Abstract
Intracranial hypertension (IH) is a clinical condition commonly encountered in the intensive care unit, which requires immediate treatment. The maintenance of normal intracranial pressure (ICP) and cerebral perfusion pressure in order to prevent secondary brain injury (SBI) is the central focus of management. SBI can be detected through clinical examination and invasive and non-invasive ICP monitoring. Progress in monitoring and understanding the pathophysiological mechanisms of IH allows the implementation of targeted interventions in order to improve the outcome of these patients. Initially, general prophylactic measures such as patient’s head elevation, fever control, adequate analgesia and sedation depth should be applied immediately to all patients with suspected IH. Based on specific indications and conditions, surgical resection of mass lesions and cerebrospinal fluid drainage should be considered as an initial treatment for lowering ICP. Hyperosmolar therapy (mannitol or hypertonic saline) represents the cornerstone of medical treatment of acute IH while hyperventilation should be limited to emergency management of life-threatening raised ICP. Therapeutic hypothermia could have a possible benefit on outcome. To control elevated ICP refractory to maximum standard medical and surgical treatment, at first, high-dose barbiturate administration and then decompressive craniectomy as a last step are recommended with unclear and probable benefit on outcomes, respectively. The therapeutic strategy should be based on a staircase approach and be individualized for each patient. Since most therapeutic interventions have an uncertain effect on neurological outcome and mortality, future research should focus on both studying the long-term benefits of current strategies and developing new ones.
Keywords: Intracranial pressure, Intracranial hypertension, Cerebral perfusion pressure, Traumatic brain injury, Osmotic agents, Neurocritical care
Introduction
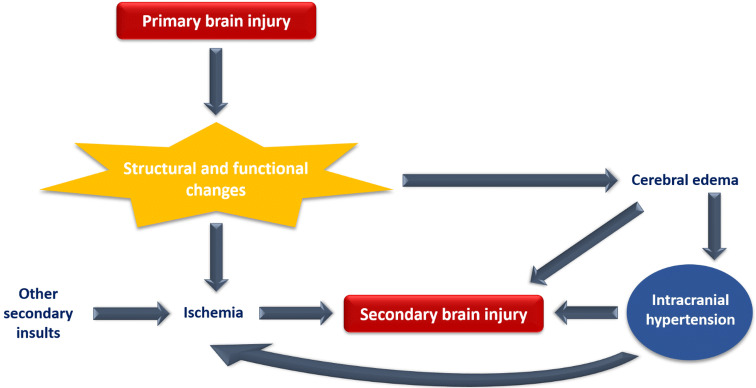
Intracranial hypertension (IH) is a common clinical problem in the intensive care unit (ICU), which requires immediate and urgent treatment. IH is the result of either primary central nervous system (CNS) lesion or a complication of co-existing systemic disease. It is caused by a variety of conditions divided into five main categories based on their pathological mechanism (Table 1). Any condition affecting the CNS, defined as acute brain injury (ABI) [(e.g. traumatic brain injury (TBI)], has two components: primary brain injury that cannot be reversed and secondary brain injury (SBI). SBI is defined as any physiological event that can occur within minutes, hours, or days after the initial injury and leads to further damage of nervous tissue. It can be detected through clinical examination and intracranial pressure (ICP) monitoring, as it is mostly due to increased ICP, and confirmed by imaging tests. Since there is a causal relationship between primary brain injury, IH, and SBI (Fig. 1), we focus on IH in this article. We conducted a literature search on MEDLINE/PubMed and Cochrane Library for studies completed in the last twenty years using the terms "intracranial hypertension" and "ICU management". We have also included guidelines from all established societies regarding IH in ABI [TBI, intracerebral hemorrhage (ICH), aneurysmal subarachnoid hemorrhage (SAH), ischemic stroke] and its management in ICU. The aim of this review article is to provide basic knowledge updated with what's new in the literature regarding the management of patient with IH.
Table 1.
Causes of intracranial hypertension based on their pathological mechanism
| Mechanism | Etiology |
|---|---|
| Venous obstruction | Sinus venous or jugular vein thrombosis |
| Increased brain volume | Brain tumor, abscess, empyema, intracerebral hemorrhage |
| Increased blood volume | Hypercapnia, anoxia, severe anemia, hyperperfusion syndrome, arteriovenous malformation, arteriovenous fistula |
| Mass effect | Subdural hematoma, epidural hematoma, empyema, tension pneumocephalus |
| Cerebral edema | |
| Cytotoxic | Ischemic stroke, anoxic encephalopathy, fulminant hepatic failure |
| Vasogenic | Hypertensive encephalopathy, brain tumor, abscess, encephalitis |
| Transependymal | Subarachnoid hemorrhage, meningitis, idiopathic intracranial hypertension |
| Osmotic | Hyponatremia, diabetic ketoacidosis, osmotherapy rebound effect |
Fig. 1.
Causal relationship between primary brain injury, intracranial hypertension and secondary brain injury
Clinical presentation of IH
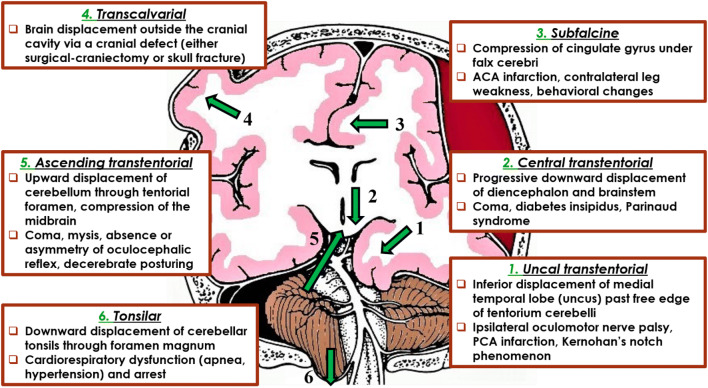
The clinical manifestations of IH are nonspecific and their severity does not correlate with the degree of IH (Table 2). The comatose patient with ABI and possible IH should be clinically evaluated using routinely either the Glasgow coma scale (GCS) (combined with assessment of pupils) or the full outline of unresponsiveness (FOUR) score, as multimodality monitoring (MMM) consensus recommend [1]. Brain herniation is a potentially fatal complication of IH. There are six types of herniation, namely the uncal transtentorial, the central transtentorial, the subfalcine, the tonsilar, the ascending transtentorial and the transcalvarial herniation (Fig. 2).
Table 2.
Clinical manifestations of intracranial hypertension
| Symptoms and signs | Comments |
|---|---|
| Headache | Often described as throbbing or bursting, exacerbated by coughing, sneezing, recumbency or exertion and in the morning |
| Nausea and vomiting | Projectile, not relieved by medication |
| Diplopia | Result of VI nerve palsy |
| Decreased level of consciousness | Drowsiness to coma, better correlation with the degree of midline shift, rather than a specific level of ICP elevation |
| Papilledema | Reliable sign but may develop after several days of increased ICP |
| Pupillary dilatation | III cranial nerve palsy |
| Downward deviation of the eyes | Due to dysfunction of the upgaze centers in the dorsal midbrain |
|
Cushing's triad Severe hypertension Bradycardia Irregular respiration |
Late and ominous sign of brain stem compression (brain herniation) |
ICP: Intracranial pressure
Fig. 2.
Types of brain herniation. ACA: Anterior cerebral artery, PCA: Posterior cerebral artery
ICP monitoring
In clinical practice, invasive and non-invasive methods of ICP monitoring are used aiming to determine the optimal cerebral perfusion pressure (CPP).
Invasive ICP monitoring
IH is associated with poor outcome and particularly with increased mortality [2], so it seems reasonable to measure ICP. The latest guidelines [3] recommend management of severe TBI patients using information from ICP monitoring to reduce in-hospital and 2-week post-injury mortality. It is difficult to demonstrate a direct association between specific monitoring and outcome improvement. Indeed, in a randomized trial [4] involving patients with severe TBI, ICP-guided therapy was not shown to be superior to care based on imaging and clinical examination. Recent studies [5–7] have also yielded conflicting results. Invasive ICP measurement is performed by specific catheters, inserted into the intraventricular, intraparenchymal, epidural, subdural or subarachnoid space [8]. The ideal ICP monitoring device should be reliable, accurate, cost-effective and be associated with minimal morbidity. Today, the intraventricular catheter remains the most reliable method (gold standard) for ICP monitoring, as it measures global ICP, provided that no obstruction of CSF flow occurs. The main features of ICP monitoring catheters are shown in Table 3. Recently, the intraparenchymal catheters used for ICP monitoring have integrated a CSF drainage catheter and catheters that detect parameters, such as brain tissue O2 partial pressure (PbtO2) and cerebral blood flow (CBF). Epidural, subdural and subarachnoid catheters are less accurate and are therefore rarely used.
Table 3.
Main features of ICP monitoring catheters
| Intraventricular catheters | Intra-parenchymal catheters |
|---|---|
| More accurate | Fairly accurate |
| Represent global ICP | May not represent global ICP |
| Lower cost | Higher cost |
| Can be recalibrated in situ | Inability to recalibrate |
| Can drain CSF as an ICP lowering therapy | Inability to drain CSF |
| Higher risk of infection | Lower risk of infection |
| Difficult to place into brains with severe cerebral edema | Easier to place |
ICP: Intracranial pressure, CSF: Cerebrospinal fluid
Non-invasive ICP monitoring
No method of non-invasive ICP monitoring can replace invasive monitoring, but may be useful either as a complementary tool or in deciding whether to initiate invasive monitoring.
Brain computed tomography (CT)
CT evaluates rapidly the presence of specific findings that enhance the diagnosis of ΙΗ. These include mass effect, midline shift, cerebral edema, hydrocephalus, compression of basal cisterns and changes in grey-white matter differentiation.
Brain magnetic resonance imaging (MRI)
MRI shows in more detail soft tissue and cerebral parenchymal lesions, which may not have been detected on CT, e.g. diffuse axonal injury. However, the prolonged screening time and stay of the patient in the supine position, which may aggravate ICP, make its use limited to patients with suspected IH.
Transcranial Doppler (TCD) ultrasonography
TCD is a useful, bedside, non-invasive technique for detecting inadequate CBF and assessing cerebral autoregulation. It may indicate the need for invasive brain monitoring and direct treatment in a multifactorial multimodal neuromonitoring approach [9]. TCD detects blood flow velocity (FV) through the major intracranial vessels, most commonly the middle cerebral artery (MCA). In cases of elevated ICP, the external pressure in the cerebral vessels increases, which is reflected by changes in FV. Detection of reduced FV indicates impediment to CBF and indirectly increased ICP. Besides the mean FV, pulsatility index (PI) and slopes of the TCD waveforms have been correlated with ICP [10–13]. It has been found that PI changes in the MCA are associated with changes in ICP, when the latter is between 5–40 mmHg. However, the accuracy of the technique depends on the experience of the operator and, in addition, 10–15% of the patients do not have adequate bone window.
