Abstract
Background
T2DM may cause increased levels of oxidative stress and cardiac apoptosis through elevated blood glucose. The present study investigated the effects of Lactobacillus plantarum (L. plantarum) as a probiotic strain and inulin as a prebiotic supplement on cardiac oxidative stress and apoptotic markers in type 2 diabetes mellitus (T2DM) rats.
Methods
A high-fat diet and a low dose of streptozotocin were used to induce type 2 diabetes. The rats were divided into six groups which were supplemented with L. plantarum, inulin, or their combination for 8 weeks.
Results
The results showed improved activity of cardiac antioxidant parameters including total antioxidant capacity (TAC), superoxide dismutase (SOD), and glutathione peroxidase (GPx) (P < 0.001, P < 0.01, and P < 0.01, respectively) and decreased level of cardiac malondialdehyde (MDA) concentration (P < 0.05). These changes were accompanied with increased protein expression of cardiac obesity receptor (Ob-R) (P = 0.05) and reduced apoptotic markers such as tumor necrosis factor-alpha (TNF-α), Fas ligand (FasL), and caspase proteins (P < 0.001, P = 0.003, and P < 0.01, respectively) in T2DM rats after concurrent L. plantarum and inulin supplementation. Moreover, a remarkable correlation of cardiac Ob-R and oxidative stress parameters with cardiac apoptotic markers was observed (P < 0.01).
Conclusion
The concurrent use of L. plantarum and inulin seems to be beneficial, as they can lead to decreased heart complications of T2DM via reducing cardiac apoptotic markers.
1. Introduction
Diabetes is a prevalent and progressive metabolic disorder. Approximately, 1 in 11 adults suffers from this disease worldwide and over 90% of diabetic patients are type 2 diabetes mellitus (T2DM) [1]. The risk of death and cardiovascular complications among diabetic patients is 2 to 4 times higher than healthy individuals [2]. Diabetic cardiomyopathy (DCM) is one of the serious complications of diabetes identified by structural and functional deficits in the heart such as cardiac tissue fibrosis and cardiomyocyte apoptosis [3, 4]. Apoptosis has been considered as a result of diabetic hyperglycemia, hyperlipidemia, inflammation, and endoplasmic reticulum (ER) stress in the cardiomyocytes of the diabetic heart [5]. In comparison to the nondiabetic individuals, diabetic individuals show an 85-fold increase in cardiomyocyte apoptosis [6].
Oxidative stress is a key factor for apoptosis and diabetic cardiomyopathy. The balance between the production of reactive oxygen species (ROS) and their elimination through antioxidants production is essential for the maintenance of cardiovascular health [7]. According to Tangvarasittichai [8], hyperglycemia leads to increased ROS generation of which ROS-induced oxidative stress may cause abnormal gene expression, defective signal transduction, and apoptosis of the cardiomyocytes [9]. Diabetes reduces the cardiac activity of superoxide dismutase (SOD) and glutathione peroxidase (GPx) as well as impairs SOD expression [10, 11]. Guo et al. [12] revealed the increased formation of malondialdehyde (MDA) and reduced activity of SOD in the cardiomyocytes exposed to a high-glucose concentration. The cardiac protein expression of caspase-9, caspase-8, and caspase-3 can increase in diabetic rats. This would lead to hyperglycemia-induced apoptosis initiated by ROS derived from high glucose levels [13]. ROS leads to the initiation of tumor necrosis factor-alpha (TNF-α) gene expression [14]. On the other hand, TNF-α causes ROS production and contributes to tissue fibrosis in the diabetic state [13].
ROS-induced TNF-α expression may cause cardiomyocyte apoptosis through the upregulation of the Fas expression in high-glucose situations [15, 16]. The role of the Fas/Fas ligand (FasL) apoptotic pathway that leads to the activation of caspase cascade has been revealed in streptozotocin- (STZ-) induced apoptosis [17]. It was demonstrated that the production of the proinflammatory cytokines can be reduced by probiotic supplementation [18]. Sadeghzadeh et al. [19] also reported that TNF-α and oxidative stress damage can be suppressed through probiotic supplementation in a rat myocardial infarction model.
Probiotics are known as live microbial food supplements or bacteria components that modify the composition of the oral and enteric microflora, reduce adipose cell size, and promote leptin/adiponectin ratio [20]. According to Castex et al. [21], a probiotic diet might lead to increased activities of some antioxidant enzymes and reduced oxidative stress. Prebiotics are defined as nondigestible food ingredients including dietary fibers that promote the growth of beneficial bacteria in the gut [22]. Inulin is a prebiotic which can mitigate oxidative status by reducing lipid peroxidation and upregulating gene expressions of various antioxidant enzymes in different tissues [23]. Moreover, inulin supplementation could significantly improve leptin sensitivity [24]. Synbiotic is a combination of probiotic and prebiotic that may be beneficial in the treatment of diabetes through modulating serum insulin levels [25]. A significant increase of SOD, GPx, and catalase (CAT) was observed after concurrent administration of Lactobacillus casei and inulin among healthy volunteers [26]. Bejar et al. [27] also reported anti-diabetic effects of L. plantarum through the inhibition of α-glucosidase activity which can decrease fasting and postprandial blood glucose, in addition to insulin resistance and oxidative stress [28]. Furthermore, L. plantarum could increase serum levels of leptin [29] which may suppress heart apoptosis [30].
Diabetes leads to weight loss and increased food intake due to reduced concentration of serum leptin [31]. Leptin promotes heart function by improving glucose and fatty acid metabolism and reducing heart apoptosis [32]. It may also be associated with decreased apoptosis and fibrosis via activating SOD [33]. In addition, leptin reduces apoptosis in beta cells at physiological concentrations, by regulating BCL-2 and Bax expression [34].
Due to the important effects of the probiotic and prebiotic supplementation on hyperglycemia-induced oxidative stress and its protective impact on the production of proinflammatory cytokines, this paper investigated the effects of separate and concurrent supplementation of Lactobacillus plantarum 1085 (ATCC 8014) and inulin on T2DM-induced cardiac apoptosis by evaluating the cardiac antioxidant parameters including MDA, total antioxidant capacity (TAC), GPx, and SOD activity and the expression of the cardiac obesity receptor (Ob-R) as well as cardiac apoptotic markers. In addition, the correlations of these antioxidant enzymes and cardiac Ob-R with protein expression of cardiac TNF-α, FasL, and caspase were examined in T2DM male rats.
2. Materials and Methods
2.1. Animal Preparation and Experimental Protocol
The study was carried out on 35 male Wistar rats (200 ± 20 gr and 6 (±1) week old) purchased from the Laboratory Animal Center of Tabriz University of Medical Sciences, Tabriz, Iran. All rats were housed in a temperature- and humidity-controlled room at 22-25°C on 40-60% (four rats per cage). The rats were maintained at 12 : 12 h light/dark cycle and allowed ad libitum access to food and water.
2.2. Diet and Induction of T2DM
After the adaptation of the animals to the new environment through maintenance under a normal diet for 7 days, the rats were randomly assigned into six groups, as follows: HC: healthy control (n = 6); DC: diabetic control (n = 6); DSh: diabetic sham (n = 6); DL: diabetics treated by L. plantarum (n = 6); DI: diabetics treated by inulin (n = 5); DLI: diabetics treated by L. plantarum and inulin (n = 6). Normal diet contained 12% fat, 22% protein, and 66% carbohydrate. A high-fat diet (HFD) consisted of 58% fat, 17% protein, and 25% carbohydrate of which the composition is described in Table 1. The healthy control group continued to receive a normal diet until the end of the study (Table 2). To induce T2DM, the other groups were fed HFD for 4 weeks (from week 2 to week 5). Then, the HFD-fed rats received a single low dose of STZ (35 mg/kg intraperitoneally) in 50 μL citrate buffer (0.1 M, pH: 4.5) [35, 36]. Three days after injection, they were grouped to begin the intervention. After 12 h fasting, the rats with fasting blood sugar (FBS) of 250 mg/dL and higher were supposed as diabetic rats. After STZ induction, all of the control and diabetic rats were fed with the normal diet until the end of the study (week 6 to 13). In addition, all intervention groups received either probiotic or prebiotic for 8 weeks (week 6 to 13).
Table 1.
