Abstract
The gasotransmitters, nitric oxide (NO), hydrogen sulfide (H2S), and carbon monoxide (CO), are endogenously-produced volatile molecules that perform signaling functions throughout the body. In biological tissues, these small, lipid-permeable molecules exist in free gaseous form for only seconds or less, and thus they are ideal for paracrine signaling that can be controlled rapidly by changes in their rates of production or consumption. In addition, tissue concentrations of the gasotransmitters are influenced by fluctuations in the level of O2 and reactive oxygen species (ROS). The normal transition from fetus to newborn involves a several-fold increase in tissue O2 tensions and ROS, and requires rapid morphological and functional adaptations to the extrauterine environment. This review summarizes the role of gasotransmitters as it pertains to newborn physiology. Particular focus is given to the vasculature, ventilatory, and gastrointestinal systems, each of which uniquely illustrate the function of gasotransmitters in the birth transition and newborn periods. Moreover, given the relative lack of studies on the role that gasotransmitters play in the newborn, particularly that of H2S and CO, important gaps in knowledge are highlighted throughout the review.
Keywords: nitric oxide, NO, hydrogen sulfide, H2S, carbon monoxide, CO, newborn, neonate, fetus, vasculature, ventilatory control, gastrointestinal tract
I. INTRODUCTION
Nitric oxide (NO), carbon monoxide (CO), and hydrogen sulfide (H2S), the three gases categorized as gasotransmitters [1], have each followed a similar course in the history of biomedical science: from initial consideration as merely environmental toxins to the eventual realization that they are endogenous signaling molecules. This revelation, and the subsequent study of the physiological role of these gasotransmitters, has extended understanding of inter- and intracellular signaling mechanisms in several ways. First, unlike canonical receptor-ligand membrane transduction pathways, these gases diffuse across lipid membranes and can thus signal between cells and intracellular compartments more freely than larger signaling molecules. Second, the gasotransmitters can interact with a longer and more varied list of targets than larger conventional signaling molecules that are often selective for only one receptor. Third, the chemical reactivity of NO and H2S, and the bioactivity of many of their reaction products, confers a level of complexity to their signaling capabilities that goes well beyond simple ligand-receptor interactions characteristic of more classical signaling molecules. Finally, oxygen exerts an influence on the activity of gasotransmitters by a variety of mechanisms, positioning them as more versatile players in oxygen-sensing than can be achieved with most other second messenger systems.
A defining characteristic of neonatal physiology is the need to adapt to extrauterine life, a transition that includes dramatic and rapid changes in cardiovascular function, several-fold increases in tissue dissolved oxygen levels, and transformative modifications in how oxygen, carbon dioxide, nutrients, and wastes are exchanged with the environment. Studies in adult humans and animals have demonstrated that gasotransmitters play an important role in regulating many of the systems that are critical to the birth transition and neonatal homeostasis, such as vascular tone and ventilatory control. In some cases the roles of gasotransmitters have actually been studied in newborns, but most work, particularly for H2S and CO, has been done in adults and can only be tenuously extrapolated to the newborn. This is despite the fact that gasotransmitters would seem to be ideal candidates as signaling molecules for the rapid, oxygen-sensitive changes that occur at birth.
In this review, we present a summary of the work that has been done to characterize the role of gasotransmitters in neonatal physiology. We also highlight understudied areas where gasotransmitters can be reasonably expected to play a key role in the newborn. It is our hope that this article will not only be useful to the neonatal physiologists looking to include gasotransmitters in their studies, but that it will also point out aspects of newborn physiology that may offer useful insights into the signaling mechanisms of gasotransmitters in general. This review provides an overview of gasotransmitters as they pertain to the newborn, and the reader interested in more detail about gasotransmitters is referred to summaries in the field [2–5].
II. DETERMINANTS OF TISSUE CONCENTRATIONS
The gasotransmitters have a relatively short half life in biological tissues, ranging from a few milliseconds in blood to seconds in most tissues. As a result, concentrations of their free gaseous forms can fluctuate rapidly in response to changes in the rates of either production or clearance. Both enzyme-dependent and enzyme-independent pathways have been found for all three gasotransmitters, although enzyme-dependent production appears to be the predominant endogenous source [6–9].
All three of the gasotransmitters rapidly react with or bind to hemoglobin, and thus the blood serves as a reservoir depot as well as a pathway for biochemical clearance. Clearance of NO and H2S can also occur by various redox reactions, while CO is chemically inert. In this section we will briefly summarize the pathways of synthesis and clearance that determine the concentrations of free NO, H2S, and CO. The rates of both production and metabolism of the gasotransmitters are also influenced by O2 concentrations, and thus we will discuss how gasotransmitter levels are affected by the dramatic changes in PO2 that occur at birth.
Nitric oxide
The concentration of free NO in circulation is believed to be at or below low nanomolar levels, while the perivascular concentration of endogenous NO is generally estimated to be at or below mid-nanomolar levels [10,11]. These concentrations are the result of a balance of several NO-producing, NO-preserving, and NO-consuming, pathways. These include the synthesis of NO by NO synthase (NOS), preservation of NO in the form of various NO adducts that can be converted back into NO, and rapid NO-scavenging reactions that oxidize NO to nitrate. In the newborn, most of these pathways are altered so as to decrease NO availability [12–18].
NO Synthesis.
De novo NO is produced endogenously by endothelial or neuronal NO synthases (eNOS or nNOS) that are present constitutively [19], or by inducible NO synthase (iNOS) that can be upregulated in various tissues in response to inflammatory stimuli [20]. NO production by NOS is of significant developmental importance, as eNOS deficiency correlates with defective angiogenesis in hypoplastic human fetal lungs [21], and mice deficient for eNOS display a range of abnormalities including fetal growth restriction, reduced survival, an increased rate of limb abnormalities [22,23], and impaired myocardial angiogenesis and morphology [24,25].
Several factors indicate that overall production of NO by NOS enzymes is decreased during the postnatal period. First, postnatal levels of all three NOS isoforms are generally reported to be decreased compared to the fetus [12–15]. Second, asymmetric dimethylarginine (ADMA), an endogenous competitive inhibitor of NOS, is increased in neonates [26], especially in preterm males [27,28]. Third, plasma concentrations of nitrite, which serve as an index of NOS activity [29–31], are similar in fetal umbilical and maternal blood, but fall by more than 50% within minutes after birth and remain low for a week or more. This phenomenon is particularly pronounced in preterm infants [17,18]. Therefore, NO biosynthesis by NOS appears to be suppressed in newborns. Availability of L-arginine, the primary substrate of all three NOS isoforms and an essential amino acid in newborns [32], is also a determinant of NOS activity. Although plasma L-arginine concentrations in term infants are comparable to those of adults, they are markedly lower in premature infants [32–34] as well as term infants with pulmonary hypertension [35,36], suggesting L-arginine deficiency may also contribute to decreased NO production in some neonatal pathologies [37,38].
NO storage and metabolism.
For some time after the discovery of NOS, it was widely held that the NO it produced was metabolized rapidly via irreversible pathways that ended with nitrite and nitrate, two anions once thought to have little physiological relevance. However, work over the past two decades has now established a number of mechanisms by which endogenous NO can be preserved in the form of NO-derived adducts that either retain NO-like bioactivity or are capable of releasing free NO again. These NO-active compounds (NOx) include nitrosothiols (SNOs), heme and iron-nitrosyls (hemeNO and FeNO), and dinitrosyl iron complexes (DNICs). Even nitrite and nitrate are now widely acknowledged to contribute to NO signaling, the former by reduction to NO in reaction with various metal-containing enzymes [39] and the latter by bacterial nitrate reductase enzymes that convert nitrate to nitrite [40]. Somewhat ironically, early experiments that detected vasodilation by what was later determined to be NO were likely stimulating the release NO from these NOx species using ultraviolet light rather than by activation of NO production by NOS [41]. These NOx species constitute a significant reservoir of NO in the circulation and within tissues, but the relative concentrations of these compounds and how they are formed and regulated is still largely uncharacterized, particularly in the fetus and newborn.
As mentioned above, although circulating NOx concentrations are very well maintained in adults [31] plasma concentrations of NOx fall abruptly at birth and are maintained at levels well below those of the fetus and adult for the first few days of life [17,18]. This phenomenon may be partly due to reduced NOS levels and activity. In addition, neonates display markedly lower dietary nitrate and nitrite intake [42], lower rates of bacterial nitrate-to-nitrite reduction [16], and greater renal nitrite and nitrate excretion [18,43,44]. The extent to which this birth-related fall in NOx levels contributes to neonatal physiology remains to be determined.
Effects of O2 on NO levels.
Oxygen availability is a key determinant of both production and consumption of NO [45], and the question thus arises how the increase in O2 concentrations at birth may affect steady-state concentrations of NO. Briefly, O2 is required for the production of NO from L-arginine by NOS. Although the Km of eNOS for O2 (23 μM) is low enough that NO production in the fetus is likely not limited by O2 availability, the Km for iNOS (135 μM) and nNOS (350 μM) [46] is markedly higher than fetal dissolved O2 concentrations (arterial ~25 to 30 μM) and thus NO production by these NOS isoforms would seem to be oxygen-limited. Nonetheless, there is evidence that nNOS does function in the sheep fetus, as selective inhibition of nNOS has been found to attenuate post-ischemic brain injury [47].
In addition to NO production by NOS, the production of NO from NOx species such as nitrite is also O2-dependent. Under hypoxic conditions the production of NO from the reaction of nitrite with deoxygenated heme iron becomes more prevalent [39]. In the case of hemoglobin, this reaction (NO2− + Hb + H+ → NO + MetHb + OH−) has been proposed to result in NO-mediated vasodilation that is maximal at oxyhemoglobin saturations of approximately 50% [48]. However, although fetal hemoglobin facilitates this reaction approximately twice as fast as adult hemoglobin [49] and deoxyhemoglobin is more prevalent in the fetus than in the adult, nitrite only produces vasodilation at supraphysiological concentrations in fetal lambs [50], calling the physiological relevance of this reaction into question. For many of the other heme-containing proteins, such as cytoglobin, myoglobin, and neuroglobin, O2-affinity is so great that availability of the deoxygenated heme for reducing nitrite would appear to be limited at physiological O2 tensions in the adult [39]. Nonetheless, although the mechanisms remain undefined, there is abundant evidence that nitrite contributes to NO-dependent signaling pathways in the adult, and that this is potentiated by hypoxia [40]. The physiological relevance of nitrite-derived NO at fetal O2 tenions, or of the markedly reduced nitrite levels in the newborn are not yet understood. They conceivably relate to the redistribution of cardiac output among organs that takes place at birth.
O2 is also required for the predominant pathways of NO consumption in biological tissues, and thus rates of NO consumption would be expected to vary by more than 10-fold across the range of fetal-to-newborn tissue O2 tensions (Figure 2). The O2-dependence of NO consumption in tissues does not appear to be due to simple NO autooxidation, as it proceeds by different kinetics and is markedly faster in biological matrices than in aqueous buffer [45,51,52]. In adult plasma, the conversion of NO to nitrite has been attributed to ceruloplasmin [53], but this may not be the predominant mechanism of NO consumption in neonates as fetal plasma NO consumption does not correlate with ceruloplasmin concentrations in sheep or humans [54]. Thus, the mechanisms of O2-dependent NO consumption and whether these pathways change developmentally, remain unanswered questions.
Figure 2. Comparison of the birth-related change in O2 tensions and dissolved O2 concentrations (x-axis) with various measures of the O2-dependence of gasotransmitter concentration.
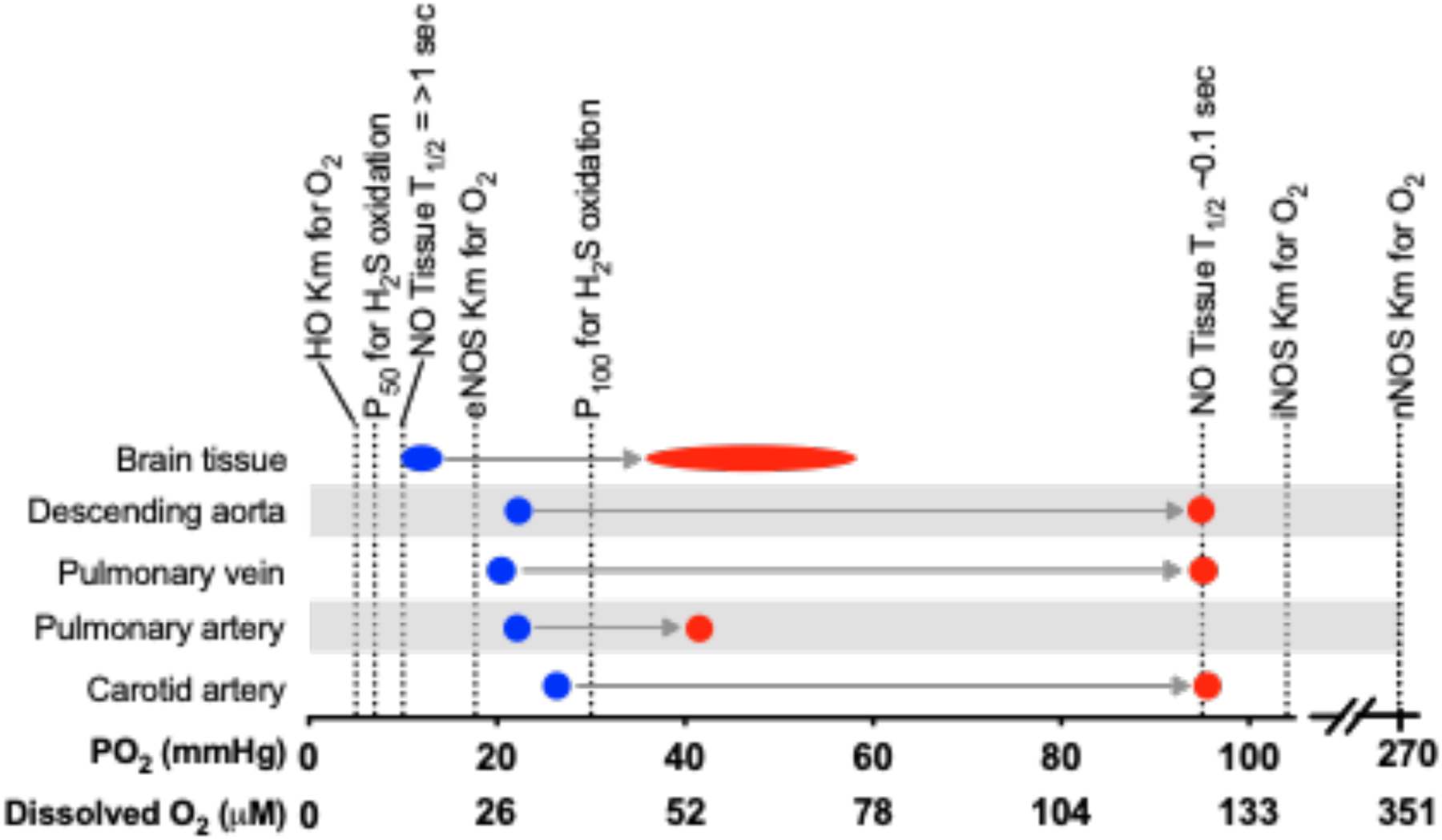
Blue markers indicate oxygen tensions in utero, red markers indicate oxygen tensions in the newborn and adult. Heme oxygenase (HO) Km for O2 from [96]. P50 and P100 for H2S oxidation from [78]. NO tissue half lives (T1/2) from [338]. NO synthase (NOS) Kms for O2 from [46].
Hydrogen Sulfide
The study of H2S is fraught with many of the same challenges as NO in that it reacts rapidly by numerous pathways in biological tissues, and assays that reliably distinguish between free H2S and its metabolites have been difficult to establish [55]. Most recent work indicates that the physiological concentration of free H2S is low nanomolar or less in blood, and tens of nanomolar in tissues [55]. These concentrations are determined by a dynamic equilibrium between rates of H2S synthesis and consumption. Likewise, although H2S production is not O2-dependent, H2S consumption is, and thus birth-related changes in O2 levels are likely to affect H2S steady-state levels.
H2S synthesis and storage.
H2S is synthesized from the sulfur in cysteine or its derivatives by the tissue specific-enzymes cystathionine-γ-lyase (CSE), cystathionine-β-lyase (CBS), and 3-mercaptopyruvate sulfurtransfurase (3-MST) (Figure 3) [9]. CSE and CBS are absent or sparse and levels of activity are relatively low in the human fetus and newborn [56]. Hepatic CSE expression and activity increase rapidly after birth reaching mature levels at about 3 months of age [57,58]. Availability of cysteine, a conditionally essential amino acid in preterm infants [59,60], may also be limited during neonatal life. Therefore, like NO, H2S biosynthesis also seems to be developmentally unfavored in the newborn. However, significant physiological importance of H2S has been indicated in neonates. Pulmonary vascular development and lung alveolarization are impaired in CSE−/− and CBS−/− mouse pups [61] and CBS−/− mice have a high mortality rate after the third and fourth postnatal weeks [62]. CBS deficiency is also associated with severe abnormalities of the eye, skeleton, vasculature, and central nervous system [63].
Figure 3. Pathways of H2S synthesis, metabolism, and clearance.
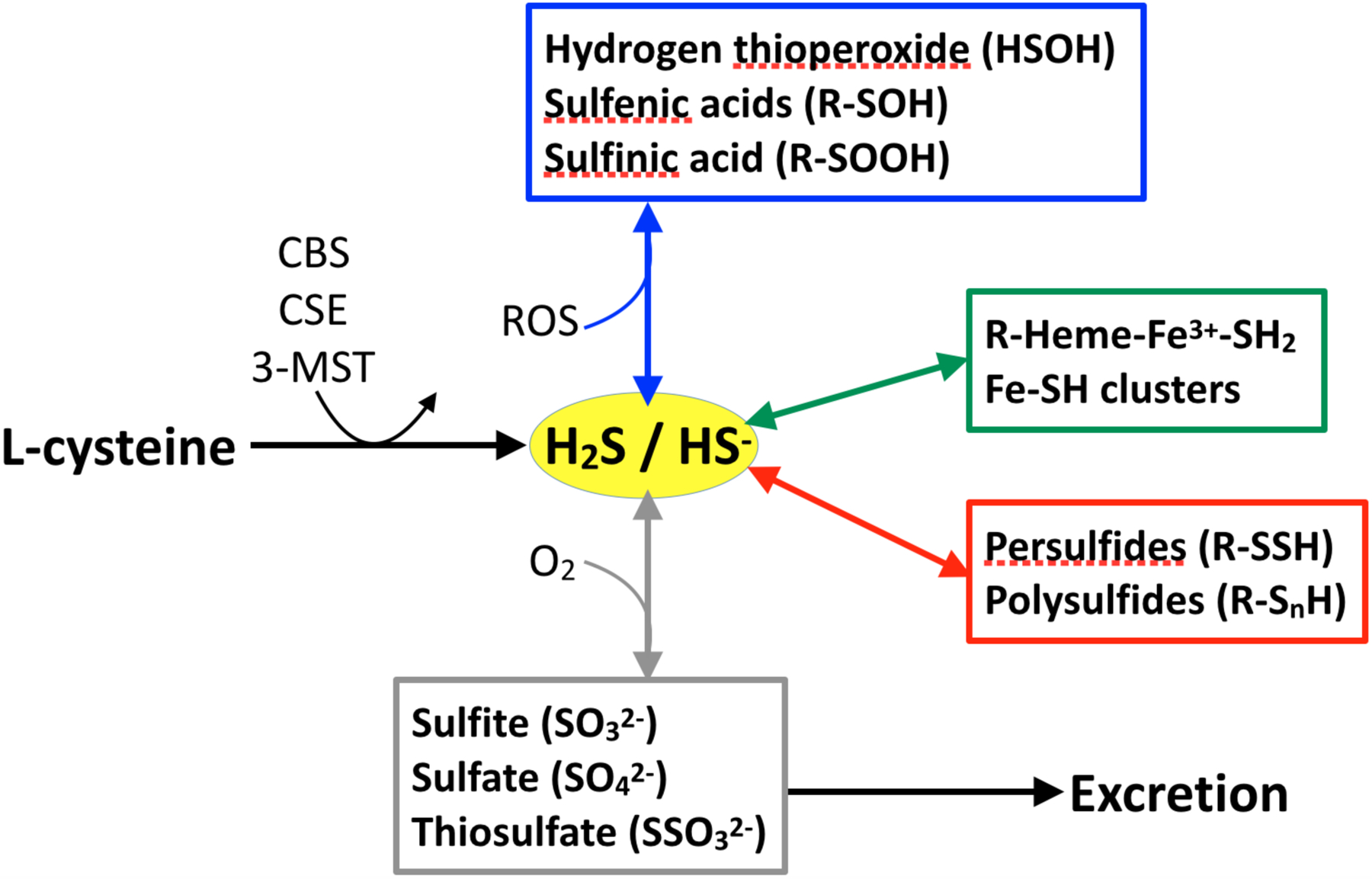
H2S is produced from L-cysteine by cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST). Oxidation reactions with reactive oxygen species (ROS) are shown in the blue box. Reactions with heme- and nonheme-iron are shown in the green box. Reactions with thiol groups are shown in the red box. Oxidation reactions with O2 to produce sulfite, sulfate, and thiosulfate are shown in the grey box. The latter constitute the major pool eliminated in the urine. Note that the many of the metabolic pathways shown are not yet well characterized in biological matrices.
