Abstract
Over the past 20 years, various identifiers of cellular senescence have been used to quantify the abundance of these cells in different tissues. These include classic markers such as p16, senescence-associated β-gal, and γH2AX, in addition to more recent markers (Sudan Black B and HMGB1). In vivo data on the usefulness of these markers in skeletal muscle are very limited and inconsistent. In the present study, we attempted to identify senescent cells in frozen human skeletal muscle biopsies using these markers to determine the effects of age and obesity on senescent cell burden; however, we were only able to assess the abundance of DNA-damaged nuclei using γH2AX immunohistochemistry. The abundance of γH2AX+ cells, including satellite cells, was not higher in muscle from old compared to young individuals; however, γH2AX+ cells were higher with obesity. Additionally, terminally differentiated, post-mitotic myofiber nuclei from obese individuals had elevated γH2AX abundance compared to muscle from lean individuals. Analyses of gene expression support the conclusion that elevated DNA damage and the senescence-associated secretory phenotype are preferentially associated with obesity in skeletal muscle. These data implicate obesity as a larger contributor to DNA-damage in skeletal muscle than aging; however, more sensitive senescence markers for human skeletal muscle are needed to determine if these cells are in fact senescent.
Keywords: γH2AX, satellite cells, post-mitotic, DNA damage, senescence
Introduction
Cellular senescence is a state of irreversible growth arrest in which a cell is unable to divide even when given an appropriate growth stimulus. Telomere shortening (replicative senescence) [1], DNA-damage [2] and cellular stress [3, 4] are some of the known causes of senescence, and lead to the upregulation of DNA-damage repair machinery and cell cycle regulatory proteins to inhibit the progression of the cell cycle. p16, a cyclin-dependent kinase inhibitor, is robustly upregulated during cellular senescence and is the most widely used biomarker of senescent cells [5-8]. Deletion of p16-expressing cells extends lifespan, prolongs healthspan, and reduces incidence of kyphosis and kidney dysfunction [9, 10], implicating accumulation of senescent cells in age-associated disease and mortality. Senescent cells release chemokines, inflammatory cytokines, and proteases to negatively affect the tissue microenvironment, dubbed the senescence-associated secretory phenotype (SASP) [11]. Accumulation of senescent cells, particularly in adipose tissue, may contribute to systemic inflammation associated with aging [12] and obesity [13].
The DNA-damage response is characteristic of senescent cells, as this occurs following single- [14] and double-strand [15, 16] DNA breaks. The phosphorylated form of the histone H2A variant gamma-H2AX (γH2AX), a marker of DNA-damage, is often used to identify senescent cells [17, 18]. γH2AX is phosphorylated at serine 139 (γH2AX) by ataxia telangiectasia mutated [19] following single and double strand breaks, effectively recruiting p53 binding protein 1 (53BP1) to initiate the DNA-damage response at the site of DNA-damage [20]. This response is also initiated through telomere shortening or uncapping, as γH2AX directly associates with chromosome ends [1]. An additional identifier of senescent cells is senescence-associated beta-galactosidase (SA β-Gal), which accumulates in senescent cells through increases of lysosomal β-Gal and is detectable using biochemical assays at pH 6.0 [21, 22]. SA β-Gal is the most widely used marker of senescence in vitro, however, there are limited reports of its use in vivo.
New senescence markers are being identified, including high mobility group protein B1 (HMGB1), which is translocated from the nucleus to the cytoplasm and then secreted following the induction of senescence [23]. When residing within the nucleus, HMGB1 remodels chromatin and promotes gene transcription [24]. When HMGB1 is secreted, it functions as a damage-associated molecular pattern and promotes inflammation through the interaction with toll-like receptor 4 [25]. Thus, cells that are devoid of nuclear HMGB1 are considered senescent, and a recent report showed an increase in HMGB1 negative nuclei in old mouse muscle [26]. Sudan Black B was recently described as having utility as a senescence marker in skeletal muscle because it interacts with lipofuscin, which may accumulate in senescent cells [26, 27].
The role of senescent cells in skeletal muscle is unclear, as there are few and conflicting reports on the in vivo abundance of senescent cells in muscle during aging [18, 26]. However, cultured muscle stem cells (satellite cells) isolated from old mouse and human muscle preferentially activate expression of either γH2AX [6, 28] or p16 [6], in addition to SA β-Gal, compared to satellite cells isolated from young muscle. As a result, it has been hypothesized that senescent satellite cells contribute to sarcopenia. There are currently no data on the effects of obesity on the prevalence of senescent cells in skeletal muscle. The purpose of this cross-sectional study was to identify reliable biomarkers of senescence in human vastus lateralis muscle biopsies and to quantify the effects of age and obesity on the abundance of senescent satellite cells.
Materials and Methods
Human subjects
Muscle biopsies and associated phenotyping data from a total of de-identified 42 men and women from the University of Kentucky Center for Muscle Biology Healthy Muscle Bank were separated into four groups with n=12 for each group; Six subjects in the young and lean groups were the same, hence the total subjects in this study was 42. Our goal was to create distinct groups, therefore the age criteria for “young” was designated age 18–35, and “old” was designated >70 yr, and all “young” and “old” subjects had a BMI <27 kg/m2. To compare “lean” and “obese”, lean subjects had a BMI<25 kg/m2, and obese had a BMI>34 kg/m2, and all were aged 18–35. Subject demographics are summarized in Tables 1 and 2. Overall, the subjects were well-matched. The obese group included more females. Vastus lateralis muscle biopsies were obtained as described previously [29] and samples for immunohistochemical analysis were mounted in tragacanth gum mixed with OCT and quickly frozen in liquid nitrogen–cooled isopentane and stored at −80°C until sectioning. Muscle for RNA isolation was immediately frozen in liquid nitrogen and stored at −80°C. All subjects had a fasting blood sample for glucose and insulin quantification and calculation of HOMA-IR [30], and body composition was measured using DXA. All protocols were approved by the Institutional Review Board of the University of Kentucky, Lexington, KY, USA and performed in accordance with the standards set forth by the Declaration of Helsinki.
Table 1:
Subject characteristics for young v. old
| Young | Old | |
|---|---|---|
| n | 12 | 12 |
| Age (Range) | 23.4 ± 3.5 (21 – 30) | 75.1 ± 3.3 * (70 – 86) |
| BMI (Range) | 22.9 ± 2.2 (20 – 26) | 24.4 ± 2.4 (20 – 27) |
| % Body Fat (Range) | 24.6 ± 7.1 (13 – 34) | 29.0 ± 6.3 (19 – 40) |
| HOMA-IR (Range) | 1.6 ± 0.9 (0.4 – 3.0) | 1.5 ± 0.8 (0.5 – 3.1) |
| Males | 6 | 6 |
| Females | 6 | 6 |
Data shown as ± the standard deviation
p<0.05 versus Young
Table 2:
Subject characteristics for lean v. obese
| Lean | Obese | |
|---|---|---|
| n | 12 | 12 |
| Age (Range) | 25.2 ± 4.0 (21 – 34) | 26.9 ± 4.4 (21 – 34) |
| BMI (Range) | 22.8 ± 1.7 (20 – 25) | 38.1 ± 3.8 * (34 – 46) |
| % Body Fat (Range) | 22.9 ± 8.1 (12 – 34) | 43.9 ± 5.6 * (32 – 49) |
| HOMA-IR (Range) | 1.5 ± 0.7 (0.4 – 3.0) | 4.9 ± 4.7 * (1.0 – 17.3) |
| Males | 6 | 4 |
| Females | 6 | 8 |
3 males and 3 females are shared between the young/lean groups
Data shown as ± the standard deviation
p<0.05 versus Lean
Cell Culture
Primary human myoblasts from a young, lean donor were used in these experiments and obtained through the Center for Muscle Biology Healthy Muscle Bank. In order to obtain a relatively pure (>90%) myoblast culture, following tissue digest with collagenase and dispase, cells were subjected to magnetic antibody cell sorting (MACS) using anti-CD56 microbeads and an Automacs Pro cell sorter (Miltenyi Biotec, Auburn, CA). The sorted cells were expanded in growth media consisting of Ham’s F-10 (Fisher Scientific, Waltham, MA), 20% fetal bovine serum (FBS, Atlanta Biologicals, Flowery Branch, GA), 1% penicillin/streptomycin (Fisher Scientific), and 10ng/ml basic fibroblast growth factor (bFGF) (Peprotech, Rocky Hill, NJ). To mimic the obese environment, myoblasts were cultured in growth media supplemented with 25 mM glucose, 100 nM insulin, and 250 Mm palmitate for 24 hours. For irradiation experiments, myoblasts were exposed to 5 Gy irradiation in a Mark I-68 137Cesium γ-irradiator (J.L Shepherd and Associates, City, State) on a rotating platform. At the designated time points (30 minutes, 2 hours, and 8 hours post-irradiation), myoblasts were fixed in 4% paraformaldehyde (PFA) and centrifuged at 1000 RPM onto slides using a cytospin (Cytospin 4; ThermoFisher, Waltham, MA). Slides were dried for 1 hour before immunocytochemical analyses.
