Highlights
-
•
Exercise training is beneficial to the cardiovascular system.
-
•
Autophagy is critical in maintaining cardiovascular homeostasis and function.
-
•
Excessive or insufficient autophagy or autophagic flux can lead to cardiovascular disease.
-
•
Exercise-mediated bidirectional regulation of autophagy can prevent cardiovascular diseases.
Keywords: Autophagy, Cardiovascular diseases, Exercise
Abstract
Cardiovascular disease is the leading cause of human death worldwide. Autophagy is an evolutionarily conserved degradation pathway, which is a highly conserved cellular degradation process in which lysosomes decompose their own organelles and recycle the resulting macromolecules. Autophagy is critical in maintaining cardiovascular homeostasis and function, and excessive or insufficient autophagy or autophagic flux can lead to cardiovascular disease. Enormous evidence indicates that exercise training plays a beneficial role in the prevention and treatment of cardiovascular diseases. The regulation of autophagy during exercise is a bidirectional process. For cardiovascular disease caused by either insufficient or excessive autophagy, exercise training restores normal autophagy function and delays the progression of cardiovascular disease. An in-depth exploration and discussion of exercise-mediated regulation of autophagy in the cardiovascular system can broaden our view about the prevention of various autophagy-related diseases through exercise training. In this article, we review autophagy and its related signaling pathways, as well as autophagy-dependent beneficial effects of exercise in cardiovascular system.
Graphical abstract
1. Introduction
Cardiovascular disease has become the leading cause of human death worldwide.1 Therefore, it is urgent to recognize and explore new targets for cardiovascular disease intervention. Autophagy is the process of transporting intracellular damaged, denatured or aging proteins and organelles into lysosomes for digestion and degradation.2 Autophagy is a physiological process that is a defense mechanism for cells in adverse environments and is involved in the pathological processes of various diseases. Normal levels of autophagy can protect cells from environmental stimuli to maintain the metabolism and balance of the organisms. However, excessive autophagy or insufficient autophagy may lead to the occurrence of various diseases. In recent years, studies have shown that autophagy is closely related to a variety of metabolic and degenerative diseases such as Alzheimer's disease, diabetes, aging, cancer, and cardiovascular diseases.3, 4, 5, 6
Numerous studies have shown that exercise training with appropriate intensity is a chronic stimulation process, which can reduce the risk of cardiovascular diseases and improve the prognosis of patients after cardiovascular events.7, 8, 9, 10, 11, 12 The beneficial effects of exercise to the cardiovascular system have been well-described in many excellent reviews.7, 8, 9 In this article, we use the terms of “exercise” and “exercise training” to generally refer to cardiac adaptations to exercise. In the detailed cases discussed below, different modes of exercise have been stated specifically. Single bout of exercise is denoted as “acute exercise”, and regular multiple bouts of exercise is referred to as “chronic exercise”. Long-term exercise can induce the heart to develop physiological hypertrophy, which can be characterized by cardiomyocytes hypertrophy and proliferation.13,14 Exercise training can also reduce the production of reactive oxygen species, reduce the inflammatory response, regulate collagen metabolism, ameliorate the imbalance of extracellular matrix synthesis and degradation, and alleviate cardiac fibrosis.8,15, 16, 17, 18, 19, 20, 21, 22, 23, 24 In addition, exercise also has protective effects on ventricular remodeling and heart failure induced by myocardial ischemia/reperfusion injury.13,25,26 Notably, exercise can benefit cardiovascular disease by moderately modulating the level of autophagy and maintaining intracellular homeostasis.27,28 In this review, we (1) summarize the mechanism of autophagy and its major signaling pathways, (2) describe the autophagy-dependent beneficial effects of exercise, and (3) discuss future perspectives and remaining questions.
2. The mechanism of autophagy and its major signaling pathways
Autophagy can be classified into macroautophagy, microautophagy, and chaperone-mediated autophagy.29,30 The main difference among them is that the substrate enters lysosome in different ways. Macroautophagy is a rapidly activated process that forms a large number of autophagosomes that encapsulate cytoplasmic degradation products, which then fuse with lysosomes to form autolysosomes for degradation of cargo contents.2,31 Microautophagy usually involves direct invagination of the lysosome membrane and delivery of the contents into lysosomes.32 Chaperone-mediated autophagy is highly specific in its degradation substrates and can selectively degrade the pentapeptide KFERQ (Lys-Phe-Glu-Arg-Gln) of substrate proteins.33 The most studied form of autophagy is macroautophagy, which is usually divided into 4 steps: induction of autophagy, autophagosome formation, degradation, and reuse.34 In this review, we focus on macroautophagy; thus, autophagy refers to macroautophagy unless otherwise stated. Induction and regulation of autophagy is a very complex process.35 Many stimulus conditions, including nutrient starvation, hypoxia, aging, microbial infection, protein folding errors or aggregation, DNA damage, and chemotherapy, can trigger autophagy.36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47 Meanwhile, a variety of autophagy-related genes (Atgs) constitute the core components of the autophagy pathway and participate in autophagy process.2,48 The microtubule-associated proteins 1A/1B light chain 3 (LC3)-binding protein, p62 is a key factor in the degradation of many proteins and mitochondria-selective autophagy, which can promote the degradation of misfolded proteins by acting on proteasomes. The accumulation of p62 in cells will inhibit the occurrence of autophagy, leading to proteasome inactivation and activation of nuclear factor kappa-B.49,50
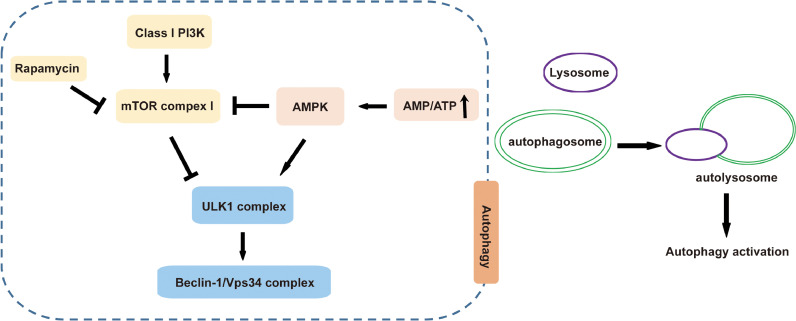
Autophagy is a complicated regulatory process that involves multiple signaling pathways (Fig. 1). Among them, the most well studied is the Class I phosphoinositide 3-kinase-mammalian target of rapamycin (mTOR) signaling pathway. There is mutual feedback regulation between autophagy and the mTOR signaling pathway. Therefore, under normal physiological conditions, mTOR is inhibited and promotes autophagy in its early stage. In the late stage of autophagy, mTOR is activated to alleviate the aggravation of autophagy. Additionally, the adenosine monophosphate-activated protein kinase (AMPK) signaling pathway is also a classical signaling pathway of autophagy. AMPK is a conserved heterotrimeric protein kinase composed of 3 subunits: 1 catalytic subunit α and 2 regulatory subunits, β and γ, which are important molecules regulating autophagy and energy metabolism. The AMPK signaling pathway plays an important role in cell growth, for example, in regulating autophagy and reducing oxidative stress damage. When the ratio of AMP/ATP is increased, AMPK can be activated to inhibit mTOR complex 1 activity, enhance autophagy, and promote mitochondrial ATP synthesis.51 AMPK can also activate vacuolar protein sorting 34 kinase.52 Similar to AMPK, glycogen synthase kinase-3β also phosphorylates tuberous sclerosis complex 1/2 to inhibit mTOR complex 1, thereby activating autophagy.52 In addition, the p38/mitogen-activated protein kinase pathway and the c-Jun N-terminal kinase pathway are also involved in the regulation of autophagy. In this process, the c-Jun N-terminal kinase 1 phosphorylates B-cell lymphoma 2, leading to the dissociation of Beclin 1 and B-cell lymphoma 2, thus activating autophagy.53,54
Fig. 1.