Composition of the high-fat diet (HFD).
| Composition | Percentage |
|---|---|
| Powdered NPD | 42 |
| Cholesterol | 1 |
| Ghee | 25 |
| Sucrose | 15 |
| Flour | 15 |
| Cholic acid | 0.5 |
| Pea flour | 0.5 |
Table 2.
Diet and intervention from the beginning to the end of the study.
| Markers | HFD | STZ injection | Normal diet | Saline gavage | Probiotic gavage | Prebiotic intake | Prebiotic and prebiotic intake |
|---|---|---|---|---|---|---|---|
| HC | — | — | Weeks 1 to 13 | — | — | — | — |
| DC | Weeks 2 to 5 | End of the week 5 | Weeks 6 to 13 | — | — | — | — |
| DSh | Weeks 2 to 5 | End of the week 5 | Weeks 6 to 13 | Weeks 6 to 13 | — | — | — |
| DL | Weeks 2 to 5 | End of the week 5 | Weeks 6 to 13 | — | Weeks 6 to 13 | — | — |
| DI | Weeks 2 to 5 | End of the week 5 | Weeks 6 to 13 | — | — | Weeks 6 to 13 | — |
| DLI | Weeks 2 to 5 | End of the week 5 | Weeks 6 to 13 | — | — | — | Weeks 6 to 13 |
HC: healthy control; DC: diabetic control; DSh: diabetic sham; DL: diabetics treated by L. plantarum; DI: diabetics treated by inulin; DLI: diabetics treated by L. plantarum and inulin; T2DM: type 2 diabetes mellitus; BDNF: brain-derived neurotrophic factor; HFD: high-fat diet; STZ: streptozotocin.
2.3. Preparation of the Supplementation
L. plantarum ATCC 8014 was acquired from the Biotechnology Research Center, TBZMED, Iran. L. plantarum was inoculated in Man-Rogosa-Sharpe (MRS) broth and cultured in aerobic conditions. The suspension was freshly prepared at a concentration of 107 colony-forming units per milliliter (CFU/mL) within 8 weeks. All rats received the suspension, using a gastric gavage once a day for 8 weeks [37]. The inulin content of the rat diet was assigned based on 5% of the daily food weight and was dissolved in drinking water.
2.4. Anesthesia and Tissue Extraction
At the end of the administration, after 12 h fasting, the animals were anesthetized with sodium pentobarbital (65 mg/kg BW IP, Sigma). Next, the left ventricle samples were hemogenized with 1 mL PBS and centrifuged for 40 min at 12000 rpm at 4°C. Then, the supernatant was separated and transferred into the microtubule and stored at −80°C for more experimental analysis.
2.5. Biochemical Assays
Cardiac levels of oxidative stress indices including SOD, GPx, and TAC were measured by biochemical kits, following the manufacturer's instruction (Randox Company, England). TAC was determined by Randox's total antioxidant status kit, as described by Miller et al. [38]. Cardiac SOD was measured, according to Breinholt et al., using a RANSOD kit (Randox Labs Crumlin, UK) [39]. Also, cardiac GPx was assayed by a RANSEL Kit (Randox Labs Crumlin, UK), as described by Paglia and Valentine [40]. Thiobarbituric acid was used to measure the MDA level; TBARS were determined by Esterbauer and Cheeseman [41]. In addition, serum glucose was evaluated spectrophotometrically using a diagnostic reagent kit (Pars Azmoon Company, Tehran, Iran). Furthermore, serum concentration of insulin was determined by the ELISA method (Shanghai Crystal Day Biotech Co., Ltd., China).
2.6. Western Blot Analysis
SDS-PAGE was performed by 10% polyacrylamide gels. 10 μL of each sample was electrophoresed at 100v and proteins were transferred to polyvinylidene difluoride (PVDF) membranes, using a transfer buffer and a Bio-Rad Scientific Instruments Transfer Unit (Sigma-Aldrich). PVDF membranes were incubated overnight at 4°C with primary antibodies against both the full-length and the cleaved fragments of caspase-9, caspase-8, and caspase-3 (Cell Signaling Technology) as well as against Ob-R, TNF-α, and FasL (Santa Crus Biotechnology) at a ratio of 1 : 200 for all of the first antibodies. This process was followed by incubation with anti-rabbit or anti-mouse secondary antibodies (Santa Crus Biotechnology) at the 1 : 5000 ratio. Blots were washed and inserted in the ECL substrate solution for 30 seconds, according to the manufacturer's instruction. The immunoblots were probed with an antibody recognizing β-actin as an internal control.
2.7. Statistical Analysis
All data were presented as the means ± SD for rats in each group. Statistical analyses were carried out by SPSS statistics software (version 25). Drawing of graphs and charts were performed, using the STATA (version 14) and GraphPad Prism (Version 8), respectively. One-way analysis of variance (ANOVA), followed by Tukey's test as a post hoc analysis, was utilized to examine the level of significance between groups. Correlations between two variables were carried out, using the Pearson correlation coefficient test. P < 0.05 was regarded as statistically significant.
3. Results
3.1. Effects on Food Intake, FBS, and Serum Insulin
As presented in Figure 1, food intake was significantly increased in the DC rats, in comparison to the HC group (P < 0.001). Also, there was a significant reduction of food intake in the treatment groups, compared with the DSh rats (P < 0.001). As compared to the HC group, a significant increase of FBS was observed in the DC rats as well as a remarkable decrease in serum insulin (P < 0.001). In addition, in comparison to the DSh rats, there was a significant reduction of FBS in the DLI group as well as a great increase in serum insulin (P = 0.01 and P = 0.005, respectively). Moreover, there was a strong correlation of food intake with cardiac apoptotic markers in the last week of the study as well as a great correlation of FBS and serum insulin with cardiac apoptotic proteins (Table 3).
Figure 1.
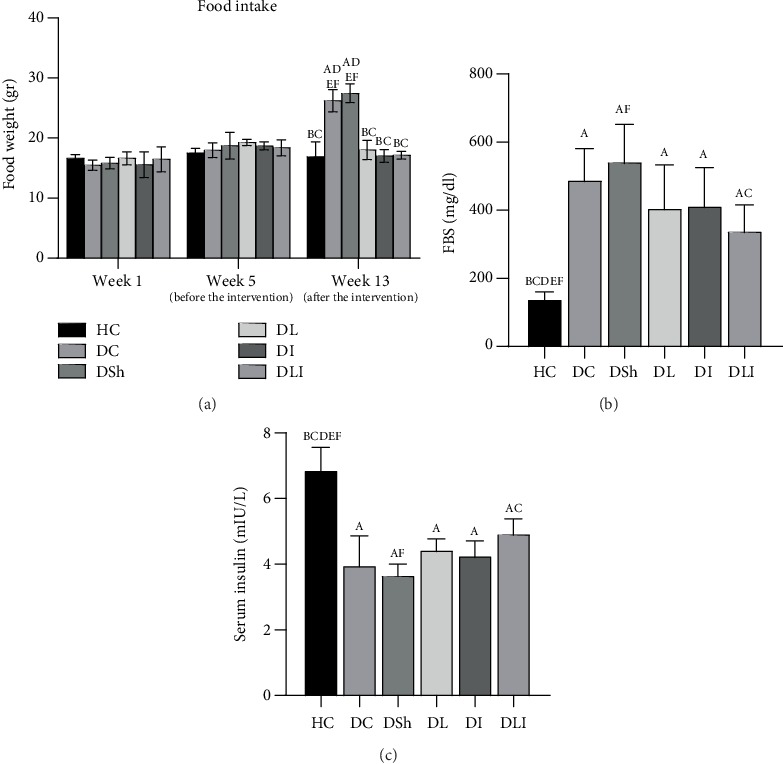
Effects of L. plantarum and inulin supplementation on (a) food intake, (b) FBS, and (c) serum insulin of the control and diabetic rats (n = 35). HC: healthy control; DC: diabetic control; DSh: diabetic sham; DL: diabetics treated by L. plantarum; DI: diabetics treated by inulin; DLI: diabetics treated by L. plantarum and inulin. Data were expressed as mean ± SD and regarded significantly different at P < 0.05 with a post hoc Tukey test.
Table 3.
