Abstract
Hutchinson-Gilford progeria syndrome (HGPS, or classical progeria) is a rare genetic disorder, characterized by premature aging, and caused by a de novo point mutation (C608G) within the lamin A/C gene (LMNA), producing an abnormal lamin A protein, termed progerin. Accumulation of progerin causes nuclear abnormalities and cell cycle arrest ultimately leading to cellular senescence. Autophagy impairment is a hallmark of cellular aging, and the rescue of this proteostasis mechanism delays aging progression in HGPS cells. We have previously shown that the endogenous Neuropeptide Y (NPY) increases autophagy in hypothalamus, a brain area already identified as a central regulator of whole-body aging. We also showed that NPY mediates caloric restriction-induced autophagy. These results are in accordance with other studies suggesting that NPY may act as a caloric restriction mimetic and plays a role as a lifespan and aging regulator. The aim of the present study was, therefore, to investigate if NPY could delay HGPS premature aging phenotype. Herein, we report that NPY increases autophagic flux and progerin clearance in primary cultures of human dermal fibroblasts from HGPS patients. NPY also rescues nuclear morphology and decreases the number of dysmorphic nuclei, a hallmark of HGPS cells. In addition, NPY decreases other hallmarks of aging as DNA damage and cellular senescence. Altogether, these results show that NPY rescues several hallmarks of cellular aging in HGPS cells, suggesting that NPY can be considered a promising strategy to delay or block the premature aging of HGPS.
Keywords: Autophagy, Cellular senescence, Human aging, Caloric restriction mimetic
Classical Hutchinson Gilford progeria syndrome (HGPS; OMIM #176670) is a rare autosomal dominant genetic disease observed in about 1 in 4–8 million live births (1,2). Children with HGPS after the first year of life begin to show growth retardation, low body weight and features of premature and accelerated aging such as alopecia, loss of subcutaneous fat, sclerodermatous skin, and bone and joint abnormalities. The cardiovascular system is severely affected and, in most cases, children succumb to myocardial infarction or stroke at an average age of 14.6 years (2).
HGPS is caused by a de novo heterozygous silent point mutation within exon 11 of LMNA gene encoding A-type lamins, namely lamins A and C (c.1824C>T; p.Gly608Gly) (3,4). This mutation activates a cryptic splicing site that causes the deletion of 150 nucleotides of exon 11 in prelamin A mRNA. When translated, it generates a truncated form of prelamin A, which lacks a 50-residue-long fragment within the carboxyl terminus containing the cleavage site for ZMPSTE24 (4), an important mediator in the processing of prelamin A. This truncated prelamin A does not undergo the normal post-translational processing and becomes permanently farnesylated, leading to the production of a mutant lamin A protein named progerin. Since it retains the farnesyl group, progerin accumulates on the inner nuclear membrane and acts as a dominant-negative protein leading to several nuclear abnormalities such as wild-type lamins aggregation, nuclear blebbing, abnormal chromatin remodeling, telomere shortening, impaired gene transcription, reduced DNA repair capacity, and cell cycle arrest. These alterations in nuclear structure and function ultimately lead to cellular senescence causing accelerated aging (1,5–7).
Neuropeptide Y (NPY), a highly conserved 36 amino acid peptide, is one of the most abundant neuropeptides in the peripheral and central nervous systems, but also in other peripheral tissues (8,9). The wide expression of NPY and its receptors (NPY Y1-Y5) underlines NPY importance in a multitude of physiological functions such as feeding behavior, body temperature, blood pressure, stress response and emotion, circadian rhythm, reproduction, learning and memory, and the release of neurotransmitters and hormones (8–10). NPY system has also been shown to have beneficial effects on several mechanisms with relevance for HGPS context. Namely, it regulates energy homeostasis, increasing body weight and adiposity, promotes bone remodeling, stimulates vascular smooth muscle cells proliferation and angiogenesis, and induces myocardial repair and stem cells proliferation (9,11–14).
Emerging evidence indicates that NPY may also influence organism senescence or aging. Aging is associated with reduced levels of NPY and its receptors in several cerebral areas which have been associated with neurodegenerative diseases (reviewed in ref. (14)). In humans, a decline in NPY plasma levels has also been correlated with increasing age (15). Interestingly, it has been shown that caloric restriction, one of the most robust antiaging interventions (16,17), does not increase lifespan in NPY knockout mice (18), suggesting that NPY plays a role as a life span and aging regulator. In fact, transgenic rats overexpressing NPY show improved resistance to stress and increased mean life span (19). NPY beneficial effects on aging may relate with its ability to stimulate autophagy (20,21). Our group has shown that NPY stimulates autophagy in hypothalamus (20), a brain area recently emerged as a central regulator of whole-body aging (22). We also observed that NPY mediates caloric restriction-induced autophagy (20,21). As autophagy is one of the key mechanisms underlying CR effects on life span (16), NPY may delay aging through autophagy activation. HGPS cells appear to share a mechanistic link with normal aging as they also show a decline in proteolytic systems activities (23,24). In addition, HGPS cells nucleome shows changes in several components involved in protein folding and quality control, proteasomal, or lysosomal degradation pathways (24), suggesting loss of proteostasis. Therefore, the identification of new strategies that can improve HGPS cells proteostasis and promote progerin degradation will certainly impact on HGPS aging phenotype. Taking into account the multitude of NPY actions, we hypothesized that NPY is a promising strategy to delay or block HGPS premature aging. In this study, we thus explored the therapeutic potential of NPY by investigating its effect on progerin accumulation and associated cellular defects in HGPS cells.
