Abstract
Background:
H3 K27M-mutant diffuse midline glioma is a fatal malignancy with no proven medical therapies. The entity predominantly occurs in children and young adults. ONC201 is a small molecule selective antagonist of dopamine receptor D2/3 (DRD2/3) with an exceptional safety profile. Following up on a durable response in the first H3 K27M-mutant diffuse midline glioma patient who received ONC201 (NCT02525692), an expanded access program was initiated.
Methods:
Patients with H3 K27M-mutant gliomas who received at least prior radiation were eligible. Patients with leptomeningeal spread were excluded. All patients received open-label ONC201 orally once every week. Safety, radiographic assessments, and overall survival were regularly assessed at least every 8 weeks by investigators. As of August 2018, a total of 18 patients with H3 K27M-mutant diffuse midline glioma or DIPG were enrolled to single patient expanded access ONC201 protocols. Among the 18 patients: seven adult (>20 years old) and seven pediatric (<20 years old) patients initiated ONC201 with recurrent disease and four pediatric patients initiated ONC201 following radiation, but prior to disease recurrence.
Findings:
Among the 14 patients with recurrent disease prior to initiation of ONC201, median progression-free survival is 14 weeks and median overall survival is 17 weeks. Three adults among the 14 recurrent patients remain on treatment progression-free with a median follow up of 49.6 (range 41–76.1) weeks. Among the 4 pediatric patients who initiated adjuvant ONC201 following radiation, two DIPG patients remain progression-free for at least 53 and 81 weeks. Radiographic regressions, including a complete response, were reported by investigators in a subset of patients with thalamic and pontine gliomas, along with improvements in disease- associated neurological symptoms.
Interpretation:
The clinical outcomes and radiographic responses in these patients provide the preliminary, and initial clinical proof-of-concept for targeting H3 K27M-mutant diffuse midline glioma with ONC201, regardless of age or location, providing rationale for robust clinical testing of the agent.
Funding:
Oncoceutics.
INTRODUCTION
The 2016 World Health Organization classification of central nervous system tumors defines diffuse midline gliomas with the histone H3 K27M mutation as a distinct Grade IV glioma, regardless of histological features, and is associated with a poor clinical prognosis.1 The H3 K27M heterozygous somatic mutation occurs at a prevalence of ~75% in pediatric diffuse intrinsic pontine glioma (DIPG) and in about 50% of other midline gliomas such as thalamic and spinal gliomas that predominantly occur in young adult patients.2 Standard therapy for H3 K27- mutant midline gliomas involves fractionated external beam radiotherapy with doses ranging from 54–60 Gy. Surgical resection of midline gliomas is often not feasible due to their location. The benefit of temozolomide, which is indicated for adult glioblastoma, is not clear as H3 K27M- mutant gliomas are MGMT unmethylated, and therefore most likely of limited to no value.3 Decades of DIPG clinical trials have failed to improve beyond a 9 to11-month median overall survival and a 2-year survival rate of <10%.4
DRD2 is a G protein-coupled receptor that promotes tumor growth and has emerged as a therapeutic target for gliomas and other tumors that overexpress this receptor.5,6 ONC201 is a selective antagonist of dopamine receptor D2/3 (DRD2/3) that crosses the blood-brain barrier and exhibits p53-independent anti-cancer efficacy in preclinical models of high-grade glioma.7–9 Downstream of DRD2 antagonism, ONC201 causes activation of the integrated stress response (ISR) combined with inactivation of Akt/ERK and other pro-survival signaling pathways.8,10,11
Results from the first Phase II clinical trial of oral ONC201 in molecularly unselected adults with recurrent, bevacizumab-naïve glioblastoma were previously reported using the recommended Phase II dose of 625mg administered orally once every three weeks (NCT02525692).12,13 Among this initial cohort of 17 patients, a 22-year-old woman with a thalamic glioma that harbored a biopsy-proven H3 K27M mutation experienced a near complete objective response (96%), including complete regression of the primary thalamic lesion. This response has remained durable as she continues on single agent ONC201 for >3 years. Based on this outlier response and emerging preclinical data indicating elevated DRD2 expression and enhanced ONC201 sensitivity in H3 K27M-mutant gliomas,14 we initiated a series of clinical investigations in this subpopulation using the weekly RP2D that was subsequently found to be equivalently well tolerated.15
Two Phase II clinical trials in adult recurrent H3 K27M-mutant diffuse midline glioma with single agent ONC201 were initiated (NCT03295396; NCT02525692), as well as a Phase I clinical trial in relapsed/refractory H3 K27M-mutant diffuse midline glioma as a single agent and in newly diagnosed DIPG in combination with radiation therapy (NCT03416530). While these clinical trials were being initiated, a small number of pediatric and adult patients with recurrent/refractory H3 K27M-mutant diffuse midline glioma received single agent ONC201 on single patient expanded access protocols.
