Abstract
Introduction: Fifty percent of women with gestational diabetes mellitus (GDM) may progress to type 2 diabetes with highest risk among black women. This study aims to characterize postpartum diabetes screening rates among U.S. women with GDM by racial and ethnic group to characterize potential disparities.
Materials and Methods: A standardized search of Ovid-Medline, Embase, Scopus, Cumulative Index of Nursing and Allied Health Literature (CINAHL), Cochrane, ProQuest, and Clinicaltrials.gov was conducted through October 12, 2018. Of 1,555 titles reviewed, 27 studies met inclusion criteria. Meta-proportion routines with random-effects models estimated pooled postpartum screening proportion effect size (ES) with 95% confidence interval (CI) by racial and ethnic group. Heterogeneity was measured using Cochrane's Q and Higgins I2 tests. Data were stratified by intervention and data source.
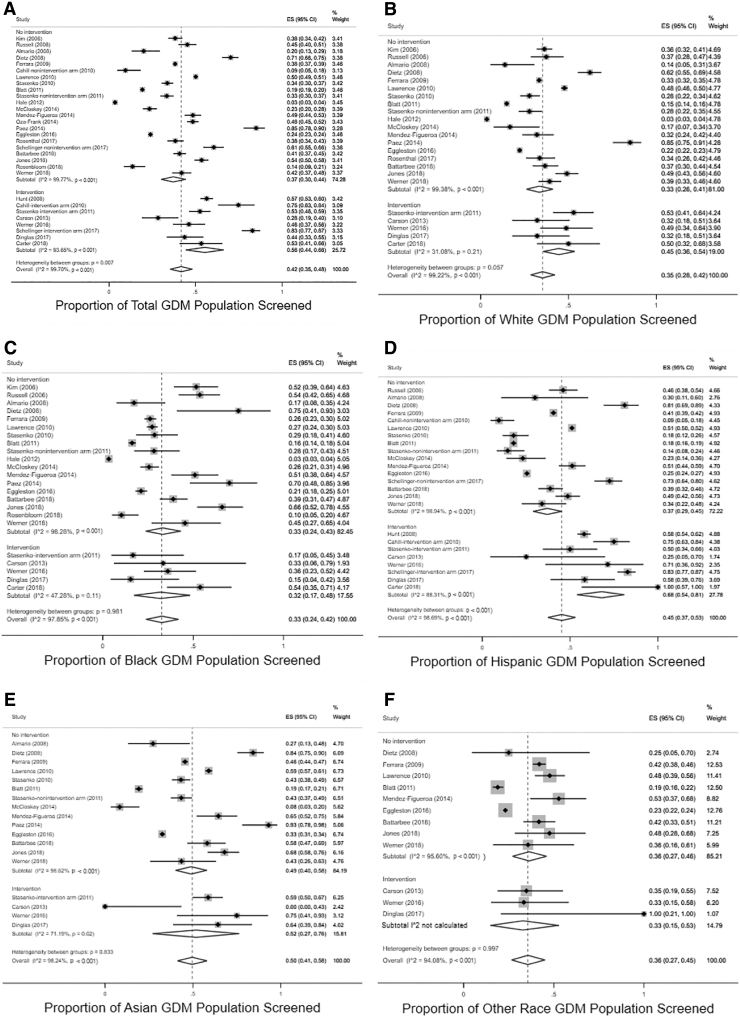
Results: There were 96,439 women, of whom 81,930 had race/ethnicity recorded. Heterogeneity was high (I2 = 99.7%). Postpartum screening rates were low (pooled ES 42% [95% CI 35%–48%]). Point estimates for pooled screening proportions were lower among white (pooled ES 35% [95% CI 28%–42%]) and black (pooled ES 33% [95% CI 24%–42%]) women than among Hispanic (pooled ES 45% [95% CI 37%–53%]) and Asian (pooled ES 50% [95% CI 41%–58%]) women. Interventions to improve screening were most common and effective among Hispanic women.
Discussion: Postpartum screening for diabetes after GDM remains low, and black women have among the lowest postpartum screening rates despite highest risk for type 2 diabetes progression. Reporting of race/ethnicity, screening methods, and screening time frames varied across studies.
