Significance
Paternal provisioning is ubiquitous in human subsistence societies and unique among apes. How could paternal provisioning have emerged from promiscuous or polygynous mating systems that characterize other apes? An anomalous provisioning male would encounter a social dilemma: Since this investment in prospective offspring can be expropriated by other males, this investment is unlikely to increase the provisioner’s fitness. We present an ecological theory of the evolution of human paternal investment. Ecological change favoring reliance on energetically rich, difficult-to-acquire resources increases payoffs to paternal provisioning due to female–male and/or male–male complementarities. Paternal provisioning becomes a viable reproductive strategy when complementarities are strong, even under high paternity uncertainty. This model illuminates additional paths for understanding the evolution of fatherhood.
Keywords: paternal care, fatherhood, human evolution, parental investment, cooperation
Abstract
Paternal provisioning among humans is puzzling because it is rare among primates and absent in nonhuman apes and because emergent provisioning would have been subject to paternity theft. A provisioning “dad” loses fitness at the hands of nonprovisioning, mate-seeking “cads.” Recent models require exacting interplay between male provisioning and female choice to overcome this social dilemma. We instead posit that ecological change favored widespread improvements in male provisioning incentives, and we show theoretically how social obstacles to male provisioning can be overcome. Greater availability of energetically rich, difficult-to-acquire foods enhances female–male and male–male complementarities, thus altering the fitness of dads versus cads. We identify a tipping point where gains from provisioning overcome costs from paternity uncertainty and the dad strategy becomes viable. Stable polymorphic states are possible, meaning that dads need not necessarily eliminate cads. Our simulations suggest that with sufficient complementarities, dads can emerge even in the face of high paternity uncertainty. Our theoretical focus on ecological change as a primary factor affecting the trade-off between male mating and parenting effort suggests different possibilities for using paleo-climatic, archaeological, and genomic evidence to establish the timing of and conditions associated with emergence of paternal provisioning in the hominin lineage.
The mating–parenting system among modern human hunter-gatherers is unique among mammals. Human hunter-gatherers also occupy a unique ecological niche. The following social and ecological characteristics are distinctive among humans: 1) They live in multimale, multifemale groups in which the majority of adults are mated in socially recognized pair bonds (mean % unions monogamous = 92%, SD = 12, n = 339 foraging societies) (1); 2) labor differs by sex, in which females specialize in childcare, gathering and food processing, and males in hunting and/or fishing (2); 3) offspring are provisioned by men and women, often parents, throughout childhood and adolescence, with men providing the majority of calories (mean % calories provided by men = 66%, SD = 18, n = 9 foraging societies) (3); and 4) families include multiple dependent young of different ages (4). This mating–parenting system differs dramatically from that of the three great ape species to which we are most closely related, chimpanzees, bonobos, and gorillas, who are either promiscuous or polygynous and lack male provisioning. If the mating–parenting system of our last common ancestor resembled that of our closest relatives (5–8), then how did extensive male provisioning evolve in our species?
Understanding the selection pressures underlying the evolutionary transition from no to extensive paternal provisioning is especially challenging in light of the “social dilemma” that would undermine fitness benefits of incipient male provisioning. Paternal care does not occur in a vacuum but instead depends on what other males do. Suppose a genetic variant arose in an ancestral nonprovisioning male, predisposing him to provision the offspring of a female with whom he mated, rather than consume the food himself. Promiscuity or dominance-based polygyny implies a low probability that he is the biological father of the offspring, meaning that his investments would increase the fitness of others who did not carry the novel genetic variant, thus preventing its spread.*
Gavrilets (7) argued that perhaps the only way this dilemma could be overcome is if females were to offer preferential mating and faithfulness to males who provision them or their offspring. Lesser-ranked males might have a comparative advantage in provisioning rather than competing for mates, but their paternal investments would somehow have to attract devoted females. Chimpanzee behavior suggests slim odds for this path to paternal provisioning. Notwithstanding reports of exchanges of meat for sex (9, 10), a detailed review (11) casts doubt on the notion that provisioning buys sexual access. Rather, prevailing evidence points to rank and aggression as key determinants of chimpanzee male reproductive success (12). Even if exchange of meat for sex were to constitute an incipient reproductive strategy, there would still be the social dilemma to contend with. This scenario also requires simultaneous evolutionary changes in both sexes.† Instead, we address the social dilemma by considering ecological factors that affect the fitness benefits and costs of alternative male reproductive strategies.
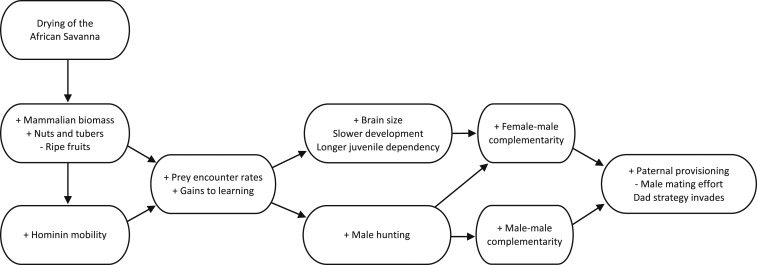
This paper presents an ecological theory of the evolution of paternal provisioning in our species that depends only on selection on males (Fig. 1). We propose that the drying of the African savanna in the Late Pliocene and Early Pleistocene—with associated increases in mammalian biomass, tubers, and nuts and decreases in ripe fruit availability—gradually resulted in obligate bipedality, increased mobility, prey encounter rates, and returns to hunting and extractive foraging. Those changes in the profitability of different ecological strategies selected for increased brain size, greater time devoted to learning and cultural innovation, and lengthening of the juvenile period (3). Those changes in ecology and life history generated new sources of female–male complementarity in producing surviving offspring and male–male complementarity in acquiring and sharing food (4, 14).
Fig. 1.
An ecological theory for the evolution of male provisioning in the hominin lineage.
Complementarities between males and females, i.e., synergistic effects that increase per capita benefits, arose from the gains to male specialization in hunting and female specialization in activities compatible with offspring care. Because hunting is not easily compatible with existing primate adaptations to lactation and carrying infants, and because hunting skill is acquired over a long period of practice, there were significant gains from a sexual division of labor (14, 15). Altriciality, prolonged development, and the simultaneous dependence of multiple offspring amplified these gains. Since hunted foods tend to be dispersed and rich in protein, fat, and certain micronutrients (e.g., zinc, B vitamins), while the foods gathered by females tend to be rich in carbohydrates and spatially segregated from hunted foods, female–male synergies also arose directly from the dietary complementarity of different macro- and micronutrient inputs to an omnivorous diet (16).
