Abstract
Objective:
To examine the associations among socioeconomic factors, depressive symptoms, and cytokines in patients diagnosed with hepatocellular carcinoma (HCC).
Methods:
A total of 266 HCC patients were administered a battery of questionnaires including a sociodemographic questionnaire and the Center for Epidemiologic Studies Depression (CES-D) scale. Blood samples were collected to assess serum levels of cytokines using Luminex™. Descriptive statistics, Mann-Whitney U, Kruskal-Wallis, linear regression, and Bonferroni corrections were performed to test the hypotheses.
Results:
Of the 266 patients, 24% reported clinically significant depressive symptoms (CES-D≥22). Females had higher CES-D score than males (Mann-Whitney U=7135, p=.014, padj=.028). Being unemployed/ disabled (Kruskal-Wallis=14.732, p=.001, padj=.005) was found to be associated with higher depressive symptoms in males, but not in females. Serum level of IL-2 (Kruskal-Wallis=17.261, p=.001, padj =.005) were found to be negatively associated with education level. Cytokines, however, were not significantly associated with depressive symptoms. Gender (β=.177, p=.035), income (β=−.252, p=.004), whether the patient’s income met their basic needs (β=.180, p=.035) and IL-1β (β=−.165, p=.045) independently-predicted depressive symptoms and together explained 19.4% of variance associated with depressive symptoms.
Conclusions:
Sociodemographic and SES factors were predictive of inflammation and depressive symptoms. Recommendations include the development of gender-targeted interventions for HCC patients who have low SES and may suffer from depressive symptoms.
Keywords: cancer, cytokines, depression, gender difference, oncology, socioeconomic disparities
Background
Socioeconomic disparities in the United States (US) have been growing since 1975.1 Socioeconomic factors, including low income, unemployment and low education, contribute significantly to poorer survival rates in cancer patients.1–3 Not only are socioeconomic disparities in cancer prevalent in the US, but they are also a rising concern worldwide. Studies in Germany, Canada, and China have demonstrated that low socioeconomic status (SES) in cancer patients predicts higher rates of cancer-related symptoms, lower health-related quality of life (HRQoL), and shorter survival.4–7 Given the prevalence of socioeconomic disparities globally, it is important to understand the role these disparities play in HRQoL and inflammation linked to survival after the diagnosis of cancer.
Socioeconomic status refers to “the placement of persons, families, households, and census tracts or other aggregates with respect to the capacity to create or consume goods that are valued in our society”.8,9 Therefore, SES can be measured by factors such as education, employment status, and income, which can also be influenced by gender, ancestry, and ethnicity.8–10
Of all cancer-related symptoms, the National Institute of Health (NIH) State of the Science consensus statement concluded that depression is one of the three most common and debilitating symptoms in cancer patients, with prevalence up to 50% (which is seven times higher than the general population).11 Depression is prevalent in patients with advanced stages of cancer, particularly in those who have pancreatic, lung or liver cancer.12 Diagnosis of depression during the treatment phase of cancer has also been associated with higher odds of emergency room visits, hospitalizations, outpatient visits and mortality.13–15
Research on disparities in cancer has focused on gender and race, but only a few studies have examined socioeconomic factors such as income and education.16–19 Low SES, as measured by income and ownership of a home or car, predicted higher levels of depressive symptoms in breast, prostate and colorectal cancer patients.16 Similarly, in a gynecologic cancer population in Thailand, lower income was associated with a higher prevalence of depression.17 A lower level of education was also reported to be associated with a higher depressive severity in lung cancer patients.18 Although there is some evidence that low SES can lead to a higher risk of depression, Maneeton and colleagues reported that having more than 13 years of education was associated with an increased risk of depression across a heterogeneous sample of cancer patients.19,20 Due to the inconsistent results regarding how SES may influence the risk of depression, further research is warranted in this area.
Several cytokines including IL-1ß, TNF-α and IFN-γ have been reported to be related to depressive disorders.21 Associations between depression and cytokines have also been found in cancer patients, but there is a lack of data available regarding the link between SES and serum levels of cytokines linked to depressive disorders.22–25 Understanding the relationship of SES and cytokine levels is vital to investigating the biological mechanisms underlying depression as healthy individuals with low SES have been reported to have an immune system dysregulation.26,27 Furthermore, studies have found that individuals with low SES consistently experience more stress and depression, which lead to increased serum levels of pro-inflammatory cytokines.28 Therefore, research is warranted to investigate whether SES and/or depressive symptoms are linked to inflammation or if SES may serve as a moderator of depressive symptoms and inflammation.
Although studies have reported that lower SES leads to higher risk of depression and lower survival in cancer patients, the biological mechanism underlying the link between socioeconomic factors and increased risk of mortality remains elusive. According to Alder and Stewart’s social model of SES and health displayed in Figure 1, four pathways including (1) access to medical care, (2) exposure to carcinogens and pathogens, (3) health-related behaviors, and (4) central nervous system and endocrine response to stress can be the mechanisms through which SES indirectly leads to poor health outcomes.9 While Alder and Stewart proposed that patients from lower SES likely have environmental restraints and psychological influences that lead to these four pathways, gaps remain in the understanding of potential interactions of SES factors and pathways involved.9
Figure 1. The model of SES on depression and inflammation (extracted from health disparities model by Alder & Stewart, 2010).
