Abstract
ALDH1A1 (aldehyde dehydrogenase 1A1) is a crucial protein in retinoids’ metabolism, and the lack of ALDH1A1 inhibits the fat deposition in mice. However, whether ALDH1A1 has a similar effect on chickens’ fat-depot is still unknown. In this study, we investigate the role of ALDH1A1 in chickens’ adipogenesis. The immortalized chicken preadipocyte 1 (ICP1) cell line and chicken primary preadipocytes isolated from abdominal fat were used to perform a series of experiments in vitro to elucidate the effects of ALDH1A1. In addition, lentivirus was used to verify the results of cell experiments in vivo. The data showed that overexpression of ALDH1A1 significantly weakened the proliferation of preadipocytes and suppressed the differentiation of preadipocytes through the PPARγ pathway, and the knockdown experiments had the opposite results. Moreover, chickens injected with overexpression lentivirus had higher abdominal fat percentage, a bigger size of lipid droplets, and higher triglyceride content in abdominal fat, and chickens injected with interfering lentivirus had the opposite situation. We proved that ALDH1A1 not only inhibited the proliferation and differentiation of chickens’ preadipocytes in vitro, but also inhibited the fat-depot of chickens in vivo, which was completely opposite the function of ALDH1A1 in mice, indicating that ALDH1A1 may have a different mechanism that is still unknown.
Keywords: ALDH1A1, preadipocytes, proliferation, differentiation, adipogenesis, PPARγ pathway, chicken
1. Introduction
Adipocyte differentiation is thought to be regulated by a series of transcription factors, including the C/EBPs protein family [1,2,3] and peroxisome proliferator-activated receptor g (PPARγ) [4]. On the one hand, retinoic acid (RA), the main product of aldehyde dehydrogenase 1A1 (ALDH1A1), inhibits adipogenesis through these transcription factors in mammals [5,6]. On the other hand, RA inhibits adipogenesis by binding to the retinoic acid receptor (RAR) [7,8,9]. Retinaldehyde dehydrogenases participate in the determination of cellular concentrations of free retinaldehyde by oxidizing retinaldehyde to RA [10].
ALDH1A1 is a member of the ALDH gene family, which plays an important role in vitamin A (retinol) and carotenoid metabolism, catalyzing a series of reactions and maintaining the normal functions of animals [11]. According to the National Center of Biotechnology Information (NCBI) data, the coding sequences (CDS) of ALDH1A1 in chicken contain 1529 bases, encoding 509 amino acids, with a total of 16 exons (www.ncbi.nlm.nih.gov/nuccore/NM_204577.4).
The aldehyde dehydrogenase 1 family catalyzes the reaction of vitamin A and its metabolites’ conversion, including retinaldehyde and retinoic acid [12]. ALDH1A1 is a key to the second step of the retinol metabolic pathway (vitamin A metabolism), which is the conversion of retinaldehyde to retinoic acid [13]. Retinol and its metabolites regulate other genes by activating some nuclear receptors, including the RAR and the retinoid X receptor (RXR), and releasing transcriptional factors, or binding to an RA response element and regulating the expression of target genes [14,15,16,17]. Thus, the ability on keep the balance between retinol and its metabolites can make ALDH1A1 involved in regulating other crucial genes that participate in important biological phenomenon like adipogenesis [18,19,20].
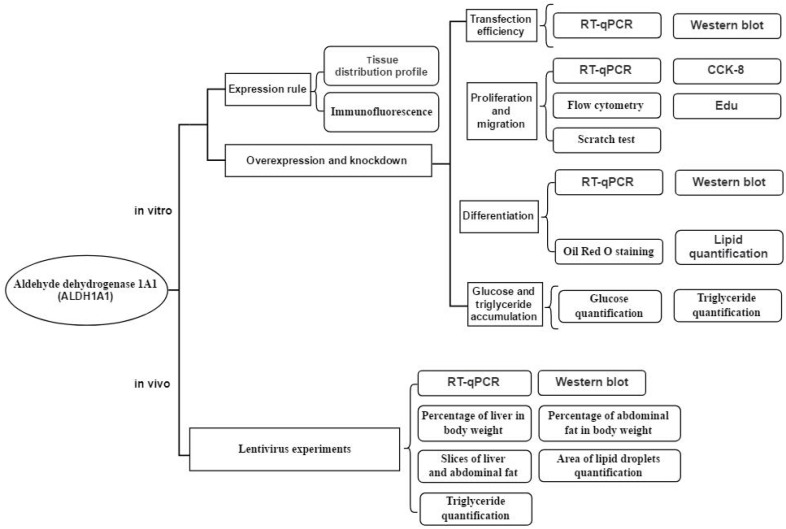
In recent years, ALDH1A1 has been found to be involved in adipogenesis through vitamin A metabolism. Research has found that ALDH1A1 deficiency inhibited high-fat diet-induced adipogenesis. At the same time, researchers observed that the lack of ALDH1A1 prevented the visceral fat deposition induced by a high-fat diet in female mice, but in males, this effect was not as great as that of females, which meant ALDH1A1 had different influences on male and female mice [18]. Other studies have shown that the lack of ALDH1A1 induces a brown adipose tissue-like transcriptional program in white adipose tissue, promoting uncoupled respiration and adaptive thermogenesis, limiting weight gain in obese mice and improving glucose homeostasis [19]. Moreover, researchers found that PPARγ expression was significantly suppressed by zinc-finger protein 423 (ZFP423) reduction, and this change induced adipogenesis damage in ALDH1A1-deficient adipocytes. In their studies, they expressed ALDH1s in ALDH1A1−/− cells and found a significant correlation between ALDH1s (including ALDH1A1 and ALDH1A3), especially ALDH1A1, ZFP423, and PPARγ expression levels. Otherwise, with low concentrations of RA, PPARγ expression in ALDH1A1−/− cells were partially recovered, indicating that ALDH1A1’s effects were dependent on RA production [20]. However, whether ALDH1A1 plays a similar role in chicken is still unknown. To answer this question, we aimed to understand the function of ALDH1A1 in chickens’ adipogenesis. In our study, we tried to find out whether ALDH1A1 improved or inhibited the proliferation and differentiation of preadipocytes in vitro and whether it accelerated or impeded the fat-depot in chickens. The experimental flow is shown below (Figure 1).
Figure 1.
The workflow of the experiments on aldehyde dehydrogenase 1A1 (ALDH1A1). The experimental flow diagram for the ALDH1A1 experiments in vitro and in vivo.
2. Results
2.1. ALDH1A1 Expression in Chickens
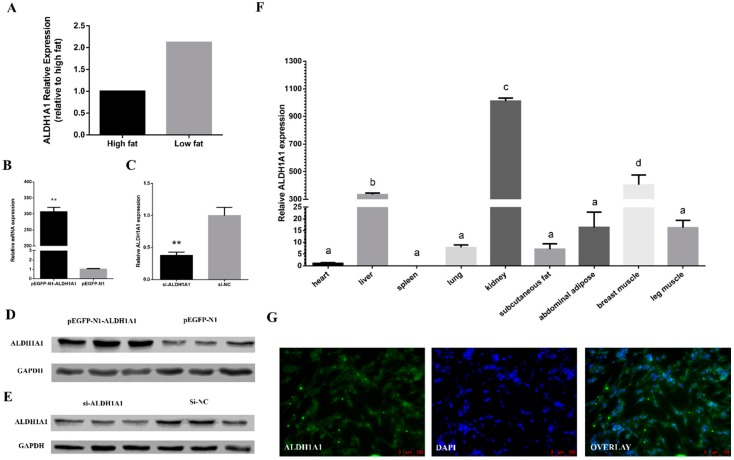
The previous transcriptome sequencing experiments showed that the mRNA expression levels of ALDH1A1 in high abdominal fat percentage chickens and low abdominal fat percentage chickens had significant differences [21]. ALDH1A1 is highly expressed in low abdominal fat chickens’ abdominal fat, which indicated that ALDH1A1 might play a negative role in the fat-depot in chickens (Figure 2A). To further investigate the function of ALDH1A1, the overexpression vector and small interfering RNA (siRNA) of ALDH1A1 were produced. Their efficiency was detected by real-time quantitative polymerase chain reaction (RT-qPCR) and Western blot in chicken primary preadipocytes differentiated for five days (Figure 2B–E).