Mechanisms involved in autophagy process. After inhibition of the mTOR or activation of the AMPK signaling pathway, the autophagy process is activated. AMPK = adenosine monophosphate-activated protein kinase; ATP = adenosine triphosphate; mTOR = mammalian target of rapamycin; PI3K = phosphatidylinositol-3-kinase; ULK1 = Unc-51 like autophagy activating kinase 1; Vps = vacuolar protein sorting.
3. Autophagy-dependent beneficial effects of exercise on the cardiovascular system
3.1. Autophagy and cardiovascular diseases
Cardiomyocytes are end-stage differentiated cells with limited regenerative capacity. Autophagy provides energy to cardiomyocytes and promotes material circulation and self-renewal of cells by degrading misfolded or dysfunctional proteins and damaged aging organelles.55 At the same time, autophagy can also selectively increase the production of ATP during oxygen deficiency and maintain myocardial energy metabolism, thus protecting cardiac function. Therefore, normal autophagy of cardiomyocytes is of great significance in maintaining cardiac function.56,57 Autophagy can eliminate damaged mitochondria and block cardiomyocytes apoptosis.57,58 In the aging heart, the level of autophagy decreases, resulting in abnormal mitochondria and harmful metabolites accumulation, eventually causing myocardial damage.59,60 Many cardiovascular diseases are associated with abnormal autophagy and changes in autophagy affect the occurrence and development of those diseases. In the ischemic heart, chronic hypoxia of cardiomyocytes can cause mitochondria to undergo biosynthesis and thus increase in number and engender other chronic adaptation processes.61 Finally, this compensatory adaption can lead to the accumulation of various morphologically irregular and impaired mitochondria in cardiomyocytes. Autophagy can clear the mitochondria with cellular dysfunction and control the number of mitochondria to maintain cardiac function.62 In addition, up-regulation of Beclin 1 during the reperfusion of myocardial ischemia/reperfusion causes excessive autophagy and leads to cardiomyocytes damage.63 Forkhead box O (FOXO) can induce autophagy by regulating the expression of some autophagy-related proteins (such as ATG8, ATG12, ATG4B, vacuolar protein sorting 34, and Beclin 1).64 In myocardial infarction, autophagy of cardiomyocytes can also prevent cell hypertrophy, increase endoplasmic reticulum stress, and restore the intracellular energy supply, consequently playing a protective role in cardiomyocytes.65 Cardiomyocytes loss is an important cause of heart failure. There are many ways of losing cardiomyocytes, including necrosis, apoptosis, and autophagic cell death.66, 67, 68 Studies have shown that, in heart failure, dilated cardiomyopathy, and hypertensive heart disease, the dying cardiomyocytes demonstrated a dramatically enhanced autophagy.69, 70, 71 Therefore, an excessive increase in autophagy induces autophagic cell death in cardiomyocytes, which may be a cause of heart failure.72,73 Non-coding RNA has also been found to be involved in the regulation of autophagy in cardiomyocytes.74, 75, 76 MicroRNA-188-3p can inhibit cardiac autophagy by inhibiting ATG7 protein translation and thus can play an important role in preventing myocardial infarction. Intriguingly, a long non-coding RNA, called autophagy-promoting factor, binds directly to microRNA-188-3p, inhibits its activity and promotes protein translation of ATG7, which in turn affects the autophagy process.75 In addition, another long non-coding RNA, called cardiac autophagy inhibitory factor, inhibits autophagy and alleviates myocardial infarction.76
3.2. Exercise regulation of autophagy to prevent cardiovascular disease
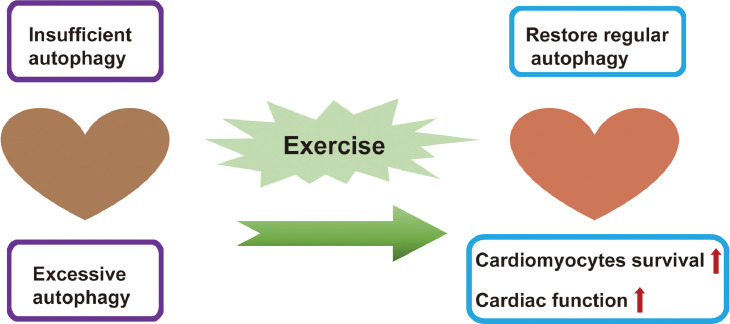
Exercise training for regulating autophagy can be bidirectional (Fig. 2).77, 78, 79, 80, 81, 82 Autophagy impairment and altered autophagy levels have been implicated in the pathogenesis of many diseases.29,83 Insufficient autophagy has been reported to contribute to multiple organ dysfunction and other adverse outcomes in autophagy-deficient mice as well as in ill patients, with an observed autophagy deficiency phenotype, evidenced by impaired autophagosome formation, accumulation of damaged proteins and mitochondria, and so on.84, 85, 86 Excessive autophagy characterized by lysosomal defects and an accumulation of autophagic vacuoles can play an important role in X-linked myopathy.29,87,88 Specifically, for cardiovascular diseases caused by insufficient autophagy, exercise training up-regulates autophagy.80, 81, 82 For cardiovascular disease caused by excessive autophagy, exercise training can inhibit autophagy, restore regular autophagy function, and delay the progression of cardiovascular disease.77, 78, 79 Additionally, it has been reported that adaptive changes in cardiac autophagy are associated with down-regulation of cardiac KATP channels underlying exercise preconditioning (5 consecutive days of treadmill exercise at 15 m/min for 10–20 min/day).89 Autophagy is critical in the maintenance of mitochondrial quality and oxidative stress during cardiovascular stress, while exercise can restore protein quality and increase the clearance of reactive aldehydes.90, 91, 92 Moreover, an increased basal level of cardiac autophagy improves myocardium resistance to subsequent ischemic injury.80,93 Aerobic exercise can inhibit the phosphorylation of mTOR by up-regulating the activity of AMPK, thereby improving cardiomyocytes autophagy and preventing cardiac aging and systolic diastolic dysfunction.27,81,82 A single bout of exercise can also activate autophagy in the heart by activating the transcription factors FOXO3 and hypoxia-inducible factor 1 and then indirectly up-regulating Beclin 1 expression.81 In addition, the carboxyl terminus of heat shock protein 70-interacting protein also plays an important role in chronic exercise (voluntary exercise for 5 weeks) that mediates autophagy-associated cardiac protection.94 Moreover, exercise-induced cardiac autophagy is dynamically regulated.82 A previous study revealed that the expression of LC3-II in the cardiac muscle of rats immediately decreased after single bout of running, while it increased during subsequent cessation, and it was found that the phosphorylation level of mTOR, which plays an important role in inhibiting autophagy, was negatively correlated with LC3-II expression in the exercise rats.82
Fig. 2.
