Abstract
Metformin is a frontline hypoglycemic agent, which is mainly prescribed to manage type 2 diabetes mellitus with obesity. Emerging evidence suggests that metformin also exerts protective effects against various kidney diseases. Some postulate that kidney disease is actually a metabolic disease, accompanied by nonresolving pathophysiologic pathways controlling oxidative stress, endoplasmic reticulum stress, inflammation, lipotoxicity, fibrosis, and senescence, as well as insufficient host defense mechanisms such as AMP-activated protein kinase (AMPK) signaling and autophagy. Metformin may interfere with these pathways by orchestrating AMPK signaling and AMPK-independent pathways to protect the kidneys from injury. Furthermore, the United States Food and Drug Administration declared metformin is safe for patients with mild or moderate kidney impairment in 2016, assuaging some conservative attitudes about metformin management in patients with renal insufficiency and broadening the scope of research on the renal protective effects of metformin. This review focuses on the molecular mechanisms by which metformin imparts renal protection and its potential in the treatment of various kidney diseases.
Keywords: metformin, renal protection, AMPK, lactic acidosis
INTRODUCTION
With an aging population, the burden of kidney disease gradually occupies more and more medical resources. Between 2013 and 2016, chronic kidney disease (CKD) was reported in 14.8% of the United States (US) general adult population. As of December 31, 2016, there are 2,160.7 patients with end stage renal disease (ESRD) per 1,000,000 US citizens, according to the US Renal Data System's latest Annual Data Report [1]. Therefore, there is an urgent demand for effective drugs to delay kidney impairment.
Beyond its hypoglycemic action, metformin has pleiotropic protective effects in various disease models, including polycystic ovary syndrome [2], cancer [3], neurological disorders [4], and kidney disease [5]. In particular, emerging evidence has demonstrated potential protective effects of metformin on acute kidney injury (AKI), CKD, diabetic kidney disease (DKD), autosomal dominant (adult) polycystic kidney disease (ADPKD), lupus nephritis (LN), renal neoplasm, and kidney transplantation [6–12].
Metformin protects the kidneys mainly via AMP-activated protein kinase (AMPK) signaling and AMPK-independent pathways. AMPK is a well-known energy and nutrient sensor, which regulates the switch from anabolic to catabolic metabolism to control energy homeostasis [13]. Many kidney diseases are intertwined with abnormal metabolic status, such as hyperglycemia, hyperlipidemia, and hyperuricemia. And many studies has explored the relationship between depressed AMPK activity and kidney disease [14–16], revealing that the AMPK agonist metformin exerts protective actions in a variety of kidney disease models, including those induced by hyperglycemia, advanced glycation end products (AGEs), proteinuria, and high fatty acid and folic acid levels, in both AMPK-dependent and -independent manners. Furthermore, its renal protective efficacy has been partly demonstrated in clinical trials. In this review, we will discuss the renal protective actions of metformin and its therapeutic benefits in different kidney diseases.
Pharmacological hallmarks of metformin
Metformin is an extract from Galega officinalis, which is primarily used to treat type 2 diabetes mellitus (T2DM), with a superior safety and efficacy profile. Metformin exerts a hypoglycemic effect by reducing hepatic glycogenesis and intestinal glucose absorption, improving glucose uptake and utilization in peripheral tissues, and increasing tissue insulin sensitivity [17]. Pharmacokinetic experiments in humans using 11C-metformin demonstrated that metformin mainly concentrates in the intestine, liver, and kidneys after oral administration [18]. Metformin is transported into hepatocytes by organic cation transporter 1 (OCT1) and OCT3, and into the renal basolateral membrane by OCT2. Metformin is primarily excreted from the kidneys via multidrug and toxin extrusion 1 and 2 by the means of prototype form [19]. Differences in transporter expression and function lead to pharmacokinetic heterogeneity between individuals, and subsequently, heterogeneous sensitivity to side effects [20]. Although its exact mechanism remains controversial, it is generally accepted that metformin activates AMPK to exert its pharmacological effects via two pathways: one, by promoting the direct phosphorylation of threonine residue 172 of AMPKα by liver kinase B1 (LKB1) [21]; and two, by inhibiting the mitochondrial respiratory chain complex I, resulting in an elevated AMP:ATP ratio and an energy crisis that results in AMPK activation [22]. Upon activation, AMPK coordinates multiple signaling networks to restore the energy balance, with protective effects on renal lesions [23]. However, there are many other mechanisms by which metformin imparts renal protection independently of AMPK, which are discussed in detail below.
Molecular mechanisms of the renal protective effects of metformin
Autophagy induction
Autophagy is an evolutionarily conserved catabolic process in eukaryotic cells, in which cells degrade senescent or dysfunctional cytoplasmic components in lysosomes and then reutilize their components. Renal cells maintain a basal level of autophagy under physiological conditions to resist multiple forms of stress. Under pathological conditions, renal cells upregulate autophagy in response to cell stress, but maladaptive autophagy can also induce apoptosis [24]. Current evidence suggests that autophagic flux is generally insufficient in various kidney disease models [25]. Therefore, many researchers are investigating whether modulating autophagy could delay the development of kidney disease.
Li et al. [26] reported that metformin markedly enhanced punctate green fluorescent protein-microtubule associated protein 1 light chain 3 formation in rat renal proximal tubular (NRK-52E) cells, implying that it can influence autophagic flux in the kidneys. Subsequently, Satriano et al. [27] showed that metformin markedly relieved insufficient autophagic flux in the rat kidney cortex in the context of ischemia/reperfusion (IR), and reduced IR damage. In a cisplatin-induced AKI model, pretreatment with metformin decreased apoptosis and induced autophagy in NRK-52E cells. Furthermore, AMPKα small interfering RNA and the autophagy inhibitor 3-methyladenine abrogated the protective effect of metformin on cisplatin-mediated apoptosis, respectively [28]. Additionally, metformin inhibits pronephros cyst formation in polycystin 2-deficient zebrafish by enhancing AMPK-tuberous sclerosis complex-dependent autophagy [29]. These results suggest that metformin-mediated autophagy has beneficial effects.
Antioxidant properties
Oxidative stress is caused by an imbalance between the production of reactive oxygen species (ROS) and the biological ability to counteract or detoxify their damage through antioxidative mechanisms. Transient increases in ROS protect cells from insult and maintain cellular homeostasis. However, excessive oxidative stress is involved in the pathogenesis of many renal diseases, including DKD [30].
Under sustained hyperglycemic conditions, massive amounts of proteins, lipids, and nucleic acids are glycated through the Maillard reaction to form AGEs. AGEs can induce the expression of oxidative, proinflammatory, and profibrotic mediators in renal cells via the advanced glycosylation end-product specific receptor (AGER) [31]. Metformin exerts its antioxidant effect by blocking the AGEs-AGER-ROS axis. Metformin negatively impacts the formation of glyceraldehyde-derived AGEs, protecting proximal tubular epithelial cells from AGEs-mediated injury [32]. In contrast to some scholars’ viewpoints, while metformin treatment reduces AGER expression, it is possible that the positive feedback effect of AGEs on AGER expression is weakened when AGEs generation is inhibited by metformin [33]. Metformin may reduce endogenous ROS generation by inhibiting nicotinamide adenine dinucleotide phosphate oxidase in high glucose-cultured podocytes [34]. In addition, metformin induces the endogenous reductants heme oxygenase 1 (HMOX1) and thioredoxin to reduce ROS generation in high glucose-cultured human kidney proximal tubular (HK-2) cells [35]. Metformin can also block damage cascades downstream of ROS. In an in vitro experiment, metformin partly alleviated oxidative stress by inhibiting ROS-induced phosphorylation of p38 mitogen-activated protein kinase (MAPK) in hyperglycemia-stimulated rat glomerular mesangial cells [36].
Aside from DKD, ROS-mediated renal tubular epithelial cell injury is an important risk factor for kidney stone formation. Metformin effectively blunts renal tubular injury resulting from oxalate and renal crystal deposition-mediated lipid peroxidation by attenuating cellular oxidative damage; however, this requires further clinical study [37]. Furthermore, gentamicin-induced nephrotoxicity is partly mediated by mitochondrial oxidative stress, and metformin ameliorated this nephrotoxicity via restoring mitochondrial function and normalizing oxidative stress [38, 39].
Altogether, metformin protects the kidneys in part by blocking ROS generation and signaling pathways downstream of oxidative stress, as well as by increasing antioxidative responses.
Attenuation of endoplasmic reticulum (ER) stress
ER stress and the course of kidney disease are mutually causal. Albumin overload [40], toxicants [41], and ischemia [42] can result in the accumulation of misfolded and unfolded proteins in the ER, resulting in the activation of ER stress responses to maintain cellular protein homeostasis. Activation of the unfolded protein response (UPR) is a protective response of ER to stress. The UPR inhibits the synthesis of new proteins, improves protein folding ability, and promotes the degradation of misfolded proteins to maintain ER function homeostasis. Notably, chronic or excessive ER stress causes a shift from prosurvival mode to proapoptotic mode, provoking programmed cell death. This occurs through the induction of the proapoptotic ER stress marker C-EBP homologous protein (CHOP), and the activation of the c-jun N-terminal kinase (JNK) and nuclear factor (NF)κB pathways, promoting inflammation, apoptosis, and fibrosis [43, 44]. Therefore, it is worth exploring whether reducing the intensity of ER stress appropriately could alleviate the deterioration of renal function.
Metformin alleviates ER stress-induced renal damage by modulating the UPR [45], partly by inhibiting ROS. Lee et al. revealed that metformin could inhibit ROS-SRC proto-oncogene, non-receptor tyrosine kinase-peroxisome proliferator activated receptor γ-mechanistic target of rapamycin kinase (mTOR) signaling by increasing the expression of endogenous thioredoxin to alleviate albumin-induced ER stress in HK-2 cells. Metformin (1 mM) downregulated glucose-regulated protein 78 (GRP78) and eukaryotic initiation factor 2 α (eIF2α) in HK-2 cells incubated with albumin (5 mg/mL) for 3 days and in the renal tissue of a rat model of proteinuria [46]. Conversely, Allouch et al. showed that cotreatment with metformin (1 mM) and albumin (10 mg/mL) increased GRP78 expression and decreased eIF2α and CHOP expression in NRK-52E cells compared to albumin alone; however, metformin had no effect on GRP78 and CHOP expression in NRK-52E cells treated with 15 mg/mL albumin [47]. The effect of metformin on ER stress may depend on the dose, manner of intervention, and injury severity. Furthermore, it remains unknown how metformin inhibits key molecules (GRP78, eIF2α, and CHOP) in the UPR pathway. Notably, untimely inhibition of the adaptive UPR by metformin can trigger cytotoxic effects [48].