Figure 2.
ALDH1A1 expression in chickens. (A) The mRNA level of ALDH1A1 in high abdominal fat chickens and low abdominal fat chickens. (B–E) The ALDH1A1 mRNA (n = 6) and protein level (n = 3) in preadipocytes differentiated for five days after being transfected with the overexpression vector and small interfering RNA (siRNA), respectively. (F) The mRNA level of ALDH1A1 in different tissues of seven weeks Xinghua chickens. Data were compared with the ALDH1A1 mRNA expression of heart (n = 6). (G) ALDH1A1 immunofluorescence of chicken primary preadipocytes in proliferation, scale bar: 100 μm. In all panels, the results are shown as the mean ± S.E.M., and the data are representative of three independent assays. The statistical significance of the differences between means was assessed using the unpaired Student’s t-test (* p < 0.05; ** p < 0.01) and Duncan’s test (p < 0.05).
In addition, six chickens in seven weeks were slaughtered, and the tissues, including heart, liver, spleen, lung, kidney, abdominal fat, subcutaneous fat, breast muscle, and leg muscle, were collected and used to detect the mRNA level of ALDH1A1 (Figure 2F). The data showed that ALDH1A1 was expressed highest in kidney and decreased in the breast muscle, liver, abdominal fat, leg muscles, lungs, subcutaneous fat, heart, and spleen in turn. Moreover, after immunofluorescence staining, ALDH1A1 was found to be expressed in both cell nucleus and cytoplasm (Figure 2G).
2.2. ALDH1A1 Represses Chicken Preadipocytes’ Proliferation and Migration
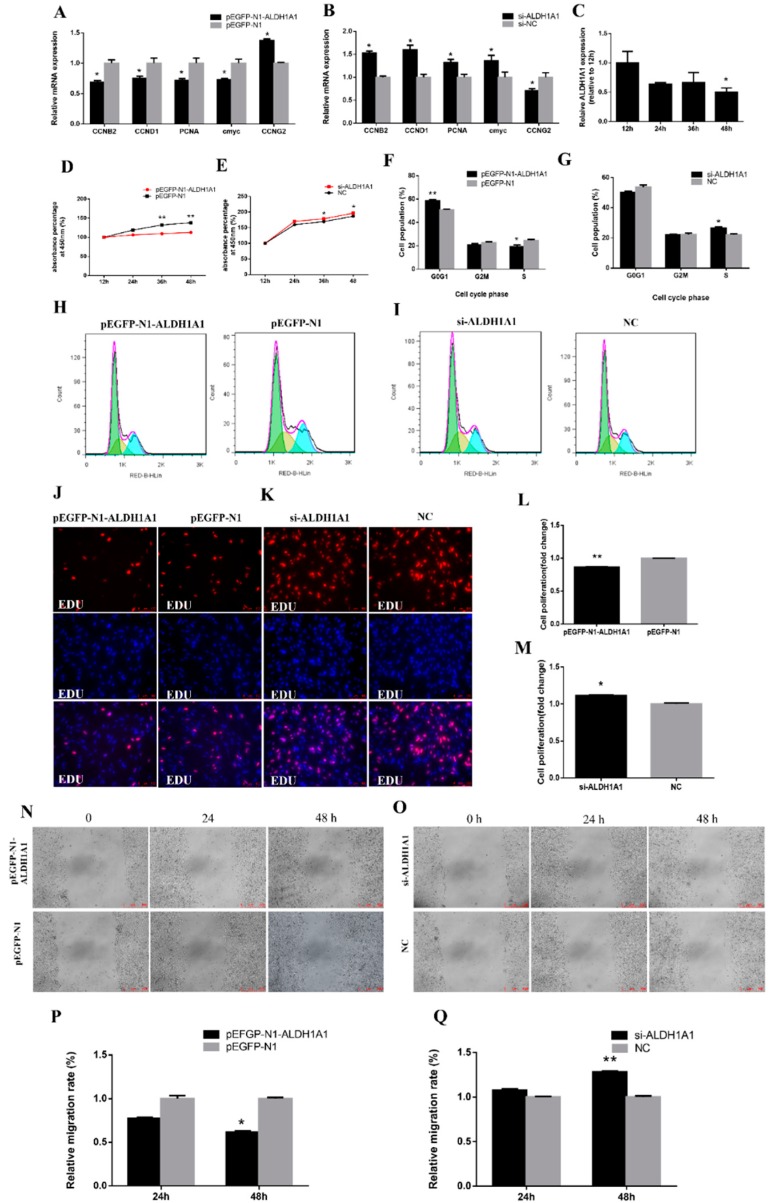
To explore the functions of ALDH1A1, we performed overexpression and knockdown experiments to assess its role in cell proliferation and viability. In immortalized chicken preadipocyte 1 (ICP1), we detected the mRNA expression of cell cycle-related gene, such as CCNB2, CCND1, PCNA, cmyc, and CCNG2. It was found that CCNB2, CCND1, PCNA, and cmyc were significantly downregulated when transfected with the overexpression vector of ALDH1A1 and were significantly upregulated when transfected with si-ALDH1A1. Meanwhile, CCNG2 was remarkably upregulated, while ALDH1A1 was overexpressed and was reduced with si-ALDH1A1 transfection (Figure 3A,B).
Figure 3.
ALDH1A1 represses chicken preadipocytes’ proliferation. (A,B) The mRNA expression of the cell cycle related gene after transfection for 48 h in chicken primary preadipocytes (n = 6). (C) The relative mRNA expression of ALDH1A1 during proliferation in immortalized chicken preadipocyte 1 (ICP1) was measured by real-time quantitative polymerase chain reaction (RT-qPCR) using Dunnett’s test (n = 6, * p < 0.05; ** p < 0.01). (D,E) Cell growth relative to the “12 h” group was measured following the transfection of the overexpression vector of ALDH1A1 and siRNA of ALDH1A1 in ICP1 (n = 6). (F–I) Cell cycle analysis of ICP1 48 h after overexpression and inhibition of ALDH1A1, using propidium iodide staining for DNA content (n = 6). (J,K) Proliferation of transfected ICP1 was assessed by 5-ethynyl-2′-deoxyuridine (EdU) incorporation, scale bar: 100 μm. (L,M) Proliferation rates of ICP1 with ALDH1A1 overexpression and inhibition (n = 6). (N,O) The scratch test detected the effect of ALDH1A1 on cell migration, scale bar: 500 μm. (P,Q) Migration rate of ICP1 with ALDH1A1 overexpression and inhibition (n = 6). In all panels, data are presented as the mean ± S.E.M. of three biological replicates. Statistical significance of differences between means was assessed using an unpaired Student’s t-test (* p < 0.05; ** p < 0.01) or Dunnett’s test (* p < 0.05; ** p < 0.01).
