Abstract
Testosterone deficiency is linked to a multitude of conditions including depression, sexual dysfunction, and cognitive impairment. Although synthetic testosterone-boosting pharmaceuticals have gained wide use, recent data suggests that vitamin D, ingested orally, may positively impact serum testosterone. Furthermore, evidence tying ultraviolet (UV) light exposure to endogenous vitamin D synthesis presents an intriguing possibility for naturally enhancing serum testosterone. This investigation sought to determine the effect of acute UV light exposure on resistance exercise-induced acute testosteronemia and vitamin D in older men. Six older adult men of varying activity levels (age 62 ± 1.79 yrs., height 179.92 ± 1.12 cm., body mass 83.79 ± 3.12 kg., BMI 25.95 ± 1.15 kg/m2) participated in two testing sessions separated by one week: 1) Resistance exercise followed by standard passive recovery (RERC) and 2) RE plus UV light exposure during the first 10-minutes of RE passive recovery (RERC-UV). The RE protocol was identical in both sessions and consisted of four sets of 10RM on leg press, chest press, and back row with 1-minute of rest between sets followed by 30-minutes of post-RE passive recovery. Serum testosterone and vitamin D were measured preand post-RE in 5-minute increments during the 30-minute recovery. Analysis of variance revealed neither RE or RERC-UV significantly affected serum testosterone or vitamin D. These findings suggest that acute UV light exposure may be insufficient to positively impact serum testosterone and vitamin D following a single bout of RE in older adult men.
Keywords: Sunlight, testosteronemia, vitamin D, hypertrophy, resistance exercise
INTRODUCTION
Preserving testosterone metabolism is critically important throughout the lifespan in order to support healthy reproductive function, skeletal muscle mass and strength, bone and fat mass and mentation (5–7). Yet, longitudinal data suggest a growing prevalence of hypogonadism (total serum T < 300 ng/dL) among aging men; at least 20% of men over 60, 30% over 70, and 50% over 80 years of age may be testosterone deficient (4, 6). Testosterone deficiency is associated with increased chances of cardiovascular disease, cerebrovascular event and all-cause mortality (6, 20, 21). Provided these negative health implications, methods for preventing testosterone deficiency have gained attention.
Various studies have demonstrated that both acute and baseline serum testosterone levels are sensitive to modulating factors beyond direct testosterone supplementation. Testosterone levels may be transiently increased by moderately intense, high volume resistance exercise (RE) coupled with short rest periods (< 1 min), a phenomenon known as a RE-induced acute testosteronemia (AT) (5, 23). Baseline serum testosterone may be modified by vitamin-D, regardless of prior vitamin-D status, as evidenced by recent studies showing elevated baseline testosterone levels following oral vitamin-D supplementation (14, 15, 24). Visible light exposure has also been shown to elevate testosterone levels (12); data from more recent reports utilizing ultra-violet light (UVL) suggest this effect may be mediated by vitamin-D (2, 16). Taken together, these findings raise the possibility that combining UVL with RE might enhance RE-induced acute testosteronemia and that UVL exposure may increase testosterone levels in a vitamin-D dependent manner. Yet, to date, these hypotheses have never been directly investigated.
Therefore, in an effort to directly examine the nature of UVL, RE and acute testosteronemia, the aim of the present study was to determine the effect of UVL exposure on RE-induced acute testosteronemia and vitamin D in older men. In an effort to maximize safety, all RE was performed using pneumatically controlled resistance machines. To our knowledge this is the first study utilize pneumatic machine resistance. A light source, similar to that used in Dabai et al. (2), was used to mimic natural UV sunlight. Central hypotheses were that participants would have elevated testosterone and vitamin-D levels upon exposure to ultraviolet light post-RE, and participants lower on the Fitzpatrick Scale (a categorical scale describing skin pigmentation) would have greater vitamin-D and testosterone responses to sunlight post-RE.
