Abstract
Birds provide ecosystem services (pest control) in many agroecosystems and have neutral or negative ecological effects (disservices) in others. Large-scale, conventional row crop agriculture is extremely widespread globally, yet few studies of bird effects take place in these agroecosystems. We studied indirect effects of insectivorous birds on corn and soybean crops in fields adjacent to a prairie in Illinois (USA). We hypothesized that prairie birds would forage for arthropods in adjacent crop fields and that the magnitude of services or disservices would decrease with distance from the prairie. We used bird-excluding cages over crops to examine the net effect of birds on corn and soybean grain yield. We also conducted DNA metabarcoding to identify arthropod prey in fecal samples from captured birds. Our exclosure experiments revealed that birds provided net services in corn and net disservices in soybeans. Distance from prairie was not a significant predictor of exclosure treatment effect in either crop. Many bird fecal samples contained DNA from both beneficial arthropods and known economically significant pests of corn, but few economically significant pests of soybeans. Song Sparrows (Melospiza melodia), one of our most captured species, most commonly consumed corn rootworms, an economically significant pest of corn crops. We estimated that birds in this system provided a service worth approximately US $275 ha−1 in corn yield gain, and a disservice valued at approximately $348 ha−1 in soybean yield loss. Our study is the first to demonstrate that birds can provide substantial and economically valuable services in field corn, and disservices in soybean crops. The contrasting findings in the 2 crop systems suggest a range of bird impacts within widespread agroecosystems and demonstrate the importance of quantifying net trophic effects.
Keywords: agroecology, biological control, corn, molecular scatology, soybeans, trophic cascade
Keywords: agroecología, cascada trófica, control biológico, escatología molecular, maíz, soja
RESUMEN
Las aves brindan servicios ecosistémicos (control de plagas) en muchos agro-ecosistemas y tienen efectos ecológicos neutrales o negativos (deservicios) en otros. La agricultura convencional a gran escala de cultivos en hilera está ampliamente distribuida a escala global, pero a pesar de esto se han realizado pocos estudios de los efectos de las aves en estos agro-ecosistemas. Estudiamos los efectos indirectos de las aves insectívoras en cultivos de maíz y soja en campos adyacentes a una pradera en Illinois (EEUU). Hipotetizamos que las aves de pradera forrajearían en busca de artrópodos en los campos de cultivo adyacentes y que la magnitud de los servicios o deservicios disminuiría con la distancia desde la pradera. Usamos jaulas de exclusión de aves sobre los cultivos para examinar el efecto neto de las aves en el rendimiento de granos de maíz y soja. También utilizamos el método de código de barras de ADN para identificar presas de artrópodos en las muestras de heces de las aves capturadas. Nuestros experimentos de exclusión revelaron que las aves brindaron servicios netos en el maíz y deservicios netos en la soja. La distancia a las praderas no fue un predictor significativo del efecto del tratamiento de exclusión en ninguno de los cultivos. Muchas muestras de heces de aves contuvieron ADN tanto de artrópodos benéficos como de plagas económicamente significativas de maíz, pero de pocas plagas económicamente significativas de soja. Melospiza melodia, una de nuestras especies más capturadas, mayormente consumió el gusano de la raíz del maíz, una plaga económicamente significativa de este cultivo. Estimamos que las aves en este sistema brindaron un servicio valuado en aproximadamente US $275 ha–1 de ganancias en rendimiento de maíz, y un deservicio valuado en aproximadamente $348 ha–1 de pérdidas en rendimiento de soja. Nuestro estudio es el primero en demostrar que las aves pueden brindar servicios substanciales y económicamente valiosos en los campos de maíz y deservicios en los cultivos de soja. Los hallazgos contrastantes en los dos sistemas de cultivo sugieren un rango de impactos de las aves dentro de los agro-ecosistemas ampliamente distribuidos y demuestra la importancia de cuantificar los efectos tróficos netos.
INTRODUCTION
Trophic cascades are well studied in agroecosystems because of their potential to produce economically valuable biological control. Agroecosystems often provide unusually strong trophic cascades for terrestrial systems owing to their simple food webs and high productivity (Halaj and Wise 2001). However, the majority of studies of trophic cascades and food web interactions in agricultural systems focus on arthropods as both pests and predators (e.g., Chaplin-Kramer et al. 2011, Liere et al. 2015) and ignore vertebrate taxa. Vertebrate taxa, however, including insectivorous and omnivorous birds, may drive substantial positive or negative effects on crops with resulting economic impacts (Sekercioglu et al. 2016). Because most insectivorous and omnivorous bird species are generalist arthropod predators (i.e. they may consume both predatory and herbivorous arthropods), they may provide a combination of both services and disservices simultaneously. Therefore, it is important to know the net effects birds provide under different agricultural conditions (Peisley et al. 2015, Pejchar et al. 2018).
A number of studies have shown that birds may provide substantial pest control services in a variety of agricultural systems through top-down control of pests (Whelan et al. 2008, Sekercioglu et al. 2016). Others have shown neutral effects (e.g., Garfinkel and Johnson 2015), direct negative effects caused by damaging or consuming crops (Gebhardt et al. 2011, Lindell and Eaton 2012, Hannay et al. 2019), or indirect negative effects when birds release pests through intraguild predation of predatory arthropods (e.g., Grass et al. 2017, Tschumi et al. 2018). Some of these studies show bird-provisioned services or disservices in terms of plant damage or pest density, without reporting resulting effects on crop yields (e.g., Van Bael et al. 2007, Koh 2008). However, plants can tolerate a certain amount of damage before they reach an economic injury level that reduces crop yield (Pedigo et al. 1986). Therefore, bird-provisioned services and disservices in agricultural systems are most meaningful when put in the context of crop yield instead of only plant damage or pest density (Whelan et al. 2008). These data can then be used to extrapolate approximate economic gains or losses due to bird services or disservices (e.g., Kellermann et al. 2008).
Few studies on bird trophic effects have taken place in large-scale conventional row crop agriculture (but see Kross et al. 2016). Instead, they have mainly been conducted on small-scale farms or agroforestry systems, often in the tropics, that tend to harbor larger or more diverse bird communities (Sekercioglu et al. 2016). In the United States, corn and soybeans are by far the most widely grown row crops, with a total combined area of over 69.7 million hectares harvested in 2017 (USDA 2018). Considering the large extent of corn and soybean agriculture, it is surprising that there is little information on the effects of birds in these systems.
While corn and soybean fields are not known for their bird abundance or diversity, fields in the Midwest region of the United States (where corn and soybean crop cultivation is concentrated) are sometimes adjacent to remnant or restored prairies and grasslands. These “natural” or uncultivated habitats may provide sources of birds that also forage within the agricultural fields (Rodenhouse and Best 1983). If prairie birds provide significant indirect effects on crops in large-scale conventional row crop agriculture, these effects may be stronger in crops close to these remnant and restored prairies. Such distance effects have been documented in other agricultural systems, including coffee (Karp et al. 2013), cacao (Maas et al. 2015), and tropical forest plantations (Roels et al. 2018).