METHODS
Study Medication and Protocol
ONC201 dihydrochloride was provided by Oncoceutics under single patient expanded access protocols that were approved by respective institutional IRBs and the FDA. The patient or the patients’ guardian provided written informed consent. Open-label ONC201 was orally administered once a week at 625 mg to patients ≥18 years of age and scaled by body weight for patients <18 years of age. All patients received single agent ONC201 until disease progression, except for one who concurrently received bevacizumab for a period of 5 months to allow for steroid tapering. Treatment was discontinued at disease progression, except for one DIPG patient with distant radiographic progression experiencing clinical benefit.
Study Evaluations
The data cutoff date for this report is April 25, 2019. History and physical examinations, complete blood count, comprehensive metabolic panel, magnesium, phosphorus, and urinalysis were performed every three or four weeks. Gadolinium-enhanced MRI and ECG were performed at baseline and subsequently every six or eight weeks. The MRI protocol included axial and sagittal T1-weighted images with coronal FLAIR, axial dual echo, coronal inversion recovery T2 and axial, sagittal and coronal T1 with gadolinium sequences. Progression-free survival was defined as time from first dose of ONC201 to radiologic or clinical progression or death. Response Assessment in Neuro-oncology (RANO) criteria were used for tumor evaluations in patients who did not have DIPG. For DIPG: radiological progression was defined as >25% increase in sum of the products of perpendicular diameters of enhancing lesions, appearance of new lesions, leptomeningeal spread; clinical progression was defined as neurologic deterioration with need for steroid dose escalation. Severe adverse events were reported per regulatory guidelines, however all adverse events were not collected as patients were treated on individual expanded access protocols. Toxicity and efficacy assessments were investigator-reported.
RESULTS
Patients
A total of 18 patients with H3 K27M-mutant diffuse midline glioma or DIPG were enrolled to single patient expanded access ONC201 protocols between June 1, 2017 and August 17, 2018 (Table 1). All patients, except for one with DIPG, had the H3 K27M mutation confirmed by immunohistochemistry or gene sequencing methods. In accordance with the prevalence of H3 K27M mutation in midline glioma, especially in children and young adults,2 the patient population was young relative to typical high grade glioma populations with a median age of 16 years (range 3–42 years). Seven patients were adult (>20 years of age) with recurrent disease at enrollment. Eleven patients were pediatric (<20 years of age): 7 patients had recurrent disease and 4 patients had stable disease following radiation at time of ONC201 initiation. Eleven patients (61%) were male and 7 (39%) were female. Primary lesions were distributed throughout the midline, predominantly thalamic and pontine locations: 8 (44.6%) pons, 6 (33%) thalamus, 1 (5.6%) spinal cord, 1 (5.6%) basal ganglia, 1 (5.6%) pineal gland, and 1 (5.6%) multifocal at diagnosis.
Table 1.
Demographics of H3 K27M-mutant diffuse gliomas treated with weekly ONC201.
| All Patients n=18 | <20 years old n=11 | >20 years old n=7 | |
|---|---|---|---|
| Gender, n | |||
| Male | 11 (61%) | 6 (55%) | 5 (71%) |
| Female | 7 (39%) | 5 (45%) | 2 (29%) |
| Age, years, median (range) | 16 (3–42) | 12 (3–19) | 29 (23–42) |
| Weight, kilogram, median (range) | 57 (11.8–130.8) | 45 (11.8–81.1) | 95.5 (63–130.8) |
| Baseline KPS, median (range) | 75 (50–80) | 80 (50–80) | 70 (50–80) |
| Primary tumor location, n | |||
| Pons | 8 (44.6%) | 7 (64%) | 1 (14%) |
| Thalamus | 6 (33%) | 2 (18%) | 4 (58%) |
| Spinal cord | 1 (5.6%) | 0 (0%) | 1 (14%) |
| Basal ganglia | 1 (5.6%) | 0( 0%) | 1 (14%) |
| Pineal | 1 (5.6%) | 1 (9%) | 0 (0%) |
| Multifocal | 1 (5.6%) | 1 (9%) | 0 (0%) |
| Prior lines of therapy, median (range) | 2 (1–4) | 2 (1–4) | 2 (1–4) |
| Prior temozolomide, n | 9 (50%) | 3 (27%) | 6 (86%) |
| Time from prior radiation, weeks, median (range) | 20 (1–87) | 16.5 (7–54) | 31.9 (1–87) |
| Baseline dexamethasone, mg/day, median (range) | 3 (0–24) | 4 (0–8) | 1.5 (0–24) |
| Time from diagnosis, weeks, median (range) | 36 (9–108) | 30.5 (15–108) | 29.4 (9–48) |
| Treatment Setting | |||
| First-line, adjuvant | 4 (22%) | 4 (36%) | 0 (0%) |
| Recurrent | 14 (78%) | 7 (64%) | 7 (100%) |
The median number of prior therapies was 2 (range 1–4). All patients received at least prior radiation. Six of 7 (86%) patients >20 years of age received prior temozolomide, in contrast to 3 of 11 (27%) patients <20 years of age in keeping with standard practices for adult malignant gliomas and DIPG, respectively.
