Abstract
Chemically defined serum-free media are increasingly used as a tool to help standardize experiments by eliminating the potential variability contributed by pooled serum. These media are formulated for the culture and expansion of specific cell types, maintaining cell viability without the need for exogenous animal proteins. Formulated serum-free media could thus help improve viability and reduce variability during sample preparation for flow cytometry, yet a thorough analysis of how such media impact fluorochrome-Ab conjugates has not been performed. In this study, we expose fluorescent Ab-labeled cells or Ab capture beads to white light in the presence of various hematopoietic cell culture media and provide evidence that formulated serum-free media permit rapid light-initiated fluorescent dye degradation in a cell-independent manner. We observed fluorescence signal loss of several dyes, which included fluorescence spillover into adjacent detectors. Finally, photostability of Ab-fluorochrome conjugates in formulated serum-free media is partially restored in the presence of either serum or vitamin C, implicating reactive oxygen species in the observed signal loss. Thus, our data indicate that formulated serum-free media designed to standardize cell culture are not currently optimized for use with fluorochrome–Ab conjugates, and thus, extreme caution should be exercised when using these media in cytometric experiments.
Cell culture using chemically defined serum-free media represents a standardized and scientifically accepted alternative to the use of pooled animal sera (1–3). Immunological studies commonly use formulated serum-free media to ensure consistent culture and expansion of hematopoietic cells, such as primary human lymphocytes and chimeric Ag receptor T cells (4–7). Varieties of formulated serum-free media are available commercially for many immune cell types, each including defined lot-to-lot combinations of amino acids, recombinant proteins, growth factors, and inorganics. Although the development and use of formulated serum-free media aims to limit technical variability in experiments, the impact of such media on Ab-conjugated fluorescent dye stability has not been well studied.
Great lengths have been taken to standardize cytometry sample preparation (8) and the efficient conjugation and proper handling of fluorescently labeled Abs (9). Although the properties of a dye are largely unchanged following Ab conjugation (10), tandem dyes, which rely on fluorescence resonance energy transfer (FRET) between donor and acceptor fluorophores, are susceptible to oxidation, leading to less efficient FRET (11). For example, indotricarbocyanine (Cy7)-conjugated PE and allophycocyanin fluorophores (12) may degrade in response to light, oxygen, or tissue fixation chemicals (11,13,14), resulting in the loss of tandem dye signal intensity and introducing additional spillover into the detector of the donor fluorophore. A quantitative assessment of PE tandem dyes in staining buffer revealed that increasing white light exposure resulted in proportionally elevated fluorochrome degradation, with the largest impact observed on Cy7 conjugates (11). The same study found a small but appreciable tandem dye signal loss during long-term temperature- and light-controlled storage, yet overall, shielding tandem dyes from light improved their long-term stability (13). Photon-induced fluorochrome degradation is thus a well-known issue; however, an analysis of flow cytometry Ab conjugate stability in formulated serum-free media has not been performed.
In this study, we evaluated fluorescent Ab photostability in formulated serum-free media compared with traditional flow cytometry buffers. Our data demonstrate that serum-free media permit rapid light-induced degradation of fluorochromes in a cell-independent manner, whereas the addition of serum or vitamin C limit fluorescence signal loss. Thus, although formulated serum-free media can standardize cell culture and reduce experimental variability, we find these media in their existing formulations are unreliable for use during flow cytometry because of their negative impact on fluorochrome photostability following even brief exposure to light.
MATERIALS AND METHODS
Human tissue and cell isolation
All human samples were obtained upon written informed consent at the Virginia Mason Medical Center (Seattle, WA). All studies were approved by the Institutional Review Board of the Benaroya Research Institute (Seattle, WA). PBMC were isolated using Ficoll-Paque (GE Healthcare, Chicago, IL) gradient separation. CD4+ T cells were enriched using CD4 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany).
Media and reagents
The following chemically defined or partially defined serum-free media were used: X-Vivo15 Hematopoietic Cell Medium (X-Vivo15) (Lonza, Bend, OR), Macrophage-SFM (Thermo Fisher Scientific, Waltham, MA), AIM V Serum-Free Medium (AIM V) (Thermo Fisher Scientific); and ImmunoCult-XF T Cell Expansion Medium (ImmunoCult-XF) (STEMCELL Technologies, Vancouver, BC, Canada). Control comparisons were performed using RPMI 1640 medium with l-glutamine and phenol red (Thermo Fisher Scientific) or 1× calcium- and magnesium-free PBS (Sigma Aldrich, St. Louis, MO).