Optic Nerve Sheath Diameter (ONSD)
The space between the optic nerve and its sheath is filled with CSF and therefore its pressure equals ICP. Thus, ONSD measured using a transocular ultrasound is increased in patients with IH. Several studies have shown that ONSD > 5 mm corresponds to ICP ≥ 20 mmHg [14, 15]. However, this association may be affected by conditions, such as tumors, inflammation, Grave’s disease and sarcoidosis, which may alter ONSD. The ONSD measurement technique is cheap, efficient and non-time consuming, but operator dependent [10].
Tympanic membrane displacement (TMD)
Because of the CSF and perilymph communication through the cochlear aqueduct, an increase in ICP is directly transmitted to the footplate of the stapes, displacing the tympanic membrane from its initial position. Inwards displacement indicates high, and outwards normal or low ICP [16]. However, this technique lacks accuracy and is an unreliable method of quantitative assessment of ICP in clinical practice.
Non-invasive methods of ICP monitoring and their basic characteristics are listed in Table 4.
Table 4.
Non-invasive methods of ICP monitoring
| Non-invasive ICP monitoring method | Comments |
|---|---|
| Brain CT | Fastest and most cost-effective method |
| Brain MRI | More accurate assessment of soft tissue and cerebral substance lesions |
| Transcranial Doppler ultrasonography | Patient bedside, highly operator dependent |
| Optic nerve sheath diameter | Cheap, efficient and not time consuming |
| Tympanic membrane displacement | Inaccurate and unreliable |
CT: Computed Tomography, MRI: Magnetic Resonance Imaging
Additional tools in ICP monitoring
Advance in understanding the pathophysiology of ABI has led to the development of various diagnostic tools that provide additional information on the adequacy of cerebral perfusion and extent of injury.
Brain tissue O2 partial pressure (PbtO2)
Measurement of PbtO2 by inserting a microcatheter in the white matter allows to unmask reduced perfusion and insufficient oxygen supply (< 10 mmHg) and also unmasks underlying hyperemia (> 30 mmHg). However, the measurement is regional, as only approximately 15 mm2 of tissue around the tip is sampled [8]. Current MMM consensus consider PbtO2 of less than 20 mmHg as threshold to consider intervention [1]. Studies have shown that low PbtO2 can be observed in combination with either high or low ICP [17], which enhances the value of brain oxygen monitoring. Thus, MMM consensus suggest its use to assist titration of medical and surgical therapies to guide ICP/CPP therapy, identify refractory IH and treatment thresholds, help manage delayed cerebral ischemia (DCI), and select patients for second-tier therapy [1]. Finally, a tendency to better outcomes with combined PbtO2 and ICP/CPP therapy compared to ICP/CPP therapy alone has been shown in severe TBI [18].
Jugular venous oxygen saturation (SjvO2)
Measurement of SjvO2 by a catheter placed in the jugular bulb could be used to estimate the balance between cerebral oxygen delivery and demand. SjvO2 differentiates insufficient oxygen supply due to impaired cerebral perfusion (SjvO2 < 50%) from reduced cerebral oxygen consumption encountered during hyperemia (SjvO2 > 80%). Increased ICP is mainly associated with reduced SjvO2 [18]. It could be part of MMM or be used in conjunction with ICP monitoring. but it is more difficult to use and less reliable than PbtO2 monitoring [1]. Due to the inherent shortcomings of the method, it can only provide information about global metabolism and its use has been limited [8].
Cerebral microdialysis
Cerebral microdialysis allows for semi-continuous measurement at the bedside of numerous parameters including glucose, glutamate, lactate, pyruvate, and glycerol concentrations. Metabolic changes of these parameters may occur before the usual cerebral physiological or pathophysiological changes [19], namely when ICP is normal. These changes may precede the clinical features of DCI and IH [20], allowing earlier therapeutic adjustments. In addition, derangements in cerebral metabolism detected by microdialysis can reveal the extent of the deleterious effect of IH on the brain [20]. However, microdialysis cannot be widely implemented yet due to its time-consuming maintenance and additional costs.
Near infrared spectroscopy (NIRS)
NIRS is a noninvasive tool that measures cerebral oxygenation by detecting oxygenated to deoxygenated hemoglobin concentrations. However, its use is limited in clinical practice since to date there are no studies to establish an absolute threshold for cerebral hypoxia and conditions such as scalp swelling and epidural/subdural hematomas lead to unreliable measurements [21].
Continuous electroencephalography (cEEG)
The use of cEEG is indicated in detection of convulsive and non‑convulsive seizures and prognosis of coma. Moreover, cEEG can be used in cases of increased ICP, as it is affected by changes in cerebral metabolism [22]. EEG patterns associated with elevated ICP include focal slowing of underlying rhythms or global EEG suppression progressing to burst suppression or flat EEG [23]. At the same time cEEG is a remarkably sensitive tool for detection of cerebral ischemia since it can reveal changes in neuronal function before structural damage. This is due to its high sensitivity to detect changes in CBF [24, 25]. Finally, cEEG can help predict outcome and titrate treatments such as barbiturates [26].
Management of IH
Progress in monitoring and understanding the pathophysiological mechanisms of IH allows the implementation of targeted interventions in order to improve the outcome of these patients. In the ICU all efforts should focus on preventing SBI although management of the primary cause of IH is the basic initial approach.
Prevention, detection and treatment of SBI are priorities of paramount importance for the clinical outcomes of patients. Several molecular and cellular pathways [27, 28] are activated in SBI. Thus, changes in ionic permeability, release of excitatory neurotransmitters and increased free radical accumulation cause mitochondrial dysfunction, which further triggers energy defects and processes of necrosis and apoptosis. These molecular and cellular changes could lead to the development of cytotoxic or vasogenic brain edema and disturbed autoregulation, resulting in an increase in the volume of intracranial components due to vasodilation or water accumulation, or both [29]. SBI is predictable and treatable and may be the result of extracranial (e.g. hypoxia, hypercapnia, arterial hypotension, fever) or intracranial (e.g. hematomas, contusions, seizures) factors (Table 5). Indeed, hypoxia and arterial hypotension trigger the systemic inflammatory response syndrome (SIRS), which may further aggravate the development of secondary damage [30]. Trauma affects the blood–brain barrier (BBB) directly, with increased permeability, favoring vasogenic edema formation and activation of a proinflammatory state [31]. Seizures may aggravate the imbalance between energy expenditure and supply [32]. The control of all these variables has been shown to improve both neurological and functional outcomes of patients [33].
Table 5.
Causes of secondary brain injury
| Causes of secondary brain injury | |
|---|---|
| Intracranial | Extracranial |
| Intracranial hematomas | Hypotension |
| Cerebral edema | Hypoxia |
| Intracranial hypertension | Hypercapnia |
| CNS infection | Electrolyte disorders |
| Seizures | Hypoglycemia |
| Hyperthermia | |
| Coagulopathy | |
| Infections | |
CNS: Central nervous system
Pathophysiologically, the management of raised ICP focuses on four main axes:
control and manipulation of vasoreactivity, CBF and flow-metabolism coupling
managing the blood/brain osmotic gradient
reducing the metabolic rate of oxygen consumption of cerebral tissue
physical/surgical modalities which affect intracranial compliance
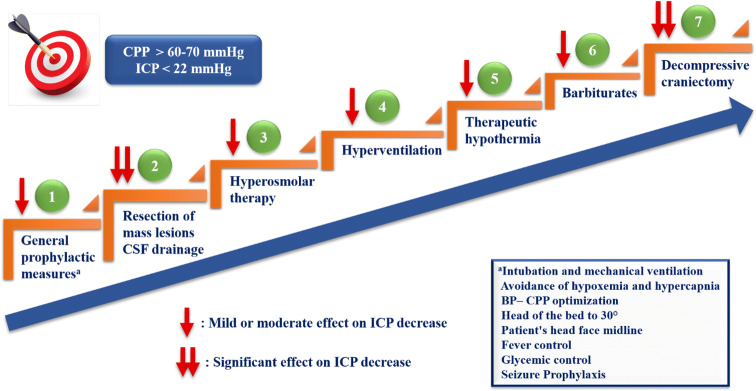
According to the last guidelines for TBI [3], the primary goal of IH treatment is to maintain ICP below 22 mmHg and CPP above 60 mmHg. Achieving these goals could be life-saving for brain's viability. The therapeutic measures for IH are distinguished in general prophylactic measures and those applied in the acute phase, in order to urgently reduce ICP and optimize CPP. All these interventions should be applied with a staircase approach tailored for each patient, as detailed below (Fig. 3).
Fig. 3.
Staircase therapeutic approach of intracranial hypertension. An optimal therapeutic strategy is considered the step-by-step escalation of available interventions [29, 129], tailored for each patient. The primary goal is to maintain ICP below 22 mmHg and CPP above 60 mmHg [3]. Initially, general prophylactic measures should be applied immediately to all patients with suspected IH. Based on specific indications and conditions, surgical resection of mass lesions [48] and CSF drainage [3, 48, 69] should be considered as an initial treatment for lowering ICP. The following steps in turn include hyperosmolar therapy (mannitol or hypertonic saline) [3], which represents the cornerstone of medical treatment of acute IH, hyperventilation and therapeutic hypothermia [107, 108]. Τo control elevated ICP refractory to maximum standard medical and surgical treatment, at first, high-dose barbiturate administration [3] and then decompressive craniectomy [3, 48, 69] as a last step are recommended. This staircase therapeutic approach is based mainly on clinical experience rather than on strong published evidence. ICP: Intracranial pressure, CPP: Cerebral pressure perfusion, IH: Intracranial hypertension, CSF: Cerebrospinal fluid, BP: Blood pressure
General prophylactic measures
General prophylactic measures aimed at optimizing various parameters [34] are an important part of the therapeutic approach of IH and are listed in Table 6.
Table 6.
Effect of general prophylactic measures and acute interventions on outcome
| Treatment of intracranial hypertension | Effect on neurological outcome | Effect on mortality |
|---|---|---|
| General prophylactic measures | ||
| Intubation and mechanical ventilation | Unclear | Unclear |
| BP – CPP optimization | Benefit | Benefit |
| Body positioning | Unclear | Unclear |
| Temperature control | Benefit | Benefit |
| Prophylactic hypothermia | No benefit | No benefit |
| Glycemic control | Benefit | Benefit |
| Seizure prophylaxis | Unclear | Unclear |
| Acute interventions | ||
| Hyperventilation | Unclear | Unclear |
| Hyperosmolar therapy | Unclear | Unclear |
| Sedation and analgesia | Unclear | Unclear |
| Barbiturates | Unclear | Unclear |
| Therapeutic hypothermia | Possible benefit | Possible benefit |
| Corticosteroids | No benefita | No benefita |
| Resection of mass lesions | Unclear | Probable benefit |
| Decompressive craniectomy | Unclear | Probable benefit |
| CSF drainage | Unclear | Unclear |
| Progesterone | No benefit | No benefit |
aExcept abscesses or neoplasms associated with vasogenic edema. BP: Blood pressure, CPP: Cerebral pressure perfusion, CSF: Cerebrospinal fluid
Intubation and mechanical ventilation
Early and rapid intubation and mechanical ventilation should be applied in comatose patients. This will help in controlling factors that may aggravate ICP, such as seizures and agitation. During intubation, adequate depth of sedation and elimination of reflexes such as cough and vomiting should be achieved.