Methods
Experimental details are provided in Supplementary Information.
Results
NPY Increases Autophagic Flux in HGPS Cells
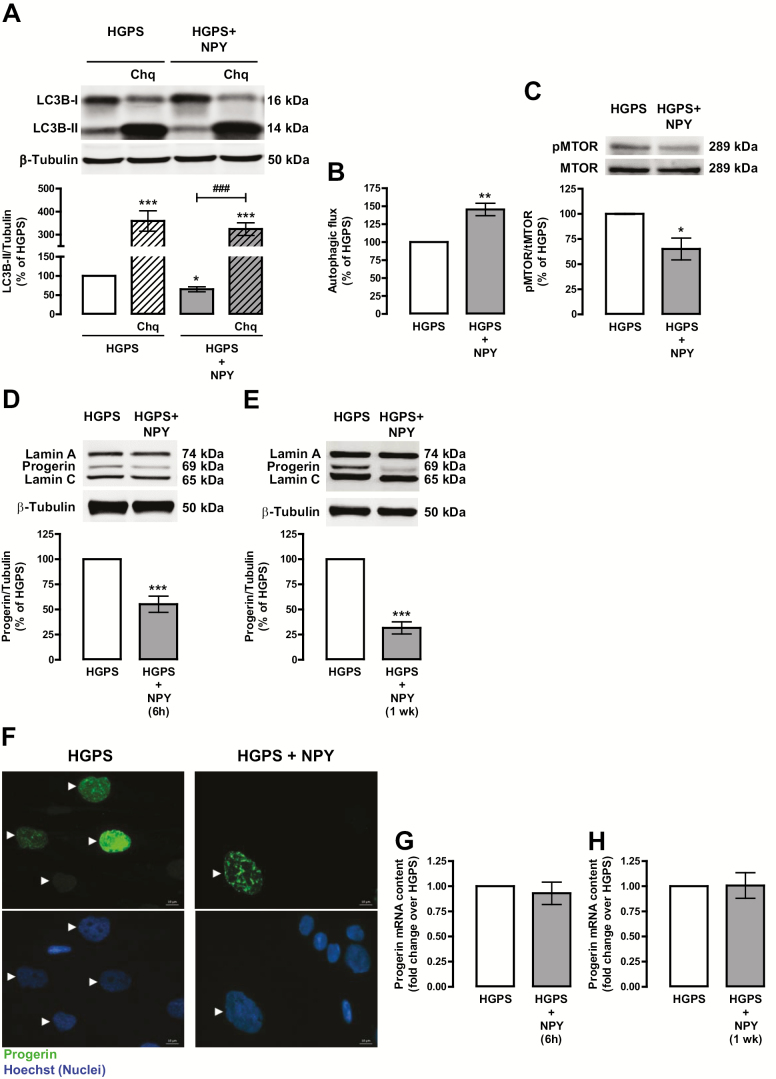
Previous studies from our group showed that NPY activates autophagy (20,21). As autophagic activity is impaired in HGPS cells (24), we hypothesized that NPY could enhance autophagy in HGPS cells and consequently prevent progerin accumulation. To assess NPY effect on autophagy regulation in HGPS cells, we measured the protein levels of the transient autophagosomal membrane-bound form of LC3B (LC3B-II), an autophagy marker (25) by western blotting. As shown in Figure 1A, NPY decreased LC3B-II protein levels in HGPS cells, suggesting autophagic degradation. To support the increase of autophagosome degradation, we determined endogenous autophagic flux by the LC3B-II turnover assay, which measures the amount of LC3B-II delivered to lysosomes in the absence and presence of chloroquine, an autophagic degradation inhibitor (25). In fact, NPY increased LC3B-II net flux in HGPS cells (Figure 1A and B). The canonical molecular switch for autophagy induction is the inhibition of mechanistic target of rapamycin complex 1 (mTORC1) (25). To evaluate whether NPY was inhibiting mTORC1 activity, we analyzed the levels of phosphorylated MTOR (Ser2448), the active kinase form. NPY significantly decreased phospho-MTOR protein levels (Figure 1C), suggesting that NPY stimulates autophagy in HGPS cells through MTOR inhibition. Rapamycin was used as positive control for autophagy induction. As expected, rapamycin increased LC3B-II protein levels, indicative of an increase in autophagosome formation (Supplementary Figure S1a), and increased autophagic flux (Supplementary Figure S1a and b) through MTOR inhibition (Supplementary Figure S1c).