Abs and labeling
Human leukocytes were stained with fluorescently labeled Abs diluted in staining buffer made up of 1× PBS and 3% FBS for 15–20 min at 37°C in the dark. Ab capture beads, UltraComp eBeads (Thermo Fisher Scientific), were stained in the dark according to manufacturer instructions using 1× PBS. For fully stained human samples, single color controls were generated using UltraComp eBeads and PBMC labeled with eBioscience Fixable Viability Dye eFluor (ef) 780 (Thermo Fisher Scientific). The following fluorescently conjugated Ab clones were used in this study: CD4–Brilliant Violet (BV) 421RPA-4, CCR7-BV605 G043H7, CD103-BV605 BerACT8, CCR6-BV650 G034E3, CD4-BV650 OKT4, CD4-BV711 OKT4, CD4-BV785 OKT4, CD4–Alexa Fluor 488 (AF488) RPA-T4, CD4-FITC SK3, CCR4-PerCP/Cy5.5 L291H4, CD3-PE HIT3a, CD4-PE/Dazzle594 OKT4, CD25-PE/Cy5 BC96, CCR6-PE/Cy7 G034E3, CD8α-AF647 SK1, anti-mouse CD45.2-Alexa Fluor 700 (AF700) 104, CD45RA-AF700 HI100, CD4-AF700 OKT4, CD4-allophycocyanin/Cy7 OKT4, and CD45RA-allophycocyanin/Cy7 HI100 (BioLegend, San Diego, CA); CD45-BUV395 H130, CD28-BUV737 CD28.2, CXCR3-BV421 1C6, CD3-v450 UCHT1, CD4-BV510 SK3, CD8α-v50, RPA-T8, CD127-BV786 HIL7RM21, CD4-PE/CF594 RPA-T4, CD127-PE/Cy7 HIL7RM21, and CD45-allophycocyanin/H7 2D1 (BD Biosciences, San Jose, CA); CD4-PerCP/ef710 SK3, CD103-PE/Cy7 B-Ly7, and CD45RA-allophycocyanin/ef780 HI100 (Thermo Fisher Scientific); and CCR10-PE 314305 (R&D Systems, Minneapolis, MN).
Strategy for modulating and measuring fluorescent signal loss
After staining, fluorochrome–Ab–labeled human T cells, PBMC, or Ab capture beads were resuspended in the indicated media or buffers. Fluorescent measurements of stained samples were recorded in the dark immediately. The same samples were then exposed to either of two sources of white light: 1) the two manufacturer-installed, user-controlled, light-emitting diodes (LED) contained within the sample injection chamber of a FACSAria II or FACSAria Fusion cell sorter or 2) using ambient fluorescent light (28 W, 40 inch fluorescent tubes; Sylvania) on the laboratory benchtop (20–25°C) for a duration of up to 1 h. The fluorescence of samples exposed to the cell sorter LEDs was measured continuously in real-time during acquisition, for a duration of 3–7 min with 100 rpm used for sample agitation. Where indicated, HyClone FBS or vitamin C reagent grade l-ascorbic acid (Sigma-Aldrich) were added to the media after stainingbut before exposure to light to determine their effects on fluorescent signal loss.
Fluorescent signal was assessed by measuring the change in mean fluorescence intensity (MFI) over the course of sample acquisition and light exposure and by monitoring increased spillover signal into the adjacent detectors for which no staining was performed.
Data acquisition and analysis
Data acquisition was performed as indicated using either a four-laser FACSAria II cell sorter (no UV laser), a FACSAria Fusion cell sorter, or a BD LSRII analytical cytometer (BD Biosciences) each equipped with five lasers: UV (355 nm), violet (405 nm), blue (488 nm), yellow-green (561 nm), and red (637 nm). Cytometer calibration and control fluorescent measurements were performed using SPHERO eight-peak Rainbow Calibration Particles (Spherotech, Lake Forest, IL). Compensation was calculated using FACS DIVA Software (BD Biosciences) acquisition-defined matrices. Data were analyzed with FlowJo 10 (TreeStar, Ashland, OR).
Statistical analysis
Significance was determined using an ordinary one-way ANOVA with the Dunnett posttest for multiple comparisons calculated using Prism 8.0 software (GraphPad).
RESULTS
Light-dependent fluorescence signal loss in defined serum-free media can occur rapidly to select dyes in a cell-independent manner
Many published immunological studies use specially formulated serum-free media to promote standardized hematopoietic cell culture and expansion (4–7), but a thorough characterization of the interaction between these media and Ab-fluorophore conjugates has not been performed. We therefore examined how different formulated serum-free media might impact the signal stability of fluorescent Ab-labeled cells.