We detected the mRNA expression of ALDH1A1 during proliferation in chicken primary preadipocytes, and the data showed that ALDH1A1 expression showed a downward trend in 12 h, 24 h, 36 h, and 48 h of proliferation, suggesting that ALDH1A1 may inhibit preadipocytes’ proliferation (Figure 3C). In addition, the Cell Counting Kit-8 (CCK-8) assay showed that cell proliferation was significantly repressed after 36 h and 48 h of transfection of the overexpression vector of ALDH1A1 compared with the control group. Conversely, cell proliferation was significantly increased when transfected with siRNA of ALDH1A1 (Figure 3D,E). Flow cytometry was used to detect the cell cycle status. It was found that overexpression of ALDH1A1 significantly increased the number of cells staying in the G0/G1 phase and significantly reduced the number of cells remaining in the S phase, while interfering ALDH1A1 expression remarkably increased the number of cells remaining in the S phase (Figure 3F–I). Moreover, the EdU (5-ethynyl-2′-deoxyuridine) assay also demonstrated that ALDH1A1 overexpression significantly repressed chicken primary preadipocytes’ viability, while its inhibition promoted their proliferation (Figure 3J–M). In the scratch test, chicken primary preadipocytes transfected with the overexpression vector showed more activity in cell migration and showed the opposite situation while transfected with siRNA (Figure 3N–Q). All assays proved that ALDH1A1 inhibited the proliferation and migration abilities of preadipocytes.
2.3. ALDH1A1 Represses Chicken Preadipocytes’ Differentiation through the PPARγ Pathway
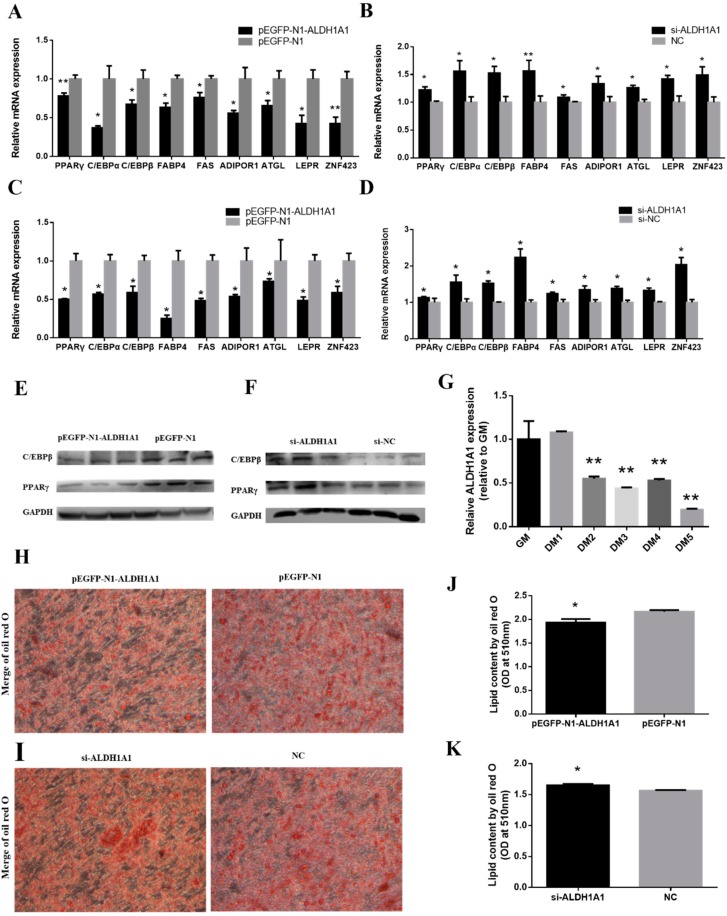
To further investigate the potential roles of ALDH1A1, chicken primary preadipocytes and the ICP cell line were induced to differentiate for three days in vitro, and the mRNA level of some preadipocyte differentiation-related genes was measured by RT-qPCR, including PPARγ, C/EBPα, C/EBPβ, FABP4, FAS, LEPR, ATGL, ZNF423, and ADIPOR1. The protein levels of PPARγ and C/EBPβ in chicken primary preadipocytes differentiated for three days were measured by Western blot at the same time. The mRNA expressions of these relative genes were all significantly downregulated in the ALDH1A1 overexpression vector transfected cells compared to control cells, especially the gene in the PPARγ pathway, including PPARγ, C/EBPα, C/EBPβ, FABP4, and ZNF423 (Figure 4A,C). Conversely, inhibition of ALDH1A1 promoted their expression (Figure 4B,D). The protein levels of PPARγ and C/EBPβ in chicken primary preadipocytes also showed similar results (Figure 4E,F). In addition, we detected the ALDH1A1 mRNA expression during differentiation and found that the expression of ALDH1A1 was downregulated from the second day, and it was significantly different from that of the proliferative stage (Figure 4G). Meanwhile, we used the Oil Red O test to analyze the lipid droplet content in different treatment groups. It was found that overexpression of ALDH1A1 could observably depress the lipid droplet depot in adipocytes, and inhibition of ALDH1A1 had the opposite effect (Figure 4H–K). All data above could prove that ALDH1A1 was involved in the differentiation of chicken preadipocytes and had a suppressive effect on preadipocytes’ differentiation.
Figure 4.
ALDH1A1 represses chicken preadipocytes’ differentiation through the PPARγ pathway. (A,B) Relative mRNA levels of adipocyte differentiation-related genes after transfection in primary preadipocytes during differentiation, determined by RT-qPCR at 48 h post-transfection (n = 6). (C,D) Relative mRNA levels of adipocyte differentiation-related genes after transfection in ICP during differentiation, determined by RT-qPCR at 48 h post-transfection (n = 6). (E,F) The protein level of adipocyte differentiation-related genes after transfection and three days differentiation in primary preadipocytes (n = 3). (G) The ALDH1A1 relative expression of primary preadipocytes during differentiation for five days was detected by RT-qPCR used Dunnett’s test (n = 6, * p < 0.05; ** p < 0.01). (H–K) Oil Red O test and lipid droplet quantification in primary preadipocytes after differentiation for five days (n = 6), scale bar: 50 μm. In all panels, data are presented as the mean ± S.E.M. of three biological replicates. The statistical significance of differences between means was assessed using an unpaired Student’s t-test (* p < 0.05; ** p < 0.01) or Dunnett’s test (* p < 0.05; ** p < 0.01).
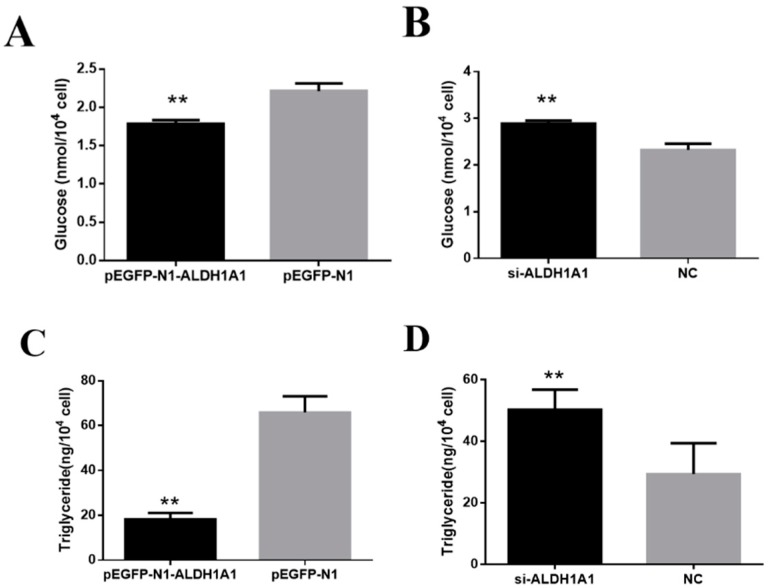
2.4. ALDH1A1 Inhibits Glucose and Triglyceride Accumulation in Chicken Preadipocytes
After transfection of the primary preadipocytes with the overexpression vector and the siRNA, the cells were induced to differentiate for three days, and the glucose content was measured. The cells were collected by protease, and ultrasonic wave was used to destroy the biological membrane under the low-temperature environment, in order to release the glucose inside the cytoplasm and accurately detected the glucose content in cells. It was found that overexpression of ALDH1A1 could significantly inhibit the accumulation of glucose in the cells, and the interference of ALDH1A1 expression showed the opposite result, accelerating the glucose deposition in cells (Figure 5A,B). These results demonstrated that, generally, ALDH1A1 had a certain inhibitory effect on glucose accumulation in differentiated preadipocytes and, to a certain extent, confirmed that ALDH1A1 had a negative effect on fat deposition in chickens.