METHODS
Participants
Six men (age 62 ± 1.79 yrs, height 179.92 ± 1.125 cm, body mass 83.79 ± 3.12 kg) volunteered to participate in this study (Table 1). Participants had to be >55 years of age, free from any major injuries, disorders, diseases, on minimal to no medication/supplements, and have no history of skin cancer. Participants taking medications that do not interact with testosterone or vitamin D synthesis required medical clearance by their physician, while those taking medication with known interactions were excluded. Additionally, participants were asked to maintain activity levels, sunlight exposure, and diet between trials. Prior to any involvement, all participants underwent a health screening and provided written consent in order to participate. The Institutional Review Board at California State University, Long Beach, approved all methods for the study. This research was carried out fully in accordance to the ethical standards of the International Journal of Exercise Science (13).
Table 1.
Individual participant descriptive data
| Participant | Age | Height (cm) | Weight (kg) | BMI | Skin Type | Chest Press 10-RM (lbs.) | Leg Press 10-RM (lbs.) | Back Row 10-RM (lbs.) |
|---|---|---|---|---|---|---|---|---|
| 1 | 59 | 180.3 | 70.4 | 21.6 | III | 95 | 400 | 105 |
| 2 | 59 | 177.8 | 93.1 | 29.4 | IV | 103 | 500 | 115 |
| 3 | 67 | 185.4 | 74 | 21.5 | III | 45 | 280 | 75 |
| 4 | 61 | 180.3 | 88.6 | 27.2 | IV | 95 | 400 | 105 |
| 5 | 70 | 180.3 | 90.9 | 27.9 | III | 75 | 415 | 100 |
| 6 | 56 | 175.2 | 85.4 | 27.8 | III | 65 | 515 | 75 |
|
| ||||||||
| Mean ± SD | 62 ± 5.3 | 179.9 ± 3.3 | 83.7± 9.3 | 25.9 ± 3.4 | 82.6 ± 22.15 | 399 ± 84.66 | 100 ± 16.86 | |
SD = Standard Deviation
Participants performed two identical RE trials: one with a passive 30-minute recovery (RERC) and one with a 30-minute passive recovery while exposed to the Sperti Vitamin D Light Box for the initial 10 minutes of recovery (RERC-UV). Prior to performing the RE trials, participants’ 10-repetition maximum (10RM) was measured using previously validated methods (1, 3). Height and body mass were measured in the beginning of each session. Capillary blood samples were collected before exercise and immediately after in 5-minute increments for 30 minutes. After each exercise trial, participants were instructed to maintain normal activity and sunlight exposure levels.
Protocol
Participants reported to the PEXS lab to obtain height, body mass and baseline serum measurements. Participants then performed a low-intensity, aerobic warm-up on either a treadmill or stationary bike for 5-minutes. The exercise protocol utilized three Keiser Air Pneumatic Machines and consisted of four sets of 10RM with 1-minute of rest between sets and exercises on in the following order: leg press, chest press, back row (22) (Air350 Biaxial Chest Press, Air300 Leg Press, Air250 Upper Back, Keiser Corporation, Fresno, CA, USA). The Keiser Pneumatic Resistance system utilizes air-pressurized resistance to maximize safety and allows for precision loading within one pound. Individual participant 10RMs were measured in a familiarization session prior to the first testing session following National Strength and Conditioning Association protocols (1, 3).