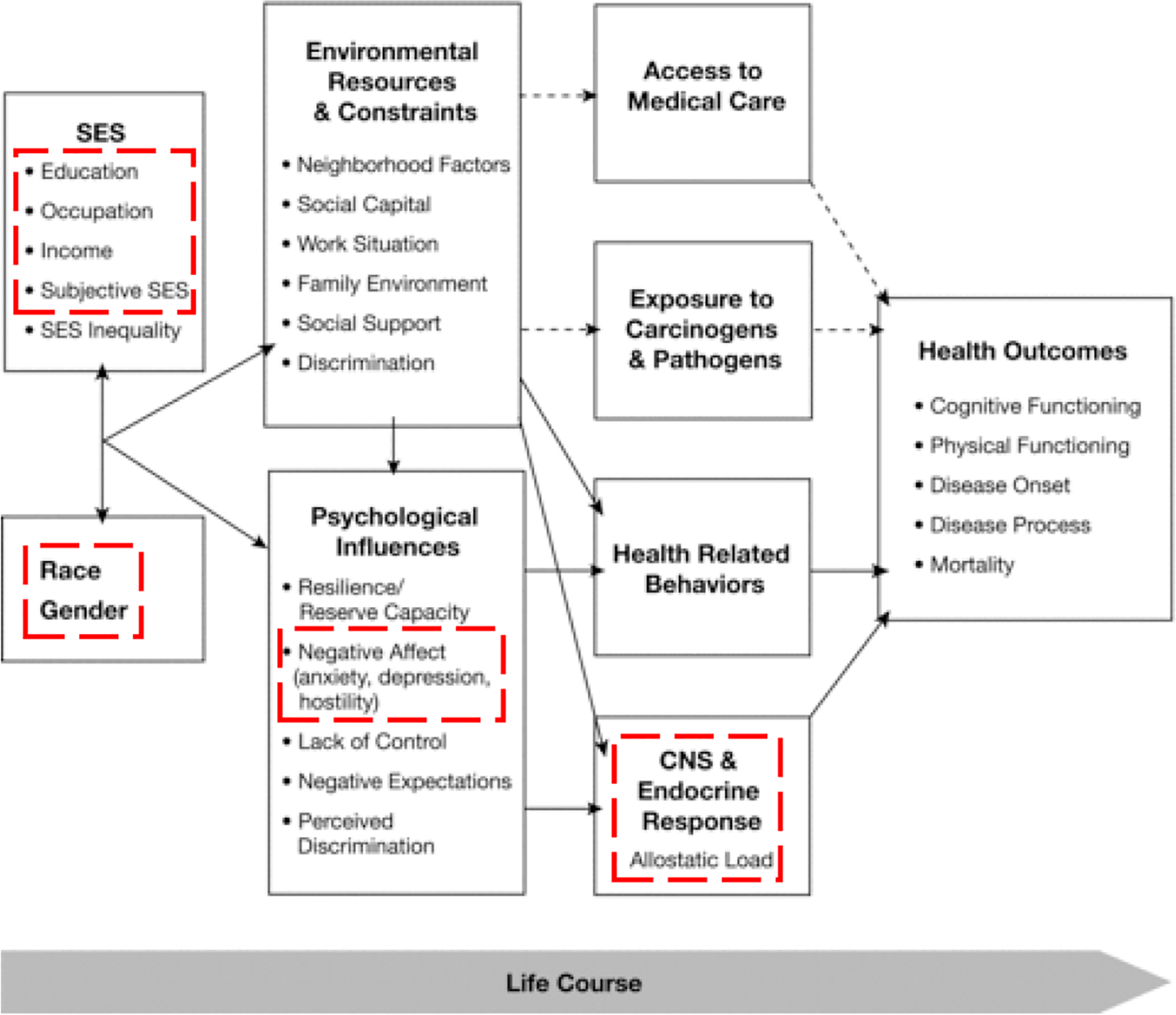
This model was originally published by Alder and Stewart in 2010 to display how SES disparities may influence health over a person’s life span. The dotted-lined regions of the diagram represent the model examined in this study.
Because it is the second leading cause of death from cancer globally and is rising in incidence in the US, patients diagnosed with hepatocellular carcinoma (HCC) were chosen for examination.29 Patients with HCC are often diagnosed at advanced stages, which is linked to poor prognosis and a higher risk for depression.29 Moreover, within the US, more HCC patients come from lower SES backgrounds due to the risk factors involved in the development of this cancer (e.g., alcohol, hepatitis C, and obesity).29 Therefore, it is urgent to understand SES disparity of depression and associated inflammation in the context of HCC to shape the development of targeted intervention. The overall aims of the study were to (1) examine the sociodemographic (age, gender, race) and socioeconomic (education, employment status, income) predictors of depressive symptoms; (2) investigate the differences in serum levels of cytokines that are linked to depression, tumor growth, or development of metastases in cancer (IL-α, IL-1β, IL-2, IL-6, IL-10, TNF-α, IFN-γ)21–27 across sociodemographic and economic factors; (3) test the link between depressive symptoms and serum levels of cytokines; and (4) determine the independent predictors of depressive symptoms among the HCC patients.
Methods
Design
Data from two previous prospective studies were used for secondary analyses (K07CA118576; R01CA176809). Although the two prospective studies enrolled participants with a wide spectrum of gastrointestinal cancer, only data from HCC patients were analyzed for this study. The recruitment periods were January 2008-November 2011 and November 2012-June 2014. For the purpose of this study, only data collected prior to cancer treatment was analyzed in order to control for dysregulation of serum cytokine levels that may be affected by treatment. Recruitment rates were 261/340 (76%) and 541/967 (56%) respectively for the two studies (K07CA118576; R01CA176809).