The cardioprotection effects of exercise via modulation of autophagy. Exercise-mediated bidirectional regulation of autophagy can prevent cardiovascular diseases.
Autophagy is a dynamic and multistep process. The level of autophagosomes can only reflect the induction of autophagy and the inhibition of autophagosome.29,95 Therefore, autophagic flux is measured to dynamically monitor autophagy and provide a much more precise interpretation of autophagic status.96, 97, 98, 99 Several studies have recently reported that exercise can affect either the cardiac autophagy level or autophagic flux to improve cardiac function and have a certain therapeutic effect on heart disease.100,101 In a post-myocardial infarction-induced heart failure model, the autophagic flux of male Wistar rats (250–300 g) with heart failure 4 weeks after myocardial infarction was impeded.100 Additionally, autophagy-related markers and damaged mitochondria accumulated in the heart, decreased levels of oxidative energy, and the opening of Ca2+-induced mitochondrial permeability transition pores were detected. In the heart failure group, the impaired autophagic flux was improved and the healthy mitochondria population increased after 8 weeks of exercise training, which slowed the further deterioration of cardiac function.100 Additional experiments found that, when autophagy inhibitors were used to suppress this elevated level of autophagy, the protective effect of exercise training against heart failure was reversed.100 That study suggests that chronic exercise training may contribute to the recovery of cardiac autophagic flux in patients with heart failure by providing better mitochondrial quality and mitigating oxidative damage, thereby alleviating cardiovascular disease.100 In addition, in a mouse model of desmin-related cardiomyopathy, chronic voluntary exercise (using male 1-month-old mice placed in long-term voluntary running conditions and monitoring their survival percentage) up-regulated autophagy. As a consequence, both cardiac hypertrophy and fibrosis were significantly reduced, and the survival time of the mice was significantly prolonged.101
Under pathological situations of excessive autophagy, exercise training can also ameliorate the aggravation of cardiovascular disease via reduction of autophagy activity.77, 78, 79 In a post-myocardial infarction model using rabbits, male New Zealand White rabbits with myocardial infarction were exposed to 4 weeks of moderate treadmill exercise (1 km/h, 30 min/day, 5 days/week). After 4 weeks, the LC3-II/LC3-I ratio in myocardium recovered to non-operation level and their cardiac function improved as well, indicating that the better cardiac function was accompanied by decreased autophagy.77 This result suggests that chronic exercise training for improving cardiac function after myocardial infarction is associated with the down-regulation of excessive autophagy and can have a positive effect on the prognosis of myocardial infarction.77 Similarly, in an acute myocardial infarction mouse model, 3 weeks of swimming training with preconditioning reduced the mice's myocardial infarct size, coordinated disordered glucose and fatty acid metabolism, alleviated excessive autophagy caused by acute myocardial infarction, and improved mitochondrial biosynthesis.79 In another study, after 4 weeks of swimming exercise, the increased expression level of LC3-I was restored in the exercise diabetic rats group, and the rats’ skeletal muscle atrophy was alleviated. Also, the body weight of the exercise group increased by 30% compared with the non-exercise diabetic rats, which indicated that exercise training had a certain therapeutic effect on skeletal muscle atrophy in diabetic rats.78
With the exception of modulation of abnormal autophagy under pathological conditions, chronic habitual exercise can maintain stable the basal autophagy in myocardium without up-regulating the autophagic flux and thereby enhance cardiac function under normal physiological conditions.102 One study, in which 2-month-old rats were subjected to regular exercise for 5 months, found that their cardiac autophagic flux was not altered but their LC3-II protein increased.102 After 8 weeks of aerobic exercise in healthy elderly, the ratio of LC3-II/LC3-I in peripheral blood mononuclear cells increased, while the level of p62 protein decreased.103 The level of proteins involved in regulating the autophagic process, including ATG12, ATG16, and Beclin 1, were significantly elevated over those in a sedentary control group, and the peak oxygen uptake was also increased.103 Collectively, this evidence shows that chronic exercise might be a factor in attenuating the declined cellular autophagy caused by aging.103
4. Conclusions and future perspectives
Regular exercise training helps to improve the body's metabolism. The protective effect of exercise on the cardiovascular system has been increasingly recognized in recent years.7,9 Exercise can improve the level of cardiac autophagy, promote cardiomyocytes proliferation, reduce local tissue inflammation, and improve cardiac function.8,13,26 Cardiac autophagy plays a crucial role in exercise-induced cardioprotection as a stress response and is a necessary process for adaptation to exercise.8,80 However, there are still many questions to be answered in the study of the protective effects and mechanisms of autophagy as they relate to exercise training. On one hand, exercise training up-regulates the level of basal autophagy and enhances cell viability, which has a positive effect on delaying aging and preventing cardiovascular diseases. On the other hand, in cardiovascular disease caused by excessive autophagy, exercise training can inhibit autophagy activity and reduce autophagic cell apoptosis, thus improving the prognosis of cardiovascular disease. However, it is worth noting that excessive exercise can also trigger excessive autophagy and have a negative impact. It has been reported that increases in exercise intensity significantly increase the autophagy level in cardiomyocytes, which may be accompanied by obvious, even necrotic cardiomyocytes damage, while the application of autophagy inhibitors at the same time could alleviate the injury of cardiomyocytes.104
In rats, intensive overload exercise is defined as placing a load of 5% of body weight on the rat's tail and having it swim for 2 h, while exhausting overload exercise is defined as placing a load of 2.5% of body weight on the rat's tail and having it swim until exhaustion.104 In addition, autophagy-deficient Atg7h&mKO mice trained with chronic exercise protocols demonstrate aggravated fibrosis and pathological hypertrophy, while normal-autophagy mice demonstrate the beneficial effects from the same exercise training protocol.105 Obviously, the intensity of exhausting overload exercise depends on the individual experimental animal itself. In humans, considering the distinct individual differences of patients should likewise be considered when exercise therapy is used in rehabilitation related to cardiovascular diseases. Several questions arise: What is the correct range and threshold of exercise intensity that will ensure that exercise-induced cardiac autophagy will be beneficial? Are specific modes of exercise training best for different age groups? When a prescription for exercise is given, should the differences between short-term training and long-term training be considered? An in-depth investigation and discussion of these issues would lead to a better understanding of the regulation of exercise-mediated autophagy in the cardiovascular system and would provide better guidance for clinical prevention of autophagy-related heart disease.
