See Kuhn and Baldermann (doi10.1093/brain/awaa108) for a scientific commentary on this article.
Deep brain stimulation for treatment-refractory OCD rapidly improves mood and anxiety. Fridgeirsson et al. report that these changes are associated with altered functional interactions in an affective network centred around the amygdala, highlighting the importance of the amygdala circuit in the pathophysiology of OCD.
Keywords: obsessive compulsive disorder, deep brain stimulation, mood, anxiety, resting state functional MRI
Abstract
Deep brain stimulation is effective for patients with treatment-refractory obsessive-compulsive disorder. Deep brain stimulation of the ventral anterior limb of the internal capsule rapidly improves mood and anxiety with optimal stimulation parameters. To understand these rapid effects, we studied functional interactions within the affective amygdala circuit. We compared resting state functional MRI data during chronic stimulation versus 1 week of stimulation discontinuation in patients, and obtained two resting state scans from matched healthy volunteers to account for test-retest effects. Imaging data were analysed using functional connectivity analysis and dynamic causal modelling. Improvement in mood and anxiety following deep brain stimulation was associated with reduced amygdala-insula functional connectivity. Directional connectivity analysis revealed that deep brain stimulation increased the impact of the ventromedial prefrontal cortex on the amygdala, and decreased the impact of the amygdala on the insula. These results highlight the importance of the amygdala circuit in the pathophysiology of obsessive-compulsive disorder, and suggest a neural systems model through which negative mood and anxiety are modulated by stimulation of the ventral anterior limb of the internal capsule for obsessive-compulsive disorder and possibly other psychiatric disorders.
See Kuhn and Baldermann (doi10.1093/brain/awaa108) for a scientific commentary on this article.
Introduction
Obsessive-compulsive disorder (OCD) is a psychiatric disorder with an estimated lifetime prevalence of 2% in the general population (Ruscio et al., 2010; Godlewska et al., 2012). The main symptoms are anxiety, obsessive thoughts (obsessions) and repetitive behaviours (compulsions). Patients are commonly treated with cognitive behavioural therapy and/or selective serotonin reuptake inhibitors (Denys, 2006). Treatment for patients who do not respond sufficiently includes clomipramine and a combination of SSRIs with antipsychotics. Approximately 10% of patients with OCD remain treatment refractory and continue to experience symptoms despite pharmacological and behavioural treatment (Denys, 2006). For those patients, deep brain stimulation (DBS) is an emerging treatment option with a ∼60% responder rate (Alonso et al., 2015).
In DBS, electrodes are implanted in brain regions that can then be selectively and focally stimulated with electrical impulses. DBS has been tested as a viable treatment option in several psychiatric conditions such as depression, anorexia nervosa and addiction (Lujan et al., 2008; Luigjes et al., 2013). In OCD, the most common target regions include striatal regions such as nucleus accumbens (NAc), ventral capsule/ventral striatum and ventral anterior limb of the internal capsule (vALIC). Other common regions are subthalamic nucleus, inferior thalamic peduncle and more recently, medial forebrain bundle (de Koning et al., 2011; Coenen et al., 2017). Once stimulation parameters have been optimized, DBS of the vALIC results in a typical sequence of symptom improvements. Patients initially experience rapid improvements of mood and anxiety, followed by more gradual decrease of obsessions and compulsion, which may take several weeks and often require additional behavioural therapy for several months (Denys et al., 2010; Mantione et al., 2014). We found that decreased obsessions and compulsions following DBS were associated with normalization of frontostriatal network function (Figee et al., 2013). However, it remains puzzling how vALIC DBS induces its rapid changes in mood and anxiety.
Here we investigated whether rapid mood and anxiety effects of vALIC-DBS are due to modulation of circuits involving a predominant role of the amygdala. The amygdalae are crucial for the detection of salient events and the initiation of anxiety (Davis and Whalen, 2001). Mood and anxiety disorders have been consistently associated with increased activity of amygdala and insula, and decreased activity of the prefrontal cortex (Etkin and Wager, 2007; Hamilton et al., 2012; Simon et al., 2014; Via et al., 2014; Taylor and Whalen, 2015). Functional connectivity between amygdala and insula is positively correlated to anxiety (Baur et al., 2013), whereas functional connectivity between amygdala and ventromedial prefrontal cortex (vmPFC) is negatively correlated to anxiety and negative affect (Kim et al., 2011; Morawetz et al., 2017). The vALIC DBS target region is strongly connected with the amygdala, insula, and vmPFC (Cho et al., 2013). We therefore hypothesized that rapid mood and anxiety effects of vALIC DBS result from modulation in connectivity between the amygdala, insula and vmPFC.
In this study, we used two methods to assess changes in connectivity as measured with resting state functional MRI. First, we used functional connectivity to measure correlations in spontaneous slow fluctuations (<0.1 Hz) in blood oxygen level-dependent signals between the amygdala and the rest of the brain. This method has shown considerable intra-subject reproducibility (Shehzad et al., 2009; Zuo et al., 2010) and has been linked to behavioural variability (Clare Kelly et al., 2008). Because the amygdala is composed of distinct nuclei that have different roles in affect regulation (Herry and Johansen, 2014), we further assessed the independent role of the centromedial and laterobasal amygdala groups. Second, we used effective connectivity as a measure of directed or causal connectivity between areas (Friston, 2011) to assess the influence of the amygdala, insula and vmPFC on one another. We also included the NAc in this model because (i) DBS was targeted at the border of the NAc and vALIC; (ii) the NAc is strongly connected with the amygdala, insula, and vmPFC (Cho et al., 2013); and (iii) we previously observed DBS-related changes in NAc connectivity (Figee et al., 2013). We used spectral dynamic causal modelling (DCM) (Friston et al., 2014; Razi et al., 2015), which is particularly suited to measure group differences in effective connectivity during the resting state. It is based on constructing a biologically plausible model that generates a predicted response in the frequency domain and fitting that to the observed response. This enables one to infer causal influences one region exerts over another. DCM provides estimation of parameters that give information on the strength of those causal influences between regions of interest, referred to as effective connectivity (Friston et al., 2003, 2014). To test the influence of DBS on these parameters, patients were investigated twice. The first resting state scan was obtained after DBS treatment for at least a year (DBS on), and the second resting state scan was obtained when stimulation was turned off for 1 week (DBS off). To control for test-retest effects on the connectivity measures, a group of healthy controls was also measured twice. Based on the positive association between anxiety and amygdala-insula connectivity and the negative association between amygdala-vmPFC connectivity, we hypothesized that the effect of DBS treatment on mood and anxiety could either be explained by decreased amygdala-insula connectivity, increased amygdala-vmPFC connectivity, or both.