H2S is a reactive molecule with a half life of seconds to minutes in the body [64,65]. At physiological pH, H2S exists at equilibrium with HS− (28% vs 72% at 25°C) [66]. Pathways for the clearance of free H2S are shown in Figure 3. They include: 1) mitochondrial oxidation to produce sulfite, thiosulfate, or sulfate; 2) binding to heme (ferric preferred over ferrous) to form an H2S complex which then leads to polysulfide and thiosulfate formation; 3) reaction with thiols, although prior oxidation of either H2S or the reactant thiol is required, to make persulfide; 4) assemblies of iron-sulfur clusters; and 5) oxidation to hydrogen thioperoxide, sulfenic acid, or sulfinic acid. Akin to NO, the concentrations of H2S metabolites are much higher than that of the free H2S itself. It was largely due to assay methods that did not distinguish between H2S and these metabolites that endogenous H2S concentrations were previously overestimated [55].
Similar to NO, some H2S metabolites can also regenerate free H2S by various pathways, and thus serve as a stable storage form of H2S [67–69]. Sulfane sulfur such as persulfide is one example of labile sulfur compounds that can release H2S by reduction [70]. So far, knowledge about the physiological role of these sulfur compounds is very limited [67,71]. Information is even more sparse with regard to the fetus and newborn, with only one study measuring urine thiosulfate as a marker of whole body H2S turnover in newborns. This work found that preterm newborns at greatest risk of microvascular dysfunction also have the highest levels of H2S turnover, suggesting that overproduction of this gasotransmitter may play a role in the pathology of these patients [72].
Effects of O2 on H2S levels.
Unlike NO and CO, O2 is not required to produce H2S. However, the predominant pathways for H2S consumption do require O2, and thus O2 tensions tend to be inversely proportional to free H2S concentrations. This has been demonstrated in tissue homogenates from several vertebrate organs including the salamander gastric tract [73], trout gill and heart [74,75], rat lung [76], seal and bovine pulmonary arteries [77], and mouse brain and liver [55]. In the adult lung, the O2-dependence of H2S consumption has an EC50 of approximately 5 mmHg PO2, and an Emax of >30 mmHg, which encompasses the physiological range of fetal tissue PO2s (~5 to 25 mmHg) [78]. Although H2S concentrations are largely unstudied in the fetus and newborn, extrapolation of what is known about O2-dependence of H2S metabolism in adult tissues would predict that H2S concentrations are markedly elevated in the fetus and then to fall rapidly within minutes after birth. Tests of this hypothesis using assays of H2S concentrations are needed.
Carbon monoxide
Interest in CO grew in the early 1900’s because of its danger in marsh gas of mines, where it was harm workers by occupying the O2 binding sites of hemoglobin. Carbon monoxide differs from NO and H2S in that it is, for the most part, chemically inert in vivo. Tissue CO concentrations are thus the result of a relatively simple equilibrium between its rate of production and the rate at which it diffuses into perfusing blood and is then eliminated from the lungs. Like NO and H2S, CO binds avidly to deoxyhemoglobin, which has an affinity for CO that is much greater than that of O2 [79]. As a result, although total concentrations of CO in blood are ~95 μM (based on 15 g/dl hemoglobin and 1% carboxyhemoglobin), concentrations of free CO are ~0.002 μM [80,81]. Likewise, mean tissue concentrations of free CO are also thought to lie in the low nanomolar range, with possible local increases as a result of surges in CO production [80]. As discussed in this section, CO also differs from NO and H2S in that overall tissue concentrations are elevated during the neonatal period.
Synthesis.
CO is generated from the oxidative degradation of heme by one of two different heme oxygenases designated HO-1 and HO-2. HO-1, also known as heat-shock protein 32, is inducible, responding to a long list of known stimuli such as oxidative stress, hypoxia, hyperoxia, ischemia, and hyperthermia [82]. The CO-producing reaction also releases free Fe2+ and biliverdin, with subsequent reduction of biliverdin to produce bilirubin (Figure 4). Newborns are exposed to many of these stresses as a result of the normal birth transition. Accordingly, circulating levels of HO-1 mRNA are upregulated during the first 2 or 3 days after birth in neonates [83], and are also found to be elevated in pig and mouse lungs [84] and rat liver [85] in the days following birth.
Figure 4. Pathways of CO synthesis, metabolism, and clearance.
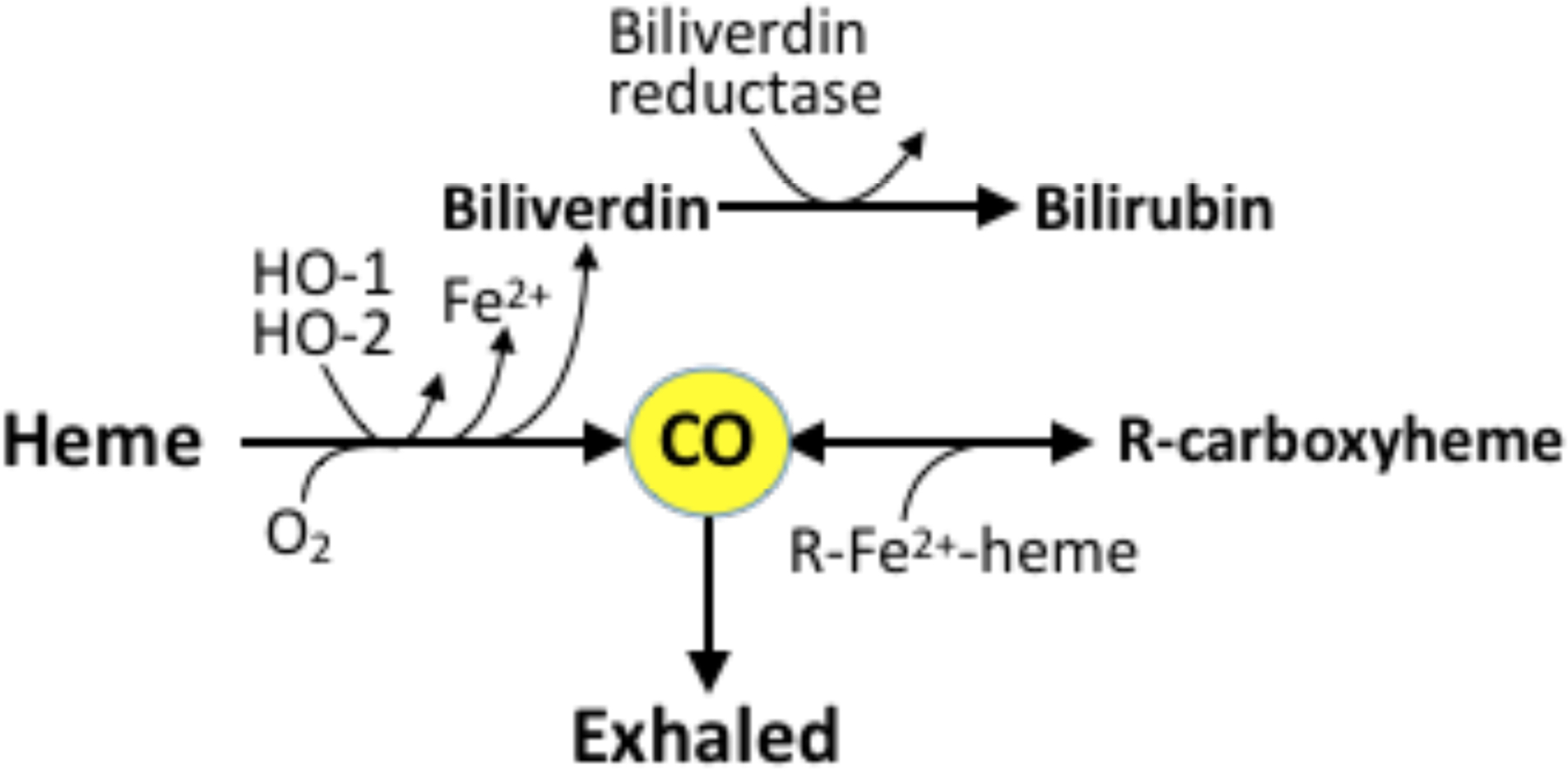
CO is formed from heme by heme oxygenases 1 and 2 (HO-1, HO-2) in a reaction that requires O2 and produces Fe2+ and biliverdin. Biliverdin is further reduced to bilirubin. CO can bind to various protein-bound heme moieties such as cytochrome oxidase c in tissues and deoxyhemoglobin in blood. CO is carried to the alveoli in the form of carboxyhemoglobin, where it can be exhaled.
The availability of heme substrate is a rate-limiting factor in heme oxygenase activity. Metabolism of heme as a result of red blood cell turnover represents the largest source of CO production in the body [86]. Accordingly, due to the higher hematocrit and shorter red blood cell half-life during the neonatal period, whole body CO production is found to be twice of that of the adult [87]. In addition, CO production in the near-term fetus is higher than the immature fetus and adult, also suggesting an augmented role for CO in the perinatal period [88,89].
CO storage and metabolism.
As mentioned above, CO is not significantly catabolized in vivo. After generation, CO rapidly equilibrates between tissue and blood by diffusion and binds to protein-bound the ferrous hemes with a half life of usually tens of minutes. The sole route of elimination of CO is via exhalation in the lung [90,91]. Heme-CO, such as carboxyhemoglobin (COHb), therefore reflects endogenous CO production and represents the main storage form of CO in the body (Figure 4). It has been suggested that COHb might be useful as a marker for high hemoglobin turnover to allow an earlier identification of newborns at risk of hyperbilirubinemia [92]. Moreover, COHb levels during the early postnatal period may serve as a practical marker for subsequent development of bronchopulmonary dysplasia, a disease associated with increased CO production [93,94]. However, COHb levels can be confounded by multiple factors such as postnatal age, gestational age, hemoglobin concentration, oxyhemoglobin saturation, ambient CO levels, and blood pH [95].
Effects of O2 on CO levels.
O2 is a substrate, along with heme, for the production of CO by both HO-1 and HO-2. However, the affinity of these enzymes for O2 is so high (~0.013 to 0.03 μM [96]) that it would seem unlikely that O2 availability is a limiting factor for HO activity even in the relatively hypoxic environment of the fetus (Figure 2). Fetal COHb concentrations are higher than those of the mother [97], although the extent to which this is due to fetal HO activity as opposed to the equilibrium of CO exchange between maternal and fetal blood has not been measured directly. However, CO competes with O2 for binding to the ferrous heme of many proteins including hemoglobin (220-fold greater affinity than O2, P50 = 0.0094 to 0.022 mmHg [79]), myoglobin (39-fold greater affinity than O2, P50 = 0.07 mmHg [79]), and cytochrome c oxidase (2.5-fold greater affinity than O2, P50 = 0.27 mmHg [98]). As a result, if tissue CO production is held constant, an increase in PO2 results in an increase in PCO due to a decrease in the availability of hemes for CO-binding [80]. Likewise, for any given PCO, an increase in PO2 results in a decrease in CO binding to heme. Due to this relationship, the rate of CO scavenging by hemoglobin is inversely proportional to blood PO2, and modeling of this phenomenon suggests that blood at normal fetal arterial oxyhemoglobin levels would scavenge tissue CO at a rate that is roughly twice that of blood at newborn oxyhemoglobin levels [80]. This interdependence of PO2 and PCO would be strongest when the PO2 is at or near the P50 of the heme for O2. Thus, the fetus would be more strongly impacted by this phenomenon than the adult, since the fetal arterial and venous PO2 gradient normally encompasses the P50 of fetal hemoglobin for O2. Experimentally, however, hypoxia in the newborn piglet results in an increase in CO concentrations in the cerebrospinal fluid [99], possibly suggesting rates of CO production are elevated in response to hypoxia.
III. CHEMICAL REACTIVITY AND SIGNALING PATHWAYS
The chemical and physical properties of gasotransmitters lay the foundation for their signaling mechanisms. This section will categorize the known biological targets of gasotransmitters according to their chemical reactivity, with emphasis on ontogeny in the newborn period.
Nitric oxide
The biological targets of NO can be classified into three main categories: free radicals, metals, and thiols. As a free radical itself, NO shows reactivity towards reactants with unpaired orbital electrons, such as other radicals and transition metals. For example, the reaction between NO and superoxide radicals approaches diffusion limited rates [100]. This reaction scavenges NO and produces the peroxynitrite intermediate which typically leads to pro-oxidant events [101]. The birth transition results in a spike in ROS and chelatable metal iron levels [102,103], particularly in preterm infants, making NO more likely to be pro-oxidant in the newborns.
Ferrous iron in heme proteins is the main metal target of NO. sGC with ferrous heme iron, is a specific receptor of NO, and is considered to be the most important biological target of NO. When bound to NO, sGC is activated and produces cGMP which leads to multiple downstream signaling cascades that finally result in vasodilation. Fetuses and neonates have higher lung expression of sGC than adults [104–107]. Likewise, inhibition of NOS activity in fetal sheep in utero significantly attenuates the fall in pulmonary artery pressure and increase in pulmonary blood flow that occurs with the onset of ventilation and increased oxygenation that occurs after birth, consistent with an essential role for NO in the birth transition [108].
Apart from activation of sGC, NO can also mediate signaling via nitrosylation of thiols [109]. Although NO free radical does not readily react with thiols, oxidation of either NO or the thiol, which may be more likely to occur during the transition from fetus to neonate [102,103], can facilitate the formation of nitrosothiols. Once formed, SNOs can readily transnitrosylate other thiol groups, whereby the NO moiety of one SNO is transferred to another thiol group. For example, nitrosoglutathione infused intravenously is found to transfer the NO moiety to plasma proteins within one circulatory transit time [110]. The extent to which SNO formation, either de novo or via transnitrosylation, is targeted to specific signaling functions is largely understudied in the newborn. At the same time, cellular pathways of SNO degradation are not well characterized but appear to involve denitrosylases such as thioredoxin, which is upregulated by oxygen in the newborn lung [111,112]. This potential of a heightened role for SNO signaling in the newborn period, together with evidence that some nitrosothiols have potent sGC-dependent vasodilatory activity that is greater in fetal vessels than in adult [113], highlights an important knowledge gap in the field.
A third category of NO-mediated signaling which has come to light recently is its effect on gene transcription via epigenetic mechanisms [114]. For example, the activity of jumonji C, an O2− and Fe2+-dependent histone demethylase, is inhibited by NO [115,116]. Histone acetylation may also be under the regulation of NO during neural crest and cranio-facial development [117,118]. There is also evidence that NO influences DNA methylation via regulation of the activity of ten-eleven translocation (TET) enzymes. Similar to jumonjiC, TETs also utilize Fe2+ and O2 [119], and thus their activities might be expected to change markedly during the birth transition when O2 levels increase and NO levels decrease, although this does not appear to have been studied.
Hydrogen sulfide
Although H2S is reactive and may play an important role in various biological functions, no specific target comparable to the importance of sGC signaling for NO has yet been identified for H2S. Metals and thiols are the main biological targets of H2S. Unlike NO, H2S does not readily react with ferrous iron in heme proteins such as oxyHb and deoxyHb, nor does it activate sGC. Instead, it binds to the ferric iron in heme and reduces it into ferrous form. For example, H2S can convert the oxidized ferric form of sGC, which does not respond to NO and might be more abundant in the oxidative neonatal environment [120,121], into the active ferrous form [122]. In addition, H2S inhibits the cGMP hydrolase activity of phosphodiesterase [123], thereby augmenting the effects of sGC activation by NO. The possibility that excessive oxidation of sGC heme may contribute to the unresponsiveness of some infants to inhaled NO for the treatment of pulmonary hypertension in infants highlights one potential therapeutic potential for H2S. In addition, methemoglobin levels tend to be higher in newborns due to relatively low plasma methemoglobin reductase levels [124–126]. As a result, H2S is more likely to react with methemoglobin in neonates, resulting in the production of thiosulfate and polysulfides, which further mediate signaling by H2S [127].
Although thiols are a main target of H2S, H2S does not react with them directly. Instead, the reaction requires prior oxidation of the reactant thiol to disulfide, sulfanic acid, or S-nitrosothiol, or oxidation of the H2S to HSOH [128]. This results in persulfidation of the reactant thiol by addition of the sulfur atom from H2S, leading to a disulfide bond. As with S-nitrosylation, addition of H2S to the thiols of proteins can lead to conformational changes that modify their activity [127]. KATP, TRPA1, and Keap1 are such targets by this pathway, as they can mediate the signaling of H2S and its polysulfide metabolites [129]. In addition, similar to transnitrosylation of SNOs, the -SH moiety of persulfides is also readily transferred to other thiol groups, leading to sulfuration of a broad spectrum of targets. In an oxidative environment, disulfides might be more abundant in neonates after the birth transition [130], potentially rendering more reactive thiol targets for H2S.
H2S has long been assumed to be an antioxidant. However, H2S does not directly react with O2 and thus this reaction is too slow to account for any significant biological effects [66]. H2S also does not readily react with ROS such as superoxide or hydrogen peroxide. Although the sequestration of oxidative iron by H2S has been proposed, autoxidation of H2S catalyzed by Fenton chemistry can also paradoxically generate considerable ROS leading to toxic effects [131,132]. Investigation is needed to test for the role of H2S in the regulation of ROS during periods of physiological oxidative stress such as labor and delivery.
Carbon monoxide
Compared with NO and H2S, CO is quite inert, and thus it is more likely to act as a reserved line of signaling. So far, the search for a specific signaling target of CO has not been successful. Only transition metals in a specific redox state, such as hemes with ferrous iron, have been identified as targets of CO [133]. It has been proposed that the signaling of CO might be mediated exclusively through its interaction, either directly or indirectly, with the transition metals of biological targets. Binding of CO to the ferrous heme of proteins leads to conformational and thus functional changes. Major proteins with high binding affinity for CO include hemoglobin, myoglobin, cytochrome P450 enzymes, and cytochrome c oxidase [134]. sGC has also been proposed to be an important target of CO. However, this activation is about 30-fold less potent than that of NO and the low binding affinity of CO to sGC requires supraphysiological concentrations of CO, raising doubt about the significance of sGC as a target of CO signaling [80].
In contrast to an apparent lack of direct targets for CO, increasing evidence suggests the importance of non-specific effects that result from inhibition of cytochrome c oxidase by competing with O2 for binding to the heme. This inhibition is proposed to account for the mitochondrial ROS generation that occurs at CO tensions at least as low as 0.19 mmHg (~0.2 μM) [135], as well as ROS-dependent signaling that activates redox-sensitive transcription factors and protein kinases to induce certain antioxidant enzyme systems at tensions as low as 0.076 mmHg (0.08 μM) [136,137]. These concentrations, as well as those observed to have biological effects in piglet pial arteries (0.09 mmHg) [138], are within the predicted range of plausible physiological tissue concentrations [80]. Notably, CO concentrations comparable to those that result from neonatal hyperbilirubinemia or routine low-flow general anesthesia have been associated with oxidative stress and impaired neuronal development in the mouse pup forebrain [139].
IV. VASOACTIVITY OF GASOTRANSMITTERS
Nitric oxide
The first and most widely-known physiological function of NO is ability to vasodilate. NO can be generated by endothelium via simulation with agonists such as acetylcholine and bradykinin, and by blood flow-induced shear stress [140]. It activates sGC to produce cGMP, which leads to modulation of L-type Ca2+ and large-conductance Ca2+-activated potassium channels via cGMP-dependent protein kinase (PKG) [113]. In newborns, the acetylcholine-dependent vasodilation in the skin of the forearm correlates directly with infant body size and head circumference, whereas vasodilation by sodium nitroprusside, which is endothelium-independent, showed no correlation with any anthropometric measures [141]. Thus, endothelial function may be decreased in low-birthweight infants, and is consistent with evidence that low birthweight results in impaired endothelial function later in life [142,143]. Both endothelium-dependent and -independent NO-mediated vasodilation also varies with postnatal age in the newborn piglet, where vasodilation in arteries isolated from animals on the first day of life is markedly reduced compared to vessels taken from animals a few days or weeks later [144,145]. In contrast to the apparent lack of NO-mediated vasodilation of pulmonary arteries from neonatal piglets, NOS inhibition in near-term fetal lambs causes a 50% reduction in birth-related pulmonary vasodilation, suggesting that a significant part of the rise in pulmonary blood flow at birth depends upon an acute release of NOS-derived NO [146–148]. Of note, it is possible that the effects of NO on overall pulmonary vascular resistance may be due to vasodilation of the pulmonary veins as well as the arteries [145,149]. In addition to the pulmonary vasculature, NO also appears to play an important role in mediating the postnatal changes in resistance of intestinal, cerebral, and skeletal vasculatures [150,151].
Due to its diffusion limited reaction with NO, superoxide is a determinant of NO bioavailability and thus vascular tone. Under physiological conditions, an increase in the ratio of superoxide and NO leads to vasoconstriction, while a decrease leads to vasodilation. In chronically instrumented fetal sheep, Giussani et al demonstrated that vascular oxidant tone plays an important role in regulating the peripheral vascular resistance via NO-dependent pathways [152–155]. The vascular oxidant tone is operational in late gestation in fetal sheep, and may contribute to the maintenance of arterial blood pressure in the perinatal period. Resetting of this vascular oxidant vascular tone at birth may also be an important factor in the physiological reduction of pulmonary vascular resistance, as exogenous superoxide dismutase results in pulmonary vasodilation in newborn lambs with pulmonary hypertension [156].
It is worth noting that, under some conditions such as hypoxia, NO can also lead to a species-dependent vasoconstriction via biased activation of sGC to produce cIMP instead of cGMP, thus favoring vasoconstriction [157–160]. To our knowledge, such NO-mediated vasoconstriction has not yet been studied in newborns.
As mentioned earlier, some metabolites of NO, such as nitrite, nitrosothiols, and DNICs [161], are capable of preserving its bioactivity and mediating vasodilation. Nitrite levels are markedly decreased in neonates [6,18]. Although we have found that nitrite itself has minimal vasoactivity at physiological concentrations in fetal [50], neonatal [162,163], and adult [164,165] sheep, we have also noted that nitrite potentiates vasodilation by nitrosothiols in adult sheep and rats [164]. We have recently observed the same potentiating effects of nitrite in neonatal lambs. Specifically, as shown in Figure 5, intravenous infusion of nitrosoglutathione (GSNO; 500 μM) produces a dose-dependent systemic hypotension that is markedly accentuated if the lambs are pre-treated with an intravenous infusion of nitrite to raise plasma nitrite concentrations by 2- to 4-fold above basal levels, well below the 100-fold increases needed for nitrite to cause vasodilation [50]. The mechanism by which nitrite potentiates vasodilation by nitrosothiols remains unclear, but may involve a vascular store of intracellular NOx species as discussed above. The effect of the birth-related fall in circulating nitrite levels on the systemic vascular constriction that is essential to maintaining arterial blood pressure after birth, and the potential role of a vascular NOx store, awaits further investigation.