Figure 1.
NPY increases autophagic flux and decreases progerin levels in HGPS fibroblasts. HGPS fibroblasts were exposed to NPY (100 nM) for 6 h (HGPS + NPY) (A, B, C, D, and G) in the presence or absence of chloroquine (Chq, 100 μM), a lysosomal degradation inhibitor, or for 1 week (E, F, and H). Untreated cells were used as control (HGPS). Whole cell extracts were assayed for LC3B (A), pMTOR/tMTOR (C), Lamin A/Progerin/Lamin C (D and E) and β-tubulin (loading control) immunoreactivity through western blotting analysis. Representative western blots for each protein are presented above each respective graph. Autophagic flux analysis in HGPS cells (B) is shown. Autophagic flux was determined in the presence of the lysosomal inhibitor chloroquine, and expressed as “Autophagic flux” calculated by subtracting the densitometric value of LC3B-II – Chq from those corresponding LC3B-II + Chq values. (F) NPY decreased progerin immunoreactivity. Cells were immunolabeled for progerin (top panels, green) and nuclei were stained with Hoechst (bottom panels, blue). Representative images of three independent experiments are shown. Scale bar, 10 µm. (G and H) Quantitative polymerase chain reaction analysis of progerin mRNA levels in HGPS fibroblasts (n = 5). The results represent the mean ± SEM of, at least, four independents experiments, and are expressed as percentage of HGPS. *p < .05, **p < .01, ***p < .001, significantly different compared to HGPS; ###p < .001, significantly different compared to NPY, as determined by analysis of variance, followed Bonferroni’s post-test, or Student’s t test. HGPS = Hutchinson-Gilford progeria syndrome; NPY = Neuropeptide Y; wk = week.
NPY Decreases Progerin Accumulation in HGPS Cells
Progerin accumulation in HGPS cells leads to nuclear membrane folding and nuclear blebbing and triggers the premature aging phenotype of HGPS cells (1,5–7). Since NPY stimulates autophagy in HGPS cells, we next evaluated whether NPY could promote progerin degradation. HGPS fibroblasts were exposed to NPY and progerin protein levels were analyzed by western blotting and immunocytochemistry. After 6 hours of treatment, the levels of progerin decreased in NPY-treated HGPS fibroblasts (55.21 ± 8.06% of HGPS; Figure 1D), suggesting that NPY enhances progerin clearance through autophagy flux stimulation. As previously described (23), we observed a decrease in progerin levels upon rapamycin treatment (Supplementary Figure S1d). To evaluate whether NPY could induce a sustained decrease of progerin accumulation, HGPS fibroblasts were exposed to NPY for 1 week. As shown in Figure 1E, progerin protein levels were significantly decreased upon NPY treatment (31.70 ± 6.01% of HGPS). A decrease in progerin immunoreactivity was also observed in NPY-treated HGPS cells (Figure 1F). We did not observe changes in progerin mRNA content upon 6 hours or 1 week of NPY treatment (Figure 1G and H), suggesting that NPY promotes progerin degradation.