Anti-inflammatory effects
Metformin may ameliorate renal lesions by abating inflammatory insults. Metformin prevents inflammatory responses through systemic immunomodulation. For example, metformin pretreatment limits immune cell infiltration into renal tissue in unilateral ureteral obstruction (UUO)- and cisplatin-induced models of AKI, thereby reducing inflammatory damage [28, 49, 50]. Christensen et al. [50] reported that metformin regulates the infiltration of microphage subpopulations in renal tissues subjected to three days of UUO. They postulated that metformin reduced microphage infiltration and elevated the ratio of anti-inflammatory M2 macrophages to proinflammatory M1 macrophages to attenuate inflammation damage in the UUO model. However, this notion should be validated using more specific biomarkers to identify microphage subtypes. Additionally, metformin reduces immune cell infiltration into the pronephric ducts of polycystin 2-deficient zebrafish, reducing inflammation-mediated cystogenesis and interfering with PKD progression [29]. In addition to modulating immune cell infiltration into renal tissue, metformin also inhibits their proinflammatory functions. For instance, it reduces the mRNA levels of proinflammatory cytokines (such as interleukin (IL)-1β, IL-6, and tumor necrosis factor α in AGEs-treated bone marrow-derived macrophages via the AMPK-NFκB pathway [51], as well as in the renal tissue of the UUO model [50]. Gu et al. [52] revealed that metformin inhibited NFκB activation and the generation of proinflammatory cytokines (such as monocyte chemoattractant protein 1, intercellular cell adhesion molecule 1, and transforming growth factor β1 (TGFβ1)) in high glucose-treated rat glomerular mesangial cells in vitro. In short, metformin reduces inflammation-induced renal injury by modulating immune cell infiltration and function.
Attenuation of lipotoxicity
Obese patients are more prone to kidney damage, partly because excess lipids ectopically accumulate in the renal tissue, resulting in lipotoxicity. This contributes to ROS generation, insulin resistance, ER stress, inflammation, and fibrosis [53]. Wang et al. [54] reported that metformin suppressed fatty acid synthesis and deposition to alleviate renal lipotoxicity in Otsuka Long-Evans Tokushima Fatty rats. Combination therapy with metformin and omega-3 polyunsaturated fatty acids improved lipid metabolism in rats with diabetes mellitus (DM) [55]. Besides regulating lipid metabolism, metformin protected mesangial cells from lipotoxicity-induced apoptosis in a diabetic nephropathy model, partially by upregulating glucagon like peptide 1 receptor [56].
Antifibrotic effects
The kidneys initiate defense responses against various injuries, and maladaptive repair processes promote the phenotypic transformation of renal cells, the proliferation of renal fibroblasts, and abnormal extracellular matrix deposition. As a result, the functional nephron is gradually replaced by connective tissue, driving the formation of interstitial fibrosis, microvascular rarefaction, and even glomerulosclerosis [57]. No effective option exists to reverse renal fibrosis; therefore, delaying renal fibrosis development is an optimal strategy to protect the residual nephron. Metformin has been suggested to affect fibrosis through several mechanisms.
TGFβ1/SMAD signaling
Dysregulation of TGFβ1 signaling is implicated in renal fibrosis. Angiotensin, glucose, and oxidative stress induce TGFβ1 overexpression in tubular epithelial cells, macrophages, and renal interstitial fibroblasts. TGFβ1/SMAD signaling increases the transcription of α-smooth muscle actin (SMA), fibronectin, collagen I, and vimentin, and decreases E-cadherin expression to promote renal fibrosis [58]. Metformin attenuated TGFβ1 expression in renal tissues from a folic acid-induced rat model of renal fibrosis [59] and a UUO mouse model [60]. Han et al. [61] revealed that metformin interacts with TGFβ1 via its receptor-binding domain, blocking the binding of TGFβ1 to its receptor, and thereby inhibiting the TGFβ1/SMAD pathway. Notably, metformin inhibited UUO-induced SMAD3 phosphorylation in AMPK alpha2 subunit knockout mice, indicating that its anti-renal fibrosis effect is not completely dependent on AMPK signaling [60].
Epithelial–mesenchymal transition (EMT)
EMT is a prominent process contributing to renal interstitial fibrosis. It is a phenotypic conversion process in which mature tubular epithelial cells transform into myofibroblasts under pathological conditions, and is characterized by the loss of epithelial markers (e.g., E-cadherin, zonula occludens-1) and the acquisition of mesenchymal markers (e.g., α-SMA, fibronectin, and vimentin) [62]. TGFβ1, angiotensin II and hypoxia are vital risks in renal tubular EMT [63]. TGFβ1-induced EMT is mediated either by SMAD2/3 or via non-SMAD (p38 MAPK, JNK, extracellular signal-regulated kinase (ERK), etc.) signaling pathways [64]. Metformin interferes with the TGFβ1-AMPK-tuberin-EMT pathway by activating AMPK and thereby inhibiting extracellular matrix synthesis and other fibrogenic responses [65]. TGFβ1 induces the expression of immediate-early response genes, such as early growth response 1 (EGR1) [66], which is involved in renal tubular EMT [67]. In a recent study, metformin attenuated TGFβ1-induced EMT by inhibiting EGR1, suggesting that this is one of the potential mechanisms behind the renal protective effects of metformin [68]. Wu et al. [69] postulated that metformin inhibits EGR1 by downregulating microRNA (miR)-34a in high glucose-stimulated rat mesangial cells. Therefore, metformin could regulate EGR1 via two pathways: through an AMPK-miR-34a-sirtuin 1 (SIRT1)-EGR1 axis, and via direct AMPK-EGR1 signaling. Furthermore, metformin promotes HMOX1 and thioredoxin expression to decrease ROS levels, thereby alleviating oxidative response-mediated EMT in vitro [35].
Fatty acid metabolism
Extensive research has demonstrated that tubulointerstitial fibrosis is associated with the reduced expression of genes required for fatty acid oxidation (FAO) in renal tubular epithelial cells. Decreased FAO is proposed to cause energy deficiency and renal fibrosis [70]. Acetyl-CoA carboxylase alpha (ACC) is one of the major regulators of FAO, which acts to increase fatty acid synthesis and decrease its oxidation. AMPK phosphorylates ACC to increase FAO, boosting ATP generation. There is evidence that the antifibrotic effect of metformin is partly dependent on its ability to increase FAO by promoting ACC phosphorylation by AMPK [71].
Hypoxia inducible factor 1
The hypoxia inducible factor (HIF) pathway is an adaptive response to renal insult; however, sustained HIF activation may promote renal fibrosis in CKD [72]. HIF1 inhibition mitigates glomerular hypertrophy, mesangial expansion, matrix accumulation, and albuminuria excretion in type I diabetic OVE26 mice [73]. HIF1 is a heterodimeric transcription factor that regulates oxygen homeostasis, which consists of the constitutively expressed HIF1β subunit and the oxygen-labile HIF1α subunit. Hypoxia prevents the proteasomal degradation of the HIF1α subunit, which then heterodimerizes with HIF1β to regulate the transcription of genes controlling erythropoiesis, angiogenesis, and nucleoside and energy metabolism [72]. Aside from hypoxia, glucose overload [74], angiotensin II [75], and albuminuria [76] also promote renal fibrosis by stabilizing HIF1α. HIF1 modulates extracellular matrix turnover, activates fibrogenic factors such as tissue inhibitor of metalloproteinases and plasminogen activator inhibitor, and promotes EMT [77]. Moreover, HIF1 can act synergistically with TGFβ1 [78].
Metformin suppresses tubular HIF1α stabilization and protects kidneys from renal injury in Zucker diabetic fatty rats independently of AMPKα signaling. It attenuates mitochondrial respiration and thereby reduces cellular oxygen consumption, subsequently enhancing the proteasomal degradation of HIF1α [79]. Notably, HIF1 promotes renal fibrosis in a cell type- and disease phase-specific manner [80].
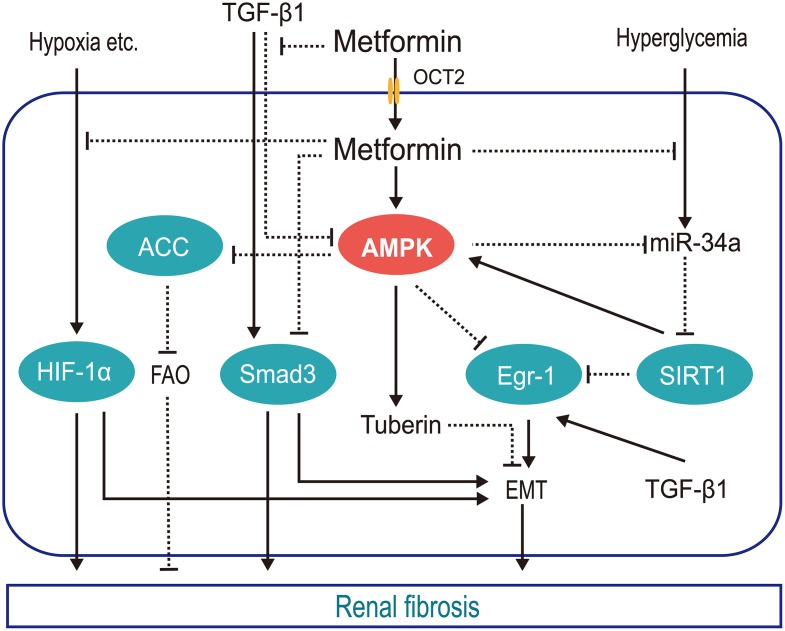
Currently, there is no effective way to reverse renal fibrosis; however, protection of the residual nephron is a worthy goal. The antifibrotic effect of metformin requires further clinical validation. The molecular mechanisms controlling this effect are depicted in Figure 1.
