The coronavirus disease 2019 (COVID-19) pandemic is likely to be the defining global health crisis of our generation. As the United Nations Development Programme highlighted in their recent call to action, the impact of this pandemic will extend beyond the immediate medical consequences to have far-reaching and long-lasting social and economic impacts, threatening to disproportionately affect poorer people in poorer countries [1]. Income losses are anticipated to exceed USD 220 billion in developing countries, where many people live day-to-day without access to social protection, and food security is precarious [1]. Strikingly, a recent United Nations study suggested that the social and economic consequences of the COVID-19 pandemic could increase the number of people living in poverty by as much as half a billion, with the majority of these newly poor people living in Africa, South-East Asia, and Central and South America [2].
Short abstract
The global health community must learn from COVID-19 and take action now on tuberculosis and its social determinants, potentially saving millions from a preventable and curable disease https://bit.ly/2LLgLgA
Introduction
The coronavirus disease 2019 (COVID-19) pandemic is likely to be the defining global health crisis of our generation. As the United Nations Development Programme highlighted in their recent call to action, the impact of this pandemic will extend beyond the immediate medical consequences to have far-reaching and long-lasting social and economic impacts, threatening to disproportionately affect poorer people in poorer countries [1]. Income losses are anticipated to exceed USD 220 billion in developing countries, where many people live day-to-day without access to social protection, and food security is precarious [1]. Strikingly, a recent United Nations study suggested that the social and economic consequences of the COVID-19 pandemic could increase the number of people living in poverty by as much as half a billion, with the majority of these newly poor people living in Africa, South-East Asia, and Central and South America [2].
COVID-19 and tuberculosis epidemiology
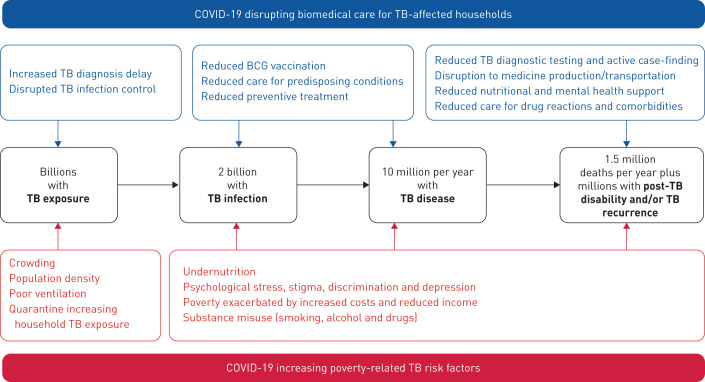
COVID-19 is likely to have catastrophic effects on tuberculosis, another global pandemic (figure 1) [3]. Tuberculosis has long been the world's leading infectious killer, until on 1 April, 2020 COVID-19 overtook tuberculosis as the infectious disease killing the most people per day. It is no coincidence that the areas of the world predicted to be most affected by the social and economic consequences of COVID-19 are also the areas with the highest tuberculosis burden [4]. This is because tuberculosis is a social as well as infectious disease: poorer, undernourished people living in densely populated areas are at higher risk of tuberculosis, and tuberculosis entrenches poverty by increasing costs, reducing income, and causing stigma and discrimination [5–7]. Indeed, poverty is the key driver of the tuberculosis pandemic, with several studies demonstrating how tuberculosis incidence rates rise and fall in association with measures of socioeconomic development and social protection [8–10]. In contrast, measures of biomedical care have had no detectable impact on tuberculosis incidence, despite helping to save millions of lives [10, 11]. If the estimates of impoverishment described above are tragically borne out, history warns us to soon expect a dramatic rise in tuberculosis incidence.
FIGURE 1.
Mechanisms by which the COVID-19 pandemic is expected to worsen the tuberculosis (TB) pandemic.