Dosing, Safety, and Pharmacokinetics
A median of 16 (range 4–88) weeks oral doses of ONC201 were administered 625mg weekly ONC201 that was scaled by body weight for patients <18 years of age. Five of 18 (27%) patients continue weekly ONC201 for a median of 53 (range 41–81.2) weeks: 3 adults who initiated ONC201 with recurrent disease and two pediatric patients who initiated adjuvant ONC201 following radiation. No dose-limiting toxicities, dose modifications, or treatment discontinuation due to drug-related toxicity occurred in any patient.
The pharmacokinetic profile of 625mg ONC201 was previously reported in adults with advanced solid tumors, including a Cmax of 1.5–7.5ug/mL and mean half-life of 11.3 hours.16 Limited pharmacokinetic sampling was obtained within 24 hours of dosing in three children with DIPG: a 3-year-old girl, a 8-year-old boy, and a 10-year-old girl (Supplemental Figure 1). While the limited sampling precluded complete PK pharmacokinetic characterization, the Cmax values were in the expected range and exceeded the targeted therapeutic threshold of 600nM.
Clinical Outcomes
Five of 18 patients continue on ONC201 progression-free for a median of 53.14 (range 41–81.9) weeks. Thirteen patients discontinued ONC201 due to clinical and/or radiographic disease progression and died due to their disease. Median time from ONC201 discontinuation to death was 3.9 (range 0.4–25) weeks.
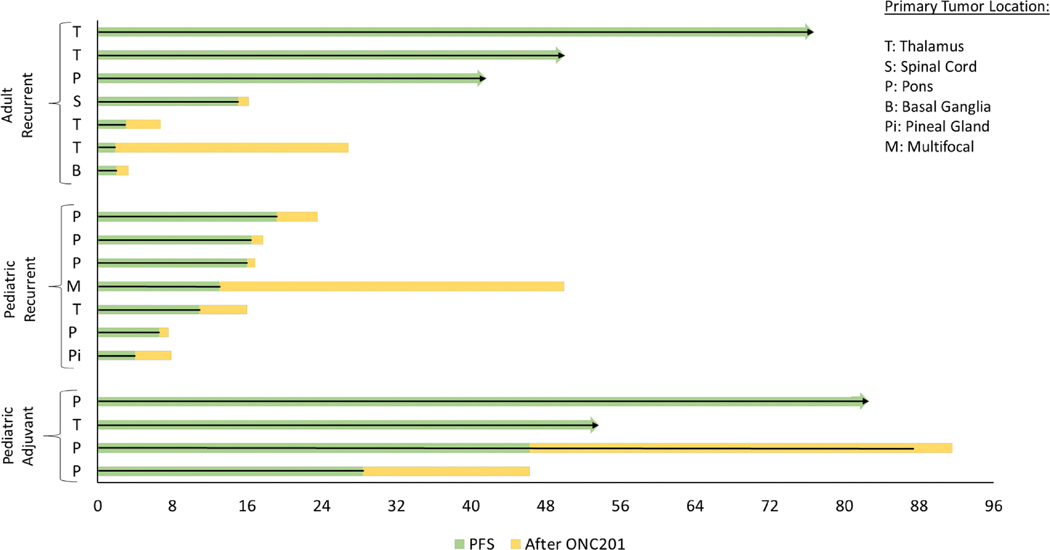
Among the 14 patients with recurrent disease, the median progression-free survival is 14 weeks: 15 weeks for the 7 adults and 13 weeks for the 7 pediatric patients (Figure 1). Three adults among the 14 recurrent patients remain on treatment progression-free with a median follow up of 49.6 (range 41–76.1) weeks.
Figure 1.
Swimmer’s plot of H3 K27M-mutant glioma patients treated with weekly ONC201. Adult patients are defined as >20 years of age and pediatric patients are defined as <20 years of age. Adjuvant denotes initiation of ONC201 following radiation, but prior to recurrence.
For the 4 pediatric patients who initiated adjuvant ONC201 after radiation, but prior to recurrence, one DIPG and one thalamic patient remain on therapy progression-free for >53 weeks and >81 weeks. Both patients who progressed had DIPG. One patient progressed at 46.3 weeks after beginning ONC201 and continued therapy an additional 41.9 weeks through progression until her death at 25.2 months from diagnosis. The other patient was progression- free for 28.4 weeks, discontinued therapy upon radiographic and clinical progression, and expired 14.1 months from diagnosis.