We used 2 approaches to examine the indirect effects of bird predation on crop pests in corn and soybean fields adjacent to prairie. First, we used bird-excluding cages (“exclosures”) over crop plants to determine whether birds provide net pest control services or disservices. If the prairie provides source habitat for birds, we hypothesized that the strength of these services or disservices would decline with distance from the prairie. Furthermore, crop field edges often host higher densities of some pest species (Nguyen and Nansen 2018), and we expected these greater prey densities would exacerbate differences between the exclosure and control treatments near the field edges. Second, we used a DNA metabarcoding diet analysis to determine whether birds captured in corn and soybean fields and adjacent prairie consume known crop pests or beneficial predatory arthropods. If birds collectively provide net services in the corn and soybean fields, we would expect to find evidence that birds predominantly consume economically significant pests. If they collectively provide net disservices, we would expect that the birds predominantly consume beneficial arthropods.
METHODS
Study Site
We conducted this study at Nachusa Grasslands, a system of restored and remnant tallgrass prairie in northern Illinois, USA (41.9048, −89.3231). Nachusa Grasslands, which is owned by The Nature Conservancy (TNC), is embedded within a landscape dominated by corn and soybean agriculture. The property is ~1,538 ha and includes some agricultural fields that are leased to farmers until TNC is ready to restore them to prairie.
We conducted experiments in 2 agricultural fields owned by Nachusa Grasslands: one planted in corn (Zea mays, ~21 ha) and another planted in soybeans (Glycine max, ~17 ha). The fields were leased to 2 different farmers (one to farm each crop) and were managed in the same ways as other fields in the region. The 2 crops were not separated by any cleared margin, and both crops shared an edge with a mature restored tallgrass prairie fragment (Figure 1). The corn variety grown in our study system was genetically modified to express Bt (Bacillus thuringiensis) toxins, and the soybeans were sprayed on July 26 with Hero (FMC Global Specialty Solutions), a broad-spectrum insecticide. The farmer raised the boom arm of the tractor over the exclosures while spraying, so the exclosures were not removed for this. Although there were some scattered shrubs along the southern border of the soybean field, and the western border of the corn and soybean fields, there were no hedgerows or treelines separating the cropland from the prairie, or within 500 m of the experimental set up.
FIGURE 1.
Study site in northern Illinois, USA. Exclosure and control plots within a pair are separated by 2 m, and exclosure representations are not sized to scale. The land to the south of our study site is grassland, and all exclosures are greater than 50 m from the southern border of the soybean field.
Exclosures
During the growing season of 2016, we placed 6 bird exclosures in the corn field and 6 in the soybean field. Each exclosure was paired with a control plot marked with small plastic plant tags and located 2 m from the exclosure. We placed half of the exclosures and control plots 5 m from the prairie/crop edge, and the other half 55 m into the field interior to test for an effect of distance from the prairie edge. We expected this distance to be outside the foraging range of most birds living in the prairie, as previous research using experimental feeders placed in other crop fields showed that most bird species foraged within 20 m of the field edge (Puckett et al. 2009).
The exclosure frames were constructed from PVC pipe covered with clear nylon monofilament netting (1.9 cm square, 3.8 cm stretch mesh). The mesh size was small enough to exclude even the smallest birds found at the site (e.g., Common Yellowthroats [Geothlypis trichas] and Field Sparrows [Spizella pusilla]) but allow access to larger arthropods such as grasshoppers (Orthoptera) and butterflies (Lepidoptera). Costamagna et al. (2008) found that exclosures using much finer mesh than ours had no direct effect on soybean grain yield, so we expected that any treatment effect would be due to bird exclusion and not changes in crop microclimate. The exclosure footprint was 1.5 × 0.6 m. The exclosures placed over soybeans were 1.5 m tall and those over corn were 3 m tall to accommodate plant growth. Each exclosure covered different numbers of plants (5–10) depending on planting density and row width. To control for this, as well as to avoid measuring the plants against the side of the exclosure that could potentially push leaves out through the netting, we focused on the central 5 plants in the exclosure and marked them with small plastic plant tags. We placed the exclosures over the crops once they had clearly sprouted in the field and were at least 5 cm tall (mid-June 2016). We removed them approximately one week before the farmers harvested the fields (early October 2016) for a total exclusion period of ~3.5 mo.
Upon removal of the exclosures, we hand-harvested the crops from the 5 marked central plants inside the exclosure, and the 5 marked plants from each control plot. We removed the corn kernels from the cobs and the soybeans from the pods, oven-dried them to remove all water weight, and recorded the total dry biomass of crop yield per plant (hereafter referred to as “grain yield”).
Collection of Fecal Samples
We operated mist nets twice in June, twice in July, and three times in August 2016 to capture birds. We placed the mist nets along a narrow, mowed path between the crop fields and the prairie, within the corn and soybean fields, and in the prairie within 10 m of the crop edge. Because we could not remove or trim plants in the cropland or prairie, we placed the nets opportunistically wherever they would not become entangled in vegetation. As a consequence, mist-netting effort varied across locations and we cannot draw conclusions about bird densities based on mist-netting data.
We placed each captured bird into a new brown paper bag for no more than 30 min (generally much less time) until it defecated, then collected the fecal sample in 90% ethyl alcohol (EtOH) and placed it on ice in an insulated cooler. We then banded the bird, collected standard measurements and demographic data, and released it. Once out of the field for the day, we stored the fecal samples at −20°C.
DNA Meta-barcoding Analysis of Fecal Samples
Fecal DNA samples were analyzed using meta-barcoding, a technique that can determine diet composition from fecal samples with a high degree of taxonomic specificity. We homogenized each raw fecal sample using a FastPrep-24 5G Homogenizer (MP Biomedicals) and extracted DNA using PowerSoil DNA Isolation Kits (MoBio). We amplified DNA with polymerase chain reactions (PCR) using the LCO1490/HCO2198 primers (Folmer et al. 1994, Hebert et al. 2003; see the Appendix and Appendix Tables 4 and 5 for PCR conditions). DNA Sequencing was performed on an Illumina MiSeq instrument employing V3 chemistry. The LCO/HCO primers gave a 710 base pair (bp) amplicon, which was too large for paired-end read merging on this instrument. Therefore, we used a set of filters and trim steps to increase the quality of the data used for analysis and annotation of the sequenced regions. First, ambiguous nucleotides were trimmed from the ends, and all reads with internal ambiguous nucleotides were discarded. Primer sequences were then trimmed from either the forward or reverse reads, and any read lacking either sequence was discarded. Subsequently, data were trimmed using a quality threshold of P = 0.01, and sequences shorter than 200 bp were discarded. We also discarded all sequences from fecal samples with fewer than 1,000 total reads, or where <10% of reads passed quality checking. The remaining 200+ base fragments were analyzed using a QIIME pipeline for clustering, annotation, and biological observation matrix formation (Caporaso et al. 2010).
