Abstract
Children living near artisanal and small‐scale gold mining (ASGM) are at risk of exposure to mercury, a neurotoxicant. It is not certain whether such exposures are harming development, as they occur in underresourced contexts entwined with other stressors, such as malnutrition and enteric infection. This study sought to investigate the association between hair‐mercury levels and visual‐motor, cognitive, and physical development among children living near ASGM in the Peruvian Amazon. Total hair‐mercury levels were measured in 164 children ages 5–12 living in Madre de Dios, Peru. Primary outcomes included Visual‐Motor Integration assessed via the Beery‐VMI Developmental Test, General Cognitive Ability assessed via the Batería‐III Woodcock‐Munoz (Spanish‐language Woodcock‐Johnson Tests of Cognitive Abilities), and Physical Health assessed via anthropometry/hemoglobin counts. Mean (SD) hair‐mercury level was 2.06 (2.43) μg/g. Fifty‐four children (32.9%) had hair‐mercury levels above the World Health Organization reference level of 2.0 μg/g. After controlling for sex, child age, maternal education, and family socioeconomic status, each one unit increase in log hair‐mercury level was associated with a 1.01 unit decrease in Visual‐Motor Integration (95%CI: −2.06, 0.05, p = 0.061), a 2.59 unit decrease in General Cognitive Ability (95%CI: −4.52, −0.66, p = 0.012), and a 2.43 unit decrease in Physical Health (95%CI: −5.34, 0.49, p = 0.096). After adjustment for covariates, children with hair‐mercury levels exceeding the World Health Organization reference level scored 4.68 IQ points lower in Cognitive Ability than their peers. Mercury exposures related to ASGM may be harming child development in the Peruvian Amazon. Children in this region may benefit from intervention to reach their full developmental potential.
Keywords: environmental pollution, mercury, child development, neurocognitive assessment, artisanal and small‐scale gold mining
Key Points
Children living near artisanal and small‐scale gold mining in the Peruvian Amazon are exposed to mercury, a neurotoxicant
Children with higher hair‐mercury levels demonstrated significantly lower General Cognitive Ability than their peers in this rural, low‐income context
Children living near artisanal and small‐scale gold mining in Peru may benefit from intervention to reach their full developmental potential
1. Introduction
Artisanal and small‐scale gold mining (ASGM) is a significant and growing economic sector in the developing world, with up to 19 million individuals across 70 countries now employed in this informal and largely unmechanized activity (United Nations Environment Programme, 2016). Most ASGM operations rely on the use of elemental mercury to extract gold from ore. Systemic underregulation of the sector has left workers and communities living near gold mining operations exposed to potentially harmful levels of mercury released during mineral extraction and discharged into waterways, soils, and the atmosphere (Esdaile & Chalker, 2018; Gibb & O'Leary, 2014).
Mercury is a toxic transition metal that, in the body, disrupts the structure of sulfur‐containing amino acids. Mercury exists in elemental, inorganic, and organic forms, with toxicity varying by form and dosage of exposure; organic forms (those combined with carbon) represent the most bioavailable. In the ASGM context mercury exposures are typically to inhaled elemental mercury vapor (for workers and those living near gold processing activities) and to ingested organic methymercury accumulated in fish tissue (for those consuming seafood from effected waterways) (Diringer et al., 2015; Harris et al., 2003). High acute exposure to inhaled elemental mercury vapor or ingested organic methylmercury (>50 μg/L blood total mercury level) results in systemic organ dysfunction, with particular harm to the kidneys and central nervous system (Bose‐O'Reilly et al., 2010). Chronic exposure below this level has been associated with diverse impairments, including muscle fatigue, weight loss, gastrointestinal disturbance, disrupted heme synthesis, peripheral nerve damage, and, in children, impaired central nervous system development (Bernhoft, 2012). Health and development effects from very low levels of chronic exposure (approximately <5–10 μg/L blood total mercury level or <1–2 μg/g hair total mercury level) are still poorly characterized (Kim et al., 2013; UNEP Chemicals, 2008), and it is not known if they ultimately influence clinical outcomes (Davidson et al., 2004; Holmes et al., 2009; Karagas et al., 2012; Ng et al., 2007; Ye et al., 2016).
Collectively, ASGM represents the largest source of mercury emissions worldwide, with nearly one fifth (18%) of global emissions originating from South America (United Nations Environment Programme, 2019). Within South America, the Madre de Dios region of Peru has become a region of particular concern for several reasons (Ashe, 2012; Asner et al., 2013; Caballero Espejo et al., 2018; Diringer et al., 2015). First, ASGM activities have expanded rapidly within the region for the past two decades, with little government monitoring or oversite. Estimates from satellite and airborne surveillance suggest that small‐scale mining in the region expanded geographically by over 400% from 1999 to 2012 (Asner et al., 2013), with continuing expansion culminating in 2017 with the greatest annual growth identified in 30 years of surveillance (Caballero Espejo et al., 2018). Second, the region is considered one of the world's most biodiverse, containing a number of important ecological parks and preserves, including Manu National Park and the Tambopata Reserve. Third, ASGM activities are now known to have contributed to mercury contamination events and high levels of methylmercury in waterways, fish tissue, and resident's blood and hair (Ashe, 2012; Diringer et al., 2015; Gonzalez et al., 2019; Hurtado et al., 2006; Weinhouse et al., 2017; Wyatt et al., 2017; Yard et al., 2012), even among individual living hundreds of miles from active mining (Wyatt et al., 2017).
Limited surveillance of mercury contamination in Madre de Dios has suggested that the communities living near small‐scale gold mining operations may be at risk of health impacts from mercury exposures (Ashe, 2012; Diringer et al., 2015; Wyatt et al., 2017; Yard et al., 2012). Indeed, a number of studies of riverine communities in the Brazilian Amazon suggest mercury exposures in the region may be of a sufficient magnitude to cause subtle neurologic, motor, and somoatosensory deficits in adults (Dolbec et al., 2000; Lebel et al., 1996, 1998; Peplow & Augustine, 2014; Yokoo et al., 2003) and neurodevelopmental delays in infants (Dórea et al., 2012; Marques et al., 2016). However, for school‐aged children, little study has yet determined whether the levels detected in this region are leading to neurodevelopmental harm. Three studies, to the best of our knowledge, have examined neurodevelopmental outcomes in school‐age children exposed to mercury in early life in the wider Amazon region owing principally to ASGM, two in Brazil (Grandjean et al., 1999; Tavares et al., 2005) and one in French Guiana (Cordier et al., 2002). Two of these studies reported neurodevelopmental deficits in the context of generally high exposures (reported mean sample hair mercury levels >10 μg/g) (Cordier et al., 2002; Grandjean et al., 1999), while the third study lacked cognitive tests that might identify subtle deficits among differentially exposed children (Tavares et al., 2005).
Additional evidence is needed to clarify the risks facing children living near ASGM activities in general and in the Peruvian Amazon in particular. Children living in rural settings in developing countries, such as those in Madre de Dios, may be uniquely vulnerable to harm from even low levels of mercury exposure owing to the presence of numerous, interrelated developmental stressors. Common threats to children's healthy development include malnutrition and anemia, low access to early learning opportunities, and exposure to enteric pathogens, other environmental pollutants, and maltreatment (e.g., abuse and neglect) (Grantham‐McGregor et al., 2007; Walker et al., 2007). In the areas where ASGM is prevalent, additive or synergistic effects may amplify the consequences of individual developmental threats. For example, mercury exposure appears to limit the absorption of key micronutrients (Peraza et al., 1998), a harm, which, in turn, may magnify the toxicity of mercury when access to nutrients is limited. Recent epidemiological research in our study region suggests a possible cyclical relationship between mercury exposure from ASGM and lower hemoglobin levels in children, even among those with exposures below the current World Health Organization (WHO) reference level for clinical concern (2.0 μg/g hair mercury) (UNEP Chemicals, 2008) (Weinhouse et al., 2017).
This study sought to determine whether mercury exposures in Madre de Dios were harming school‐age child development by assessing the association between hair mercury levels and visual‐motor, cognitive, and physical development among school‐age children living near ASGM activities. We examined the distribution of child mercury exposure across population subgroups within the region and tested the hypothesis that children with elevated hair mercury levels would demonstrate poorer visual‐motor integration, cognitive ability, and physical health relative to their less exposed peers. Our study is unique given its inclusion of mining and nonmining rural communities in a region undergoing rapid ASGM expansion. It is, however, emblematic of the conditions in which thousands of other small‐scale gold mining centers around the world operate.
