Abstract
Despite the strong demand for orally-delivered fish vaccines and the deficient response of those currently available in the market, little is known about how teleost B cells differentiate to antibody secreting cells (ASCs) in response to antigens delivered to the intestinal mucosa. To fill this gap, in the current study, we have studied the dynamics of B cell differentiation in spleen and kidney of rainbow trout (Oncorhynchus mykiss) anally immunized with antigens catalogued in mammals as thymus dependent (TD) or thymus-independent (TI). Our results show that, in the absence of additional adjuvants, rainbow trout preferentially responded to a model TI antigen such as TNP-LPS (2,4,6-trinitrophenyl hapten conjugated to lipopolysaccharide). The anal administration of TNP-LPS elicited TNP-specific serum antibodies, and a significant increase in the number of total and TNP-specific ASCs in both spleen and kidney, being the kidney the site where most ASCs are found at later time points. In the spleen, a proliferative response of both IgM+ B and T cells was also clearly visible, while the proliferative response was weaker in the kidney. Finally, TNP-LPS also provoked a transcriptional regulation of some immune genes in the spleen and the intestine, including a decreased transcription of foxp3a and foxp3b in intestine that suggests a breach in tolerogenic responses in response to TI stimulation. These results contribute to a better understanding of how intestinal immunity is regulated in teleost and will aid in the future design of effective oral strategies for aquaculture.
Keywords: Rainbow trout, Anal immunization, Thymus-independent (TI) antigens, B cells, IgM, Spleen
Highlights
-
•
After anal immunization of trout, a specific IgM response is only mounted in response to TI antigens.
-
•
Specific antibody secreting cells were found in spleen and kidney.
-
•
In response to anal immunization, IgM+ B cells only increase in size and proliferate in the spleen.
-
•
Genes related to B cell differentiation are not transcriptionally regulated in the kidney but in the spleen.
-
•
TNP-LPS down-regulated the transcription of foxp3a and foxp3b in intestine suggesting a breach in tolerogenic responses.
1. Introduction
Vaccination is, from all points of view, the most effective method to control the impact of infectious diseases on aquaculture. However, to date, most commercially available vaccines require an intraperitoneal injection in combination with an emulsion-based adjuvant. Alternatively, for some pathogens, DNA vaccination has also proven as an effective strategy. In this case, although no adjuvant is required, the DNA vaccine has to be administered by intramuscular injection (Corbeil et al., 2000). Nonetheless, vaccinating fish by injection is a labor-intensive process that requires individual handling of fish, provoking handling mortalities and a great amount of stress in the vaccinated animals, known to condition the immune response mounted (Plant and Lapatra, 2011). For this reason, oral vaccination is viewed as the most desirable vaccination strategy for aquaculture (Embregts and Forlenza, 2016; Mutoloki et al., 2015).
Despite this high demand, oral vaccines usually perform poorly when compared to vaccines delivered by injection, and only a few of them are available in the market for use in fish (Embregts and Forlenza, 2016; Mutoloki et al., 2015). An initial aspect that needs to be taken into account is that orally-delivered antigens have to be prevented from degradation until they reach the more posterior sections of the digestive tract where immune induction is known to take place (Rombout et al., 2011). However, many antigens that have been shown to be effectively delivered to the intestinal mucosa have still elicited deficient immune responses at both local and systemic levels and no protection (Embregts et al., 2019: Embregts et al., 2019 #6708). To understand why this occurs, we have to consider that the intestinal mucosa is in direct contact with the outer medium, having to balance immune surveillance and tolerance to commensals and food-borne antigens. The cells involved in antigen uptake within the epithelial barrier initially play a role in maintaining intestinal homeostasis as reviewed in fish by Lokka and Koppang (2016). Additionally, regulatory elements of the local immune system guarantee that harmless antigens presented in the gut result in systemic unresponsiveness. In this context, vaccine formulations delivered through the intestinal mucosa have to overcome the complex regulatory network deployed by the gut immune system to avoid unwanted immune reactions to harmless antigens.
Upon antigen encounter, B cells commence a differentiation program that eventually leads them to acquire either a memory or an antibody-secreting phenotype. Among antibody-secreting cells (ASCs), different subpopulations coexist in mammals including plasmablasts, short-lived plasma cells or long-lived plasma cells (Boothby et al., 2019), being the capacity of a specific vaccine formulation to induce the differentiation of B cells to each of these subpopulations one of the main factors that conditions a long-term vaccine efficacy. Despite the important structural differences between the mammalian and the teleost immune system that include the lack of lymph nodes, bone marrow (with the kidney being the main hematopoietic organ) or cognate germinal centers, the presence of these functionally and phenotypically different B cell subsets has been demonstrated in teleost (Ye et al., 2011). In many teleost species, including rainbow trout (Oncorhynchus mykiss), B cells have also been identified in the intestinal mucosa both within the lamina propria and as intraepithelial lymphocytes (Rombout et al., 2011; Ballesteros et al., 2013). However, to date, little work has focused on how teleost B cells differentiate to ASCs upon encounter with different antigens and how these cells in diverse differentiation stages are distributed throughout the teleost immune tissues during the immune response (Bromage et al., 2004; Ma et al., 2013). Unfortunately, the information available regarding the dynamics of B cell responses is even more limited when referring to antigens that are delivered through a mucosal compartment. Hence, in the current study, we have investigated the compartmentalization of ASCs to anally delivered model antigens in rainbow trout. Anal immunization, a strategy previously used by other research groups in the past (Chen et al., 2015; Joosten et al., 1996; Villumsen et al., 2014), allowed us to evaluate the response to antigens that are presented at the intestinal mucosa without the need of establishing an encapsulation method that avoids antigen degradation in the stomach. To immunize the fish, we have used 2,4,6-Trinitrophenyl Keyhole Limpet Hemocyanin (TNP-KLH) and TNP-lipopolysaccharide (TNP-LPS) as model thymus-dependent (TD) and thymus-independent (TI) antigens respectively. Our results demonstrate that after the stimulation of the intestinal mucosa with a TI antigen, antigen-specific ASCs are detected both in the spleen and kidney, with the kidney being the site where most ASCs are located at later time points. Additionally, we investigated the proliferation of both IgM+ B and T (ZAP70+) cells in these two sites at both time points. These experiments demonstrated a significant increase in the number of proliferating IgM+ B and T cells in the spleen in response to TNP-LPS, which was not evident in the kidney. The results obtained contribute to a better understanding of the dynamics of B cell populations in teleost upon intestinal immunization and will aid in the future design of effective oral vaccines for aquaculture.
2. Materials and Methods
2.1. Fish
Rainbow trout (Oncorhynchus mykiss) of ~50 g were obtained from Piscifactoria Cifuentes (Guadalajara, Spain) and maintained at the animal facilities of the Animal Health Research Center (CISA-INIA) in a recirculating water system at 14 °C, with a 12:12-h light/dark photoperiod. Fish were fed twice a day with a commercial diet (Skretting). Prior to any experimental procedure, fish were acclimatized to laboratory conditions for at least 2 weeks. All of the experiments described comply with the Guidelines of the European Union Council (2010/63/EU) for use of laboratory animals and have been approved by the Instituto Nacional de Investigación Agraria y Alimentaria (INIA) Ethics Committee (CEEA PROEX002/17).
2.2. Anal immunization procedure and sampling
2,4,6-Trinitrophenyl Keyhole Limpet Hemocyanin (TNP-KLH) and TNP-lipopolysaccharide (TNP-LPS) obtained from Biosearch technologies were used as model TD and TI antigens, respectively. Each fish then received 50 μg of TNP-KLH or 50 μg of TNP-LPS in 100 μl of saline solution (0.9% ClNa) into the posterior segment of the gut, by introducing a pippete tip through the anus. A mock immunized group (control) received 100 μl of saline solution. Prior to the immunization, fish were starved for three days and sedated using benzocaine at 10 mg/l (Sigma Aldrich).