We generated operational taxonomic unit (OTU) clusters de novo using the UCLUST method with a 97% sequence similarity threshold. Taxonomic annotations for each OTU were determined using a BLAST search of the NCBI nt nucleotide database (Benson et al. 2012) and used only OTUs identified to the species level in our subsequent analyses. The results from the BLAST search were then processed using the program MEGAN to generate the taxonomic consensus at each taxonomic level (Huson et al. 2007). Because we did not use a sterile technique in the field while collecting fecal samples, we expected our samples to be contaminated with bacterial, fungal, and human DNA (although we used new materials for each sample to avoid cross-contamination). Although our primers are arthropod-specific, non-arthropod DNA may still be found in samples after PCR. Therefore, we then discarded all OTUs that were not placed in either class Arachnida or Insecta, and samples with fewer than 100 reads assigned to phylum Arthropoda. Because we were interested in species likely to have been directly consumed as prey by birds, we further narrowed our dataset to include only Arachnids in the order Araneae (spiders). This allowed us to exclude species such as feather mites that were present but not of interest in this study.
We compared the taxonomic lists of bird diet components produced by the DNA analysis to lists of arthropod pests of field crops in the Illinois Field Crop Scouting Manual (Bissonnette 2010). We identified arthropods as potential economically significant pest species if they appeared in this manual, even if they are generally pests of field crops other than corn or soybeans (e.g., alfalfa) because these field crops are all often grown in close proximity in Illinois. All pests listed in the manual have the potential to cause yield-reducing (“economically significant”) damage to field crops. We were further able to assign the main crop affected by these pests based on recommendations within the Scouting Manual: some pests are known to cause economically significant damage to corn, soybean, or other crops, while generalist pests may affect multiple crop types. We also assigned general feeding guilds to each arthropod species detected, using Triplehorn and Johnson (2005) as a guide, and Parr et al. (2014) when further information was needed. We assigned arthropods to the following guilds: herbivores, omnivores, natural enemies (predators and parasitoids), and other (for detritivores and species that fall into multiple feeding guilds during different life stages).
We cannot reliably determine the proportional components of an individual bird’s diet due to the biases introduced in PCR and the metabarcoding process (Jedlicka et al. 2017), so we instead calculated the percentages of fecal samples that contained DNA from various arthropod species. We normalized sequence read counts by dividing the number of reads per OTU by the total count of OTUs assigned to phylum Arthropoda for each sample. We considered species to be present if they represented at least 1% of the reads per sample (sensu McInnes et al. 2017b) and at least 5 reads per OTU. This may be considered a fairly conservative approach to assigning presence: Deagle et al. (2019) suggested that a 1% presence threshold is suitable for many situations except where the diet was extremely diverse, in which case a much lower threshold was warranted. Because we were interested in the collective effects of the bird assemblage, we pooled data from fecal samples across all bird species for the majority of our analyses, but also compared dietary components from bird species from which we were able to obtain at least 10 fecal samples.
Exclosure Data Analysis
We conducted separate analyses for corn and soybean crops. We checked response variables (grain yield per plant) for normality using Shapiro-Wilk tests (Shapiro and Wilk 1965). Because these variables were normally distributed, we fit linear mixed effects models and used F-tests to check for an effect of exclosure treatment, distance from field edge, and a treatment*distance interaction on total grain yield. We modeled exclosure/control replicates and plant replicates within exclosures/control plots as nested random effects, and exclosure treatment, distance, and the treatment*distance interaction as fixed effects. We included these nested random effects in our models to account for the lack of independence among sampled plants within exclosures and matching controls (Millar and Anderson 2004, Harrison et al. 2018). We ran a single full model for each crop type (including random effects and treatment, distance, and interaction fixed effects). We then applied a stepwise approach, and removed any nonsignificant predictor variables (P > 0.05 or 95% confidence intervals overlap 0) and re-ran the model with only significant predictor variables. We used the coefficient of the treatment effect from the final model (including only significant predictor variables) to calculate the economic value of bird effects as described below. All exclosure analyses were conducted with R 3.4.3 (R Core Team 2017).
Economic Value of Bird Effects
To calculate the approximate economic value per hectare of bird-provisioned services and disservices, we applied the average estimated differences in crop yield between exclosures and control plots for both corn and soybeans to Equation (1):
| (1) |
Because field corn and soybeans are generally not grown for direct human consumption in this agroecosystem, we used the dry mass of crop grain yield in this equation. We did not account for cosmetically damaged crops as this generally does not decrease crop value. We used the average grain price per bushel from Illinois during the 2016 marketing year (USDA 2018) and average planting densities obtained from the farmers of our study site (values differ by crop type and are listed in Appendix Table 6).
RESULTS
Exclosures
We found significant, although opposite, effects of exclosure treatment on both corn and soybean grain yield (Table 1). Corn yield was significantly greater in control plots while soybean grain yield was significantly greater in exclosures. Neither distance from field edge nor the interaction between treatment and distance from field edge had a significant effect on yield for either crop type (P > 0.05 and 95% confidence intervals [CI] overlap 0), so we removed these predictor variables from our final models (as presented in Table 1).
TABLE 1.
Final linear mixed effects models of grain yield as a function of exclosure treatment for 2 crop types. Plant replicate nested within exclosure replicate are modeled as random effects. n = 6 exclosure/control plots, 60 plants.
| Estimate | SE | LCI a | UCI a | P | |
|---|---|---|---|---|---|
| Soybeans | |||||
| Intercept | 11.60 | 1.14 | 9.31 | 13.89 | 1.13 × 10−6 |
| Exclosure treatment | 4.05 | 1.27 | 1.54 | 6.57 | 0.002 |
| Corn | |||||
| Intercept | 177.43 | 20.76 | 134.17 | 220.68 | 1.48 × 10−4 |
| Exclosure treatment | −27.45 | 12.01 | −51.36 | −3.54 | 0.030 |
a 95% lower and upper confidence intervals (CIs) for estimate.