Materials and methods
Participants
Sixteen patients with treatment refractory OCD were recruited from the outpatient clinic for DBS at the Department of Psychiatry of the Academic Medical Center in Amsterdam, the Netherlands. Symptom severity was assessed using the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) (Goodman et al., 1989a, b), the Hamilton Depression Rating Scale (HAM-D) (Hamilton, 1960) and the Hamilton Anxiety Rating Scale (HAM-A) (Hamilton, 1959). Two quadripolar electrodes (Model 3389; Medtronics Inc.) with four contact points of 1.5 mm long and intersected by 0.5 mm spaces were implanted bilaterally through the anterior limb of the internal capsule with the deepest contact point located in the NAc in the plane 3 mm anterior to the anterior commissure and the three upper contact points positioned in the vALIC. Patients were included only if they had undergone an optimization phase of at least 1 year. During this phase, patients were evaluated every 2 weeks and stimulation parameters were adjusted in order to obtain the optimal clinical response. For all patients the optimal stimulation was monopolar using the two middle contact points superior to the NAc, in the vALIC. We could not collect all resting state functional MRI data for three patients, one patient had a deviating electrode placement, and data from two patients were excluded due to excessive head motion during scanning (max movement > 2.5 mm or 2.5 degrees of rotation), leaving a final sample for data analysis of 10 patients. At the commencement of the study the mean stimulation voltage was 4.8 V (3.5–6.2 V), frequency was 130 Hz (nine patients) or 185 Hz (one patient). The pulse width was 90 μs (seven patients) or 150 μs (three patients). We recruited 16 healthy control participants from the community via local advertisements. Exclusion criteria were the presence of a mental disorder according to DSM-IV (Diagnostic and Statistical Manual of Mental Disorders IV) as assessed with the Mini International Neuropsychiatric Inventory (Sheehan et al., 1998; van Vliet and de Beurs, 2007), a family history of psychiatric disease, a history of head trauma, any neurological or other medical disorders, a history of substance abuse, or a contraindication for MRI. Data from one session of one patient were missing and data of four controls were excluded because of excessive head motion during scanning, leaving a final sample size of 11 controls. During the two scanning days, the participants did not use cigarettes, caffeine or sedatives. The study was approved by the Medical Ethics Committee of the Academic Medical Center in Amsterdam and all participants signed an informed consent form before participation.
Study design
Each subject underwent two resting state functional MRI scans. Subjects were instructed to keep their eyes open during the scan. The first scan (DBS on) was performed after DBS had been constantly turned on for at least 1 year. After the first scan, patients entered the DBS off phase, and the second scan was performed 1 week later (DBS off). Symptom severity was assessed on each scanning day through the use of Y-BOCS, HAM-D and HAM-A. The healthy controls were scanned with 1 week in-between sessions.
Image acquisition
Data were acquired using a 1.5 T Siemens MAGNETOM Avanto scanner. A transmit receive head coil was used to minimize exposure of DBS electrodes to the pulsed radiofrequency field. The DBS was turned off 2 min prior to scanning and programmed at 0 V in bipolar mode. The subject’s head was held in place with padding and straps. Specific absorption rate was limited to 0.1 W/kg. Structural images were acquired with 1 × 1 × 1 mm resolution using a 3D sagittal MPRAGE with repetition time of 1.9 s, echo time of 3.08 ms, flip angle of 8° and inversion time of 1.1 s. Functional MRI data were acquired with 2D echo-planar imaging with repetition time = 2000 ms, echo time = 30 ms, flip angle = 90°. Each scan consisted of 25 transverse slices of 4-mm thick with in plane voxel size of 3.6 × 3.6 mm and slice gap of 0.4 mm. The first 10 volumes were discarded to allow for magnetization stabilization and the subsequent 180 volumes were analysed.
Image preprocessing
Image processing was carried out using Nipype, a pipeline tool for neuroimaging data processing (Gorgolewski et al., 2011) and SPM12 (http://www.fil.ion.ucl.ac.uk/spm). The functional data were first realigned to correct for motion using rigid body realignment to the first functional image. Subjects exhibiting more than 2.5 mm movement in any direction were excluded, resulting in 10 patients and 11 controls. After motion correction the brain extracted structural data were coregistered to functional space. The structural data were normalized to MNI152 (Montreal Neurological Institute) space using SPM’s unified segmentation approach (Ashburner and Friston, 2005), which is robust to brain lesions (Crinion et al., 2007) to accommodate the signal dropout due to the DBS system. The resulting non-linear warps were then used to normalize the functional data, which were resampled into 2-mm isotropic voxels and subsequently smoothed using an 8-mm Gaussian kernel and bandpass filtered from 0.01 to 0.1 Hz.