The experiment was repeated several times, sampling tissues for different techniques in each case as described in Table S1. In each experiment, sampling was performed after 15 and 30 days, collecting 5–8 rainbow trout from each group, depending on the experiment. For this, rainbow trout were killed by benzocaine overdose (50 mg/l). Before collecting the organs, blood was extracted from the caudal vein. Spleen and kidney were removed and fixed in 4% paraformaldehyde for immunofluorescence and confocal microscopy. In other experiments, leukocytes were isolated from spleen and kidney to quantify the number of total and TNP-specific trout IgM-secreting cells by ELISPOT and to perform flow cytometry analysis of the IgM+ B cell population. In other experiments, spleen, kidney and gut were placed on TRI Reagent solution (Invitrogen) for RNA extraction and subsequent evaluation of the transcriptomic response. In the case of the gut, the segment sampled included the sections previously defined as mid-intestine posterior to pyloric caeca and the posterior segment (Lokka et al., 2013).
2.3. ELISA
Blood removed from the caudal vein of experimental fish was let to clot at 4 °C overnight. Serum extraction was then performed by centrifugation at 4000×g for 10 min at 4 °C. Supernatants were collected and centrifuged again at 10000×g for 10 min at 4 °C. Serum was stored at −80 °C until use. The presence of total and TNP-specific trout IgM in serum was estimated by ELISA. For this, microtitre plates were coated with 2 μg/ml of an anti-trout IgM or 5 μg/ml of TNP-BSA (Biosearch technologies) in a volume of 100 μl PBS (phosphate buffered saline) overnight at 4 °C to detect total or TNP-specific IgM, respectively. Thereafter, non-specific binding sites were blocked by incubation with 1% BSA (bovine serum albumin) in PBS with 0.05% Tween 20 (PBT) for 1 h at room temperature (RT). Plates were then washed with PBT and serial dilutions of each serum sample in PBS 1% BSA added to each well and incubated for 1 h at RT. Serum samples from all groups were analyzed in duplicate wells. After washing three times with PBT, each well was incubated with 1 μg/ml biotinyilated anti-trout IgM mAb (clone 4C10) diluted in PBS 1% BSA for 1 h at RT. The plates were washed again three times in PBT and 100 ng/ml of HRP-streptavidin (Thermo Fisher Scientific) added to each well in 100 μl PBS 1% BSA. After incubation at RT for 1 h, 100 μl of o-phenylenediamine dihydrochloride substrate reagent (Sigma-Aldrich) were added to each well. The reaction was stopped after 15 min by adding 50 μl of 2.5 M H2SO4. Absorbances were recorded at 490 nm using a FLUOstar Omega (BMG Labtech) plate reader. Internal positive and negative control samples were included in all assays.
2.4. Leukocyte isolation
Spleen and kidney were collected from experimental fish and single cell suspensions were obtained using 100 μm nylon cell strainers (BD Biosciences) and Leibovitz medium (L-15, Invitrogen) supplemented with 100 IU/ml penicillin and 100 μg/ml streptomycin (P/S, Life Technologies), 5% fetal calf serum (FCS, Life Technologies) and 10 U/ml heparin (Sigma). Cell suspensions were placed onto 30/51% discontinuous Percoll (GE Healthcare) density gradients and centrifuged at 500×g for 30 min at 4 °C, without brake. Cells at the interface were collected and washed in L-15 containing P/S and 5% FCS. The viable cell concentration was determined by trypan blue (Sigma-Aldrich) exclusion.
2.5. ELISPOT
ELISPOT was used to quantify the number of total and TNP-specific IgM-secreting B cells in spleen and kidney of experimental fish. For this, ELISPOT plates containing Immobilon-P membranes (Millipore) were activated with 70% ethanol and coated with 2 μg/ml of an anti-trout IgM mAb (clone 4C10) or with 5 μg/ml of TNP-BSA (Biosearch Technologies) overnight at 4 °C in agitation. Non-specific binding sites were blocked by incubation with 2% BSA in PBS for 2 h at RT. After that, leukocytes from rainbow trout kidney and spleen were added to the wells in triplicate at a concentration of 5 × 104 cells per well. After 24 h of incubation at 20 °C, cells were washed 5 times with PBS and plates blocked with 2% BSA in PBS for 1 h at RT. After blocking, biotinylated anti-trout IgM mAb (clone 4C10) was added to the plates (1 μg/ml) that were subsequently incubated for 1 h at RT. Following additional washing steps (5 times in PBS), the plates were developed using streptavidin-HRP (Thermo Fisher Scientific) (100 ng/ml) for 1 h at RT, washed again with PBS and incubated with 3-amino 9-ethylcarbazole (Sigma-Aldrich) for 30 min at RT in the dark. The substrate reaction was stopped by washing the plates with tap water. Once the membranes were dried, the number of spots in each well was determined using an AID iSpot Reader System (Autoimmun Diagnostika GMBH).
2.6. Flow cytometry analysis
Spleen and kidney leukocytes (4 × 105 cells) obtained as described above were incubated with an anti-trout IgM [1.14 mAb mouse IgG1 coupled to R-phycoerythrin (R-PE), 1 μg/ml] in staining buffer (phenol red-free L-15 medium supplemented with 2% FCS) for 1 h at 4 °C. After the staining, cells were washed twice with staining buffer and analyzed on a FACS Celesta flow cytometer (BD Biosciences) equipped with BD FACSDiva™ software. Only viable cells were analyzed after staining the cells with 4′,6-diamine-2′-phenylindole dihydrochlorid (DAPI, 0.2 μg/ml). Flow cytometry analysis was performed with FlowJo® v.10 (FlowJo LLC, Tree Star).
2.7. Immunofluorescence and confocal microscopy
Spleen and kidney obtained from immunized fish were fixed in 4% paraformaldehyde and processed for paraffin embedding following routine histological procedures. Thereafter, 4 μm-thick tissue sections were mounted on Superfrost Plus slides (Menzel- Gläser) to determine the percentage of proliferating IgM+ B cells or zeta-chain-associated protein kinase 70+ (ZAP70) which account for T cells. In these assays, a mouse monoclonal antibody directed against IgM or a rabbit polyclonal antibody directed against ZAP70 (99F2, Cell Signaling Technology) previously used in fish (Yoon et al., 2015) were co-incubated with an antibody directed against the proliferating cell nuclear antigen (PCNA), an intracellular molecule whose expression and synthesis is linked with cellular proliferation (Miyachi et al., 1978). Antigen retrieval was performed heating in Tris–EDTA buffer (10 mM Tris base, 1 mM EDTA, pH 9) in a microwave oven for 5 min at 800 W and 5 min at 450 W. Thereafter, non-specific binding was blocked with 5% bovine serum albumin (BSA) in Tris-buffered saline (TBS). Tissues were then incubated with the corresponding primary antibodies, either anti-trout-IgM (Abos et al., 2018b) diluted 1:10 or anti-ZAP70 (Cell Signaling) diluted 1:50 in blocking buffer (5% BSA/TBS). Incubation with a secondary anti-mouse IgG1 antibody conjugated with AlexaFluor®488 (ThermoFisher) or anti-rabbit IgG antibody conjugated with AlexaFluor®488 (Life Technologies) was followed by a further incubation with a mouse IgG2 anti-PCNA antibody conjugated with AlexaFluor®647 (BioLegend) and counterstained with DAPI (1 μg/ml, Sigma). All the incubation steps were performed for 1 h at RT in the dark. Tissue autofluorescence was then blocked by incubation with 0.3% Sudan black B in 70% ethanol for 10 min, sections were rinsed with TBS and mounted with Fluoromount (Sigma-Aldrich) for microscopy. Laser scanning confocal microscopy images were acquired with an inverted Zeiss Axiovert LSM 880 microscope with Zeiss Zen software. Tissue images were then analyzed in 10 digital fields at 400x magnification of each tissue section from at least 4 different individuals from the 3 different experimental conditions (control, TNP-KLH and TNP-LPS) and processed with Zeiss Zen and Adobe Photoshop CS6 software packages.