Figure 1.
Schematic of the mechanisms by which metformin protects against renal fibrosis. Metformin inhibits hypoxia-mediated renal fibrosis by inhibiting HIF1α stabilization via blocking the mitochondrial oxidative respiratory chain, reducing renal oxygen consumption; inhibits TGFβ1 generation and receptor binding to prevent TGFβ1-mediated renal fibrosis; promotes AMPK phosphorylation of ACC to increase FAO and slow renal fibrosis; inhibits hyperglycemia-induced expression of mi-R34a, which negatively regulates AMPK both directly and by downregulating SIRT1, reducing the pro-EMT factor EGR1. (HIF1α, hypoxia inducible factor 1α; TGFβ1, transforming growth factor β1; AMPK, AMP-activated kinase protein; ACC, acetyl-CoA carboxylase; FAO, fatty acid oxidation; miR-34a, microRNA-34a; EGR1, early growth response 1; EMT, epithelial-mesenchymal transition; OCT2, organic cation transporter 2).
Antiaging effects
Physiological or stress-induced aging weakens the ability of intrinsic renal cells to resist injury and self-repair, increasing AKI risk and accelerating CKD progression. The US FDA has been conducting the randomized double-blind clinical experiment, ‘‘Targeting Aging with Metformin’’ since 2015, extending the uses of metformin into the field of antiaging. Metformin restored expression of the high glucose-downregulates antiaging gene klotho in serum, urine, and renal tissues [81]. Furthermore, metformin downregulates senescence-associated-β-galactosidase and cyclin dependent kinase inhibitors 1A and 2A during hyperglycemia-induced premature aging of glomerular mesangial cells and proximal tubular epithelial cells via the AMPK/mTOR pathway [82, 83]. However, the antiaging effects of metformin have only been demonstrated in some small trails [84], and its precise antiaging effects on the kidneys should be investigated in further basic and clinical studies.
The pros and cons of metformin: adverse effects and renal protective actions
Lactic acidosis
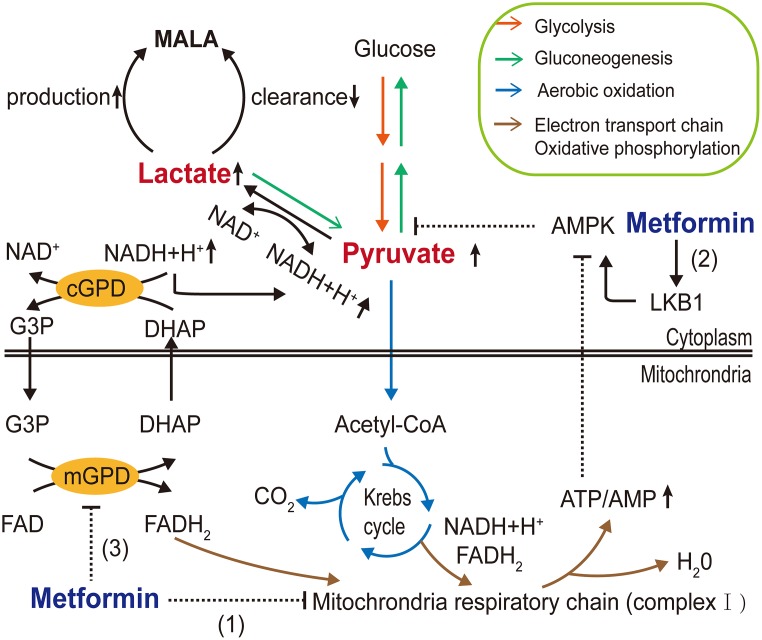
The major adverse side effect of metformin exposure is gastrointestinal irritation, including diarrhea, nausea, vomiting, flatulence, and cramps [85]. In addition, the US FDA has given metformin a black box warning regarding lactic acidosis. Because of this, metformin use is restricted for patients with severe renal impairment. Biguanide exposure is related to increased plasma lactic acid levels. Metformin may block the Krebs cycle by inhibiting mitochondrial oxidative respiratory chain complex I, thereby facilitating the Pasteur effect. Lactic acid, a byproduct of glycolysis, accumulates in the body as a result of lactate overproduction or decreased removal [5]. Moreover, in addition to its effects on AMPK-dependent inhibition of gluconeogenesis [86], Madiraju et al. [87, 88] reported that metformin might reduce the conversion of lactate and glycerol to glucose and suppress hepatic gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase (mGPD) at clinically relevant plasma concentrations. Hence, metformin may interfere with the oxidative pathway and gluconeogenesis pathway of pyruvate, the only precursor of lactate. This would increase the reduction of pyruvate to lactate and decrease the conversion of lactate to pyruvate, resulting in increased theoretical buildup of lactate concentrations (Figure 2). Furthermore, DeFronzo et al. [89] postulated that disease itself exaggerates the risk of metformin-associated lactic acidosis (MALA).
Figure 2.
Molecular mechanisms of metformin-associated lactic acidosis (MALA). Metformin (1) inhibits mitochondrial respiratory chain complex I, reducing Krebs cycle flux and shifting metabolism toward glycolysis, increasing the pyruvate level; (2) partly inhibits gluconeogenesis through the AMPK pathway, further contributing to pyruvate accumulation and increasing the conversion of accumulated pyruvate to lactate; and (3) inhibits mGPD, blocking the G3P pathway and altering the cytoplasmic redox state, inhibiting the conversion of lactate to pyruvate, resulting in MALA. (AMPK, AMP-activated protein kinase; LKB1, liver kinase B1; G3P, glycerol-3-phosphate; DHAP, dihydroxyacetone phosphate; mGPD, mitochondrial glycerophosphate dehydrogenase; cGPD, cytosolic glycerophosphate dehydrogenase).
Given the risk of lactic acidosis, how do we weigh the pros and cons of metformin use? MALA is a low probability event. Its incidence varies in different backgrounds, but is generally < 10 per 100,000 patient-years [90]. However, the mortality rate approaches 25% in patients with MALA [91]. In 2016, the US FDA revised metformin safe in patients with mild to moderate renal impairment (estimated glomerular filtration rate (eGFR): 30-60 mL·min-1·1.73 m-2), but metformin use is contraindicated in patients with eGFR values < 30 mL·min-1·1.73 m-2. There is no convincing evidence that the label change has increased the MALA rate [92]. The FDA suggests that the initiation and withdrawal of metformin treatment should be based on a comprehensive assessment of the eGFR and risk factors such as hepatic insufficiency, alcoholism, heart impairment, and intra-arterial iodinated contrast exposure. Notably, patient eGFR levels are volatile, and should therefore be periodically monitored [93].
AKI and CKD
AKI is characterized by a rapid and abrupt reduction of renal function, within days or even a few hours [94], while CKD is defined by a gradual decrease in kidney function over 3 months [95]. However, they are interrelated and cannot simply be classified into two separate diseases. Currently, therapies for AKI and CKD are limited to mitigating etiological factors and treating symptoms, and treatment breakthroughs for these conditions are needed.
Metformin may exert protective effects on AKI and CKD. Li et al. [28] reported that metformin protected a cisplatin-induced AKI model through AMPKα-regulated autophagy induction. Furthermore metformin corrected renal metabolic disorders, suppressed renal fibrosis, and improved renal function in an ablation and infarction rat model of subtotal or five-sixths nephrectomy [96]. Neven et al. [7] revealed that metformin treatment slowed the progression of severe CKD and maintained mineral homeostasis, which reduced the risks of vascular calcification and high bone turnover in CKD-Mineral and Bone Disorder.
In 2017, an observational cohort study of patients with AKI in the Tayside region of Scotland (n= 25,148) revealed that metformin did not affect AKI incidence, but was associated with a 28-day increase in survival [6]. A meta-analysis of 17 observational studies revealed that metformin administration was associated with reduced all-cause mortality in patients with CKD (eGFR= 30-60 mL·min-1·1.73 m-2) [97].
More recently, Lalau et al. [98] investigated the safety and efficacy of metformin in patients with T2DM and CKD and provided metformin management strategies. The dose-finding study, involving 69 patients, suggested daily doses of 1,500, 1,000, and 500 mg for stages 3A, 3B, and 4 CKD, respectively (Table 1). They suggest that the eGFR and plasma lactate concentration be monitored to evaluate to need to withdraw metformin treatment.
Table 1. Metformin management in patients with T2DM with stage 3A, 3B, and 4 CKD.
| CKD stage | eGFR (mL·min-1·1.73 m-2) | Recommended daily dose |
| 3A | 45-59 | 0.5 g in the morning + 1 g in the evening |
| 3B | 30-44 | 0.5 g in the morning + 0.5 g in the evening |
| 4 | 15-29 | 500 mg in the morning |
T2DM: type 2 diabetes mellitus; CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate.
Taken together, these studies suggest that metformin has renal protective effects against AKI and CKD. However, this notion will require validation through further prospective randomized controlled trials.
DKD
DKD is a chronic microvascular complication of diabetes mellitus (DM), which is the major cause of ESRD in the US [1]. Reversing high glucose-induced inactivation of AMPK alleviated renal hypertrophy, glomerular basement thickening, podocyte loss, and foot process effacement in OVE26 mice [99], suggesting that AMPK agonist metformin can relieve kidney damage in patients with DM. Aside from its hypoglycemic effect, metformin also delays DKD progression by modulating metabolic dysfunctions, such as insulin resistance, autophagy, oxidative stress, ER stress, inflammation, and renal fibrosis. Insulin resistance, caused by defective insulin signaling in target cells, is a key factor in diabetic glomerulopathy [100]. Podocyte-specific insulin receptor knockout mice develop symptoms of DKD [101]. SIRT1 downregulation in DM is positively associated with insulin resistance [102]. Metformin can overcome hyperglycemia-induced insulin resistance in podocytes by promoting the expression and function of SIRT1 and AMPK [103]. Furthermore, metformin attenuates DM-induced renal medullary tissue hypoxia by inhibiting uncoupling protein 2 in insulinopenic type 1 diabetes rats [104]. As mentioned above, metformin also modulates the interplay between oxidative stress, lipotoxicity, fibrosis, and aging in DKD to delay renal exacerbation.
