Abstract
A personalized medicine approach seems to be particularly applicable to psychiatry. Indeed, considering mental illness as deregulation, unique to each patient, of molecular pathways, governing the development and functioning of the brain, seems to be the most justified way to understand and treat disorders of this medical category. In order to extract correct information about the implicated molecular pathways, data can be drawn from sampling phenotypic and genetic biomarkers and then analyzed by a machine learning algorithm. This review describes current difficulties in the field of personalized psychiatry and gives several examples of possibly actionable biomarkers of psychotic and other psychiatric disorders, including several examples of genetic studies relevant to personalized psychiatry. Most of these biomarkers are not yet ready to be introduced in clinical practice. In a next step, a perspective on the path personalized psychiatry may take in the future is given, paying particular attention to machine learning algorithms that can be used with the goal of handling multidimensional datasets.
Keywords: Neuroscience, Bioinformatics, Genetics, Pharmaceutical science, Molecular biology, Pathophysiology, Mathematical biosciences, Psychiatry, Evidence-based medicine, Biomarker, Human brain, Machine learning, Pharmacotherapy, RDoC, Schizophrenia
Neuroscience; Bioinformatics; Genetics; Pharmaceutical Science; Molecular Biology; Pathophysiology; Mathematical Biosciences; Psychiatry; Evidence-Based Medicine; Biomarker; human brain; Machine Learning; Pharmacotherapy; RDoC; schizophrenia
1. Introduction
The human brain is by far the most complex structure of the body. It is so exquisite we may still be far from fully understanding the way it functions, especially in regard to brain circuits that determine the human mind. Deviations of this tremendously intricate system from the normal function are often translated as psychiatric symptoms. It comes as no surprise that it is extremely challenging for a clinician to classify all existing manifestations of these deviations, in particular because defective brain circuits are always under influence of other factors, like healthy brain circuits, which are also different in different individuals [1]. The function of the brain is also altered, during the lifetime, by different molecular factors brought by epigenetics, stages of development and aging, and endocrine and immune systems. From the viewpoint of genetics, genes with pathogenic variants, associated with mental disorders, always interact with other genes, bearing functional, but not necessarily pathogenic variants. The overall result of the deviations from normal functioning under additional influences is thus truly unique to each psychiatric patient.
Psychiatric disorders have the heaviest toll on population health, in terms of Years Lived with Disability (YLDs), compared to any other medical category [2, 3]. Despite the pressing need for improved standards of care, the currently existing traditional diagnostic criteria, the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) and the International Statistical Classification of Diseases and Related Health Problems (ICD-10), fail to reflect the actual biology behind mental illnesses [4, 5, 6, 7]. If we do not understand the nature of the pathological phenomena we are dealing with, it is hard to imagine how we will be able to efficiently treat and prevent these disorders.
This general informative review describes current difficulties in personalized psychiatry, followed by several examples of biomarkers of psychotic and other psychiatric disorders, including genetic biomarkers. These biomarkers, with exception of a few pharmacogenetic-guided decision support tools, have not been yet introduced in clinical practice. In addition to the brief overview of the current status of clinical and biomedical research in psychiatry, the review gives a perspective on future developments, including the translation of scientific discoveries into clinical practice and machine learning algorithms that can be used with the goal of handling multidimensional datasets. The present review is different from previously published reviews on personalized psychiatry in three ways: (1) it is aimed at readers outside of the field of psychiatric research and is meant to be accessible to the general biological and medical community; (2) it gives examples of and discusses both genetic and phenotypic biomarkers, avoiding giving preference to one type of biomarkers over another; (3) contrary to other reviews dealing separately with biomarkers or with machine learning domain applications, the present review integrates both these aspects.
2. Current issues in psychiatry and possible solutions
2.1. Categorical classification systems in psychiatry are not based on etiology
Traditional diagnostic tools, DSM-5 and ICD-10, propose symptom-based categories into which we may “fit” the patients [6]. However, the actual clinical picture in each patient is more complex than the existing nosological entities: a patient may simultaneously “fit” into different diagnoses [8], present most but not all “required” signs and symptoms, and drift from one diagnosis to another. This may be explained in part by the technical difficulty diagnosing psychiatric illnesses, stemming from the absence of valid tests that can unambiguously indicate the diagnosis. However, insufficient power of existing diagnostic methods cannot be the only reason for these systematization difficulties. Molecular factors are shared between different nosological entities [9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22] and, at the same time, within one nosological entity there is a great variability [23, 24, 25, 26, 27, 28, 29, 30]. For example, schizophrenia (SCZ), while sharing symptoms with other psychiatric disorders [31], is a questionable umbrella term incorporating etiologically and pathophysiologically distinct syndromes [32, 33, 34, 35]. In other words, the existing psychiatric diagnoses are too narrow and too broad at the same time [5, 36].
2.2. Research Domain Criteria
Despite the apparent complexity of the biologically informed and personalized approach in psychiatry, this approach seems particularly relevant to this branch of medicine [23, 24, 25, 28, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56]. As an answer to the existing demand, a new experimental framework in psychiatry was proposed in 2009 by an internal workgroup of the National Institute of Mental Health staff: Research Domain Criteria (RDoC) that adopt a personalized approach based on dimensions, rather than categories [4, 6, 7, 57, 58, 59]. RDoC is a research initiative aimed at informing future versions of diagnostic systems (ICD and DSM) [4, 6, 57, 58, 59]. According to the NIMH Strategic Plan, it is “a classification system based upon dimensions of observable behavior and neurobiological measures” (http://www.nimh.nih.gov/about/strategic-planning-reports/index.shtml). Although it is not designed as an independent diagnostic system aimed at replacing the current systems, nor it is fully developed at present, the arrival of RDoC seems timely, because it is about to develop a long-awaited etiology-based framework [6]. RDoC considers six domains that encompass several constructs, represented by different faculties of the human brain (https://www.nimh.nih.gov/research-priorities/rdoc/constructs/rdoc-matrix.shtml). Examples are social communication, cognitive control, and circadian rhythms. These constructs are perceived as dimensions, and the patient is thus characterized in this “multidimensional space”. Each construct is defined by seven units of analysis that can be applied to evaluate it. The units of analysis are defined by quantitative evaluations, such as levels of hormones, gene expression levels, neuroimaging, or neurocognitive tests. In this manner, a particular faculty of the human brain is described as on a scale, ranging from “normal” to different degrees of “abnormal”.
RDoC principles have been put in action in clinical studies in order to define particular dimensions in groups of patients with different classical categorical diagnoses. For example, a continuum in severity of cognitive deficits was found when bipolar disorder (BD) type I and schizophrenic patients were characterized using the RDoC dimension “cognitive control” measured with a cognitive test and functional magnetic resonance imaging [60].
2.3. Biomarkers
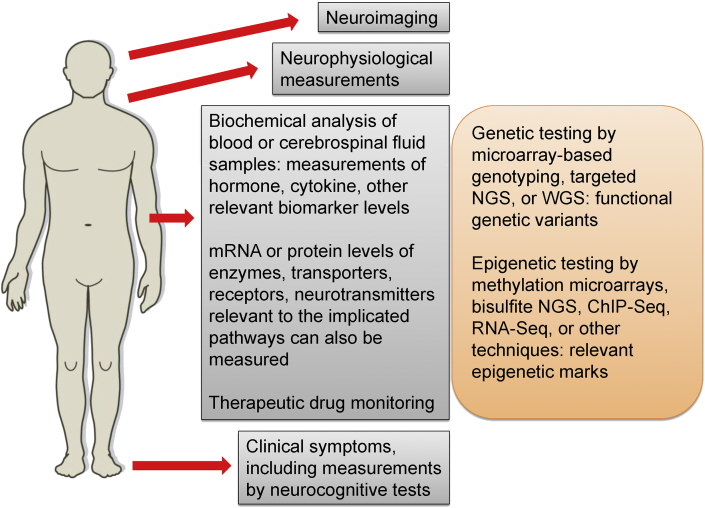
The framework of RDoC is just one potential path personalized psychiatry may take in the upcoming years. Another approach is based on a search for biomarkers of psychiatric disorders. According to the Biomarkers Definitions Working Group, a biomarker is “a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” [61]. In other words, a biomarker is a relevant biological characteristic that can be unambiguously evaluated with a test [62, 63, 64] (Figure 1). An example is the blood glucose level in diabetes. Biomarkers do not answer to all questions about health and disease, but they indicate particular aspects of biological processes.
Figure 1.
A possible case scenario of personalized approach in psychiatry. A number of biomarkers, including transcriptomic and proteomic profiling and response to medication, along with relevant genetic and epigenetic factors are considered for the following data analysis.
The quest for biomarkers in psychiatry has not been very successful [23, 24, 25, 28, 49, 51, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74]: as mentioned earlier, no biomarkers, with exception of a few pharmacogenetic-guided decision support tools, have been introduced in clinical practice. This might stem from the fact that researches try to discover a biomarker that would be present in all or in the majority of patients with a particular “diagnosis”. Given that the current nosology in psychiatry might not reflect the actual molecular etiologies, the quest for unifying biomarkers in what is defined as SCZ or major depressive disorder (MDD) seems like an unachievable goal [6]. Although a minority of psychiatric cases, such as SCZ (9.9%) and MDD (2.8%), were found to be cases of autoimmune encephalitis [75, 76], that could be neoplastic or viral in its origin [77], the etiology of the vast majority of psychiatric cases cannot be determined with one clear agent and instead these disorders are considered a result of a very complex interaction of a myriad of molecular factors [78, 79].
This does not mean biomarkers do not apply to personalized psychiatry. Quite the opposite, it is crucial to discover actionable biomarkers that will inform us about the molecular basis of the illness. These biomarkers would act as pieces of a puzzle that constitute the entire clinical picture in one particular individual. The pieces would inform us about the precise pathological processes and indicate an appropriate treatment. Despite the difficulties, there have been studies that indicated a number of potentially actionable biomarkers in psychiatry [23, 24, 25, 28, 49, 51, 65, 66, 67, 69, 70, 71, 72, 73, 80, 81, 82, 83, 84]. Described in the next sections are only several examples of such biomarkers of psychotic and other psychiatric disorders, including genetic biomarkers, and therefore a brief overview of the existing body of knowledge. This brief list is not meant to name only fully studied and reliable biomarkers that are ready to be introduced into clinic, because at this point such biomarkers, with exception of few pharmacogenetic biomarkers [45, 85, 86, 87, 88], have not been discovered.
Epigenetics is another root cause of the processes taking place in the cell that is not necessarily defined by changes in the DNA sequence. The reader is referred to reviews dealing with the subject of epigenetic biomarkers in psychiatry [23, 89, 90, 91, 92], as the present review will describe only genetic aspects, mentioning epigenetic aspects only briefly.
It is also worth mentioning that the present review will discuss only the pharmacological approach to treatment. Apart from pharmacology and another widely-used treatment approach, psychotherapy, various brain stimulation (neuromodulation) techniques (electroconvulsive therapy [93], non-invasive methods [94, 95, 96, 97], and deep brain stimulation [94, 98, 99, 100, 101, 102, 103, 104]) as well as gene therapy [105, 106] are potential approaches to treat psychiatric disorders. While some brain stimulation methods were approved for clinical use [96, 97, 107], gene therapy is an approach still limited to biomedical research laboratory settings, with the hope it will be translated into clinical settings sometime in the future.
3. Examples of potential phenotypic biomarkers of psychotic and other psychiatric disorders
Examples of potential neurophysiological, immune, and endocrine biomarkers are given below. Other proteomic [23, 49, 108, 109, 110], gene expression (transcriptomic) [20, 23, 49, 108], and neuroimaging [24, 73, 111, 112, 113, 114, 115, 116, 117, 118] biomarkers are additional important examples that are described elsewhere. These biomarkers have the potential to be used as diagnostic instruments and indicators of disease prognosis and treatment outcome (diagnostic, prognostic and predictive biomarkers, respectively [119]), but they have not yet been validated [23, 24, 25, 51, 64, 71, 72]. For these reasons, these potential biomarkers continue to be investigated in experimental settings either aimed at revealing molecular mechanisms behind them, or aimed at validating them as robust biomarkers.
3.1. Neurophysiological biomarkers
3.1.1. Mismatch negativity
The mismatch negativity (MMN) is a component of event-related potentials (ERP), measured with electroencephalography [25, 120, 121, 122]. MMN is a negative voltage deflection that is observed when among frequently presented standard auditory stimuli there is an infrequent deviant stimulus. This deflection is weaker in individuals with SCZ or those who later present psychotic symptoms [123, 124, 125, 126, 127, 128], which means that it can be used to identify high-risk individuals for preventive interventions. MMN is associated with poor functioning of patients in the community [129, 130, 131, 132, 133, 134] and reduced cognitive function in patients and healthy subjects [131, 132, 134, 135, 136, 137, 138].
These ERP deficits seem to be related to an N-methyl-D-aspartate (NMDA) receptor hypofunction [139, 140], because insufficient MMN can be corrected and clinical symptoms improved by treating schizophrenic patients with N-acetyl-cysteine that is subsequently converted to glutathione [141, 142]. Glutathione potentiates the NMDA receptor through its redox activity [143], in a similar manner NMDA receptor antagonists provoke psychotic symptoms [141]. NMDA receptor is a constituent of the postsynaptic density that is believed to be a hotspot of pathogenic alterations in a number of psychiatric disorders, such as SCZ, autism spectrum disorders (ASD), mental retardation, mood disorders, and obsessive-compulsive disorder (OCD) [23, 144, 145, 146].
This encephalographic measure appears to be an easy-to-use, cost-effective, reliable, and informative potential diagnostic instrument and an indicator of prognosis and treatment outcome for psychotic disorders [121]. It is also ready for use in large-scale multisite clinical studies of SCZ [147].
3.1.2. Retinal anomalies
Other potentially actionable diagnostic and prognostic biomarkers in psychiatry are retinal anomalies, measured by an electroretinogram, including reduction of cone and rod wave amplitudes, detected in BD and SCZ patients [148]. In addition, these waves were studied using mouse strains with either increased or decreased/absent levels of expression of GSK3α and GSK3β [149], whose protein products play a very important role in molecular pathways, implicated in psychiatric disorders, AKT/GSK3 and WNT [150, 151, 152]. In particular, the WNT pathway seems to be implicated in the pathogenesis of SCZ, ASD, and mental retardation [153, 154, 155, 156, 157, 158]. Expression levels of GSK3α and GSK3β were inversely correlated with cone and rod wave amplitudes, which underlines the pathogenic role of GSK3α and GSK3β excessive activity [149] that leads downstream to reduced levels of β-catenin in psychiatric pathology [150, 151, 152]. Furthermore, multiple retinal anomalies were observed in mouse models of decreased serotonin and increased dopamine functions that represent neurochemical pathologies described in MDD and SCZ, respectively [159]. The model relevant to MDD was Arg439His tryptophan hydroxylase 2 knockin, containing the analog of the human mutation Arg441His that inactivates the serotonin-synthesizing enzyme and decreases brain serotonin levels by ~80% [160]. The model relevant to SCZ was dopamine transporter knockout that results in increased extracellular levels of dopamine in the striatum [161]. In the model of decreased serotonin function an increase in cone b-wave implicit time was observed, whereas the model of hyperdopaminergia was characterized by a decrease in rod sensitivity; these retinal anomalies may therefore be biomarkers of MDD and SCZ [159].
3.1.3. Prepulse inhibition
A further example of potential diagnostic instrument and indicator of treatment outcome is prepulse inhibition (PPI) of the startle reflex, a capacity of the brain to filter out irrelevant stimuli that is determined by the limbic cortico-striato-pallido-pontine circuitry [25, 162]. Because a muscle reflex is measured, it is a sensorimotor gating measure. PPI is insufficient in schizophrenic patients, which seems to explain the nature of hallucinations and delusions as insufficient filtering of irrelevant stimuli. PPI was also found to be impaired in patients with OCD and ASD [162]. It is important to note however that PPI should be eventually used in clinical settings in combination with other biomarkers due to its low sensitivity and specificity [25, 162].
