Abstract
Grapevine leafroll-associated virus 3 (GLRaV-3) is one of the most important viruses of grapevine but, despite this, there remain several gaps in our understanding of its biology. Because of its narrow host range - limited to Vitis species - and because the virus is restricted to the phloem, most GLRaV-3 research has concentrated on epidemiology and the development of detection assays. The recent discovery that GLRaV-3 can infect Nicotiana benthamiana, a plant model organism, makes new opportunities available for research in this field. We used RNA-seq to compare both V. vinifera and P1/HC-Pro N. benthamiana host responses to GLRaV-3 infection. Our analysis revealed that the majority of DEGs observed between the two hosts were unique although responses between the two hosts also showed several shared gene expression results. When comparing gene expression patterns that were shared between the two hosts, we observed the downregulation of genes associated with stress chaperones, and the induction of gene families involved in primary plant physiological processes. This is the first analysis of gene expression profiles beyond Vitis to mealybug-transmitted GLRaV-3 and demonstrates that N. benthamiana could serve as a useful tool for future studies of GLRaV-3-host interactions.
Subject terms: Microbiology, Plant sciences
Introduction
One of the gaps in our knowledge of plant virology remains our understanding of how virus infection affects whole plants physiologically and biochemically, especially at the cellular level. Viruses are biotrophs, and plants respond with a highly polymorphic innate immune response to infection1. Determining how the plant responds to virus infection and which sets of genes are differentially expressed represents a first step to better understanding the mechanisms behind the regulatory pathways involved2. High throughput sequencing (HTS) based research has been increasingly carried out with model as well as in non-model plants for gene expression studies to aid in understanding host and virus responses during infection cycles3. Model organisms are indispensable to scientific progress because they are well studied and provide a biological setting to undertake experiments when ethics, costs, and technical difficulties can otherwise impair research. In the context of understanding virus infection in plants and in determining the usefulness of a model organism for future work, a critical comparison is of host response to virus infection between its original non-model host and a novel potential model organism.
Grapevine leafroll-associated virus 3 (GLRaV-3) was previously thought to be limited to Vitis species, but was recently demonstrated also to infect Nicotiana benthamiana4. GLRaV-3, a ssRNA virus within the family Closteroviridae, is regarded as the most important agent of grapevine leafroll disease (GLD) that results in substantial economic losses (20–40%) to the wine, table grape, raisin, and nursery industries5. The virus is transmitted primarily by phloem-sap sucking mealybugs (Hemiptera, Pseudococcidae) in a semi-persistent manner 6. It infects the phloem tissues of both V. vinifera and N. benthamiana hosts4,6. The finding that GLRaV-3 infects N. benthamiana provides advantages of working with this model organism when comparing time from infection to detection, relative ease of virion purification, as well as visualization of viral particles and virion structural proteins. Importantly, for N. benthamiana to be used as a model host for GLRaV-3 research it must share key responses with the natural grapevine host4,7.
Plant viruses cause significant changes in host gene expression in response to infection8. In recent years, RNA-sequencing technology has progressed rapidly, providing a more sensitive method to detect low-abundance host gene expression changes due to stresses induced by viral infection than previously observed using microarray technologies9,10. RNA-seq has quickly become the preferred tool for gene expression analyses in important model hosts like N. benthamiana. This popular experimental host has become an indispensable tool in plant virology because of its susceptibility to infection by a large number of diverse plant viruses, perhaps because of a naturally occurring mutation in an RNA-dependent RNA polymerase gene7. This, combined with the recent release of the draft genome sequence for N. benthamiana, has made it a particularly useful for host–pathogen studies focused on innate immunity and defense signaling, protein localization and interactions, and a system for protein expression and purification research11,12.
In contrast, gene expression studies in V. vinifera in response to viral infection are relatively limited. Most RNA-sequencing work on V. vinifera as a host has focused on differential gene expression analysis during berry development and other developmental stages of plant growth3,13. HTS has also been adapted for detection of virus infection in V. vinifera14,15. Transcriptome analyses and differential expression profiles of small RNAs associated with GLRaV-3 infection in grapes was recently found16. It is our hope that together with these studies, the gene expression profiles in response to GLRaV-3 infection in two distinct hosts will help gain a better understanding of host-pathogen interactions of GLD.
We used RNA-seq to analyze the gene expression profiles of N. benthamiana and V. vinifera responses to GLRaV-3 infection. This is a first assessment of how a novel host, other than V. vinifera, responds to GLRaV-3 infection. Responses between the two hosts show several shared gene expression results. This, together with the small number of shared gene expression differences, demonstrates that N. benthamiana could serve as a useful tool for future studies of GLRaV-3-host interactions.
Results
RNAseq data were mapped to the respective N. benthamiana or V. vinifera genome and the results of that mapping assessed (Table 1). In all cases, the majority of sequenced reads (54–78%) mapped to the appropriate genome, although a proportion of reads mapped to multiple loci probably because of repeats or gene families. A substantial proportion of reads (22–46%) did not map to the genome. To determine whether RNA from other species had been included in the RNAseq data, analysis of ribosomal RNA was conducted, which demonstrated that cross-contamination between other organisms was not a major source of unmapped reads, and poor-quality sequence was removed as part of pre-processing.
Table 1.
Mapping of RNA sequences to Nicotiana benthamiana and Vitis vinifera.
| Species | Sample | Total reads | % mapped (uniquely) | % unmapped |
|---|---|---|---|---|
| V. vinifera | Healthy | 17.5 Mbp | 71 (60) | 29 |
| Infected-1 | 43.3 Mbp | 74 (67) | 26 | |
| Infected-2 | 6.5 Mbp | 54 (46) | 46 | |
| N. benthamiana | Healthy-1 | 52.7 Mbp | 78 (66) | 22 |
| Healthy-2 | 49.4 Mbp | 76 (64) | 24 | |
| Infected | 6.6 Mbp | 63 (53) | 37 |
Some sequencing libraries produced substantially less sequence than others, because of the difficulty of obtaining high quality RNA. Furthermore, not all treatments had replicates. However, differential gene expression analysis was performed using edgeR, with appropriate normalization, allowing for statistically valid gene expression comparisons. To determine if other viruses were present in our plant samples, we mapped reads from our sample sequences against a custom library of grapevine-infecting virus species and viroids (summarized in Supplementary Table S5). Interestingly, we observed a co-infection of GLRaV-3 with grapevine virus A in the infected N. benthamiana sample used in this study.