We first labeled freshly isolated human CD4+ T cells with a staining panel designed to discriminate CD4+ naive and memory Thelper subsets. Fluorescently labeled CD4+ T cells were resuspended in formulated serum-free hematopoietic media, X-Vivo15, and their fluorescence intensity was recorded immediately in the dark (Fig. 1A, t = 0 m). These cells were then exposed to the LED within the sample injection chamber of the cell sorter for a minimum of 5 min, and fluorescence intensity was recorded again (Fig. 1A, t = 5 m). All analyses were performed on live cells, indicated by an absence of signal for fixable amine-reactive dyes (data not shown). We observed an ~10-fold loss of staining intensity for CD45RA–AF700. CD127-PE/Cy7 signal loss was similar and included a reciprocal increase of signal into the detector for the PE donor fluorophore, which greatly impacted Thelper subset discrimination. Other dyes, such as the polymers BV605 and BV650, remained intact following exposure to the LED. These data suggest that formulated serum-free media reduce the photostability of select fluorochrome–Ab conjugates, even in response to abbreviated light exposure.
FIGURE 1. Rapid light-initiated, cell-independent fluorochrome degradation in formulated serum-free media.
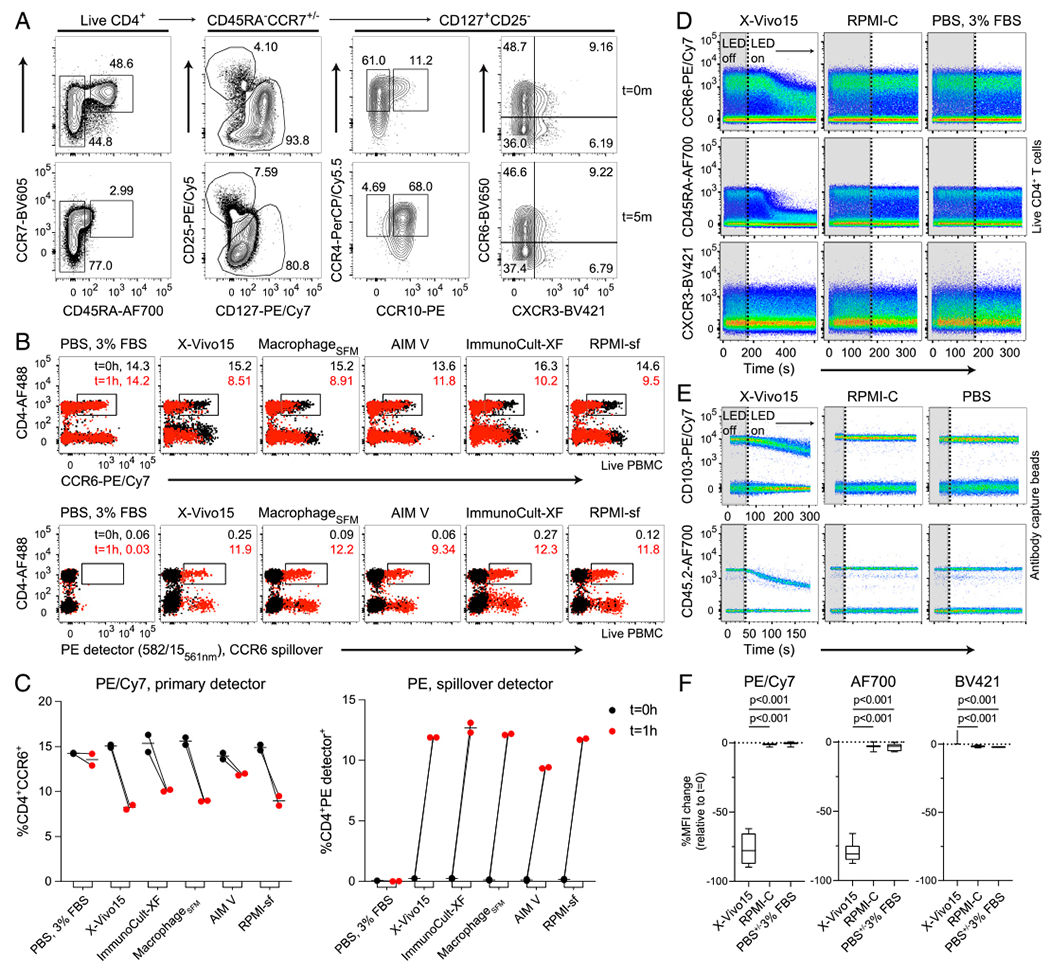
(A) Fluorescence measurements of labeled CD4+ T cells resuspended in formulated serum-free medium, X-Vivo15, either before (t = 0 m) or after exposure to the LEDs (t = 5 m) in the cell sorter sample injection chamber. Cells gated as indicated inside each plot. (B and C) Fluorescence measurements of labeled, live gated PBMC resuspended in the indicated media were taken before (t = 0 h, black dots) or after (t = 1 h, red dots) exposure to ambient fluorescent light on the benchtop. The gated frequency for each timepoint is shown. Simultaneous measurements were performed for (top) CCR6-PE/Cy7 into its primary detector and (bottom) spillover into the PE detector. The bandpass filter and laser line of the spillover detector are indicated. (C) A graphical summary of the data showing changes to CCR6-PE/Cy7 signal and concomitant spillover into the PE detector across both timepoints. (D–F) Real-time fluorochrome photostability of either single-labeled (D) CD4+ T cells or (E) Ab capture beads was compared across the indicated media or buffer. Surface protein expression was measured first briefly in the dark (LED off, gray boxes ending in vertical dotted line) and then in response to light (LED on). (F) A statistical comparison of the percentage of MFI change following up to 5 min of light exposure is shown for pooled cells and Ab capture beads. Data are representative of at least two independent experiments. Significance was determined by ordinary one-way ANOVA with Dunnett posttest for multiple comparisons.