Functional connectivity analysis
Data from regions of interest were extracted using SPM’s Anatomy toolbox (Eickhoff et al., 2005). For each amygdala, the centromedial and laterobasal subregions were extracted. The mean signal in the region of interest was computed from the unsmoothed but bandpass filtered functional data. For the subregions the mean signal was weighted with the probability at each voxel as was done previously (Roy et al., 2009). This gives stronger weight to those voxels more probable to belong to each subregion. Noise correction followed the procedure described by Muschelli et al. (2014). Principal components were extracted from the CSF and white matter signals. The CSF tissue mask from SPM’s unified segmentation was confined to the ventricles using the ALVIN mask (Kempton et al., 2011) and including only voxels with a 99% probability or higher as being CSF. The white matter mask was confined to a 99% probability or higher and eroded to minimize the risk of capturing signals from the nearby grey matter regions. For the signal at each voxel the voxel mean was removed and the results divided by the voxel standard deviation. Singular value decomposition was then used to generate principal components. The components accounting for the 50% of variance from each tissue class were included in the nuisance regression as covariates along with the six motion parameters and derivatives (computed using backward differences). The motion parameters were bandpass filtered to prevent inadvertent reintroduction of nuisance-related variation into frequencies previously suppressed by bandpass filtering (Hallquist et al., 2013). For each analysis, the other subregion from the same hemisphere was included in the nuisance regression. After nuisance covariates were regressed out, the region of interest signal was correlated with the remaining residuals from the whole brain. This provided a map of correlation coefficients that were then transformed to z-scores using Fisher’s transformation.
The individual statistical maps were entered into a 2 × 2 flexible factorial design using GLM Flex (http://mrtools.mgh.harvard.edu/) with partitioned error terms for the within and between subject factors. The factors included were Group (patients versus controls) and Condition (DBS on versus off). All main effects and interactions were computed. Voxel-wise statistical tests were familywise error-corrected for multiple comparisons at the cluster level (P < 0.05) using a cluster forming threshold of P = 0.001 (Eklund et al., 2016) using peak_nii (https://www.nitrc.org/projects/peak_nii) for the whole brain or the regions of interest. The insula was extracted from the automatic anatomical labelling atlas (Tzourio-Mazoyer et al., 2002) using wfu Pickatlas (Maldjian et al., 2003) and the vmPFC was defined as a 10-mm sphere centred on (−1, 49, −5) MNI coordinates (Fox et al., 2005). Post hoc t-tests were performed in the presence of significant interactions between factors. Data were extracted from the entire region of interest for brain regions that showed significant clusters for subsequent correlation analyses with clinical scores.
Dynamic causal modelling
Because functional connectivity analyses do not provide information about the direction of connectivity, we subsequently performed an effective connectivity analysis using spectral DCM. First a general linear model was set up in SPM with cosine basis functions from 1/128 Hz to 0.1 Hz as effects of interest and the movement parameters, white matter and CSF signals as nuisance regressors. This way the resulting effects of interest contrast over the basis functions reveals the resting state fluctuations in that frequency range. For model simplicity only regions of interest from the left hemisphere were included since the functional connectivity results were with the left amygdala. We included the amygdala and insula as regions of interest but also the NAc and vmPFC, because all of these regions are anatomically connected (Cho et al., 2013) and we previously showed that vALIC DBS also influences NAc vmPFC connectivity (Figee et al., 2013). The left amygdala was specified based on an anatomical atlas using the SPM anatomy toolbox. The NAc was defined as the caudate nucleus from the automatic anatomical atlas (Eickhoff et al., 2005) below z = 0 and excluding the signal dropout due to the DBS electrodes. The vmPFC region of interest was specified as a 10-mm sphere centred on (−1, 49, −5) MNI coordinates (Fox et al., 2005). The left insula region of interest was specified as a 10-mm sphere centred on the peak value in the region of interest from the seed-based correlation analysis. When extracting the signal, the spheres were allowed to shift to the nearest local maxima according to the effects of interest contrast defined above, but within the 10-mm sphere. From each region of interest the first principal eigenvariate, corrected for confounds, was used to represent the region of interest.
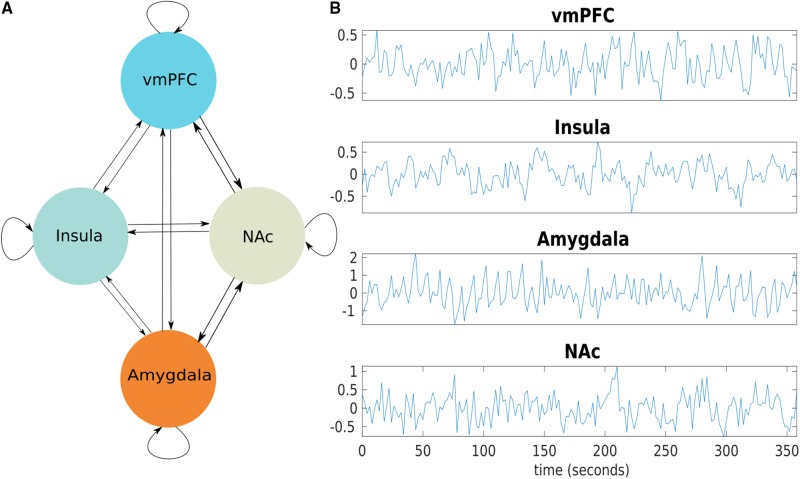
A DCM model was constructed with the four regions of interest as nodes. Bilateral connections between all nodes were defined, resulting in 16 connections including each node’s self-connection (Fig. 1). Then for each subject this full model was inverted using spectral DCM in SPM (DCM12 revision 6662). The resulting posterior probabilities of each subject’s connection coefficients were then entered into a repeated measures mixed ANOVA in MATLAB and solved for significant interactions between DBS on and off in patients and controls.
Figure 1.
The causal neural model for the effects of DBS. (A) A graph model showing the fully connected model with four regions, the vmPFC, insula, amygdala, and NAc. In a fully connected model, each region is reciprocally connected and each region has a self-inhibitory connection. (B) Blood oxygen level-dependent time series data from regions of interest from one subject.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Results
Deep brain stimulation effects on mood and anxiety
Demographics and clinical characteristics are summarized in Table 1. All participants were right-handed. The patients and controls did not differ in age, sex ratio, years of education and head motion during functional MRI scanning (all P > 0.05; Table 1). All patients had OCD as primary diagnosis, four patients had co-morbid major depressive disorder, one had co-morbid panic disorder, and three had co-morbid obsessive-compulsive personality disorder. In line with our previous report on the clinical outcome of DBS (Denys et al., 2010), turning off DBS increased anxiety symptoms [HAM-A; t(9) = 2.84, P = 0.019, paired t-test], increased mood symptoms [HAM-D; t(9) = 3.31, P = 0.009, paired t-test], and increased obsessive-compulsive symptoms [Y-BOCS; t(9) = 3.46, P = 0.007].
