Abstract
Background
Despite demonstrated efficacy, patient decision aids (DAs) are rarely used in clinical practice in the absence of coverage mandates. Deciding whether to pursue a left ventricular assist device (LVAD) is a major, preference-sensitive decision—ideal for exploring implementation of a DA.
Methods
We conducted a type II effectiveness-implementation hybrid trial at 6 LVAD programs using a stepped-wedge cluster-randomized design. Using the RE-AIM framework, we collected both quantitative and qualitative outcomes, including a checklist collected by study staff for each enrolled patient regarding DA use and interviews with LVAD program clinicians pre-intervention, 6-months post-intervention, and at the conclusion of the study.
Results
From June 2015 to January 2017, 248 patients and their caregivers were enrolled. A total of 69 interviews were conducted with 48 clinicians at three time points. The DA reached 95% of eligible patients. Adoption was 100%, as all sites approached agreed to participate in the trial. Interviews revealed several themes related to the implementation of the DA: clinicians had a strong desire to ensure patients were informed and embraced the DA. Despite this, they reported communication challenges amongst their team that impeded implementation. Five of the 6 sites have maintained use of the DA following the trial; one site reported concerns about decreased procedural volume with use of the DA as a reason for discontinuation.
Conclusions
In this hybrid trial, a DA for patients considering LVADs and their caregivers demonstrated high reach. Adoption and implementation were facilitated by a strong desire to assure that patients were well informed. Future dissemination research and practice should attend to concerns about procedure volume, coverage mandates and facilitate ongoing communication at sites using the DA.
Patient decision aids (DAs) are an evidence-based strategy to support patients, families, and clinicians in shared decision-making. DAs have consistently been shown to improve knowledge, satisfaction, patient-clinician communication, patient involvement in decision making, decisional conflict, and regret.1, 2 Despite their established efficacy, DAs are rarely implemented outside research settings.3, 4 A systematic review of DA implementation identified a host of logistical barriers, including clinicians’ perception of time necessary to use DAs, lack of reimbursement, and perceived bias inherent in the DAs themselves.5
Patients with end-stage heart failure are increasingly offered therapies to prolong survival, including implantation of left ventricular assist devices (LVADs). LVADs are implantable pumps that require a major surgery and come with major tradeoffs and significant lifestyle changes including the need for patients to be connected to battery power or electricity at all times.6, 7 The high-risk, high-reward decision-making process is complex and preference-sensitive, making it an ideal scenario for shared decision making.
The Multicenter Trial of a Shared Decision Support Intervention for Patients and their Caregivers Offered Destination Therapy for End-Stage Heart Failure (DECIDE-LVAD trial) was a pragmatic study performed to simultaneously assess the effectiveness and implementation of a DA for patients, who had advanced heart failure and were not eligible for heart transplant, and their caregivers considering a destination therapy (DT) LVAD as permanent treatment. The design of the DA and its evaluation had a strong focus on making a tool that would fit the implementation context and thus be adopted and implemented in real world settings. The DECIDE-LVAD trial demonstrated the DA improved decision quality (knowledge and value-treatment concordance) for both patients and caregivers.8, 9 As it was designed as a type II effectiveness-implementation hybrid trial,10, 11 understanding how sites implemented the tools was a co-primary aim of the study. The following is the rigorous implementation evaluation that was conducted as part of the DECIDE-LVAD trial.
METHODS
Trial Design
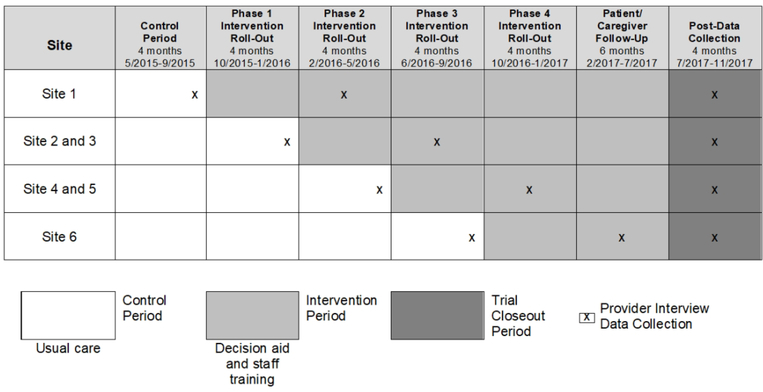
To evaluate both effectiveness and implementation simultaneously, we utilized the stepped-wedge trial design, where phased rollout of the DA intervention occurred at the hospital level (Figure 1). The order in which the hospitals rolled out the DA was randomized, with the constraint that the coordinating site begin the intervention first. The study was overseen by the institutional review board at the University of Colorado and approved by institutional review boards at all sites. The trial design and effectiveness papers have been published previously.8–10
FIGURE 1.
Timeline of Stepped Wedge Intervention Rollout and Interview Data Collection
Settings
Implementation occurred at 6 diverse LVAD centers across the United States, including a not-for-profit Catholic medical center, a state university medical center, and 4 private university-based medical centers, drawing from both urban and rural populations spread throughout the country (Table 1).
Table 1.