The ubiquitin-proteasome system (UPS) also plays major role in maintaining cellular protein quality.106 Importantly, UPS and the autophagy-lysosomal pathway are functionally connected and have compensatory effects in cells.107,108 Among cardiomyocytes, p62 and ubiquitinated unfolded proteins accumulate in autophagy-deficient mice.109 In addition, when the function of UPS is impaired, increased the autophagy-lysosomal pathway activity can be detected.110, 111, 112 Exercise also induces an increase in intracellular ubiquitinated protein expression and has a positive effect on protein quality control.91,113,114 It has been reported that UPS shows improvement and autophagy is activated after aerobic exercise training.115,116 Importantly, exercise provides beneficial effects in relation to autophagy not only for the human heart and other organs, but also for other tissues, such as skeletal muscle, which is often affected by the same systemic degeneration seen in cardiovascular diseases.117 Notably, the molecular mechanisms that mediating autophagy regulated by exercise are still poorly understood, and which molecules play essential roles during this process is unknown. In the future, the exploration of these underlying mechanisms will provide a theoretical basis for clinical exercise training and the prevention of various autophagy-related diseases.
Acknowledgments
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81722008, 91639101, and 81570362 to J. Xiao, and 81800358 to L. Wang), from the Innovation Program of Shanghai Municipal Education Commission (2017-01-07-00-09-E00042 to J. Xiao), from the Science and Technology Commission of Shanghai Municipality (17010500100 and 18410722200 to J. Xiao), and from the development fund for Shanghai talents (to J. Xiao).
Authors’ contributions
LW and JX performed the literature search and drafted the manuscript, JW drew the figures, and DC and GL discussed the content and contributed to the writing of the manuscript. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Appendix. Supplementary materials
References
- 1.GBD 2016 Causes of Death Collaborators Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet. 2017;390:1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine B., Kroemer G. Biological functions of autophagy genes: a disease perspective. Cell. 2019;176:11–42. doi: 10.1016/j.cell.2018.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levine B., Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lassen K.G., Xavier R.J. Mechanisms and function of autophagy in intestinal disease. Autophagy. 2018;14:216–220. doi: 10.1080/15548627.2017.1389358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ueno T., Komatsu M. Autophagy in the liver: functions in health and disease. Nat Rev Gastroenterol Hepatol. 2017;14:170–184. doi: 10.1038/nrgastro.2016.185. [DOI] [PubMed] [Google Scholar]
- 6.Zare-Shahabadi A., Masliah E., Johnson G.V., Rezaei N. Autophagy in Alzheimer's disease. Rev Neurosci. 2015;26:385–395. doi: 10.1515/revneuro-2014-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiuza-Luces C., Santos-Lozano A., Joyner M., Carrera-Bastos P., Picazo O., Zugaza J.L. Exercise benefits in cardiovascular disease: beyond attenuation of traditional risk factors. Nat Rev Cardiol. 2018;15:731–743. doi: 10.1038/s41569-018-0065-1. [DOI] [PubMed] [Google Scholar]
- 8.Bernardo B.C., Ooi J.Y.Y., Weeks K.L., Patterson N.L., McMullen J.R. Understanding key mechanisms of exercise-induced cardiac protection to mitigate disease: current knowledge and emerging concepts. Physiol Rev. 2018;98:419–475. doi: 10.1152/physrev.00043.2016. [DOI] [PubMed] [Google Scholar]
- 9.Vega R.B., Konhilas J.P., Kelly D.P., Leinwand L.A. Molecular mechanisms underlying cardiac adaptation to exercise. Cell Metab. 2017;25:1012–1026. doi: 10.1016/j.cmet.2017.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L., Lv Y., Li G., Xiao J. MicroRNAs in heart and circulation during physical exercise. J Sport Health Sci. 2018;7:433–441. doi: 10.1016/j.jshs.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Z., Yan W., Mao Y., Ni Y., Zhou L., Song H. Effect of aerobic exercise on Treg and Th17 of rats with ischemic cardiomyopathy. J Cardiovasc Transl Res. 2018;11:230–235. doi: 10.1007/s12265-018-9794-0. [DOI] [PubMed] [Google Scholar]
- 12.Cai Y., Xie K.L., Zheng F., Liu S.X. Aerobic exercise prevents insulin resistance through the regulation of miR-492/Resistin axis in aortic endothelium. J Cardiovasc Transl Res. 2018;11:450–458. doi: 10.1007/s12265-018-9828-7. [DOI] [PubMed] [Google Scholar]
- 13.Boström P., Mann N., Wu J., Quintero P.A., Plovie E.R., Panáková D. C/EBPβ controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell. 2010;143:1072–1083. doi: 10.1016/j.cell.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vujic A., Lerchenmüller C., Wu T.D., Guillermier C., Rabolli C.P., Gonzalez E. Exercise induces new cardiomyocyte generation in the adult mammalian heart. Nat Commun. 2018;9:1659. doi: 10.1038/s41467-018-04083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai C., Ho T.J., Kuo W.W., Day C.H., Pai P.Y., Chung L.C. Exercise training enhanced SIRT1 longevity signaling replaces the IGF1 survival pathway to attenuate aging-induced rat heart apoptosis. Age (Dordr) 2014;36:9706. doi: 10.1007/s11357-014-9706-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin H., Yang R., Li W., Lu H., Ryan A.M., Ogasawara A.K. Effects of exercise training on cardiac function, gene expression, and apoptosis in rats. Am J Physiol Heart Circ Physiol. 2000;279:H2994–H3002. doi: 10.1152/ajpheart.2000.279.6.H2994. [DOI] [PubMed] [Google Scholar]
- 17.Siu P.M., Bryner R.W., Martyn J.K., Alway S.E. Apoptotic adaptations from exercise training in skeletal and cardiac muscles. FASEB J. 2004;18:1150–1152. doi: 10.1096/fj.03-1291fje. [DOI] [PubMed] [Google Scholar]
