, phosphatase and tensin homolog (PTEN), which initiates a slow positive feedback loop stabilizing the level of p53 [27]. If DNA damage is large and its repair takes a longer time, PTEN accumulates to high levels and inhibits AKT, which may no longer phosphorylate Mdm2. Unphosphorylated Mdm2 remains in cytoplasm and may not target nuclear p53 for degradation. Thus, accumulation of PTEN results in disconnection of negative feedback loop between p53 and Mdm2. The slow positive feedback loop acts as a clock, giving cells time to repair DNA and initiating apoptosis if DNA repair takes too long. The apoptotic module, where transcription of pro-apoptotic proteins is induced, is controlled by p53Killer and Akt that suppresses the apoptosis. When Akt is inhibited by increased level of PTEN, it will no longer suppress the apoptotic module. Thus, the p53Killer will initiate activation of cysteine-aspartic proteases (caspases), enzymes having an essential role in cell death (Fig. 1). Since we are interested in the analysis of the signaling outcomes which affect whether the cell survives or triggers apoptosis, we do not consider the cell cycle arrest module (regulated by p53Arrester) and focus on the apoptotic module. Simulation files can be found in Hat et al. [9], and more detailed information about the p53 system and each component and interaction there can be found in Hat et al. [9] and Bogdal et al. [29]. More specifically, interested readers can refer to the Supporting Information S1 Text of [9], which includes a summary of mathematical models of the p53 system, a detailed description of the model, a notation guide and lists of parameters and reactions.
Figure 1.
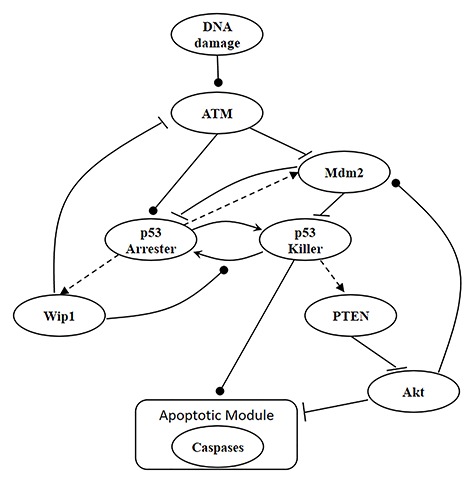
A p53 system model [9]. Arrow-headed dashed lines represent positive transcriptional regulations, arrow-headed solid lines stand for protein transformations, circle-headed solid lines are activatory regulations, and hammer-headed solid lines represent inhibitory regulations. All the molecules and the interactions between them are described in the main body of the paper.
DECISION-MAKING AND OUTCOME ANALYSIS: HYPOTHESIS TESTING ON INPUT SIGNALS AND OPTIMAL DECISIONS WITH MINIMUM ERRORS
When cells are exposed to radiation, each cell may respond differently due to noise or some other factors. One may decide to survive, whereas another may trigger apoptosis, both under the same IR dose. Given the probabilistic nature of such decisions [1], we can formulate p53-based decision-making as a binary hypothesis testing problem, where the decision-making system is going to test which of the following two hypotheses is true regarding the applied IR dose, to trigger an action accordingly:
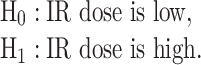 |
(1) |
Binary hypothesis testing is observed in other systems, e.g. the TNF/NF-κB system [1].
In response to an IR dose, two types of incorrect decisions can be made. One is deciding that the input IR level is high, whereas in fact it is low (deciding H1 when H0 is true), which may falsely trigger apoptosis. The other one is deciding that the input IR level is low, whereas in fact it is high (deciding H0 when H1 is true), which may result in missing apoptosis. These two erroneous decisions can be called as ‘false alarm’ and ‘miss event’, respectively, and their probabilities can be defined as:
 |
(2) |
The overall error probability 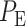 of making decisions is a combination of
of making decisions is a combination of 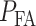 and
and 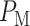 :
:
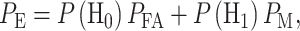 |
(3) |
where 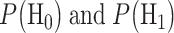 are prior probabilities of H0 and H1, respectively. Note that as mentioned in the Introduction section, IR causes DNA damage. Therefore, one can instead formulate the p53-based decision-making process as a binary hypothesis testing on DNA damage being low or high and define the associated false alarm and miss event probabilities accordingly.
are prior probabilities of H0 and H1, respectively. Note that as mentioned in the Introduction section, IR causes DNA damage. Therefore, one can instead formulate the p53-based decision-making process as a binary hypothesis testing on DNA damage being low or high and define the associated false alarm and miss event probabilities accordingly.
The optimal decision-making system which minimizes the above 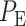 is the one that compares probabilities of observed data under the hypotheses H0 and H1 [30]. More precisely, suppose that
is the one that compares probabilities of observed data under the hypotheses H0 and H1 [30]. More precisely, suppose that 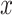 is the observation and
is the observation and 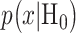 and
and 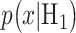 are the conditional probability density functions (PDFs) of
are the conditional probability density functions (PDFs) of 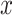 under H0 and H1, respectively. Also consider equi-probable hypotheses, i.e.
under H0 and H1, respectively. Also consider equi-probable hypotheses, i.e. 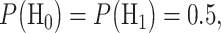 which is a reasonable assumption in the absence of prior information on the possibilities of H0 and H1. Then, the optimal system decides H1 if
which is a reasonable assumption in the absence of prior information on the possibilities of H0 and H1. Then, the optimal system decides H1 if 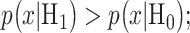 otherwise, it decides the H0. This means that the hypothesis with the highest likelihood is decided. This decision is called the maximum likelihood decision [30].
otherwise, it decides the H0. This means that the hypothesis with the highest likelihood is decided. This decision is called the maximum likelihood decision [30].
SINGLE CELL DATA OF THE P53 SYSTEM EXPOSED TO IR
To calculate the error probabilities in Equation (2) , we use PTEN level as the decision variable because when unrepairable DNA damage occurs, the activated p53 triggers pro-apoptotic phosphatase PTEN [27], and PTEN initiates apoptosis [28]. It has also been shown by Hat et al. [9] that PTEN is a decent predictor of cell fate. After specifying the decision variable, we use the stochastic simulator of Hat et al. [9] to generate 5000 cells for each IR dose. The stochastic simulation has three phases. The first phase is the ‘equilibrium phase’ where we simulate 2 weeks of cell behavior when no IR dose is applied. The second phase is called ‘irradiation phase’ in which 10 minutes of IR dose is applied. The last phase is called ‘relaxation phase’ in which we simulate 72 hours of cell behavior after it is exposed to 10 minutes of IR. When IR dose increases, apoptotic cell percentage increases as well [9] (Fig. 2). For more details on the simulation phases, see supporting files of Hat et al. [9]. In order to decide whether a cell is apoptotic or not, we check active caspase level 72 hours after the irradiation phase and compare it with the threshold of 0.5 × 105 suggested in Hat et al. [9]. Cells with the level of active caspase higher (or lower) than the threshold of 0.5 × 105 are considered to be apoptotic (or surviving).
Figure 2.
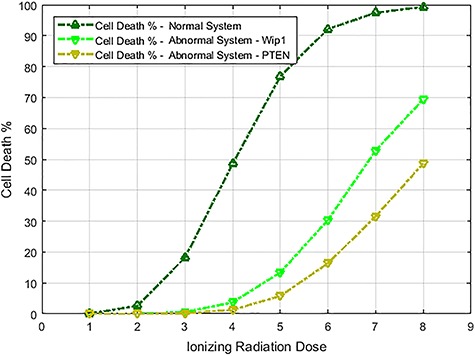
Cell death percentage versus IR dose in both normal and abnormal p53 systems. The dark green curve at the top represents a normal p53 system with no perturbation, whereas the other two curves correspond to p53 systems behaving abnormally due to Wip1 or PTEN perturbations.
The data of normal cells includes eight sets of PTEN levels in 5000 cells, which correspond to eight doses of IR = 1, 2, 3, 4, 5, 6, 7 and 8 Gy. Here Gy stands for Gray, the unit of radiation dose, and 1 Gy is 1 Joule of energy absorbed by 1 kg of tissue. We focus our analysis on low IR versus high IR hypothesis testing, to see how accurately it can be decided whether the applied radiation level is low or high. We consider IR = 1 Gy as the low dose, whereas the higher dose can be IR = 2, 3, 4, 5, 6, 7 or 8 Gy. More specifically, scenarios in which signaling outcomes are analyzed are 1 vs. 2 Gy, 1 vs. 3 Gy, 1 vs. 4 Gy, 1 vs. 5 Gy, 1 vs. 6 Gy, 1 vs. 7 Gy and 1 vs. 8 Gy. We quantitatively study in which of these scenarios more erroneous decisions are made. We also determine to what extent decision between responses to low and high IR levels depends on the input IR separation. We conduct these studies by computing the optimal decision threshold in each scenario using the PTEN data, following the maximum likelihood principle that provides the best decisions, i.e. smallest decision error probabilities. We also compute numerical values of the decision error probabilities using the PTEN data.
In addition to the analysis of erroneous decision-making and incorrect signaling outcomes in normal cells mentioned above, we analyze them in abnormal cells as well, where there is a dysfunctional molecule in the p53 system. Wip1 is one of the key regulatory pro-survival phosphatases [23] in the p53 system (Fig. 1). If the DNA damage can be repaired, then Wip1 expression returns the cell to the pre-stress state from cell-cycle arrest [23, 32]. It has been observed that elevated Wip1 level exists in multiple human cancer types such as breast, lung, pancreas, bladder and liver cancer [33–38]. Therefore, to obtain abnormal cells, we generate cells with increased Wip1 synthesis rate. In normal cells, Wip1 synthesis rate is about 0.1 [9], and here we increase it to 0.15, a 50% increase, to reproduce abnormality. This increase in the Wip1 synthesis rate causes a significant decrease in the cell death percentage (Fig. 2), which can be considered as an abnormal cell state. In addition to Wip1, we analyze abnormal cellular state caused by PTEN abnormalities. It has been observed that attenuated PTEN levels exist in MCF-7, a noninvasive form of human breast cancer cells [39]. Therefore, it is of interest to see how the abnormal PTEN level affects signaling outcomes in the p53 system. To study this, we generate abnormal cells by decreasing PTEN synthesis rate. In healthy cells, the PTEN synthesis rate is about 0.03 [9]. Here we decrease it to 0.015, a 50% decrease, to reproduce abnormality. We observe a considerable decrease in the cell death percentage (Fig. 2), representing an abnormal cellular state.
UNIVARIATE ANALYSIS: METHODS FOR COMPUTING DECISION THRESHOLDS AND DECISION ERROR RATES USING ‘SINGLE’ TIME POINT MEASUREMENTS IN INDIVIDUAL CELLS
In this section, we analyze PTEN levels of 5000 cells measured in 72 hours after the irradiation phase. It has been observed that PTEN levels of both apoptotic and surviving cells become very distinct in 72 hours after 10 minutes of IR application [9] (decision analysis based on PTEN levels at other time instants and multiple time instants are presented in other sections).