3.2. Immune and endocrine biomarkers
3.2.1. Pro-inflammatory signaling factors
Multiple studies indicated anomalies in the immune system in patients diagnosed with MDD, BD, SCZ, and ASD [23, 26, 29, 51, 63, 64, 69, 163, 164, 165, 166, 167, 168, 169, 170]. The overall finding is deregulation of pro-inflammatory and anti-inflammatory factors, but the most consistent finding is an increase of pro-inflammatory cytokines and other immune signaling factors (Table 1). Examples are interleukin 6 (IL-6), tumor necrosis factor α (TNF-α), C reactive protein (CRP), and the interferon α/β (IFN-α/β) signaling pathway components in MDD [49, 51, 171, 172, 173, 174]; IL-6, IL-1β, TNF-α, soluble TNF receptors 1 and 2 (sTNFR1 and 2), and CRP in BD [69, 70, 71, 163, 170]; IL-1β, IL-6, IL-12, TNF-α, and transforming growth factor β (TGF-β) in SCZ [165, 170, 175, 176, 177, 178, 179]; and sTNFR2, IL-4, IL-5, and IL-13 in ASD [180, 181]. It is not clear however whether the immune system deregulation in patients shares the etiology with mental disorders [182, 183, 184], for example, via the kynurenine pathway [23, 163, 185, 186, 187, 188], or is a consequence of a chronic illness [189, 190].
Table 1.
Examples of immune signaling factors and hormones that are increased in psychiatric patients.
| Biomarker | Disorder | References |
|---|---|---|
| IL-1β | BD, SCZ | [70, 71, 163, 165, 170, 175, 177, 214, 215] |
| IL-4, IL-5, IL-13 | ASD | [181] |
| IL-6 | MDD, BD, SCZ | [49, 51, 70, 71, 163, 172, 173, 174, 176, 177, 179] |
| IL-12 | SCZ | [177] |
| TNF-α | MDD, BD, SCZ | [49, 51, 69, 70, 71, 163, 172, 174, 177] |
| sTNFR1 | BD | [69, 71] |
| sTNFR2 | BD, ASD | [69, 180] |
| TGF-β | SCZ | [177] |
| CRP | MDD, BD | [49, 69, 70, 71, 173, 192] |
| IFN-α/β pathway | MDD | [171] |
| cortisol | MDD, BD, SCZ | [30, 51, 179, 195, 196, 197, 198, 199, 200] |
| insulin | SCZ | [30, 199, 200, 207, 213] |
Clinical studies also indicated potential usefulness of these biomarkers in predicting treatment outcomes [191]. For example, baseline serum CRP levels in patients with MDD could predict antidepressant treatment response [192].
There are many ways to evaluate immune factors in blood serum and cerebrospinal fluid [193], examples being a multiplex immunoassay [166] and liquid chromatography tandem mass spectrometry [194].
3.2.2. Hypothalamic-pituitary-adrenal axis and insulin
The hypothalamic-pituitary-adrenal axis in MDD, BD, and SCZ is overly active [30, 50, 51, 195, 196], and the patients are afflicted with elevated cortisol levels [30, 51, 179, 195, 196, 197, 198, 199, 200] (Table 1). In addition, these patients are often diagnosed with insulin resistance, accompanied with hyperinsulinemia [199, 200, 201, 202, 203, 204, 205, 206, 207, 208]. Cortisol is produced by the adrenal gland in response to stress and is known to cause insulin resistance [209]. These endocrine abnormalities could share a common etiology with mental illnesses, because reduced stress tolerance was observed in psychiatric patients [196, 198]. Additionally, insulin resistance, that can result in type 2 diabetes, and hyperinsulinemia, that can result in hypoglycemia, both have a negative impact on the neuronal function [210, 211, 212].
Although endocrine biomarkers in psychiatry sill await clinical validation, clinical studies indicate promising candidates, such as insulin levels in schizophrenic patients that may be predicting response to antipsychotic treatment and the time until patients in remission would relapse [213].
4. Examples of genetic studies relevant to personalized psychiatry
Single nucleotide polymorphism (SNP) genotyping is followed by statistical analyses of association between variants and phenotypes [21, 216, 217, 218, 219], including polygenic risk score analyses [217, 220, 221]. Despite impressive sample sizes and robust statistics, only a minority of these studies, unfortunately, is followed by functional assays for the associated variants, using in vitro and in vivo models [222].
Rare single nucleotide variants (SNVs) and small indels are discovered by sequencing [11, 12, 13, 157, 223, 224] and may be used to conduct population association studies. While rare and predicted to be deleterious protein-truncating and missense variants in 26 genes were found to be associated with ASD [224], at present, rare and predicted to be loss-of-function variants in just one gene, SETD1A, were found to be associated with SCZ [12]. No such genes were discovered in population association studies of other psychiatric disorders. The main reason for this lack of association with rare and deleterious variants is that psychiatric disorders are common and highly polygenic, so in order to detect association with rare variants, sample sizes exceeding what is presently used in genomic studies (10 000–130 000 cases) are needed [78].
Copy number variants (CNVs) are another category of rare variants of large effect on the pathogenesis and the reader is referred to other articles discussing the role of these genetic variants in psychiatry [225, 226, 227, 228].
These potential genetic biomarkers, with exception of a few pharmacogenetic-guided decision support tools, still awaits clinical validation and is not introduced in routine clinical practice. Same as in the previous section, below are given several examples of genetic biomarkers holding promise for future clinical use.
4.1. Association studies
There have been a number of association studies aimed at discovering genetic biomarkers in psychiatry [27, 28, 49, 50, 51, 229]. Despite the impressive amount of data, the results obtained are often inconsistent [27, 28, 49, 51]. This could be explained by the fact that symptom-based diagnostic categories used to group patients do not reflect biological reality [6].
One of these genetic studies confirmed association of interacting GSK3β and fragile X mental retardation-related protein 1 (FXR1) [230] with the level of mania in acute periods and depression in stabilized periods in BD patients [231]. Another study revealed the haplotype A-G-T in the promoter of the IL-1β gene that is associated not only with higher levels of expression of this cytokine in the dorsolateral prefrontal cortex (DLPFC) and a reduction of the total grey matter volume in schizophrenic patients and healthy controls [215], but also with transition to psychosis [214], which makes it a potential prognostic genetic biomarker. A further study investigated association between 1536 SNPs in 94 candidate genes and 16 primary and secondary neurophysiological and neurocognitive measurements in patients with SCZ [232, 233]. SNPs in 40 genes, including α-catenin CTNNA2 [234] and neuregulin NRG1 [235], were found to be associated with some of these measurements. Also, association between NRG1 and positive symptoms of SCZ [236], smooth pursuit eye movements [237], and parameters of an ERP component P300 [238] was revealed. Further studies showed association between the α7 nicotinic receptor gene CHRNA7 and the neurophysiological biomarker sensory gating/P50 suppression, but not with SCZ per se [239, 240, 241]. The SNP rs1344706 in the zinc-finger protein (ZNF) gene ZNF804A, associated with BD and SCZ in genome-wide association studies (GWAS) [242], was also associated with P300 parameters [243]. Another study estimated association between functional magnetic resonance imaging measurements and the same SNP [244]. This study revealed disturbed connectivity in the DLPFC, hippocampus, and amygdala in healthy carriers of the associated allele, a finding that recapitulated results in psychiatric patients. Similar results were obtained for neuroimaging data in BD, MDD, anorexia nervosa, attention deficit hyperactivity disorder (ADHD), ASD, and SCZ [19, 220].
4.2. Functional studies
The importance of functional studies is paramount because they are the most convincing evidence the link between a genetic variant and a biomarker is real. Statistical test results, no matter how significant they are and how many times replicated in independent samples, do not prove there is a cause and effect relationship. In other words, genetic biomarkers with evidence confirmed only by statistical association should be used in clinic to make decisions with great caution, because in such case we still do not understand the precise molecular mechanisms leading from a given genetic variant to the measurable phenotype. Meanwhile, functional alleles in several individual genes, discovered by sequencing or statistical association, were studied in detail, and results indicate particular pathological processes determined by these variants. Three examples are given below.
4.2.1. Kalirin
KALRN, coding for a Rac1 guanine nucleotide exchange factor kalirin, was found to bear a rare variant p.Asp1338Asn in two siblings, one with SCZ and the other with MDD and drug and alcohol addiction [245]. The presence of the variant was correlated with reduced cortical volume in the superior temporal sulcus in these patients. The mutation is found in the protein domain that catalyzes activation of the GTPase Rac1 and reduces this activation. Overexpression of the mutated variant in mature cortical pyramidal neurons resulted in abnormalities of dendritic spines. Furthermore, a murine model with reduced expression of the gene showed a reduced number of neurons in the murine brain region corresponding to the human superior temporal sulcus [245].
4.2.2. G protein-coupled receptor kinase interacting ArfGAP 1
Another rare variant p.Arg283Trp in G protein-coupled receptor kinase interacting ArfGAP 1, GIT1, was discovered in four unrelated patients with SCZ, in addition to six other rare coding variants in this gene in schizophrenic patients [246]. GIT1 is active in both presynaptic and postsynaptic neurons and in both excitatory and inhibitory synapses [247, 248, 249, 250, 251]. This protein controls synaptic vesicle dynamics, dendritic spine growth and localization of neurotransmitter receptors. As was demonstrated by in vitro studies using human cell lines, including hippocampal neurons, the variants resulted in reduced activation of p21 protein (Cdc42/Rac)-activated kinase 3 (PAK3) and mitogen-activated protein kinase (MAPK), a finding correlated with synaptic deficits [246].
4.2.3. Complement component 4
Two paralogous genes of complement component 4, C4A and C4B, are associated with SCZ [217]. The allele that is characterized by two long (presence of a human endogenous retroviral insert) copies in tandem of C4A is associated with the greatest risk of SCZ [222]. This allele is also associated with the highest level of C4A expression in the brain, a finding also true for schizophrenic patients when compared to controls. This gene is expressed in basically all compartments of human neurons: synapses, dendrites, axons, and cell bodies. In a murine model, C4 mediated synapse elimination (pruning) during postnatal development [222], a molecular mechanism believed to determine, when excessive, the pathogenesis of SCZ, a neurodevelopmental disorder with an insufficient number of synapses [252, 253, 254].
4.3. Pharmacogenetic studies
An important number of studies reported the statistical association of genes with response to treatment by antidepressants, mood stabilizers, antipsychotics, and antiepileptics, the results that were also supported by some functional studies [28, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 56, 255, 256, 257, 258].
4.3.1. Cytochrome P450 enzymes
The cytochrome P450 (CYP) enzymes are known drug-metabolizing enzymes that can determine drug concentration in the blood, depending on their genotype, and therefore drug availability, a pharmacokinetic aspect. These biomarkers thus indicate both the drug efficacy and safety. One gene is CYP2D6 whose protein product metabolizes 40% of antipsychotics, as well as drugs for ADHD and various antidepressants, such as tricyclics or tetracyclics, that are serotonin and/or norepinephrine reuptake inhibitors, and selective serotonin reuptake inhibitors [44, 45, 259]. The gene is characterized by a number of functional genetic variants that determine the catalytic activity of the enzyme. In particular, alleles that determine “poor metabolizers” are associated with increased availability of drugs such as haloperidol and risperidone and, as a consequence, higher rates of some undesirable side effects, such as tardive dyskinesia and weight gain [259]. Other CYP genes, such as CYP2C19, have analogous effects on pharmacokinetics [44, 45, 259]. CYP2D6 and CYP2C19 genotyping was shown to be cost-effective [260, 261] and is already available in some commercial pharmacogenetic tests [45, 85, 86, 87, 88], although considerable improvements of these tests are still needed, due to the fact that the testing results do not always agree with each other and the test design does not always follow reporting recommendations [262, 263].
4.3.2. Major histocompatibility complex proteins
Pharmacodynamic genetic biomarkers HLA-B∗15:02 and HLA-A∗31:01 indicate adverse reactions to carbamazepine [257, 264, 265], an antiepileptic drug also used to treat BD and other psychiatric disorders [266]. These biomarkers thus indicate the drug safety. HLA-B and HLA-A are genes coding for the major histocompatibility complex proteins that take part in the presentation of antigens to T-lymphocytes. The allele HLA-B∗15:02 codes for a protein with a particular three-dimensional configuration that allows binding carbamazepine; the result of this binding is a cytotoxic immune reaction [267, 268]. A similar mechanism may take place in case of HLA-A∗31:01. As a consequence, the carriers of these alleles are susceptible to develop severe cutaneous reactions [264, 265, 269, 270, 271], such as Stevens–Johnson syndrome and toxic epidermal necrolysis. Genotyping HLA-B and HLA-A genes in patients before prescribing carbamazepine is therefore advised in order to alleviate these life-threatening adverse reactions [272, 273, 274] and was shown to be cost-effective [260]. As a result, genetic testing of these alleles is currently available [274, 275], although, as mentioned above, the commercial tests need major improvements [263].
4.3.3. Glycogen synthase kinase 3-beta
A potential pharmacodynamic genetic biomarker is the functional SNP rs334558 [276] in the gene GSK3β [150] coding for glycogen synthase kinase 3-beta that is associated with response to antidepressant medication in patients with depressive disorders [277, 278] and lithium treatment in patients with BD [279, 280, 281, 282, 283, 284]. The variant rs334558, found in the promoter, determines the expression level of GSK3β; in particular, the allele T is associated with 1.4-fold increased transcriptional strength, compared to the ancestral allele C, apparently because the nucleotide T creates a new binding site for the transcription factor AP4 [276]. The SNP rs334558 could therefore be viewed as a pharmacogenetic biomarker for mood disorders [53, 258], but it should be used in conjunction with other biomarkers due to its low specificity [278]. This potential predictive biomarker should be further studied in clinical settings, before a reliable genetic test is developed.
5. A possible route that personalized psychiatry may take in the future
5.1. Translation of scientific discoveries into clinical practice
Genetic, epigenetic, and phenotypic data may be used to determine the appropriate treatment for an individual. The genotyping in clinic could be based on microarray technologies, if only previously known genetic variants are considered, and sequencing may be based on next-generation sequencing (NGS) technologies. NGS can be either whole genome (WGS), or targeted to all or particular exons and regulatory sequences. Epigenetic testing may be performed by methylation microarrays, bisulfite NGS, chromatin immunoprecipitation sequencing (ChIP-Seq), RNA-Seq, or other relevant techniques. All relevant, informative biomarkers, such as neurophysiological, neuroimaging, and biochemical measurements, should be sampled. In addition, transcriptomic and proteomic profiling could be made, using appropriate gene expression microarray, RNA-Seq, protein microarray, or mass spectrometry techniques (Figure 1). Examples of early attempts to apply such multidimensional analyses to sets of biomarkers in order to better characterize patients include a study that combined genetic, neuroimaging and clinical biomarkers thus defining three subtypes of schizophrenic patients [285].
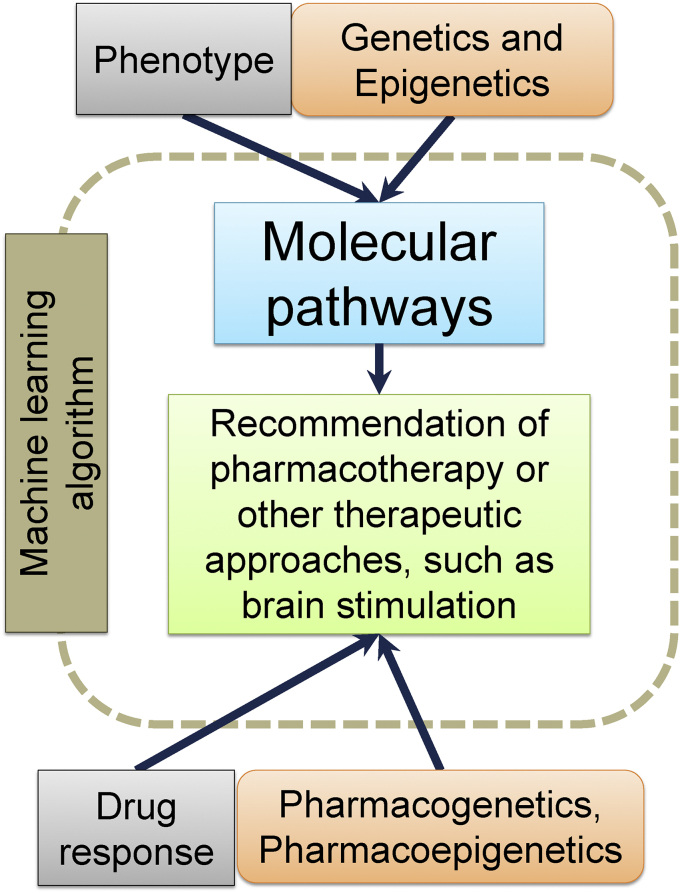
The most important information to be deduced from biomarkers is molecular pathways implicated in the pathogenesis in relevant cell types [15, 41, 286] (Figure 2). The pathways encompass all DNA, RNA, and protein interactions in the cell, from gene expression regulation to exocytosis. Genetics and epigenetics will indicate the possible etiology and information drawn from the sampling of phenotypic biomarkers will indicate the phenotypic consequences; between the two are molecular pathways that are altered. An example of the pathway is the NMDA receptor signaling complex found in the postsynaptic density of glutamatergic neurons that is believed to be one of the hotspots where pathogenic events resulting in a number of psychiatric disorders take place [144].