Correlation coefficients of food intake, FBS, and serum insulin with the expression of cardiac TNF-α and apoptotic proteins at the last week of the study.
| TNF-α/β-actin | FasL/β-actin | Cleaved caspase-9/procaspase-9 | Cleaved caspase-8/procaspase-8 | Cleaved caspase-3/procaspase-3 | |
|---|---|---|---|---|---|
| Food intake (week 13) | 0.698∗ | 0.746∗ | 0.786∗ | 0.682∗ | 0.619∗ |
| FBS | 0.570∗ | 0.740∗ | 0.684∗ | 0.682∗ | 0.751∗ |
| Serum insulin | -0.576∗ | -0.755∗ | -0.639∗ | -0.759∗ | -0.781∗ |
TNF-α: tumor necrosis factor-α; FasL: Fas ligand; BW: body weight; FI: food intake; FBS: fasting blood glucose. P based on the Pearson correlation test (n = 35). P < 0.05 was regarded as statistically significant. ∗P < 0.001.
3.2. Effects on the Cardiac TAC, SOD, and GPx
As compared to the HC group, there was a significant decrease in the cardiac TAC, SOD, and GPx in the DC rats (P < 0.001). In comparison to the DSh group, there was a significant increase of the cardiac TAC, SOD, and GPx in the DL (P < 0.001, P = 0.013, and P = 0.001, respectively) as well as the DLI (P < 0.001, P = 0.001, and P = 0.001, respectively) group. In addition, the cardiac TAC and SOD increased significantly in the DI rats, compared to the DSh group (P < 0.001 and P = 0.001, respectively). In contrast, insignificant differences were observed in the cardiac GPx of the DI rats (Table 4, Figure 2). Also, cardiac TAC, SOD, and GPx were significantly correlated with cardiac apoptotic markers (Table 5). Furthermore, there was a strong correlation of the cardiac TAC, SOD, and GPx with cardiac TNF-α expression (P < 0.001, P < 0.001, and P < 0.01, respectively) (Figure 3).
Table 4.
.Effects of L. plantarum and inulin supplementation on cardiac oxidative stress markers in T2DM rats.
| Markers | HC | DC | DSh | DL | DI | DLI |
|---|---|---|---|---|---|---|
| Cardiac TAC (nmol/mg pro) | 3.76 (0.40) | 1.57 (0.18)## | 1.35 (0.31)## | 2.91 (0.40)∗∗∗ | 3.21 (0.56)∗∗∗ | 3.36 (0.43)∗∗∗ |
| Cardiac SOD (U/mgr pro) | 6.03 (0.88) | 3.65 (1.15)## | 3.61 (0.64)## | 5.26 (0.93)∗ | 5.75 (0.25)∗∗ | 5.67 (0.38)∗∗ |
| Cardiac GPx (U/gr pro) | 0.37 (0.04) | 0.16 (0.02)## | 0.16 (0.03)## | 0.30 (0.07)∗∗ | 0.25 (0.06) | 0.30 (0.06)∗∗ |
| Cardiac MDA (nmol/mg pro) | 0.13 (0.01) | 0.25 (0.02)# | 0.23 (0.03)# | 0.17 (0.06) | 0.21 (0.07) | 0.14 (0.03)∗ |
Data are expressed as means (SD). HC: healthy control; DC: diabetic control; DSh: diabetic sham; DL: diabetics treated by L. plantarum; DI: diabetics treated by inulin; DLI: diabetics treated by L. plantarum and inulin; TAC: total antioxidant capacity; SOD: superoxide dismutase; GPx: glutathione peroxidase; MDA: malondialdehyde.##P < 0.001, as compared to the HC group.#P < 0.01 as compared to the HC group. ∗∗∗P < 0.001 as compared to the DSh group. ∗∗P < 0.01 as compared to the DSh group. ∗P < 0.05 as compared to the DSh group.
Figure 2.
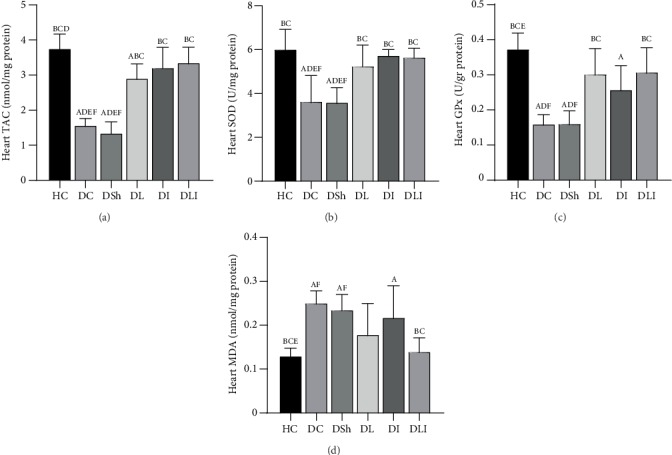
Effects of L. plantarum and inulin supplementation on oxidative stress markers in the left ventricles of excided hearts from the control and diabetic rats (n = 35). (a–d) The level of TAC, SOD activity, GPx activity, and MDA concentration in the left ventricles of the rats, respectively. HC: healthy control; DC: diabetic control; DSh: diabetic sham; DL: diabetics treated by L. plantarum; DI: diabetics treated by inulin; DLI: diabetics treated by L. plantarum and inulin. Data were expressed as mean ± SD and regarded significantly different at P < 0.05 with a post hoc Tukey test.
Table 5.
Correlation coefficients of cardiac oxidative stress markers and TNF-α with cardiac apoptotic markers.
| Cardiac markers | FasL/β-actin | Cleaved caspase-9/procaspase-9 | Cleaved caspase-8/procaspase-8 | Cleaved caspase-3/procaspase-3 |
|---|---|---|---|---|
| Cardiac TAC | -0.802∗∗ | -0.873∗∗ | -0.756∗∗ | -0.708∗∗ |
| Cardiac SOD | -0.673∗∗ | -0.711∗∗ | -0.618∗∗ | -0.576∗∗ |
| Cardiac GPx | -0.761∗∗ | -.0727∗∗ | -0.717∗∗ | -0.737∗∗ |
| Cardiac MDA | 0.663∗∗ | 0.712∗∗ | 0.583∗∗ | 0.616∗∗ |
| TNF-α/β-actin | 0.614∗∗ | 0.767∗∗ | 0.662∗∗ | 0.529∗ |
TAC: total antioxidant capacity; SOD: superoxide dismutase; GPx: glutathione peroxidase; MDA: malondialdehyde; TNF-α: tumor necrosis factor-α; FasL: Fas ligand. P based on the Pearson correlation test (n = 35). P < 0.05 was regarded as statistically significant. ∗∗P < 0.001 and ∗P < 0.01.
Figure 3.
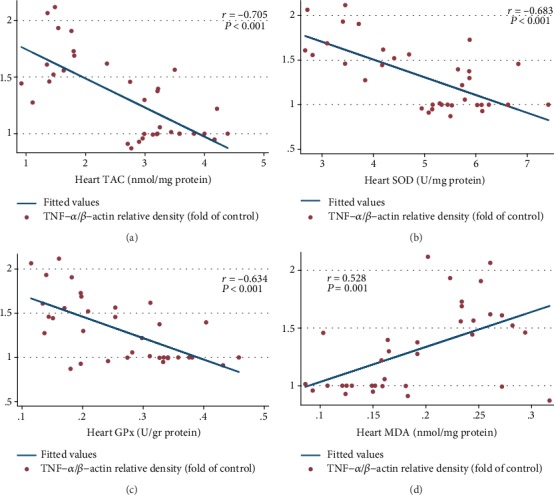
Inhibiting impacts of L. plantarum and inulin administration on the TNF-α expression in the left ventricles of the rats through the promotion of the cardiac oxidative status. (a–d) Correlation of the cardiac TAC, SOD activity, GPx activity, and MDA concentration with protein expression of TNF-α, respectively (n = 35). Correlation between two variables was determined, using the Pearson correlation coefficient; P < 0.05 was considered statistically significant.
3.3. Effects on the Cardiac MDA Concentration
Cardiac MDA concentration significantly increased in the DC group, compared with the HC rats (P = 0.001). There was no significant decrease in the cardiac MDA concentration of the DL and DI groups, compared with the DSh rats after supplementation. In contrast, post hoc analyses revealed a significant decrease of the cardiac MDA concentration in the DLI group, in comparison to the DSh rats (P = 0.013). However, the test showed insignificant differences in the cardiac MDA concentration among the treatment groups (Table 4, Figure 2). The Pearson correlation test showed a strong correlation between the cardiac MDA concentration and apoptotic markers (Table 5). In addition, a significant correlation was observed between the cardiac MDA concentration and TNF-α expression (P < 0.01) (Figure 3).