Histograms of natural logarithm, ln, of PTEN levels for IR = 1 and 2 Gy data sets and IR = 1 and 8 Gy data sets are shown in Fig. 3A and C, respectively. As presented in Fig. 3B and D, Gaussian PDFs whose means and variances are estimated from the data reasonably represent the histograms. This indicates that the PTEN data can be reasonably approximated by lognormal PDF. Due to the mathematical convenience of working with Gaussian PDFs and variables, especially for multivariate analysis of multiple time point data discussed later, we continue working with the logarithm of the PTEN data. Let 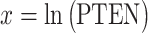 be the Gaussian variable of interest with mean
be the Gaussian variable of interest with mean 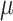 and variance
and variance 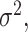 i.e.,
i.e., 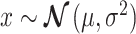 where
where 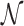 stands for the following normal or Gaussian PDF:
stands for the following normal or Gaussian PDF:
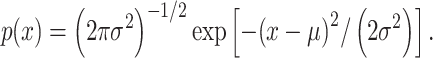 |
Figure 3.
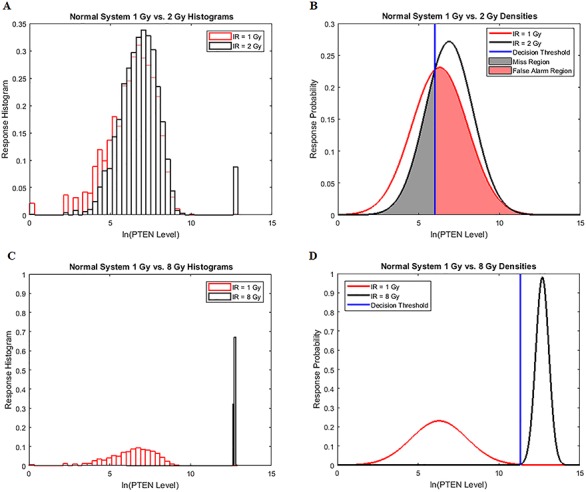
Univariate decision-making and signaling outcome analysis in the normal p53 system based on PTEN response distributions. (A) Histograms of PTEN levels of cells under IR = 1 and 2 Gy doses. (B) Gaussian PDFs for PTEN levels of cells under IR = 1 and 2 Gy doses, together with the optimal maximum likelihood decision threshold which minimizes the total decision error probability. (C) Histograms of PTEN levels of cells under IR = 1 and 8 Gy doses. (D) Gaussian PDFs for PTEN levels of cells under IR = 1 and 8 Gy doses, together with the optimal maximum likelihood decision threshold which minimizes the total decision error probability.
The Gaussian PDFs shown in Fig. 3 are indeed the conditional PDFs 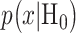 and
and 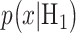 under the hypotheses H0 and H1 defined earlier in Equation (1). For example, in Fig. 3B, H0 and H1 correspond to IR = 1 and 2 Gy doses, respectively, and the red and black curves in there are the conditional PDFs
under the hypotheses H0 and H1 defined earlier in Equation (1). For example, in Fig. 3B, H0 and H1 correspond to IR = 1 and 2 Gy doses, respectively, and the red and black curves in there are the conditional PDFs 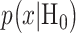 and
and 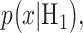 respectively.
respectively.
The optimal maximum likelihood decision-making system
Recall our two hypotheses previously defined in (1). The optimal decision-maker, which minimizes the overall error probability 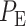 in (3), compares the conditional likelihood ratio
in (3), compares the conditional likelihood ratio 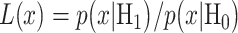 with the ratio
with the ratio 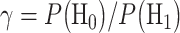 [1]. The system decides H1 if
[1]. The system decides H1 if 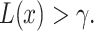 If the hypotheses are equi-probable, i.e.
If the hypotheses are equi-probable, i.e. 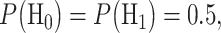 then the optimal system decides H1 if
then the optimal system decides H1 if 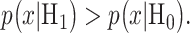
The optimal decision threshold
To find the optimal decision threshold, we need to solve the equation 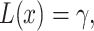 i.e.
i.e. 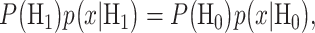 for x. When H0 and H1 are equi-probable, the threshold equation to be solved simplifies to
for x. When H0 and H1 are equi-probable, the threshold equation to be solved simplifies to 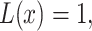 i.e.
i.e. 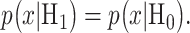
The decision error probabilities
Once the optimal decision threshold is determined, it can be used to compute false alarm and miss decision error probabilities, by integrating the conditional PDFs of data over error regions. More specifically, using the conditional PDFs 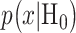 and
and 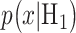 representing the response probabilities of the ln of PTEN levels under the two hypotheses, Equation (2) can be written as [1]:
representing the response probabilities of the ln of PTEN levels under the two hypotheses, Equation (2) can be written as [1]:
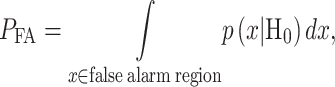 |
(4) |
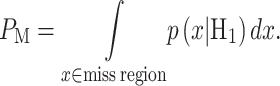 |
(5) |
The false alarm region in (4) is defined by 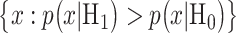 when H0 is true, whereas the miss region in (5) is defined by
when H0 is true, whereas the miss region in (5) is defined by 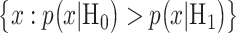 when H1 is true. By substituting
when H1 is true. By substituting  and
and  in Equation (3), the overall error probability
in Equation (3), the overall error probability 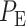 can be obtained.
can be obtained.
The Gaussian data model to compute the optimal decision threshold
Here we focus on Fig. 3B as an example, where two Gaussian PDFs are shown for 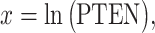 the natural logarithm of PTEN levels in the two data sets of IR = 1 and 2 Gy, with each data set consisting of 5000 cells. Let
the natural logarithm of PTEN levels in the two data sets of IR = 1 and 2 Gy, with each data set consisting of 5000 cells. Let 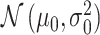 and
and 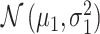 represent the Gaussian PDFs that correspond to the IR = 1 and 2 Gy data sets, respectively, where
represent the Gaussian PDFs that correspond to the IR = 1 and 2 Gy data sets, respectively, where 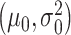 and
and 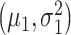 are mean/variance pairs estimated from their associated data sets. The optimal maximum likelihood decision threshold in Fig. 3B is at the intersection of the two PDFs and can be computed by solving the equation
are mean/variance pairs estimated from their associated data sets. The optimal maximum likelihood decision threshold in Fig. 3B is at the intersection of the two PDFs and can be computed by solving the equation 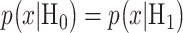 written below:
written below:
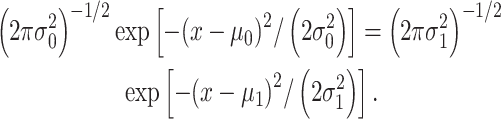 |
(6) |
By multiplying both sides by 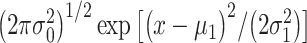 and then taking natural logarithm of both sides, (6) can be written in the following quadratic equation form [1]:
and then taking natural logarithm of both sides, (6) can be written in the following quadratic equation form [1]:
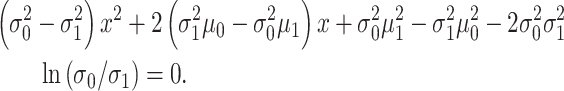 |
(7) |
Equation (7) is derived assuming our hypotheses are equi-probable, i.e. 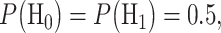 as mentioned before. The solution of Equation (7) gives the optimal decision threshold PTENth, located at the intersection of the two PDFs for IR = 1 and 2 Gy doses in Fig. 3B (the italic style is adopted to clarify that the threshold is related to the logarithm of PTEN data). Interestingly, for equal variances, the solution of Equation (7) for the optimal decision threshold simplifies to the average of the means, i.e.
as mentioned before. The solution of Equation (7) gives the optimal decision threshold PTENth, located at the intersection of the two PDFs for IR = 1 and 2 Gy doses in Fig. 3B (the italic style is adopted to clarify that the threshold is related to the logarithm of PTEN data). Interestingly, for equal variances, the solution of Equation (7) for the optimal decision threshold simplifies to the average of the means, i.e. 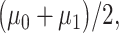 which intuitively makes sense. For other prior probabilities and PDF models, the optimal threshold can be obtained similarly, by solving the equation
which intuitively makes sense. For other prior probabilities and PDF models, the optimal threshold can be obtained similarly, by solving the equation 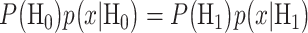 for
for 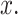
The Gaussian data model to compute the decision error probabilities
Using the PTENth obtained by solving Equation (7) and using the Gaussian PDFs, Equations (4) and (5) for the false alarm and miss error probabilities can be written as:
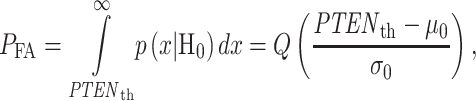 |
(8) |
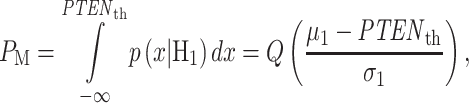 |
(9) |
where 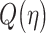 is tail probability of the standard Gaussian PDF:
is tail probability of the standard Gaussian PDF:
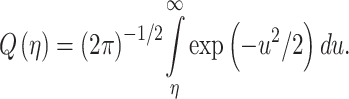 |
Equation (8) represents area of the pink region in Fig. 3B under the tail of the IR = 1 Gy PDF, beyond the PTENth threshold. In this region of 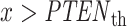 , we have
, we have 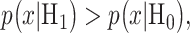 while H0 is true. This is the ‘false alarm’ region for which we have computed
while H0 is true. This is the ‘false alarm’ region for which we have computed 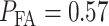 in Fig. 3B. On the other hand, Equation (9) represents the area of the gray region in Fig. 3B under the tail of the IR = 2 Gy PDF, below the PTENth threshold. In this region of
in Fig. 3B. On the other hand, Equation (9) represents the area of the gray region in Fig. 3B under the tail of the IR = 2 Gy PDF, below the PTENth threshold. In this region of 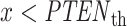 , we have
, we have 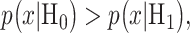 while H1 is true. This is the ‘miss’ region for which we have computed
while H1 is true. This is the ‘miss’ region for which we have computed 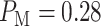 in Fig. 3B. After computing
in Fig. 3B. After computing 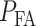 and
and 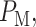 we can now compute the overall error probability
we can now compute the overall error probability 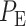 using Equation (3), which results in
using Equation (3), which results in 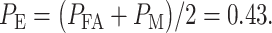 Similarly, by computing Equations (8) and (9) for the 1 vs. 8 Gy scenario, we obtain
Similarly, by computing Equations (8) and (9) for the 1 vs. 8 Gy scenario, we obtain 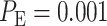 (Fig. 3D). Based on the results of 1 vs. 2 Gy and 1 vs. 8 Gy decision scenarios, it can be concluded that when the difference between the two applied IR doses increases, the overall decision error probability
(Fig. 3D). Based on the results of 1 vs. 2 Gy and 1 vs. 8 Gy decision scenarios, it can be concluded that when the difference between the two applied IR doses increases, the overall decision error probability 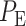 decreases. This is mainly because the two response PDFs become more distinct with less overlap, as the difference between the two applied IR doses increases.
decreases. This is mainly because the two response PDFs become more distinct with less overlap, as the difference between the two applied IR doses increases.
For some cases such as 1 vs. 3, 4, 5 and 6 Gy IR doses, some data sets need to be modeled by a mixture of Gaussian PDFs due to the bistable behavior of p53 system and hence cells’ bimodal histograms. Still the same underlying theory and proposed framework hold. Nevertheless, in what follows we explain how to determine the optimal decision thresholds and how to compute the decision error probabilities when using a mixture model, for the 1 vs. 4 Gy scenario.