Radiographic, clinical symptoms, and quality of life improvements were reported for two of the adult patients with recurrent thalamic disease and two DIPG patients treated in the adjuvant setting after radiation.
Clinical Benefit in Adults
One adult with recurrent H3 K27M-mutant diffuse midline glioma exhibited a complete response of their disease involving the thalamus and other sites (Figure 2). This 38-year-old patient was diagnosed with a left thalamic WHO grade III anaplastic astrocytoma, subsequently revised to a WHO grade IV H3 K27M-mutant diffuse midline glioma. The patient was started on temozolomide (75mg/m2/day for 42 consecutive days) and radiation (60 Gy at 2 Gy per daily fraction) shortly after diagnosis. His radiotherapy prescription was modified to 2.5 Gy per fraction after receiving 30 Gy in 15 fractions due to clinical and neurological progression. He then received 5 cycles of adjuvant temozolomide (dosed at 150–200 mg/m2 daily for 5 consecutive days of every 28 day cycle) until radiographic progression was observed on a brain MRI. The patient then began CCNU at 110mg/m2 every 6 weeks for 2 cycles until further radiographic progression was observed on a brain MRI. Progression involved contrast- enhancing lesions, with MR spectroscopy and MR perfusion patterns characteristic of neoplasm rather than pseudoprogression.
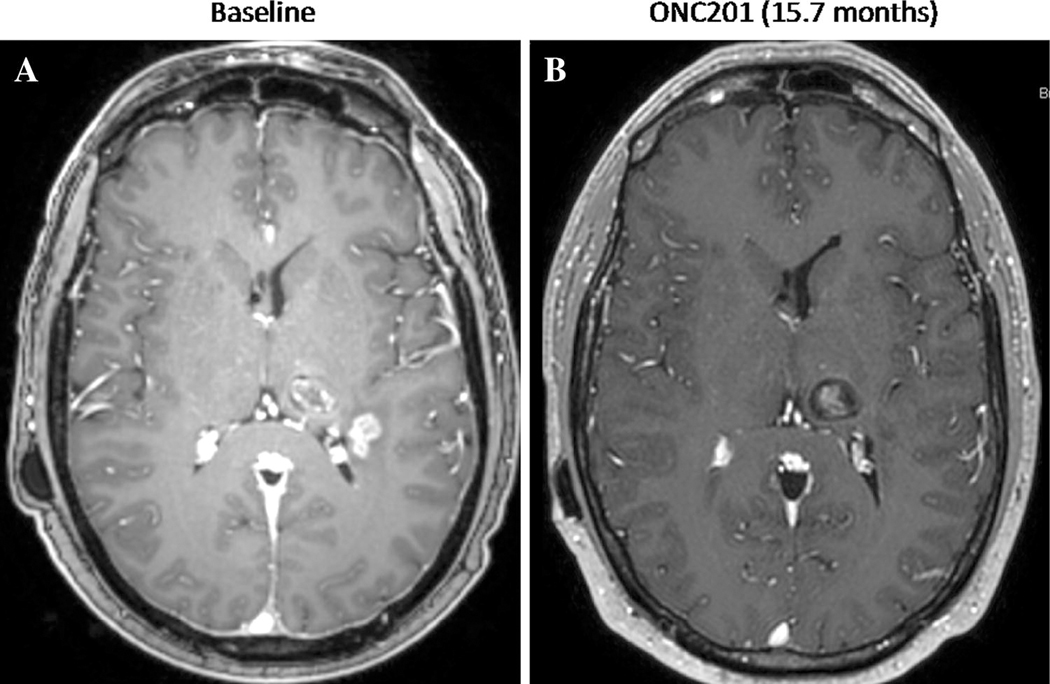
Figure 2.
Gadolinium-enhanced MRI of adult recurrent H3 K27M-mutant glioma patient at (A) baseline and (B) 15.7 months after initiating ONC201 (625mg PO, weekly).
The patient then began ONC201 via a compassionate use emergency protocol one month after completion of CCNU, receiving 625mg ONC201 orally once every week. At baseline, the patient was on 60mg hydrocortisone daily plus 10mg prednisone, and took dexamethasone for headaches as needed up to 8mg daily. His first brain MRI 6 weeks after initiating ONC201 showed 34% overall regression of the left lateral parietal enhancing tumor and improved associated edema. Multiple subsequent interval MRIs have confirmed >50% regression, representing a partial response by RANO criteria, that continued to deepen until a complete response was achieved after 10 months of ONC201. The patient has reported clinical improvements in disease-related symptoms such as headaches, nausea, and right-sided numbness. The patient continues on ONC201 for >17 months. The weight of radiographic findings supporting true progression at baseline, rather than pseudo-progression, and the achievement of a complete response in this context is a rare event that provides early clinical support for activity of the agent.