3.4. Effects on Cardiac Protein Expression of the Ob-R, FasL, and TNF-α
As compared with the HC group, there was a significant decrease of the Ob-R expression in DC rats (P = 0.005). Also, FasL and TNF-α protein expressions were significantly higher in the DC rats, in comparison with the HC group (P < 0.001). L. plantarum supplementation significantly decreased the cardiac protein expression of FasL, but not TNF-α, compared with the DSh group (P < 0.001). However, inulin supplementation decreased protein expression of FasL and TNF-α, compared with the DSh rats (P = 0.001 and P = 0.002, respectively). Such a decrease was also observed in the cardiac protein expression of FasL and TNF-α in the hearts of the DLI group, in comparison to the DSh rats (P = 0.003 and P < 0.001, respectively) (Figure 4). In addition, the increase of Ob-R expression in DLI rats was critically significant, compared to the DSh group (P = 0.05). In contrast, there was no significant increase in protein expression of the Ob-R in DL and DI rats (Figure 4). Moreover, a strong correlation was found between the cardiac TNF-α expression and apoptotic markers (Table 5) as well as a great correlation between the cardiac Ob-R expression and cardiac expression of the caspase proteins (Figure 5).
Figure 4.
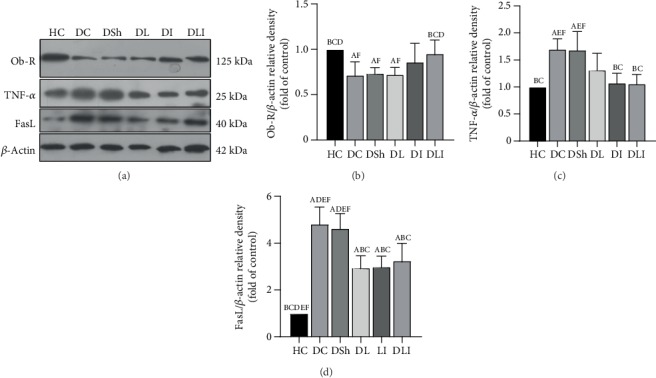
Effects of L. plantarum and inulin supplementation on the protein expression of Ob-R, TNF-α, and FasL in the left ventricles of excided hearts from the control and diabetic rats. (a) The protein levels of Ob-R, TNF-α, and FasL in the left ventricles of the rats, as determined by western blot analysis. (b–d) Relative protein quantification of Ob-R, TNF-α, and FasL on the basis of β-actin. HC: healthy control; DC: diabetic control; DSh: diabetic sham; DL: diabetics treated by L. plantarum; DI: diabetics treated by inulin; DLI: diabetics treated by L. plantarum and inulin. Values are mean ± SD and regarded significantly different at P < 0.05 with a post hoc Tukey test.
Figure 5.
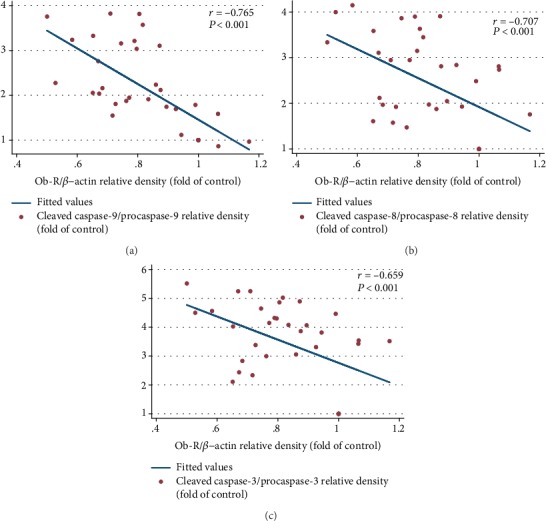
Inhibiting effects of L. plantarum and inulin supplementation on expression of the caspase proteins in the left ventricles of the rats through the increased expression of the Ob-R. (a–c) Correlation of the cardiac protein expression of the Ob-R with the cardiac expression of the activated caspase-9, caspase-8, and caspase-3, respectively (n = 35). Correlation between two variables was determined, using the Pearson correlation coefficient; P < 0.05 was considered statistically significant.
3.5. Effects on Protein Expression of Cleaved Caspase-9, Caspase-8, and Caspase-3 in the Hearts of the Control and Diabetic Rats
Western blot analysis showed a significant increase in the protein expression of cleaved caspase-9, caspase-8, and caspase-3 in the DC rats, compared with the HC group (P < 0.001). In comparison to the DSh group, there was a significant reduction in the cardiac protein expression of cleaved caspase-9, caspase-8, and caspase-3 in the DL (P = 0.002, P < 0.001, and P < 0.001, respectively) as well as the DLI rats (P < 0.001, P = 0.001, and P = 0.002, respectively). Inulin supplementation caused a significant reduction in the protein expression of cleaved caspase-9, compared to the DSh rats (P < 0.001) (Figure 6). However, there were no significant differences in the cardiac protein expression of cleaved caspase-8 and caspase-3 in the DI rats, in comparison with the DSh group (Figure 7). In addition, the protein expression of cleaved caspase-3 was significantly lower in the DL rats, compared with the DI and DLI groups (P < 0.001). Also, there was a significant decrease in the protein expression of cleaved caspase-8 in the DL (but not DLI) rats, in comparison to the DI group (P = 0.006) (Figure 7).
Figure 6.
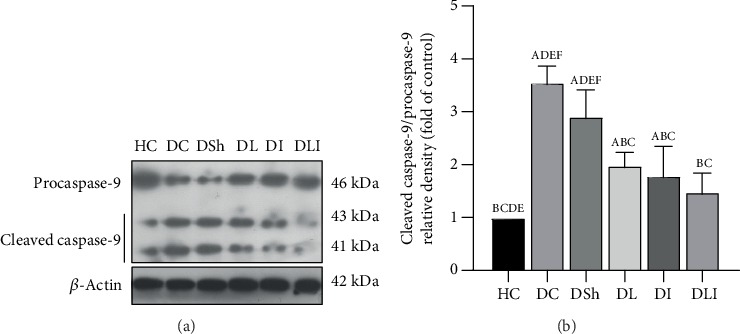
Effects of L. plantarum and inulin supplementation on protein expression of the cardiac caspase-9. (a) The protein level of caspase-9 in the left ventricles of excided hearts from the control and diabetic rats, as determined by western blot analysis. (b) Relative protein quantification of cleaved caspase-9, on the basis of procaspase-9. HC: healthy control; DC: diabetic control; DSh: diabetic sham; DL: diabetics treated by L. plantarum; DI: diabetics treated by inulin; DLI: diabetics treated by L. plantarum and inulin. Values are means ± SD and regarded significantly different at P < 0.05 with a post hoc Tukey test.
Figure 7.
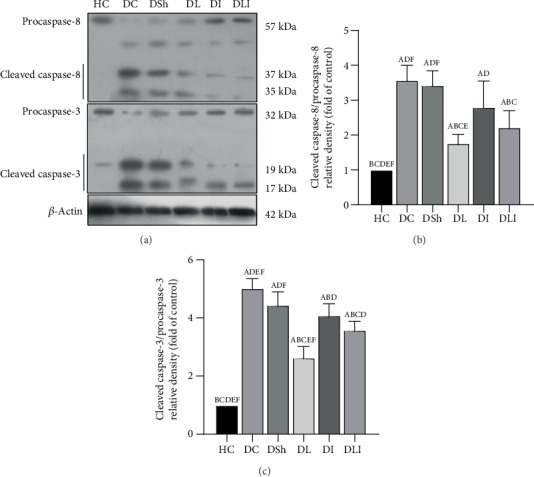
Effects of L. plantarum and inulin supplementation on protein expression of the cardiac caspase-8 and caspase-3. (a) The protein levels of caspase-8 and caspase-3 in the left ventricles of excided hearts from the control and diabetic rats, as determined by western blot analysis. (b) Relative protein quantification of cleaved caspase-8, on the basis of procaspase-8. (c) Relative protein quantification of cleaved caspase-3, on the basis of procaspase-3. HC: healthy control; DC: diabetic control; DSh: diabetic sham; DL: diabetics treated by L. plantarum; DI: diabetics treated by inulin; DLI: diabetics treated by L. plantarum and inulin. Values are means ± SD and regarded significantly different at P < 0.05 with a post hoc Tukey test.