Description of Advanced Heart Failure Programs and Participants Involved in the DECIDE-LVAD Trial
| Count | |||||
|---|---|---|---|---|---|
| Site # | Hospital Type | Region | DT LVAD Volume | Program Team Members | Study Interviews Conducted |
| Site 1 | State university, tertiary care | West, serving urban and rural population | • In 2013: 13 • In 2016: 22 |
• AHF Cardiologist: 7 • Cardiothoracic Surgeon: 3 • AHF Nurse Practitioner: 4 • LVAD Coordinator: 3 • Social Worker: 1 |
• Pre-Intervention: 8 • Post-Intervention: 5 • Maintenance: 1 |
| Site 2* | Private university-based, tertiary care | Northeast, serving urban population | • In 2013: 23 • In 2016: 11 |
• AHF Cardiologist: 8 • Cardiothoracic Surgeon: 4 • AHF Nurse Practitioner: 5 • LVAD Coordinator: 3 • Social Worker: 2 |
• Pre-Intervention: 5 • Post-Intervention: 8 • Maintenance: 2 |
| Site 3 | Not-for-profit catholic, tertiary care | Midwest, serving urban and rural populations | • In 2013: 32 • In 2016: 24 |
• AHF Cardiologist: 6 • Cardiothoracic Surgeon: 6 • AHF Nurse Practitioner: 5 • LVAD Coordinator: 2 • Social Worker: 3 |
• Pre-Intervention: 4 • Post-Intervention: 5 • Maintenance: 2 |
| Site 4 | Private university-based, tertiary care | Midwest, serving rural populations | • In 2013: 18 • In 2016: 30 |
• AHF Cardiologist: 10 • Cardiothoracic Surgeon: 7 • AHF Nurse Practitioner: 2 • LVAD Coordinator: 7 • Social Worker: 3 |
• Pre-Intervention: 6 • Post-Intervention: 7 • Maintenance: 2 |
| Site 5 | Private university-based, tertiary care | Midwest, serving urban and rural populations | • In 2013: 33 • In 2016: 47 |
• AHF Cardiologist: 7 • Cardiothoracic Surgeon: 8 • AHF Nurse Practitioner: 3 • LVAD Coordinator: 4 • Social Worker: 2 |
• Pre-Intervention: 5 • Post-Intervention: 7 • Maintenance: 2 |
| Site 6 | Private university-based, tertiary care | South, serving urban and rural populations | • In 2013: 19 • In 2016: 95 |
• AHF Cardiologist: 7 • Cardiothoracic Surgeon: 2 • AHF Nurse Practitioner: 10 • LVAD Coordinator: 3 • Social Worker: 2 |
• Pre-Intervention: 4 • Post-Intervention: 3 • Maintenance: 2 |
AHF, advanced heart failure; DT, destination therapy; LVAD, left ventricular assist device.
Site that did not maintain use of the patient decision aids at end of study.
Patients and Caregivers Participants
Patient and caregiver dyads were enrolled. Eligibility criteria for patients included 18 years of age or older, English speaking and being considered for DT LVAD. Patients were identified at the time of the medical team member’s request for financial approval for DT LVAD evaluation or request for formal DT LVAD education. Caregivers were identified by patients as the individual who would serve as the primary caregiver if the patient were to receive the LVAD. Inclusion criteria for the caregivers included 18 years of age or older, English speaking, and non-paid family or friend of patient considered for DT LVAD.
Clinician Participants
Multiple clinical team members from each of the 6 LVAD programs were enrolled, including heart failure cardiologists, cardiothoracic surgeons, heart failure nurse practitioners, LVAD coordinators (typically registered nurses), and social workers. Inclusion criteria for the implementation evaluation included involvement in the DT LVAD evaluation and education process at their program. Potential participants were identified by the site’s principal investigator. Participants were recruited via email.
Patient Decision Aid Intervention
Prior to intervention rollout, patients received usual care. This included existing educational materials at each program, such as industry pamphlets and videos and program-specific documents. The DA intervention was an 8-page pamphlet and a 26-minute video, and provided as either a packet with the pamphlet professionally printed and video on a DVD, or digital copies of both on a study iPad. A full description of the DAs is presented elsewhere,12 and they can be found online at patientdecisionaid.org. Several design features were specifically included to enhance implementation and dissemination: use of testimonials (as many patients and caregivers desire discussions with other patients); acknowledgement of emotion (as many patients and caregivers were frightened); and multiple formats (video and pamphlet versions to address the various cognitive needs of critically ill patients).13–15
Implementation Strategy
At the time of intervention implementation, each site participated in: (1) a grand rounds-style presentation for the entire heart failure and mechanical circulatory support staff, which provided background, pilot work, and objectives; and (2) a 60-minute coaching session for staff directly involved in LVAD patient education, which included review of the DA materials and communication training. To emphasize that shared decision making for LVADs was about the conversation and not just a DA, four short animated videos were used to demonstrate key challenges in communication: introducing the option of an LVAD, framing of the options, responding to emotions, and providing recommendations.
The trial had few requirements for fidelity to the intervention and allowed flexibility in implementation procedures (e.g. who introduced the DA). Sites integrated the DA into the existing educational processes for all patients undergoing DT LVAD evaluation as the new standard of care, with the one implementation requirement being that the DA be provided to patients by a clinician (not study personnel) prior to the final treatment decision. However, fidelity to a certain delivery method was not mandated, and an explicitly empiric research question was to study if and how sites adapted the delivery.
Implementation Framework
We used the RE-AIM framework for our planning and implementation evaluation.16, 17 The RE-AIM framework assesses an intervention’s potential for dissemination and public health impact using five criteria: Reach, Effectiveness, Adoption, Implementation, and Maintenance. RE-AIM has been used to translate research into practice and to help plan programs and improve their chances of working in real-world settings.
Data collection
Patient and caregiver data collection have been described previously.8, 9 To evaluate the implementation process in real-time, the site study coordinator completed a checklist for each enrolled patient directly following formal LVAD education (see Supplemental Material 1). This checklist included information about which materials were provided, who was present during material delivery, and in what format patients viewed the DA.