2.8. RNA isolation, cDNA synthesis and real time PCR
Total RNA was extracted from rainbow trout spleen, kidney and gut using TRI Reagent solution (Invitrogen) according to the manufacturer's instructions and then quantified using a NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific). cDNA synthesis was performed as explained elsewhere (Munoz-Atienza et al., 2019). The resultant cDNA was diluted in nuclease-free water and stored at −20 °C until use. The levels of transcription of Pax 5, Blimp 1, IRF4, IRF8, AID, CD9, CD27, CD80/86, Foxp3a, Foxp3b, CD4 and CD8 (Table S2) were determined by real time PCR as explained previously (Munoz-Atienza et al., 2019) and were normalized to the relative expression of the rainbow trout elongation factor (EF-1α) gene. Expression levels were calculated using the 2−ΔCt method, where ΔCt is determined by subtracting the EF-1 α value from the target cycle threshold. Negative controls with no template and minus-reverse transcriptase (−RT) controls were included in all experiments.
2.9. Statistical analysis
Data was analyzed using Microsoft Office Excel 2010. Statistical analyses were performed using a two-tailed Student's t-test with Welch's correction when the F test indicated that the variances of both groups differed significantly. The differences between the mean values were considered significant on different degrees, where * means p ≤ 0.05, ** means p ≤ 0.01, and *** means p ≤ 0.005.
3. Results
3.1. Total and TNP-specific IgM titers in sera of anally immunized fish
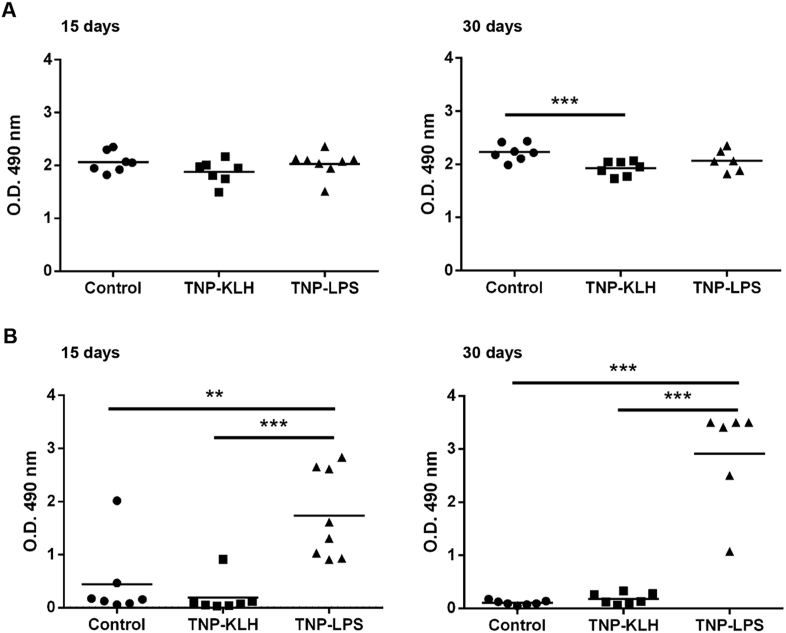
We first determined whether fish anally immunized with the different antigens, were able to mount a systemic specific IgM response. For this, we evaluated the levels of total and TNP-specific IgM in the serum of immunized fish after 15 or 30 days. At day 15 post-immunization, although the levels of total IgM were not significantly different among groups (Fig. 1A), the levels of TNP-specific antibodies were already significantly higher in fish that were immunized with TNP-LPS when compared to controls (Fig. 1B). At day 30 post-immunization, a decrease in total IgM titers was detected in fish immunized with TNP-KLH when compared to controls (Fig. 1A). At this point, TNP-specific titers were further increased in TNP-LPS-immunized fish, at levels significantly higher than those of other groups (Fig. 1B).
Fig. 1.
Detection of total and specific IgM in fish serum 15 and 30 days after the anal administration of antigens. The concentration of total (A) and TNP-specific (B) IgMs was evaluated in serum dilutions (1/1000 for total IgM and 1/50 for TNP-specific IgM) by ELISA as described in Materials and Methods. Results are shown as mean values of absorbance at 490 nm (n = 7–8). Asterisks denote significantly different values among groups as indicated (**p ≤ 0.01 and ***p ≤ 0.005).
3.2. Quantification of total and TNP-specific IgM-secreting cells in anally immunized fish
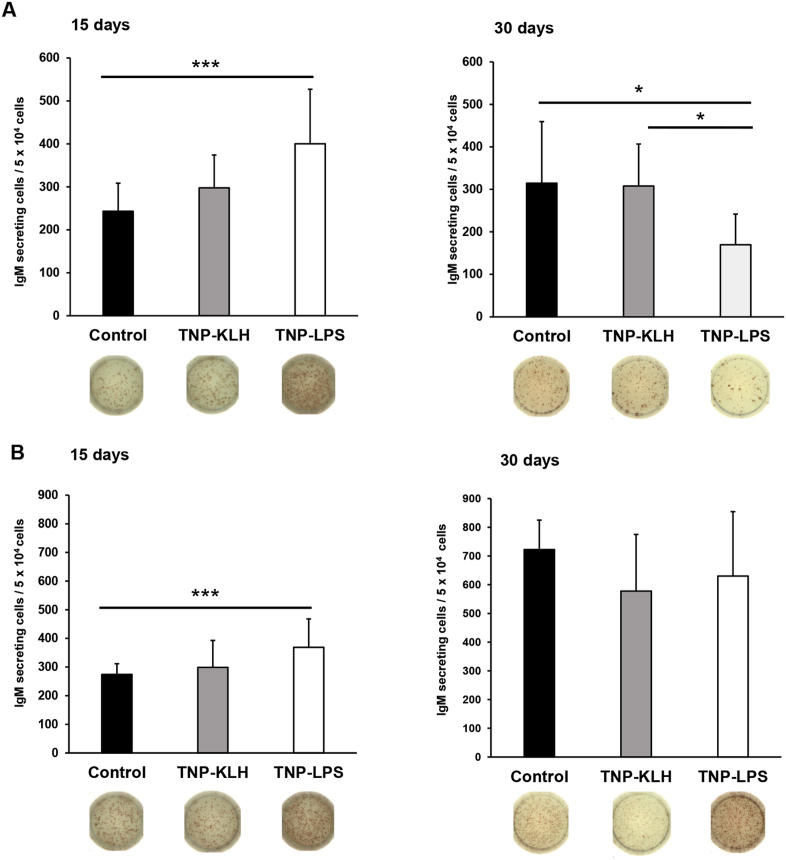
Having established that TNP-LPS-immunized fish were able to mount a systemic TNP-specific IgM response, we next wanted to study whether total or TNP-specific IgM-secreting cells could be detected in different central immune organs (spleen and kidney). In the spleen, although the number of IgM-secreting cells significantly increased in fish immunized with TNP-LPS at day 15 post-immunization when compared to control fish (Fig. 2A); after 30 days of immunization, the number of IgM-secreting cells in this group was significantly lower than that of control or TNP-KLH-immunized fish (Fig. 2A). In the case of the kidney, only a significant increase in the number of IgM-secreting cells in fish immunized with TNP-LPS was observed at day 15 post-immunization (Fig. 2B).
Fig. 2.
Detection of total IgM-secreting 15 and 30 days after the anal administration of the antigens. To determine the amount of total IgM-secreting cells in (A) spleen and (B) kidney, ELISPOT plates were coated with an anti-trout IgM mAb (clone 4C10) and developed using the same mAb biotinylated. Results are shown as mean number of IgM-secreting cells per 5 × 104 cells +SD (n = 7). Representative wells for each condition are shown. Asterisks denote significantly different values among groups as indicated (*p ≤ 0.05 and ***p ≤ 0.005).