Participants
A total of 266 participants were recruited from the University of Pittsburgh’s Liver Cancer Center by physician referral during their initial visit in the clinic. Inclusion criteria included patients who were (1) diagnosed with HCC, (2) older than 21, and (3) fluent in English. Exclusion criteria included patients who had (1) already initiated treatments for cancer and (2) evidence of psychiatric symptoms including thought disorders, hallucinations and suicidal ideation.
Instruments/ Assessment
Sociodemographic factors
A 19-item sociodemographic questionnaire was used to collect information regarding patients’ age, gender, race, and SES. Socioeconomic status was measured by education, employment status and income (gross household income/ whether income met basic needs).
Depression
A 20-item Center for Epidemiologic Studies Depression (CES-D) scale was used to evaluate levels of depressive symptoms of participants in the week prior to assessment. Single items like “I felt depressed” were rated on a 4-point Likert scale, from 0 = “rarely/none of the time” to 3 = “most/all of the time”. The total score ranged from 0 to 60, with a higher score indicating higher presence of depressive symptoms quantitatively.30–31 Clinically significant level of depression was set at a score ≥22, as it has previously demonstrated high sensitivity.32 The CES-D is valid and has a high level of internal consistency and reliability across culture and languages, and has been used in clinical settings.30–32
Cytokines
Blood samples of participants were collected within two weeks of their initial assessment with instruments described above. Samples were processed, aliquoted and frozen at −80°C. Serum levels of cytokines of interest were assessed using Luminex™ beadset (Millipore Corporation, Billerica MA and Luminex 100 IS apparatus, Austin TX). A standard curve using recombinant cytokines in the Millipore multi-plex kit (HCYTOMAG-60K) were generated for each assay and compared to normal controls (collected from previous studies). The Millipore multi-plex kit uses a standard curve range of 3.2 to 10,000 pg/ml for all cytokines/chemokines. Standard curve concentrations and Minimum Detectable Concentration (MinDC) were calculated using Milliplex Analyst 5.1 software. MinDC is determined by calculating the lowest detection limit assuming an infinite number of standards run under the same assay conditions. The MinDC for individual cytokines measured in the study are 9.4pg/ml (IL-1α), 0.8pg/ml (IL-1β), 1pg/ml (IL-2), 0.9pg/ml (IL-6), 1.1pg/ml (IL-10), 0.7pg/ml (TNF-α) and 0.8pg/ml (IFN-γ).
Procedures
The protocol from which data were derived was approved by the University of Pittsburgh Institutional Review Board (PR007050143 and PR012060036). If the patient was visiting the clinic as a new patient and met inclusion criteria, he/she was asked by the physician to speak with the study team. If the patient agreed, he/she was enrolled following Good Clinical Practice (GCP) and asked to complete a battery of questionnaires and provide blood samples. Each patient was compensated $25 for each set of questionnaires and each blood sample.
Data Analyses
Statistical Package for the Social Sciences Version 22 (Armonk, NY) was used to enter, verify and analyze all data. Race was coded into “White” and “minorities” (which included African American, Native American, Pacific Islander, and Asian). Education was coded into “less than high school”, “high school or GED graduate”, and “some college or above”. Gross household income data was coded into five ranges: “under $10,000”, “$10,000-$19,999”, “$20,000 to $29,999”, “$30,000-$49,999”, and “$50,000 or over” per year. Responses to whether income meets basic needs were coded into “yes” and “no”. Current employment status was categorized into “employed”, “unemployed/disabled” and “retired”.
Descriptive statistics were used to provide a summary of central tendency as well as dispersion of data. Differences in the level of depressive symptoms were first analyzed separately by age using Kruskal-Wallis test and by gender/race using Mann-Whitney U test. Since there were statistically significant differences on depressive symptoms by gender, subsequent analyses of depressive symptoms and other socioeconomic variables were analyzedby gender. Since the CES-D score was non-normally distributed, Mann-Whitney U and Kruskal-Wallis tests were performed to examine between-group differences in CES-D scores by socioeconomic factors. Cytokine levels were also first analyzed separately by age using Kruskal- Wallis test and by gender/race using Mann-Whitney U test. Since there was no statistical difference on levels of cytokines by age, gender, or race, subsequent analyses on biomarkers were performed without covarying age, gender, or race. Mann-Whitney U tests were used to analyze differences in cytokines across socioeconomic groups as well as associations between clinical levels of depression and cytokines. Univariate and multivariate linear regression models were used to test sociodemographic predictors of depressive symptoms. Bonferroni corrections were done on all significant p-values to correct for multiplicity.
Results
Descriptive statistics of SES factors and HCC etiology of participants
A total of 266 patients diagnosed with HCC were eligible for this study. The average age of the participants was 63 (SD=10.26, range:30–93 years). Of the 266 patients, 56 (21.1%) were female, 67 (25.2%) had education less than high school, 86 (32.2%) were unemployed or disabled, 35 (13.2%) had income less than $10,000, and 50 (18.8%) had income that did not meet their basic needs. In terms of disease-specific factors, the majority of patients had hepatitis C virus (HCV) induced HCC (n=73, 26.9%), cirrhosis (n=221, 81.5%), one liver lesion (n=94, 34.7%), and no vascular invasion (n=183, 67.5%). Detailed descriptive statistics regarding the SES and disease-specific factors are displayed in Table 1. A significant difference was found across risk factors for SES including current employment status (χ2=18.78, p=0.005), with hepatitis C patients being more likely to be unemployed or disabled (50%) than patients with other risk factors such as hepatitis B (12%), alcohol (20.8%), or non-alcoholic steatohepatitis (NASH) (37.1%).
Table 1.