4. Discussion
To our knowledge, the results of the present study demonstrated inhibiting effects of L. plantarum and inulin supplementation on the cardiac apoptotic markers by increasing the expression of Ob-R and antioxidant parameters in the diabetic rat hearts for the first time. DCM is one of the main heart complications in T2DM which increases the risk of heart failure and mortality in diabetic patients [42]. Myocardial cell apoptosis occurring in the DCM may play an important role in the development of the disease [43].
In the present study, the levels of antioxidant enzymes were evaluated in the cardiac tissue. Similar to previous researches [44, 45], we observed a significant reduction of cardiac TAC, GPx, and SOD activities and a remarkable increase of the cardiac MDA in the T2DM rats which were reversed after supplementation with L. plantarum and inulin.
Hyperglycemia condition in T2DM can lead to increased ROS production [8, 14] which upregulates TNF-α expression and induces cardiomyocyte apoptosis [46] via increasing the expression of the Fas [16]. Higher production of glucose-induced ROS plays an important role in the intrinsic apoptosis pathway by increasing cytochrome c release and activating caspase-9 and caspase-3 [47]. On the other hand, TNF-α provokes the activation of caspase-8 in the cardiomyocytes, leading to ROS generation [48] and subsequent apoptosis [49]. According to Wang et al. [50], antioxidant enzymes such as SOD are key factors in the reduction of the diabetes-induced cardiac apoptosis in mice. In a human research, Aouacheri et al. [44] illustrated decreased serum levels of GSH, GPx, and SOD activities as well as increased concentration of serum MDA in T2DM.
In the present research, increased activity of the cardiac SOD, GPx, and TAC was observed in the synbiotic group, accompanied by a reduced concentration of the cardiac MDA. Moreover, L. plantarum improved the cardiac activity of SOD, GPx, and TAC significantly. As described in our previous study by Morshedi et al. [51], there was also a considerable increase of serum TAC, SOD, and GPx activities and reduced concentration of serum MDA in the T2DM rats after a separate and concurrent supplementation of L. plantarum and inulin. However, in contrast with our results, Tunapong et al. [45] showed insignificant changes in the cardiac SOD by a separate and concurrent administration of the probiotics and prebiotics in the male obese insulin-resistant rats. But they revealed decreased cardiac MDA concentration in the rats treated by probiotics, prebiotics, and synbiotics. The reason for the observed difference seems to be due to the fashion by which diabetes was induced in the rats. The diabetic rats in our study were STZ induced, whereas those of the mentioned research were high-fat-diet-fed obese rats. Also, the composition of their supplement was different from the present study. We administered L. plantarum and inulin while they used L. paracasei and xylooligosaccharides in their study. Aluwong et al. [52] revealed the decreased concentration of serum MDA in Saccharomyces cerevisiae-supplemented diabetic rats with insignificant changes in the SOD activity. The observed controversy may be due to the type of diabetes or administered probiotic being studied. Unlike our research, Aluwong et al. studied on alloxan-induced T1DM rats supplemented with Saccharomyces cerevisiae. Moreover, their supplementation continued for four weeks which was shorter than ours. In a similar study by Zhang et al. [53], supplementation of Lacobacillus casei significantly reduced serum and hepatic levels of MDA and increased SOD and GSH-Px activities in hyperlipidemic rats. In vivo results of a research by Li et al. [54] indicated the ameliorative effects of L. plantarum C88 administration on the antioxidant status of the D-gal-induced oxidatively stressed mice including improved serum SOD activity and decreased concentration of hepatic MDA. In addition, Li et al. [28] revealed that L. plantarum X1 and L. plantarum CCFM30 can elevate SOD and GPx activities and ameliorate MDA level. Another similar study showed beneficial effects of L. plantarum BL0021 supplementation on hepatic and renal oxidative stress and apoptosis accompanied by reduced hepatic and renal MDA in pregnant rats [55].
In the present study, the western blot analysis revealed increased expression of TNF-α protein in the cardiomyocytes of the diabetic rats. As described above, TNF-α is an important mediator of the apoptotic pathway in the diabetic heart which stimulates the expression of the FasL protein. Mellado-Gil and Aguilar-Diosdado [17] demonstrated the role of the Fas/FasL system in the STZ-induced apoptosis in rats. Fas ligand provokes caspase-8 activation and causes cardiomyocyte apoptosis [56]. Similar to these studies, expression of the FasL was upregulated in the hearts of the T2DM rats in the present research. Also, there was a significant increase in the expression of caspase-9, caspase-8, and caspase-3 in the diabetic rats. In this regard, some studies demonstrated increased cardiac levels of the activated caspase-3 in the STZ-induced diabetic rats [57].
The results of the present research demonstrated reduced cardiac TNF-α and FasL expressions in the prebiotic and synbiotic groups. Additionally, it demonstrated a decreased expression of cleaved caspase-9, caspase-8, and caspase-3 in the hearts of the probiotic- and synbiotic-supplemented rats. Wang et al. [58] also reported anti-inflammatory and anti-apoptotic effects of Lactobacillus paracasei in the cardiomyocytes of the ovalbumin-induced allergic mice by decreasing the expression of cardiac TNF-α, caspase-3, and proapoptotic proteins such as Bax. In the present work, we also observed a strong correlation between the cardiac oxidative parameters and cardiac apoptotic markers which reveals the beneficial effects of the improved oxidative status on the prevention of cardiac apoptosis. Furthermore, there was a great correlation of the cardiac TNF-α expression with cardiac oxidative status and apoptotic proteins. A strong negative correlation of the cardiac TAC, SOD, and GPx with cardiac TNF-α expression was observed as well as a significant positive correlation between the cardiac MDA and TNF-α expression that indicates the inhibiting impacts of L. plantarum and inulin administration on TNF-α expression through the promotion of the cardiac oxidative status. However, the correlation of the cardiac apoptotic proteins with cardiac inflammatory and oxidative markers was not investigated in the mentioned previous studies.
In the present research, a high-fat diet and a single dose of STZ injection were used to develop hyperglycemia and insulin resistance [35, 36]. In fact, HFD was used to help induce T2DM before STZ injection [36]. Insulin resistance which occurs in type 2 diabetes mellitus is a situation that higher circulating insulin levels are necessary to achieve the integrated glucose-lowering response [59]. On the other hand, insulin secretion is severely impaired in poorly controlled type 2 diabetes which leads to the lower concentration of the serum insulin [60]. Impaired levels of insulin occur in diabetes which results in the disability of the cells to uptake glucose from the blood [29]. However, in the present study, the supplementation resulted in normalized levels of insulin and FBS in the intervention groups and prevented excessive food intake in the supplemented rats. In addition, as described in our previous study [29], serum leptin was significantly decreased in diabetic patients which was modulated after simultaneous administration of L. plantarum and inulin. In a similar study by Havel et al. [31], diabetes resulted in increased food intake by reducing circulating leptin levels. On the other hand, leptin inhibits the heart apoptosis by activating SOD or other possible manners [33]. Barouch et al. [30] demonstrated increased cardiac apoptosis in leptin-deficient ob/ob and leptin-resistant db/db mice. Moreover, McGaffin et al. [61] reported higher rates of caspase-3 activity and cardiac apoptosis as well as reduced expression of Bcl-2 in the infarcted tissue of lean and obese ob/ob mice, compared with the wild-type mice. Leptin is a survival cytokine for human neutrophils by inhibiting the activation of Bid, Bax, caspase-8, and caspase-3 and decreasing the mitochondrial release of cytochrome c [62]. Furthermore, Fruhbeck et al. [63] demonstrated that leptin reduces the expression of genes involved in inflammation and oxidative stress in the adipose tissue and skeletal muscle of ob/ob mice. Also, it has a protective effect against ethanol-induced oxidative stress [64].
According to our results, a significant increase in cardiac Ob-R expression was observed by the simultaneous use of inulin and L. plantarum. Also, there was a strong positive correlation of food intake and serum glucose with cardiac apoptotic markers in the last week of the study along with a great negative correlation of serum insulin and cardiac Ob receptor expression with the activation of caspase proteins. These results indicate beneficial effects of inulin and L. plantarum supplementation on cardiac apoptosis through controlling food intake, FBS, serum insulin, and enhanced expression of cardiac Ob receptor in diabetes (Figure 8).