Immunocytochemistry
γH2AX, DAPI:
Slides that contained myoblasts from the irradiation experiments were incubated in 0.5% Triton X-100 diluted in phosphate buffered saline (PBS) for 10 minutes and then washed in PBS. Cells were then incubated in blocking reagent (2% BSA, 0.1% Triton X-100 diluted in PBS) for 1 hour. Following blocking, cells were incubated in primary antibodies overnight for rabbit IgG anti-p16 (1:250, ab108349; Abcam, Cambridge, MA) and mouse IgG1 anti-γH2AX (1:250, 05–636; Millipore Sigma, Burlington, MA) diluted in blocking reagent. The following morning, cells were washed in PBS and then incubated in goat anti-rabbit IgG biotin (1:1000, 111–065-144; Jackson ImmunoResearch, West Grove, PA) diluted in blocking reagent for 75 minutes. Cells were then washed and incubated in streptavidin-conjugated AF594 (1:250, S11227; Invitrogen, Waltham, MA) and goat anti-mouse IgG1 AF488 (1:250, A21121; Invitrogen) diluted in PBS with 0.1% Triton X-100 for 75 minutes. Cells were then washed in PBS, stained with DAPI (1:10,000, D35471; Invitrogen) for 10 minutes and mounted with VectaShield fluorescent mounting media (H1000; Vector Labs, Burlingame, CA).
p16, MyHC, DAPI:
Cells were fixed in 4% paraformaldehyde (PFA) for 10 minutes, wash in phosphate buffer saline (PBS), and then incubated in 0.5% Triton X-100 diluted in PBS for 10 minutes. Cells were then incubated in blocking reagent (2% BSA, 0.1% Triton X-100 diluted in PBS) for 1 hour. Following blocking, cells were incubated in primary antibodies overnight for rabbit IgG anti-p16 (1:250, ab108349; Abcam, Cambridge, MA) and mouse IgG2a anti-myosin heavy chain (1:100, A4.1025; Developmental Studies Hybridoma Bank, Iowa City, IA) diluted in blocking reagent. The following morning, cells were washed in PBS and then incubated in goat anti-rabbit IgG biotin (1:1000, 111–065-144; Jackson ImmunoResearch, West Grove, PA) diluted in blocking reagent for 75 minutes. Cells were washed and incubated in streptavidin-conjugated AF594 (1:250, S11227; Invitrogen, Waltham, MA) and goat anti-mouse IgG2a AF488 (1:250, A21121; Invitrogen) diluted in PBS with 0.1% Triton X-100 for 75 minutes. Cells were then washed in PBS, stained with DAPI (1:10,000, D35471; Invitrogen) for 10 minutes and mounted with VectaShield fluorescent mounting media (H1000; Vector Labs, Burlingame, CA).
Immunohistochemistry
All immunohistochemistry (IHC) was performed on fresh frozen muscle cross-sections. Muscle blocks were removed from −80°C storage and placed into a cryostat (HM525 NX; ThermoFisher) set at −23°C. After the tissue warmed to −23°C, 7μm sections were cut and allowed to dry for 1 hour before IHC or stored at −20°C. Muscle sections were fixed in 4% PFA for 10 minutes, washed in PBS, then incubated in 0.5% Triton X-100 diluted in PBS for 10 minutes. For all immunohistochemical experiments, secondary-only antibody controls were used to determine background signal.
γH2AX, dystrophin, DAPI:
Fixed sections were rinsed in PBS and blocked in 2.5% normal horse serum (NHS) (S-2012; Vector Labs) for 90 minutes. Following blocking, sections were incubated in primary antibodies overnight for mouse IgG1 anti-γH2AX (1:250; Millipore Sigma) and mouse IgG2b anti-dystrophin (1:200, D8168 Sigma; Millipore Sigma) diluted in NHS. The following morning, sections were rinsed in PBS and incubated in goat anti-mouse IgG1 AF488 (1:250; Invitrogen) and goat anti-mouse IgG2b AF647 (1:250, A21242; Invitrogen) diluted in PBS for 75 minutes, rinsed in PBS, stained with DAPI (1:10,000; Invitrogen) for 10 minutes and mounted with VectaShield fluorescent mounting media (Vector Labs).
γH2AX, Pax7, DAPI:
Fixed sections were rinsed in PBS and incubated with 3% H2O2 for 10 minutes to block endogenous peroxidases. After being washed with PBS, muscle sections underwent heat-mediated antigen retrieval in sodium citrate (10 mM, pH 6.5) for 20 min at 92 °C. Sections were then washed in PBS and block in 2.5% NHS for 90 minutes. Following blocking, sections were incubated in primary antibody overnight for mouse IgG1 anti-Pax7 (1:100; DHSB) diluted in 2.5% NHS. The next day, sections were washed with PBS, incubated for 75 minutes in goat anti-mouse IgG1 biotinylated secondary antibody (1:1000, 115–065-205; Jackson ImmunoResearch) diluted in NHS, washed in PBS, and then incubated for 75 minutes in streptavidin horseradish peroxidase (1:500, S-911, Invitrogen) diluted in PBS. Sections were washed again in PBS, then incubated for 15 minutes in TSA AF594 (1:500, B40957, Invitrogen) diluted in PBS. Sections were then washed and incubated in primary antibody overnight for mouse IgG1 anti-γH2AX (1:250; Millipore Sigma) diluted in NHS. The following morning, sections were rinsed in PBS, incubated in goat anti-mouse IgG1 AF488 (1:250; Invitrogen) diluted in PBS for 75 minutes, rinsed in PBS, stained with DAPI (1:10,000; Invitrogen) for 10 minutes and mounted with VectaShield fluorescent mounting media (Vector Labs).
p16, dystrophin, DAPI:
Muscle sections were fixed in 4% PFA for 10 minutes, washed in PBS, then incubated in 0.5% Triton X-100 diluted in PBS for 10 minutes. Sections were then rinsed in PBS and blocked in 2.5% normal horse serum (NHS) (S-2012; Vector Labs) for 90 minutes. Following blocking, sections were incubated in primary antibodies overnight for rabbit IgG anti-p16 (1:250, ab108349; Abcam) and mouse IgG2b anti-dystrophin (1:200, D8168 Sigma; Millipore Sigma) diluted in NHS. For p16, two additional antibodies were used (A0262, ABclonal; MAB4133, Millipore Sigma), however, they did not result in any positive staining. The following morning, sections were rinsed in PBS and then incubated in goat anti-rabbit IgG biotin (1:1000; Jackson ImmunoResearch) diluted in NHS for 75 minutes. Sections were then rinse in PBS and incubated in streptavidin-conjugated AF594 (1:250; Invitrogen) and goat anti-mouse IgG2b AF488 (1:250, A21141; Invitrogen) diluted in PBS for 75 minutes. Sections were then rinsed in PBS, stained with DAPI (1:10,000; Invitrogen) for 10 minutes and mounted with VectaShield fluorescent mounting media (Vector Labs).
HMGB1, dystrophin, DAPI:
Muscle sections were fixed in 4% PFA for 10 minutes, washed in PBS, then incubated in 0.5% Triton X-100 diluted in PBS for 10 minutes. Sections were rinsed in PBS and incubated with 3% H2O2 for 10 minutes to block endogenous peroxidases. After being washed with PBS, muscle sections underwent heat-mediated antigen retrieval in sodium citrate (10 mM, pH 6.5) for 20 minutes at 92 °C. Sections were then washed in PBS and block in 2.5% NHS for 90 minutes. Following blocking, sections were incubated in primary antibody overnight for Rb IgG anti-HMGB1 (1:250, ab18256; Abcam) and mouse IgG2b anti-dystrophin (1:200, D8168 Sigma; Millipore Sigma) diluted in NHS. The next day, sections were washed with PBS, incubated for 75 minutes in goat anti-rabbit IgG biotinylated secondary antibody (1:1000; Jackson ImmunoResearch) diluted in NHS, washed in PBS, and then incubated for 75 minutes in streptavidin-conjugated AF594 (1:250; Invitrogen) and goat anti-mouse IgG2b AF488 (1:250, A21141; Invitrogen) diluted in PBS for 75 minutes. Sections were then rinsed in PBS, stained with DAPI (1:10,000; Invitrogen) for 10 minutes and mounted with VectaShield fluorescent mounting media (Vector Labs).
Senescence-associated (SA) β-Gal
Tibialis anterior muscle sections from a 24 month old male C57Bl/6J mouse were used. Muscle sections were cut and then allowed to dry for 30 minutes. Sections were then fixed in 0.5% glutaraldehyde in PBS for 5 minutes at room temperature and washed in PBS. After washing, sections were incubated in fresh made staining solution that contained: 1mg/X-gal in DMF, 5mM potassium ferrocyanide, 5mM potassium ferricyanide, 5M sodium chloride, 1M magnesium chloride, and 0.2M citric acid/Na phosphate buffer. Citric acid/Na phosphate buffer should be pH 6.0. Muscle sections were incubated in staining solution for 48 hours at 37°C in the dark, with fresh solution added after the first 24 hours. Do not use cell culture incubator because the CO2 will change the pH of the staining solution.
Sudan Black B, Type 1 MyHC, laminin
Our Sudan Black B (SBB) protocol is adapted from da Silva et al. [26]. SBB powder was dissolved in 100% ethanol (7 mg/mL), covered with parafilm, and mixed overnight. The solution was then filtered and stored in an airtight container. Sections were fixed in 1% PFA for 5 min, washed three times in distilled water and then incubated in 100% ethanol for 5 min. 100 μl of SBB solution were then dropped on to a clean glass slide and the slide containing the muscle sections was placed faced down on the SBB glass slide for 90 minutes at room temperature. The slide with the muscle sections was then lifted off, and rinsed with distilled water. Immediately following SBB staining, sections were placed in primary antibodies for Rb anti-laminin (1:250, L9393; Sigma) and MyHC Type 1 (1:100, BA.D5; DSHB), diluted in PBS, for 90 minutes, washed in PBS, and then put in secondary antibodies for Rb IgG AMCA (1:100, CI-1000; Vector Labs) and Ms IgG2b AF647 (1:250, A-21242; Invitrogen) for 60 minutes. Sections were then washed and coversliped in Vectashield (Vector Labs).