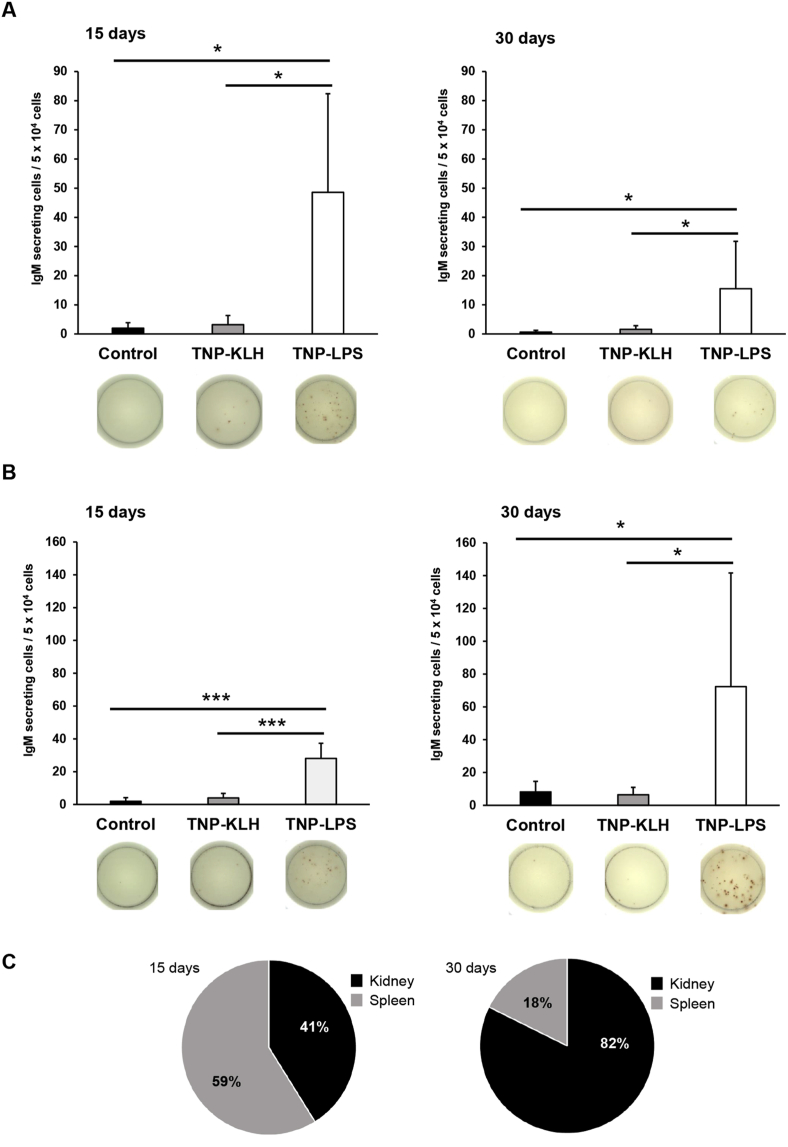
We next determined the number of cells secreting TNP-specific IgMs. In this case, fish immunized with TNP-LPS contained a significantly higher number of cells secreting TNP-specific IgMs than fish in the other two groups (Fig. 3). Although this difference was evident after both 15 and 30 days post-immunization, the number of cells secreting TNP-specific IgMs in the spleen was higher after 15 days (Fig. 3A). On the other hand, although the number of cells secreting TNP-specific IgMs was also significantly higher in the kidney in fish immunized with TNP-LPS after 15 and 30 days (Fig. 3B), in this case, the number of cells increased with time (Fig. 3B). Thus, at day 15 post-immunization, it is the spleen that bears a higher percentage of cells secreting TNP-specific IgMs, while these cells are mostly found in the kidney at later time points (Fig. 3C).
Fig. 3.
Quantification of cells secreting TNP-specific IgMs in (A) spleen and (B) kidney 15 and 30 days after the anal administration of the antigens. To determine the amount of cells secreting TNP-specific IgMs, ELISPOT plates were coated with TNP-BSA and developed using a biotinylated anti-trout IgM mAb (clone 4C10). Results are shown as mean number of IgM-secreting cells per 5 × 104 cells +SD (n = 7). Representative wells for each condition are shown. Asterisks denote significantly different values among groups as indicated (*p ≤ 0.05 and ***p ≤ 0.005). (C) Pie charts show the ratio of cells secreting TNP-specific IgM between spleen and kidney at different time points in fish anally immunized with TNP-LPS.
3.3. Flow cytometry analysis of IgM+ B cells in anally immunized fish
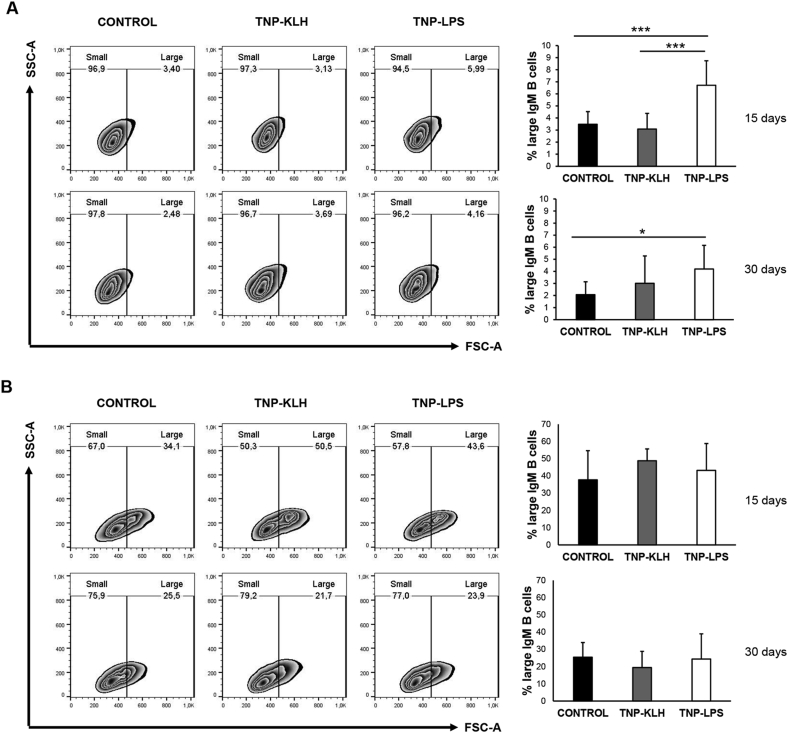
It has been previously established that as B cells differentiate towards a plasmablast/plasma cell profile, they increase their size (Granja and Tafalla, 2019; Zhang et al., 2010). For this reason, we evaluated the percentage of large IgM+ B cells that were present in the spleen and kidney of fish anally immunized with the different antigens. In the spleen, the percentage of large IgM+ B cells among total IgM+ B cells was significantly higher in fish immunized with TNP-LPS than in fish immunized with TNP-KLH or control fish (Fig. 4A). After 30 days, the percentage of large IgM+ B cells in fish immunized with TNP-LPS was still significantly higher than that of control fish but was not significantly different than that found in fish immunized with TNP-KLH (Fig. 4A). In the kidney, no differences in the percentage of large cells were found among groups at any time point (Fig. 4B).
Fig. 4.
Percentage of large and small IgM+ B cells in (A) spleen and (B) kidney 15 and 30 days after the anal administration of antigens. Leukocytes isolated from spleen and kidney of rainbow trout stimulated anally with the different antigens were incubated with an anti-trout IgM mAb conjugated to phycoerythrin (PE) and analyzed by flow cytometry. Cells were gated as lymphoid on the basis of their FSC and SSC and percentages of small (naïve) and large (activated) IgM+ B cells were determined on singlet, live (DAPI negative) IgM+ B cells. Representative dot plots showing the percentage of small and large IgM+ B cells are included for each group and tissue at both sampling times. Graphs on the right show the percentage of large IgM+ B cells (mean +SD, n = 7). Asterisks denote significantly different values between TNP stimulated groups compared with control group (*p ≤ 0.05 and ***p ≤ 0.001).