Mechanical ventilation should aim at avoiding hypoxemia, hypercapnia and hypocapnia. Hypoxemia and hypercapnia should be avoided because of linear increase in CBF and hence ICP. Conversely, hypocapnia leads to an increased risk of ischemia by inducing cerebral vasoconstriction and reducing CBF. Consequently, PCO2 should be maintained at values between 35 and 40 mmHg.
The use of positive end-expiratory pressure (PEEP) during mechanical ventilation in patients with ABI has the risk of ICP elevation and CPP reduction [35] due to increased intrathoracic pressure and decreased cerebral venous drainage from the superior vena cava. However, in clinical trials, these effects occurred only when applying PEEP > 15 cmH2O in hypovolemic patients [36, 37]. Caricato et al. [38] concluded that the level of applied PEEP had no effect on the intracranial system in patients with low respiratory system compliance. Also, there are data [39, 40] claiming that the effect of PEEP on ICP depends on whether it causes alveolar hyperinflation or recruitment. In particular, if PEEP does not achieve effective alveolar recruitment but causes hyperinflation, it results in a significant increase in ICP due to impediment of cerebral venous return [40].
Blood pressure (BP) – CPP optimization
During BP monitoring hypotension should be avoided because it is an independent risk factor for poor outcome in patients with ABI [41]. The consequences of low BP are determined by the state of cerebral autoregulation. In patients with intact autoregulation, hypotension triggers reflex cerebral vasodilation and increases cerebral blood volume (CBV). In contrast, in patients with impaired autoregulation, hypotension leads to cerebral ischemia due to CPP reduction. Nearly all patients with severe TBI exhibit hypotension, even in the absence of hemorrhage. This is regarded as a result of both administration of sedation / analgesia and severe SIRS. SIRS is induced by trauma and increases endothelial permeability, favoring volume shift and volume loss into the "third space" [42]. This hypovolemia may lead to inadequate CPP and subsequent ICP increase [43].
According to a large retrospective study of 15,733 patients with isolated moderate to severe TBI, patients with systolic blood pressure (SBP) < 110 mmHg should be considered hypotensive [44]. Brain Trauma Foundation (BTF) guidelines [3] suggest that SBP ≥ 100 mmHg should be maintained for patients 50 to 69 years old or ≥ 110 mmHg for patients 15 to 49 or > 70 years old to decrease mortality and improve outcomes.
Strict monitoring of fluid balance is necessary in order to prevent hypovolemia – hypotension. Isotonic fluids should only be used and hypotonic fluids, such as 5% dextrose or 0.45% saline, should be strictly avoided. Systemic hypoosmolality (< 280 mOsm/L) should be aggressively reversed [45]. Regarding the type of fluids (crystalloids vs colloids), the optimal choice remains controversial. However, the SAFE study [46] involving 460 patients with TBI, compared fluid resuscitation with albumin or saline and concluded that the former may be harmful and should be avoided, as it was associated with higher mortality rates.
If mean arterial pressure (MAP) is > 110 mmHg and ICP > 20 mmHg, systemic BP should be carefully lowered in order not to decrease CPP significantly. Therefore, it is suggested to use short-acting titratable agents, such as labetalol and nicardipine [47]. The guidelines for ICH [48] recommend the use of antihypertensive drugs if SBP is > 150 mmHg as it has been associated with improvement in functional outcome, namely significantly better functional recovery based on the modified Rankin scale (mRS) and better physical and mental health–related quality of life based on the EQ-5D scale.
The optimal value—target for CPP is a matter of debate. The minimum CPP value required to prevent cerebral ischemia is generally acceptable at 50–60 mmHg [49]. However, two distinct approaches have developed with differing views on whether CPP should be maintained at a higher or lower level. The Rosner concept [50] advocates an increased MAP, aiming at a higher CPP value in order to maintain adequate CBF. In contrast, the Lund concept [51] advocates reducing intravascular resistance and hydrostatic pressure and reducing CBV, thereby increasing CBF and making a lower CPP acceptable. According to the last guidelines for TBI [3], the recommended target CPP value is between 60 and 70 mmHg, as it has been associated with improved survival and favorable outcomes. However, whether 60 or 70 mmHg is the minimum optimal CPP threshold is unclear and may depend upon the patient’s autoregulatory status. At the same time, aggressive efforts to maintain CPP > 70 mmHg with fluids and vasopressors should be avoided because of the risk of acute respiratory distress syndrome (ARDS). Moreover, excessive elevation of CPP favors edema formation by increasing capillary hydrostatic pressure across the BBB [52]. Therefore, it is worth noting that the optimal CPP depends on the particularities of each patient and should be individualized based on MMM. Advanced monitoring techniques such as PbtO2 and SjvO2 measurement, EEG and microdialysis may eventually allow clinicians to optimize CPP based on specific physiological circumstances in a particular patient at any given point in time.
Body positioning
The head of the bed should be elevated to 30° and the patient's head face midline so that internal jugular vein is not compressed and cerebral venous drainage is facilitated. Head elevation may reduce ICP without adversely affecting either CBF or CPP [53]. However, head elevation in excess of 45° should generally be avoided because paradoxical increases in ICP may occur in response to the excessive CPP reduction [54]. Important maneuvers that protect against ICP increases include reducing excessive flexion or rotation of the neck, avoiding restrictive neck taping, and minimizing stimuli that could induce cough and Valsalva responses, such as endotracheal suctioning [45].
Temperature control
The bundle of prophylactic measures to treat IH includes fever control. As it is known, elevated temperature affects ICP, by increasing cerebral metabolic demands and CBF [55]. It has been shown that patients with ICH, who develop a body temperature > 37.5 °C within the first 72 h, have significantly worse outcomes determined as Glasgow outcome scale (GOS) 1 or 2 [56]. In addition, in a later study of 110 TBI patients Stocchetti et al. demonstrated that fever within the first week was associated with increased ICP, significant neurologic impairment and prolonged ICU stay [57]. Due to the harmful effect of increased temperature on the cerebral parenchyma, it is recommended that it should not exceed 37 °C. To this end, early aggressive measures to control temperature in the patient with ABI should be implemented. These include intravenous and enteral antipyretic medications, control of room temperature, and cooling blankets or pads. A French study involving patients with septic shock showed that fever control using external cooling was safe and decreased vasopressor requirements and early mortality [58]. Regarding early hypothermia induction as a primary neuroprotective strategy in severe TBI patients, two recent randomized, multicenter clinical trials [59, 60] did not confirm its usefulness, given that prophylactic hypothermia was associated with poor outcomes (no difference on GOS at 6 months). Thus, the TBI guidelines [3] do not recommend early (within 2.5 h), short-term (48 h post-injury) prophylactic hypothermia to improve outcomes in patients with diffuse brain injury.
Glycemic control
Hyperglycemia is associated with increased mortality in patients with ABI [61, 62]. However, it remains unclear what are the optimal blood glucose (BG) values. Initially, van den Berghe et al. [63] showed that normal BG levels between 80 and 110 mg/dL were associated with decreased morbidity and mortality, decreased hospitalization and cost-effectiveness. However, these results were not confirmed in later studies [64–66]. Thus, the guidelines for the treatment of hyperglycemia in critically ill patients [67] suggest that BG < 100 mg/dL should be avoided during insulin infusion for patients with ABI. In addition, they suggest that BG ≥ 150 mg/dL triggers initiation of insulin therapy for most patients admitted to an ICU with the diagnoses of ischemic stroke, intraparenchymal hemorrhage, SAH, or TBI, titrated to achieve BG values absolutely < 180 mg/dL [67].
Seizure prophylaxis
Seizures can exacerbate IH by increasing cerebral metabolic rate of oxygen (CMRO2) and CBF. Patients with ABI are at increased risk of seizures because of a reduction of threshold for epileptic discharges by the underlying structural and functional injuries. The use of prophylactic antiepileptic treatment for the prevention of SBI was a topic of investigation for many years. In a randomized, double-blind study Temkin et al. [68] examined the role of phenytoin in the prevention of early and late post-traumatic seizures (PTS). The results showed a statistically significant difference in the rate of early PTS in phenytoin group compared with placebo group (3.6 vs 14.2%, P < 0.001). In contrast, there was no significant difference between the two groups in PTS rates from day 8 until the end of follow-up. The recent BTF guidelines [3] are in accordance with these results and do not recommend the prophylactic use of phenytoin or valproate for preventing late PTS. Phenytoin is recommended to decrease the incidence of early PTS (within 7 days of injury), when the overall benefit is felt to outweigh the complications associated with such treatment. Prophylactic antiepileptic therapy in other acute neurological conditions (e.g. spontaneous ICH [48], ischemic stroke [69]) is not recommended.
Acute interventions
Interventions performed in acute phase, in order to reduce ICP, can be categorized as medical or surgical (Table 6).
Hyperventilation
Hyperventilation is an effective and rapid method of treating IH. Reduction of PCO2 induces vasoconstriction of cerebral arterioles and a decrease in CBF, resulting in ICP reduction. The effect is almost immediate, but generally lasts less than 24 h, as the CSF pH rapidly equilibrates to the new PaCO2 level [49]. However, prolonged, aggressive hyperventilation may lead to a critical decrease in local cerebral perfusion and cerebral ischemia, potentially resulting in worsening of neurologic injury, particularly in the first 24 to 48 h [70, 71]. Therefore, hyperventilation may have a role as a temporizing measure for the reduction of elevated ICP. Meanwhile, SjvO2 or PbtO2 measurements can be used to monitor oxygen delivery. Finally, it is worth noting that hyperventilation should not be abruptly discontinued but should be tapered slowly over 4–6 h to avoid vasodilatation of cerebral arterioles and rebound increases in ICP [72].
Hyperosmolar therapy
Hyperosmolar therapy is the cornerstone of medical treatment of acute IH. The most commonly used medications are mannitol and hypertonic saline (HS). Osmotic agents reduce brain tissue volume by drawing free water out of brain tissue and into the systemic circulation, where it is then excreted by the kidneys [73]. The beneficial effect of hyperosmolar therapy requires an intact BBB. Otherwise, as in traumatic contusion, BBB disruption allows equilibration of molecules between blood and interstitial fluid of the brain. Thus, osmotic agents exert their effect largely by removing water from the remaining normal brain tissue [74].