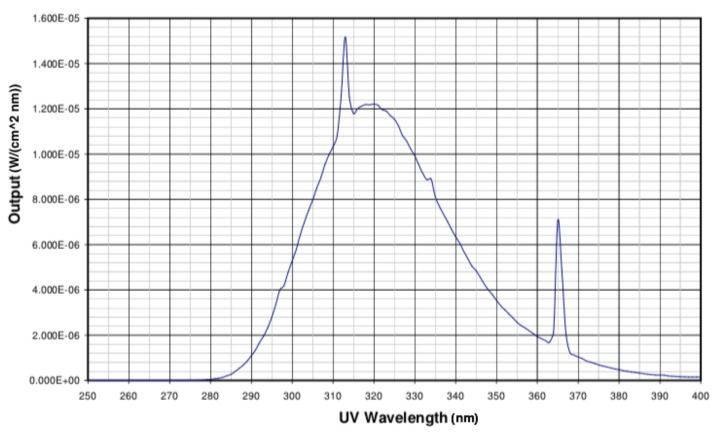
Ultraviolet Light Application: Following completion of the RERC protocol, participants returned to the PEXS lab for a 30-minute monitored recovery. The RERC-UV condition exposed ~21% of participants’ skin to the Sperti Vitamin D Light Box for the initial 10 minutes of the 30-minute recovery (KBD, Inc., Sperti Sunlamps, Crescent Springs, KY, USA). The exact spectral output of the light box is illustrated in Figure 1 and the box was placed 44 inches away from the participant in order to allow for maximum skin exposure for 10 minutes. Subjects wore above-the-knee shorts and t-shirts throughout all testing, so that light was shown on anterior surfaces of the lower leg, lower arm, neck and face.
Figure 1.
Established light emission from the Sperti Vitamin D Light Box.
Biochemical Analyses: Capillary blood was collected into CB 300 microvette tubes with clotting agent (Microvette®CB 300 K2E, SARSTEDT Aktiengeselischaft & Co., Sarstedt, Germany) and centrifuged at 5000 rpm for 5 mins at 23 °C. Serum was separated into 200 μl PCR tubes (0.2 mL flat top, ThermoFisher Scientific, Waltham, MA) and stored at −20 °C until analyzed. Total serum testosterone and vitamin D levels were assessed by Enzyme-Linked Immunosorbent Assays (ELISA) (Eagle Biosciences, INC., Nashua, NH, USA; Abcam, Cambridge, MA, USA). All procedures were followed in accordance with the manufacturer directions.
Statistical Analysis
Repeated measures ANOVA was performed to examine the differences in testosterone and vitamin D between RERC and RERC-UV at time points 0, 5, 10, 15, 20, 25, and 30 minutes. All data analyses were performed with JMP Pro 12 SAS Institute Inc., Cary, NC, USA) with significance set a priori at p ≤ 0.05.
RESULTS
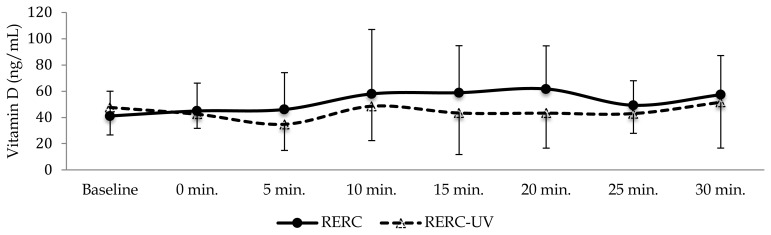
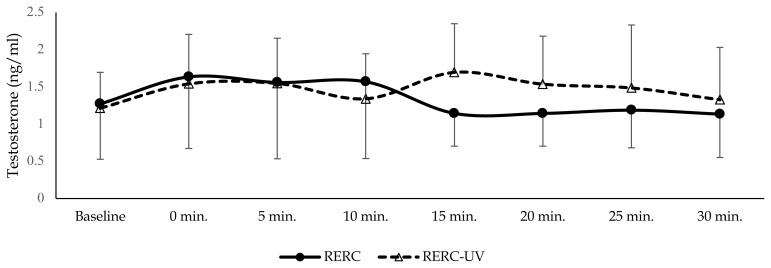
All six participants completed both RERC and RERC-UV trials. Individual participants’ descriptive data are presented in Table 1. Neither serum testosterone nor vitamin-D differed significantly (p > 0.05) between conditions or time points (p > 0.05) as illustrated in Figure 2 and Figure 3.