V. vinifera response to GLRaV-3 infection
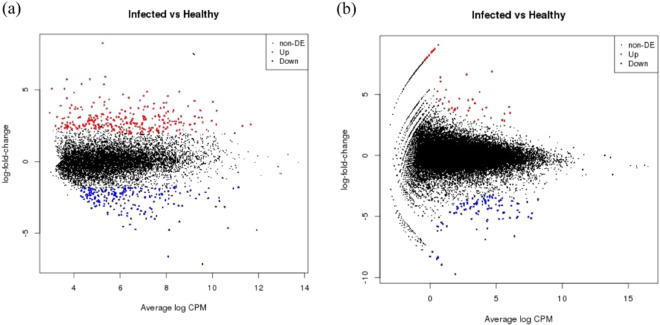
In our analyses, 494 genes were differentially expressed in response to GRLaV-3 infection in V. vinifera (Fig. 1a, Supplementary Table S1). Of these differentially expressed genes (DEGs), 222 were downregulated and 272 were upregulated. Additionally, 44 genes were shown to be stably expressed when comparing infected versus healthy plants. Gene ontology (GO) analysis of the most commonly observed DEGs, revealed metabolic processes (biological process), binding activity (molecular function), and membrane and integral part of membrane (cellular component) to be the most common processes differentially expressed (Fig. 2a).
Figure 1.
MA-plot of differential expression for (a) Vitis vinifera and (b) Nicotiana benthamiana, M-values, i.e. the log of the ratio of level counts for each gene between two samples against A-values. A-values, i.e. the average level counts for each gene across the two samples. (a) GLRaV-3 infected V. vinifera versus healthy V. vinifera. (b) GLRaV-3 infected N. benthamiana versus healthy N. benthamiana. Figure key: Non-DE, UP, and Down refer to Non-differentially expressed genes (black), Up regulated genes (red), and Down regulated genes (blue), respectively.
Figure 2.
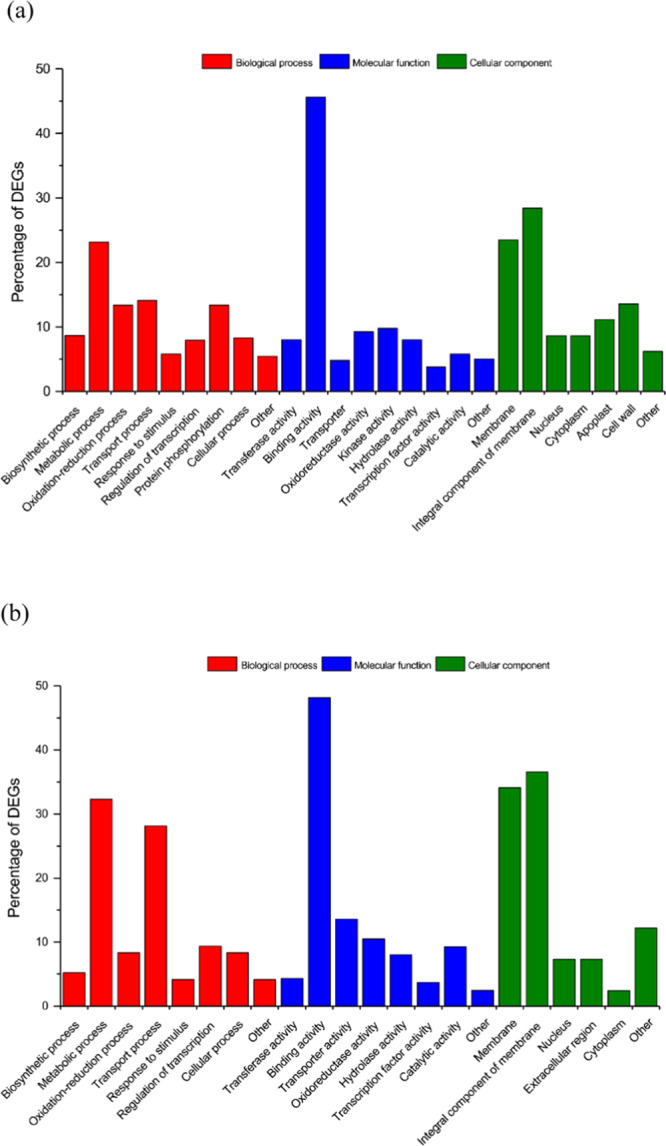
Functional categorization of up- and downregulated genes in (a) Vitis vinifera and (b) Nicotiana benthamiana in response to GLRaV-3 infection based on gene ontology annotations. For both V. vinifera and N. benthamiana, the differentially expressed genes (DEGs) were primarily related to “binding activity” molecular functions, mediate metabolic processes, and associated with the cellular membrane components.
N. benthamiana response to GLRaV-3 infection
In the herbaceous host, 157 genes were shown to be differentially expressed (Fig. 1b, Supplementary Table S1), of these, 107 were downregulated and 49 were upregulated, while 28 genes were stably expressed. In comparison to the trends observed in V. vinifera GO analysis, metabolic and transport processes were common biological processes observed, binding activity was a common molecular function, followed by membrane and integral part of membrane (cellular component) as the most common differentially expressed processes (Fig. 2b).
Comparison of differential gene expression between N. benthamiana and V. vinifera
To determine if N. benthamiana could serve as a suitable model organism for this host-pathogen system, we determined how similarly it responded to GLRaV-3 infection compared with V. vinifera. General families of DEGs common in both hosts also followed similar expression changes, with six upregulated, seven downregulated, and two stably expressed genes in common (summarized in Table 2 with specific gene ID, transcript accumulation summarized in Supplementary Table S2). Only 1% of the shared DEGs observed in both hosts showed up- or downregulation patterns that were different from each other (Table 3). Shared genes were categorized into general groups and annotated with functions associated with expression based on literature searches. Several DEGs were shared but present in up (n = 10), down (n = 13), and/or stable (n = 3) categories concurrently (Supplementary Table S3).