Figure 5.
ALDH1A1 inhibits glucose accumulation in chicken preadipocytes. (A,B) Glucose content in chicken primary preadipocytes after transfection and differentiation for three days (n = 6). (C,D) Triglyceride accumulation in the preadipocytes after differentiation for five days was detected under 420 nm (n = 6). Statistical significance of the differences between the means was assessed using an unpaired Student’s t-test (** p < 0.01) vs. negative control (NC).
Moreover, we analyzed the triglyceride content after five days of differentiation of adipocytes, and the data showed that ALDH1A1 repressed the triglyceride accumulation in the preadipocytes during early differentiation (Figure 5C,D), demonstrating that ALDH1A1 could inhibit the accumulation of triglyceride in preadipocytes.
2.5. ALDH1A1 Inhibits Fat-Depot in Chickens
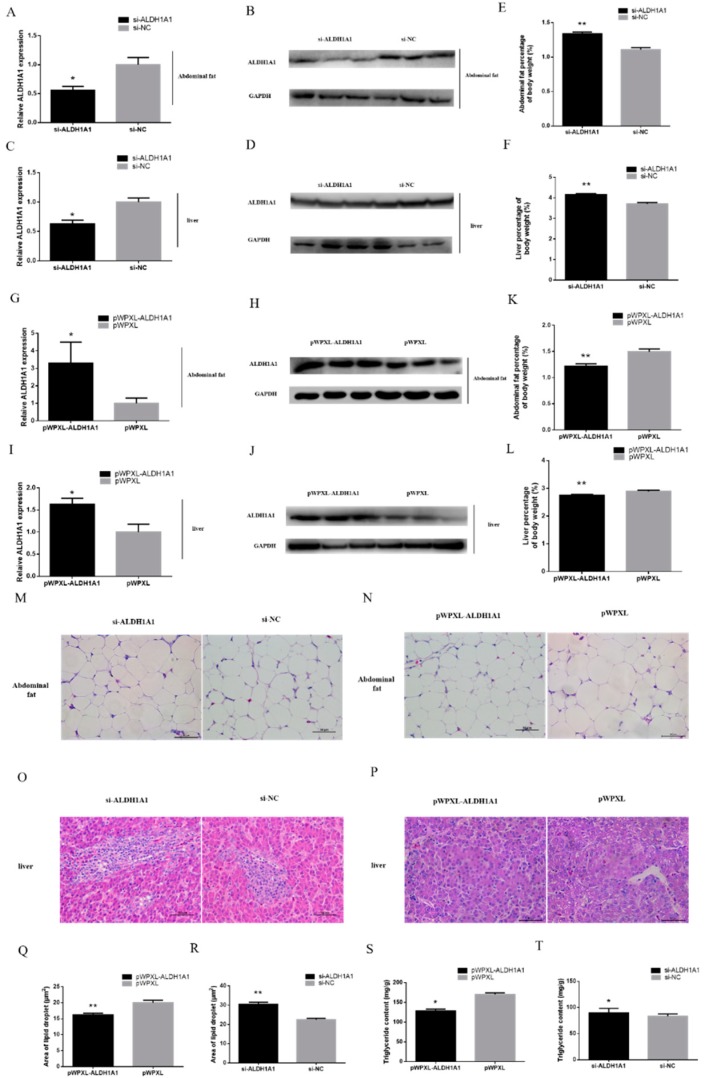
To find out whether ALDH1A1 inhibited fat-depot in chickens, we injected lentivirus into 42 two-weeks-old chickens at liver and peritoneal cavity. Chickens were divided into four groups, including the pWPXL-N1-ALDH1A1 group (n = 9), the pWPXL group (n = 9), the si-ALDH1A1 group (n = 12), and the si-NC group (n = 12) and were slaughtered at three weeks old. The data of the body weight, the mass of liver, and the mass of abdominal fat are shown in Table S1. Liver and abdominal fat were collected and were used to detect the size of the lipid droplet, triglyceride content, and the mRNA and protein level of ALDH1A1. All chickens were female and were under free feed intake.
The result of Western blot and RT-qPCR proved that the administration of lentivirus did reduce or increase the expression of ALDH1A1 in liver and abdominal fat (Figure 6A–D,G–J).
Figure 6.
ALDH1A1 inhibits fat-depot in chickens. (A–D) ALDH1A1 mRNA relative expression (n = 12) and protein level in abdominal fat and liver after interfering lentivirus injection for a week. (E,F) Abdominal fat percentage and mass of liver after interfering lentivirus injection for a week (n = 12). (G–J) ALDH1A1 mRNA relative expression (n = 9) and protein level in abdominal fat and liver after overexpression lentivirus injection for a week. (K,L) Abdominal fat percentage and mass of liver after overexpression lentivirus injection for a week (n = 9). (M–P) The slices of abdominal fat and liver from different groups, scale bar: 50 μm. (Q,R) The area of lipid droplets in abdominal fat from different groups analyzed by ImageJ. (S,T) The triglyceride accumulation in abdominal fat from different groups. In all panels, data are presented as the mean ± S.E.M. of three biological replicates. The statistical significance of differences between means was assessed using an unpaired Student’s t-test (* p < 0.05; ** p < 0.01) vs. NC.
We found that ALDH1A1 could inhibit the fat-depot in abdominal fat. The chickens injected with interference lentivirus had a higher abdominal fat percentage and a bigger liver, while overexpression of ALDH1A1 showed the opposite situation (Figure 6E,F,K,L). Moreover, we captured the slices of their abdominal fat and calculated the area of lipid droplets by ImageJ, and the results showed that chickens injected with interference lentivirus had bigger lipid droplets and a small tendency for adiposis hepatica. However, the chickens injected with overexpression lentivirus had smaller lipid droplets, indicating that ALDH1A1 expression could significantly reduce the fat deposition in chickens (Figure 6M–R). In addition, we analyzed the triglyceride content in abdominal fat and found that ALDH1A1 suppressed the triglyceride accumulation (Figure 6S,T).
3. Discussion
Chicken is the second most consumed meat in China, and the quality of chicken has become a more important tissue that determines the commercial value of chicken. Abdominal fat, one of the important issues that affects the price of chicken, is significantly influenced by RA [22]. Reports showed that RA could significantly inhibit the adipogenesis in mice through the PPARγ pathway [23]. ALDH1A1 is the crucial protease in the reaction of the conversion of retinol to RA [16], indicating that ALDH1A1 may play an important role in fat deposition. The previous study revealed the role of ALDH1A1 in preadipocytes’ proliferation and differentiation in mice. Moreover, in recent years, many reports also proved that ALDH1A1 promoted the fat-depot in mice [20,24]. The nuclear hormone receptor PPARγ promotes adipogenesis [25], and it was found that PPARγ was affected by ZFP423 expression, showing prominent reduction in ALDH1A1 knock-down adipocytes [20].
