Abstract
Since discovering that ketamine has robust antidepressant effects, the glutamatergic system has been proposed as an attractive target for the development of novel antidepressants. Among the glutamatergic system, metabotropic glutamate (mGlu) receptors are of interest because mGlu receptors play modulatory roles in glutamatergic transmission, consequently, agents acting on mGlu receptors might not exert the adverse effects associated with ketamine. mGlu receptors have eight subtypes that are classified into three groups, and the roles of each mGlu receptor subtype in depression are being investigated. To date, the potential use of mGlu5 receptor antagonists and mGlu2/3 receptor antagonists as antidepressants has been actively investigated, and the mechanisms underlying these antidepressant effects are being delineated. Although the outcomes of clinical trials using an mGlu5 receptor negative allosteric modulator and an mGlu2/3 receptor negative allosteric modulator have not been encouraging, these trials have been inconclusive, and additional trials using other compounds with more appropriate profiles are needed. In contrast, the roles of group III mGlu receptors have not yet been fully elucidated because of a lack of suitable pharmacological tools. Nonetheless, investigations of the use of mGlu4 and mGlu7 receptors as drug targets for the development of antidepressants have been ongoing, and some interesting evidence has been obtained.
Keywords: mGlu2 receptor, mGlu3 receptor, mGlu4 receptor, mGlu5 receptor, mGlu7 receptor, antidepressant, ketamine
Introduction
Major depressive disorder (MDD) is a highly prevalent, recurrent, and debilitating disorder that affects millions of people worldwide. Clinically available medications such as selective serotonin reuptake inhibitors (SSRIs) and serotonin and noradrenaline reuptake inhibitors (SNRIs) only improve symptoms in about two thirds of patients after several weeks of treatment.1,2 This implies that the dysfunction of other neurotransmitter systems besides monoaminergic systems is important for the manifestation of depression. Glutamate, the major excitatory neurotransmitter in the mammalian central nervous system, plays a number of important roles in physiological conditions but also in the pathophysiology of depression.3
Glutamate is basically released presynaptically into the synaptic cleft and acts via two distinct classes of receptors: ionotropic glutamate (iGlu) receptors and metabotropic glutamate (mGlu) receptors. iGlu receptors are pharmacologically divided into three receptor types (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), kainate, and N-methyl-D-aspartate (NMDA)), each of which is formed by heteromeric assemblies of multiple subunit proteins (AMPA: GluA1-4; kainate: GluK1-5; and NMDA: GluN1, GluN2A-D, GluN3A, B). mGlu receptors, which are seven-transmembrane domain G-protein-coupled receptors are divided into three major functional subgroups. mGlu receptors regulate intracellular signals via both cAMP and phosphatidyl inositol cascades and modulate the capacity of the neuronal membrane potential. Group I mGlu receptors, which include the mGlu1 receptor and the mGlu5 receptor, are predominantly expressed at the postsynaptic terminal and can activate the inositol-1,4,5-trisphosphate-calcium and diacylglycerol-protein kinase C cascades. In addition, postsynaptic mGlu5 receptors are functionally linked to NMDA receptors to modulate their activity. The presynaptic group I mGlu receptor can facilitate neurotransmitter release upon activation. Group II mGlu receptors include the mGlu2 and mGlu3 receptors that reside predominantly on the presynaptic terminal and can inhibit presynaptic glutamate release through the inhibition of adenylyl cyclase. Astrocytes also express the mGlu3 receptor, but its function in neurotransmission has not been fully investigated. Group III mGlu receptors include the mGlu4, 6, 7, and 8 receptors, which have a negative feedback function in presynaptic glutamate release via the inhibition of adenylyl cyclase. The localization and pharmacological properties of each mGlu receptor subtype are summarized in Table 1.
Table 1.
Distribution, signaling and pharmacological properties of mGlu receptors.
| Group I |
Group II |
Group III |
||||||
|---|---|---|---|---|---|---|---|---|
| mGlu1 | mGlu5 | mGlu2 | mGlu3 | mGlu4 | mGlu6 | mGlu7 | mGlu8 | |
| Signaling | Gq/11 | Gq/11 | Gi/o | Gi/o | Gi/o | Gi/o | Gi/o | Gi/o |
| Distribution | Cerebellum olfactory bulb hippocampus | Cortex hippocampus caudate-putamen | Cortex hippocampus | Cortex hippocampus amygdala | Cerebellum | Retina | Cortex hippocampus amygdala | Olfactory bulb cortex |
| Cell type | Neurons | Neurons glial cells | Neurons | Neurons glial cells | Neurons | ON bipolar cells | Neurons | Neurons |
| Representative agonists or PAMs | DHPG | CHPG CDPPB ADX47273 | LY404039 LY354740 MGS0008 MGS0028 | LY404039 LY354740 MGS0008 MGS0028 | LSP4-2022 ADX88178 Lu AF21934 | HomoAMPA | LSP4-2022 AMN082 VU0155094 VU0422288 | LSP4-2022 ADX88178 |
| Representative antagonists or NAMs | JNJ16567083 LY367385 | basimglurant MPEP MTEP | decoglurant LY341495 MGS0039 | decoglurant LY341495 MGS0039 | CPPG | CPPG | XAP044 MMPIP ADX71743 | LY341495 |
NAM: negative allosteric modulator.
In addition to the excitatory synaptic transmission mentioned above, the activation of glutamatergic receptors contributes to many forms of synaptic plasticity. The activity-dependent modifications of the strength and efficacy of synaptic transmission at synapses are thought to play a key role in learning and memory. Synaptic plasticity is also considered to be a potential target for neuropsychiatric disorders including depression.
The glutamatergic system has recently received much attention as a potential therapeutic target for depression since the discovery of the antidepressant effect of ketamine, a non-competitive NMDA receptor antagonist;4 this discovery stands out as one of the most important findings in the history of the discovery of antidepressants. Ketamine shows rapid and robust antidepressant effects that have been reproduced across institutes and even in patients with treatment-resistant depression who have failed to respond to currently available treatments multiple times.5,6 However, ketamine has some adverse effects such as the induction of psychotomimetic/dissociative symptoms and the potential for abuse. Therefore, much effort has been invested in finding an alternate treatment that has a similar therapeutic action but avoids the adverse effects of ketamine. Given that each of the mGlu receptors has a unique expression pattern and function, mGlu receptors have been recognized as attractive and potential therapeutic targets for depression.
Group I mGlu Receptors for Depression
Antidepressant Effects in Rodents
Negative allosteric modulators (NAMs) of the mGlu5 receptor, including MPEP, MTEP, GRN-529 and basimglurant, have been reported to induce antidepressant effects in rodent models of depression.7–10 Consistent with these pharmacological studies, the genetic deletion of the mGlu5 receptor also results in an antidepressant-like phenotype, supporting the hypothesis that mGlu5 receptor blockade can exert antidepressant effects.11Moreover, a seven-day treatment with DSR-98776, an mGlu5 receptor NAM, induced antidepressant effects in a forced swimming test (FST) using male Wistar rats, while paroxetine, an SSRI, required two weeks of treatment before the manifestation of its effects,12 suggesting that DSR-98776 has a faster acting antidepressant efficacy. On the other hand, although repeated treatments with MTEP have exerted antidepressant effects in an olfactory bulbectomy model of depression in rats and treatments with basimglurant have exerted antidepressant effects in a chronic mild stress model of depression in mice, either of these studies provided any evidence that mGlu5 receptor NAMs exert their effects faster than typical antidepressant agents.8,9 Thus, more studies are needed to elucidate whether mGlu5 receptor NAMs have a faster onset of antidepressant action, compared with currently prescribed antidepressants. In addition to their antidepressant effects, mGlu5 receptor NAMs have been reported to improve the comorbidities of MDD, including anxiety and pain as well as daytime sleepiness, without affecting locomotion in rodents.7,8,13 Of note, effective treatment for comorbidities of MDD is also beneficial for the treatment of depression. Collectively, these findings suggest that mGlu5 receptor NAMs could be potent agents for the treatment of depression.