Socioeconomic and disease specific characteristics of participants (N = 266).
| Age (years) | 62.91 (SD = 10.26, range 30 – 93) |
|---|---|
| Gender (n (%)) | |
| Male | 210 (78.9) |
| Female | 56 (21.1) |
| Race (n (%)) | |
| White | 219 (82.3) |
| Minorities | 38 (14.3) |
| Education level (n (%)) | |
| Less than high school | 67 (25.2) |
| High school of GED graduate | 68 (25.6) |
| Some college or above | 119 (44.7) |
| Employment status (n (%)) | |
| Employed; full time/ part time | 53 (19.9) |
| Unemployed/ disabled | 86 (32.3) |
| Retired | 101 (38.0) |
| Gross household income (n (%)) | |
| Less than $10,000 | 35 (13.2) |
| $10,000-$19,999 | 31 (11.7) |
| $20,000-$29,999 | 32 (12.0) |
| $30,000-$49,999 | 54 (20.3) |
| Over $50,000 | 77 (28.9) |
| Income meets basic needs (n (%)) | |
| Yes | 202 (75.9) |
| No | 50 (18.8) |
| HCC etiology (n (%)) | |
| Hepatitis C | 73 (26.9) |
| Hepatitis B | 9 (3.3) |
| Alcohol | 30 (11.1) |
| NASH | 24 (8.9) |
| Cryptogenic | 61 (22.5) |
| Hepatitis C & alcohol | 30 (11.1) |
| Hepatitis C & NASH | 1 (0.4) |
| Alcohol & NASH | 1 (0.4) |
| Hepatitis B & Hepatitis C | 2 (0.7) |
| Hepatitis B & Hepatitis C & alcohol | 1 (0.4) |
| Hepatitis B & alcohol | 2 (0.7) |
| Hemochromatosis | 7 (2.6) |
| Cryptogenic & NASH | 2 (0.7) |
| Recurrence | 2 (0.7) |
| Autoimmune hepatitis | 1 (0.4) |
| Other | 9 (3.3) |
| Cirrhosis (n (%)) | |
| Yes | 221 (81.5) |
| No | 50 (18.5) |
| Number of lesions (n (%)) | |
| 1 | 94 (34.7) |
| 2 | 47 (17.3) |
| 3 | 28 (10.3) |
| 4 | 11 (4.1) |
| 5 | 7 (2.6) |
| More than 5 | 54 (19.9) |
| None | 16 (6.9) |
| Vascular invasion (n (%)) | |
| Yes | 58 (21.4) |
| No | 183 (67.5) |
Difference in depressive symptoms across socioeconomic groups
The mean CES-D total score was 14.5 (SD=10.64, range: 0–47). Among the 266 patients, a total of 64 (24%) reported clinically-significant depressive symptoms (CES-D total ≥22). Of all female patients, 33.9% reported clinically-significant depressive symptoms as opposed to only 21.4% in male patients. When depressive symptoms were analyzed, gender (Mann-Whitney U=7135, p=.014, padj=.028), but not age or race, was associated with CES-D scores. Therefore, the association of all socioeconomic factors and depressive symptoms were analyzed separately by gender.
For male HCC patients, a significant association was observed between employment status and depressive symptoms (Kruskal-Wallis =14.732, p=.001, padj=.005). Male patients who were unemployed/disabled reported the highest number of depressive symptoms when compared to those who were employed or retired. Gross household income (Kruskal-Wallis=11.429, p=.022, padj=.11) and whether income met the patient’s basic needs (Mann-Whitney U=3621, p=.028, padj=.14) were initially found to be significantly associated with depressive symptoms in males, but after Bonferroni adjustment these relationships were no longer significant. Similarly, education level was not found to be associated with depressive symptoms in males (Kruskal-Wallis=1.61, p=0.45). The significant differences in depressive symptoms across gender and employment status (in male) are illustrated in Figure 2.
Figure 2. Significant association of various socioeconomic factors and CES-D total.
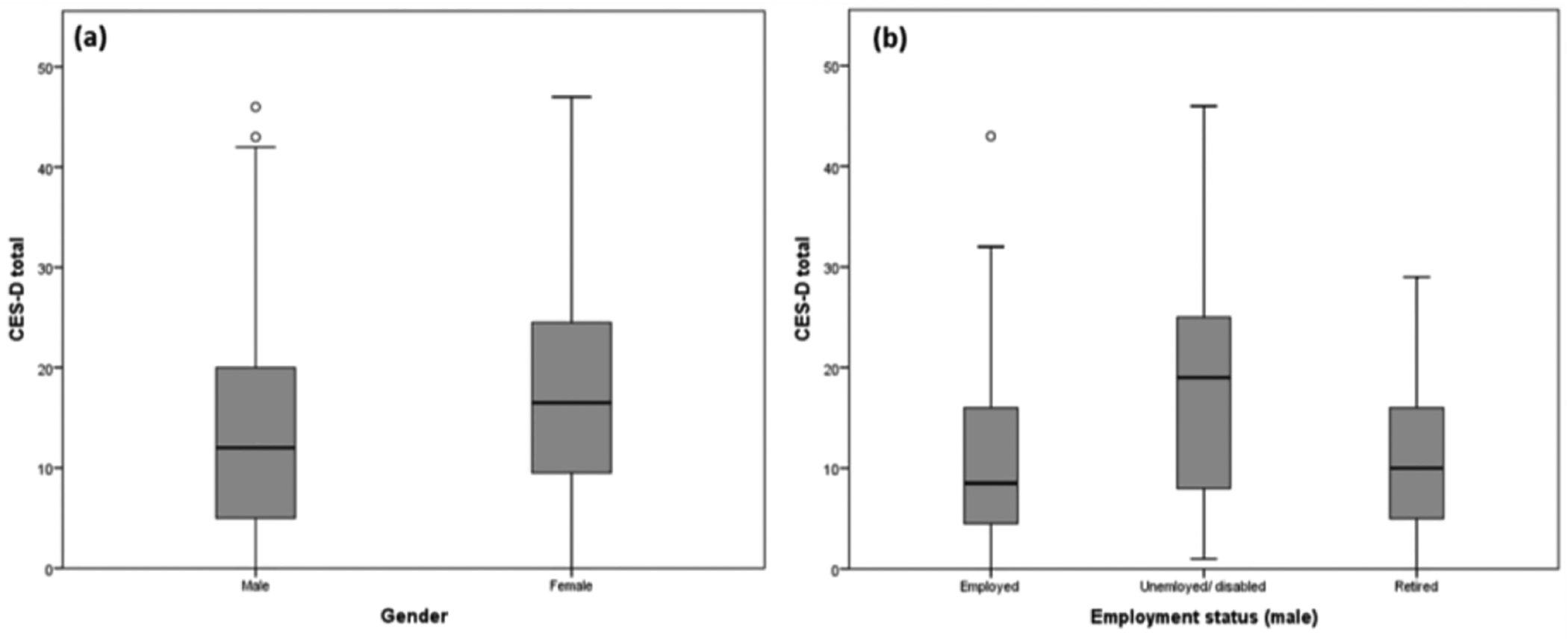
(a) Gender was found to be significantly associated with CES-D total. (b) Employment status was found to be significantly associated with CES-D total in male.