Table 1.
Demographics of the study sample and clinical scales
| Patients (n = 10) |
Controls (n = 11) |
Difference |
|||
|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | P-value | |
| Age, years | 44.1 (9.7) | 27–56 | 44.7 (9.1) | 25–56 | 0.88a |
| Gender, % males | 50 | 63.6 | 0.37b | ||
| Education, years | 13.7 (1.56) | 12–16 | 15.7 (4.07) | 12–23 | 0.16a |
| Smoking, % yes | 30 | 54.5 | 0.76b | ||
| Illness duration, years | 27.6 (13.4) | 8–48 | |||
| Motion, mm (mean framewise displacement) | 0.26 (0.13) | 0.09–0.55 | 0.21 (0.08) | 0.09–0.36 | 0.16 |
| Clinical scales patients | DBS off d | DBS on | |||
| Y-BOCS total | 28.5 (6.3) | 15–38 | 18.9 (7.7) | 6–32 | <0.01c,* |
| Y-BOCS obsessions | 13.7 (3.3) | 9–19 | 9.0 (4.1) | 0–15 | <0.01c* |
| Y-BOCS compulsions | 14.8 (4.0) | 6–20 | 9.9 (4.0) | 6–18 | <0.05c,* |
| HAM-D | 30 (9.2) | 13–40 | 16.7 (10.4) | 0–30 | <0.01c,* |
| HAM-A | 38.3 (11.3) | 11–51 | 17.7 (9.2) | 4–31 | <0.05c,* |
Significant (two-tailed).
aIndependent sample t-test.
bChi-square test.
cPaired t-test.
dAfter 1 week of DBS off.
Y-BOCS = Yale-Brown Obsessive-Compulsive Scale.
The impact of deep brain stimulation on functional connectivity of the amygdala
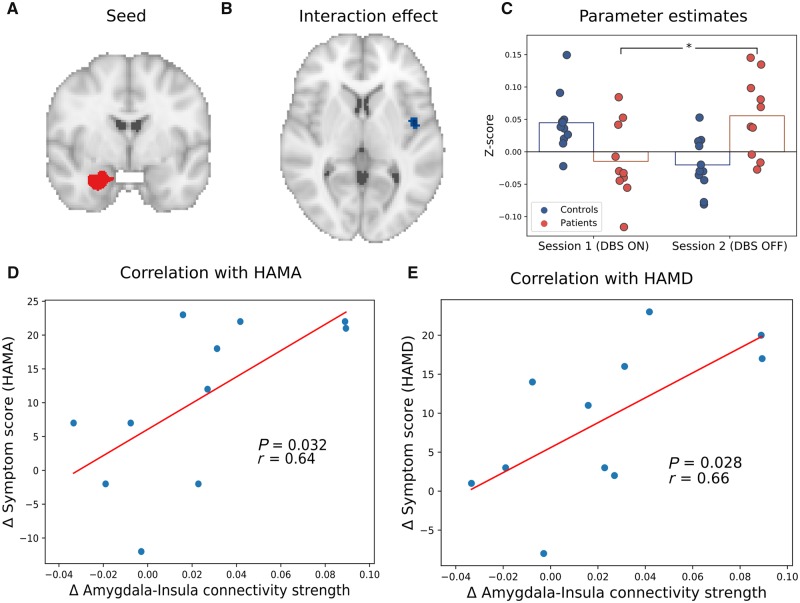
Functional connectivity analysis showed a significant interaction between group (patients versus controls) and session (DBS on versus off) of the left laterobasal amygdala groups with the right insula [P = 0.014, MNI: (44, −2, 4), size: 248 mm3, familywise error-corrected; Fig. 2]. Left amygdala functional connectivity with the right insula increased from DBS on to DBS off in OCD patients, whereas it decreased between sessions in controls. Post hoc tests showed that the interaction was primarily driven by an increase in laterobasal amygdala-insula connectivity when DBS was switched off in the patient groups (P = 0.009). Further, connectivity tended to be higher in patients than controls when DBS was switched off (P = 0.078), and was not significantly different when DBS was on (P = 0.51).
Figure 2.
The effects of DBS on functional connectivity of the laterobasal amygdala with the insula. (A) The left laterobasal amygdala seed region. (B) The significant interaction cluster in the right insula. (C) Parameter estimates for the significant interaction cluster (for illustrative purposes). (D) Correlation between changes in laterobasal amygdala-insula connectivity and HAM-A scores in OCD patients. The blue dots are data from each patient while the red line is a fitted regression line. (E) Correlation between changes in laterobasal amygdala-insula connectivity and HAM-D scores in OCD patients. *P < 0.05.
The DBS-induced change in connectivity was positively correlated to changes in anxiety (r = 0.67, P = 0.035) and mood (r = 0.67, P = 0.033), such that a larger DBS-related increase in laterobasal amygdala-insula connectivity was associated with higher increase in mood and anxiety symptoms. The correlation between DBS-induced changes in mood and anxiety symptoms was 0.9 (P = 0.0004), precluding further partial correlations and suggesting that the influence of DBS on these symptoms cannot be dissociated.