Greater dietary reliance on animal products also generated complementarities between males in producing and sharing food. Returns to scale in cooperative prey capture allowed hunters to achieve higher per capita return rates (14). Highly variable success rates in hunting also generated returns to food sharing that buffered risk and smoothed variability in consumption over time (4). Reducing risk through food sharing was essential to the long-term sustainability of omnivorous forager diets that include high-risk, high-reward foods such as large game.
We present a simple evolutionary game theoretic model that shows how these complementarities between women and men and among men can select for male provisioning, even when paternity is not fully certain. The model connects ecology to male reproductive strategies to predict the effect of ecological change on the evolution of paternal provisioning. Reproductive strategies are modeled by imagining the behavior of pairs of male neighbors. Males are of two types, “cads,” who do not provision offspring and who mate with multiple females, and “dads,” who do provision offspring and who mate with only one female. In an initial population predominated by cads, a variant dad paired with a cad would have lower reproductive success than his neighbor. Following ecological change that increases the synergistic benefits of cooperation (complementarities) between sexes and between males, changes in the fitness of dads and cads select for male provisioning. We show that these forces can be strong enough to coexist with an appreciable degree of extrapair mating, so that complete monogamy is not necessary for the initiation and maintenance of paternal provisioning.
Model
We model a population in which males and females in each generation interact in groups; each female has a mate, but she can also copulate with other males. For analytical simplicity, we consider groups with two males and two females each. The males forage together and share the outcome equally. We envision two types of males, dads (nonphilandering provisioners) and cads (philandering nonprovisioners). A dad favors his mate’s offspring’s survival by sharing some of his food with them and his mate. In contrast, a cad uses extrapair matings rather than provisioning to increase his reproductive success. This is the primary tension in the model. Complex foraging enhances dads’ reproductive success (i.e., offspring survivorship) because dads are more adept at exploiting its inherent complementarities. The model examines whether enhanced offspring survival prospects can allow the dad trait to spread in an ancestral environment of promiscuity, i.e., in a population where almost all dads have a cad as a neighbor.
Importantly, we suppress the evolution of female faithfulness to focus on the interplay between ecological change and male reproductive behavior in overcoming the social dilemma. Specifically, we take all females to be identical within and across generations in terms of fertility, ability to care for offspring, and openness to extrapair matings. (It is reasonable to imagine scenarios, not considered here, whereby male provisioning and female faithfulness coevolve, but only after the initial social dilemma has been overcome.).
Male–Male Interactions.
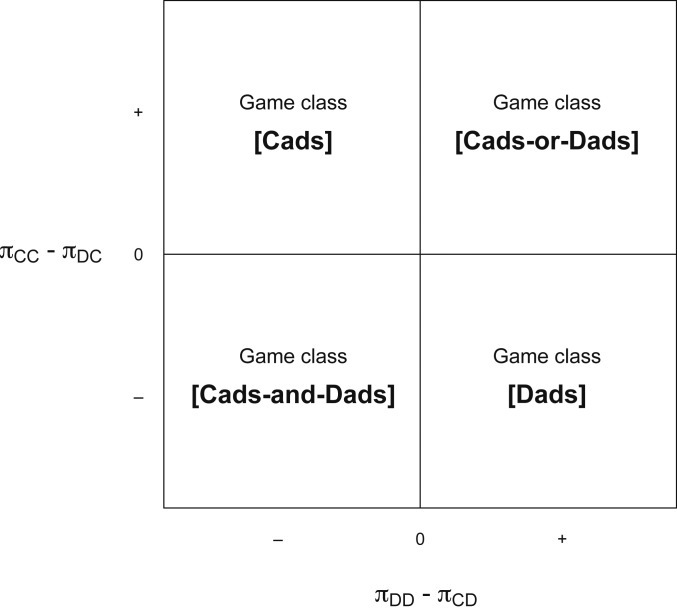
The model focuses entirely on variation in male reproductive strategies. Assuming that the groups of males and females are randomly formed, we formalize the interaction between neighboring males as an evolutionary game with two strategies or types—the aforementioned dad () and cad (). Let denote the reproductive success of a male of type whose neighbor is of type . The payoff matrix of the evolutionary game is shown in Fig. 2 (since the game is symmetric, the matrix shows only the reproductive success of the row player).
Fig. 2.
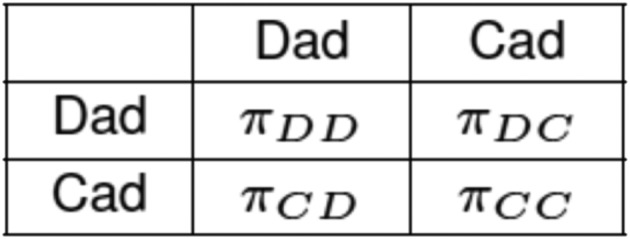
The payoff matrix of the dad–cad game.
Assume, for simplicity, that each adult female gives birth to the same number of offspring ( males and females). All of the females being identical, a male’s reproductive success—the number of his biological offspring who survive to sexual maturity—is then wholly determined by offspring survival probabilities and female openness to extrapair matings. Since neighboring males forage together, there may be male–male complementarities in production (described in detail below). Hence, while the survival probability of a male’s mate’s offspring depends on whether he is a provisioning dad or a philandering cad, it may also depend on whether his neighbor is a dad or a cad. Accordingly, we write for the survival probability of the offspring of a female whose mate has type and whose neighboring male has type . Turning to extrapair matings, dads spend less time philandering than do cads. Here we make the extreme assumption that dads engage in no extrapair copulations at all (although unrealistic, the assumption is conservative since it reduces the odds that dad genes spread). Thus, let denote the share of copulatary acts that a female concedes to the neighbor if he is a cad, while 1 − is the share alloted to her mate. The reproductive successes , , are then given by the following expressions:
| [1] |
| [2] |
| [3] |
| [4] |
A cad matched with another cad poaches as much paternity from his neighbor as his neighbor does from him, on average (Eq. 1). A cad matched with a dad steals, on average, a share of the offspring born to his neighbor and faces no paternity uncertainty himself (Eqs. 2 and 3). A dad matched with a dad faces no paternity uncertainty. Each dad devotes all of his time to producing food and other types of care for his offspring; this explains Eq. 4. [Note that the Fisher condition, sensu Houston and McNamara (17), holds.]