Compared with other hypoglycemic agents, metformin has obvious renal protective functions. For example, compared with sulfonylureas, metformin administration is associated with lower risks of kidney function decline and death, independent of changes in systolic blood pressure, body mass index, and glycated hemoglobin levels over time [105]. An open cohort study of patients with T2DM in primary care (n= 469,688) revealed that metformin decreased the risk of severe T2DM complications, including blindness and severe kidney failure, compared to a group not administered metformin [106]. These results suggest that metformin may be a better choice for DM patients with DKD.
Autosomal dominant polycystic kidney disease
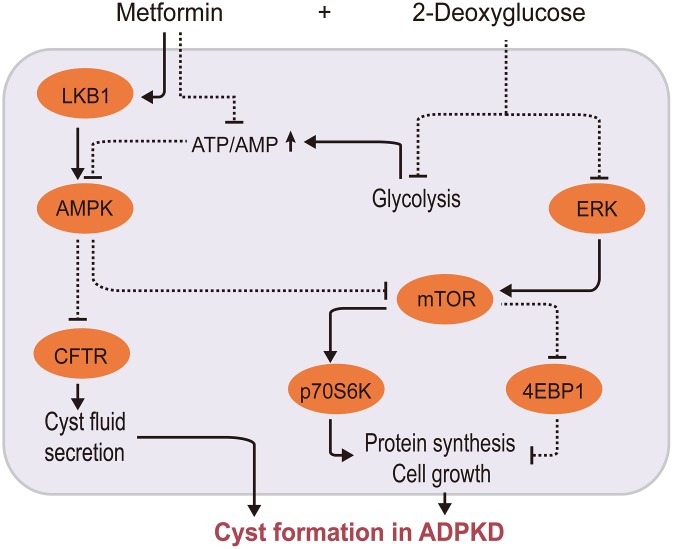
ADPKD is a monogenically inherited cystic kidney disease characterized by renal cysts and extrarenal multisystem manifestations [107]. Cystic cells switch their energy metabolism to glycolysis accompanied with AMPK downregulation, mTOR overactivation, and ERK activation [108, 109]. Therefore, researchers have hypothesized that it may be possible to influence the proliferation and cell cycle progression of cystic cells by regulating their cellular energy metabolism pathways in order to delay ADPKD progression. Cystic fibrosis transmembrane conductance regulator (CFTR) is involved in cyst fluid and electrolyte secretion, and mTOR participates in the proliferation of cyst epithelial cells. Metformin negatively regulates CFTR and mTOR via the AMPK pathway in vitro [110]. Interestingly, metformin and 2-deoxyglucose (2DG), a competitive inhibitor of the rate-limiting glycolytic enzyme hexokinase, synergistically inhibit mTOR signaling to inhibit ADPKD cell proliferation by activating AMPK and suppressing ERK [111, 112], as depicted in Figure 3. Moreover, a few clinical trials have demonstrated the safety and effectiveness of metformin in ADPKD, althought these had small sample sizes [9, 113, 114].
Figure 3.
Metformin/2-deoxyglucose cotreatment delays ADPKD progression. Metformin interferes with ADPKD cell proliferation by inhibiting CFTR and mTOR signaling via AMPK 2-DG inhibits mTOR via two pathways: by suppressing ERK, an upstream activator of mTOR, and by competitively inhibiting glycolysis, leading to energy imbalance and AMPK activation. This further inhibits CFTR and mTOR, thereby synergistically inhibiting ADPKD proliferation. (2-DG, 2-deoxyglucose; CFTR, cystic fibrosis transmembrane conductance regulator; AMPK, AMP-activated protein kinase; mTOR, mechanistic target of rapamycin kinase; ERK: extracellular signal-regulated kinase; ADPKD, autosomal dominant polycystic kidney disease).
Metformin combined with 2DG may represent a novel intervention strategy for ADPKD, but some researchers have questioned whether clinical trials will have the same inspiring effect, considering the difference in blood drug concentrations between the experimental conditions and those required for clinical treatment [115]. The outcomes of a recent phase 2 placebo-controlled trial regarding the effects of metformin on ADPKD are greatly anticipated.
LN
Systemic lupus erythematosus (SLE) is a chronic inflammatory autoimmune disease. Aberrant activation of the immune system results in a large number of autoreactive antibodies that attack autoantigens, forming circulating and in situ immune complexes that are deposited in multiple organs, particularly the kidneys.
Metformin exhibits a potential immune regulatory function. Metformin restores immune homeostasis by interfering with T cell subtype differentiation, decreasing autoreactive marginal zone B cells and antibody-secreting plasma cells, and reducing germinal center formation via the AMPK-mTOR-STAT3 pathway in Roquinsan/san mice, along with reduced inflammatory mediators and antibodies [116]. A proof-of-concept trial reported that metformin downregulates neutrophil extracellular trap (NETs) mitochondrial DNA (mt-DNA) mediated interferon (IFN)α generation in plasmacytoid dendritic cells (PDCs) to inhibit SLE progression [117]. The dual upregulation of glycolysis and mitochondrial oxidative metabolism is involved in the effector function, activation and proliferation of autoreactive CD4+ T cells. Metformin combined with 2DG normalized T cell metabolism, and reversed the lupus phenotype and renal disease in lupus-prone mouse models; however these drugs had no effect alone [118]. Taken together, these studies indicate that metformin improves SLE by inhibiting AMPK/mTOR/STAT3 signaling, NET mtDNA-PDCs-IFNα signaling, and oxidative phosphorylation. However, the efficacy of metformin on lupus nephritis requires further clinical study.
Renal neoplasms
Renal cell carcinoma (RCC) accounts for 90-95% of renal neoplasms [119]. A recent popular viewpoint has defined cancer as a genetic and metabolic disorder, which establishes high anabolic and catabolic activity to meet proliferation, growth, and survival demands [120]. Consistent with this idea, RCC is accompanied by reduced AMPK levels and dysregulation of proliferation-related mTOR signaling [121]. Therefore, pharmacologically targeting AMPK and mTOR may be a potential therapeutic strategy.
Metformin suppresses RCC progression mainly by promoting apoptosis, as well as inhibiting proliferation and viability in a dose- and time-dependent manner. It has been reported that metformin promotes apoptosis in human RCC (A498) cells [122]. Metformin inhibits RCC cell proliferation and viability by inducing G0/G1 cell cycle arrest [123], and by upregulating cell growth-related miRNAs such as miR-34a [124], miR-26a [125], and miR-21 [126]. Notably, the effects of metformin on apoptosis, G0/G1 phase cell cycle arrest and viability differ between RCC cell lines [127]. Furthermore, metformin promotes RCC cell proliferation under nutrient restriction. Energy stress increases AMPK nuclear translocation, which recruits pyruvate kinase M2 (PKM2) and its downstream effector β-catenin to the nucleus, activating the transcription of proliferation-related genes such as CCND1 and MYC proto-oncogene, bHLH transcription factor [128]. This suggests that combining metformin with a PKM2 inhibitor may be a promising strategy to suppress RCC growth.
In 2017, a meta-analysis (n= 254,329) suggested that metformin administration could improve overall and cancer-specific survival in patients with kidney cancer [129]. A retrospective analysis (n= 1,528 RCC patients) reveled that metformin administration improved survival in patients with localized RCC, but not in those with metastatic RCC [130]. However, a retrospective study (n= 158 patients with diabetes undergoing nephrectomy for kidney cancer) during the same period provided dissenting results [131].
Recently, increasing evidence has revealed a potential therapeutic effect for metformin combined with calorie restriction in colon cancer cell lines or with hemin in breast cancer cell lines [3, 132]. However, the exact action of metformin in renal cancer remains unknown. The antitumor actions of metformin have thus far been demonstrated at experimental doses far exceeding its clinical plasms concentration, sparking speculation as to whether metformin will display clinical effects.
Renal transplantation
The use of metformin in kidney transplant recipients lacks authoritative clinical criteria. Considering its potential for adverse side effects, metformin requires further assessment regarding the risk of hypoglycemia in these patients. Some studies have indicated that metformin is safe for kidney transplant recipients, improving the survival rate [6, 12, 133–136]. However, due to a series of clinical research limitations, such as small sample sizes and questionable data integrity, the reliability of these studies is unclear. The safety and efficacy of metformin in post-transplantation DM requires further clinical retrospective study and randomized controlled trials with larger sample sizes.
CONCLUSIONS
Severe kidney damage is irreversible and can develop into ESRD, which requires renal replacement therapy (dialysis or renal transplantation). Kidney transplant surgery is not vigorously promoted because of the rarity of locating matching donors, and early protection of residual nephrons and prevention of further renal deterioration could not only alleviate patient suffering, but also reduce the health and economic burdens of kidney disease worldwide.
In the past few decades, many preclinical and clinical studies have reported the renal protective effects of metformin. At the same time, controversial outcomes of metformin treatment have sparked debate regarding its therapeutic efficacy in some kidney diseases. The renal effects of metformin are complex and dependent on the disease type, as well as the nature and timing of the injury. The clinical efficacy of metformin should be validated in well-designed randomized controlled trials with larger sample sizes, and the precise renal protective mechanisms of metformin should be further explored.
Footnotes
AUTHOR CONTRIBUTIONS: Conceptualization, QJP and HFL; Writing—original draft preparation, QJP, XL, and CFZ; Writing—review and editing, SZL, XQC, FBG, CY., and HFL.; Funding acquisition, QJP.
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
FUNDING: This research was funded by grants from Department of Education of Guangdong Province (2017 Key Platform and Scientific Research project, No. 2017KTSCX077), and the project of “Dengfeng Plan” from Affiliated Hospital of Guangdong Medical University.