Table 2.
Summary of differentially expressed genes in common between Nicotiana benthamiana and Vitis vinifera.
| Gene Name | Gene Function | Reference |
|---|---|---|
| Upregulated | ||
| NAD(P)H-quinone oxidoreductase | Protect organisms from oxidative stress | Heyno et al.49 |
| Subtilisin-like serine protease | Immune response | Figueiredo et al.50 |
| Sugar transporter protein | Sugar transport, drought response | Yıldırım et al.33 |
| U-box domain-containing protein | Degradation of aberrant proteins induced by stress | Azevedo et al.51 |
| Beta-glucosidase | Initiate cell division | Brzobohaty et al.52 |
| MLP-like protein | Drought tolerance | Wang et al.53 |
| Downregulated | ||
| BAG family molecular chaperone regulator | Regulate apoptosis-like processes | Doukhanina et al.54 |
| Chaperone protein | Thermotolerance, protein disaggregation | Lee et al.55 |
| Copper chaperone | Intracellular delivery of copper to target proteins | Harrison et al.56 |
| Ethylene-responsive transcription factor | Transcription regulation | Fujimoto et al.57 |
| Heat shock cognate 70 kDa | molecular chaperone, non-covalent folding/unfolding | Chen et al.58 |
| Homeobox-leucine zipper protein | Drought tolerance | Lee & Chun59 |
| Peptidyl-prolyl cis-trans isomerase | Thermotolerance, pH stabilization, biotic stress | Pogorelko et al.60 |
| Stable | ||
| 60 S ribosomal protein | Defense against viral infection | Carvalho et al.61 |
| Eukaryotic translation initiation factor | Abiotic stress tolerance | Gallino et al.62 |
Table 3.
Summary of shared differentially expressed genes with different expression levels between Nicotiana benthamiana and Vitis vinifera.
| Transcript ID | Gene Name | log2 FC | Gene Description | Reference |
|---|---|---|---|---|
| GSVIVG01007719001 | Glutaredoxin | 2.925504 | Regulate cellular processes through dithiol–disulfide exchanges with many target proteins | Rouhier et al.21 |
| GSVIVG01020614001 | Glutaredoxin-C9 | 2.931628 | ||
| Niben101Scf06504g01026 | Glutaredoxin family protein | −4.23343 | ||
| GSVIVG01019824001 | RHOMBOID-like protein 2 | 3.539202 | Catalyzes intramembrane proteolysis. May function in pollen elongation, signaling, development, apoptosis, and mitochondrial integrity | Knopf & Adam22 |
| Niben101Scf18513g00008 | RHOMBOID-like protein 14 | −3.99537 | ||
| Niben101Scf03770g02001 | RHOMBOID-like protein 14 | −3.39187 | ||
| GSVIVG01009928001 | Thaumatin-like protein 1b | −2.069703 | Involved in anti-fungal response | Vigers et al.20 |
| Niben101Scf01400g00014 | Thaumatin-like protein | 3.664741 | ||
| GSVIVG01026054001 | UDP-glycosyltransferase 88F3 | 5.085967 | Biosyntheses of cell-wall polysaccharides, the addition of N-linked glycans to glycoproteins, attachment of sugar moieties to various small molecules | Keegstra & Raikhel23 |
| Niben101Scf02751g02006 | UDP-Glycosyltransferase superfamily | −8.9506 | ||
| Niben101Scf04875g02008 | UDP-Glycosyltransferase superfamily | −5.80191 | ||
| Niben101Scf06112g01008 | UDP-Glycosyltransferase superfamily | −4.73772 |
RT-qPCR validation of selected genes
Validation of differentially expressed genes was performed by RT-qPCR. In total, eleven DEGs (seven grapevine and four N. benthamiana) were verified. Based on the transcriptome data, selection of genes whose expression did not vary between healthy and GLRaV-3-infected plants were used as references in RT-qPCR. All gene expression levels were consistent with the results of the transcriptome analysis for both hosts (Supplementary Fig. S1). Four of the grapevine genes (V8856.2, V8031, V6014, V9000) were consistently downregulated, while three grapevine genes (V2636, V1578, V9187) showed upregulation consistent with the transcriptome results. Of the four N. benthamiana genes verified, two genes (B1018, B2016) showed downregulation while the other two genes (B0002, B0006) showed upregulation consistent with the transcriptome analysis.
Discussion
The discovery that GLRaV-3 could infect the model host N. benthamiana is an important finding for a research field where studies have been limited by a difficult host-pathogen system4. In this work, we compared the host responses between GLRaV-3-infected V. vinifera and P1/HC-Pro N. benthamiana to determine if gene expression profiles were similar. These are also the first gene expression data for GLRaV-3 infection in a host outside of V. vinifera.
Reads that did not map to either the V. vinifera or N. benthamiana genomes could be a result of the presence of RNA from other sources, e.g. RNA from GLRaV-3, other viruses, bacteria or fungi, or may be a consequence of poor quality sequence, or sequences that are correct but not present in the genome. The rRNA analysis to detect contamination from other organisms showed this as only a small contributor to unmatched reads. Therefore, it is likely that most unmapped reads do not map to the respective genome because that part of the genome is missing or is significantly different.
Data showed transcriptional changes in both GLRaV-3-infected vs healthy V. vinifera and infected vs healthy N. benthamiana. We observed 494 DEGs in infected V. vinifera compared with healthy plants. In contrast, only 157 DEGs were observed in GLRaV-3-infected N. benthamiana. Several variables could explain the increase in the number of DEGs expressed in V. vinifera compared with N. benthamiana. One limitation is the lack of a well-annotated genome for N. benthamiana relative to the more extensively annotated V. vinifera genome. As future research continues to improve available annotations for the N. benthamiana genome, more DEGs could be uncovered that were missed in our analysis. In other gene expression studies on N. benthamiana response to virus infection, smaller numbers of DEGs have been associated with infection of virus-resistant plant varieties as well as infected hosts with less severe observable symptoms17–19. In GLRaV-3-infected N. benthamiana, no clear symptoms were observed that could be attributed to GLRaV-3 infection when petioles were collected for analysis at two months post-inoculation. The smaller number of DEGs in N. benthamiana could be attributed to a lack of severe plant responses, as has been previously described in other virus infections in this host17–19. In addition, our results provide a limited snapshot of plant host response to GLRaV-3 infection taken during one timepoint. Future studies looking at multiple timepoints of virus infection as well as an increase in replicates used for sequencing could help to decipher this trend.