A series of qualitative interviews, using interview guides based on the RE-AIM framework, were conducted with LVAD team members from each of the 6 LVAD programs (Supplemental Material 2–4). Interviews were conducted at three different time points: 1) pre-intervention: 1–8 weeks prior to intervention initiation to understand the current LVAD evaluation and educational process; 2) post-intervention: 4–7 months after intervention initiation to understand the intervention implementation and how the LVAD evaluation and educational process has changed; and 3) maintenance: 7–9 months after patient and caregiver enrollment was complete to explore ongoing maintenance of the intervention. The post-intervention interview also included a site visit. Two study personnel (DDM and JST) visited the site, conducted data quality checks, and learned about the implementation procedures in person. Many participants were interviewed at multiple time points. Interviews were semi-structured and conducted by study personnel from the main site (DDM and JST) over the phone or in-person. Interviews were audio recorded and transcribed verbatim.
Analysis
Coordinator checklist data were summarized descriptively by site. For the qualitative interviews, we used qualitative Content Analysis methodology, where interview transcriptions were inductively coded by 3 qualitatively-trained study personnel, including the interviewer and a PhD-trained qualitative methodologist (JST, MAM, GV). Study personnel met weekly to develop and refine the codebook, reconcile coded transcripts, and discuss emerging themes. Once a final codebook was developed, 25% of the remaining transcripts were double coded to ensure consistency. A team-based approach was utilized throughout analysis, where the codebook and emerging themes were discussed with other study personnel (DDM). The team determined thematic saturation was reached once additional interview data created little or no change to the codebook and no new patterns or themes emerged. Coded data were analyzed within and across programs to develop themes that described the DT LVAD evaluation and education process at each program and experiences across each team member type. ATLAS.ti software was used throughout the coding and analysis process. To establish credibility and trustworthiness of the data,18, 19 we triangulated the preliminary findings with the quantitative data and with a multidisciplinary team that included clinicians specializing in LVAD and implementation science experts.20 We also conducted member checking, where we presented preliminary findings to our participants in order to obtain feedback, and participants agreed with our findings. The findings from member checking were included in the final analysis.21
RESULTS
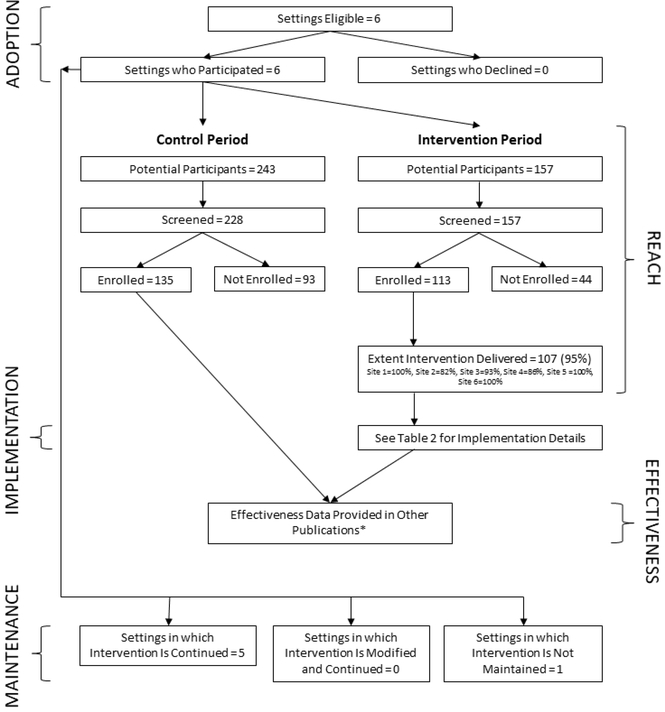
Between June 2015 and January 2017, a total of 400 patients were evaluated for a DT LVAD and 385 of those were screened for the study across all 6 sites. Of those, 248 patients were enrolled; 135 in the control and 113 in the intervention period (Figure 2). Of the 248 patients, 166 of their primary caregivers (105 control, 61 intervention) consented to be in the study. We include an expanded CONSORT diagram22 to show the addition of adoption, reach, and maintenance assessments to the traditional CONSORT diagram. For the qualitative portion of the implementation evaluation, 69 interviews with clinicians occurred across the 6 sites. Several clinicians were interviewed at two or three time points, with a total of 48 unique clinicians (Table 1). This included 13 advanced heart failure cardiologists, 3 cardiothoracic surgeons, 6 nurse practitioners, 17 LVAD coordinators, 8 social workers, and 1 palliative care physician. Of the total interviews, 36 were conducted in person and 33 were conducted over the phone.
FIGURE 2.
RE-AIM Extended CONSORT Diagram
This figure contains most but not all elements of the Expanded CONSORT Figure (22) and was customized to fit this study. Some of the qualitative information on representativeness at different stages is not available and was not the focus of this paper.
Reach
Overall, the DA was delivered to nearly 95% of eligible patients (99 patients received both pamphlet and video, 2 received pamphlet only, 6 received video only, and 6 received neither). The use of the pamphlet and video varied across each of the 6 sites (Table 2). Among caregivers enrolled in the study, 95% received the DA; among total caregivers (enrolled and non-enrolled), 82% received the DA (Table 2).
Table 2.