REFERENCES
- 1.Saran R, Robinson B, Abbott KC, Agodoa LY, Bragg-Gresham J, Balkrishnan R, Bhave N, Dietrich X, Ding Z, Eggers PW, Gaipov A, Gillen D, Gipson D, et al. US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2019. (Suppl 1); 73:A7–8. 10.1053/j.ajkd.2019.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Løvvik TS, Carlsen SM, Salvesen Ø, Steffensen B, Bixo M, Gómez-Real F, Lønnebotn M, Hestvold KV, Zabielska R, Hirschberg AL, Trouva A, Thorarinsdottir S, Hjelle S, et al. Use of metformin to treat pregnant women with polycystic ovary syndrome (PregMet2): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019; 7:256–66. 10.1016/S2213-8587(19)30002-6 [DOI] [PubMed] [Google Scholar]
- 3.Elgendy M, Cirò M, Hosseini A, Weiszmann J, Mazzarella L, Ferrari E, Cazzoli R, Curigliano G, DeCensi A, Bonanni B, Budillon A, Pelicci PG, Janssens V, et al. Combination of Hypoglycemia and Metformin Impairs Tumor Metabolic Plasticity and Growth by Modulating the PP2A-GSK3β-MCL-1 Axis. Cancer Cell. 2019; 35:798–815.e5. 10.1016/j.ccell.2019.03.007 [DOI] [PubMed] [Google Scholar]
- 4.Gantois I, Popic J, Khoutorsky A, Sonenberg N. Metformin for Treatment of Fragile X Syndrome and Other Neurological Disorders. Annu Rev Med. 2019; 70:167–81. 10.1146/annurev-med-081117-041238 [DOI] [PubMed] [Google Scholar]
- 5.Corremans R, Vervaet BA, D’Haese PC, Neven E, Verhulst A. Metformin: A Candidate Drug for Renal Diseases. Int J Mol Sci. 2018; 20:20. 10.3390/ijms20010042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell S, Farran B, McGurnaghan S, McCrimmon RJ, Leese GP, Petrie JR, McKeigue P, Sattar N, Wild S, McKnight J, Lindsay R, Colhoun HM, Looker H. Risk of acute kidney injury and survival in patients treated with Metformin: an observational cohort study. BMC Nephrol. 2017; 18:163. 10.1186/s12882-017-0579-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neven E, Vervaet B, Brand K, Gottwald-Hostalek U, Opdebeeck B, De Maré A, Verhulst A, Lalau JD, Kamel S, De Broe ME, D’Haese PC. Metformin prevents the development of severe chronic kidney disease and its associated mineral and bone disorder. Kidney Int. 2018; 94:102–13. 10.1016/j.kint.2018.01.027 [DOI] [PubMed] [Google Scholar]
- 8.Ravindran S, Kuruvilla V, Wilbur K, Munusamy S. Nephroprotective Effects of Metformin in Diabetic Nephropathy. J Cell Physiol. 2017; 232:731–42. 10.1002/jcp.25598 [DOI] [PubMed] [Google Scholar]
- 9.Seliger SL, Abebe KZ, Hallows KR, Miskulin DC, Perrone RD, Watnick T, Bae KT. A Randomized Clinical Trial of Metformin to Treat Autosomal Dominant Polycystic Kidney Disease. Am J Nephrol. 2018; 47:352–60. 10.1159/000488807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan MK, Heng TY, Mak A. The Potential Use of Metformin, Dipyridamole, N-Acetylcysteine and Statins as Adjunctive Therapy for Systemic Lupus Erythematosus. Cells. 2019; 8:8. 10.3390/cells8040323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tseng CH. Use of metformin and risk of kidney cancer in patients with type 2 diabetes. Eur J Cancer. 2016; 52:19–25. 10.1016/j.ejca.2015.09.027 [DOI] [PubMed] [Google Scholar]
- 12.Stephen J, Anderson-Haag TL, Gustafson S, Snyder JJ, Kasiske BL, Israni AK. Metformin use in kidney transplant recipients in the United States: an observational study. Am J Nephrol. 2014; 40:546–53. 10.1159/000370034 [DOI] [PubMed] [Google Scholar]
- 13.Hardie DG. AMPK—sensing energy while talking to other signaling pathways. Cell Metab. 2014; 20:939–52. 10.1016/j.cmet.2014.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Oliveira AA, de Oliveira TF, Bobadilla LL, Garcia CC, Berra CM, de Souza-Pinto NC, Medeiros MH, Di Mascio P, Zatz R, de M Loureiro AP. Sustained kidney biochemical derangement in treated experimental diabetes: a clue to metabolic memory. Sci Rep. 2017; 7:40544. 10.1038/srep40544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Declèves AE, Mathew AV, Cunard R, Sharma K. AMPK mediates the initiation of kidney disease induced by a high-fat diet. J Am Soc Nephrol. 2011; 22:1846–55. 10.1681/ASN.2011010026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kume S, Koya D, Uzu T, Maegawa H. Role of nutrient-sensing signals in the pathogenesis of diabetic nephropathy. Biomed Res Int. 2014; 2014:315494. 10.1155/2014/315494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou T, Xu X, Du M, Zhao T, Wang J. A preclinical overview of metformin for the treatment of type 2 diabetes. Biomed Pharmacother. 2018; 106:1227–35. 10.1016/j.biopha.2018.07.085 [DOI] [PubMed] [Google Scholar]
- 18.Gormsen LC, Sundelin EI, Jensen JB, Vendelbo MH, Jakobsen S, Munk OL, Hougaard Christensen MM, Brøsen K, Frøkiær J, Jessen N. In Vivo Imaging of Human 11C-Metformin in Peripheral Organs: Dosimetry, Biodistribution, and Kinetic Analyses. J Nucl Med. 2016; 57:1920–26. 10.2967/jnumed.116.177774 [DOI] [PubMed] [Google Scholar]
- 19.Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab. 2014; 20:953–66. 10.1016/j.cmet.2014.09.018 [DOI] [PubMed] [Google Scholar]
- 20.Florez JC. The pharmacogenetics of metformin. Diabetologia. 2017; 60:1648–55. 10.1007/s00125-017-4335-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie Z, Dong Y, Scholz R, Neumann D, Zou MH. Phosphorylation of LKB1 at serine 428 by protein kinase C-zeta is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells. Circulation. 2008; 117:952–62. 10.1161/CIRCULATIONAHA.107.744490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stephenne X, Foretz M, Taleux N, van der Zon GC, Sokal E, Hue L, Viollet B, Guigas B. Metformin activates AMP-activated protein kinase in primary human hepatocytes by decreasing cellular energy status. Diabetologia. 2011; 54:3101–10. 10.1007/s00125-011-2311-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017; 60:1577–85. 10.1007/s00125-017-4342-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huber TB, Edelstein CL, Hartleben B, Inoki K, Jiang M, Koya D, Kume S, Lieberthal W, Pallet N, Quiroga A, Ravichandran K, Susztak K, Yoshida S, Dong Z. Emerging role of autophagy in kidney function, diseases and aging. Autophagy. 2012; 8:1009–31. 10.4161/auto.19821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin TA, Wu VC, Wang CY. Autophagy in Chronic Kidney Diseases. Cells. 2019; 8:8. 10.3390/cells8010061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Xu Z, Jiang L, Mao J, Zeng Z, Fang L, He W, Yuan W, Yang J, Dai C. Rictor/mTORC2 protects against cisplatin-induced tubular cell death and acute kidney injury. Kidney Int. 2014; 86:86–102. 10.1038/ki.2013.559 [DOI] [PubMed] [Google Scholar]
- 27.Declèves AE, Sharma K, Satriano J. Beneficial Effects of AMP-Activated Protein Kinase Agonists in Kidney Ischemia-Reperfusion: Autophagy and Cellular Stress Markers. Nephron, Exp Nephrol. 2014; 128:98–110. 10.1159/000368932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Gui Y, Ren J, Liu X, Feng Y, Zeng Z, He W, Yang J, Dai C. Metformin Protects Against Cisplatin-Induced Tubular Cell Apoptosis and Acute Kidney Injury via AMPKα-regulated Autophagy Induction. Sci Rep. 2016; 6:23975. 10.1038/srep23975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang MY, Ma TL, Hung CC, Tian YC, Chen YC, Yang CW, Cheng YC. Metformin Inhibits Cyst Formation in a Zebrafish Model of Polycystin-2 Deficiency. Sci Rep. 2017; 7:7161. 10.1038/s41598-017-07300-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krata N, Zagożdżon R, Foroncewicz B, Mucha K. Oxidative Stress in Kidney Diseases: The Cause or the Consequence? Arch Immunol Ther Exp (Warsz). 2018; 66:211–20. 10.1007/s00005-017-0496-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamagishi S, Matsui T. Advanced glycation end products, oxidative stress and diabetic nephropathy. Oxid Med Cell Longev. 2010; 3:101–08. 10.4161/oxim.3.2.11148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishibashi Y, Matsui T, Takeuchi M, Yamagishi S. Beneficial effects of metformin and irbesartan on advanced glycation end products (AGEs)-RAGE-induced proximal tubular cell injury. Pharmacol Res. 2012; 65:297–302. 10.1016/j.phrs.2011.11.001 [DOI] [PubMed] [Google Scholar]
- 33.Ishibashi Y, Matsui T, Takeuchi M, Yamagishi S. Metformin inhibits advanced glycation end products (AGEs)-induced renal tubular cell injury by suppressing reactive oxygen species generation via reducing receptor for AGEs (RAGE) expression. Horm Metab Res. 2012; 44:891–95. 10.1055/s-0032-1321878 [DOI] [PubMed] [Google Scholar]
- 34.Piwkowska A, Rogacka D, Jankowski M, Dominiczak MH, Stepiński JK, Angielski S. Metformin induces suppression of NAD(P)H oxidase activity in podocytes. Biochem Biophys Res Commun. 2010; 393:268–73. 10.1016/j.bbrc.2010.01.119 [DOI] [PubMed] [Google Scholar]
- 35.Lee JH, Kim JH, Kim JS, Chang JW, Kim SB, Park JS, Lee SK. AMP-activated protein kinase inhibits TGF-β-, angiotensin II-, aldosterone-, high glucose-, and albumin-induced epithelial-mesenchymal transition. Am J Physiol Renal Physiol. 2013; 304:F686–97. 10.1152/ajprenal.00148.2012 [DOI] [PubMed] [Google Scholar]