However, full blockade or inverse agonist activity by full mGlu5 NAMs has been reported to induce adverse effects, including psychosis in humans and psychotomimetic-like effects in animals.14,15 Recent studies have demonstrated that MTEP, a full mGlu5 receptor NAM, potentiated phencyclidine (PCP)-induced hyperlocomotion in rodents, which was presumably associated with the blockade of the NMDA receptor, while partial NAMs, which reduce the maximal glutamate response by 50% in vitro, did not exacerbate the psychotomimetic-like effects even at doses at which nearly all mGlu5 receptors were occupied.14,16 Importantly, partial NAMs of the mGlu5 receptor exerted their antidepressant effects without affecting PCP-induced hyperlocomotion.14 Therefore, these findings suggest that mGlu5 receptor partial NAMs are more suitable as new antidepressants than full NAMs.
Synaptic and Neural Basis of the Antidepressant Effects of mGlu5 Receptor Antagonists
The mechanisms of the antidepressant effects of mGlu5 receptor NAMs reportedly differ from those of ketamine. Unlike the effects of ketamine, the antidepressant/anxiolytic effects of mGlu5 receptor NAMs were not fully blocked by a tropomyosin receptor kinase B (TrkB) inhibitor, a mechanistic target of rapamycin complex 1 (mTORC1) inhibitor,13 nor by an AMPA receptor antagonist.17 Moreover, an mGlu5 receptor NAM did not change the mTORC1 signal and synaptic protein levels.17 Therefore, the activation of AMPA receptor/TrkB/mTORC1 signaling is not involved in the antidepressant effects of mGlu5 receptor NAMs. This suggestion is also supported by the finding that an mGlu5 receptor NAM, unlike ketamine, did not induce sustained antidepressant effects in the FST.17,18
In contrast to synaptic mechanisms, serotonergic systems have been reported to play an important role in the antidepressant effects of mGlu5 receptor NAMs. The antidepressant/anxiolytic effects of mGlu5 receptor NAMs were blocked by 5-HT depletion and a 5-HT2A/2C receptor antagonist, suggesting that the stimulation of 5-HT2A/2C is involved in the actions of mGlu5 receptor NAMs.19,20 However, there is an inconsistent finding: the antidepressant effects of an mGlu5 receptor NAM were not blocked by a 5-HT-depleting agent.12 Therefore, further research is needed to elucidate the involvement of the serotonergic systems in the antidepressant effects of mGlu5 receptor NAMs. Notably, we reported that ketamine induced its antidepressant effects through stimulation of the 5-HT1A receptor but not the 5-HT2A/2C receptor. Therefore, serotonergic systems are involved in the antidepressant effects of mGlu5 receptor NAMs in a manner that differs from that which occurs in the case of ketamine.19,21
The cell-specific deletion of the mGlu5 receptor in parvalbumin-positive gamma-aminobutyric acid (GABA) interneurons, which negatively regulate glutamatergic neurons, has been reported to induce antidepressant effects, presumably through an increase in glutamatergic activity in the medial prefrontal cortex (mPFC).22 Interestingly, glutamatergic activation has been considered to be involved in the antidepressant actions of ketamine.23 However, there are opposite reports that mGluR5 antagonists inhibit glutamate release and that mGluR5 NAMs downregulate excessive glutamate transmission.24,25 These differences in the effects of mGlu5 receptor NAMs on glutamatergic transmission may reflect the expression diversity of mGlu5 receptors and their modulatory functions in addition to differences in their blockade efficacies.
Notably, transplantation of bone marrow from knockout mice lacking mGlu5 receptor has recently been reported to exhibit antidepressant effects, and the effects may be ascribed to reduced releases of pro-inflammatory cytokines such as interleukin (IL)-1β, tumor necrosis factor α, and IL-6.26 Therefore, reduced inflammatory response may also be involved in the antidepressant actions of mGlu5 receptor antagonists.
Clinical Studies
Studies using positron emission tomography (PET) and postmortem brain have been performed to examine the involvement of the mGlu5 receptor in the pathophysiology of depression and antidepressant effects. Although the mGlu5 receptor level was found to be unchanged in postmortem brain27–29 and in another PET study30 in MDD patients, lower levels of mGlu5 receptor binding in several brain regions have been observed in patients with MDD, and this change is considered to be a compensatory change.27 Likewise, in postmortem studies, lower levels of mGlu5 receptor protein have been reported in the PFC (Brodmann's area 10 [BA10]) and cerebellum in patients with MDD,27,28 although regional variability has been recognized. Consistent with these studies, recent study, by combination of a selective mGlu5 receptor PET ligand and resting-state functional magnetic resonance imaging, also indicated lower mGlu5 receptor availability and related functional connectivity alterations in drug-naïve young adults with MDD without comorbidity.31 Recently, ketamine has been reported to reduce mGlu5 receptor availability, which is associated with an antidepressant response in patients with MDD, suggesting that the attenuation of mGlu5 receptor activity plays an important role in the antidepressant effects of ketamine.32
The mGlu5 receptor NAM basimglurant, which has been used as an adjunctive therapy to ongoing SSRI or SNRI treatment, has been reported to not have any effect on the change in the primary end point of the clinician-rated Montgomery-Åsberg Depression Rating Scale (MADRS) between baseline and the end of treatment when adjunctive basimglurant versus a placebo were compared in a six-week, double-blind, placebo-controlled study in MDD patients.33 In contrast, the same study demonstrated that basimglurant enabled an improvement across secondary and exploratory end points, particularly in patient-rated measures.33 However, another mGlu5 NAM AZD2066 did not induce significant effects on the MADRS change between baseline and week 6, compared with a placebo, in a phase II clinical trial in patients with MDD, although this trial can be regarded as having failed because duloxetine, which was used as a positive comparator, also did not have any effect (see results at the http://clinicaltrials.gov website: NCT01145755). Collectively, whether mGlu5 receptor NAMs are effective clinically has yet to be determined, and further clinical studies are needed.
Group II mGlu Receptor for Depression
Antidepressant Effects in Rodents
Unique antidepressant effects of mGlu2/3 receptor blockade have been demonstrated in rodent models of depression. LY341495, an orthosteric mGlu2/3 receptor antagonist, reversed the decreased sucrose preference caused by chronic unpredictable stress (CUS) in rats within 48 h after a single dose.34 Another orthosteric mGlu2/3 receptor antagonist, MGS0039, ameliorated depressive-like behaviors observed in socially defeated mice within 24 h after a single injection.35 Low doses of ketamine also exerted antidepressant effects within 24 h after drug administration in both models.35,36 On the other hand, traditional antidepressants took weeks to reach antidepressant efficacy.37,38 These findings suggest that mGlu2/3 receptor antagonists have rapid antidepressant effects. In addition, the reversal of depression-like behaviors was still observed for at least 7 to 10 days after a single dose of ketamine and mGlu2/3 receptor antagonists in both models,34,35 indicating that mGlu2/3 receptor antagonists also have the ability to produce long-lasting antidepressant actions. Interestingly, similar to ketamine, orthosteric mGlu2/3 receptor antagonists were shown to exert antidepressant effects in animal models resistant to current antidepressants,39–41 suggesting that orthosteric mGlu2/3 receptor antagonists potentially produce antidepressant effects similar to those of ketamine for treatment-resistant depression. The antidepressant effects of mGlu2/3 receptor antagonists have also been observed in several behavioral assays using naïve rodents. Ketamine and mGlu2/3 receptor antagonists reportedly decreased the immobility time in the tail suspension test (TST) and FST at 30 min as well as at 24 h after injection.36,42–46 Both ketamine and LY341495 have been shown to shorten the latency to feed in the novelty-suppressed feeding test within 30 min, and the effects lasted for at least 24 h after treatment.21,36,47 The opportunity to detect the unique antidepressant effects of ketamine and mGlu2/3 receptor antagonists even in naïve rodents is an additional impetus to facilitate investigations of the mechanism underlying those antidepressant actions.