Implementation of Decision Aids by Site
| Site | |||||||
|---|---|---|---|---|---|---|---|
| Total | 1 | 2* | 3 | 4 | 5 | 6 | |
| Control Period | N=135 | N=13 | N=11 | N=13 | N=28 | N=25 | N=45 |
| Educational Materials Delivered to Patient: Industry Materials | 37% | 62% | 46% | 92% | 0% | 100% | 0% |
| Intervention Period | N=113 | N=28 | N=11 | N=42 | N=7 | N=11 | N=14 |
| Educational Materials Delivered to Patient: | |||||||
| DECIDE Pamphlet | 89% | 100% | 46% | 93% | 71% | 91% | 100% |
| DECIDE Video | 93% | 100% | 82% | 93% | 71% | 91% | 100% |
| Industry Materials | 43% | 0% | 0% | 93% | 29% | 73% | 0% |
| Other Materials | 70% | 64% | 36% | 83% | 86% | 18% | 100% |
| Materials Provided by Clinician | 97% | 100% | 100% | 98% | 100% | 82% | 93% |
| Caregiver (Enrolled and Non-Enrolled) Received Materials | 82% | 79% | 73% | 86% | 86% | 64% | 100% |
| Other Family/Friend(s) Received Materials | 28% | 14% | 64% | 21% | 43% | 27% | 43% |
| Patient Kept Materials | 93% | 96% | 64% | 93% | 100% | 100% | 100% |
| Time Patient Spent with Materials: | |||||||
| Less than 10 minutes | 2% | 4% | 0% | 0% | 0% | 9% | 0% |
| 10–30 minutes | 3% | 4% | 18% | 0% | 0% | 0% | 0% |
| 30–60 minutes | 16% | 36% | 27% | 0% | 43% | 9% | 7% |
| 60–90 minutes | 18% | 43% | 18% | 0% | 29% | 36% | 0% |
| More than 90 minutes | 7% | 4% | 0% | 2% | 0% | 18% | 29% |
| Unknown | 55% | 11% | 36% | 98% | 29% | 27% | 64% |
| Clinician Went Through DECIDE | 23% | 14% | 0% | 0% | 57% | 46% | 93% |
| Pamphlet with Patient | |||||||
| How Patient Watched DECIDE Video:† | |||||||
| In-Hospital Television with DVD | 19% | 71% | 9% | 0% | 0% | 0% | 0% |
| In-Hospital on Tablet/Computer | 23% | 4% | 55% | 10% | 0% | 27% | 86% |
| In-Clinic on Tablet/Computer | 4% | 0% | 0% | 2% | 14% | 9% | 14% |
| Took Home DVD (Did Not Watch in Clinic/Hospital) | 33% | 21% | 0% | 62% | 43% | 18% | 0% |
| Other | 6% | 0% | 9% | 10% | 0% | 18% | 0% |
| Other Education Conducted with Patient: | |||||||
| Formal Education with LVAD Coordinator | 67% | 89% | 82% | 31% | 100% | 73% | 100% |
| Met an LVAD Patient | 32% | 32% | 46% | 17% | 14% | 18% | 57% |
| Other‡ | 23% | 11% | 9% | 50% | 0% | 0% | 7% |
| None | 7% | 11% | 9% | 7% | 0% | 9% | 0% |
| Patient Demographics | N=248 | N=41 | N=22 | N=55 | N=35 | N=36 | N=59 |
| Age, mean | 63.4 | 64.1 | 68.4 | 61.4 | 61.3 | 62.9 | 64.3 |
| Male | 84% | 93% | 86% | 85% | 83% | 80% | 80% |
| White Non-Hispanic§ | 76% | 68% | 95% | 91% | 85% | 91% | 68% |
| INTERMACS score, mean||# | 3.1 | 3.1 | 3.2 | 4.2 | 2.4 | 2.8 | 2.7 |
| Enrollment location | |||||||
| Outpatient | 23% | 17% | 0% | 44% | 0% | 29% | 29% |
| Inpatient (non-ICU) | 53% | 34% | 95% | 45% | 89% | 54% | 34% |
| ICU | 24% | 49% | 5% | 11% | 11% | 17% | 27% |
| Heart failure diagnosis for ≥4 years** | 68% | 58% | 77% | 74% | 67% | 87% | 75% |
DECIDE, Multicenter Trial of a Shared Decision Support Intervention for Patients and their Caregivers Offered Destination Therapy for End-Stage Heart Failure; ICU, intensive care unit; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; LVAD, left ventricular assist device.
Site that did not maintain use of the patient decision aids at end of study.
Missing/unknown for 17 participants.
Includes “group visit”, “wore sample LVAD equipment”, and “other”.
Missing/unknown for 15 participants.
Missing/unknown for 32 participants.
INTERMACS score profile descriptions: 1=critical cardiogenic shock, 2=progressive decline, 3=stable but inotrope dependent, 4=resting symptoms, 5=exertion intolerant, 6=exertion limited, 7=Advanced New York Heart Association function class III.
Missing/unknown for 16 participants.
Effectiveness and Adoption
The intervention was shown to be effective in improving decision quality for both patients and caregivers in previously published manuscripts.8, 9 Adoption is somewhat artificial in this controlled trial as sites were invited specifically related to investigators’ prior relationships. All 6 sites approached agreed to participate, giving 100% adoption among this highly selected cohort (Figure 2).
Implementation
Detailed data on how each site implemented the DA are summarized in Table 2. There was strong fidelity to the mandate that clinicians at the sites must deliver the decision aids (98%). In parallel, there was important variation by sites in terms of whether they continued to use industry materials in addition to the DA, whether caregivers received the materials, and whether a clinician went through the DA with the patient. Over half of the time, it was not clear exactly how long each patient spent with the decision aids.
Through the interviews, several themes were elucidated which were organized around three domains: 1) clinician-level interactions with the DA; 2) team-level experiences with implementation, and 3) system-level observations.
1). Clinician-level interactions with the DA
At the individual clinician level, 3 themes emerged, all of which reinforced implementation: a) a strong need for shared decision making with this patient population; b) a desire for unbiased materials; and c) a generally positive experience with the DA, with some variability in opinions about the DA.