Our findings were partly based on the function of ALDH1A1 on fat-depot, but surprisingly found that ALDH1A1 had a completely different influence on chicken fat-depot. In our previous RNA sequencing data, we found that ALDH1A1 had higher expression in chickens with lower abdominal fat percentage. In subsequent experiments, we detected the mRNA level of ALDH1A1 in primary preadipocytes during proliferation and differentiation. It was found that ALDH1A1 was downregulated during the proliferation and early differentiation stage. To further investigate the biological functions of ALDH1A1, we performed a series of in vitro and in vivo experiments and found that ALDH1A1 inhibited the proliferation and differentiation of preadipocytes and the fat deposition in chicken.
To perform the in vitro experiments, we constructed an overexpression vector and synthesized siRNA to overexpress and knockdown the expression of ALDH1A1. With the series of experiments, our results showed that ALDH1A1 was highly expressed in abdominal fat and expressed in both cell nucleus and cytoplasm. Moreover, in preadipocytes, ALDH1A1 inhibited the genes promoting cell proliferation and upregulated genes suppressing cell proliferation [26,27]. The differentiation-related genes [28], including PPARγ, C/EBPα, C/EBPβ, FABP4, FAS, LEPR, ATGL, ZNF423, and ADIPOR1, were repressed as well. The process of adipogenesis is controlled by diverse factors, and one of the famous ones is PPARγ, which is known to promote fat cell differentiation in vitro [29]. Upon activation, PPARγ heterodimerizes with the retinoid X receptor, recruiting factors, combining with the peculiar DNA structure, so as to activate the adipogenesis target genes [30]. Interestingly, in a previous study, ALDH1A1′s deficiency significantly reduced the mRNA expression of PPARγ and ZNF423 in mice [20], which was different from the results of experiments on chicken. The differences indicated that one of the reasons that ALDH1A1 had a different effect between mice and chicken might be the changes of PPARγ and ZNF423.
In our study, on the one hand, overexpression of ALDH1A1 significantly reduced the number of cells in the S phase, while transfection with siRNA remarkably increased the number of cells in the S phase, proving that ALDH1A1 inhibited the cell cycle. On the other hand, the EdU and CCK-8 assays showed that ALDH1A1 also downregulated the proliferative activity of preadipocytes. In addition, we found that ALDH1A1 inhibited the accumulation of lipogenesis. The cell transfected with pEGFP-N1-ALDH1A1 had a small size of the lipid droplets and a lower content of triglyceride, while the cell transfected with siRNA had the completely opposite situation, which meant that ALDH1A1 blocked the lipogenesis in vitro. Above all, we drew the conclusion that ALDH1A1 inhibited the proliferation and differentiation of preadipocytes, which blocked the adipogenesis in chicken.
To further investigate whether ALDH1A1 inhibited the adipogenesis in vivo, we performed the lentivirus assay, injecting lentivirus into chickens’ liver and enterocoelia [31,32,33] and identified the transfection efficiency by RT-qPCR and Western blot. We used the tissues, which were successfully injected by lentivirus, to perform the following experiments. Moreover, we detected the abdominal fat percentage, size of lipid droplets, mass of liver, and triglyceride content of abdominal fat. The results showed that ALDH1A1 inhibited the adipogenesis in chickens.
4. Materials and Methods
4.1. Ethics Statement
Animal experiments in the research were under the rigorous supervision of the Administration of Laboratory Animals in Guangdong Province. All operations involved in animals aimed to reduce the pain of the animals. The experimental operations were approved by the Institutional Animal Care and Use Committee at South China Agricultural University (approval ID: SCAU#0011, 3 June 2019).
4.2. Experimental Animals and Tissues
In the laboratory, prior to transcriptome sequencing, one-hundred-fifty-eight 100 days old female chickens were supplied by Wens Co., Ltd. (Yunfu, China) with free water and food. Chickens were divided into two groups according to the abdominal fat percentage (high-fat group and low-fat group). The chickens were slaughtered at 100 days old after 12 h of fasting, and the abdominal fat was collected and stored in liquid nitrogen to avoid the degradation of RNA. The HiPure Total RNA Plus Mini Kit (Megen, Guangzhou, China) was used to exact the total RNA, and the RNA was sent to the Beijing Genomics Institution (Beijing, China) as the pooled samples for quality testing and RNA sequencing [21]. In this research, we chose ALDH1A1′s different expression data in abdominal fat. The data of these two groups are recorded in Table 1.
Table 1.
Slaughter data of female chickens.
| Groups | Body Weight (g) | Mass of Abdominal Fat (g) | Abdominal Fat Percentage (%) |
|---|---|---|---|
| High Fat | 1659.93 ± 60.61 | 146.95 ± 8.58 ** | 8.83 ± 0.28 ** |
| Low Fat | 1472.63 ± 68.49 | 53.67 ± 4.35 | 3.62 ± 0.17 |
** Represents that the mass of abdominal fat and abdominal fat percentage of the high-fat group were significantly higher than low-fat group. The statistical significance of differences between means was assessed using an unpaired Student’s t-test (** p < 0.01).
Six 7-weeks-old chickens were fed at South China Agriculture University (Guangzhou, China). Nine tissues, heart, liver, spleen, lung, kidney, abdominal fat, subcutaneous fat, breast muscle. and leg muscle, were collected and stored in liquid nitrogen immediately in order to protect the RNA from degrading. TRIzol reagent (TaKaRa, Kyoto, Japan) was used to extract the total RNA, and the PrimeScript RT Reagent Kit (TaKaRa, Kyoto, Japan) was used to synthesize cDNA to detect the mRNA level of ALDH1A1. The Bio-Rad Real-Time Detection Machine (Bio-Rad, Hercules, CA, USA) was utilized to process the RT-qPCR and calculate the fold change.
Forty-two chickens at one day old, which were bought from Yuhe Agriculture and Animal Husbandry Co., Ltd. (Zhuhai, China), were housed in the same room with free food and water. Overexpression lentivirus, control lentivirus, interference lentivirus, or interference negative control lentivirus (3 × 107 infectious lentivirus particles per chicken) were delivered by liver injection and intraperitoneal injection to 2-weeks-old chickens. The chickens were slaughtered at 3 weeks old, and the liver and abdominal fat were collected and used to detect the mRNA expression of ALDH1A1. Only tissues that had a significant difference from the negative control groups on ALDH1A1 mRNA expression were used for subsequent experiments. The selected tissues were used to analyze the abdominal fat percentage, mass of liver, triglyceride content, and protein level of ALDH1A1.
4.3. Lentivirus Production and Transduction
To generate lentivirus, the full-length sequence of ALDH1A1 was inserted into pWPXL (Addgene, Watertown, MA, USA), and the recombinant lentiviral expression vectors were co-transfected with PMD 2.4G (Addgene, Watertown, MA, USA) and psPAX2 (Addgene, Watertown, MA, USA) into 293T cell by using Lipofectamin 3000 reagent (Gibco, Grand Island, NY, USA). The supernatant was collected after 48 h and 72 h of transfection, filtered through 0.45 μm PVDF membranes (Millipore, Boston, MA, USA), and concentrated by ultracentrifugation. The viral titer was evaluated by gradient dilution. The sequence of si-ALDH1A1 was given to Hanbio Biotechnology (Shanghai, China) to produce interfering lentivirus. The viral titers of these lentiviruses were 5.0 × 108 TU/mL and 5.2 × 108 TU/mL, respectively.