DNA Metabarcoding Diet Analysis
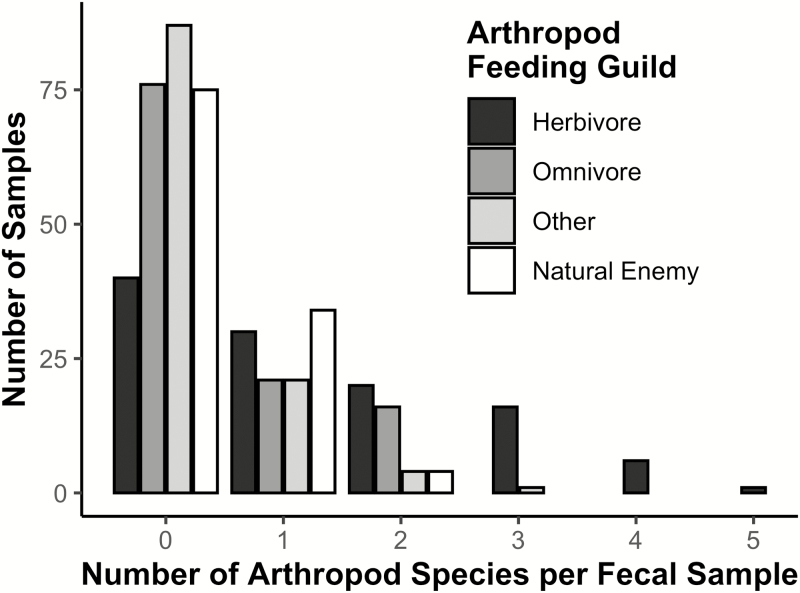
We amplified arthropod DNA from 113 fecal samples collected from 19 bird species (Table 2). Of the 113 samples, 23 did not have any OTUs that met our criteria for quality, minimum number of reads, and/or taxonomy (see “DNA Meta-barcoding Analysis of Fecal Samples” in Methods). Across all 113 fecal samples, we identified DNA from 61 arthropod species, representing 8 orders in Class Insecta and 1 order in Class Arachnida (Araneae) ( = 2.42 ± 2.38 SD species per sample, range: 0–9 species per sample; Table 3). We detected DNA from 6 field crop pest species listed in the Illinois Field Crop Scouting Manual: 3 species that affect corn crops (northern and western corn rootworms, Diabrotica barberi and D. virgifera, and a sap beetle, Carpophilus antiquus), one that mainly affects soybeans (a stink bug, Euschistus variolarius), one that generally affects alfalfa (tarnished plant bug, Lygus lineolaris), and one generalist species that affects many crop types (a grasshopper, Encoptolophus costalis; Figure 2). We also detected DNA from at least one species of natural enemy arthropod in 22.1% of fecal samples. We generally detected more species of herbivorous arthropods than arthropods of other feeding guilds per sample (Figure 3). Northern corn rootworm (detected in 34.5% of samples) was the most commonly detected of the corn or soybean pest species, while spiders (order Araneae, at least one species detected in 19.5% of samples) were the most commonly detected natural enemy (Table 3).
TABLE 2.
Quantity of fecal samples collected and DNA successfully sequenced from 19 bird species.
| Location where bird was captured a | ||||||
|---|---|---|---|---|---|---|
| Common name | Scientific name | Crop edge | Crop interior | Prairie edge | Prairie interior | Total samples |
| Common Yellowthroat | Geothlypis trichas | 5 | 2 | 17 | 5 | 29 |
| Song Sparrow | Melospiza melodia | 3 | 2 | 9 | 3 | 17 |
| Gray Catbird | Dumetella carolinensis | 7 | 0 | 6 | 0 | 13 |
| Dickcissel | Spiza americana | 1 | 4 | 2 | 5 | 12 |
| House Wren | Troglodytes aedon | 3 | 0 | 5 | 0 | 8 |
| American Goldfinch | Carduelis tristis | 1 | 2 | 2 | 2 | 7 |
| Field Sparrow | Spizella pusilla | 2 | 2 | 3 | 0 | 7 |
| American Robin | Turdus migratorius | 1 | 1 | 0 | 1 | 3 |
| Indigo Bunting | Passerina cyanea | 3 | 0 | 0 | 0 | 3 |
| Red-winged Blackbird | Agelaius phoeniceus | 0 | 2 | 0 | 1 | 3 |
| Savannah Sparrow | Passerculus sandwichensis | 2 | 0 | 0 | 0 | 2 |
| Willow Flycatcher | Empidonax traillii | 2 | 0 | 0 | 0 | 2 |
| Brown-headed Cowbird | Molothrus ater | 1 | 0 | 0 | 0 | 1 |
| Brown Thrasher | Toxostoma rufum | 0 | 1 | 0 | 0 | 1 |
| Downy Woodpecker | Dryobates pubescens | 0 | 0 | 0 | 1 | 1 |
| Eastern Kingbird | Tyrannus tyrannus | 0 | 0 | 1 | 0 | 1 |
| Eastern Meadowlark | Sturnella magna | 0 | 1 | 0 | 0 | 1 |
| Eastern Towhee | Pipilo erythrophthalmus | 1 | 0 | 0 | 0 | 1 |
| Northern Flicker | Colaptes auratus | 1 | 0 | 0 | 0 | 1 |
| Total | 33 | 17 | 46 | 18 | 113 |
a Mist-net effort was unequal between locations. Crop edge includes corn or soybeans within 5 m of the cultivated field edge. Crop interior includes corn or soybeans >5 m from the cultivated field edge. Prairie edge includes prairie habitat within 5 m of the cultivated field edge. Prairie interior includes prairie habitat 5–10 m from the cultivated field edge.
TABLE 3.
Arthropod species detected in bird fecal samples through DNA barcoding, and their general feeding guilds; n = 113 fecal samples. Species in bold were detected in at least 5% of the fecal samples.
| Order | Family | Genus | Species | Feeding guild a | Count samples | Percent samples |
|---|---|---|---|---|---|---|
| Araneae | Araneidae | Argiope | trifasciata | natural enemy | 1 | 0.9% |
| Clubionidae | Clubiona | abboti | natural enemy | 7 | 6.2% | |
| Corinnidae | Trachelas | tranquillus | natural enemy | 2 | 1.8% | |
| Linyphiidae | Diplostyla | concolor | natural enemy | 1 | 0.9% | |
| Grammonota | angusta | natural enemy | 1 | 0.9% | ||
| Lycosidae | Pardosa | milvina | natural enemy | 6 | 5.3% | |
| Trochosa | ruricola | natural enemy | 1 | 0.9% | ||
| Salticidae | Phidippus | clarus | natural enemy | 2 | 1.8% | |
| Tutelina | similis | natural enemy | 2 | 1.8% | ||
| Tetragnathidae | Pachygnatha | autumnalis | natural enemy | 1 | 0.9% | |
| Theridiidae | Theridion | frondeum | natural enemy | 2 | 1.8% | |
| Thomisidae | Ozyptila | praticola | natural enemy | 1 | 0.9% | |
| Xysticus | ferox | natural enemy | 1 | 0.9% | ||
| Coleoptera | Brentidae | Perapion | curtirostre | herbivore | 1 | 0.9% |
| Carabidae | Bembidion | quadrimaculatum | natural enemy | 1 | 0.9% | |
| Pterostichus | melanarius | natural enemy | 1 | 0.9% | ||
| Chrysomelidae | Diabrotica | barberi | herbivore | 39 | 34.5% | |
| Diabrotica | virgifera | herbivore | 1 | 0.9% | ||
| Epitrix | fasciata | herbivore | 1 | 0.9% | ||
| Curculionidae | Larinus | planus | herbivore | 1 | 0.9% | |
| Rhinoncus | castor | herbivore | 1 | 0.9% | ||
| Nitidulidae | Carpophilus | antiquus | herbivore | 2 | 1.8% | |
| Silphidae | Ptomascopus | morio | other | 10 | 8.8% | |
| Diptera | Tephritidae | Rhagoletis | cingulata | herbivore | 2 | 1.8% |
| Agromyzidae | Liriomyza | brassicae | herbivore | 1 | 0.9% | |
| Chloropidae | Malloewia | sp. | other | 1 | 0.9% | |
| Drosophilidae | Drosophila | melanogaster | other | 1 | 0.9% | |
| Calliphoridae | Pollenia | pediculata | other | 25 | 22.1% | |
| Chironomidae | Orthocladius | oblidens | other | 1 | 0.9% | |
| Limoniidae | Helius | flavipes | other | 1 | 0.9% | |
| Limonia | novaeangliae | other | 1 | 0.9% | ||
| Hemiptera | Aphididae | Capitophorus | elaeagni | herbivore | 1 | 0.9% |
| Chaitophorus | nigrae | herbivore | 1 | 0.9% | ||
| Cicadidae | Tibicen | lyricen | herbivore | 1 | 0.9% | |
| Coccidae | Parthenolecanium | corni | herbivore | 3 | 2.7% | |
| Dictyopharidae | Nersia | florida | herbivore | 3 | 2.7% | |
| Miridae | Lygus | lineolarus | herbivore | 44 | 38.9% | |
| Neurocolpus | sp. | herbivore | 1 | 0.9% | ||
| Pentatomidae | Euschistus | variolarius | herbivore | 15 | 13.3% | |
| Alydidae | Alydus | sp. | herbivore | 1 | 0.9% | |
| Lygaeidae | Neortholomus | scolopax | herbivore | 1 | 0.9% | |
| Rhyparochromidae | Ligyrocoris | sylvestris | herbivore | 1 | 0.9% | |