Figure 5. Effect of nitrite on hypotensive response to intravenous infusion of nitrosoglutathione (GSNO) in anesthetized one-day-old lambs.
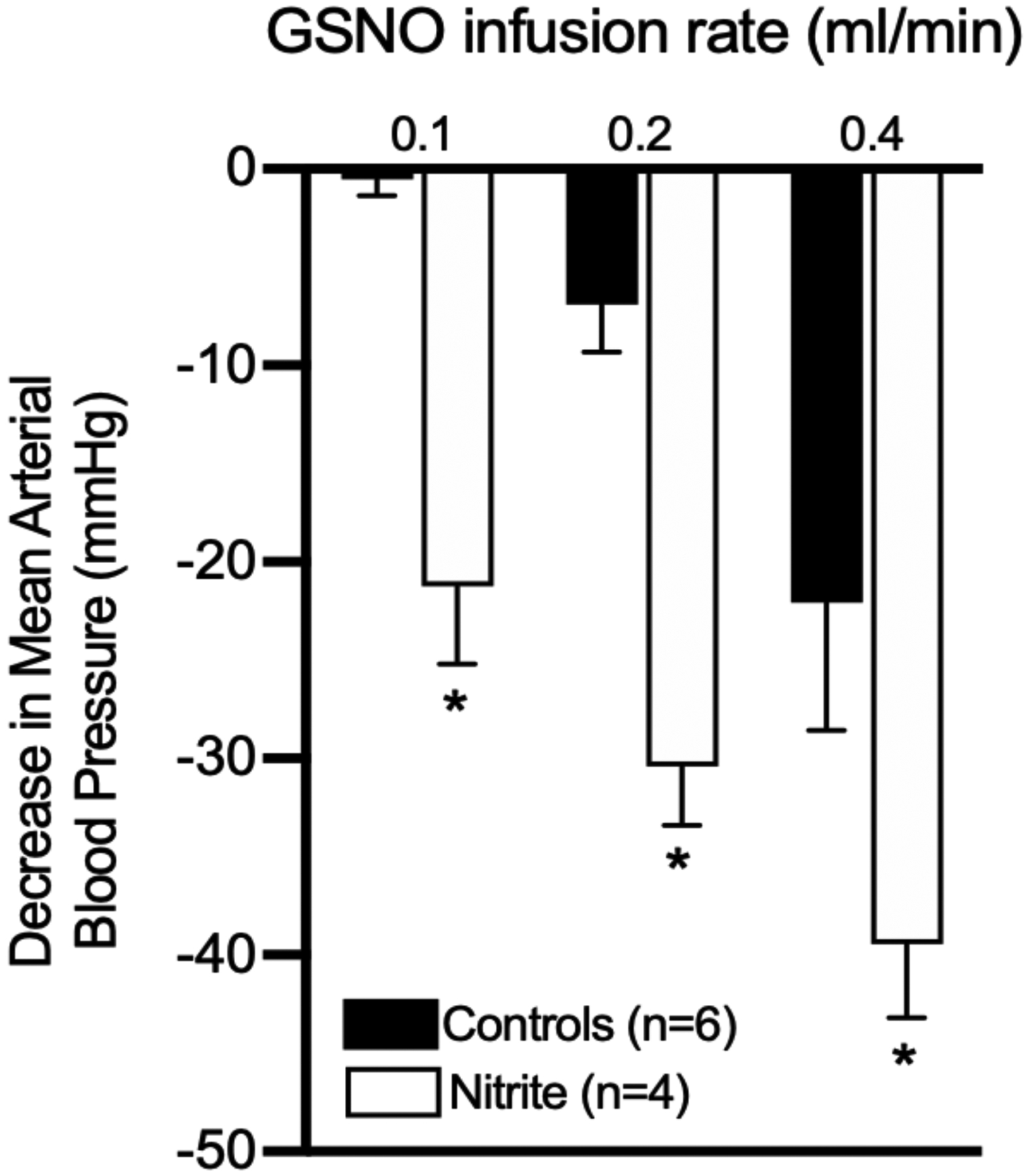
An intravenous bolus of sodium nitrite to achieve ~2 μM in the blood, which alone does not cause vasodilation [50,165], resulted in a significant potentiation of the hypotensive response to GSNO compared to lambs that received a saline bolus instead of nitrite. These results are consistent with previous observations in adult sheep and rats [164]. (P < 0.05 for nitrite vs controls, 2-way ANOVA with Dunnett’s post hoc test.)
Hydrogen sulfide
The vasoactivity of H2S is not as well-defined as that of NO. H2S has been demonstrated to induce either dilation or contraction, depending on the species, organ bed, and experimental conditions such as type and concentration of H2S donor used, oxygen tensions, presence of endothelium, substance used for precontraction, and composition of the organ bath buffer [166]. The long list of mechanisms suggested to be involved in H2S-mediated vasodilation includes opening of ATP- and voltage-sensitive potassium channels (KATP and KV, respectively) and Ca2+-dependent potassium channels (SKCa, IKCa, BKCa), closing of voltage-dependent Ca2+ channels, release of endothelial NO and hyperpolarizing factor, alteration of intracellular pH and ATP levels, oxidation of protein kinase G 1α, activation of transient receptor potential cation channel, release of calcitonin gene-related peptide, inhibition of phosphodiesterase, reduction of renin release, and inhibition of ACE activity [123,166–170]. The contractile effects of H2S have been proposed to involve reduction of cAMP concentrations, inhibition of beta-adrenergic vasodilation, inhibition of prostanoid-mediated vasodilation, opening of L-type Ca2+ channels, and activation of Na+,K+,2Cl−-cotransport [167,171,172].
Investigation of the vasoactivity of H2S in human vessels is very limited [173–177]. While there are no reports in infants, a few reports from neonates of other species exist. Using a cranial window preparation in piglets, Leffler et al showed that H2S production by endogenous CSE results in vasodilation of pial arteries [178,179]. They also found that vasodilation by endothelin-1 is mediated by H2S activation of KATP and BKCa channels [180]. In the ductus arteriosus of mice, H2S is proposed to be an endothelium-derived hyperpolarizing factor [181]. However, no significant ductus arteriosus reactivity was found for endogenous H2S in chickens [182]. Intriguingly, H2S has also been proposed as an oxygen sensor in hypoxic vasoconstriction and hypoxic vasodilation of various adult vertebrate species [77,78,183], capable of responding to changes in arterial PO2 that are within the fetal-to-neonatal range (Figure 2). It is therefore reasonable to speculate that H2S may play a role in the cardiovascular transition at birth.
Carbon monoxide
Vasodilation is one of the most characterized functions of CO, although it is significantly less potent than NO [5]. The supraphysiological concentrations of exogenous CO necessary to achieve vasodilation have cast some doubt on its role as an endogenous vasodilator. Early studies with cerebral arteries from adult rabbits and dogs found no response to exogenous CO at concentrations more than three orders of magnitude above physiological levels [184]. However, Leffler et al observed significant dilation of piglet pial arteries in response to addition of nanomolar concentrations of CO, and suggested HO-2 could produce adequate amounts of CO to act as an endogenous dilator [185]. Subsequent work indicates that newborn piglet vessels are more responsive to CO than those of older animals [186,187]. While the vasodilating potency of NO increased over the first few days of life, that of CO decreased [145], suggesting CO may play a compensatory vasodilating role. Using the closed cranial window model in 3 to 7-day-old piglets, endogenous CO has been identified as a cerebral vasodilator during hyperemia, hypotension, and seizures [188–191]. Carbon monoxide also mediates the cerebral vasodilation induced by arachidonic acid, prostaglandin E2, and ADP [192–194]. Furthermore, work by Parfenova and Leffler has demonstrated that coupling of neuronal activity to cerebral blood flow is facilitated by activation of glutamate receptors leading to increased intracellular Ca2+ in astrocytes that then results in activation of calmodulin-dependent HO-2 to produce CO. The resulting CO then stimulates arteriole myocyte KCa channels to dilate cerebral arterioles [195–201]. Endothelial NO also appears to play a permissive role in CO-induced cerebrovascular dilation in the piglets [202,203]. However, prolonged production of CO can inhibit NOS and reduce NO to elevate cerebrovascular tone [138].
In the lungs, CO might play an important role in reducing pulmonary vascular tone in highland-adapted species. In studies comparing newborn lambs and llamas, a species acclimated to high altitude for over two million years, Llanos et al found pulmonary hypertension at high altitude only in the lambs, despite greater pulmonary NO production. In contrast, llamas displayed enhanced production of CO, rather than NO, implicating CO as a key vasodilator in the newborns of highland-adapted species [204–207].
V. GASOTRANSMITTERS IN THE NEONATAL GASTROINTESTINAL TRACT
This section will cover the role of NO, H2S, and CO in the neonatal gastrointestinal (GI) tract. The GI tract is of particular importance since it has historically emerged as the center of NO recycling, as well as non-enzymatic NO generation. The GI tract also contains microbiota that contribute to gasotransmitter production and elimination. We will therefore explore the enzymatic and non-enzymatic sources of these gasotransmitters in the GI tract, as well as delineate their known roles and mechanisms of action in the normal function of the neonatal GI tract. Where the neonatal-specific research is sparse, as is the case for CO and H2S, we will point out the known functions in the adult GI tract, then speculate on the potential contributions of these gases in the neonate, as well as highlight the need for more neonatal research.
Nitric Oxide
NOS-independent NO Generation in the GI Tract.
The GI tract is a prominent source of NOS-independent NO production. The rat and adult human oral cavity contain nitrate-reducing bacteria that reduce the physiologically inert nitrate to nitrite, a portion of which subsequently disproportionates into NO and nitrosating species such as N2O3 and NO+ upon acidification in the low pH stomach environment [208], or can circulate in the blood where it may be reduced to NO and contribute to vasodilation, particularly at low oxygen tensions [209–213]. Plasma nitrate can be derived from dietary sources such as beets, radishes, and leafy vegetables. The salivary glands actively concentrate nitrate from plasma into saliva, raising salivary concentrations by as much as ten-fold [214].
Historically, dietary intake of nitrate/nitrite was viewed as detrimental due to the ability of nitrite to oxidize hemoglobin to methemoglobin, leading to cyanosis, a problem that particularly affects newborn infants. Intake of nitrite-treated meats was also shown to be associated with GI cancer, possibly through the intermediacy of N-nitrosamine formation. Recent research is however increasingly demonstrating more favorable, physiological roles of dietary nitrate/nitrite; dietary nitrate intake is associated with better indices of cardiovascular health such as lower blood pressure, and improved exercise performance as has been reviewed more comprehensively elsewhere [212,215]. Vitamin C and other phenolic compounds have been shown to favor the formation of NO over the potentially detrimental nitrosating species by catalyzing nitrous acid reduction to NO and also reducing the nitrosating species to NO [216,217]. Besides the benefit of protecting from nitrosating species, the NO that is produced in the gastric lumen can easily diffuse into the adjacent epithelium and affect GI function.
Though dietary nitrate can be a significant source of NO or NO-like bioactivity in the adult, it does not seem to be a major source of NO in the neonate. There is essentially very little nitrate-to-nitrite-to-NO conversion in the human newborn in the first weeks of life [6,16]. There are multiple factors that ensure neonatal nitrite levels remain low. First, the infant dietary intake of nitrate and nitrite is only 5% and 0.6% of the normal adult intake, respectively [42,218]. In addition, although the infant salivary glands are able to concentrate nitrate into the saliva similar to the adult, the oral commensal bacteria that are essential for reducing nitrate to nitrite are largely absent during the first few weeks after birth [16]. Furthermore, neonatal saliva production is markedly lower than the adult [16], and preventing adults from swallowing saliva essentially blocks the physiological effects of dietary nitrate [212].
Nitrite can be protonated to HNO2 (pK = 3.3), and then quickly disproportionates to produce NO. Thus, swallowed nitrite becomes an important source of NO in the stomach when pH is low, and NO concentrations there are found to be orders of magnitude higher than in other portions of the GI tract [219]. However, the neonatal stomach, particularly that of preterm infants, is less acidic than that of the adult [220–222], and thus stomach NO levels would be expected to be much lower.
The intestinal microbiome also appears to play an important role in regulating NO bioavailability. Lactobacilli can act as a nitrite sink in the GI tract [223], and dietary supplementation with both lactobacilli and nitrate in rats led to a 3 to 8 fold increase in NO in the small intestine and cecum, but not in the colon [218]. Lactobacilli and bifidobacteria are very prevalent in the diet and GI tract of infants fed breast milk [224], and their prevalence in stool samples increases gradually over the first few weeks of life [225]. In vitro, these bacterial strains are able to convert nitrite into NO, while other strains of bacteria (E. coli and S. aureus) can act as an NO sink to suppress NO concentrations [218]. Intestinal NO gas has also been measured in human preterm infants, and was found to be decreased by antibiotic treatment [226]. Altogether, these results demonstrate how bacteria are important contributors to NOS-independent NO-generation, and NO metabolism in the human infant GI tract. Moreover, NO-generation by commensal bacteria in the human neonate is even more relevant considering the increasingly common use of probiotics, especially in preterm infants, to minimize risk for necrotizing enterocolitis [227,228].
NO and GI Function.
NO generated from eNOS, iNOS and nNOS contributes to almost all aspects of GI processes and functions such as gastric motility, gastric secretions, preservation of mucosal barrier integrity, mesenteric and intestinal perfusion, and innate immunity against enteropathogens as well as protection against epithelial injury. One of the most prominent roles of NO in the GI tract is its modulation of mesenteric vascular tone in both the fasted state to support basal GI function, and in the postprandial state when blood flow to the gut and intestines increases in support of digestion and absorption.
Normally, the adult small intestine receives about 10 % of cardiac output, about 80 % of which passes to the mucosal and submucosal layers. Total blood flow to the small intestine can increase by almost 100 % in the postprandial phase [229]. Interestingly, blood volume per gram of tissue is higher in the neonatal rat and piglet intestines compared to adults, increasing rapidly in piglets between days 1 and 3 post-birth and then falling gradually with increasing age [230,231]. The fetal-to-neonatal increase in intestinal blood flow is also observed in lambs, with an ~100 % increase in blood flow in 2 to 10-day-old lambs compared to fetal lambs at 90% gestation [232–234]. These flow changes are accompanied by decreases in intestinal postprandial hyperemia in both piglets and newborn lambs [232].
These fetal-to-neonatal-to-adult blood flow and vascular resistance changes have multifactorial causes, but have been shown to be mediated partly through changes in the eNOS → sGC → cGMP vasodilation pathway in the mesenteric arteries. In the piglet, whose GI system closely matches the human system [232], decreases in intestinal vascular resistance are flow-stimulated and NO-mediated. However, the eNOS → sGC → cGMP pathway contributes to the regulation of intestinal vascular resistance to a greater extent in 3-day-old vs. 35-day-old swine as determined by eNOS inhibition in both whole animal and isolated artery experiments [235–237]. In these studies, flow-mediated decreases in vascular resistance were eliminated by L-NAME only in the 3-day-old piglets, which also had greater acetylcholine and substance P-induced relaxation. Furthermore, the cGMP content of 3-day-old piglets was also higher than in 35-day-old piglets following acetylcholine/substance-P-induced relaxation. Of note, NO-mediated vasodilation serves to counterbalance the myogenic vasoconstriction exhibited by mesenteric terminal arteries shortly after birth (1-day-old), in contrast to postnatal day 40 when myogenic vasoconstriction is no longer observed [238,239]. Subsequent studies revealed that the eNOS protein levels are increased two-fold in 1-day-old piglets, compared to fetal or 1-day-old non-fed piglets, and eNOS levels continue to increase until day 10, after which protein levels decreased to day 1 levels [240], correlating with increased sensitivity to NO-mediated vasodilation. Together, these studies indicate that eNOS-dependent vasodilation is of more importance in the newborn piglet than in the fetus or adult.
The mesenteric endothelium regulates blood flow in response to endocrine, neural, and physiologic stimuli most of which utilize the eNOS-dependent pathway as a major determinant to effect an increase in mucosal blood flow. Neurohormones such as serotonin, acetylcholine, adenosine diphosphate, bradykinin [241], gastrin [242] and cholecystokinin [243] can all bind to luminal endothelial cell receptors, and increase mucosal blood flow in an eNOS-dependent manner, under both basal and postprandial hyperemic conditions. In adult rats, glucose-mediated hyperemia was inhibited by L-NAME, but reversed by L-arginine and L-NAME co-infusion [244], and the NO production requires activation of the adenosine-A2b receptor [245]. Postprandial hyperemia has also been specifically studied in neonatal pigs (3-week old), in which the hyperemia is also inhibited by NO-blockade [246].
Submucosal sympathetic nerves, which are the primary determinants of intestinal blood flow in the adult, contain both adrenergic vasoconstrictor fibers and non-adrenergic non-cholinergic (NANC) vasodilatory fibers. NANC fibers contain nNOS and produce NO as their primary neurotransmitter, and help to increase blood flow through an sGC-dependent mechanism. nNOS derived NO is a significant mediator of gut motility by inhibiting smooth muscle contractility directly, and through inhibiting the release of excitatory mediators. Loss of nNOS signaling is a key feature in the gastroparesis associated with diabetes, showcasing the importance of NO in normal gastric function [247,248]. The importance of nNOS in the human neonate is also apparent in Hirschsprung disease, a common cause of neonatal and infantile large gut obstruction due to dysfunctions in the development of nNOS-containing nerves, involving reduced expression of nNOS mRNA and protein [249,250], and dysfunctional NO transmission to the target cells [251,252]. Studies have consistently described decreases in nNOS-containing myenteric nitrergic cell density from fetus to birth, with continued decreases during the first few years of life [15,253]. The biological relevance of these changes are not well understood.
Hydrogen sulfide and carbon monoxide
Compared to NO, there is a large gap in knowledge about the physiological involvement of CO and H2S in the adult GI tract, particularly so in the neonate. Of the few studies that are available, most have been performed using exogenous donor compounds and under pathophysiological conditions. While it is still possible to gain hints about the normal physiological function of these molecules from these studies, any conclusions should be made with caution as more research is performed to obtain a clearer picture of all gasotransmitters in both adult and neonatal physiology.
Metabolism of H2S in the GI tract.
Due to the presence of mainly sulfate-reducing bacteria, which reduce inorganic sulfate (SO42−) to H2S, and other bacteria that reduce cysteine and methionine to H2S, the adult human gastrointestinal tract, especially the colon, is a site of significant H2S metabolism and bioactivity [254–256]. The GI tract also contains the CSE and CBS, which produce H2S from cysteine, especially in the colonic epithelial, smooth muscle, and neuronal cells, and interstitial cells of Cajal, in both animals and humans [257–260]. Similar to both NO and CO, H2S was historically regarded as a toxin in the GI tract. In fact, since bacteria-derived H2S is still being implicated in the etiology and pathogenesis of inflammatory bowel diseases such as Crohn’s disease and ulcerative colitis, and also in colorectal cancer [256]. Though there are many conflicting reports in the literature, endogenous H2S does seem to have some physiological relevance in the GI tract where it has mainly been implicated in the modulation of gastric motility through its hyperpolarization and relaxation of enteric smooth muscle cells [261,262], involving the S-sulfhydration and activation of KATP channels, inhibition of Rho/RhoA, [258], inhibition of both L-type Ca2+ and BK channels [263], or through crosstalk with NO [264,265]. Similar to NOS and HO-2, expression of CSE and CBS is reduced in Hirschsprung’s disease [257], demonstrating the possible importance of H2S to normal GI motility function in a neonatal or pediatric population group.
Sulfate-reducing bacteria have been observed in the feces of human infants as early as two weeks following delivery, with a prevalence that increases with developmental age [266,267]. The physiological contributions of endogenous and bacterial H2S to neonatal and infant gastrointestinal health are otherwise unknown due to very limited study, but it is likely that H2S at low concentrations coordinates with NO and CO to effect the fetal-to-neonatal decrease in intestinal vascular resistance at birth, and as a modulator of both gastric motility and protection. Studies utilizing already-established animal models of neonatal GI disease would likely make an important contribution to understanding the role of H2S in the neonatal GI tract.
Metabolism of CO in the GI tract.
Unlike its role in NO generation, the GI tract does not seem to be a significant source of bacteria-facilitated CO production. There are however a few reports about some enteric bacteria, specifically some strains of E. coli, which express a protein (chuS) with heme oxygenase-like activity [268,269], and might contribute to heme degradation, in vivo CO generation, and to the modulation of the host immune response when added to the GI tract of the adult mouse [270]. This study showed that endogenous production of CO by gut bacteria is feasible, especially in the context of probiotic supplementation, but the relevance of this phenomenon to the human adult and neonate still needs to be studied. Metabolism of CO by the colonic flora has been observed in the adult human, where bacteria play a role in regulating CO levels within the GI tract [271], but the physiological relevance of this potential CO sink and source of CO for the circulation is uncertain. The adult gut lumen is also known to have increased CO due to the action of mucosal heme oxygenases on luminal heme after its uptake into the intestinal mucosa [272]. In the adult, ingestion of meat has been reported to increase intestinal CO levels since the mucosal heme oxygenases can also act on exogenous, meat-derived heme [273]. Since the normal neonate diet does not contain appreciable heme, meat-derived heme is not a feasible source of CO production in the neonate. The differential postnatal changes in intestinal mucosa HO-1/2 expression from the fetal to neonatal to the adult age have also not been characterized, therefore it is not clear how much endogenous CO levels would increase in the event of heme supplied by gastrointestinal bleeding, a common neonatal and pediatric disorder with an incidence of about 6.4% [274]. In neonatal rats, however, enteral heme administration increases CO levels [275].