Conclusion: Future studies must standardize racial/ethnic data reporting and examine interventions that address postpartum diabetes screening and prevention.
Keywords: gestational diabetes, race and ethnicity, postpartum diabetes screening, health disparities
Introduction
Gestational diabetes mellitus (GDM) complicates 5%–9% of pregnancies in the United States, and risk increases with age, body mass index (BMI), nonwhite race, and lower socioeconomic status.1–4 One third of women with GDM have persistent glucose abnormalities at 6–12 weeks postpartum, and more than half may develop type 2 diabetes during their lifetime.5–7 Furthermore, the risk of developing type 2 diabetes appears to be highest among black women.4,8 Thus, GDM is associated with significant risk of future chronic disease among young women, and postpartum follow-up is critical.3,5
Type 2 diabetes screening after a pregnancy complicated by GDM is critical because hyperglycemia, even in a relatively asymptomatic patient, increases the risk for complications, such as neuropathy, retinopathy, and nephropathy.9,10 Furthermore, impaired glucose metabolism increases risk of maternal obstetric and child developmental complications in future pregnancies.5 With early detection of at risk patients, lifestyle interventions and metformin can reduce the risk for progression to type 2 diabetes by 50%.11–13
The American College of Obstetricians and Gynecologists and the American Diabetes Association recommend diabetes screening at 4–12 weeks postpartum and every 1–3 years thereafter among women with GDM.3,5 Fasting plasma glucose and the 2 hours 75 g oral glucose tolerance test (oGTT) are acceptable tests in the 4–12 weeks postpartum, but the oGTT is most sensitive. A hemoglobin A1C (Hb A1C) is acceptable for screening after 12 weeks postpartum.
However, estimates from prior studies suggest that rates of screening are low, particularly in populations served by Medicaid.14–17 A prior systematic review, including single center studies and a single large prospective cohort study, estimated median postpartum screening rates to be 48% (range 34%–73%).14 Race was mentioned as one of many factors that could affect screening rates, but this variation was not systematically examined. In women on Medicaid during pregnancy, receipt of recommended screening appears to be under 10% in the first 12 weeks and under 20% at 1 year.15,17
Systematic reviews and meta-analyses quantify the rates of progression to type 2 diabetes from GDM7,18 as well as the recurrence of GDM.19 The utility of reminder systems for screening after pregnancy is well documented20,21 as is the sensitivity and specificity of different tests for screening.22,23 Another systematic review in 2013 stratified studies by screening time frame and active intervention compared to usual care, but again did not look specifically at variation by race/ethnicity.24 Thus, while screening rates and strategies to improve screening have been documented, there has not been a systematic examination to date of screening rates and interventions to improve screening across racial and ethnic groups which is critical for addressing health disparities.25
In the current systematic review, we expand upon existing reviews to include studies published after 2013, incorporating one large retrospective cohort study and several other smaller studies, with a specific aim to describe racial and ethnic differences in screening rates. To accomplish this unique objective, we selected studies that specifically reported screening rates by racial and ethnic group, and we utilized meta-analysis techniques to determine how postpartum diabetes screening rates vary by race/ethnicity among women with a history of GDM in the United States.
Materials and Methods
This systematic review was registered on March 7, 2017 in PROSPERO (CRD42017068383). The search strategy was intentionally broad seeking to capture all literature that addressed any postpartum diabetes screening in women with GDM. The initial search was not limited to studies that addressed U.S. populations or racial and ethnic disparities because there were concerns that this would eliminate studies with race data in the full text that were not highlighted in titles and abstracts. Prospective and retrospective cohort studies, cross-sectional studies, case–control studies, and randomized controlled trials were considered for inclusion. Case reports, case series, and abstracts were excluded.
Published and gray literature was searched using strategies designed by a medical librarian for the concepts of GDM and postpartum diabetes screening. These strategies were established using a combination of standardized terms and keywords, and were executed in Ovid Medline 1946-, Embase 1947-, Scopus 1823-, Cumulative Index of Nursing and Allied Health Literature (CINAHL) 1937-, Cochrane Database of Systematic Reviews, Cochrane Register of Controlled Trials, Database of Abstracts of Reviews of Effects, ProQuest Dissertations and Theses, and Clinicaltrials.gov. Database-supplied English language limits were applied.