Figure 8.
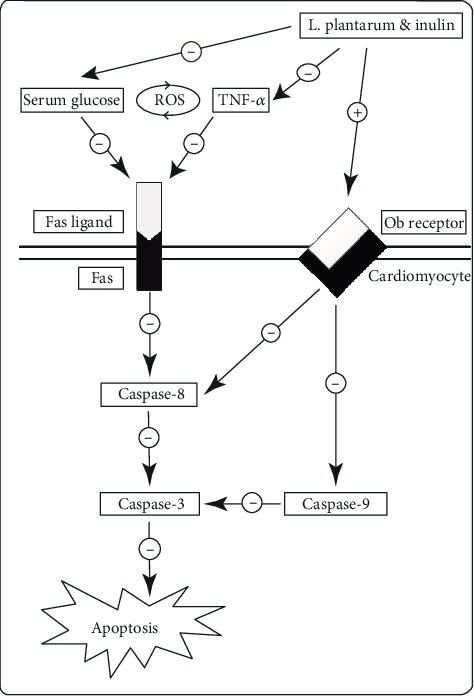
Suggested hypothesis of STZ-induced cardiac apoptosis, inhibited by L. plantarum and inulin supplementation in T2DM rats.
According to our previous work [29], improved lipid profile and metabolic status including modulated serum leptin can be achieved in T2DM rats by the administration of L. plantarum and inulin. This improved metabolic status and reduced blood glucose level can lead to decreased weight loss and food intake, followed by lower cardiac apoptotic markers observed in T2DM. L. plantarum and inulin supplementation may suppress cardiac apoptosis via direct and indirect mechanisms. L. plantarum and inulin supplementation could indirectly decrease apoptosis in diabetic hearts via improving the metabolic status and reducing serum glucose and food intake. In addition, it could inhibit cardiac apoptosis directly through different mechanisms such as the upregulated concentration of antioxidants and Ob-R as well as the downregulated expression of TNF-α in the cardiac tissue. Although no study has suggested the exact suitable dose of L. plantarum in order to reap benefits for cardiac disease, L. plantarum is safe at high doses. Fuentes et al. [65] illustrated that patients treated by daily consumption of L. plantarum-containing capsules (100 mg per capsule) for 12 weeks showed a significant reduction of LDL-C and improved other lipid parameters. Furthermore, L. plantarum administration leads to a reduction in cardiovascular disease risk factors such as systolic blood pressure, leptin, and fibrinogen in smokers consuming 400 mL/d of a rose-hip drink containing L. plantarum 299v (5 × 107 CFU/mL) for 6 weeks [66].
In summary, L. plantarum, inulin, or their combination can modulate cardiac antioxidant enzymes which result in lower protein expression of the cardiac apoptotic markers. Moreover, stronger effects of the concurrent supplementation of L. plantarum and inulin than their separate administration were revealed. In addition to the useful effects of the supplements, the present study had some limitations. One of the major limitations of the present research was that we could not examine the microbial population directly due to financial and time constraints. Also, the duration of the supplementation was a bit shorter which was due to the consideration of the higher mortality rate of the animals, proposed to be tested over a longer period of time. In the present research, the effect of L. plantarum and inulin on other apoptotic pathways was not investigated which is suggested to be investigated in future studies. Furthermore, we did not study the possible effects of our research on female rats. More animal and human researches are recommended to evaluate the possible effects of our interventions on female rats, other species, and human subjects.
5. Conclusion
Our findings provided the first evidence that Lactobacillus plantarum 1085 (ATCC 8014) and inulin supplementation could lead to a significant decrease of TNF-α-induced cardiac apoptosis in T2DM. The results also revealed decreased expression of cardiac apoptotic proteins including TNF-α, FasL, and caspase proteins after supplementation. In addition, the two supplements could reduce cardiac apoptotic markers by modulating cardiac Ob-R and antioxidant parameters. Moreover, the correlation of cardiac Ob-R and oxidative stress markers with the expression of the apoptotic proteins was noticed in the hearts of the T2DM male rats. Finally, we demonstrated L. plantarum accompanied by inulin as an effective useful supplement that may have beneficial effects on cardiac apoptosis inhibition in T2DM. Further studies are warranted to obtain more comprehensive results.
Acknowledgments
Special thanks is given to the Laboratory Animal Center of Drug Applied Research Center and Nutrition Research Center, Tabriz University of Medical Sciences, Tabriz, Iran. This study was financially supported by Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
Abbreviations
- Bax:
Bcl-2-associated X protein
- CAT:
Catalase
- DCM:
Diabetic cardiomyopathy
- ER:
Endoplasmic reticulum
- FasL:
Fas ligand
- GPx:
Glutathione peroxidase
- GSH:
Glutathione
- HFD:
High-fat diet
- MDA:
Malondialdehyde
- Ob-R:
Leptin or obesity receptor
- ROS:
Reactive oxygen species
- SOD:
Superoxide dismutase
- STZ:
Streptozotocin
- T2DM:
Type 2 diabetes mellitus
- TAC:
Total antioxidant capacity
- TNF-α:
Tumor necrosis factor-alpha.
Data Availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Ethical Approval
All experimental procedures were performed, according to the guidelines of the Principles of Laboratory Animal Care (NIH Publication, revised 1996) and the protocol was approved by the Institutional Animal Ethics Committee (IR.TBZMED. VCR.REC.1398.187).
Conflicts of Interest
The authors declare that they have no competing interests.
Authors' Contributions
MSA and SSA wrote the study protocol and study design. MM and KBV helped with the preparation and replication of bacterial and inulin solutions and performing intervention phases. SSA and MSA analyzed and interpreted the data and drew graphs. MM, KBV, and SM helped with keeping rats and supplementing. LR, PK, SSA, and MRH assisted in preparation or harvesting tissues. MSA supervised the whole stages of the research. SSA and MSA were involved in drafting the manuscript or revising it critically for content. All Authors have given final approval for the version to be published.