- 18.Werner C., Hanhoun M., Widmann T., Kazakov A., Semenov A., Poss J. Effects of physical exercise on myocardial telomere-regulating proteins, survival pathways, and apoptosis. J Am Coll Cardiol. 2008;52:470–482. doi: 10.1016/j.jacc.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 19.Ma X., Fu Y., Xiao H., Song Y., Chen R., Shen J. Cardiac fibrosis alleviated by exercise training is AMPK-dependent. PLoS One. 2015;10 doi: 10.1371/journal.pone.0129971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwak H.B., Kim J.H., Joshi K., Yeh A., Martinez D.A., Lawler J.M. Exercise training reduces fibrosis and matrix metalloproteinase dysregulation in the aging rat heart. FASEB J. 2011;25:1106–1117. doi: 10.1096/fj.10-172924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas D.P., Cotter T.A., Li X., McCormick R.J., Gosselin L.E. Exercise training attenuates aging-associated increases in collagen and collagen crosslinking of the left but not the right ventricle in the rat. Eur J Appl Physiol. 2001;85:164–169. doi: 10.1007/s004210100447. [DOI] [PubMed] [Google Scholar]
- 22.Weeks K.L., Gao X., Du X.J., Boey E.J., Matsumoto A., Bernardo B.C. Phosphoinositide 3-kinase p110α is a master regulator of exercise-induced cardioprotection and PI3K gene therapy rescues cardiac dysfunction. Circ Heart Fail. 2012;5:523–534. doi: 10.1161/CIRCHEARTFAILURE.112.966622. [DOI] [PubMed] [Google Scholar]
- 23.Serra A.J., Santos M.H., Bocalini D.S., Antônio E.L., Levy R.F., Santos A.A. Exercise training inhibits inflammatory cytokines and more than prevents myocardial dysfunction in rats with sustained beta-adrenergic hyperactivity. J Physiol. 2010;588:2431–2442. doi: 10.1113/jphysiol.2010.187310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santos-Parker J.R., LaRocca T.J., Seals D.R. Aerobic exercise and other healthy lifestyle factors that influence vascular aging. Adv Physiol Educ. 2014;38:296–307. doi: 10.1152/advan.00088.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi J., Bei Y., Kong X., Liu X., Lei Z., Xu T. miR-17-3p contributes to exercise-induced cardiac growth and protects against myocardial ischemia-reperfusion injury. Theranostics. 2017;7:664–676. doi: 10.7150/thno.15162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X., Xiao J., Zhu H., Wei X., Platt C., Damilano F. miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab. 2015;21:584–595. doi: 10.1016/j.cmet.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garber K. Autophagy. Explaining exercise. Science. 2012;335:281. doi: 10.1126/science.335.6066.281. [DOI] [PubMed] [Google Scholar]
- 28.Halling J.F., Pilegaard H. Autophagy-dependent beneficial effects of exercise. Cold Spring Harb Perspect Med. 2017;7 doi: 10.1101/cshperspect.a029777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klionsky D.J., Abdelmohsen K., Abe A., Abedin M.J., Abeliovich H., Acevedo Arozena A. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galluzzi L., Green D.R. Autophagy-independent functions of the autophagy machinery. Cell. 2019;177:1682–1699. doi: 10.1016/j.cell.2019.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galluzzi L., Baehrecke E.H., Ballabio A., Boya P., Bravo-San Pedro J.M., Cecconi F. Molecular definitions of autophagy and related processes. EMBO J. 2017;36:1811–1836. doi: 10.15252/embj.201796697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li W.W., Li J., Bao J.K. Microautophagy: lesser-known self-eating. Cell Mol Life Sci. 2012;69:1125–1136. doi: 10.1007/s00018-011-0865-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaushik S., Cuervo A.M. The coming of age of chaperone-mediated autophagy. Nat Rev Mol Cell Biol. 2018;19:365–381. doi: 10.1038/s41580-018-0001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 35.Lahiri V., Hawkins W.D., Klionsky D.J. Watch what you (self-) eat: autophagic mechanisms that modulate metabolism. Cell Metab. 2019;29:803–826. doi: 10.1016/j.cmet.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madeo F., Zimmermann A., Maiuri M.C., Kroemer G. Essential role for autophagy in life span extension. J Clin Invest. 2015;125:85–93. doi: 10.1172/JCI73946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morselli E., Maiuri M.C., Markaki M., Megalou E., Pasparaki A., Palikaras K. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis. 2010;1:e10. doi: 10.1038/cddis.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi A.M., Ryter S.W., Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:651–662. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- 39.Rubinsztein D.C., Mariño G., Kroemer G. Autophagy and aging. Cell. 2011;146:682–695. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 40.Lipinski M.M., Zheng B., Lu T., Yan Z., Py B.F., Ng A. Genome-wide analysis reveals mechanisms modulating autophagy in normal brain aging and in Alzheimer's disease. Proc Natl Acad Sci U S A. 2010;107:14164–14169. doi: 10.1073/pnas.1009485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mizushima N., Klionsky D.J. Protein turnover via autophagy: implications for metabolism. Annu Rev Nutr. 2007;27:19–40. doi: 10.1146/annurev.nutr.27.061406.093749. [DOI] [PubMed] [Google Scholar]
- 42.Song H., Feng X., Zhang H., Luo Y., Huang J., Lin M. METTL3 and ALKBH5 oppositely regulate m6A modification of TFEB mRNA, which dictates the fate of hypoxia/reoxygenation-treated cardiomyocytes. Autophagy. 2019;15:1419–1437. doi: 10.1080/15548627.2019.1586246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khandia R., Dadar M., Munjal A., Dhama K., Karthik K., Tiwari R. A comprehensive review of autophagy and its various roles in infectious, non-infectious, and lifestyle diseases: current knowledge and prospects for disease prevention, novel drug design, and therapy. Cells. 2019;8 doi: 10.3390/cells8070674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Umar S.A., Tanveer M.A., Nazir L.A., Divya G., Vishwakarma R.A., Tasduq S.A. Glycyrrhizic acid prevents oxidative stress mediated DNA damage response through modulation of autophagy in ultraviolet-B-irradiated human primary dermal fibroblasts. Cell Physiol Biochem. 2019;53:242–257. doi: 10.33594/000000133. [DOI] [PubMed] [Google Scholar]