2. Materials and Methods
2.1. Sample
Participants in this study are members of the Amarakaeri Cohort Study, which enrolled 1,221 households across 23 communities (N = 4,083) surrounding the Amarakaeri Communal Reserve, a large protected area in Madre de Dios, to study the impact of resource extraction on human and environmental health (Figure 1). Four communities were classified as urban (≥100 households) and 19 as rural (<100 households) following the Peruvian National Census designations (Intituto Nacional de Estadistica e Informatica, n.d.). Further, 11 of 19 rural communities were classified as native, or ethnically indigenous (Native Federation of the Madre de Dios River and Tributaries, 2014), where inhabitants typically speak both Spanish and an indigenous tongue. Native languages included Yine, Harakbut, and Machiguenga. Households with at least one woman of childbearing age (aged 15–49) were enrolled. Two rounds of data collection were performed: baseline collection between March and May 2015 and follow‐up of 458 of 667 nuclear families between February and April 2016 (i.e., families consisting of a women of childbearing age, their spouse/partner, and child under 12; families were randomly selected). All household members were offered testing for hemoglobin and total hair mercury at each round. Informed consent was obtained from parents for each child, with children aged 9 and above giving written consent and children below age 9 giving verbal assent. Approval to conduct research on human subjects was obtained through the Universidad Peruana Cayetano‐Heredia Comité Institucional de Ética para Humanos (SIDISI 63056) and the Regional Health Directorate of Madre de Dios.
Figure 1.
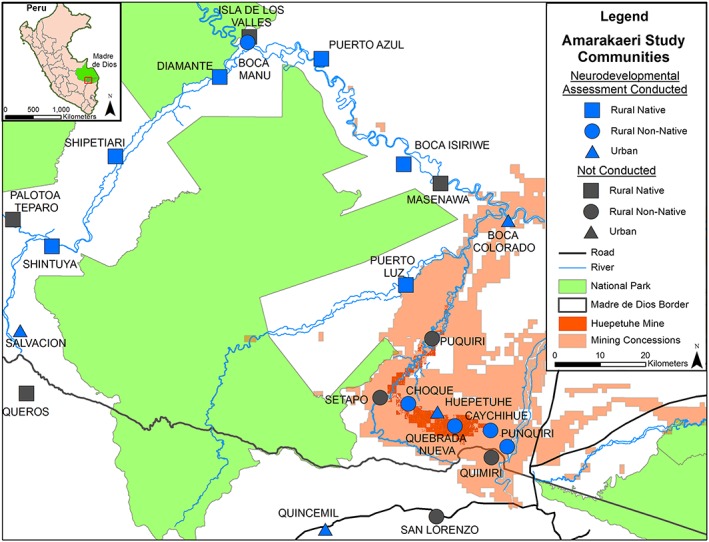
Sampled communities in the Amarakaeri Cohort Study, Madre de Dios, Peru.
Neurodevelopmental screening was conducted during 2016 follow‐up on school‐aged children. Two hundred twelve children across 14 communities were eligible a priori (aged 5–12 years) and provided a hair mercury sample at baseline. Data were collected from 164 children (57% female) aged 5–12 years (mean age = 8.0, SD = 2.0), who represented 77% of those eligible. Those who did not respond tended to be traveling at the time of the follow‐up visit; no children or parents refused participation.
2.2. Mercury Exposure
Child mercury exposure was assessed via measurement of total mercury content in hair. The methods of hair sample collection, storage, quality assurance, and analysis procedures have been previously described (Weinhouse et al., 2017). Briefly, hair samples were collected by cutting three tufts of hair from the occipital region of the scalp. Each tuft of hair was stored individually in paper envelopes, held at ambient conditions (e.g., room temperature), and transported to Duke University for total mercury analysis. Following standard protocols endorsed by the U.S. Environmental Protection Agency (McDowell et al., 2004), hair samples were not washed prior to processing. Total mercury could contain both direct adsorption of inorganic mercury and incorporated organic mercury from inside the body. Total mercury content in the proximal 2‐cm segment of hair was determined by direct combustion, gold amalgamation, and atomic absorption spectrometry (Milestone DMA‐80). The instrument was calibrated with a certified dissolved Hg (NO3)2 in 1% HNO3. The lower limit of quantification was 0.5 ng, calculated as 10 times the instrument detection limit, which corresponds to 0.05 ug/g for hair. The instrument calibration was verified by analysis of a hair standard reference material (ERM‐DB001) every 10 samples in a batch run. Accepted measurements were within 10% of certified value. This segment length approximates mercury exposure over 2 months before sampling. The lower limit of quantification was 1 ng total mercury (approximately 0.05 μg/g in hair). Total hair mercury content was measured as μg/g hair.
Hair mercury levels from each assessment wave were averaged to produce a school‐age mercury load score (r = 0.53, p < 0.0001 for the correlation between the two assessments). The mercury exposure variable was highly kurtotic in this sample (12.64); thus, the values were transformed using the natural logarithm for statistical analyses. The log‐transformed mercury variable was normally distributed and was utilized for all analyses, although descriptive statistics present mercury hair levels as the untransformed values (i.e., in μg/g). Hair mercury content was treated as a continuous variable for all statistical tests.
2.3. Developmental Outcomes
2.3.1. Visual‐Motor Integration
Visual‐Motor Integration describes a child's ability to interpret visual information, direct coordinated movements, and integrate these two processes together. Visual‐Motor Integration (primary outcome) was assessed via the full‐form 30‐item Beery VMI Developmental Test of Visual‐Motor Integration 6th Edition (Beery & Beery, 2004) using standard testing procedures. The Beery VMI asks children to reproduce increasingly complex drawings, with the reproductions scored for accuracy. The test demonstrates good internal consistency (Cronbach's alpha > 0.80) and construct validity (Beery & Beery, 2004; Brown & Hockey, 2013; Harvey et al., 2017). Following standard procedure, scores were standardized within the sample to M = 100, SD = 15.
2.3.2. Cognitive Ability
Cognitive ability was measured using the Batería III Woodcock‐Munoz Pruebas de Habilidades Cognitivas (Batería III COG) (Mather et al., 2008), the Spanish‐language version of the Woodcock‐Johnson III Tests of Cognitive Abilities (WJ III COG) (Woodcock et al., 2001). The Batería III COG measures general intellectual ability, broad and narrow cognitive abilities, and aspects of executive functioning. The Batería III COG yields a General Cognitive Ability score, which is analogous to an intelligence quotient (IQ), using either the standard scale (Tests 1–10), the extended scale (Tests 11–20), or the brief scale, which uses a subset of tests from the standard and extended scales (Tests 1, 2, 5, 7, 9, and 20). The brief scale was administered to produce the General Cognitive Ability score and to measure the following domains of narrow cognitive ability: Verbal Comprehension, Working Memory, Processing Speed, Auditory Processing, and Fluid Reasoning.
The Batería III COG has been separately validated for use in Madre de Dios within this sample using exploratory and confirmatory factor analysis (Reuben et al., 2020). Details on the validation of the Batería III COG are provided in the supporting information. Confirmatory factor analysis results supported a bifactor structure, involving the four cognitive domains of Verbal Comprehension, Working Memory, Processing Speed, and Fluid Reasoning, and a General Cognitive Ability factor. To aid in model identification and estimation convergence, Auditory Processing was not included in the factor analysis tests. All administered tests loaded highly onto a General Cognitive Ability factor, while tests contributing to specific cognitive domains loaded only weakly onto factors hypothesized to represent those domains. Findings supported the use of the Batería III COG as a measure of General Cognitive Ability in children living in the Peruvian Amazon but not necessarily as a measure of the more narrow domains of cognitive ability. Hence, General Cognitive Ability, calculated as an average of domain‐level scores, was used as a primary outcome in this study, while narrow cognitive domain scores were utilized as secondary, exploratory outcomes that should be interpreted with caution (Vetter & Mascha, 2017).
The Batería III COG was administered by trained research staff using standard protocols (McGrew & Woodcock, 2001). Children were tested at their home between February and April 2016. The General Cognitive Ability and narrow cognitive domain factor scores were standardized to M = 100, SD = 15 to make them comparable to IQ scales reported in other studies of child mercury exposure (e.g., the Wechsler Adult Intelligence Scale). Internal reliabilities of the General Cognitive Ability and narrow cognitive domain scores were high (Cronbach's α statistics from 0.92 to 0.97).