Figure 2.
Machine learning algorithm applications. Multidimensional data may be analyzed by a machine learning algorithm that integrates the genotype, epigenotype, and phenotype of an individual to extract information about molecular pathways implicated in the pathogenesis. Genetic, epigenetic, and therapeutic drug monitoring data from the individual is also used to choose the safest and most efficient pharmacological agents, and, at the same time, information about the pathways is used to select the most appropriate pharmacological agents. Alternative therapeutic approaches, such as brain stimulation techniques, can also be chosen.
In order to create a link between genetics, epigenetics, phenotype, and molecular pathways, it is crucial to carefully study all existing functional genetic variants and epigenetic marks that can result in significant alterations in protein or non-coding RNA function. We are still far from this complete knowledge, as the genetic and epigenetic basis of the vast majority of biomarkers is still unknown, and for the vast majority of discovered genetic variants and epigenetic marks, statistically associated with mental illnesses, there are no described pathogenic molecular mechanisms. Functional studies revealing the link between genetic, epigenetic, and phenotypic biomarkers are therefore essential for the development of a personalized approach in psychiatry [78]. Novel approaches, such as multiple assays of variant effects [287] and integrative systems like ClinGen [288], could be useful in establishing a list of actionable variant candidates.
When the picture of altered molecular pathways is drawn, it will be possible to choose the most correctly targeted therapeutic approach, including pharmacotherapy, i.e. biologically active molecules that will be able to correct faults in the pathways in the most efficient way. At this point, pharmacogenetics and pharmacoepigenetics will be taken into account, because genotyping or sequencing will indicate the patient's pharmacokinetic and pharmacodynamic particularities, which will help choosing the treatment correctly. At the same time, therapeutic drug monitoring [289] should be applied in order to integrate pharmacogenetic and pharmacoepigenetic parameters with actual clinical measurements (Figures 1 and 2). Again, we are still far from full understanding of all aspects of pharmacogenetics and pharmacoepigenetics, and, because we still have not fully understood the existing pathogenic mechanisms behind mental illnesses, we have not discovered all relevant pharmacological agents. Functional pharmacogenetic, pharmacoepigenetic, and drug development studies are therefore also essential for the advancement of a personalized approach in psychiatry. In addition, not all pharmacogenetic (or pharmacoepigenetic) testing in clinic has yet been shown convincingly to be cost-effective [261, 290], so more prospective studies evaluating cost-effectiveness, together with development of new cost-effective treatment schemes, are needed. Furthermore, considerable improvements of these commercial tests are warranted, because the testing results do not always agree with each other, and the test designs do not always follow reporting recommendations [262, 263].
5.2. Data analysis using machine learning algorithms
The scenario described above actually signifies a tremendous amount of multidimensional, heterogeneous (and often sparse) data that will be very challenging to analyze. In particular, it will be necessary to take account of all other genetic and epigenetic factors, not only the pathogenic ones. To accomplish this goal, it seems appropriate to apply data-driven analysis methods from machine learning domain [291, 292, 293], including deep learning [294], as their power to translate complex pattern discovery in large and varied data into clinically relevant practices has been recently demonstrated in several neuroscience research projects, including PsychENCODE [295, 296, 297]. Since these approaches are generally agnostic as to the underlying mechanisms, they facilitate both the de novo discovery and statistical modeling of molecular pathways in relevant cell types. Thus, in order to generate the most justified answer in a form of a treatment strategy, such models incorporate biomarker measurements, therapeutic drug monitoring and electronic health records (Figure 2).
5.2.1. Supervised learning
Machine learning may be supervised or unsupervised. Supervised learning (e.g. decision trees, k-nearest neighbor algorithm, support vector machines, some of neural-network algorithms) is the machine learning task type associated with learning functional relations based on input-output data pairs (also called features versus target variables) [298]. By training on example pairs, the algorithm infers a function which allows predicting the best-possible outcome (i.e. a target variable) for mapping a new unseen input data point (i.e. a single patient).
Supervised machine learning may be associated with several diagnostic applications, such as prevention and early diagnosis of mental health issues [299], exemplified by measurements of symptom progression in individuals at ultra-high risk for developing psychosis based on magnetic resonance imaging (MRI) data [300, 301]. This technique can also be used for diagnosis confirmation. For example, 70% of BD patients are misdiagnosed, most often with MDD [302, 303]. This problem may be solved by applying the support vector machine, a multivariable supervised learning task and a sensitive classification algorithm, to MRI scans of patients with BD and MDD. This classification algorithm achieved an accuracy approaching 80% in differentiating the two categories of patients [304].
Another promising application for supervised machine learning in psychiatry is long-term clinical prognoses, in particular, with the help of electronic health records (EHRs) [305]. EHRs can be also used for extraction of RDoC dimensions that demonstrated accurate predictions regarding length of hospital stay and hospital readmission [306]. A multivariate pattern recognition study of MDD which used a combination of clinical data and structural/functional MRI indicated that different disease trajectories, i.e. chronic, improving, and fast remission, could be discriminated with accuracies near 70% over two-month period [307]. Similar results of the functional outcome prediction over four weeks and over a year have been obtained for patients with first-episode psychosis, with accuracies surpassing 70% [308]. These applications can potentially be extended for predicting remissions, relapses, symptoms severity and quality of life in general.
Another issue which can be successfully solved by supervised machine learning algorithms is the determination of optimal treatment and dosage. Current success rate of treatment choices is dramatically low: only one third of individuals with a mental disorder who receive either drug treatment or psychotherapy fell into the category of ‘at least minimally adequate treatment’ [309]. Examples of successful supervised machine learning applications include predictions of clinical remission in response to drug treatments with escitalopram, citalopram, or sertraline in MDD patients [310, 311]. In these studies predictive models were generated from patient-reported clinical data [311] or behavioral tests of cognitive and emotional capacities [310]. An additional study also found that pre-treatment electroencephalogram measurements can be used for estimating response to antipsychotic treatment with clozapine in schizophrenic patients with total classification accuracy of 81.4% [312]. A further useful application for predictive modeling is assessment of drug side effects and of their severity. For example, three classification algorithms, i.e. artificial neural-network, support vector machine, and logistic regression, predicted metabolic syndrome in SCZ and schizoaffective disorder patients treated with atypical antipsychotics with accuracies of about 80% [313].
5.2.2. Unsupervised learning
As mentioned earlier, nosological classifications of psychiatric disorders in DSM and ICD need to be revised. All kinds of data from genomics and circuits to behavior and self-reports may be used to provide a new classification representing both underlying molecular basis of disease and existing clinical groups of patients. Since unsupervised machine learning, including cluster analysis (e.g. k-means, affinity propagation and DBSCAN algorithms), finds hidden patterns in data, it can be used for re-working existing mental health nosological systems [314, 315, 316, 317, 318]. Importantly, cluster analysis should be used in combination with dimensionality reduction techniques that transform a high-dimensional dataset into a smaller one [315, 319]. Cluster identification may also be based on the provisions of the RDoC initiative [315, 318]. Examples of applications include analysis of neuroimaging and physiological recordings that revealed three subtypes of ADHD with different types of emotional regulation labeled as mild, surgent and irritable [316]. Also, clinical characterization using cognitive and electrophysiological data of schizophrenic [317] and cognitive and neuroimaging data of BD [318] patients suggested distinct biologically validated subtypes of these disorders.
5.2.3. Limitations of machine learning in psychiatry
Although machine learning approaches are effective in application to clinical computational psychiatry, that is, in stratifying individuals in reference to diagnostic, prognostic, and treatment decisions, there are pitfalls and obstacles which also need to be examined carefully. Obvious limitations for deployment of state-of-the-art algorithms for psychiatric care are probably the size of available datasets, their heterogeneity, insufficient phenotypic annotation and incomplete medical records. Compared to other non-medical domains where, as it has been demonstrated, the sufficient predictive power for deep learning can be achieved if a sample size exceeds 106 samples [320], the typical cohort size in psychiatric research is far less than this threshold. Also, there are very few studies where all elements of the data triad (i.e. genotype, epigenotype, and phenotype) essential for understanding the disease trajectories and clinical guidance development are available. Furthermore, the confounding influences of different experimental protocols, standard operating procedures, standards of care, cohort and population biases may contribute to inflated prediction performance and irreproducibility of the results, thus highlighting the importance of accurate accounting for confounding variables in machine learning applications for mental health research.
Last but not least, application of many machine learning tools and more importantly interpretation of results (e.g. why a sample is classified and how a model works) require substantial expertise and are frequently opaque to clinical researchers and clinicians. This creates a major barrier in the translation of statistical machine learning methods into routine clinical practice, which emphasizes the importance of educational initiatives that facilitate knowledge exchange between clinical researchers, experimentalists and data scientists.
6. Conclusions
This review described current challenges and possible future developments in psychiatry. The description of the multidimensional approach was intentionally simplified: epigenetic aspects and other therapeutic approaches, such as brain stimulation techniques, were only briefly mentioned. But, if personalized medicine in psychiatry one day becomes reality, genetic (including functional common SNPs, rare SNVs, as well as CNVs), epigenetic, and phenotypic biomarkers, pharmacology, psychotherapy, brain stimulation techniques, and gene therapy must all be taken into picture with the goal of translating scientific discoveries into clinical practice. Machine learning algorithms will be the only possible way to analyze these multidimensional datasets, in order to extract meaningful information from them. In this sense, psychiatrists will have to learn to work with these algorithms, and one of the roles of a psychiatrist in the process of determining the diagnosis will be to confirm the choices and treatment recommendations, made by the machine.
In order to make this scenario possible, functional studies aiming at understanding molecular networks implicated in the pathogenesis and response to treatment are of paramount importance [78]. Further development of new methods, allowing multiplex functional studies, is therefore needed [287, 321]. This research will make much more sense if sets of biomarkers or RDoC dimensions are considered, instead of the presently existing categorical nosological entities.
Declarations
Author contribution statement
Anastasia Levchenko: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Timur Nurgaliev: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Alexander Kanapin, Anastasia Samsonova: Analyzed and interpreted the data; Wrote the paper.
Raul R. Gainetdinov: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Russian Science Foundation (grant number 19-75-30008).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Alexander Kanapin and Anastasia Samsonova were supported by funding for the project number 51148284 from Saint Petersburg State University, Saint Petersburg, Russia.