Search terms included variations and combinations of the following terms: gestational diabetes, pregnancy-induced diabetes, pregnancy diabetes, diabetes mellitus gravidarum, postpartum period, puerperium, puerperal, postdelivery, postnatal, glucose tolerance test, fasting plasma glucose, oral glucose tolerance, glycemic, HbA1C, A1C, and hemoglobin A1C. Full search strategies for each database are delineated in Supplementary Data S1. All searches were initially completed in February 2017 and updated in October 2018 with results exported to EndNote. Automated duplicate finding was used and 1339 duplicates were assumed to be accurately identified and removed for a total of 1555 citations. Manual review of the reference list for each incorporated study was completed to identify any studies missing from our search strategy. Institutional Review Board approval was not required for this systematic review and meta-analysis of deidentified published data.
Two authors individually reviewed titles and abstracts against inclusion and exclusion criteria. Full text articles were retrieved where necessary to further evaluate relevant articles for inclusion. As differences by race and ethnicity are likely unique to each country and this was the focus of our review, only studies performed in the United States were included in the final review and meta-analysis. Non-U.S. studies were excluded at the title and abstract review stage if it was possible to identify study location. Studies that discussed postpartum screening but did not include sufficiently stratified publicly available race/ethnicity data were excluded at the full-text review stage.
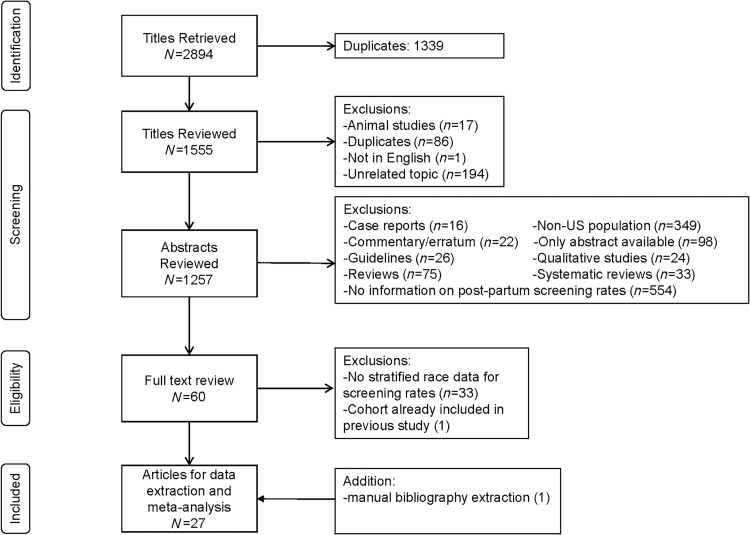
Of the 1,555 citations derived with the above search strategy, a further 298 studies were excluded because they were animal studies, duplicates, studies on an unrelated topic, or not in English. The remaining 1,257 abstracts were reviewed and a total of 1,197 studies were excluded because they were abstract only, case reports, commentaries/errata, guidelines, reviews, systematic reviews, non-U.S. population-based studies, qualitative studies, and studies for which no information on postpartum screening rates was available. Sixty articles were obtained for full-text review. Additional studies were excluded because there were no stratified race/ethnicity data for screening rates or because the same cohort had been used in a previously included study. Ultimately, 27 articles met inclusion criteria and contributed data to the analysis.15,26,31,32–51 Three of the studies were divided into intervention and non-intervention groups in the meta-analysis.39,41,42 Figure 1 illustrates the PRISMA diagram for study selection.
FIG. 1.
Flowchart depicting selection of studies included in the systematic review and meta-analysis, according to PRISMA criteria.
Abstracted data included publication year, data collection time frame, data source (electronic medical record [EMR], laboratory data warehouse, survey, and administrative claims), study setting and location, study method, method for identifying GDM, and the time frame for postpartum screening assessment. In addition, it was noted if an intervention to improve screening was studied and if only recommended screening tests or all tests were considered. Finally, total numbers of women with GDM and total numbers of women with GDM who were screened for diabetes postpartum both overall and within each racial and ethnic group (white, black, Hispanic, Asian, and other) were abstracted. To standardize our data collection, when screening percentages were presented between racial and ethnic groups, we calculated screening rates within racial and ethnic groups. A template for data extraction is presented in Supplementary Data S2.