Histograms of natural logarithm of PTEN levels for IR = 1 and 4 Gy data sets are shown in Fig. 4A. We notice that while 1 Gy data histogram is unimodal, histogram of 4 Gy data is bimodal. Therefore, for the 1 Gy data, we use a single Gaussian PDF as before, whereas for the 4 Gy data, we utilize a mixture of two Gaussian PDFs. More specifically, we consider 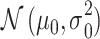 for
for 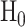 to represent the single Gaussian PDF that corresponds to the IR = 1 Gy data, whereas we use
to represent the single Gaussian PDF that corresponds to the IR = 1 Gy data, whereas we use 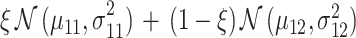 for
for 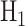 , with
, with 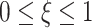 being the mixing parameter, to represent the mixture of two Gaussian PDFs which correspond to the IR = 4 Gy data set. The mean and variance
being the mixing parameter, to represent the mixture of two Gaussian PDFs which correspond to the IR = 4 Gy data set. The mean and variance 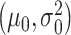 are estimated from the 1 Gy data, and the associated single Gaussian PDF is shown in Fig. 4B. Furthermore, the means and variances
are estimated from the 1 Gy data, and the associated single Gaussian PDF is shown in Fig. 4B. Furthermore, the means and variances 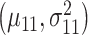 and
and 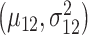 and the mixing parameter
and the mixing parameter 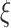 are estimated from the 4 Gy data using the MATLAB command ‘fitgmdist’ which implements the iterative expectation–maximization algorithm. The resulting mixture of two Gaussian PDFs is shown in Fig. 4B.
are estimated from the 4 Gy data using the MATLAB command ‘fitgmdist’ which implements the iterative expectation–maximization algorithm. The resulting mixture of two Gaussian PDFs is shown in Fig. 4B.
Figure 4.
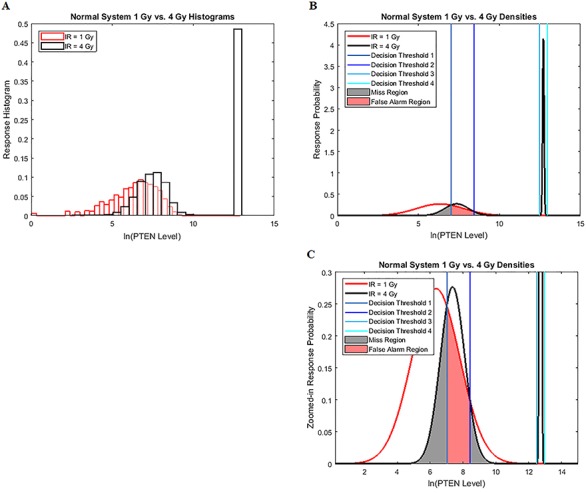
Univariate decision making and signaling outcome analysis in the normal p53 system when a PTEN response distribution is bimodal. (A) Histograms of PTEN levels of cells under IR = 1 and 4 Gy doses. (B) A Gaussian PDF for PTEN levels of cells under IR = 1 Gy and a mixture of two Gaussian PDFs for PTEN levels of cells under IR = 4 Gy doses, together with the optimal maximum likelihood decision thresholds which minimize the total decision error probability. (C) Zoomed-in view of panel B.
Similar to the previous scenarios, the optimal maximum likelihood decision thresholds shown in Fig. 4B for equi-probable hypotheses are at the intersections of the conditional PDFs 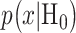 and
and 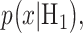 the latter being a Gaussian mixture for the 4 Gy data. Note that here solving the equation
the latter being a Gaussian mixture for the 4 Gy data. Note that here solving the equation 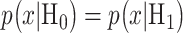 results in four solutions for x; that is why there are four decision thresholds,
results in four solutions for x; that is why there are four decision thresholds, 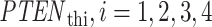 in Fig. 4B (note that each decision threshold
in Fig. 4B (note that each decision threshold 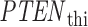 is listed as ‘Decision Threshold i’ in Fig. 4).
is listed as ‘Decision Threshold i’ in Fig. 4).
To compute the decision error probabilities, the false alarm and miss probabilities 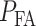 and
and 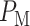 need to be calculated using Equations (4) and (5), respectively. Since there are four decision thresholds in this case, integration has to be performed over multiple regions, which results in lengthy expressions. However, note that as can be seen in Fig. 4B and its zoomed-in view in Fig. 4C, PDFs for low dose (red) and the lower Gaussian mode for the high dose (black) assume very small values as they reach the third threshold. Therefore, their contributions to possible error events around the third and fourth thresholds are negligible (later this is shown numerically). Similarly, given the very small variance of the higher Gaussian mode of the PDF for the high dose, this PDF is substantially different from zero only between the third and fourth thresholds. Consequently, the contribution of the PDF of this mode to possible errors around the third and fourth thresholds is negligible as well. Overall, as just explained, optimal decision when
need to be calculated using Equations (4) and (5), respectively. Since there are four decision thresholds in this case, integration has to be performed over multiple regions, which results in lengthy expressions. However, note that as can be seen in Fig. 4B and its zoomed-in view in Fig. 4C, PDFs for low dose (red) and the lower Gaussian mode for the high dose (black) assume very small values as they reach the third threshold. Therefore, their contributions to possible error events around the third and fourth thresholds are negligible (later this is shown numerically). Similarly, given the very small variance of the higher Gaussian mode of the PDF for the high dose, this PDF is substantially different from zero only between the third and fourth thresholds. Consequently, the contribution of the PDF of this mode to possible errors around the third and fourth thresholds is negligible as well. Overall, as just explained, optimal decision when 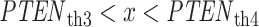 is
is 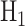 with no decision error, whereas for
with no decision error, whereas for 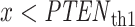 ,
, 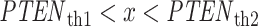 and
and 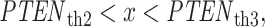 optimal decisions are
optimal decisions are 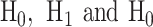 , respectively, with the following decision error probabilities:
, respectively, with the following decision error probabilities:
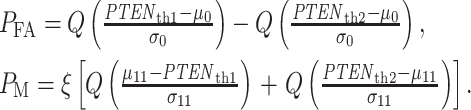 |
The 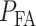 expression corresponds to the pink region in Fig. 4C, whereas the two Q functions in the
expression corresponds to the pink region in Fig. 4C, whereas the two Q functions in the 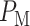 expression correspond to the two gray regions in Fig. 4C, respectively. Using the data, computed numerical values are
expression correspond to the two gray regions in Fig. 4C, respectively. Using the data, computed numerical values are 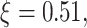
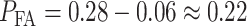 ,
, 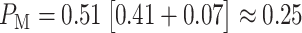 and
and 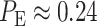 , the last one being calculated using Equation (3).
, the last one being calculated using Equation (3).
As an example of a negligible decision error probability around the third and fourth thresholds mentioned earlier, consider the area under the red Gaussian PDF 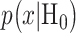 in Fig. 4C for
in Fig. 4C for 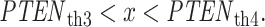 While not visible due to being very small, it can be understood that the aforementioned area is a false alarm probability of deciding
While not visible due to being very small, it can be understood that the aforementioned area is a false alarm probability of deciding 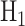 , although
, although 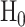 is true. The numerical value of this false alarm probability is:
is true. The numerical value of this false alarm probability is: 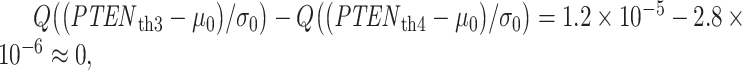 which is negligible compared to
which is negligible compared to 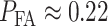 calculated in the previous paragraph.
calculated in the previous paragraph.
Abnormal p53 systems
To see how an abnormality in the p53 system affects the decision-making and signaling outcomes, we calculate 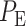 values when Wip1 synthesis rate is elevated by 50% from 0.1 to 0.15 (Fig. 5), as mentioned previously. As suggested by Habibi et al. [1], decision thresholds are modeled to be those of the normal cells. This implies that abnormal cells are not aware of the abnormality and therefore erroneously use the previous threshold. As we see later, this increases decision error probabilities, a behavior that can be anticipated from abnormal cells. Using Equations (3), (8) and (9),
values when Wip1 synthesis rate is elevated by 50% from 0.1 to 0.15 (Fig. 5), as mentioned previously. As suggested by Habibi et al. [1], decision thresholds are modeled to be those of the normal cells. This implies that abnormal cells are not aware of the abnormality and therefore erroneously use the previous threshold. As we see later, this increases decision error probabilities, a behavior that can be anticipated from abnormal cells. Using Equations (3), (8) and (9),  are computed:
are computed: 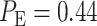 is obtained for 1 vs. 2 Gy scenario (Fig. 5A), and
is obtained for 1 vs. 2 Gy scenario (Fig. 5A), and 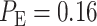 is obtained for 1 vs. 8 Gy scenario (Fig. 5B). Compared to the normal system results, the overall error probability is significantly higher for the abnormal system under the 1 vs. 8 Gy scenario (we observe that
is obtained for 1 vs. 8 Gy scenario (Fig. 5B). Compared to the normal system results, the overall error probability is significantly higher for the abnormal system under the 1 vs. 8 Gy scenario (we observe that 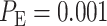 of normal cells markedly increases to
of normal cells markedly increases to 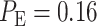 in abnormal cells). The reason is that when the Wip1 synthesis rate is increased, the two response PDF curves significantly overlap (notice the overlap between the left-side component of the IR = 8 Gy PDF with the IR = 1 Gy PDF in Fig. 5B). This is while in normal cells they had almost no overlap (Fig. 3D).
in abnormal cells). The reason is that when the Wip1 synthesis rate is increased, the two response PDF curves significantly overlap (notice the overlap between the left-side component of the IR = 8 Gy PDF with the IR = 1 Gy PDF in Fig. 5B). This is while in normal cells they had almost no overlap (Fig. 3D).
Figure 5.
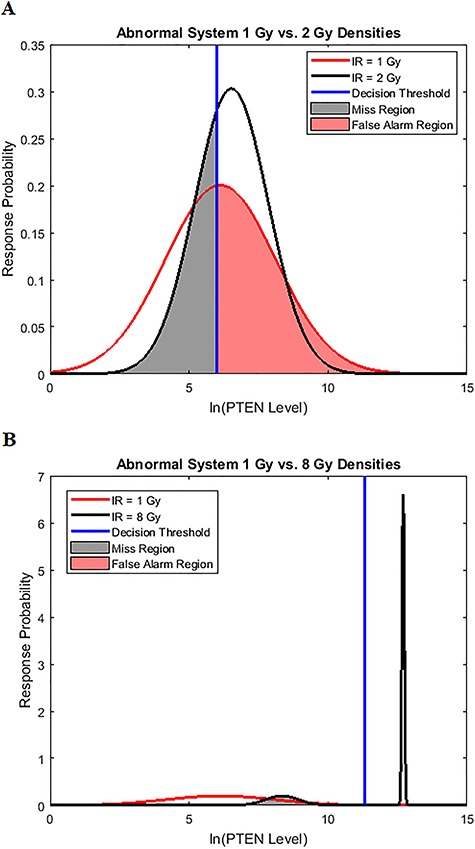
Univariate decision-making and signaling outcome analysis in an abnormal p53 system, with increased Wip1 synthesis rate, based on PTEN response distributions. (A) Gaussian PDFs for PTEN levels of abnormal cells under IR = 1 and 2 Gy doses, together with the decision threshold of normal cells. This implies that in abnormal cells the previous decision threshold is erroneously used [1]. As discussed later, this increases decision error probabilities, a behavior that can be anticipated from abnormal cells. (B) A Gaussian PDF for PTEN levels of abnormal cells under IR = 1 Gy dose and a mixture of two Gaussian PDFs for PTEN levels of abnormal cells under IR = 8 Gy dose, together with the decision threshold of normal cells.