We next asked whether serum-free hematopoietic media formulated for use with different immune cell populations also impact dye photostability. For this, CCR6-PE/Cy7 and CD4-AF488 dual-labeled PBMC were resuspended in either staining buffer made of PBS–FBS, one of four distinct formulated serum-free media, or serum-free RPMI. PE/Cy7 and AF488 fluorescence intensity as well as CCR6-PE/Cy7 spillover signal into the PE detector (Fig. 1B, t = 0 h) were measured immediately in the dark using an analytical cytometer. These cells were not stained with PE conjugates. Labeled cells were then exposed to ambient light on the benchtop for 1 h at room temperature. Measurements were then repeated for PE/Cy7 and spillover into its donor fluorophore detector, PE (Fig. 1B, t = 1 h). When cells were kept in staining buffer during 1 h of fluorescent light exposure, signal intensity remained constant for CCR6-PE/Cy7 and CD4–AF488, with no spillover signal increase in the PE detector. In contrast, PE/Cy7 photostability was reduced by varying degrees depending on whether Ab-labeled cells were kept in either serum-free X-Vivo15, Macrophage-SFM, AIM V, ImmunoCult-XF, or serum-free RPMI (Fig. 1B, 1C). In some serum-free media, for example, AIM V, tandem dye signal spillover into the adjacent PE detector was extensive, despite a limited change in CCR6-PE/Cy7+ signal (Fig. 1B, 1C), indicating that increased donor fluorophore signal could occur in the absence of much noticeable loss of FRET. These data demonstrate that formulated serum-free media permitted light-induced fluorochrome–Ab signal loss to a subset of tested dyes, including substantial spillover into adjacent detectors.
We next wanted to assess the rate of light-initiated fluorescence loss in formulated serum-free media. Fluorescence measurements of stained human T cells were recorded in real-time on a cell sorter, first for 3 min in the dark, followed by an additional 3–7 min of LED exposure in the sample injection chamber. We observed no signal loss on labeled T cells during the dark period; however, drastic reductions to the signal of PE/Cy7 and AF700 conjugates were detected within 60 s of LED exposure in formulated serum-free X-Vivo15 media (Fig. 1D). By comparison, BV421 conjugates were unaffected by light.
Tandem dye degradation can be impacted by metabolic processes and cell viability (14). We thus wanted to test whether the fluorescence signal loss we observed (Fig. 1A–C) was cell dependent. To do this, we measured the fluorescence stability of single-stained Ab capture beads in real time for up to 1 min in the dark, followed by 3–5 min of LED exposure in the cell sorter as described above. CD103-PE/Cy7– and CD45.2–AF700–labeled beads remained photostable in the dark, but a rapid signal reduction of both conjugates was observed after LED exposure (Fig. 1E). Whereas PE/Cy7 and AF700 conjugates were susceptible to light-mediated degradation in formulated serum-free medium compared with photostable BV421 conjugates, all dyes were photostable in complete RPMI containing serum or in PBS, which was evident using either stained cells or Ab capture beads (Fig 1F). To investigate photostability over the full visible spectrum, we single labeled Ab capture beads with 25 different fluorescent Ab conjugates, resuspended them in X-Vivo15 serum-free media, and tested dye photostability in real time (Fig. 2A). We observed varying degrees of light-induced signal loss to several tandem dyes including CCR6-PE/Cy7, as well as conjugates of allophycocyanin/Cy7, BV785, and BV786, whereas base fluorophores, such as PE, allophycocyanin, and BV421, were largely photostable. In addition, tandem dyes previously reported to be susceptible to light-mediated degradation in other systems such as PE/Cy5 (11) were unaffected in our experimental conditions. Unexpectedly, conjugates of AF700, which is not a tandem dye, were highly susceptible to light-induced signal loss in formulated serum-free media, as indicated by decreasing MFI signal over time (Fig. 2B). These data collectively demonstrate that photostability of fluorescent Ab-labeled samples is reduced in formulated serum-free media. This phenomenon is cell independent and impacts a select range of both tandem and base fluorophores.
FIGURE 2. Broadly assessing fluorochrome photostability in formulated serum-free media.