As the exposure of interest for our review, we collected the method by which race and ethnicity were assessed (self-report, birth certificate, medical record extraction, geographic variables, or not reported). Studies were examined for assessment of confounding, and when assessed, incorporated confounders were extracted. Study quality was also assessed using the 14 question Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies from the National Heart, Lung, and Blood Institute (NHLBI) (Supplementary Table S1).
This tool assesses study quality indicators such as prespecification of the research question and population, inclusion of >50% of eligible participants, utilization of inclusion and exclusion criteria, and sample size justification. It also considers assessment of exposure before outcome, time frame sufficient for outcome assessment, exposure and outcome measurement validity and consistent application, outcome assessor blinding, <20% loss to follow-up, and confounder assessment. Questions on this tool related to exposure dose and frequency were not applicable to our research question considering race and ethnicity as the exposure.
All analyses were performed using Stata 15.1 (College Station, TX). A meta-analysis of proportions (routine metaprop) was utilized to combine proportion estimates across studies and approximate a pooled proportion effect size with 95% confidence interval (CI) for postpartum screening in the overall population and each racial and ethnic group. The effect of intervention to improve screening was examined through stratification of analyses by populations in intervention and nonintervention groups. Stratified analyses are also presented according to data source (EMR vs. other [administrative claims, laboratory data warehouse, or survey]). Heterogeneity was assessed using the Cochran's Q and Higgins I2 statistic. Given substantial heterogeneity between studies, a random effects model was appropriate. Publication bias was assessed utilizing a funnel plot and Begg and Egger's tests for small study effects. In addition, a sensitivity analysis was conducted, eliminating studies with under 200 participants.
Results
Study characteristics
The publication dates of the studies included in this systematic review and meta-analysis range from 2006 to 2018, representing deliveries from 1995 to 2016. Table 1 summarizes study characteristics, including author, year and study data sources, setting, and location. There were no randomized controlled studies and most included studies were retrospective cohorts. Studies differed in the way GDM was identified (Table 1, column 4). In most studies, GDM diagnosis was established by a 3 hours oGTT that met either the Carpenter-Coustan criteria or National Diabetes Data Group criteria (NDDG) or by coding from the International Classification of Diseases, 9th Revision (ICD-9). Some studies used ICD-9 classification combined with medication or test strip prescription. Other studies only specified medical chart review or use of procedure codes for prenatal glucose screening for GDM (CPT 82950 or 82951), and survey studies utilized self-report to identify GDM.
Table 1.
Summary of Studies Included in the Systematic Review and Meta-Analysis
| Author (publication year) | Study method | Study setting, State, Institution, Data source | GDM identification | Postpartum screening time frame and test(s) | Women with at least 1 visit after delivery n (%) | Overall postpartum diabetes screening n (%) |
Postpartum diabetes screening by race/ethnicity n (%)a |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 96,439 | White n = 36,807 | Black n = 7,315 | Hispanic n = 18,336 | Asian n = 12,269 | Other n = 7,203b | ||||||