3.4. Proliferation of IgM+ B and T cells in spleen and kidney of immunized fish
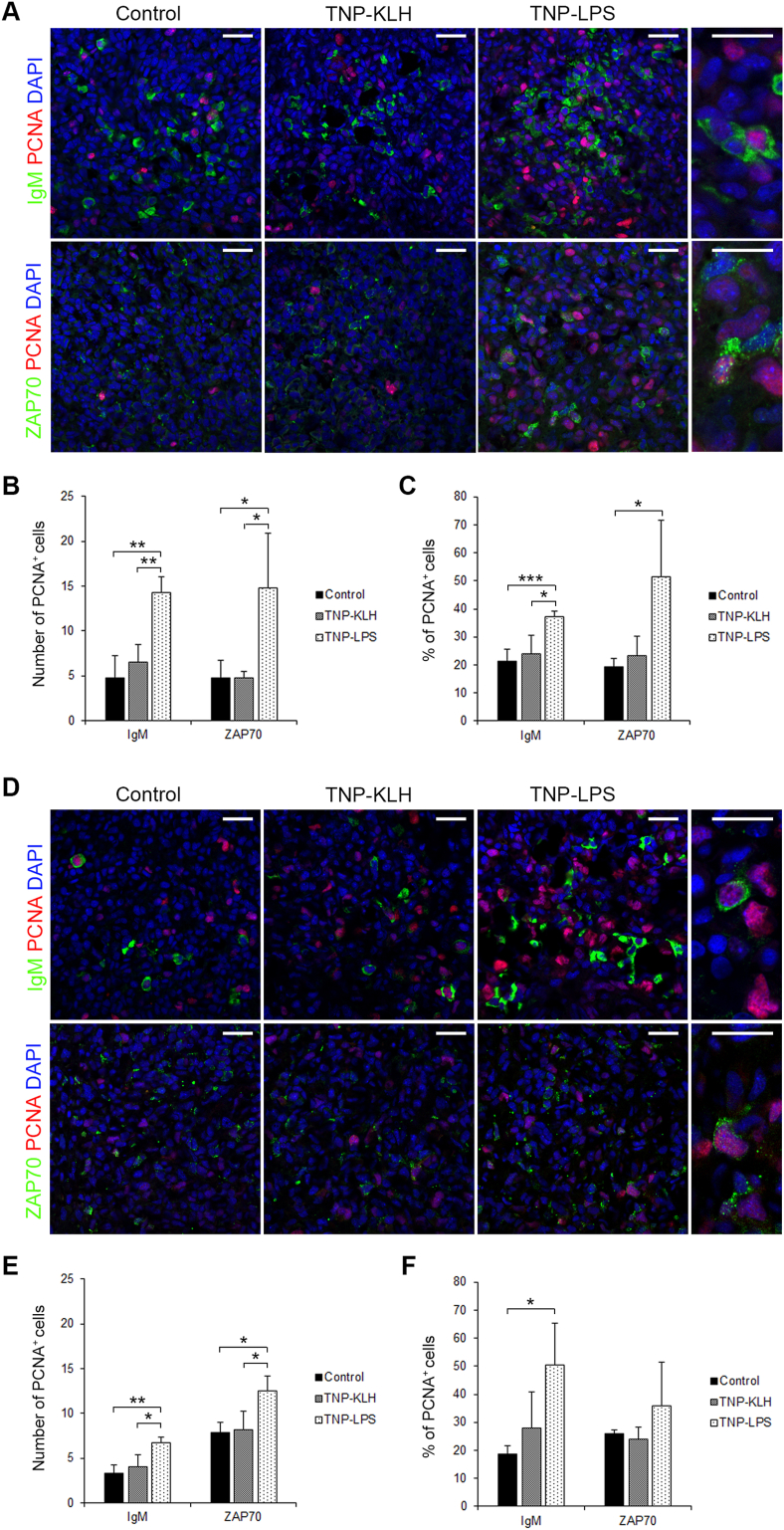
We also established whether IgM+ B cells or T cells (ZAP70+ cells) were proliferating in the spleen and kidney of fish immunized with the different antigens. For this, we used confocal microscopy, combining anti-IgM or anti-ZAP70 with an antibody directed against PCNA, an intracellular molecule whose expression and synthesis is linked with cellular proliferation (Miyachi et al., 1978). In the spleen, we found that TNP-LPS increased the total number of proliferating IgM+ B and T cells when compared to those found in other groups both after 15 (Fig. 5A and B) and 30 days (Fig. 5D and E) of immunization. These results referred to total numbers of IgM+PCNA+ or ZAP70+PCNA+ cells found in 10 digital fields. Additionally, we calculated the percentage of IgM+PCNA+ or ZAP70+PCNA+ cells among the total number of IgM+ or ZAP70+ cells in these fields. In this case, fish immunized with TNP-LPS showed significantly higher percentages of IgM+PCNA+ cells at both time points (Fig. 5C, F), while the percentages of ZAP70+PCNA+ cells was only significantly higher in the spleen of TNP-LPS immunized fish after 15 days of immunization (Fig. 5C, F).
Fig. 5.
Proliferation of IgM+ and ZAP70+ cells in spleen 15 and 30 days after the anal administration of antigens. Confocal microscopy images of rainbow trout spleen sections obtained from fish anally immunized with the different antigens at days 15 (A) and 30 (D) post-immunization. Sections were labeled with anti-IgM (green), or anti-ZAP70 (green) in combination with anti-proliferating cell nuclear antigen (PCNA) (red). All sections were also counterstained with DAPI (blue). Representative images of each condition are shown (scale bars = 20 μm) along with representative images from TNP-LPS immunized spleens at higher magnification (right; scale bars = 5 μm). Note that in non-proliferating cells, nuclei appear blue whereas they appear violet in proliferating cells. Mean number (B, E) and mean percentage (C, F) of proliferating IgM+ and ZAP70+ cells were calculated in 10 digital fields (400 × magnification) from at least 4 different individuals. Asterisks denote significantly different values between indicated groups (*p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.005). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
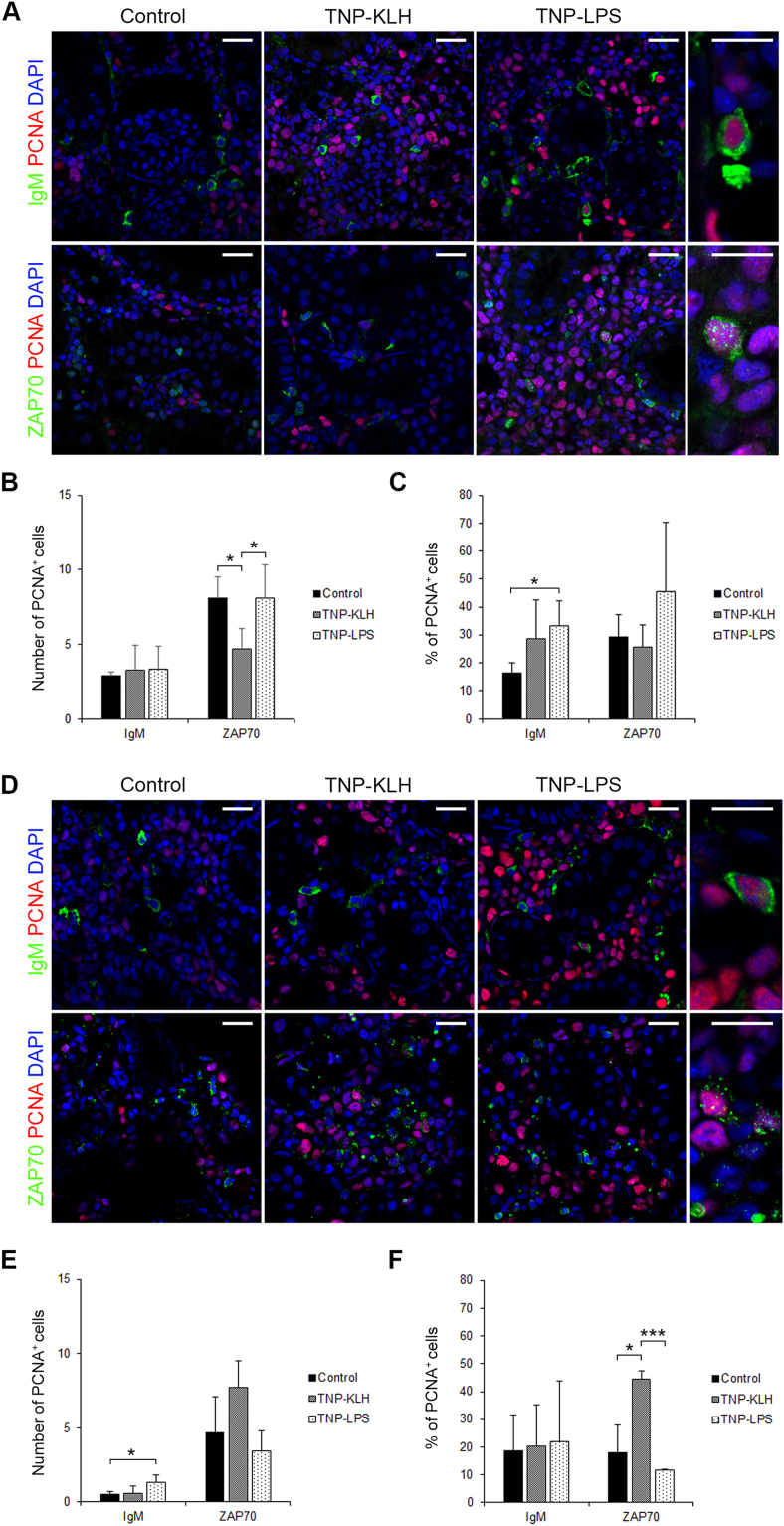
In the kidney, at day 15 post-immunization, although no significant differences were found among the number of IgM+PCNA+ cells in the different groups (Fig. 6A and B), the percentage of IgM+PCNA+ cells among total IgM+ B cells was significantly higher in TNP-LPS-immunized fish than in control fish (Fig. 6C). At this point, a significant decrease in the total number of ZAP70+PCNA+ cells was found in fish immunized with TNP-KLH when compared to other groups (Fig. 6B), however no significant differences were found among the percentages of ZAP70+PCNA+ cells among total ZAP70+ cells (Fig. 6C). At day 30 post-immunization, although the total number of IgM+PCNA+ cells was significantly higher in fish immunized with TNP-LPS than in those of other groups (Fig. 6D and E), these differences were not significant when expressed as percentage of proliferating IgM+ cells among the total IgM+ population (Fig. 6F). In the case of ZAP70+ cells, although no significant differences were obtained when total numbers of ZAP70+ cells were compared (Fig. 6D and E), the percentage of ZAP70+PCNA+ cells among total ZAP70+ cells was significantly higher in fish immunized with TNP-KLH at this point (Fig. 6F).