References
- 1.Lydon-Staley D.M., Bassett D.S. Network neuroscience: a framework for developing biomarkers in psychiatry. Curr. Top Behav. Neurosci. 2018:1–31. doi: 10.1007/7854_2018_41. [DOI] [PubMed] [Google Scholar]
- 2.Prince M., Patel V., Saxena S., Maj M., Maselko J., Phillips M.R. No health without mental health. Lancet. 2007;370:859–877. doi: 10.1016/S0140-6736(07)61238-0. [DOI] [PubMed] [Google Scholar]
- 3.Vigo D., Thornicroft G., Atun R. Estimating the true global burden of mental illness. Lancet Psychiatr. 2016;3:171–178. doi: 10.1016/S2215-0366(15)00505-2. [DOI] [PubMed] [Google Scholar]
- 4.Morris S.E., Cuthbert B.N. Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin. Neurosci. 2012;14:29–37. doi: 10.31887/DCNS.2012.14.1/smorris. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smoller J.W., Andreassen O.A., Edenberg H.J., Faraone S.V., Glatt S.J., Kendler K.S. Psychiatric genetics and the structure of psychopathology. Mol. Psychiatr. 2018 doi: 10.1038/s41380-017-0010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark L.A., Cuthbert B., Lewis-Fernandez R., Narrow W.E., Reed G.M. Three approaches to understanding and classifying mental disorder: ICD-11, DSM-5, and the national Institute of mental health's research domain criteria (RDoC) Psychol. Sci. Publ. Interest. 2017;18:72–145. doi: 10.1177/1529100617727266. [DOI] [PubMed] [Google Scholar]
- 7.Insel T.R., Cuthbert B.N. Medicine. Brain disorders? Precisely. Science. 2015;348:499–500. doi: 10.1126/science.aab2358. [DOI] [PubMed] [Google Scholar]
- 8.Kessler R.C., McGonagle K.A., Zhao S., Nelson C.B., Hughes M., Eshleman S. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the national comorbidity survey. Arch. Gen. Psychiatr. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 9.Doherty J.L., Owen M.J. Genomic insights into the overlap between psychiatric disorders: implications for research and clinical practice. Genome Med. 2014;6:29. doi: 10.1186/gm546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talkowski M.E., Rosenfeld J.A., Blumenthal I., Pillalamarri V., Chiang C., Heilbut A. Sequencing chromosomal abnormalities reveals neurodevelopmental loci that confer risk across diagnostic boundaries. Cell. 2012;149:525–537. doi: 10.1016/j.cell.2012.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarthy S.E., Gillis J., Kramer M., Lihm J., Yoon S., Berstein Y. De novo mutations in schizophrenia implicate chromatin remodeling and support a genetic overlap with autism and intellectual disability. Mol. Psychiatr. 2014;19:652–658. doi: 10.1038/mp.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh T., Kurki M.I., Curtis D., Purcell S.M., Crooks L., McRae J. Rare loss-of-function variants in SETD1A are associated with schizophrenia and developmental disorders. Nat. Neurosci. 2016;19:571–577. doi: 10.1038/nn.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fromer M., Pocklington A.J., Kavanagh D.H., Williams H.J., Dwyer S., Gormley P. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506:179–184. doi: 10.1038/nature12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International Schizophrenia Consortium. Purcell S.M., Wray N.R., Stone J.L., Visscher P.M., O'Donovan M.C. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Network, Pathway Analysis Subgroup of Psychiatric Genomics Consortium Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nat. Neurosci. 2015;18:199–209. doi: 10.1038/nn.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kendler K.S., Aggen S.H., Knudsen G.P., Roysamb E., Neale M.C., Reichborn-Kjennerud T. The structure of genetic and environmental risk factors for syndromal and subsyndromal common DSM-IV axis I and all axis II disorders. Am. J. Psychiatr. 2011;168:29–39. doi: 10.1176/appi.ajp.2010.10030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hormozdiari F., Penn O., Borenstein E., Eichler E.E. The discovery of integrated gene networks for autism and related disorders. Genome Res. 2015;25:142–154. doi: 10.1101/gr.178855.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cross-Disorder Group of the Psychiatric Genomics Consortium. Lee S.H., Ripke S., Neale B.M., Faraone S.V., Purcell S.M. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat. Genet. 2013;45:984–994. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee P.H., Baker J.T., Holmes A.J., Jahanshad N., Ge T., Jung J.Y. Partitioning heritability analysis reveals a shared genetic basis of brain anatomy and schizophrenia. Mol. Psychiatr. 2016;21:1680–1689. doi: 10.1038/mp.2016.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gandal M.J., Haney J.R., Parikshak N.N., Leppa V., Ramaswami G., Hartl C. Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science. 2018;359:693–697. doi: 10.1126/science.aad6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brainstorm Consortium. Anttila V., Bulik-Sullivan B., Finucane H.K., Walters R.K., Bras J. Analysis of shared heritability in common disorders of the brain. Science. 2018;360 doi: 10.1126/science.aap8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cross-Disorder Group of the Psychiatric Genomics Consortium Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381:1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitt A., Martins-de-Souza D., Akbarian S., Cassoli J.S., Ehrenreich H., Fischer A. Consensus paper of the WFSBP task force on biological markers: criteria for biomarkers and endophenotypes of schizophrenia, part III: molecular mechanisms. World J. Biol. Psychiatr. 2017;18:330–356. doi: 10.1080/15622975.2016.1224929. [DOI] [PubMed] [Google Scholar]
- 24.Schmitt A., Rujescu D., Gawlik M., Hasan A., Hashimoto K., Iceta S. Consensus paper of the WFSBP Task Force on Biological Markers: criteria for biomarkers and endophenotypes of schizophrenia part II: cognition, neuroimaging and genetics. World J. Biol. Psychiatr. 2016;17:406–428. doi: 10.1080/15622975.2016.1183043. [DOI] [PubMed] [Google Scholar]
- 25.Thibaut F., Boutros N.N., Jarema M., Oranje B., Hasan A., Daskalakis Z.J. Consensus paper of the WFSBP task force on biological markers: criteria for biomarkers and endophenotypes of schizophrenia part I: neurophysiology. World J. Biol. Psychiatr. 2015;16:280–290. doi: 10.3109/15622975.2015.1050061. [DOI] [PubMed] [Google Scholar]
- 26.Guest P.C., Chan M.K., Gottschalk M.G., Bahn S. The use of proteomic biomarkers for improved diagnosis and stratification of schizophrenia patients. Biomarkers Med. 2014;8:15–27. doi: 10.2217/bmm.13.83. [DOI] [PubMed] [Google Scholar]
- 27.Zai G., Robbins T.W., Sahakian B.J., Kennedy J.L. A review of molecular genetic studies of neurocognitive deficits in schizophrenia. Neurosci. Biobehav. Rev. 2017;72:50–67. doi: 10.1016/j.neubiorev.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 28.Ozomaro U., Wahlestedt C., Nemeroff C.B. Personalized medicine in psychiatry: problems and promises. BMC Med. 2013;11:132. doi: 10.1186/1741-7015-11-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan M.K., Guest P.C., Levin Y., Umrania Y., Schwarz E., Bahn S. Converging evidence of blood-based biomarkers for schizophrenia: an update. Int. Rev. Neurobiol. 2011;101:95–144. doi: 10.1016/B978-0-12-387718-5.00005-5. [DOI] [PubMed] [Google Scholar]
- 30.Guest P.C., Martins-de-Souza D., Vanattou-Saifoudine N., Harris L.W., Bahn S. Abnormalities in metabolism and hypothalamic-pituitary-adrenal axis function in schizophrenia. Int. Rev. Neurobiol. 2011;101:145–168. doi: 10.1016/B978-0-12-387718-5.00006-7. [DOI] [PubMed] [Google Scholar]
- 31.Tandon R., Nasrallah H.A., Keshavan M.S. Schizophrenia, "just the facts" 4. Clinical features and conceptualization. Schizophr. Res. 2009;110:1–23. doi: 10.1016/j.schres.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Tandon R. The nosology of schizophrenia: toward DSM-5 and ICD-11. Psychiatr. Clin. North Am. 2012;35:557–569. doi: 10.1016/j.psc.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 33.Tandon R., Keshavan M.S., Nasrallah H.A. Schizophrenia, "just the facts" what we know in 2008. 2. Epidemiology and etiology. Schizophr. Res. 2008;102:1–18. doi: 10.1016/j.schres.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 34.Keshavan M.S., Tandon R., Boutros N.N., Nasrallah H.A. Schizophrenia, "just the facts": what we know in 2008 Part 3: neurobiology. Schizophr. Res. 2008;106:89–107. doi: 10.1016/j.schres.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 35.Tandon R., Maj M. Nosological status and definition of schizophrenia: some considerations for DSM-V and ICD-11. Asian J. Psychiatr. 2008;1:22–27. doi: 10.1016/j.ajp.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Owen M.J. New approaches to psychiatric diagnostic classification. Neuron. 2014;84:564–571. doi: 10.1016/j.neuron.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 37.Smoller J.W. Psychiatric genetics and the future of personalized treatment. Depress. Anxiety. 2014;31:893–898. doi: 10.1002/da.22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alda M. Personalized psychiatry: many questions, fewer answers. J. Psychiatry Neurosci. 2013;38:363–365. doi: 10.1503/jpn.130221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wium-Andersen I.K., Vinberg M., Kessing L.V., McIntyre R.S. Personalized medicine in psychiatry. Nord. J. Psychiatr. 2017;71:12–19. doi: 10.1080/08039488.2016.1216163. [DOI] [PubMed] [Google Scholar]
- 40.Madan A., Walker C.R., Weinstein B., Fowler J.C. Pharmacogenomics in practice: a case report of personalized inpatient psychiatric care. Pharmacogenomics. 2015;16:433–439. doi: 10.2217/pgs.15.9. [DOI] [PubMed] [Google Scholar]
- 41.Demkow U., Wolanczyk T. Genetic tests in major psychiatric disorders-integrating molecular medicine with clinical psychiatry-why is it so difficult? Transl. Psychiatry. 2017;7:e1151. doi: 10.1038/tp.2017.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McMahon F.J., Insel T.R. Pharmacogenomics and personalized medicine in neuropsychiatry. Neuron. 2012;74:773–776. doi: 10.1016/j.neuron.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamilton S.P. The promise of psychiatric pharmacogenomics. Biol. Psychiatr. 2015;77:29–35. doi: 10.1016/j.biopsych.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 44.Eap C.B. Personalized prescribing: a new medical model for clinical implementation of psychotropic drugs. Dialogues Clin. Neurosci. 2016;18:313–322. doi: 10.31887/DCNS.2016.18.3/ceap. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pouget J.G., Shams T.A., Tiwari A.K., Muller D.J. Pharmacogenetics and outcome with antipsychotic drugs. Dialogues Clin. Neurosci. 2014;16:555–566. doi: 10.31887/DCNS.2014.16.4/jpouget. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fabbri C., Serretti A. Pharmacogenetics of major depressive disorder: top genes and pathways toward clinical applications. Curr. Psychiatr. Rep. 2015;17:50. doi: 10.1007/s11920-015-0594-9. [DOI] [PubMed] [Google Scholar]
- 47.Fabbri C., Crisafulli C., Calabro M., Spina E., Serretti A. Progress and prospects in pharmacogenetics of antidepressant drugs. Expet Opin. Drug Metabol. Toxicol. 2016;12:1157–1168. doi: 10.1080/17425255.2016.1202237. [DOI] [PubMed] [Google Scholar]
- 48.Ramos M., Berrogain C., Concha J., Lomba L., Garcia C.B., Ribate M.P. Pharmacogenetic studies: a tool to improve antidepressant therapy. Drug Metabol. Person. Therapy. 2016;31:197–204. doi: 10.1515/dmpt-2016-0019. [DOI] [PubMed] [Google Scholar]
- 49.Fabbri C., Hosak L., Mossner R., Giegling I., Mandelli L., Bellivier F. Consensus paper of the WFSBP Task Force on Genetics: genetics, epigenetics and gene expression markers of major depressive disorder and antidepressant response. World J. Biol. Psychiatr. 2017;18:5–28. doi: 10.1080/15622975.2016.1208843. [DOI] [PubMed] [Google Scholar]
- 50.Alhajji L., Nemeroff C.B. Personalized medicine and mood disorders. Psychiatr. Clin. 2015;38:395–403. doi: 10.1016/j.psc.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 51.Huang T.L., Lin C.C. Advances in biomarkers of major depressive disorder. Adv. Clin. Chem. 2015;68:177–204. doi: 10.1016/bs.acc.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 52.Serretti A., Gibiino S., Olgiati P. Pharmacogenetics of antidepressants and mood stabilizers. Handb. Clin. Neurol. 2012;106:715–744. doi: 10.1016/B978-0-444-52002-9.00043-7. [DOI] [PubMed] [Google Scholar]
- 53.Salloum N.C., McCarthy M.J., Leckband S.G., Kelsoe J.R. Towards the clinical implementation of pharmacogenetics in bipolar disorder. BMC Med. 2014;12:90. doi: 10.1186/1741-7015-12-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Budde M., Degner D., Brockmoller J., Schulze T.G. Pharmacogenomic aspects of bipolar disorder: an update. Eur. Neuropsychopharmacol. 2017;27:599–609. doi: 10.1016/j.euroneuro.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 55.Papakostas G.I., Shelton R.C., Kinrys G., Henry M.E., Bakow B.R., Lipkin S.H. Assessment of a multi-assay, serum-based biological diagnostic test for major depressive disorder: a pilot and replication study. Mol. Psychiatr. 2013;18:332–339. doi: 10.1038/mp.2011.166. [DOI] [PubMed] [Google Scholar]
- 56.Goes F.S. Genetics of bipolar disorder: recent update and future directions. Psychiatr. Clin. 2016;39:139–155. doi: 10.1016/j.psc.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cuthbert B.N. Research Domain Criteria: toward future psychiatric nosologies. Dialogues Clin. Neurosci. 2015;17:89–97. doi: 10.31887/DCNS.2015.17.1/bcuthbert. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Insel T., Cuthbert B., Garvey M., Heinssen R., Pine D.S., Quinn K. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatr. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 59.Kozak M.J., Cuthbert B.N. The NIMH research domain criteria initiative: background, issues, and pragmatics. Psychophysiology. 2016;53:286–297. doi: 10.1111/psyp.12518. [DOI] [PubMed] [Google Scholar]
- 60.Smucny J., Lesh T.A., Newton K., Niendam T.A., Ragland J.D., Carter C.S. Levels of cognitive control: a functional magnetic resonance imaging-based test of an RDoC domain across bipolar disorder and schizophrenia. Neuropsychopharmacology. 2018;43:598–606. doi: 10.1038/npp.2017.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Biomarkers Definitions Working Group Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 62.Filiou M.D., Turck C.W. General overview: biomarkers in neuroscience research. Int. Rev. Neurobiol. 2011;101:1–17. doi: 10.1016/B978-0-12-387718-5.00001-8. [DOI] [PubMed] [Google Scholar]
- 63.Sokolowska I., Ngounou Wetie A.G., Wormwood K., Thome J., Darie C.C., Woods A.G. The potential of biomarkers in psychiatry: focus on proteomics. J. Neural. Transm. (Vienna) 2015;122(Suppl 1):S9–18. doi: 10.1007/s00702-013-1134-6. [DOI] [PubMed] [Google Scholar]
- 64.Redei E.E., Mehta N.S. The promise of biomarkers in diagnosing major depression in primary care: the present and future. Curr. Psychiatr. Rep. 2015;17:601. doi: 10.1007/s11920-015-0601-1. [DOI] [PubMed] [Google Scholar]
- 65.Fonseka T.M., MacQueen G.M., Kennedy S.H. Neuroimaging biomarkers as predictors of treatment outcome in Major Depressive Disorder. J. Affect. Disord. 2018;233:21–35. doi: 10.1016/j.jad.2017.10.049. [DOI] [PubMed] [Google Scholar]
- 66.Strawbridge R., Young A.H., Cleare A.J. Biomarkers for depression: recent insights, current challenges and future prospects. Neuropsychiatric Dis. Treat. 2017;13:1245–1262. doi: 10.2147/NDT.S114542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gadad B.S., Jha M.K., Czysz A., Furman J.L., Mayes T.L., Emslie M.P. Peripheral biomarkers of major depression and antidepressant treatment response: current knowledge and future outlooks. J. Affect. Disord. 2018;233:3–14. doi: 10.1016/j.jad.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vieta E., Berk M., Schulze T.G., Carvalho A.F., Suppes T., Calabrese J.R. Bipolar disorders. Nat. Rev. Dis. Prim. 2018;4:18008. doi: 10.1038/nrdp.2018.8. [DOI] [PubMed] [Google Scholar]