1a. Need for shared decision making
Respondents universally felt that patient education and shared decision making is highly important in the context of LVAD evaluation and medical decision making. They noted that clinicians were on the “frontline” and saw both the good and bad sides of the therapy. There was a strong theme that clinicians would not want to facilitate LVAD implantation in a patient who was not informed. The DA supported the respondents’ need for materials that could help with education and decision making.
“You know, I’m here to convince you [patients] why you shouldn’t have an LVAD. Not necessarily in those words. But I want them to be clear that I need them to really, really, really, really want this therapy because it’s a really tough life-changing decision.” (Site 1 – LVAD coordinator)
“What is our purpose? Is our purpose to VAD everyone so we can get our numbers up, or is our purpose to make sure that we VAD the right people and help patients make decisions so that their decision on quality will be better and they won’t have buyer’s remorse or decision remorse right afterwards? And I said I think it’s the latter.” (Site 2 – Cardiologist)
1b. Desire for unbiased materials
In addition to needing more educational materials in general, respondents also reported wanting materials that were unbiased. Many reported that they previously relied on industry materials, which they perceived as overly biased. They felt that the study DA was objective, and so were more comfortable providing the tool to patients.
“I think that it’s valuable as a decision-making tool. Just having seen some of the other materials that were available to patients that had come from like a manufacturer. Kind of seen them like a lot of propaganda that wasn’t really you know, impartial. So I would really like to see something like this continued.” (Site 5 – Social worker)
1c. Positive experience with the DA
Lastly, respondents reported an overall positive experience using the DA. The DA helped focus and guide subsequent discussions with patients, which made the education sessions shorter and more fruitful. Patients were more likely to ask “good” questions during discussions with providers, and many patients reported back to respondents that they liked the materials.
“This is kind of their first introduction to everything so I think that it’s helpful for them to get a good idea. And I’ve had patients say that, you know, after reading the information that they come up with questions so it’s obviously helping them understand to the point where they can come up with some thoughtful questions about the devices.” (Site 5 – Nurse practitioner)
“They [patients] said – I think almost too uniformly, they’ve all said that it’s helped them get through the information that they were having a hard time grasping, and that they felt like things were a little bit more clear sometimes, but that it just reinforced a lot of what we said. They kind of tended to thank our team for being as comprehensive with the approach that we take. So in some ways it’s validated us a little bit.” (Site 3 – Cardiologist)
Respondents’ personal opinions of the tone of the DA were mixed, where some felt it was somber, while others felt it was a realistic depiction of LVAD.
“But I think there are some patients for whom it’s what they expected. There are some patients for whom it’s more frightening than they expected. In most cases, I think it catalyzes a conversation with the clinicians.” (Site 2 – Cardiologist)
Respondents reported a general preference for the video portion of the DA.
“I think everybody loves the video. I think everybody feels like it’s the most realistic picture for somebody who’s getting a VAD. I think it presents the best information.” (Site 2 – Nurse practitioner)
“…so being able to see real people that aren’t acting, aren’t doing, they’re just telling you their story, might be helpful because most people don’t have an ability to go somewhere and ask other patients who’ve had it or decided not to have it, so it’s kind of the next best thing.” (Site 4 – LVAD coordinator)
2). Team level experiences with implementation
At the team level, we observed three themes: a) delivery of the DA was facilitated by an already established education process inherent to the LVAD evaluation context; b) teams expanded the use of the DA beyond the study intended use; and c) implementation challenges arose due to minimal or miscommunication at the team level.
2a. Built-in delivery process
All sites already had a formal patient education process in place, which was usually led by the LVAD coordinator prior to the patient’s decision on treatment and the clinical team’s determination of patient eligibility, and included a sit-down education session and delivery of tangible materials. Education occurred in both the inpatient and outpatient setting. Thus, during the trial, the DA was easily integrated into this existing education process. When an LVAD evaluation was initiated, the LVAD coordinator would either (1) provide the DA for viewing before the education session so that the patient would have a baseline knowledge, (2) go through the DA with the patient during the education session, or (3) provide the DA to view after the education session as more information. Since the education session was already standard practice at all programs, and other materials were already being given to patients, use of the DA became part of the existing structure to enhance education without adding extra time to the process.
The existing process provided an obvious avenue for incorporation of the DA, and LVAD coordinator respondents reported it being natural for them to use the materials.
“I feel like a chunk of that is our responsibility per se, I mean we’re the ones who are kind of doing that preoperative education…” (Site 5 – Nurse practitioner)
2b. Adaptations
The primary adaptation of the use of the DA from its intended purpose was expansion of use into populations other than DT patients. Namely, the majority of the sites were using the DA with all patients being considered for an LVAD, including patients who were being considered for bridge to transplant and those who were not enrolled in the trial. The DA was being used as standard of care, not strictly as part of the trial.
“…because there’s always a chance that they will end up with the LVAD forever….so pretty much everybody gets a video.” (Site 1 – LVAD coordinator)
A second adaptation was use of the DA earlier in the process; some sites used the DA earlier with patients who might not yet be eligible for DT LVAD. One respondent reported:
“So I think the upstream use of it, to me, is valuable…I think it’s a good place to start for a lot of people.” (Site 5 – Cardiologist)
2c. Communication challenges
Respondents reported a variety of challenges that stemmed from minimal communication amongst team members after the initial implementation. This led to variation of the DA’s delivery within sites; how the materials were used depended on who was delivering them (for instance, whether the DA was given prior to or during the education, whether it was simply provided or gone through in detail with the LVAD coordinator). This inconsistency led to different patients receiving different types of care, rather than having a standard procedure for every patient education encounter. Without consistent communication and group consensus on delivery model, clinicians continued to use the DA in whichever ways they saw most fit, with some LVAD coordinators expressing confusion on what “should” be occurring. This, at times, led to some gaps, such as when a new LVAD coordinator was hired and not informed by the other LVAD coordinators on how to use the DA, creating a threat to long-term sustainability. The new LVAD Coordinator who had begun in her position 6 months prior described this challenge:
“Maybe it’s just I don’t know who’s giving them [patients] the information, but I can tell you that I haven’t been, not because I don’t want to but just because maybe I didn’t understand the process.” (Site 1 – LVAD coordinator)
The lack of communication also led to issues with timing at some sites, where the LVAD coordinators felt they had to give the DA prior to any patient education, which was not actually required.