To determine if N. benthamiana could serve as a suitable model host for future studies, we wanted to determine how similar it responded to GLRaV-3 infection compared with V. vinifera. This also meant determining if there were any major differences in expression profiles between the two hosts. The majority of DEGs observed between the two hosts were unique. It should be noted that the comparison between hosts was limited by replication number available for sequencing. Additionally, P1/HC-Pro N. benthamiana was used in these studies because it was relatively easier to infect with GLRaV-3 than wild-type tobacco4. It is likely that the expression of a silencing suppressor interferes with how GLRaV-3 establishes infection and our results reflect the host response from the transgenic host. The infected N. benthamiana was co-infected with GLRaV-3 with grapevine virus A. It is possible that the co-infection also impacted host gene regulation. Among the shared DEGs between the two hosts, only 1% in N. benthamiana showed up- or downregulation that was different from those observed in V. vinifera. Only one of these, thaumatin-like protein, which was downregulated in V. vinifera and upregulated in N. benthamiana, is found to be associated with response to biotic stress and in this case is thought to be antifungal20. The other three gene families detected - glutaredoxin, RHOMBOID-like protein, and UDP-glycosyltransferase - are involved in other plant cellular processes21–23. This small number of genes observed to have differing gene expression, and the fact that only one appears to be related to biotic stress, is encouraging for future work to determine the use of N. benthamiana as a model host for this system.
Response to GLRaV-3 infection resulted in a common set of genes being differentially expressed in both hosts. When comparing gene expression patterns that were shared between the two hosts, two outcomes were observed: (i) the downregulation of genes associated with stress chaperones, and (ii) the induction of gene families involved in primary plant physiological processes. We observed a shared pattern of downregulated genes associated with drought stress. Among these downregulated genes were molecular chaperones and specifically HSP70, which is part of a larger group of heat shock proteins. Chaperone synthesis is a common aspect of plant virus infection, and the induction of biotic and abiotic stress response genes can be associated with exposure to other various stressors, including thermal changes, heavy metal accumulation, pH variation, and hypoxia19,24–26. Our samples used for sequencing were collected at two months post-inoculation when virus infection would have been well established throughout the plant. It is possible that downregulation of genes associated with a stress response could be the result of a successful long-term GLRaV-3 infection, as a strategy to escape plant defenses. The downregulation of stress-related genes has also been described in prunus necrotic ringspot ilarvirus infection of N. benthamiana, where mild symptoms were observed in comparison with responses to other viruses infecting this host19. The lack of observable symptoms associated with GLRaV-3 infection at two months post-inoculation in both hosts could perhaps be attributed to the escape of plant stress responses.
The shared downregulation of HSP70s observed in both hosts is another example of a molecular chaperone commonly associated with host response to stress and viral infection being repressed. Recently, it has been shown that heat shock proteins are associated with regulation of the plant immune response, and HSP70 as well as other chaperones have been shown to be transiently up- or downregulated in a dynamic manner through different stages of viral infection27–29. Many plant and animal viruses recruit host cellular HSP70s for replication, and cytoplasmic HSP70 has also been shown to enhance infection of N. benthamiana by tobacco mosaic virus, potato virus X, and watermelon mosaic virus29. Although HSP70s appear to be an important factor for plant-virus infections, viruses do not typically encode their own HSP70s and must rely on their availability in an infected host30. In contrast, viruses from the family Closteroviridae encode their own homologs of HSP70s (HSP70h)30. For GLRaV-3, HSP70h is thought to be associated with cell-to-cell movement and virion assembly31. Because GLRaV-3 encodes its own customized HSP70h, it does not have to rely on the host to provide this protein, thus explaining a possible model for the downregulation of these genes in N. benthamiana and V. vinifera. This could also be another strategy used by the virus to avoid host stress responses.
Both V. vinifera and N. benthamiana share upregulation of a few genes associated with general plant or cellular processes, such as sugar transport and ubiquitination proteins. The induction of U-box domain-containing proteins in both hosts could be interpreted as a mechanism to cope with GLRaV-3 infection. The initiation of plant immune responses requires ubiquitination for positive and negative regulation, and is also involved in hormone signaling required for cellular integration of biotic stress cues32. Recent studies have also demonstrated that ubiquitination-associated proteins are targeted by pathogen virulence effectors, emphasizing their importance in immunity32.
Another commonly upregulated family of proteins was associated with sugar transport. This group of proteins is associated with drought stress as well as cellular sugar transport33. Accumulation of soluble sugars, decreased photosynthesis and increased respiration have been linked to virus infection in plants34. Previous work has also demonstrated that GLRaV-3 infection induces genes related to sugar metabolism, such as sugar transporters and glycosyl transferases35. In GLRaV-3 infection of grapevines, it is common to observe symptoms like leaf curling that are associated with decreased photosynthesis36. This is thought to be due to the movement and accumulation of sugar to the roots of the plant. Since GLRaV-3 affects sugar accumulation37, it is therefore not surprising to see an upregulation of sugar transport proteins in hosts infected by GLRaV-3. The upregulation of genes involved in this pathway in N. benthamiana demonstrates that the virus also has an effect on sugar transport in the herbaceous host. Future experiments should measure the sugar content of roots in GLRaV-3-infected N. benthamiana.