| Studies with no intervention to improve screening | |||||||||||
| Kim et al.33 | Retrospective cohort | Academic MI University of Michigan EMR |
ICD9 code + Chart review +5% check of 3 hours oGTT (C/C) | ≥6 weeks Any labs with glucose, Hb A1C, FPG, oGTT |
447/533 (84) | 204/533 (38) | 159/439 (36) | 31/60 (52) | NR | NR | 14/34 (41) |
| Russell et al.38 | Retrospective cohort | Academic DIP clinic RI Brown University EMR |
3 hours oGTT (C/C) | After delivery 5–8.5 weeks IQR 2 hours oGTT, FPG |
265/344 (77) | 156/344 (45) | 37/99 (37) | 37/69 (54) | 64/139 (46) | NR | 18/37 (49) |
| Almario et al.26 | Retrospective cohort | Academic PA Thomas Jefferson University EMR + Quest, Labcorp data |
3 hours oGTT (C/C) + clinician dx | 5–12 weeks 2 hours oGTT, FPG |
90/90 (100) | 18/90 (20) | 4/29 (14) | 5/29 (17) | 3/10 (30) | 6/22 (27) | NR |
| Dietz et al.29 | Retrospective cohort | Integrated health system OR, WA Kaiser NW EMR |
FPG ≥126 or 1 hour GCT ≥200 or 3 hours oGTT (NDDG), or ICD-9 + insulin or glyburide | ≤12 weeks 2 hours oGTT, FPG |
356/356 (100) | 251/356 (71) | 122/196 (62) | 6/8 (75) | 47/58 (81) | 75/89 (84) | 1/4 (25) |
| Ferrara et al.31 | Prospective cohort | Integrated health system Northern CA Kaiser EMR |
3 hours oGTT (NDDG) | 6 weeks to 12 months 2 hours oGTT, FPG, Hb A1C after 12 weeks |
NR | 5,524/14,448 (38) | 1,547/4,652 (33) | 177/677 (26) | 1,497/3,692 (41) | 1,729/3,799 (46) | 309/737 (42) |
| Lawrence et al.34 | Retrospective cohort | Integrated health system Southern CA Kaiser EMR |
3 hours oGTT (C/C) | 7 days to 6 months 2 hours oGTT or FPG |
11,164/11,825 (94) | 5,939/11,825 (50) | 1,184/2,484 (48) | 219/804 (27) | 3,139/6,144 (51) | 1,333/2,259 (59) | 64/134 (48) |
| Stasenko et al.40 | Retrospective cohort | Academic Northern CA UCSF EMR |
3 hours oGTT (C/C) | 6 months 2 hours oGTT or FPG |
NR | 251/745 (34) | 66/238 (28) | 16/56 (29) | 18/101 (18) | 146/338 (43) | NR |
| Blatt et al.27 | Retrospective cohort | Quest Data Warehouse U.S. National |
2 hours oGTT (IADPSG) | 6 months 2 hours oGTT, 3 hours oGTT, FPG, RPG, Hb A1C |
NR | 4,486/23,299 (19) | 774/5,236 (15) | 209/1,291 (16) | 393/2,209 (18) | 244/1,272 (19) | 125/663 (19) |
| Hale et al.15 | Retrospective cohort | Medicaid SC Administrative claims, birth certificate, hospital discharge data |
ICD9 for GDM + CPT code for oGTT in pregnancy | 5–13 weeks 2 hours oGTT, FPG, RPG |
5,144/6,239 (83) | 214/6,239 (3) | 102/2,949 (3) | 98/2,949 (3) | NR | NR | 12/276 (4) |
| McCloskey et al.35 | Cross sectional | Academic MA Boston Medical Center EMR ± Administrative claims |
ICD9 | 6 months 2 hours oGTT, FPG, Hb A1C |
333/415 (80) | 97/415 (23) | 5/30 (17) | 70/274 (26) | 12/52 (23) | 4/48 (8) | NR |
| Mendez-Figueroa et al.43 | Retrospective cohort | Academic DIP clinic RI Brown University EMR |
3 hours oGTT (C/C) | 8–159 days 2 hours oGTT |
NR | 201/414 (49) | 32/100 (32) | 27/53 (51) | 83/162 (51) | 40/62 (65) | 19/36 (53) |
| Oza-Frank36,c | Cross sectional | PRAMS CO, MN, UT, NYC Survey |
Self-report | <4 months NR |
NR | 402/829 (48) | (51) | (55) | (43) | NR | (51) |
| Paez et al.37 | Retrospective cohort | Medical group MA Harvard Vanguard EMR |
ICD9 + 3 hours oGTT or test strip script | ≥6 weeks 2 hours oGTT, FPG; Hb A1C after 12 weeks |
NR | 118/139 (85) | 63/74 (85) | 14/20 (70) | NR | 27/29 (93) | 12/14 (86) |
| Eggleston et al.30 | Retrospective cohort | Private health plan U.S. National Administrative claims |
ICD9 (1 inpatient or 2 outpatient) | 12 months Any labs with glucose 2 hours oGTT, FPG, Hb A1C |
26,125/32,253 (81) | 7,722/32,253 (24) | 4,252/19,161 (22) | 126/597 (21) | 1,039/4,086 (25) | 1,219/3,743 (33) | 1,061/4,588 (23) |