COVID-19 and tuberculosis diagnosis
The COVID-19 pandemic is also likely to have a significant impact on the provision of biomedical care for tuberculosis-affected households. Access to diagnostic testing is likely to be reduced, partly because of limited human and material resources, but also because of the social stigma of having a cough or being unwell. This stigma has always been important for tuberculosis and has been exacerbated by the COVID-19 pandemic, potentially driving people with tuberculosis to hide their illness from others and delay accessing healthcare until disease and infectiousness are advanced [12]. The World Health Organization (WHO) already estimates that approximately a third of people living with tuberculosis are either not diagnosed, treated, or reported [4]. The COVID-19 pandemic might be expected to increase the number of these “missing” people, who are a major source of ongoing transmission and have a high risk of tuberculosis-related morbidity and mortality [13, 14].
COVID-19 and tuberculosis treatment and prevention
Provision of appropriate treatment for people who are diagnosed with tuberculosis might also be affected, particularly for people with drug-resistant tuberculosis, due to disruptions in the production and transportation of medicines and supplies, reduced nutritional and mental health support, limited access to healthcare facilities, and reduced clinical care to manage adverse drug reactions and comorbidities, such as HIV, diabetes and cancer. Furthermore, impaired management of these comorbidities is also likely to significantly increase the risk of progression from latent tuberculosis infection to active disease in the general population. Relatedly, tuberculosis preventive treatment for household members is likely to be severely weakened, as strained health systems focus their limited resources on diagnosis and treatment, and visits to health facilities for non-emergencies are minimised. This is particularly alarming because transmission of tuberculosis to household members is likely to be increased by COVID-19, mediated by delayed tuberculosis diagnosis and heavier household tuberculosis exposure during household quarantine [15, 16]. Unfortunately, isolation and quarantine of unwell individuals within households is unfeasible for much of the world's population living in crowded housing in the densely populated urban areas where most of the world's tuberculosis occurs. This increased tuberculosis transmission is likely to be worsened by COVID-19-associated economic challenges, such as undernutrition, increasing tuberculosis susceptibility [15, 16].
Preventing COVID-19 from worsening tuberculosis
Taken together, the social, economic and biomedical consequences of the COVID-19 pandemic are likely to combine to create a perfect storm with respect to tuberculosis. What can be done to address this evolving crisis? Many of us wish that we had done more, sooner to address the current COVID-19 pandemic; what lessons can be learned to prevent COVID-19 from causing a secondary tuberculosis emergency? Already, the WHO has issued an information note urging the continuity of essential services for people with tuberculosis during the pandemic [17]. However, if much of the progress gained in tuberculosis care and prevention is not to be rapidly undone, further steps must be urgently taken to mitigate some of the broader impacts discussed above.
Social protection interventions
It is socioeconomic development and poverty that drive tuberculosis rates globally, so fighting tuberculosis in the context of COVID-19 demands that we address social determinants as well as biomedical care [5, 6]. Whilst people are unable to work, national and local governments need to be able to access funds to provide social protection to vulnerable populations at high risk of impoverishment, and therefore COVID-19 and tuberculosis, to reduce their risk [8, 18]. Provision should also be made for tuberculosis-specific social protection, which could take the form of cash transfers or food parcels for tuberculosis-affected households [5, 6]. Importantly, any economic support should involve engagement with patient civil society organisations, as they have a potentially critical role in providing psychosocial support to tuberculosis-affected households, reducing stigma and discrimination [19, 20]. This may harness digital technology to improve equity and efficiency, and overcome the infection control challenges associated with both tuberculosis and COVID-19 [21]. Tuberculosis-specific social protection should improve equitable access to tuberculosis care and prevention, reduce poverty-related tuberculosis risk factors, and therefore improve outcomes [22–24].
Biomedical interventions
Non-governmental organisations may partner with governments and national tuberculosis programmes to mitigate the effects of the COVID-19 pandemic on the provision of biomedical care for tuberculosis-affected households. This might include sharing diagnostic and laboratory capacity and strengthening caregiver and community health worker roles to support care delivery. To further optimise the use of resources, national tuberculosis programmes could use locally derived, simple risk stratification tools to focus interventions such as active case finding and preventive treatment to members of the highest risk households to increase their impact and cost-effectiveness [15, 16].