The emerging contributions of CO to the adult gastrointestinal physiology are covered in a number of in-depth reviews [276,277]. Most of the actions of CO parallel those of NO in the GI tract. HO-2 is constitutively expressed in various cells of the GI tract of animals and humans, especially the myenteric interstitial cells of Cajal, and myenteric neuronal cells, both of which are the gut pacemaker cells and mediate gut motility [278–281]. HO-1 tends to only be induced under injury or inflammation, and can help in gastroprotection [282]. Endogenous CO has been shown to contribute to mesenteric vasodilation since an HO-substrate was shown to vasodilate endothelium-intact, isolated rat mesenteric arteries in the presence of a NOS inhibitor [283]. HO-2-derived CO is also a critical mediator of gastric motility by acting as a hyperpolarizing factor [281,284,285], and by acting as a critical determinant of the membrane potential across myenteric smooth muscle cells [286]. The studies of CO involvement in gastroprotection are of a more pharmacological nature, but do suggest that CO may play a role in the protection of the gut from injury or inflammation [287,288]. Interestingly, HO-2 expression often co-localizes with NOS expression [15,280,289], and in myenteric NANC neurons, is more highly expressed than nNOS [290]. Accordingly, the effects of CO on both gut motility and protection of the gastric barrier are co-mediated by NO [288,291].
Carbon monoxide also seems to play a significant role in the neonatal GI tract, although there are only a few comparative studies between neonates and adults, or in neonates alone. In the neonatal suckling rat, the production of CO by isolated small intestine is not inhibited by tin-protoporphyrin, a competitive inhibitor of HO-1 and HO-2, while the CO production is significantly decreased in the adult rat, suggesting a possible alternative means of CO production in the neonatal intestine. In the same model, gradients of CO production across the intestinal length (duodenum to ileum) have an opposite pattern in the neonatal (ileum > duodenum) vs. adult intestine (duodenum > ileum) [292]. Postnatal changes in HO-2 and nNOS expression in myenteric neurons have also been reported in the pig at fetal, 1 to 2-day-old, and 5 to 6-week-old stages of development. These studies reveal that HO-2 expression increases with age in the neonatal period, and has an opposite pattern to nNOS expression, which decreases with developmental stage [293]. The physiological importance of these differential developmental changes are not yet clear.
VENTILATORY CONTROL
Pulmonary ventilation is an automatic process that begins at birth and usually proceeds without conscious intervention [294]. The control of ventilation is performed by a complex, highly integrative system that maintains the homeostasis of blood O2, CO2, and pH within narrow limits by modulating ventilatory rate. This complex system involves the sensing of blood PO2 or PCO2/pH by peripheral (aortic and carotid body) and central (medullary) chemoreceptors, as well as input from pulmonary stretch receptors. Gasotransmitters are found to be involved in many aspects of ventilatory control in studies of adults, and likely also play an important role in the initiation and maintenance of ventilation in the neonate, as described in the following sections.
Nitric oxide
The control of ventilation is carefully integrated, mainly in the medullary and pontine portions of the brainstem, into a modulated transmission to effector organs via the involvement of many afferent and efferent neurons, and motor output by respiratory muscles, facial structures and airway effectors [295]. Gasotransmitters, especially NO, have been implicated in these complex networks of ventilatory control, especially in the observed reflex increase in ventilatory responses to hypoxia and hypercapnia.
These inputs are carefully integrated, mainly in the medullary and pontine portions of the brainstem, into a modulated transmission to effector organs via the involvement of many afferent and efferent neurons, and motor output by respiratory muscles, facial structures and airway effectors [295]. Gasotransmitters, especially NO, have been implicated in these complex networks of ventilatory control especially in the observed reflex increase in ventilation following acute hypoxia (hypoxic ventilatory response, HVR), or the time-dependent increase in ventilation and ventilatory response following chronic hypoxia (ventilatory acclimatization to hypoxia, VAH), and also the increase in ventilation following a rise in PaCO2 (hypercapnic ventilatory response).
NOS has been localized in both the peripheral and central regions associated with the control of breathing. NOS expression has been reported within the nerve fibers innervating the glomus tissue of the carotid bodies, which are the primary peripheral chemoreceptors, in which NO plays an inhibitory role. Accordingly, NOS inhibition results in an increase in the hypoxic ventilatory response [296–298]. The carotid body response, however, may depend on the specific NOS isoform, as nNOS inhibition augments the hypoxic ventilatory response, while eNOS inhibition attenuates it [297]. NOS has also been localized to the areas of the brainstem that are involved in ventilatory control such as the nucleus tractus solitarius — where carotid body efferents terminate [299,300]. Here, NO plays an excitatory role which can be blunted by NOS inhibition resulting in a depression of the hypoxic ventilatory response. NOS also plays an excitatory role in the rostral ventrolateral medulla, where the central chemoreceptors sensitive to low pH are located, and in the nucleus raphe magnus, in which NOS inhibition attenuates the hypoxic ventilatory response [301,302]. NOS has also been localized to the locus coeruleus, which has been reported to have an inhibitory effect on the ventilatory response but can be inhibited by NO, giving NO an overall stimulatory effect towards the ventilatory response [303–305]. NO has also been suggested to play a role in the ventilatory acclimatization to hypoxia (VAH), in which ventilation and ventilatory-oxygen sensitivity is time-dependently increased following chronic hypoxia [306], but a few studies in rats have mostly yielded null results, showing no effect of NOS inhibition on VAH [307–309]. It is important to note that systemic administration of NOS inhibitors might yield confounding results due to opposing effects on the peripheral and central chemoreceptors [310].
The role of NO in ventilatory control is understudied in neonatal subjects. The few studies that exist indicate that NO plays a similar role as in adults. NO is suggested to have an inhibitory role on the carotid body-mediated ventilatory response to hypoxia following endotoxin administration in a newborn piglet model [310,311]. The role of NO has also been explored in the biphasic hypoxic ventilatory response characteristic of newborn mammals, in which the initial increase in ventilation is quickly followed by a depression of the ventilatory rate to levels less than or equal to baseline. The ventilatory depression decreases with increasing developmental age to yield a sustained ventilatory increase with hypoxia. In neonatal rats the transition from biphasic to sustained hypoxic ventilatory response correlates with increased NOS protein expression, and is reversed by NOS inhibition [312]. At least one study, however, has yielded a null result since NOS inhibition did not promote ventilatory depression following hypoxia in hyperoxia-reared neonatal rats [313]. There is the limitation that this study used systemic NOS inhibition, which can confound results due to opposing effects on carotid body and central hypoxic response.
Overall, there is a need for more studies about the role of NO in neonatal ventilatory control, and in both adult and neonatal groups there is still a dearth of knowledge about the molecular pathways through which NO modulates ventilatory control, and also little information about the possible contributions of the metabolites of nitric oxide such as nitrite, nitrosothiols and dinitrosyl iron complexes to the control of ventilation. Nitrosothiols, especially ones with low molecular weight, have however been reported to increase the basal ventilatory rate upon direct administration to the nucleus tractus solitarius, and are a possible candidate for the molecular effector of NOS-mediated ventilatory increase during hypoxia [314,315].
Hydrogen sulfide and carbon monoxide
Endogenous H2S has also been shown to play a role in the central regulation of respiratory rhythm and in both the central and peripheral response to hypoxia. Both CBS and CSE are expressed in the glomus cells of the carotid body, where H2S levels are increased by hypoxia, and the H2S has been suggested to augment sensory excitation by low oxygen [316], although the exact mechanism is still debated [78]. The physiological role of endogenous H2S in O2-sensing in the carotid body has also been challenged, since most of the in vitro work depends on exogenous H2S at concentrations higher than those generated endogenously [317]. None of the research on the role of H2S on oxygen-sensing in the carotid body appears to have been conducted in a neonatal population for any animal model, which presents an avenue for continued research.
Within the brain, CBS and 3-MST are the predominant H2S-producing enzymes, and have both been implicated in ventilatory control with specific effects that seem to be dependent on the exact brainstem region. CBS expression has been reported in the rat nucleus tractus solitarius (NTS), where exogenous and endogenous H2S has been reported to have an excitatory effect on isolated neurons [318,319]. Increased H2S production has also been reported following hypercapnia in the rat NTS, along with the observation that CBS blockade attenuates the ventilatory response to hypercapnia, further demonstrating the excitatory role of H2S [319]. CBS expression has also been observed in the rostral ventrolateral medulla, where H2S has been reported to have a down-modulatory role in the response to hypoxia, a role that is also reported in the anteroventral preoptic region [320,321]. Endogenous H2S, in contrast, has an excitatory role in both the adult and neonatal pre-Bötzinger complex where it has been suggested to modulate inspiratory rhythm since CBS-inhibition reduces inspiratory burst frequency of brainstem spinal cord (BSSC) and medullary slice preparations from both newborn and adult rats, and also decreases basal inspiratory rhythm of anaesthetized adult rats [322]. Exogenous H2S and L-cysteine were also shown to increase the burst frequency of neonatal rat medullary brain slices containing the pre-Bötzinger complex after a brief depression, and the L-cysteine activity was abolished by CBS inhibition, and enhanced by S-adenosyl-L-methionine, an allosteric activator of CBS. In this study, the biphasic effects of H2S in the neonatal rat medulla were shown to be mediated through KATP channels and cAMP [323], in different region of the neonatal medulla [323,324]. 3-MST expression has been reported in the adult rat medulla oblongata, and has been shown to be increased with chronic intermittent hypoxia [325], but similar studies have not been reported in the neonate. Overall, there is need for more studies of the contributions of the CBS/3-MST-H2S pathway in neonatal ventilatory control, and in general more mechanistic studies on the molecular pathways of demonstrated H2S-mediated modulation in both adults and neonates.
Similar to H2S, most studies have historically focused on the toxic effect of CO on ventilation, as CO exposure is frequently employed to model hypoxia [326,327]. However, similar to H2S, endogenous CO production by constitutive HO-2 expression, has been shown in the brain medullary centers such as the NTS [328–330]. Though studies are fewer, a physiological role for HO/CO and ventilatory control has also been suggested mainly due to proposed crosstalk between CO and H2S in carotid body O2-sensing; an inverse relationship has been observed between HO-2/CO and H2S levels in the carotid body during hypoxia. This relationship involves a reduction in CO levels which allows an increase in H2S levels leading to stimulation of the carotid body of adult rats [331–334]. Electrophysiological experiments using medullary slices from neonatal rats with and without HO-2 inhibition or HO-2 substrate (hemin) supplementation suggest that endogenous CO has a role in maintaining respiratory frequency, expiratory duration, and respiratory magnitude [335]. These effects are partially mediated by nNOS [336], and can be partially abolished by BKCA channel inhibition [337]. However, more research is needed to persuasively implicate the HO/CO pathway in central ventilatory control, which can be done by using in vivo models, and also studying differences in CO response by different brainstem regions responsible for respiratory control, as has been documented for both NO and H2S.
CONCLUSION
The revelation four decades ago that NO is produced endogenously and leads to vasodilation sparked a field of study that has now established that NO plays a role in cell signaling throughout the body. While NO production by NOS enzymes and sGC-mediated signaling pathways have been relatively well-characterized, much remains to be learned regarding how NO bioactivity is preserved and regulated in the form of its many metabolites. The same appears to be true for the bioactivity of H2S and its metabolites. In both cases, progress in the field is hindered by a lack of reliable assay methods capable of distinguishing between specific metabolites, particularly in living tissues where the more important adducts may also be the least stable and challenging to measure. The development of novel tools for determining levels of the specific NO and H2S adducts in living tissues is critical to gaining a better understanding of this important aspect of gasotransmitter signaling.
As we have highlighted throughout this review, the biological roles of all three of the gasotransmitters are intimately linked to O2 concentrations, whether it be by the effects of O2 on their production and metabolism, or by virtue of the fact that they often compete with O2 for the same heme targets that are critical to the O2-mediated functions. The physiology of the neonate provides a unique model for studying the effects of gasotransmitters in various organ systems under broad range of O2 tensions. Previous advances in understanding the effects of NO on vascular tone, and the role that it plays in the fetus-to-neonate transition led to significant improvements in neonatal care, such as the use of inhaled NO gas for treating pulmonary hypertension. It is likely that as the complexities of modes of storage and action of these gasotransmitters are further characterized, particularly with respect to the influence of changes in O2 concentrations on these pathways, additional novel modes of therapy will appear.
Figure 1. Major pathways of NO synthesis, metabolism, and clearance.
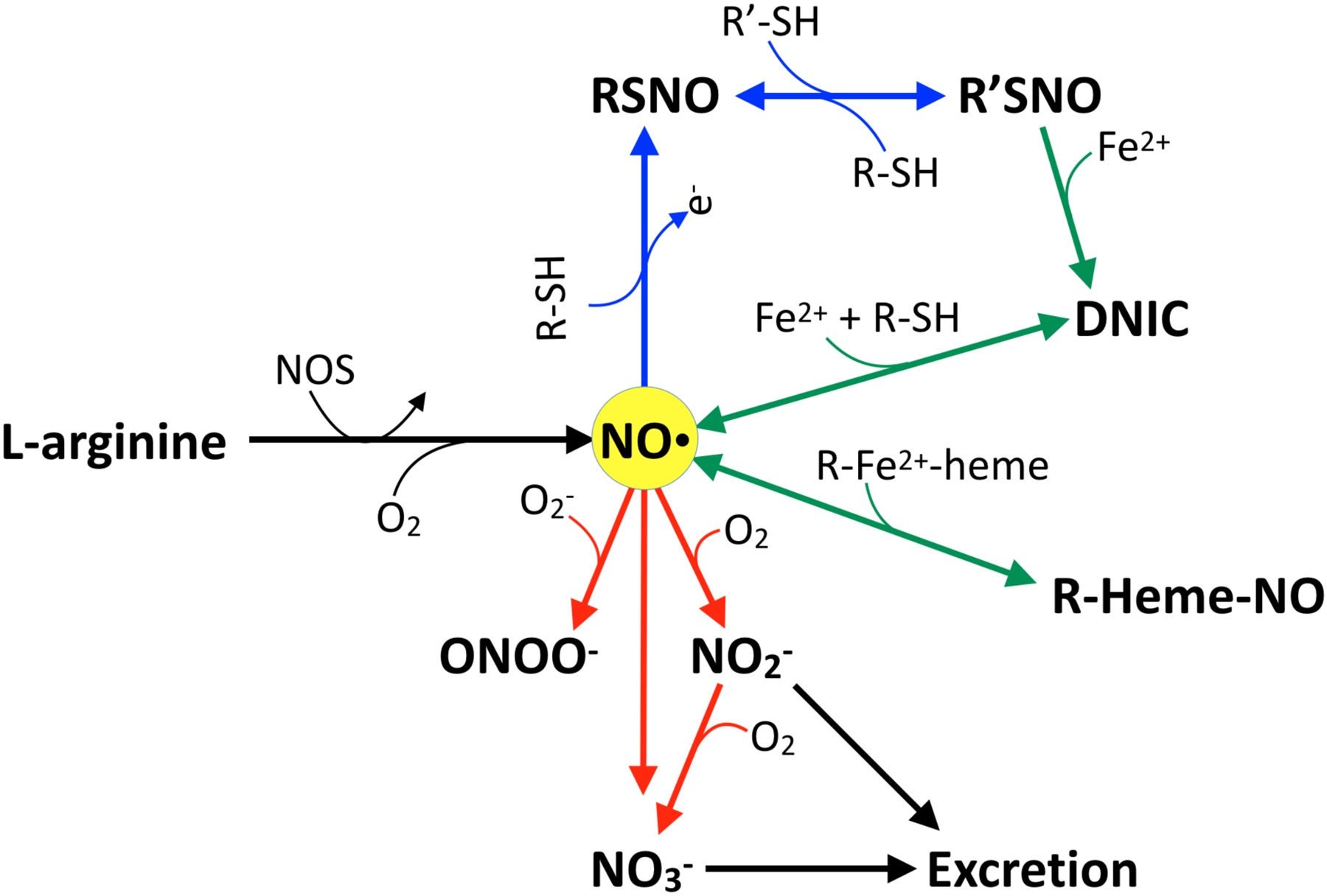
De novo NO is produced from L-arginine by NO synthases (NOS), a reaction that requires O2. If oxidized, NO free radical (NO·) can form nitrosothiols (RSNO), which are capable of transferring the NO moiety from one thiol to another (blue arrows). NO· and nitrosothiols can also react with heme and nonheme iron to produce heme iron nitrosyl compounds (R-heme-NO) and dinitrosyl iron complexes (DNIC) (green arrows). Finally, NO· can react with superoxide (O2−) to produce peroxynitrite (ONOO−), or with O2 to produce nitrite (NO2−) or nitrate (NO3−), both of which are excreted in the urine (red arrows). Note that NO3− can also be reduced back into NO2− by oral bacterial reductases, and that NO2− can be reduced back into NO under hypoxic and acidic conditions as described in the text.
FUNDING
This work was supported by the National Institutes of Health (HD083132 to ABB) and by research funding from the Loma Linda University Department of Pediatrics, Division of Neonatology.