53BP1, γH2AX, dystrophin, DAPI:
Muscle sections were fixed in 4% PFA for 10 minutes, washed in PBS, then incubated in 0.5% Triton X-100 diluted in PBS for 10 minutes. Sections were then washed in PBS and block in 2.5% NHS for 90 minutes. Following blocking, sections were incubated in primary antibody overnight for Rb IgG anti-53BP1 (1:250, ab175933; Abcam), mouse IgG1 anti-γH2AX (1:250; Millipore Sigma), and mouse IgG2b anti-dystrophin (1:200, Millipore Sigma) diluted in NHS. The next day, sections were washed with PBS, incubated for 75 minutes in goat anti-rabbit IgG AF647 (1:250; Invitrogen), goat anti-mouse IgG1 AF488 (1:250; Invitrogen), and goat anti-mouse IgG2b AF594 (1:250; Invitrogen) diluted in PBS for 75 minutes. Sections were then rinsed in PBS, stained with DAPI (1:10,000; Invitrogen) for 10 minutes and mounted with VectaShield fluorescent mounting media (Vector Labs).
Fiber Size Analysis
Average myofiber cross sectional area (CSA) quantification was performed using MyoVision [31]. The dystrophin labeled image was used as the reference and myofiber CSA was automatically determined by MyoVision’s region of interest algorithm. Myofibers with CSAs below 500 μm2 and above 20,000 μm2 were excluded from the analysis. Parts of the cross section that appeared to be folded on top of another region or appeared to be damaged during cryosectioning were manually excluded from the analysis.
Image capture
An Axio Imager M1 upright microscope (Zeiss, City, Country) equipped with both ZEN software (blue edition, v2.3) and AxioVision (4.8.2) was used to capture stitched images of entire tissue cross sections for cell counts. Images were minimally post-processed for color balance, contrast, and brightness.
RNA Isolation
Muscle tissue was homogenized in QIAzol Lysis Reagent (QIAGEN, Hilden, Germany, 79306) and RNA isolated using the RNeasy kit (QIAGEN, 74104). RNA quality and integrity were assessed using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). Reverse transcription (RT) was performed with the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Quantitative RT-PCR was performed using PowerUp™ SYBR™ Green Master Mix (Thermo-Fisher). Gene expression, normalized to the geometric mean of 3 housekeeping genes (β2M, PGK1, and VCP), was quantified using the 2^-(∆Ct) method and expressed as fold-difference in the obese group relative to the lean group. RNA primer pairs are shown in Supplemental Table 1.
RNAseq and Pathway Analysis
RNAseq was performed on 6 lean and 6 obese subjects by Novogene using a read depth of 60 million reads. Differential expression analysis was conducted to compare the obese and lean groups. Read counts from Novogene were used. Among the original 58,676 genes, 22,463 had all non-zero reads. Those genes were excluded from the analysis. We analyzed the expression of the remaining genes with DESeq2 (Version 1.24.0) [32], in R (Version 3.6.0). The output of DESeq2 was inputted into ConsensusPathDB [33, 34]. Genes with p-value less than 0.05 and log2 fold change larger than 0.3 were considered up-regulated genes. And those with p-value less than 0.05 and log2 fold change less than −0.3 were considered down-regulated genes. 898 up-regulated and 938 down-regulated genes identified using the above criteria were subjected to pathway analysis.
Statistical Analysis
For analyses comparing two groups (young v. old; lean v. obese), a two-tailed t-test was performed with significance set at p<0.05. For analyses correlating percent γH2AX+ cells and myonuclei against HOMA-IR, a two-tailed Pearson correlation was performed. For analyses correlating γH2AX+ cells and myonuclei against percent body fat and ALM/BMI, analysis of covariance (ANCOVA) was used to generate statistical models with sex and percent fat used as independent variables. Statistics were performed using Prism 8 software for Mac or JMP 14.
Results
Subject Characteristics.
Young v. Old:
Subjects in the young/old comparison did not differ by BMI or percent body fat, indicating good matching between groups (Table 1). DXA scans revealed that appendicular lean mass (ALM) was not significantly different between young and old subjects, however, ALM relative to BMI (ALM/BMI) was significantly lower in the old subjects (Supplemental Table 1). Mean myofiber cross-sectional area (CSA) was significantly lower in the old when compared to the young individuals (Supplemental Table 2), suggesting age-associated myofiber atrophy.
Lean v. Obese:
Subjects in the lean/obese groups did not differ by age, indicating good matching between groups (Table 1). With obesity, there was no significant difference in ALM, although ALM/BMI was lower in obese individuals (Supplementary Table 3). There was no group difference in mean myofiber CSA.
Commonly used reagents to quantify senescence markers are mostly ineffective in human skeletal muscle.
The goal of this study was to use multiple different markers of senescence (p16, γH2AX, SA β-Gal, HMGB1, Sudan Black B) to quantify the abundance of senescent cells in muscle. We first attempted to quantify p16 expression. Although many p16 antibodies used in previous studies of senescence are no longer available [5, 6, 35], we utilized 3 different commercially available p16 antibodies. One, used by various labs for immunohistochemical analysis of p16 [36-39], labeled >60% of all nuclei in both young and old muscle (Supplemental Figure 1), in spite of the fact that p16 mRNA expression is low in muscle, as is p21 mRNA (CT values 35–36; data not shown). Consistent with mRNA results, 2 of the antibodies detected no p16+ cells in human skeletal muscle, regardless of age (data not shown). We utilized 2 SA β-Gal kits, in addition to making an inhouse staining cocktail, however, we were unable to detect senescent cells in old human muscle (data not shown). To determine if our lack of SA β-Gal staining was due to a technical issue, we stained old mouse muscle, as this has been done successfully in the past by other labs [40, 41]. We were able to label senescent cells in old mouse muscle, which were rare (~1 SA β-Gal+ cell per 1000 fibers, Supplemental Figure 2). The HMGB1 antibody provided consistent results, but nearly 50% of all nuclei in young, healthy individuals were devoid of HMGB1, so likely was not indicative of senescence (Supplemental Figure 3) [26]. Supplemental Figure 4 shows that Sudan Black B preferentially labels Type 1 muscle fibers, consistent with previous reports [42, 43], and with the fact that aging is characterized by loss of Type 2 fibers and Type 1 fiber type grouping [44]. γH2AX provided consistent, reliable results on isolated myoblasts in vitro following γ-irradiation and on muscle tissue cross sections (see below). γH2AX is best known as a indicator of DNA damage, and, according to PubMed, γH2AX has been used as an indicator of senescence in nearly 500 manuscripts, including several studies [28, 45-47] that use γH2AX as the sole marker of senescence. However, due to the importance of using multiple markers to quantify senescent cells (reviewed by [48]), we can only speculate that the γH2AX+ cells are senescent.
γH2AX+ cell abundance is unchanged with aging.
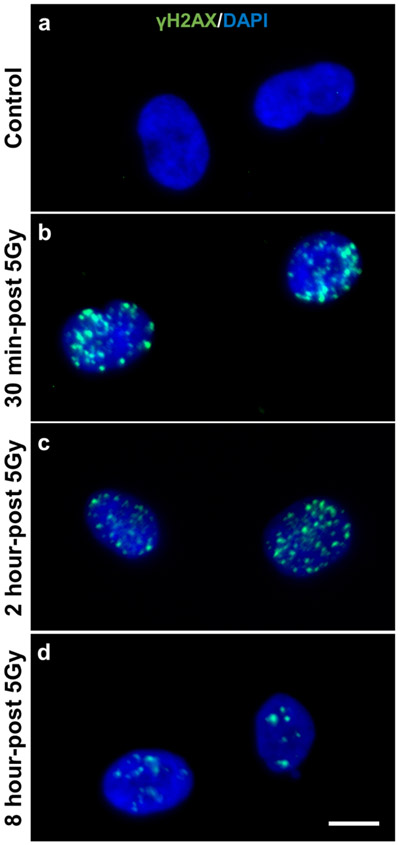
Prior to immunohistochemical staining of human muscle tissue, we validated the γH2AX antibody by performing an immunocytochemistry time course on 5Gy γ-irradiated human primary myoblasts prior to and 30 minutes, 2 hours, and 8 hours post irradiation (Figure 1a-d). γH2AX foci were detectable in the nucleus within 30 minutes of irradiation (Figure 1a) and peaked 2 hours post-irradiation (Figure 1c). After 8 hours, the number of γH2AX foci were reduced, likely due to DNA-damage repair (Figure 1d). Using immunohistochemistry (IHC), we observed a consistent staining pattern of 53BP1 overlaying with γH2AX in skeletal muscle (Supplemental Figure 5).
Figure 1.
5Gy γ-irradiation increases abundance of γH2AX in human primary myoblast nuclei. a) Non-irradiated primary myoblasts, b) 30 minutes, c) 2 hours and d) 8 hours-post irradiation. Scale bar is 10 μm.
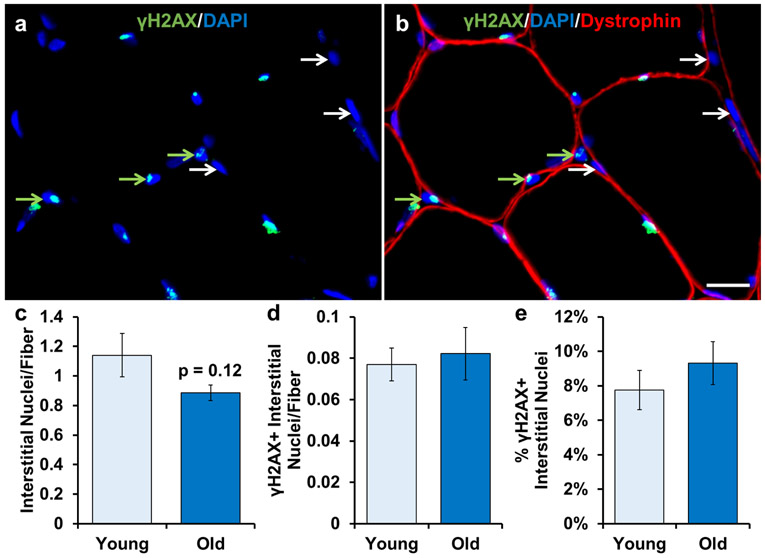
We performed IHC on fresh frozen human muscle tissue to quantify the number of γH2AX+ nuclei residing outside the post mitotic myofiber, delineated by the dystrophin border (Figure 2a-b), as these interstitial cells have the potential to undergo senescence. The relative abundance of γH2AX+ interstitial cell nuclei trended to be lower in old muscle compared to young (Figure 2c; p=0.12). The relative number of γH2AX+ cells per fiber was low in muscle from young individuals and unchanged with aging (Figure 2d). Approximately ~8% of interstitial cell nuclei were γH2AX+, with no significant effect of age (Figure 2e). These results indicate that γH2AX+ cells in skeletal muscle from sedentary, healthy young adults are of relatively low abundance and are not higher in muscle from adults over the age of 70 years old.