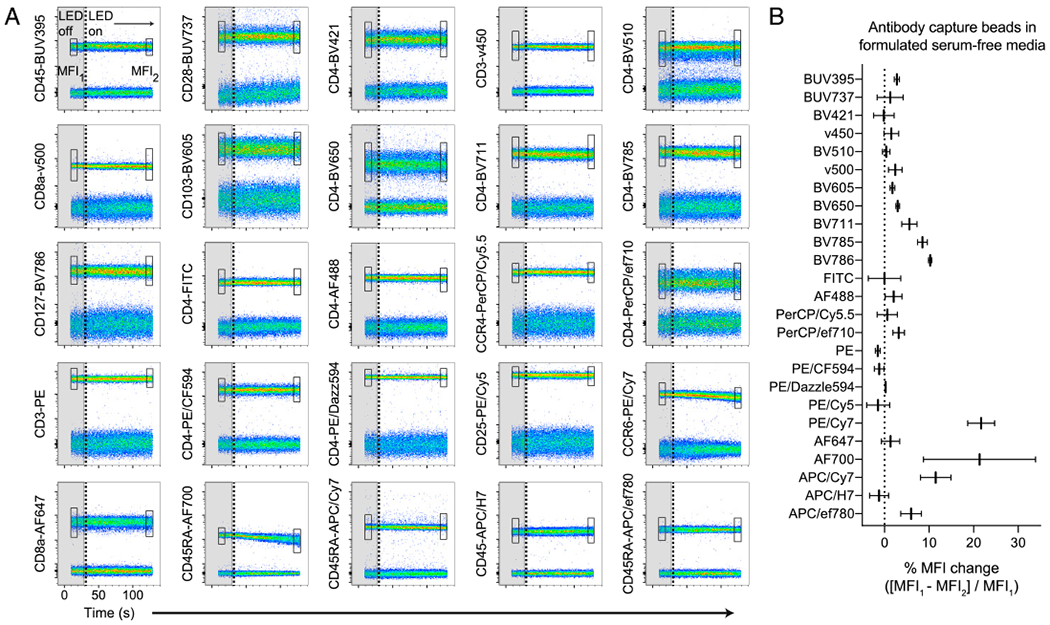
(A) Ab capture beads single stained with each of the 25 indicated Ab–dye conjugates were resuspended in X-Vivo15 serum-free media. Real-time fluorochrome photostability was measured, first briefly in the dark (LED off, gray boxes ending in vertical dotted line) and then in response to light (LED on). The MFI of the positively stained bead population was calculated for the beginning (box MFI1) and end (box MFI2) of the time course. (B) Graph of the percentage of MFI change calculated from (A), following 2–3 min of light exposure. Data are representative of at least two independent experiments.
Serum and vitamin C can limit light-induced fluorochrome degradation in formulated serum-free media
Our data indicate that fluorescent Ab conjugates are photostable in complete RPMI containing serum (Fig. 1D–F), whereas a previously published study demonstrated that vitamin C could prevent allophycocyanin tandem dye signal loss on stained cells (14). To test the impact of serum and vitamin C on cell-independent fluorescence signal loss, we generated single-stained Ab capture beads using PE/Cy7, allophycocyanin/Cy7, or AF700 conjugates. Stained beads were placed in formulated serum-free media alone or in the presence of either 1 mM vitamin C or 3% FBS and exposed to ambient light on the benchtop for 1 h at room temperature. Following light exposure, we observed fluorescence signal loss of all tested Ab conjugates and substantial spillover into the base fluorophore detectors for PE/Cy7 and allophycocyanin/Cy7 dyes when in formulated serum-free medium alone (Fig. 3A, 3B). Signal loss and concomitant spillover was reduced in the presence of vitamin C or FBS, although the degree to which these media additives increased photostability varied among dyes. We were unable to detect AF700 spillover into any other fluorescence detectors, despite marked signal loss in the primary detector (Fig. 3B).
FIGURE 3. Serum and vitamin C limit light-initiated fluorochrome degradation in formulated serum-free media.