Dynamic causal modelling for deep brain stimulation effects
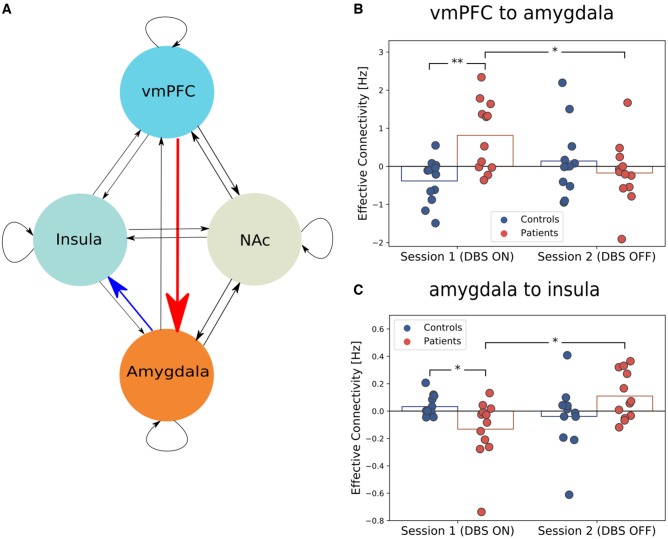
Our analysis showed an interaction between group and session for the connection between the left vmPFC and left amygdala [P = 0.045, FDR (false discovery rate) corrected], and between the left amygdala and left insula (P = 0.045, FDR corrected). The impact of the vmPFC over the amygdala was higher during DBS on compared to DBS off in patients (P = 0.0122) and was higher during DBS on compared to controls (P = 0.0012). The connection parameter for the impact of amygdala on insula was positive during DBS off, indicating an excitatory connection, whereas it was negative during DBS on, indicating an inhibitory connection (P = 0.0052). Moreover, the connection parameter was lower in patients during DBS on compared to controls (P = 0.033, Fig. 3).
Figure 3.
The influence of DBS on effective connectivity between the amygdala, insula, vmPFC, and NAc. (A) A graph model showing the fully connected model with four regions, the vmPFC, insula, amygdala, and NAc. In a fully connected model, each region is reciprocally connected and each region has a self-inhibitory connection. (B) The connection from vmPFC to the amygdala is higher when the DBS is on and is higher than in controls. (C) The connection from amygdala to insula reverses direction from inhibitory influence to excitatory when DBS is turned off. *P < 0.05, **P < 0.001.
Discussion
When undergoing vALIC DBS for OCD, the most prominent and rapid changes in symptoms are improvements in mood and anxiety. To understand the underlying mechanism, we investigated functional connectivity within the amygdala network and found that turning DBS off increased functional connectivity between amygdala and insula. This increase was correlated to an increase of both anxiety and mood symptoms. When analysing directional connectivity within the network, we found that during DBS the impact of vmPFC on amygdala was higher than when DBS was switched off and turning DBS off reversed the impact of amygdala on insula from inhibitory to excitatory.
This affective prefrontal-limbic network has primarily been linked to mood and anxiety disorders (Etkin and Wager, 2007; Kim et al., 2011; Hamilton et al., 2012; Baur et al., 2013; Taylor and Whalen, 2015), but it is also associated with OCD. In particular, OCD symptom-provoking stimuli induce exaggerated amygdala responses (van den Heuvel et al., 2004; Simon et al., 2010, 2014). It has been suggested that elevated fear and anxiety, and associated frontolimbic impairments, may be causal to, or driving some of the compulsions (Milad and Rauch, 2012). This is supported by a recent meta-analysis (Thorsen et al., 2018) that found increased activation of the amygdala during emotional processing in OCD, and that co-morbidity with mood and anxiety disorders was associated with even higher activations of the right amygdala, putamen and insula as well as lower activations in the left amygdala and right vmPFC. The insula is involved in perception of internal feelings (Craig, 2009) and is suggested to have an important role in anxiety (Paulus and Stein, 2006). In OCD, the insula has been primarily associated with disgust sensitivity (Shapira et al., 2003), but animal studies suggest that it also has a crucial role in the development of compulsive behaviour (Belin-Rauscent et al., 2016). Our results suggest that the effects of vALIC DBS on mood and anxiety are primarily driven by changes in connectivity between insula and amygdala, demonstrating the importance of these brain regions in OCD symptomatology.
Further, we showed that DBS alters the top-down control of vmPFC on amygdala and reverses amygdala drive on insula from excitatory to inhibitory. It has been shown that the strength of amygdala coupling with the vmPFC predicts the extent of attenuation of negative affect by reappraisal in healthy subjects (Banks et al., 2007), indicating that normal top-down control of the amygdala can lower anxiety and negative mood. OCD patients show reduced vmPFC-amygdala coupling during appraisal and passive viewing of symptom stimuli (Heinzel et al., 2018). In addition, reduced activity in the vmPFC is also associated with impaired recall of fear extinction in OCD (Milad et al., 2013). The retention of extinction memory is crucial for the success of extinction training, and is therefore also thought to underlie the success of exposure therapy. So the change in top-down control of the vmPFC on the amygdala might mean that DBS restores reappraisal mechanisms and facilitates fear extinction, and can thereby improve mood and anxiety to enable successful cognitive behavioural therapy (Mantione et al., 2014).
The current pathophysiological model for OCD is centred around hyperactivity in cortico-striatal-thalamic loops and does not fully explain negative mood and anxiety. In line with previous suggestions (Milad and Rauch, 2012), our results suggest that abnormal frontolimbic connectivity needs to be incorporated into that model. In fact, the temporal sequence of symptom changes following DBS shows that improvements in mood and anxiety happen before improvements in obsessions and compulsions (Denys et al., 2010). The changes in frontolimbic connectivity might therefore precede and enable the changes in frontostriatal circuits that are associated with obsessive-compulsive symptoms (Figee et al., 2013). The influence of DBS on the frontolimbic circuit may also underlie the restoration of self-confidence, as is typically reported by patients. We recently hypothesized that, in particular, anxiety fuels low self-confidence in OCD, which could be related to insufficient vmPFC control over the amygdala (Kiverstein et al., 2019).