Similarly, we compute error probabilities for the other abnormal p53 system we mentioned previously, generated by the PTEN synthesis rate reduced from 0.03 to 0.015 (50% reduction). Error probabilities for this abnormality for all different radiation exposure scenarios of 1 vs. 2 Gy up to 1 vs. 8 Gy are shown in Fig. 6. For comparison, error probabilities for the Wip1-perturbed abnormal p53 system and also the normal p53 system are provided in Fig. 6 as well. We observe that as the difference between the two applied IR doses increases, decision error probability in normal cells drops significantly. This is while, in abnormal cells, decision error probabilities remain high. These signaling outcomes might be correlated with the observation that cell death percentages in abnormal systems are considerably lower than the normal system, even when the radiation dose increases (Fig. 2). This could indicate that abnormal cells do not respond to IR levels properly and, hence, decisions and signaling outcomes affecting apoptosis and survival become more erroneous. Care should be taken that these specific observations are based on the low versus high IR, e.g. d0 vs. d1 IR hypothesis testing formulation, where the low IR dose is fixed to 1 Gy (d0 = 1 Gy) and the high IR dose is ranging from 2 up to 8 Gy (d1 = 2, 3, …, 8 Gy) in the p53 system, that is considered in this paper as an example. These observations may not be generalized to other selections of the low d0 and high d1 IR doses or other hypothesis testing formulations, case studies or signaling networks. However, the proposed framework and its analytical tools, whose introduction has been the main goal of this paper, can be similarly used.
Figure 6.
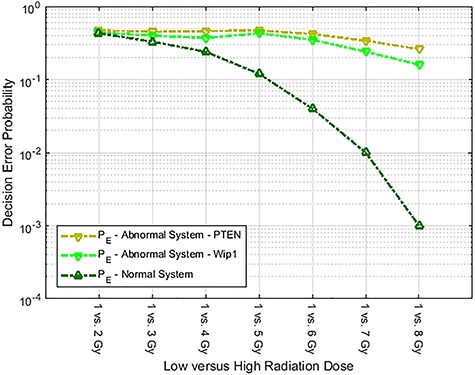
Decision error probabilities for several low IR versus high IR scenarios. The ‘Abnormal System—PTEN’ legend refers to a p53 system whose PTEN synthesis rate is decreased by 50%, compared to its nominal value. The ‘Abnormal System—Wip1’ legend refers to a p53 system whose Wip1 synthesis rate is increased by 50%, compared to its nominal value. Smaller decision error probabilities in the normal system are noteworthy.
DECISION AND SIGNALING OUTCOME ANALYSIS USING ROC CURVES
In this subsection, we show how to analyze performance of a decision-maker using ROC curves. The ROC curve is developed to visualize the performance of decision-making systems [30, 40] and is a graph of probability of detection, 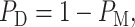 versus the probability of false alarm,
versus the probability of false alarm, 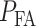 . In Fig. 7 we present ROC curves for both the normal p53 system (Fig. 7A) and the abnormal p53 system (Fig. 7B) whose Wip1 synthesis rate is elevated, for these two low versus high IR decision-making scenarios: 1 vs. 2 Gy and 1 vs. 8 Gy. The theoretical ROC curves in Fig. 7 are graphed using the false alarm and miss decision error probability formulas in Equations (8) and (9), respectively, with μs, σs and the thresholds estimated from the data. The empirical ROC curves in Fig. 7 are graphed by using the data sets directly, using the MATLAB command ‘perfcurve’. We observe that the theoretical and empirical ROCs are nearly the same. Therefore, in what follows, we focus on the theoretical ROC curves, to explain concepts and results.
. In Fig. 7 we present ROC curves for both the normal p53 system (Fig. 7A) and the abnormal p53 system (Fig. 7B) whose Wip1 synthesis rate is elevated, for these two low versus high IR decision-making scenarios: 1 vs. 2 Gy and 1 vs. 8 Gy. The theoretical ROC curves in Fig. 7 are graphed using the false alarm and miss decision error probability formulas in Equations (8) and (9), respectively, with μs, σs and the thresholds estimated from the data. The empirical ROC curves in Fig. 7 are graphed by using the data sets directly, using the MATLAB command ‘perfcurve’. We observe that the theoretical and empirical ROCs are nearly the same. Therefore, in what follows, we focus on the theoretical ROC curves, to explain concepts and results.
Figure 7.
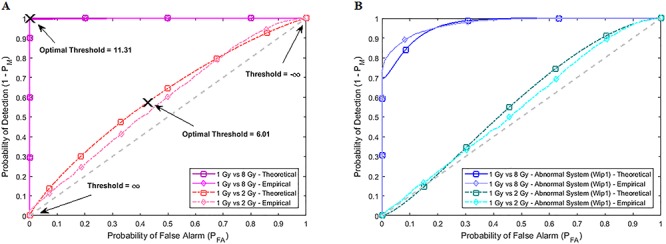
Empirical and theoretical ROC curves for both normal and abnormal p53 systems. The theoretical ROC curves labeled by □ are obtained from the Gaussian and mixture of Gaussian data models and formulas whose parameters are estimated from the data, whereas the empirical ROC curves labeled by ◊ are obtained directly from the data. We observe that the theoretical and empirical ROCs are nearly the same. Note that Threshold = ln(PTEN Level) in the figures. (A) ROC curves of 1 vs. 2 Gy and 1 vs. 8 Gy radiation scenarios for the normal system. (B) ROC curves of 1 vs. 2 Gy and 1 vs. 8 Gy radiation scenarios for the Wip1-perturbed abnormal system.
A ROC curve is above a 45° diagonal line [30], the gray dashed line in Fig. 7. In our study it represents the worst possible decision-maker, i.e. a decision-making system that does not use the data and instead randomly decides if the applied IR dose is low or high, by just flipping a coin. The 45° line is indeed a reference to judge the performance of a decision-making system. A ROC curve far away from the 45° reference line indicates a good decision-maker. Each point on a ROC curve represents a 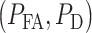 pair that corresponds to a certain decision threshold. Other properties of ROC curves can be found in Van Trees et al. [40]. The ‘×’ marks in Fig. 7A show the optimal
pair that corresponds to a certain decision threshold. Other properties of ROC curves can be found in Van Trees et al. [40]. The ‘×’ marks in Fig. 7A show the optimal 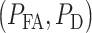 points that correspond to the optimal decision thresholds shown in Fig. 3B and D, previously computed using Equation (7) for the 1 vs. 2 Gy and 1 vs. 8 Gy scenarios, respectively.
points that correspond to the optimal decision thresholds shown in Fig. 3B and D, previously computed using Equation (7) for the 1 vs. 2 Gy and 1 vs. 8 Gy scenarios, respectively.
Based on the normal p53 system ROC curves in Fig. 7A, we observe that decisions are made better under the 1 vs. 8 Gy scenario, because of its ROC curve being very far from the 45° reference line, compared to the 1 vs. 2 Gy case whose ROC curve is much closer to the 45° reference line. This finding supports our results presented in Fig. 6, showing the smaller decision error probability of 0.001 for 1 vs. 8 Gy, compared to the larger decision error probability of 0.43 for 1 vs. 2 Gy. ROC curves also show that abnormalities in the p53 system can cause decision precision loss. Comparing the normal (Fig. 7A) and abnormal system ROC curves (Fig. 7B), we observe that the abnormal system ROC curves are closer to the 45° reference line, meaning that more erroneous decisions are made, when there is an abnormality in the system.
BIVARIATE ANALYSIS: METHODS FOR COMPUTING DECISION THRESHOLDS AND DECISION ERROR RATES USING ‘TWO’ TIME POINT MEASUREMENTS IN INDIVIDUAL CELLS
In this section, we analyze PTEN levels of 5000 cells measured in 1 hour and 30 hours after the irradiation phase. Using two variables instead of one allows to study the effect of temporal dynamical changes on decision-making and signaling outcomes and paves the way for analyzing decisions based on multiple time point data. Suppose 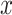 and y represent the ln(PTEN) levels in 1 hour and 30 hours, respectively, after radiation. Joint Gaussian PDF for
and y represent the ln(PTEN) levels in 1 hour and 30 hours, respectively, after radiation. Joint Gaussian PDF for 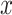 and
and 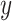 can be written as [41]:
can be written as [41]:
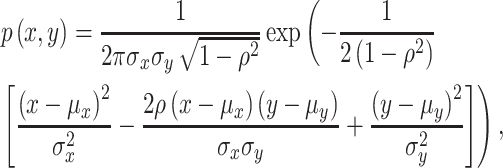 |
(10) |
where 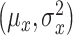 and
and 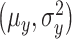 are means and variances of
are means and variances of  and
and  and
and 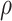 is the correlation coefficient between
is the correlation coefficient between 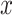 and
and 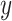 . Bivariate conditional likelihood ratio is given by
. Bivariate conditional likelihood ratio is given by 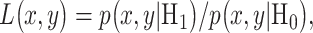 and the optimal decision-maker which minimizes the overall error probability
and the optimal decision-maker which minimizes the overall error probability 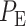 compares
compares 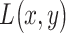 with the ratio
with the ratio 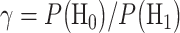 . The system decides the H1 if
. The system decides the H1 if 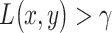 . If the hypotheses are equi-probable, i.e.
. If the hypotheses are equi-probable, i.e. 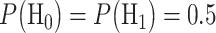 , then the optimal system decides the H1 if
, then the optimal system decides the H1 if 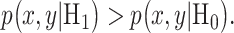 To find the optimal decision threshold curve, we need to solve the equation
To find the optimal decision threshold curve, we need to solve the equation 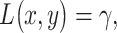 i.e.
i.e. 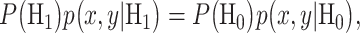 for x and y. When H0 and H1 are equi-probable, the threshold equation to be solved simplifies to
for x and y. When H0 and H1 are equi-probable, the threshold equation to be solved simplifies to 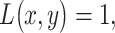 i.e.
i.e. 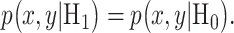 To find false alarm and miss probabilities, Equations (4) and (5) can be extended to two variables as follows:
To find false alarm and miss probabilities, Equations (4) and (5) can be extended to two variables as follows:
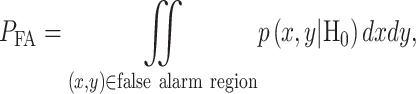 |
(11) |
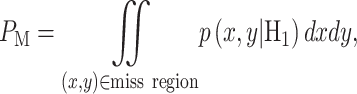 |
(12) |
where 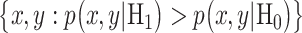 defines the false alarm region when H0 is true and
defines the false alarm region when H0 is true and 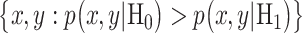 specifies the miss region when H1 is true. After computing
specifies the miss region when H1 is true. After computing 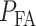 and
and 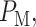 the overall decision error probability
the overall decision error probability 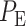 can be calculated using Equation (3).
can be calculated using Equation (3).