Figure 2.
γH2AX+ cells are not different in skeletal muscle from old compared to young subjects. Representative images of a) γH2AX and DAPI and b) dystrophin, γH2AX, and DAPI merged. Any nucleus that was outside dystrophin (red muscle fiber border) was classified as an interstitial nucleus. White arrows indicate γH2AX- interstitial nuclei. Green arrows indicate γH2AX+ interstitial nuclei. Light blue bars represent young subjects; dark blue bars represent old subjects. Quantification of: c) number of total interstitial nuclei relative to the number of muscle fibers, d) number of γH2AX+ interstitial nuclei relative to the number of muscle fibers, and e) percentage of γH2AX+ interstitial nuclei. Error bars indicate −/+ the standard error of the mean (SEM). A two-tailed t-test was performed to determine significance between groups. * indicates p=0.05. n=12 for both groups. Scale bar is 20 μm.
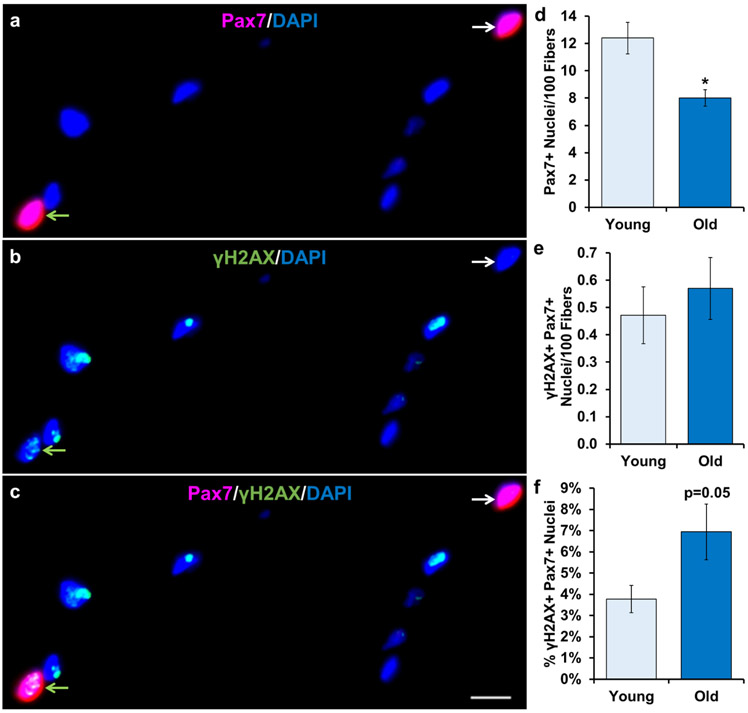
Much of the senescence literature in skeletal muscle is focused on satellite cells, so we aimed to determine their abundance and frequency in young and old muscle. Using IHC detection of γH2AX and paired box 7 (Pax7; Figure 3a-c), a well-established marker of satellite cells, we confirmed that satellite cell frequency was significantly lower with aging (Figure 3d). Moreover, we determined that there was no difference in the frequency of DNA-damaged (γH2AX+/Pax7+) satellite cells in muscle from young and older individuals (Figure 3e). This equates to approximately 1 per 200 myofibers (Figure 3e), which is 3.5% of total satellite cells in young and 7% in old (Figure 3f) muscle, due to the fact that total satellite cell number was lower in old.
Figure 3.
Relative γH2AX+ satellite cell abundance does not increase with aging. Representative image of a) Pax7 and DAPI, b) γH2AX and DAPI, and c) Pax7, γH2AX and DAPI merged. White arrows indicate γH2AX-/Pax7+ nuclei. Green arrows indicate γH2AX+/Pax7+ nuclei. Light blue bars represent young subjects; dark blue bars represent old subjects. Quantification of: d) number of Pax7+ nuclei per 100 muscle fibers, e) number of γH2AX+/Pax7+ nuclei per 100 muscle fibers and f) percentage of γH2AX+/Pax7+ nuclei. Error bars indicate −/+ SEM. A two-tailed t-test was performed to determine significance between groups. * indicates p=0.05. n=12 for both groups. Scale bar is 10 μm.
Abundance of γH2AX+ post-mitotic myonuclei is not affected by age.
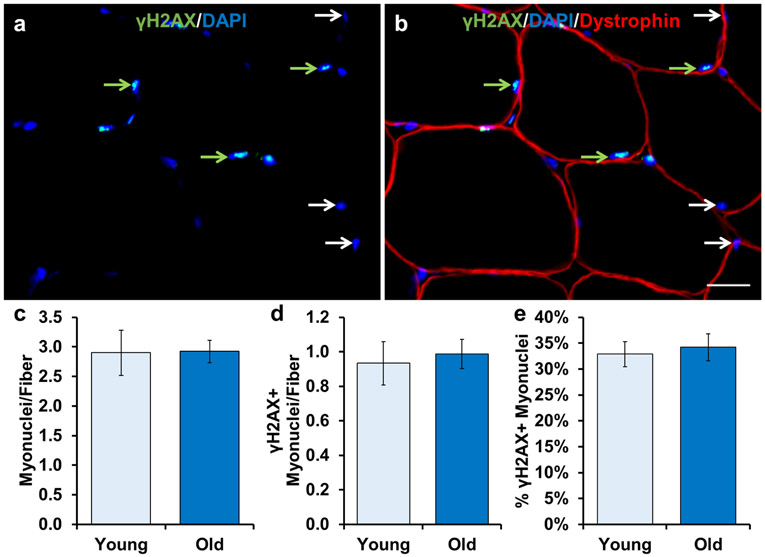
During our quantification of γH2AX+ interstitial cells, we noted robust γH2AX expression in myonuclei (nuclei within myofibers delineated by dystrophin; Figure 4a-c). As myonuclei comprise the largest pool of skeletal muscle nuclei, we quantified the relative abundance of γH2AX+ myonuclei. Overall myonuclear abundance was not different with age (Figure 4d). Both young and old muscle had approximately one γH2AX+ myonucleus per myofiber in cross-section (Figure 4e), which represents 36% and 34% of young and old myonuclei, respectively (Figure 4f).
Figure 4.
No difference in DNA-damaged myonuclei between young and old subjects. Representative images of a) γH2AX and DAPI, and b) dystrophin, γH2AX and DAPI merged. Any nucleus that was inside dystrophin was classified as a myonucleus. White arrows indicate γH2AX- myonuclei. Green arrows indicate γH2AX+ myonuclei. Light blue bars represent young subjects; dark blue bars represent old subjects. Quantification of: c) number of myonuclei relative to the number of muscle fibers, d) number of γH2AX+ myonuclei relative to the number of muscle fibers, and e) percentage of γH2AX+ myonuclei. Error bars indicate −/+ SEM. A two-tailed t-test was performed to determine significance between groups. * indicates p=0.05. n=12 for both groups. Scale bar is 20 μm.
γH2AX+ cells are higher in skeletal muscle of obese compared to lean individuals.
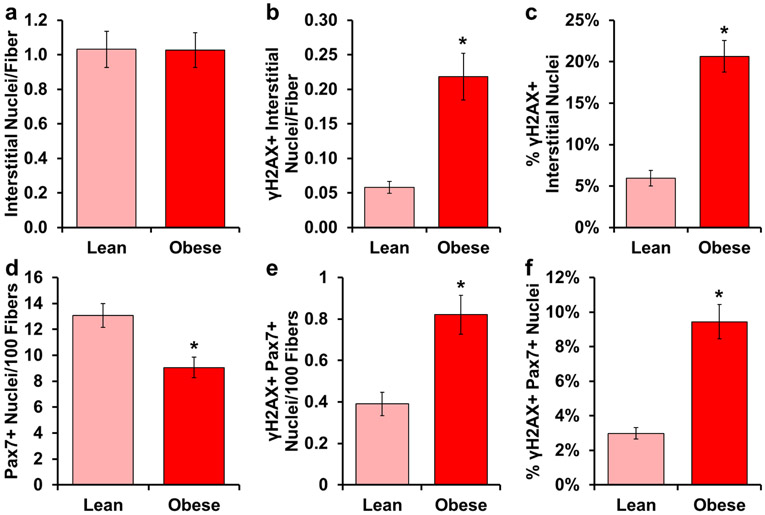
A recent report showed a positive correlation between senescent cells in adipose and percent body fat, while higher abundance of senescent cells was also associated with poor physical function [49]. Similarly, obese mice have nearly 4 times as many γH2AX+ neurons compared to lean mice [47]. Our goal was to examine the abundance of cells expressing γH2AX+ in obese skeletal muscle. There was no difference in the total number of interstitial cell nuclei between lean and obese individuals (Figure 5a). Both the number of γH2AX+ cells per myofiber in cross-section (Figure 5b) and the percentage of γH2AX+ cells (Figure 5c) were 3–4 times higher with obesity. Similar to old individuals, we observed significantly fewer satellite cells in obese muscle (Figure 5d). Additionally, there was a larger number of γH2AX+ satellite cells per myofiber with obesity (Figure 5e), and a greater percentage of γH2AX+ satellite cells (Figure 5f).
Figure 5.