- 69.Castano-Ramirez O.M., Sepulveda-Arias J.C., Duica K., Diaz Zuluaga A.M., Vargas C., Lopez-Jaramillo C. Inflammatory markers in the staging of bipolar disorder: a systematic review of the literature. Rev. Colomb. Psiquiatr. 2018;47:119–128. doi: 10.1016/j.rcp.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 70.Sayana P., Colpo G.D., Simoes L.R., Giridharan V.V., Teixeira A.L., Quevedo J. A systematic review of evidence for the role of inflammatory biomarkers in bipolar patients. J. Psychiatr. Res. 2017;92:160–182. doi: 10.1016/j.jpsychires.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 71.Teixeira A.L., Salem H., Frey B.N., Barbosa I.G., Machado-Vieira R. Update on bipolar disorder biomarker candidates. Expert Rev. Mol. Diagn. 2016;16:1209–1220. doi: 10.1080/14737159.2016.1248413. [DOI] [PubMed] [Google Scholar]
- 72.Kalia M., Costa E.S.J. Biomarkers of psychiatric diseases: current status and future prospects. Metabolism. 2015;64:S11–S15. doi: 10.1016/j.metabol.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 73.Teixeira A.L., Colpo G.D., Fries G.R., Bauer I.E., Selvaraj S. Biomarkers for bipolar disorder: current status and challenges ahead. Expert Rev. Neurother. 2019;19:67–81. doi: 10.1080/14737175.2019.1550361. [DOI] [PubMed] [Google Scholar]
- 74.Venkatasubramanian G., Keshavan M.S. Biomarkers in psychiatry - a critique. Ann. Neurosci. 2016;23:3–5. doi: 10.1159/000443549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steiner J., Walter M., Glanz W., Sarnyai Z., Bernstein H.G., Vielhaber S. Increased prevalence of diverse N-methyl-D-aspartate glutamate receptor antibodies in patients with an initial diagnosis of schizophrenia: specific relevance of IgG NR1a antibodies for distinction from N-methyl-D-aspartate glutamate receptor encephalitis. JAMA Psychiatr. 2013;70:271–278. doi: 10.1001/2013.jamapsychiatry.86. [DOI] [PubMed] [Google Scholar]
- 76.Maneta E., Garcia G. Psychiatric manifestations of anti-NMDA receptor encephalitis: neurobiological underpinnings and differential diagnostic implications. Psychosomatics. 2014;55:37–44. doi: 10.1016/j.psym.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 77.Venkatesan A., Adatia K. Anti-NMDA-receptor encephalitis: from bench to clinic. ACS Chem. Neurosci. 2017;8:2586–2595. doi: 10.1021/acschemneuro.7b00319. [DOI] [PubMed] [Google Scholar]
- 78.Sullivan P.F., Geschwind D.H. Defining the genetic, genomic, cellular, and diagnostic architectures of psychiatric disorders. Cell. 2019;177:162–183. doi: 10.1016/j.cell.2019.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kendler K.S. From many to one to many-the search for causes of psychiatric illness. JAMA Psychiatr. 2019 doi: 10.1001/jamapsychiatry.2019.1200. [DOI] [PubMed] [Google Scholar]
- 80.Lai C.Y., Scarr E., Udawela M., Everall I., Chen W.J., Dean B. Biomarkers in schizophrenia: a focus on blood based diagnostics and theranostics. World J. Psychiatr. 2016;6:102–117. doi: 10.5498/wjp.v6.i1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lozupone M., La Montagna M., D'Urso F., Daniele A., Greco A., Seripa D. The role of biomarkers in psychiatry. Adv. Exp. Med. Biol. 2019;1118:135–162. doi: 10.1007/978-3-030-05542-4_7. [DOI] [PubMed] [Google Scholar]
- 82.Gandal M.J., Zhang P., Hadjimichael E., Walker R.L., Chen C., Liu S. Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science. 2018;362 doi: 10.1126/science.aat8127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Naghavi-Gargari B., Zahirodin A., Ghaderian S.M.H., Shirvani-Farsani Z. Significant increasing of DISC2 long non-coding RNA expression as a potential biomarker in bipolar disorder. Neurosci. Lett. 2019;696:206–211. doi: 10.1016/j.neulet.2018.12.044. [DOI] [PubMed] [Google Scholar]
- 84.Sayad A., Taheri M., Omrani M.D., Fallah H., Kholghi Oskooei V., Ghafouri-Fard S. Peripheral expression of long non-coding RNAs in bipolar patients. J. Affect. Disord. 2019;249:169–174. doi: 10.1016/j.jad.2019.02.034. [DOI] [PubMed] [Google Scholar]
- 85.Nassan M., Nicholson W.T., Elliott M.A., Rohrer Vitek C.R., Black J.L., Frye M.A. Pharmacokinetic pharmacogenetic prescribing guidelines for antidepressants: a template for psychiatric precision medicine. Mayo Clin. Proc. 2016;91:897–907. doi: 10.1016/j.mayocp.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 86.Bousman C.A., Forbes M., Jayaram M., Eyre H., Reynolds C.F., Berk M. Antidepressant prescribing in the precision medicine era: a prescriber's primer on pharmacogenetic tools. BMC Psychiatr. 2017;17:60. doi: 10.1186/s12888-017-1230-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moore T.R., Hill A.M., Panguluri S.K. Pharmacogenomics in psychiatry: implications for practice. Recent Pat. Biotechnol. 2014;8:152–159. doi: 10.2174/1872208309666140904113615. [DOI] [PubMed] [Google Scholar]
- 88.Bousman C.A., Arandjelovic K., Mancuso S.G., Eyre H.A., Dunlop B.W. Pharmacogenetic tests and depressive symptom remission: a meta-analysis of randomized controlled trials. Pharmacogenomics. 2019;20:37–47. doi: 10.2217/pgs-2018-0142. [DOI] [PubMed] [Google Scholar]
- 89.Sweatt J.D., Tamminga C.A. An epigenomics approach to individual differences and its translation to neuropsychiatric conditions. Dialogues Clin. Neurosci. 2016;18:289–298. doi: 10.31887/DCNS.2016.18.3/dsweatt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kular L., Kular S. Epigenetics applied to psychiatry: clinical opportunities and future challenges. Psychiatr. Clin. Neurosci. 2018;72:195–211. doi: 10.1111/pcn.12634. [DOI] [PubMed] [Google Scholar]
- 91.Pena C.J., Nestler E.J. Progress in epigenetics of depression. Prog. Mol. Biol. Transl. Sci. 2018;157:41–66. doi: 10.1016/bs.pmbts.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fries G.R., Zhang W., Benevenuto D., Quevedo J. MicroRNAs in major depressive disorder. Adv. Exp. Med. Biol. 2019;1118:175–190. doi: 10.1007/978-3-030-05542-4_9. [DOI] [PubMed] [Google Scholar]
- 93.Read J., Cunliffe S., Jauhar S., McLoughlin D.M. Should we stop using electroconvulsive therapy? BMJ. 2019;364:k5233. doi: 10.1136/bmj.k5233. [DOI] [PubMed] [Google Scholar]
- 94.Lee D.J., Elias G.J.B., Lozano A.M. Neuromodulation for the treatment of eating disorders and obesity. Therap. Adv. Psychopharmacol. 2018;8:73–92. doi: 10.1177/2045125317743435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sagliano L., Atripaldi D., De Vita D., D'Olimpio F., Trojano L. Non-invasive brain stimulation in generalized anxiety disorder: a systematic review. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2019;93:31–38. doi: 10.1016/j.pnpbp.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 96.Becker J.E., Shultz E.K.B., Maley C.T. Transcranial magnetic stimulation in conditions other than major depressive disorder. Child Adolesc. Psychiatr. Clin. N. Am. 2019;28:45–52. doi: 10.1016/j.chc.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 97.Wani A., Trevino K., Marnell P., Husain M.M. Advances in brain stimulation for depression. Ann. Clin. Psychiatr. 2013;25:217–224. [PubMed] [Google Scholar]
- 98.Gault J.M., Davis R., Cascella N.G., Saks E.R., Corripio-Collado I., Anderson W.S. Approaches to neuromodulation for schizophrenia. J. Neurol. Neurosurg. Psychiatry. 2018;89:777–787. doi: 10.1136/jnnp-2017-316946. [DOI] [PubMed] [Google Scholar]
- 99.Barrett K. Psychiatric neurosurgery in the 21st century: overview and the growth of deep brain stimulation. BJPsych Bull. 2017;41:281–286. doi: 10.1192/pb.bp.116.055772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lo M.C., Widge A.S. Closed-loop neuromodulation systems: next-generation treatments for psychiatric illness. Int. Rev. Psychiatr. 2017;29:191–204. doi: 10.1080/09540261.2017.1282438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Widge A.S., Malone D.A., Jr., Dougherty D.D. Closing the loop on deep brain stimulation for treatment-resistant depression. Front. Neurosci. 2018;12:175. doi: 10.3389/fnins.2018.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kohl S., Baldermann J.C. Progress and challenges in deep brain stimulation for obsessive-compulsive disorder. Pharmacol. Ther. 2018;186:168–175. doi: 10.1016/j.pharmthera.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 103.Graat I., Figee M., Denys D. The application of deep brain stimulation in the treatment of psychiatric disorders. Int. Rev. Psychiatr. 2017;29:178–190. doi: 10.1080/09540261.2017.1282439. [DOI] [PubMed] [Google Scholar]
- 104.Ward H.E., Hwynn N., Okun M.S. Update on deep brain stimulation for neuropsychiatric disorders. Neurobiol. Dis. 2010;38:346–353. doi: 10.1016/j.nbd.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 105.Naldini L. Gene therapy returns to centre stage. Nature. 2015;526:351–360. doi: 10.1038/nature15818. [DOI] [PubMed] [Google Scholar]
- 106.Dunbar C.E., High K.A., Joung J.K., Kohn D.B., Ozawa K., Sadelain M. Gene therapy comes of age. Science. 2018;359 doi: 10.1126/science.aan4672. [DOI] [PubMed] [Google Scholar]
- 107.Lesage A., Lemasson M., Medina K., Tsopmo J., Sebti N., Potvin S. The prevalence of electroconvulsive therapy use since 1973: a meta-analysis. J. ECT. 2016;32:236–242. doi: 10.1097/YCT.0000000000000320. [DOI] [PubMed] [Google Scholar]
- 108.Park D.I., Turck C.W. Interactome studies of psychiatric disorders. Adv. Exp. Med. Biol. 2019;1118:163–173. doi: 10.1007/978-3-030-05542-4_8. [DOI] [PubMed] [Google Scholar]
- 109.Silva-Costa L.C., Carlson P.T., Guest P.C., de Almeida V., Martins-de-Souza D. Proteomic markers for depression. Adv. Exp. Med. Biol. 2019;1118:191–206. doi: 10.1007/978-3-030-05542-4_10. [DOI] [PubMed] [Google Scholar]
- 110.Abraham J., Szoko N., Natowicz M.R. Proteomic investigations of autism spectrum disorder: past findings, current challenges, and future prospects. Adv. Exp. Med. Biol. 2019;1118:235–252. doi: 10.1007/978-3-030-05542-4_12. [DOI] [PubMed] [Google Scholar]
- 111.Haijma S.V., Van Haren N., Cahn W., Koolschijn P.C., Hulshoff Pol H.E., Kahn R.S. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr. Bull. 2013;39:1129–1138. doi: 10.1093/schbul/sbs118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mueller S., Keeser D., Reiser M.F., Teipel S., Meindl T. Functional and structural MR imaging in neuropsychiatric disorders, part 2: application in schizophrenia and autism. AJNR Am. J. Neuroradiol. 2012;33:2033–2037. doi: 10.3174/ajnr.A2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Arnone D., Cavanagh J., Gerber D., Lawrie S.M., Ebmeier K.P., McIntosh A.M. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. Br. J. Psychiatry. 2009;195:194–201. doi: 10.1192/bjp.bp.108.059717. [DOI] [PubMed] [Google Scholar]
- 114.Pezzoli S., Emsell L., Yip S.W., Dima D., Giannakopoulos P., Zarei M. Meta-analysis of regional white matter volume in bipolar disorder with replication in an independent sample using coordinates, T-maps, and individual MRI data. Neurosci. Biobehav. Rev. 2018;84:162–170. doi: 10.1016/j.neubiorev.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nortje G., Stein D.J., Radua J., Mataix-Cols D., Horn N. Systematic review and voxel-based meta-analysis of diffusion tensor imaging studies in bipolar disorder. J. Affect. Disord. 2013;150:192–200. doi: 10.1016/j.jad.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 116.Graham J., Salimi-Khorshidi G., Hagan C., Walsh N., Goodyer I., Lennox B. Meta-analytic evidence for neuroimaging models of depression: state or trait? J. Affect. Disord. 2013;151:423–431. doi: 10.1016/j.jad.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 117.Fujiwara H., Yassin W., Murai T. Neuroimaging studies of social cognition in schizophrenia. Psychiatr. Clin. Neurosci. 2015;69:259–267. doi: 10.1111/pcn.12258. [DOI] [PubMed] [Google Scholar]
- 118.Minzenberg M.J., Laird A.R., Thelen S., Carter C.S., Glahn D.C. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch. Gen. Psychiatr. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.FDA-NIH Biomarker Working Group BEST (biomarkers, EndpointS, and other tools) resource. Silver Spring (MD): Food Drug Adm. (US) 2016 [PubMed] [Google Scholar]
- 120.Mirzakhanian H., Singh F., Cadenhead K.S. Biomarkers in psychosis: an approach to early identification and individualized treatment. Biomarkers Med. 2014;8:51–57. doi: 10.2217/bmm.13.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Light G.A., Swerdlow N.R. Future clinical uses of neurophysiological biomarkers to predict and monitor treatment response for schizophrenia. Ann. N. Y. Acad. Sci. 2015;1344:105–119. doi: 10.1111/nyas.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Owens E.M., Bachman P., Glahn D.C., Bearden C.E. Electrophysiological endophenotypes for schizophrenia. Harv. Rev. Psychiatr. 2016;24:129–147. doi: 10.1097/HRP.0000000000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Higuchi Y., Sumiyoshi T., Seo T., Miyanishi T., Kawasaki Y., Suzuki M. Mismatch negativity and cognitive performance for the prediction of psychosis in subjects with at-risk mental state. PLoS One. 2013;8:e54080. doi: 10.1371/journal.pone.0054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bodatsch M., Ruhrmann S., Wagner M., Muller R., Schultze-Lutter F., Frommann I. Prediction of psychosis by mismatch negativity. Biol. Psychiatr. 2011;69:959–966. doi: 10.1016/j.biopsych.2010.09.057. [DOI] [PubMed] [Google Scholar]
- 125.Jahshan C., Cadenhead K.S., Rissling A.J., Kirihara K., Braff D.L., Light G.A. Automatic sensory information processing abnormalities across the illness course of schizophrenia. Psychol. Med. 2012;42:85–97. doi: 10.1017/S0033291711001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Atkinson R.J., Michie P.T., Schall U. Duration mismatch negativity and P3a in first-episode psychosis and individuals at ultra-high risk of psychosis. Biol. Psychiatr. 2012;71:98–104. doi: 10.1016/j.biopsych.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 127.Perez V.B., Woods S.W., Roach B.J., Ford J.M., McGlashan T.H., Srihari V.H. Automatic auditory processing deficits in schizophrenia and clinical high-risk patients: forecasting psychosis risk with mismatch negativity. Biol. Psychiatr. 2014;75:459–469. doi: 10.1016/j.biopsych.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shaikh M., Valmaggia L., Broome M.R., Dutt A., Lappin J., Day F. Reduced mismatch negativity predates the onset of psychosis. Schizophr. Res. 2012;134:42–48. doi: 10.1016/j.schres.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 129.Light G.A., Braff D.L. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Arch. Gen. Psychiatr. 2005;62:127–136. doi: 10.1001/archpsyc.62.2.127. [DOI] [PubMed] [Google Scholar]
- 130.Light G.A., Braff D.L. Stability of mismatch negativity deficits and their relationship to functional impairments in chronic schizophrenia. Am. J. Psychiatr. 2005;162:1741–1743. doi: 10.1176/appi.ajp.162.9.1741. [DOI] [PubMed] [Google Scholar]
- 131.Rissling A.J., Miyakoshi M., Sugar C.A., Braff D.L., Makeig S., Light G.A. Cortical substrates and functional correlates of auditory deviance processing deficits in schizophrenia. NeuroImage Clin. 2014;6:424–437. doi: 10.1016/j.nicl.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wynn J.K., Sugar C., Horan W.P., Kern R., Green M.F. Mismatch negativity, social cognition, and functioning in schizophrenia patients. Biol. Psychiatr. 2010;67:940–947. doi: 10.1016/j.biopsych.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rasser P.E., Schall U., Todd J., Michie P.T., Ward P.B., Johnston P. Gray matter deficits, mismatch negativity, and outcomes in schizophrenia. Schizophr. Bull. 2011;37:131–140. doi: 10.1093/schbul/sbp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lee S.H., Sung K., Lee K.S., Moon E., Kim C.G. Mismatch negativity is a stronger indicator of functional outcomes than neurocognition or theory of mind in patients with schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2014;48:213–219. doi: 10.1016/j.pnpbp.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 135.Baldeweg T., Klugman A., Gruzelier J., Hirsch S.R. Mismatch negativity potentials and cognitive impairment in schizophrenia. Schizophr. Res. 2004;69:203–217. doi: 10.1016/j.schres.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 136.Naatanen R., Kujala T., Kreegipuu K., Carlson S., Escera C., Baldeweg T. The mismatch negativity: an index of cognitive decline in neuropsychiatric and neurological diseases and in ageing. Brain. 2011;134:3435–3453. doi: 10.1093/brain/awr064. [DOI] [PubMed] [Google Scholar]