Mannitol acts by increasing serum osmolality, resulting in an osmotic gradient from interstitial to intravascular space, reduction of cerebral edema and, consequently, ICP. Its strong osmotic force is due to its high reflection coefficient (σ = 0, 9). Mannitol also acts by other mechanisms, such as induction of reflex cerebral arteriolar vasoconstriction, improvement in blood rheology, reduction of CSF formation [75], and free radicals scavenging [76]. Its effect is dose-dependent [75], since a positive correlation has been demonstrated between dose and magnitude of ICP reduction. The recommended ICP lowering dose of mannitol (usually 20%) is 0.25 to 1 g/kg every 6 h [77, 78], although doses < 0.5 g/kg are usually considered less effective. Serum osmolality should be maintained between 310 and 320 mOsm/l, while some researchers advocate that even higher levels can be cautiously tolerated [74, 79]. Mannitol is excreted entirely in urine and there is a risk of acute tubular necrosis if serum osmolality exceeds these recommended levels. Other adverse effects of mannitol include hypotension, electrolyte disturbances (hyperkalemia, hypokalemia, hypomagnesemia, hypophosphatemia) and rebound cerebral edema after prolonged use. Mannitol is contraindicated in patients with renal failure [80] due to the risk of osmotic nephrosis [81] and possible pulmonary edema and heart failure.
HS is used alternatively to mannitol. Compared to mannitol, it has a higher reflection coefficient (1.0 vs. 0.9, respectively). Therefore, HS is less able to cross the BBB and may have a stronger osmotic action. Thus, it reduces ICP by decreasing cerebral edema while at the same time increases CPP by improving MAP. Other mechanisms of action include induction of reflex cerebral arteriolar vasoconstriction, improved deformability of erythrocytes with enhanced microcirculation, and an anti-inflammatory effect due to reduced adhesion of polymononuclear cells in the cerebral microvasculature [82–84]. In literature, concentrations of HS used to treat IH, were ranging from 3% to 23.4%. Bolus doses are usually administered in response to a measured ICP and may be repeated as needed until either the ICP is in an acceptable range or serum sodium concentrations have risen above normal (> 145–155 mEq/L) [85]. Possible adverse effects of HS include rebound cerebral edema, electrolyte disturbances (hypokalemia), congestive heart failure, renal failure, hyperchloremic metabolic acidosis, phlebitis, transient hypotension, hemolysis, osmotic demyelination, subarachnoid bleeding, seizures and muscle twitching [86].
Clinical evidence demonstrates the efficacy of mannitol and HS for acute IH in the setting of TBI, edema secondary to tumor, ICH, SAH, and stroke [87]. Kamel et al. [88] performed a meta-analysis of 5 randomized controlled trials (RCT), comparing the above osmotic agents in the treatment of IH from a variety of causes. HS appeared to have greater efficacy in managing elevated ICP, but the effect on clinical outcomes was not assessed. Mortazavi et al. [89] reached the same conclusion, noting the absence of clear neurological outcome benefit. A more recent meta-analysis [90], including 7 RCT and 191 patients, also highlighted the superiority of HS when compared to mannitol in the treatment of elevated ICP. Regarding the 6-month mortality, no difference was observed, with limited adverse reactions reported. Conversely, Cochrane analysis [91] concluded that mannitol treatment for IH may have a detrimental effect on mortality when compared to HS. It is worth noting that, in a study by Sakellaridis et al. [92], there was no significant difference in the extent of reduction of ICP or duration of action between the two medications. Therefore, in recent years, the characterization of mannitol as a "gold standard" is controversial and the role of HS in IH [93] is upgraded. Hence, the last TBI guidelines in contrast with the previous ones advocate that there are insufficient evidence about effects on clinical outcomes to support use of any specific hyperosmolar agent [3]. In particular, there is no difference on GOS at 6 months and mortality.
Sedation and analgesia
Sedation and analgesia are an integral part of medical treatment of IH. Patient-ventilator dyssynchrony and agitation increases intrathoracic pressure, causing decreased thoracic venous return, which increases CBV and thus ICP. In addition, agitation contributes to increased ICP by elevating systemic BP especially in patients on the extreme of autoregulatory curve [47]. Under these conditions, CMRO2 and brain tissue oxygen demands are increased, leading to vasodilation and a consequent increase in CBF, CBV and ICP.
Propofol is one of the preferred drugs for sedation in patients with IH, although there is no evidence that it improves mortality or 6-month outcomes [3]. It has a relatively quick onset and offset of action, allowing for more rapid assessment of the neurological status once stopped. Conversely, reduced clearance of benzodiazepines (e.g. midazolam) after prolonged infusion can significantly delay arousal, especially in the elderly [94]. Additional benefits of propofol include increased seizure threshold and a better quality of sedation when compared to midazolam [95]. However, one should be aware of its hemodynamic effects, as it can cause a reduction in MAP, which may require fluid resuscitation or even vasopressors to maintain the CPP. Finally, propofol can induce the lethal “propofol-infusion syndrome,” characterized by lactic acidosis, rhabdomyolysis, renal insufficiency/failure, arrhythmias, and cardiac failure [96].
Given that pain is often a contributor to elevated ICP, especially in TBI patients, coadministration of fentanyl can work synergistically with propofol to reach the sedation goal. However, paradoxical rises in ICP may occur following a bolus injection of fentanyl, due to the transient MAP lowering and the reflex cerebral vasodilation to maintain CBF [47]. Remifentanil has more favorable pharmacokinetic properties and in particular lower volume of distribution and very short half-life. ICP may decrease without substantial changes of the CPP, but the exact effect on cerebral hemodynamics remains to be elucidated [97–99].
Neuromuscular blocking agents (e.g. vecuronium, cisatracurium) could be useful in the control of refractory IH in specific conditions such as very severe agitation, shivering and difficult ventilation [94]. However, few studies exist to support this as a routine practice.
Barbiturates
Barbiturate therapy is supported by the literature [100–102] on failure of the other conservative measures of treatment of IH and includes the use of pentobarbital or thiopental. Barbiturates suppress brain metabolism, reduce CBF, and improve oxygenation of the cerebral tissue. Marshall et al. [100] in a study of 55 patients with severe TBI and refractory IH, treated with barbiturates, showed that 40% of them survived at discharge and 68% of survivors had good functional outcome (GOS 4 or 5 at ≥ 1 year). Later, Cochrane analysis [103] did not reach the same conclusion, noting that barbiturates may reduce ICP, but there is no difference in death or disability, measured using the GOS, in patients with acute ΤΒΙ. However, according to the BTF guidelines [3], high-dose barbiturate administration is recommended to control elevated ICP refractory to maximum standard medical and surgical treatment. They also emphasize that hemodynamic stability is essential before and during therapy.
Due to their long duration of action, barbiturates limit the ability to perform frequent neurological assessments. This limitation warrants the need for cEEG monitoring. Other important adverse effects of barbiturates include hypotension, myocardial and respiratory depression, infections, liver and renal dysfunction, thrombocytopenia, metabolic acidosis and gastric stasis [29].
Therapeutic hypothermia
Hypothermia decreases cerebral metabolism and may reduce CBF and ICP. However, to date literature data are inadequate to support its neuroprotective effect. Indeed, favorable neurologic outcome and reduced mortality using therapeutic hypothermia have only been demonstrated after ventricular fibrillation and ventricular tachycardia cardiac arrest [104, 105]. Recently, a study of Nielsen et al. [106] which involved patients with out-of-hospital cardiac arrest showed different results. In particular, there was no difference in neurological outcome or survival, at a targeted temperature of 33 °C vs 36 °C. Regarding the treatment of TBI patients, a systematic review and meta-analysis [107] showed that therapeutic hypothermia may be of benefit in reducing rates of death, vegetative state, and long-term disability. However, there remains a need for more, high quality RCT in this patient population. Lastly, Andrews et al. [108] compared therapeutic hypothermia (32–35 ºC) plus standard care, with standard care alone, in 387 patients with ICP > 20 mmHg after TBI. They concluded that hypothermia does not improve long-term outcome, determined as extended GOS (GOS-E) 1 to 4 at 6 months, although it appears to reduce ICP and PbtO2 [109].
Serious adverse effects are associated with hypothermia, especially if used for a prolonged period of time. So, hypokalemia, atrial and ventricular arrhythmias, hypotension, coagulopathy may occur, while risk of infection, particularly ventilator associated and nosocomial pneumonia, is elevated.
Corticosteroids
The role of corticosteroids in the treatment of elevated ICP is limited. MRC CRASH trial [110], which included approximately 10,000 TBI patients, evaluated methylprednisolone versus placebo. The steroid treated patients had a significantly higher mortality compared with the placebo group (25.7 vs. 22.3%, P = 0.0001). Recently, the BTF guidelines [3] do not recommend the use of steroids for improving outcome or reducing ICP. Similarly, corticosteroids are not recommended for the treatment of cerebral edema and increased ICP complicating ischemic stroke, because of a lack of evidence of efficacy and the potential to increase the risk of infectious complications [69]. Except infections, corticosteroids also carry additional risks, such as hyperglycemia, impaired wound healing, muscle catabolism, and psychosis. Finally, steroid use is only indicated for reducing ICP in abscesses or neoplasms associated with vasogenic edema [45].
Resection of mass lesions
Based on specific indications and conditions, resection of mass lesions may immediately lower ICP. The large, international, multicenter, randomized STICH II trial [111] included 601 patients with spontaneous supratentorial lobar ICH without intraventricular hemorrhage or hydrocephalus, treating them either with early surgery or conservatively. It showed that early surgery did not increase the rate of death or disability at 6 months and might have a small but clinically relevant survival advantage for patients with spontaneous superficial ICH without intraventricular hemorrhage. Recent guidelines for spontaneous ICH [48] recommend surgical removal of the hemorrhage as soon as possible for patients with cerebellar hemorrhage who are deteriorating neurologically or who have brainstem compression and/or hydrocephalus from ventricular obstruction. In addition, they emphasize that supratentorial hematoma evacuation in deteriorating patients might be considered as a life-saving measure [48]. Other acceptable indications for removal of a mass lesion include evacuation of subdural and epidural hematomas, brain abscesses, and resection of brain tumors. Recently, RCT STITCH (Trauma) [112] compared early surgery with the initial conservative treatment in 170 patients with traumatic ICH. It concluded that early surgery was associated with significantly fewer deaths in the first 6 months, compared with conservative treatment (15 vs 33%, P = 0.006). However, further well-designed trials are required to assess whether this encouraging signal can be confirmed in TBI patients.
Decompressive craniectomy (DC)
DC, which consists in creating a large bone window, provides a larger reserve to the swollen brain, negating the Monroe-Kellie doctrine of fixed intracranial volume. In this way, DC alone lowers ICP by 15% and with dural opening by 70%. The main indication for DC is massive hemispheric ischemic stroke. A meta-analysis [113] of 3 RCT (DECIMAL, HAMLET, DESTINY) showed that early (< 48 h) decompressive hemicraniectomy in massive ischemic stroke significantly improved survival (78 vs 29%) and resulted in better neurological outcomes, measured using mRS at 1 year, compared to conservative therapy. In literature, two more recent reviews and meta-analyses [114, 115] reached the same conclusions. The guidelines for ischemic stroke [69] note that decompressive surgery may be the only effective option for very severe cases but decision making should include patient-centered preferences. In patients with spontaneous ICH, DC with or without hematoma evacuation might reduce mortality for patients with supratentorial ICH who are in a coma, have large hematomas with significant midline shift, or have elevated ICP refractory to medical management [48]. Regarding TBI, the recent BTF guidelines [3] do not recommend bifrontal DC to improve outcomes, as measured by the GOS-E at 6 months post-injury, although this procedure has been demonstrated to reduce ICP and to minimize days in the ICU [3]. These guidelines were based on DECRA trial [116], which involved 155 patients with severe diffuse TBI and refractory IH. In this trial, early bifrontotemporoparietal DC decreased ICP and the length of stay in the ICU, but was associated with more unfavorable outcomes indicated by the GOS-E at 6 months after the injury. However, the study faced intense criticism and some authorities claimed that it should have no influence on clinical practice. Later, RESCUEicp trial [117] showed that DC had more favorable results on outcome. 408 patients with TBI and refractory IH (ICP > 25 mmHg), underwent DC or received ongoing medical care were randomized. At 6 months, DC resulted in lower mortality (26.9 vs 48.9%) but higher rates of vegetative state and severe disability than medical care. However, more studies are required to determine which patients will benefit with mortality reduction but minimize risk for vegetative state and poor functional outcomes [118].