- 45.Espinoza J.A., Zisi A., Kanellis D.C., Carreras-Puigvert J., Henriksson M., Hühn D. The antimalarial drug amodiaquine stabilizes p53 through ribosome biogenesis stress, independently of its autophagy-inhibitory activity. Cell Death Differ. 2020;27:773–789. doi: 10.1038/s41418-019-0387-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X., Li C., Wang Q., Li W., Guo D., Zhang X. Tanshinone IIA restores dynamic balance of autophagosome/autolysosome in doxorubicin-induced cardiotoxicity via targeting Beclin1/LAMP1. Cancers (Basel) 2019;11 doi: 10.3390/cancers11070910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu Y.F., Li Z.Y., Dong L.L., Li W.J., Wu Y.P., Wang J. Inactivation of MTOR promotes autophagy-mediated epithelial injury in particulate matter-induced airway inflammation. Autophagy. 2020;16:435–450. doi: 10.1080/15548627.2019.1628536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dikic I., Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19:349–364. doi: 10.1038/s41580-018-0003-4. [DOI] [PubMed] [Google Scholar]
- 49.Norman J.M., Cohen G.M., Bampton E.T. The in vitro cleavage of the hAtg proteins by cell death proteases. Autophagy. 2010;6:1042–1056. doi: 10.4161/auto.6.8.13337. [DOI] [PubMed] [Google Scholar]
- 50.Fan L., Yin S., Zhang E., Hu H. Role of p62 in the regulation of cell death induction. Apoptosis. 2018;23:187–193. doi: 10.1007/s10495-018-1445-z. [DOI] [PubMed] [Google Scholar]
- 51.Costa R., Morrison A., Wang J., Manithody C., Li J., Rezaie A.R. Activated protein C modulates cardiac metabolism and augments autophagy in the ischemic heart. J Thromb Haemost. 2012;10:1736–1744. doi: 10.1111/j.1538-7836.2012.04833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim J., Kim Y.C., Fang C., Russell R.C., Kim J.H., Fan W. Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell. 2013;152:290–303. doi: 10.1016/j.cell.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sui X., Kong N., Ye L., Han W., Zhou J., Zhang Q. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014;344:174–179. doi: 10.1016/j.canlet.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 54.Zhou F., Yang Y., Xing D. Bcl-2 and Bcl-xL play important roles in the crosstalk between autophagy and apoptosis. FEBS J. 2011;278:403–413. doi: 10.1111/j.1742-4658.2010.07965.x. [DOI] [PubMed] [Google Scholar]
- 55.Saito T., Sadoshima J. Molecular mechanisms of mitochondrial autophagy/mitophagy in the heart. Circ Res. 2015;116:1477–1490. doi: 10.1161/CIRCRESAHA.116.303790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Delbridge L.M.D., Mellor K.M., Taylor D.J., Gottlieb R.A. Myocardial stress and autophagy: mechanisms and potential therapies. Nat Rev Cardiol. 2017;14:412–425. doi: 10.1038/nrcardio.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bravo-San Pedro J.M., Kroemer G., Galluzzi L. Autophagy and mitophagy in cardiovascular disease. Circ Res. 2017;120:1812–1824. doi: 10.1161/CIRCRESAHA.117.311082. [DOI] [PubMed] [Google Scholar]
- 58.Kim I., Rodriguez-Enriquez S., Lemasters J.J. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys. 2007;462:245–253. doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shirakabe A., Ikeda Y., Sciarretta S., Zablocki D.K., Sadoshima J. Aging and autophagy in the heart. Circ Res. 2016;118:1563–1576. doi: 10.1161/CIRCRESAHA.116.307474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sasaki Y., Ikeda Y., Iwabayashi M., Akasaki Y., Ohishi M. The Impact of autophagy on cardiovascular senescence and diseases. Int Heart J. 2017;58:666–673. doi: 10.1536/ihj.17-246. [DOI] [PubMed] [Google Scholar]
- 61.Takagi H., Matsui Y., Hirotani S., Sakoda H., Asano T., Sadoshima J. AMPK mediates autophagy during myocardial ischemia in vivo. Autophagy. 2007;3:405–407. doi: 10.4161/auto.4281. [DOI] [PubMed] [Google Scholar]
- 62.Gustafsson A.B., Gottlieb R.A. Autophagy in ischemic heart disease. Circ Res. 2009;104:150–158. doi: 10.1161/CIRCRESAHA.108.187427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matsui Y., Takagi H., Qu X., Abdellatif M., Sakoda H., Asano T. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 64.Ferdous A., Battiprolu P.K., Ni Y.G., Rothermel B.A., Hill J.A. FoxO, autophagy, and cardiac remodeling. J Cardiovasc Transl Res. 2010;3:355–364. doi: 10.1007/s12265-010-9200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buss S.J., Riffel J.H., Katus H.A., Hardt S.E. Augmentation of autophagy by mTOR-inhibition in myocardial infarction: when size matters. Autophagy. 2010;6:304–306. doi: 10.4161/auto.6.2.11135. [DOI] [PubMed] [Google Scholar]
- 66.Moe G.W., Marín-García J. Role of cell death in the progression of heart failure. Heart Fail Rev. 2016;21:157–167. doi: 10.1007/s10741-016-9532-0. [DOI] [PubMed] [Google Scholar]
- 67.Dong Y., Liu C., Zhao Y., Ponnusamy M., Li P., Wang K. Role of noncoding RNAs in regulation of cardiac cell death and cardiovascular diseases. Cell Mol Life Sci. 2018;75:291–300. doi: 10.1007/s00018-017-2640-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adameova A., Goncalvesova E., Szobi A., Dhalla N.S. Necroptotic cell death in failing heart: relevance and proposed mechanisms. Heart Fail Rev. 2016;21:213–221. doi: 10.1007/s10741-016-9537-8. [DOI] [PubMed] [Google Scholar]
- 69.Takemura G., Miyata S., Kawase Y., Okada H., Maruyama R., Fujiwara H. Autophagic degeneration and death of cardiomyocytes in heart failure. Autophagy. 2006;2:212–214. doi: 10.4161/auto.2608. [DOI] [PubMed] [Google Scholar]