Shares of Dads and Cads.
Let denote the share of dads among males at time . In an infinitely large population, the replicator dynamic (18, 19)—the rate of change in the share of dads at time —is written
| [5] |
where the term in the square brackets indicates that the ultimate evolutionary success of dads versus cads depends on the average reproductive success of each strategy, given by
| [6] |
and
| [7] |
In a population with both male types present (i.e., where ), the share of dads thus increases () if dads achieve a higher reproductive success on average than cads; it decreases () if cads achieve a higher average reproductive success than dads; and it remains constant () if both types achieve the same average reproductive success. Rewriting the difference as
| [8] |
reveals that the signs of and are crucial. For example, if and , then a cad’s reproductive success exceeds that of a dad regardless of whether the neighbor is a dad or a cad, and the share of dads will decrease. By contrast, if and , then dads always outperform cads and the share of dads will increase.
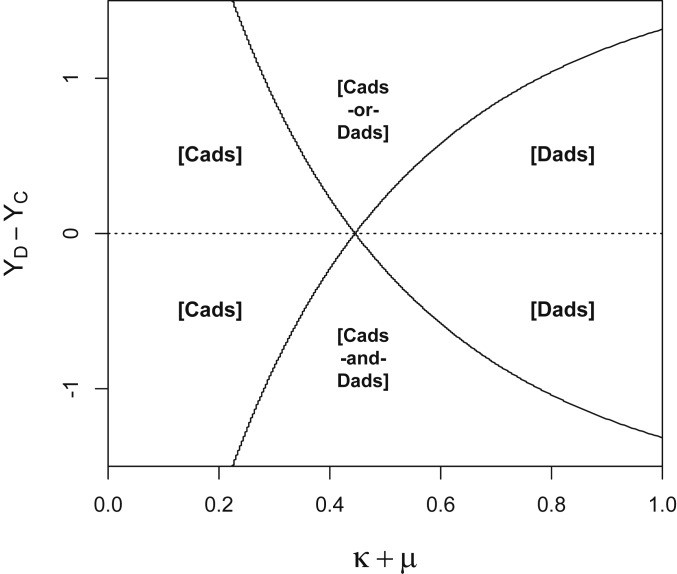
We are interested in values of that are asymptotically stable states; the replicator dynamic brings the share of dads back toward these states if the share of dads is slightly perturbed away from (ref. 20, p. 243). From the reproductive success values given in Eqs. 1–4, and ignoring knife-edge cases where and/or , the replicator dynamic gives rise to four distinct game classes, each with qualitatively different asymptotically stable states (Fig. 3):
Game class [cads] obtains if and . Regardless of the neighbor’s type, a cad’s reproductive success exceeds that of a dad, and the unique asymptotically stable state has only cads: .
Game class [dads] obtains if and . Regardless of the neighbor’s type, a dad’s reproductive success exceeds that of a cad, and the unique asymptotically stable state has only dads: .
Game class [cads and dads] obtains if and . It is preferable to be a cad when matched with a dad and a dad when matched with a cad. This asymmetry implies that there is a unique asymptotically stable state, which is polymorphic: .
Game class [cads or dads] obtains if and . Being matched with a male of the same type is always better than being matched with a male of a different type. Both monomorphic states are asymptotically stable: or .
Fig. 3.
The evolutionary game class, which determines whether the share of dads in the population increases or decreases over time, depends on the difference (the horizontal axis) and the difference (the vertical axis).
With these concepts in hand, our question can be expressed formally as follows: In a population initially predominated by cads, and where is an asymptotically stable state, what could cause the share of dads to increase over time? If is asymptotically stable, and initially , then the share of dads will decline over time, getting closer to . This will remain true as long as the reproductive successes of dads versus cads remain unchanged. However, changes in reproductive successes can alter the prospects for dads; if large enough, they can stimulate transitions between game classes that favor increases in the share of dads. We illustrate this with two examples.
First, suppose that initially the game class is [cads] and that suddenly the inequality switches to while the inequality continues to hold. This switch is literally “game changing”: The game class transitions from [cads] to [cads and dads]. The replicator dynamic, whose sign hinges on the sign of [8], switches from negative to positive. The share of dads will keep increasing toward the asymptotically stable state .
Second, again start with but now suppose the initial game class is [cads or dads] and that suddenly the inequality switches to while the inequality continues to hold. Then the game class transitions from [cads or dads] to [dads]: The replicator dynamic becomes positive, and the share of dads will increase toward the asymptotically stable state .
Given that female openness to extrapair copulations is constant, such transitions between game classes can only be driven by changes in offspring survival probabilities. The key point of our analysis below is that enhanced female–male and male–male complementarities in production can trigger precisely such changes. We now describe how we model these complementarities.
Complementarities and Survival.
Let denote the survival probability of an offspring who receives resources from its mother and whose mother’s mate brings the amount of food back to the mother–offspring pair. Central to our model is that dads provision more than cads. For simplicity, and without losing anything essential, we assume that cads do no provisioning at all.‡
To highlight the role of complementarities, as distinct from forces such as increasing or decreasing returns to inputs, we adopt a survival function with constant returns to male and female inputs,
| [9] |
where is a constant large enough for to be smaller than one, and where (this ensures that the female’s contribution cannot reduce the value of the male’s contribution). The parameter measures the female–male complementarity. There is no complementarity if : Only the sum of and matters. The complementarity is present and increasing in if . This is revealed by the sign of the cross-partial derivative
| [10] |
The positive sign of this expression implies that when , resources provided by one sex increase the value of resources provided by the other.
Neighboring males forage together and equally share the collectively produced food. Using and to denote the amount of food that a dad brings to his mate and her offspring when his neighbor is a dad and a cad, respectively, let
| [11] |
| [12] |
and measure the productivity of the two types of males in procuring food: While all males may spend the same amount of time producing food together, we allow for the possibility that time spent by cads and dads could differ in productivity.
The parameter measures the male–male complementarity in food production. If , there is no complementarity: Only the sum of and matters. If , the complementarity is maximal: Only their product matters (since when and , the product , , is at least as large as the sum). The 2 in the denominator of Eqs. 11 and 12 means that the food is shared equally between the two males.