4.4. Cell Culture
ICP1 cell culture: The ICP1 cell line was bought from Northeast Agricultural University (Harbin, China) and cultured with Dulbecco’s Modified Eagle Medium (DMEM, Gibco, Grand Island, NY, USA). Ten percent fetal bovine serum (FBS, Hyclone, Logan, UT, USA) and 0.2% penicillin and streptomycin (Invitrogen, Carlsbad, CA, USA) were supplemented in DMEM.
Chicken primary preadipocytes’ isolation and culture: Primary preadipocytes were isolated from the abdominal fat of 3 weeks old chickens. Firstly, the abdominal fat was dissected away from the enterocoelia and washed by using DMEM. Secondly, the abdominal fat was cut into pieces by surgery scissors and digested by collagenase I for 30 min at 37 °C in order to acquire a single-cell suspension. Then, the suspension was centrifuged at 1300× g to collect the preadipocytes. After this protocol, cells were seeded in petri dishes with complete medium. The ingredients in the complete medium of primary preadipocytes were the same as those for ICP1.
The differentiation of preadipocytes was induced by DMEM/F12 medium supplemented with 0.2% penicillin/streptomycin and 0.05% sodium oleate [34,35,36,37].
All cells were cultured at 37 °C in a 5% CO2 humidified atmosphere.
4.5. RNA Isolation, Complementary DNA Synthesis, and RT-qPCR Analysis
RNA in tissues and cells was extracted by TRIzol reagent (TaKaRa, Kyoto, Japan) under the guidance of the manufacturer’s instructions. The quality of RNA was examined by 1.5% agarose gel electrophoresis and detected by Nanodrop 2000c spectrophotometer (Thermo, Waltham, MA, USA). The PrimeScript RT Reagent Kit (TaKaRa, Kyoto, Japan) was used to synthesize cDNA and get rid of genomic DNA (gDNA). The Bio-Rad Real-Time Detection Machine (Bio-Rad, Hercules, CA, USA) was utilized to detect and amplify cDNA with the iTaq Universal SYBR Green Supermix Kit (Toyobo, Osaka, Japan). GAPDH was used as the internal control. Data analysis was carried out with the comparative 2−ΔΔCT method [38].
4.6. Primers
Premier Primer 5.0 (Premier Biosoft International, Palo Alto, CA, USA) and Oligo 7 (Molecular Biology Insights Inc., Cascade, CO, USA) were utilized to devise the sequences of primers. Primers were sent to Sangon Biotech (Shanghai, China) and Tsingke Biotech (Beijing, China) for synthesis. Primers used for vector construction are listed in Table S2. Primers for RT-qPCR are shown in Tables S3 and S4.
4.7. RNA Oligonucleotides, Plasmids Construction, and Lentivirus Construction
siRNA used for the knockdown of ALDH1A1 was devised and sent to RiboBio (Guangzhou, China) for synthesis. The sequence of siRNA is listed in Table S5. For RNA oligonucleotides, a concentration of 100 nM was used.
For ALDH1A1 overexpression plasmid construction, the full-length sequence of ALDH1A1 was amplified from chicken abdominal fat cDNA by PCR and cloned into the expression plasmid, the pEGFP-N1 vector (Promega, Madison, WI, USA), by using the NheI and XhoI restriction sites.
For lentivirus, the same cDNA cloned before was cloned into the pWPXL vector (Promega, Madison, WI, USA) by using the SmaI and MIuI restriction sites. Then, the overexpression plasmid and the sequence of si-ALDH1A1 were given to Hanbio Biotechnology (Shanghai, China) to produce overexpression and interfering lentivirus.
4.8. Western Blotting Assay
Radio immune precipitation assay buffer (Beyotime, Shanghai, China) supplemented with 10% phenylmethane sulfonyl fluoride protease inhibitor (Beyotime, Shanghai, China) was used to extract protein in tissues and cells under the guidance of the manufacturer’s direction. Proteins after denaturation were loaded into the sample holes of 10% SDS-PAGE and separated under a stable voltage. Nitrocellulose membranes (Whatman, Maidstone, UK) were put on the surface of 10% SDS-PAGE and transferred protein under a constant electricity. Then, antibodies were utilized to combine the antigens.
The antibodies and their dilutions used for Western blots were as follows: Rabbit Anti-ALDH1A1 antibody (GTX123973; GeneTex, San Antonio, TX, USA; 1:5000), Rabbit Anti-CEBP Beta Polyclonal Antibody (bs-1396R; Bioss, Beijing, China; 1:500) [39,40], Rabbit Anti-PPAR Gamma Polyclonal Antibody (bs-0530R; Bioss, China; 1:500), Rabbit Anti-Adiponectin Polyclonal Antibody (bs-0471R; Bioss, Beijing, China; 1:500) [41], Rabbit Anti-GAPDH (AB-P-R 001; Hangzhou Goodhere Biotechnology Co. Ltd., Hangzhou, China; 1:10,000), Goat Anti-rabbit IgG-HRP(BA1054; Boster, Wuhan, China; 1:10,000), and Peroxidase-goat Anti-mouse IgG (BA1051; Boster, Wuhan, China; 1:10,000).
4.9. EdU Assay
Cells seeded in 12 well plates were cultured to 70% density and then transfected. Forty-eight hours after transfection with siRNAs or the overexpression vector, the cells were fixed and stained with a C10310 EdU Apollo In Vitro Imaging Kit (RiboBio, China). A fluorescence microscope (TE2000-U; Nikon, Tokyo, Japan) was used to capture three randomly selected fields, and the ImageJ software (National Institutes of Health, Bethesda, MD, USA) was used to count the number of EdU-stained cells.
4.10. Analysis of the Cell Cycle
Flow cytometry (FCM) was used to detect the cell cycle of preadipocytes. Cells after different treatments were collected and stored with 70% ethanol for more than 2 h under −20 °C. Then, the preadipocytes were dyed with 50 μg/mL propidium iodide (Sigma, Saint Louis, MO, USA), 0.2% Triton X-100 (Sigma, Saint Louis, MO, USA), and 10 μg/mL RNase A (Takara, Kyoto, Japan). After that, a 37 °C incubator was used to incubate the stained cells for 30 min. A flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) was used to perform FCM, and the data were analyzed by FlowJo7.6 software (BD Biosciences, Franklin Lakes, NJ, USA).
4.11. CCK-8 Experiments
Preadipocytes were transferred to the 96 well culture plate and transfected with the overexpression vector and siRNA. A Model 680 Microplate Reader (Bio-Rad) was used to detect the absorbance of cells at 450 nm after being incubated with TransDetect CCK (TransGen Biotech, Beijing, China) for 1 h. The data were detected at 12 h, 24 h, 36 h, and 48 h after transfection [42].
4.12. Immunofluorescence Assay
Preadipocytes were seeded in 12 well culture plates and transfected with the overexpression vector and siRNA. After 48 h, preadipocytes were incubated with 4% formaldehyde for 20 min for fixation. Then, cells were washed by phosphate buffer saline (PBS) several times and the biological membrane damaged with 0.1% Triton X-100 for 15 min. After that, the preadipocytes were blocked by goat serum for 30 min and incubated with Rabbit Anti-ALDH1A1 antibody overnight at 4 °C. The Fluorescein (FITC)-conjugated AffiniPure Goat Anti-Rabbit IgG (H + L) (BS10950; Bioworld, USA; 1:50) was used to combine the antibody/antigen complex at normal temperature for 1 h. The preadipocytes’ nuclei were dyed by 4’,6-diamidino-2-phenylindole (DAPI) for 5 min, and a fluorescence microscope (Nikon, Tokyo, Japan) was utilized to capture the pictures of immunofluorescence.