Just as DBS treatment improves mood and anxiety, its cessation of 1 week was associated with a worsening of symptoms. We previously found that mood and anxiety scores even tend to be higher than before the initiation of treatment (Denys et al., 2010). This rebound effect can be sudden, even in patients who do not experience a clear anxiolytic effect of DBS (Huys et al., 2019). Fortunately, the clinical effects are reinstated rapidly once DBS is turned on again (de Koning et al., 2016). These clinical observations indicate that the effects of DBS on the amygdala network may be acute and that DBS has limited effects on neural plasticity.
The frontolimbic network has primarily been implicated in other anxiety disorders and depression (Taylor and Whalen, 2015). VALIC DBS is also beneficial for treatment resistant depression, suggesting that DBS-induced changes in vmPFC-amygdala-insula connectivity might also be important for its clinical effects in major depression (Bergfeld et al., 2016). At the same time, this implies that vALIC DBS might be beneficial for other treatment-resistant anxiety disorders for which DBS is not yet a treatment option. A case study in a patient with post-traumatic stress disorder suggests that direct amygdala DBS is promising (Langevin et al., 2016), but our results suggests that vALIC stimulation could also be used to target the amygdala network. Besides DBS, similar neural network changes may underlie clinical improvement induced by other treatment options such as pharmacotherapy and psychotherapy, which could be explored in future studies.
We stimulated the patients within the vALIC that consists of two fibre bundles. The anterior thalamic radiation (ATR) connects the thalamus to the prefrontal cortex, and the supero-lateral branch of the medial forebrain bundle (slMFB) connects the ventral tegmental area to the prefrontal cortex. We previously found that stimulation closer to the slMFB is associated with a better clinical outcome, based on tracking of white matter bundles of each patient using diffusion MRI (Liebrand et al., 2019). In contrast, another recent study reported that better outcome is associated with stimulation of tracts that presumably overlap with the ATR, based on an average white matter tract model of healthy individuals (Baldermann et al., 2019). Furthermore, a recent study reported that another tract that connects the amygdala to the prefrontal cortex runs ventral to the ALIC (Folloni et al., 2019). As all these pathways are integrated at the striatal and amygdala level (Cho et al., 2013), the contribution of each of these pathways to the DBS effects on the amygdala circuitry we reported here remains unclear, which requires further investigation.
There are a few limitations to our study. First the sample size is small as can be expected for a neuroimaging study with fully implanted DBS electrodes, which previously has only been investigated in several cases (Rauch et al., 2006). The small sample size did not allow us to assess the influence of clinical heterogeneity or concurrent medication use. Second, spectral DCM is a technique that is hard to validate in the absence of known causal network changes; however, it has been shown to be able to recover those changes in synthetic data (Razi et al., 2015) and was found to be more sensitive to group differences than conventional DCM. Further, a recent study shows that spectral DCM has good inter-subject and inter-session reliability when studying the default mode network (Almgren et al., 2018). Third, a relatively small number of nodes can be used in spectral DCM due to computational reasons, requiring a priori specification of regions of interest. We therefore only selected those nodes that had shown DBS-related changes in functional connectivity in the current and a previous study (Figee et al., 2013). Fourth, in our study we cannot disentangle the effects of DBS on mood or anxiety separately due to the high correlation between them. Further work is needed to see if DBS is affecting one more than the other or if mood and anxiety change concurrently.
In conclusion, our results reveal a neural network model for how vALIC DBS could exert its rapid effects on mood and anxiety, which may enable patients to challenge their obsessive-compulsive symptoms with behavioural therapy. Mood and anxiety symptoms may therefore be more important in OCD than often appreciated, or at least they are critical symptoms targeted by effective vALIC DBS for OCD. In fact, the initial modulation of the frontolimbic circuit may enable later alterations in the frontostriatal circuit, which we found is related to eventual changes in compulsivity (Figee et al., 2013). Beyond OCD, the frontolimbic network also has an important role in other anxiety disorders and depression (Hamilton et al., 2012; Taylor and Whalen, 2015). This suggests that modulation of the vmPFC-amygdala-insula circuit may also have a role in the clinical effects of vALIC DBS in depression (Bergfeld et al., 2016) and highlights its potential as a novel treatment option for patients with other treatment-resistant anxiety disorders. Future studies may investigate whether other treatment modalities exert their antidepressant and anxiolytic effects through similar neural mechanisms.
Funding
This study was supported by an unrestricted investigator-initiated research grant by Medtronic Inc (D.D. and P.R.S.), which provided the devices used herein. The sponsor had no role in the design or conduct of the study; in the collection, management, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript.
Competing interests
The authors report no competing interests.