NPY Rescued Nuclear Morphology and Decreased DNA Damage in HGPS Cells
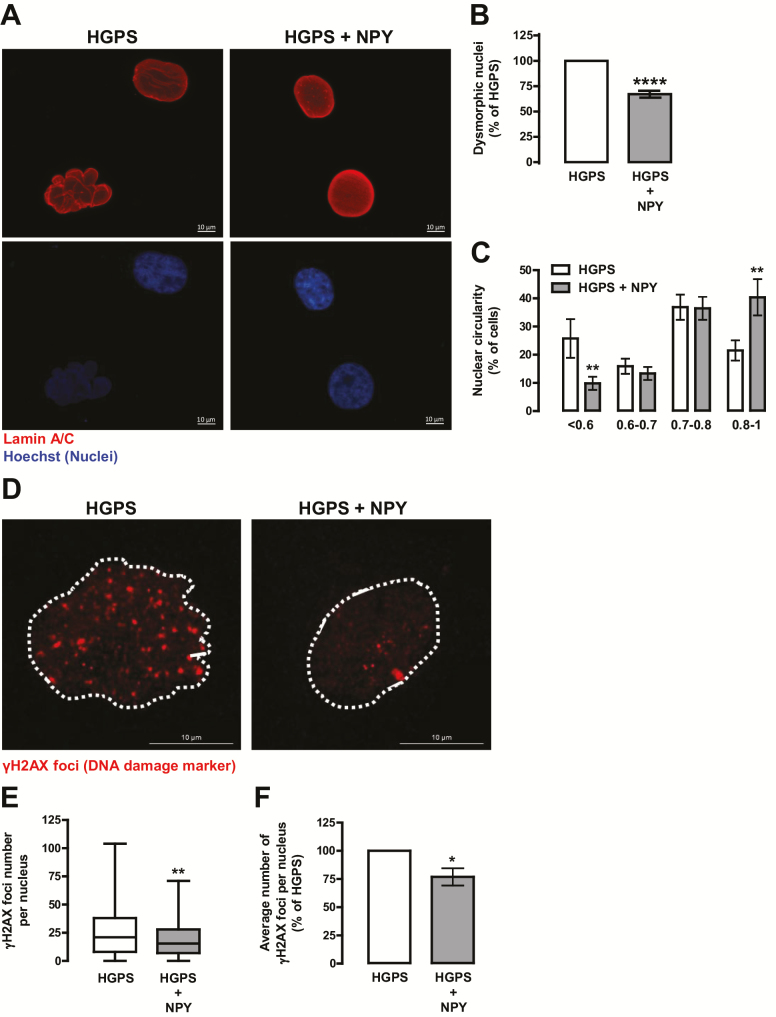
We next evaluated NPY effect on HGPS cells nuclear abnormalities, a hallmark of HGPS cells phenotype. NPY decreased the number of abnormal nuclei in HGPS fibroblasts (67.08 ± 3.40% of HGPS; Figure 2A and B). In addition, when analyzing the circularity index of these nuclei, we observed that NPY decreased the frequency of aberrant nuclei (circularity < 0.6; 25.77 ± 6.87% in HGPS vs 9.84 ± 2.30% in HGPS+NPY) and increased the frequency of normal shaped nuclei (circularity >0.8; 21.49 ± 3.57% in HGPS vs 40.37 ± 6.41% in HGPS+NPY; Figure 2C). Interestingly, we also observed that NPY decreased the number of abnormal nuclei in primary cultures of fibroblasts from a healthy individual (61.33 ± 14.34% of Control; Supplementary Figure 2a–c). Progerin is known to affect DNA damage repair systems leading to DNA damage (7,26). As NPY decreased progerin accumulation in HGPS cells, we hypothesized that NPY could also decrease DNA damage in these cells. To test our hypothesis, we evaluated the effect of NPY on DNA damage by evaluating a DNA damage marker, the γH2AX foci. As shown in Figure 3D–F, γH2AX immunoreactivity and γH2AX foci number decrease in NPY-treated cells, suggesting that NPY decreases DNA damage in HGPS cells.
Figure 2.
NPY rescues nuclear morphology and decreases DNA damage in HGPS fibroblasts. HGPS fibroblasts were exposed to NPY (100 nM; HGPS + NPY) for 1 week. Untreated cells were used as control (HGPS). (A) HGPS fibroblasts were immunolabeled for Lamin A/C (top panels, red) and nuclei were stained with Hoechst (bottom panels, blue). Representative images of four independent experiments are shown. Scale bar, 10 µm. Quantification of the number of abnormal nuclei (B) and nuclear circularity (C) upon NPY treatment. For each condition, an equal number of nuclei (>400) were randomly analyzed. Circularity (defined as: 4*π*area/perimeter^2) was measured using ImageJ. A circularity value equal to 1 corresponds to perfectly circular nuclei. (D–F) NPY decreased γH2AX immunoreactivity. Cells were immunolabeled for γH2AX (red) and nuclei were stained with Hoechst (blue). Representative images of three independent experiments are shown. Scale bar, 10 µm. (E and F) Quantification of γH2AX foci number using ImageJ analysis and customized macros of three independent experiments; >200 cells analyzed) (E) Boxplot of the number of γH2AX foci per nucleus in all the cells for each experimental group. (F) Average number of γH2AX foci per nucleus. The results represent the mean ± SEM of, at least, three independents experiments, and are expressed as percentage of HGPS. *p < .05, **p < .01, ****p < .0001, significantly different from HGPS, as determined by Student’s t test. HGPS = Hutchinson-Gilford progeria syndrome; NPY = Neuropeptide Y.