| Hymenoptera | Agaonidae | Ceratosolen | n. sp. | herbivore | 1 | 0.9% |
| Cynipidae | Antistrophus | silphii | herbivore | 1 | 0.9% | |
| Formicidae | Lasius | alienus | omnivore | 28 | 24.8% | |
| Lasius | neoniger | omnivore | 23 | 20.4% | ||
| Camponotus | pennsylvanicus | omnivore | 1 | 0.9% | ||
| Stenamma | wheelerorum | omnivore | 1 | 0.9% | ||
| Scelionidae | Telenomus | podisi | natural enemy | 1 | 0.9% | |
| Tenthredinidae | Caliroa | fasciata | other | 1 | 0.9% | |
| Lepidoptera | Geometridae | Eulithis | sp. | herbivore | 3 | 2.7% |
| Eupithecia | miserulata | herbivore | 2 | 1.8% | ||
| Noctuidae | Apamea | sordens | herbivore | 1 | 0.9% | |
| Galgula | partita | herbivore | 1 | 0.9% | ||
| Pterophoridae | Emmelina | monodactyla | herbivore | 1 | 0.9% | |
| Crambidae | Neodactria | sp. | herbivore | 1 | 0.9% | |
| Orthoptera | Acrididae | Encoptolophus | costalis | herbivore | 1 | 0.9% |
| Gryllidae | Eunemobius | carolinus | herbivore | 1 | 0.9% | |
| Gryllus | pennsylvanicus | herbivore | 1 | 0.9% | ||
| Thysanoptera | Thripidae | Frankliniella | tritici | herbivore | 13 | 11.5% |
| Trichoptera | Hydropsychidae | Potamyia | flava | other | 1 | 0.9% |
a Feeding guilds determined from Triplehorn and Johnson (2005). “Natural enemy” includes predators and parasitoids; “other” includes detritivores and species that are in different feeding guilds during different life stages.
FIGURE 2.
Percent of fecal samples (from all bird species) containing DNA from arthropod pests of field crops (see text for explanation of pest criteria); n = 113 fecal samples. Arthropod pests are grouped by the primary crop type affected by the pest species; “other” category includes multiple crop types or field crops other than corn or soybeans. Genera indicated are Carpophilus, Diabrotica, Encoptolophus, Euschistus, Diabrotica, and Lygus.
FIGURE 3.
Categorized histogram of the number of arthropod species detected per bird fecal sample, grouped by arthropod feeding guild; n = 113 fecal samples. “Natural Enemy” category includes predators and parasitoids. “Other” category includes detritivores, as well as species that change feeding guild between life stages.
We obtained at least 10 fecal samples each from 4 bird species (Table 2). Of those species, Song Sparrows (Melospiza melodia) had the highest percentage of samples that contained DNA from northern corn rootworms (Figure 4A) and also tarnished plant bugs (Figure 4B), the 2 most commonly encountered pests in our diet study.
FIGURE 4.
Number of bird fecal samples containing DNA from (A) the northern corn rootworm (Diabrotica barberi), a corn pest, and (B) the tarnished plant bug (Lygus lineolarus), an alfalfa pest, from the 4 bird species with at least 10 fecal samples. Bird species indicated are Dickcissel (Spiza americana), Gray Catbird (Dumetella carolinensis), Song Sparrow (Melospiza melodia), and Common Yellowthroat (Geothlypis trichas). Percentages in bars indicate the percent of fecal samples per bird species in which pest DNA was present.
Economic Value of Bird Effects
Birds provided a service worth approximately US $275 ha−1 in the corn crop, and a disservice valued at approximately US $348 ha−1 in the soybean crop. These extrapolated values are relevant to crop yield within 55 m from a prairie edge, and do not necessarily apply to agricultural areas farther from prairie if there is a distance threshold at which bird foraging decreases.
DISCUSSION
This study is the first to demonstrate that birds can provide substantial services in field corn, and disservices in soybean crops. We documented a higher corn yield, and a lower soybean yield, when birds were allowed access to crops. DNA diet analysis showed that many birds captured in experimental fields and nearby prairie consumed an economically significant pest of corn, northern corn rootworm (34.5% of samples), and both predatory spiders (Araneae, 19.5% of samples) and predatory beetles (Carabidae, 1.8% of samples). The fact that many individual birds consumed corn pests that can cause significant economic damage may explain the net positive services provided by birds in the corn field. Conversely, only 13.3% of fecal samples contained DNA from an economically significant soybean pest (Figure 2). The net disservices in soybeans may be due to birds consuming natural enemy arthropods that would otherwise provide biological control, while rarely consuming the pests themselves. Furthermore, bird disservices may be more pronounced than services if birds eat predatory arthropods that naturally occur in lower densities than herbivores. Together, these results suggest that birds have the potential for more substantial effects on conventionally grown field crops than previously expected.
Pests of field crops vary by year and by crop type. While corn rootworms are the main corn-specific pest that birds consumed in our study system, tarnished plant bugs were detected in more fecal samples than any other pest species (Figure 2). Although the crop scouting manual lists tarnished plant bugs as pests of alfalfa crops, these bugs can make use of an extremely wide variety of plant hosts, including soybeans (Snodgrass et al. 2010). Bird consumption of tarnished plant bugs also may have consequences across the larger landscape because alfalfa fields are common within the agricultural matrix in Illinois and much of the Midwest of the United States.
In many systems, pest removal services may be driven disproportionately by a single or a few species of predator (Letourneau et al. 2009, Maas et al. 2015). Of the 4 bird species for which we tested at least 10 fecal samples, Song Sparrows had the highest proportion of fecal samples containing DNA from corn rootworm and tarnished plant bug (Figure 4A, B). Song Sparrows are primarily insectivorous during the breeding season, and they can consume a wide range of sizes of arthropod prey (Arcese et al. 2002). Because Song Sparrows are found in a wide variety of habitats and are more generalist in habitat affiliation than many grassland bird species, they have the potential to provide extensive services across many landscapes. Indeed, they are often found along the shrubby edges of agricultural fields. Furthermore, as a resident species across much of its range, the Song Sparrow has potential to consume pests both earlier and later into the growing season than migratory species.