References
- 1.Zheng Y., Ley S. H., Hu F. B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nature Reviews. Endocrinology. 2018;14(2):88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 2.Rawshani A., Rawshani A., Franzén S., et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. The New England Journal of Medicine. 2017;376(15):1407–1418. doi: 10.1056/NEJMoa1608664. [DOI] [PubMed] [Google Scholar]
- 3.Yin L., Fang Y., Song T., et al. FBXL10 regulates cardiac dysfunction in diabetic cardiomyopathy via the PKC β2 pathway. Journal of Cellular and Molecular Medicine. 2019;23(4):2558–2567. doi: 10.1111/jcmm.14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miki T., Yuda S., Kouzu H., Miura T. Diabetic cardiomyopathy: pathophysiology and clinical features. Heart Failure Reviews. 2013;18(2):149–166. doi: 10.1007/s10741-012-9313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Q., Wang S., Cai L. Diabetic cardiomyopathy and its mechanisms: role of oxidative stress and damage. J Diabetes Investig. 2014;5(6):623–634. doi: 10.1111/jdi.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ouyang C., You J., Xie Z. The interplay between autophagy and apoptosis in the diabetic heart. Journal of Molecular and Cellular Cardiology. 2014;71:71–80. doi: 10.1016/j.yjmcc.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 7.Huynh K., Bernardo B. C., McMullen J. R., Ritchie R. H. Diabetic cardiomyopathy: mechanisms and new treatment strategies targeting antioxidant signaling pathways. Pharmacology & Therapeutics. 2014;142(3):375–415. doi: 10.1016/j.pharmthera.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World Journal of Diabetes. 2015;6(3):456–480. doi: 10.4239/wjd.v6.i3.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuethe F., Sigusch H., Bornstein S., Hilbig K., Kamvissi V., Figulla H. Apoptosis in patients with dilated cardiomyopathy and diabetes: a feature of diabetic cardiomyopathy? Hormone and Metabolic Research. 2007;39(9):672–676. doi: 10.1055/s-2007-985823. [DOI] [PubMed] [Google Scholar]
- 10.Kaul N., Siveski-Iliskovic N., Thomas T. P., Hill M., Khaper N., Singal P. K. Probucol improves antioxidant activity and modulates development of diabetic cardiomyopathy. Nutrition. 1995;11(5 Supplement):551–554. [PubMed] [Google Scholar]
- 11.Ghattas M. H., Abo-Elmatty D. M. Association of polymorphic markers of the catalase and superoxide dismutase genes with type 2 diabetes mellitus. DNA and Cell Biology. 2012;31(11):1598–1603. doi: 10.1089/dna.2012.1739. [DOI] [PubMed] [Google Scholar]
- 12.Guo S., Yao Q., Ke Z., Chen H., Wu J., Liu C. Resveratrol attenuates high glucose-induced oxidative stress and cardiomyocyte apoptosis through AMPK. Molecular and Cellular Endocrinology. 2015;412:85–94. doi: 10.1016/j.mce.2015.05.034. [DOI] [PubMed] [Google Scholar]
- 13.Amin A. H., El-Missiry M. A., Othman A. I. Melatonin ameliorates metabolic risk factors, modulates apoptotic proteins, and protects the rat heart against diabetes-induced apoptosis. European Journal of Pharmacology. 2015;747:166–173. doi: 10.1016/j.ejphar.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Quan Y., Jiang C. T., Xue B., Zhu S. G., Wang X. High glucose stimulates TNFα and MCP-1 expression in rat microglia via ROS and NF-κB pathways. Acta Pharmacologica Sinica. 2011;32(2):188–193. doi: 10.1038/aps.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cherng S.-H., Huang C.-Y., Kuo W.-W., et al. GABA tea prevents cardiac fibrosis by attenuating TNF-alpha and Fas/FasL-mediated apoptosis in streptozotocin-induced diabetic rats. Food and Chemical Toxicology. 2014;65:90–96. doi: 10.1016/j.fct.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 16.Kageyama S.-i., Yokoo H., Tomita K., et al. High glucose-induced apoptosis in human coronary artery endothelial cells involves up-regulation of death receptors. Cardiovasc Diabetol. 2011;10(1):p. 73. doi: 10.1186/1475-2840-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mellado-Gil J., Aguilar-Diosdado M. High glucose potentiates cytokine-and streptozotocin-induced apoptosis of rat islet cells: effect on apoptosis-related genes. The Journal of Endocrinology. 2004;183(1):155–162. doi: 10.1677/joe.1.05542. [DOI] [PubMed] [Google Scholar]
- 18.Wang X., Li Z., Xu Z., Wang Z., Feng J. Probiotics prevent Hirschsprung’s disease-associated enterocolitis: a prospective multicenter randomized controlled trial. International Journal of Colorectal Disease. 2015;30(1):105–110. doi: 10.1007/s00384-014-2054-0. [DOI] [PubMed] [Google Scholar]
- 19.Sadeghzadeh J., Students' Research Center, School of Medicine, Semnan University of Medical Sciences, Semnan, Iran, Vakili A., et al. The effect of oral consumption of probiotics in prevention of heart injury in a rat myocardial infarction model: a histopathological, hemodynamic and biochemical evaluation. Iranian Biomedical Journal. 2017;21(3):174–181. doi: 10.18869/acadpub.ibj.21.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalogeris T., Baines C. P., Krenz M., Korthuis R. J. Ischemia/reperfusion. Comprehensive Physiology. 2011;7(1):113–170. doi: 10.1002/cphy.c160006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castex M., Lemaire P., Wabete N., Chim L. Effect of dietary probiotic Pediococcus acidilactici on antioxidant defences and oxidative stress status of shrimp Litopenaeus stylirostris. Aquaculture. 2009;294(3-4):306–313. doi: 10.1016/j.aquaculture.2009.06.016. [DOI] [Google Scholar]
- 22.Kavitha K., Reddy A. G., Reddy K. K., Kumar C. S., Boobalan G., Jayakanth K. Hypoglycemic, hypolipidemic and antioxidant effects of pioglitazone, insulin and synbiotic in diabetic rats. Veterinary World. 2016;9(2):118–122. doi: 10.14202/vetworld.2016.118-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu W.-T., Chen H.-L. Konjac glucomannan and inulin systematically modulate antioxidant defense in rats fed a high-fat fiber-free diet. Journal of Agricultural and Food Chemistry. 2011;59(17):9194–9200. doi: 10.1021/jf202060p. [DOI] [PubMed] [Google Scholar]
- 24.Erejuwa O., Sulaiman S., Wahab M. Modulation of gut microbiota in the management of metabolic disorders: the prospects and challenges. International Journal of Molecular Sciences. 2014;15(3):4158–4188. doi: 10.3390/ijms15034158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asemi Z., Khorrami-Rad A., Alizadeh S.-A., Shakeri H., Esmaillzadeh A. Effects of synbiotic food consumption on metabolic status of diabetic patients: a double-blind randomized cross-over controlled clinical trial. Clinical Nutrition. 2014;33(2):198–203. doi: 10.1016/j.clnu.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 26.Kleniewska P., Hoffmann A., Pniewska E., Pawliczak R. The influence of probiotic Lactobacillus casei in combination with prebiotic inulin on the antioxidant capacity of human plasma. Oxidative Medicine and Cellular Longevity. 2016;2016:10. doi: 10.1155/2016/1340903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bejar W., Hamden K., Ben Salah R., Chouayekh H. Lactobacillus plantarum TN627 significantly reduces complications of alloxan- induced diabetes in rats. Anaerobe. 2013;24:4–11. doi: 10.1016/j.anaerobe.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Li X., Wang N., Yin B., et al. Lactobacillus plantarum X1 with α-glucosidase inhibitory activity ameliorates type 2 diabetes in mice. RSC Advances. 2016;6(68):63536–63547. doi: 10.1039/C6RA10858J. [DOI] [Google Scholar]
- 29.Valenlia K. B., Morshedi M., Saghafi-Asl M., Shahabi P., Abbasi M. M. Beneficial impacts of Lactobacillus plantarum and inulin on hypothalamic levels of insulin, leptin, and oxidative markers in diabetic rats. Journal of Functional Foods. 2018;46:529–537. doi: 10.1016/j.jff.2018.04.069. [DOI] [Google Scholar]
- 30.Barouch L. A., Gao D., Chen L., et al. Cardiac myocyte apoptosis is associated with increased DNA damage and decreased survival in murine models of obesity. Circulation Research. 2006;98(1):119–124. doi: 10.1161/01.RES.0000199348.10580.1d. [DOI] [PubMed] [Google Scholar]
- 31.Havel P. J., Uriu-Hare J. Y., Liu T., et al. Marked and rapid decreases of circulating leptin in streptozotocin diabetic rats: reversal by insulin. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1998;274(5):R1482–R1491. doi: 10.1152/ajpregu.1998.274.5.R1482. [DOI] [PubMed] [Google Scholar]
- 32.Hall M. E., Harmancey R., Stec D. E. Lean heart: role of leptin in cardiac hypertrophy and metabolism. World Journal of Cardiology. 2015;7(9):511–524. doi: 10.4330/wjc.v7.i9.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng J., Fang J., Yin Y. J., et al. Leptin protects cardiomyocytes from serum-deprivation-induced apoptosis by increasing anti-oxidant defence. Clinical and Experimental Pharmacology & Physiology. 2010;37(10):955–962. doi: 10.1111/j.1440-1681.2010.05415.x. [DOI] [PubMed] [Google Scholar]