2.3.3. Physical Health
A physical health composite indicator (primary outcome) was derived from two independent indicators of child physical development: height‐for‐age z‐score (HAZ) and hemoglobin level. HAZ is a standard measure of growth that is used to evaluate chronic malnutrition and classify children as stunted (HAZ < −2) (Black et al., 2013; Reinhardt & Fanzo, 2014). HAZ was calculated using exact month of age and standing height using the WHO child growth standards and the WHO Anthro program, V3.2.2. Hemoglobin concentration in whole blood is a reliable measure of anemia and child nutritional status. Capillary blood was obtained from each individual with a finger prick by a disposable lancet, dispensed in a microcuvette sample holder, and immediately measured for hemoglobin using a portable Hemocue Hb201 Analyzer (Hemocue AB, Angelholm, Sweden). Hemoglobin was measured as g/dL of blood and analyzed as a continuous measure (sample M = 11.9 g/dL, SD = 1.1, range 8.4 to 15.1).
To create the primary physical health outcome, the hemoglobin measure was z‐score transformed to match the HAZ scale and the two indicator measures (HAZ and z‐scored hemoglobin count) were then averaged together. Hemoglobin z‐score transformation used population mean and standard deviations reported for Peruvian children living at the general altitudes of the study communities (Ocas‐Córdova et al., 2018). The primary physical health outcome was then scaled to M = 100, SD = 15 for comparability to the other primary outcomes.
2.4. Statistical Analysis
We tested the association between child hair mercury levels and developmental outcomes in the three primary outcome domains (Visual‐Motor Integration, General Cognitive Ability, and Physical Health) using Ordinary Least Squares multiple linear regression. We also tested for specificity in the relationship between child hair mercury levels and developmental outcomes by examining mercury associations with the secondary outcomes including (1) narrow domains of cognitive ability: Verbal Comprehension, Working Memory Ability, Processing Speed, Auditory Processing, and Fluid Reasoning and (2) the measured subcomponents of the primary Physical Health outcome: HAZ and hemoglobin count. Each outcome was examined using two models: (1) a “sex‐adjusted” model in which the outcome was regressed on the child mercury variable and sex and (2) a “fully adjusted” model in which the outcome was regressed on the child mercury variable and the covariates of sex, child age at follow‐up, maternal education (as reported by the mother at baseline), and family socioeconomic status (a composite of family monthly income and total family assets, as reported by the head of household at baseline). Finally, for descriptive purposes group mean levels on the developmental outcomes were also compared for individuals above and below the WHO reference level for clinical concern (2.0 μg/g) using analysis of covariance tests.
Following the primary study analyses, additional post hoc tests were added to evaluate whether associations between hair mercury and child developmental outcomes were driven by the presence of native children with high mercury exposures. These tests repeated the main study analyses, specified above, adding a native‐status indicator (dummy) variable to the fully adjusted regression models and analysis of variance tests.
Only participants who had complete data on all covariates for each outcome were included in each model; no data were imputed. For Visual‐Motor Integration, 163 (99%) participants were analyzed; for General Cognitive Ability, 161 (98%) participants were analyzed; for Physical Health, 162 (99%) participants were analyzed. Hair mercury level was natural‐log transformed and analyzed as a continuous measure. Analyses were conducted in STATA v.15.1. The nonindependence of data from children living within the same communities was accounted for at each analysis step by adjusting the standard errors using the STATA Robust Cluster command. Power sensitivity analysis of the smallest analytic sample (n = 161) conducted using the G*Power package (v3.1) revealed sensitivity to detect effect sizes as low as Cohen's f 2 = 0.05 at power = 0.80 in the unadjusted model and as low as Cohen's f 2 = 0.08 in the fully adjusted model; both are considered small effects, accounting for approximately 5% to 7% of the variance in the outcome variable (given by the equation: proportion of variance = ) (Cohen, 1988).
3. Results
One hundred sixty‐four children were enrolled for this study, with 97 (59%) from urban communities, 39 (24%) from rural nonnative communities, and 28 (17%) from rural native communities (Table 1). Slightly more than half the children were female (57%). Children in native communities lived in homes with the lowest socioeconomic levels and had mothers with the lowest educational levels compared to children in urban and rural nonnative communities (Table 1). 20 (71%) of the native children spoke Spanish as a second language. All other children spoke Spanish as a first language.
Table 1.
Comparison of Participants Living in Urban, Rural Nonnative, and Rural Native Communities on Primary Study Variables
| Full sample (N = 164) | Urban (n = 97) | Rural nonnative (n = 39) | Rural native (n = 28) | p value for native versus nonnative | |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| % female | 57.3% | 58.8% | 43.6% | 71.4% | 0.099 |
| Age | 8.0 ± 2.0 | 8.0 ± 2.1 | 7.9 ± 1.9 | 8.1 ± 2.0 | 0.794 |
| Socioeconomic status | 0.01 ± 1.0 | 0.24 ± 0.9 | 0.02 ± 0.9 | −0.80 ± 1.1 | <0.001 |
| Maternal education | 8.3 ± 3.8 | 8.9 ± 3.8 | 8.2 ± 4.1 | 6.6 ± 3.1 | 0.010 |
| Child hair mercury level | 2.1 ± 2.4 | 1.6 ± 2.4 | 1.7 ± 2.0 | 4.1 ± 2.2 | <0.001 |
| % over WHO standard (2.0 μg/g) | 32.9% | 22.7% | 20.5% | 85.7% | |
| % over U.S. NRC standard (1.2 μg/g) | 48.8% | 37.1% | 43.6% | 96.4% | |
| Visual‐Motor Integration | 99.9 ± 15.0 | 100.8 ± 15.8 | 98.2 ± 12.5 | 98.8 ± 15.9 | 0.676 |
| Cognitive Ability | 100.2 ± 15.1 | 102.3 ± 15.5 | 101.1 ± 13.4 | 91.6 ± 13.4 | <0.001 |
| Physical Health | 100.0 ± 15.0 | 102.0 ± 14.3 | 99.8 ± 17.5 | 94.2 ± 12.1 | 0.024 |
Note. Socioeconomic status is a composite of household income and assets as reported on by the mother at baseline, presented in z‐score units (M = 0, SD = 1). Maternal education was measured in years of formal schooling. Child hair mercury level was measured in μg/g hair. WHO and U.S. National Research Council (U.S. NRC) guideline mercury levels are 2.0 μg/g and 1.2 μg/g hair, respectively. Visual‐Motor Integration was assessed via the Beery VMI Developmental Test of Visual‐Motor Integration 6th Edition and scaled within sample to M = 100, SD = 15. Cognitive Ability was assessed via Batería III Woodcock‐Munoz Pruebas de Habilidades Cognitivas, scaled within sample to M = 100, SD = 15. Physical Health was assessed as a composite of height‐for‐age z‐score and hemoglobin count and scaled within sample to M = 100, SD = 15.
Mean (SD) hair mercury was 2.06 μg/g (2.43) and ranged 0.08 to 14.61 μg/g (Figure 2). Fifty‐four children (33%) had hair mercury levels above the WHO reference value of 2.0 μg/g (UNEP Chemicals, 2008) and 80 children (49%) had mercury levels above the U.S. National Research Council (NRC) reference level of 1.2 μg/g (U.S. National Research Council, 2000).
Figure 2.
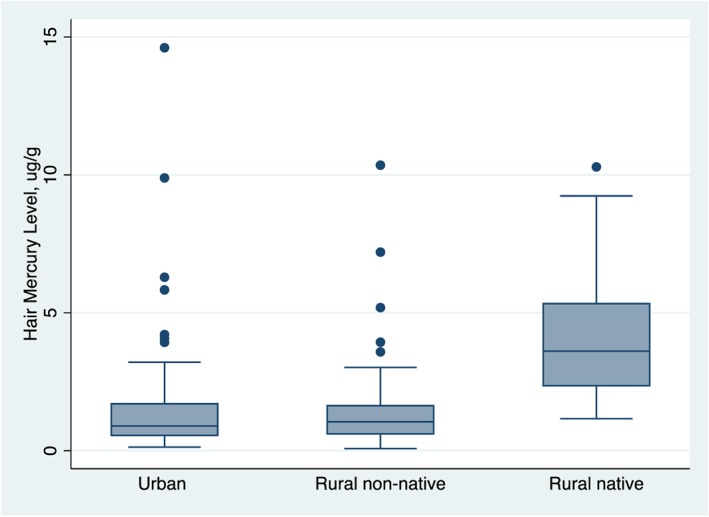
Distribution of hair mercury levels (μg/g) among participants living in urban, rural nonnative, and rural native communities.