Cerebrospinal fluid drainage
CSF drainage, even 5–10 ml, may lead to a significant ICP reduction in patients with a low intracranial compliance. This can be accomplished with an external ventricular drainage device (EVD), lumbar drain, or serial lumbar punctures. CSF removal via an EVD is preferred over the use of a lumbar drain due to the risk of transtentorial herniation. EVD placement with continuous drainage of CSF for acute hydrocephalus treatment is also supported by the guidelines for TBI [3], spontaneous ICH [48] and ischemic stroke [69]. Except obstructive hydrocephalus, other indications of CSF drainage include diffuse cerebral edema, or mass effect due to a space-occupying lesion. Finally, it should be noted that the major risks of EVD placement include infection (especially ventriculitis and meningitis) and hemorrhage [119].
Other treatment options
Other options to manage raised ICP are under investigation, but they have no place yet in clinical practice. The use of hyperbaric oxygen therapy in TBI has been shown to reduce ICP, may reduce the risk of death and improve GCS, but there is little evidence that survivors have a good outcome. Therefore, its application to these patients cannot be supported [120].
Progesterone has been shown to have potentially protective properties since it may slow the development of malignant cerebral edema and ICP elevation [121, 122]. However, two recent large Phase III RCT (SYNAPSE [123], PROTECT III [124]) in TBI treatment did not confirm any clinical benefit of progesterone in GOS at 6 months.
Ketamine has been previously reported to cause an increase in ICP. However, current literature support that it does not increase ICP in severe TBI patients that are sedated and ventilated, and in fact may lower it in selected cases [125, 126].
Deciphering what is new in the literature
Based on all the above, we have distinguished and listed below what is new in the management of IH:
The recommended ICP (treating ICP > 22 mmHg) and CPP (between 60 and 70 mmHg) thresholds [3] have been revised from previous guidelines [41].
Patients with SBP < 110 mmHg should be considered hypotensive [3], in contrast to previous guidelines [41].
Prophylactic hypothermia has not been established as a primary neuroprotective strategy, according to additional recent data [60].
Τhere are insufficient evidence about effects on clinical outcomes to support use of any specific hyperosmolar agent [3].
Although therapeutic hypothermia reduces ICP [109], it does not appear to improve long-term outcome [108].
Except spontaneous ICH [111], encouraging recent data emerge on the positive effect of early surgery on mortality in patients with traumatic ICH [112].
In RESCUEicp trial [117], DC for IH refractory to conventional therapy decreased mortality but did not improve functional outcomes in TBI patients.
Conclusion
Despite conflicting evidence, ICP monitoring remains the cornerstone of IH treatment, contributing to reduce mortality [2, 127]. This is supported by new observational studies and meta-analyses, although ICP monitoring itself does not seem to affect outcome [4]. The intensivists can optimize IH treatment by modifying factors such as vasoreactivity and CBF, blood/brain osmotic gradient, CMRO2 and issues of intracranial compliance. To date, many interventions, such as surgical decompression, hypothermia, barbiturates and osmotic agents, have been shown to be effective in reducing ICP but with controversial effects on outcome (Table 6) [127, 128]. The risks and benefits of any medical and surgical intervention must be carefully evaluated. An optimal therapeutic strategy is considered the step-by-step escalation of available interventions [29, 129], tailored for each patient. Prophylactic measures are common for almost all patients with IH, while acute interventions, in order to reduce ICP, should be individualized (Fig. 3).
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Le Roux P, Menon DK, Citerio G, Vespa P, Bader MK, Brophy GM, Diringer MN, Stocchetti N, Videtta W, Armonda R, Badjatia N, Böesel J, Chesnut R, Chou S, Claassen J, Czosnyka M, De Georgia M, Figaji A, Fugate J, Helbok R, Horowitz D, Hutchinson P, Kumar M, McNett M, Miller C, Naidech A, Oddo M, Olson D, O'Phelan K, Provencio JJ, Puppo C, Riker R, Robertson C, Schmidt M, Taccone F. Consensus summary statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Neurocrit Care. 2014; 21(2):1–26. [DOI] [PMC free article] [PubMed]
- 2.Güiza F, Depreitere B, Piper I, Citerio G, Chambers I, Jones PA, Lo TY, Enblad P, Nillson P, Feyen B, Jorens P, Maas A, Schuhmann MU, Donald R, Moss L, Van den Berghe G, Meyfroidt G. Visualizing the pressure and time burden of intracranial hypertension in adult and paediatric traumatic brain injury. Intensive Care Med. 2015;41(6):1067–1076. doi: 10.1007/s00134-015-3806-1. [DOI] [PubMed] [Google Scholar]
- 3.Carney N, Totten AM, OʼReilly C, Ullman JS, Hawryluk GW, Bell MJ, Bratton SL, Chesnut R, Harris OA, Kissoon N, Rubiano AM, Shutter L, Tasker RC, Vavilala MS, Wilberger J, Wright DW, Ghajar J. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery. 2017; 80(1):6–15. [DOI] [PubMed]
- 4.Chesnut RM, Temkin N, Carney N, Dikmen S, Rondina C, Videtta W, Petroni G, Lujan S, Pridgeon J, Barber J, Machamer J, Chaddock K, Celix J, Cherner M, Hendrix T. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. 2012;367(26):2471–2481. doi: 10.1056/NEJMoa1207363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agrawal D, Raghavendran K, Schaubel DE, Mishra MC, Rajajee V. A propensity score analysis of the impact of invasive intracranial pressure monitoring on outcomes after severe traumatic brain injury. J Neurotrauma. 2016;33(9):853–858. doi: 10.1089/neu.2015.4015. [DOI] [PubMed] [Google Scholar]
- 6.Yuan Q, Wu X, Cheng H, Yang C, Wang Y, Wang E, Qiu B, Fei Z, Lan Q, Wu S, Jiang Y, Feng H, Liu J, Liu K, Zhang F, Jiang R, Zhang J, Tu Y, Wu X, Zhou L, Hu J. Is Intracranial Pressure Monitoring of Patients with Diffuse Traumatic Brain Injury Valuable? An Observational Multicenter Study Neurosurgery. 2016;78(3):361–368. doi: 10.1227/NEU.0000000000001050. [DOI] [PubMed] [Google Scholar]
- 7.You W, Feng J, Tang Q, Cao J, Wang L, Lei J, Mao Q, Gao G, Jiang J. Intraventricular intracranial pressure monitoring improves the outcome of older adults with severe traumatic brain injury: An observational, prospective study. BMC Anesthesiol. 2016;16(1):1–8. doi: 10.1186/s12871-016-0199-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ristic A, Sutter R, Steiner L. Current neuromonitoring techniques in critical care. J Neuroanaesth Crit Care. 2015;02(02):097–103. [Google Scholar]
- 9.Bouzat P, Oddo M, Payen JF. Transcranial Doppler after traumatic brain injury: is there a role? Curr Opin Crit Care. 2014;20(2):153–160. doi: 10.1097/MCC.0000000000000071. [DOI] [PubMed] [Google Scholar]
- 10.Abraham M, Singhal V. Intracranial pressure monitoring. J Neuroanaesthesiol Crit Care. 2015;2:193–203. [Google Scholar]
- 11.Bellner J, Romner B, Reinstrup P, Kristiansson KA, Ryding E, Brandt L. Transcranial Doppler sonography pulsatility index (PI) reflects intracranial pressure (ICP) Surg Neurol. 2004;62(1):45–51. doi: 10.1016/j.surneu.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Wakerley BR, Kusuma Y, Yeo LL, Liang S, Kumar K, Sharma AK, Sharma VK. Usefulness of transcranial Doppler-derived cerebral hemodynamic parameters in the noninvasive assessment of intracranial pressure. J Neuroimaging. 2015;25(1):111–116. doi: 10.1111/jon.12100. [DOI] [PubMed] [Google Scholar]
- 13.Nag DS, Sahu S, Swain A, Kant S. Intracranial pressure monitoring: Gold standard and recent innovations. World J Clin Cases. 2019;7(13):1535–1553. doi: 10.12998/wjcc.v7.i13.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajajee V, Vanaman M, Fletcher JJ, Jacobs TL. Optic nerve ultrasound for the detection of raised intracranial pressure. Neurocrit Care. 2011;15(3):506–515. doi: 10.1007/s12028-011-9606-8. [DOI] [PubMed] [Google Scholar]
- 15.Dubourg J, Javouhey E, Geeraerts T, Messerer M, Kassai B. Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: a systematic review and meta-analysis. Intensive Care Med. 2011;37(7):1059–1068. doi: 10.1007/s00134-011-2224-2. [DOI] [PubMed] [Google Scholar]
- 16.Reid A, Marchbanks RJ, Burge DM, Martin AM, Bateman DE, Pickard JD, Brightwell AP. The relationship between intracranial pressure and tympanic membrane displacement. Br J Audiol. 1990;24(2):123–129. doi: 10.3109/03005369009077853. [DOI] [PubMed] [Google Scholar]
- 17.Al-Mufti F, Lander M, Smith B, Morris NA, Nuoman R, Gupta R, Lissauer ME, Gupta G, Lee K. Multimodality Monitoring in Neurocritical Care: Decision-Making Utilizing Direct And Indirect Surrogate Markers. J Intensive Care Med. 2019;34(6):449–463. doi: 10.1177/0885066618788022. [DOI] [PubMed] [Google Scholar]
- 18.Oddo M, Bösel J; Participants in the International Multidisciplinary Consensus Conference on Multimodality Monitoring. Monitoring of brain and systemic oxygenation in neurocritical care patients. Neurocrit Care. 2014;21 Suppl 2:S103–20. [DOI] [PubMed]