- 70.Kostin S., Pool L., Elsässer A., Hein S., Drexler H.C., Arnon E. Myocytes die by multiple mechanisms in failing human hearts. Circ Res. 2003;92:715–724. doi: 10.1161/01.RES.0000067471.95890.5C. [DOI] [PubMed] [Google Scholar]
- 71.Rothermel B.A., Hill J.A. Autophagy in load-induced heart disease. Circ Res. 2008;103:1363–1369. doi: 10.1161/CIRCRESAHA.108.186551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fidziańska A., Bilińska Z.T., Walczak E., Witkowski A., Chojnowska L. Autophagy in transition from hypertrophic cardiomyopathy to heart failure. J Electron Microsc (Tokyo) 2010;59:181–183. doi: 10.1093/jmicro/dfp048. [DOI] [PubMed] [Google Scholar]
- 73.Yu P., Zhang Y., Li C., Li Y., Jiang S., Zhang X. Class III PI3K-mediated prolonged activation of autophagy plays a critical role in the transition of cardiac hypertrophy to heart failure. J Cell Mol Med. 2015;19:1710–1719. doi: 10.1111/jcmm.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou S., Lei D., Bu F., Han H., Zhao S., Wang Y. MicroRNA-29b-3p targets SPARC gene to protect cardiocytes against autophagy and apoptosis in hypoxic-induced H9c2 cells. J Cardiovasc Transl Res. 2019;12:358–365. doi: 10.1007/s12265-018-9858-1. [DOI] [PubMed] [Google Scholar]
- 75.Wang K., Liu C.Y., Zhou L.Y., Wang J.X., Wang M., Zhao B. APF lncRNA regulates autophagy and myocardial infarction by targeting miR-188-3p. Nat Commun. 2015;6:6779. doi: 10.1038/ncomms7779. [DOI] [PubMed] [Google Scholar]
- 76.Liu C.Y., Zhang Y.H., Li R.B., Zhou L.Y., An T., Zhang R.C. LncRNA CAIF inhibits autophagy and attenuates myocardial infarction by blocking p53-mediated myocardin transcription. Nat Commun. 2018;9:29. doi: 10.1038/s41467-017-02280-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen C.Y., Hsu H.C., Lee B.C., Lin H.J., Chen Y.H., Huang H.C. Exercise training improves cardiac function in infarcted rabbits: involvement of autophagic function and fatty acid utilization. Eur J Heart Fail. 2010;12:323–330. doi: 10.1093/eurjhf/hfq028. [DOI] [PubMed] [Google Scholar]
- 78.Lee Y., Kim J.H., Hong Y., Lee S.R., Chang K.T., Hong Y. Prophylactic effects of swimming exercise on autophagy-induced muscle atrophy in diabetic rats. Lab Anim Res. 2012;28:171–179. doi: 10.5625/lar.2012.28.3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tao L., Bei Y., Lin S., Zhang H., Zhou Y., Jiang J. Exercise training protects against acute myocardial infarction via improving myocardial energy metabolism and mitochondrial biogenesis. Cell Physiol Biochem. 2015;37:162–175. doi: 10.1159/000430342. [DOI] [PubMed] [Google Scholar]
- 80.He C., Bassik M.C., Moresi V., Sun K., Wei Y., Zou Z. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee Y., Kang E.B., Kwon I., Cosio-Lima L., Cavnar P., Javan G.T. Cardiac kinetophagy coincides with activation of anabolic signaling. Med Sci Sports Exerc. 2016;48:219–226. doi: 10.1249/MSS.0000000000000774. [DOI] [PubMed] [Google Scholar]
- 82.Ogura Y., Iemitsu M., Naito H., Kakigi R., Kakehashi C., Maeda S. Single bout of running exercise changes LC3-II expression in rat cardiac muscle. Biochem Biophys Res Commun. 2011;414:756–760. doi: 10.1016/j.bbrc.2011.09.152. [DOI] [PubMed] [Google Scholar]
- 83.Lin N.Y., Stefanica A., Distler J.H. Autophagy: a key pathway of TNF-induced inflammatory bone loss. Autophagy. 2013;9:1253–1255. doi: 10.4161/auto.25467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vanhorebeek I., Gunst J., Derde S., Derese I., Boussemaere M., Güiza F. Insufficient activation of autophagy allows cellular damage to accumulate in critically ill patients. J Clin Endocrinol Metab. 2011;96:E633–E645. doi: 10.1210/jc.2010-2563. [DOI] [PubMed] [Google Scholar]
- 85.Komatsu M., Waguri S., Ueno T., Iwata J., Murata S., Tanida I. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169:425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Masiero E., Agatea L., Mammucari C., Blaauw B., Loro E., Komatsu M. Autophagy is required to maintain muscle mass. Cell Metab. 2009;10:507–515. doi: 10.1016/j.cmet.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 87.Nishino I. Autophagic vacuolar myopathy. Semin Pediatr Neurol. 2006;13:90–95. doi: 10.1016/j.spen.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 88.Ruggieri A., Ramachandran N., Wang P., Haan E., Kneebone C., Manavis J. Non-coding VMA21 deletions cause X-linked myopathy with excessive autophagy. Neuromuscul Disord. 2015;25:207–211. doi: 10.1016/j.nmd.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 89.Lu J., Pan S.S., Wang Q.T., Yuan Y. Alterations of cardiac KATP channels and autophagy contribute in the late cardioprotective phase of exercise preconditioning. Int Heart J. 2018;59:1106–1115. doi: 10.1536/ihj.17-003. [DOI] [PubMed] [Google Scholar]
- 90.Campos J.C., Queliconi B.B., Dourado P.M., Cunha T.F., Zambelli V.O., Bechara L.R. Exercise training restores cardiac protein quality control in heart failure. PLoS One. 2012;7:e52764. doi: 10.1371/journal.pone.0052764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Campos J.C., Fernandes T., Bechara L.R., da Paixão N.A., Brum P.C., de Oliveira E.M. Increased clearance of reactive aldehydes and damaged proteins in hypertension-induced compensated cardiac hypertrophy: impact of exercise training. Oxid Med Cell Longev. 2015;2015 doi: 10.1155/2015/464195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Disatnik M.H., Hwang S., Ferreira J.C., Mochly-Rosen D. New therapeutics to modulate mitochondrial dynamics and mitophagy in cardiac diseases. J Mol Med (Berl) 2015;93:279–287. doi: 10.1007/s00109-015-1256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Klionsky D.J., Saltiel A.R. Autophagy works out. Cell Metab. 2012;15:273–274. doi: 10.1016/j.cmet.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Willis M.S., Min J.N., Wang S., McDonough H., Lockyer P., Wadosky K.M. Carboxyl terminus of Hsp70-interacting protein (CHIP) is required to modulate cardiac hypertrophy and attenuate autophagy during exercise. Cell Biochem Funct. 