How do ecological changes in and confer an evolutionary advantage to dads? And what are the effects of paternity uncertainty () and cad–dad differences in productivity (), if any? The next section (Results) answers these questions by reporting results based on analysis in SI Appendix, Lemmas 1 and 2 and Propositions 1–10. We also conduct simulations of finite populations to illustrate the plausible emergence of dads as a result of ecological change.
Results
Complementarities Favor the Spread of Dads.
Keeping the parameters for male contributions ( and ) and female extrapair copulations () fixed, increases in female–male complementarity () and/or male–male complementarity () can cause the share of dads to increase over time in a population initially predominated by cads. This is easiest to see when dads and dads are equally productive (). In this case, a dad brings to his mate–offspring pair the same amount of food whether matched with a dad or a cad (), so that . We show in SI Appendix, Lemma 1 that the game class is then either [cads] or [dads] and that the game class switches from [cads] to [dads] if the following expression switches from being negative to being positive:
| [13] |
A shift from [cads] to [dads] thus requires either an increase in or a decrease in .
Can an increase in tip outcomes in favor of dads and achieve such a change? Yes. Because nonpaternity is held constant, and cads do not invest in a mate or her offspring, neither nor changes with . In contrast, for any given amount provisioning from the mother, , and from the dad, , the survival of a dad’s mate’s offspring, , is strictly increasing in :
| [14] |
(SI Appendix, Lemmas 1 and 2, Proposition 1, and Fig. S2). Thus, all else equal, when , a sufficient increase in female–male complementarity, , is enough for dads to outcompete cads. This is intuitive: An increase in renders the food contribution from dads more valuable, and the fitness of dads relative to cads increases.
Can increasing tip the balance in favor of dads? Yes. An increase in entails an increase in dads’ provisioning, , and this has a positive impact on offspring survival, :
| [15] |
(SI Appendix, Lemmas 1 and 2, Proposition 1, and Proof of Propositions 7 and 8). As a result, all else equal, when , a sufficient increase in male–male complementarity, , is enough for dads to spread. In other words, greater male–male complementarity increases the food contributed by dads to a mate and her offspring, resulting in higher fitness for dads relative to cads.
More generally, whether cads and dads are equally productive or not (, , or ), an increase in and/or increases the survival probability of offspring provisioned by dads. This generally contributes to dads’ reproductive success in the sense that if the effect is pronounced enough, the game will switch from class [cads] to one of the other three game classes. While this is intuitive, it is by no means trivial, since an increase in the survival probability of offspring provisioned by dads also means that the value of stealing paternity increases. In fact, when both and are large, an increase in and/or can favor cads by inducing a switch from game class [dads] to [cads and dads] (but not to [cads]). However, this is the only exception to the rule that complementarities favor dads. (See SI Appendix, Propositions 4 and 5 and Propositions 7–10, which describe a slightly more general model that includes a parameter that represents the share of the collected food that a dad brings back to his mate and her offspring; an increase in has effects in the same direction as increases in or .
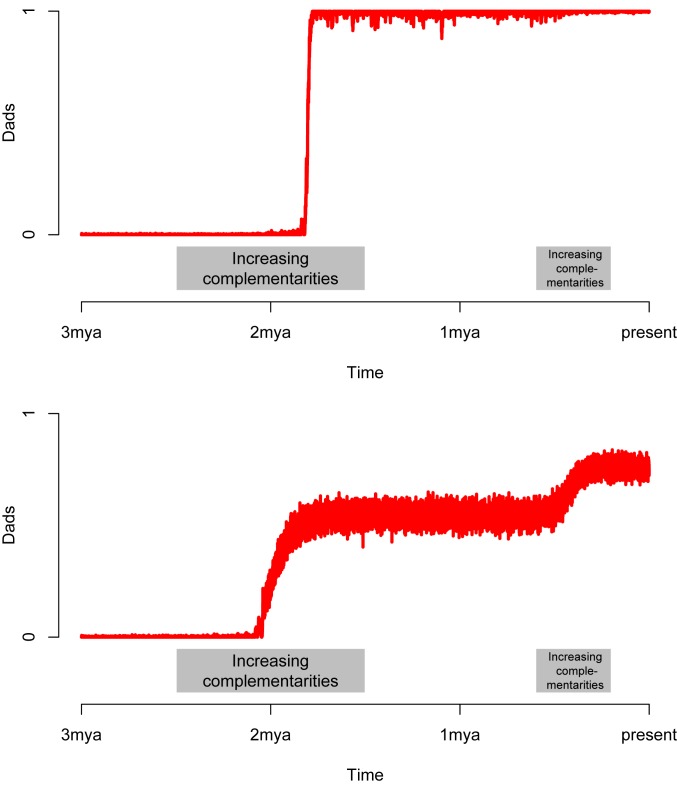
The potential for complementarities to underwrite the evolution of dads is borne out in numerical simulations of finite populations. Fig. 4 shows two simulations of the evolution of dads over 100,000 generations. Each simulation begins with a population of all cads (), no complementarities (), and a small () probability of mutation between male types. In rough correspondence with existing prehistoric evidence (Discussion), the simulation posits that complementarities and increase at two points in time: first around 2 Mya (corresponding roughly to an increase by Homo in dietary reliance on animal products from hunting and scavenging) and again around 400 kya (emergence of Homo sapiens and habitual use of technology [e.g., controlled fire, bifacial hand axes] to more efficiently harvest and process faunal remains).
Fig. 4.
Increases in complementarities allow the evolution of dads. The plot shows the frequency of dads in two simulated populations of 2,000 males over 100,000 generations with nonpaternity held fixed at . Complementarities ( and ) begin at 0 and increase at two points in time: from 0 to around 2 Mya and from to around 400 kya. (Top) . (Bottom) , . In both Top and Bottom, , , mutation probability = .
Fig. 4, Top shows the evolutionary trajectory of a population in which cads and dads have equal productivity (). Once and approach around 2 Mya, the sign of Eq. 13 switches from negative to positive, and selection begins to favor dads, despite their relative scarcity. Within a number of generations, dads compose the near entirety of the population. Fig. 4, Bottom illustrates a case in which cads contribute relatively more to group production (). Under this condition, the first ecological and behavioral shift around 2 Mya (increased availability, acquisition, and consumption of animal products) induces the game class to transition from [cads] to [cads and dads]. As a result, the share of dads increases and then fluctuates around the asymptotically stable state . The second shift around 400 kya (emergence of H. sapiens and more efficient acquisition of animal products) further enhances the viability of dads, but the game remains in the class [cads and dads]: The share of dads increases and then stabilizes at the new asymptotically stable state . These results confirm that sufficient increases in complementarity push populations toward more dad-favorable states within reasonable evolutionary timescales (even, as in the simulations, in a finite population and in the presence of stochastic reproduction and mutations).