For female HCC patients, none of the socioeconomic factors including education (Kruskal-Wallis=3.747, p=.154), employment status (Kruskal-Wallis=1.123, p=.57), gross household income (Kruskal-Wallis=7.02, p=.135) or whether income met the patients’ basic needs (Mann Whitney U=330, p=.25) was found to be significantly linked to depressive symptoms.
Socioeconomic factors and cytokines
The descriptive statistics of serum levels of cytokines in pg/mL among the 266 participants are IL −1α (mean=223,SD=1738,range=0–20758); IL-1β (mean=284,SD= 1170,range=0–11655); IL-6 (mean=119.59, median=23, range=3–6346); IL-2 (mean=69,SD=336,range=0–3965); IL-10 (mean=15,SD=27,range=0–234); IFN-γ (mean=107,SD=1052,range=0–12864); TNF-α (mean=62,SD=228,range=2–2179). Spearman correlation was used to test the correlation between SES factors, and a significant correlation was observed between the number of years of education and income level (rho=0.290, p=0.01). Kruskal-Wallis tests were employed to analyze the association between socioeconomic variables and serum levels of cytokines without differentiating by age, gender or race, as these factors were not significantly associated with cytokines in the study cohort. Of all socioeconomic factors, only education was found to be significantly associated with serum levels of IL-1α , IL-1β, IL-2, and IL-10. Hepatocellular carcinoma patients who had an education of less than high school had significant elevations in serum levels of cytokines including IL-1α , IL-1β, IL-2, and IL-10, in comparison to those who were high school/GED graduates or had an education of at least some college. However, with a Bonferroni correction, only serum levels of IL-2 remained significantly associated with education level. Moreover, there was no significant association between education and serum levels of IL-6, TNF-α or IFN-γ. Furthermore, employment status, gross household income and whether income met the patients’ basic needs were not significantly associated with any cytokine. Similar results were observed using multiple regression in log-transformed cytokines data.
Association of depressive symptoms and cytokines
Mann-Whitney U tests revealed no significant difference between those with or without clinical levels of depressive symptoms and levels of IL-1α (Mann-Whitney U=2481, p=.320), IL-1-β (Mann-Whitney U=3160, p=.388), IL-2 (Mann-Whitney U=2861, p=.465 ), IL-6 (Mann-Whitney U=523.5, p=.359), IL-10 (Mann-Whitney U=3171, p=.399), TNF-α (Mann-Whitney U=1791.5, p=.863) or IFN-γ (Mann-Whitney U=2381, p=.805) when analyses were performed by gender.
Predictors of depressive symptoms
Linear regression analysis was performed to examine predictors of depressive symptoms in HCC patients. Socio-demographic and economic factors that were significantly related to depressive symptoms (gender, employment status, income, whether income met basic needs) or cytokines (education, and IL-1β, etc.) in univariate analyses were entered into the multivariable linear regression. Altogether, these factors accounted for 19.4% of the variance of depressive symptoms in patients diagnosed with HCC. The factors that independently predicted an increase of depressive symptoms included female gender (β=.177, p=.035), gross household income less than $10,000 (β=−.252, p=.004), income that did not meet patients’ and families’ basic needs (β=.180, p=.035) and elevation of IL-1β (β=−.165, p=.045).