- 137.Light G.A., Swerdlow N.R., Braff D.L. Preattentive sensory processing as indexed by the MMN and P3a brain responses is associated with cognitive and psychosocial functioning in healthy adults. J. Cognit. Neurosci. 2007;19:1624–1632. doi: 10.1162/jocn.2007.19.10.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kawakubo Y., Kasai K., Kudo N., Rogers M.A., Nakagome K., Itoh K. Phonetic mismatch negativity predicts verbal memory deficits in schizophrenia. Neuroreport. 2006;17:1043–1046. doi: 10.1097/01.wnr.0000221828.10846.ba. [DOI] [PubMed] [Google Scholar]
- 139.Javitt D.C., Spencer K.M., Thaker G.K., Winterer G., Hajos M. Neurophysiological biomarkers for drug development in schizophrenia. Nat. Rev. Drug Discov. 2008;7:68–83. doi: 10.1038/nrd2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Javitt D.C., Zukin S.R., Heresco-Levy U., Umbricht D. Has an angel shown the way? Etiological and therapeutic implications of the PCP/NMDA model of schizophrenia. Schizophr. Bull. 2012;38:958–966. doi: 10.1093/schbul/sbs069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lavoie S., Murray M.M., Deppen P., Knyazeva M.G., Berk M., Boulat O. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology. 2008;33:2187–2199. doi: 10.1038/sj.npp.1301624. [DOI] [PubMed] [Google Scholar]
- 142.Berk M., Copolov D., Dean O., Lu K., Jeavons S., Schapkaitz I. N-acetyl cysteine as a glutathione precursor for schizophrenia--a double-blind, randomized, placebo-controlled trial. Biol. Psychiatr. 2008;64:361–368. doi: 10.1016/j.biopsych.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 143.Choi Y.B., Lipton S.A. Redox modulation of the NMDA receptor. Cell. Mol. Life Sci. 2000;57:1535–1541. doi: 10.1007/PL00000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hall J., Trent S., Thomas K.L., O'Donovan M.C., Owen M.J. Genetic risk for schizophrenia: convergence on synaptic pathways involved in plasticity. Biol. Psychiatr. 2015;77:52–58. doi: 10.1016/j.biopsych.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 145.Lee S.E., Kim J.A., Chang S. nArgBP2-SAPAP-SHANK, the core postsynaptic triad associated with psychiatric disorders. Exp. Mol. Med. 2018;50:2. doi: 10.1038/s12276-017-0018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Verpelli C., Heise C., Sala C. Chapter four - structural and functional organization of the postsynaptic density. In: Pickel V., Segal M., editors. The Synapse. Academic Press; Boston: 2014. pp. 129–153. [Google Scholar]
- 147.Light G.A., Swerdlow N.R., Thomas M.L., Calkins M.E., Green M.F., Greenwood T.A. Validation of mismatch negativity and P3a for use in multi-site studies of schizophrenia: characterization of demographic, clinical, cognitive, and functional correlates in COGS-2. Schizophr. Res. 2015;163:63–72. doi: 10.1016/j.schres.2014.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Adams S.A., Nasrallah H.A. Multiple retinal anomalies in schizophrenia. Schizophr. Res. 2017 doi: 10.1016/j.schres.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 149.Lavoie J., Hebert M., Beaulieu J.M. Glycogen synthase kinase-3 overexpression replicates electroretinogram anomalies of offspring at high genetic risk for schizophrenia and bipolar disorder. Biol. Psychiatr. 2014;76:93–100. doi: 10.1016/j.biopsych.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 150.Beaulieu J.M., Gainetdinov R.R., Caron M.G. Akt/GSK3 signaling in the action of psychotropic drugs. Annu. Rev. Pharmacol. Toxicol. 2009;49:327–347. doi: 10.1146/annurev.pharmtox.011008.145634. [DOI] [PubMed] [Google Scholar]
- 151.Freyberg Z., Ferrando S.J., Javitch J.A. Roles of the Akt/GSK-3 and Wnt signaling pathways in schizophrenia and antipsychotic drug action. Am. J. Psychiatr. 2010;167:388–396. doi: 10.1176/appi.ajp.2009.08121873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lovestone S., Killick R., Di Forti M., Murray R. Schizophrenia as a GSK-3 dysregulation disorder. Trends Neurosci. 2007;30:142–149. doi: 10.1016/j.tins.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 153.Levchenko A., Davtian S., Freylichman O., Zagrivnaya M., Kostareva A., Malashichev Y. Beta-catenin in schizophrenia: possibly deleterious novel mutation. Psychiatr. Res. 2015;228:843–848. doi: 10.1016/j.psychres.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 154.de Ligt J., Willemsen M.H., van Bon B.W., Kleefstra T., Yntema H.G., Kroes T. Diagnostic exome sequencing in persons with severe intellectual disability. N. Engl. J. Med. 2012;367:1921–1929. doi: 10.1056/NEJMoa1206524. [DOI] [PubMed] [Google Scholar]
- 155.Kharbanda M., Pilz D.T., Tomkins S., Chandler K., Saggar A., Fryer A. Clinical features associated with CTNNB1 de novo loss of function mutations in ten individuals. Eur. J. Med. Genet. 2017;60:130–135. doi: 10.1016/j.ejmg.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li N., Xu Y., Li G., Yu T., Yao R.E., Wang X. Exome sequencing identifies a de novo mutation of CTNNB1 gene in a patient mainly presented with retinal detachment, lens and vitreous opacities, microcephaly, and developmental delay: case report and literature review. Medicine (Baltim.) 2017;96:e6914. doi: 10.1097/MD.0000000000006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.O'Roak B.J., Vives L., Girirajan S., Karakoc E., Krumm N., Coe B.P. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012;485:246–250. doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tucci V., Kleefstra T., Hardy A., Heise I., Maggi S., Willemsen M.H. Dominant beta-catenin mutations cause intellectual disability with recognizable syndromic features. J. Clin. Invest. 2014;124:1468–1482. doi: 10.1172/JCI70372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lavoie J., Illiano P., Sotnikova T.D., Gainetdinov R.R., Beaulieu J.M., Hebert M. The electroretinogram as a biomarker of central dopamine and serotonin: potential relevance to psychiatric disorders. Biol. Psychiatr. 2014;75:479–486. doi: 10.1016/j.biopsych.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 160.Zhang X., Gainetdinov R.R., Beaulieu J.M., Sotnikova T.D., Burch L.H., Williams R.B. Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron. 2005;45:11–16. doi: 10.1016/j.neuron.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 161.Kesby J.P., Eyles D.W., McGrath J.J., Scott J.G. Dopamine, psychosis and schizophrenia: the widening gap between basic and clinical neuroscience. Transl. Psychiatry. 2018;8:30. doi: 10.1038/s41398-017-0071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Swerdlow N.R., Braff D.L., Geyer M.A. Sensorimotor gating of the startle reflex: what we said 25 years ago, what has happened since then, and what comes next. J. Psychopharmacol. 2016;30:1072–1081. doi: 10.1177/0269881116661075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Berk M., Kapczinski F., Andreazza A.C., Dean O.M., Giorlando F., Maes M. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci. Biobehav. Rev. 2011;35:804–817. doi: 10.1016/j.neubiorev.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 164.Leighton S.P., Nerurkar L., Krishnadas R., Johnman C., Graham G.J., Cavanagh J. Chemokines in depression in health and in inflammatory illness: a systematic review and meta-analysis. Mol. Psychiatr. 2018;23:48–58. doi: 10.1038/mp.2017.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Schwarz E., van Beveren N.J., Ramsey J., Leweke F.M., Rothermundt M., Bogerts B. Identification of subgroups of schizophrenia patients with changes in either immune or growth factor and hormonal pathways. Schizophr. Bull. 2014;40:787–795. doi: 10.1093/schbul/sbt105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schwarz E., Guest P.C., Rahmoune H., Harris L.W., Wang L., Leweke F.M. Identification of a biological signature for schizophrenia in serum. Mol. Psychiatr. 2012;17:494–502. doi: 10.1038/mp.2011.42. [DOI] [PubMed] [Google Scholar]
- 167.Krishnadas R., Cavanagh J. Depression: an inflammatory illness? J. Neurol. Neurosurg. Psychiatry. 2012;83:495–502. doi: 10.1136/jnnp-2011-301779. [DOI] [PubMed] [Google Scholar]
- 168.Felger J.C., Lotrich F.E. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229. doi: 10.1016/j.neuroscience.2013.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lichtblau N., Schmidt F.M., Schumann R., Kirkby K.C., Himmerich H. Cytokines as biomarkers in depressive disorder: current standing and prospects. Int. Rev. Psychiatr. 2013;25:592–603. doi: 10.3109/09540261.2013.813442. [DOI] [PubMed] [Google Scholar]
- 170.Drexhage R.C., Weigelt K., van Beveren N., Cohen D., Versnel M.A., Nolen W.A. Immune and neuroimmune alterations in mood disorders and schizophrenia. Int. Rev. Neurobiol. 2011;101:169–201. doi: 10.1016/B978-0-12-387718-5.00007-9. [DOI] [PubMed] [Google Scholar]
- 171.Mostafavi S., Battle A., Zhu X., Potash J.B., Weissman M.M., Shi J. Type I interferon signaling genes in recurrent major depression: increased expression detected by whole-blood RNA sequencing. Mol. Psychiatr. 2014;19:1267–1274. doi: 10.1038/mp.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dowlati Y., Herrmann N., Swardfager W., Liu H., Sham L., Reim E.K. A meta-analysis of cytokines in major depression. Biol. Psychiatr. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 173.Valkanova V., Ebmeier K.P., Allan C.L. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J. Affect. Disord. 2013;150:736–744. doi: 10.1016/j.jad.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 174.Liu Y., Ho R.C., Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-alpha) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J. Affect. Disord. 2012;139:230–239. doi: 10.1016/j.jad.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 175.Soderlund J., Schroder J., Nordin C., Samuelsson M., Walther-Jallow L., Karlsson H. Activation of brain interleukin-1beta in schizophrenia. Mol. Psychiatr. 2009;14:1069–1071. doi: 10.1038/mp.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pedrini M., Massuda R., Fries G.R., de Bittencourt Pasquali M.A., Schnorr C.E., Moreira J.C. Similarities in serum oxidative stress markers and inflammatory cytokines in patients with overt schizophrenia at early and late stages of chronicity. J. Psychiatr. Res. 2012;46:819–824. doi: 10.1016/j.jpsychires.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 177.Miller B.J., Buckley P., Seabolt W., Mellor A., Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol. Psychiatr. 2011;70:663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.de Witte L., Tomasik J., Schwarz E., Guest P.C., Rahmoune H., Kahn R.S. Cytokine alterations in first-episode schizophrenia patients before and after antipsychotic treatment. Schizophr. Res. 2014;154:23–29. doi: 10.1016/j.schres.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 179.Zhang X.Y., Zhou D.F., Cao L.Y., Wu G.Y., Shen Y.C. Cortisol and cytokines in chronic and treatment-resistant patients with schizophrenia: association with psychopathology and response to antipsychotics. Neuropsychopharmacology. 2005;30:1532–1538. doi: 10.1038/sj.npp.1300756. [DOI] [PubMed] [Google Scholar]
- 180.Zimmerman A.W., Jyonouchi H., Comi A.M., Connors S.L., Milstien S., Varsou A. Cerebrospinal fluid and serum markers of inflammation in autism. Pediatr. Neurol. 2005;33:195–201. doi: 10.1016/j.pediatrneurol.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 181.Molloy C.A., Morrow A.L., Meinzen-Derr J., Schleifer K., Dienger K., Manning-Courtney P. Elevated cytokine levels in children with autism spectrum disorder. J. Neuroimmunol. 2006;172:198–205. doi: 10.1016/j.jneuroim.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 182.Samuelsson A.M., Jennische E., Hansson H.A., Holmang A. Prenatal exposure to interleukin-6 results in inflammatory neurodegeneration in hippocampus with NMDA/GABA(A) dysregulation and impaired spatial learning. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R1345–R1356. doi: 10.1152/ajpregu.00268.2005. [DOI] [PubMed] [Google Scholar]
- 183.Brown A.S., Derkits E.J. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am. J. Psychiatr. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Na K.S., Jung H.Y., Kim Y.K. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2014;48:277–286. doi: 10.1016/j.pnpbp.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 185.Muller N., Myint A.M., Schwarz M.J. Kynurenine pathway in schizophrenia: pathophysiological and therapeutic aspects. Curr. Pharmaceut. Des. 2011;17:130–136. doi: 10.2174/138161211795049552. [DOI] [PubMed] [Google Scholar]
- 186.Schwarcz R., Bruno J.P., Muchowski P.J., Wu H.Q. Kynurenines in the mammalian brain: when physiology meets pathology. Nat. Rev. Neurosci. 2012;13:465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Campbell B.M., Charych E., Lee A.W., Moller T. Kynurenines in CNS disease: regulation by inflammatory cytokines. Front. Neurosci. 2014;8:12. doi: 10.3389/fnins.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sellgren C.M., Kegel M.E., Bergen S.E., Ekman C.J., Olsson S., Larsson M. A genome-wide association study of kynurenic acid in cerebrospinal fluid: implications for psychosis and cognitive impairment in bipolar disorder. Mol. Psychiatr. 2016;21:1342–1350. doi: 10.1038/mp.2015.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor-mediated neuronal injury. Glia. 2010;58:253–263. doi: 10.1002/glia.20928. [DOI] [PubMed] [Google Scholar]
- 190.Holden J.M., Meyers-Manor J.E., Overmier J.B., Gahtan E., Sweeney W., Miller H. Lipopolysaccharide-induced immune activation impairs attention but has little effect on short-term working memory. Behav. Brain Res. 2008;194:138–145. doi: 10.1016/j.bbr.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 191.Perlman K., Benrimoh D., Israel S., Rollins C., Brown E., Tunteng J.F. A systematic meta-review of predictors of antidepressant treatment outcome in major depressive disorder. J. Affect. Disord. 2019;243:503–515. doi: 10.1016/j.jad.2018.09.067. [DOI] [PubMed] [Google Scholar]
- 192.Zhang J., Yue Y., Thapa A., Fang J., Zhao S., Shi W. Baseline serum C-reactive protein levels may predict antidepressant treatment responses in patients with major depressive disorder. J. Affect. Disord. 2019;250:432–438. doi: 10.1016/j.jad.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 193.Martins-de-Souza D., Guest P.C., Vanattou-Saifoudine N., Harris L.W., Bahn S. Proteomic technologies for biomarker studies in psychiatry: advances and needs. Int. Rev. Neurobiol. 2011;101:65–94. doi: 10.1016/B978-0-12-387718-5.00004-3. [DOI] [PubMed] [Google Scholar]
- 194.Woods A.G., Sokolowska I., Taurines R., Gerlach M., Dudley E., Thome J. Potential biomarkers in psychiatry: focus on the cholesterol system. J. Cell Mol. Med. 2012;16:1184–1195. doi: 10.1111/j.1582-4934.2012.01543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Stelzhammer V., Haenisch F., Chan M.K., Cooper J.D., Steiner J., Steeb H. Proteomic changes in serum of first onset, antidepressant drug-naive major depression patients. Int. J. Neuropsychopharmacol. 2014;17:1599–1608. doi: 10.1017/S1461145714000819. [DOI] [PubMed] [Google Scholar]
- 196.Watson S., Gallagher P., Ritchie J.C., Ferrier I.N., Young A.H. Hypothalamic-pituitary-adrenal axis function in patients with bipolar disorder. Br. J. Psychiatry. 2004;184:496–502. doi: 10.1192/bjp.184.6.496. [DOI] [PubMed] [Google Scholar]
- 197.Handwerger K. Differential patterns of HPA activity and reactivity in adult posttraumatic stress disorder and major depressive disorder. Harv. Rev. Psychiatr. 2009;17:184–205. doi: 10.1080/10673220902996775. [DOI] [PubMed] [Google Scholar]
- 198.Corcoran C.M., Smith C., McLaughlin D., Auther A., Malaspina D., Cornblatt B. HPA axis function and symptoms in adolescents at clinical high risk for schizophrenia. Schizophr. Res. 2012;135:170–174. doi: 10.1016/j.schres.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ryan M.C., Collins P., Thakore J.H. Impaired fasting glucose tolerance in first-episode, drug-naive patients with schizophrenia. Am. J. Psychiatr. 2003;160:284–289. doi: 10.1176/appi.ajp.160.2.284. [DOI] [PubMed] [Google Scholar]