In this study group, child hair mercury level was significantly related to family socioeconomic status (r = −0.23, p = 0.003; Figure 3) but was unrelated to child age (r = 0.13, p = 0.102) or maternal education (r = 0.02, p = 0.805). Females had higher average hair mercury levels than males (2.21 μg/g vs. 1.85 μg/g, respectively), but the difference was not statistically significant (t(162) = 0.943, p = 0.347).
Figure 3.
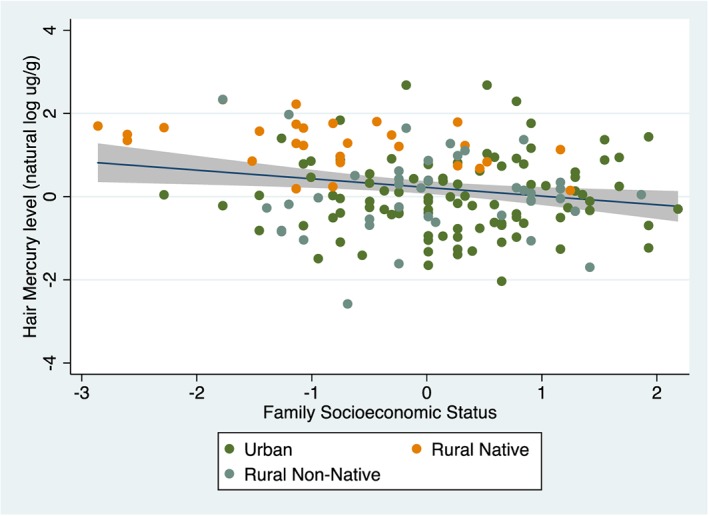
Association of child hair mercury level with family socioeconomic status.
Child hair mercury level differed significantly across the sampled communities (p < 0.001, Table 1). Children from native communities had higher mean hair mercury levels than nonnative children (4.1 μg/g native vs. 1.6 μg/g urban and rural nonnative children, respectively, t(162) = −5.214, p < 0.001). This group difference remained significant even after removing children with >3SD from the mean hair mercury level. In addition to having high mercury levels, native children also scored significantly worse on some primary outcomes compared to their nonnative peers (Table 1), including General Cognitive Ability (M = 91.6 IQ points for native children versus 102.0 for nonnative children, t(162) = 3.420, p < 0.001) and Physical Health (94.20 for native children versus 101.24 for nonnative children, t(161) = 2.282, p = 0.024). Group differences in Visual‐Motor Integration were not significant (t(162) = 0.418, p = 0.676).
3.1. Hair Mercury Level Versus Visual‐Motor Integration, General Cognitive Ability, and Physical Health
Regression models show that children with higher hair mercury levels performed more poorly on all primary outcome measures, although the associations were nonsignificant for the Visual‐Motor and Physical Health outcomes (Table 2 and Figure 4). After controlling for sex, child age at follow‐up, maternal education, and family socioeconomic status, each 1 unit higher natural‐log hair mercury level was associated with a 1.01 point deficit in Visual‐Motor Integration (95%CI: −2.06, 0.05; p = 0.061), a 2.59 IQ point deficit in General Cognitive Ability (95%CI: −4.52, −0.66; p = 0.012), and a 2.43 unit deficit in Physical Health (95%CI: −5.34, 0.49; p = 0.096) (Table 2). After adjustment for covariates, children with hair Hg levels above the WHO guideline (2.0 μg/g hair) scored, on average, 2.40 points lower in Visual‐Motor Integration (F 1,156 = 2.177, p = 0.142), 4.68 points lower in Cognitive Ability (F 1,156 = 10.497, p = 0.001), and 6.24 points lower in Physical Health (F 1,155 = 5.960, p = 0.016) than cohort peers.
Table 2.
Association of Hair Hg Levels With Child Development Outcomes
| Sex adjusted | Fully adjusted | Fully adjusted model with a native status variable added | |||||||
|---|---|---|---|---|---|---|---|---|---|
| b | 95% CI | p value | b | 95% CI | p value | b | 95% CI | p value | |
| Visual‐Motor Integration | −2.06 | (−4.20, 0.07) | 0.057 | −1.01 | (−2.06, 0.05) | 0.061 | −0.98 | (−2.38, 0.41) | 0.151 |
| Cognitive Ability | −3.71 | (−6.11, −1.32) | 0.005 | −2.59 | (−4.52, −0.66) | 0.012 | −1.35 | (−2.63, −0.67) | 0.041 |
| Physical Health | −2.36 | (−5.18, 0.46) | 0.095 | −2.43 | (−5.34, 0.49) | 0.096 | −1.70 | (−5.21, 1.81) | 0.317 |
Note. CI = Confidence interval. Child hair mercury level (μg/g) was subject to a natural‐log transformation. Covariates in the fully adjusted model were sex, child age at follow‐up, maternal education, and family socioeconomic status. N = 159–163. One hundred sixty‐three study members had present data on all the covariates and the Visual‐Motor Integration outcome. One hundred sixty‐one study members had present data on all the covariates and the Cognitive Ability outcome. One hundred sixty‐two study members had present data on all the covariates and the Physical Health outcome. All primary outcomes are scaled within the sample to M = 100, SD = 15. Regression estimates present change in the outcomes per 1 unit increase in natural log hair mercury level.
Figure 4.
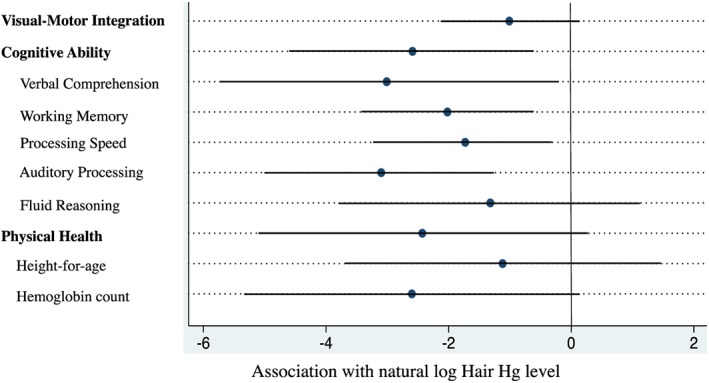
Association of hair Hg levels with developmental outcomes after full adjustment for study covariates.
Prespecified exploratory analyses of the five narrow domains of cognitive ability comprising the general Cognitive Ability outcome measure revealed no obvious specificity in mercury‐related cognitive deficits (Figure 4). Specifically, after adjustment for study covariates, children with higher levels of hair mercury performed significantly worse in all cognitive domains, save Fluid Reasoning ability. Effect size estimates are presented in supporting information Table S1). Prespecified exploratory analyses of the two physical health indicators (hemoglobin count and HAZ) revealed slightly larger effect sizes for hemoglobin count than for the HAZs (b = −0.15 for hemoglobin count and b = −0.08 for HAZ).
3.2. Native Community Effect
As native children had higher mercury exposures, on average, following the primary study analyses, we evaluated whether the associations between hair mercury and poor developmental outcomes were driven by the presence of native children with high mercury exposures by adding a native‐status indicator (dummy) variable to the fully adjusted models. The effect of mercury exposure remained negative for all of the primary (Table 2) and secondary outcomes (Figure 4 and supporting information Table S1), with the effect on General Cognitive Ability still significant after adjustment for child native‐status (b = −1.35, p = 0.041). Although consistent in direction of the effect, the mean effect size of mercury exposure was attenuated for all secondary outcomes, becoming a nonsignificant association for Verbal Comprehension and Processing Speed but retaining significance for Auditory Processing (b = −2.39, p = 0.005) and Working Memory (b = −1.22, p = 0.022). After adjustment for study covariates and child native‐status, children with hair mercury levels above the WHO reference level scored 1.56 IQ points lower in General Cognitive Ability, 3.16 points lower in Auditory Processing ability, and 1.72 points lower in Working Memory ability.
As native children had higher mercury burden and lower socioeconomic status on average than their nonnative peers, post hoc exploratory tests were also conducted to examine possible differences in mercury‐health associations between native and nonnative children. Models testing the primary study outcomes were rerun with a mercury‐level‐by‐native‐status interaction term included; these terms were nonsignificant in all models (p values ranged from 0.133 to 0.656).