γH2AX+ cells accumulate in skeletal muscle from obese subjects. Light red bars represent lean subjects; dark red bars represent obese subjects. Quantification of: a) number of interstitial nuclei relative to the number of muscle fibers, b) number of γH2AX+ interstitial nuclei relative to the number of muscle fibers, c) percentage of γH2AX+ interstitial nuclei, d) number of Pax7+ nuclei per 100 muscle fibers, e) number of γH2AX+/Pax7+ nuclei per 100 muscle fibers and f) percentage of γH2AX+/Pax7+ nuclei. Error bars indicate −/+ SEM. A two-tailed t-test was performed to determine significance between groups. * indicates p=0.05. n=12 for both groups.
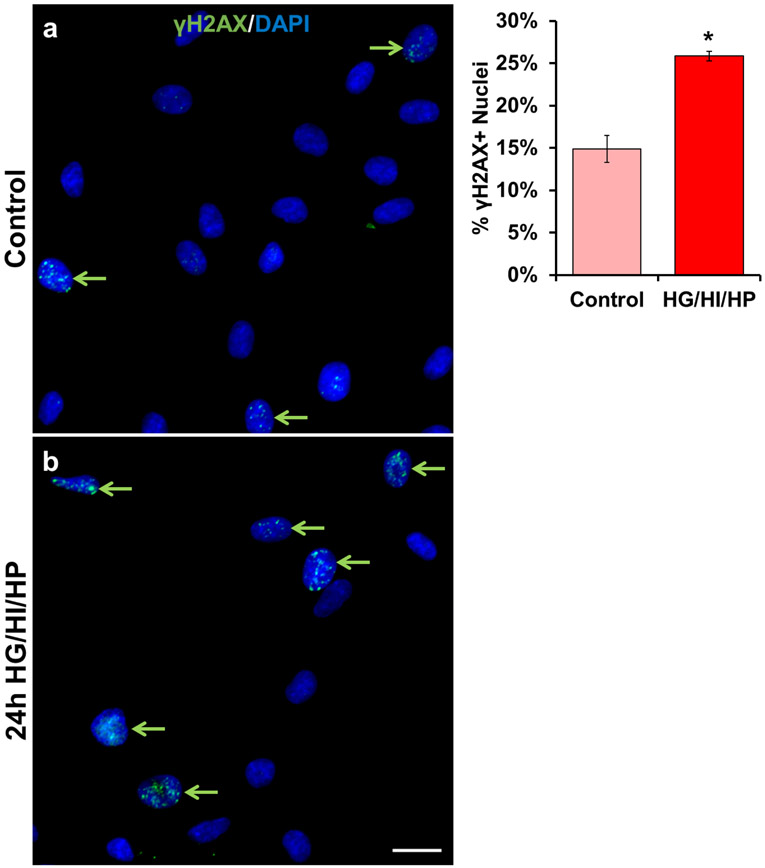
To demonstrate a direct effect of the obese environment on γH2AX accumulation in activated satellite cells/myoblasts, we treated human primary myoblasts with high glucose, insulin, and palmitate for 24 hours to mimic the obesogenic environment. Immunocytochemistry showed that this treatment caused a 74% increase in γH2AX+ positive cells (Figure 6a-c).
Figure 6.
Exposure to an obesity-like environment induces DNA-damage in human primary myoblasts. Human primary myoblasts were treated with a) growth media (GM, control) or b) GM supplemented with high concentrations of glucose (25mM), insulin (100nM), and palmitate (250μM) for 24 hours. White arrows indicate γH2AX- cells. Green arrows indicate γH2AX+ cells. Error bars indicate −/+ SEM. A two-tailed t-test was performed to determine significance between groups. * indicates p=0.05. n=3 technical replicates. Scale bar is 20 μm.
Abundance of γH2AX+ post-mitotic myonuclei is elevated in muscle from obese individuals.
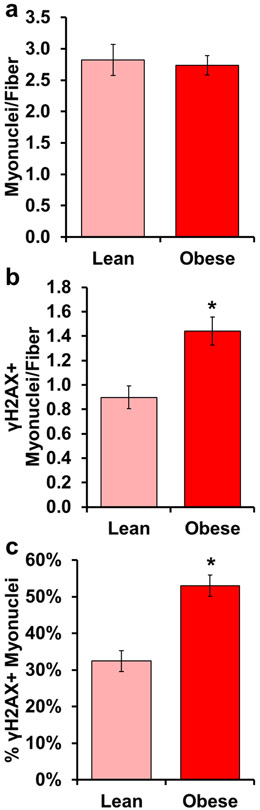
Considering interstitial cell populations in obese individuals displayed elevated γH2AX accumulation (Figure 5b-c), we assessed the relative abundance of γH2AX+ myonuclei. There was no difference in the number of total myonuclei per myofiber in cross-section between lean and obese individuals (Figure 7a). However, the frequency of γH2AX+ myonuclei per myofiber (Figure 7b) and the percentage of γH2AX+ myonuclei (Figure 7c) were approximately 50% higher with obesity.
Figure 7.
DNA-damaged myonuclei are higher in obese, compared to lean subjects. Light red bars represent lean subjects; dark red bars represent obese subjects. Quantification of: a) number of myonuclei relative to the number of muscle fibers, b) number of γH2AX+ myonuclei relative to the number of muscle fibers, and c) percentage of γH2AX+ myonuclei. Error bars indicate −/+ SEM. A two-tailed t-test was performed to determine significance between groups. * indicates p=0.05. n=12 for both groups.
Deep RNAseq analysis reveals up regulation of DNA-damage repair pathways in obese muscle.
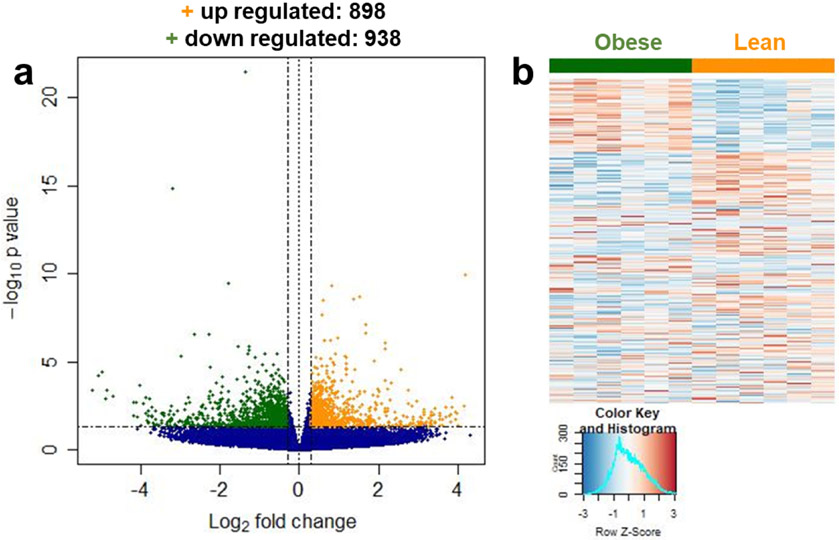
We performed deep RNAseq on a subset of our lean and obese subjects (6 lean and 6 obese; Supplemental Table 4), and identified 1,836 differentially expressed genes (DEG) between groups, 938 that were down regulated and 898 that were up regulated with obesity (Figure 8a-b). Pathway analysis of the down regulated genes revealed 3 pathways that were, in general, involved in gene transcription (Table 3). There were over 50 pathways represented from the up regulated DEG analysis. Upon further interrogation of the top 25 pathways, 8 are involved in DNA-damage repair (Table 3, bold), which is consistent with our IHC observations of elevated γH2AX+ nuclei with obesity. The SASP pathway also appeared on the list of up-regulated genes with obesity (Table 3, italicized).
Figure 8.
Deep RNAseq analysis reveals that DNA-damage repair pathways are upregulated in skeletal muscle from obese individuals. a) Volcano plot of genes that are up regulated (orange +) and down regulated (green +) in obese v. lean muscle. b) Heat map of the 1836 differentially expressed genes. n=6 for both groups.