- 36.Yao XM, Ye SD, Xiao CC, Gu JF, Yang D, Wang S. Metformin alleviates high glucose-mediated oxidative stress in rat glomerular mesangial cells by modulation of p38 mitogen-activated protein kinase expression in vitro. Mol Med Rep. 2015; 12:520–26. 10.3892/mmr.2015.3446 [DOI] [PubMed] [Google Scholar]
- 37.Yang X, Ding H, Qin Z, Zhang C, Qi S, Zhang H, Yang T, He Z, Yang K, Du E, Liu C, Xu Y, Zhang Z. Metformin Prevents Renal Stone Formation through an Antioxidant Mechanism In Vitro and In Vivo. Oxid Med Cell Longev. 2016; 2016:4156075. 10.1155/2016/4156075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morales AI, Detaille D, Prieto M, Puente A, Briones E, Arévalo M, Leverve X, López-Novoa JM, El-Mir MY. Metformin prevents experimental gentamicin-induced nephropathy by a mitochondria-dependent pathway. Kidney Int. 2010; 77:861–69. 10.1038/ki.2010.11 [DOI] [PubMed] [Google Scholar]
- 39.Zorov DB. Amelioration of aminoglycoside nephrotoxicity requires protection of renal mitochondria. Kidney Int. 2010; 77:841–43. 10.1038/ki.2010.20 [DOI] [PubMed] [Google Scholar]
- 40.Cheng YC, Chen CA, Chang JM, Chen HC. Albumin overload down-regulates integrin-β1 through reactive oxygen species-endoplasmic reticulum stress pathway in podocytes. J Biochem. 2015; 158:101–08. 10.1093/jb/mvv020 [DOI] [PubMed] [Google Scholar]
- 41.Chen ZJ, Chen JX, Wu LK, Li BY, Tian YF, Xian M, Huang ZP, Yu RA. Induction of Endoplasmic Reticulum Stress by Cadmium and Its Regulation on Nrf2 Signaling Pathway in Kidneys of Rats. Biomed Environ Sci. 2019; 32:1–10. 10.3967/bes2019.001 [DOI] [PubMed] [Google Scholar]
- 42.Shu S, Zhu J, Liu Z, Tang C, Cai J, Dong Z. Endoplasmic reticulum stress is activated in post-ischemic kidneys to promote chronic kidney disease. EBioMedicine. 2018; 37:269–80. 10.1016/j.ebiom.2018.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inoue T, Maekawa H, Inagi R. Organelle crosstalk in the kidney. Kidney Int. 2019; 95:1318–25. 10.1016/j.kint.2018.11.035 [DOI] [PubMed] [Google Scholar]
- 44.Cybulsky AV. Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases. Nat Rev Nephrol. 2017; 13:681–96. 10.1038/nrneph.2017.129 [DOI] [PubMed] [Google Scholar]
- 45.Thériault JR, Palmer HJ, Pittman DD. Inhibition of the Unfolded Protein Response by metformin in renal proximal tubular epithelial cells. Biochem Biophys Res Commun. 2011; 409:500–05. 10.1016/j.bbrc.2011.05.034 [DOI] [PubMed] [Google Scholar]
- 46.Lee EK, Jeong JU, Chang JW, Yang WS, Kim SB, Park SK, Park JS, Lee SK. Activation of AMP-activated protein kinase inhibits albumin-induced endoplasmic reticulum stress and apoptosis through inhibition of reactive oxygen species. Nephron, Exp Nephrol. 2012; 121:e38–48. 10.1159/000342802 [DOI] [PubMed] [Google Scholar]
- 47.Allouch S, Munusamy S. Metformin attenuates albumin-induced alterations in renal tubular cells in vitro. J Cell Physiol. 2017; 232:3652–63. 10.1002/jcp.25838 [DOI] [PubMed] [Google Scholar]
- 48.Granato M, Gilardini Montani MS, Romeo MA, Santarelli R, Gonnella R, D’Orazi G, Faggioni A, Cirone M. Metformin triggers apoptosis in PEL cells and alters bortezomib-induced Unfolded Protein Response increasing its cytotoxicity and inhibiting KSHV lytic cycle activation. Cell Signal. 2017; 40:239–47. 10.1016/j.cellsig.2017.09.020 [DOI] [PubMed] [Google Scholar]
- 49.Christensen M, Nørgård MØ, Jensen MS, Møller BK, Nørregaard R. Metformin modulates immune cell infiltration into the kidney during unilateral ureteral obstruction in mice. Physiol Rep. 2019; 7:e14141. 10.14814/phy2.14141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Christensen M, Jensen JB, Jakobsen S, Jessen N, Frøkiær J, Kemp BE, Marciszyn AL, Li H, Pastor-Soler NM, Hallows KR, Nørregaard R. Renoprotective Effects of Metformin are Independent of Organic Cation Transporters 1 &2 and AMP-activated Protein Kinase in the Kidney. Sci Rep. 2016; 6:35952. 10.1038/srep35952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Z, Tang Y, Jin X, Chen C, Lu Y, Liu L, Shen C. Metformin Inhibits Advanced Glycation End Products-Induced Inflammatory Response in Murine Macrophages Partly through AMPK Activation and RAGE/NFκB Pathway Suppression. J Diabetes Res. 2016; 2016:4847812. 10.1155/2016/4847812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gu J, Ye S, Wang S, Sun W, Hu Y. Metformin inhibits nuclear factor-κB activation and inflammatory cytokines expression induced by high glucose via adenosine monophosphate-activated protein kinase activation in rat glomerular mesangial cells in vitro. Chin Med J (Engl). 2014; 127:1755–60. [PubMed] [Google Scholar]
- 53.Izquierdo-Lahuerta A, Martínez-García C, Medina-Gómez G. Lipotoxicity as a trigger factor of renal disease. J Nephrol. 2016; 29:603–10. 10.1007/s40620-016-0278-5 [DOI] [PubMed] [Google Scholar]
- 54.Wang W, Guo XH, Wu HH, Wang NH, Xu XS. [Effect of fenofibrate and metformin on lipotoxicity in OLETF rat kidney]. Beijing Da Xue Xue Bao Yi Xue Ban. 2006; 38:170–75. [PubMed] [Google Scholar]
- 55.Ghadge A, Harsulkar A, Karandikar M, Pandit V, Kuvalekar A. Comparative anti-inflammatory and lipid-normalizing effects of metformin and omega-3 fatty acids through modulation of transcription factors in diabetic rats. Genes Nutr. 2016; 11:10. 10.1186/s12263-016-0518-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim DI, Park MJ, Heo YR, Park SH. Metformin ameliorates lipotoxicity-induced mesangial cell apoptosis partly via upregulation of glucagon like peptide-1 receptor (GLP-1R). Arch Biochem Biophys. 2015; 584:90–97. 10.1016/j.abb.2015.08.009 [DOI] [PubMed] [Google Scholar]
- 57.Nogueira A, Pires MJ, Oliveira PA. Pathophysiological Mechanisms of Renal Fibrosis: A Review of Animal Models and Therapeutic Strategies. In Vivo. 2017; 31:1–22. 10.21873/invivo.11019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016; 12:325–38. 10.1038/nrneph.2016.48 [DOI] [PubMed] [Google Scholar]
- 59.Yi H, Huang C, Shi Y, Cao Q, Zhao Y, Zhang L, Chen J, Pollock CA, Chen XM. Metformin attenuates folic-acid induced renal fibrosis in mice. J Cell Physiol. 2018; 233:7045–54. 10.1002/jcp.26505 [DOI] [PubMed] [Google Scholar]
- 60.Feng Y, Wang S, Zhang Y, Xiao H. Metformin attenuates renal fibrosis in both AMPKα2-dependent and independent manners. Clin Exp Pharmacol Physiol. 2017; 44:648–55. 10.1111/1440-1681.12748 [DOI] [PubMed] [Google Scholar]
- 61.Xiao H, Zhang J, Xu Z, Feng Y, Zhang M, Liu J, Chen R, Shen J, Wu J, Lu Z, Fang X, Li J, Zhang Y. Metformin is a novel suppressor for transforming growth factor (TGF)-β1. Sci Rep. 2016; 6:28597. 10.1038/srep28597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.He J, Xu Y, Koya D, Kanasaki K. Role of the endothelial-to-mesenchymal transition in renal fibrosis of chronic kidney disease. Clin Exp Nephrol. 2013; 17:488–97. 10.1007/s10157-013-0781-0 [DOI] [PubMed] [Google Scholar]
- 63.Xavier S, Vasko R, Matsumoto K, Zullo JA, Chen R, Maizel J, Chander PN, Goligorsky MS. Curtailing endothelial TGF-β signaling is sufficient to reduce endothelial-mesenchymal transition and fibrosis in CKD. J Am Soc Nephrol. 2015; 26:817–29. 10.1681/ASN.2013101137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heldin CH, Landström M, Moustakas A. Mechanism of TGF-beta signaling to growth arrest, apoptosis, and epithelial-mesenchymal transition. Curr Opin Cell Biol. 2009; 21:166–76. 10.1016/j.ceb.2009.01.021 [DOI] [PubMed] [Google Scholar]
- 65.Thakur S, Viswanadhapalli S, Kopp JB, Shi Q, Barnes JL, Block K, Gorin Y, Abboud HE. Activation of AMP-activated protein kinase prevents TGF-β1-induced epithelial-mesenchymal transition and myofibroblast activation. Am J Pathol. 2015; 185:2168–80. 10.1016/j.ajpath.2015.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ohba M, Shibanuma M, Kuroki T, Nose K. Production of hydrogen peroxide by transforming growth factor-beta 1 and its involvement in induction of egr-1 in mouse osteoblastic cells. J Cell Biol. 1994; 126:1079–88. 10.1083/jcb.126.4.1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun S, Ning X, Zhai Y, Du R, Lu Y, He L, Li R, Wu W, Sun W, Wang H. Egr-1 mediates chronic hypoxia-induced renal interstitial fibrosis via the PKC/ERK pathway. Am J Nephrol. 2014; 39:436–48. 10.1159/000362249 [DOI] [PubMed] [Google Scholar]