N. benthamiana has already shown several promising advantages over V. vinifera to serve as a model organism, including a shortened time from infection to detection, relative ease of virion purifications, as well as visualization of viral particles and structural proteins4. Model systems are essential to the propagation of knowledge when ethics, costs, and technical difficulties can be impediments to experiments38,39. In addition, N. benthamiana is an herbaceous plant, capable of being grown in greenhouse conditions year around, compared with V. vinifera, a deciduous, woody host, where transmission experiments are limited by growing season. Ease of genetic transformations methods and use for virus-induced gene silencing or transient protein expression, make N. benthamiana a popular choice as a tool in plant biology7. Further experiments are needed to increase efficiencies in the initial virus transmission experiments to wild-type and/or transgenic N. benthamiana to allow for comparisons with non-transgenic plants and with larger sample sizes.
In conclusion, our results indicate that N. benthamiana and V. vinifera show a promising degree of similar gene expression patterns in response to GLRaV-3 infection, although many of the DEGs observed were unique to each respective host. As research on genome annotation of both organisms progresses, we expect further interesting insights into host response to GLRaV-3 infection in the future. To help to continue our understanding of this disease, future work could isolate proteins of interest for reverse genetic experiments to test for roles in viral pathogenesis and to provide insights into signaling pathways that are affected by GLRaV-3 infection.
Materials and Methods
Plants used for the analysis
Planococcus ficus (Hemiptera, Pseudococcidae) colonies were maintained on butternut squash (Cucurbita moschata) at 22 °C, with a 16:8-h photoperiod. First instar mealybugs were used for all experiments because they were shown to be the most efficient life stages for transmitting GLRaV-36. Wet Whatman filter papers were placed on top of mealybug colonies. After 30 min the papers were pinned to GLRaV-3 source vine cuttings (accession LR101 (cv. Italia-3), group I) provided by Foundation Plant Services, University of California Davis, CA. After a 24-h acquisition access period (AAP), approximately 20 first instars were transferred manually with a small paintbrush to either healthy V. vinifera (cv. Cabernet Sauvignon) or to N. benthamiana expressing the turnip mosaic virus P1/HC-Pro, kindly supplied by B. Falk (University of California, Davis). Transgenic tobacco were used because transmission efficiencies to wildtype tobacco were very low and previously published studies showed increased numbers of P1/HC-Pro N. benthamiana plants infected (n = 11 total over the course of experiments) when compared to wildtype N. benthamiana (n = 1)4. After 4 days, any visible mealybugs were removed and plants were sprayed with insecticide before being moved to the greenhouse. To test for GLRaV-3 infection, petiole samples were collected from plants at two months post-inoculation and RNA extractions were completed on 100 mg of petiole tissue40. One-step reverse transcription-polymerase chain reaction (RT-PCR) was then performed and PCR products were analyzed using fragment analysis as described previously40. At two months post inoculation for this experiment, petioles from four non-infected V. vinifera, four GLRaV-3-infected V. vinifera, three non-infected N. benthamiana, and four GLRaV-3-infected N. benthamiana were collected for RNA extractions and HTS submission.
RNA extractions
0.1 g of petioles from a known GLRaV-3-infected N. benthamiana plant and 0.1 g petioles from the original GLRaV-3 source V. vinifera were used for next generation sequencing. For RNA extractions, petioles were ground in liquid nitrogen and added to 5 mL of Guanidine extraction buffer (4 M Guanidine thiocyanate, 0.2 M sodium acetate, 25 mM EDTA, 2.5% polyvinylpyrrolidone-40) and 1% beta-mercaptoethanol. 20% sarcosyl buffer was added followed by vigorous mixing and incubation in a 57 °C water bath for 12 minutes, vortexing every 3 minutes for better lysis efficiency. The extract was then added to QIAshredder columns (Qiagen) and the remainder of the protocol was followed according to Qiagen RNeasy Plant Mini Kit instructions as previously described41. RNA concentration and quality were evaluated by measuring the absorbance at 260 nm and the absorbance ratio 260/280 with a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, USA) and stored at −80 °C until further analysis.
RNASeq library preparation and sequencing
Sequencing libraries were constructed at the Functional Genomics Lab (FGL), a QB3-Berkeley Core Research Facility (University of California, Berkeley). Quality of RNA was checked on a 2100 Bioanalyzer (Agilent Technologies, CA, USA). The library preparation was done using RiboZero and Apollo 324 with PrepX RNAseq Library Prep Kits (WaferGen Biosystems, Fremont, CA) and 15 cycles of PCR amplification was used for index addition and library fragment enrichment. RNA samples with >RIN 8 were selected for sequencing. From this selection, one healthy V. vinifera, two GRLaV-3-infected V. vinifera, two healthy N. benthamiana, and one GLRaV-3-infected samples were sequenced.
Genomic sequencing (150 bp paired-end) was done using the Illumina platform (Illumina, Inc., CA, USA) at Vincent J. Coates Genomics Sequencing Laboratory at University of California, Berkeley. The raw data are publicly available as NCBI BioSample accession numbers: SAMN10753963, SAMN10753964, SAMN10753965, SAMN10753966, SAMN10753967, SAMN10753968. To determine if viruses other than GLRaV-3 were present in our samples, trimmed sequences (as described below) against a custom library of 57 grapevine virus species (included representatives of each GLRaV-3 genotype known to-date) and four viroids found in grapevine42. Briefly, trimmed reads were aligned to a grapevine or N. benthamiana genome and the unaligned reads were mapped against the virus and viroid library using Bowtie2 ver. 2.2.943.
Quality assessment and pre-processing
Sequence data were assessed for overall quality using FastQC (0.11.7) and MultiQC (1.5), before and after every pre-processing step. Ribosomal RNA was removed using SortMeRNA (2.1), and the samples checked for the presence of contaminant ribosomal RNA. Sequences were trimmed by quality and Illumina adaptors removed using Trimmomatic (0.36). This workflow (including MultiQC reports) is described in full in the github repository https://github.com/PlantandFoodResearch/bioinf_Vitis_Nicotiana_RNAseq (Refer to Jupyter notebook and MultiQC report files: 01_RNAseq_Quality_Control_Vitis_Nicotiana-Manuscript.ipynb).