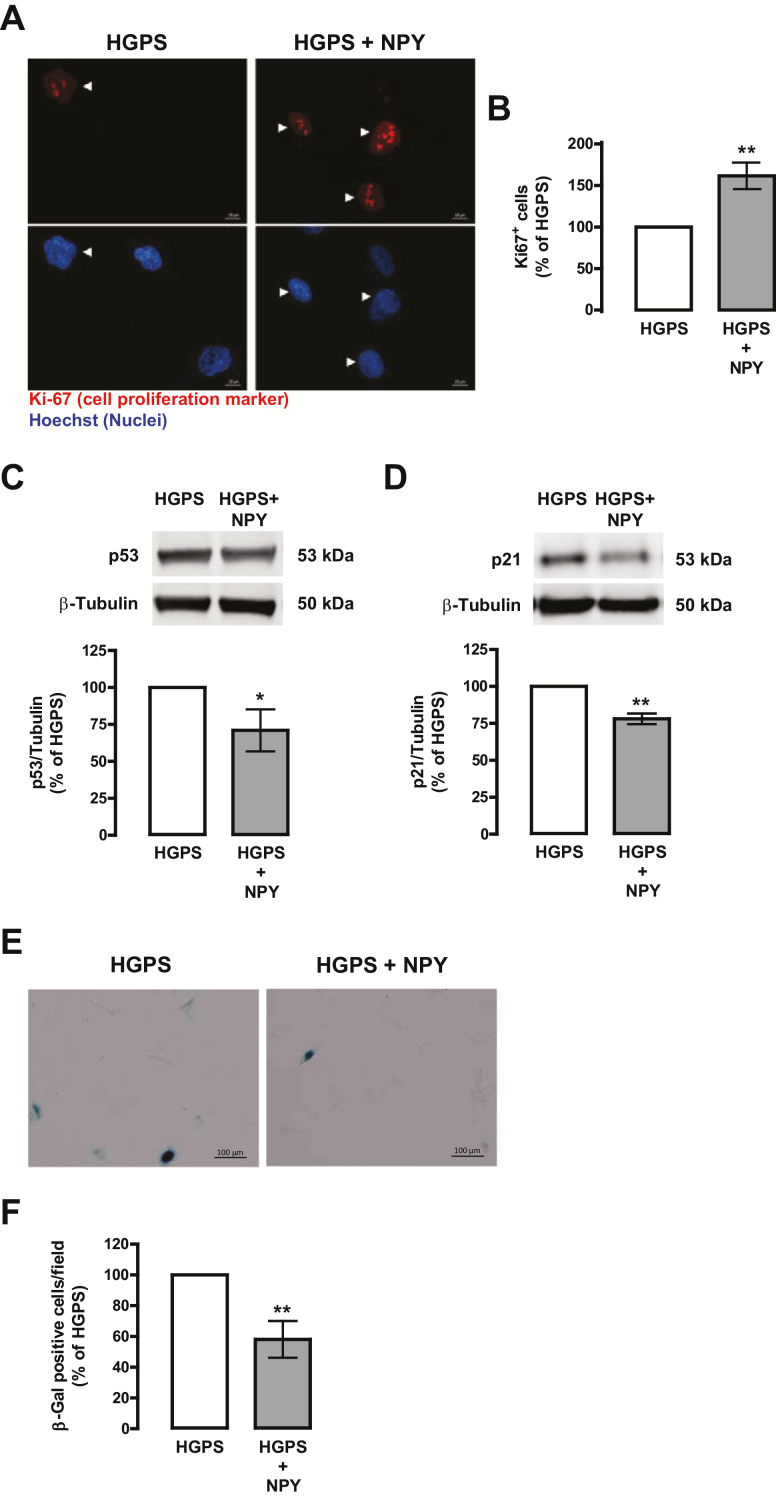
Figure 3.
NPY enhances cell proliferation and delays cellular senescence in HGPS fibroblasts. HGPS fibroblasts were exposed to NPY (100 nM; HGPS + NPY) for 1 week. Untreated cells were used as control (HGPS). (A) NPY increases cell proliferation, as determined by Ki-67 immunoreactivity. Cells were immunolabeled for Ki-67 (top panels, red) and nuclei were stained with Hoechst (bottom panels, blue). Representative images of five independent experiments are shown. Scale bar, 10 µm. (B) Quantification of the number of Ki-67-positive cells in HGPS and NPY-treated HGPS cells. (C and D) Whole cell extracts were assayed for p53 (C), p21 (D), and β-tubulin (loading control) immunoreactivity through western blotting analysis. Representative western blots for each protein are presented above each respective graph. (E–F) NPY decreases cellular senescence, as determined by SA-β-Gal activity. Representative images of four independent experiments are shown. Scale bar, 100 µm. (F) Quantification of SA-β-Gal-positive cells. All the results represent the mean ± SEM of, at least, four independents experiments, and are expressed as percentage of HGPS. *p < .05, **p < .01, significantly different from HGPS, as determined by Student’s t test. HGPS = Hutchinson-Gilford progeria syndrome; NPY = Neuropeptide Y.