| Rosenthal et al.51 | Retrospective cohort | Academic DIP clinic MA Brigham & Womens EMR |
3 hours oGTT (C/C) or clinician dx | 6–12 weeks 2 hours oGTT |
296/404 (73) | 155/404 (38) | 41/122 (34) | NR | NR | NR | 114/282 (40) |
| Battarbee and Yee44 | Retrospective case-control | Academic IL Northwestern EMR |
NR | 4 months 2 hours oGTT |
560/683 (82) | 279/683 (41) | 60/164 (37) | 58/149 (39) | 70/181 (39) | 43/74 (58) | 48/115 (42) |
| Jones et al.47 | Cross sectional | PRAMS CO, MA Survey |
Self-report | <4 months NR |
NR | 313/584 (54) | 109/222 (49) | 33/50 (66) | 93/191 (49) | 68/100 (68) | 10/21 (48) |
| Rosenbloom et al.48 | Retrospective cohort | Academic MD University of Maryland EMR |
3 hours oGTT (C/C) | ≥6 weeks 2 hours oGTT |
98/118 (83) | 16/118 (14) | NR/36 | 7/68 (10) | NR | NR/7 | NR/7 |
| Werner et al.49 | Prospective cohort | Academic RI Brown University EMR |
3 hours oGTT (C/C) or GCT ≥200 | 12 weeks 2 hours oGTT |
258/300 (86) | 126/300 (42) | 84/214 (39) | 10/22 (45) | 17/50 (34) | 10/23 (43) | 5/14 (36) |
| Studies with intervention to improve screening with a non-intervention “control” | |||||||||||
| Cahill et al.42 | Retrospective cohort (historical control) | FQHC NJ EMR |
NR | Before postpartum visit 2 hours oGTT, FPG |
52/71 (70) | 7/74 (9) | NA | NA | 7/74 (9) | NA | NA |
| Prospective cohort intervention: nurse counseling 1 session | 55/64 (86) | 48/64 (75) | NA | NA | 48/64 (75) | NA | NA | ||||
| Stasenko et al.41 | Retrospective cohort (historical control) | Academic Northern CA UCSF EMR |
3 hours oGTT (C/C) | 6 months 2 hours oGTT or FPG |
NR | 187/560 (33) | 48/173 (28) | 13/46 (28) | 11/76 (14) | 111/256 (43) | NR |
| Retrospective cohort intervention: CDE counseling 1 session—with verbal and written information | NR | 129/245 (53) | 36/68 (53) | 2/12 (17) | 18/36 (50) | 71/121 (59) | NR | ||||
| Schellinger et al.39 | Retrospective cohort (control) | County hospital Midwest EMR |
NR | NR 2 hours oGTT |
220/257 (86) | 156/257 (61) | NR | NR | 87/120 (73) | NR | 69/137 (50) |
| Retrospective cohort intervention: Centering© Pregnancy 4 sessions with one on postpartum risk | 187/203 (92) | 168/203 (83) | NR | NR | 168/203 (83) | NR | NR | ||||
| Studies with intervention to improve screening without a non-intervention “control” | |||||||||||
| Hunt et al.32 | Prospective cohort intervention: nurse case manager three contacts | Academic TX UT-San Antonio EMR |
3 hours oGTT (C/C) | 4–12 weeks 2 hours oGTT, FPG |
NR | 400/707 (57) | NR | NR | 376/648 (58) | NR | 24/59 (41) |
| Carson et al.28 | Prospective cohort intervention: home fingerstick testing | Private practice NJ EMR |
NR | 6 weeks 2 hours oGTT ± FSBS 4 × /day × 2 days |
NR | 20/67 (30) | 9/28 (32) | 1/3 (33) | 1/4 (25) | 0/5 (0) | 8/23 (35) |
| Werner et al.50 | Prospective cohort intervention: reminder calls | Academic MD, RI Johns Hopkins Brown University EMR |
3 hours oGTT (C/C) or GCT ≥200 | 6–12 weeks 2 hours oGTT |
NR | 49/106 (46) | 19/39 (49) | 14/39 (36) | 5/7 (71) | 6/8 (75) | 5/15 (33) |
| Dinglas et al.46 | Prospective cohort intervention: reminder calls | Academic NY NYU-Winthrop EMR |
3 hours oGTT (C/C) or GCT ≥200 | 6–12 weeks 2 hours oGTT |
NR | 35/80 (44) | 9/28 (32) | 2/13 (15) | 14/24 (58) | 9/14 (64) | 1/1 (100) |
| Carter et al.45 | Prospective cohort intervention: reminder calls | Academic MO Washington University in St. Louis EMR |
3 hours oGTT (NDDG) or GCT ≥200 | 6–12 weeks 2 hours oGTT |
43/48 (74) | 31/58 (53) | 13/26 (50) | 14/26 (54) | 5/5 (100) | NR | 4/6 (67) |
Not all studies had race/ethnicity data on the whole population. The sum of women with GDM in all race/ethnicity groups does not always equal the total women with GDM.