Reference-based gene expression
Gene expression in both hosts was assessed in reference to existing genomes and annotations. In N. benthamiana, the Niben 1.0.1 genome and annotations were obtained from solgenomics.net (https://solgenomics.net/organism/Nicotiana_benthamiana/genome)44. For V. vinifera, the Genoscope 12X genome and annotations were obtained from Genoscope (www.genoscope.cns.fr/externe/GenomeBrowser/Vitis/). For both species, pre-processed RNAseq reads were mapped to the appropriate genome using STAR (2.6.1a), deriving raw read counts for each annotated gene in each sample. These workflows are described in full (Refer to Jupyter notebook, Files 02a_RNAseq_Alignment_to_Reference_Nicotinia_benthamiana-Manuscript.ipynb, 02b_RNAseq_Alignment_to_Reference_Vitis_vinifera-Manuscript.ipynb).
Differential gene expression
Differential gene expression between healthy and infected samples was assessed for both species using edgeR45. Briefly, read counts are normalized for library size using the trimmed mean of m-values45, genes that showed very low abundance were removed, and differential expression assessed using both the likelihood ratio and quasi-likelihood F-test methods. These workflows are described in full (Refer to R markdown notebooks, Files GRLaV_Nb_EdgeR.Rmd, GRLaV_Vv_EdgeR.Rmd).
Quantitative RT-PCR
A total of 11 DEGs (seven V. vinifera and four N. benthamiana genes), and four stable expressed genes (two per plant host) identified by RNA-Seq were selected for validating differential expression analysis by RT-qPCR (Supplementary Table S4). Primers were designed using GenScript Real-time PCR (TaqMan) Primer Design online tool (https://www.genscript.com/tools/real-time-pcr-tagman-primer-design-tool) requiring one primer pair to cross an exon-exon junction. The OligoAnalyzer 3.1 program (Integrated DNA Technologies, Coralville, USA) was used to analyze the likelihood of prospective primers generating secondary structures.
Total RNAs from petioles from four non-infected V. vinifera, four GLRaV-3-infected V. vinifera, three non-infected N. benthamiana, and four GLRaV-3-infected N. benthamiana were collected for RNA extractions and isolated using a modified Qiagen kit protocol, described above. Amplicons were synthesized using two-step qualitative RT-PCR (RT-qPCR), with Superscript III Reverse Transcriptase (Invitrogen, Carlsbad, CA) used to synthesize the first-strand cDNA, following treatment with Invitrogen RNaseOUT Recombinant Ribonuclease Inhibitor (Invitrogen, Carlsbad, CA). qPCR was carried out using PerfeCTa SYBR Green SuperMix (Quanta Biosciences, Inc., Gaithersburg, MD) on a LightCycler 480 System (Roche Diagnostics, Penzberg, Germany) under the following conditions: 95 °C for 5 min, 40 cycles of 95 °C for 10 s, 60 °C for 20 s and 72 °C for 20 s, followed by a dissociation step. The Ct values and amplification curve data for each reaction generated by the Lightcycler 480 software were exported. Relative quantification (RQ) values for each sample were calculated using the method described by Pfaffl, and the geometric average of the plant genes showing stable expression by HTS was used to normalize the data, instead of using a single reference gene46–48. Calculated RQ values were log2 transformed and averaged for each of the technical replications of each sample and primer pair combination.
Supplementary information
Acknowledgements
We are grateful to Foundation Plant Services at the University of California, Davis, for donating known infected virus source material and known virus-free propagation material. This research was supported in the University of California Agricultural Experiment Station. C.A. Prator was supported by a National Science Foundation fellowship. This work is part of the Plant & Food Research Wine Research programme, funded by the New Zealand MBIE Strategic Science Investment Fund.This research used the Vincent J. Coates Genomics Sequencing Laboratory at UC Berkeley, supported by NIH S10 OD018174 Instrumentation Grant.
Author contributions
Conceptualization, C.A.P., K.M.C., R.P.P.A., methodology, C.A.P., K.M.C., D.J., M.W.D., software, D.J., M.W.D., validation, C.A.P., K.M.C., formal analysis, C.A.P., K.M.C., D.J., M.W.D., investigation, C.A.P., K.M.C., resources, R.P.P.A., R.M.M., data curation, C.A.P., D.J., writing—original draft preparation, C.A.P., writing—review and editing, C.A.P., K.M.C., D.J., M.W.D., R.M.M., R.P.P.A., visualization, C.A.P., K.M.C., D.J., M.W.D., supervision, R.P.P.A., R.M., project administration, R.P.P.A., funding acquisition, R.P.P.A., K.M.C., R.M.M.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-64972-8.