4. Discussion
This observation of hair mercury levels and visual‐motor, cognitive, and physical health outcomes in a sample of children living near small‐scale gold mining operations in the Peruvian Amazon revealed three findings. First, many children living in the region tended to have hair mercury levels above recommended levels. Roughly one third of the sample exceeded WHO international mercury reference values and half exceeded U.S. NRC reference values. On average, children in this study had mean hair mercury levels nearly 20 times greater than those reported from a representative 1999–2000 sample of U.S. children (average hair mercury in this sample was 2.1 μg/g vs. 0.1 μg/g in the U.S. sample). These levels were similar, however, to a subset of children from the U.S. sample who ate fish ≥3 times a month (McDowell et al., 2004; Nuttall, 2006).
Second, mean hair mercury levels varied significantly by community. The risk of mercury exposure in the Peruvian Amazon is not shared equally across the region. In particular, children from native communities had significantly greater hair mercury levels than their peers living in nonnative communities (mean hair mercury level was 4.1 μg/g for native children vs. 1.6 μg/g for nonnative children). All but one of the native children had hair mercury levels greater than the U.S. NRC reference value. This is likely a consequence of the high fish consumption documented in native communities in Madre de Dios (Wyatt et al., 2017) and the wider Amazon region (e.g., Farias et al., 2006; Piperata et al., 2013; Roche et al., 2011).
Third, children with greater hair mercury levels demonstrated poorer performance on all primary study outcomes, including Visual‐Motor Integration, General Cognitive Ability, and Physical Health, although the associations only achieved statistical significance for the cognitive outcome after adjustment for covariates. Analysis of secondary outcomes comprising the primary outcomes suggested no specificity in the association between hair mercury level and negative developmental outcomes, although the associations were greater for some cognitive domains (Verbal Comprehension and Auditory Processing) than for others (Fluid Reasoning and Processing Speed) and for one physical health domain (hemoglobin count) than the other (HAZ).
Taken together, these results suggest that mercury exposures may be harming the development of children living in an ASGM region and that the effects may be most pronounced among native children. Although this study does not directly link child mercury exposure to gold mining, research in Madre de Dios has suggested a strong causal link between artisanal mining, increased release of mercury into the watershed (i.e., either directly from mining or increased erosion and release of natural mercury), and increased bioaccumulation of methylmercury in fish (e.g., Diringer et al., 2015; Martinez et al., 2018). This, combined with studies linking increased hair mercury levels to fish consumption in the same communities (e.g., Feingold et al., 2019; Fréry et al., 2001; Wyatt et al., 2017), provides consistent evidence for the link between neurocognitive impairment originating from mercury exposure and ASGM in the region. While percent contribution of methylmercury to total hair mercury levels was not assessed in this study, evidence suggests that the majority of the mercury (>80%) will have been to this form (Diringer et al., 2015; George et al., 2010; Nuttall, 2006).
Our results support the growing literature relating mercury exposure to impaired neurocognitive function in children, which has primarily been reported outside of ASGM contexts. Notably, seminal research from the Faroe Islands, New Zealand, and the Seychelles reported a decline of 0.18 IQ points in children for each 1 μg/g increase in maternal hair mercury (Axelrad et al., 2007). Our results also support the small but growing toxic‐metal‐cognition research among school‐aged children living in artisanal gold mining areas of the Amazon. In Brazil, hair mercury levels have been associated with lower motor, attention, and visual‐spatial function among children aged 7–12 years old (N = 351 children tested via the finger‐tapping task, Santa Ana form board, and the Stanford‐Binet copying test; geometric mean hair mercury 11.0 μg/g) (Grandjean et al., 1999). In French Guiana, maternal hair mercury levels have been associated with increased deep tendon reflexes, poorer coordination of the legs, and decreased performance on the Stanford‐Binet copying test among children aged 5–12 years old (N = 156; geometric mean maternal hair mercury 12.7 μg/g) (Cordier et al., 2002). And in Ecuador, elevated mining‐metal exposure (particularly to manganese) has been associated with impaired neurocognitive function among children aged 5–11 years old (N = 73 children tested via the Raven's Colored Progressive Matrices) (Betancourt et al., 2015). Impairments to neurological development have long‐term cognitive, mental, and physical health consequences (Bellinger, 2008; Brubaker et al., 2009; Reuben et al., 2017, 2019; Taber & Hurley, 2008) and can significantly reduce economic productivity. Notably, loss of neurocognitive function due to methylmercury exposure has been associated with a net population decline of 600,000 IQ points across Europe and economic losses of $9B–$10B per year (Bellanger et al., 2013). Similar research in the United States estimated losses of $8.7B annually due to methylmercury exposure (Trasande et al., 2005).
In this study, the effects of mercury exposure may have been disproportionally borne by native children, who had higher mercury levels on average and poorer performance on study outcomes than their nonnative peers. Adjusting the associations between mercury levels and the development outcomes for child native‐status reduced the strength of the association for all outcomes and attenuated many to nonsignificance. However, mercury levels did remain a significant predictor of poor performance on the primary General Cognitive Ability outcome (b = −1.35, p = 0.041) and several secondary cognitive subdomains (Working Memory and Auditory Processing ability) even after adjustment for child native status. The lack of a statistically significant interaction between mercury exposure and child native status suggests that the strength of associations between mercury levels and the primary outcomes were similar between native and nonnative children.
This study had limitations. First, with a relatively small sample size, we were underpowered to detect very subtle effects, although power analysis suggested power to detect small effects as low as Cohen's f 2 = 0.08. Notably, this limitation is common to cognitive‐development studies undertaken in isolated, rural communities in developing countries. Second, we lacked a measure of school‐quality and community‐level socioeconomic status. Although we adjusted statistically for the nonindependence of observations from children living within the same communities, we may not have been able to fully adjust for residual confounding by socioeconomic factors potentially associated with both mercury exposure and developmental outcomes at the community level. This may have been particularly true for children from native communities, who tended to have higher hair mercury levels and lower family socioeconomic status. Future studies in this region, and other similar low‐resource rural communities living near small‐scale gold mining operations, should seek to incorporate measures of socioeconomic status at the community level, particularly when large inequalities exist among communities. Third, a significant portion (71%) of native children in the study spoke Spanish, the language of cognitive testing, as a second language. While the cognitive tasks were specifically validated for use in this sample, the lower‐on‐average cognitive scores recorded among native children could reflect some residual measurement error, particularly in the Verbal Comprehension domain, which relies, in part, on Spanish‐language fluency. Fourth, we did not have a measure of prenatal mercury exposure, which is considered the most vulnerable developmental stage (Bose‐O'Reilly et al., 2010). It is possible that our findings of adverse effects of school‐age mercury exposure may also reflect the influence of past, prenatal exposures if family fish consumption patterns were consistent across time, which they appear to be (Wyatt et al., 2017). Fifth, while ASGM activity has been found to be the primary source of mercury contamination among fish in this region (Diringer et al., 2015), and the consumption of fish the primary driver of mercury exposure among residents of the area (Wyatt et al., 2017), fish tissue and fish consumption were not directly measured in this study. Finally, this study was observational and does not establish causation.
Notwithstanding its limitations, this study holds implications for research and policy within the Amazon region and, more globally, for other regions with ASGM operations. First, our findings suggest that child health monitoring programs such as the Demographic and Health Surveys (La Encuesta Demográfica y de Salud Familiar, or ENDES, in Peru) should incorporate measures of cognitive performance as well as chemical exposure into their annual assessments. Second, research into the health and neurocognitive impacts of child mercury exposure should consider giving special attention to populations, such as indigenous communities living in isolated areas, who may experience greater harm owing to additive effects from other stressors, including maltreatment, malnutrition, and low access to healthcare, sanitation, and education. Third, our results provide additional evidence to support the need to limit the release of mercury into the environment from small‐scale gold mining activities, either from mercury alternatives or better mercury capture and control procedures (U.S. Environmental Protection Agency, 2014). Impaired cognition owing to widespread mercury exposure in Madre de Dios and in communities living near watersheds with legacy mercury contamination may result in challenges for families, communities, and regional governments hoping to follow sustainable development trajectories. Investments into alternative livelihoods outside of artisanal gold mining to lift people out of poverty may be needed to slow the pace of mining in Peru and other developing regions.