As an example, here we focus on Fig. 8A, where two bivariate Gaussian PDFs are shown for 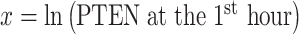 and
and 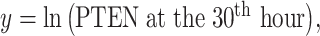 logarithms of PTEN levels in the two data sets of IR = 1 and 2 Gy, with each data set consisting of 5000 cells. The mean and variance parameters of each bivariate response PDF are estimated from the associated data set. The overlap between the two bivariate PDFs in response to IR = 1 and 2 Gy can be better seen in the top view shown in Fig. 8B. This figure also demonstrates that the decision threshold between the two PDFs is going to be a curve in the x–y plane, where the two PDFs intersect. The equation for this optimal threshold curve which minimizes the total decision error probability is given by
logarithms of PTEN levels in the two data sets of IR = 1 and 2 Gy, with each data set consisting of 5000 cells. The mean and variance parameters of each bivariate response PDF are estimated from the associated data set. The overlap between the two bivariate PDFs in response to IR = 1 and 2 Gy can be better seen in the top view shown in Fig. 8B. This figure also demonstrates that the decision threshold between the two PDFs is going to be a curve in the x–y plane, where the two PDFs intersect. The equation for this optimal threshold curve which minimizes the total decision error probability is given by 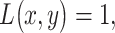 where L is the bivariate conditional likelihood ratio defined previously. This decision threshold curve curveth is shown together with contour plots of the two bivariate PDFs in Fig. 8C. To compute the decision error probabilities using the decision threshold curveth, Equations (11) and (12) for the false alarm and miss error probabilities can be written as:
where L is the bivariate conditional likelihood ratio defined previously. This decision threshold curve curveth is shown together with contour plots of the two bivariate PDFs in Fig. 8C. To compute the decision error probabilities using the decision threshold curveth, Equations (11) and (12) for the false alarm and miss error probabilities can be written as:
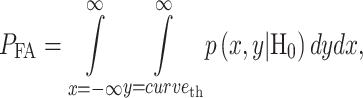 |
(13) |
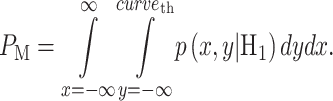 |
(14) |
Figure 8.
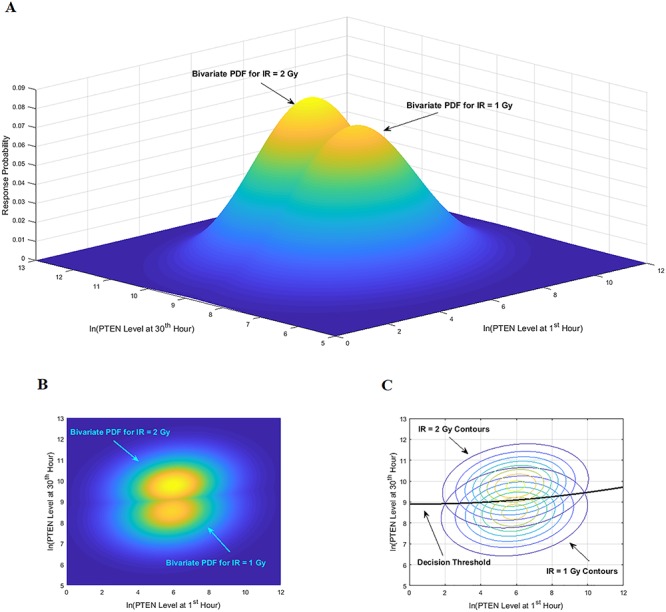
Bivariate decision-making and signaling outcome analysis in the normal p53 system based on PTEN response distributions. (A) Bivariate Gaussian PDFs for PTEN levels of cells at the first hour and the 30th hour, under IR = 1 and 2 Gy doses. (B) Top view of the two bivariate Gaussian PDFs. (C) Top contour view of the two bivariate Gaussian PDFs, together with the optimal maximum likelihood decision threshold curve which minimizes the total decision error probability.
After computing the integrals in Equations (13) and (14) numerically, we obtain 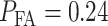 and
and 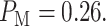 Upon their substitution in Equation (3) and with equi-probable hypotheses, we obtain
Upon their substitution in Equation (3) and with equi-probable hypotheses, we obtain 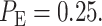
To compare the above two time point decision with individual one time point decisions, we compute decision error probabilities based on the first hour data and the 30th hour data, individually, for the IR = 1 vs. 2 Gy scenario. We obtain  and
and 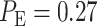 for individual univariate decisions in 1 hour and 30 hours after the radiation, respectively. We observe that the bivariate decision offers significant improvement over the 1 hour decision and slight improvement over the 30 hour decision. Univariate decision error probabilities at different time points are discussed in the next section, as well as how multivariate decision error probability changes, as the data of more time points are added to the decision process in a sequential manner.
for individual univariate decisions in 1 hour and 30 hours after the radiation, respectively. We observe that the bivariate decision offers significant improvement over the 1 hour decision and slight improvement over the 30 hour decision. Univariate decision error probabilities at different time points are discussed in the next section, as well as how multivariate decision error probability changes, as the data of more time points are added to the decision process in a sequential manner.
MULTIVARIATE ANALYSIS: METHODS FOR COMPUTING DECISION THRESHOLDS AND DECISION ERROR RATES USING ‘MULTIPLE’ TIME POINT MEASUREMENTS IN INDIVIDUAL CELLS
In this section, we further study the effect of system dynamics on decision-making and signaling outcomes, by considering multiple time point data. More specifically, we consider PTEN levels of 5000 cells measured in 1, 10, 20, 30, 40, 50, 60 and 70 hours after the irradiation phase. Let 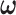 be an
be an 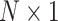 column vector that represents the ln(PTEN) levels at a subset or all of the aforementioned time instants. Joint Gaussian PDF for all the decision variables in
column vector that represents the ln(PTEN) levels at a subset or all of the aforementioned time instants. Joint Gaussian PDF for all the decision variables in 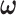 can be written as [31, 40]:
can be written as [31, 40]:
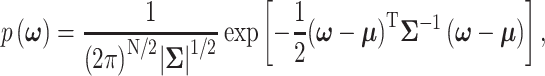 |
(15) |
where 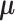 is the
is the 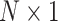 mean vector,
mean vector, 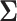 is the
is the 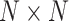 covariance matrix,
covariance matrix, 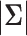 and
and 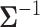 denote the determinant and inverse of
denote the determinant and inverse of 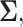 respectively, and T represents matrix transpose. This multivariate Gaussian or normal PDF for the decision vector
respectively, and T represents matrix transpose. This multivariate Gaussian or normal PDF for the decision vector 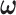 can be symbolically shown by
can be symbolically shown by 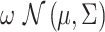 . For
. For 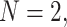 Equation (15) simplifies to the bivariate PDF in Equation (10), such that
Equation (15) simplifies to the bivariate PDF in Equation (10), such that
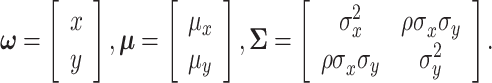 |
Computation of the decision error probabilities using multiple decision variables can be accomplished using discriminant functions [31, 40]:
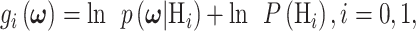 |
(16) |
where 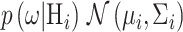 and i is index of the discriminant function associated with the hypothesis
and i is index of the discriminant function associated with the hypothesis 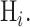 In our case we have
In our case we have 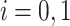 , referring to our two hypotheses in Equation (1). For any hypothesis
, referring to our two hypotheses in Equation (1). For any hypothesis 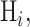 substitution of (15) in (16) simplifies its discriminant function to
substitution of (15) in (16) simplifies its discriminant function to
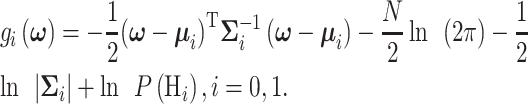 |
(17) |
Using the discriminant functions in (17) and for a given 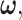 the optimal decision-making system decides the H0 if
the optimal decision-making system decides the H0 if 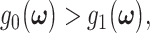 and decides the H1 if
and decides the H1 if 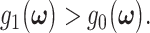 The false alarm probability
The false alarm probability 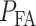 is the probability of deciding H1, i.e.
is the probability of deciding H1, i.e. 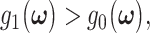 whereas in fact H0 is true. On the other hand, the miss probability
whereas in fact H0 is true. On the other hand, the miss probability 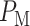 is the probability of deciding the H0, i.e.
is the probability of deciding the H0, i.e. 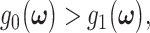 although indeed H1 is true. Computing
although indeed H1 is true. Computing 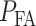 and
and 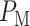 using multivariate PDFs directly entail multivariate integrations over regions defined by decision surfaces. Given the complexities of such computations, as a simpler alternative, we calculate
using multivariate PDFs directly entail multivariate integrations over regions defined by decision surfaces. Given the complexities of such computations, as a simpler alternative, we calculate 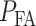 and
and 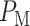 using the data directly, by counting the number of times that false alarm and miss event occur, respectively, after comparing the discriminant function values
using the data directly, by counting the number of times that false alarm and miss event occur, respectively, after comparing the discriminant function values 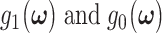 for each
for each 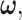 and then divide them by the total number of data points. The overall decision error probability
and then divide them by the total number of data points. The overall decision error probability 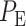 can be calculated using Equation (3). Another method for computing
can be calculated using Equation (3). Another method for computing 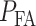 and
and 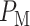 relies on characteristic functions [42].
relies on characteristic functions [42].
Single-variable decision-making and signaling outcome analysis as time evolves
To understand how decision-making and signaling outcomes may change over time, first we look at the decision error probability 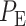 using PTEN levels measured at individual consecutive time instants (Fig. 9A), for the 1 vs. 2 Gy scenario. A noteworthy observation is that the decision error exhibits a minimum value. The minimum occurs in 20 hours after the radiation. This can be visually explained by the amount of overlap of PTEN histograms at each individual time point. For instance, we provide histograms of PTEN levels at the 20th and the 70th hours in Fig. 10, for IR = 1 and 2 Gy doses. We observe that the 20th hour histograms have less overlap than the 70th hour histograms, shown in Fig. 10A and B, respectively, which results in the smaller
using PTEN levels measured at individual consecutive time instants (Fig. 9A), for the 1 vs. 2 Gy scenario. A noteworthy observation is that the decision error exhibits a minimum value. The minimum occurs in 20 hours after the radiation. This can be visually explained by the amount of overlap of PTEN histograms at each individual time point. For instance, we provide histograms of PTEN levels at the 20th and the 70th hours in Fig. 10, for IR = 1 and 2 Gy doses. We observe that the 20th hour histograms have less overlap than the 70th hour histograms, shown in Fig. 10A and B, respectively, which results in the smaller 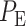 at the 20th hour in Fig. 9A.
at the 20th hour in Fig. 9A.
Figure 9.