Table 3:
Up and down regulated pathways for DEGs
| Pathway Name – Down Regulated | p-value |
|---|---|
| GLI proteins bind promoters of Hh responsive genes to promote transcription | 0.00105 |
| Generic Transcription Pathway | 0.00772 |
| Nitric oxide stimulates guanylate cyclase | 0.00781 |
| Pathway Name – Up Regulated | p-value |
| Signaling by Nuclear Receptors | 0.00013 |
| RNA Polymerase I Chain Elongation | 0.00013 |
| Muscle contraction | 0.00015 |
| mRNA Splicing - Minor Pathway | 0.0002 |
| Transcription-Coupled Nucleotide Excision Repair (TC-NER) | 0.00022 |
| Gap-filling DNA repair synthesis and ligation in TC-NER | 0.00027 |
| Dual incision in TC-NER | 0.0003 |
| NoRC negatively regulates rRNA expression | 0.00055 |
| RA biosynthesis pathway | 0.00064 |
| 0.0007 | |
| RNA Polymerase I Promoter Clearance | 0.00075 |
| RHO GTPases activate PKNs | 0.00081 |
| RNA Polymerase I Transcription | 0.00088 |
| Nucleotide Excision Repair | 0.00088 |
| Cardiac conduction | 0.00089 |
| Telomere Maintenance | 0.00119 |
| CLEC7A (Dectin-1) signaling | 0.00147 |
| Dual Incision in GG-NER | 0.00168 |
| Transcriptional regulation by small RNAs | 0.00188 |
| Formation of TC-NER Pre-Incision Complex | 0.00201 |
| B-WICH complex positively regulates rRNA expression | 0.00263 |
| Senescence-Associated Secretory Phenotype (SASP) | 0.00265 |
| Chromosome Maintenance | 0.00265 |
| Signaling by Retinoic Acid | 0.00273 |
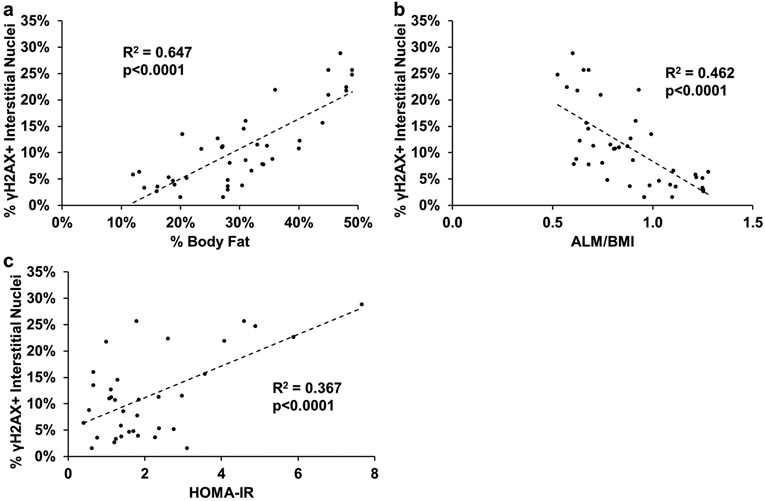
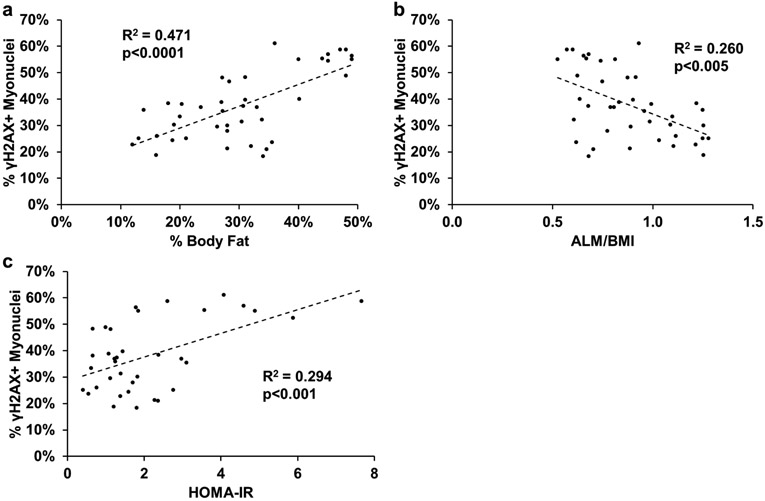
γH2AX+ cells and myonuclei are strongly correlated to measures of whole-body and metabolic health.
To examine the relationship between γH2AX+ cells and measures of whole-body and metabolic health, we grouped all of our subjects together to look at the relationship between the percentage of γH2AX+ interstitial cell nuclei and myonuclei and measures of whole-body and metabolic health. There was a positive correlation between percent γH2AX+ cells and percent body fat and HOMA-IR, and a negative correlation between percent γH2AX+ cells and appendicular lean mass relative to BMI (ALM/BMI; Figure 9a-c). There was significant interaction between sex and percent body fat (Figure 9a; p<0.05). The same association was observed between percent γH2AX+ myonuclei and percent body fat, ALM/BMI, and HOMA-IR (Figure 10a-c). There was no effect of sex.
Figure 9.
Significant correlations between the percent γH2AX+ interstitial nuclei and markers of metabolic health for all subjects. Pearson correlations between: a) percent γH2AX+ interstitial cells and percent body fat, b) percent γH2AX+ interstitial cells and appendicular lean mass normalized to BMI (ALM/BMI), and c) percent γH2AX+ interstitial cells and HOMA-IR. A 2-tailed Pearson correlation was performed to determine significance. n=38–42.
Figure 10.
Significant correlations between the percent γH2AX+ myonuclei and markers of physical and metabolic health for all subjects. Pearson correlations between: a) percent γH2AX+ myonuclei and percent body fat, b) percent γH2AX+ myonuclei and appendicular lean mass normalized to BMI (ALM/BMI), and c) percent γH2AX+ myonuclei and HOMA-IR. A 2-tailed Pearson correlation was performed to determine significance. n=38–42.
Discussion
Senescent cells have been shown to increase with aging in a variety of tissues and negatively contribute to the local environment through induction of a SASP [6, 9, 28]. Specific to skeletal muscle, satellite cells isolated from old mice and humans display increased p16 and γH2AX expression, while also exhibiting a SASP [6]. The major drawback with those analyses is that cells continue to proliferate after being isolated from muscle tissue and may undergo replicative senescence in vitro. Clearly, the transcriptome of isolated satellite cells differs from those in vivo [50]. Additionally, obesity is associated with increased senescent cells and inflammation in adipose tissue, but the prevalence of senescent cells in skeletal muscle with obesity has not been described. Therefore, we sought to quantify senescent cell abundance in human skeletal muscle biopsies using multiple validated senescence markers, p16, γH2AX, SA β-Gal, HMGB1, and Sudan Black B. Only γH2AX provided reliable results in human skeletal muscle.
An indepth review by the International Cell Senescence Association provided guidelines for assessing and quantifying senescent cells in vivo [48]. They state that, while no marker is completely specific for senescent cells, multiple markers (cytoplasmic, nuclear, and cell-type-specific) should be used to assess the senescence burden of a given tissue. In the present manuscript, we attempted to utilize 5 markers of senescence; however, due to the reasons outlined in the results section, only γH2AX has the potential to serve as a senescence marker in human skeletal muscle. Therefore, we can only assess the abundance of cells that are experiencing a DNA damage reponse in muscle from old and obese individuals, and speculate that these cells may also be senescent. Although this is a limitation to our study, it highlights an important issue within the senescence field. Identifying senescent cells in skeletal muscle can be particularly challenging because many classic markers are either lowly expressed or have other functions. For example, interleukin-6 is a hallmark SASP marker [11], but is induced by muscle contraction and important for muscle growth [51-53]. Plasminogen Activator Inhibitor-1 is a marker and mediator of cellular senescence [54], but is induced by TGFβ in muscle, independent of senescence, and contributes to fibrosis [53, 55, 56]. p21 inhibits cell cycle progression in senescent cells, but also promotes apoptosis [57, 58], assists in maintaining cells in quiescence [59-61], is necessary for differentiation of satellite cells to myofibers [62-64], and is essential for skeletal muscle regeneration [65, 66]. Further, some of the commonly used senescence markers appear to be tissue specific. A recent paper by Yousefzadeh and colleagues [67] quantified p16 and p21 expression in various tissues using two animal models: 1) mice that developed senescence at a rapid rate (Ercc1-/Δ) and 2) young and old wild-type mice. Relative to 10 other tissues, p16 and p21 expression in muscle tissues (heart, gastrocnemius, quadriceps) were, overall, unchanged in the Ercc1-/Δ model, whereas fat, aorta, kidney, liver, and large intestine had >10-folder higher expression of p16 and p21 [67]. Moreover, p16 and p21 expression were the lowest in the gastrocnemius and quadriceps, and were not higher in old wild-type mice. These data indicate that either senescent cells do not accumulate (or are not very abundant) in skeletal muscle with aging or that p16 and p21 are not good markers of senescence in muscle. Thus, the senescence field, particularly within the context of skeletal muscle, is in desperate need of bona fide senescence markers.
We observed a trend for fewer interstitial cells per myofiber in old muscle, and old subjects had significantly fewer satellite cells compared to young, which is consistent with previous reports [68-70]. Thus, in skeletal muscle, cells with replicative potential that experience DNA damage with age may be lost as opposed to enter senescence and accumulate. This possibility is supported by the observation that accumulation of DNA-damaged fibroblasts from skin, but not muscle, is higher in old primates [5]. The older individuals in our study had lower satellite cell abundance and reduced muscle fiber size compared to the young cohort, suggesting they were experiencing age associated muscle loss, but their percent body and thigh fat were not significantly different than the young. It is possible that DNA-damaged and senescent cells will accumulate in human muscle at very old age to negatively impact muscle function, as in mice, where the increase in isolated senescent satellite cells only occurred in geriatric (>28 month old) mice [6]. Our old subjects, with an age range of 70–86, may not have reached the age at which DNA-damaged or senescent cells accumulate, however, the average lifespan for an American is 79 years. Our results suggest that age-associated loss in muscle mass cannot be attributed to an increase in DNA-damaged or senescent cell load. Additional studies need to be conducted on individuals >90 years old to determine if these cells accumulate in skeletal muscle much later in life, although these individuals are likely to have a different senescence profile due to inherent differences in genetics, endocrine function, signaling pathways, etc. that exist in long-lived organisms [71].
We found that the number of γH2AX+ satellite cells is not significantly higher in muscle with aging. Consistent with this finding, a paper by Wang et al. showed a significant increase in γH2AX+ cells with age in liver, spleen, skin, lung and small intestine, but not in skeletal or cardiac muscle comparing 12 and 42 month old mice [18]. Similarly, there was no increase in the number of γH2AX+ satellite cells, or the number of γH2AX foci in each satellite cell nucleus, between 2- and 22-month old mice before or after cardiotoxin-mediated muscle damage [72]. In satellite cells isolated from young (15–24) and old (72–80) humans, the rate of division, maximum number of divisions, p16 mRNA, and telomere length are not different [70, 73]. Further, skeletal muscle from aged wild type and progeroid mice do not exhibit an increase in p16 or p21 expression [67]. However, although aging does not increase the absolute number of γH2AX+ satellite cells in human muscle, the percentage of satellite cells that are γH2AX+ is higher with age (3.5% compared to 7%), due to lower satellite cell abundance in aged muscle. A higher percentage of DNA-damaged satellite cells could influence the ability of muscle to adapt and regenerate. However, given that a recent report showed that senescent cells, specifically fibro-adipogenic progenitor cells, are actually required for muscle regeneration [74], the relative impact, if any, of satellite cell abundance compared to the percentage that are senescent on muscle mass loss with age remains to be determined. Overall, our results show that γH2AX+ satellite cells are rare in resting muscle in vivo (1 per every 200 myofibers) regardless of age.