ABBREVIATIONS
- CSE
cystathionine-γ-lyase
- CBS
cystathionine-β-lyase
- 3-MST
3-mercaptopyruvate sulfurtransferase
- sGC
soluble guanylate cyclase
- cGMP
cycling guanosine monophosphate
- NANC
non-adrenergic non-cholinergic
REFERENCES
- [1].Wang R, Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter?, FASEB J. 16 (2002) 1792–1798. [DOI] [PubMed] [Google Scholar]
- [2].Mustafa AK, Gadalla MM, Snyder SH, Signaling by gasotransmitters, Sci. Signal 2 (2009) re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cebová M, Košútová M, Pecháňová O, Cardiovascular effects of gasotransmitter donors, Physiol. Res 65 (2016) S291–S307. [DOI] [PubMed] [Google Scholar]
- [4].Liu Y-H, Lu M, Hu L-F, Wong PT-H, Webb GD, Bian J-S, Hydrogen sulfide in the mammalian cardiovascular system, Antioxid. Redox Signal 17 (2012) 141–185. [DOI] [PubMed] [Google Scholar]
- [5].Leffler CW, Parfenova H, Jaggar JH, Carbon monoxide as an endogenous vascular modulator, Am. J. Physiol. Heart Circ. Physiol 301 (2011) H1–H11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jones JA, Hopper AO, Power GG, Blood AB, Dietary intake and bio-activation of nitrite and nitrate in newborn infants, Pediatr. Res 77 (2015) 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Flannigan KL, McCoy KD, Wallace JL, Eukaryotic and prokaryotic contributions to colonic hydrogen sulfide synthesis, Am. J. Physiol. Gastrointest. Liver Physiol 301 (2011) G188–93. [DOI] [PubMed] [Google Scholar]
- [8].Shen X, Carlström M, Borniquel S, Jädert C, Kevil CG, Lundberg JO, Microbial regulation of host hydrogen sulfide bioavailability and metabolism, Free Radic. Biol. Med 60 (2013) 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Olson KR, H2S and polysulfide metabolism: Conventional and unconventional pathways, Biochemical Pharmacology. 149 (2018) 77–90. doi: 10.1016/j.bcp.2017.12.010. [DOI] [PubMed] [Google Scholar]
- [10].Polhemus DJ, Lefer DJ, Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease, Circ. Res 114 (2014) 730–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chen K, Pittman RN, Popel AS, Nitric oxide in the vasculature: where does it come from and where does it go? A quantitative perspective, Antioxid. Redox Signal 10 (2008) 1185–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].North AJ, Star RA, Brannon TS, Ujiie K, Wells LB, Lowenstein CJ, Snyder SH, Shaul PW, Nitric oxide synthase type I and type III gene expression are developmentally regulated in rat lung, Am. J. Physiol 266 (1994) L635–41. [DOI] [PubMed] [Google Scholar]
- [13].Parker TA, le Cras TD, Kinsella JP, Abman SH, Developmental changes in endothelial nitric oxide synthase expression and activity in ovine fetal lung, Am. J. Physiol. Lung Cell. Mol. Physiol 278 (2000) L202–8. [DOI] [PubMed] [Google Scholar]
- [14].Aikio O, Vuopala K, Pokela ML, Hallman M, Diminished inducible nitric oxide synthase expression in fulminant early-onset neonatal pneumonia, Pediatrics. 105 (2000) 1013–1019. [DOI] [PubMed] [Google Scholar]
- [15].Van Ginneken C, Van Meir F, Sys S, Weyns A, Developmental changes in heme-oxygenase-2 and bNOS expression in enteric neurons in the pig duodenum, Auton. Neurosci 91 (2001) 16–25. [DOI] [PubMed] [Google Scholar]
- [16].Kanady JA, Aruni AW, Ninnis JR, Hopper AO, Blood JD, Byrd BL, Holley LR, Staker MR, Hutson S, Fletcher HM, Power GG, Blood AB, Nitrate reductase activity of bacteria in saliva of term and preterm infants, Nitric Oxide. 27 (2012) 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ibrahim YI, Ninnis JR, Hopper AO, Deming DD, Zhang AX, Herring JL, Sowers LC, McMahon TJ, Power GG, Blood AB, Inhaled nitric oxide therapy increases blood nitrite, nitrate, and s-nitrosohemoglobin concentrations in infants with pulmonary hypertension, J. Pediatr 160 (2012) 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pun P, Jones J, Wolfe C, Deming DD, Power GG, Blood AB, Changes in plasma and urinary nitrite after birth in premature infants at risk for necrotizing enterocolitis, Pediatr. Res 79 (2016) 432–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Forstermann U, Li H, Schwarz PM, Kleinert H, NO Synthesis and NOS Regulation, in: Forman HJ, Fukuto J, Torres M (Eds.), Signal Transduction by Reactive Oxygen and Nitrogen Species: Pathways and Chemical Principles, Springer; Netherlands, Dordrecht, 2003: pp. 119–154. [Google Scholar]
- [20].Pautz A, Art J, Hahn S, Nowag S, Voss C, Kleinert H, Regulation of the expression of inducible nitric oxide synthase, Nitric Oxide. 23 (2010) 75–93. [DOI] [PubMed] [Google Scholar]
- [21].Boucherat O, Franco-Montoya M-L, Delacourt C, Martinovic J, Masse V, Elie C, Thébaud B, Benachi A, Bourbon JR, Defective angiogenesis in hypoplastic human fetal lungs correlates with nitric oxide synthase deficiency that occurs despite enhanced angiopoietin-2 and VEGF, American Journal of Physiology-Lung Cellular and Molecular Physiology. 298 (2010) L849–L856. doi: 10.1152/ajplung.00333.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hefler LA, Reyes CA, O’Brien WE, Gregg AR, Perinatal development of endothelial nitric oxide synthase-deficient mice, Biol. Reprod 64 (2001) 666–673. [DOI] [PubMed] [Google Scholar]
- [23].Pallares P, Garcia-Fernandez RA, Criado LM, Letelier CA, Esteban D, Fernandez-Toro JM, Flores JM, Gonzalez-Bulnes A, Disruption of the endothelial nitric oxide synthase gene affects ovulation, fertilization and early embryo survival in a knockout mouse model, Reproduction. 136 (2008) 573–579. [DOI] [PubMed] [Google Scholar]
- [24].Zhao X, Lu X, Feng Q, Deficiency in endothelial nitric oxide synthase impairs myocardial angiogenesis, Am. J. Physiol. Heart Circ. Physiol 283 (2002) H2371–8. [DOI] [PubMed] [Google Scholar]
- [25].Lee TC, Zhao YD, Courtman DW, Stewart DJ, Abnormal aortic valve development in mice lacking endothelial nitric oxide synthase, Circulation. 101 (2000) 2345–2348. [DOI] [PubMed] [Google Scholar]
- [26].Maeda T, Yoshimura T, Okamura H, Asymmetric Dimethylarginine, an Endogenous Inhibitor of Nitric Oxide Synthase, in Maternal and Fetal Circulation, Journal of the Society for Gynecologic Investigation. 10 (2003) 2–4. doi: 10.1177/107155760301000102. [DOI] [PubMed] [Google Scholar]
- [27].Tsukahara H, Ohta N, Tokuriki S, Nishijima K, Kotsuji F, Kawakami H, Ohta N, Sekine K, Nagasaka H, Mayumi M, Determination of asymmetric dimethylarginine, an endogenous nitric oxide synthase inhibitor, in umbilical blood, Metabolism. 57 (2008) 215–220. [DOI] [PubMed] [Google Scholar]
- [28].Mittermayer F, Prusa A-R, Pollak A, Wolzt M, Umbilical vein plasma concentrations of asymmetrical dimethylarginine are increased in male but not female neonates delivered preterm: a pilot study, Early Hum. Dev 82 (2006) 421–424. [DOI] [PubMed] [Google Scholar]
- [29].Lauer T, Preik M, Rassaf T, Strauer BE, Deussen A, Feelisch M, Kelm M, Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action, Proc. Natl. Acad. Sci. U. S. A 98 (2001) 12814–12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rhodes P, Leone AM, Francis PL, Struthers AD, Moncada S, P.]. Rhodes PM [corrected to Rhodes, The L-arginine:nitric oxide pathway is the major source of plasma nitrite in fasted humans, Biochem. Biophys. Res. Commun 209 (1995) 590–596. [DOI] [PubMed] [Google Scholar]
- [31].Milsom AB, Fernandez BO, Garcia-Saura MF, Rodriguez J, Feelisch M, Contributions of nitric oxide synthases, dietary nitrite/nitrate, and other sources to the formation of NO signaling products, Antioxid. Redox Signal 17 (2012) 422–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wu G, Jaeger LA, Bazer FW, Rhoads JM, Arginine deficiency in preterm infants: biochemical mechanisms and nutritional implications, J. Nutr. Biochem 15 (2004) 442–451. [DOI] [PubMed] [Google Scholar]
- [33].Zamora SA, Amin HJ, McMillan DD, Fick GH, Butzner JD, Parsons HG, Scott RB, Plasma L-arginine concentration, oxygenation index, and systemic blood pressure in premature infants, Crit. Care Med 26 (1998) 1271–1276. [DOI] [PubMed] [Google Scholar]
- [34].Becker RM, Wu G, Galanko JA, Chen W, Maynor AR, Bose CL, Rhoads JM, Reduced serum amino acid concentrations in infants with necrotizing enterocolitis, J. Pediatr 137 (2000) 785–793. [DOI] [PubMed] [Google Scholar]
- [35].Vosatka RJ, Kashyap S, Trifiletti RR, Arginine deficiency accompanies persistent pulmonary hypertension of the newborn, Biol. Neonate 66 (1994) 65–70. [DOI] [PubMed] [Google Scholar]
- [36].Castillo L, DeRojas-Walker T, Yu YM, Sanchez M, Chapman TE, Shannon D, Tannenbaum S, Burke JF, Young VR, Whole body arginine metabolism and nitric oxide synthesis in newborns with persistent pulmonary hypertension, Pediatr. Res 38 (1995) 17–24. [DOI] [PubMed] [Google Scholar]
- [37].Shah PS, Shah VS, Kelly LE, Arginine supplementation for prevention of necrotising enterocolitis in preterm infants, Cochrane Database Syst. Rev 4 (2017) CD004339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pearson DL, Dawling S, Walsh WF, Haines JL, Christman BW, Bazyk A, Scott N, Summar ML, Neonatal pulmonary hypertension--urea-cycle intermediates, nitric oxide production, and carbamoyl-phosphate synthetase function, N. Engl. J. Med 344 (2001) 1832–1838. [DOI] [PubMed] [Google Scholar]
- [39].Kim-Shapiro DB, Gladwin MT, Mechanisms of nitrite bioactivation, Nitric Oxide. 38 (2014) 58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lundberg JO, Carlström M, Weitzberg E, Metabolic Effects of Dietary Nitrate in Health and Disease, Cell Metab. 28 (2018) 9–22. [DOI] [PubMed] [Google Scholar]
- [41].Furchgott RF, Sleator W, McCaman MW and Elchlepp J, Relaxation of Arterial Strips by Light, and the Influence of Drugs on This Photodynamic Effect, J. Pharmacol. Exp. Ther 113 (1955) 22–23. [Google Scholar]
- [42].Jones JA, Ninnis JR, Hopper AO, Ibrahim Y, Merritt TA, Wan K-W, Power GG, Blood AB, Nitrite and nitrate concentrations and metabolism in breast milk, infant formula, and parenteral nutrition, JPEN J. Parenter. Enteral Nutr 38 (2014) 856–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tsukahara H, Hiraoka M, Hori C, Miyanomae T, Kikuchi K, Sudo M, Age-related changes of urinary nitrite/nitrate excretion in normal children, Nephron. 76 (1997) 307–309. [DOI] [PubMed] [Google Scholar]
- [44].Elli M, Söylemezoglu O, Erbas D, Bakkaloglu SA, Buyan N, Ozkaya O, Hasanoglu E, Plasma and urine nitric oxide levels in healthy Turkish children, Pediatr. Nephrol 20 (2005) 1605–1609. [DOI] [PubMed] [Google Scholar]
- [45].Thomas DD, Breathing new life into nitric oxide signaling: A brief overview of the interplay between oxygen and nitric oxide, Redox Biol. 5 (2015) 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Stuehr DJ, Santolini J, Wang Z-Q, Wei C-C, Adak S, Update on mechanism and catalytic regulation in the NO synthases, J. Biol. Chem 279 (2004) 36167–36170. [DOI] [PubMed] [Google Scholar]
- [47].Drury PP, Davidson JO, van den Heuij LG, Tan S, Silverman RB, Ji H, Blood AB, Fraser M, Bennet L, Gunn AJ, Partial neuroprotection by nNOS inhibition during profound asphyxia in preterm fetal sheep, Exp. Neurol 250 (2013) 282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gladwin MT, Raat NJH, Shiva S, Dezfulian C, Hogg N, Kim-Shapiro DB, Patel RP, Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection, and vasodilation, Am. J. Physiol. Heart Circ. Physiol 291 (2006) H2026–35. [DOI] [PubMed] [Google Scholar]
- [49].Blood AB, Tiso M, Verma ST, Lo J, Joshi MS, Azarov I, Longo LD, Gladwin MT, Kim-Shapiro DB, Power GG, Increased nitrite reductase activity of fetal versus adult ovine hemoglobin, Am. J. Physiol. Heart Circ. Physiol 296 (2009) H237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Truong GT, Schröder HJ, Liu T, Zhang M, Kanda E, Bragg S, Power GG, Blood AB, Role of nitrite in regulation of fetal cephalic circulation in sheep, J. Physiol 592 (2014) 1785–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Thomas DD, Liu X, Kantrow SP, Lancaster JR Jr, The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2, Proc. Natl. Acad. Sci. U. S. A 98 (2001) 355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Vrancken K, Schroeder HJ, Longo LD, Power GG, Blood AB, Postprandial lipids accelerate and redirect nitric oxide consumption in plasma, Nitric Oxide. 55–56 (2016) 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Shiva S, Wang X, Ringwood LA, Xu X, Yuditskaya S, Annavajjhala V, Miyajima H, Hogg N, Harris ZL, Gladwin MT, Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis, Nat. Chem. Biol 2 (2006) 486–493. [DOI] [PubMed] [Google Scholar]
- [54].Vrancken K, Schroeder HJ, Longo LD, Power GG, Blood AB, Role of ceruloplasmin in nitric oxide metabolism in plasma of humans and sheep: a comparison of adults and fetuses, Am. J. Physiol. Regul. Integr. Comp. Physiol 305 (2013) R1401–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Furne J, Saeed A, Levitt MD, Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values, Am. J. Physiol. Regul. Integr. Comp. Physiol 295 (2008) R1479–85. [DOI] [PubMed] [Google Scholar]
- [56].Thomas B, Gruca LL, Bennett C, Parimi PS, Hanson RW, Kalhan SC, Metabolism of methionine in the newborn infant: response to the parenteral and enteral administration of nutrients, Pediatr. Res 64 (2008) 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zlotkin SH, Anderson GH, The development of cystathionase activity during the first year of life, Pediatr. Res 16 (1982) 65–68. [DOI] [PubMed] [Google Scholar]
- [58].Martin JA, Pereda J, Martínez-López I, Escrig R, Miralles V, Pallardó FV, Vina JR, Vento M, Vina J, Sastre J, Oxidative stress as a signal to up-regulate gamma-cystathionase in the fetal-to-neonatal transition in rats, Cell. Mol. Biol . 53 (2007) OL1010–7. [PubMed] [Google Scholar]
- [59].Riedijk MA, van Beek RHT, Voortman G, de Bie HMA, Dassel ACM, van Goudoever JB, Cysteine: a conditionally essential amino acid in low-birth-weight preterm infants?, Am. J. Clin. Nutr 86 (2007) 1120–1125. [DOI] [PubMed] [Google Scholar]
- [60].Pallardo FV, Sastre J, Asensi M, Rodrigo F, Estrela JM, Viña J, Physiological changes in glutathione metabolism in foetal and newborn rat liver, Biochem. J 274 (Pt 3) (1991) 891–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Madurga A, Golec A, Pozarska A, Ishii I, Mižíková I, Nardiello C, Vadász I, Herold S, Mayer K, Reichenberger F, Fehrenbach H, Seeger W, Morty RE, The H2S-generating enzymes cystathionine β-synthase and cystathionine γ-lyase play a role in vascular development during normal lung alveolarization, Am. J. Physiol. Lung Cell. Mol. Physiol 309 (2015) L710–24. [DOI] [PubMed] [Google Scholar]
- [62].Watanabe M, Osada J, Aratani Y, Kluckman K, Reddick R, Malinow MR, Maeda N, Mice deficient in cystathionine beta-synthase: animal models for mild and severe homocyst(e)inemia, Proceedings of the National Academy of Sciences. 92 (1995) 1585–1589. doi: 10.1073/pnas.92.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Andria G, Fowler B, Sebastio G, Disorders of Sulfur Amino Acid Metabolism, in: Saudubray J-M, van den Berghe G, Walter JH (Eds.), Inborn Metabolic Diseases: Diagnosis and Treatment, Springer Berlin Heidelberg, Berlin, Heidelberg, 2012: pp. 311–321. [Google Scholar]
- [64].Wintner EA, Deckwerth TL, Langston W, Bengtsson A, Leviten D, Hill P, Insko MA, Dumpit R, VandenEkart E, Toombs CF, Others A monobromobimane-based assay to measure the pharmacokinetic profile of reactive sulphide species in blood, Br. J. Pharmacol 160 (2010) 941–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Olson KR, A practical look at the chemistry and biology of hydrogen sulfide, Antioxid. Redox Signal 17 (2012) 32–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Li Q, Lancaster JR Jr, Chemical foundations of hydrogen sulfide biology, Nitric Oxide. 35 (2013) 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Kolluru GK, Shen X, Bir SC, Kevil CG, Hydrogen sulfide chemical biology: pathophysiological roles and detection, Nitric Oxide. 35 (2013) 5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Marutani E, Yamada M, Ida T, Tokuda K, Ikeda K, Kai S, Shirozu K, Hayashida K, Kosugi S, Hanaoka K, Kaneki M, Akaike T, Ichinose F, Thiosulfate Mediates Cytoprotective Effects of Hydrogen Sulfide Against Neuronal Ischemia, J. Am. Heart Assoc 4 (2015). doi: 10.1161/JAHA.115.002125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Toohey JI, Sulfur signaling: is the agent sulfide or sulfane?, Anal. Biochem 413 (2011) 1–7. [DOI] [PubMed] [Google Scholar]
- [70].Ubuka T, Assay methods and biological roles of labile sulfur in animal tissues, J. Chromatogr. B Analyt. Technol. Biomed. Life Sci 781 (2002) 227–249. [DOI] [PubMed] [Google Scholar]
- [71].Tan B, Jin S, Sun J, Gu Z, Sun X, Zhu Y, Huo K, Cao Z, Yang P, Xin X, Liu X, Pan L, Qiu F, Jiang J, Jia Y, Ye F, Xie Y, Zhu YZ, New method for quantification of gasotransmitter hydrogen sulfide in biological matrices by LC-MS/MS, Sci. Rep 7 (2017) 46278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Dyson RM, Palliser HK, Latter JL, Chwatko G, Glowacki R, Wright IMR, A role for H2S in the microcirculation of newborns: the major metabolite of H2S (thiosulphate) is increased in preterm infants, PLoS One. 9 (2014) e105085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Dombkowski RA, Naylor MG, Shoemaker E, Smith M, DeLeon ER, Stoy GF, Gao Y, Olson KR, Hydrogen sulfide (H2S) and hypoxia inhibit salmonid gastrointestinal motility: evidence for H2S as an oxygen sensor, J. Exp. Biol 214 (2011) 4030–4040. [DOI] [PubMed] [Google Scholar]
- [74].Whitfield NL, Kreimier EL, Verdial FC, Skovgaard N, Olson KR, Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in ischemic preconditioning and vascular signaling, Am. J. Physiol. Regul. Integr. Comp. Physiol 294 (2008) R1930–7. [DOI] [PubMed] [Google Scholar]
- [75].Olson KR, Healy MJ, Qin Z, Skovgaard N, Vulesevic B, Duff DW, Whitfield NL, Yang G, Wang R, Perry SF, Hydrogen sulfide as an oxygen sensor in trout gill chemoreceptors, Am. J. Physiol. Regul. Integr. Comp. Physiol 295 (2008) R669–80. [DOI] [PubMed] [Google Scholar]
- [76].Madden JA, Ahlf SB, Dantuma MW, Olson KR, Roerig DL, Precursors and inhibitors of hydrogen sulfide synthesis affect acute hypoxic pulmonary vasoconstriction in the intact lung, J. Appl. Physiol 112 (2012) 411–418. [DOI] [PubMed] [Google Scholar]
- [77].Olson KR, Whitfield NL, Bearden SE, St Leger J, Nilson E, Gao Y, Madden JA, Hypoxic pulmonary vasodilation: a paradigm shift with a hydrogen sulfide mechanism, Am. J. Physiol. Regul. Integr. Comp. Physiol 298 (2010) R51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Olson KR, Hydrogen sulfide as an oxygen sensor, Antioxid. Redox Signal 22 (2015) 377–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Agostoni A, Stabilini R, Viggiano G, Luzzana M, Samaja M, Influence of capillary and tissue PO2 on carbon monoxide binding to myoglobin: a theoretical evaluation, Microvasc. Res 20 (1980) 81–87. [DOI] [PubMed] [Google Scholar]
- [80].Levitt DG, Levitt MD, Carbon monoxide: a critical quantitative analysis and review of the extent and limitations of its second messenger function, Clin. Pharmacol 7 (2015) 37–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Coburn RF, Forster RE, Kane PB, Considerations of the physiological variables that determine the blood carboxyhemoglobin concentration in man, J. Clin. Invest 44 (1965) 1899–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Abraham NG, Kappas A, Pharmacological and clinical aspects of heme oxygenase, Pharmacol. Rev 60 (2008) 79–127. [DOI] [PubMed] [Google Scholar]
- [83].Maróti Z, Katona M, Orvos H, Németh I, Farkas I, Túri S, Heme oxygenase-1 expression in premature and mature neonates during the first week of life, Eur. J. Pediatr 166 (2007) 1033–1038. [DOI] [PubMed] [Google Scholar]
- [84].Stanford SJ, Hislop AA, Oltmanns U, Nabel EG, Sang H, Haworth SG, Mitchell JA, Transition from placental to air breathing stimulates haem-oxygenase-1 expression without functional consequence for pulmonary vascular adaptation in pigs and mice, British Journal of Pharmacology. 144 (2005) 467–476. doi: 10.1038/sj.bjp.0705988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Lin JH, Villalon P, Nelson JC, Abraham NG, Expression of rat liver heme oxygenase gene during development, Arch. Biochem. Biophys 270 (1989) 623–629. [DOI] [PubMed] [Google Scholar]