Fig. 6.
Proliferation of IgM+ and ZAP70+ cells in kidney 15 and 30 days after the anal administration of antigens. Confocal microscopy images of rainbow trout kidney sections obtained from fish anally immunized with the different antigens at days 15 (A) and 30 (D) post-immunization. Sections were labeled with anti-IgM (green), or anti-ZAP70 (green) in combination with anti-proliferating cell nuclear antigen (PCNA) (red). All sections were also counterstained with DAPI (blue). Representative images of each condition are shown (scale bars = 20 μm) along with representative images from TNP-LPS immunized spleens at higher magnification (right; scale bars = 5 μm). Note that in non-proliferating cells, nuclei appear blue whereas they appear violet in proliferating cells. Mean number (B, E) and mean percentage (C, F) of proliferating IgM+ and ZAP70+ cells were calculated in 10 digital fields (400 × magnification) from at least 4 different individuals. Asterisks denote significantly different values between indicated groups (*p ≤ 0.05 and ***p ≤ 0.005). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.5. Transcriptional response in spleen, kidney and gut of anally immunized fish
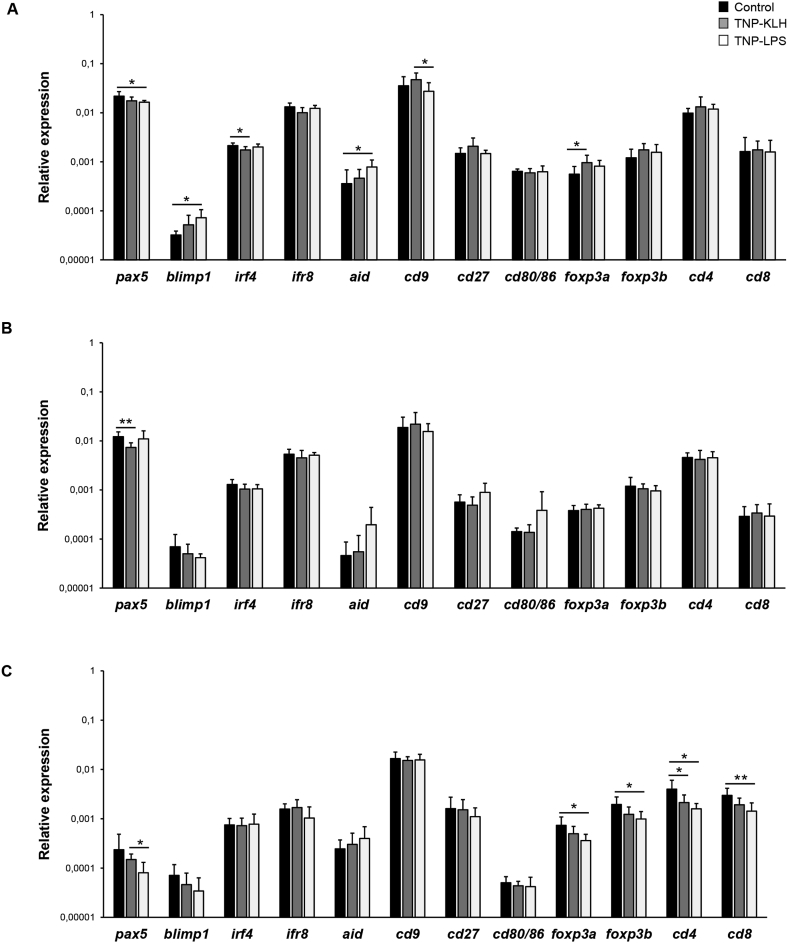
Finally, we studied the levels of transcription of a range of genes related to B and T cell functionality in the spleen, kidney and hindgut of fish anally immunized with the different antigens after 15 days. In the spleen, we found that fish immunized with TNP-LPS showed significantly higher blimp1 and aid mRNA levels than control fish, as well as significantly lower pax5 transcription levels (Fig. 7A). Fish immunized with TNP-KLH, on the other hand, exhibited a decreased irf4 and an increased foxP3a transcription in the spleen when compared to control fish (Fig. 7A). In the case of the kidney, only fish immunized with TNP-KLH showed a significantly lower level of pax5 transcription than control fish (Fig. 7B). In the intestine, changes were only detected in TNP-LPS-immunized fish when compared to controls, with significant decreases in foxP3a, foxP3b, cd4 and cd8 transcription (Fig. 7C).
Fig. 7.
Transcription of genes related to B and T cell activity in spleen (A), kidney (B) and gut (C) 15 days after the anal administration of antigens. Total RNA was extracted from the tissues obtained from vaccinated fish sacrificed at day 15 post-immunization and used to determine the levels of transcription of different genes related to B and T cell activity by real time PCR. Results are shown as the gene expression relative to the expression of endogenous control EF-1α (mean +SD, n = 7). Asterisks denote significantly different values among groups as indicated (*p ≤ 0.05 and **p ≤ 0.01).