- 34.Brown J. E. P., Dunmore S. J. Leptin decreases apoptosis and alters BCL-2: Bax ratio in clonal rodent pancreatic beta-cells. Diabetes/Metabolism Research and Reviews. 2007;23(6):497–502. doi: 10.1002/dmrr.726. [DOI] [PubMed] [Google Scholar]
- 35.Srinivasan K., Viswanad B., Asrat L., Kaul C., Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacological Research. 2005;52(4):313–320. doi: 10.1016/j.phrs.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Skovsø S. Modeling type 2 diabetes in rats using high fat diet and streptozotocin. Journal of Diabetes Investigation. 2014;5(4):349–358. doi: 10.1111/jdi.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X., Wang E., Yin B., et al. Effects of Lactobacillus casei CCFM419 on insulin resistance and gut microbiota in type 2 diabetic mice. Beneficial Microbes. 2017;8(3):421–432. doi: 10.3920/BM2016.0167. [DOI] [PubMed] [Google Scholar]
- 38.Miller N. J., Rice-Evans C., Davies M. J., Gopinathan V., Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clinical Science. 1993;84(4):407–412. doi: 10.1042/cs0840407. [DOI] [PubMed] [Google Scholar]
- 39.Breinholt V., Lauridsen S., Dragsted L. Differential effects of dietary flavonoids on drug metabolizing and antioxidant enzymes in female rat. Xenobiotica. 1999;29(12):1227–1240. doi: 10.1080/004982599237903. [DOI] [PubMed] [Google Scholar]
- 40.Paglia D. E., Valentine W. N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. The Journal of Laboratory and Clinical Medicine. 1967;70(1):158–169. [PubMed] [Google Scholar]
- 41.Esterbauer H., Cheeseman K. H. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods in Enzymology. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi S., Liang Q. Autophagy and mitophagy in diabetic cardiomyopathy. Biochimica et Biophysica Acta. 2015;1852(2):252–261. doi: 10.1016/j.bbadis.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 43.Qin W.-d., Liu G.-l., Wang J., et al. Poly (ADP-ribose) polymerase 1 inhibition protects cardiomyocytes from inflammation and apoptosis in diabetic cardiomyopathy. Oncotarget. 2016;7(24):35618–35631. doi: 10.18632/oncotarget.8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aouacheri O., Saka S., Krim M., Messaadia A., Maidi I. The investigation of the oxidative stress-related parameters in type 2 diabetes mellitus. Canadian Journal of Diabetes. 2015;39(1):44–49. doi: 10.1016/j.jcjd.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 45.Tunapong W., Apaijai N., Yasom S., et al. Chronic treatment with prebiotics, probiotics and synbiotics attenuated cardiac dysfunction by improving cardiac mitochondrial dysfunction in male obese insulin-resistant rats. European Journal of Nutrition. 2018;57(6):2091–2104. doi: 10.1007/s00394-017-1482-3. [DOI] [PubMed] [Google Scholar]
- 46.Aikawa R., Nitta-Komatsubara Y., Kudoh S., et al. Reactive oxygen species induce cardiomyocyte apoptosis partly through TNF-α. Cytokine. 2002;18(4):179–183. doi: 10.1006/cyto.2001.1007. [DOI] [PubMed] [Google Scholar]
- 47.Das S., Babick A. P., Xu Y. J., Takeda N., Rodriguez-Levya D., Dhalla N. S. TNF-alpha-mediated signal transduction pathway is a major determinant of apoptosis in dilated cardiomyopathy. Journal of Cellular and Molecular Medicine. 2010;14(7):1988–1997. doi: 10.1111/j.1582-4934.2009.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberge S., Roussel J., Andersson D. C., et al. TNF-α-mediated caspase-8 activation induces ROS production and TRPM2 activation in adult ventricular myocytes. Cardiovascular Research. 2014;103(1):90–99. doi: 10.1093/cvr/cvu112. [DOI] [PubMed] [Google Scholar]
- 49.Tsai C.-Y., Wang C.-C., Lai T.-Y., et al. Antioxidant effects of diallyl trisulfide on high glucose-induced apoptosis are mediated by the PI3K/Akt-dependent activation of Nrf 2 in cardiomyocytes. International Journal of Cardiology. 2013;168(2):1286–1297. doi: 10.1016/j.ijcard.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 50.Wang F., Fisher S. A., Zhong J., Wu Y., Yang P. Superoxide dismutase 1 in vivo ameliorates maternal diabetes mellitus–induced apoptosis and heart defects through restoration of impaired Wnt signaling. Circulation. Cardiovascular Genetics. 2015;8(5):665–676. doi: 10.1161/CIRCGENETICS.115.001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morshedi M., Valenlia K. B., Hosseinifard E. S., et al. Beneficial psychological effects of novel psychobiotics in diabetic rats: the interaction among the gut, blood, and amygdala. The Journal of Nutritional Biochemistry. 2018;57:145–152. doi: 10.1016/j.jnutbio.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 52.Aluwong T., Ayo J., Kpukple A., Oladipo O. Amelioration of hyperglycaemia, oxidative stress and dyslipidaemia in alloxan-induced diabetic Wistar rats treated with probiotic and vitamin C. Nutrients. 2016;8(5):p. 151. doi: 10.3390/nu8050151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y., du R., Wang L., Zhang H. The antioxidative effects of probiotic Lactobacillus casei Zhang on the hyperlipidemic rats. European Food Research and Technology. 2010;231(1):151–158. doi: 10.1007/s00217-010-1255-1. [DOI] [Google Scholar]
- 54.Li S., Zhao Y., Zhang L., et al. Antioxidant activity of Lactobacillus plantarum strains isolated from traditional Chinese fermented foods. Food Chemistry. 2012;135(3):1914–1919. doi: 10.1016/j.foodchem.2012.06.048. [DOI] [PubMed] [Google Scholar]
- 55.Bouhafs L., Moudilou E. N., Exbrayat J. M., Lahouel M., Idoui T. Protective effects of probiotic Lactobacillus plantarum BJ0021 on liver and kidney oxidative stress and apoptosis induced by endosulfan in pregnant rats. Renal Failure. 2015;37(8):p. 1370. doi: 10.3109/0886022X.2015.1073543. [DOI] [PubMed] [Google Scholar]
- 56.Lee S. D., Tzang B. S., Kuo W. W., et al. Cardiac fas receptor-dependent apoptotic pathway in obese Zucker rats. Obesity. 2007;15(10):2407–2415. doi: 10.1038/oby.2007.286. [DOI] [PubMed] [Google Scholar]
- 57.Cheng S.-M., Ho T.-J., Yang A.-L., et al. Exercise training enhances cardiac IGFI-R/PI3K/Akt and Bcl-2 family associated pro-survival pathways in streptozotocin-induced diabetic rats. International Journal of Cardiology. 2013;167(2):478–485. doi: 10.1016/j.ijcard.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 58.Wang H.-F., Tseng C.-Y., Chang M.-H., et al. Anti-inflammatory effects of probiotic Lactobacillus paracasi on ventricles of BALB/C mice treated with ovalbumin. The Chinese Journal of Physiology. 2012;55(1):37–46. doi: 10.4077/CJP.2012.AMM107. [DOI] [PubMed] [Google Scholar]
- 59.Petersen M. C., Shulman G. I. Mechanisms of insulin action and insulin resistance. Physiological Reviews. 2018;98(4):2133–2223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guillausseau P.-J., Meas T., Virally M., Laloi-Michelin M., Médeau V., Kevorkian J. P. Abnormalities in insulin secretion in type 2 diabetes mellitus. Diabetes & Metabolism. 2008;34:S43–S48. doi: 10.1016/S1262-3636(08)73394-9. [DOI] [PubMed] [Google Scholar]
- 61.McGaffin K. R., Zou B., McTiernan C. F., O’Donnell C. P. Leptin attenuates cardiac apoptosis after chronic ischaemic injury. Cardiovascular Research. 2009;83(2):313–324. doi: 10.1093/cvr/cvp071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bruno A., Conus S., Schmid I., Simon H. U. Apoptotic pathways are inhibited by leptin receptor activation in neutrophils. Journal of Immunology. 2005;174(12):8090–8096. doi: 10.4049/jimmunol.174.12.8090. [DOI] [PubMed] [Google Scholar]
- 63.Frühbeck G., Catalán V., Rodríguez A., et al. Normalization of adiponectin concentrations by leptin replacement in ob/ob mice is accompanied by reductions in systemic oxidative stress and inflammation. Scientific Reports. 2017;7(1):1–12. doi: 10.1038/s41598-017-02848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Balasubramaniyan V., Shukla R., Murugaiyan G., Bhonde R. R., Nalini N. Mouse recombinant leptin protects human hepatoma HepG2 against apoptosis, TNF-α response and oxidative stress induced by the hepatotoxin–ethanol. Biochimica et Biophysica Acta - General Subjects. 2007;1770(8):1136–1144. doi: 10.1016/j.bbagen.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 65.Fuentes M. C., Lajo T., Carrión J. M., Cuñé J. A randomized clinical trial evaluating a proprietary mixture of Lactobacillus plantarum strains for lowering cholesterol 1. Mediterranean Journal of Nutrition and Metabolism. 2016;9(2):125–135. doi: 10.3233/MNM-160065. [DOI] [Google Scholar]
- 66.Naruszewicz M., Johansson M. L., Zapolska-Downar D., Bukowska H. Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. The American Journal of Clinical Nutrition. 2002;76(6):1249–1255. doi: 10.1093/ajcn/76.6.1249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.