NPY Enhances Cell Proliferation and Delays Cellular Senescence in HGPS Cells
Senescence, a hallmark of aged fibroblasts, is characterized by a loss of proliferation and an increase in senescence‐associated β‐galactosidase (SA‐β‐Gal) activity. We observed that NPY increased HGPS cells proliferative capacity, as determined by an increase in the number of Ki-67-positive cells (Ki-67 is a marker of cell proliferation; 161.60 ± 15.96% of HGPS; Figure 3A and B). A similar effect was observed in NPY-treated fibroblasts from a healthy individual (167.75 ± 17.37% of Control; Supplementary Figure 2d and e). We next evaluated whether an increase in cell proliferation was correlated with inhibition of p53 and its downstream effector p21, well known cell cycle inhibitors. In fact, NPY decreased both p53 (Figure 3C) and p21 (Figure 3D) protein levels. Moreover, NPY decreased the number of SA-β-Gal–positive cells (Figure 3E and F), indicating that NPY slowed down the progression of cellular senescence.
Discussion
In this study, we show, for the first time, that NPY delays cellular HGPS aging phenotype, by stimulating autophagy and decreasing progerin accumulation, rescuing nuclear abnormalities, and delaying cellular senescence.
HGPS, a rare and fatal genetic disorder caused by a de novo point mutation in the LMNA gene, which results in the production of a truncated lamin A, named progerin (3,4). Progerin accumulation within the nucleus causes nuclear and mitotic abnormalities and cell cycle arrest, ultimately leading to cellular senescence (5,6) contributing to HGPS premature and accelerated aging phenotype (1). HGPS cells show a decline in protein degradation systems activities and loss of proteostasis (23,24), which may contribute to progerin accumulation. In line with this, some treatment strategies, namely rapamycin, sulforaphane, and MG132, have been proposed to enhance proteostasis and progerin clearance and, thus, ameliorate aging defects in HGPS cells (23,24,27). Although these compounds may be promising for HGPS treatment, in vivo studies should be performed before translation into clinical trials and, thus, safe strategies are still needed (28,29). In this study, we explored, therefore, the potential of NPY, an endogenous molecule that we previously showed to stimulate autophagy (20,21), which is known to decline with aging, contributing to age-related loss of proteostasis (30).
Herein, we show that NPY stimulates autophagy, through MTOR inhibition, and enhances progerin clearance in HGPS cells, leading to a significant reduction of progerin levels and its nuclear accumulation.
Irregular nuclei shape is one of the most prominent features of HGPS due to the accumulation of progerin in the nuclear envelope (5,6). Since NPY promotes progerin degradation, we hypothesized that nuclear morphology could be improved in the absence of progerin. In fact, our results show that NPY rescued nuclear circularity, decreasing the number of dysmorphic nuclei in HGPS cells. By decreasing progerin accumulation within the nucleus, NPY may restore the nucleus scaffold conferring to these cells nuclear steadiness and integrity. Several studies reported that progerin is expressed in low amounts in normal cells and accumulates with age (5,31), establishing a link between progerin and normal aging. Interestingly, we also observed that NPY ameliorated nuclear morphology of normal fibroblasts, further supporting NPY role on the molecular mechanisms underlying cellular senescence and normal aging (14).
It is well accepted that cellular DNA damage accumulation is a hallmark step leading to premature aging (30). A common feature of progeria syndrome is a premature aging phenotype accompanied by an accumulation of DNA damage arising from a compromised repair system (7,26). Progerin accumulation causes chromatin alterations which result in the formation of DNA double-strand breaks and an abnormal DNA-damage response. The accumulation of DSBs causes genome instability, eventually leading to cellular senescence (7). In HGPS cells, NPY enhanced DNA damage repair, as indicated by the reduced number of γH2AX foci (a DNA damage marker), probably by decreasing progerin accumulation.
Since NPY ameliorates HGPS cells phenotype by promoting progerin clearance and consequently rescuing nuclear morphology, we then investigated whether NPY could delay cellular senescence. In fact, NPY increased cell proliferation and delayed cellular senescence in HGPS fibroblasts through decreased activation of p53/p21 pathway, rescuing the senescent phenotype of HGPS fibroblasts. In recent studies, p53 pathway has been highlighted as a common denominator between cellular senescence and DNA damage (32). It is known that in HGPS patients, p53 is chronically activated by permanent DNA damage (33). In NPY-treated cells, the reduction of progerin and, consequently, nuclear abnormalities may release the activation of the checkpoint response to nuclear abnormalities, arresting the stress signaling pathways stimulated by p53 leading to an augmentation in the DNA damage response machinery and to reactivation of the cell cycle (32,34,35).
Altogether, these results show that NPY reverses cellular hallmarks of premature aging of HGPS fibroblasts and strongly support that NPY can be considered a promising strategy to delay or block the premature aging of HGPS as well as normal cellular aging.