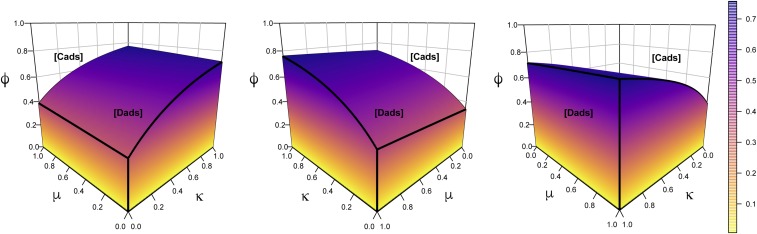
Dads Can Thrive Despite High Paternity Uncertainty.
The results reported above do not require low female openness to extrapair copulations. The simulations in Fig. 4, for example, hold constant at , meaning that of the fertility of any dad whose neighbor is a cad is diverted to cads. Fig. 5 generalizes this point. Fig. 5 assumes equal productivity of cads and dads (), which means that the game class is either [cads] or [dads], depending on complementarities and nonpaternity (SI Appendix, Lemma 1). The blank space in the top part of each cube in Fig. 5 represents parameter combinations that result in game class [cads], where the evolution of dads is suppressed. The multicolored hill, on the other hand, shows the parameter combinations of , , and that result in game class [dads], where dads are able to evolve. For any given values of and , the height of the hill indicates the maximum degree of paternity uncertainty () that would still allow the invasion of dads. It is clear that as either or increases, the range of allowable paternity uncertainty grows; in other words, dads can evolve at a higher degree of paternity uncertainty when there are greater complementarities (SI Appendix, Proposition 6).
Fig. 5.
The greater the female–male complementarity () and/or the male–male complementarity (), the higher the nonpaternity () that dads can confront yet still prevail. Points inside the multicolored hill favor the evolution of dads, while those in the blank space above it do not. As either female–male or male–male complementarities increase, dads can evolve at higher levels of nonpaternity. The same plot is displayed from three different angles to enhance clarity. ; ; .
Dad–Cad Polymorphism.
The model encompasses the possibility that a dad’s reproductive success is higher when matched with a cad than with a dad. Dads gain from cads if cads procure enough additional food () to compensate for the paternity they steal from dads. Such payoffs can lead to a stable dad–cad polymorphism, as illustrated in Fig. 4, Bottom. More generally, Fig. 6 shows that must strictly exceed for such a stable polymorphism to arise: If , the game is never in class [cads and dads] (SI Appendix, Propositions 2 and 3). Fig. 6 further shows that a stable dad–cad polymorphism is possible only if complementarities are strong enough but not too strong (i.e., is neither too small nor too large). Interestingly, when ecological conditions favor such a mutualism between dads and cads, cads implicitly “trade” food for sex, but in contrast to the standard food-for-sex scenario between a male and a female, here the trade is between males: A cad’s food production increases the amount of food a dad can provide his mate and her offspring, and in return the dad is willing to lose paternity.
Fig. 6.
Complementarities (here shown as the sum of the male–female complementarity and the male–male complementarity ) and differential production of dads versus cads () determine the game class and thus the asymptotically stable states and evolutionary outcomes. When at intermediate levels of complementarity, the game class is [cads and dads], meaning that dads and cads coexist in a polymorphic stable state. When , any stable state is monomorphic. ; ; ; .
In our view, or is the most plausible scenario based on empirical observations of modern human hunter-gatherers. Hence, our model would need to be enriched to help understand the factors that can sustain stable dad–cad polymorphisms over hominin evolutionary history. Nonetheless, the polymorphism described above may be relevant for understanding the evolution of provisioning in other ecological contexts.
Discussion
Our theory is that ecological changes in the availability of difficult-to-acquire, energy-dense foods interacted with existing traits in ancestral hominins to promote foraging specialization by sex—with males largely acquiring protein- and fat-rich animal products and females largely gathering carbohydrate-rich plant-based foods while providing offspring care. This specialization in turn generated complementarities—both between males and females and between males—which would have given a reproductive advantage to provisioning males. The model reveals the following sufficient conditions for the evolution of paternal provisioning: 1) Male hunting reliably complements female gathering and offspring care and/or 2) males cooperate with each other in the pursuit and/or sharing of hunted game, generating complementarities in their efforts. The model results, as displayed in Figs. 4–6, show that sufficiently high female–male and/or male–male complementarities can enable dads to evolve amid even high probabilities of nonpaternity. The next sections discuss available paleontological and archeological evidence relevant to the model and the predictions generated by the theory. The final sections of the paper discuss the relationship between this model and other models of paternal care and additional directions for theory development.
Evidence for Complementarities from the Paleontological and Archeological Record.
The fossil and paleo-climatic record provides clues about specific ecological forces generating complementarities in the hominin lineage. Since the last shared common ancestor with chimpanzees (8 to 5 Mya), hominins increasingly inhabited ecologies characterized by high habitat instability; heterogeneous patterns of vegetation; and long-term trends toward aridity, open habitat, and greater C4 biomass (primarily grasses and sedges) (21). These ecologies promoted the evolution of hominin morphologies allowing incipient and ultimately obligate bipedality, including adaptations for thermoregulation, locomotor efficiency, and endurance activity. Varying conditions also favored traits facilitating survival in shifting environments, including dietary flexibility, migration to new habitats, and finding novel solutions to environmental problems (22).
Isotopic studies identify a diverse array of hominin diets incorporating a broad range of plant foods during the Mid to Late Pliocene (3.5 Mya) (23, 24). During the Plio-Pleistocene transition a radiation of hominin species is evident, with species varying in body size, tooth morphology, brain size, and likely other features of life histories and social behavior. Some hominin species would have experienced ecological conditions favoring greater gains to hunting and learning, which in turn would have increased inter- and intrasexual complementarities (Fig. 1).