Figure 2.
Serum Testosterone concentration (mean ± SD) starting at baseline (immediately prior to exercise), immediately following exercise (0 min) and 5, 10, 15, 20 and 30 mins into recovery from RE.
Figure 3.
Serum vitamin-D concentration (mean ± SD) starting at baseline (immediately prior to exercise), immediately following exercise (0 min) and 5, 10, 15, 20 and 30 mins into recovery from RE.
DISCUSSION
In order to tie together previous findings on UV light, testosterone and vitamin D, the present investigation aimed to determine the effect of an acute bout of UV light exposure on acute testosteronemia and vitamin D in older men. The present findings indicate that neither resistance exercise (RERC) or resistance exercise with UV during recovery (RERC-UV) induced acute testosteronemia. Furthermore, vitamin-D was not affected by either condition.
Despite previous evidence of acute testosteronemia (AT) (9–11, 22), subjects in the present study did not experience AT. Although our resistance exercise parameters (intensity, volume and rest periods) were similar to those used by studies that reported AT, the advanced age of our chosen sample population and mode of resistance exercise likely limited the potential for AT. As noted by Kraemer et al. (9), who also found blunted AT responses in older males, basal testosterone levels, which are typically lower in older men, may drive post-RE AT. Furthermore, the novel use of pneumatic machine resistance may have played a role in limiting an AT response. Compared to free weight resistance, similar machine weight exercises generally require less muscle activation, which reduces acute endocrine responses (8, 17–19, 23).
To the best of our knowledge, the only known study to analyze ultraviolet light exposure and testosterone was performed by Myerson and Neustadt (12). They reported that repetitive UV exposure to the face, neck, chest, abdomen, back, and genitalia enhanced serum testosterone (12). Noteworthy, however, is that Myerson and Neustadt exposed areas of the body (genitalia) that do not mimic real-life exposure, and they used young adult males. The present study differed from Myerson and Neustadt’s exploration in the age of the participants, amount and areas of skin exposed, the duration to which participants were exposed, and the number of UV exposures. Although we exposed less total skin area to shorter and fewer duration exposures than Myerson and Neustadt, our chosen parameters for UV exposure reflect an effort to investigate acute effects of UV light efficaciously and realistically while maximizing the health and safety of our older male sample population.
Our data reveal that vitamin D levels remained unaffected by post-RE UV exposure. In contrast, Dabai et al. (2) found that UV exposure with the same light box used in the present study boosted vitamin D in both in vitro and in vivo models. In their in vivo model, human participants were exposed to UV light on their abdomen and back at a distance of 15 inches, three-times a week over the course of four weeks (2). Rhodes et al. (16) observed increased serum vitamin-D levels, but they used a whole-body irradiation cabinet and exposed participants to UV light for 6.5 minutes, three times a week, for six weeks. The current study differed from the aforementioned studies in several key ways, including: the combination of UV exposure with resistance exercise to examine post-RE AT; the increased UV light-to-participant distance of 44 inches, which was done to maximally expose the anterior portions of the participant’s face, neck, lower arms, lower legs; lastly and crucially, this study sought to examine the effect of acute, not repetitive, sunlight exposure compounded by moderate-high intensity resistance exercise. Future research examining the effect of UV light on metabolic and endocrine responses to RE should explore the optimal distance, choice/amount of skin exposure and size of the UV source. Additionally, acute RE parameters (intensity, volume, exercise choice, rest periods) should be varied and a more chronic application of RE (> 6 weeks) would be beneficial to explore adaptive responses.
In conclusion, we demonstrate preliminary evidence suggesting that resistance exercise does not necessarily induce a post-RE AT response in older adults. Furthermore, acute exposure to UV light following RE does not appear to improve the likelihood of an AT response in older adults. The acute nature and time of exposure to the light box (10 minutes), the distance participants sat from the light (44 inches) and the amount of skin exposed may have all limited a potential vitamin-D response. If future research is to use older participants, free weight resistance exercises may be preferable over machine resistance to induce AT during recovery; additionally, longer UV exposure times combined with repetitive exposures, instead of single exposures, may improve the likelihood of a vitamin-D response. However, future research must responsibly design both RE and UV parameters to account for maximal safety of participants.