The corn variety grown in our study system was genetically modified (GM) to express Bt toxins, and the soybeans were sprayed with broad-spectrum insecticides. These are extremely common pest management strategies for corn and soybeans, respectively, in the United States. Although we do not have data on pest densities before and after insecticide treatment, it is possible that bird services were undervalued in soybean crops due to insecticide use. Nevertheless, we found that bird trophic interactions in this system were strong enough to be detectable despite those pest management strategies. Because some species of corn rootworms are beginning to show resistance to Bt transgenic corn crops (Gassmann et al. 2014), our results take on added significance. We expect that these indirect bird effects may be even stronger in organic crop systems, or those that do not employ chemical or GM-produced insecticides (but see Garfinkel and Johnson 2015).
While studies have found that birds consume corn pests (e.g., Bendell et al. 1981, Bollinger and Caslick 1985), the only other study that quantified bird indirect effects in field corn (Tremblay et al. 2001) found depression of corn pests, but no cascading increase of corn grain yield. Exclosure effects may be highly dependent on many variables including the surrounding landscape (Boesing et al. 2017) and local prey or pest conditions (Halaj and Wise 2001, Salo et al. 2010), which vary with location, time, and management practices. The disparity of our findings from those of Tremblay et al. (2001) may result from differences in such conditions.
We know of no other study that examined indirect bird effects in soybean crops. Results from our DNA analysis suggest that the disservice in soybeans resulted from intraguild predation of birds on arthropod predators, thus releasing pest species. Birds have also been shown to provide indirect disservices in other systems such as non-maize grain (Grass et al. 2017, Tschumi et al. 2018) and cabbage crops (Martin et al. 2013). Those studies, like ours, suggested that bird disservices were caused by intraguild predation, where birds consumed predatory or parasitoid arthropods.
DNA metabarcoding of scat is an evolving, minimally invasive technique that provides highly specific data on prey identity that would otherwise be difficult to ascertain using older methods of diet analysis (e.g., emetics or stomach sampling; McInnes et al. 2017a). Like many other diet analysis techniques, however, we cannot determine where the bird captured prey items, or the life stage of the prey items consumed. While the sampled birds almost certainly foraged in the prairie, certain prey items, such as crop pests, most likely originated in the crop fields. Moreover, any potential crop pest that is consumed in prairie habitat is no longer able to spill over into the adjacent crop (Tscharntke et al. 2012). Therefore, we believe that identifying prey species helps explain the mechanisms behind the bird service and disservice provision at the larger multi-field scale.
Although our estimated economic values of bird effects (+US $275 ha−1 in corn and −US $348 ha−1 in soybeans) are only approximations, these results suggest that birds provide previously unexpected but substantial economic consequences in conventional agriculture. These net effects deserve further exploration because the perceived costs and/or benefits from wildlife such as birds are among the strongest drivers of farmers adopting conservation practices (Kross et al. 2018). Indeed, new technologies such as precision agriculture may allow farmers in the future to take low-yield areas out of production and replace them with native plantings that encourage birds and their services, and potentially increase their overall crop yield and biodiversity (see Lindell et al. 2018). Because the majority of farmers rotate corn and soybean crops (Wiebe and Gollehon 2006), it will be important to determine overall economic effects of bird populations over a multi-year rotational schedule. This may be best addressed with models that are beyond the scope of the current paper.
Contrary to our hypothesis, we found that distance from prairie/field edge was not a significant predictor of grain yield or exclosure treatment effect for either crop. This may indicate that our “field interior” exclosures, placed 55 m into the field, had not yet reached an interior threshold where bird foraging declined compared to the field edge, provided that such an edge effect exists. It is also possible that our study fields are not representative of crop fields in terms of size, and larger crop fields (with a smaller edge:area ratio) would show a stronger distance effect. Because of the low levels of replication in our study, future research should expand specifically on both the spatial and temporal scale of our study to determine if these net effects in corn and soybean crops are consistent across space and time. Future studies should also incorporate within-year changes in bird diets to determine whether net services vs. disservices may differ throughout a growing season (Grass et al. 2017).
Our study provides the first evidence of a previously unquantified, but potentially ecologically and economically important, process within a widespread agroecosystem. Although our study was conducted across a small scale, these results show that bird communities have the potential to produce real economic effects even in large-scale, conventional monocrop systems. While we caution using these results to generalize across all similar systems, the substantial effects we found in this study indicate a need for further research to better explicate net bird effects in conventional corn and soybean agriculture.
Funding statement: Funding was provided to MBG by Friends of Nachusa Grasslands, The American Ornithological Society, Annie’s Homegrown, University of Illinois at Chicago (UIC) Institute for Environmental Science and Policy, and UIC’s Elmer Hadley Graduate Research Award. Bioinformatics analysis in the project described was performed in part by the UIC Research Informatics Core, supported in part by NCATS through Grant UL1TR002003.
Ethics statement: This study was conducted with the approval of UIC’s Institutional Animal Care and Use Committee (ACC protocol 16–043).
Author contributions: MBG, ESM, and CJW conceived the ideas and designed methodology; MBG and CJW collected the data; MBG analyzed the data and led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Conflict of interest statement: The authors declare no conflict of interests.
Data depository: Analyses reported in this article can be reproduced using the data provided by Garfinkel et al. (2020).
ACKNOWLEDGMENTS
We thank farmers Dave Didier and Ed Bettner, as well as Bill Kleiman and the staff of Nachusa Grasslands. Special thanks to Daryl Coldren, Stephanie Kadej, Elisabeth Tapoi, and Jessica Bonomo for field and lab assistance.
APPENDIX
PCR Conditions for Amplicon Sequencing
Primers without CS tags used for sequencing (Folmer et al. 1994):
LCO1490 5′-GGTCAACAAATCATAAAGATATTGG-3′
HC02198 5′-TAAACTTCAGGGTGACCAAAAAATCA-3′
PCR was run twice to facilitate addition of CS tags (first time using primers without tags, and second time with the tags). The gDNA samples from fecal DNA extraction were diluted 1:10 due to the presence of inhibitors in the samples. PCR was not replicated and products were pooled (see Smith and Peay [2014] for a discussion of a study indicating that PCR replication did not alter observed ecological data). The PCR conditions were as follows:
APPENDIX TABLE 4.
First PCR using primers without CS tags.
| Temperature | Time | Number of cycles |
|---|---|---|
| 95°C | 3 min | |
| 95°C | 1 min | 27 |
| 40°C | 1 min | |
| 72°C | 1.5 min | |
| 4°C | ∞ |
APPENDIX TABLE 5.