In addition to orthosteric mGlu2/3 receptor antagonists, RO4491533, an mGlu2 receptor NAM, binds to the allosteric site of the mGlu2/3 receptor and has been shown to exert antidepressant effects in rodents,48 although the potencies of mGlu2/3 receptor NAMs are weaker than those of orthosteric antagonists.49 The reason for this might be at least partly due to the different mechanisms of antagonistic action against the mGlu2/3 receptor (orthosteric vs. allosteric). Indeed, different numbers of bindings of mGlu2/3 receptor-radiolabeled ligands were reported between ligands that bind to orthosteric sites and those that bind to allosteric sites.50
Notably, LY3020371, an orthosteric mGlu2/3 receptor antagonist, showed an antidepressant effect in preclinical assays but did not produce ketamine-like adverse effects, such as psychotomimetic-like behavior and the potential for abuse.51 Moreover, MGS0039 and RO4432717 enhanced social and object recognition in rats, respectively.52,53 In addition to pro-cognitive effects, mGlu2/3 receptor antagonists also exert anxiolytic effects in some animal models.54 Therefore, mGlu2/3 receptor antagonism could prove to be a safer therapeutic approach, compared with ketamine. Furthermore, mGlu2/3 receptor antagonists may have additional benefits, as cognitive impairments and anxiety symptoms are often observed in depressive patients. Interestingly, LY341495 reportedly enhanced the antidepressant effects of ketamine,55 suggesting that a combination therapy consisting of ketamine and mGlu2/3 receptor antagonists might be able to reduce the adverse effects of ketamine by decreasing the required dose of ketamine. Collectively, these results suggest that blockade of the mGlu2/3 receptor can produce ketamine-like antidepressant actions while enabling the unpleasant adverse effects of ketamine to be avoided.
Synaptic and Neural Basis of the Antidepressant Effects of mGlu2/3 Receptor Antagonists
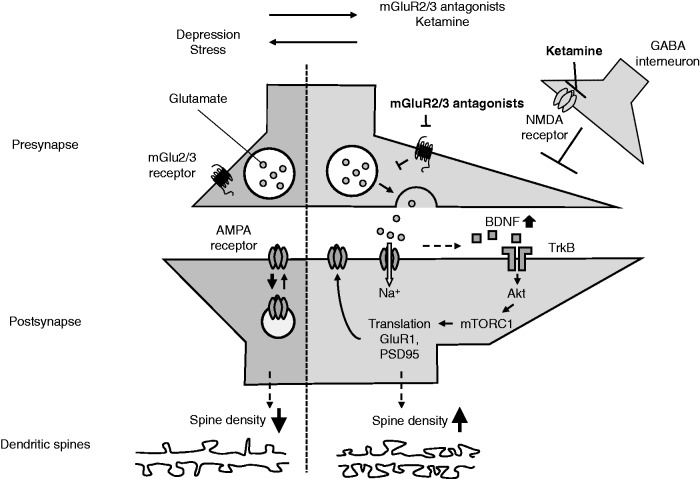
Studies using rodents have revealed several molecular and structural alternations that contribute to the antidepressant effects of mGlu2/3 receptor antagonists. These overlapping mechanisms underlie the antidepressant effects of low subanesthetic doses of ketamine. The facilitation of glutamate signaling is thought to be a trigger that induces the antidepressant effects of mGlu2/3 receptor antagonists, since the mGlu2/3 receptor negatively regulates glutamate release at presynaptic terminals. Extracellular glutamate within the mPFC of rodents is transiently increased by ketamine,56 possibly via the disinhibition of pyramidal neurons upon the blockade of NMDA receptors on GABA interneurons.57Pretreatment with NBQX, an AMPA receptor antagonist, has been reported to prevent the antidepressant effects of both mGlu2/3 receptor antagonists and ketamine.43,45,46 The necessity of AMPA receptor stimulation, presumably through glutamate release, suggests that the antidepressant effects of those compounds are activity dependent. Preclinical investigations have reported that postsynaptic AMPA receptor stimulation increases brain-derived neurotrophic factor (BDNF) release and subsequently activates mTORC1 signaling and extracellular regulated kinase, leading to the upregulation of synaptic proteins in the mPFC.58 These signal cascades triggered by AMPA receptor stimulation are necessary for the sustained antidepressant effects of mGlu2/3 receptor antagonists and ketamine as demonstrated by the pharmacological and genetic inhibition of those effectors, which blocked the antidepressant effects of both compounds.36,44,47,59,60 Interestingly, the expression of GluR1, an AMPA receptor subunit, in the mPFC and hippocampus was upregulated within 24 h after treatment with ketamine and mGlu2/3 receptor antagonists.35,59,61 Consistent with the expression change, the AMPA receptor-mediated current was enhanced in the mPFC of ketamine-treated rats at 24 h after injection,62 suggesting that upregulated GluR1 is functionally integrated into the excitatory transmission in the mPFC. This strengthened the assumption that AMPA receptor-mediated transmission in the mPFC contributes to the antidepressant effects of ketamine and mGlu2/3 receptor antagonists. Indeed, NBQX injection 30 min before the FST prevented the antidepressant effects of ketamine and LY341495, the effects of which were assessed 24 h after treatment.45,63 Therefore, these findings suggest that AMPA receptor stimulation is responsible for the antidepressant effects of ketamine and mGlu2/3 receptor antagonists in two different ways: one is via glutamate release immediately after the injection and the other is via the upregulation of AMPA receptor expression and neurotransmission, though the former triggers the latter. In addition to GluR1, increases in the expressions of other synaptic proteins that are proposed to induce synaptic remodeling, such as synaptogenesis, are critical for the antidepressant effects, as the inhibition of BDNF and mTORC1 signaling prevents the increased expression of synaptic proteins and spine density.59,60 The causal relevance has yet to be demonstrated. However, a key role in synaptic remodeling in the mPFC is supported by the findings that a decreased dendritic spine density in the PFC in animal models of depression was associated with depressive behaviors and that these behaviors were ameliorated by MGS0039 and ketamine.35,59 The proposed synaptic mechanisms of the mGlu2/3 receptor described above are illustrated in Figure 1.
Figure 1.
Synaptic mechanisms underlying the antidepressant effects of mGlu2/3 receptor antagonists. mGlu2/3 receptor antagonists, which act via the blockade of perisynaptic autoreceptors, increase glutamate release into the synaptic craft and stimulate postsynaptic AMPA receptors, while ketamine is proposed to increase glutamate release by disinhibition of GABA interneurons. Stimulation of the AMPA receptor increases BDNF synthesis or secretion, which subsequently activates TrkB receptor signaling. The activation of TrkB signaling then activates AKT/mTORC1 signaling, leading to an increase in the synthesis of synaptic proteins, such as GluR1 and PSD95. The increased expression of GluR1 (and its insertion into the membrane) is also necessary for mGlu2/3 receptor antagonists to exert sustained antidepressant effects. In a depressed state, it is hypothesized that these pathways are disrupted, which eventually reduces the dendritic spine density. mGlu2/3 receptor antagonists reverse these spine deficits by allowing the disrupted pathways to resume. AMPA: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; NMDA: N-methyl-D-aspartate; GABA: gamma-aminobutyric acid; BDNF: brain-derived neurotrophic factor.