4.13. Oil Red O Staining and Quantification
Preadipocytes were seeded in 12 well culture plates. After transfection and differentiation for 5 days, differentiated preadipocytes were washed by PBS and incubated with 4% formaldehyde for 20 min for fixation. Then, differentiated preadipocytes were dyed with Oil Red O working solution (Solarbio, Beijing, China) under the direction of the manufacturer’s specification for 30 min at room temperature and washed five times with distilled water. Then, DAPI was added and incubated for 5 min. After washing, a fluorescence inverted light microscope (Leica DMi8, Wetzlar, Germany) was used to capture the images. In the end, the stain in the cells was extracted by isopropanol and analyzed at 510 nm absorbance with a NanoDrop 2000C Spectrophotometers (Thermo).
4.14. Triglyceride Quantification
Cells were seeded in 24 well plates and transfected when cells were cultured to 70% density. After 5 days of differentiation, cells were collected by 0.25% trypsin-EDTA (Gibco, USA), disrupted by an Ultrasonic Cell Crusher (Biolon, Shanghai, China), and the triglyceride content detected by using the Triglyceride Assay Kit (Solarbio, China), according to the manufacturer’s protocol.
4.15. Glucose Quantification
Cells were seeded in 12 well plates and transfected when cells were cultured to 70% density. After 3 days of differentiation, cells were collected and disrupted by ultrasonic waves at 4 °C. The cell suspension was collected and used to continue subsequent detection by the Glucose Assay Kit (Solarbio, China), according to the manufacturer’s protocol.
5. Conclusions
In summary, ALDH1A1 not only inhibited the proliferation of preadipocytes, but also inhibited the differentiation of preadipocytes, blocking the adipogenesis in vitro. In addition, ALDH1A1 had a similar effect on adipogenesis in vivo as well, which was completely different from the effect of ALDH1A1 on mice, indicating that the molecular mechanism of ALDH1A1 in adipogenesis may have a tremendous difference from that of mice, and it is worthwhile for us to make a further investigation.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/21/9/3150/s1. Table S1: Data of slaughtered chickens used in the lentivirus experiments. Table S2: Primer used for vector construction. Table S3: Cell cycle gene primer and ALDH1A1 primer used for RT-qPCR. Table S4: Differentiation regulated gene primer used for RT-qPCR. Table S5: Oligonucleotides sequences in this study.
Author Contributions
Conceptualization, J.Z.; data curation, J.Z. and B.C.; formal analysis, J.Z. and B.C.; funding acquisition, Q.N.; investigation, J.Z. and B.C.; methodology, J.Z. and B.C.; project administration, Q.N.; resources, X.Z. and Q.N.; software, J.Z., W.L., and Z.Z.; supervision, J.Z., B.C. and Q.N.; validation, B.C. and M.M.; visualization, J.Z.; writing, original draft, J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Key-Area Research and Development Program of Guangdong Province (2018B020203001), Science and Technology Planning Project of Guangzhou City (201504010017), Ten-Thousand Talents Program (W03020593), China Agriculture Research System (CARS-41-G03), College students’ innovation and entrepreneurship training program (201910564214), and the Graduate Students Overseas Study Program of South China Agricultural University (2018LHPY015).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Freytag O., Paielli L., Gilbert D. Ectopic expression of the CCAAT/enhancer-binding protein alpha promotes the adipogenic program in a variety of mouse fibroblastic cells. Genes Dev. 1994;8:1654–1663. doi: 10.1101/gad.8.14.1654. [DOI] [PubMed] [Google Scholar]
- 2.Lin F., Lane D. CCAAT/enhancer binding protein alpha is sufficient to initiate the 3T3-L1 adipocyte differentiation program. Proc. Natl. Acad. Sci. USA. 1994;91:8757–8761. doi: 10.1073/pnas.91.19.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeh W., Cao Z., Classon M., McKnight L. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev. 1995;9:168–181. doi: 10.1101/gad.9.2.168. [DOI] [PubMed] [Google Scholar]
- 4.Tontonoz P., Hu E., Graves A., Budavari I., Spiegelman M. MPPAR-gamma-2: Tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 5.Schwarz J., Reginato J., Shao D., Krakow L., Lazar A. Retinoic acid blocks adipogenesis by inhibiting C/EBP-beta-mediated transcription. Mol. Cell. Biol. 1997;17:1552–1561. doi: 10.1128/MCB.17.3.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stone L., Bernlohr A. The molecular basis for inhibition of adipose conversion of murine 3T3-L1 cells by retinoic acid. Differentiation. 1990;45:119–127. doi: 10.1111/j.1432-0436.1990.tb00465.x. [DOI] [PubMed] [Google Scholar]
- 7.Chawla A., Lazar A. Peroxisome proliferator and retinoid signaling pathways co-regulate preadipocyte phenotype and survival. Proc. Natl. Acad. Sci. USA. 1994;91:1786–1790. doi: 10.1073/pnas.91.5.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuri-Harcuch W. Differentiation of 3T3-F442A cells into adipocytes is inhibited by retinoic acid. Differentiation. 1982;23:164–169. doi: 10.1111/j.1432-0436.1982.tb01279.x. [DOI] [PubMed] [Google Scholar]
- 9.Xue J., Schwarz J., Chawla A., Lazar A. Distinct stages in adipogenesis revealed by retinoid inhibition of differentiation after induction of PPAR-gamma. Mol. Cell. Biol. 1996;16:1567–1575. doi: 10.1128/MCB.16.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duester G., Mic A., Molotkov A. Cytosolic retinoid dehydrogenases govern ubiquitous metabolism of retinol to retinaldehyde followed by tissue-specific metabolism to retinoic acid. Chem. Interact. 2003;143:201–210. doi: 10.1016/S0009-2797(02)00204-1. [DOI] [PubMed] [Google Scholar]
- 11.Bowles J., Feng C., Knight D., Smith A., Roeszler N., Bagheri-Fam S., Harley R., Sinclair H., Koopman P. Male-Specific Expression of Aldh1a1 in Mouse and Chicken Fetal Testes: Implications for Retinoid Balance in Gonad Development. Dev. Dynam. 2009;238:2073–2080. doi: 10.1002/dvdy.22024. [DOI] [PubMed] [Google Scholar]
- 12.Yasmeen R., Jeyakumar M., Reichert B., Yang F., Ziouzenkova O. The contribution of vitamin A to autocrine regulation of fat depots. BBA Mol. Cell Biol. Lipids. 2012;1821:190–197. doi: 10.1016/j.bbalip.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molotkov A., Duester G. Genetic evidence that retinaldehyde dehydrogenase Raldh1 (Aldh1a1) functions downstream of alcohol dehydrogenase Adh1 in metabolism of retinol to retinoic acid. J. Biol. Chem. 2003;278:36085–36090. doi: 10.1074/jbc.M303709200. [DOI] [PubMed] [Google Scholar]
- 14.Ziouzenkova O., Orasanu G., Sharlach M., Akiyama E., Berger P., Viereck J., Hamilton A., Tang G., Dolnikowski G., Vogel S., et al. Retinaldehyde represses adipogenesis and diet-induced obesity. Nat. Med. 2007;13:695–702. doi: 10.1038/nm1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chambon P. A decade of molecular biology of retinoic acid receptors. Faseb J. 1996;10:940–954. doi: 10.1096/fasebj.10.9.8801176. [DOI] [PubMed] [Google Scholar]