2013;31:724–735. doi: 10.1002/cbf.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Klionsky D.J. The autophagosome is overrated! Autophagy. 2011;7:353–354. doi: 10.4161/auto.7.4.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Klionsky D.J., Abeliovich H., Agostinis P., Agrawal D.K., Aliev G., Askew D.S. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ju J.S., Varadhachary A.S., Miller S.E., Weihl C.C. Quantitation of “autophagic flux” in mature skeletal muscle. Autophagy. 2010;6:929–935. doi: 10.4161/auto.6.7.12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou C., Zhong W., Zhou J., Sheng F., Fang Z., Wei Y. Monitoring autophagic flux by an improved tandem fluorescent-tagged LC3 (mTagRFP-mWasabi-LC3) reveals that high-dose rapamycin impairs autophagic flux in cancer cells. Autophagy. 2012;8:1215–1226. doi: 10.4161/auto.20284. [DOI] [PubMed] [Google Scholar]
- 99.Iwai-Kanai E., Yuan H., Huang C., Sayen M.R., Perry-Garza C.N., Kim L. A method to measure cardiac autophagic flux in vivo. Autophagy. 2008;4:322–329. doi: 10.4161/auto.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Campos J.C., Queliconi B.B., Bozi L.H.M., Bechara L.R.G., Dourado P.M.M., Andres A.M. Exercise reestablishes autophagic flux and mitochondrial quality control in heart failure. Autophagy. 2017;13:1304–1317. doi: 10.1080/15548627.2017.1325062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bhuiyan M.S., Pattison J.S., Osinska H., James J., Gulick J., McLendon P.M. Enhanced autophagy ameliorates cardiac proteinopathy. J Clin Invest. 2013;123:5284–5297. doi: 10.1172/JCI70877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tam B.T., Pei X.M., Yung B.Y., Yip S.P., Chan L.W., Wong C.S. Autophagic adaptations to long-term habitual exercise in cardiac muscle. Int J Sports Med. 2015;36:526–534. doi: 10.1055/s-0034-1398494. [DOI] [PubMed] [Google Scholar]
- 103.Mejías-Peña Y., Rodriguez-Miguelez P., Fernandez-Gonzalo R., Martínez-Flórez S., Almar M., de Paz J.A. Effects of aerobic training on markers of autophagy in the elderly. Age (Dordr) 2016;38:33. doi: 10.1007/s11357-016-9897-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu H., Lei H., Shi Y., Wang J.J., Chen N., Li Z.H. Autophagy inhibitor 3-methyladenine alleviates overload-exercise-induced cardiac injury in rats. Acta Pharmacol Sin. 2017;38:990–997. doi: 10.1038/aps.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yan Z., Kronemberger A., Blomme J., Call J.A., Caster H.M., Pereira R.O. Exercise leads to unfavourable cardiac remodelling and enhanced metabolic homeostasis in obese mice with cardiac and skeletal muscle autophagy deficiency. Sci Rep. 2017;7:7894. doi: 10.1038/s41598-017-08480-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dikic I. Proteasomal and autophagic degradation systems. Annu Rev Biochem. 2017;86:193–224. doi: 10.1146/annurev-biochem-061516-044908. [DOI] [PubMed] [Google Scholar]
- 107.Wang X.J., Yu J., Wong S.H., Cheng A.S., Chan F.K., Ng S.S. A novel crosstalk between two major protein degradation systems: regulation of proteasomal activity by autophagy. Autophagy. 2013;9:1500–1508. doi: 10.4161/auto.25573. [DOI] [PubMed] [Google Scholar]
- 108.Korolchuk V.I., Mansilla A., Menzies F.M., Rubinsztein D.C. Autophagy inhibition compromises degradation of ubiquitin-proteasome pathway substrates. Mol Cell. 2009;33:517–527. doi: 10.1016/j.molcel.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nakai A., Yamaguchi O., Takeda T., Higuchi Y., Hikoso S., Taniike M. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 110.Zheng Q., Su H., Tian Z., Wang X. Proteasome malfunction activates macroautophagy in the heart. Am J Cardiovasc Dis. 2011;1:214–226. [PMC free article] [PubMed] [Google Scholar]
- 111.Rothermel B.A., Hill J.A. The heart of autophagy: deconstructing cardiac proteotoxicity. Autophagy. 2008;4:932–935. doi: 10.4161/auto.6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tannous P., Zhu H., Nemchenko A., Berry J.M., Johnstone J.L., Shelton J.M. Intracellular protein aggregation is a proximal trigger of cardiomyocyte autophagy. Circulation. 2008;117:3070–3078. doi: 10.1161/CIRCULATIONAHA.107.763870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jamart C., Francaux M., Millet G.Y., Deldicque L., Frère D., Féasson L. Modulation of autophagy and ubiquitin-proteasome pathways during ultra-endurance running. J Appl Physiol (1985) 2012;112:1529–1537. doi: 10.1152/japplphysiol.00952.2011. [DOI] [PubMed] [Google Scholar]
- 114.De Moraes W., de Souza P.R.M., da Paixão N.A., de Sousa L.G.O., Ribeiro D.A., Marshall A.G. Aerobic exercise training rescues protein quality control disruption on white skeletal muscle induced by chronic kidney disease in rats. J Cell Mol Med. 2018;22:1452–1463. doi: 10.1111/jcmm.13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cunha T.F., Moreira J.B., Paixão N.A., Campos J.C., Monteiro A.W., Bacurau A.V. Aerobic exercise training upregulates skeletal muscle calpain and ubiquitin-proteasome systems in healthy mice. J Appl Physiol (1985) 2012;112:1839–1846. doi: 10.1152/japplphysiol.00346.2011. [DOI] [PubMed] [Google Scholar]
- 116.Campos J.C., Baehr L.M., Gomes K.M.S., Bechara L.R.G., Voltarelli V.A., Bozi L.H.M. Exercise prevents impaired autophagy and proteostasis in a model of neurogenic myopathy. Sci Rep. 2018;8:11818. doi: 10.1038/s41598-018-30365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brum P.C., Bacurau A.V., Medeiros A., Ferreira J.C., Vanzelli A.S., Negrão C.E. Aerobic exercise training in heart failure: impact on sympathetic hyperactivity and cardiac and skeletal muscle function. Braz J Med Biol Res. 2011;44:827–835. doi: 10.1590/s0100-879x2011007500075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.