Conclusions
Few studies have examined the associations between SES, depression, and biomarkers of inflammation in the context of cancer. In our study, 1.5 times as many female HCC patients demonstrated clinically relevant depressive symptoms when compared to male HCC patients, which is slightly less than in the general population.33,34 We found that employment status was only significantly associated with depression in males, but not in female patients, which has not been previously reported. This finding may reflect the increased stress that males may experience due to gender roles, particularly being the primary provider for the family. Based on these findings, targeted and tailored interventions to reduce depressive symptoms are warranted to treat those from underserved populations to maintain HRQoL and potentially improve survival.35
An alternative explanation for the lower percentage of depressive symptoms reported in this study cohort than in the general population may be the higher CES-D cutoff used in this study. The original cutoff for depression measured by the self-report instrument was CES-D≥16.30–31 In a later study, researchers found that a cutoff of CES-D≥ 22 is more sensitive to clinically significant depression (sensitivity 84%, specificity 60%).32 While study patients may not have high level of depressive symptoms or Major Depressive Disorder (MDD), some of them may experience subclinical levels of depression (16 ≤CES-D ≤21) . Furthermore, CES-D used in this study measures depressive symptoms with an instrument and cutoff set by previous research, but is not a clinical diagnosis of MDD or Mood Disorder due to Medical Condition. A structured clinical interview is recommended for future studies to obtain a more accurate diagnosis of depression. Due to the complexity of depression and the difference in symptoms displayed, studying the link between depression and inflammation by including the somatic, cognitive, and emotional symptoms of depression may provide insights into the mechanisms that underlie depression.36
Consistent with prior research in the general population and in colorectal cancer patients, serum level of IL-1β independently predicted depressive symptoms after adjusting for demographic and SES factors.21,25 In addition to its association with depressive symptoms, one explanation of such dysregulation can be due to HCC secondary to hepatitis B virus (HBV) and HCV infections, which make up 80% of HCC etiologies globally and approximately 50% of this sample.29 Alcohol dependence, which leads to alcoholic cirrhosis and serves as a contributing factor to HCC, has also been reported to significantly elevate chronic level of IL-1β which may explain the elevation of IL-1β in this cohort.37
On the other hand, some commonly elevated cytokines in depressed cancer patients were not detected in this study. One explanation may be differences in the inflammatory processes by cancer type. For example, elevation of IL-6 was reported in lung and breast cancer patients, yet dysregulation in IL-6, IL-1β and IL8 was observed in colorectal cancer patients.22,24–25 Since HCC in the US is a rare cancer type, few studies have a large enough sample to investigate these factors. Another explanation of undetected inflammation may be due to unaccounted for tumor-associated inflammation, which may fluctuate as the disease progresses. Therefore, regulatory T cells or myeloid-derived suppressor cells should be included in future studies to adjust for global elevations in cytokines due to tumor. Lastly, a recent study on cytokine levels of patients with MDD and chronic illness (compared with those of matched controls) found that there is no significant association between measures of systemic inflammation.38 The authors of the study hypothesized that MDD can be induced by comorbid inflammatory processes mediated by elevated serum cytokines.38 Future studies should recruit otherwise healthy MDD patients as matched controls to study the change of inflammatory cytokines in depressed cancer patients.
Even though multiple studies reported that low SES is associated with overall inflammation in the general population, we found that only education was robustly related to serum levels of IL-2.39 These findings reflect that cytokine levels in the serum may not be explained by current SES but rather the early-life SES of an individual as it has been previously reported that lower early-life SES can lead to general elevation of cytokine levels in adulthood.40 Since lower educational attainment of parents, which is reflective of an individual’s early life SES, is often linked to lower educational attainment of their children, early life SES may explain the significant association between IL-1α, IL-1β, IL-2 and IL-10 and educational levels, but not other socioeconomic factors.
Study limitations
One major limitation to the current study is the absence of covariates for lifestyle factors such as smoking, alcohol consumption and obesity. Since these lifestyle factors have previously been reported to predict the risk of HCC, they should be included in multivariate regression models to better understand the relationship among depression, inflammation and SES factors in HCC populations.
Another limitation is the under-representation of some sociodemographic (e.g. minorities) and socioeconomic (e.g. low education/income, unemployed) groups, which does not represent HCC populations worldwide. The few patients from racial minority backgrounds is alarming as it may signal the lack of access to health care. In the future, multi-site studies can be done to improve diversity of the study cohort. Furthermore, longitudinal analyses could be performed to examine how depressive symptoms and cytokine levels change over the course of the disease.
Clinical implications
Overall, future investigations on the impact of SES on depressive symptoms and on the immune system in cancer patients is warranted, as findings may have several clinical implications. First, understanding differences with regard to socioeconomic factors and depression among cancer patients across SES can facilitate the targeting of interventions to at-risk populations to improve HRQoL and potentially survival. Targeting those most at risk for poor health outcomes is cost-effective. Since most studies examining the association between depressive symptoms and serum levels of cytokines were conducted in healthy individuals, studies that focus on depressive symptoms and cytokines may provide insights to the development of immune- or pharmacological therapies that target cancer patients with comorbid depression. Finally, studies on SES, depression, and immunity will enhance the emerging research concerning heath disparities to understand the interaction of multiple socioeconomic factors as well as other sociodemographic factors which may facilitate the prevention of these disparities as well as to strategically target the health consequences of these disparities
Table 2.
Association of various SES factors and cytokine levels.