- [86].Blanckaert N, Fevery J, Physiology and pathophysiology of bilirubin metabolism, Hepatology. A Textbook of Liver Disease 1 (1990) 254–303. [Google Scholar]
- [87].Maisels MJ, Pathak A, Nelson NM, Nathan DG, Smith CA, Endogenous production of carbon monoxide in normal and erythroblastotic newborn infants, J. Clin. Invest 50 (1971) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Cook MN, Marks GS, Vreman HJ, Nakatsu K, Stevenson DK, Brien JF, Ontogeny of heme oxygenase activity in the hippocampus, frontal cerebral cortex, and cerebellum of the guinea pig, Brain Res. Dev. Brain Res 92 (1996) 18–23. [DOI] [PubMed] [Google Scholar]
- [89].Bartoletti AL, Stevenson DK, Ostrander CR, Johnson JD, Pulmonary excretion of carbon monoxide in the human infant as an index of bilirubin production. I. Effects of gestational and postnatal age and some common neonatal abnormalities, J. Pediatr 94 (1979) 952–955. [DOI] [PubMed] [Google Scholar]
- [90].Shimazu T, Ikeuchi H, Sugimoto H, Goodwin CW, Mason AD Jr, Pruitt BA Jr, Half-life of blood carboxyhemoglobin after short-term and long-term exposure to carbon monoxide, J. Trauma 49 (2000) 126–131. [DOI] [PubMed] [Google Scholar]
- [91].Weaver LK, Howe S, Hopkins R, Chan KJ, Carboxyhemoglobin half-life in carbon monoxide-poisoned patients treated with 100% oxygen at atmospheric pressure, Chest. 117 (2000) 801–808. [DOI] [PubMed] [Google Scholar]
- [92].Bailey DGN, Fuchs H, Hentschel R, Carboxyhemoglobin--the forgotten parameter of neonatal hyperbilirubinemia, J. Perinat. Med 45 (2017) 613–617. [DOI] [PubMed] [Google Scholar]
- [93].Tokuriki S, Okuno T, Ohta G, Ohshima Y, Carboxyhemoglobin Formation in Preterm Infants Is Related to the Subsequent Development of Bronchopulmonary Dysplasia, Dis. Markers 2015 (2015) 620921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].May C, Patel S, Peacock J, Milner A, Rafferty GF, Greenough A, End-tidal carbon monoxide levels in prematurely born infants developing bronchopulmonary dysplasia, Pediatr. Res 61 (2007) 474–478. [DOI] [PubMed] [Google Scholar]
- [95].McArdle AJ, Webbe J, Sim K, Parrish G, Hoggart C, Wang Y, Kroll JS, Godambe S, Cunnington AJ, Determinants of Carboxyhemoglobin Levels and Relationship with Sepsis in a Retrospective Cohort of Preterm Neonates, PLoS One. 11 (2016) e0161784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Migita CT, Matera KM, Ikeda-Saito M, Olson JS, Fujii H, Yoshimura T, Zhou H, Yoshida T, The oxygen and carbon monoxide reactions of heme oxygenase, J. Biol. Chem 273 (1998) 945–949. [DOI] [PubMed] [Google Scholar]
- [97].Longo LD, Power GG, Forster RE 2nd, Respiratory function of the placenta as determined with carbon monoxide in sheep and dogs, J. Clin. Invest 46 (1967) 812–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Petersen LC, The effect of inhibitors on the oxygen kinetics of cytochrome c oxidase, Biochim. Biophys. Acta 460 (1977) 299–307. [DOI] [PubMed] [Google Scholar]
- [99].Kanu A, Leffler CW, Carbon monoxide and Ca2+-activated K+ channels in cerebral arteriolar responses to glutamate and hypoxia in newborn pigs, Am. J. Physiol. Heart Circ. Physiol 293 (2007) H3193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Nauser T, Koppenol WH, The Rate Constant of the Reaction of Superoxide with Nitrogen Monoxide: Approaching the Diffusion Limit, J. Phys. Chem. A 106 (2002) 4084–4086. [Google Scholar]
- [101].Radi R, Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine, Proc. Natl. Acad. Sci. U. S. A 115 (2018) 5839–5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Perrone S, Laschi E, Buonocore G, Biomarkers of oxidative stress in the fetus and in the newborn, Free Radic. Biol. Med (2019). doi: 10.1016/j.freeradbiomed.2019.03.034. [DOI] [PubMed] [Google Scholar]
- [103].Saugstad OD, Oxidative stress in the newborn--a 30-year perspective, Neonatology. 88 (2005) 228–236. [DOI] [PubMed] [Google Scholar]
- [104].Bloch KD, Filippov G, Sanchez LS, Nakane M, de la Monte SM, Pulmonary soluble guanylate cyclase, a nitric oxide receptor, is increased during the perinatal period, Am. J. Physiol 272 (1997) L400–6. [DOI] [PubMed] [Google Scholar]
- [105].Behrends S, Kempfert J, Mietens A, Koglin M, Scholz H, Middendorff R, Developmental changes of nitric oxide-sensitive guanylyl cyclase expression in pulmonary arteries, Biochem. Biophys. Res. Commun 283 (2001) 883–887. [DOI] [PubMed] [Google Scholar]
- [106].Budworth J, Meillerais S, Charles I, Powell K, Tissue distribution of the human soluble guanylate cyclases, Biochem. Biophys. Res. Commun 263 (1999) 696–701. [DOI] [PubMed] [Google Scholar]
- [107].Protas L, DiFrancesco D, Robinson RB, L-type but not T-type calcium current changes during postnatal development in rabbit sinoatrial node, American Journal of Physiology-Heart and Circulatory Physiology. 281 (2001) H1252–H1259. doi: 10.1152/ajpheart.2001.281.3.h1252. [DOI] [PubMed] [Google Scholar]
- [108].Cornfield DN, Chatfield BA, McQueston JA, McMurtry IF, Abman SH, Effects of birth-related stimuli on L-arginine-dependent pulmonary vasodilation in ovine fetus, Am. J. Physiol 262 (1992) H1474–81. [DOI] [PubMed] [Google Scholar]
- [109].Wynia-Smith SL, Smith BC, Nitrosothiol formation and S-nitrosation signaling through nitric oxide synthases, Nitric Oxide. 63 (2017) 52–60. [DOI] [PubMed] [Google Scholar]
- [110].Liu T, Schroeder HJ, Wilson SM, Terry MH, Romero M, Longo LD, Power GG, Blood AB, Local and systemic vasodilatory effects of low molecular weight S-nitrosothiols, Free Radic. Biol. Med 91 (2016) 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Das KC, Guo XL, White CW, Induction of thioredoxin and thioredoxin reductase gene expression in lungs of newborn primates by oxygen, Am. J. Physiol 276 (1999) L530–9. [DOI] [PubMed] [Google Scholar]
- [112].Benhar M, Thompson JW, Moseley MA, Stamler JS, Identification of S-nitrosylated targets of thioredoxin using a quantitative proteomic approach, Biochemistry. 49 (2010) 6963–6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Liu T, Schroeder HJ, Zhang M, Wilson SM, Terry MH, Longo LD, Power GG, Blood AB, S-nitrosothiols dilate the mesenteric artery more potently than the femoral artery by a cGMP and L-type calcium channel-dependent mechanism, Nitric Oxide. 58 (2016) 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Socco S, Bovee RC, Palczewski MB, Hickok JR, Thomas DD, Epigenetics: The third pillar of nitric oxide signaling, Pharmacol. Res 121 (2017) 52–58. [DOI] [PubMed] [Google Scholar]
- [115].Vasudevan D, Hickok JR, Bovee RC, Pham V, Mantell LL, Bahroos N, Kanabar P, Cao X-J, Maienschein-Cline M, Garcia BA, Thomas DD, Nitric Oxide Regulates Gene Expression in Cancers by Controlling Histone Posttranslational Modifications, Cancer Res. 75 (2015) 5299–5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Hickok JR, Vasudevan D, Antholine WE, Thomas DD, Nitric oxide modifies global histone methylation by inhibiting Jumonji C domain-containing demethylases, J. Biol. Chem 288 (2013) 16004–16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Kong Y, Grimaldi M, Curtin E, Dougherty M, Kaufman C, White RM, Zon LI, Liao EC, Neural crest development and craniofacial morphogenesis is coordinated by nitric oxide and histone acetylation, Chem. Biol 21 (2014) 488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Berghella L, Puri PL, Nitric oxide and histone acetylation-shaping craniofacial development, Chem. Biol 21 (2014) 565–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Kohli RM, Zhang Y, TET enzymes TDG and the dynamics of DNA demethylation, Nature. 502 (2013) 472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Stasch J-P, Pacher P, Evgenov OV, Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease, Circulation. 123 (2011) 2263–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Evgenov OV, Kohane DS, Bloch KD, Stasch J-P, Volpato GP, Bellas E, Evgenov NV, Buys ES, Gnoth MJ, Graveline AR, Liu R, Hess DR, Langer R, Zapol WM, Inhaled agonists of soluble guanylate cyclase induce selective pulmonary vasodilation, Am. J. Respir. Crit. Care Med 176 (2007) 1138–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Zhou Z, Martin E, Sharina I, Esposito I, Szabo C, Bucci M, Cirino G, Papapetropoulos A, Regulation of soluble guanylyl cyclase redox state by hydrogen sulfide, Pharmacol. Res 111 (2016) 556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Bucci M, Papapetropoulos A, Vellecco V, Zhou Z, Pyriochou A, Roussos C, Roviezzo F, Brancaleone V, Cirino G, Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity, Arterioscler. Thromb. Vasc. Biol 30 (2010) 1998–2004. [DOI] [PubMed] [Google Scholar]
- [124].Naoum PC, Methemoglobinemia in children: how to explain the results?, Rev. Bras. Hematol. Hemoter 34 (2012) 5–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Herzog P, Feig SA, Methaemoglobinaemia in the newborn infant, Clin. Haematol 7 (1978) 75–83. [PubMed] [Google Scholar]
- [126].Power GG, Bragg SL, Oshiro BT, Dejam A, Hunter CJ, Blood AB, A novel method of measuring reduction of nitrite-induced methemoglobin applied to fetal and adult blood of humans and sheep, J. Appl. Physiol 103 (2007) 1359–1365. [DOI] [PubMed] [Google Scholar]
- [127].Kimura H, Hydrogen Sulfide and Polysulfide Signaling, Antioxid. Redox Signal 27 (2017) 619–621. [DOI] [PubMed] [Google Scholar]
- [128].Mishanina TV, Libiad M, Banerjee R, Biogenesis of reactive sulfur species for signaling by hydrogen sulfide oxidation pathways, Nat. Chem. Biol 11 (2015) 457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Kimura H, Hydrogen sulfide and polysulfides as biological mediators, Molecules. 19 (2014) 16146–16157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Küster A, Tea I, Ferchaud-Roucher V, Le Borgne S, Plouzennec C, Winer N, Rozé J-C, Robins RJ, Darmaun D, Cord blood glutathione depletion in preterm infants: correlation with maternal cysteine depletion, PLoS One. 6 (2011) e27626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Mironov A, Seregina T, Nagornykh M, Luhachack LG, Korolkova N, Lopes LE, Kotova V, Zavilgelsky G, Shakulov R, Shatalin K, Nudler E, Mechanism of H2S-mediated protection against oxidative stress in Escherichia coli, Proc. Natl. Acad. Sci. U. S. A 114 (2017) 6022–6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Hoffman M, Rajapakse A, Shen X, Gates KS, Generation of DNA-damaging reactive oxygen species via the autoxidation of hydrogen sulfide under physiologically relevant conditions: chemistry relevant to both the genotoxic and cell signaling properties of H2S, Chem. Res. Toxicol 25 (2012) 1609–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Rana A, Amanullah S, Das PK, McQuarters AB, Lehnert N, Dey A, Formally Ferric Heme Carbon Monoxide Adduct, Journal of the American Chemical Society. 141 (2019) 5073–5077. doi: 10.1021/jacs.8b09067. [DOI] [PubMed] [Google Scholar]
- [134].Piantadosi CA, Biological chemistry of carbon monoxide, Antioxid. Redox Signal 4 (2002) 259–270. [DOI] [PubMed] [Google Scholar]
- [135].Zuckerbraun BS, Chin BY, Bilban M, de C. d’Avila J, Rao J, Billiar TR, Otterbein LE, Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species, FASEB J. 21 (2007) 1099–1106. [DOI] [PubMed] [Google Scholar]
- [136].Choi YK, Por ED, Kwon Y-G, Kim Y-M, Regulation of ROS production and vascular function by carbon monoxide, Oxid. Med. Cell. Longev 2012 (2012) 794237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Thom SR, Fisher D, Xu YA, Notarfrancesco K, Ischiropoulos H, Adaptive responses and apoptosis in endothelial cells exposed to carbon monoxide, Proc. Natl. Acad. Sci. U. S. A 97 (2000) 1305–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Knecht KR, Milam S, Wilkinson DA, Fedinec AL, Leffler CW, Time-dependent action of carbon monoxide on the newborn cerebrovascular circulation, Am. J. Physiol. Heart Circ. Physiol 299 (2010) H70–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Cheng Y, Mitchell-Flack MJ, Wang A, Levy RJ, Carbon monoxide modulates cytochrome oxidase activity and oxidative stress in the developing murine brain during isoflurane exposure, Free Radic. Biol. Med 86 (2015) 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Furchgott RF, Vanhoutte PM, Endothelium-derived relaxing and contracting factors, FASEB J. 3 (1989) 2007–2018. [PubMed] [Google Scholar]
- [141].Touwslager RNH, Alfons JH, Gielen M, Zeegers MP, Stehouwer CDA, Zimmermann LJ, Kessels AGH, Gerver W-JM, Blanco CE, Mulder ALM, Endothelial vasodilatation in newborns is related to body size and maternal hypertension, Journal of Hypertension. 30 (2012) 124–131. doi: 10.1097/hjh.0b013e32834d75c6. [DOI] [PubMed] [Google Scholar]
- [142].Martin H, Hu J, Gennser G, Norman M, Impaired endothelial function and increased carotid stiffness in 9-year-old children with low birthweight, Circulation. 102 (2000) 2739–2744. [DOI] [PubMed] [Google Scholar]
- [143].Leeson CP, Kattenhorn M, Morley R, Lucas A, Deanfield JE, Impact of low birth weight and cardiovascular risk factors on endothelial function in early adult life, Circulation. 103 (2001) 1264–1268. [DOI] [PubMed] [Google Scholar]
- [144].Liu SF, Hislop AA, Haworth SG, Barnes PJ, Developmental changes in endothelium-dependent pulmonary vasodilatation in pigs, Br. J. Pharmacol 106 (1992) 324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Villamor E, Pérez-Vizcaíno F, Cogolludo AL, Conde-Oviedo J, Zaragozá-Arnáez F, López-López JG, Tamargo J, Relaxant effects of carbon monoxide compared with nitric oxide in pulmonary and systemic vessels of newborn piglets, Pediatr. Res 48 (2000) 546–553. [DOI] [PubMed] [Google Scholar]
- [146].Abman SH, Abnormal vasoreactivity in the pathophysiology of persistent pulmonary hypertension of the newborn, Pediatr. Rev 20 (1999) e103–9. [PubMed] [Google Scholar]
- [147].Rairigh RL, Parker TA, Ivy DD, Kinsella JP, Fan ID, Abman SH, Role of inducible nitric oxide synthase in the pulmonary vascular response to birth-related stimuli in the ovine fetus, Circ. Res 88 (2001) 721–726. [DOI] [PubMed] [Google Scholar]
- [148].Cornfield DN, Chatfield BA, McQueston JA, McMurtry IF, Abman SH, Effects of birth-related stimuli on L-arginine-dependent pulmonary vasodilation in ovine fetus, American Journal of Physiology-Heart and Circulatory Physiology. 262 (1992) H1474–H1481. doi: 10.1152/ajpheart.1992.262.5.h1474. [DOI] [PubMed] [Google Scholar]
- [149].Gao Y, Zhou H, Usha Raj J, Endothelium-Derived Nitric Oxide Plays a Larger Role in Pulmonary Veins Than in Arteries of Newborn Lambs, Circulation Research. 76 (1995) 559–565. doi: 10.1161/01.res.76.4.559. [DOI] [PubMed] [Google Scholar]
- [150].Boegehold MA, Endothelium-dependent control of vascular tone during early postnatal and juvenile growth, Microcirculation. 17 (2010) 394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Nankervis CA, Reber KM, Nowicki PT, Age-Dependent Changes in the Postnatal Intestinal Microcirculation, Microcirculation. 8 (2001) 377–387. doi: 10.1111/j.1549-8719.2001.tb00185.x. [DOI] [PubMed] [Google Scholar]
- [152].Thakor AS, Herrera EA, Serón-Ferré M, Giussani DA, Melatonin and vitamin C increase umbilical blood flow via nitric oxide-dependent mechanisms, J. Pineal Res 49 (2010) 399–406. [DOI] [PubMed] [Google Scholar]
- [153].Thakor AS, Richter HG, Kane AD, Dunster C, Kelly FJ, Poston L, Giussani DA, Redox modulation of the fetal cardiovascular defence to hypoxaemia, J. Physiol 588 (2010) 4235–4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Kane AD, Herrera EA, Hansell JA, Giussani DA, Statin treatment depresses the fetal defence to acute hypoxia via increasing nitric oxide bioavailability, J. Physiol 590 (2012) 323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Kane AD, Hansell JA, Herrera EA, Allison BJ, Niu Y, Brain KL, Kaandorp JJ, Derks JB, Giussani DA, Xanthine oxidase and the fetal cardiovascular defence to hypoxia in late gestation ovine pregnancy, J. Physiol 592 (2014) 475–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Steinhorn RH, Albert G, Swartz DD, Russell JA, Levine CR, Davis JM, Recombinant human superoxide dismutase enhances the effect of inhaled nitric oxide in persistent pulmonary hypertension, Am. J. Respir. Crit. Care Med 164 (2001) 834–839. [DOI] [PubMed] [Google Scholar]
- [157].Gao Y, Chen Z, Leung SWS, Vanhoutte PM, Hypoxic Vasospasm Mediated by cIMP: When Soluble Guanylyl Cyclase Turns Bad, J. Cardiovasc. Pharmacol 65 (2015) 545–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Detremmerie CM, Chen Z, Li Z, Alkharfy KM, Leung SWS, Xu A, Gao Y, Vanhoutte PM, Endothelium-Dependent Contractions of Isolated Arteries to Thymoquinone Require Biased Activity of Soluble Guanylyl Cyclase with Subsequent Cyclic IMP Production, J. Pharmacol. Exp. Ther 358 (2016) 558–568. [DOI] [PubMed] [Google Scholar]
- [159].Seifert R, Is cIMP a second messenger with functions opposite to those of cGMP?, Naunyn. Schmiedebergs. Arch. Pharmacol 387 (2014) 897–899. [DOI] [PubMed] [Google Scholar]
- [160].Chen Z, Zhang X, Ying L, Dou D, Li Y, Bai Y, Liu J, Liu L, Feng H, Yu X, Leung SW-S, Vanhoutte PM, Gao Y, cIMP synthesized by sGC as a mediator of hypoxic contraction of coronary arteries, Am. J. Physiol. Heart Circ. Physiol 307 (2014) H328–36. [DOI] [PubMed] [Google Scholar]
- [161].Liu T, Zhang M, Terry MH, Schroeder H, Wilson SM, Power GG, Li Q, Tipple TE, Borchardt D, Blood AB, Hemodynamic Effects of Glutathione-Liganded Binuclear Dinitrosyl Iron Complex: Evidence for Nitroxyl Generation and Modulation by Plasma Albumin, Mol. Pharmacol 93 (2018) 427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Blood AB, Schroeder HJ, Terry MH, Merrill-Henry J, Bragg SL, Vrancken K, Liu T, Herring JL, Sowers LC, Wilson SM, Power GG, Inhaled nitrite reverses hemolysis-induced pulmonary vasoconstriction in newborn lambs without blood participation, Circulation. 123 (2011) 605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Blood AB, Power GG, In vitro and in vivo kinetic handling of nitrite in blood: effects of varying hemoglobin oxygen saturation, Am. J. Physiol. Heart Circ. Physiol 293 (2007) H1508–17. [DOI] [PubMed] [Google Scholar]
- [164].Liu T, Zhang M, Terry MH, Schroeder H, Wilson SM, Power GG, Li Q, Tipple TE, Borchardt D, Blood AB, Nitrite potentiates the vasodilatory signaling of S-nitrosothiols, Nitric Oxide. 75 (2018) 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Liu T, Schroeder HJ, Barcelo L, Bragg SL, Terry MH, Wilson SM, Power GG, Blood AB, Role of blood and vascular smooth muscle in the vasoactivity of nitrite, Am. J. Physiol. Heart Circ. Physiol 307 (2014) H976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Dongó E, Beliczai-Marosi G, Dybvig AS, Kiss L, The mechanism of action and role of hydrogen sulfide in the control of vascular tone, Nitric Oxide. 81 (2018) 75–87. [DOI] [PubMed] [Google Scholar]
- [167].Cacanyiova S, Berenyiova A, Kristek F, The role of hydrogen sulphide in blood pressure regulation, Physiol. Res 65 (2016) S273–S289. [DOI] [PubMed] [Google Scholar]
- [168].Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, Barodka VM, Gazi FK, Barrow RK, Wang R, Amzel LM, Berkowitz DE, Snyder SH, Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels, Circ. Res 109 (2011) 1259–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Bucci M, Papapetropoulos A, Vellecco V, Zhou Z, Zaid A, Giannogonas P, Cantalupo A, Dhayade S, Karalis KP, Wang R, Feil R, Cirino G, cGMP-dependent protein kinase contributes to hydrogen sulfide-stimulated vasorelaxation, PLoS One. 7 (2012) e53319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Minagawa T, Aizawa N, Igawa Y, Wyndaele J-J, The role of transient receptor potential ankyrin 1 (TRPA1) channel in activation of single unit mechanosensitive bladder afferent activities in the rat, Neurourology and Urodynamics. 33 (2014) 544–549. doi: 10.1002/nau.22449. [DOI] [PubMed] [Google Scholar]