- 68.Guan M, Li W, Xu L, Zeng Y, Wang D, Zheng Z, Lyv F, Xue Y. Metformin Improves Epithelial-to-Mesenchymal Transition Induced by TGF-β1 in Renal Tubular Epithelial NRK-52E Cells via Inhibiting Egr-1. J Diabetes Res. 2018; 2018:1031367. 10.1155/2018/1031367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu C, Qin N, Ren H, Yang M, Liu S, Wang Q. Metformin Regulating miR-34a Pathway to Inhibit Egr1 in Rat Mesangial Cells Cultured with High Glucose. Int J Endocrinol. 2018; 2018:6462793. 10.1155/2018/6462793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F, Park AS, Tao J, Sharma K, Pullman J, Bottinger EP, Goldberg IJ, Susztak K. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med. 2015; 21:37–46. 10.1038/nm.3762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee M, Katerelos M, Gleich K, Galic S, Kemp BE, Mount PF, Power DA. Phosphorylation of Acetyl-CoA Carboxylase by AMPK Reduces Renal Fibrosis and Is Essential for the Anti-Fibrotic Effect of Metformin. J Am Soc Nephrol. 2018; 29:2326–36. 10.1681/ASN.2018010050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haase VH. The sweet side of HIF. Kidney Int. 2010; 78:10–13. 10.1038/ki.2010.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nayak BK, Shanmugasundaram K, Friedrichs WE, Cavaglierii RC, Patel M, Barnes J, Block K. HIF-1 Mediates Renal Fibrosis in OVE26 Type 1 Diabetic Mice. Diabetes. 2016; 65:1387–97. 10.2337/db15-0519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Isoe T, Makino Y, Mizumoto K, Sakagami H, Fujita Y, Honjo J, Takiyama Y, Itoh H, Haneda M. High glucose activates HIF-1-mediated signal transduction in glomerular mesangial cells through a carbohydrate response element binding protein. Kidney Int. 2010; 78:48–59. 10.1038/ki.2010.99 [DOI] [PubMed] [Google Scholar]
- 75.Luo R, Zhang W, Zhao C, Zhang Y, Wu H, Jin J, Zhang W, Grenz A, Eltzschig HK, Tao L, Kellems RE, Xia Y. Elevated Endothelial Hypoxia-Inducible Factor-1α Contributes to Glomerular Injury and Promotes Hypertensive Chronic Kidney Disease. Hypertension. 2015; 66:75–84. 10.1161/HYPERTENSIONAHA.115.05578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu J, Wang W, Zhang F, Li PL, Boini KM, Yi F, Li N. Hypoxia inducible factor-1α mediates the profibrotic effect of albumin in renal tubular cells. Sci Rep. 2017; 7:15878. 10.1038/s41598-017-15972-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, Eckardt KU, Iwano M, Haase VH. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007; 117:3810–20. 10.1172/JCI30487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baumann B, Hayashida T, Liang X, Schnaper HW. Hypoxia-inducible factor-1α promotes glomerulosclerosis and regulates COL1A2 expression through interactions with Smad3. Kidney Int. 2016; 90:797–808. 10.1016/j.kint.2016.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takiyama Y, Harumi T, Watanabe J, Fujita Y, Honjo J, Shimizu N, Makino Y, Haneda M. Tubular injury in a rat model of type 2 diabetes is prevented by metformin: a possible role of HIF-1α expression and oxygen metabolism. Diabetes. 2011; 60:981–92. 10.2337/db10-0655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kabei K, Tateishi Y, Nozaki M, Tanaka M, Shiota M, Osada-Oka M, Nishide S, Uchida J, Nakatani T, Tomita S, Miura K. Role of hypoxia-inducible factor-1 in the development of renal fibrosis in mouse obstructed kidney: special references to HIF-1 dependent gene expression of profibrogenic molecules. J Pharmacol Sci. 2018; 136:31–38. 10.1016/j.jphs.2017.12.004 [DOI] [PubMed] [Google Scholar]
- 81.Xue J, Wang L, Sun Z, Xing C. Basic Research in Diabetic Nephropathy Health Care: A study of the Renoprotective Mechanism of Metformin. J Med Syst. 2019; 43:266. 10.1007/s10916-019-1412-4 [DOI] [PubMed] [Google Scholar]
- 82.Guo YN, Wang JC, Cai GY, Hu X, Cui SY, Lv Y, Yin Z, Fu B, Hong Q, Chen XM. AMPK-mediated downregulation of connexin43 and premature senescence of mesangial cells under high-glucose conditions. Exp Gerontol. 2014; 51:71–81. 10.1016/j.exger.2013.12.016 [DOI] [PubMed] [Google Scholar]
- 83.Dong D, Cai GY, Ning YC, Wang JC, Lv Y, Hong Q, Cui SY, Fu B, Guo YN, Chen XM. Alleviation of senescence and epithelial-mesenchymal transition in aging kidney by short-term caloric restriction and caloric restriction mimetics via modulation of AMPK/mTOR signaling. Oncotarget. 2017; 8:16109–21. 10.18632/oncotarget.14884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abbott A. First hint that body’s ‘biological age’ can be reversed. Nature. 2019; 573:173. 10.1038/d41586-019-02638-w [DOI] [PubMed] [Google Scholar]
- 85.Bonnet F, Scheen A. Understanding and overcoming metformin gastrointestinal intolerance. Diabetes Obes Metab. 2017; 19:473–81. 10.1111/dom.12854 [DOI] [PubMed] [Google Scholar]
- 86.Cao J, Meng S, Chang E, Beckwith-Fickas K, Xiong L, Cole RN, Radovick S, Wondisford FE, He L. Low concentrations of metformin suppress glucose production in hepatocytes through AMP-activated protein kinase (AMPK). J Biol Chem. 2014; 289:20435–46. 10.1074/jbc.M114.567271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Madiraju AK, Erion DM, Rahimi Y, Zhang XM, Braddock DT, Albright RA, Prigaro BJ, Wood JL, Bhanot S, MacDonald MJ, Jurczak MJ, Camporez JP, Lee HY, et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014; 510:542–46. 10.1038/nature13270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Madiraju AK, Qiu Y, Perry RJ, Rahimi Y, Zhang XM, Zhang D, Camporez JG, Cline GW, Butrico GM, Kemp BE, Casals G, Steinberg GR, Vatner DF, et al. Metformin inhibits gluconeogenesis via a redox-dependent mechanism in vivo. Nat Med. 2018; 24:1384–94. 10.1038/s41591-018-0125-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.DeFronzo R, Fleming GA, Chen K, Bicsak TA. Metformin-associated lactic acidosis: current perspectives on causes and risk. Metabolism. 2016; 65:20–29. 10.1016/j.metabol.2015.10.014 [DOI] [PubMed] [Google Scholar]
- 90.Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA. 2014; 312:2668–75. 10.1001/jama.2014.15298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kajbaf F, Lalau JD. Mortality rate in so-called “metformin-associated lactic acidosis”: a review of the data since the 1960s. Pharmacoepidemiol Drug Saf. 2014; 23:1123–27. 10.1002/pds.3689 [DOI] [PubMed] [Google Scholar]
- 92.Flory JH, Hennessy S, Bailey CJ, Inzucchi SE. Reports of Lactic Acidosis Attributed to Metformin, 2015–2018. Diabetes Care. 2020; 43:244–46. 10.2337/dc19-0923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. US Food and Drug Administration. https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-drug-safety-podcast-fda-revises-warnings-regarding-use-diabetes-medicine-metformin-certain
- 94.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012; 380:756–66. 10.1016/S0140-6736(11)61454-2 [DOI] [PubMed] [Google Scholar]
- 95.Webster AC, Nagler EV, Morton RL, Masson P. Chronic Kidney Disease. Lancet. 2017; 389:1238–52. 10.1016/S0140-6736(16)32064-5 [DOI] [PubMed] [Google Scholar]
- 96.Satriano J, Sharma K, Blantz RC, Deng A. Induction of AMPK activity corrects early pathophysiological alterations in the subtotal nephrectomy model of chronic kidney disease. Am J Physiol Renal Physiol. 2013; 305:F727–33. 10.1152/ajprenal.00293.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Crowley MJ, Diamantidis CJ, McDuffie JR, Cameron CB, Stanifer JW, Mock CK, Wang X, Tang S, Nagi A, Kosinski AS, Williams JW Jr. Clinical Outcomes of Metformin Use in Populations With Chronic Kidney Disease, Congestive Heart Failure, or Chronic Liver Disease: A Systematic Review. Ann Intern Med. 2017; 166:191–200. 10.7326/M16-1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lalau JD, Kajbaf F, Bennis Y, Hurtel-Lemaire AS, Belpaire F, De Broe ME. Metformin Treatment in Patients With Type 2 Diabetes and Chronic Kidney Disease Stages 3A, 3B, or 4. Diabetes Care. 2018; 41:547–53. 10.2337/dc17-2231 [DOI] [PubMed] [Google Scholar]
- 99.Eid AA, Ford BM, Block K, Kasinath BS, Gorin Y, Ghosh-Choudhury G, Barnes JL, Abboud HE. AMP-activated protein kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. J Biol Chem. 2010; 285:37503–12. 10.1074/jbc.M110.136796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Petersen MC, Shulman GI. Mechanisms of Insulin Action and Insulin Resistance. Physiol Rev. 2018; 98:2133–223. 10.1152/physrev.00063.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Welsh GI, Hale LJ, Eremina V, Jeansson M, Maezawa Y, Lennon R, Pons DA, Owen RJ, Satchell SC, Miles MJ, Caunt CJ, McArdle CA, Pavenstädt H, et al. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab. 2010; 12:329–40. 10.1016/j.cmet.2010.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang W, Sun W, Cheng Y, Xu Z, Cai L. Role of sirtuin-1 in diabetic nephropathy. J Mol Med (Berl). 2019; 97:291–309. 10.1007/s00109-019-01743-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rogacka D, Audzeyenka I, Rychłowski M, Rachubik P, Szrejder M, Angielski S, Piwkowska A. Metformin overcomes high glucose-induced insulin resistance of podocytes by pleiotropic effects on SIRT1 and AMPK. Biochim Biophys Acta Mol Basis Dis. 2018; 1864:115–25. 10.1016/j.bbadis.2017.10.014 [DOI] [PubMed] [Google Scholar]
- 104.Christensen M, Schiffer TA, Gustafsson H, Krag SP, Nørregaard R, Palm F. Metformin attenuates renal medullary hypoxia in diabetic nephropathy through inhibition uncoupling protein-2. Diabetes Metab Res Rev. 2019; 35:e3091. 10.1002/dmrr.3091 [DOI] [PubMed] [Google Scholar]