Glossary
- DBS =
deep brain stimulation
- DCM =
dynamic causal modelling
- HAM-A/D =
Hamilton Rating Scale-Anxiety/Depression
- NAc =
nucleus accumbens
- OCD =
obsessive-compulsive disorder
- vALIC =
ventral anterior limb of the internal capsule
- vmPFC =
ventromedial prefrontal cortex
References
- Almgren H, Van de Steen F, Kühn S, Razi A, Friston K, Marinazzo D.. Variability and reliability of effective connectivity within the core default mode network: a multi-site longitudinal spectral DCM study. Neuroimage 2018; 183: 757–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso P, Cuadras D, Gabriels L, Denys D, Goodman W, Greenberg BD, et al. Deep brain stimulation for obsessive-compulsive disorder: a meta-analysis of treatment outcome and predictors of response. PLoS One 2015; 10: e0133591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ.. Unified segmentation. Neuroimage 2005; 26: 839–51. [DOI] [PubMed] [Google Scholar]
- Baldermann JC, Melzer C, Zapf A, Kohl S, Timmermann L, Tittgemeyer M, et al. Connectivity profile predictive of effective deep brain stimulation in obsessive-compulsive disorder. Biol Psychiatry 2019; 9: 735–43. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Luan Phan K.. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci 2007; 2: 303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur V, Hänggi J, Langer N, Jäncke L.. Resting-state functional and structural connectivity within an insula–amygdala route specifically index state and trait anxiety. Biol Psychiatry 2013; 73: 85–92. [DOI] [PubMed] [Google Scholar]
- Belin-Rauscent A, Daniel M-L, Puaud M, Jupp B, Sawiak S, Howett D, et al. . From impulses to maladaptive actions: the insula is a neurobiological gate for the development of compulsive behavior. Mol Psychiatry 2016; 21: 491–9. [DOI] [PubMed] [Google Scholar]
- Bergfeld IO, Mantione M, Hoogendoorn MLC, Ruhé HG, Notten P, van Laarhoven J, et al. Deep brain stimulation of the ventral anterior limb of the internal capsule for treatment-resistant depression. JAMA Psychiatry 2016; 73: 456. [DOI] [PubMed] [Google Scholar]
- Cho YT, Ernst M, Fudge JL.. Cortico-amygdala-striatal circuits are organized as hierarchical subsystems through the primate amygdala. J Neurosci 2013; 33: 14017–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clare Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP.. Competition between functional brain networks mediates behavioral variability. Neuroimage 2008; 39: 527–37. [DOI] [PubMed] [Google Scholar]
- Coenen VA, Schlaepfer TE, Goll P, Reinacher PC, Voderholzer U, Tebartz van Elst L, et al. The medial forebrain bundle as a target for deep brain stimulation for obsessive-compulsive disorder. CNS Spectr 2017; 22: 282–9. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel-now? The anterior insula and human awareness. Nat Rev Neurosci 2009; 10: 59–70. [DOI] [PubMed] [Google Scholar]
- Crinion J, Ashburner J, Leff A, Brett M, Price C, Friston K.. Spatial normalization of lesioned brains: performance evaluation and impact on fMRI analyses. Neuroimage 2007; 37: 866–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ.. The amygdala: vigilance and emotion. Mol Psychiatry 2001; 6: 13–34. [DOI] [PubMed] [Google Scholar]
- de Koning PP, Figee M, Endert E, van den Munckhof P, Schuurman PR, Storosum JG, et al. Rapid effects of deep brain stimulation reactivation on symptoms and neuroendocrine parameters in obsessive-compulsive disorder. Transl Psychiatry 2016; 6: e722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Koning PP, Figee M, van den Munckhof P, Schuurman PR, Denys D.. Current status of deep brain stimulation for obsessive-compulsive disorder: a clinical review of different targets. Curr Psychiatry Rep 2011; 13: 274–82. [DOI] [PubMed] [Google Scholar]
- Denys D. Pharmacotherapy of obsessive-compulsive disorder and obsessive-compulsive spectrum disorders. Psychiatr Clin North Am 2006; 29: 553–84. [DOI] [PubMed] [Google Scholar]
- Denys D, Mantione M, Figee M, van den Munckhof P, Koerselman F, Westenberg H, et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry 2010; 67: 1061–8. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 2005; 25: 1325–35. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H.. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci 2016; 113: 7900–05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD.. Functional neuroimaging of anxiety: a meta-ana lysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 2007; 164: 1476–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figee M, Luigjes J, Smolders R, Valencia-Alfonso C-E, van Wingen G, de Kwaasteniet B, et al. Deep brain stimulation restores frontostriatal network activity in obsessive-compulsive disorder. Nat Neurosci 2013; 16: 386–7. [DOI] [PubMed] [Google Scholar]
- Folloni D, Sallet J, Khrapitchev AA, Sibson N, Verhagen L, Mars RB.. Dichotomous organization of amygdala/temporal-prefrontal bundles in both humans and monkeys. Elife 2019; 8: e47175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME.. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 2005; 102: 9673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. Functional and effective connectivity: a review. Brain Connect 2011; 1: 13–36. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W.. Dynamic causal modelling. Neuroimage 2003; 19: 1273–302. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Kahan J, Biswal B, Razi A.. A DCM for resting state fMRI. Neuroimage 2014; 94: 396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godlewska BR, Norbury R, Selvaraj S, Cowen PJ, Harmer CJ.. Short-term SSRI treatment normalises amygdala hyperactivity in depressed patients. Psychol Med 2012; 42: 2609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, et al. The Yale-Brown Obsessive Compulsive Scale. II. Validity. Arch Gen Psychiatry 1989b; 46: 1012–6. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen S A, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry 1989a; 46: 1006–11. [DOI] [PubMed] [Google Scholar]
- Gorgolewski K, Burns CD, Madison C, Clark D, Halchenko YO, Waskom ML, et al. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front Neuroinform 2011; 5: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallquist MN, Hwang K, Luna B.. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. Neuroimage 2013; 82: 208–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH.. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry 2012; 169: 693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. Hamilton Anxiety Rating Scale (HAM-A). J Med 1959; 61: 81–2. [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatr 1960; 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel S, Paul S, Beucke JC, Kaufmann C, Mersov A, Kathmann N, et al. Amygdala-prefrontal connectivity during appraisal of symptom-related stimuli in obsessive-compulsive disorder. Psychol Med 2018; 49: 278–86. [DOI] [PubMed] [Google Scholar]