References
- 1.Dangl JL, Jones JD. Plant pathogens and integrated defence responses to infection. Nature. 2001;411:826. doi: 10.1038/35081161. [DOI] [PubMed] [Google Scholar]
- 2.Ekblom R, Galindo J. Applications of next generation sequencing in molecular ecology of non-model organisms. Heredity. 2010;107:1. doi: 10.1038/hdy.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pervaiz T, et al. Transcriptomic analysis of grapevine (cv. Summer Black) leaf, using the Illumina platform. PLoS One. 2016;11:e0147369. doi: 10.1371/journal.pone.0147369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prator CA, Kashiwagi CM, Voncina D, Almeida RPP. Infection and colonization of Nicotiana benthamiana by Grapevine leafroll-associated virus 3. Virology. 2017;510:60–66. doi: 10.1016/j.virol.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Bester R, Burger JT, Maree HJ. Differential expression of miRNAs and associated gene targets in grapevine leafroll-associated virus 3-infected plants. Archives of Virology. 2017;162:987–996. doi: 10.1007/s00705-016-3197-9. [DOI] [PubMed] [Google Scholar]
- 6.Tsai CW, et al. Transmission of Grapevine leafroll-associated virus 3 by the Vine Mealybug (Planococcus ficus) Phytopathology. 2008;98:1093–1098. doi: 10.1094/PHYTO-98-10-1093. [DOI] [PubMed] [Google Scholar]
- 7.Goodin MM, Zaitlin D, Naidu RA, Lommel SA. Nicotiana benthamiana: its history and future as a model for plant–pathogen interactions. Molecular plant-microbe interactions. 2008;21:1015–1026. doi: 10.1094/MPMI-21-8-1015. [DOI] [PubMed] [Google Scholar]
- 8.Barba M, Czosnek H, Hadidi A. Historical Perspective, Development and Applications of Next-Generation Sequencing in Plant Virology. Viruses. 2014;6:106. doi: 10.3390/v6010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nature reviews genetics. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garber M, Grabherr MG, Guttman M, Trapnell C. Computational methods for transcriptome annotation and quantification using RNA-seq. Nature methods. 2011;8:469–477. doi: 10.1038/nmeth.1613. [DOI] [PubMed] [Google Scholar]
- 11.Bally J, et al. The extremophile Nicotiana benthamiana has traded viral defence for early vigour. Nature plants. 2015;1:15165. doi: 10.1038/nplants.2015.165. [DOI] [PubMed] [Google Scholar]
- 12.Nakasugi K, et al. De novo transcriptome sequence assembly and analysis of RNA silencing genes of Nicotiana benthamiana. PLoS one. 2013;8:e59534. doi: 10.1371/journal.pone.0059534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sweetman C, Wong DCJ, Ford CM, Drew DP. Transcriptome analysis at four developmental stages of grape berry (Vitis vinifera cv. Shiraz) provides insights into regulated and coordinated gene expression. BMC Genomics. 2012;13:691–691. doi: 10.1186/1471-2164-13-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Visser M, Bester R, Burger JT, Maree HJ. Next-generation sequencing for virus detection: covering all the bases. Virology journal. 2016;13:85. doi: 10.1186/s12985-016-0539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coetzee B, et al. Deep sequencing analysis of viruses infecting grapevines: virome of a vineyard. Virology. 2010;400:157–163. doi: 10.1016/j.virol.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 16.Bester R, Burger JT, Maree HJ. Transcriptome analysis reveals differentially expressed small RNAs and genes associated with grapevine leafroll-associated virus 3 infections. Physiological and Molecular Plant Pathology. 2017;100:220–236. doi: 10.1016/j.pmpp.2017.10.006. [DOI] [Google Scholar]
- 17.Fan H, et al. Deep Sequencing–based transcriptome profiling reveals comprehensive insights into the responses of Nicotiana benthamiana to Beet necrotic yellow vein virus infections containing or lacking RNA4. PLoS one. 2014;9:e85284. doi: 10.1371/journal.pone.0085284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Senthil G, et al. Specific and common changes in Nicotiana benthamiana gene expression in response to infection by enveloped viruses. Journal of General Virology. 2005;86:2615–2625. doi: 10.1099/vir.0.81043-0. [DOI] [PubMed] [Google Scholar]
- 19.Dardick C. Comparative expression profiling of Nicotiana benthamiana leaves systemically infected with three fruit tree viruses. Molecular plant-microbe interactions. 2007;20:1004–1017. doi: 10.1094/MPMI-20-8-1004. [DOI] [PubMed] [Google Scholar]
- 20.Vigers AJ, et al. Thaumatin-like pathogenesis-related proteins are antifungal. Plant Science. 1992;83:155–161. doi: 10.1016/0168-9452(92)90074-V. [DOI] [Google Scholar]
- 21.Rouhier N, et al. Identification of plant glutaredoxin targets. Antioxidants & redox signaling. 2005;7:919–929. doi: 10.1089/ars.2005.7.919. [DOI] [PubMed] [Google Scholar]
- 22.Knopf RR, Adam Z. Rhomboid proteases in plants - still in square one? Physiologia plantarum. 2012;145:41–51. doi: 10.1111/j.1399-3054.2011.01532.x. [DOI] [PubMed] [Google Scholar]
- 23.Keegstra K, Raikhel N. Plant glycosyltransferases. Current opinion in plant biology. 2001;4:219–224. doi: 10.1016/S1369-5266(00)00164-3. [DOI] [PubMed] [Google Scholar]
- 24.Qian, S.-B. & Patterson, C. Up and down regulation of the stress response by the co-chaperone ubiquitin ligase CHIP. In Cell Stress Proteins 313–325 (Springer, 2007).
- 25.Lindquist S, Craig E. The heat-shock proteins. Annual review of genetics. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 26.Pockley AG. Heat shock proteins as regulators of the immune response. The Lancet. 2003;362:469–476. doi: 10.1016/S0140-6736(03)14075-5. [DOI] [PubMed] [Google Scholar]
- 27.Wainberg Z, Oliveira M, Lerner S, Tao Y, Brenner BG. Modulation of stress protein (hsp27 and hsp70) expression in CD4+ lymphocytic cells following acute infection with human immunodeficiency virus type-1. Virology. 1997;233:364–373. doi: 10.1006/viro.1997.8618. [DOI] [PubMed] [Google Scholar]
- 28.Phillips B, Abravaya K, Morimoto RI. Analysis of the specificity and mechanism of transcriptional activation of the human hsp70 gene during infection by DNA viruses. Journal of Virology. 1991;65:5680–5692. doi: 10.1128/JVI.65.11.5680-5692.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park C-J, Seo Y-S. Heat shock proteins: a review of the molecular chaperones for plant immunity. The Plant Pathology Journal. 2015;31:323–333. doi: 10.5423/PPJ.RW.08.2015.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peremyslov VV, Hagiwara Y, Dolja VV. HSP70 homolog functions in cell-to-cell movement of a plant virus. Proceedings of the National Academy of Sciences. 1999;96:14771–14776. doi: 10.1073/pnas.96.26.14771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maree HJ, et al. Grapevine leafroll-associated virus 3. Frontiers in Microbiology. 2013;4:82. doi: 10.3389/fmicb.2013.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trujillo M, Shirasu K. Ubiquitination in plant immunity. Current Opinion in Plant Biology. 2010;13:402–408. doi: 10.1016/j.pbi.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Yıldırım K, Yağcı A, Sucu S, Tunç S. Responses of grapevine rootstocks to drought through altered root system architecture and root transcriptomic regulations. Plant Physiology and Biochemistry. 2018;127:256–268. doi: 10.1016/j.plaphy.2018.03.034. [DOI] [PubMed] [Google Scholar]
- 34.Lemoine R, et al. Source-to-sink transport of sugar and regulation by environmental factors. Frontiers in plant science. 2013;4:272. doi: 10.3389/fpls.2013.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Espinoza C, et al. Gene expression associated with compatible viral diseases in grapevine cultivars. Functional & integrative genomics. 2007;7:95–110. doi: 10.1007/s10142-006-0031-6. [DOI] [PubMed] [Google Scholar]
- 36.Almeida R, et al. Ecology and management of grapevine leafroll disease. Frontiers in Microbiology. 2013;4:94. doi: 10.3389/fmicb.2013.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blaisdell GK, et al. Disease progression of vector-mediated Grapevine leafroll-associated virus 3 infection of mature plants under commercial vineyard conditions. European journal of plant pathology. 2016;146:105–116. doi: 10.1007/s10658-016-0896-8. [DOI] [Google Scholar]
- 38.Li R, Zanin M, Xia X, Yang Z. The tree shrew as a model for infectious diseases research. Journal of thoracic disease. 2018;10:S2272. doi: 10.21037/jtd.2017.12.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaw, A. E. et al. Drosophila melanogaster as a model organism for bluetongue virus replication and tropism. Journal of virology, JVI. 00131-00112 (2012). [DOI] [PMC free article] [PubMed]
- 40.Sharma AM, et al. Occurrence of grapevine leafroll-associated virus complex in Napa valley. PLOS ONE. 2011;6:e26227. doi: 10.1371/journal.pone.0026227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santos, D. S. Análise de expressão gênica de Xylella fastidiosa in planta PhD thesis, Universidade de Mogi das Cruzes, (2013).