| SES factors | Cytokine | Kruskal-Wallis | Mann Whitney U | p | padj (Bonferroni) |
|---|---|---|---|---|---|
| Education | IL-1α | 7.83 | - | .02* | .14 |
| IL-1β | 9.59 | - | .008* | .056 | |
| IL-2 | 17.26 | - | <.001* | <.007* | |
| IL-10 | 6.46 | - | .04* | .28 | |
| IL-6 | .89 | - | .64 | 4.48 | |
| TNF-α | .04 | - | .98 | 6.86 | |
| IFN-γ | 5.51 | - | .06 | .42 | |
| Employment status | IL-1α | 1.02 | - | .6 | 4.2 |
| IL-1β | 2.10 | - | .35 | 2.45 | |
| IL-2 | 2.17 | - | .34 | 2.38 | |
| IL-10 | 1.83 | - | .57 | 3.99 | |
| IL-6 | 5.20 | - | .45 | 3.15 | |
| TNF-α | 0.27 | - | .32 | 2.24 | |
| IFN-γ | 2.77 | - | .85 | 5.95 | |
| Gross household income | IL-1α | 5.64 | - | .24 | 1.68 |
| IL-1β | 5.71 | - | .23 | 1.61 | |
| IL-2 | 7.35 | - | .14 | .98 | |
| IL-10 | 5.04 | - | .28 | 1.96 | |
| IL-6 | 2.21 | - | .07 | .49 | |
| TNF-α | 0.32 | - | .99 | 6.93 | |
| IFN-γ | 8.77 | - | .08 | .56 | |
| Whether income meets patients’ basic needs | IL-1α | - | 1695 | .85 | 5.95 |
| IL-1β | - | 2079 | .59 | 4.13 | |
| IL-2 | - | 1838 | .57 | 3.99 | |
| IL-10 | - | 2162 | .45 | 3.15 | |
| IL-6 | - | 669.5 | .32 | 2.24 | |
| TNF-α | - | 1863 | .57 | 3.99 | |
| IFN-γ | - | 1771 | .73 | 5.11 |
Footnotes
Conflict of Interest Statement
All authors of this article declare they have no conflicts of interest.
Contributor Information
Hoyee H. Cheng, University of Pittsburgh, Department of Surgery
Thomas W. Kamarck, University of Pittsburgh, Department of Psychology
Peter J. Gianaros, University of Pittsburgh, Department of Psychology
Kathryn A. Roecklein, University of Pittsburgh, Department of Psychology
Yanet Vanegas, University of Pittsburgh, Department of Surgery.
Allan Tsung, University of Pittsburgh, Department of Surgery.
David A. Geller, University of Pittsburgh, Department of Surgery
James W. Marsh, West Virginia University, Department of Surgery
Nadia S. Ahmed, University of Pittsburgh, Department of Surgery
Jennifer. L. Steel, University of Pittsburgh, Department of Surgery, Psychiatry, and Psychology
References
- 1.Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004. Mar-Apr;54(2):78–93. [DOI] [PubMed] [Google Scholar]
- 2.Rosengren A, Wilhelmsen L. Cancer incidence, mortality from cancer and survival in men of different occupational classes. Eur J Epidemiol. 2004;19(6):533–40. [DOI] [PubMed] [Google Scholar]
- 3.Parise CA, Caggiano V. Disparities in race/ethnicity and socioeconomic status: risk of mortality of breast cancer patients in the California Cancer Registry, 2000–2010. BMC Cancer. 2013. October 2;13:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jansen L, Eberle A, Emrich K, et al. Socioeconomic deprivation and cancer survival in Germany: an ecological analysis in 200 districts in Germany. Int J Cancer. 2014. June 15;134(12):2951–60. [DOI] [PubMed] [Google Scholar]
- 5.Coleman MP, Rachet B, Woods LM, et al. Trends and socioeconomic inequalities in cancer survival in England and Wales up to 2001. Br J Cancer. 2004. April 5;90(7):1367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackillop WJ, Zhang-Salomons J, Groome PA, et al. Socioeconomic status and cancer survival in Ontario. J Clin Oncol. 1997. April;15(4):1680–9. [DOI] [PubMed] [Google Scholar]
- 7.Shen FR, Liu M, Zhang X, et al. Health-related quality of life among breast cancer patients and its influencing factor in a Chinese population. Asian Pac J Cancer Prev. 2012;13(8):3747– 50. [DOI] [PubMed] [Google Scholar]
- 8.Shavers VL. Measurement of socioeconomic status in health disparities research. J Natl Med Assoc 2007;99:1013–23. [PMC free article] [PubMed] [Google Scholar]
- 9.Adler NE, Stewart J. Health disparities across the lifespan: meaning, methods, and mechanisms. Ann N Y Acad Sci. 2010. February;1186:5–23. [DOI] [PubMed] [Google Scholar]
- 10.Calixto OJ, Anaya JM. Socioeconomic status. The relationship with health and autoimmune diseases. Autoimmun Rev. 2014. June;13(6):641–54. [DOI] [PubMed] [Google Scholar]
- 11.Patrick DL, Ferketich SL, Frame PS, et al. National Institutes of Health State-of-the-Science Conference Statement: Symptom Management in Cancer: Pain, Depression, and Fatigue, July 15–17, 2002. J Natl Cancer Inst. 2003. August 6;95(15):1110–7. [DOI] [PubMed] [Google Scholar]
- 12.Brintzenhofe-Szoc KM, Levin TT, Li Y, et al. Mixed anxiety/depression symptoms in a large cancer cohort: prevalence by cancer type. Psychosomatics. 2009. Jul-Aug;50(4):383–91. [DOI] [PubMed] [Google Scholar]
- 13.Jayadevappa R, Malkowicz SB, Chhatre S, et al. The burden of depression in prostate cancer. Psychooncology. 2012. December;21(12):1338–45. [DOI] [PubMed] [Google Scholar]
- 14.Teng PR, Yeh CJ, Lee MC, et al. Depressive symptoms as an independent risk factor for mortality in elderly persons: results of a national longitudinal study. Aging Ment Health. 2013;17(4):470–8. [DOI] [PubMed] [Google Scholar]
- 15.Arrieta O, Angulo LP, Nunez-Valencia C et al. , Association of depression and anxiety on quality of life, treatment adherence, and prognosis in patients with advanced non-small cell lung cancer. Ann Surg Oncol. 2013. June;20(6):1941–8. [DOI] [PubMed] [Google Scholar]
- 16.Simon AE, Wardle J. Socioeconomic disparities in psychosocial wellbeing in cancer patients. Eur J Cancer. 2008. March;44(4):572–8. [DOI] [PubMed] [Google Scholar]