The burgeoning of grazing mammals in eastern and southern Africa is evident by 2.5 to 2 Mya (25, 26) and coincides with a relative decline in arboreal and frugivorous animals. More open, arid environments offered a new distribution of nutrients, with increased abundance of protected plant foods (e.g., nuts, tubers) and large grassland-adapted mammals (27). Tool cut marks have been found on large animal bones before 3 Mya (28), although evidence of stone tool-assisted foraging is intermittent before 2 Mya. Compared to plant foods, animal foods tended to be less predictable, require greater foraging distances, and entail increased energy expenditures. Greater hominin mobility is suggested by the appearance of elongated legs and taller stature beginning 2 Mya.
The first evidence of the cluster of characteristics associated with complementarities conducive to hominin fatherhood may be in Homo erectus. In early H. erectus (1.8 Mya), the combination of smaller incisors and molars, a wide range of dental microwear textural complexity, smaller jaw and gut, and larger body and brain size (29–31) implies a diverse and high-quality diet. This diet included a greater proportion of animal products (32) and plant-based foods requiring tools to acquire and prepare. The fact that omnivorous hominins absorbed the energetic costs of transporting stones for processing carcasses over long distances (>10 km) (33) suggests substantial investment in accessing animal tissues. Brain sizes >700 cc are found after 1.8 Mya, along with evidence of reductions in sexual dimorphism (34) and a modest slowing of development (22).
H. erectus ontogeny was likely somewhat slower than that of Australopithecus and modern chimpanzees, but considerably faster than in modern humans (22, 35, 36). Slower maturation might imply postlactational offspring provisioning, particularly if coupled with an earlier age at weaning (37). Given a lack of evidence for protracted growth (e.g., ref. 38) or an adolescent growth spurt, it is possible that fully modern human life histories had yet to evolve by 1.5 Mya. Given evidence of very late first molar eruption in Neanderthal fossils (but see ref. 38) and typically modern human hunter-gatherer base camps by 500 to 450 kya (39), it is more certain that extended juvenile dependence and near-modern brain size were in place by the time of archaic H. sapiens 400 kya. Viewed through the lens of the model, these developments imply that paternal provisioning would also have evolved in our lineage by this time.
New Research Directions and Predictions Generated by the Model.
Our theory directs attention to critical pieces of evidence that are missing from the archeological and paleontological record, providing guidance for additional research. More evidence is needed on sexual dimorphism in behavior and diet. Contemporary foragers show reduced dimorphism in body size relative to nonhuman apes, very little dimorphism in diet due to food sharing, and pronounced dimorphism with respect to hunting and gathering. We need evidence regarding changes in those dimorphisms and especially when extensive intersexual food sharing became prevalent. Similarly, we need additional evidence regarding juvenile development beyond what is known about molar eruption, which may provide evidence about weaning but not sources of food or dietary composition. Evidence regarding when meat becomes an important part of juvenile diets is critical to determining when paternal provisioning emerges.
Increasingly sophisticated methods for assessing dietary composition (e.g., radioisotope analysis) can be applied to existing and newly discovered fossils to test for sex and age differences in diet. Paleogenomic data on rates of development and aging will also shed crucial light on the timing of these changes in the hominin lineage. There is now a growing body of evidence of joint neural, hormonal, and behavioral responses underlying transition to fatherhood in modern humans, including lower testosterone production and sexual drive, higher oxytocin production to facilitate bonding, and increased activation in brain regions important for face emotion processing (40). As we better understand the genetic bases of those changes, we can look to paleogenomics to investigate the emergence of those traits as well.
Applications to Nonhuman Species.
The model provides insights into the question of why paternal provisioning evolved in humans but not in other species exposed to the same environmental conditions. The answer begins with the features of the last common ancestor: intelligent and tool using, with females committed to carrying offspring and investing in offspring over several years. Bipedality freed the hands for greater tool use and expanded the day range for hunting and foraging. Ecological shifts resulted in a greater abundance of concentrated, valuable food packages that are difficult and dangerous to obtain. Without sharp canines, claws, or projectile weapons, early hominin carnivory would have been limited by the ability to utilize creative strategies to scavenge and/or kill game, selecting for increased intelligence and learning about animal behavior. At the same time, a commitment to caring for and carrying vulnerable young, coupled with lengthy time requirements for learning complex and flexible foraging strategies, would have rendered hunting unprofitable for females and increased the benefits of male provisioning. These same conditions would also have favored slowing development to facilitate the learning process. This is a special constellation of conditions that gave rise to an exceptional level of paternal provisioning in our species relative to other primates and mammals.
This model can also be applied to understand more general conditions associated with paternal investment, which are still far from well understood (41). It can help explain, for example, why male provisioning is so uncommon in mammals and so common in birds. It is interesting to note that the mammalian adaptation is associated with increased investment in offspring survival: Internal gestation and lactation provide a protective environment for development, and allow females to combine feeding with direct offspring care. Those adaptations, however, lower the scope for female–male complementarity and effectively “crowd out” male investment. Most species of flying birds, on the other hand, face a trade-off between acquiring food and protecting vulnerable eggs and hatchlings. The young are often vulnerable for extended periods after hatching until they reach adult body size and can fly. This situation generates gains from complementary in the form of turn taking or specialization in feeding and offspring care. Our theory predicts that variation in paternal investment within the mammalian and avian classes will be associated with variation in complementarity and perhaps opportunities for extrapair mating.
Our theory also provides insight into why we still find male parental investment in birds, despite evidence of extrapair mating and paternity. Penguins provide an exception that proves the rule. Penguins have converged with fish-eating mammals in body form and have lost the ability to fly. However, they still evidence male parental investment and pair bonding in response to the need for biparental protection of vulnerable eggs and young.
Comparison with Other Models of Paternal Investment.
In addition to the model by Gavrilets (7), there is a rich set of theoretical models of the evolution of parental care—both maternal and paternal—or lack thereof (42–48; see ref. 49 for a survey). As in our model, in these models the opportunity cost of providing care (42) appears as lost mating opportunities (in our model, provisioning dads have no extrapair copulations while nonprovisioning dads do). Compared to this literature, our model innovates in two major ways.
First, in almost all existing models, offspring survival depends only on the sum of maternal and paternal care. By contrast, in our model there are female–male complementarities. While such complementarities are also present in refs. 43–45, our model is distinct in that it considers two types of males and also includes male–male complementarities in food production. Our model thus sheds light on the interplay between male–female and male–male complementarities in the evolution of paternal care. Moreover, an unexpected insight appears, namely, that dads can benefit from the presence of cads, which can give rise to a stable polymorphism including both dads and cads.