Integrated healthcare for tuberculosis and COVID-19
Importantly, reflecting upon the findings of Tadolini et al. [25] in their first description of patients with both tuberculosis and COVID-19 in this issue of the European Respiratory Journal highlights opportunities for how healthcare might be integrated for both diseases [26]. First, people living with tuberculosis and tuberculosis survivors are likely to be at high risk of COVID-19 and associated adverse outcomes due to chronic lung damage [13], highlighting the importance of COVID-19 testing for this population. Second, survivors of severe COVID-19 with lung damage might be at high risk of tuberculosis, and SARS-CoV-2 infection itself might increase the risk of progression from latent tuberculosis infection to active disease. Longitudinal studies are needed to explore these possibilities in further detail, but COVID-19 diagnosis could represent an opportunity for concurrent latent tuberculosis infection testing and provision of preventive treatment [27]. Third, acute COVID-19 symptoms may cause chronic sub-clinical tuberculosis to be diagnosed, probably because of overlapping symptoms, but also potentially because COVID-19 may cause people to access healthcare before tuberculosis symptoms develop, or before they become severe enough to cause care seeking [12, 28]. Therefore, in high burden areas, healthcare for people with respiratory symptoms might involve integrated testing for both diseases, potentially increasing the number of people tested for tuberculosis. Finally, given the similarities between the two diseases, there is also a clear opportunity to leverage the extensive knowledge, experience and infrastructure of tuberculosis healthcare workers and researchers on infection control and contact investigation for the control of COVID-19, and vice versa.
Research
It seems clear that taking a “business as usual” approach to tuberculosis care and prevention in the face of COVID-19 is destined for failure. In the context of tuberculosis, research is often considered to refer to the biomedical: developing new drugs, vaccines and tests. Whilst this research remains critical to the long-term prospects for tuberculosis elimination, more than ever, funding organisations and policy makers must prioritise and invest in operational research to learn how to effectively and efficiently tackle tuberculosis and its social determinants through the lens of the new reality of the COVID-19 pandemic and associated economic collapse.
Advocacy and community mobilisation
It is critical that alongside all of the above, the scientific and wider global health communities, including civil society representatives, advocate for the rights of tuberculosis-affected households in the context of the COVID-19 pandemic. The collective response of these communities to COVID-19, a pandemic currently principally affecting wealthier countries, has been extraordinary and should be seen as an inspiration for the chronically underfunded and relatively neglected effort to control tuberculosis, the world's oldest pandemic that causes an enormous burden of morbidity and mortality in poorer countries [29]. Indeed, WHO estimates suggest that tuberculosis is likely to have already caused more than double the number of deaths of COVID-19 in 2020, yet has received only a small fraction of the equivalent attention and research funding [4].
Using the “perfect storm” analogy to conceptualise public health emergencies has been criticised for emphasising the power of chance over the efficacy of public health surveillance and prevention efforts [30]. Instead, the global health community must be proactive and anticipate the potentially destructive synergism between COVID-19, tuberculosis, and poverty, which is both predictable and preventable. If we have the foresight and vision to act now through investment, research and strong leadership for tuberculosis, we will avoid finding ourselves in the eye of this storm and potentially save millions from a preventable and curable disease.
Shareable PDF
Supplementary Material
Footnotes
Support statement: M.J. Saunders and C.A. Evans acknowledge funding from: The Wellcome Trust (awards 057434/Z/99/Z, 070005/Z/02/Z, 078340/Z/05/Z, 105788/Z/14/Z and 201251/Z/16/Z); DFID-CSCF; the Joint Global Health Trials consortium (MRC, DFID, and Wellcome Trust award MR/K007467/1); the Bill and Melinda Gates Foundation (award OPP1118545); the Sir Halley Stewart Trust; the World Health Organization; the STOP TB partnership's TB REACH initiative funded by the Government of Canada and the Bill & Melinda Gates Foundation (W5_PER_CDT1_PRISMA); and the charity IFHAD: Innovation For Health And Development. None of these organisations had any role in or placed any restrictions on the preparation or publication of this manuscript. This manuscript represents the opinion of the authors and not of any of these funding organisations.