- 17.Hengrasmee P, Padungsutt P, Boriboonhirunsarn D. Depression among gynecologic cancer patients at Siriraj Hospital: prevalence and associated factors. J Med Assoc Thai. 2004. October;87 Suppl 3:S74–9. [PubMed] [Google Scholar]
- 18.Fagundes C, Jones D, Vichaya E, et al. Socioeconomic Status Is Associated with Depressive Severity Among Patients with Advanced Non-Small-Cell Lung Cancer: Treatment Setting and Minority Status Do Not Make a Difference. J Thorac Oncol. 2014. August 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maneeton B, Maneeton N, Mahathep P. Prevalence of depression and its correlations: a cross-sectional study in Thai cancer patients. Asian Pac J Cancer Prev. 2012;13(5):2039–43. [DOI] [PubMed] [Google Scholar]
- 20.Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005. February;29(2):201–17. [DOI] [PubMed] [Google Scholar]
- 21.Lichtblau N, Schmidt FM, Schumann R et al. Cytokines as biomarkers in depressive disorder: current standing and prospects. Int Rev Psychiatry. 2013. October;25(5):592–603. [DOI] [PubMed] [Google Scholar]
- 22.Du YJ, Zhang HY, Li B, et al. Sputum interleukin-6, tumor necrosis factor-α and Salivary cortisol as new biomarkers of depression in lung cancer patients. Prog Neuropsychopharmacol Biol Psychiatry. 2013. December 2;47:69–76. [DOI] [PubMed] [Google Scholar]
- 23.Musselman DL, Miller AH, Porter MR. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. Am J Psychiatry. 2001. August;158(8):1252–7. [DOI] [PubMed] [Google Scholar]
- 24.Soygur H, Palaoglu O, Akarsu ES. Interleukin-6 levels and HPA axis activation in breast cancer patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007. August 15;31(6):1242–7. [DOI] [PubMed] [Google Scholar]
- 25.Oliveira Miranda D, Soares de Lima TA, Ribeiro Azevedo L. Proinflammatory cytokines correlate with depression and anxiety in colorectal cancer patients. Biomed Res Int. 2014;2014:739650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gruenewald TL, Cohen S, Matthews KA. Association of socioeconomic status with inflammation markers in black and white men and women in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Soc Sci Med. 2009. August;69(3):451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller GE, Chen E, Fok AK. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci U S A. 2009. August 25;106(34):14716–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Godbout JP, Glaser R. Stress-induced immune dysregulation: implications for wound healing, infectious disease and cancer. J Neuroimmune Pharmacol. 2006. December;1(4):421–7. [DOI] [PubMed] [Google Scholar]
- 29.McGlynn KA, London WT. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis. 2011. May;15(2):223–43, vii-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radloff SL. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement 1977. 1: 385 [Google Scholar]
- 31.Hann D, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients: evaluation of the Center for Epidemiological Studies Depression Scale (CES-D). J Psychosom Res. 1999. May;46(5):437.-. [DOI] [PubMed] [Google Scholar]
- 32.Haringsma R, Engels GI, Beekman AT, et al. The criterion validity of the Center for Epidemiological Studies Depression Scale (CES-D) in a sample of self-referred elders with depressive symptomatology. Int J Geriatr Psychiatry. 2004. June;19(6):558–63. [DOI] [PubMed] [Google Scholar]
- 33.Accortt EE, Freeman MP, Allen JJ. Women and major depressive disorder: clinical perspectives on causal pathways. J Womens Health (Larchmt). 2008. December;17(10):1583–90. [DOI] [PubMed] [Google Scholar]
- 34.Linden W, Vodermaier A, Mackenzie R, et al. Anxiety and depression after cancer diagnosis: prevalence rates by cancer type, gender, and age. J Affect Disord. 2012. December 10;141(2–3):343–51. [DOI] [PubMed] [Google Scholar]
- 35.Steel JL, Geller DA, Robinson TL et al. Health-related quality of life as a prognostic factor in patients with advanced cancer. Cancer. 2014. December 1;120(23):3717–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reuter K, Raugust S, Bengel J et al. Depressive symptom patterns and their consequences for diagnosis of affective disorders in cancer patients. Support Care Cancer (2004) 12: 864. [DOI] [PubMed] [Google Scholar]
- 37.Liu L, Hutchinson MR, White JM et al. Association of IL-β genetic polymorphisms with an increased risk of opioid and alcohol dependence. Pharmacogenet Genomics. 2009. November;19(11):869–76 [DOI] [PubMed] [Google Scholar]
- 38.Cassano P, Bui E, Rogers AH, Walton ZE, Ross R, Zeng M, Nadal-Vicens M, Mischoulon D, Baker AW , Keshaviah A, Worthington J, Hoge EA, Alpert J, Fava M, Wong KK, Simon NM. Inflammatory cytokines in major depressive disorder: A case-control study. Aust N Z J Psychiatry. 2017. January;51(1):23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fraga S, Marques-Vidal P, Vollenweider P et al. Association of socioeconomic status with inflammatory markers: A two cohort comparison. Prev Med. 2015. February;71:12–9. [DOI] [PubMed] [Google Scholar]
- 40.Carroll JE, Cohen S, Marsland AL. Early childhood socioeconomic status is associated with circulating interleukin-6 among mid-life adults. Brain Behav Immun. 2011. October;25(7):1468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]