Second, while we follow in the footsteps of Maynard Smith (46) and use evolutionary game theory to study the evolution of paternal care, we do so in a different way. The original parental care game studied a population where males and females are matched to interact and where males and females are either a guarding type or a deserting type; the intuition was that deserting males would stand to gain more than deserting females (46). This modeling strategy, however, was difficult to reconcile with the Fisher condition (47), which requires that males and females must on average have the same number of offspring (see refs. 17 and 48 for excellent discussions). The Fisher condition is fulfilled in our model because each male spends an equal amount of time with his mate, and this time may be spent provisioning or seducing his neighbor’s mate. This allows a straightforward definition of reproductive success that does not require tracking the time spent in and out of the mating pool. Moreover, framing the evolutionary game as an interaction between males opens the door to studying male–male complementarities in food production and sharing.
Directions for Future Research.
We have proposed an evolutionary game theoretic model to study the evolution of paternal investment. This model sheds light on how ecological changes in the distant past may have altered the trade-off between mating effort and paternal investment and promoted the evolution of human fatherhood. While this model delivers a rich set of original insights, we see several directions in which it could be further extended.
First, our model focuses on paternal investment in the form of food provisioning. Male–female and male–male complementarities in other domains, e.g., tool production and domestic tasks, may be modeled using a similar approach.
Second, our model disregards sexual selection. But what would happen if females could actively select their mates? The observed polymorphic equilibrium opens the door to female choice by generating heterogeneity in male contributions to offspring. Relatedly, in our model females do not gain anything from extrapair copulations: What if they obtained extra resources or genetic benefits that conferred survival advantages for their offspring? What level of female willingness to engage in extrapair copulations would be expected if these traits were subject to selection? The same ecological conditions that increase the fitness benefits to males from investing in offspring also increase the benefits to females from receiving that investment. Concealing ovulation is a potential female strategy that could have reduced rates of male mating competition at the time of ovulation, thereby reducing nonpaternity as the primary brake on male investment.
Third, since our model disregards relatedness, it would be interesting to embed the male–male interaction we examine here in a model with population structure. Moreover, since variance in male ability and reliability in cooperation could motivate assortment among males, models which allow for male partner choice are warranted.
Additional questions are, Would model results change if males interacted in groups of individuals rather than in pairs? How would the presence of grandparents or other alloparents affect the key trade-offs?
Finally, the model may also be applicable to modern, culturally variable trends in men’s parenting behavior. Both within and among populations there is significant variation in family forms, and the matrifocal family, with limited male paternal investment, is becoming increasingly prevalent in sectors of societies worldwide. This variation invites analysis and assessment of whether variable complementarities may help explain it.
Data Availability.
The simulation code is available to readers at https://github.com/systemsscience/paternal.
Supplementary Material
Acknowledgments
I.A. acknowledges funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (Grant 789111; ERC Evolving Economics). I.A. and J.S. acknowledge Institute for Advanced Study in Toulouse (IAST) funding from the French National Research Agency (ANR) under Grant ANR-17-EURE-0010 (Investissements d’Avenir program).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The simulation code is available to readers on GitHub at https://github.com/systemsscience/paternal.
*The title of ref. 5 summarizes this predicament: “The male’s dilemma: Increased offspring production is more paternity to steal.”
†Indeed, some argue that the complex clockwork of adaptations needed to generate paternal provisioning casts doubt on the notion that human reproductive behavior has its origins in chimpanzee-like ancestors (13).
Having cads provision a positive amount complicates the analysis without affecting the qualitative results. Furthermore, no paternal provisioning is arguably a reasonable ancestral benchmark given its absence in nonhuman apes.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1917166117/-/DCSupplemental.
References
- 1.Binford L. R., Human ancestors: Changing views of their behavior. J. Anthropol. Archaeol. 4, 292–327 (1985). [Google Scholar]
- 2.Ember C. R., Myths about hunter-gatherers. Ethnology 17, 439–448 (1978). [Google Scholar]
- 3.Kaplan H., Hill K., Lancaster J., Hurtado A. M., A theory of human life history evolution: Diet, intelligence, and longevity. Evol. Anthropol. 9, 156–185 (2000). [Google Scholar]
- 4.Hooper P. L., Gurven M., Kaplan H., “Social and economic underpinnings of human biodemography” in Sociality, Hierarchy, Health: Comparative Biodemography, Weinstein M., Lane M. A., Eds. (National Academies Press, 2014), pp. 169–195. [PubMed] [Google Scholar]
- 5.Hawkes K., Rogers A. R., Charnov E. L., The male’s dilemma: Increased offspring production is more paternity to steal. Evol. Ecol. 9, 662–677 (1995). [Google Scholar]
- 6.Shultz S., Opie C., Atkinson Q. D., Stepwise evolution of stable sociality in primates. Nature 479, 219–222 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Gavrilets S., Human origins and the transition from promiscuity to pair-bonding. Proc. Natl. Acad. Sci. U.S.A. 109, 9923–9928 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaeggi A. V., et al. , “Cooperation between the sexes” in Chimpanzees and Human Evolution, Muller M. N., Wrangham R. W., Pilbeam D. R., Eds. (Belknap Press of Harvard University Press, 2017), pp. 548–571. [Google Scholar]
- 9.Stanford C. B., The hunting ecology of wild chimpanzees: Implications for the evolutionary ecology of Pliocene hominids. Am. Anthropol. 98, 96–113 (1996). [Google Scholar]