- [171].Orlov SN, Gusakova SV, Smaglii LV, Koltsova SV, Sidorenko SV, Vasoconstriction triggered by hydrogen sulfide: Evidence for Na+,K+,2Cl-cotransport and L-type Ca2+ channel-mediated pathway, Biochem Biophys Rep. 12 (2017) 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Lim JJ, Liu Y-H, Khin ESW, Bian J-S, Vasoconstrictive effect of hydrogen sulfide involves downregulation of cAMP in vascular smooth muscle cells, Am. J. Physiol. Cell Physiol 295 (2008) C1261–70. [DOI] [PubMed] [Google Scholar]
- [173].Materazzi S, Zagli G, Nassini R, Bartolini I, Romagnoli S, Chelazzi C, Benemei S, Coratti A, De Gaudio AR, Patacchini R, Vasodilator activity of hydrogen sulfide (H2S) in human mesenteric arteries, Microvasc. Res 109 (2017) 38–44. [DOI] [PubMed] [Google Scholar]
- [174].Webb GD, Lim LH, Oh VMS, Yeo SB, Cheong YP, Ali MY, El Oakley R, Lee CN, Wong PS, Caleb MG, Salto-Tellez M, Bhatia M, Chan ESY, Taylor EA, Moore PK, Contractile and vasorelaxant effects of hydrogen sulfide and its biosynthesis in the human internal mammary artery, J. Pharmacol. Exp. Ther 324 (2008) 876–882. [DOI] [PubMed] [Google Scholar]
- [175].Kutz JL, Greaney JL, Santhanam L, Alexander LM, Evidence for a functional vasodilatatory role for hydrogen sulphide in the human cutaneous microvasculature, J. Physiol 593 (2015) 2121–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Eberhardt M, Dux M, Namer B, Miljkovic J, Cordasic N, Will C, Kichko TI, de la Roche J, Fischer M, Suárez SA, Bikiel D, Dorsch K, Leffler A, Babes A, Lampert A, Lennerz JK, Jacobi J, Martí MA, Doctorovich F, Högestätt ED, Zygmunt PM, Ivanovic-Burmazovic I, Messlinger K, Reeh P, Filipovic MR, H2S and NO cooperatively regulate vascular tone by activating a neuroendocrine HNO–TRPA1–CGRP signalling pathway, Nat. Commun 5 (2014) 4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Mohammed R, Provitera L, Cavallaro G, Lattuada D, Ercoli G, Mosca F, Villamor E, Vasomotor effects of hydrogen sulfide in human umbilical vessels, J. Physiol. Pharmacol 68 (2017) 737–747. [PubMed] [Google Scholar]
- [178].Leffler CW, Parfenova H, Basuroy S, Jaggar JH, Umstot ES, Fedinec AL, Hydrogen sulfide and cerebral microvascular tone in newborn pigs, Am. J. Physiol. Heart Circ. Physiol 300 (2011) H440–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Liang GH, Adebiyi A, Leo MD, McNally EM, Leffler CW, Jaggar JH, Hydrogen sulfide dilates cerebral arterioles by activating smooth muscle cell plasma membrane KATP channels, Am. J. Physiol. Heart Circ. Physiol 300 (2011) H2088–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Patel S, Fedinec AL, Liu J, Weiss MA, Pourcyrous M, Harsono M, Parfenova H, Leffler CW, H2S mediates the vasodilator effect of endothelin-1 in the cerebral circulation, Am. J. Physiol. Heart Circ. Physiol 315 (2018) H1759–H1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Baragatti B, Ciofini E, Sodini D, Luin S, Scebba F, Coceani F, Hydrogen sulfide in the mouse ductus arteriosus: a naturally occurring relaxant with potential EDHF function, Am. J. Physiol. Heart Circ. Physiol 304 (2013) H927–34. [DOI] [PubMed] [Google Scholar]
- [182].Van der Sterren S, Kleikers P, Zimmermann LJI, Villamor E, Vasoactivity of the gasotransmitters hydrogen sulfide and carbon monoxide in the chicken ductus arteriosus, American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 301 (2011) R1186–R1198. [DOI] [PubMed] [Google Scholar]
- [183].Olson KR, Dombkowski RA, Russell MJ, Doellman MM, Head SK, Whitfield NL, Madden JA, Hydrogen sulfide as an oxygen sensor/transducer in vertebrate hypoxic vasoconstriction and hypoxic vasodilation, J. Exp. Biol 209 (2006) 4011–4023. [DOI] [PubMed] [Google Scholar]
- [184].Brian JE Jr, Heistad DD, Faraci FM, Effect of carbon monoxide on rabbit cerebral arteries, Stroke. 25 (1994) 639–43; discussion 643–4. [DOI] [PubMed] [Google Scholar]
- [185].Leffler CW, Nasjletti A, Yu C, Johnson RA, Fedinec AL, Walker N, Carbon monoxide and cerebral microvascular tone in newborn pigs, Am. J. Physiol 276 (1999) H1641–6. [DOI] [PubMed] [Google Scholar]
- [186].Holt DC, Fedinec AL, Vaughn AN, Leffler CW, Age and species dependence of pial arteriolar responses to topical carbon monoxide in vivo, Exp. Biol. Med . 232 (2007) 1465–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Leffler CW, Parfenova H, Jaggar JH, Wang R, Carbon monoxide and hydrogen sulfide: gaseous messengers in cerebrovascular circulation, J. Appl. Physiol 100 (2006) 1065–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Carratu P, Pourcyrous M, Fedinec A, Leffler CW, Parfenova H, Endogenous heme oxygenase prevents impairment of cerebral vascular functions caused by seizures, Am. J. Physiol. Heart Circ. Physiol 285 (2003) H1148–57. [DOI] [PubMed] [Google Scholar]
- [189].Kanu A, Whitfield J, Leffler CW, Carbon monoxide contributes to hypotension-induced cerebrovascular vasodilation in piglets, Am. J. Physiol. Heart Circ. Physiol 291 (2006) H2409–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Pourcyrous M, Bada HS, Parfenova H, Daley ML, Korones SB, Leffler CW, Cerebrovasodilatory contribution of endogenous carbon monoxide during seizures in newborn pigs, Pediatr. Res 51 (2002) 579–585. [DOI] [PubMed] [Google Scholar]
- [191].Winestone JS, Bonner C, Leffler CW, Carbon Monoxide as an Attenuator of Vasoconstriction in Piglet Cerebral Arterioles1, Exp. Biol. Med . 228 (2003) 46–50. [DOI] [PubMed] [Google Scholar]
- [192].Kanu A, Gilpin D, Fedinec AL, Leffler CW, Cyclooxygenase products stimulate carbon monoxide production by piglet cerebral microvessels, Exp. Biol. Med . 231 (2006) 181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Kanu A, Leffler CW, Arachidonic acid- and prostaglandin E2-induced cerebral vasodilation is mediated by carbon monoxide, independent of reactive oxygen species in piglets, American Journal of Physiology-Heart and Circulatory Physiology. 301 (2011) H2482–H2487. doi: 10.1152/ajpheart.00628.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Kanu A, Leffler CW, Roles of glia limitans astrocytes and carbon monoxide in adenosine diphosphate-induced pial arteriolar dilation in newborn pigs, Stroke. 40 (2009) 930–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Robinson JS, Fedinec AL, Leffler CW, Role of carbon monoxide in glutamate receptor-induced dilation of newborn pig pial arterioles, Am. J. Physiol. Heart Circ. Physiol 282 (2002) H2371–6. [DOI] [PubMed] [Google Scholar]
- [196].Parfenova H, Fedinec A, Leffler CW, Ionotropic glutamate receptors in cerebral microvascular endothelium are functionally linked to heme oxygenase, J. Cereb. Blood Flow Metab 23 (2003) 190–197. [DOI] [PubMed] [Google Scholar]
- [197].Fiumana E, Parfenova H, Jaggar JH, Leffler CW, Carbon monoxide mediates vasodilator effects of glutamate in isolated pressurized cerebral arterioles of newborn pigs, Am. J. Physiol. Heart Circ. Physiol 284 (2003) H1073–9. [DOI] [PubMed] [Google Scholar]
- [198].Li A, Xi Q, Umstot ES, Bellner L, Schwartzman ML, Jaggar JH, Leffler CW, Astrocyte-derived CO is a diffusible messenger that mediates glutamate-induced cerebral arteriolar dilation by activating smooth muscle Cell KCa channels, Circ. Res 102 (2008) 234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Xi Q, Umstot E, Zhao G, Narayanan D, Leffler CW, Jaggar JH, Glutamate regulates Ca2 signals in smooth muscle cells of newborn piglet brain slice arterioles through astrocyte- and heme oxygenase-dependent mechanisms, American Journal of Physiology-Heart and Circulatory Physiology. 298 (2010) H562–H569. doi: 10.1152/ajpheart.00823.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Xi Q, Tcheranova D, Basuroy S, Parfenova H, Jaggar JH, Leffler CW, Glutamate-induced calcium signals stimulate CO production in piglet astrocytes, Am. J. Physiol. Heart Circ. Physiol 301 (2011) H428–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Parfenova H, Tcheranova D, Basuroy S, Fedinec AL, Liu J, Leffler CW, Functional role of astrocyte glutamate receptors and carbon monoxide in cerebral vasodilation response to glutamate, Am. J. Physiol. Heart Circ. Physiol 302 (2012) H2257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Barkoudah E, Jaggar JH, Leffler CW, The permissive role of endothelial NO in CO-induced cerebrovascular dilation, Am. J. Physiol. Heart Circ. Physiol 287 (2004) H1459–65. [DOI] [PubMed] [Google Scholar]
- [203].Leffler CW, Balabanova L, Fedinec AL, Parfenova H, Nitric oxide increases carbon monoxide production by piglet cerebral microvessels, Am. J. Physiol. Heart Circ. Physiol 289 (2005) H1442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Llanos AJ, Ebensperger G, Herrera EA, Reyes RV, Cabello G, Díaz M, Giussani DA, Parer JT, The heme oxygenase–carbon monoxide system in the regulation of cardiorespiratory function at high altitude, Respiratory Physiology & Neurobiology. 184 (2012) 186–191. doi: 10.1016/j.resp.2012.05.003. [DOI] [PubMed] [Google Scholar]
- [205].Herrera EA, Reyes RV, Giussani DA, Riquelme RA, Sanhueza EM, Ebensperger G, Casanello P, Méndez N, Ebensperger R, Sepúlveda-Kattan E, Pulgar VM, Cabello G, Blanco CE, Hanson MA, Parer JT, Llanos AJ, Carbon monoxide: a novel pulmonary artery vasodilator in neonatal llamas of the Andean altiplano, Cardiovasc. Res 77 (2008) 197–201. [DOI] [PubMed] [Google Scholar]
- [206].Herrera EA, Ebensperger G, Hernández I, Sanhueza EM, Llanos AJ, Reyes RV, The role of nitric oxide signaling in pulmonary circulation of high- and low-altitude newborn sheep under basal and acute hypoxic conditions, Nitric Oxide. 89 (2019) 71–80. [DOI] [PubMed] [Google Scholar]
- [207].Reyes RV, Díaz M, Ebensperger G, Herrera EA, Quezada SA, Hernandez I, Sanhueza EM, Parer JT, Giussani DA, Llanos AJ, The role of nitric oxide in the cardiopulmonary response to hypoxia in highland and lowland newborn llamas, J. Physiol 596 (2018) 5907–5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Duncan C, Dougall H, Johnston P, Green S, Brogan R, Leifert C, Smith L, Golden M, Benjamin N, Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate, Nat. Med 1 (1995) 546–551. [DOI] [PubMed] [Google Scholar]
- [209].Totzeck M, Hendgen-Cotta UB, Luedike P, Berenbrink M, Klare JP, Steinhoff H-J, Semmler D, Shiva S, Williams D, Kipar A, Gladwin MT, Schrader J, Kelm M, Cossins AR, Rassaf T, Nitrite regulates hypoxic vasodilation via myoglobin-dependent nitric oxide generation, Circulation. 126 (2012) 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Hunter CJ, Dejam A, Blood AB, Shields H, Kim-Shapiro DB, Machado RF, Tarekegn S, Mulla N, Hopper AO, Schechter AN, Power GG, Gladwin MT, Inhaled nebulized nitrite is a hypoxia-sensitive NO-dependent selective pulmonary vasodilator, Nat. Med 10 (2004) 1122–1127. [DOI] [PubMed] [Google Scholar]
- [211].Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO 3rd, Gladwin MT, Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation, Nat. Med 9 (2003) 1498–1505. [DOI] [PubMed] [Google Scholar]
- [212].Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, MacAllister R, Hobbs AJ, Ahluwalia A, Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite, Hypertension. 51 (2008) 784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Kapil V, Milsom AB, Okorie M, Maleki-Toyserkani S, Akram F, Rehman F, Arghandawi S, Pearl V, Benjamin N, Loukogeorgakis S, MacAllister R, Hobbs AJ, Webb AJ, Ahluwalia A, Inorganic Nitrate Supplementation Lowers Blood Pressure in Humans, Hypertension. 56 (2010) 274–281. [DOI] [PubMed] [Google Scholar]
- [214].Qin L, Liu X, Sun Q, Fan Z, Xia D, Ding G, Ong HL, Adams D, Gahl WA, Zheng C, Qi S, Jin L, Zhang C, Gu L, He J, Deng D, Ambudkar IS, Wang S, Sialin (SLC17A5) functions as a nitrate transporter in the plasma membrane, Proc. Natl. Acad. Sci. U. S. A 109 (2012) 13434–13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Jones AM, Dietary Nitrate Supplementation and Exercise Performance, Sports Medicine. 44 (2014) 35–45. doi: 10.1007/s40279-014-0149-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Suzuki H, Iijima K, Scobie G, Fyfe V, McColl KEL, Nitrate and nitrosative chemistry within Barrett’s oesophagus during acid reflux, Gut. 54 (2005) 1527–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Iijima K, Grant J, McElroy K, Fyfe V, Preston T, McColl KEL, Novel mechanism of nitrosative stress from dietary nitrate with relevance to gastro-oesophageal junction cancers, Carcinogenesis. 24 (2003) 1951–1960. [DOI] [PubMed] [Google Scholar]
- [218].Sobko T, Huang L, Midtvedt T, Norin E, Gustafsson LE, Norman M, Jansson EA, Lundberg JO, Generation of NO by probiotic bacteria in the gastrointestinal tract, Free Radic. Biol. Med 41 (2006) 985–991. [DOI] [PubMed] [Google Scholar]
- [219].Sobko T, Reinders C, Norin E, Midtvedt T, Gustafsson LE, Lundberg JO, Gastrointestinal nitric oxide generation in germ-free and conventional rats, Am. J. Physiol. Gastrointest. Liver Physiol 287 (2004) G993–7. [DOI] [PubMed] [Google Scholar]
- [220].Harries JT, Fraser AJ, The acidity of the gastric contents of premature babies during the first fourteen days of life, Biol. Neonat 12 (1968) 186–193. [DOI] [PubMed] [Google Scholar]
- [221].Ward RM, Kearns GL, Proton Pump Inhibitors in Pediatrics, Pediatric Drugs. 15 (2013) 119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Miclat NN, Hodgkinson R, Marx GF, Neonatal gastric pH, Anesth. Analg 57 (1978) 98–101. [DOI] [PubMed] [Google Scholar]
- [223].Lin JK, Lai CC, Effects of lactobacillus, antacids and antibiotics on the levels of nitrite in the gastro-intestinal tracts of rats fed sodium nitrate, Food Chem. Toxicol 20 (1982) 197–204. [DOI] [PubMed] [Google Scholar]
- [224].Fanaro S, Chierici R, Guerrini P, Vigi V, Intestinal microflora in early infancy: composition and development, Acta Paediatr. Suppl 91 (2003) 48–55. [DOI] [PubMed] [Google Scholar]
- [225].Ahrné S, Lönnermark E, Wold AE, Aberg N, Hesselmar B, Saalman R, Strannegård I-L, Molin G, Adlerberth I, Lactobacilli in the intestinal microbiota of Swedish infants, Microbes Infect. 7 (2005) 1256–1262. [DOI] [PubMed] [Google Scholar]
- [226].Sobko T, Elfström K, Navér L, Lundberg JO, Norman M, Intestinal hydrogen and nitric oxide gases in preterm infants--effects of antibiotic therapy, Neonatology. 95 (2009) 68–73. [DOI] [PubMed] [Google Scholar]
- [227].Frost BL, Modi BP, Jaksic T, Caplan MS, New Medical and Surgical Insights Into Neonatal Necrotizing Enterocolitis: A Review, JAMA Pediatr. 171 (2017) 83–88. [DOI] [PubMed] [Google Scholar]
- [228].Baldassarre ME, Palladino V, Amoruso A, Pindinelli S, Mastromarino P, Fanelli M, Di Mauro A, Laforgia N, Rationale of Probiotic Supplementation during Pregnancy and Neonatal Period, Nutrients. 10 (2018). doi: 10.3390/nu10111693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Hansen MB, Dresner LS, Wait RB, Profile of neurohumoral agents on mesenteric and intestinal blood flow in health and disease, Physiol. Res 47 (1998) 307–327. [PubMed] [Google Scholar]
- [230].Linderkamp O, Berg D, Betke K, Köferl F, Kriegel H, Riegel KP, Blood volume and hematocrit in various organs in newborn piglets, Pediatr. Res 14 (1980) 1324–1327. [DOI] [PubMed] [Google Scholar]
- [231].Gaynullina DK, Schubert R, Tarasova OS, Changes in Endothelial Nitric Oxide Production in Systemic Vessels during Early Ontogenesis-A Key Mechanism for the Perinatal Adaptation of the Circulatory System, Int. J. Mol. Sci 20 (2019). doi: 10.3390/ijms20061421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Nankervis CA, Reber KM, Nowicki PT, Age-dependent changes in the postnatal intestinal microcirculation, Microcirculation. 8 (2001) 377–387. [DOI] [PubMed] [Google Scholar]
- [233].Edelstone DI, Holzman IR, Fetal intestinal oxygen consumption at various levels of oxygenation, Am. J. Physiol 242 (1982) H50–4. [DOI] [PubMed] [Google Scholar]
- [234].Holzman IR, Tabata B, Edelstone DI, Effects of varying hematocrit on intestinal oxygen uptake in neonatal lambs, Am. J. Physiol 248 (1985) G432–6. [DOI] [PubMed] [Google Scholar]
- [235].Nankervis CA, Nowicki PT, Role of nitric oxide in regulation of vascular resistance in postnatal intestine, Am. J. Physiol 268 (1995) G949–58. [DOI] [PubMed] [Google Scholar]
- [236].Nowicki PT, Effects of sustained low-flow perfusion on the response to vasoconstrictor agents in postnatal intestine, Am. J. Physiol 276 (1999) G1408–16. [DOI] [PubMed] [Google Scholar]
- [237].Nowicki PT, Effects of sustained flow reduction on postnatal intestinal circulation, Am. J. Physiol 275 (1998) G758–68. [DOI] [PubMed] [Google Scholar]
- [238].Nankervis CA, Dunaway DJ, Nowicki PT, Determinants of terminal mesenteric artery resistance during the first postnatal month, Am. J. Physiol. Gastrointest. Liver Physiol 280 (2001) G678–86. [DOI] [PubMed] [Google Scholar]
- [239].Su BY, Reber KM, Nankervis CA, Nowicki PT, Development of the myogenic response in postnatal intestine: role of PKC, Am. J. Physiol. Gastrointest. Liver Physiol 284 (2003) G445–52. [DOI] [PubMed] [Google Scholar]
- [240].Reber KM, Su BY, Clark KR, Pohlman DL, Miller CE, Nowicki PT, Developmental expression of eNOS in postnatal swine mesenteric artery, Am. J. Physiol. Gastrointest. Liver Physiol 283 (2002) G1328–35. [DOI] [PubMed] [Google Scholar]
- [241].Bohlen HG, Zhou X, Unthank JL, Miller SJ, Bills R, Transfer of nitric oxide by blood from upstream to downstream resistance vessels causes microvascular dilation, Am. J. Physiol. Heart Circ. Physiol 297 (2009) H1337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Walder CE, Thiemermann C, Vane JR, Endothelium-derived relaxing factor participates in the increased blood flow in response to pentagastrin in the rat stomach mucosa, Proc. Biol. Sci 241 (1990) 195–200. [DOI] [PubMed] [Google Scholar]
- [243].West SD, Helmer KS, Chang LK, Cui Y, Greeley GH, Mercer DW, Cholecystokinin secretagogue-induced gastroprotection: role of nitric oxide and blood flow, Am. J. Physiol. Gastrointest. Liver Physiol 284 (2003) G399–410. [DOI] [PubMed] [Google Scholar]
- [244].Matheson PJ, Wilson MA, Spain DA, Harris PD, Anderson GL, Garrison RN, Glucose-induced intestinal hyperemia is mediated by nitric oxide, J. Surg. Res 72 (1997) 146–154. [DOI] [PubMed] [Google Scholar]
- [245].Matheson PJ, Spain DA, Harris PD, Garrison RN, Wilson MA, Glucose and glutamine gavage increase portal vein nitric oxide metabolite levels via adenosine A2b activation, J. Surg. Res 84 (1999) 57–63. [DOI] [PubMed] [Google Scholar]
- [246].Alemany CA, Oh W, Stonestreet BS, Effects of nitric oxide synthesis inhibition on mesenteric perfusion in young pigs, Am. J. Physiol 272 (1997) G612–6. [DOI] [PubMed] [Google Scholar]
- [247].Gangula PRR, Mukhopadhyay S, Pasricha PJ, Ravella K, Sepiapterin reverses the changes in gastric nNOS dimerization and function in diabetic gastroparesis, Neurogastroenterol. Motil 22 (2010) 1325–31, e351–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Lu H-L, Huang X, Wu Y-S, Zhang C-M, Meng X-M, Liu D-H, Kim Y-C, Xu W-X, Gastric nNOS reduction accompanied by natriuretic peptides signaling pathway upregulation in diabetic mice, World J. Gastroenterol 20 (2014) 4626–4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Kusafuka T, Puri P, Altered mRNA expression of the neuronal nitric oxide synthase gene in Hirschsprung’s disease, J. Pediatr. Surg 32 (1997) 1054–1058. [DOI] [PubMed] [Google Scholar]
- [250].Coyle D, O’Donnell AM, Gillick J, Puri P, Altered neurotransmitter expression profile in the ganglionic bowel in Hirschsprung’s disease, J. Pediatr. Surg 51 (2016) 762–769. [DOI] [PubMed] [Google Scholar]
- [251].Rivera LR, Poole DP, Thacker M, Furness JB, The involvement of nitric oxide synthase neurons in enteric neuropathies, Neurogastroenterol. Motil 23 (2011) 980–988. [DOI] [PubMed] [Google Scholar]
- [252].Bealer JF, Natuzzi ES, Buscher C, Ursell PC, Flake AW, Adzick NS, Harrison MR, Nitric oxide synthase is deficient in the aganglionic colon of patients with Hirschsprung’s disease, Pediatrics. 93 (1994) 647–651. [PubMed] [Google Scholar]
- [253].Wester T, O’Briain DS, Puri P, Notable postnatal alterations in the myenteric plexus of normal human bowel, Gut. 44 (1999) 666–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Pochart P, Doré J, Lémann F, Goderel I, Rambaud JC, Interrelations between populations of methanogenic archaea and sulfate-reducing bacteria in the human colon, FEMS Microbiol. Lett 77 (1992) 225–228. [DOI] [PubMed] [Google Scholar]