4. Discussion
In fish, as in other animals including mammals, most infections occur or initiate at mucosal surfaces. To prevent these infectious diseases, it is therefore important to induce a strong mucosal immune response through vaccination that can neutralize the pathogen at these early replication sites. In this context, and knowing that, when successful, mucosal vaccination provides a superior ability than systemic vaccination to induce local mucosal immune responses (Chen and Cerutti, 2010), it is imperative that we investigate how the teleost immune system responds to antigens presented at mucosal surfaces and to what degree they are able to overcome mucosal tolerance. Therefore, understanding for example how B cells differentiate and respond to orally- or anally-delivered antigens will be helpful for the future design of effective oral vaccines.
Thus, in the current study, we have investigated the differentiation and proliferation of IgM+ B cells in spleen and kidney after the anal administration of model TD or TI antigens. For this, the antigens were delivered without additional adjuvants. In these conditions, we found that only TI antigens were able to induce a specific humoral response. Similarly, rainbow trout splenic B cells were also found to preferentially respond to TI antigens in the absence of additional adjuvants (Granja et al., 2019), suggesting that the engagement of innate receptors that takes place during the recognition of the pathogen motifs present in TI antigens is an essential step to achieve a full activation of B cells. On the other hand, it seems that for trout B cells the signals received in response to TD antigens through the BCR (signal 1) in combination with cognate help from T cells (signal 2) are not sufficient to induce a robust antibody response. Although evidence gathered in the past years supports that activation of B cells through innate receptors (signal 3) is important to achieve a complete activation state also in mammals (Rawlings et al., 2012; Ruprecht and Lanzavecchia, 2006), it seems that this early recognition of innate patterns is even more decisive in fish where signaling through the B cell receptor is limited in comparison to mammalian conventional B cells (Abos et al., 2018a; Granja et al., 2019) and where naïve B cells express a wider range of innate receptors (Abos et al., 2013). Interestingly, when TNP-KLH was delivered anally to coho salmon in the form of enteric coated antigen microspheres (ECAM), humoral anti-TNP antibodies were detected (Piganelli et al., 1994), thus it might be possible that the co-polymer used to obtain the microspheres was acting as an adjuvant/innate stimulator thus facilitating a full B cell activation.
When the presence of antigen-specific ASCs was studied, similar results were obtained as TNP-specific ASCs were exclusively detected in fish anally immunized with TNP-LPS and were not identified in fish immunized with the TD antigen TNP-KLH. These ASCs were found in both the spleen and the kidney at day 15 post-immunization, while at later time points it was the kidney that accommodated most of these cells. These results are in concordance with the model proposed by Bromage and collaborators (Bromage et al., 2004) in which B cells developed in the anterior kidney and were then distributed to the periphery (blood or spleen) where they differentiated into plasmablasts and plasma cells upon antigen encounter. After some time, these plasmablasts or plasma cells that had differentiated within the periphery home to the anterior kidney, wherein plasma cells are maintained for long time periods. Our results suggest that similar B cell dynamics take place after antigen encounter in the intestinal mucosa, with the spleen responding at earlier time points and the kidney maintaining the antigen-specific ASCs for longer time periods. In our experiments, given the size of the fish, we did not distinguish between anterior and posterior kidney. However, previous studies have demonstrated that it is the anterior kidney that contains most long lived plasma cells while the posterior kidney houses activated B cells and plasmablasts (Zwollo et al., 2005). Due to the fact that very low numbers of leukocytes are obtained from the gut of fish of the size used in these studies, we were not able to study the local activation and/or differentiation of B cells upon antigen encounter. Thus, although it is highly probable that specific ASCs are also found within the intestinal mucosa, this is something that should be confirmed in future studies. These studies would also help us establish whether intestinal B cells travel to the spleen and later on to the kidney, or if splenic B cells are activated due to antigen arrival to this organ.
Previous studies undertaken in rainbow trout demonstrated that ASC differentiation is dependent on cell proliferation (Bromage et al., 2004), therefore, we also studied if IgM+ B cells and T cells (defined as ZAP70+ cells) were proliferating in spleen and kidney after anal immunization with either TD and TI antigens. As occurred when the presence of TNP-specific responses were determined, it was in the fish that had been immunized with TNP-LPS and not in those immunized with TNP-KLH that most of the proliferative responses were found. Thus, the total number and the percentage of proliferating IgM+ B cells and T cells was significantly higher in the spleen of fish immunized with TNP-LPS, being the differences higher after 15 days than at later time points. These changes were not so evident in the kidney, confirming again that it is in the spleen where B and T cells differentiate to ASC through a proliferative process, while these cells just travel to the kidney afterwards once they have already differentiated. Interestingly, the percentage of proliferating ZAP70+ T cells significantly increased in the kidney after 30 days in fish immunized with TNP-KLH. This result confirms that TNP-KLH is in fact inducing a response in the fish, even if it does not lead to the production of TNP-specific antibodies. However, the biological significance of this up-regulation of ZAP70+ cells in the kidney is still unknown and should be further investigated.
Finally, we also performed a transcriptional analysis at day 15 post-immunization in spleen and kidney, this time also comparing the results to those obtained in the hindgut. Most of the gene regulations observed in response to TNP-LPS were detected in the spleen and were consistent with a differentiation from naïve B cells to plasmablasts/plasma cells (i.e. pax5 downregulation and blimp1 and aid upregulations). In the kidney, these genes were not regulated in response to TNP-LPS and only a transcriptional down-regulation of pax5 was detected in response to TNP-KLH. These results also seem to support that a differentiation program does not take place in the kidney. In the hindgut, most of the genes regulated by TNP-LPS locally were genes related to T cells, such as cd4, cd8 and the two isoforms of foxp3 found in rainbow trout. As FoxP3 is expressed in a subset of CD4+ T cells that play a suppressive role in the immune system (Kim, 2009), the downregulation of their mRNA levels in response to TNP-LPS might be indicative of a breach in peripheral tolerance through the down-regulation of these regulatory T cells. In response to TNP-KLH all these genes were down-regulated when compared to control animals, but the reduction was not significant suggesting that only TI antigens are capable of efficiently overcoming this peripheral tolerance.
In conclusion, we have demonstrated that the anal administration of TI antigens induces in rainbow trout an efficient B cell response that results in the production of serum antigen-specific IgMs and the differentiation of antigen-specific ASCs in the spleen and kidney. This response was not achieved when TD antigens were administered in the absence of additional adjuvants, suggesting that an engagement of innate immune receptors is required to achieve a full activation. Our results also support the previously formulated hypothesis that teleost B cells differentiate in the spleen or the periphery and then migrate to the kidney where they are maintained for longer time periods. This is in concordance with the following findings: antigen-specific ASCs are found preferentially in the kidney at later time points; IgM+ B cells only increase in size in the spleen; the proliferation of IgM+ B cells is mostly observed in the spleen; and genes related to B cell differentiation are not transcriptionally regulated in the kidney. Finally, the local regulation of genes related to T cells including foxp3 in response to TNP-LPS points to a required local down-regulation of regulatory T cells to overcome tolerance and achieve a full activation of B cells.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgements
This work was supported by the European Research Council (ERC Consolidator Grant 725061), by the Spanish Ministry of Science, Innovation and Universities (project AGL2017-85494-C2-1-R) and by the Comunidad de Madrid (grant 2016-T1/BIO-1672).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dci.2020.103715.
Contributor Information
Patricia Díaz-Rosales, Email: diaz.patricia@inia.es.