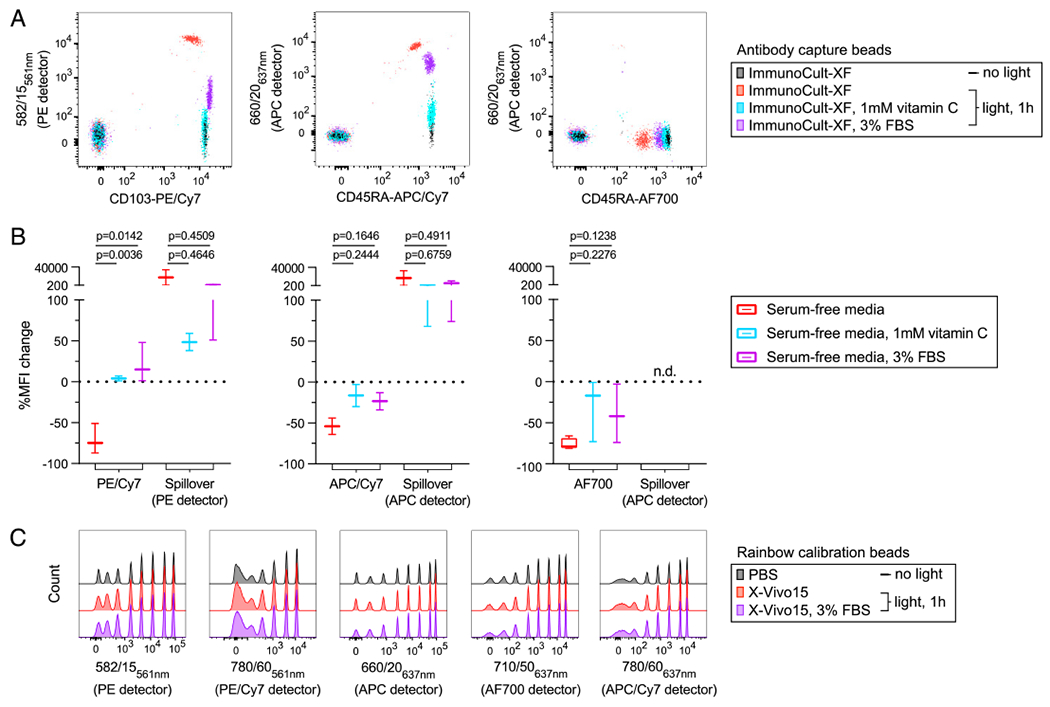
(A and B) Ab capture beads single stained with the indicated conjugates were resuspended in formulated serum-free media, either ImmunoCult-XF or X-Vivo15. (A) Fluorescence signal and spillover into adjacent detectors was measured immediately (no light) and again following exposure to ambient fluorescent light (light, 1 h) with or without either vitamin C or FBS. (B) A statistical comparison of the percentage of MFI change following light exposure is shown for data pooled from stained cells and Ab capture beads resuspended in either ImmunoCult-XF or X-Vivo15 serum-free media. (C) Rainbow calibration eight-peak beads were placed in PBS or formulated serum-free media, X-Vivo15 with or without FBS. Fluorescence signal was measured before and after light exposure as in (A) and for the same five detectors. Detector bandpass filter and laser line are shown. Significance was determined by ordinary one-way ANOVA with Dunnett posttest for multiple comparisons. Data are representative of at least two independent experiments. n.d., not detectable.
To rule out that the observed fluorescence signal changes were not artifacts of formulated serum-free media, Ab lot, or the light source used and to confirm normal junction of our cytometers, we measured the fluorescence signal of eight-peak rainbow calibration beads resuspended in PBS or serum-free media. Because the calibration beads include highly stable fluorescent particles contained within the beads themselves, they should be immune to light-dependent fluorescence decay. Consistent with this notion, rainbow calibration particles were unaffected by exposure to 1 h of ambient fluorescent light, irrespective of the buffer or media used or the presence of serum (Fig. 3C).
DISCUSSION
Chemically defined serum-free media help standardize cell culture, circumventing lot-to-lot variability inherent to pooled human or animal serum. Cell type–specific formulations of serum-free media are now commonly used to permit immune cell culture without sacrificing cell viability or junction. For example, these media have become a critical tool for the culture and expansion of chimeric Ag receptor–T cells (6, 7). Cytometry core facilities and technical manuals on cell sorting suggest using buffered, serum-supplemented media to help maintain cell viability during the sorting and collection of live stained cells (15), but few, if any, manufacturer guidelines or publications address the use of formulated serum-free media for these procedures. Our study thus aimed to characterize the stability of fluorescent Abs in formulated serum-free media.
Fluorescent Ab conjugates are susceptible to signal loss from light exposure or reactive oxygen species (ROS) (11, 14). In this study, we found that white light caused rapid signal loss in serum-free media to Cy7 and select BV tandem dyes as well as the nontandem AF700 (Fig. 1). Other tandem conjugates such as Cy5, which are subject to photostability issues, were not impacted under our tested conditions. Our data suggest this fluorescence signal loss is cell independent as it occurs not only to stained cells (Fig. 1A–D) but also to labeled Ab capture beads (Figs. 1E, 2). Importantly, signal loss occurred even after brief periods of light exposure that might be encountered during normal sample preparation and handling.