- 19.Adamides AA, Rosenfeldt FL, Winter CD, Pratt NM, Tippett NJ, Lewis PM, Bailey MJ, Cooper DJ, Rosenfeld JV. Brain tissue lactate elevations predict episodes of intracranial hypertension in patients with traumatic brain injury. J Am Coll Surg. 2009;209(4):531–539. doi: 10.1016/j.jamcollsurg.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 20.Hutchinson P, O'Phelan K; Participants in the International Multidisciplinary Consensus Conference on Multimodality Monitoring. International multidisciplinary consensus conference on multimodality monitoring: cerebral metabolism. Neurocrit Care. 2014;21 Suppl 2:S148–58. [DOI] [PubMed]
- 21.Tasneem N, Samaniego EA, Pieper C, Leira EC, Adams HP, Hasan D, Ortega-Gutierrez S. Brain Multimodality Monitoring: A New Tool in Neurocritical Care of Comatose Patients. Crit Care Res Pract. 2017;2017:6097265. doi: 10.1155/2017/6097265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurtz P, Hanafy KA, Claassen J. Continuous EEG monitoring: is it ready for prime time? Curr Opin Crit Care. 2009;15(2):99–109. doi: 10.1097/MCC.0b013e3283294947. [DOI] [PubMed] [Google Scholar]
- 23.Newey CR, Sarwal A, Hantus S. Continuous electroencephalography (cEEG) changes precede clinical changes in a case of progressive cerebral edema. Neurocrit Care. 2013;18(2):261–265. doi: 10.1007/s12028-011-9650-4. [DOI] [PubMed] [Google Scholar]
- 24.Foreman B, Claassen J. Quantitative EEG for the detection of brain ischemia. Crit Care. 2012;16(2):216. doi: 10.1186/cc11230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Putten MJ, Hofmeijer J. EEG monitoring in cerebral ischemia: Basic concepts and clinical applications. J Clin Neurophysiol. 2016;33(3):203–210. doi: 10.1097/WNP.0000000000000272. [DOI] [PubMed] [Google Scholar]
- 26.Peacock SH, Tomlinson AD. Multimodal Neuromonitoring in Neurocritical Care. AACN Adv Crit Care. 2018;29(2):183–194. doi: 10.4037/aacnacc2018632. [DOI] [PubMed] [Google Scholar]
- 27.Kochanek PM, Jackson TC, Ferguson NM, Carlson SW, Simon DW, Brockman EC, Ji J, Bayır H, Poloyac SM, Wagner AK, Kline AE, Empey PE, Clark RS, Jackson EK, Dixon CE. Emerging therapies in traumatic brain injury. Semin Neurol. 2015;35:83–100. doi: 10.1055/s-0035-1544237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pearn ML, Niesman IR, Egawa J, Sawada A, Almenar-Queralt A, Shah SB, Duckworth JL, Head BP. Pathophysiology associated with traumatic brain injury: current treatments and potential novel therapeutics. Cell Mol Neurobiol. 2017;37(4):571–585. doi: 10.1007/s10571-016-0400-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stocchetti N, Maas AI. Traumatic intracranial hypertension. N Engl J Med. 2014;370:2121–2130. doi: 10.1056/NEJMra1208708. [DOI] [PubMed] [Google Scholar]
- 30.McDonald SJ, Sun M, Agoston DV, Shultz SR. The effect of concomitant peripheral injury on traumatic brain injury pathobiology and outcome. J Neuroinflammation. 2016;13:90. doi: 10.1186/s12974-016-0555-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corps KN, Roth TL, McGavern DB. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 2015;72:355–362. doi: 10.1001/jamaneurol.2014.3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vespa P, Tubi M, Claassen J, Buitrago-Blanco M, McArthur D, Velazquez AG, Tu B, Prins M, Nuwer M. Metabolic crisis occurs with seizures and periodic discharges after brain trauma. Ann Neurol. 2016;79:579–590. doi: 10.1002/ana.24606. [DOI] [PubMed] [Google Scholar]
- 33.Patel HC, Bouamra O, Woodford M, King AT, Yates DW, Lecky FE; Trauma Audit and Research Network. Trends in head injury outcome from to 2003 and the effect of neurosurgical care: An observational study. Lancet. 1989;2005(366):1538–1544. doi: 10.1016/S0140-6736(05)67626-X. [DOI] [PubMed] [Google Scholar]
- 34.Lee K, Mayer SA. Management of increased intracranial pressure. In: Kiwon L, editor. The NeuroICU Book. 1. New York, NY: McGraw-Hill; 2012. pp. 213–225. [Google Scholar]
- 35.Lapinsky SE, Posadas-Colleja JG, McCullagh I. Clinical review: ventilatory strategies for obstetric, brain-injured and obese patients. Crit Care. 2009;13(2):206. doi: 10.1186/cc7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Videtta W, Villarejo F, Cohen M, Domeniconi G, Santa Cruz R, Pinillos O, Rios F, Maskin B. Effects of positive end-expiratory pressure on intracranial pressure and cerebral perfusion pressure. Acta Neurochir Suppl. 2002;81:93–97. doi: 10.1007/978-3-7091-6738-0_25. [DOI] [PubMed] [Google Scholar]
- 37.Huynh T, Messer M, Sing RF, Miles W, Jacobs DG, Thomason MH. Positive end-expiratory pressure alters intracranial and cerebral perfusion pressure in severe traumatic brain injury. J Trauma. 2002;53:488. doi: 10.1097/00005373-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 38.Caricato A, Conti G, Della Corte F, Mancino A, Santilli F, Sandroni C, Proietti R, Antonelli M. Effects of PEEP on the intracranial system of patients with head injury and subarachnoid hemorrhage: the role of respiratory system compliance. J Trauma. 2005;58(3):571–576. doi: 10.1097/01.ta.0000152806.19198.db. [DOI] [PubMed] [Google Scholar]
- 39.Mascia L, Grasso S, Fiore T, Bruno F, Berardino M, Ducati A. Cerebro-pulmonary interactions during the application of low levels of positive end-expiratory pressure. Intensive Care Med. 2005;31(3):373–379. doi: 10.1007/s00134-004-2491-2. [DOI] [PubMed] [Google Scholar]
- 40.Oddo M, Citerio G. ARDS in the brain-injured patient: what’s different? Intensive Care Med. 2016;42(5):790–793. doi: 10.1007/s00134-016-4298-3. [DOI] [PubMed] [Google Scholar]
- 41.Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care, AANS/CNS, Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R, Manley GT, Nemecek A, Newell DW, Rosenthal G, Schouten J, Shutter L, Timmons SD, Ullman JS, Videtta W, Wilberger JE, Wright DW. Guidelines for the management of severe traumatic brain injury. IX. Cerebral perfusion thresholds. J Neurotrauma. 2007; 24 Suppl 1:S59–64. [DOI] [PubMed]
- 42.Stover JF, Stocker R. Intensive care treatment options of elevated intracranial pressure following severe traumatic brain injury. In: Oestern HJ, Trentz O, Uranues S, editors. Head, thoracic, abdominal, and vascular, injuries. 1. Berlin: Springer-Verlag; 2011. pp. 93–152. [Google Scholar]
- 43.Rosner MJ, Rosner SD, Johnson AH. Cerebral perfusion pressure: Management protocol and clinical results. J Neurosurg. 1995;83(6):949–962. doi: 10.3171/jns.1995.83.6.0949. [DOI] [PubMed] [Google Scholar]
- 44.Berry C, Ley EJ, Bukur M, Malinoski D, Margulies DR, Mirocha J, Salim A. Redefining hypotension in traumatic brain injury. Injury. 2012;43(11):1833–1837. doi: 10.1016/j.injury.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 45.Sadoughi A, Rybinnik I, Cohen R. Measurement and Management of Increased Intracranial Pressure. Open Crit Care Med J. 2013;6:56–65. [Google Scholar]
- 46.SAFE Study Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group; Australian Red Cross Blood Service; George Institute for International Health, Myburgh J, Cooper DJ, Finfer S, Bellomo R, Norton R, Bishop N, Kai Lo S, Vallance S. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007; 357:874. [DOI] [PubMed]
- 47.Ragland J, Lee K. Critical Care Management and Monitoring of Intracranial Pressure. J Neurocritical Care. 2016;9(2):105–112. [Google Scholar]
- 48.Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, Fung GL, Goldstein JN, Macdonald RL, Mitchell PH, Scott PA, Selim MH, Woo D; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2015; 46(7):2032–60. [DOI] [PubMed]
- 49.Ropper AH. Management of intracranial hypertension and mass effect. In: Ropper AH, editor. Neurological and Neurosurgical Intensive Care. 4. Charlottesville, VA: Lippincott Williams & Wilkins; 2004. pp. 26–51. [Google Scholar]
- 50.Rosner MJ, Becker DP. Origin and evolution of plateau waves. Experimental observations and a theoretical model. J Neurosurg. 1984; 60(2):312–24. [DOI] [PubMed]
- 51.Grande PO. The “Lund Concept” for the treatment of severe head trauma–physiological principles and clinical application. Intensive Care Med. 2006;32:1475–1484. doi: 10.1007/s00134-006-0294-3. [DOI] [PubMed] [Google Scholar]
- 52.Nordström CH. Physiological and biochemical principles underlying volume-targeted therapy - The “lund concept”. Neurocrit Care. 2005;2(1):83–95. doi: 10.1385/NCC:2:1:083. [DOI] [PubMed] [Google Scholar]
- 53.Feldman Z, Kanter M, Robertson C, Contant CF, Hayes C, Sheinberg MA, Villareal CA, Narayan RK, Grossman RG. Effect of head elevation on intracranial pressure, cerebral perfusion pressure, and cerebral blood flow in head injured patients. J Neurosurg. 1992;76(2):207–211. doi: 10.3171/jns.1992.76.2.0207. [DOI] [PubMed] [Google Scholar]
- 54.Moraine JJ, Berré J, Mélot C. Is cerebral perfusion pressure a major determinant of cerebral blood flow during head elevation in comatose patients with severe intracranial lesions? J Neurosurg. 2000;92(4):606–614. doi: 10.3171/jns.2000.92.4.0606. [DOI] [PubMed] [Google Scholar]
- 55.Rossi S, Roncati Zanier E, Mauri I, Columbo A, Stocchetti N. Brain temperature, body core temperature, and intracranial pressure in acute cerebral damage. J Neurol Neurosurg Psychiatry. 2001;71(4):448–454. doi: 10.1136/jnnp.71.4.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwarz S, Hafner K, Aschoff A, Schwab S. Incidence and prognostic significance of fever following intracerebral hemorrhage. Neurology. 2000;54:354–361. doi: 10.1212/wnl.54.2.354. [DOI] [PubMed] [Google Scholar]
- 57.Stocchetti N, Rossi S, Zanier ER, Colombo A, Beretta L, Citerio G. Pyrexia in head-injured patients admitted to intensive care. Intensive Care Med. 2002;28(11):1555–1562. doi: 10.1007/s00134-002-1513-1. [DOI] [PubMed] [Google Scholar]