Currently prescribed antidepressants improve depressive symptoms by globally increasing the concentrations of monoamine neurotransmitters in the brain. Both serotoninergic and dopaminergic systems have also been proposed to mediate the antidepressant effects of the mGlu2/3 receptor antagonists and ketamine, but their cellular and circuit mechanisms are different from those of current antidepressants. mGlu2/3 receptor antagonists increased the spontaneous firing rates of serotonergic neurons in the dorsal raphe nucleus (DRN) and 5-HT release in the mPFC.64 The antidepressant effects of LY341495 and ketamine were blocked by the pharmacological depletion of 5-HT and a 5-HT1A receptor antagonist, suggesting that the serotonergic system, especially 5-HT1A receptor stimulation, is necessary for mGlu2/3 receptor antagonists to exert their antidepressant effects.18 The local injection of LY341495 and NBQX in the mPFC of mice revealed that mGlu2/3 receptor blockade within the mPFC activated the subpopulation of 5-HT neurons in the DRN, the activation of which was caused by AMPA receptor stimulation in the mPFC.18 Furthermore, a 5-HT1A receptor antagonist injected in the mPFC prevented the antidepressant effects of LY341495.65 Of note, these neural mechanisms are also necessary for the antidepressant effects of ketamine.18,66 These findings suggest that mGlu2/3 receptor antagonist and ketamine initially activate mPFC neurons in an AMPA receptor-dependent manner, resulting in the activation of DRN serotonergic neurons projecting to the mPFC; the subsequent release of 5-HT in the mPFC activates postsynaptic 5-HT1A receptors to exert their antidepressant effects. In addition, mGlu2/3 receptor antagonists and ketamine have been reported to increase the number of spontaneously active dopamine neurons in the ventral tegmental area and, accordingly, to elevate dopamine release in the mPFC and nucleus accumbens.41,67 NBQX prevented these events, indicating an AMPA receptor stimulation dependency.41,67 However, although the stimulation of dopamine D1 receptor on the pyramidal neurons in the mPFC was required for the antidepressant effect of ketamine in the FST and NFST,68 to date, no direct evidence exists demonstrating that dopaminergic transmission contributes to the antidepressant actions of mGlu2/3 receptor antagonists. Collectively, these findings suggest that not only the synaptic mechanisms but also the neural mechanisms of mGlu2/3 receptor antagonists are shared with ketamine.
Although mGlu2 receptor blockade is likely involved in the antidepressant effects of mGlu2/3 receptor antagonists, the roles of the individual subtypes (mGlu2 or mGlu3 receptors) are still under discussion. LY341495 no longer exerted antidepressant effects in knockout mice lacking the mGlu2 receptor but not possessing the mGlu3 receptor,49 suggesting that the mGlu2 receptor rather than the mGlu3 receptor plays a critical role in the actions of mGlu2/3 receptor antagonists. This finding is consistent with the finding that knockout mice lacking the mGlu2 receptor displayed increased reward activity and an antidepressant-like phenotype in the FST.69 In contrast, selective mGlu3 receptor NAMs and mGlu2 NAMs have been recently synthesized, and interesting results were reported using these pharmacological tools. A selective mGlu3 NAM exerted an antidepressant effect in the FST and anxiolytic effects on marble-burying behavior.70 It was further reported that a selective mGlu3 receptor NAM exerted antidepressant effects in the TST comparable to those of ketamine, while a selective mGlu2 receptor NAM did not have a significant effect at an appropriate exposure level.71 These findings suggest that mGlu3 blockade has a larger contribution to the antidepressant effects of mGlu2/3 receptor NAMs. It would be interesting to further investigate how mGlu3 receptor blockade leads to these antidepressant effects.
Clinical Studies
The etiological link between mGlu2/3 receptor levels and depression has been investigated using postmortem samples from MDD patients. An autoradiographic binding study using [3H]LY341495 reported reduced binding levels to mGlu2/3 receptors in the anterior cingulate cortex, BA24, in subjects with MDD but not in those with schizophrenia or bipolar disorder.72 In contrast, a study using western blotting demonstrated increased levels of mGlu2/3 receptor expression in the PFC containing BA10 in MDD patients.73 Thus, there have been mixed findings across brain regions in MDD but selective changes in the expression of the mGlu2/3 receptor across psychiatric disorders. Of note, these alterations cannot be ascribed to antidepressant treatment, as no obvious differences in the mGlu2/3 receptor expression levels in monkey and rat brains were observed following the chronic administration of antidepressants, such as fluoxetine and imipramine.72,73
The antidepressant effects of the mGlu2 NAM decoglurant administered as an adjunct to ongoing treatment have been evaluated in patients with MDD who were inadequately treated with SSRIs and SNRIs. In a phase II double-blinded, placebo-controlled study, decoglurant demonstrated an insufficient efficacy on both primary (mean change in MADRS score) and secondary outcomes (proportion of patients exhibiting remission or response based on the MADRS score).74 Consequently, decoglurant was withdrawn from the Roche pipeline. However, whether appropriate doses were used in this trial were not clarified, making the results of the trial inconclusive in terms of the potential of mGlu2/3 receptor antagonists. A human study using magnetic resonance spectroscopy recently showed the putative presence of a glutamate surge in human PFC after ketamine infusion,75 possibly contributing to synaptic plasticity. Indeed, ketamine has also been reported to increase global signal regression in the PFC at 24 h post-infusion and to show a positive relationship with the treatment response in MDD patients.76 Therefore, mGlu2/3 receptor antagonists may exert antidepressant actions at optimized doses affecting a glutamate surge in the PFC of MDD patients.
Antidepressant Potential of mGlu2 Receptor Stimulation
In contrast to the finding that mGlu2/3 receptor blockade exerts the antidepressant effects, some reports have shown that mGlu2 receptor stimulation may lead to antidepressant effects. THIIC, which is an mGlu2 receptor positive allosteric modulator (PAM), has been reported to exert antidepressant effects in three animal models of depression (mouse FST, the rat differential reinforcement of a low-rate 72-s assay, and the rat dominant-submissive test).77 The antidepressant effects of THIIC were no longer observed in knockout mice lacking the mGlu2 receptor, suggesting that this effect is mediated through the mGlu2 receptor. Notably, this is the only mGlu2 receptor PAM to demonstrate an antidepressant efficacy, and antidepressant effects have not been reported for other mGlu2 receptor PAMs or mGlu2/3 receptor agonists. The potential of mGlu2 receptor enhancement for antidepressant activity has been demonstrated by a series of studies on L-acetylcarnitine (LAC). LAC exerted antidepressant effects earlier (after 3 days of treatment) than current medications (14 days of treatment) in both genetic (Flinders sensitive line) and environmental (CUS model) animal models.78 LAC increased mGlu2 receptor expression in the hippocampus and PFC by increasing the levels of acetylated H3K27 bound to the GRM2 promoter, thereby enhancing the transcription of the GRM2 gene that encodes for the mGlu2 receptor. Moreover, the antidepressant effects of LAC were partially attenuated in mGlu2 receptor-knockout mice or mGlu2/3 receptor antagonist LY341495-treated mice. Collectively, LAC may exert its antidepressant effects at least partly through an increase in mGlu2 receptor expression. Consistent with this result, Cuccurazzu et al.79 also reported that chronic treatment for three weeks with LAC reversed the depressive-like behavior induced by chronic unpredictable mild stress (CUMS), and the treatment with LAC increased mGlu2 receptor expression in the hippocampus without changing the expression of the mGlu3 receptor. Moreover, a lower level of mGlu2 receptor has been proposed to play a critical role in susceptibility to stress. Knockout mice lacking the mGlu2 receptor are more susceptible to CUS, and a lower level of mGlu2 receptor expression in the hippocampus was observed in stress-susceptible mice than in resilient mice.80 Stimulation of the mineral corticoid receptor, but not the glucocorticoid receptor, has been proposed to mediate the stress-induced reduction in hippocampal mGlu2 receptor expression via a decrease in the level of acetylated H3K27 bound to the GRM2 promoter.80 The role of LAC in depression is interesting since the plasma LAC level was recently reported to be reduced in patients with MDD, compared with age- and sex-matched healthy controls, at two independent study centers.81 However, increased mGlu2 receptor expression in the hippocampus may not be solely responsible for the effects of LAC, and evidence for the antidepressant effects of mGlu2 receptor agonists/PAMs is limited. Therefore, the role of mGlu2 receptor stimulation in antidepressant actions needs to be further investigated.
Group III mGlu Receptors for Depression
The roles of group III mGlu receptors in depression and the antidepressant potential of agents acting on group III mGlu receptors have not been fully explored, mostly because of the limited access to selective ligands for each receptor. Among the group III mGlu receptors, nonetheless, both the mGlu4 receptor and the mGlu7 receptor have been proposed as potential therapeutic targets for depression.