5. Conclusion
In this sample of Peruvian children living in the Amazon Basin, children with greater hair mercury levels demonstrated poorer performance on measures of General Cognitive Ability than their less exposed peers. A significant percentage of tested children within the region displayed mercury exposure levels above current international standards. Children currently living near ASGM operations in the Peruvian Amazon may require intervention to reach their full developmental potential, including regular biomonitoring to identify children at risk and expanded education on how families can limit exposure.
Abbreviations
ASGMArtisanal and small‐scale gold mining.
- Batería III COG
The Batería III Woodcock‐Munoz Pruebas de Habilidades Cognitivas, the Spanish‐language version of the Woodcock‐Johnson III Tests of Cognitive Abilities.
- ENDES
La Encuesta Demográfica y de Salud Familiar (the Peruvian Demographic and Health Survey).
- HAZ
Height‐for‐age z‐score.
- U.S. NRC
The U.S. National Research Council.
- WHO
The World Health Organization.
Ethics Approval and Consent to Participate
Informed consent was obtained from parents of each child, with, in addition, children aged 9 and above giving written consent and children below age 9 giving verbal assent. Approval to conduct research on human subjects was obtained through the Universidad Peruana Cayetano‐Heredia Comité Institucional de Ética para Humanos (SIDISI 63056) and the Regional Health Directorate of Madre de Dios.
Funding
Funding for this study was provided by Hunt Oil Peru LLC to Duke University (HOEP‐QEHSS‐140003, W. P.). The funders had no role in study design, data collection and analysis, interpretation, decision to publish, or preparation of the manuscript. H. F. received support as trainee, under the mentorship of W. P., from the Doris Duke Foundation. A. R. was supported by the U.S. National Institute of Environmental Health Sciences Grant F31ES029358. W. P. acknowledges additional support from Bass Connections and the Center for Latin American Studies at Duke University, the U.S. National Aeronautics and Space Administration (NNX15AP74G), and the U.S. National Institute of Environmental Health Sciences (R21ES026960).
Conflict of Interest
The authors declare no conflicts of interest relevant to this study.
Supporting information
Supporting Information S1
Acknowledgments
The authors would like to acknowledge support from the Dirección Regional de Salud de Madre de Dios and the aid of the healthcare posts and the field technicians who assisted this study in each of the assessed communities. The authors would also like to thank Minerva Cartagena for her invaluable assistance. Special thanks to the children and families who participated in this study.
Reuben, A. , Frischtak, H. , Berky, A. , Ortiz, E. J. , Morales, A. M. , Hsu‐Kim, H. , et al. (2020). Elevated hair mercury levels are associated with neurodevelopmental deficits in children living near artisanal and small‐scale gold mining in Peru. GeoHealth, 4, e2019GH000222 10.1029/2019GH000222
Aaron Reuben and Helena Frischtak contributed equally to this work.
Contributor Information
Aaron Reuben, Email: aaron.reuben@duke.edu.
William K. Pan, Email: william.pan@duke.edu.
Data Availability Statement
Data for this research represent deidentified human subject data available for download and use by verifiable users with academic credentials and institutional affiliations. Data are archived via the Harvard University Dataverse (https://doi.org/10.7910/DVN/9947LY).
References
- Ashe, K. (2012). Elevated mercury concentrations in humans of Madre de Dios, Peru. PLoS ONE, 7(3), e33305 10.1371/journal.pone.0033305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asner, G. P. , Llactayo, W. , Tupayachi, R. , & Luna, E. R. (2013). Elevated rates of gold mining in the Amazon revealed through high‐resolution monitoring. Proceedings of the National Academy of Sciences of the United States of America, 110(46), 18454–18459. 10.1073/pnas.1318271110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrad, D. A. , Bellinger, D. C. , Ryan, L. M. , & Woodruff, T. J. (2007). Dose–response relationship of prenatal mercury exposure and IQ: An integrative analysis of epidemiologic data. Environmental Health Perspectives, 115(4), 609–615. 10.1289/ehp.9303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery, K. E. , & Beery, N. A. (2004). The Beery‐Buktenica developmental test of visual‐motor integration: Administration, scoring, and teaching manual, (5th ed.). Bloomington, MN: NCS Pearson. [Google Scholar]
- Bellanger, M. , Pichery, C. , Aerts, D. , Berglund, M. , Castaño, A. , Čejchanová, M. , Crettaz, P. , Davidson, F. , Esteban, M. , Fischer, M. E. , Gurzau, A. E. , Halzlova, K. , Katsonouri, A. , Knudsen, L. E. , Kolossa‐Gehring, M. , Koppen, G. , Ligocka, D. , Miklavčič, A. , Reis, M. , & ccccFátima, … DEMO/COPHES (2013). Economic benefits of methylmercury exposure control in Europe: Monetary value of neurotoxicity prevention. Environmental Health, 12(1), 3 10.1186/1476-069X-12-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger, D. C. (2008). Very low lead exposures and children's neurodevelopment. Current Opinion in Pediatrics, 20(2), 172–177. 10.1097/MOP.0b013e3282f4f97b [DOI] [PubMed] [Google Scholar]
- Bernhoft, R. A. (2012). Mercury toxicity and treatment: A review of the literature. Journal of Environmental and Public Health, 2012, 1–10. 10.1155/2012/460508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancourt, Ó. , Tapia, M. , & Méndez, I. (2015). Decline of general intelligence in children exposed to manganese from mining contamination in Puyango river basin, southern Ecuador. EcoHealth, 12(3), 453–460. 10.1007/s10393-015-1027-2 [DOI] [PubMed] [Google Scholar]
- Black, R. E. , Victora, C. G. , Walker, S. P. , Bhutta, Z. A. , Christian, P. , de Onis, M. , Ezzati, M. , Grantham‐McGregor, S. , Katz, J. , Martorell, R. , Uauy, R. , & Maternal and Child Nutrition Study Group (2013). Maternal and child undernutrition and overweight in low‐income and middle‐income countries. Lancet (London, England), 382(9890), 427–451. 10.1016/S0140-6736(13)60937-X [DOI] [PubMed] [Google Scholar]
- Bose‐O'Reilly, S. , McCarty, K. M. , Steckling, N. , & Lettmeier, B. (2010). Mercury exposure and children's health. Current Problems in Pediatric and Adolescent Health Care, 40(8), 186–215. 10.1016/j.cppeds.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, T. , & Hockey, S. C. (2013). The validity and reliability of developmental test of visual perception—2nd edition (DTVP‐2). Physical & Occupational Therapy in Pediatrics, 33(4), 426–439. 10.3109/01942638.2012.757573 [DOI] [PubMed] [Google Scholar]
- Brubaker, C. J. , Schmithorst, V. J. , Haynes, E. N. , Dietrich, K. N. , Egelhoff, J. C. , Lindquist, D. M. , Lanphear, B. P. , & Cecil, K. M. (2009). Altered myelination and axonal integrity in adults with childhood lead exposure: A diffusion tensor imaging study. Neurotoxicology, 30(6), 867–875. 10.1016/j.neuro.2009.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero Espejo, J. , Messinger, M. , Román‐Dañobeytia, F. , Ascorra, C. , Fernandez, L. E. , & Silman, M. (2018). Deforestation and forest degradation due to gold mining in the Peruvian Amazon: A 34‐year perspective. Remote Sensing, 10(12), 1903 10.3390/rs10121903 [DOI] [Google Scholar]
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences, (2nd ed.). Mahwah, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Cordier, S. , Garel, M. , Mandereau, L. , Morcel, H. , Doineau, P. , Gosme‐Seguret, S. , Josse, D. , White, R. , & Amiel‐Tison, C. (2002). Neurodevelopmental investigations among methylmercury‐exposed children in French Guiana. Environmental Research, 89(1), 1–11. 10.1006/enrs.2002.4349 [DOI] [PubMed] [Google Scholar]
- Davidson, P. W. , Myers, G. J. , & Weiss, B. (2004). Mercury exposure and child development outcomes. Pediatrics, 113(4 Suppl), 1023–1029. [PubMed] [Google Scholar]