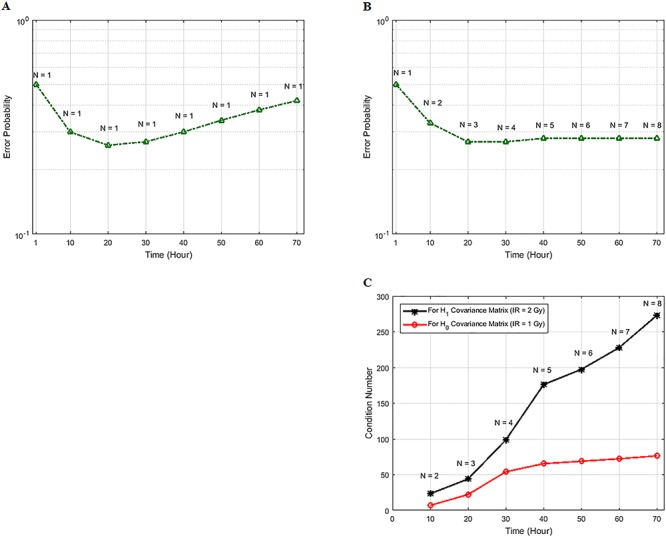
Decision error probabilities versus time in the normal p53 system: a single versus multiple time point study. (A) PE as a function of time for the 1 vs. 2 Gy radiation scenario, computed using only the PTEN data of a single, N = 1, individual time instant. This assumes at any given time, decision is made based on the data of that time only. Having a minimum error probability at the 20th hour is noteworthy. (B) PE as a function of time for the 1 vs. 2 Gy radiation scenario, computed using the PTEN data of N time instants, N = 1, 2,…, 8 (N = 1 means the PTEN data of the first hour, N = 2 refers to the PTEN data of the first and the 10th hours, N = 3 indicates the PTEN data of the first, 10th, 20th hours, etc.). This assumes, at any given time, decision is made based on the data of that time, plus the data of the previous time instants, which means accumulating the data to make a decision. It is observed that PE first decreases, and after a certain point, it remains nearly constant. (C) Condition numbers of 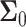 and
and  the
the 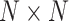 covariance matrices of the data for the two hypotheses H0 and H1, for IR = 1 and 2 Gy, respectively, as N increases from 2 to 8. When N increases, condition numbers of both of the covariance matrices
covariance matrices of the data for the two hypotheses H0 and H1, for IR = 1 and 2 Gy, respectively, as N increases from 2 to 8. When N increases, condition numbers of both of the covariance matrices 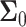 and
and 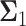 increase. On the other hand, a large condition number for a covariance matrix implies large correlations among some of its random variables. Therefore, as time evolves after a certain point, the suggested sequential decision-maker incorporates a new observation that is correlated with the previously used observations. The correlation does not allow the decision error probability PE to decrease beyond a certain point, although N constantly increases.
increase. On the other hand, a large condition number for a covariance matrix implies large correlations among some of its random variables. Therefore, as time evolves after a certain point, the suggested sequential decision-maker incorporates a new observation that is correlated with the previously used observations. The correlation does not allow the decision error probability PE to decrease beyond a certain point, although N constantly increases.
Figure 10.
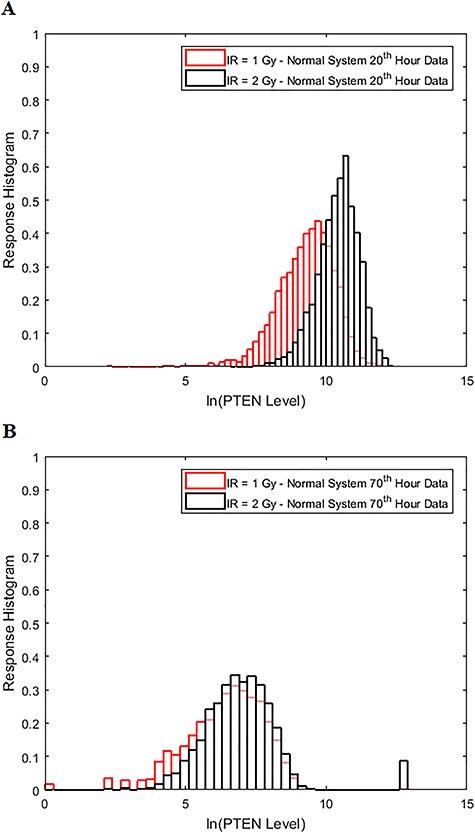
Comparison of the histograms of cell PTEN levels at the 20th and 70th hours under IR = 1 and 2 Gy doses in the normal p53 system. (A) Histograms of the 20th hour PTEN data under IR = 1 and 2 Gy doses, which show less overlap. (B) Histograms of the 70th hour PTEN data under IR = 1 and 2 Gy doses, which show more overlap.
Multivariable decision-making and signaling outcome analysis as time evolves
Now we focus on studying how decision-making works, if data of N time instants are utilized, such that N = 1, 2, …, 8 (Fig. 9B). In the figure, N = 1 means the PTEN data of the first hour, N = 2 refers to the PTEN data of the first and the 10th hours, and N = 3 indicates the PTEN data of the first, the 10th, the 20th hours, etc. This assumes at any given time, decision is made based on the data of that given time, plus the data of the previous time instants, which means progressively accumulating the data to make decisions. It is observed in Fig. 9B that PE first decreases, and after a certain point, it remains nearly constant. To understand this behavior, we note that if the data collected at various time instants are independent, then error probability of a decision-making system that performs sequential hypothesis testing decreases, as the number of observations N increases [42]. This property of a multivariate sequential decision-maker is intuitively appealing. However, if the data collected at various time instants are correlated, the performance of the multivariate sequential decision-maker can significantly degrade, and its error probability does not necessarily decrease, as N increases [42].
To examine possible temporal correlations among the data that the suggested sequential decision strategy employs, we compute condition numbers of 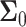 and
and 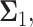 the
the 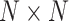 covariance matrices of the data for the two hypotheses H0 and H1, for IR = 1 and 2 Gy, respectively, as N increases from 2 to 8 (Fig. 9C). The condition number of a matrix is the ratio of its largest singular value to its smallest. A large condition number indicates that the matrix is nearly singular. On the other hand, a near singular covariance matrix of several random variables means that some of the random variables are highly correlated. Therefore, a large condition number for a covariance matrix implies large correlations among some of its random variables. We observe in Fig. 9C that as N increases, condition numbers of both of the covariance matrices
covariance matrices of the data for the two hypotheses H0 and H1, for IR = 1 and 2 Gy, respectively, as N increases from 2 to 8 (Fig. 9C). The condition number of a matrix is the ratio of its largest singular value to its smallest. A large condition number indicates that the matrix is nearly singular. On the other hand, a near singular covariance matrix of several random variables means that some of the random variables are highly correlated. Therefore, a large condition number for a covariance matrix implies large correlations among some of its random variables. We observe in Fig. 9C that as N increases, condition numbers of both of the covariance matrices 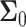 and
and 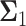 increase. This means as time evolves after a certain point, the suggested sequential decision-maker incorporates a new observation that is correlated with the previously used observations. The correlation does not allow the decision error probability to decrease beyond a certain point, although N constantly increases (Fig. 9B).
increase. This means as time evolves after a certain point, the suggested sequential decision-maker incorporates a new observation that is correlated with the previously used observations. The correlation does not allow the decision error probability to decrease beyond a certain point, although N constantly increases (Fig. 9B).
Multivariable analysis of two or more molecules: methods for computing decision thresholds and decision error rates using their concentration measurements in individual cells
So far we have focused on multivariable decision-making and signaling outcome analysis for one molecule at different time instants. However, the introduced methods and algorithms are not limited to the outcome analyses for just one molecule, and they can be applied to various other scenarios and studies. In fact, they can be used to analyze and compute decision error rates based on concentration levels of two or more molecules, measured simultaneously or even at different time instants. For example, if decision and outcome analysis are going to be conducted based on simultaneous concentration level measurements of two molecules labeled by x and y, then Equations (10–14) can be used to find the maximum likelihood bivariate decision strategy and its minimum error probability. As a more elaborate example, suppose concentration levels of molecule A measured at time instants t1 and t2 are labeled as variables x and y, respectively, concentration levels of molecule B measured at t1 and t2 are labeled as variables v and w, respectively, and finally concentration levels of molecule C measured at t1 and t2 are labeled as variables 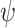 and
and 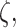 respectively. The
respectively. The 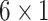 decision vector
decision vector 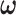 including all these six decision variables can be defined as
including all these six decision variables can be defined as 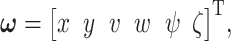 where T stands for transpose. Now Equations (15–17) can be used to find the maximum likelihood six-variate decision strategy and its minimum decision error probability.
where T stands for transpose. Now Equations (15–17) can be used to find the maximum likelihood six-variate decision strategy and its minimum decision error probability.
Effect of heterogeneity of initial values and reaction rates on cell response histograms
In addition to stochasticity in dynamic processes, it is natural to consider that the initial level of each protein is not the same in a cell population (heterogeneity of cells). Additionally, there are pseudo-first-order dephosphorylation reactions for which reaction rate coefficients depend on the levels of implicit phosphatases. Thus, it is also natural to assume that reaction rate coefficients corresponding to pseudo-first-order dephosphorylations may vary from cell to cell. To see the effect of heterogeneous initial values and parameters on cell response distributions, we generated new PTEN data for IR = 2 Gy in 5000 cells, assuming that the initial values and parameters of the p53 system are coming from lognormal distributions with means equal to their default values, and ran simulations for different standard deviations, i.e. σ = 0.2, 0.5 and 1 [43]. Cell response histograms of the new data for different σ values are shown in Fig. 11 and compared against the 2 Gy data of homogenous cells (σ = 0). In this system and example, we observe that PTEN histograms undergo some change as σ increases, i.e. more cell heterogeneity, which may result in some changes in decision error probabilities. Nevertheless, one can still use the exact same methods and algorithms introduced in the paper, to conduct signaling outcome analyses of interest for inhomogeneous cells.
Figure 11.
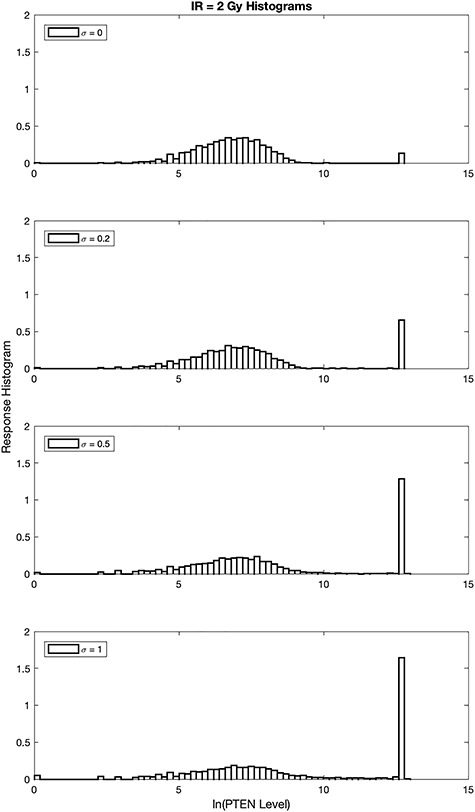
Effect of heterogeneity of initial values and pseudo-first-order dephosphorylation reaction rates on PTEN histograms. Histograms of PTEN levels of cells under IR = 2 Gy dose, with σ = 0, 0.2, 0.5 and 1.