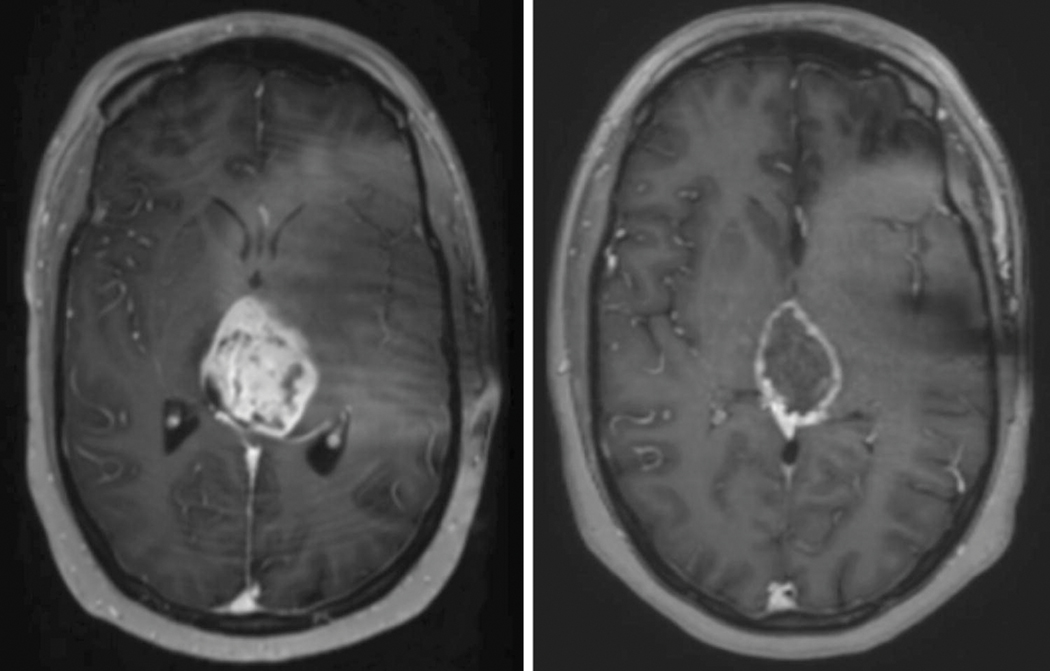
Another adult with recurrent H3 K27M-mutant diffuse midline glioma exhibited stabilization and subsequent reduction in tumor size by RANO criteria (Figure 3) while on ONC201. This patient was diagnosed with WHO Grade IV diffuse midline glioma, treated with 60 Gy in 30 fractions in combination with temozolomide 75 mg/m2 beginning 1 month after diagnosis. MRI evaluation indicated continued increase in tumor burden. The patient began 625mg ONC201 orally once weekly 4 months after diagnosis and 1.5 months after completion of radiation at the time of recurrence. Distinguishing pseudo-progression versus true progression is always a challenge post-radiation, however true progression was suspected as progressive radiographic and clinical findings did not respond to high-dose steroids. The first on-treatment scan 6 weeks after initiating ONC201 treatment was the first scan that indicated stable disease. The patient began bevacizumab 15 mg/kg every three weeks due to continued steroid requirement (6mg dexamethasone). Due to the lack of evidence in bevacizumab to prolong overall survival in grade IV gliomas, bevacizumab was discontinued after the patient was successfully tapered off of dexamethasone. The patient received a total of 7 doses of bevacizumab in a period of 5 months. The patient continues on ONC201 for >12 months with significant improvement in ability to partake in day to day activity since initiating ONC201, and remains clinically and radiographically improved from baseline. While psuedo-progression at baseline cannot be categorically excluded, the clinical improvement and response sustained >12 month is more likley to be associated with therapeutic effect, rather than pseudoprogression stabilizaton, in an unresected H3K27M mutant midline malignant glioma patient.
Figure 3.
Gadolinium-enhanced MRI of adult recurrent H3 K27M-mutant glioma patient at baseline (left) and one year (right) after initiating ONC201 (625mg PO, weekly). The on-treatment scan was taken 50 weeks after initiation of ONC201 and 22.5 weeks since the last dose of bevacizumab.