- 105.Hung AM, Roumie CL, Greevy RA, Liu X, Grijalva CG, Murff HJ, Griffin MR. Kidney function decline in metformin versus sulfonylurea initiators: assessment of time-dependent contribution of weight, blood pressure, and glycemic control. Pharmacoepidemiol Drug Saf. 2013; 22:623–31. 10.1002/pds.3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hippisley-Cox J, Coupland C. Diabetes treatments and risk of amputation, blindness, severe kidney failure, hyperglycaemia, and hypoglycaemia: open cohort study in primary care. BMJ. 2016; 352:i1450. 10.1136/bmj.i1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cornec-Le Gall E, Alam A, Perrone RD. Autosomal dominant polycystic kidney disease. Lancet. 2019; 393:919–35. 10.1016/S0140-6736(18)32782-X [DOI] [PubMed] [Google Scholar]
- 108.Padovano V, Podrini C, Boletta A, Caplan MJ. Metabolism and mitochondria in polycystic kidney disease research and therapy. Nat Rev Nephrol. 2018; 14:678–87. 10.1038/s41581-018-0051-1 [DOI] [PubMed] [Google Scholar]
- 109.Rowe I, Chiaravalli M, Mannella V, Ulisse V, Quilici G, Pema M, Song XW, Xu H, Mari S, Qian F, Pei Y, Musco G, Boletta A. Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat Med. 2013; 19:488–93. 10.1038/nm.3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Takiar V, Nishio S, Seo-Mayer P, King JD Jr, Li H, Zhang L, Karihaloo A, Hallows KR, Somlo S, Caplan MJ. Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc Natl Acad Sci USA. 2011; 108:2462–67. 10.1073/pnas.1011498108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhao J, Ma Y, Zhang Y, Fu B, Wu X, Li Q, Cai G, Chen X, Bai XY. Low-dose 2-deoxyglucose and metformin synergically inhibit proliferation of human polycystic kidney cells by modulating glucose metabolism. Cell Death Discov. 2019; 5:76. 10.1038/s41420-019-0156-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lian X, Wu X, Li Z, Zhang Y, Song K, Cai G, Li Q, Lin S, Chen X, Bai XY. The combination of metformin and 2-deoxyglucose significantly inhibits cyst formation in miniature pigs with polycystic kidney disease. Br J Pharmacol. 2019; 176:711–24. 10.1111/bph.14558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pisani A, Riccio E, Bruzzese D, Sabbatini M. Metformin in autosomal dominant polycystic kidney disease: experimental hypothesis or clinical fact? BMC Nephrol. 2018; 19:282. 10.1186/s12882-018-1090-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Capuano I, Riccio E, Caccavallo S, De Simone I, Pisani A. ADPKD and metformin: from bench to bedside. Clin Exp Nephrol. 2019; 23:1341–42. 10.1007/s10157-019-01770-1 [DOI] [PubMed] [Google Scholar]
- 115.Leonhard WN, Song X, Kanhai AA, Iliuta IA, Bozovic A, Steinberg GR, Peters DJ, Pei Y. Salsalate, but not metformin or canagliflozin, slows kidney cyst growth in an adult-onset mouse model of polycystic kidney disease. EBioMedicine. 2019; 47:436–45. 10.1016/j.ebiom.2019.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee SY, Moon SJ, Kim EK, Seo HB, Yang EJ, Son HJ, Kim JK, Min JK, Park SH, Cho ML. Metformin Suppresses Systemic Autoimmunity in Mice through Inhibiting B Cell Differentiation into Plasma Cells via Regulation of AMPK/mTOR/STAT3. J Immunol. 2017; 198:2661–70. 10.4049/jimmunol.1403088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang H, Li T, Chen S, Gu Y, Ye S. Neutrophil Extracellular Trap Mitochondrial DNA and Its Autoantibody in Systemic Lupus Erythematosus and a Proof-of-Concept Trial of Metformin. Arthritis Rheumatol. 2015; 67:3190–200. 10.1002/art.39296 [DOI] [PubMed] [Google Scholar]
- 118.Yin Y, Choi SC, Xu Z, Perry DJ, Seay H, Croker BP, Sobel ES, Brusko TM, Morel L. Normalization of CD4+ T cell metabolism reverses lupus. Sci Transl Med. 2015; 7:274ra18. 10.1126/scitranslmed.aaa0835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sachdeva K, Jana BRP, Curti B. Renal Cell Carcinoma. Medscape. 2014. https://emedicine.medscape.com/article/281340-overview#showall.
- 120.Martinez-Outschoorn UE, Peiris-Pagés M, Pestell RG, Sotgia F, Lisanti MP. Cancer metabolism: a therapeutic perspective. Nat Rev Clin Oncol. 2017; 14:11–31. 10.1038/nrclinonc.2016.60 [DOI] [PubMed] [Google Scholar]
- 121.Massari F, Ciccarese C, Santoni M, Brunelli M, Piva F, Modena A, Bimbatti D, Fantinel E, Santini D, Cheng L, Cascinu S, Montironi R, Tortora G. Metabolic alterations in renal cell carcinoma. Cancer Treat Rev. 2015; 41:767–76. 10.1016/j.ctrv.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 122.Jang JH, Song IH, Sung EG, Lee TJ, Kim JY. Metformin-induced apoptosis facilitates degradation of the cellular caspase 8 (FLICE)-like inhibitory protein through a caspase-dependent pathway in human renal cell carcinoma A498 cells. Oncol Lett. 2018; 16:2030–38. 10.3892/ol.2018.8832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu J, Li M, Song B, Jia C, Zhang L, Bai X, Hu W. Metformin inhibits renal cell carcinoma in vitro and in vivo xenograft. Urol Oncol. 2013; 31:264–70. 10.1016/j.urolonc.2011.01.003 [DOI] [PubMed] [Google Scholar]
- 124.Xie W, Wang L, Sheng H, Qiu J, Zhang D, Zhang L, Yang F, Tang D, Zhang K. Metformin Induces Growth Inhibition and Cell Cycle Arrest by Upregulating MicroRNA34a in Renal Cancer Cells. Med Sci Monit. 2017; 23:29–37. 10.12659/MSM.898710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang FQ, Wang JJ, Yan JS, Huang JH, Li W, Che JP, Wang GC, Liu M, Zheng JH. Metformin inhibits cell growth by upregulating microRNA-26a in renal cancer cells. Int J Clin Exp Med. 2014; 7:3289–96. [PMC free article] [PubMed] [Google Scholar]
- 126.Kalogirou C, Schäfer D, Krebs M, Kurz F, Schneider A, Riedmiller H, Kneitz B, Vergho D. Metformin-Derived Growth Inhibition in Renal Cell Carcinoma Depends on miR-21-Mediated PTEN Expression. Urol Int. 2016; 96:106–15. 10.1159/000441011 [DOI] [PubMed] [Google Scholar]
- 127.Pasha M, Sivaraman SK, Frantz R, Agouni A, Munusamy S. Metformin Induces Different Responses in Clear Cell Renal Cell Carcinoma Caki Cell Lines. Biomolecules. 2019; 9:9. 10.3390/biom9030113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu M, Zhang Z, Wang H, Chen X, Jin C. Activation of AMPK by metformin promotes renal cancer cell proliferation under glucose deprivation through its interaction with PKM2. Int J Biol Sci. 2019; 15:617–27. 10.7150/ijbs.29689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li Y, Hu L, Xia Q, Yuan Y, Mi Y. The impact of metformin use on survival in kidney cancer patients with diabetes: a meta-analysis. Int Urol Nephrol. 2017; 49:975–81. 10.1007/s11255-017-1548-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cheng JJ, Li H, Tan HS, Tan PH, Ng LG, Ng QS, Toh CK, Kanesvaran R, Tan MH. Metformin Use in Relation With Survival Outcomes of Patients With Renal Cell Carcinoma. Clin Genitourin Cancer. 2016; 14:168–75. 10.1016/j.clgc.2015.12.012 [DOI] [PubMed] [Google Scholar]
- 131.Nayan M, Finelli A, Jewett MA, Juurlink DN, Austin PC, Kulkarni GS, Hamilton RJ. Metformin Use and Kidney Cancer Outcomes in Patients With Diabetes: A Propensity Score Analysis. Clin Genitourin Cancer. 2017; 15:300–05. 10.1016/j.clgc.2016.06.008 [DOI] [PubMed] [Google Scholar]
- 132.Lee J, Yesilkanal AE, Wynne JP, Frankenberger C, Liu J, Yan J, Elbaz M, Rabe DC, Rustandy FD, Tiwari P, Grossman EA, Hart PC, Kang C, et al. Effective breast cancer combination therapy targeting BACH1 and mitochondrial metabolism. Nature. 2019; 568:254–58. 10.1038/s41586-019-1005-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vest LS, Koraishy FM, Zhang Z, Lam NN, Schnitzler MA, Dharnidharka VR, Axelrod D, Naik AS, Alhamad TA, Kasiske BL, Hess GP, Lentine KL. Metformin use in the first year after kidney transplant, correlates, and associated outcomes in diabetic transplant recipients: A retrospective analysis of integrated registry and pharmacy claims data. Clin Transplant. 2018; 32:e13302. 10.1111/ctr.13302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kurian B, Joshi R, Helmuth A. Effectiveness and long-term safety of thiazolidinediones and metformin in renal transplant recipients. Endocr Pract. 2008; 14:979–84. 10.4158/EP.14.8.979 [DOI] [PubMed] [Google Scholar]
- 135.Alnasrallah B, Goh TL, Chan LW, Manley P, Pilmore H. Transplantation and diabetes (Transdiab): a pilot randomised controlled trial of metformin in impaired glucose tolerance after kidney transplantation. BMC Nephrol. 2019; 20:147. 10.1186/s12882-019-1321-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Alnasrallah B, Pilmore H, Manley P. Protocol for a pilot randomised controlled trial of metformin in pre-diabetes after kidney transplantation: the Transplantation and Diabetes (Transdiab) study. BMJ Open. 2017; 7:e016813. 10.1136/bmjopen-2017-016813 [DOI] [PMC free article] [PubMed] [Google Scholar]