BEYOND BINARY DECISIONS: TERNARY DECISIONS AND SIGNALING OUTCOMES AND TERNARY ERROR PROBABILITIES
While the focus of this paper is on binary hypothesis testing, it is possible to develop a multiple hypothesis testing model for outcome analysis, where there exist more than two possible outcomes. This entails more erroneous decisions than false alarm and miss events. Optimal decision thresholds and error probabilities for all the incorrect decisions can be similarly computed. For example, assume there are three different signaling outcomes depending on concentration level of a hypothetical molecule called MOL, whose level can fall within one of three regions, which results in the following three possible hypotheses:
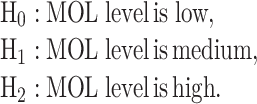 |
(18) |
Let us assume under each condition, PDF of the MOL level represented by x is normal or Gaussian, i.e. 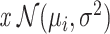 such that
such that 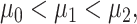 where variances are assumed to be equal, to simplify the notation. These PDFs are shown in Fig. 12, with
where variances are assumed to be equal, to simplify the notation. These PDFs are shown in Fig. 12, with 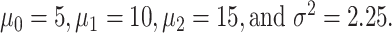 By extending the binary decision errors presented earlier in Equations (8) and (9), ternary decision errors for the three hypotheses can be written as
By extending the binary decision errors presented earlier in Equations (8) and (9), ternary decision errors for the three hypotheses can be written as
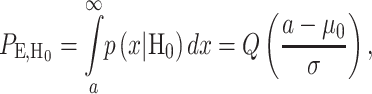 |
(19) |
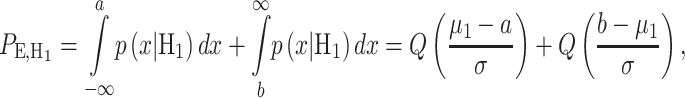 |
(20) |
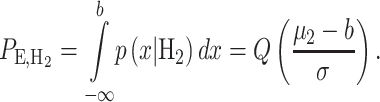 |
(21) |
Figure 12.
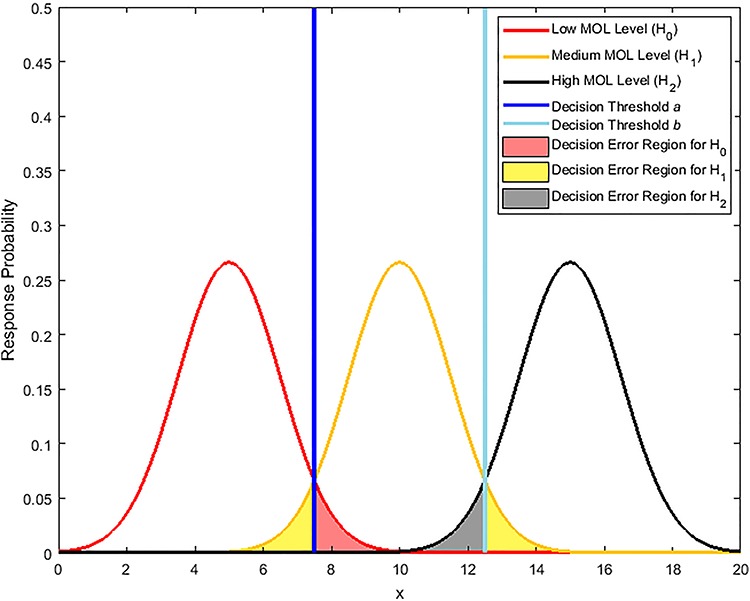
Response PDFs of a hypothetical molecule called MOL whose level entails a ternary decision-making process with three signaling outcomes. Shaded tail areas with the same color represent decision error regions associated with each specific hypothesis. Assuming equi-probable hypotheses, optimal maximum likelihood decision thresholds which minimize the total decision error probability are shown by vertical blue lines at the points of intersection of the probability density functions.
In the above equations, a and b are thresholds to decide between H0 and H1 and between H1 and H2, respectively. This means the decision regions for the three hypotheses can be written as:
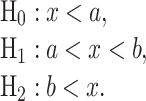 |
(22) |
For equi-probable hypotheses and similar to the derivation that leads to Equation (7), optimal decision thresholds which minimize the total decision error probability can be shown to be
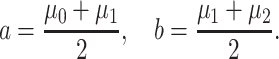 |
(23) |
Upon substituting (23) in (19–21), the total error probability in making ternary decisions can be written as:
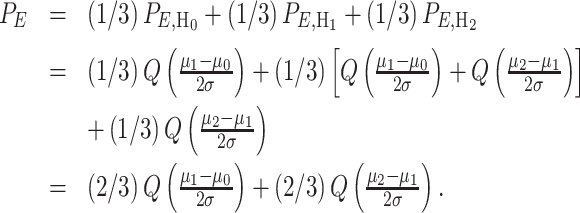 |
(24) |
As a reference, for the binary decision-making problems and outcome analysis studied earlier in the paper and using Equations (8) and (9), the total error probability in making binary decisions with equal variances simplifies to
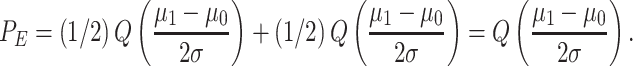 |
(25) |
To compare ternary and binary error probabilities, let us assume 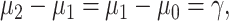 which reduces Equations (24) and (25) to
which reduces Equations (24) and (25) to 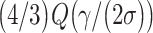 and
and 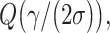 respectively. This indicates that the ternary decision error rate can be higher than the binary decision error rate, under the assumed conditions.
respectively. This indicates that the ternary decision error rate can be higher than the binary decision error rate, under the assumed conditions.
ON THE COSTS OF CORRECT AND INCORRECT DECISIONS
In decision theory, there can be some costs associated with correct or incorrect decisions. Let 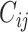 be the cost of deciding
be the cost of deciding  when
when 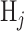 is true. To minimize the expected cost,
is true. To minimize the expected cost, 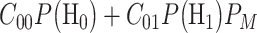
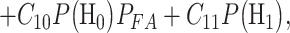 the decision-making system decides
the decision-making system decides 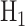 if [30]
if [30]
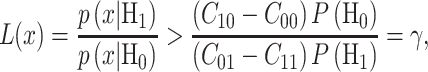 |
(26) |
where 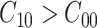 and
and 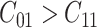 . Usually the costs associated with correct decisions are zero, i.e.
. Usually the costs associated with correct decisions are zero, i.e. 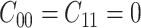 . Additionally, if there is no preference in assigning different costs to different incorrect decisions, one can choose
. Additionally, if there is no preference in assigning different costs to different incorrect decisions, one can choose 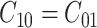 . This is what we would consider as well, since we do not have a knowledge of the costs of incorrect decisions in the studied cellular system. Upon substituting
. This is what we would consider as well, since we do not have a knowledge of the costs of incorrect decisions in the studied cellular system. Upon substituting 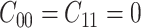 and
and 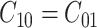 in the above equation, it simplifies to the following equation, which is the optimal maximum likelihood decision rule presented earlier in the paper:
in the above equation, it simplifies to the following equation, which is the optimal maximum likelihood decision rule presented earlier in the paper:
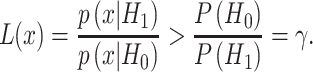 |
(27) |
CONCLUSION
This study presents a set of decision-theoretic, statistical signal processing and machine learning methods and metrics for modeling and measurement of decision-making processes and signaling outcomes under normal and abnormal conditions and in the presence of noise and other uncertainties. Due to the noise, signaling malfunctions or other factors, cells may respond differently to the same input signal. Some of these responses can be erroneous and unexpected. Here we present univariate and multivariate models and methods for decision-making processes and signaling outcome analyses and, as an example, apply them to an important system that is involved in cell survival and death, i.e. the p53 system shown in Fig. 1 (another decision analysis example can be found in the paper by Habibi et al. [1]). The p53 system becomes active due to DNA damage caused by IR, and as a result, a cell can take two different actions: it can either survive by repairing the DNA or trigger apoptosis. In this context, we model decisions and signaling outcomes triggered by the p53 system as a binary hypothesis testing problem, where two hypotheses are introduced in Equation (1). Regarding these two hypotheses, our approach identifies that there can be two types of incorrect decisions: ‘false alarm’ and ‘miss’. To compute the likelihood of these decisions, we employ the simulator of Hat et al. [9], to obtain single cell data of the p53 system, by exposing the cells to different radiation doses. We consider PTEN levels in cells as the decision variable, since it is a good predictor of cell fate [9]. Our analysis focuses on low radiation dose versus high radiation dose scenarios, where we fix the low IR dose at 1 Gy, whereas we set the high IR dose at 2–8 Gy. We also analyze decision-making events and signaling outcomes when an abnormality is present in the p53 system.
The incorrect decision probabilities provided in Equation (2) and the overall decision error probability in Equation (3) are computed after determining an optimal decision threshold. We obtain this decision threshold using the maximum likelihood principle which states that the best decision can be made by selecting the hypothesis that has the maximum probability of occurrence. We compute decision threshold and error probabilities using single time point data of PTEN levels in both normal and abnormal p53 systems. For 1 vs. 2 Gy and 1 vs. 8 Gy case studies, we present histograms, response distributions, decision thresholds and false alarm and miss decision regions in normal and abnormal p53 systems in Figs. 3 and 5, respectively. Our decision analysis reveals and quantifies that more erroneous decisions are made when deciding between two nearly the same radiation doses in the normal p53 system (Fig. 6). On the other hand, the difference between responses is easily identifiable for very low versus very high IR doses. This feature seems not to be present in the abnormal p53 systems (Fig. 6), according to our decision modeling approach. Our decision and outcome analyses and observations are further visualized and confirmed by using the ROC curves (Fig. 7), which are useful graphical tools to study the performance of decision-making systems. We would like to note that these observations are specifically made based on the low versus high IR case studies, e.g. d0 vs. d1 IRs introduced in the paper for the p53 system, as an example of a signaling network, in which the low IR dose is fixed to 1 Gy (d0 = 1 Gy) and the high IR dose is ranging from 2 up to 8 Gy (d1 = 2, 3, …, 8 Gy). Such conclusions may not be generalized to other biological hypotheses and systems, while the proposed framework and its analytical tools, whose introduction has been the main goal of this paper, can be similarly used.
In addition to the above univariate single time point analysis, we extend our signaling outcome modeling framework to dynamical multi-time point measurements and multi-dimensional decision making algorithms, to see how the number of decision variables affects the decisions and signaling outcomes over time. To introduce the concepts, first we conduct a bivariate analysis, for which bivariate response distributions of cell PTEN levels measured at two different time instants are shown in Fig. 8, as well as the optimal maximum likelihood decision boundary. Then we introduce a multivariate dynamic decision modeling framework, for the general scenario where there are more than two decision variables over time. This allows to model and understand how decision error probability changes over time, if at any time the decision is made based on the current observation, together with the previous observations. We observe in Fig. 9B that as the decision-making strategy incorporates more and more PTEN data of various time instants into its decisions, for the p53 system exposed to two radiation doses of 1 and 2 Gy, the decision error probability reaches its smallest value at a certain time instant. However, adding more data afterwards does not necessarily improve the decision precision, i.e. the decision error probability does not necessarily decrease as N increases with time (Fig. 9B). We show that this behavior can be related to the correlations that exist among the PTEN levels measured at different times (Fig. 9C).
Although we focus on multivariable decision-making and signaling outcome analysis for one molecule at different time instants, the introduced methods and algorithms are not limited to the outcome analyses for just one molecule. They can be applied to various other scenarios and studies. For instance, they can be used to analyze decision strategies and compute decision error rates based on concentration levels of two or more molecules, measured simultaneously or even at different time instants.
We finally show how the introduced binary decision-making and signaling outcome analysis models can be extended to more than two decisions, i.e. more than two hypotheses. A ternary scenario with three signaling outcomes is analyzed as an example, and it is shown that under certain conditions, ternary decision error probability can be higher than the binary one.