One possible limitation is the fact that subjects did not come into the study with abnormally low vitamin-D. Therefore, the likelihood of observing any increases in serum vitamin-D was inherently lower than it might have been if subjects with abnormally low vitamin-D were examined. An additional limitation is the low samples size of this pilot study (n = 6), which necessitates future research on a larger scale.
REFERENCES
- 1.Baechle TR, Earle RW. Essentials of strength and conditioning. Champaign: Human Kinetics; 2008. [Google Scholar]
- 2.Dabai NS, Pramyothin P, Holick MF. The effect of ultraviolet radiation from a novel portable fluorescent lamp on serum 25-hydroxyvitamin d3 levels in healthy adults with fitzpatrick skin types ii and iii. Photodermatol Photoimmunol Photomed. 2012;28(6):307–311. doi: 10.1111/phpp.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haff G, Triplett NT. Essentials of strength training and conditioning. 4th ed. Champaign: Human Kinetics; 2016. [Google Scholar]
- 4.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol and Metab. 2001;86(2):724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 5.Hooper DR, Kraemer WJ, Focht BC, Volek JS, DuPont WH, Caldwell LK, Maresh CM. Endocrinological roles for testosterone in resistance exercise responses and adaptations. Sports Med. 2017;47(9):1709–1720. doi: 10.1007/s40279-017-0698-y. [DOI] [PubMed] [Google Scholar]
- 6.Jia H, Sullivan CT, McCoy SC, Yarrow JF, Morrow M, Borst SE. Review of health risks of low testosterone and testosterone administration. World J Clin Cases. 2015;3(4):338–344. doi: 10.12998/wjcc.v3.i4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26(6):833–876. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- 8.Kraemer WJ, Marchitelli L, Gordon SE, Harman E, Dziados JE, Mello R, Frykman P, McCurry D, Fleck SJ. Hormonal and growth factor responses to heavy resistance exercise protocols. J Appl Physiol. 1990;69(4):1442–1450. doi: 10.1152/jappl.1990.69.4.1442. [DOI] [PubMed] [Google Scholar]
- 9.Kraemer WJ, Ratamess NA, Nindl BC. Recovery responses of testosterone, growth hormone, and igf-1 after resistance exercise. J Appl Physiol. 2017;122(3):549–558. doi: 10.1152/japplphysiol.00599.2016. [DOI] [PubMed] [Google Scholar]
- 10.Kraemer WJ, Spiering BA, Volek JS, Ratamess NA, Sharman MJ, Rubin MR, French DN, Silvestre R, Hatfield DL, Van Heest JL, Vingren JL, Judelson DA, Deschenes MR, Maresh CM. Androgenic responses to resistance exercise: Effects of feeding and l-carnitine. Med Sci Sports Exerc. 2006;38(7):1288–1296. doi: 10.1249/01.mss.0000227314.85728.35. [DOI] [PubMed] [Google Scholar]
- 11.Kraemer WJ, Staron RS, Hagerman FC, Hikida RS, Fry AC, Gordon SE, Nindl BC, Gothshalk LA, Volek JS, Marx JO, Newton RU, Hakkinen K. The effects of short-term resistance training on endocrine function in men and women. Eur J Appl Physiol. 1998;78(1):69–76. doi: 10.1007/s004210050389. [DOI] [PubMed] [Google Scholar]
- 12.Myerson A, Neustadt R. Influence of ultraviolet irradiation upon excretion of sex hormones in the male. Endocr J. 1939;25(1):7–12. [Google Scholar]