- 200.Spelman L.M., Walsh P.I., Sharifi N., Collins P., Thakore J.H. Impaired glucose tolerance in first-episode drug-naive patients with schizophrenia. Diabet. Med. 2007;24:481–485. doi: 10.1111/j.1464-5491.2007.02092.x. [DOI] [PubMed] [Google Scholar]
- 201.van Nimwegen L.J., Storosum J.G., Blumer R.M., Allick G., Venema H.W., de Haan L. Hepatic insulin resistance in antipsychotic naive schizophrenic patients: stable isotope studies of glucose metabolism. J. Clin. Endocrinol. Metab. 2008;93:572–577. doi: 10.1210/jc.2007-1167. [DOI] [PubMed] [Google Scholar]
- 202.Cohn T.A., Remington G., Zipursky R.B., Azad A., Connolly P., Wolever T.M. Insulin resistance and adiponectin levels in drug-free patients with schizophrenia: a preliminary report. Can. J. Psychiatr. 2006;51:382–386. doi: 10.1177/070674370605100608. [DOI] [PubMed] [Google Scholar]
- 203.Chen D.C., Du X.D., Yin G.Z., Yang K.B., Nie Y., Wang N. Impaired glucose tolerance in first-episode drug-naive patients with schizophrenia: relationships with clinical phenotypes and cognitive deficits. Psychol. Med. 2016;46:3219–3230. doi: 10.1017/S0033291716001902. [DOI] [PubMed] [Google Scholar]
- 204.Licinio J., Wong M.L. The interface of obesity and depression: risk factors for the metabolic syndrome. Rev. Bras. Psiquiatr. 2003;25:196–197. doi: 10.1590/s1516-44462003000400002. [DOI] [PubMed] [Google Scholar]
- 205.Rasgon N.L., Kenna H.A. Insulin resistance in depressive disorders and Alzheimer's disease: revisiting the missing link hypothesis. Neurobiol. Aging. 2005;26(Suppl 1):103–107. doi: 10.1016/j.neurobiolaging.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 206.Fagiolini A., Frank E., Scott J.A., Turkin S., Kupfer D.J. Metabolic syndrome in bipolar disorder: findings from the bipolar disorder center for pennsylvanians. Bipolar Disord. 2005;7:424–430. doi: 10.1111/j.1399-5618.2005.00234.x. [DOI] [PubMed] [Google Scholar]
- 207.Guest P.C., Schwarz E., Krishnamurthy D., Harris L.W., Leweke F.M., Rothermundt M. Altered levels of circulating insulin and other neuroendocrine hormones associated with the onset of schizophrenia. Psychoneuroendocrinology. 2011;36:1092–1096. doi: 10.1016/j.psyneuen.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 208.Buoli M., Serati M., Altamura A.C. Biological aspects and candidate biomarkers for rapid-cycling in bipolar disorder: a systematic review. Psychiatr. Res. 2017;258:565–575. doi: 10.1016/j.psychres.2017.08.059. [DOI] [PubMed] [Google Scholar]
- 209.Nosadini R., Del Prato S., Tiengo A., Valerio A., Muggeo M., Opocher G. Insulin resistance in Cushing's syndrome. J. Clin. Endocrinol. Metab. 1983;57:529–536. doi: 10.1210/jcem-57-3-529. [DOI] [PubMed] [Google Scholar]
- 210.Bruehl H., Sweat V., Hassenstab J., Polyakov V., Convit A. Cognitive impairment in nondiabetic middle-aged and older adults is associated with insulin resistance. J. Clin. Exp. Neuropsychol. 2010;32:487–493. doi: 10.1080/13803390903224928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bello N.T., Hajnal A. Alterations in blood glucose levels under hyperinsulinemia affect accumbens dopamine. Physiol. Behav. 2006;88:138–145. doi: 10.1016/j.physbeh.2006.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Convit A. Links between cognitive impairment in insulin resistance: an explanatory model. Neurobiol. Aging. 2005;26(Suppl 1):31–35. doi: 10.1016/j.neurobiolaging.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 213.Schwarz E., Guest P.C., Steiner J., Bogerts B., Bahn S. Identification of blood-based molecular signatures for prediction of response and relapse in schizophrenia patients. Transl. Psychiatry. 2012;2:e82. doi: 10.1038/tp.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bousman C.A., Lee T.Y., Kim M., Lee J., Mostaid M.S., Bang M. Genetic variation in cytokine genes and risk for transition to psychosis among individuals at ultra-high risk. Schizophr. Res. 2018;195:589–590. doi: 10.1016/j.schres.2017.08.040. [DOI] [PubMed] [Google Scholar]
- 215.Mostaid M.S., Dimitrakopoulos S., Wannan C., Cropley V., Weickert C.S., Everall I.P. An Interleukin-1 beta (IL1B) haplotype linked with psychosis transition is associated with IL1B gene expression and brain structure. Schizophr. Res. 2018 doi: 10.1016/j.schres.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 216.Wray N.R., Ripke S., Mattheisen M., Trzaskowski M., Byrne E.M., Abdellaoui A. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 2018;50:668–681. doi: 10.1038/s41588-018-0090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Schizophrenia Working Group of the Psychiatric Genomics Consortium Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Demontis D., Walters R.K., Martin J., Mattheisen M., Als T.D., Agerbo E. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat. Genet. 2019;51:63–75. doi: 10.1038/s41588-018-0269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Autism Spectrum Disorders Working Group of The Psychiatric Genomics Consortium Meta-analysis of GWAS of over 16,000 individuals with autism spectrum disorder highlights a novel locus at 10q24.32 and a significant overlap with schizophrenia. Mol. Autism. 2017;8:21. doi: 10.1186/s13229-017-0137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Dezhina Z., Ranlund S., Kyriakopoulos M., Williams S.C.R., Dima D. A systematic review of associations between functional MRI activity and polygenic risk for schizophrenia and bipolar disorder. Brain Imag. Beh. 2018:1–16. doi: 10.1007/s11682-018-9879-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Li Z., Chen J., Yu H., He L., Xu Y., Zhang D. Genome-wide association analysis identifies 30 new susceptibility loci for schizophrenia. Nat. Genet. 2017;49:1576–1583. doi: 10.1038/ng.3973. [DOI] [PubMed] [Google Scholar]
- 222.Sekar A., Bialas A.R., de Rivera H., Davis A., Hammond T.R., Kamitaki N. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–183. doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Purcell S.M., Moran J.L., Fromer M., Ruderfer D., Solovieff N., Roussos P. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506:185–190. doi: 10.1038/nature12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Satterstrom F.K., Kosmicki J.A., Wang J., Breen M.S., De Rubeis S., An J.-Y. Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Cell. 2020;180(3) doi: 10.1016/j.cell.2019.12.036. 568.e23–584.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Malhotra D., Sebat J. CNVs: harbingers of a rare variant revolution in psychiatric genetics. Cell. 2012;148:1223–1241. doi: 10.1016/j.cell.2012.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Takumi T., Tamada K. CNV biology in neurodevelopmental disorders. Curr. Opin. Neurobiol. 2018;48:183–192. doi: 10.1016/j.conb.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 227.Shishido E., Aleksic B., Ozaki N. Copy-number variation in the pathogenesis of autism spectrum disorder. Psychiatr. Clin. Neurosci. 2014;68:85–95. doi: 10.1111/pcn.12128. [DOI] [PubMed] [Google Scholar]
- 228.Marshall C.R., Howrigan D.P., Merico D., Thiruvahindrapuram B., Wu W., Greer D.S. Contribution of copy number variants to schizophrenia from a genome-wide study of 41,321 subjects. Nat. Genet. 2017;49:27–35. doi: 10.1038/ng.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Thompson P.M., Andreassen O.A., Arias-Vasquez A., Bearden C.E., Boedhoe P.S., Brouwer R.M. ENIGMA and the individual: predicting factors that affect the brain in 35 countries worldwide. Neuroimage. 2017;145:389–408. doi: 10.1016/j.neuroimage.2015.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Del'Guidice T., Latapy C., Rampino A., Khlghatyan J., Lemasson M., Gelao B. FXR1P is a GSK3beta substrate regulating mood and emotion processing. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E4610–E4619. doi: 10.1073/pnas.1506491112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bureau A., Beaulieu J.M., Paccalet T., Chagnon Y.C., Maziade M. The interaction of GSK3B and FXR1 genotypes may influence the mania and depression dimensions in mood disorders. J. Affect. Disord. 2017;213:172–177. doi: 10.1016/j.jad.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 232.Greenwood T.A., Light G.A., Swerdlow N.R., Radant A.D., Braff D.L. Association analysis of 94 candidate genes and schizophrenia-related endophenotypes. PLoS One. 2012;7:e29630. doi: 10.1371/journal.pone.0029630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Greenwood T.A., Lazzeroni L.C., Calkins M.E., Freedman R., Green M.F., Gur R.E. Genetic assessment of additional endophenotypes from the consortium on the genetics of schizophrenia family study. Schizophr. Res. 2016;170:30–40. doi: 10.1016/j.schres.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Stocker A.M., Chenn A. The role of adherens junctions in the developing neocortex. Cell Adhes. Migrat. 2015;9:167–174. doi: 10.1080/19336918.2015.1027478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Mostaid M.S., Lloyd D., Liberg B., Sundram S., Pereira A., Pantelis C. Neuregulin-1 and schizophrenia in the genome-wide association study era. Neurosci. Biobehav. Rev. 2016;68:387–409. doi: 10.1016/j.neubiorev.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 236.Edwards A.C., Bigdeli T.B., Docherty A.R., Bacanu S., Lee D., de Candia T.R. Meta-analysis of positive and negative symptoms reveals schizophrenia modifier genes. Schizophr. Bull. 2016;42:279–287. doi: 10.1093/schbul/sbv119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Schmechtig A., Vassos E., Kumari V., Hutton S.B., Collier D.A., Morris R.G. Association of Neuregulin 1 rs3924999 genotype with antisaccades and smooth pursuit eye movements. Gene Brain Behav. 2010;9:621–627. doi: 10.1111/j.1601-183X.2010.00594.x. [DOI] [PubMed] [Google Scholar]
- 238.Kang C., Yang X., Xu X., Liu H., Su P., Yang J. Association study of neuregulin 1 gene polymorphisms with auditory P300 in schizophrenia. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2012;159B:422–428. doi: 10.1002/ajmg.b.32045. [DOI] [PubMed] [Google Scholar]
- 239.Liu X., Hong X., Chan R.C., Kong F., Peng Z., Wan X. Association study of polymorphisms in the alpha 7 nicotinic acetylcholine receptor subunit and catechol-o-methyl transferase genes with sensory gating in first-episode schizophrenia. Psychiatr. Res. 2013;209:431–438. doi: 10.1016/j.psychres.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 240.Cabranes J.A., Ancin I., Santos J.L., Sanchez-Morla E., Garcia-Jimenez M.A., Lopez-Ibor J.J. No effect of polymorphisms in the non-duplicated region of the CHRNA7 gene on sensory gating P50 ratios in patients with schizophrenia and bipolar disorder. Psychiatr. Res. 2013;205:276–278. doi: 10.1016/j.psychres.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 241.Leonard S., Freedman R. Genetics of chromosome 15q13-q14 in schizophrenia. Biol. Psychiatr. 2006;60:115–122. doi: 10.1016/j.biopsych.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 242.O'Donovan M.C., Craddock N., Norton N., Williams H., Peirce T., Moskvina V. Identification of loci associated with schizophrenia by genome-wide association and follow-up. Nat. Genet. 2008;40:1053–1055. doi: 10.1038/ng.201. [DOI] [PubMed] [Google Scholar]
- 243.Del Re E.C., Bergen S.E., Mesholam-Gately R.I., Niznikiewicz M.A., Goldstein J.M., Woo T.U. Analysis of schizophrenia-related genes and electrophysiological measures reveals ZNF804A association with amplitude of P300b elicited by novel sounds. Transl. Psychiatry. 2014;4:e346. doi: 10.1038/tp.2013.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Esslinger C., Walter H., Kirsch P., Erk S., Schnell K., Arnold C. Neural mechanisms of a genome-wide supported psychosis variant. Science. 2009;324:605. doi: 10.1126/science.1167768. [DOI] [PubMed] [Google Scholar]
- 245.Russell T.A., Blizinsky K.D., Cobia D.J., Cahill M.E., Xie Z., Sweet R.A. A sequence variant in human KALRN impairs protein function and coincides with reduced cortical thickness. Nat. Commun. 2014;5:4858. doi: 10.1038/ncomms5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kim M.J., Biag J., Fass D.M., Lewis M.C., Zhang Q., Fleishman M. Functional analysis of rare variants found in schizophrenia implicates a critical role for GIT1-PAK3 signaling in neuroplasticity. Mol. Psychiatr. 2017;22:417–429. doi: 10.1038/mp.2016.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Smith K.R., Davenport E.C., Wei J., Li X., Pathania M., Vaccaro V. GIT1 and betaPIX are essential for GABA(A) receptor synaptic stability and inhibitory neurotransmission. Cell Rep. 2014;9:298–310. doi: 10.1016/j.celrep.2014.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Podufall J., Tian R., Knoche E., Puchkov D., Walter A.M., Rosa S. A presynaptic role for the cytomatrix protein GIT in synaptic vesicle recycling. Cell Rep. 2014;7:1417–1425. doi: 10.1016/j.celrep.2014.04.051. [DOI] [PubMed] [Google Scholar]
- 249.Zhang H., Webb D.J., Asmussen H., Horwitz A.F. Synapse formation is regulated by the signaling adaptor GIT1. J. Cell Biol. 2003;161:131–142. doi: 10.1083/jcb.200211002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ko J., Kim S., Valtschanoff J.G., Shin H., Lee J.R., Sheng M. Interaction between liprin-alpha and GIT1 is required for AMPA receptor targeting. J. Neurosci. 2003;23:1667–1677. doi: 10.1523/JNEUROSCI.23-05-01667.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kim S., Ko J., Shin H., Lee J.R., Lim C., Han J.H. The GIT family of proteins forms multimers and associates with the presynaptic cytomatrix protein Piccolo. J. Biol. Chem. 2003;278:6291–6300. doi: 10.1074/jbc.M212287200. [DOI] [PubMed] [Google Scholar]
- 252.Schmitt A., Hasan A., Gruber O., Falkai P. Schizophrenia as a disorder of disconnectivity. Eur. Arch. Psychiatr. Clin. Neurosci. 2011;261(Suppl 2):S150–S154. doi: 10.1007/s00406-011-0242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.van den Heuvel M.P., Fornito A. Brain networks in schizophrenia. Neuropsychol. Rev. 2014;24:32–48. doi: 10.1007/s11065-014-9248-7. [DOI] [PubMed] [Google Scholar]
- 254.Birnbaum R., Weinberger D.R. Genetic insights into the neurodevelopmental origins of schizophrenia. Nat. Rev. Neurosci. 2017;18:727–740. doi: 10.1038/nrn.2017.125. [DOI] [PubMed] [Google Scholar]
- 255.Lin E., Lane H.Y. Genome-wide association studies in pharmacogenomics of antidepressants. Pharmacogenomics. 2015;16:555–566. doi: 10.2217/pgs.15.5. [DOI] [PubMed] [Google Scholar]
- 256.Pickard B.S. Genomics of lithium action and response. Neurotherapeutics. 2017;14:582–587. doi: 10.1007/s13311-017-0554-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Franco V., Perucca E. The pharmacogenomics of epilepsy. Expert Rev. Neurother. 2015;15:1161–1170. doi: 10.1586/14737175.2015.1083424. [DOI] [PubMed] [Google Scholar]
- 258.Corponi F., Fabbri C., Serretti A. Pharmacogenetics in psychiatry. Adv. Pharmacol. 2018;83:297–331. doi: 10.1016/bs.apha.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 259.Ravyn D., Ravyn V., Lowney R., Nasrallah H.A. CYP450 pharmacogenetic treatment strategies for antipsychotics: a review of the evidence. Schizophr. Res. 2013;149:1–14. doi: 10.1016/j.schres.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 260.Plumpton C.O., Roberts D., Pirmohamed M., Hughes D.A. A systematic review of economic evaluations of pharmacogenetic testing for prevention of adverse drug reactions. Pharmacoeconomics. 2016;34:771–793. doi: 10.1007/s40273-016-0397-9. [DOI] [PubMed] [Google Scholar]
- 261.Peterson K., Dieperink E., Anderson J., Boundy E., Ferguson L., Helfand M. Rapid evidence review of the comparative effectiveness, harms, and cost-effectiveness of pharmacogenomics-guided antidepressant treatment versus usual care for major depressive disorder. Psychopharmacology (Berl) 2017;234:1649–1661. doi: 10.1007/s00213-017-4622-9. [DOI] [PubMed] [Google Scholar]
- 262.Bousman C.A., Jaksa P., Pantelis C. Systematic evaluation of commercial pharmacogenetic testing in psychiatry: a focus on CYP2D6 and CYP2C19 allele coverage and results reporting. Pharmacogenetics Genom. 2017;27:387–393. doi: 10.1097/FPC.0000000000000303. [DOI] [PubMed] [Google Scholar]