Clinical Benefit in Children
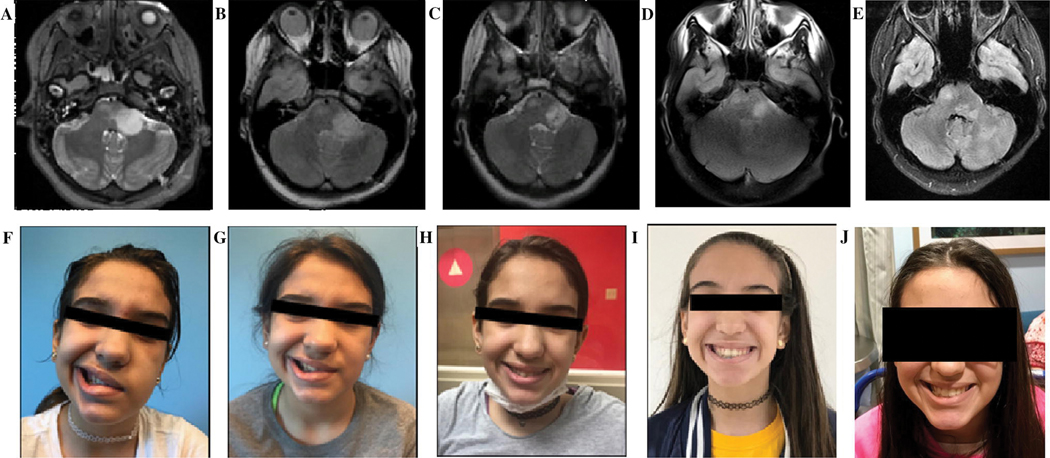
The first DIPG patient to receive ONC201 was a 10-years-old at enrollment. Her biopsied tumor exhibited the H3 K27M mutation and she was treated with ONC201 on an expanded access protocol approximately 7 weeks following completion of radiotherapy. Tumor volume on MRIs every 8 weeks sequentially decreased by 26%, 40%, and 44% relative to the pre-ONC201 MRI (Figure 4). Ipsilateral hearing was restored and facial palsy improved from House-Brackmann Grade IV to Grade I by 16 weeks after starting ONC201. Following initiation of ONC201, the patient was able to continue school attendance and participate in her normal activities for the next year. After almost 12 months on ONC201, the patient developed new right hemiparesis. MRI revealed that that the primary tumor remained relatively stable in size, however, new lesions in the thalamus and cerebellum located outside of the high dose target volume from the first course of radiotherapy consistent with tumor were reported. The patient received 39 Gy in 13 fractions to these new lesions in addition to dexamethasone, bevacizumab, and continuation of ONC201. After reirradiation, the patient continued ONC201 for another 8 months before developing symptomatic progression. MRI demonstrated progression of both the primary tumor and in the cerebellum. ONC201 was discontinued and the patient died of disease approximately one month later.
Figure 4.
MRI and cranial nerve palsy of H3 K27M-mutant DIPG patient. MRI at (A) diagnosis, (B) one month after radiation therapy, and (C) six months after beginning ONC201, (D) twelve months after beginning ONC201, and (E) eighteen months after beginning ONC201. Of note, the patient developed sites of tumor progression outside of the high dose region twelve months after beginning ONC201. Facial palsy at (F) diagnosis, (G) post-radiation, (H) four months after beginning ONC201, and (I) eight months after beginning ONC201, and (J) eighteen months after beginning ONC201.
A 33-month-old patient presented with headaches, right 6th nerve palsy and MRI findings consistent with a DIPG. A biopsy revealed an H3.3 K27M-mutant diffuse midline glioma. A patient-derived DIPG tumorsphere cell line was created in serum-free, neural stem cell media from the biopsy sample and in-vitro studies revealed potent reduction in cell viability following 5 days of treatment with ONC201, with an IC50 of approximately 600nM. She received involved field irradiation to a total dose of 54 Gy. One month following completion of irradiation, weekly single agent ONC201 on weight-based dosing was initiated. Radiographic improvements in FLAIR and stable tumor size have been documented over her time on treatment and she continues on ONC201 beyond 18 months with no treatment-related adverse events. Clinically her 6th nerve palsy and left hemiparesis have improved significantly, and she is otherwise asymptomatic. On account of slight enhancements in FLAIR, the patient underwent gamma knife treatment 18 months after initiating ONC201.
DISCUSSION
H3 K27M-mutant diffuse midline glioma is a lethal disease with no medical therapies that have shown survival benefit to date except radiation that offers only transient benefit. The identification of the H3 K27M mutation in DIPG and other diffuse midline gliomas has reignited enthusiasm for targeted therapies in this population. However, clinically effective therapies remain elusive.