- 58.Schortgen F, Clabault K, Katsahian S, Devaquet J, Mercat A, Deye N, Dellamonica J, Bouadma L, Cook F, Beji O, Brun-Buisson C, Lemaire F, Brochard L. Fever control using external cooling in septic shock: A randomized controlled trial. Am J Respir Crit Care Med. 2012;185(10):1088–1095. doi: 10.1164/rccm.201110-1820OC. [DOI] [PubMed] [Google Scholar]
- 59.Clifton GL, Valadka A, Zygun D, Coffey CS, Drever P, Fourwinds S, Janis LS, Wilde E, Taylor P, Harshman K, Conley A, Puccio A, Levin HS, McCauley SR, Bucholz RD, Smith KR, Schmidt JH, Scott JN, Yonas H, Okonkwo DO. Very early hypothermia induction in patients with severe brain injury (the National Acute Brain Injury Study: Hypothermia II): A randomised trial. Lancet Neurol. 2011;10(2):131–139. doi: 10.1016/S1474-4422(10)70300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cooper DJ, Nichol AD, Bailey M, Bernard S, Cameron PA, Pili-Floury S, Forbes A, Gantner D, Higgins AM, Huet O, Kasza J, Murray L, Newby L, Presneill JJ, Rashford S, Rosenfeld JV, Stephenson M, Vallance S, Varma D, Webb SAR, Trapani T, McArthur C; POLAR Trial Investigators and the ANZICS Clinical Trials Group. Effect of Early Sustained Prophylactic Hypothermia on Neurologic Outcomes Among Patients With Severe Traumatic Brain Injury: The POLAR Randomized Clinical Trial. JAMA. 2018; 320(21):2211–20. [DOI] [PMC free article] [PubMed]
- 61.Jeremitsky E, Omert LA, Dunham CM, Wilberger J, Rodriguez A. The impact of hyperglycemia on patients with severe brain injury. J Trauma. 2005;58(1):47–50. doi: 10.1097/01.ta.0000135158.42242.b1. [DOI] [PubMed] [Google Scholar]
- 62.Godoy DA, Piñero GR, Svampa S, Papa F, Di Napoli M. Hyperglycemia and short-term outcome in patients with spontaneous intracerebral hemorrhage. Neurocrit Care. 2008;9(2):217–229. doi: 10.1007/s12028-008-9063-1. [DOI] [PubMed] [Google Scholar]
- 63.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 64.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 65.Van Den Berghe G, Schoonheydt K, Becx P, Bruyninckx F, Wouters PJ. Insulin therapy protects the central and peripheral nervous system of intensive care patients. Neurology. 2005;64(8):1348–1353. doi: 10.1212/01.WNL.0000158442.08857.FC. [DOI] [PubMed] [Google Scholar]
- 66.Bilotta F, Caramia R, Cernak I, Paoloni FP, Doronzio A, Cuzzone V, Santoro A, Rosa G. Intensive insulin therapy after severe traumatic brain injury: A randomized clinical trial. Neurocrit Care. 2008;9(2):159–166. doi: 10.1007/s12028-008-9084-9. [DOI] [PubMed] [Google Scholar]
- 67.Jacobi J, Bircher N, Krinsley J, Agus M, Braithwaite SS, Deutschman C, Freire AX, Geehan D, Kohl B, Nasraway SA, Rigby M, Sands K, Schallom L, Taylor B, Umpierrez G, Mazuski J, Schunemann H. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med. 2012;40(12):3251–3276. doi: 10.1097/CCM.0b013e3182653269. [DOI] [PubMed] [Google Scholar]
- 68.Temkin NR, Dikmen SS, Wilensky AJ, Keihm J, Chabal S, Winn HR. A randomized, double-blind study of phenytoin for the prevention of post-traumatic seizures. N Engl J Med. 1990;323(8):497–502. doi: 10.1056/NEJM199008233230801. [DOI] [PubMed] [Google Scholar]
- 69.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL; American Heart Association Stroke Council. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018; 49(3):e46-e110. [DOI] [PubMed]
- 70.Stocchetti N, Maas AIR, Chieregato A, Van Der Plas AA. Hyperventilation in head injury: A review. Chest. 2005;127(5):1812–1827. doi: 10.1378/chest.127.5.1812. [DOI] [PubMed] [Google Scholar]
- 71.Godoy DA, Seifi A, Garza D, Lubillo-Montenegro S, Murillo-Cabezas F. Hyperventilation therapy for control of posttraumatic intracranial hypertension. Front Neurol. 2017;8:250. doi: 10.3389/fneur.2017.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mayer SA, Chong JY. Critical Care Management of Increased Intracranial Pressure. J Intensive Care Med. 2002;17(2):55–67. [Google Scholar]
- 73.Paczynski RP. Osmotherapy: Basic concepts and controversies. Crit Care Clin. 1997;13(1):105–129. doi: 10.1016/s0749-0704(05)70298-0. [DOI] [PubMed] [Google Scholar]
- 74.Ropper AH. Hyperosmolar therapy for raised intracranial pressure. N Engl J Med. 2012;367(8):746–752. doi: 10.1056/NEJMct1206321. [DOI] [PubMed] [Google Scholar]
- 75.Tan G, Zhou J, Yuan D, Sun S. Formula for use of mannitol in patients with intracerebral haemorrhage and high intracranial pressure. Clin Drug Investig. 2008;28(2):81–87. doi: 10.2165/00044011-200828020-00002. [DOI] [PubMed] [Google Scholar]
- 76.Rangel-Castillo L, Robertson CS. Management of Intracranial Hypertension. Crit Care Clin. 2006;22(4):713–732. doi: 10.1016/j.ccc.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 77.Raslan A, Bhardwaj A. Medical management of cerebral edema. Neurosurg Focus. 2007;22(5):1–12. doi: 10.3171/foc.2007.22.5.13. [DOI] [PubMed] [Google Scholar]
- 78.Sakowitz OW, Stover JF, Sarrafzadeh AS, Unterberg AW, Kiening KL. Effects of mannitol bolus administration on intracranial pressure, cerebral extracellular metabolites, and tissue oxygenation in severely head-injured patients. J Trauma. 2007;62(2):292–298. doi: 10.1097/01.ta.0000203560.03937.2d. [DOI] [PubMed] [Google Scholar]
- 79.Marko N. Hyperosmolar therapy for intracranial hypertension: Time to dispel antiquated myths. Am J Respir Crit Care Med. 2012;185:467–468. doi: 10.1164/rccm.201109-1698ED. [DOI] [PubMed] [Google Scholar]
- 80.Better OS, Rubinstein I, Winaver JM, Knochel JP. Mannitol therapy revisited (1940–1997) Kidney Int. 1997;52(4):886–894. doi: 10.1038/ki.1997.409. [DOI] [PubMed] [Google Scholar]
- 81.Visweswaran P, Massin EK, Dubose TD., Jr Mannitol-induced acute renal failure. J Am Soc Nephrol. 1997;8(6):1028–1033. doi: 10.1681/ASN.V861028. [DOI] [PubMed] [Google Scholar]
- 82.Qureshi AI, Suarez JI. Use of hypertonic saline solutions in treatment of cerebral edema and intracranial hypertension. Crit Care Med. 2000;28(9):3301–3313. doi: 10.1097/00003246-200009000-00032. [DOI] [PubMed] [Google Scholar]
- 83.Qureshi AI, Suarez JI, Bhardwaj A, Mirski M, Schnitzer MS, Hanley DF, Ulatowski JA. Use of hypertonic (3%) saline/acetate infusion in the treatment of cerebral edema: Effect on intracranial pressure and lateral displacement of the brain. Crit Care Med. 1998;26(3):440–446. doi: 10.1097/00003246-199803000-00011. [DOI] [PubMed] [Google Scholar]
- 84.Suarez JI. Hypertonic saline for cerebral edema and elevated intracranial pressure. Cleve Clin J Med. 2004;71(S1):S9–S13. doi: 10.3949/ccjm.71.suppl_1.s9. [DOI] [PubMed] [Google Scholar]
- 85.Ennis KM, Brophy GM. Management of intracranial hypertension: Focus on pharmacologic strategies. AACN Adv Crit Care. 2011;22(3):177–182. doi: 10.1097/NCI.0b013e318214564b. [DOI] [PubMed] [Google Scholar]
- 86.Georgiadis AL, Suarez JI. Hypertonic saline for cerebral edema. Curr Neurol Neurosci Rep. 2003;3(6):524–530. doi: 10.1007/s11910-003-0058-1. [DOI] [PubMed] [Google Scholar]
- 87.Torre-Healy A, Marko NF, Weil RJ. Hyperosmolar therapy for intracranial hypertension. Neurocrit Care. 2012;17(1):117–130. doi: 10.1007/s12028-011-9649-x. [DOI] [PubMed] [Google Scholar]
- 88.Kamel H, Navi BB, Nakagawa K, Hemphill JC, Ko NU. Hypertonic saline versus mannitol for the treatment of elevated intracranial pressure: A meta-analysis of randomized clinical trials. Crit Care Med. 2011;39(3):554–559. doi: 10.1097/CCM.0b013e318206b9be. [DOI] [PubMed] [Google Scholar]
- 89.Mortazavi MM, Romeo AK, Deep A, Griessenauer CJ, Shoja MM, Tubbs RS, Fisher W. Hypertonic saline for treating raised intracranial pressure: Literature review with meta-analysis: A review. J Neurosurg. 2012;116(1):210–221. doi: 10.3171/2011.7.JNS102142. [DOI] [PubMed] [Google Scholar]
- 90.Burgess S, Abu-Laban RB, Slavik RS, Vu EN, Zed PJ. A Systematic Review of Randomized Controlled Trials Comparing Hypertonic Sodium Solutions and Mannitol for Traumatic Brain Injury: Implications for Emergency Department Management. Ann Pharmacother. 2016;50(4):291–300. doi: 10.1177/1060028016628893. [DOI] [PubMed] [Google Scholar]
- 91.Wakai A, Mccabe A, Roberts I, Schierhout G. Mannitol for acute traumatic brain injury. Cochrane Database Syst Rev. 2013; 8:CD001049. [DOI] [PMC free article] [PubMed]
- 92.Sakellaridis N, Pavlou E, Karatzas S, Chroni D, Vlachos K, Chatzopoulos K, Dimopoulou E, Kelesis C, Karaouli V. Comparison of mannitol and hypertonic saline in the treatment of severe brain injuries. J Neurosurg. 2011;114(2):545–548. doi: 10.3171/2010.5.JNS091685. [DOI] [PubMed] [Google Scholar]
- 93.Marko NF. Hypertonic saline, not mannitol, should be considered gold-standard medical therapy for intracranial hypertension. Crit Care. 2012;16(1):2010–2012. doi: 10.1186/cc11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Godoy DA, Lubillo S, Rabinstein AA. Pathophysiology and Management of Intracranial Hypertension and Tissular Brain Hypoxia After Severe Traumatic Brain Injury: An Integrative Approach. Neurosurg Clin N Am. 2018;29(2):195–212. doi: 10.1016/j.nec.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 95.Changoor NR, Haider AH. Pharmacological and Surgical Treatment of Intracranial Hypertension. Curr Trauma Rep. 2015;1(3):155–159. [Google Scholar]
- 96.Otterspoor LC, Kalkman CJ, Cremer OL. Update on the propofol infusion syndrome in ICU management of patients with head injury. Curr Opin Anaesthesiol. 2008;21(5):544–551. doi: 10.1097/ACO.0b013e32830f44fb. [DOI] [PubMed] [Google Scholar]