Conflict of interest: M.J. Saunders has nothing to disclose.
Conflict of interest: C.A. Evans has nothing to disclose.
References
- 1.United Nations Development Programme COVID-19: Looming Crisis in Developing Countries Threatens to Devastate Economies and Ramp Up Inequality. 2020. www.undp.org/content/undp/en/home/presscenter/pressreleases/2020/COVID19_Crisis_in_developing_countries_threatens_devastate_economies.html Date last accessed: 9 April, 2020.
- 2.Sumner A, Hoy C, Ortiz-Juarez E. Estimates of the Impact of COVID-19 on Global Poverty. WIDER Working Paper 2020/43 Helsinki, UNU-WIDER, 2020. [Google Scholar]
- 3.Hogan AB, Jewell B, Sherrard-smith E, et al. The Potential Impact of the COVID-19 Epidemic on HIV, TB and Malaria in Low- and Middle-Income Countries. London, Imperial College London, 2020. doi: 10.25561/78670 [DOI] [Google Scholar]
- 4.World Health Organization Global Tuberculosis Report 2019. Geneva, World Health Organization, 2019. [Google Scholar]
- 5.Wingfield T, Tovar MA, Datta S, et al. Addressing social determinants to end tuberculosis. Lancet 2018; 391: 1129–1132. doi: 10.1016/S0140-6736(18)30484-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saunders MJ, Evans CA. Fighting poverty to prevent tuberculosis. Lancet Infect Dis 2016; 16: 395–396. doi: 10.1016/S1473-3099(15)00434-X [DOI] [PubMed] [Google Scholar]
- 7.Wingfield T, Boccia D, Tovar MA, et al. Defining catastrophic costs and comparing their importance for adverse tuberculosis outcome with multi-drug resistance: a prospective cohort study, Peru. PLoS Med 2014; 11: e1001675. doi: 10.1371/journal.pmed.1001675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter DJ, Glaziou P, Lönnroth K, et al. The impact of social protection and poverty elimination on global tuberculosis incidence: a statistical modelling analysis of Sustainable Development Goal 1. Lancet Glob Heal 2018; 6: e514–e522. doi: 10.1016/S2214-109X(18)30195-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siroka A, Ponce NA, Lönnroth K. Association between spending on social protection and tuberculosis burden: a global analysis. Lancet Infect Dis 2016; 16: 473–479. doi: 10.1016/S1473-3099(15)00401-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dye C, Lönnroth K, Jaramillo E, et al. Trends in tuberculosis incidence and their determinants in 134 countries. Bull World Health Organ 2009; 87: 683–691. doi: 10.2471/BLT.08.058453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saunders MJ, Evans CA. Ending tuberculosis through prevention. N Engl J Med 2019; 380: 1073–1074. doi: 10.1056/NEJMe1901656 [DOI] [PubMed] [Google Scholar]
- 12.Bonadonna LV, Saunders MJ, Zegarra R, et al. Why wait? The social determinants underlying tuberculosis diagnostic delay. PLoS One 2017; 12: e0185018. doi: 10.1371/journal.pone.0185018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Datta S, Evans CA. Healthy survival after tuberculosis. Lancet Infect Dis 2019; 19: 1045–1047. doi: 10.1016/S1473-3099(19)30387-1 [DOI] [PubMed] [Google Scholar]
- 14.Yuen CM, Amanullah F, Dharmadhikari A, et al. Turning off the tap: stopping tuberculosis transmission through active case-finding and prompt effective treatment. Lancet 2015; 386: 2334–2343. doi: 10.1016/S0140-6736(15)00322-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saunders MJ, Wingfield T, Tovar MA, et al. A score to predict and stratify risk of tuberculosis in adult contacts of tuberculosis index cases: a prospective derivation and external validation cohort study. Lancet Infect Dis 2017; 17: 1190–1199. doi: 10.1016/S1473-3099(17)30447-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saunders MJ, Wingfield T, Datta S, et al. A household-level score to predict the risk of tuberculosis among contacts of patients with tuberculosis: a derivation and external validation prospective cohort study. Lancet Infect Dis 2020; 20: 110–122. doi: 10.1016/S1473-3099(19)30423-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization Information Note: Tuberculosis and COVID-19. Geneva, World Health Organization, 2020. [Google Scholar]