- Diringer, S. E. , Feingold, B. J. , Ortiz, E. J. , Gallis, J. A. , Araújo‐Flores, J. M. , Berky, A. , Pan, W. K. Y. , & Hsu‐Kim, H. (2015). River transport of mercury from artisanal and small‐scale gold mining and risks for dietary mercury exposure in Madre de Dios, Peru. Environmental Science. Processes & Impacts, 17(2), 478–487. 10.1039/c4em00567h [DOI] [PubMed] [Google Scholar]
- Dolbec, J. , Mergler, D. , Sousa Passos, C. J. , Sousa de Morais, S. , & Lebel, J. (2000). Methylmercury exposure affects motor performance of a riverine population of the Tapajós river, Brazilian Amazon. International Archives of Occupational and Environmental Health, 73(3), 195–203. 10.1007/s004200050027 [DOI] [PubMed] [Google Scholar]
- Dórea, J. G. , Marques, R. C. , & Isejima, C. (2012). Neurodevelopment of Amazonian infants: Antenatal and postnatal exposure to methyl‐ and ethylmercury. Journal of Biomedicine & Biotechnology, 2012, 132,876 10.1155/2012/132876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esdaile, L. J. , & Chalker, J. M. (2018). The mercury problem in artisanal and small‐scale gold mining. Chemistry (Weinheim an Der Bergstrasse, Germany), 24(27), 6905–6916. 10.1002/chem.201704840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias, L. A. , Favaro, D. I. T. , Maihara, V. A. , M. B. A., V. , Yuyama, L. K. , Aguiar, J. P. L. , & Alencar, F. J. (2006). Assessment of daily dietary intake of Hg and some essential elements in diets of children from the Amazon region. Journal of Radioanalytical and Nuclear Chemistry, 270(1), 217–223. 10.1007/s10967-006-0341-0 [DOI] [Google Scholar]
- Feingold, B. J. , Berky, A. , Hsu‐Kim, H. , Rojas Jurado, E. , & Pan, W. K. (2019). Population‐based dietary exposure to mercury through fish consumption in the Southern Peruvian Amazon. Environmental Research. 10.1016/j.envres.2019.108720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fréry, N. , Maury‐Brachet, R. , Maillot, E. , Deheeger, M. , de Mérona, B. , & Boudou, A. (2001). Gold‐mining activities and mercury contamination of native amerindian communities in French Guiana: Key role of fish in dietary uptake. Environmental Health Perspectives, 109(5), 449–456. 10.1289/ehp.109-1240303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George, G. N. , Singh, S. P. , Myers, G. J. , Watson, G. E. , & Pickering, I. J. (2010). The chemical forms of mercury in human hair: A study using X‐ray absorption spectroscopy. Journal of Biological Inorganic Chemistry: JBIC: A Publication of the Society of Biological Inorganic Chemistry, 15(5), 709–715. 10.1007/s00775-010-0638-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb, H. , & O'Leary, K. G. (2014). Mercury exposure and health impacts among individuals in the artisanal and small‐scale gold mining community: A comprehensive review. Environmental Health Perspectives, 122(7), 667–672. 10.1289/ehp.1307864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez, D. J. X. , Arain, A. , & Fernandez, L. E. (2019). Mercury exposure, risk factors, and perceptions among women of childbearing age in an artisanal gold mining region of the Peruvian Amazon. Environmental Research, 179(Pt A). 10.1016/j.envres.2019.108786 [DOI] [PubMed] [Google Scholar]
- Grandjean, P. , White, R. F. , Nielsen, A. , Cleary, D. , & de Oliveira Santos, E. C. (1999). Methylmercury neurotoxicity in Amazonian children downstream from gold mining. Environmental Health Perspectives, 107(7), 587–591. 10.1289/ehp.99107587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham‐McGregor, S. , Cheung, Y. B. , Cueto, S. , Glewwe, P. , Richter, L. , & Strupp, B. (2007). Developmental potential in the first 5 years for children in developing countries. The Lancet, 369(9555), 60–70. 10.1016/S0140-6736(07)60032-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, H. H. , Pickering, I. J. , & George, G. N. (2003). The chemical form of mercury in fish. Science, 301(5637), 1203–1203. 10.1126/science.1085941 [DOI] [PubMed] [Google Scholar]
- Harvey, E. M. , Leonard‐Green, T. K. , Mohan, K. M. , Kulp, M. T. , Davis, A. L. , Miller, J. M. , Twelker, J. D. , Campus, I. , & Dennis, L. K. (2017). Inter‐rater and test‐retest reliability of the Beery VMI in schoolchildren. Optometry and Vision Science: Official Publication of the American Academy of Optometry, 94(5), 598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes, P. , James, K. A. F. , & Levy, L. S. (2009). Is low‐level environmental mercury exposure of concern to human health? Science of the Total Environment, 408(2), 171–182. 10.1016/j.scitotenv.2009.09.043 [DOI] [PubMed] [Google Scholar]
- Hurtado, J. , Gonzales, G. F. , & Steenland, K. (2006). Mercury exposures in informal gold miners and relatives in southern Peru. International Journal of Occupational and Environmental Health, 12(4), 340–345. 10.1179/oeh.2006.12.4.340 [DOI] [PubMed] [Google Scholar]
- Intituto Nacional de Estadistica e Informatica . (n.d.). Variables contextuales. https://www.inei.gob.pe/media/MenuRecursivo/publicaciones_digitales/Est/Lib0014/varicont.htm.
- Karagas, M. R. , Choi, A. L. , Oken, E. , Horvat, M. , Schoeny, R. , Kamai, E. , Cowell, W. , Grandjean, P. , & Korrick, S. (2012). Evidence on the human health effects of low‐level methylmercury exposure. Environmental Health Perspectives, 120(6), 799–806. 10.1289/ehp.1104494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, R. B. , Kim, B.‐G. , Kim, Y.‐M. , Hong, Y.‐S. , You, C.‐H. , & Kim, D.‐S. (2013). Association between low‐level mercury exposure and neurobehavioral functions in Korean adults living in a coastal city. Environmental Health and Toxicology, 28 10.5620/eht.2013.28.e2013015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel, J. , Mergler, D. , Branches, F. , Lucotte, M. , Amorim, M. , Larribe, F. , & Dolbec, J. (1998). Neurotoxic effects of low‐level methylmercury contamination in the Amazonian Basin. Environmental Research, 79(1), 20–32. 10.1006/enrs.1998.3846 [DOI] [PubMed] [Google Scholar]
- Lebel, J. , Mergler, D. , Lucotte, M. , Amorim, M. , Dolbec, J. , Miranda, D. , Arantès, G. , Rheault, I. , & Pichet, P. (1996). Evidence of early nervous system dysfunction in Amazonian populations exposed to low‐levels of methylmercury. Neurotoxicology, 17(1), 157–167. [PubMed] [Google Scholar]
- Marques, R. C. , Abreu, L. , Bernardi, J. V. E. , & Dórea, J. G. (2016). Neurodevelopment of Amazonian children exposed to ethylmercury (from Thimerosal in vaccines) and methylmercury (from fish). Environmental Research, 149, 259–265. 10.1016/j.envres.2015.12.022 [DOI] [PubMed] [Google Scholar]
- Martinez, G. , McCord, S. A. , Driscoll, C. T. , Todorova, S. , Wu, S. , Araújo, J. F. , Vega, C. M. , & Fernandez, L. E. (2018). Mercury contamination in riverine sediments and fish associated with artisanal and small‐scale gold mining in Madre de Dios, Peru. International Journal of Environmental Research and Public Health, 15(8), 1584 10.3390/ijerph15081584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather, N. , Woodcock, R. W. , Wolfson, L. , McGrew, K. S. , Schrank, F. A. , Ruef, M. L. , Alvarado, C. G. , Muñoz‐Sandoval, A. F. , Wendling, B. J. , Marshall, A. , & Riverside Publishing Company (2008). Batería III Woodcock‐Muñoz. Rolling Meadows, IL: Riverside Pub. [Google Scholar]
- McDowell, M. A. , Dillon, C. F. , Osterloh, J. , Bolger, P. M. , Pellizzari, E. , Fernando, R. , de Oca, R. M. , Schober, S. E. , Sinks, T. , Jones, R. L. , & Mahaffey, K. R. (2004). Hair mercury levels in U.S. children and women of childbearing Age: Reference range data from NHANES 1999–2000. Environmental Health Perspectives, 112(11), 1165–1171. 10.1289/ehp.7046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrew, K. S. , & Woodcock, R. W. (2001). Technical manual: Woodcock‐Johnson III. Itasca, IL: Riverside. [Google Scholar]
- Native Federation of the Madre de Dios River and Tributaries . (2014). Native Federation of the Madre de Dios River and Tributaries. http://www.fenamad.org.pe/