LSP4-2022, an mGlu4 receptor agonist, has been reported to induce a pro-depressive effect, which was no longer observed in knockout mice lacking the mGlu4 receptor but not in knockout mice lacking the mGlu7 receptor, suggesting that the stimulation of the mGlu4 receptor may induce depressive-like behavior.82 In contrast, a selective and brain-penetrant mGlu4 receptor PAM, ADX88178, exerted an antidepressant effect.83 Moreover, an mGlu4 receptor agonist (LSP1-2111) and PAM (Lu AF21934) did not have any antidepressant effects in the mouse FST and TST, while they exhibited anxiolytic effects in rodents.84,85 Since LSP1-2111 decreased the basal locomotor activity at the doses that were used, the results need to be cautiously assessed. Therefore, the roles of the mGlu4 receptor in depression/antidepressant actions need to be studied further using potent and selective ligands for the mGlu4 receptor. Other than pharmacological studies, the roles of the mGlu4 receptor in depression have been reported from studies using microRNAs (miRNAs), which constitute a class of small non-coding RNA molecules whose function is to regulate the transcription and translation of downstream-related genes. miR-1202 was identified as the most dysregulated miRNA, with decreased expression in the ventrolateral PFC of depressed subjects. In addition, the gene encoding metabotropic glutamate receptor 4 (GRM4) was found to be negatively correlated with the expression of miR-1202 in depression. Indeed, GRM4 was upregulated in the PFC of depressed subjects.86 A role for the mGlu4 receptor in the action of ketamine has been suggested.87 Although CUMS, which caused depressive-like behavior in rats, decreased miR-29b-3 p in the PFC of rats, ketamine administration reversed the depressive-like behavior as well as the reduction of miR-29b-3 p. Consistent with the fact that miR-29b-3 p negatively regulates the gene expression of GRM4, both the mRNA and protein levels of GRM4 in the PFC were significantly increased in the CUMS rats, and these levels decreased after ketamine treatment. Interestingly, the overexpression of miR-29b-3 p relieved the depressive behaviors in the CUMS rats and lowered the expression of GRM4. Collectively, the increased mGlu4 receptor level may be related to depressive-like behavior and depressive symptoms, which is supported by the finding that mGlu4 receptor stimulation results in pro-depressive-like behavior,82 while mGlu4 receptor blockade may possibly exert antidepressant effects.
An mGlu7 receptor allosteric agonist, AMN082, has been reported to exert an antidepressant effect in some animal models,88–91 and the antidepressant effects of AMN082 were blocked by the mGlu7 receptor antagonist MMPIP92 or by mGlu7 receptor-knockout,90 suggesting that mGlu7 receptor stimulation may play an important role in the antidepressant effects. Notably, a major metabolite of AMN082 has been reported to exhibit affinity for monoamine transporters,93 and MMPIP only partially blocked the antidepressant effects of AMN082.89 Therefore, the antidepressant actions of AMN082 should be interpreted with caution.
The synaptic and neural mechanisms underlying the antidepressant effects of AMN082 have not been fully characterized. The rapid antidepressant effect of AMN082 was reported to be mediated by mTOR signaling, leading to an increase in the levels of the synaptic proteins in the PFC, similar to the case for ketamine.17 However, unlike ketamine, the antidepressant effect of AMN082 was not observed at 23 h after administration.17 Moreover, the role of the AMPA receptor in the effect of AMN082 remains controversial, since contradictory results that its antidepressant effect was blocked88 or not blocked17 by NBQX have been reported. Therefore, the underlying mechanisms of mGlu7 receptor agonists may differ from those of ketamine and mGlu2/3 receptor antagonists. Regardless of the similar functional property that mGlu2/3, mGlu4, and mGlu7 receptors work as presynaptic glutamate autoreceptors, the dissimilarity lies in the fact that while mGlu2/3 receptor antagonists (and possibly mGlu4 receptor antagonists) exert antidepressant effects, mGlu7 receptor agonists show antidepressant effects. This discrepancy may be explained by the differential cell-type expressions of these receptors. mGlu7 receptors are predominantly expressed on GABAergic terminals,94 where mGlu7 receptor stimulation inhibits GABA release.95 As a result, this event may result in the activation of glutamatergic transmission via the disinhibition of GABAergic inputs. Therefore, both mGlu7 receptor activation and mGlu2/3 receptor blockade (and possibly mGlu4 receptor blockade) may converge the activation of pyramidal neurons, although this hypothesis needs to be proven. Moreover, the mGlu7 receptor expressed on glial cells has recently been reported to exert neuroprotective effects,96 which may partly contribute to the antidepressant effects of mGlu7 receptor stimulation. The proposed synaptic mechanisms described above are illustrated in Figure 2.
Figure 2.

Proposed synaptic mechanisms underlying the antidepressant effects of an agent acting on each mGlu receptor subtype. mGlu2/3, mGlu4, and mGlu7 receptors are located in presynaptic terminals, and the blockade of these receptors leads to an increase in release of neurotransmitters. Both mGlu2/3 receptor antagonists and mGlu4 receptor antagonists increase glutamate release via autoreceptor blockade, while mGlu7 receptor agonists may predominantly act on heteroreceptors on GABAergic terminals to reduce GABA release, leading to the disinhibition of inhibitory inputs to the pyramidal neurons. On the other hand, mGlu5 receptor antagonists block extrasynaptic mGlu5 receptors, which positively regulate extrasynaptic NMDA receptor function to reduce BDNF expression. As a result, all of these mGlu receptor ligands (mGlu2/3 receptor antagonists, mGlu4 receptor antagonists, mGlu7 receptor agonists, mGlu5 receptor antagonists) may converge to increase BDNF signaling, which eventually enhances synaptic transmissions. NMDA: N-methyl-D-aspartate; GABA: gamma-aminobutyric acid; BDNF: brain-derived neurotrophic factor; AMPA: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid.
Conclusion and Future Directions
As described above, accumulating evidence suggests the antidepressant potential of both mGlu5 receptor antagonists and mGlu2/3 receptor antagonists. In particular, mGlu2/3 receptor antagonists have been demonstrated to exert antidepressant effects via underlying mechanisms that are similar to those of ketamine. On the other hand, mGlu2/3 receptor antagonists may be devoid of ketamine-like adverse effects, such as psychotomimetic/dissociated behavior and the potential for abuse. Therefore, mGlu2/3 receptor antagonists may provide a useful means of pharmacotherapy, retaining ketamine’s rapid and sustained antidepressant effects without the drawbacks associated with ketamine treatment. An mGlu2/3 receptor NAM, decoglurant, was tested in clinical trials for patients with depression who had been inadequately treated with ongoing antidepressants, but the results were not encouraging. However, the appropriateness of this study, including the properties of decoglurant, the doses used, and patient stratifications, must be carefully examined, and differences in the pharmacological properties between orthosteric and allosteric antagonists need to be considered. Therefore, clinical trials with appropriate compounds and optimized study designs are needed before a conclusion can be made regarding the efficacy of mGlu2/3 receptor antagonists.
Regarding mGlu5 receptor NAMs, although basimglurant failed to show any efficacy in terms of the primary outcomes in clinical trials, recent studies have provided alternative approaches. mGlu5 receptor partial NAMs have been reported to exhibit the same antidepressant potency as mGlu5 receptor full NAMs but with a lower likelihood of causing psychotomimetic-like behavior. Therefore, the efficacy of mGlu5 receptor partial NAMs at doses capable of achieving a sufficient receptor occupancy in clinical trials needs to be tested.
Compared with mGlu5 receptor antagonists and mGlu2/3 receptor antagonists, the roles of group III mGlu receptors in depression have not been evaluated because of the lack of selective pharmacological tools. In future studies, it will be important to clarify whether stimulation or blockade is required for each receptor subtype and whether agents acting on these receptors are likely to have an antidepressant potential similar to that of ketamine.