Obesity is linked to accelerated T cell [75] and preadipocyte [76] senescence, and our results show that muscle from young obese individuals has nearly 4 times the number of γH2AX+ cells than young lean muscle, comparable to the 6-fold increase in the number of senescent cells in adipose during obesity [76]. The greater abundance of γH2AX+ cells in muscle from obese individuals seems likely due to DNA-damage from oxidative stress via reactive oxygen species (ROS), as oxidative stress is elevated during obesity [77] and has been shown to induce senescence [78, 79]. Alternatively, the γH2AX+ cells might not be senescent, but instead are γH2AX+ because of acute DNA-damage. Most of our obese subjects were insulin resistant (elevated HOMA-IR) and skeletal muscle from obese individuals display elevated intramuscular triglycerides [80]. To mimic this obesogenic environment in vitro, we showed that short term treatment with high glucose, insulin, and palmitate is sufficient to induce DNA-damage in human primary myoblasts. There was a trending reduction in the overall number of satellite cells in obese muscle, which is consistent with diet-induced obesity in mice [81]. Collectively, the larger number of γH2AX+ satellite cells, along with a reduction in the number of satellite cells, could explain why diet-induced obesity has been shown to impair regeneration of skeletal muscle and increase collagen deposition following cardiotoxin injection [82].
In the classical sense, cellular senescence occurs only in proliferating cell populations where a cell permanently exits the cell cycle through robust expression of cell cycle inhibitory proteins. These senescent cells can negatively affect the surrounding environment and cell populations through the SASP. There is emerging evidence that post-mitotic cells can also become “senescent” and promote a SASP-like phenotype [83-85], dubbed post-mitotic senescent cells (PoMiSCs) [86]. Myonuclei are post-mitotic and are the most abundant pool of nuclei in skeletal muscle; however, we did not observe a higher number of γH2AX+ myonuclei with aging. Increased DNA-damage, as measured by OH8dG, has been previously reported in old muscle [87]; however, this measurement quantifies the amount of DNA-damage in a given sample and does not take into account the amount of damage in an individual nucleus. The number of γH2AX foci is often used as a rough estimation of the number of DNA-lesions in a nucleus. For example, a nucleus with 9 γH2AX foci would have 3 times the amount of damage as a nucleus with 3 γH2AX foci. This method is commonly used in vitro because it is possible to visualize the entire nucleus and get an accurate measure of the number of γH2AX foci. For the current study, it is challenging to use this approach because we are only visualizing a single cross-sectional view of each nucleus. For our in vivo studies, we defined an γH2AX+ nucleus as having 3+ foci or >25% of the nucleus positive for γH2AX. Therefore, even though we did not observe greater numbers of DNA-damaged myonuclei with aging, each individual myonucleus could have increased γH2AX foci, culminating in a global increase in DNA-damage with aging. To our knowledge, this is the first report to quantify the abundance of DNA-damaged myonuclei, although we did not quantify the specific number of γH2AX+ foci per nucleus and thus could not test the effect of age on a per nucleus basis.
In addition to higher γH2AX interstitial cells, obese individuals had significantly more γH2AX+ myonuclei compared to lean, which was accompanied by a SASP based on RNAseq analyses. The SASP genes contained within our pathway analysis are likely part of the hundreds of genes that contribute to the SASP [88], and reaffirm that the SASP has tissue specificity. In addition, the mean age of our obese subjects was 26.9 years, which highlights how rapidly a SASP can develop. The SASP might be exacerbated in middle-aged or old obese individuals. Alternatively, the elevated number of γH2AX+ cells in skeletal muscle from obese individuals might not be due to senescence, but acute DNA damage. We showed that acute (24 hours) treatment with an obesity-like environment (high glucose, insulin, and palmitate) leads to an increase in DNA-damaged cells. Cytokine secretion does not occur following transient DNA-damage but after chronic DNA-damage signaling [2]. Future studies will investigate the contribution of DNA-damage in myonuclei on global skeletal muscle inflammation (SASP) in middle-aged and elderly obese individuals, in addition to the role of myonuclear DNA-damage on glucose uptake and indices of metabolic health.
Taken together, our data suggest that obesity, even at a young age, is associated with DNA-damage in skeletal muscle, whereas aging is not. Our findings also support the conclusion that obesity in old age may be particularly detrimental to muscle function. However, there is a critical need to identify additional markers, and establish detailed protocols, to help identify senescent cells in skeletal muscle.
Supplementary Material
Acknowledgements
The authors would like thank Drs. Ying Liang and Cuiping Zhang for their assistance with the cell irradiation experiments, and Dr. Kevin Murach for the thoughtful discussions about this project. This research was supported by NIH grants AG049806 to C.A.P, P.A.K and M.M.B., AR060701 to C.A.P., and CTSA grant UL1 TR001998.
Nonstandard Abbreviations
- ALM
appendicular lean mass
- BMI
body mass index
- BSA
bovine serum albumin
- CSA
cross-sectional area
- DAP
4′,6-diamidino-2-phenylindole
- DEG
differentially expressed gene
- FBS
fetal bovine serum
- HMGB1
high mobility group protein B1
- γH2AX
histone H2A variant gamma-H2AX
- IHC
immunohistochemistry
- NHS
normal horse serum
- PFA
paraformaldehyde
- PBS
phosphate buffered saline
- PoMiSCs
post-mitotic senescent cells
- RT-PCR
reverse transcription polymerase chain reaction
- SA β-Gal
senescence-associated beta-galactosidase
- SASP
senescence-associated secretory phenotype
- SBB
Sudan Black B
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- 1.d’Adda di Fagagna F, et al. , A DNA damage checkpoint response in telomere-initiated senescence. Nature, 2003. 426(6963): p. 194–8. [DOI] [PubMed] [Google Scholar]
- 2.Rodier F, et al. , Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol, 2009. 11(8): p. 973–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee AC, et al. , Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. J Biol Chem, 1999. 274(12): p. 7936–40. [DOI] [PubMed] [Google Scholar]
- 4.Wang CY, et al. , Obesity increases vascular senescence and susceptibility to ischemic injury through chronic activation of Akt and mTOR. Sci Signal, 2009. 2(62): p. ra11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeyapalan JC, et al. , Accumulation of senescent cells in mitotic tissue of aging primates. Mech Ageing Dev, 2007. 128(1): p. 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sousa-Victor P, et al. , Geriatric muscle stem cells switch reversible quiescence into senescence. Nature, 2014. 506(7488): p. 316–21. [DOI] [PubMed] [Google Scholar]
- 7.Alcorta DA, et al. , Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc Natl Acad Sci U S A, 1996. 93(24): p. 13742–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burd CE, et al. , Monitoring tumorigenesis and senescence in vivo with a p16(INK4a)-luciferase model. Cell, 2013. 152(1–2): p. 340–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker DJ, et al. , Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature, 2016. 530(7589): p. 184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker DJ, et al. , Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature, 2011. 479(7372): p. 232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coppe JP, et al. , The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol, 2010. 5: p. 99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beyer I, Mets T, and Bautmans I, Chronic low-grade inflammation and age-related sarcopenia. Curr Opin Clin Nutr Metab Care, 2012. 15(1): p. 12–22. [DOI] [PubMed] [Google Scholar]
- 13.Fontana L, et al. , Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes, 2007. 56(4): p. 1010–3. [DOI] [PubMed] [Google Scholar]
- 14.Fortini P, et al. , DNA damage response by single-strand breaks in terminally differentiated muscle cells and the control of muscle integrity. Cell Death Differ, 2012. 19(11): p. 1741–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogakou EP, et al. , DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem, 1998. 273(10): p. 5858–68. [DOI] [PubMed] [Google Scholar]
- 16.Riballo E, et al. , A pathway of double-strand break rejoining dependent upon ATM, Artemis, and proteins locating to gamma-H2AX foci. Mol Cell, 2004. 16(5): p. 715–24. [DOI] [PubMed] [Google Scholar]
- 17.Bernadotte A, Mikhelson VM, and Spivak IM, Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging (Albany NY), 2016. 8(1): p. 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang C, et al. , DNA damage response and cellular senescence in tissues of aging mice. Aging Cell, 2009. 8(3): p. 311–23. [DOI] [PubMed] [Google Scholar]
- 19.Burma S, et al. , ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem, 2001. 276(45): p. 42462–7. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Capetillo O, et al. , DNA damage-induced G2-M checkpoint activation by histone H2AX and 53BP1. Nat Cell Biol, 2002. 4(12): p. 993–7. [DOI] [PubMed] [Google Scholar]
- 21.Lee BY, et al. , Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell, 2006. 5(2): p. 187–95. [DOI] [PubMed] [Google Scholar]
- 22.Dimri GP, et al. , A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A, 1995. 92(20): p. 9363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gardella S, et al. , The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep, 2002. 3(10): p. 995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El Gazzar M, et al. , Chromatin-specific remodeling by HMGB1 and linker histone H1 silences proinflammatory genes during endotoxin tolerance. Mol Cell Biol, 2009. 29(7): p. 1959–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan J, et al. , Hemorrhagic shock induces NAD(P)H oxidase activation in neutrophils: role of HMGB1-TLR4 signaling. J Immunol, 2007. 178(10): p. 6573–80. [DOI] [PubMed] [Google Scholar]
- 26.da Silva PFL, et al. , The bystander effect contributes to the accumulation of senescent cells in vivo. Aging Cell, 2019. 18(1): p. e12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evangelou K and Gorgoulis VG, Sudan Black B, The Specific Histochemical Stain for Lipofuscin: A Novel Method to Detect Senescent Cells. Methods Mol Biol, 2017. 1534: p. 111–119. [DOI] [PubMed] [Google Scholar]
- 28.Zhang H, et al. , NAD(+) repletion improves mitochondrial and stem cell function and enhances life span in mice. Science, 2016. 352(6292): p. 1436–43. [DOI] [PubMed] [Google Scholar]