Individuals represented in the “Other” group are indicated here exactly as they are reported in each study. For studies where rates were not reported for white, black, Hispanic and Asian populations, this “Other” category could include some of these groups.
Number of individuals (total and screened) in each ethnic/racial group were not reported in this study; only percentages were reported.
C/C, Carpenter and Coustan criteria; CPT, Current Procedural Terminology; DIP, Diabetes in Pregnancy Specialty clinic; EMR, Electronic Medical Record; FPG, fasting plasma glucose; FQHC, Federally Qualified Health Center; FSBS, finger stick blood sugar; GCT, glucose challenge test; GDM, gestational diabetes mellitus; Hb A1C, hemoglobin A1C; IADPSG, International Association of Diabetes and Pregnancy Study Groups; ICD9, International Classification of Diseases, 9th edition; IQR, interquartile range; NA, not applicable; NDDG, National Diabetes Data Group; NR, not reported; oGTT, oral glucose tolerance test; PRAMS, Pregnancy Risk Assessment Monitoring System; RPG, random plasma glucose.
In addition to identifying GDM in different ways, studies included in the meta-analysis also differed regarding size of the population with GDM. The total number of women with GDM in the studies included in our meta-analysis was 96,439. Race and ethnicity data were available on 81,930 of these women. Study populations ranged from 58 to 32,253 women with GDM. Nineteen studies did not include an intervention,15,26,27,29–31,33–38,40,43,44,47–49,51 five had only an intervention arm,28,32,45,46,50 and three studies had both nonintervention and intervention arms.39,41,42 Interventions varied and included nurse education,41,42 reminder calls,45,46,50 home glucose testing,28 nurse case management,32 and Centering Pregnancy© group prenatal care.39
Two studies in the meta-analysis utilized claims data,15,30 one study used a large laboratory data warehouse,27 two studies utilized national survey data,36,47 and others used EMR data from single, predominantly academic, medical centers, or integrated health systems (Table 1, column 3). Four studies had only privately insured patients,28–31 one had Medicaid only15 and others had a mix of payer sources or failed to report this variable. Maternal age, parity, insurance status, education, BMI, postpartum visits, and diabetes medication use in pregnancy were commonly collected confounders among the included studies. Among the studies that reported attendance at the postpartum visit, all reported attendance rates ≥70% (Table 1, column 6).
Study quality as assessed by the NHLBI quality assessment tool was generally fair to good. All studies had defined the research question, specified the population under study, clearly applied inclusion and exclusion criteria, and had exposure assessed before outcome (for noncross-sectional studies). While all studies collected at least some demographic and clinical information that could be considered confounders, six studies did not include a method, such as multivariable regression, to directly assess the effect of confounders on the screening outcome.28,32,42,45,46,50 Four studies had <50% of the eligible population participating27,37,43,46 and three did not specify how many women were eligible.42,45,50 Loss to follow-up was <20% in 11 of the 24 studies that were not cross-sectional.15,26,29,30,33,34,37,39,44,48,49 Only five studies did an a priori sample size calculation,38,43,45,46,49 and assessors were not blinded to outcome in any study. Only two studies included a self-reported postpartum screening outcome.36,47 All other outcomes were laboratory results documented in medical records or administrative codes for laboratories completed. Race and ethnicity were typically self-reported or extracted from birth certificate or medical records. One study utilized a method to estimate racial and ethnic distribution in the sample through geographic information systems,30 and four studies did not record how race and ethnicity were assessed.27,36,37,51
Findings
Overall, postpartum screening rates for diabetes after a pregnancy complicated by GDM were low. As shown in Table 2, the pooled proportion estimate of screening among all women with GDM was 42% (95% CI 35%–48%). Screening rates were relatively low among individual racial and ethnic groups as well. The overall pooled proportion estimates of screening among different racial and ethnic groups follow: white 35% (95% CI 28%–42%), black 33% (95% CI 24%–42%), Hispanic 45% (95% CI 37%–53%), Asian 50% (95% CI 41%–58%), and other 36% (95% CI 27%–45%) (Table 2). Point estimates for screening were at least 10% higher in Asian and Hispanic populations than in white and black populations, although CIs overlapped.