Since mGlu receptors play critical roles regulating glutamatergic tones which may be disrupted in depression, and some mGlu receptors are uniquely distributed in brain regions associated with depression, targeting mGlu receptors for the development of novel antidepressants is a very exciting field that deserves further attention.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Shigeyuki Chaki, Hiroyuki Koike, and Kenichi Fukumoto are employees of Taisho Pharmaceutical Co., Ltd.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006; 163: 1905–1917. [DOI] [PubMed] [Google Scholar]
- 2.Trivedi MH, Rush AJ, Wisniewski SR, et al. STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 2006; 163: 28–40. [DOI] [PubMed] [Google Scholar]
- 3.Niciu MJ, Ionescu DF, Richards EM, et al. Glutamate and its receptors in the pathophysiology and treatment of major depressive disorder. J Neural Transm (Vienna) 2014; 121: 907–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 2000; 47: 351–354. [DOI] [PubMed] [Google Scholar]
- 5.Murrough JW, Iosifescu DV, Chang LC, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry 2013; 170: 1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zarate CA, Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 2006; 63: 856–864. [DOI] [PubMed] [Google Scholar]
- 7.Hughes ZA, Neal SJ, Smith DL, et al. Negative allosteric modulation of metabotropic glutamate receptor 5 results in broad spectrum activity relevant to treatment resistant depression. Neuropharmacology 2013; 66: 202–214. [DOI] [PubMed] [Google Scholar]
- 8.Lindemann L, Porter RH, Scharf SH, et al. Pharmacology of basimglurant (RO4917523, RG7090), a unique metabotropic glutamate receptor 5 negative allosteric modulator in clinical development for depression. J Pharmacol Exp Ther 2015; 353: 213–233. [DOI] [PubMed] [Google Scholar]
- 9.Palucha A, Branski P, Szewczyk B, et al. Potential antidepressant-like effect of MTEP, a potent and highly selective mGluR5 antagonist. Pharmacol Biochem Behav 2005; 81: 901–906. [DOI] [PubMed] [Google Scholar]
- 10.Tatarczyńska E, Klodzińska A, Chojnacka-Wójcik E, et al. Potential anxiolytic- and antidepressant-like effects of MPEP, a potent, selective and systemically active mGlu5 receptor antagonist. Br J Pharmacol 2001; 132: 1423–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Need AB, Baez M, et al. Metabotropic glutamate 5 receptor antagonism is associated with antidepressant-like effects in mice. J Pharmacol Exp Ther 2006; 319: 254–259. [DOI] [PubMed] [Google Scholar]
- 12.Kato T, Takata M, Kitaichi M, et al. DSR-98776, a novel selective mGlu5 receptor negative allosteric modulator with potent antidepressant and antimanic activity. Eur J Pharmacol 2015; 757: 11–20. [DOI] [PubMed] [Google Scholar]
- 13.Iijima M, Fukumoto K, Chaki S. Acute and sustained effects of a metabotropic glutamate 5 receptor antagonist in the novelty-suppressed feeding test. Behav Brain Res 2012; 235: 287–292. [DOI] [PubMed] [Google Scholar]
- 14.Gould RW, Amato RJ, Bubser M, et al. Partial mGlu5 negative allosteric modulators attenuate cocaine-mediated behaviors and lack psychotomimetic-like effects. Neuropsychopharmacology 2016; 41: 1166–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pecknold JC, McClure DJ, Appeltauer L, et al. Treatment of anxiety using fenobam (a nonbenzodiazepine) in a double-blind standard (diazepam) placebo-controlled study. J Clin Psychopharmacol 1982; 2: 129–133. [PubMed] [Google Scholar]
- 16.Pisani A, Gubellini P, Bonsi P, et al. Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-D-aspartate responses in medium spiny striatal neurons. Neuroscience 2001; 106: 579–587. [DOI] [PubMed] [Google Scholar]
- 17.Pałucha-Poniewiera A, Szewczyk B, Pilc A. Activation of the mTOR signaling pathway in the antidepressant-like activity of the mGlu5 antagonist MTEP and the mGlu7 agonist AMN082 in the FST in rats. Neuropharmacology 2014; 82: 59–68. [DOI] [PubMed] [Google Scholar]
- 18.Fukumoto K, Iijima M, Chaki S. The antidepressant effects of an mGlu2/3 Receptor antagonist and ketamine require AMPA receptor stimulation in the mPFC and subsequent activation of the 5-HT neurons in the DRN. Neuropsychopharmacology 2016; 41: 1046–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukumoto K, Chaki S. Involvement of serotonergic system in the effect of a metabotropic glutamate 5 receptor antagonist in the novelty-suppressed feeding test. J Pharmacol Sci 2015; 127: 57–61. [DOI] [PubMed] [Google Scholar]
- 20.Pałucha-Poniewiera A, Brański P, Wierońska JM, et al. The antidepressant-like action of mGlu5 receptor antagonist, MTEP, in the tail suspension test in mice is serotonin dependent. Psychopharmacology (Berl) 2014; 231: 97–107. [DOI] [PubMed] [Google Scholar]
- 21.Fukumoto K, Iijima M, Chaki S. Serotonin-1A receptor stimulation mediates effects of a metabotropic glutamate 2/3 receptor antagonist, 2S-2-amino-2-(1S,2S-2-carboxycycloprop-1-yl)-3-(xanth-9-yl)propanoic acid (LY341495), and an N-methyl-D-aspartate receptor antagonist, ketamine, in the novelty-suppressed feeding test. Psychopharmacology (Berl) 2014; 231: 2291–2298. [DOI] [PubMed] [Google Scholar]
- 22.Lee KW, Westin L, Kim J, et al. Alteration by p11 of mGluR5 localization regulates depression-like behaviors. Mol Psychiatry 2015; 20: 1546–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerhard DM, Wohleb ES, Duman RS. Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug Discov Today 2016; 21: 454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morin N, Morissette M, Grégoire L, et al. Chronic treatment with MPEP, an mGlu5 receptor antagonist, normalizes basal ganglia glutamate neurotransmission in L-DOPA-treated parkinsonian monkeys. Neuropharmacology 2013; 73: 216–231. [DOI] [PubMed] [Google Scholar]
- 25.Thomas LS, Jane DE, Harris JR, et al. Metabotropic glutamate autoreceptors of the mGlu(5) subtype positively modulate neuronal glutamate release in the rat forebrain in vitro. Neuropharmacology 2000; 39: 1554–1566. [DOI] [PubMed] [Google Scholar]
- 26.Liu YW, Zhao L, Zhou M, et al. Transplantation with mGluR5 deficiency bone marrow displays antidepressant-like effect in C57BL/6J mice [published online ahead of print January 22, 2019]. Brain Behav Immun. doi:10.1016/j.bbi.2019.01.022. [DOI] [PubMed]
- 27.Deschwanden A, Karolewicz B, Feyissa AM, et al. Reduced metabotropic glutamate receptor 5 density in major depression determined by [(11)C]ABP688 PET and postmortem study. Am J Psychiatry 2011; 168: 727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fatemi SH, Folsom TD, Rooney RJ, et al. mRNA and protein expression for novel GABAA receptors theta and rho2 are altered in schizophrenia and mood disorders; relevance to FMRP-mGluR5 signaling pathway. Translational psychiatry 2013; 3: e271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matosin N, Fernandez-Enright F, Frank E, et al. Metabotropic glutamate receptor mGluR2/3 and mGluR5 binding in the anterior cingulate cortex in psychotic and nonpsychotic depression, bipolar disorder and schizophrenia: implications for novel mGluR-based therapeutics. J Psychiatry Neurosci 2014; 39: 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdallah CG, Hannestad J, Mason GF, et al. Metabotropic glutamate receptor 5 and glutamate involvement in major depressive disorder: a multimodal imaging study. Biol Psychiatry Cogn Neurosci Neuroimaging 2017; 2: 449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim JH, Joo YH, Son YD, et al. In vivo metabotropic glutamate receptor 5 availability-associated functional connectivity alterations in drug-naïve young adults with major depression. Eur Neuropsychopharmacol 2019; 29: 278–290. [DOI] [PubMed] [Google Scholar]