- 13.Navalta JW, Stone WJ, Lyons TS. Ethical issues relating to scientific discovery in exercise science. Int J Exerc Sci. 2019;12(1):1–8. doi: 10.70252/EYCD6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nimptsch K, Platz EA, Willett WC, Giovannucci E. Association between plasma 25-oh vitamin d and testosterone levels in men. Clin Endocrinol. 2012;77(1):106–112. doi: 10.1111/j.1365-2265.2012.04332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pilz S, Frisch S, Koertke H, Kuhn J, Dreier J, Obermayer-Pietsch B, Wehr E, Zittermann A. Effect of vitamin d supplementation on testosterone levels in men. Horm Metab Res. 2011;43(3):223–225. doi: 10.1055/s-0030-1269854. [DOI] [PubMed] [Google Scholar]
- 16.Rhodes LE, Webb AR, Fraser HI, Kift R, Durkin MT, Allan D, O’Brien SJ, Vail A, Berry JL. Recommended summer sunlight exposure levels can produce sufficient (> or =20 ng ml(−1)) but not the proposed optimal (> or =32 ng ml(−1)) 25(oh)d levels at uk latitudes. J Invest Dermatol. 2010;130(5):1411–1418. doi: 10.1038/jid.2009.417. [DOI] [PubMed] [Google Scholar]
- 17.Schick EE, Coburn JW, Brown LE, Judelson DA, Khamoui AV, Tran TT, Uribe BP. A comparison of muscle activation between a smith machine and free weight bench press. J Strength Cond Res. 2010;24(3):779–784. doi: 10.1519/JSC.0b013e3181cc2237. [DOI] [PubMed] [Google Scholar]
- 18.Schwanbeck S, Chilibeck PD, Binsted G. A comparison of free weight squat to smith machine squat using electromyography. J Strength Cond Res. 2009;23(9):2588–2591. doi: 10.1519/JSC.0b013e3181b1b181. [DOI] [PubMed] [Google Scholar]
- 19.Shaner AA, Vingren JL, Hatfield DL, Budnar RG, Jr, Duplanty AA, Hill DW. The acute hormonal response to free weight and machine weight resistance exercise. J Strength cond Res. 2014;28(4):1032–1040. doi: 10.1519/JSC.0000000000000317. [DOI] [PubMed] [Google Scholar]
- 20.Shores MM, Arnold AM, Biggs ML, Longstreth WT, Jr, Smith NL, Kizer JR, Cappola AR, Hirsch CH, Marck BT, Matsumoto AM. Testosterone and dihydrotestosterone and incident ischaemic stroke in men in the cardiovascular health study. Clin Endocrinol. 2014;81(5):746–753. doi: 10.1111/cen.12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shores MM, Matsumoto AM, Sloan KL, Kivlahan DR. Low serum testosterone and mortality in male veterans. Arch Intern Med. 2006;166(15):1660–1665. doi: 10.1001/archinte.166.15.1660. [DOI] [PubMed] [Google Scholar]
- 22.Spiering BA, Kraemer WJ, Vingren JL, Ratamess NA, Anderson JM, Armstrong LE, Nindl BC, Volek JS, Hakkinen K, Maresh CM. Elevated endogenous testosterone concentrations potentiate muscle androgen receptor responses to resistance exercise. J Steroid Biochem Mol Biol. 2009;114(3–5):195–199. doi: 10.1016/j.jsbmb.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Vingren JL, Kraemer WJ, Ratamess NA, Anderson JM, Volek JS, Maresh CM. Testosterone physiology in resistance exercise and training: The up-stream regulatory elements. Sports Med. 2010;40(12):1037–1053. doi: 10.2165/11536910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 24.Wehr E, Pilz S, Boehm BO, Marz W, Obermayer-Pietsch B. Association of vitamin d status with serum androgen levels in men. Clin Endocrinol. 2010;73(2):243–248. doi: 10.1111/j.1365-2265.2009.03777.x. [DOI] [PubMed] [Google Scholar]