- 18.Rudgard WE, Evans CA, Sweeney S, et al. Comparison of two cash transfer strategies to prevent catastrophic costs for poor tuberculosis-affected households in low- and middle-income countries: an economic modelling study. PLoS Med 2017; 14: e1002418. doi: 10.1371/journal.pmed.1002418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wingfield T, Boccia D, Tovar MA, et al. Designing and implementing a socioeconomic intervention to enhance TB control: operational evidence from the CRESIPT project in Peru. BMC Public Health 2015; 15: 810. doi: 10.1186/s12889-015-2128-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Datta S, Gilman R, Montoya R, et al. Quality of life, tuberculosis and treatment outcome; a case-control and nested cohort study. Eur Respir J 2020; in press [https://doi.org/10.1183/13993003.00495-2019]. doi: 10.1183/13993003.00495-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saunders MJ, Wingfield T, Tovar MA, et al. Mobile phone interventions for tuberculosis should ensure access to mobile phones to enhance equity ′ a prospective, observational cohort study in Peruvian shantytowns. Trop Med Int Health 2018; 23: 850–859. doi: 10.1111/tmi.13087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wingfield T, Tovar MA, Huff D, et al. A randomized controlled study of socioeconomic support to enhance tuberculosis prevention and treatment, Peru. Bull World Health Organ 2017; 95: 270–280. doi: 10.2471/BLT.16.170167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saunders MJ, Datta S, Wingfield T, et al. Socioeconomic support to improve tuberculosis screening and preventive therapy completion in tuberculosis-affected households in Peru: a cluster randomised trial. Int J Tuberc Lung Dis 2019; 23: S581. [Google Scholar]
- 24.Wingfield T, Tovar MA, Huff D, et al. The economic effects of supporting tuberculosis-affected households in Peru. Eur Respir J 2016; 48: 1396–1410. doi: 10.1183/13993003.00066-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tadolini M, Codecasa L, Garcia-Garcia J, et al. Active tuberculosis, sequelae and COVID-19 co-infection: first cohort of 49 cases. Eur Respir J 2020; 56: 2001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Datta S, Saunders MJ, Tovar MA, et al. Improving tuberculosis diagnosis: better tests or better healthcare? PLoS Med 2017; 14: 4–7. doi: 10.1371/journal.pmed.1002406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rangaka MX, Cavalcante SC, Marais BJ, et al. Controlling the seedbeds of tuberculosis: diagnosis and treatment of tuberculosis infection. Lancet 2015; 386: 2344–2353. doi: 10.1016/S0140-6736(15)00323-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saunders MJ, Tovar MA, Collier D, et al. Active and passive case-finding in tuberculosis-affected households in Peru: a 10-year prospective cohort study. Lancet Infect Dis 2019; 19: 519–528. doi: 10.1016/S1473-3099(18)30753-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Treatment Action Group, Stop TB Partnership Tuberculosis Research Funding Trends 2005–2017. New York, Treatment Action Group, 2018. [Google Scholar]
- 30.Brandt A, Botelho A. Not a perfect storm – COVID-19 and the importance of language. N Engl J Med 2020; 382: 1493–1495. doi: 10.1056/NEJMp2005032 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01348-2020.Shareable (241.6KB, pdf)