- 32.Esterlis I, DellaGioia N, Pietrzak RH, et al. Ketamine-induced reduction in mGluR5 availability is associated with an antidepressant response: an [11C]ABP688 and PET imaging study in depression. Mol Psychiatry 2018; 23: 824–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quiroz JA, Tamburri P, Deptula D, et al. Efficacy and safety of basimglurant as adjunctive therapy for major depression: a randomized clinical trial. JAMA Psychiatry 2016; 73: 675–684. [DOI] [PubMed] [Google Scholar]
- 34.Dwyer JM, Lepack AE, Duman RS. mGluR2/3 blockade produces rapid and long-lasting reversal of anhedonia caused by chronic stress exposure. J Mol Psychiatry 2013; 1: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong C, Zhang JC, Yao W, et al. Rapid and sustained antidepressant action of the mGlu2/3 receptor antagonist MGS0039 in the social defeat stress model: comparison with ketamine. Int J Neuropsychopharmacol 2017; 20: 228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li N, Lee B, Liu RJ, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010; 329: 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsankova NM, Berton O, Renthal W, et al. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci 2006; 9: 519–525. [DOI] [PubMed] [Google Scholar]
- 38.Willner P. The chronic mild stress (CMS) model of depression: history, evaluation and usage. Neurobiol Stress 2016; 6: 78–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ago Y, Yano K, Araki R, et al. Metabotropic glutamate 2/3 receptor antagonists improve behavioral and prefrontal dopaminergic alterations in the chronic corticosterone-induced depression model in mice. Neuropharmacology 2013; 65: 29–38. [DOI] [PubMed] [Google Scholar]
- 40.Koike H, Iijima M, Chaki S. Effects of ketamine and LY341495 on the depressive-like behavior of repeated corticosterone-injected rats. Pharmacol Biochem Behav 2013; 107: 20–23. [DOI] [PubMed] [Google Scholar]
- 41.Witkin JM, Monn JA, Schoepp DD, et al. The Rapidly Acting Antidepressant Ketamine and the mGlu2/3 Receptor Antagonist LY341495 Rapidly Engage Dopaminergic Mood Circuits. J Pharmacol Exp Ther 2016; 358: 71–82. [DOI] [PubMed] [Google Scholar]
- 42.Chaki S, Yoshikawa R, Hirota S, et al. MGS0039: a potent and selective group II metabotropic glutamate receptor antagonist with antidepressant-like activity. Neuropharmacology 2004; 46: 457–467. [DOI] [PubMed] [Google Scholar]
- 43.Karasawa J, Shimazaki T, Kawashima N, et al. AMPA receptor stimulation mediates the antidepressant-like effect of a group II metabotropic glutamate receptor antagonist. Brain Res 2005; 1042: 92–98. [DOI] [PubMed] [Google Scholar]
- 44.Koike H, Iijima M, Chaki S. Involvement of the mammalian target of rapamycin signaling in the antidepressant-like effect of group II metabotropic glutamate receptor antagonists. Neuropharmacology 2011; 61: 1419–1423. [DOI] [PubMed] [Google Scholar]
- 45.Koike H, Chaki S. Requirement of AMPA receptor stimulation for the sustained antidepressant activity of ketamine and LY341495 during the forced swim test in rats. Behav Brain Res 2014; 271: 111–115. [DOI] [PubMed] [Google Scholar]
- 46.Maeng S, Zarate CA, Jr, Du J, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry 2008; 63: 349–352. [DOI] [PubMed] [Google Scholar]
- 47.Koike H, Fukumoto K, Iijima M, et al. Role of BDNF/TrkB signaling in antidepressant-like effects of a group II metabotropic glutamate receptor antagonist in animal models of depression. Behav Brain Res 2013; 238: 48–52. [DOI] [PubMed] [Google Scholar]
- 48.Campo B, Kalinichev M, Lambeng N, et al. Characterization of an mGluR2/3 negative allosteric modulator in rodent models of depression. J Neurogenet 2011; 25: 152–166. [DOI] [PubMed] [Google Scholar]
- 49.Gleason SD, Li X, Smith IA, et al. mGlu2/3 agonist-induced hyperthermia: an in vivo assay for detection of mGlu2/3 receptor antagonism and its relation to antidepressant-like efficacy in mice. CNS Neurol Disord Drug Targets 2013; 12: 554–566. [DOI] [PubMed] [Google Scholar]
- 50.Lavreysen H, Langlois X, Ahnaou A, et al. Pharmacological characterization of JNJ-40068782, a new potent, selective, and systemically active positive allosteric modulator of the mGlu2 receptor and its radioligand [3H]JNJ-40068782. J Pharmacol Exp Ther 2013; 346: 514–527. [DOI] [PubMed] [Google Scholar]
- 51.Witkin JM, Monn JA, Li J, et al. Preclinical predictors that the orthosteric mGlu2/3 receptor antagonist LY3020371 will not engender ketamine-associated neurotoxic, motor, cognitive, subjective, or abuse-liability-related effects. Pharmacol Biochem Behav 2017; 155: 43–55. [DOI] [PubMed] [Google Scholar]
- 52.Goeldner C, Ballard TM, Knoflach F, et al. Cognitive impairment in major depression and the mGlu2 receptor as a therapeutic target. Neuropharmacology 2013; 64: 337–346. [DOI] [PubMed] [Google Scholar]
- 53.Shimazaki T, Kaku A, Chaki S. Blockade of the metabotropic glutamate 2/3 receptors enhances social memory via the AMPA receptor in rats. Eur J Pharmacol 2007; 575: 94–97. [DOI] [PubMed] [Google Scholar]
- 54.Chaki S. mGlu2/3 Receptor Antagonists as Novel Antidepressants. Trends Pharmacol Sci 2017; 38: 569–580. [DOI] [PubMed] [Google Scholar]
- 55.Podkowa K, Pochwat B, Brański P, et al. Group II mGlu receptor antagonist LY341495 enhances the antidepressant-like effects of ketamine in the forced swim test in rats. Psychopharmacology (Berl) 2016; 233: 2901–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moghaddam B, Adams B, Verma A, et al. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 1997; 17: 2921–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Widman AJ, McMahon LL. Disinhibition of CA1 pyramidal cells by low-dose ketamine and other antagonists with rapid antidepressant efficacy. Proc Natl Acad Sci USA 2018; 115: E3007–E3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wohleb ES, Gerhard D, Thomas A, et al. Molecular and Cellular Mechanisms of Rapid-Acting Antidepressants Ketamine and Scopolamine. Curr Neuropharmacol 2017; 15: 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li N, Liu RJ, Dwyer JM, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 2011; 69(8): 754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu RJ, Lee FS, Li XY, et al. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry 2012; 71: 996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dwyer JM, Lepack AE, Duman RS. mTOR activation is required for the antidepressant effects of mGluR2/3 blockade. Int J Neuropsychopharmacol 2012; 15: 429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.El Iskandrani KS, Oosterhof CA, El Mansari M, et al. Impact of subanesthetic doses of ketamine on AMPA-mediated responses in rats: an in vivo electrophysiological study on monoaminergic and glutamatergic neurons. J Psychopharmacol 2015; 29: 792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zanos P, Moaddel R, Morris PJ, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 2016; 533: 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kawashima N, Karasawa J, Shimazaki T, et al. Neuropharmacological profiles of antagonists of group II metabotropic glutamate receptors. Neurosci Lett 2005; 378: 131–134. [DOI] [PubMed] [Google Scholar]
- 65.Fukumoto K, Iijima M, Funakoshi T, et al. 5-HT1A receptor stimulation in the medial prefrontal cortex mediates the antidepressant effects of mGlu2/3 receptor antagonist in mice. Neuropharmacology 2018; 137: 96–103. [DOI] [PubMed] [Google Scholar]