Funding
This work was funded by Progeria Research Foundation (PRF2014-53 and PRF2015-60) and European Regional Development Fund (ERDF), through the Centro 2020 Regional Operational Programme under project CENTRO-01-0145-FEDER-000012-HealthyAging and through the COMPETE 2020 - Operational Programme for Competitiveness and Internationalization and Portuguese national funds via FCT – Fundação para a Ciência e a Tecnologia, under projects POCI-01-0145-FEDER-007440, PTDC/MED-FAR/30167/2017 (POCI-01-0145-FEDER-030167), and UID/NEU/04539/2019, FCT Investigator Programme (IF/00825/2015), and SFRH/BD/120023/2016 fellowship.
Conflict of Interest
The authors disclose any financial conflict of interest that might be construed to influence the results or interpretation of their manuscript.
Supplementary Material
Acknowledgments
C.A.A. and C.C. contributed to study conception and design. C.A.A., M.F.M., L.C., and D.P. performed experiments; J.V. and L.C. contributed with analytical tools; C.A.A. carried out experiments analysis and interpretation of data, and wrote the manuscript; C.C. and L.P.A. critically reviewed the manuscript. All authors commented and approved the manuscript.
References
- 1. Gordon LB, Rothman FG, López-Otín C, Misteli T. Progeria: a paradigm for translational medicine. Cell. 2014;156:400–407. doi: 10.1016/j.cell.2013.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ullrich NJ, Gordon LB. Hutchinson-Gilford progeria syndrome. Handb Clin Neurol. 2015;132:249–264. doi: 10.1016/B978-0-444-62702-5.00018-4 [DOI] [PubMed] [Google Scholar]
- 3. De Sandre-Giovannoli A, Bernard R, Cau P, et al. Lamin a truncation in Hutchinson-Gilford progeria. Science. 2003;300:2055. doi: 10.1126/science.1084125 [DOI] [PubMed] [Google Scholar]
- 4. Eriksson M, Brown WT, Gordon LB, et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423:293–298. doi: 10.1038/nature01629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cao K, Capell BC, Erdos MR, Djabali K, Collins FS. A lamin A protein isoform overexpressed in Hutchinson-Gilford progeria syndrome interferes with mitosis in progeria and normal cells. Proc Natl Acad Sci USA 2007;104:4949–4954. doi: 10.1073/pnas.0611640104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goldman RD, Shumaker DK, Erdos MR, et al. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci USA 2004;101:8963–8968. doi: 10.1073/pnas.0402943101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Musich PR, Zou Y. Genomic instability and DNA damage responses in progeria arising from defective maturation of prelamin A. Aging (Albany NY). 2009;1:28–37. doi: 10.18632/aging.100012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wettstein JG, Earley B, Junien JL. Central nervous system pharmacology of neuropeptide Y. Pharmacol Ther. 1995;65:397–414. doi: 10.1016/0163-7258(95)98598-k [DOI] [PubMed] [Google Scholar]
- 9. Silva AP, Cavadas C, Grouzmann E. Neuropeptide Y and its receptors as potential therapeutic drug targets. Clin Chim Acta. 2002;326:3–25. doi: 10.1016/s0009-8981(02)00301-7 [DOI] [PubMed] [Google Scholar]
- 10. Nguyen AD, Herzog H, Sainsbury A. Neuropeptide Y and peptide YY: important regulators of energy metabolism. Curr Opin Endocrinol Diabetes Obes. 2011;18:56–60. doi: 10.1097/MED.0b013e3283422f0a [DOI] [PubMed] [Google Scholar]
- 11. Baldock PA, Lin S, Zhang L, et al. Neuropeptide y attenuates stress-induced bone loss through suppression of noradrenaline circuits. J Bone Miner Res. 2014;29:2238–2249. doi: 10.1002/jbmr.2205 [DOI] [PubMed] [Google Scholar]
- 12. Erlinge D, Brunkwall J, Edvinsson L. Neuropeptide Y stimulates proliferation of human vascular smooth muscle cells: cooperation with noradrenaline and ATP. Regul Pept. 1994;50:259–265. doi: 10.1016/0167-0115(94)90006-x [DOI] [PubMed] [Google Scholar]
- 13. Zukowska-Grojec Z, Karwatowska-Prokopczuk E, Rose W, et al. Neuropeptide Y: a novel angiogenic factor from the sympathetic nerves and endothelium. Circ Res. 1998;83:187–195. doi: 10.1161/01.res.83.2.187 [DOI] [PubMed] [Google Scholar]
- 14. Botelho M, Cavadas C. Neuropeptide Y: an anti-aging player? Trends Neurosci. 2015;38:701–711. doi: 10.1016/j.tins.2015.08.012 [DOI] [PubMed] [Google Scholar]
- 15. Chiodera P, Volpi R, Pilla S, Cataldo S, Coiro V. Decline in circulating neuropeptide Y levels in normal elderly human subjects. Eur J Endocrinol. 2000;143:715–716. doi: 10.1530/eje.0.1430715 [DOI] [PubMed] [Google Scholar]