In addition, communication challenges amongst the team members also occurred with assigning who would deliver the DA. Respondents across all clinician types were mixed in whether they viewed it as their responsibility to deliver the DA. Non-physician respondents reported a disconnect between those who saw it as their role to provide the DA (typically the LVAD coordinators) and those who did not see education as their role (typically physicians), which led most of the implementation work to fall on the LVAD coordinators. While some physician respondents expressed that they would sometimes use the DA, the LVAD coordinators stated that it was part of their job to use the DA at all times. While physicians pushed the use of the DA, they often left the actual use of them to the LVAD coordinators without much discussion between the team members.
“Some cardiologists know we have it and they’ll sometimes see people in the clinic and call us and say, ‘Hey, what can you give me to give to this patient?’ or something, but as far as, I mean, there’s kind of sporadic people in different areas…I don’t know if many of the physicians would be aware what’s going on…I mean, I don’t think they – we do the education… I mean maybe they do, but I’ve never had discussion with them about it.” (Site 4 – LVAD Coordinator)
3). System level experiences
At the system level, two themes emerged as challenges: a) logistical issues using the DA; and b) institutional pressures to maintain volume of LVAD implants.
3a. Logistical issues
While many respondents preferred the video DA, most expressed logistical frustrations with utilizing it. Some sites did not have a reliable method for playing the video to patients due to a lack of DVD players in the hospital rooms or accessible devices to show the video online. While they were sometimes able to troubleshoot by using the study-provided iPad or accessing the video online on a clinic computer or a patients’ own device, these means were not always available.
3b. Program pressures
Respondents reported a system level pressure on the LVAD team to expand their program. Sites in areas where there were other LVAD programs nearby felt pressure institutionally to compete, and respondents mentioned a need to keep their numbers high enough to maintain quality and accreditation. Some mentioned that the DA could be helpful as part of their outreach efforts.
“The hospital wants us to be busy here, and I want us to be busy here. I think epidemiologically we’re not even hitting the tip of the iceberg. But I don’t think that that means – we shouldn’t change our proportions of the patients we see. We need to increase the volume of the patients we see. That’s the difference. So we need to increase our implants. That doesn’t mean take on poor candidates, or take on people who aren’t necessarily interested in the device and implant devices in them. It means increasing our referrals and growing our business that way, where we see more patients, more candidates, and letting those proportions continue to fall out in that normal way.” (Site 1 – LVAD coordinator)
The pressure to maintain LVAD numbers had an impact on some providers’ view of the DA. At one site, a patient declined an LVAD after viewing the video DA, which led to some physicians at the program not wanting their patients to view the DA. This resulted in the site temporarily stopping use of the DA.
“… the particular reason that it ended up being an issue in our group was that the clinician, I think, was very eager for this patient to move forward with LVAD therapy, and the patient was kind of excited about it until after seeing the video and then was a bit turned off and there was a lot of fallout.” (Site 2 – Cardiologist)
Maintenance
When maintenance was assessed at the end of the study, 5 of the 6 sites continued using the DA. The 5 sites used hard copies and online versions of the pamphlet and video. Programs provided the DA to all LVAD patients regardless of indication. Use of the DA was maintained as in the study period, with no modifications made. Participants felt that the more education and resources available to patients, the better, and the DA helped provide unbiased, easy to understand information. One of the 5 sites expressed some concern with requirements of branding the materials at their institution, but ultimately was able to use the DA in the current form.
The site that did not maintain the use of the DA reported the reason as clinical leadership having concerns about patients declining the device. This was the same site that temporarily stopped using the DA during the study.
DISCUSSION
The DECIDE-LVAD trial is a robust randomized (stepped wedge) pragmatic trial using a Type 2 hybrid design, with implementation measures informed by the RE-AIM conceptual model. The mixed methods evaluation facilitates understanding of how and why the implementation results occurred. The trial’s implementation of LVAD DAs in 6 experienced LVAD centers had significant reach to eligible patients, consistent implementation, and ongoing maintenance. Combined with the positive effectiveness data for both patients and caregivers, this was a doubly successful trial.8, 9
Implementation of DAs in other settings has been a challenge.1, 5 Frequent barriers include clinicians’ concern for the lack of time that may be required, the lack of available staff to deliver the DAs, and concerns among physicians about the content of the DAs.3–5, 23 However, in contrast to many other settings, the implementation of the DAs for patients considering LVADs was relatively straight forward and successful due to a variety of factors. First, the majority of previous implementation experiences occur in settings where there is not an established team available to deliver tools. Consequently, it takes additional resources to develop a successful delivery strategy. Given the significant amount of personnel involved in LVAD patient education, there was an existing delivery process within which the DA could embed. Thus, the implementation in this setting was relatively streamlined.