- Ng, D. K.‐K. , Chan, C.‐H. , Soo, M.‐T. , & Lee, R. S.‐Y. (2007). Low‐level chronic mercury exposure in children and adolescents: Meta‐analysis. Pediatrics International: Official Journal of the Japan Pediatric Society, 49(1), 80–87. 10.1111/j.1442-200X.2007.02303.x [DOI] [PubMed] [Google Scholar]
- Nuttall, K. L. (2006). Interpreting hair mercury levels in individual patients. Annals of Clinical & Laboratory Science, 36(3), 248–261. [PubMed] [Google Scholar]
- Ocas‐Córdova, S. , Tapia, V. , & Gonzales, G. F. (2018). Hemoglobin concentration in children at different altitudes in Peru: Proposal for [Hb] correction for altitude to diagnose anemia and polycythemia. High Altitude Medicine & Biology, 19(4), 398–403. 10.1089/ham.2018.0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peplow, D. , & Augustine, S. (2014). Neurological abnormalities in a mercury exposed population among indigenous Wayana in Southeast Suriname. Environmental Science. Processes & Impacts, 16(10), 2415–2422. 10.1039/c4em00268g [DOI] [PubMed] [Google Scholar]
- Peraza, M. A. , Ayala‐Fierro, F. , Barber, D. S. , Casarez, E. , & Rael, L. T. (1998). Effects of micronutrients on metal toxicity. Environmental Health Perspectives, 106(Suppl 1), 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperata, B. A. , Schmeer, K. K. , Hadley, C. , & Ritchie‐Ewing, G. (2013). Dietary inequalities of mother–child pairs in the rural Amazon: Evidence of maternal‐child buffering? Social Science & Medicine, 96, 183–191. 10.1016/j.socscimed.2013.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt, K. , & Fanzo, J. (2014). Addressing chronic malnutrition through multi‐sectoral, sustainable approaches: A review of the causes and consequences. Frontiers in Nutrition, 1, 13–13. 10.3389/fnut.2014.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben, A. , Caspi, A. , Belsky, D. W. , Broadbent, J. , Harrington, H. , Sugden, K. , Houts, R. M. , Ramrakha, S. , Poulton, R. , & Moffitt, T. E. (2017). Association of childhood blood lead levels with cognitive function and socioeconomic status at age 38 years and with IQ change and socioeconomic mobility between childhood and adulthood. JAMA, 317(12), 1244–1251. 10.1001/jama.2017.1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben, A. , Frischtak, H. , Mehta, P. , Wertz, J. , Bullins, P. , Ortiz, E. J. , Berky, A. J. , Morales, A. M. , Pan, W. K. Y. , & Pendergast, L. L. (2020). Validation of the Batería III Woodcock‐Munoz Pruebas de Habilidades Cognitivas for assessment of cognitive ability among children living in the Peruvian Amazon. PsyArXiv. 10.31234/osf.io/5jduq [DOI]
- Reuben, A. , Schaefer, J. D. , Moffitt, T. E. , Broadbent, J. , Harrington, H. , Houts, R. M. , Ramrakha, S. , Poulton, R. , & Caspi, A. (2019). Association of childhood lead exposure with adult personality traits and lifelong mental health. JAMA Psychiatry, 76(4), 418–425. 10.1001/jamapsychiatry.2018.4192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche, M. L. , Creed‐Kanashiro, H. M. , Tuesta, I. , & Kuhnlein, H. V. (2011). Infant and young child feeding in the Peruvian Amazon: The need to promote exclusive breastfeeding and nutrient‐dense traditional complementary foods. Maternal & Child Nutrition, 7(3), 284–294. 10.1111/j.1740-8709.2009.00234.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber, K. H. , & Hurley, R. A. (2008). Mercury exposure: Effects across the lifespan. The Journal of Neuropsychiatry and Clinical Neurosciences, 20(4), iv–389. 10.1176/jnp.2008.20.4.iv [DOI] [PubMed] [Google Scholar]
- Tavares, L. M. B. , Câmara, V. M. , Malm, O. , & Santos, E. C. de O. (2005). Performance on neurological development tests by riverine children with moderate mercury exposure in Amazonia, Brazil. Cadernos de Saúde Pública, 21(4), 1160–1167. 10.1590/S0102-311X2005000400018 [DOI] [PubMed] [Google Scholar]
- Trasande, L. , Landrigan, P. J. , & Schechter, C. (2005). Public health and economic consequences of methyl mercury toxicity to the developing brain. Environmental Health Perspectives, 113(5), 590–596. 10.1289/ehp.7743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Environment Programme . (2019). Global Mercury Assessment 2018. United Nations Environment Programme, Chemicals and Health Branch. http://www.unenvironment.org/resources/publication/global-mercury-assessment-2018
- UNEP Chemicals (2008). Guidance for identifying populations at risk from mercury exposure, (pp. 1–167). The Inter‐Organisation: Programme for the Sound Management of Chemicals. [Google Scholar]
- United Nations Environment Programme (2016). Introduction to mercury in ASGM. http://web.unep.org/globalmercurypartnership/introduction-mercury-asgm
- U.S. Environmental Protection Agency (2014). Reducing mercury pollution from artisanal and small‐scale gold mining [Overviews and Factsheets]. US EPA. https://www.epa.gov/international-cooperation/reducing-mercury-pollution-artisanal-and-small-scale-gold-mining
- U.S. National Research Council (2000). Toxicological effects of methylmercury. National Academies Press (US). http://www.ncbi.nlm.nih.gov/books/NBK225778/ [PubMed]
- Vetter, T. R. , & Mascha, E. J. (2017). Defining the primary outcomes and justifying secondary outcomes of a study: Usually, the fewer, the better. Anesthesia and Analgesia, 125(2), 678–681. 10.1213/ANE.0000000000002224 [DOI] [PubMed] [Google Scholar]
- Walker, S. P. , Wachs, T. D. , Gardner, J. M. , Lozoff, B. , Wasserman, G. A. , Pollitt, E. , & Carter, J. A. (2007). Child development: Risk factors for adverse outcomes in developing countries. The Lancet, 369(9556), 145–157. 10.1016/S0140-6736(07)60076-2 [DOI] [PubMed] [Google Scholar]
- Weinhouse, C. , Ortiz, E. J. , Berky, A. J. , Bullins, P. , Hare‐Grogg, J. , Rogers, L. , Morales, A.‐M. , Hsu‐Kim, H. , & Pan, W. K. (2017). Hair mercury level is associated with anemia and micronutrient status in children living near artisanal and small‐scale gold mining in the Peruvian Amazon. The American Journal of Tropical Medicine and Hygiene, 97(6), 1886–1897. 10.4269/ajtmh.17-0269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock, R. W. , McGrew, K. S. , & Mather, N. (2001). Woodcock‐Johnson III. Riverside Publishing. [Google Scholar]
- Wyatt, L. , Ortiz, E. J. , Feingold, B. , Berky, A. , Diringer, S. , Morales, A. M. , Jurado, E. R. , Hsu‐Kim, H. , & Pan, W. (2017). Spatial, temporal, and dietary variables associated with elevated mercury exposure in Peruvian riverine communities upstream and downstream of artisanal and small‐scale gold mining. International Journal of Environmental Research and Public Health, 14(12), 1582 10.3390/ijerph14121582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yard, E. E. , Horton, J. , Schier, J. G. , Caldwell, K. , Sanchez, C. , Lewis, L. , & Gastaňaga, C. (2012). Mercury exposure among artisanal gold miners in Madre de Dios, Peru: A cross‐sectional study. Journal of Medical Toxicology, 8(4), 441–448. 10.1007/s13181-012-0252-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, B.‐J. , Kim, B.‐G. , Jeon, M.‐J. , Kim, S.‐Y. , Kim, H.‐C. , Jang, T.‐W. , Chae, H.‐J. , Choi, W.‐J. , Ha, M.‐N. , & Hong, Y.‐S. (2016). Evaluation of mercury exposure level, clinical diagnosis and treatment for mercury intoxication. Annals of Occupational and Environmental Medicine, 28 10.1186/s40557-015-0086-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoo, E. M. , Valente, J. G. , Grattan, L. , Schmidt, S. L. , Platt, I. , & Silbergeld, E. K. (2003). Low level methylmercury exposure affects neuropsychological function in adults. Environmental Health, 2(1), 8 10.1186/1476-069X-2-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
Data for this research represent deidentified human subject data available for download and use by verifiable users with academic credentials and institutional affiliations. Data are archived via the Harvard University Dataverse (https://doi.org/10.7910/DVN/9947LY).