- [255].Morvan B, Bonnemoy F, Fonty G, Gouet P, Quantitative determination of H2-utilizing acetogenic and sulfate-reducing bacteria and methanogenic archaea from digestive tract of different mammals, Curr. Microbiol 32 (1996) 129–133. [DOI] [PubMed] [Google Scholar]
- [256].Ijssennagger N, van der Meer R, van Mil SWC, Sulfide as a Mucus Barrier-Breaker in Inflammatory Bowel Disease?, Trends Mol. Med 22 (2016) 190–199. [DOI] [PubMed] [Google Scholar]
- [257].Tomuschat C, O’Donnell AM, Coyle D, Puri P, Reduction of hydrogen sulfide synthesis enzymes cystathionine-β-synthase and cystathionine-γ-lyase in the colon of patients with Hirschsprungs disease, J. Pediatr. Surg 53 (2018) 525–530. [DOI] [PubMed] [Google Scholar]
- [258].Nalli AD, Wang H, Bhattacharya S, Blakeney BA, Murthy KS, Inhibition of RhoA/Rho kinase pathway and smooth muscle contraction by hydrogen sulfide, Pharmacol Res Perspect. 5 (2017). doi: 10.1002/prp2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Schicho R, Krueger D, Zeller F, Von Weyhern CWH, Frieling T, Kimura H, Ishii I, De Giorgio R, Campi B, Schemann M, Hydrogen sulfide is a novel prosecretory neuromodulator in the Guinea-pig and human colon, Gastroenterology. 131 (2006) 1542–1552. [DOI] [PubMed] [Google Scholar]
- [260].Xiao A, Li J, Liu T, Liu Z, Wei C, Xu X, Li Q, Li J, L-Cysteine enhances nutrient absorption via a cystathionine-β-synthase-derived H2 S pathway in rodent jejunum, Clin. Exp. Pharmacol. Physiol 43 (2016) 562–568. [DOI] [PubMed] [Google Scholar]
- [261].Linden DR, Hydrogen sulfide signaling in the gastrointestinal tract, Antioxid. Redox Signal 20 (2014) 818–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Sha L, Linden DR, Farrugia G, Szurszewski JH, Effect of endogenous hydrogen sulfide on the transwall gradient of the mouse colon circular smooth muscle, J. Physiol 592 (2014) 1077–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Quan X, Luo H, Liu Y, Xia H, Chen W, Tang Q, Hydrogen sulfide regulates the colonic motility by inhibiting both L-type calcium channels and BKCa channels in smooth muscle cells of rat colon, PLoS One. 10 (2015) e0121331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Nalli AD, Bhattacharya S, Wang H, Kendig DM, Grider JR, Murthy KS, Augmentation of cGMP/PKG pathway and colonic motility by hydrogen sulfide, Am. J. Physiol. Gastrointest. Liver Physiol 313 (2017) G330–G341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Martinez-Cutillas M, Gil V, Mañé N, Clavé P, Gallego D, Martin MT, Jimenez M, Potential role of the gaseous mediator hydrogen sulphide (H2S) in inhibition of human colonic contractility, Pharmacol. Res 93 (2015) 52–63. [DOI] [PubMed] [Google Scholar]
- [266].Pham VT, Lacroix C, Braegger CP, Chassard C, Early colonization of functional groups of microbes in the infant gut, Environ. Microbiol 18 (2016) 2246–2258. [DOI] [PubMed] [Google Scholar]
- [267].Hopkins MJ, Macfarlane GT, Furrie E, Fite A, Macfarlane S, Characterisation of intestinal bacteria in infant stools using real-time PCR and northern hybridisation analyses, FEMS Microbiol. Ecol 54 (2005) 77–85. [DOI] [PubMed] [Google Scholar]
- [268].Ouellet YH, Ndiaye CT, Gagné SM, Sebilo A, Suits MDL, Jubinville É, Jia Z, Ivancich A, Couture M, An alternative reaction for heme degradation catalyzed by the Escherichia coli O157:H7 ChuS protein: Release of hematinic acid, tripyrrole and Fe(III), J. Inorg. Biochem 154 (2016) 103–113. [DOI] [PubMed] [Google Scholar]
- [269].Suits MDL, Pal GP, Nakatsu K, Matte A, Cygler M, Jia Z, Identification of an Escherichia coli O157:H7 heme oxygenase with tandem functional repeats, Proc. Natl. Acad. Sci. U. S. A 102 (2005) 16955–16960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Maharshak N, Ryu HS, Fan T-J, Onyiah JC, Schulz S, Otterbein SL, Wong R, Hansen JJ, Otterbein LE, Carroll IM, Plevy SE, Escherichia coli heme oxygenase modulates host innate immune responses, Microbiol. Immunol 59 (2015) 452–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Levine AS, Bond JH, Prentiss RA, Levitt MD, Metabolism of carbon monoxide by the colonic flora of humans, Gastroenterology. 83 (1982) 633–637. [PubMed] [Google Scholar]
- [272].Raffin SB, Woo CH, Roost KT, Price DC, Schmid R, Intestinal absorption of hemoglobin iron-heme cleavage by mucosal heme oxygenase, J. Clin. Invest 54 (1974) 1344–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Brouillard RP, Conrad ME, Bensinger TA, Effect of blood in the gut on measurements of endogenous carbon monoxide production, Blood. 45 (1975) 67–69. [PubMed] [Google Scholar]
- [274].Romano C, Oliva S, Martellossi S, Miele E, Arrigo S, Graziani MG, Cardile S, Gaiani F, de’Angelis GL, Torroni F, Pediatric gastrointestinal bleeding: Perspectives from the Italian Society of Pediatric Gastroenterology, World J. Gastroenterol 23 (2017) 1328–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Stevenson DK, Hamori CJ, Carlton RR, Castillo RO, Kerner JA Jr, Vreman HJ, Carbon monoxide production by nonbacterial sources after heme feeding of neonatal rats, Biol. Neonate 57 (1990) 238–242. [DOI] [PubMed] [Google Scholar]
- [276].Gibbons SJ, Farrugia G, The role of carbon monoxide in the gastrointestinal tract, J. Physiol 556 (2004) 325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Farrugia G, Szurszewski JH, Carbon monoxide, hydrogen sulfide, and nitric oxide as signaling molecules in the gastrointestinal tract, Gastroenterology. 147 (2014) 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Miller SM, Farrugia G, Schmalz PF, Ermilov LG, Maines MD, Szurszewski JH, Heme oxygenase 2 is present in interstitial cell networks of the mouse small intestine, Gastroenterology. 114 (1998) 239–244. [DOI] [PubMed] [Google Scholar]
- [279].Porcher C, Orsoni P, Berdah S, Monges G, Mazet B, Distribution of heme oxygenase 2 in nerves and c-kit(+) interstitial cells in human stomach, Histochem. Cell Biol 112 (1999) 317–322. [DOI] [PubMed] [Google Scholar]
- [280].Miller SM, Reed D, Sarr MG, Farrugia G, Szurszewski JH, Haem oxygenase in enteric nervous system of human stomach and jejunum and co-localization with nitric oxide synthase, Neurogastroenterol. Motil 13 (2001) 121–131. [DOI] [PubMed] [Google Scholar]
- [281].Piotrowska AP, Solari V, de Caluwé D, Puri P, Immunocolocalization of the heme oxygenase-2 and interstitial cells of Cajal in normal and aganglionic colon, J. Pediatr. Surg 38 (2003) 73–77. [DOI] [PubMed] [Google Scholar]
- [282].Liao Y-F, Zhu W, Li D-P, Zhu X, Heme oxygenase-1 and gut ischemia/reperfusion injury: A short review, World J. Gastroenterol 19 (2013) 3555–3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Naik JS, O’Donaughy TL, Walker BR, Endogenous carbon monoxide is an endothelial-derived vasodilator factor in the mesenteric circulation, Am. J. Physiol. Heart Circ. Physiol 284 (2003) H838–45. [DOI] [PubMed] [Google Scholar]
- [284].Farrugia G, Lei S, Lin X, Miller SM, Nath KA, Ferris CD, Levitt M, Szurszewski JH, A major role for carbon monoxide as an endogenous hyperpolarizing factor in the gastrointestinal tract, Proc. Natl. Acad. Sci. U. S. A 100 (2003) 8567–8570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Szurszewski JH, Farrugia G, Carbon monoxide is an endogenous hyperpolarizing factor in the gastrointestinal tract, Neurogastroenterol. Motil 16 Suppl 1 (2004) 81–85. [DOI] [PubMed] [Google Scholar]
- [286].Sha L, Farrugia G, Linden DR, Szurszewski JH, The transwall gradient across the mouse colonic circular muscle layer is carbon monoxide dependent, FASEB J. 24 (2010) 3840–3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Colpaert EE, Timmermans JP, Lefebvre RA, Immunohistochemical localization of the antioxidant enzymes biliverdin reductase and heme oxygenase-2 in human and pig gastric fundus, Free Radic. Biol. Med 32 (2002) 630–637. [DOI] [PubMed] [Google Scholar]
- [288].de Araújo S, Oliveira AP, Sousa FBM, Souza LKM, Pacheco G, Filgueiras MC, Nicolau LAD, Brito GAC, Cerqueira GS, Silva RO, Souza MHLP, Medeiros JVR, AMPK activation promotes gastroprotection through mutual interaction with the gaseous mediators HS, NO, and CO, Nitric Oxide. 78 (2018) 60–71. [DOI] [PubMed] [Google Scholar]
- [289].Colpaert EE, Timmermans J-P, Lefebvre RA, Investigation of the potential modulatory effect of biliverdin, carbon monoxide and bilirubin on nitrergic neurotransmission in the pig gastric fundus, Eur. J. Pharmacol 457 (2002) 177–186. [DOI] [PubMed] [Google Scholar]
- [290].Chen Y, Lui VCH, Sham MH, Tam PKH, Distribution of carbon monoxide-producing neurons in human colon and in Hirschsprung’s disease patients, Hum. Pathol 33 (2002) 1030–1036. [DOI] [PubMed] [Google Scholar]
- [291].Xue L, Farrugia G, Miller SM, Ferris CD, Snyder SH, Szurszewski JH, Carbon monoxide and nitric oxide as coneurotransmitters in the enteric nervous system: evidence from genomic deletion of biosynthetic enzymes, Proc. Natl. Acad. Sci. U. S. A 97 (2000) 1851–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Kim CB, Hintz SR, Vreman HJ, Stevenson DK, In vitro carbon monoxide production by the small intestine of suckling and adult Wistar rats: effect of parenteral tin-protoporphyrin, Dev. Pharmacol. Ther 11 (1988) 166–172. [DOI] [PubMed] [Google Scholar]
- [293].van Ginneken C, van Meir F, Sys S, Weyns A, Stereologic description of the changing expression of constitutive nitric oxide synthase and heme oxygenase in the enteric plexuses of the pig small intestine during development, J. Comp. Neurol 437 (2001) 118–128. [DOI] [PubMed] [Google Scholar]
- [294].Mitchell GS, Baker-Herman TL, McCrimmon DR, Feldman JL, Respiration, Encyclopedia of Neuroscience. (2009) 121–130. doi: 10.1016/b978-008045046-9.00479-4. [DOI] [Google Scholar]
- [295].Biagas K, Naran N, Fuhrman BP, Overview of Breathing Failure, Pediatric Critical Care. (2011) 520–527. doi: 10.1016/b978-0-323-07307-3.10040-0. [DOI] [Google Scholar]
- [296].Di Giulio C, Grilli A, De Lutiis MA, Di Natale F, Sabatino G, Felaco M, Does chronic hypoxia increase rat carotid body nitric oxide?, Comp. Biochem. Physiol. A Mol. Integr. Physiol 120 (1998) 243–247. [DOI] [PubMed] [Google Scholar]
- [297].Kline DD, Prabhakar NR, Peripheral chemosensitivity in mutant mice deficient in nitric oxide synthase, Adv. Exp. Med. Biol 475 (2000) 571–579. [DOI] [PubMed] [Google Scholar]
- [298].Pichon A, Zhenzhong B, Favret F, Jin G, Shufeng H, Marchant D, Richalet J-P, Ge R-L, Long-term ventilatory adaptation and ventilatory response to hypoxia in plateau pika (Ochotona curzoniae): role of nNOS and dopamine, Am. J. Physiol. Regul. Integr. Comp. Physiol 297 (2009) R978–87. [DOI] [PubMed] [Google Scholar]
- [299].Lin LH, Emson PC, Talman WT, Apposition of neuronal elements containing nitric oxide synthase and glutamate in the nucleus tractus solitarii of rat: a confocal microscopic analysis, Neuroscience. 96 (2000) 341–350. [DOI] [PubMed] [Google Scholar]
- [300].Torres JE, Kreisman NR, Gozal D, Nitric oxide modulates in vitro intrinsic optical signal and neural activity in the nucleus tractus solitarius of the rat, Neurosci. Lett 232 (1997) 175–178. [DOI] [PubMed] [Google Scholar]
- [301].de Paula PM, Branco LGS, Nitric oxide in the rostral ventrolateral medulla modulates hyperpnea but not anapyrexia induced by hypoxia, Brain Res. 977 (2003) 231–238. [DOI] [PubMed] [Google Scholar]
- [302].Nucci TB, Branco LGS, Gargaglioni LH, Nitric oxide pathway in the nucleus raphe magnus modulates hypoxic ventilatory response but not anapyrexia in rats, Brain Res. 1017 (2004) 39–45. [DOI] [PubMed] [Google Scholar]
- [303].Fabris G, Anselmo-Franci JA, Branco LG, Role of nitric oxide in hypoxia-induced hyperventilation and hypothermia: participation of the locus coeruleus, Braz. J. Med. Biol. Res 32 (1999) 1389–1398. [DOI] [PubMed] [Google Scholar]
- [304].Fabris G, Steiner AA, Anselmo-Franci JA, Branco LG, Role of nitric oxide in rat locus coeruleus in hypoxia-induced hyperventilation and hypothermia, Neuroreport. 11 (2000) 2991–2995. [DOI] [PubMed] [Google Scholar]
- [305].Xu ZQ, Pieribone VA, Zhang X, Grillner S, Hökfelt T, A functional role for nitric oxide in locus coeruleus: immunohistochemical and electrophysiological studies, Exp. Brain Res 98 (1994) 75–83. [DOI] [PubMed] [Google Scholar]
- [306].Powell FL, Fu Z, HIF-1 and ventilatory acclimatization to chronic hypoxia, Respir. Physiol. Neurobiol 164 (2008) 282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [307].Pamenter ME, Go A, Fu Z, Powell FL, No evidence of a role for neuronal nitric oxide synthase in the nucleus tractus solitarius in ventilatory responses to acute or chronic hypoxia in awake rats, J. Appl. Physiol 118 (2015) 750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [308].Schwenke DO, Pearson JT, Kangawa K, Shirai M, Does central nitric oxide chronically modulate the acute hypoxic ventilatory response in conscious rats?, Acta Physiol. . 186 (2006) 309–318. [DOI] [PubMed] [Google Scholar]
- [309].Bai Z, Voituron N, Wuren T, Jeton F, Jin G, Marchant D, Richalet J-P, Ge R-L, Pichon AP, Role of glutamate and serotonin on the hypoxic ventilatory response in high-altitude-adapted plateau Pika, Respir. Physiol. Neurobiol 212–214 (2015) 39–45. [DOI] [PubMed] [Google Scholar]
- [310].Gautier H, NO and the hypometabolic and hypothermic responses to hypoxia in the rat, Respir. Physiol 126 (2001) 201–209. [DOI] [PubMed] [Google Scholar]
- [311].Ladino J, Bancalari E, Suguihara C, Ventilatory response to hypoxia during endotoxemia in young rats: role of nitric oxide, Pediatr. Res 62 (2007) 134–138. [DOI] [PubMed] [Google Scholar]
- [312].Gozal D, Gozal E, Torres JE, Gozal YM, Nuckton TJ, Hornby PJ, Nitric oxide modulates ventilatory responses to hypoxia in the developing rat, Am. J. Respir. Crit. Care Med 155 (1997) 1755–1762. [DOI] [PubMed] [Google Scholar]
- [313].Bavis RW, DeAngelis KJ, Horowitz TC, Reedich LM, March RJ, Hyperoxia-induced developmental plasticity of the hypoxic ventilatory response in neonatal rats: contributions of glutamate-dependent and PDGF-dependent mechanisms, Respir. Physiol. Neurobiol 191 (2014) 84–94. [DOI] [PubMed] [Google Scholar]
- [314].Lipton AJ, Johnson MA, Macdonald T, Lieberman MW, Gozal D, Gaston B, S-nitrosothiols signal the ventilatory response to hypoxia, Nature. 413 (2001) 171–174. [DOI] [PubMed] [Google Scholar]
- [315].Gaston B, May WJ, Sullivan S, Yemen S, Marozkina NV, Palmer LA, Bates JN, Lewis SJ, Essential role of hemoglobin beta-93-cysteine in posthypoxia facilitation of breathing in conscious mice, J. Appl. Physiol 116 (2014) 1290–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [316].Prabhakar NR, Hydrogen sulfide (H(2)S): a physiologic mediator of carotid body response to hypoxia, Adv. Exp. Med. Biol 758 (2012) 109–113. [DOI] [PubMed] [Google Scholar]
- [317].Haouzi P, Bell H, Van de Louw A, Hypoxia-induced arterial chemoreceptor stimulation and hydrogen sulfide: too much or too little?, Respir. Physiol. Neurobiol 179 (2011) 97–102. [DOI] [PubMed] [Google Scholar]
- [318].Malik R, Ferguson AV, Hydrogen sulfide depolarizes neurons in the nucleus of the solitary tract of the rat, Brain Res. 1633 (2016) 1–9. [DOI] [PubMed] [Google Scholar]
- [319].da Silva GSF, Soriano RN, Kwiatkoski M, Giusti H, Glass ML, Branco LGS, Central hydrogen sulphide mediates ventilatory responses to hypercapnia in adult conscious rats, Acta Physiol. . 212 (2014) 239–247. [DOI] [PubMed] [Google Scholar]
- [320].Donatti AF, Soriano RN, Sabino JP, Branco LGS, Endogenous hydrogen sulfide in the rostral ventrolateral medulla/Bötzinger complex downregulates ventilatory responses to hypoxia, Respir. Physiol. Neurobiol 200 (2014) 97–104. [DOI] [PubMed] [Google Scholar]
- [321].Kwiatkoski M, Soriano RN, da Silva GSF, Francescato HD, Coimbra TM, Glass ML, Carnio EC, Branco LGS, Endogenous preoptic hydrogen sulphide attenuates hypoxia-induced hyperventilation, Acta Physiol. . 210 (2014) 913–927. [DOI] [PubMed] [Google Scholar]
- [322].da Silva GSF, Sabino JPJ, Rajani V, Alvares TS, Pagliardini S, Branco LGS, Funk GD, Excitatory Modulation of the preBötzinger Complex Inspiratory Rhythm Generating Network by Endogenous Hydrogen Sulfide, Front. Physiol 8 (2017) 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [323].Hu H, Shi Y, Chen Q, Yang W, Zhou H, Chen L, Tang Y, Zheng Y, Endogenous hydrogen sulfide is involved in regulation of respiration in medullary slice of neonatal rats, Neuroscience. 156 (2008) 1074–1082. [DOI] [PubMed] [Google Scholar]
- [324].Chen L, Zhang J, Ding Y, Li H, Nie L, Zhou H, Tang Y, Zheng Y, Site-specific hydrogen sulfide-mediated central regulation of respiratory rhythm in medullary slices of neonatal rats, Neuroscience. 233 (2013) 118–126. [DOI] [PubMed] [Google Scholar]
- [325].Li M, Nie L, Hu Y, Yan X, Xue L, Chen L, Zhou H, Zheng Y, Chronic intermittent hypoxia promotes expression of 3-mercaptopyruvate sulfurtransferase in adult rat medulla oblongata, Auton. Neurosci 179 (2013) 84–89. [DOI] [PubMed] [Google Scholar]
- [326].Wang W, Fung ML, St John WM, Pontile regulation of ventilatory activity in the adult rat, J. Appl. Physiol 74 (1993) 2801–2811. [DOI] [PubMed] [Google Scholar]
- [327].Fitzgerald RS, Dehghani GA, Kiihl S, Autonomic control of the cardiovascular system in the cat during hypoxemia, Auton. Neurosci 174 (2013) 21–30. [DOI] [PubMed] [Google Scholar]
- [328].Lin C-H, Lo W-C, Hsiao M, Tung C-S, Tseng C-J, Interactions of carbon monoxide and metabotropic glutamate receptor groups in the nucleus tractus solitarii of rats, J. Pharmacol. Exp. Ther 308 (2004) 1213–1218. [DOI] [PubMed] [Google Scholar]
- [329].Lin C-H, Lo W-C, Hsiao M, Tseng C-J, Interaction of carbon monoxide and adenosine in the nucleus tractus solitarii of rats, Hypertension. 42 (2003) 380–385. [DOI] [PubMed] [Google Scholar]
- [330].Silva CC, Almeida VA, Haibara AS, Johnson RA, Colombari E, Role of carbon monoxide in L-glutamate-induced cardiovascular responses in nucleus tractus solitarius of conscious rats, Brain Res. 824 (1999) 147–152. [DOI] [PubMed] [Google Scholar]
- [331].Prabhakar NR, Endogenous carbon monoxide in control of respiration, Respir. Physiol 114 (1998) 57–64. [DOI] [PubMed] [Google Scholar]
- [332].Prabhakar NR, Carbon monoxide (CO) and hydrogen sulfide (H(2)S) in hypoxic sensing by the carotid body, Respir. Physiol. Neurobiol 184 (2012) 165–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [333].Peng Y-J, Makarenko VV, Nanduri J, Vasavda C, Raghuraman G, Yuan G, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR, Inherent variations in CO-H2S-mediated carotid body O2 sensing mediate hypertension and pulmonary edema, Proc. Natl. Acad. Sci. U. S. A 111 (2014) 1174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [334].Peng Y-J, Zhang X, Gridina A, Chupikova I, McCormick DL, Thomas RJ, Scammell TE, Kim G, Vasavda C, Nanduri J, Kumar GK, Semenza GL, Snyder SH, Prabhakar NR, Complementary roles of gasotransmitters CO and H2S in sleep apnea, Proc. Natl. Acad. Sci. U. S. A 114 (2017) 1413–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [335].Yang W, Zhang Q, Zhou H, Sun X, Chen Q, Zheng Y, Heme oxygenase-carbon monoxide pathway is involved in regulation of respiration in medullary slice of neonatal rats, Neurosci. Lett 426 (2007) 128–132. [DOI] [PubMed] [Google Scholar]
- [336].Zhang J, He Y, Ding Y, Zhou H, Tang Y, Chen L, Zheng Y, Nitric oxide synthase expression in the medullary respiratory related nuclei and its involvement in CO-mediated central respiratory effects in neonatal rats, Brain Res. Bull 84 (2011) 258–263. [DOI] [PubMed] [Google Scholar]
- [337].Chen L, Zhang J, He Y, Pan J, Zhou H, Li H, Tang Y, Zheng Y, Contribution of BK(Ca) channels of neurons in rostral ventrolateral medulla to CO-mediated central regulation of respiratory rhythm in medullary slices of neonatal rats, Respir. Physiol. Neurobiol 182 (2012) 93–99. [DOI] [PubMed] [Google Scholar]
- [338].Thomas DD, Liu X, Kantrow SP, Lancaster JR, The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2, Proceedings of the National Academy of Sciences. 98 (2001) 355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]