Carolina Tafalla, Email: tafalla@inia.es.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abos B., Bird S., Granja A.G., Morel E., More Bayona J.A., Barreda D.R., Tafalla C. Identification of the first teleost CD5 molecule: additional evidence on phenotypical and functional similarities between fish IgM(+) B cells and mammalian B1 cells. J. Immunol. 2018;201:465–480. doi: 10.4049/jimmunol.1701546. [DOI] [PubMed] [Google Scholar]
- Abos B., Castro R., Pignatelli J., Luque A., Gonzalez L., Tafalla C. Transcriptional heterogeneity of IgM(+) cells in rainbow trout (Oncorhynchus mykiss) tissues. PloS One. 2013;8 doi: 10.1371/journal.pone.0082737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abos B., Estensoro I., Perdiguero P., Faber M., Hu Y., Diaz Rosales P., Granja A.G., Secombes C.J., Holland J.W., Tafalla C. Dysregulation of B cell activity during proliferative kidney disease in rainbow trout. Front. Immunol. 2018;9:1203. doi: 10.3389/fimmu.2018.01203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros N.A., Castro R., Abos B., Rodríguez Saint-Jean S.S., Pérez-Prieto S.I., Tafalla C. The pyloric caeca area is a major site for IgM(+) and IgT(+) B cell recruitment in response to oral vaccination in rainbow trout. PloS One. 2013;8(6) doi: 10.1371/journal.pone.0066118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothby M.R., Hodges E., Thomas J.W. Molecular regulation of peripheral B cells and their progeny in immunity. Genes Dev. 2019;33:26–48. doi: 10.1101/gad.320192.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromage E.S., Kaattari I.M., Zwollo P., Kaattari S.L. Plasmablast and plasma cell production and distribution in trout immune tissues. J. Immunol. 2004;173:7317–7323. doi: 10.4049/jimmunol.173.12.7317. [DOI] [PubMed] [Google Scholar]
- Corbeil S., Kurath G., LaPatra S.E. Fish DNA vaccine against infectious hematopoietic necrosis virus: efficacy of various routes of immunisation. Fish Shellfish Immunol. 2000;10:711–723. doi: 10.1006/fsim.2000.0286. [DOI] [PubMed] [Google Scholar]
- Chen K., Cerutti A. Vaccination strategies to promote mucosal antibody responses. Immunity. 2010;33:479–491. doi: 10.1016/j.immuni.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Evensen O., Mutoloki S. IPNV Antigen uptake and distribution in Atlantic salmon following oral administration. Viruses. 2015;7:2507–2517. doi: 10.3390/v7052507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embregts C.W., Forlenza M. Oral vaccination of fish: lessons from humans and veterinary species. Dev. Comp. Immunol. 2016;64:118–137. doi: 10.1016/j.dci.2016.03.024. [DOI] [PubMed] [Google Scholar]
- Embregts C.W.E., Tadmor-Levi R., Vesely T., Pokorova D., David L., Wiegertjes G.F., Forlenza M. Intra-muscular and oral vaccination using a Koi Herpesvirus ORF25 DNA vaccine does not confer protection in common carp (Cyprinus carpio L.) Fish Shellfish Immunol. 2019;85:90–98. doi: 10.1016/j.fsi.2018.03.037. [DOI] [PubMed] [Google Scholar]
- Granja A.G., Perdiguero P., Martin-Martin A., Diaz-Rosales P., Soleto I., Tafalla C. Rainbow trout IgM(+) B cells preferentially respond to thymus-independent antigens but are activated by CD40L. Front. Immunol. 2019;10:2902. doi: 10.3389/fimmu.2019.02902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granja A.G., Tafalla C. Different IgM(+) B cell subpopulations residing within the peritoneal cavity of vaccinated rainbow trout are differently regulated by BAFF. Fish Shellfish Immunol. 2019;85:9–17. doi: 10.1016/j.fsi.2017.10.003. [DOI] [PubMed] [Google Scholar]
- Joosten P.H.M., Kruijer W.J., Rombout J.H. Anal immunisation of carp and rainbow trout with different fractions of a Vibrio anguillarum bacterin. Fish Shellfish Immunol. 1996;6:541–551. [Google Scholar]
- Kim C.H. FOXP3 and its role in the immune system. Adv. Exp. Med. Biol. 2009;665:17–29. doi: 10.1007/978-1-4419-1599-3_2. [DOI] [PubMed] [Google Scholar]
- Lokka G., Austbo L., Falk K., Bjerkas I., Koppang E.O. Intestinal morphology of the wild Atlantic salmon (Salmo salar) J. Morphol. 2013;274:859–876. doi: 10.1002/jmor.20142. [DOI] [PubMed] [Google Scholar]
- Lokka G., Koppang E.O. Antigen sampling in the fish intestine. Dev. Comp. Immunol. 2016;64:138–149. doi: 10.1016/j.dci.2016.02.014. [DOI] [PubMed] [Google Scholar]
- Ma C., Ye J., Kaattari S.L. Differential compartmentalization of memory B cells versus plasma cells in salmonid fish. Eur. J. Immunol. 2013;43:360–370. doi: 10.1002/eji.201242570. [DOI] [PubMed] [Google Scholar]
- Miyachi K., Fritzler M.J., Tan E.M. Autoantibody to a nuclear antigen in proliferating cells. J. Immunol. 1978;121:2228–2234. [PubMed] [Google Scholar]
- Munoz-Atienza E., Tavara C., Diaz-Rosales P., Llanco L., Serrano-Martinez E., Tafalla C. Local regulation of immune genes in rainbow trout (Oncorhynchus mykiss) naturally infected with Flavobacterium psychrophilum. Fish Shellfish Immunol. 2019;86:25–34. doi: 10.1016/j.fsi.2018.11.027. [DOI] [PubMed] [Google Scholar]
- Mutoloki S., Munang'andu H.M., Evensen O. Oral vaccination of fish - antigen preparations, uptake, and immune induction. Front. Immunol. 2015;6:519. doi: 10.3389/fimmu.2015.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piganelli J.D., Zhang J.A., Christensen J.M., Kaattari S.L. Enteric coated microspheres as an oral method for antigen delivery to salmonids. Fish Shellfish Immunol. 1994;4:179–188. [Google Scholar]
- Plant K.P., Lapatra S.E. Advances in fish vaccine delivery. Dev. Comp. Immunol. 2011;35:1256–1262. doi: 10.1016/j.dci.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Rawlings D.J., Schwartz M.A., Jackson S.W., Meyer-Bahlburg A. Integration of B cell responses through Toll-like receptors and antigen receptors. Nat. Rev. Immunol. 2012;12:282–294. doi: 10.1038/nri3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombout J.H., Abelli L., Picchietti S., Scapigliati G., Kiron V. Teleost intestinal immunology. Fish Shellfish Immunol. 2011;31:616–626. doi: 10.1016/j.fsi.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Ruprecht C.R., Lanzavecchia A. Toll-like receptor stimulation as a third signal required for activation of human naive B cells. Eur. J. Immunol. 2006;36:810–816. doi: 10.1002/eji.200535744. [DOI] [PubMed] [Google Scholar]
- Villumsen K.R., Neumann L., Ohtani M., Strom H.K., Raida M.K. Oral and anal vaccination confers full protection against enteric redmouth disease (ERM) in rainbow trout. PloS One. 2014;9 doi: 10.1371/journal.pone.0093845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J., Kaattari I., Kaattari S. Plasmablasts and plasma cells: reconsidering teleost immune system organization. Dev. Comp. Immunol. 2011;35:1273–1281. doi: 10.1016/j.dci.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Yoon S., Mitra S., Wyse C., Alnabulsi A., Zou J., Weerdenburg E.M., van der Sar A.M., Wang D., Secombes C.J., Bird S. First demonstration of antigen induced cytokine expression by CD4-1+ lymphocytes in a poikilotherm: studies in zebrafish (Danio rerio) PloS One. 2015;10 doi: 10.1371/journal.pone.0126378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.A., Salinas I., Li J., Parra D., Bjork S., Xu Z., LaPatra S.E., Bartholomew J., Sunyer J.O. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat. Immunol. 2010;11:827–835. doi: 10.1038/ni.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwollo P., Cole S., Bromage E., Kaattari S. B cell heterogeneity in the teleost kidney: evidence for a maturation gradient from anterior to posterior kidney. J. Immunol. 2005;174:6608–6616. doi: 10.4049/jimmunol.174.11.6608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.