Not all tandem dyes behaved similarly when exposed to light in formulated serum-free media. For example, Ab capture beads labeled with the polymer tandems BV605 and BV650 were more photostable in serum-free media when compared with BV785/6 (Fig. 2). The reason for this variable dye stability remains unclear but could be connected to differences in their specific chemistry. Surprisingly, we found rapid and substantial light-induced signal loss of multiple AF700 conjugates despite the known photostability of the Alexa Fluor family of dyes (16). No other Alexa Fluor dyes we tested exhibited this effect. It remains unclear why AF700, which is not a tandem dye, was so strongly impacted by light in formulated serum-free media. Our understanding of how sample handling can impact fluorochrome degradation will be aided by the advent of spectral cytometers, which can measure the full fluorescence profile of dyes instead of only instrument-specific bandpass filters.
Two recent studies showed that cell sorting can induce oxidative stress, altering the basal metabolic state of cells (17), and that the presence of photo-sensitive molecules within standard serum-supplemented cell culture medium could drive morphological changes or cell death via oxidative stress (18). In each case, the addition of serum or antioxidants reduced measurable cell stress. We observed that by using AIM V medium, there was only a marginal loss of PE/Cy7 FRET efficiency compared with other serum-free formulations, although extensive spillover into the PE detector remained. AIM V contains human serum albumin, a protein component similar to serum, with noted antioxidant capacity (19), which could explain the small photostability increase we observed in this media. However, as the commercially manufactured media we used contain proprietary preparations, it is unclear if other formulations tested contain human serum albumin, and thus, it is difficult to resolve the noted difference in signal loss.
Vitamin C can also reduce oxidative stress by scavenging free oxygen radicals and is sufficient to prevent cell-dependent allophycocyanin tandem dye degradation (14). We find that RPMI containing serum maintains the photostability of stained cells (Fig. 1D–F), and supplementing formulated serum-free media with serum or vitamin C can reduce but does not eliminate signal loss and spillover into adjacent detectors (Fig. 3A, 3B). Although we did not test for the presence of ROS in our experiments, the effect of vitamin C we observe, and its published role as a scavenger of these compounds (14), suggests a light-dependent mechanism through which components of formulated serum-free media are transformed to promote fluorochrome degradation. Although we did not test cell viability after photodegradation occurred, we demonstrate that signal loss in this study is cell independent, suggesting that any deleterious role for ROS persists independently of the effects on cell viability. Further study is required to validate and dissect these mechanisms and, if necessary, address the matter of light-induced oxidative stress in serum-free media. However, care must be taken in the selection and addition of new components to sorting media. For example, vitamin C has been shown to interact with components of cell culture to produce detrimental byproducts (20). Other chemicals can also protect against oxidative stress, including selenium and 2-ME (1), although in our hands, the addition of 2-ME to X-Vivo15 did not prevent light-induced fluorescence signal loss (data not shown). Additional work is required to identify specific additives that can safely and effectively address the issue of light-dependent fluorophore degradation in formulated serum-free media.
Collectively, our findings suggest that chemically defined and formulated serum-free media in their current manufactured forms do not sufficiently support the photostability of fluorochrome–Ab conjugates. We conclude that these media should be avoided during cytometric experiments as even supplementing with serum did not fully maintain dye photostability. Even sorting cells into formulated serum-free media could prove problematic as some sorters are equipped with lights near the collection tubes that cannot be turned off by the user and could thus impact the reliability of postsort purity checks. Future modification of formulated serum-free media to support dye photostability would help reduce the variability of flow cytometry sample preparation and collection by eliminating the need for pooled serum and other undefined additives that could impact downstream readouts.
ACKNOWLEDGMENTS
We thank Jessica Hamerman, Sabine Spath, and Liliane Khoryati for helpful discussion and review of the manuscript.
This work was supported by National Institutes of Health Grant 5R01AI127726-02.
P.A.M. and D.J.C. designed the experiments. P.A.M. developed the methods and wrote the manuscript. P.A.M. and S.J.M. performed experiments. P.A.M., S.J.M., and D.J.C. reviewed and edited the manuscript. D.J.C. supervised the project.
Abbreviations used in this article:
- AF488
Alexa Fluor 488
- AF700
Alexa Fluor 700
- AIM V
AIM V Serum-Free Medium
- BV
Brilliant Violet
- Cy7
indotricarbocyanine
- ef
eFluor
- FRET
fluorescence resonance energy transfer
- ImmunoCult-XF
ImmunoCult-XF T Cell Expansion Medium
- LED
light-emitting diode
- MFI
mean fluorescence intensity
- ROS
reactive oxygen species
- X-Vivo15
X-Vivo15 Hematopoietic Cell Medium
Footnotes
DISCLOSURES
The authors have no financial conflicts of interest.