- 66.Fukumoto K, Iijima M, Funakoshi T, et al. Role of 5-HT1A receptor stimulation in the medial prefrontal cortex in the sustained antidepressant effects of ketamine. Int J Neuropsychopharmacol 2018; 21: 371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karasawa J, Kotani M, Kambe D, et al. AMPA receptor mediates mGlu 2/3 receptor antagonist-induced dopamine release in the rat nucleus accumbens shell. Neurochem Int 2010; 57: 615–619. [DOI] [PubMed] [Google Scholar]
- 68.Hare BD, Shinohara R, Liu RJ, et al. Optogenetic stimulation of medial prefrontal cortex Drd1 neurons produces rapid and long-lasting antidepressant effects. Nat Commun 2019; 10: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morishima Y, Miyakawa T, Furuyashiki T, et al. Enhanced cocaine responsiveness and impaired motor coordination in metabotropic glutamate receptor subtype 2 knockout mice. Proc Natl Acad Sci USA 2005; 102: 4170–4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Engers JL, Rodriguez AL, Konkol LC, et al. Discovery of a selective and CNS penetrant negative allosteric modulator of metabotropic glutamate receptor subtype 3 with antidepressant and anxiolytic activity in rodents. J Med Chem 2015; 58: 7485–7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Engers JL, Bollinger KA, Weiner RL, et al. Design and synthesis of N-Aryl phenoxyethoxy pyridinones as highly selective and CNS penetrant mGlu3 NAMs. ACS Med Chem Lett 2017; 8: 925–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McOmish CE, Pavey G, Gibbons A, et al. Lower [3H]LY341495 binding to mGlu2/3 receptors in the anterior cingulate of subjects with major depressive disorder but not bipolar disorder or schizophrenia. J Affect Disord 2016; 190: 241–248. [DOI] [PubMed] [Google Scholar]
- 73.Feyissa AM, Woolverton WL, Miguel-Hidalgo JJ, et al. Elevated level of metabotropic glutamate receptor 2/3 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry 2010; 34: 279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Umbricht D, Niggli M, Sanwald-Ducray P, et al. Results of a double-blind placebo-controlled study of the antidepressant effects of the mGLU2 negative allosteric modulator RG1578. European Neuropsychopharmacology 2015; 25(Supp. 2): S447. [Google Scholar]
- 75.Abdallah CG, De Feyter HM, Averill LA, et al. The effects of ketamine on prefrontal glutamate neurotransmission in healthy and depressed subjects. Neuropsychopharmacology 2018; 43: 2154–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abdallah CG, Dutta A, Averill CL, et al. Ketamine, but not the NMDAR antagonist lanicemine, increases prefrontal global connectivity in depressed patients [published online ahead of print September 21, 2018]. Chronic Stress (Thousand Oaks). doi:10.1177/2470547018796102. [DOI] [PMC free article] [PubMed]
- 77.Fell MJ, Witkin JM, Falcone JF, et al. N-(4-((2-(trifluoromethyl)-3-hydroxy-4-(isobutyryl)phenoxy)methyl)benzyl)-1-methyl-1H-imidazole-4-carboxamide (THIIC), a novel metabotropic glutamate 2 potentiator with potential anxiolytic/antidepressant properties: in vivo profiling suggests a link between behavioral and central nervous system neurochemical changes. J Pharmacol Exp Ther 2011; 336: 165–177. [DOI] [PubMed] [Google Scholar]
- 78.Nasca C, Xenos D, Barone Y, et al. L-acetylcarnitine causes rapid antidepressant effects through the epigenetic induction of mGlu2 receptors. Proc Natl Acad Sci USA 2013; 110: 4804–4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cuccurazzu B, Bortolotto V, Valente MM, et al. Upregulation of mGlu2 receptors via NF-κB p65 acetylation is involved in the Proneurogenic and antidepressant effects of acetyl-L-carnitine. Neuropsychopharmacology 2013; 38: 2220–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nasca C, Bigio B, Zelli D, et al. Mind the gap: glucocorticoids modulate hippocampal glutamate tone underlying individual differences in stress susceptibility. Mol Psychiatry 2015; 20: 755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nasca C, Bigio B, Lee FS, et al. Acetyl-l-carnitine deficiency in patients with major depressive disorder. Proc Natl Acad Sci USA 2018; 115: 8627–8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Podkowa K, Rzeźniczek S, Marciniak M, et al. A novel mGlu4 selective agonist LSP4-2022 increases behavioral despair in mouse models of antidepressant action. Neuropharmacology 2015; 97: 338–345. [DOI] [PubMed] [Google Scholar]
- 83.Kalinichev M, Le Poul E, Boléa C, et al. Characterization of the novel positive allosteric modulator of the metabotropic glutamate receptor 4 ADX88178 in rodent models of neuropsychiatric disorders. J Pharmacol Exp Ther 2014; 350: 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sławińska A, Wierońska JM, Stachowicz K, et al. Anxiolytic- but not antidepressant-like activity of Lu AF21934, a novel, selective positive allosteric modulator of the mGlu4 receptor. Neuropharmacology 2013; 66: 225–235. [DOI] [PubMed] [Google Scholar]
- 85.Wierońska JM, Stachowicz K, Pałucha-Poniewiera A, et al. Metabotropic glutamate receptor 4 novel agonist LSP1-2111 with anxiolytic, but not antidepressant-like activity, mediated by serotonergic and GABAergic systems. Neuropharmacology 2010; 59: 627–634. [DOI] [PubMed] [Google Scholar]
- 86.Lopez JP, Lim R, Cruceanu C, et al. miR-1202 is a primate-specific and brain-enriched microRNA involved in major depression and antidepressant treatment. Nat Med 2014; 20: 764–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wan YQ, Feng JG, Li M, et al. Prefrontal cortex miR-29b-3p plays a key role in the antidepressant-like effect of ketamine in rats. Exp Mol Med 2018; 50: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bradley SR, Uslaner JM, Flick RB, et al. The mGluR7 allosteric agonist AMN082 produces antidepressant-like effects by modulating glutamatergic signaling. Pharmacol Biochem Behav 2012; 101: 35–40. [DOI] [PubMed] [Google Scholar]
- 89.O'Connor RM, Cryan JF. The effects of mGlu7 receptor modulation in behavioural models sensitive to antidepressant action in two mouse strains. Behav Pharmacol 2013; 24: 105–113. [DOI] [PubMed] [Google Scholar]
- 90.Palucha A, Klak K, Branski P, et al. Activation of the mGlu7 receptor elicits antidepressant-like effects in mice. Psychopharmacology (Berl) 2007; 194: 555–562. [DOI] [PubMed] [Google Scholar]
- 91.Pałucha-Poniewiera A, Brański P, Lenda T, et al. The antidepressant-like action of metabotropic glutamate 7 receptor agonist N,N'-bis(diphenylmethyl)-1,2-ethanediamine (AMN082) is serotonin-dependent. J Pharmacol Exp Ther 2010; 334: 1066–1074. [DOI] [PubMed] [Google Scholar]
- 92.Pałucha-Poniewiera A, Pilc A. A selective mGlu7 receptor antagonist MMPIP reversed antidepressant-like effects of AMN082 in rats. Behav Brain Res 2013; 238: 109–112. [DOI] [PubMed] [Google Scholar]
- 93.Sukoff Rizzo SJ, Leonard SK, Gilbert A, et al. The metabotropic glutamate receptor 7 allosteric modulator AMN082: a monoaminergic agent in disguise? J Pharmacol Exp Ther 2011; 338: 345–352. [DOI] [PubMed] [Google Scholar]
- 94.Somogyi P, Dalezios Y, Luján R, et al. High level of mGluR7 in the presynaptic active zones of select populations of GABAergic terminals innervating interneurons in the rat hippocampus. Eur J Neurosci 2003; 17: 2503–2520. [DOI] [PubMed] [Google Scholar]
- 95.Summa M, Di Prisco S, Grilli M, et al. Presynaptic mGlu7 receptors control GABA release in mouse hippocampus. Neuropharmacology 2013; 66: 215–224. [DOI] [PubMed] [Google Scholar]
- 96.Jantas D, Lech T, Gołda S, et al. New evidences for a role of mGluR7 in astrocyte survival: possible implications for neuroprotection. Neuropharmacology 2018; 141: 223–237. [DOI] [PubMed] [Google Scholar]