Lysine 27, located in the conserved N-terminal tail of histone H3, is a component of the nucleosome, and is responsible for broad epigenetic regulation of gene expression or silencing through its acetylation or methylation, respectively. The H3 K27M mutation often co-occurs with mutations in TP53, PDGFR, or ACVR1 and is mutually exclusive to genetic alterations conferring more favorable prognosis, including IDH1/2 mutations and 1p/19q co-deletion.17,18 Gene silencing occurs through trimethylation of H3 K27 by the polycomb repressor complex 2 (PRC2) that is catalytically inhibited by H3 K27M.19,20 This leads to hyperacetylation of the remaining wild-type histone H3 variants and enhanced oncogenic transcription associated with heterotypic H3K27M/H3K27-acetylated nucleosome. Prior efforts to target this epigenetic aberrancy have focused on the HDAC inhibitors – such as panobinostat,21 inhibition of EZH2,22 or inhibition of CDK7 or BRD4, which have demonstrated activity in preclinical models but supportive clinical efficacy results have yet to emerge. Other approaches focus on H3 K27M as a neoantigen, such as the peptide vaccine against H3.3 K27M currently in early phase trials.23 H3 K27M-mutant diffuse midline gliomas have been reported to exhibit elevated expression and dependency on DRD2 as a downstream epigenetic consequence of the mutation.14 ONC201 is well-suited to address this potential vulnerability as a selective DRD2 antagonist that exhibits blood-brain-barrier penetrance26 and p53-independent anti-cancer efficacy.8
In addition to responses to single agent ONC201 reported in two adult patients with recurrent H3 K27M-mutant thalamic glioma, we provide initial proof-of-concept for ONC201 as a potential targeted therapy for H3 K27M-mutant DIPG. The first DIPG patient treated with adjuvant ONC201 derived a radiographic response, near complete resolution of her grade IV facial palsy, and hearing restoration. This patient received ONC201 monotherapy for approximately 12 months, before progressing out of field. She received additional radiotherapy for these new lesions and continued on ONC201 for another 8 months until progression. She discontinued ONC201 and expired approximately 1 month later. The potential of ONC201 as a therapeutic strategy for DIPG is further supported by the other DIPG case reported here who continues progression-free beyond 18 months with radiographic and clinical improvements, along with the recurrent pediatric cases with progression-free survival exceeding expected outcomes for recurrent DIPG of ~2 months.24 The anecdotal clinical benefit reported by investigators and limitations of comparable radiographic response based on RANO criteria underscores the need for novel response endpoints for infratentorial/midline gliomas in order to adequately capture clinical benefit.
This expanded access cohort is almost entirely restricted to H3 K27M-mutant diffuse midline gliomas. Interestingly, DRD2 expression within the central nervous system is highest in midline structures of the brain. Preclinical activity of ONC201 has been shown in the tumor microenvironment through TRAIL induction in proximal fibroblasts8 and activated NK cells.25 A dedicated arm in an ongoing Phase II trial is evaluating the activity of ONC201 in midline gliomas without the H3 K27M mutation (NCT02525692).
This report is limited by several factors, as it describes single patient experiences derived from a cohort of heterogenous patients with limited follow up time. Of note, the anecdotal clinical benefit reported by investigators and inability of investigators to use RANO for several patients underscores the need to create endpoints for infratentorial/midline gliomas in order to adequately capture clinical benefit. The effect of prior therapy in some patients treated immediately after radiation and prior to recurrence also limits interpretation, as the possibility of pseudoprogression could confound interpretation. Nevertheless, these results support further clinical investigation of ONC201 for H3 K27M-mutant diffuse midline gliomas (NCT03416530; NCT03295396; NCT02525692) in view of the absence of any meaningful therapy for this uniformly lethal disease, the mechanistic rationale for this agent, the ability to readily identify H3 K27M as an actionable mutation, and the radiographic regressions observed with single agent ONC201 in some patients with recurrent H3 K27M-mutant glioma.
Supplementary Material
Footnotes
COI Disclosure:
RST, KM, LS, MS, JEA and WO have ownership/employment relationships with Oncoceutics that develops ONC201.
LS, MS, WO and MPM serves on the Board of Directors of Oncoceutics.