Second PCR using primers with CS tags.
| Temperature | Time | Number of cycles |
|---|---|---|
| 95°C | 3 min | |
| 95°C | 1 min | 8 |
| 40°C | 1 min | |
| 72°C | 1.5 min | |
| 4°C | ∞ |
APPENDIX TABLE 6.
Values used in equation (1) to perform economic calculations.
| Value | Corn | Soybeans |
|---|---|---|
| US $/bushel, Illinois, 2016 marketing year a | 3.43 | 9.78 |
| Pounds/gram | 0.0022 | 0.0022 |
| Bushels/pound a | 0.0179 | 0.0167 |
| Treatment effect (grams/plant) b | 27.45 | −4.05 |
| Average number plants/acre c | 30,000 | 100,000 |
| Acres/hectare | 2.47 | 2.47 |
a From USDA (2018).
b Values from bird exclosure treatment effects reported in this study.
c Information provided by farmers of study fields.
LITERATURE CITED
- Arcese P., Sogge M. K., Marr A. B., and Patten M. A.(2002). Song Sparrow (Melospiza melodia), version 2.0. In The Birds of North America (Poole A. F. and Gill F. B., Editors). Cornell Lab of Ornithology, Ithaca, NY, USA. https://doi.org/10.2173/bna.704 [Google Scholar]
- Bendell B. E., Weatherhead P. J., and Stewart R. K.(1981). The impact of predation by Red-winged Blackbirds on European corn borer populations. Canadian Journal of Zoology 59:1535–1538. [Google Scholar]
- Benson D. A., Cavanaugh M., Clark K., Karsch-Mizrachi I., Lipman D. J., Ostell J., and Sayers E. W.(2012). GenBank. Nucleic Acids Research 41:D36–D42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonnette S. M. (2010). Field Crop Scouting Manual. University of Illinois Extension, Urbana, IL, USA. [Google Scholar]
- Boesing A. L., Nichols E., and Metzger J. P.(2017). Effects of landscape structure on avian-mediated insect pest control services: A review. Landscape Ecology 32:931–944. [Google Scholar]
- Bollinger E. K., and Caslick J. W.(1985). Red-winged Blackbird predation on northern corn rootworm beetles in field corn. The Journal of Applied Ecology 22:39–48. [Google Scholar]
- Caporaso J. G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F. D., Costello E. K., Fierer N., Peña A. G., Goodrich J. K., Gordon J. I., . et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nature Methods 7:335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin-Kramer R., O’Rourke M. E., Blitzer E. J., and Kremen C.(2011). A meta-analysis of crop pest and natural enemy response to landscape complexity. Ecology Letters 14:922–932. [DOI] [PubMed] [Google Scholar]
- Costamagna A. C., Landis D. A., and Brewer M. J.(2008). The role of natural enemy guilds in Aphis glycines suppression. Biological Control 45:368–379. [Google Scholar]
- Deagle B. E., Thomas A. C., McInnes J. C., Clarke L. J., Vesterinen E. J., Clare E. L., Kartzinel T. R., and Eveson J. P.(2019). Counting with DNA in metabarcoding studies: How should we convert sequence reads to dietary data? Molecular Ecology 28:391–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folmer O., Black M., Hoeh W., Lutz R., and Vrijenhoek R.(1994). DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3:294–299. [PubMed] [Google Scholar]
- Garfinkel M., and Johnson M.(2015). Pest-removal services provided by birds on small organic farms in northern California. Agriculture, Ecosystems and Environment 211:24–31. [Google Scholar]
- Garfinkel M. B., Minor E. S., and Whelan C. J.(2020). Data from: Birds suppress pests in corn but release them in soybean crops within a mixed prairie/agriculture system. The Condor: Ornithological Applications 122:1–12. doi: 10.5061/dryad.z34tmpg8t [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann A. J., Petzold-Maxwell J. L., Clifton E. H., Dunbar M. W., Hoffmann A. M., Ingber D. A., and Keweshan R. S.(2014). Field-evolved resistance by western corn rootworm to multiple Bacillus thuringiensis toxins in transgenic maize. Proceedings of the National Academy of Sciences USA 111:5141–5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt K., Anderson A. M., Kirkpatrick K. N., and Shwiff S. A.(2011). A review and synthesis of bird and rodent damage estimates to select California crops. Crop Protection 30:1109–1116. [Google Scholar]
- Grass I., Lehmann K., Thies C., and Tscharntke T.(2017). Insectivorous birds disrupt biological control of cereal aphids. Ecology 98:1583–1590. [DOI] [PubMed] [Google Scholar]
- Halaj J., and Wise D. H.(2001). Terrestrial trophic cascades: How much do they trickle? The American Naturalist 157:262–281. [DOI] [PubMed] [Google Scholar]
- Hannay M. B., Boulanger J. R., Curtis P. D., Eaton R. A., Hawes B. C., Leigh D. K., Rossetti C. A., Steensma K. M. M., and Lindell C. A.(2019). Bird species and abundances in fruit crops and implications for bird management. Crop Protection 120:43–49. [Google Scholar]
- Harrison X. A., Donaldson L., Correa-Cano M. E., Evans J., Fisher D. N., Goodwin C. E. D., Robinson B. S., Hodgson D. J., and Inger R.(2018). A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 6:e4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert P. D. N., Cywinska A., Ball S. L., and deWaard J. R.(2003). Biological identifications through DNA barcodes. Proceedings of the Royal Society B: Biological Sciences 270:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huson D. H., Auch A. F., Qi J., and Schuster S. C.(2007). MEGAN analysis of metagenomic data. Genome Research 17:377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedlicka J. A., Vo A.-T. E., and Almeida R. P. P.(2017). Molecular scatology and high-throughput sequencing reveal predominately herbivorous insects in the diets of adult and nestling Western Bluebirds (Sialia mexicana) in California vineyards. The Auk: Ornithological Advances 134:116–127. [Google Scholar]
- Karp D. S., Mendenhall C. D., Sandí R. F., Chaumont N., Ehrlich P. R., Hadly E. A., and Daily G. C.(2013). Forest bolsters bird abundance, pest control and coffee yield. Ecology Letters 16:1339–1347. [DOI] [PubMed] [Google Scholar]
- Kellermann J. L., Johnson M. D., Stercho A. M., and Hackett S. C.(2008). Ecological and economic services provided by birds on Jamaican Blue Mountain coffee farms. Conservation Biology 22:1177–1185. [DOI] [PubMed] [Google Scholar]
- Koh L. P. (2008). Birds defend oil palms from herbivorous insects. Ecological Applications 18:821–825. [DOI] [PubMed] [Google Scholar]
- Kross S. M., Ingram K. P., Long R. F., and Niles M. T.(2018). Farmer perceptions and behaviors related to wildlife and on-farm conservation actions: Farmer perceptions of wildlife. Conservation Letters 11:e12364. [Google Scholar]
- Kross S. M., Kelsey T. R., McColl C. J., and Townsend J. M.(2016). Field-scale habitat complexity enhances avian conservation and avian-mediated pest-control services in an intensive agricultural crop. Agriculture, Ecosystems and Environment 225:140–149. [Google Scholar]