Second, there are few examples of an intervention where clinicians have been so receptive of the concept of shared decision making from the beginning. This is likely due to the high-risk, high-reward nature of the LVAD decision—patients are weighing large potential benefits and large potential harms and burdens, and clinicians appeared to understand these risks, benefits, and burdens. Thus, the clinicians view this decision as truly preference sensitive.15, 24 Additionally, clinicians desired to limit downstream “buyer’s remorse” if things did not go well, often citing difficult personal experiences.
Third, the DAs themselves were designed using a user-centered approach with significant input from both patients and clinicians. Some design decisions were made for the purpose of implementation even if they violated the International Patient Decision Aid Standard (IPDAS) criteria.25 For example, the IPDAS criteria suggest that presentation of all harms be presented with the same risk communication strategy for both getting and not getting a procedure, but presenting things like stroke rate and infection to people without an LVAD did not make any sense to the end users. This user-centered design approach with rapid prototyping and empathy to the end users is also consistent with current “design for dissemination” principles.
Two notable problems that threaten sustainability emerged in our evaluation. First, there was very little communication about the use of these DA among team members. While it may be good that the intervention was implemented without much disruption to clinical workflow, it raises concern that sites may have a problem maintaining the intervention if the initial clinical champions leave their program. Additionally, lack of communication across the team could threaten ongoing implementation and sustainability. Encouraging ongoing communication at a program level regarding the use of the DA is an important maintenance aspect and an aspect future research should address. Programs could utilize the weekly multidisciplinary committee meetings, where all team members gather to discuss evaluated patients, as a standard time and place to check-in on DA use and provide a feedback and monitoring forum. This could ensure consistent DA delivery across team members, troubleshoot communication challenges, and help make the non-physician team members feel heard. Second, one site had significant concerns about the potential volume reduction with the use of the DA. Indeed, in the primary effectiveness trial, there was a reduction in patients going on to accept an LVAD (80% vs. 54%) after using the DA.8 Going forward, careful messaging around the tension between patient-centered care and pressures on procedure volume will be important to assure broader dissemination. Furthermore, changes in healthcare policy that reward value over volume would better align care decisions with shared decision making and better promote patient-centeredness.
Limitations
A number of limitations should be recognized. First, the sites invited to participate and involved in the study were eager to be involved and all had an engaged site-investigator who was also part of the clinical team. As such, the positive implementation results seen here are likely greater than what might be seen in the average LVAD program. Second, hard copies the DAs were provided to sites for free as part of a funded research project, an artificial aspect of the implementation. While the authors have a commitment to keep the DA available for free online, there are not guaranteed resources going forward to provide hard copies to all sites who might want them. While this may diminish the generalizability of this implementation effort, we have instituted a purchasing process for sites who wish to have hard copies of DAs. Furthermore, it has been our experience that cloud-based PDF download and video streaming onto personal devices is becoming more and more ubiquitous. Third, patients were not exposed to the DA until they were referred for an LVAD. Knowing the optimal timing for DA delivery is one of the major challenges in DA implementation and it is possible that patients would benefit from the DA more if they received them further upstream. Indeed, later in the trial, sites were delivering tools to some patients before they were being evaluated for the LVAD. Fourth, how the DA was used by the patients and families was not well documented and there were minimal data on the amount of time the patients and families spent using the DA.
Conclusions
A DA for patients and caregivers considering DT LVAD was successfully implemented. Adoption was facilitated by a strong desire to assure that patients and caregivers were well informed. The majority of eligible patients and caregivers were reached by the intervention. Implementation was successful but could have been enhanced with greater facilitation of communication among team members during implementation. Notably, the intervention was maintained even after the study funding ended. Perhaps the biggest lesson of this effectiveness-implementation hybrid is that the LVAD context is uniquely situated for the use of a DA to support shared decision making. The built-in delivery combined with a strong desire to assure that patients and caregivers were informed can be leveraged in broader implementation and dissemination activities.
Supplementary Material
Acknowledgments
Sources of Funding: This work was supported through a Patient-Centered Outcomes Research Institute (PCORI) Program Award (CDR-1310–06998). All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee. This work was also supported in part by the National Heart, Lung and Blood Institute (1K23HL105896–01, Allen), the Heart Failure Society of America (McIlvennan), the National Institute on Aging (1K23AG040696, Matlock), REDCap database hosting through University of Colorado supported by NIH/NCRR Colorado CTSI (Grant Number UL1 TR001082).
FUNDING: Work primarily supported through a Patient-Centered Outcomes Research Institute (PCORI) Program Award (CDR-1310–06998), but also in part through the National Heart, Lung and Blood Institute, the Heart Failure Society of America, the National Institute on Aging.
Footnotes
Declaration of Conflicting Interests: There are no conflicts of interest to disclose.
LOCATION: Work completed at the Adult and Child Consortium for Outcomes Research and Delivery Science, University of Colorado School of Medicine, Aurora, CO.