- 263.Bousman C.A., Dunlop B.W. Genotype, phenotype, and medication recommendation agreement among commercial pharmacogenetic-based decision support tools. Pharmacogenomics J. 2018;18:613–622. doi: 10.1038/s41397-018-0027-3. [DOI] [PubMed] [Google Scholar]
- 264.Cheng C.Y., Su S.C., Chen C.H., Chen W.L., Deng S.T., Chung W.H. HLA associations and clinical implications in T-cell mediated drug hypersensitivity reactions: an updated review. J. Immunol. Res. 2014;2014:1–8. doi: 10.1155/2014/565320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Genin E., Chen D.P., Hung S.I., Sekula P., Schumacher M., Chang P.Y. HLA-A∗31:01 and different types of carbamazepine-induced severe cutaneous adverse reactions: an international study and meta-analysis. Pharmacogenomics J. 2014;14:281–288. doi: 10.1038/tpj.2013.40. [DOI] [PubMed] [Google Scholar]
- 266.Alrashood S.T. Carbamazepine. Profiles of Drug Substances, Excipients, and Related Methodology. 2016;41:133–321. doi: 10.1016/bs.podrm.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 267.Jaruthamsophon K., Tipmanee V., Sangiemchoey A., Sukasem C., Limprasert P. HLA-B∗15:21 and carbamazepine-induced Stevens-Johnson syndrome: pooled-data and in silico analysis. Sci. Rep. 2017;7:45553. doi: 10.1038/srep45553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Wei C.Y., Chung W.H., Huang H.W., Chen Y.T., Hung S.I. Direct interaction between HLA-B and carbamazepine activates T cells in patients with Stevens-Johnson syndrome. J. Allergy Clin. Immunol. 2012;129:1562–1569. doi: 10.1016/j.jaci.2011.12.990. e5. [DOI] [PubMed] [Google Scholar]
- 269.Chung W.H., Hung S.I., Hong H.S., Hsih M.S., Yang L.C., Ho H.C. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. 2004;428:486. doi: 10.1038/428486a. [DOI] [PubMed] [Google Scholar]
- 270.McCormack M., Alfirevic A., Bourgeois S., Farrell J.J., Kasperaviciute D., Carrington M. HLA-A∗3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N. Engl. J. Med. 2011;364:1134–1143. doi: 10.1056/NEJMoa1013297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Ozeki T., Mushiroda T., Yowang A., Takahashi A., Kubo M., Shirakata Y. Genome-wide association study identifies HLA-A∗3101 allele as a genetic risk factor for carbamazepine-induced cutaneous adverse drug reactions in Japanese population. Hum. Mol. Genet. 2011;20:1034–1041. doi: 10.1093/hmg/ddq537. [DOI] [PubMed] [Google Scholar]
- 272.Amstutz U., Shear N.H., Rieder M.J., Hwang S., Fung V., Nakamura H. Recommendations for HLA-B∗15:02 and HLA-A∗31:01 genetic testing to reduce the risk of carbamazepine-induced hypersensitivity reactions. Epilepsia. 2014;55:496–506. doi: 10.1111/epi.12564. [DOI] [PubMed] [Google Scholar]
- 273.Leckband S.G., Kelsoe J.R., Dunnenberger H.M., George A.L., Jr., Tran E., Berger R. Clinical Pharmacogenetics Implementation Consortium guidelines for HLA-B genotype and carbamazepine dosing. Clin. Pharmacol. Ther. 2013;94:324–328. doi: 10.1038/clpt.2013.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Dean L. Carbamazepine therapy and HLA genotype. In: Pratt V., McLeod H., Rubinstein W., Dean L., Kattman B., Malheiro A., editors. Medical Genetics Summaries. Bethesda (MD) 2012. [Google Scholar]
- 275.Hicks J.K., Stowe D., Willner M.A., Wai M., Daly T., Gordon S.M. Implementation of clinical pharmacogenomics within a large health system: from electronic health record decision support to consultation services. Pharmacotherapy. 2016;36:940–948. doi: 10.1002/phar.1786. [DOI] [PubMed] [Google Scholar]
- 276.Kwok J.B., Hallupp M., Loy C.T., Chan D.K., Woo J., Mellick G.D. GSK3B polymorphisms alter transcription and splicing in Parkinson's disease. Ann. Neurol. 2005;58:829–839. doi: 10.1002/ana.20691. [DOI] [PubMed] [Google Scholar]
- 277.Tsai S.J., Liou Y.J., Hong C.J., Yu Y.W., Chen T.J. Glycogen synthase kinase-3beta gene is associated with antidepressant treatment response in Chinese major depressive disorder. Pharmacogenomics J. 2008;8:384–390. doi: 10.1038/sj.tpj.6500486. [DOI] [PubMed] [Google Scholar]
- 278.Levchenko A., Losenkov I.S., Vyalova N.M., Simutkin G.G., Bokhan N.A., Wilffert B. The functional variant rs334558 of GSK3B is associated with remission in patients with depressive disorders. Pharmgenomics Pers. Med. 2018;11:121–126. doi: 10.2147/PGPM.S171423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Lin Y.F., Huang M.C., Liu H.C. Glycogen synthase kinase 3beta gene polymorphisms may be associated with bipolar I disorder and the therapeutic response to lithium. J. Affect. Disord. 2013;147:401–406. doi: 10.1016/j.jad.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 280.Benedetti F., Bollettini I., Barberi I., Radaelli D., Poletti S., Locatelli C. Lithium and GSK3-beta promoter gene variants influence white matter microstructure in bipolar disorder. Neuropsychopharmacology. 2013;38:313–327. doi: 10.1038/npp.2012.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Benedetti F., Poletti S., Radaelli D., Locatelli C., Pirovano A., Lorenzi C. Lithium and GSK-3beta promoter gene variants influence cortical gray matter volumes in bipolar disorder. Psychopharmacology (Berl) 2015;232:1325–1336. doi: 10.1007/s00213-014-3770-4. [DOI] [PubMed] [Google Scholar]
- 282.Adli M., Hollinde D.L., Stamm T., Wiethoff K., Tsahuridu M., Kirchheiner J. Response to lithium augmentation in depression is associated with the glycogen synthase kinase 3-beta -50T/C single nucleotide polymorphism. Biol. Psychiatr. 2007;62:1295–1302. doi: 10.1016/j.biopsych.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 283.Benedetti F., Serretti A., Pontiggia A., Bernasconi A., Lorenzi C., Colombo C. Long-term response to lithium salts in bipolar illness is influenced by the glycogen synthase kinase 3-beta -50 T/C SNP. Neurosci. Lett. 2005;376:51–55. doi: 10.1016/j.neulet.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 284.Iwahashi K., Nishizawa D., Narita S., Numajiri M., Murayama O., Yoshihara E. Haplotype analysis of GSK-3beta gene polymorphisms in bipolar disorder lithium responders and nonresponders. Clin. Neuropharmacol. 2014;37:108–110. doi: 10.1097/WNF.0000000000000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Ma L., Rolls E.T., Liu X., Liu Y., Jiao Z., Wang Y. Multi-scale analysis of schizophrenia risk genes, brain structure, and clinical symptoms reveals integrative clues for subtyping schizophrenia patients. J. Mol. Cell Biol. 2018 doi: 10.1093/jmcb/mjy071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Willsey A.J., Morris M.T., Wang S., Willsey H.R., Sun N., Teerikorpi N. The psychiatric cell map initiative: a convergent systems biological approach to illuminating key molecular pathways in neuropsychiatric disorders. Cell. 2018;174:505–520. doi: 10.1016/j.cell.2018.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Starita L.M., Ahituv N., Dunham M.J., Kitzman J.O., Roth F.P., Seelig G. Variant interpretation: functional assays to the rescue. Am. J. Hum. Genet. 2017;101:315–325. doi: 10.1016/j.ajhg.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Patel R.Y., Shah N., Jackson A.R., Ghosh R., Pawliczek P., Paithankar S. ClinGen Pathogenicity Calculator: a configurable system for assessing pathogenicity of genetic variants. Genome Med. 2017;9:3. doi: 10.1186/s13073-016-0391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Hiemke C., Baumann P., Bergemann N., Conca A., Dietmaier O., Egberts K. AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry. 2011;44:195–235. doi: 10.1055/s-0031-1286287. [DOI] [PubMed] [Google Scholar]
- 290.Rosenblat J.D., Lee Y., McIntyre R.S. Does pharmacogenomic testing improve clinical outcomes for major depressive disorder? A systematic review of clinical trials and cost-effectiveness studies. J. Clin. Psychiatr. 2017;78:720–729. doi: 10.4088/JCP.15r10583. [DOI] [PubMed] [Google Scholar]
- 291.Adams R.A., Huys Q.J., Roiser J.P. Computational Psychiatry: towards a mathematically informed understanding of mental illness. J. Neurol. Neurosurg. Psychiatry. 2016;87:53–63. doi: 10.1136/jnnp-2015-310737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Hahn T., Nierenberg A.A., Whitfield-Gabrieli S. Predictive analytics in mental health: applications, guidelines, challenges and perspectives. Mol. Psychiatr. 2017;22:37–43. doi: 10.1038/mp.2016.201. [DOI] [PubMed] [Google Scholar]
- 293.Huys Q.J., Maia T.V., Frank M.J. Computational psychiatry as a bridge from neuroscience to clinical applications. Nat. Neurosci. 2016;19:404–413. doi: 10.1038/nn.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Esteva A., Robicquet A., Ramsundar B., Kuleshov V., DePristo M., Chou K. A guide to deep learning in healthcare. Nat. Med. 2019;25:24–29. doi: 10.1038/s41591-018-0316-z. [DOI] [PubMed] [Google Scholar]
- 295.Woo C.W., Chang L.J., Lindquist M.A., Wager T.D. Building better biomarkers: brain models in translational neuroimaging. Nat. Neurosci. 2017;20:365–377. doi: 10.1038/nn.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Zhang X., Mormino E.C., Sun N., Sperling R.A., Sabuncu M.R., Yeo B.T. Bayesian model reveals latent atrophy factors with dissociable cognitive trajectories in Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E6535–E6544. doi: 10.1073/pnas.1611073113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Wang D., Liu S., Warrell J., Won H., Shi X., Navarro F.C.P. Comprehensive functional genomic resource and integrative model for the human brain. Science. 2018;362 doi: 10.1126/science.aat8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Bzdok D., Meyer-Lindenberg A. Machine learning for precision psychiatry: opportunities and challenges. Biol. Psychiatr. Cogn. Neurosci. Neuroimag. 2018;3:223–230. doi: 10.1016/j.bpsc.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 299.Durstewitz D., Koppe G., Meyer-Lindenberg A. Deep neural networks in psychiatry. Mol. Psychiatr. 2019 doi: 10.1038/s41380-019-0365-9. [DOI] [PubMed] [Google Scholar]
- 300.de Wit S., Ziermans T.B., Nieuwenhuis M., Schothorst P.F., van Engeland H., Kahn R.S. Individual prediction of long-term outcome in adolescents at ultra-high risk for psychosis: applying machine learning techniques to brain imaging data. Hum. Brain Mapp. 2017;38:704–714. doi: 10.1002/hbm.23410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Tognin S., Pettersson-Yeo W., Valli I., Hutton C., Woolley J., Allen P. Using structural neuroimaging to make quantitative predictions of symptom progression in individuals at ultra-high risk for psychosis. Front. Psychiatr. 2013;4:187. doi: 10.3389/fpsyt.2013.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Hirschfeld R.M., Lewis L., Vornik L.A. Perceptions and impact of bipolar disorder: how far have we really come? Results of the national depressive and manic-depressive association 2000 survey of individuals with bipolar disorder. J. Clin. Psychiatr. 2003;64:161–174. [PubMed] [Google Scholar]
- 303.Fajutrao L., Locklear J., Priaulx J., Heyes A. A systematic review of the evidence of the burden of bipolar disorder in Europe. Clin. Pract. Epidemiol. Ment. Health. 2009;5:3. doi: 10.1186/1745-0179-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Redlich R., Almeida J.J., Grotegerd D., Opel N., Kugel H., Heindel W. Brain morphometric biomarkers distinguishing unipolar and bipolar depression. A voxel-based morphometry-pattern classification approach. JAMA Psychiatr. 2014;71:1222–1230. doi: 10.1001/jamapsychiatry.2014.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Miotto R., Li L., Kidd B.A., Dudley J.T. Deep patient: an unsupervised representation to predict the future of patients from the electronic health records. Sci. Rep. 2016;6:26094. doi: 10.1038/srep26094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.McCoy T.H., Castro V.M., Rosenfield H.R., Cagan A., Kohane I.S., Perlis R.H. A clinical perspective on the relevance of research domain criteria in electronic health records. Am. J. Psychiatr. 2015;172:316–320. doi: 10.1176/appi.ajp.2014.14091177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Schmaal L., Marquand A.F., Rhebergen D., van Tol M.J., Ruhe H.G., van der Wee N.J. Predicting the naturalistic course of major depressive disorder using clinical and multimodal neuroimaging information: a multivariate pattern recognition study. Biol. Psychiatr. 2015;78:278–286. doi: 10.1016/j.biopsych.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Koutsouleris N., Kahn R.S., Chekroud A.M., Leucht S., Falkai P., Wobrock T. Multisite prediction of 4-week and 52-week treatment outcomes in patients with first-episode psychosis: a machine learning approach. Lancet Psychiatr. 2016;3:935–946. doi: 10.1016/S2215-0366(16)30171-7. [DOI] [PubMed] [Google Scholar]
- 309.Wang P.S., Lane M., Olfson M., Pincus H.A., Wells K.B., Kessler R.C. Twelve-month use of mental health services in the United States: results from the National Comorbidity Survey Replication. Arch. Gen. Psychiatr. 2005;62:629–640. doi: 10.1001/archpsyc.62.6.629. [DOI] [PubMed] [Google Scholar]
- 310.Etkin A., Patenaude B., Song Y.J., Usherwood T., Rekshan W., Schatzberg A.F. A cognitive-emotional biomarker for predicting remission with antidepressant medications: a report from the iSPOT-D trial. Neuropsychopharmacology. 2015;40:1332–1342. doi: 10.1038/npp.2014.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Chekroud A.M., Zotti R.J., Shehzad Z., Gueorguieva R., Johnson M.K., Trivedi M.H. Cross-trial prediction of treatment outcome in depression: a machine learning approach. Lancet Psychiatr. 2016;3:243–250. doi: 10.1016/S2215-0366(15)00471-X. [DOI] [PubMed] [Google Scholar]
- 312.Ravan M., Hasey G., Reilly J.P., MacCrimmon D., Khodayari-Rostamabad A. A machine learning approach using auditory odd-ball responses to investigate the effect of Clozapine therapy. Clin. Neurophysiol. 2015;126:721–730. doi: 10.1016/j.clinph.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 313.Van Schependom J., Yu W., Gielen J., Laton J., De Keyser J., De Hert M. Do advanced statistical techniques really help in the diagnosis of the metabolic syndrome in patients treated with second-generation antipsychotics? J. Clin. Psychiatr. 2015;76:e1292–e1299. doi: 10.4088/JCP.14m09367. [DOI] [PubMed] [Google Scholar]
- 314.Chen G., Ward B.D., Xie C., Li W., Chen G., Goveas J.S. A clustering-based method to detect functional connectivity differences. Neuroimage. 2012;61:56–61. doi: 10.1016/j.neuroimage.2012.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Zhao X., Rangaprakash D., Yuan B., Denney T.S., Jr., Katz J.S., Dretsch M.N. Investigating the correspondence of clinical diagnostic grouping with underlying neurobiological and phenotypic clusters using unsupervised machine learning. Front. Appl. Mathemat. Statist. 2018;4 doi: 10.3389/fams.2018.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Karalunas S.L., Fair D., Musser E.D., Aykes K., Iyer S.P., Nigg J.T. Subtyping attention-deficit/hyperactivity disorder using temperament dimensions: toward biologically based nosologic criteria. JAMA Psychiatr. 2014;71:1015–1024. doi: 10.1001/jamapsychiatry.2014.763. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 317.Bak N., Ebdrup B.H., Oranje B., Fagerlund B., Jensen M.H., During S.W. Two subgroups of antipsychotic-naive, first-episode schizophrenia patients identified with a Gaussian mixture model on cognition and electrophysiology. Transl. Psychiatry. 2017;7:e1087. doi: 10.1038/tp.2017.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Wu M.J., Mwangi B., Bauer I.E., Passos I.C., Sanches M., Zunta-Soares G.B. Identification and individualized prediction of clinical phenotypes in bipolar disorders using neurocognitive data, neuroimaging scans and machine learning. Neuroimage. 2017;145:254–264. doi: 10.1016/j.neuroimage.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Sorzano C.O.S., Vargas J., Pascual Montano A. arXiv. Cornell University; 2014. A Survey of Dimensionality Reduction Techniques. [Google Scholar]
- 320.Goodfellow I.J., Bengio Y., Courville A. MIT Press; Cambridge, MA: 2016. Deep Learning. [Google Scholar]
- 321.Gasperini M., Starita L., Shendure J. The power of multiplexed functional analysis of genetic variants. Nat. Protoc. 2016;11:1782–1787. doi: 10.1038/nprot.2016.135. [DOI] [PMC free article] [PubMed] [Google Scholar]