- 97.Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, Davidson JE, Devlin JW, Kress JP, Joffe AM, Coursin DB, Herr DL, Tung A, Robinson BR, Fontaine DK, Ramsay MA, Riker RR, Sessler CN, Pun B, Skrobik Y, Jaeschke R; American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013; 41(1):263–306. [DOI] [PubMed]
- 98.Mirski MA, Lewin JJ. Sedation and pain management in acute neurological disease. Semin Neurol. 2008;28(5):611–630. doi: 10.1055/s-0028-1105970. [DOI] [PubMed] [Google Scholar]
- 99.Celis-Rodríguez E, Birchenall C, de la Cal MÁ, Castorena Arellano G, Hernández A, Ceraso D, Díaz Cortés JC, Dueñas Castell C, Jimenez EJ, Meza JC, Muñoz Martínez T, Sosa García JO, Pacheco Tovar C, Pálizas F, Pardo Oviedo JM, Pinilla DI, Raffán-Sanabria F, Raimondi N, Righy Shinotsuka C, Suárez M, Ugarte S, Rubiano S; Federación Panamericana e Ibérica de Sociedades de Medicina Crítica y Terapia Intensiva. Clinical practice guidelines for evidence-based management of sedoanalgesia in critically ill adult patients. Med Intensiva. 2013; 37(8):519–74. [DOI] [PubMed]
- 100.Marshall GT, James RF, Landman MP, O'Neill PJ, Cotton BA, Hansen EN, Morris JA, Jr, May AK. Pentobarbital coma for refractory intra-cranial hypertension after severe traumatic brain injury: Mortality predictions and one-year outcomes in 55 patients. J Trauma. 2010;69(2):275–283. doi: 10.1097/TA.0b013e3181de74c7. [DOI] [PubMed] [Google Scholar]
- 101.Roberts DJ, Hall RI, Kramer AH, Robertson HL, Gallagher CN, Zygun DA. Sedation for critically ill adults with severe traumatic brain injury: A systematic review of randomized controlled trials. Crit Care Med. 2011;39(12):2743–2751. doi: 10.1097/CCM.0b013e318228236f. [DOI] [PubMed] [Google Scholar]
- 102.Stocchetti N, Zanaboni C, Colombo A, Citerio G, Beretta L, Ghisoni L, Zanier ER, Canavesi K. Refractory intracranial hypertension and “second-tier” therapies in traumatic brain injury. Intensive Care Med. 2008;34(3):461–467. doi: 10.1007/s00134-007-0948-9. [DOI] [PubMed] [Google Scholar]
- 103.Roberts I, Sydenham E. Barbiturates for acute traumatic brain injury. Cochrane Database Syst Rev. 2012; 12:CD000033. [DOI] [PMC free article] [PubMed]
- 104.Hypothermia after Cardiac Arrest Study Group Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 105.Elmer J, Polderman KH. Emergency Neurological Life Support: Resuscitation Following Cardiac Arrest. Neurocrit Care. 2017;27:134–143. doi: 10.1007/s12028-017-0457-9. [DOI] [PubMed] [Google Scholar]
- 106.Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J, Kuiper M, Pellis T, Stammet P, Wanscher M, Wise MP, Aneman A, Al-Subaie N, Boesgaard S, Bro-Jeppesen J, Brunetti I, Bugge JF, Hingston CD, Juffermans NP, Koopmans M, Køber L, Langørgen J, Lilja G, Møller JE, Rundgren M, Rylander C, Smid O, Werer C, Winkel P, Friberg H; TTM Trial Investigators. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013; 369(23):2197–206.
- 107.Crossley S, Reid J, McLatchie R, Hayton J, Clark C, MacDougall M, Andrews PJ. A systematic review of therapeutic hypothermia for adult patients following traumatic brain injury. Crit Care. 2014;18(2):R75. doi: 10.1186/cc13835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Andrews PJ, Sinclair HL, Rodriguez A, Harris BA, Battison CG, Rhodes JK, Murray GD; Eurotherm3235 Trial Collaborators. Hypothermia for intracranial hypertension after traumatic brain injury. N Engl J Med. 2015; 373(25):2403–12. [DOI] [PubMed]
- 109.Flynn LM, Rhodes J, Andrews PJ. Therapeutic hypothermia reduces intracranial pressure and partial brain oxygen tension in patients with severe traumatic brain injury: preliminary data from the Eurotherm3235 trial. Ther Hypothermia Temp Manag. 2015;5(3):143–151. doi: 10.1089/ther.2015.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Edwards P, Arango M, Balica L, Cottingham R, El-Sayed H, Farrell B, Fernandes J, Gogichaisvili T, Golden N, Hartzenberg B, Husain M, Ulloa MI, Jerbi Z, Khamis H, Komolafe E, Laloë V, Lomas G, Ludwig S, Mazairac G, Muñoz Sanchéz Mde L, Nasi L, Olldashi F, Plunkett P, Roberts I, Sandercock P, Shakur H, Soler C, Stocker R, Svoboda P, Trenkler S, Venkataramana NK, Wasserberg J, Yates D, Yutthakasemsunt S; CRASH trial collaborators. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet. 2005; 365(9475):1957–9. [DOI] [PubMed]
- 111.Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM; STICH II Investigators. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. 2013; 382(9890):397–408. [DOI] [PMC free article] [PubMed]
- 112.Mendelow AD, Gregson BA, Rowan EN, Francis R, McColl E, McNamee P, Chambers IR, Unterberg A, Boyers D, Mitchell PM; STITCH(Trauma) Investigators. Early Surgery versus Initial Conservative Treatment in Patients with Traumatic Intracerebral Hemorrhage (STITCH[Trauma]): The First Randomized Trial. J Neurotrauma. 2015; 32(17):1312–23. [DOI] [PMC free article] [PubMed]
- 113.Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, Amelink GJ, Schmiedeck P, Schwab S, Rothwell PM, Bousser MG, van der Worp HB, Hacke W; DECIMAL, DESTINY, and HAMLET investigators. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. 2007; 6(3):215–22. [DOI] [PubMed]
- 114.Alexander P, Heels-Ansdell D, Siemieniuk R, Bhatnagar N, Chang Y, Fei Y, Zhang Y, McLeod S, Prasad K, Guyatt G. Hemicraniectomy versus medical treatment with large MCA infarct: A review and meta-analysis. BMJ Open. 2016;6(11):e014390. doi: 10.1136/bmjopen-2016-014390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang MH, Lin HY, Fu J, Roodrajeetsing G, Shi SL, Xiao SW. Decompressive hemicraniectomy in patients with malignant middle cerebral artery infarction: A systematic review and meta-analysis. Surgeon. 2015;13(4):230–240. doi: 10.1016/j.surge.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 116.Cooper DJ, Roseneld JV, Murray L, Arabi YM, Davies AR, D'Urso P, Kossmann T, Ponsford J, Seppelt I, Reilly P, Wolfe R; DECRA Trial Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group. Decompressive Craniectomy in Diffuse Traumatic Brain Injury. N Engl J Med. 2011; 364:1493–1502. [DOI] [PubMed]
- 117.Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J, Anderson I, Bulters DO, Belli A, Eynon CA, Wadley J, Mendelow AD, Mitchell PM, Wilson MH, Critchley G, Sahuquillo J, Unterberg A, Servadei F, Teasdale GM, Pickard JD, Menon DK, Murray GD, Kirkpatrick PJ; RESCUEicp Trial Collaborators. Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med. 2016; 375(12):1119–30.
- 118.Wang JW, Li JP, Song YL, Tan K, Wang Y, Li T, Guo P, Li X, Wang Y, Zhao QH. Decompressive craniectomy in neurocritical care. J Clin Neurosci. 2016;27:1–7. doi: 10.1016/j.jocn.2015.06.037. [DOI] [PubMed] [Google Scholar]
- 119.Bershad EM, Humphreis WE, Suarez JI. Intracranial hypertension. Semin Neurol. 2008;28(5):690–702. doi: 10.1055/s-0028-1105968. [DOI] [PubMed] [Google Scholar]
- 120.Bennett MH, Trytko B, Jonker B. Hyperbaric oxygen therapy for the adjunctive treatment of traumatic brain injury. Cochrane Database Syst Rev. 2012; 12:CD004609. [DOI] [PMC free article] [PubMed]
- 121.Maghool F, Khaksari M, Siahposht KA. Differences in brain edema and intracranial pressure following traumatic brain injury across the estrous cycle: Involvement of female sex steroid hormones. Brain Res. 2013;1497:61–72. doi: 10.1016/j.brainres.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 122.Shahrokhi N, Khaksari M, Soltani Z, Mahmoodi M, Nakhaee N. Effect of sex steroid hormones on brain edema, intracranial pressure, and neurologic outcomes after traumatic brain injury. Can J Physiol Pharmacol. 2010;88(4):414–421. doi: 10.1139/y09-126. [DOI] [PubMed] [Google Scholar]
- 123.Skolnick BE, Maas AI, Narayan RK, van der Hoop RG, MacAllister T, Ward JD, Nelson NR, Stocchetti N; SYNAPSE Trial Investigators. A clinical trial of progesterone for severe traumatic brain injury. N Engl J Med. 2014; 371(26):2467–76. [DOI] [PubMed]
- 124.Wright DW, Yeatts SD, Silbergleit R, Palesch YY, Hertzberg VS, Frankel M, Goldstein FC, Caveney AF, Howlett-Smith H, Bengelink EM, Manley GT, Merck LH, Janis LS, Barsan WG; NETT Investigators. Very early administration of progesterone for acute traumatic brain injury. N Engl J Med. 2014; 371(26):2457–66. [DOI] [PMC free article] [PubMed]
- 125.Zeiler FA, Teitelbaum J, West M, Gillman LM. The ketamine effect on ICP in traumatic brain injury. Neurocrit Care. 2014;21(1):163–173. doi: 10.1007/s12028-013-9950-y. [DOI] [PubMed] [Google Scholar]
- 126.Bourgoin A, Albanèse J, Léone M, Sampol-Manos E, Viviand X, Martin C. Effects of sufentanil or ketamine administered in target-controlled infusion on the cerebral hemodynamics of severely brain-injured patients. Crit Care Med. 2005;33(5):1109–1113. doi: 10.1097/01.ccm.0000162491.26292.98. [DOI] [PubMed] [Google Scholar]
- 127.Stocchetti N, Zoerle T, Carbonara M. Intracranial pressure management in patients with traumatic brain injury: An update. Curr Opin Crit Care. 2017;23(2):110–114. doi: 10.1097/MCC.0000000000000393. [DOI] [PubMed] [Google Scholar]
- 128.Zhang Z, Guo Q, Wang E. Hyperventilation in neurological patients: From physiology to outcome evidence. Curr Opin Anaesthesiol. 2019;32(5):568–573. doi: 10.1097/ACO.0000000000000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Robba C, Citerio G. Focus on brain injury. Intensive Care Med. 2017;43(9):1418–1420. doi: 10.1007/s00134-017-4869-y. [DOI] [PubMed] [Google Scholar]