CLINICAL TRIAL REGISTRY: Clinicaltrial.gov identifier: NCT02344576 https://clinicaltrials.gov/ct2/show/NCT02344576?term=NCT02344576&rank=1
REFERENCES
- 1.O’Connor AM, Wennberg JE, Legare F, Llewellyn-Thomas HA, Moulton BW, Sepucha KR, Sodano AG, King JS. Toward the ‘tipping point’: decision aids and informed patient choice. Health Aff (Millwood). 2007;26:716–25. [DOI] [PubMed] [Google Scholar]
- 2.Stacey D, Legare F, Lewis KB. Patient decision aids to engage adults in treatment or screening decisions. JAMA. 2017;318:657–658. [DOI] [PubMed] [Google Scholar]
- 3.Gravel K, Legare F, Graham ID. Barriers and facilitators to implementing shared decision-making in clinical practice: a systematic review of health professionals’ perceptions. Implement Sci. 2006;1:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Legare F, Witteman HO. Shared decision making: examining key elements and barriers to adoption into routine clinical practice. Health Aff (Millwood). 2013;32:276–84. [DOI] [PubMed] [Google Scholar]
- 5.Elwyn G, Scholl I, Tietbohl C, Mann M, Edwards AG, Clay C, Legare F, van der Weijden T, Lewis CL, Wexler RM, et al. “Many miles to go …”: a systematic review of the implementation of patient decision support interventions into routine clinical practice. BMC Med Inform Decis Mak. 2013;13 Suppl 2:S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McIlvennan CK, Magid KH, Ambardekar AV, Thompson JS, Matlock DD, Allen LA. Clinical outcomes after continuous-flow left ventricular assist device: a systematic review. Circ Heart Fail. 2014;7:1003–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grady KL, Meyer PM, Dressler D, White-Williams C, Kaan A, Mattea A, Ormaza S, Chillcott S, Loo A, Todd B, et al. Change in quality of life from after left ventricular assist device implantation to after heart transplantation. J Heart Lung Transplant. 2003;22:1254–67. [DOI] [PubMed] [Google Scholar]
- 8.Allen LA, McIlvennan CK, Thompson JS, Dunlay SM, LaRue SJ, Lewis EF, Patel CB, Blue L, Fairclough DL, Leister EC, et al. Effectiveness of an intervention supporting shared decision making for destination therapy left ventricular assist device: the DECIDE-LVAD randomized clinical trial. JAMA Intern Med. 2018;178:520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McIlvennan CK, Matlock DD, Thompson JS, Dunlay SM, Blue L, LaRue SJ, Lewis EF, Patel CB, Fairclough DL, Leister EC, et al. Caregivers of patients considering a destination therapy left ventricular assist device and a shared decision-making intervention: the DECIDE-LVAD trial. JACC Heart Fail. 2018;6:904–913. [DOI] [PubMed] [Google Scholar]
- 10.McIlvennan CK, Thompson JS, Matlock DD, Cleveland JC Jr., Dunlay SM, LaRue SJ, Lewis EF, Patel CB, Walsh MN, Allen LA. A multicenter trial of a shared decision support intervention for patients and their caregivers offered destination therapy for advanced heart failure: DECIDE-LVAD: rationale, design, and pilot data. J Cardiovasc Nurs. 2016;31:E8–E20. [DOI] [PubMed] [Google Scholar]
- 11.Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 2012;50:217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson JS, Matlock DD, McIlvennan CK, Jenkins AR, Allen LA. Development of a decision aid for patients with advanced heart failure considering a destination therapy left ventricular assist device. JACC Heart Fail. 2015;3:965–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McIlvennan CK, Allen LA, Nowels C, Brieke A, Cleveland JC, Matlock DD. Decision making for destination therapy left ventricular assist devices: “there was no choice” versus “I thought about it an awful lot”. Circ Cardiovasc Qual Outcomes. 2014;7:374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McIlvennan CK, Jones J, Allen LA, Lindenfeld J, Swetz KM, Nowels C, Matlock DD. Decision-making for destination therapy left ventricular assist devices: implications for caregivers. Circ Cardiovasc Qual Outcomes. 2015;8:172–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McIlvennan CK, Matlock DD, Narayan MP, Nowels C, Thompson JS, Cannon A, Bradley WJ, Allen LA. Perspectives from mechanical circulatory support coordinators on the pre-implantation decision process for destination therapy left ventricular assist devices. Heart Lung. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glasgow RE, Harden SM, Gaglio B, Rabin B, Smith ML, Porter GC, Ory MG, Estabrooks PA. RE-AIM planning and evaluation framework: adapting to new science and practice with a 20-year review. Front Public Health. 2019;7:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harden SM, Smith ML, Ory MG, Smith-Ray RL, Estabrooks PA, Glasgow RE. RE-AIM in clinical, community, and corporate settings: perspectives, strategies, and recommendations to enhance public health impact. Front Public Health. 2018;6:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19:349–57. [DOI] [PubMed] [Google Scholar]
- 19.Miles MB, Huberman AM, Saldana J. Qualitative Data Analysis. 3rd ed. Thousand Oaks, CA: Sage Publications; 2013. [Google Scholar]
- 20.Holtrop JS, Rabin BA, Glasgow RE. Qualitative approaches to use of the RE-AIM framework: rationale and methods. BMC Health Serv Res. 2018;18:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Creswell JW. Qualitative Inquiry and Research Design: Choosing Among Five Traditions Thousand Oaks, CA: SAGE Publications, Inc.; 1998. [Google Scholar]
- 22.Glasgow RE, Huebschmann AG, Brownson RC. Expanding the CONSORT figure: increasing transparency in reporting on external validity. Am J Prev Med. 2018;55:422–430. [DOI] [PubMed] [Google Scholar]
- 23.Legare F, Stacey D, Turcotte S, Cossi MJ, Kryworuchko J, Graham ID, Lyddiatt A, Politi MC, Thomson R, Elwyn G, et al. Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database Syst Rev. 2014:CD006732. [DOI] [PubMed] [Google Scholar]
- 24.Thompson JS, Matlock DD, Morris MA, McIlvennan CK, Allen LA. Organic dissemination and real-world implementation of patient decision aids for left ventricular assist device. MDM Policy Pract. 2018;3:2381468318767658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joseph-Williams N, Newcombe R, Politi M, Durand MA, Sivell S, Stacey D, O’Connor A, Volk RJ, Edwards A, Bennett C, et al. Toward minimum standards for certifying patient decision aids: a modified delphi consensus process. Med Decis Making. 2013;34:699–710. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.