The methods and models presented here can be expanded to describe the performance and precision of more complex systems and networks such as the ones whose inputs are multiple ligands or secondary messengers and whose outputs are several transcription factors involved in certain cellular functions. Analyzing concentration levels of these transcription factors over time using the proposed approaches can model various decisions and signaling outcomes, and their probabilities, in the presence of noise or some cellular abnormalities and in response to the input signals.
The methods and formalism developed in this study are applicable to a wide variety of signaling outcome analyses, decision-makings and signal transduction processes where there are two or more possible outcomes. For example, in the context of Escherichia coli chemotaxis, binary decisions (influencing all chemotaxis processes) are either to continue motion in the same direction or to change the flagellum operation mode from run, counterclockwise, to tumble, clockwise, resulting in random direction changes [44]. Based on the network or system of interest and the available data, the hypotheses in Equation (1) can be revised, and subsequently the same mathematical framework and algorithms and methods can be applied, using the underlying probability distributions of data.
Overall, these decision-theoretic models and signaling outcome analysis methods can be beneficial for better understanding of transition from physiological to pathological conditions such as inflammatory diseases, various cancers and autoimmune diseases.
Funding
T.L. was supported by the National Science Center (Poland) [grant number 2018/29/B/NZ2/00668]. A.L. was supported by the National Institutes of Health [grant numbers GM072024, GM123011].
Conflict of interest statement
The authors declare that no conflicts of interest exist.
Author contributions
M.O. developed the methods, performed the simulations and computations and wrote the manuscript. T.L. contributed to and discussed the research topics and edited the manuscript. A.L. contributed to and discussed the research topics and edited the manuscript. E.S.E. contributed to and discussed the research topics and edited the manuscript. A.A. conceived the ideas and concepts, developed the methods and wrote the manuscript.
Contributor Information
Mustafa Ozen, Email: mo292@njit.edu.
Tomasz Lipniacki, Email: tlipnia@ippt.pan.pl.
Andre Levchenko, Email: andre.levchenko@yale.edu.
Effat S Emamian, Email: emame@atnt-usa.com.
Ali Abdi, Email: ali.abdi@njit.edu.
References
- 1. Habibi I, Cheong R, Lipniacki T et al. Computation and measurement of cell decision making errors using single cell data. PLoS Comput Biol 2014;13:e1005436. doi: 10.1371/journal.pcbi.1005436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kolitz SE, Lauffenburger DA. Measurement and modeling of signaling at the single-cell level. Biochemistry 2012;51:7433–43. doi: 10.1021/bi300846pPMID: 22954137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheong R, Rhee A, Wang CJ et al. Information transduction capacity of noisy biochemical signaling networks. Science 2011;334:354–8. doi: 10.1126/science.1204553PMID: 21921160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Balazsi G, Oudenaarden A, Collins JJ. Cellular decision making and biological noise: from microbes to mammals. Cell 2011;144:910–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell 2009;137:413–31. doi: 10.1016/j.cell.2009.04.037PMID: 19410540. [DOI] [PubMed] [Google Scholar]
- 6. Levine AJ. p53, the cellular gatekeeper for growth and division. Cell 1997;88:323–31PMID: 9039259. [DOI] [PubMed] [Google Scholar]
- 7. Kastan MB, Onyekwere O, Sidransky D et al. Participation of p53 protein in the cellular response to DNA damage. Cancer Res 1991;51:6304–11PMID: 1933891. [PubMed] [Google Scholar]
- 8. Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol 2007;35:495–516. doi: 10.1080/01926230701320337PMID: 17562483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hat B, Kochańczyk M, Bogdał MN et al. Feedbacks, bifurcations, and cell fate decision-making in the p53 system. PLoS Comput Biol 2016;12:e1004787. doi: 10.1371/journal.pcbi.1004787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Siliciano JD, Canman CE, Taya Y et al. DNA damage induces phosphorylation of the amino terminus of p53. Genes Dev 1997;11:3471–81PMID: 9407038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature 2000;408:307–10. [DOI] [PubMed] [Google Scholar]
- 12. Rothkamm K, Krüger I, Thompson LH et al. Pathways of DNA double-strand break repair during the mammalian cell cycle. Mol Cell Biol 2003;23:5706–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alberts B, Johnson A, Lewis J et al. Molecular Biology of the Cell, 4th edn. New York: Garland Science, 2002. [Google Scholar]
- 14. Iliakis G. The role of DNA double strand breaks in ionizing radiation-induced killing of eukaryotic cells. Bioessays 1991;13:641–8. doi: 10.1002/bies.950131204PMID: 1789781. [DOI] [PubMed] [Google Scholar]
- 15. Vilenchik MM, Knudson AG. Endogenous DNA double-strand breaks: production, fidelity of repair, and induction of cancer. Proc Natl Acad Sci U S A 2003;100:12871–6. doi: 10.1073/pnas.2135498100PMID: 14566050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saito S, Goodarzi AA, Higashimoto Y et al. ATM mediates phosphorylation at multiple p53 sites, including Ser46, in response to ionizing radiation. J Biol Chem 2002;277:12491–4PMID: 11875057. [DOI] [PubMed] [Google Scholar]
- 17. Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 2003;421:499–506. doi: 10.1038/nature01368PMID: 12556884. [DOI] [PubMed] [Google Scholar]
- 18. Maya R, Balass M, Kim S-T et al. ATM-dependent phosphorylation of Mdm2 on serine 395: role in p53 activation by DNA damage. Genes Dev 2001;15:1067–77. doi: 10.1101/gad.886901PMID: 11331603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shieh SY, Taya Y, Prives C. DNA damage-inducible phosphorylation of p53 at N-terminal sites including a novel site, Ser20, requires tetramerization. EMBO J 1999;18:1815–23. doi: 10.1093/emboj/18.7.1815PMID: 10202145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Banin S, Moyal L, Shieh S et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 1998;281:1674–7PMID: 9733514. [DOI] [PubMed] [Google Scholar]
- 21. Canman CE, Lim D-S, Cimprich KA et al. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 1998;281:1677–9. doi: 10.1126/science.281.5383.1677PMID: 9733515. [DOI] [PubMed] [Google Scholar]
- 22. Barak Y, Juven T, Haffner R et al. mdm2 expression is induced by wild type p53 activity. EMBO J 1993;12:461–8PMID: 8440237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fiscella M, Zhang H, Fan S et al. Wip1, a novel human protein phosphatase that is induced in response to ionizing radiation in a p53-dependent manner. Proc Natl Acad Sci U S A 1997;94:6048–53PMID: 9177166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Choi J, Nannenga B, Demidov ON et al. Mice deficient for the wildtype p53-induced phosphatase gene (Wip1) exhibit defects in reproductive organs, immune function, and cell cycle control. Mol Cell Biol 2002;22:1094–105PMID: 11809801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shreeram S, Hee WK, Demidov ON et al. Regulation of ATM/p53-dependent suppression of myc-induced lymphomas by Wip1 phosphatase. J Exp Med 2006;203:2793–9. doi: 10.1084/jem.20061563PMID: 17158963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Takekawa M, Adachi M, Nakahata A et al. p53-inducible Wip1 phosphatase mediates a negative feedback regulation of p38 MAPK-p53 signaling in response to UV radiation. EMBO J 2000;19:6517–26. doi: 10.1093/emboj/19.23.6517PMID: 11101524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stambolic V, MacPherson D, Sas D et al. Regulation of PTEN transcription by p53. Mol Cell 2001;8:317–25PMID: 11545734. [DOI] [PubMed] [Google Scholar]
- 28. Hlobilkova A, Knillova J, Svachova M et al. Tumour suppressor PTEN regulates cell cycle and protein kinase B/Akt pathway in breast cancer cells. Anticancer Res 2006;26:1015–22PMID: 16619501. [PubMed] [Google Scholar]
- 29. Bogdał MN, Hat B, Kochańczyk M et al. Levels of pro-apoptotic regulator bad and anti-apoptotic regulator Bcl-xL determine the type of the apoptotic logic gate. BMC Syst Biol 2013;7:67. doi: 10.1186/1752-0509-7-67PMID: 23883471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kay SM. Fundamentals of Statistical Signal Processing: Detection Theory. New Jersey: PTR Prentice-Hall, 1998. [Google Scholar]
- 31. Duda RO, Hart PE, Stork DG. Pattern Classification. New York: John Wiley & Sons, 2001. [Google Scholar]
- 32. Lu X, Ma O, Nguyen T-A et al. The Wip1 phosphatase acts as a gatekeeper in the p53-Mdm2 autoregulatory loop. Cancer Cell 2007;12:342–54. doi: 10.1016/j.ccr.2007.08.033PMID: 17936559. [DOI] [PubMed] [Google Scholar]
- 33. Li J, Yang Y, Peng Y et al. Oncogenic properties of PPM1D located within a breast cancer amplification epicenter at 17q23. Nat Genet 2002;31:133–4. doi: 10.1038/ng888PMID: 12021784. [DOI] [PubMed] [Google Scholar]
- 34. Saito-Ohara F, Imoto I, Inoue J et al. PPM1D is a potential target for 17q gain in neuroblastoma. Cancer Res 2003;63:1876–83PMID: 12702577. [PubMed] [Google Scholar]
- 35. Bulavin DV, Demidov ON, Saito S et al. Amplification of PPM1D in human tumors abrogates p53 tumor-suppressor activity. Nat Genet 2002;31:210–5. doi: 10.1038/ng894PMID: 12021785. [DOI] [PubMed] [Google Scholar]
- 36. Castellino RC, Bortoli MD, Lu X et al. Medulloblastomas overexpress the p53-inactivating oncogene WIP1/PPM1D. J Neurooncol 2007;86:245–56. doi: 10.1007/s11060-007-9470-8PMID: 17932621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hirasawa A, Saito-Ohara F, Inoue J et al. Association of 17q21-q24 gain in ovarian clear cell adenocarcinomas with poor prognosis and identification of PPM1D and APPBP2 as likely amplification targets. Clin Cancer Res 2003;9:1995–2004PMID: 12796361. [PubMed] [Google Scholar]
- 38. Rauta J, Alarmo E-L, Kauraniemi P et al. The serine-threonine protein phosphatase PPM1D is frequently activated through amplification in aggressive primary breast tumours. Breast Cancer Res Treat 2006;95:257–63. doi: 10.1007/s10549-005-9017-7PMID: 16254685. [DOI] [PubMed] [Google Scholar]
- 39. Geva-Zatorsky N, Rosenfeld N, Itzkovitz S et al. Oscillations and variability in the p53 system. Mol Syst Biol 2006;2:2006.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Van Trees HL, Bell KL, Tian Z. Detection, Estimation and Modulation Theory, Part I: Detection, Estimation, and Filtering Theory, 2nd edn. New Jersey: Wiley, 2013. [Google Scholar]
- 41. Papoulis A. Probability, Random Variables, and Stochastic Processes, 3rd edn. New York: McGraw-Hill, 1991. [Google Scholar]
- 42. Fukunaga K. Introduction to Statistical Pattern Recognition, 2nd edn. San Diego: Academic Press, 1990. [Google Scholar]
- 43. Grabowski F, Czyż P, Kochańczyk M et al. Limits to the rate of information transmission through the MAPK pathway. J R Soc Interface 2019;16:20180792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Watari N, Larson RG. The hydrodynamics of a run-and-tumble bacterium propelled by polymorphic helical flagella. Biophys J 2010;98:12–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