- Herry C, Johansen JP.. Encoding of fear learning and memory in distributed neuronal circuits. Nat Neurosci 2014; 17: 1644–54. [DOI] [PubMed] [Google Scholar]
- Huys D, Kohl S, Baldermann JC, Timmermann L, Sturm V, Visser-Vandewalle V, et al. Open-label trial of anterior limb of internal capsule-nucleus accumbens deep brain stimulation for obsessive-compulsive disorder: insights gained. J Neurol Neurosurg Psychiatry 2019; 90: 805–12. [DOI] [PubMed] [Google Scholar]
- Kempton MJ, Underwood TSA, Brunton S, Stylios F, Schmechtig A, Ettinger U, et al. A comprehensive testing protocol for MRI neuroanatomical segmentation techniques: evaluation of a novel lateral ventricle segmentation method. Neuroimage 2011; 58: 1051–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ.. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex 2011; 21: 1667–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiverstein J, Rietveld E, Slagter HA, Denys D.. Obsessive compulsive disorder: a pathology of self-confidence? Trends Cogn Sci 2019; 23: 369–72. [DOI] [PubMed] [Google Scholar]
- Langevin JP, Koek RJ, Schwartz HN, Chen JWY, Sultzer DL, Mandelkern MA, et al. Deep brain stimulation of the basolateral amygdala for treatment-refractory posttraumatic stress disorder. Biol Psychiatry 2016; 79: e82–4. [DOI] [PubMed] [Google Scholar]
- Liebrand LC, Caan MWA, Schuurman PR, van den Munckhof P, Figee M, Denys D, et al. Individual white matter bundle trajectories are associated with deep brain stimulation response in obsessive-compulsive disorder. Brain Stimul 2019; 12: 353–60. [DOI] [PubMed] [Google Scholar]
- Luigjes J, de Kwaasteniet BP, de Koning PP, Oudijn MS, van den Munckhof P, Schuurman PR, et al. Surgery for psychiatric disorders. World Neurosurg 2013; 80: S31.e17–28. [DOI] [PubMed] [Google Scholar]
- Lujan JL, Chaturvedi A, McIntyre CC.. Tracking the mechanisms of deep brain stimulation for neuropsychiatric disorders. Front Biosci 2008; 13: 5892–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdettea JH.. An automated method for neuroanatomic and cytoarchitectonic\ratlas-based interrogation of fMRI data sets. Neuroimage 2003; 19: 1233–9. [DOI] [PubMed] [Google Scholar]
- Mantione M, Nieman DH, Figee M, Denys D.. Cognitive–behavioural therapy augments the effects of deep brain stimulation in obsessive–compulsive disorder. Psychol Med 2014; 44: 3515–22. [DOI] [PubMed] [Google Scholar]
- Milad MR, Furtak SC, Greenberg JL, Keshaviah A, Im JJ, Falkenstein MJ, et al. Deficits in conditioned fear extinction in obsessive-compulsive disorder and neurobiological changes in the fear circuit. JAMA Psychiatry 2013; 70: 608–18. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL.. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci 2012; 16: 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawetz C, Bode S, Baudewig J, Heekeren HR.. Effective amygdala-prefrontal connectivity predicts individual differences in successful emotion regulation. Soc Cogn Affect Neurosci 2017; 12: 569–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschelli J, Nebel MB, Caffo BS, Barber AD, Pekar JJ, Mostofsky SH.. Reduction of motion-related artifacts in resting state fMRI using aCompCor. Neuroimage 2014; 96: 22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Stein MB.. An insular view of anxiety. Biol Psychiatry 2006; 60: 383–7. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Dougherty DD, Malone D, Rezai A, Friehs G, Fischman AJ, et al. A functional neuroimaging investigation of deep brain stimulation in patients with obsessive–compulsive disorder. J. Neurosurg 2006. [DOI] [PubMed] [Google Scholar]
- Razi A, Kahan J, Rees G, Friston KJ.. Construct validation of a DCM for resting state fMRI. Neuroimage 2015; 106: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AMC, Uddin LQ, Gotimer K, et al. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage 2009; 45: 614–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscio AM, Stein DJ, Chiu WT, Kessler RC.. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry 2010; 15: 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira NA, Liu Y, He AG, Bradley MM, Lessig MC, James GA, et al. Brain activation by disgust-inducing pictures in obsessive-compulsive disorder. Biol Psychiatry 2003. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59: 22–33. [PubMed] [Google Scholar]
- Shehzad Z, Kelly AMC, Reiss PT, Gee DG, Gotimer K, Uddin LQ, et al. The resting brain: unconstrained yet reliable. Cereb Cortex 2009; 19: 2209–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D, Adler N, Kaufmann C, Kathmann N.. Amygdala hyperactivation during symptom provocation in obsessive-compulsive disorder and its modulation by distraction. NeuroImage Clin 2014; 4: 549–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D, Kaufmann C, Müsch K, Kischkel E, Kathmann N.. Fronto-striato-limbic hyperactivation in obsessive-compulsive disorder during individually tailored symptom provocation. Psychophysiology 2010. [DOI] [PubMed] [Google Scholar]
- Taylor JM, Whalen PJ.. Neuroimaging and anxiety: the neural substrates of pathological and non-pathological anxiety. Curr Psychiatry Rep 2015; 17: 49. [DOI] [PubMed] [Google Scholar]
- Thorsen AL, Hagland P, Radua J, Mataix-Cols D, Kvale G, Hansen B, et al. Emotional processing in obsessive-compulsive disorder: a systematic review and meta-analysis of 25 functional neuroimaging studies. Biol Psychiatry Cogn Neurosci Neuroimaging 2018; 3: 563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002; 15: 273–89. [DOI] [PubMed] [Google Scholar]
- van den Heuvel OA, Veltman DJ, Groenewegen HJ, Dolan RJ, Cath DC, Boellaard R, et al. Amygdala activity in obsessive-compulsive disorder with contamination fear: a study with oxygen-15 water positron emission tomography. Psychiatry Res-Neuroimaging 2004; 132: 225–37. [DOI] [PubMed] [Google Scholar]
- van Vliet IM, de Beurs E. [ The MINI-International Neuropsychiatric Interview. A brief structured diagnostic psychiatric interview for DSM-IV en ICD-10 psychiatric disorders]. Tijdschr Psychiatr 2007; 49: 393–7. [PubMed] [Google Scholar]
- Via E, Cardoner N, Pujol J, Alonso P, López-Solà M, Real E, et al. Amygdala activation and symptom dimensions in obsessive-compulsive disorder. Br J Psychiatry 2014; 204: 61–8. [DOI] [PubMed] [Google Scholar]
- Zuo XN, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP.. Reliable intrinsic connectivity networks: test-retest evaluation using ICA and dual regression approach. Neuroimage 2010; 49: 2163–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.