- 16.Heyman A., Mangelsdorf J., Dyck A., Stein B., Eichele G., Evans M., Thaller C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992;68:397–406. doi: 10.1016/0092-8674(92)90479-V. [DOI] [PubMed] [Google Scholar]
- 17.Hegele A. Retinoid X receptor heterodimers in the metabolic syndrome. N. Engl. J. Med. 2005;353:2088. doi: 10.1056/NEJM200511103531921. [DOI] [PubMed] [Google Scholar]
- 18.Yasmeen R., Reichert B., Deiuliis J., Yang F., Lynch A., Meyers J., Sharlach M., Shin S., Volz S., Green B., et al. Autocrinefunction of aldehyde dehydrogenase 1 as a determinant of diet- and sex-specific differences in visceral adiposity. Diabetes. 2013;62:124–136. doi: 10.2337/db11-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiefer W., Vernochet C., O’Brien P., Spoerl S., Brown D., Nallamshetty S., Zeyda M., Stulnig M., Cohen E., Kahn R., et al. Retinaldehyde dehydrogenase 1 regulates a thermogenic program in white adipose tissue. Nat. Med. 2012;18:918–925. doi: 10.1038/nm.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reichert B., Yasmeen R., Jeyakumar S.M., Yang F., Thomou T., Alder H., Duester G., Maiseyeu A., Mihai G., Harrison H., et al. Concerted Action of Aldehyde Dehydrogenases Influences Depot-Specific Fat Formation. Mol. Endocrinol. 2011;25:799–809. doi: 10.1210/me.2010-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q. Master’s Thesis. South China Agricultural University; Guangzhou, China: 2019. RNA-Seq Screening for Genes and miRNAs Associated with Chicken Abdominal Fat Deposition. [Google Scholar]
- 22.Malladi P., Xu Y., Yang P., Longaker T. Functions of vitamin D, retinoic acid, and dexamethasone in mouse adipose-derived mesenchymal cells. Tissue Eng. 2006;12:2031–2040. doi: 10.1089/ten.2006.12.2031. [DOI] [PubMed] [Google Scholar]
- 23.Mercader J., Ribot J., Murano I., Felipe F., Cinti S., Bonet L., Palou A. Remodeling of white adipose tissue after retinoic acid administration in mice. Endocrinology. 2006;147:5325–5332. doi: 10.1210/en.2006-0760. [DOI] [PubMed] [Google Scholar]
- 24.Yang D., Krois R., Huang P., Wang J., Min J., Yoo S., Deng Y., Napoli L. Raldh1 promotes adiposity during adolescence independently of retinal signaling. PLoS ONE. 2017;12:e018766911. doi: 10.1371/journal.pone.0187669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren L., Collingwood N., Rebar J., Wolffe P., Camp S. PPAR gamma knockdown by engineered transcription factors: Exogenous PPAR gamma 2 but not PPAR gamma 1 reactivates adipogenesis. Gene. Dev. 2002;16:27–32. doi: 10.1101/gad.953802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piastowska-Ciesielska W., Gajewska M., Wagner W., Dominska K., Ochedalski T. Modulatory effect of selenium on cell-cycle regulatory genes in the prostate adenocarcinoma cell line. J. Appl. Biomed. 2014;12:87–95. doi: 10.1016/j.jab.2013.02.002. [DOI] [Google Scholar]
- 27.Meloche S., Pouyssegur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26:3227–3239. doi: 10.1038/sj.onc.1210414. [DOI] [PubMed] [Google Scholar]
- 28.Abdalla B.A., Li Z., Ouyang H., Jebessa E., Sun L., Yu J., Cai B., Chen B., Nie Q., Zhang X. A Novel Dnmt3a1 Transcript Inhibits Adipogenesis. Frotiers Physiol. 2018;9:1270. doi: 10.3389/fphys.2018.01270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosen D., Sarraf P., Troy E., Bradwin G., Moore K., Milstone S., Spiegelman M., Mortensen M. PPARgamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell. 1999;4:611–617. doi: 10.1016/S1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 30.Frederick P., Johan A. PPARγ and glucose homeostasis. Annu. Rev. Nutr. 2002;22:167–197. doi: 10.1146/annurev.nutr.22.010402.102808. [DOI] [PubMed] [Google Scholar]
- 31.Wu W., Zhang J., Zhao C., Sun Y., Yin Y., Peng Y., Pang W., Yang G. Lentivirus-mediated CTRP6 silencing ameliorates diet-induced obesity in mice. Exp. Cell Res. 2018;367:15–23. doi: 10.1016/j.yexcr.2018.01.027. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Z.-W., Fan X.-X., Song J.-J., Xu M., Chen M.-J., Tu J.-F., Wu F.-Z., Zhang D.-K., Liu L., Chen L., et al. ShRNA knock-down of CXCR7 inhibits tumour invasion and metastasis in hepatocellular carcinoma after transcatheter arterial chemoembolization. J. Cell. Mol. Med. 2017;21:1989–1999. doi: 10.1111/jcmm.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng J., Li Y., Wu G., Zheng J., Lu H., Shi X.-E., Yang G. Ectopic expression of RBP4 impairs the insulin pathway and inguinal fat deposition in mice. J. Physiol. Biochem. 2014;70:479–486. doi: 10.1007/s13105-014-0326-3. [DOI] [PubMed] [Google Scholar]
- 34.Fan H., Wu D., Tian W., Ma X. Inhibitory effects of tannic acid on fatty acid synthase and 3T3-L1 preadipocyte. Biochim. Biophys. Acta. 2013;1831:1260–1266. doi: 10.1016/j.bbalip.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Cryer J., Woodhead B., Cryer A. The isolation and characterization of a putative adipocyte precursor cell type from the white adipose tissue of the chicken (Gallus domesticus) Comp. Biochem. Physiol. A. 1987;86:515–521. doi: 10.1016/0300-9629(87)90535-4. [DOI] [PubMed] [Google Scholar]
- 36.Butterwith S., Griffin H. The effects of macrophage-derived cytokines on lipid metabolism in chicken (Gallus domesticus) hepatocytes and adipocytes. Comp. Biochem. Physiol. 1989;94:721–724. doi: 10.1016/0300-9629(89)90623-3. [DOI] [PubMed] [Google Scholar]
- 37.Yusuke M., Kan S., Hiroshi I., Yukio A. Changes in mRNA expression of regulatory factors involved in adipocyte differentiation during fatty acid induced adipogenesis in chicken. Comp. Biochem. Physiol. 2005;141:108–115. doi: 10.1016/j.cbpb.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 38.Livak J., Schmittgen D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-DELTADELTACT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 39.Ting Z., Buhao D., Ruixin Z., Xuze Q., Jianxin Z., Junxing Z. Dietary Sea Buckthorn Pomace Induces Beige Adipocyte Formation in Inguinal White Adipose Tissue in Lambs. Animals. 2019;9:193. doi: 10.3390/ani9040193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang K., Wang F., Huang J., Lou Y., Xie J., Li H., Cao D., Huang X. Insulin-like growth factor 2 promotes the adipogenesis of hemangioma-derived stem cells. Exp. Ther. Med. 2018;17:1663–1669. doi: 10.3892/etm.2018.7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du J., Zhang P., Gan M., Zhao X., Xu Y., Li Q., Jiang Y., Tang G., Li M., Wang J., et al. MicroRNA-204-5p regulates 3T3-L1 preadipocyte proliferation, apoptosis and differentiation. Gene. 2018;668:1–7. doi: 10.1016/j.gene.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 42.Cai B., Li Z., Ma M., Wang Z., Han P., Abdalla B.A., Nie Q., Zhang X. LncRNA-Six1 Encodes a Micropeptide to Activate Six1 in Cis and Is Involved in Cell Proliferation and Muscle Growth. Front. Physiol. 2017;8:595. doi: 10.3389/fphys.2017.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.