- 10.Gomes C. M., Boesch C., Wild chimpanzees exchange meat for sex on a long-term basis. PLoS One 4, e5116 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilby I. C., Thompson M. E., Ruane J. D., Wrangham R., No evidence of short-term exchange of meat for sex among chimpanzees. J. Hum. Evol. 59, 44–53 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Feldblum J., et al. , Sexually coercive male chimpanzees sire more offspring. Curr. Biol. 24, 2855–2860 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geary D. C., Evolution of Fatherhood in Family Relationships: An Evolutionary Perspective, Salmon CA, Shackelford TK., Eds. (Oxford University Press, Oxford, UK, 2007), pp. 115–144. [Google Scholar]
- 14.Hooper P. L., Demps K., Gurven M., Gerkey D., Kaplan H. S., Skills, division of labour and economies of scale among Amazonian hunters and South Indian honey collectors. Philos. Trans. Roy. Soc. B 370, 20150008 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan H., Lancaster J., Robson A., Embodied capital and the evolutionary economics of the human life span. Popul. Dev. Rev. 29, 152–182 (2003). [Google Scholar]
- 16.Gurven M., Hill K., Why do men hunt? A reevaluation of “man the hunter” and the sexual division of labor. Curr. Anthropol. 50, 51–74 (2009). [DOI] [PubMed] [Google Scholar]
- 17.Houston A. I., McNamara J. M., John Maynard Smith and the importance of consistency in evolutionary game theory. Biol. Philos. 20, 933–950 (2005). [Google Scholar]
- 18.Taylor P. D., Jonker L. B., Evolutionary stable strategies and game dynamics. Math. Biosci. 40, 145–156 (1978). [Google Scholar]
- 19.Maynard Smith J., Evolution and the Theory of Games (Cambridge University Press, New York, NY, 1982). [Google Scholar]
- 20.Weibull J. W., Evolutionary Game Theory (MIT Press, Cambridge, MA, 1995). [Google Scholar]
- 21.Cerling T. E., et al. , Woody cover and hominin environments in the past 6 million years. Nature 476, 51–56 (2011). [DOI] [PubMed] [Google Scholar]
- 22.Antón S. C., Potts R., Aiello L. C., Evolution of early Homo: An integrated biological perspective. Science 345, 1236828 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Cerling T. E., et al. , Stable isotope-based diet reconstructions of Turkana basin hominins. Proc. Natl. Acad. Sci. U.S.A. 110, 10501–10506 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sponheimer M., et al. , Isotopic evidence of early hominin diets. Proc. Natl. Acad. Sci. U.S.A. 110, 10513–10518 (2013). [Google Scholar]
- 25.Reed K. E., Early hominid evolution and ecological change through the African Plio-Pleistocene. J. Hum. Evol. 32, 289–322 (1997). [DOI] [PubMed] [Google Scholar]
- 26.Bobe R., Behrensmeyer A. K., The expansion of grassland ecosystems in Africa in relation to mammalian evolution and the origin of the genus Homo. Palaeogeogr. Palaeoclimatol. Palaeoecol. 207, 399–420 (2004). [Google Scholar]
- 27.Faith J. T., Rowan J., Du A., Early hominins evolved within non-analog ecosystems. Proc. Natl. Acad. Sci. U.S.A. 116, 21478–21483 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dominguez-Rodrigo M., Pickering T. R., Semaw S., Rogers M. J., Cutmarked bones from Pliocene archaeological sites at Gona, Afar, Ethiopia: Implications for the function of the world’s oldest stone tools. J. Hum. Evol. 48, 109–121 (2005). [DOI] [PubMed] [Google Scholar]
- 29.Aiello L. C., Wheeler P., The expensive-tissue hypothesis: The brain and the digestive system in human and primate evolution. Curr. Anthropol. 36, 199–221 (1995). [Google Scholar]
- 30.Ungar P. S., Dental evidence for the reconstruction of diet in African early Homo. Curr. Anthropol. 53 (suppl. 6), S318–S329 (2012). [Google Scholar]
- 31.Antón SC., Snodgrass J. J., Origins and evolution of genus Homo: New perspectives. Curr. Anthropol. 53 (suppl. 6), S479–S496 (2012). [Google Scholar]
- 32.Thompson J. C., Carvalho S., Marean C. W., Alemseged Z., Origins of the human predatory pattern: The transition to large-animal exploitation by early hominins. Curr. Anthropol. 60, 1–23 (2019). [Google Scholar]
- 33.Braun D. R., et al. , Oldowan behavior and raw material transport: Perspectives from the Kanjera formation. J. Archaeol. Sci. 35, 2329–2345 (2008). [Google Scholar]
- 34.Grabowski M., Hatala K. G., Jungers W. L., Richmond B. G., Body mass estimates of hominin fossils and the evolution of human body size. J. Hum. Evol. 85, 75–93 (2015) [DOI] [PubMed] [Google Scholar]
- 35.Dean C., et al. , Growth processes in teeth distinguish modern humans from Homo erectus and earlier hominins. Nature 414, 628–631 (2001). [DOI] [PubMed] [Google Scholar]
- 36.Kelley J., Schwartz G. T., Life-history inference in the early hominins Australopithecus and Paranthropus. Int. J. Primatol. 33, 1332–1363 (2012). [Google Scholar]
- 37.Smith B. H., Dental development as a measure of life history in primates. Evolution 43, 683–688 (1989). [DOI] [PubMed] [Google Scholar]
- 38.Smith T. M., et al. , First molar eruption, weaning, and life history in living wild chimpanzees. Proc. Natl. Acad. Sci. U.S.A. 110, 2787–2791 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuhn S. L., Stiner M. C., Hearth and home in the middle Pleistocene. J. Anthropol. Res. 75, 305–327 (2019). [Google Scholar]
- 40.Mascaro J. S., Hackett P. D., Rilling J. K., Differential neural responses to child and sexual stimuli in human fathers and non-fathers and their hormonal correlates. Psychoneuroendocrinology 46, 153–163 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alonzo S. H., Social and coevolutionary feedbacks between mating and parental investment. Trends Ecol. Evol. 25, 99–108 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Trivers R. L., “Parental investment and sexual selection” in Sexual Selection and the Descent of Man, Campbell B., Ed. (Aldine, Chicago, IL, 1972), pp. 136–179. [Google Scholar]
- 43.Grafen A., Sibly R. M., A model of mate desertion. Anim. Behav. 26, 645–652 (1978). [Google Scholar]
- 44.Kokko H., Johnstone R. A., Why is mutual mate choice not the norm? Operational sex ratios, sex roles and the evolution of sexually dimorphic and monomorphic signalling. Philos. Trans. Roy. Soc. B 357, 319–330 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fromhage L., Jennions M. D., Coevolution of parental investment and sexually selected traits drives sex-role divergence. Nat. Commun. 7, 12517 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith J. M., Parental investment: A prospective analysis. Anim. Behav. 25, 1–9 (1977). [Google Scholar]
- 47.Fisher R. A., The Genetical Theory of Natural Selection (Oxford University Press, Oxford, UK, 1930). [Google Scholar]
- 48.Kokko H., Jennions M., Parental investment, sexual selection and sex ratios. J. Evol. Biol. 21, 919–948 (2008). [DOI] [PubMed] [Google Scholar]
- 49.Alger I., Cox D., The evolution of altruistic preferences: Mothers versus fathers. Rev. Econ. Househ. 11, 421–446 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The simulation code is available to readers at https://github.com/systemsscience/paternal.