- 42.Voncina D, Almeida RPP. Screening of some Croatian autochthonous grapevine varieties reveals a multitude of viruses, including novel ones. Archives of Virology. 2018;163:2239–2243. doi: 10.1007/s00705-018-3850-6. [DOI] [PubMed] [Google Scholar]
- 43.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nature methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bombarely A, et al. A draft genome sequence of Nicotiana benthamiana to enhance molecular plant-microbe biology research. Molecular Plant-Microbe Interactions. 2012;25:1523–1530. doi: 10.1094/MPMI-06-12-0148-TA. [DOI] [PubMed] [Google Scholar]
- 45.Robinson MD, McCarthy DJ, Smyth G. K. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pfaffl MW. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic acids research. 2001;29:e45–e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome biology. 2007;8:R19. doi: 10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vandesompele J, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome biology. 2002;3:research0034. 0031. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heyno E, Alkan N, Fluhr R. A dual role for plant quinone reductases in host-fungus interaction. Physiologia plantarum. 2013;149:340–353. doi: 10.1111/ppl.12042. [DOI] [PubMed] [Google Scholar]
- 50.Figueiredo J, et al. Revisiting Vitis vinifera subtilase gene family: a possible role in grapevine resistance against Plasmopara viticola. Frontiers in plant science. 2016;7:1783. doi: 10.3389/fpls.2016.01783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Azevedo C, Santos-Rosa MJ, Shirasu K. The U-box protein family in plants. Trends in plant science. 2001;6:354–358. doi: 10.1016/S1360-1385(01)01960-4. [DOI] [PubMed] [Google Scholar]
- 52.Brzobohaty B, et al. Release of active cytokinin by a beta-glucosidase localized to the maize root meristem. Science. 1993;262:1051–1054. doi: 10.1126/science.8235622. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, et al. Major latex protein-like protein 43 (MLP43) functions as a positive regulator during abscisic acid responses and confers drought tolerance in Arabidopsis thaliana. Journal of experimental botany. 2015;67:421–434. doi: 10.1093/jxb/erv477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doukhanina EV, et al. Identification and functional characterization of the BAG protein family in Arabidopsis thaliana. Journal of Biological Chemistry. 2006;281:18793–18801. doi: 10.1074/jbc.M511794200. [DOI] [PubMed] [Google Scholar]
- 55.Lee S, Sowa ME, Choi J-M, Tsai FT. The ClpB/Hsp104 molecular chaperone—a protein disaggregating machine. Journal of structural biology. 2004;146:99–105. doi: 10.1016/j.jsb.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 56.Harrison MD, Jones CE, Solioz M, Dameron CT. Intracellular copper routing: the role of copper chaperones. Trends in biochemical sciences. 2000;25:29–32. doi: 10.1016/S0968-0004(99)01492-9. [DOI] [PubMed] [Google Scholar]
- 57.Fujimoto SY, Ohta M, Usui A, Shinshi H, Ohme-Takagi M. Arabidopsis ethylene-responsive element binding factors act as transcriptional activators or repressors of GCC box–mediated gene expression. The Plant Cell. 2000;12:393–404. doi: 10.1105/tpc.12.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen Z, et al. Influence of cytoplasmic heat shock protein 70 on viral infection of Nicotiana benthamiana. Molecular plant pathology. 2008;9:809–817. doi: 10.1111/j.1364-3703.2008.00505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee Y-H, Chun J-Y. A new homeodomain-leucine zipper gene from Arabidopsis thaliana induced by water stress and abscisic acid treatment. Plant Molecular Biology. 1998;37:377–384. doi: 10.1023/A:1006084305012. [DOI] [PubMed] [Google Scholar]
- 60.Pogorelko GV, et al. Characterization of three Arabidopsis thaliana immunophilin genes involved in the plant defense response against Pseudomonas syringae. Gene. 2014;538:12–22. doi: 10.1016/j.gene.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 61.Carvalho CM, et al. Regulated nuclear trafficking of rpL10A mediated by NIK1 represents a defense strategy of plant cells against virus. PLoS pathogens. 2008;4:e1000247. doi: 10.1371/journal.ppat.1000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gallino JP, et al. A Dehydration-induced eukaryotic translation initiation factor iso4g identified in a slow wilting soybean cultivar enhances abiotic stress tolerance in Arabidopsis. Frontiers in plant science. 2018;9:262. doi: 10.3389/fpls.2018.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.