- 29.Murach KA, et al. , Cycle training modulates satellite cell and transcriptional responses to a bout of resistance exercise. Physiol Rep, 2016. 4(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muniyappa R, et al. , Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab, 2008. 294(1): p. E15–26. [DOI] [PubMed] [Google Scholar]
- 31.Wen Y, et al. , MyoVision: software for automated high-content analysis of skeletal muscle immunohistochemistry. J Appl Physiol (1985), 2018. 124(1): p. 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Love MI, Huber W, and Anders S, Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol, 2014. 15(12): p. 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herwig R, et al. , Analyzing and interpreting genome data at the network level with ConsensusPathDB. Nat Protoc, 2016. 11(10): p. 1889–907. [DOI] [PubMed] [Google Scholar]
- 34.Kamburov A, et al. , The ConsensusPathDB interaction database: 2013 update. Nucleic Acids Res, 2013. 41(Database issue): p. D793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Helman A, et al. , p16(Ink4a)-induced senescence of pancreatic beta cells enhances insulin secretion. Nat Med, 2016. 22(4): p. 412–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murer A, et al. , EBV persistence without its EBNA3A and 3C oncogenes in vivo. PLoS Pathog, 2018. 14(4): p. e1007039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H, et al. , Mangiferin induces islet regeneration in aged mice through regulating p16INK4a. Int J Mol Med, 2018. 41(6): p. 3231–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang C, et al. , Aged cells in human skeletal muscle after resistance exercise. Aging (Albany NY), 2018. 10(6): p. 1356–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lessard F, et al. , Senescence-associated ribosome biogenesis defects contributes to cell cycle arrest through the Rb pathway. Nat Cell Biol, 2018. 20(7): p. 789–799. [DOI] [PubMed] [Google Scholar]
- 40.Cazin C, Chiche A, and Li H, Evaluation of Injury-induced Senescence and In Vivo Reprogramming in the Skeletal Muscle. J Vis Exp, 2017(128). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He MY, et al. , Hsp90beta interacts with MDM2 to suppress p53-dependent senescence during skeletal muscle regeneration. Aging Cell, 2019. 18(5): p. e13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts CJ, Turfrey BA, and Bland AP, Lipid deposition in different fiber types of skeletal muscle of periparturient dairy cows. Vet Pathol, 1983. 20(1): p. 23–31. [DOI] [PubMed] [Google Scholar]
- 43.Gauthier GF and Padykula HA, Cytological studies of fiber types in skeletal muscle. A comparative study of the mammalian diaphragm. J Cell Biol, 1966. 28(2): p. 333–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelly NA, et al. , Quantification and characterization of grouped type I myofibers in human aging. Muscle Nerve, 2018. 57(1): p. E52–E59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He ZY, et al. , Gamma-H2AX upregulation caused by Wip1 deficiency increases depression-related cellular senescence in hippocampus. Sci Rep, 2016. 6: p. 34558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ibler AEM, et al. , Typhoid toxin exhausts the RPA response to DNA replication stress driving senescence and Salmonella infection. Nat Commun, 2019. 10(1): p. 4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu H, Harrison FE, and Xia F, Altered DNA repair; an early pathogenic pathway in Alzheimer’s disease and obesity. Sci Rep, 2018. 8(1): p. 5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gorgoulis V, et al. , Cellular Senescence: Defining a Path Forward. Cell, 2019. 179(4): p. 813–827. [DOI] [PubMed] [Google Scholar]
- 49.Justice JN, et al. , Cellular senescence biomarker p16INK4a+ cell burden in thigh adipose is associated with poor physical function in older women. J Gerontol A Biol Sci Med Sci, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Velthoven CTJ, et al. , Transcriptional Profiling of Quiescent Muscle Stem Cells In Vivo. Cell Rep, 2017. 21(7): p. 1994–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pedersen BK, Steensberg A, and Schjerling P, Muscle-derived interleukin-6: possible biological effects. J Physiol, 2001. 536(Pt 2): p. 329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Munoz-Canoves P, et al. , Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? FEBS J, 2013. 280(17): p. 4131–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mann CJ, et al. , Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle, 2011. 1(1): p. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vaughan DE, et al. , Plasminogen Activator Inhibitor-1 Is a Marker and a Mediator of Senescence. Arterioscler Thromb Vasc Biol, 2017. 37(8): p. 1446–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naderi J, et al. , Plasminogen activator inhibitor type 1 up-regulation is associated with skeletal muscle atrophy and associated fibrosis. Am J Pathol, 2009. 175(2): p. 763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghosh AK and Vaughan DE, PAI-1 in tissue fibrosis. J Cell Physiol, 2012. 227(2): p. 493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gartel AL and Tyner AL, The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther, 2002. 1(8): p. 639–49. [PubMed] [Google Scholar]
- 58.Karimian A, Ahmadi Y, and Yousefi B, Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair (Amst), 2016. 42: p. 63–71. [DOI] [PubMed] [Google Scholar]
- 59.Kippin TE, Martens DJ, and van der Kooy D, p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity. Genes Dev, 2005. 19(6): p. 756–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perucca P, et al. , Loss of p21 CDKN1A impairs entry to quiescence and activates a DNA damage response in normal fibroblasts induced to quiescence. Cell Cycle, 2009. 8(1): p. 105–14. [DOI] [PubMed] [Google Scholar]
- 61.Barr AR, et al. , DNA damage during S-phase mediates the proliferation-quiescence decision in the subsequent G1 via p21 expression. Nat Commun, 2017. 8: p. 14728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang P, et al. , p21(CIP1) and p57(KIP2) control muscle differentiation at the myogenin step. Genes Dev, 1999. 13(2): p. 213–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Halevy O, et al. , Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science, 1995. 267(5200): p. 1018–21. [DOI] [PubMed] [Google Scholar]
- 64.Marroncelli N, et al. , HDAC4 regulates satellite cell proliferation and differentiation by targeting P21 and Sharp1 genes. Sci Rep, 2018. 8(1): p. 3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hawke TJ, et al. , p21 is essential for normal myogenic progenitor cell function in regenerating skeletal muscle. Am J Physiol Cell Physiol, 2003. 285(5): p. C1019–27. [DOI] [PubMed] [Google Scholar]
- 66.Chinzei N, et al. , P21 deficiency delays regeneration of skeletal muscular tissue. PLoS One, 2015. 10(5): p. e0125765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yousefzadeh MJ, et al. , Tissue specificity of senescent cell accumulation during physiologic and accelerated aging of mice. Aging Cell, 2020: p. e13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shefer G, et al. , Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol, 2006. 294(1): p. 50–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Verdijk LB, et al. , Satellite cells in human skeletal muscle; from birth to old age. Age (Dordr), 2014. 36(2): p. 545–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Renault V, et al. , Regenerative potential of human skeletal muscle during aging. Aging Cell, 2002. 1(2): p. 132–9. [DOI] [PubMed] [Google Scholar]
- 71.Kenyon CJ, The genetics of ageing. Nature, 2010. 464(7288): p. 504–12. [DOI] [PubMed] [Google Scholar]
- 72.Cousin W, et al. , Regenerative capacity of old muscle stem cells declines without significant accumulation of DNA damage. PLoS One, 2013. 8(5): p. e63528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bigot A, et al. , Age-Associated Methylation Suppresses SPRY1, Leading to a Failure of Re-quiescence and Loss of the Reserve Stem Cell Pool in Elderly Muscle. Cell Rep, 2015. 13(6): p. 1172–1182. [DOI] [PubMed] [Google Scholar]
- 74.Saito Y, et al. , Exercise enhances skeletal muscle regeneration by promoting senescence in fibro-adipogenic progenitors. Nat Commun, 2020. 11(1): p. 889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shirakawa K, et al. , Obesity accelerates T cell senescence in murine visceral adipose tissue. J Clin Invest, 2016. 126(12): p. 4626–4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schafer MJ, et al. , Exercise Prevents Diet-Induced Cellular Senescence in Adipose Tissue. Diabetes, 2016. 65(6): p. 1606–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Furukawa S, et al. , Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest, 2004. 114(12): p. 1752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Borodkina A, et al. , Interaction between ROS dependent DNA damage, mitochondria and p38 MAPK underlies senescence of human adult stem cells. Aging (Albany NY), 2014. 6(6): p. 481–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weyemi U, et al. , ROS-generating NADPH oxidase NOX4 is a critical mediator in oncogenic H-Ras-induced DNA damage and subsequent senescence. Oncogene, 2012. 31(9): p. 1117–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coen PM, et al. , Reduced skeletal muscle oxidative capacity and elevated ceramide but not diacylglycerol content in severe obesity. Obesity (Silver Spring), 2013. 21(11): p. 2362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Woo M, et al. , Early life nutrition modulates muscle stem cell number: implications for muscle mass and repair. Stem Cells Dev, 2011. 20(10): p. 1763–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hu Z, et al. , PTEN inhibition improves muscle regeneration in mice fed a high-fat diet. Diabetes, 2010. 59(6): p. 1312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jurk D, et al. , Postmitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response. Aging Cell, 2012. 11(6): p. 996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Farr JN, et al. , Identification of Senescent Cells in the Bone Microenvironment. J Bone Miner Res, 2016. 31(11): p. 1920–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Minamino T, et al. , A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med, 2009. 15(9): p. 1082–7. [DOI] [PubMed] [Google Scholar]
- 86.Sapieha P and Mallette FA, Cellular Senescence in Postmitotic Cells: Beyond Growth Arrest. Trends Cell Biol, 2018. [DOI] [PubMed]
- 87.Mecocci P, et al. , Age-dependent increases in oxidative damage to DNA, lipids, and proteins in human skeletal muscle. Free Radic Biol Med, 1999. 26(3–4): p. 303–8. [DOI] [PubMed] [Google Scholar]
- 88.Ozcan S, et al. , Unbiased analysis of senescence associated secretory phenotype (SASP) to identify common components following different genotoxic stresses. Aging (Albany NY), 2016. 8(7): p. 1316–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.