REFERENCES
- 1.van der Valk J, Brunner D, De Smet K, Fex Svenningsen A, Honegger P, Knudsen LE, Lindl T, Noraberg J, Price A, Scarino ML, and Gstraunthaler G. 2010. Optimization of chemically defined cell culture media-replacing fetal bovine serum in mammalian in vitro methods. Toxicol. In Vitro 24: 1053–1063. [DOI] [PubMed] [Google Scholar]
- 2.Landecker H 2016. It is what it eats: chemically defined media and the history of surrounds. Stud Hist Philos Biol Biomed Sci 57: 148–160. [DOI] [PubMed] [Google Scholar]
- 3.Gstraunthaler G 2003. Alternatives to the use of fetal bovine serum: serum-free cell culture. ALTEX 20: 275–281. [PubMed] [Google Scholar]
- 4.Cerezo M, Guemiri R, Druillennec S, Girault I, Malka-Mahieu H, Shen S, Allard D, Martineau S, Welsch C, Agoussi S, et al. 2018. Translational control of tumor immune escape via the eIF4F-STAT1-PD-L1 axis in melanoma. Nat. Med 24: 1877–1886. [DOI] [PubMed] [Google Scholar]
- 5.James JR 2018. Tuning ITAM multiplicity on T cell receptors can control potency and selectivity to ligand density. Sci Signal 11: eaan1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brentjens RJ, Rivière I, Park JH, Davila ML, Wang X, Stefanski J, Taylor C, Yeh R, Bartido S, Borquez-Ojeda O, et al. 2011. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 118: 4817–4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moon EK, Carpenito C, Sun J, Wang L-CS, Kapoor V, Predina J, Powell DJ Jr., Riley JL, June CH, and Albelda SM. 2011. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin. Cancer Res 17: 4719–4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Owens MA, Vall HG, Hurley AA, and Wormsley SB. 2000. Validation and quality control of immunophenotyping in clinical flow cytometry. J. Immunol. Methods 243: 33–50. [DOI] [PubMed] [Google Scholar]
- 9.Maecker HT, Frey T, Nomura LE, and Trotter J. 2004. Selecting fluorochrome conjugates for maximum sensitivity. Cytometry A 62: 169–173. [DOI] [PubMed] [Google Scholar]
- 10.Schauenstein K, Schauenstein E, and Wick G. 1978. Fluorescence properties of free and protein bound fluorescein dyes. I. Macro-spectrofluorometric measurements. J. Histochem. Cytochem 26: 277–283. [DOI] [PubMed] [Google Scholar]
- 11.Hulspas R, Dombkowski D, Preffer F, Douglas D, Kildew-Shah B, and Gilbert J. 2009. Flow cytometry and the stability of phycoerythrin-tandem dye conjugates. Cytometry A 75: 966–972. [DOI] [PubMed] [Google Scholar]
- 12.Roederer M, Kantor AB, Parks DR, and Herzenberg LA. 1996. Cy7PE and Cy7APC: bright new probes for immunofluorescence. Cytometry 24: 191–197. [DOI] [PubMed] [Google Scholar]
- 13.Forman MA, and Gupta RK. 2007. Tandem dyes for flow cytometry: can we overcome quality concerns? MLO Med. Lab. Obs 39: 24, 26. [PubMed] [Google Scholar]
- 14.Le Roy C, Varin-Blank N, Ajchenbaum-Cymbalista F, and Letestu R. 2009. Flow cytometry APC-tandem dyes are degraded through a cell-dependent mechanism. Cytometry A 75: 882–890. [DOI] [PubMed] [Google Scholar]
- 15.Houtz B, Trotter J, and Sasaki D. 2004. BD FACService TECHNOTES. 9: 3. [Google Scholar]
- 16.Panchuk-Voloshina N, Haugland RP, Bishop-Stewart J, Bhalgat MK, Millard PJ, Mao F, Leung WY, and Haugland RP. 1999. Alexa dyes, a series of new fluorescent dyes that yield exceptionally bright, photostable conjugates. J. Histochem. Cytochem 47: 1179–1188. [DOI] [PubMed] [Google Scholar]
- 17.Llufrio EM, Wang L, Naser FJ, and Patti GJ. 2018. Sorting cells alters their redox state and cellular metabolome. Redox Biol. 16: 381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stockley JH, Evans K, Matthey M, Volbracht K, Agathou S, Mukanowa J, Burrone J, and Karadottir RT. 2017. Surpassing light-induced cell damage in vitro with novel cell culture media. Sci. Rep 7: 849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taverna M, Marie A-L, Mira J-P, and Guidet B. 2013. Specific antioxidant properties of human serum albumin. Ann. Intensive Care 3: 4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chumsae C, Hossler P, Raharimampionona H, Zhou Y, McDermott S, Racicot C, Radziejewski C, and Zhou ZS. 2015. When good intentions go awry: modification of a recombinant monoclonal antibody in chemically defined cell culture by xylosone, an oxidative product of ascorbic acid. Anal. Chem 87: 7529–7534. [DOI] [PubMed] [Google Scholar]