MPM has served as a consultant to Varian, Astra-Zeneca, Celgene, Tocagen, and Abbvie Research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748 to Memorial Sloan Kettering Cancer Center
MAK has served as a consultant (medical advisory board) to Bayer over the preceding 2 years.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta neuropathologica 2016;131:803–20. [DOI] [PubMed] [Google Scholar]
- 2.Solomon DA, Wood MD, Tihan T, et al. Diffuse Midline Gliomas with Histone H3-K27M Mutation: A Series of 47 Cases Assessing the Spectrum of Morphologic Variation and Associated Genetic Alterations. Brain Pathology 2016;26:569–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen KJ, Pollack IF, Zhou T, et al. Temozolomide in the treatment of high-grade gliomas in children: a report from the Children’s Oncology Group. Neuro-oncology 2011;13:317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones C, Karajannis MA, Jones DTW, et al. Pediatric high-grade glioma: biologically and clinically in need of new thinking. Neuro-oncology 2017;19:153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, Zhu S, Kozono D, et al. Genome-wide shRNA screen revealed integrated mitogenic signaling between dopamine receptor D2 (DRD2) and epidermal growth factor receptor (EGFR) in glioblastoma. Oncotarget 2014;5:882–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caragher SP, Hall RR, 3rd, Ahsan R, Ahmed AU. Monoamines in Glioblastoma: complex biology with therapeutic potential. Neuro-oncology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madhukar Neel, Elemento Olivier, Benes Cyril, et al. D2-like dopamine receptor antagonism by ONC201 identified by confluence of computational, receptor binding, and clinical studies. Annual Meeting of the American Association for Cancer Research (AACR) 2016. [Google Scholar]
- 8.Allen JE, Krigsfeld G, Mayes PA, et al. Dual inactivation of Akt and ERK by TIC10 signals Foxo3a nuclear translocation, TRAIL gene induction, and potent antitumor effects. Science translational medicine 2013;5:171ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishida CT, Zhang Y, Bianchetti E, et al. Metabolic Reprogramming by Dual AKT/ERK Inhibition through Imipridones Elicits Unique Vulnerabilities in Glioblastoma. Clinical Cancer Research 2018;24:5392–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kline CLB, Van den Heuvel APJ, Allen JE, Prabhu VV, Dicker DT, El-Deiry WS. ONC201 kills solid tumor cells by triggering an integrated stress response dependent on ATF4 activation by specific eIF2α kinases. Science Signaling 2016;9:ra18–ra. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishizawa J, Kojima K, Chachad D, et al. ATF4 induction through an atypical integrated stress response to ONC201 triggers p53-independent apoptosis in hematological malignancies. Science Signaling 2016;9:ra17–ra. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arrillaga-Romany I, Chi AS, Allen JE, Oster W, Wen PY, Batchelor TT. A phase 2 study of the first imipridone ONC201, a selective DRD2 antagonist for oncology, administered every three weeks in recurrent glioblastoma. Oncotarget 2017;8:79298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stein MN, Bertino JR, Kaufman HL, et al. First-in-Human Clinical Trial of Oral ONC201 in Patients with Refractory Solid Tumors. Clinical cancer research : an official journal of the American Association for Cancer Research 2017;23:4163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chi A, Gardner S, Arrillaga-Romany I, et al. ACTR-34. INTEGRATED CLINICAL EXPERIENCE WITH ONC201 IN PREVIOUSLY-TREATED H3 K27M-MUTANT GLIOMA PATIENTS. Neuro-oncology 2018;20:vi19–vi. [Google Scholar]
- 15.Stein MN, Malhotra J, Malhotra U, et al. Safety and pharmacodynamics of the DRD2 antagonist ONC201 in advanced solid tumor patients with weekly oral administration. Journal of Clinical Oncology 2018;36:2595-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stein MN, Bertino JR, Kaufman HL, et al. First-in-Human Clinical Trial of Oral ONC201 in Patients with Refractory Solid Tumors. Clinical Cancer Research 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buczkowicz P, Hoeman C, Rakopoulos P, et al. Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nature genetics 2014;46:451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.the St. Jude Children’s Research Hospital–Washington University Pediatric Cancer Genome P, Wu G, Diaz AK, et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nature genetics 2014;46:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis PW, Müller MM, Koletsky MS, et al. Inhibition of PRC2 Activity by a Gain-of- Function H3 Mutation Found in Pediatric Glioblastoma. Science 2013;340:857–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Justin N, Zhang Y, Tarricone C, et al. Structural basis of oncogenic histone H3K27M inhibition of human polycomb repressive complex 2. Nature Communications 2016;7:11316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grasso CS, Tang Y, Truffaux N, et al. Functionally defined therapeutic targets in diffuse intrinsic pontine glioma. Nature medicine 2015;21:555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohammad F, Weissmann S, Leblanc B, et al. EZH2 is a potential therapeutic target for H3K27M-mutant pediatric gliomas. Nature medicine 2017;23:483. [DOI] [PubMed] [Google Scholar]
- 23.Chheda ZS, Kohanbash G, Okada K, et al. Novel and shared neoantigen derived from histone 3 variant H3.3K27M mutation for glioma T cell therapy. The Journal of Experimental Medicine 2018;215:141–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolff JE, Rytting M, Vats T, et al. Treatment of Recurrent Diffuse Intrinsic Pontine Glioma, Experience of MDAnderson Cancer Center. Journal of neuro-oncology 2012;106:391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner J, Kline CL, Zhou L, et al. Dose intensification of TRAIL-inducing ONC201 inhibits metastasis and promotes intratumoral NK cell recruitment. The Journal of clinical investigation 2018;128:2325–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.