- 16. Blagosklonny MV. Linking calorie restriction to longevity through sirtuins and autophagy: any role for TOR. Cell Death Dis. 2010;1:e12. doi: 10.1038/cddis.2009.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ravussin E, Redman LM, Rochon J, et al. ; CALERIE Study Group A 2-Year Randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevity. J Gerontol A Biol Sci Med Sci. 2015;70:1097–1104. doi: 10.1093/gerona/glv057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chiba T, Tamashiro Y, Park D, et al. A key role for neuropeptide Y in lifespan extension and cancer suppression via dietary restriction. Sci Rep. 2014;4:4517. doi: 10.1038/srep04517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Michalkiewicz M, Knestaut KM, Bytchkova EY, Michalkiewicz T. Hypotension and reduced catecholamines in neuropeptide Y transgenic rats. Hypertension. 2003;41:1056–1062. doi: 10.1161/01.HYP.0000066623.64368.4E [DOI] [PubMed] [Google Scholar]
- 20. Aveleira CA, Botelho M, Carmo-Silva S, et al. Neuropeptide Y stimulates autophagy in hypothalamic neurons. Proc Natl Acad Sci USA 2015;112:E1642–E1651. doi: 10.1073/pnas.1416609112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ferreira-Marques M, Aveleira CA, Carmo-Silva S, Botelho M, Pereira de Almeida L, Cavadas C. Caloric restriction stimulates autophagy in rat cortical neurons through neuropeptide Y and ghrelin receptors activation. Aging (Albany NY). 2016;8:1470–1484. doi: 10.18632/aging.100996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang G, Li J, Purkayastha S, et al. Hypothalamic programming of systemic ageing involving IKK-beta, NF-kappaB and GnRH. Nature. 2013;497(7448):211–216. doi: 10.1038/nature12143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cao K, Graziotto JJ, Blair CD, et al. Rapamycin reverses cellular phenotypes and enhances mutant protein clearance in Hutchinson-Gilford progeria syndrome cells. Sci Transl Med. 2011;3:89ra58. doi: 10.1126/scitranslmed.3002346 [DOI] [PubMed] [Google Scholar]
- 24. Gabriel D, Roedl D, Gordon LB, Djabali K. Sulforaphane enhances progerin clearance in Hutchinson-Gilford progeria fibroblasts. Aging Cell. 2015;14:78–91. doi: 10.1111/acel.12300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klionsky DJ, Abdelmohsen K, Abe A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Musich PR, Zou Y. DNA-damage accumulation and replicative arrest in Hutchinson-Gilford progeria syndrome. Biochem Soc Trans. 2011;39:1764–1769. doi: 10.1042/BST20110687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harhouri K, Navarro C, Depetris D, et al. MG132-induced progerin clearance is mediated by autophagy activation and splicing regulation. EMBO Mol Med. 2017;9:1294–1313. doi: 10.15252/emmm.201607315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dumas SN, Lamming DW. Next generation strategies for geroprotection via mTORC1 inhibition. J Gerontol A Biol Sci Med Sci. 2019. doi: 10.1093/gerona/glz056, [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee JY, Kennedy BK, Liao CY. mTOR signaling in mouse models of accelerated aging. J Gerontol A Biol Sci Med Sci. 2019. doi: 10.1093/gerona/glz059, [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Scaffidi P, Misteli T. Lamin A-dependent nuclear defects in human aging. Science. 2006;312:1059–1063. doi: 10.1126/science.1127168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Benson EK, Lee SW, Aaronson SA. Role of progerin-induced telomere dysfunction in HGPS premature cellular senescence. J Cell Sci. 2010;123(Pt 15):2605–2612. doi: 10.1242/jcs.067306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu Y, Rusinol A, Sinensky M, Wang Y, Zou Y. DNA damage responses in progeroid syndromes arise from defective maturation of prelamin A. J Cell Sci. 2006;119(Pt 22):4644–4649. doi: 10.1242/jcs.03263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Varela I, Cadiñanos J, Pendás AM, et al. Accelerated ageing in mice deficient in Zmpste24 protease is linked to p53 signalling activation. Nature. 2005;437:564–568. doi: 10.1038/nature04019 [DOI] [PubMed] [Google Scholar]
- 35. Wang C, Jurk D, Maddick M, Nelson G, Martin-Ruiz C, von Zglinicki T. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell. 2009;8:311–323. doi: 10.1111/j.1474-9726.2009.00481.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.