- Letourneau D. K., Jedlicka J. A., Bothwell S. G., and Moreno C. R.(2009). Effects of natural enemy biodiversity on the suppression of arthropod herbivores in terrestrial ecosystems. Annual Review of Ecology, Evolution, and Systematics 40:573–592. [Google Scholar]
- Liere H., Kim T. N., Werling B. P., Meehan T. D., Landis D. A., and Gratton C.(2015). Trophic cascades in agricultural landscapes: Indirect effects of landscape composition on crop yield. Ecological Applications 25:652–661. [DOI] [PubMed] [Google Scholar]
- Lindell C. A., and Eaton R. A.(2012). Bird consumption of sweet and tart cherries. Human–Wildlife Interactions 6:283–290. [Google Scholar]
- Lindell C., Eaton R. A., Howard P. H., Roels S. M., and Shave M. E.(2018). Enhancing agricultural landscapes to increase crop pest reduction by vertebrates. Agriculture, Ecosystems and Environment 257:1–11. [Google Scholar]
- Maas B., Tscharntke T., Saleh S., Dwi Putra D., and Clough Y.(2015). Avian species identity drives predation success in tropical cacao agroforestry. Journal of Applied Ecology 52:735–743. [Google Scholar]
- Martin E. A., Reineking B., Seo B., and Steffan-Dewenter I.(2013). Natural enemy interactions constrain pest control in complex agricultural landscapes. Proceedings of the National Academy of Sciences USA 110:5534–5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes J. C., Alderman R., Deagle B. E., Lea M.-A., Raymond B., and Jarman S. N.(2017a). Optimised scat collection protocols for dietary DNA metabarcoding in vertebrates. Methods in Ecology and Evolution 8:192–202. [Google Scholar]
- McInnes J. C., Alderman R., Lea M.-A., Raymond B., Deagle B. E., Phillips R. A., Stanworth A., Thompson D. R., Catry P., Weimerskirch H., . et al. (2017b). High occurrence of jellyfish predation by Black-browed and Campbell albatross identified by DNA metabarcoding. Molecular Ecology 26:4831–4845. [DOI] [PubMed] [Google Scholar]
- Millar R. B., and Anderson M. J.(2004). Remedies for pseudoreplication. Fisheries Research 70:397–407. [Google Scholar]
- Nguyen H. D. D., and Nansen C.(2018). Edge-biased distributions of insects. A review. Agronomy for Sustainable Development 38:11. [Google Scholar]
- Parr C. S., Wilson N., Leary P., Schulz K., Lans K., Walley L., Hammock J., Goddard A., Rice J., Studer M., . et al. (2014). The Encyclopedia of Life v2: Providing global access to knowledge about life on Earth. Biodiversity Data Journal 2:e1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedigo L. P., Hutchins S. H., and Higley L. G.(1986). Economic injury levels in theory and practice. Annual Review of Entomology 31:341–368. [Google Scholar]
- Peisley R. K., Saunders M. E., and Luck G. W.(2015). A systematic review of the benefits and costs of bird and insect activity in agroecosystems. Springer Science Reviews 3:113–125. [Google Scholar]
- Pejchar L., Clough Y., Ekroos J., Nicholas K., Olsson O., Ram D., Tschumi M., and Smith H. G.(2018). Net effects of birds in agroecosystems. BioScience 68:896–904. [Google Scholar]
- Puckett H. L., Brandle J. R., Johnson R. J., and Blankenship E. E.(2009). Avian foraging patterns in crop field edges adjacent to woody habitat. Agriculture Ecosystems and Environment 131:9–15. [Google Scholar]
- R Core Team (2017). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing,Vienna, Austria: http://www.r-project.org/ [Google Scholar]
- Rodenhouse N. L., and Best L. B.(1983). Breeding ecology of Vesper Sparrows in corn and soybean fields. American Midland Naturalist 110:265–275. [Google Scholar]
- Roels S. M., Porter J. L., and Lindell C. A.(2018). Predation pressure by birds and arthropods on herbivorous insects affected by tropical forest restoration strategy: Predation pressure in tropical forest restorations. Restoration Ecology 26:1203–1211. [Google Scholar]
- Salo P., Banks P. B., Dickman C. R., and Korpimäki E.(2010). Predator manipulation experiments: Impacts on populations of terrestrial vertebrate prey. Ecological Monographs 80:531–546. [Google Scholar]
- Sekercioglu C. H., Wenny D. G., and Whelan C. J.(Editors) (2016). Why Birds Matter: Avian Ecological Function and Ecosystem Services. University of Chicago Press, Chicago, IL, USA. [Google Scholar]
- Shapiro S. S., and Wilk M. B.(1965). An analysis of variance test for normality (complete samples). Biometrika 52:591–611. [Google Scholar]
- Smith D. P., and Peay K. G.(2014). Sequence depth, not PCR replication, improves ecological inference from next generation DNA sequencing. PLOS One 9:e90234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass G. L., Jackson R. E., Abel C. A., and Perera O. P.(2010). Utilization of early soybeans for food and reproduction by the tarnished plant bug (Hemiptera: Miridae) in the delta of Mississippi. Environmental Entomology 39:1111–1121. [DOI] [PubMed] [Google Scholar]
- Tremblay A., Mineau P., and Stewart R. K.(2001). Effects of bird predation on some pest insect populations in corn. Agriculture Ecosystems and Environment 83:143–152. [Google Scholar]
- Triplehorn C. A., and Johnson N. F.(2005). Borror and Delong’s Introduction to the Study of Insects. Thompson Brooks/Cole,Belmont, CA, USA. [Google Scholar]
- Tscharntke T., Tylianakis J. M., Rand T. A., Didham R. K., Fahrig L., Batáry P., Bengtsson J., Clough Y., Crist T. O., Dormann C. F., . et al. (2012). Landscape moderation of biodiversity patterns and processes—Eight hypotheses. Biological Reviews 87:661–685. [DOI] [PubMed] [Google Scholar]
- Tschumi M., Ekroos J., Hjort C., Smith H. G., and Birkhofer K.(2018). Predation-mediated ecosystem services and disservices in agricultural landscapes. Ecological Applications 28:2109–2118. [DOI] [PubMed] [Google Scholar]
- [USDA] United States Department of Agriculture (2018). National Agricultural Statistics Service. http://www.nass.usda.gov/ [Google Scholar]
- Van Bael S. A., Bichier P., and Greenberg R.(2007). Bird predation on insects reduces damage to the foliage of cocoa trees (Theobroma cacao) in western Panama. Journal of Tropical Ecology 23:715–719. [Google Scholar]
- Whelan C. J., Wenny D. G., and Marquis R. J.(2008). Ecosystem services provided by birds. Annals of the New York Academy of Sciences 1134:25–60. [DOI] [PubMed] [Google Scholar]
- Wiebe K., and Gollehon N.(2006). Agricultural resources and environmental indicators (No. EIB-16). Economic Research Service/USDA; https://www.ers.usda.gov/webdocs/publications/44107/30222_eib16.pdf?v=0 [Google Scholar]