Table 2.
Pooled Estimates of Postpartum Diabetes Screening by Race and Ethnicity from Metaproportion Routines
| Overall |
Sensitivity analysis (Studies with total samples size >200 participants) |
|||||
|---|---|---|---|---|---|---|
| Included studies | No. of women with GDM | Pooled proportion point estimate of postpartum screening ES (95% CI) | Included studies | No. of women with GDM | Pooled proportion point estimate of postpartum screening ES (95% CI) | |
| Totala | 27 | 96,439 | 0.42 (0.35–0.48) | 19 | 95,643 | 0.42 (0.35–0.50) |
| White | 22 | 36,807 | 0.35 (0.28–0.42) | 16 | 36,547 | 0.33 (0.25–0.40) |
| Black | 22 | 7,315 | 0.33 (0.24–0.42) | 15 | 7,117 | 0.33 (0.23–0.44) |
| Hispanic | 21 | 18,336 | 0.45 (0.37–0.53) | 15 | 18,148 | 0.44 (0.36–0.52) |
| Asian | 17 | 12,269 | 0.50 (0.41–0.58) | 12 | 12,184 | 0.48 (0.39–0.57) |
| Otherb | 12 | 6,351 | 0.36 (0.27–0.45) | 9 | 6,312 | 0.36 (0.27–0.46) |
Oza-Frank36 was only included in the total. Although screening rates were reported for racial and ethnic groups, exact numbers to calculate screening rate were only reported on the total.
Studies included in the meta-analysis of proportions for the “Other” racial and ethnic group must have specified screening rates for all four racial and ethnic groups (white, black, Hispanic, and Asian), such that the “Other” group would not include information about screening in any of these groups. There were 10 studies that had data for an “Other” group (including a total of 852 women) that did not have data stratified for all white, black, Hispanic, and Asian populations as well and are not included in the meta-analysis here. This discrepancy accounts for the 1% of the population not represented in this table.
CI, confidence interval; ES, effect size.
Studies in which there was an intervention to improve screening reported higher pooled proportions screened 56% (95% CI 44%–66%) than those without intervention 37% (95% CI 30%–44%) (Fig. 2). When data were stratified by intervention and no intervention, only Hispanic women demonstrated a significant effect of intervention on pooled proportion estimates of screening (intervention 68% [95% CI 54%–81%] vs. nonintervention groups 37% [95% CI 29%–45%]). There were also more studies reporting on interventions in Hispanic women than any other group (eight intervention studies among Hispanic women,28,32,39,41,42,45,46,50 five for white and black women,28,41,45,46,50 four for Asian women,28,41,46,50 and three for other racial and ethnic groups).28,46,50 Other racial and ethnic subgroups did not demonstrate consistent effects of intervention on screening. Studies with interventions to improve screening are reported in Table 1 with information about type of intervention and screening point estimates by racial and ethnic group. A meta-analysis of intervention effect in the three studies with a control arm was not possible given heterogeneity. Stratification by study quality, study design, method for identifying GDM, or screening time frame did not result in differences in pooled proportion screened overall or in any racial and ethnic subgroup.
FIG. 2.
Forest plots depicting pooled proportions for postpartum diabetes screening in each racial and ethnic group (A: Total, B: White, C: Black, D: Hispanic, E: Asian, F: Other Race), stratified by no intervention versus intervention.
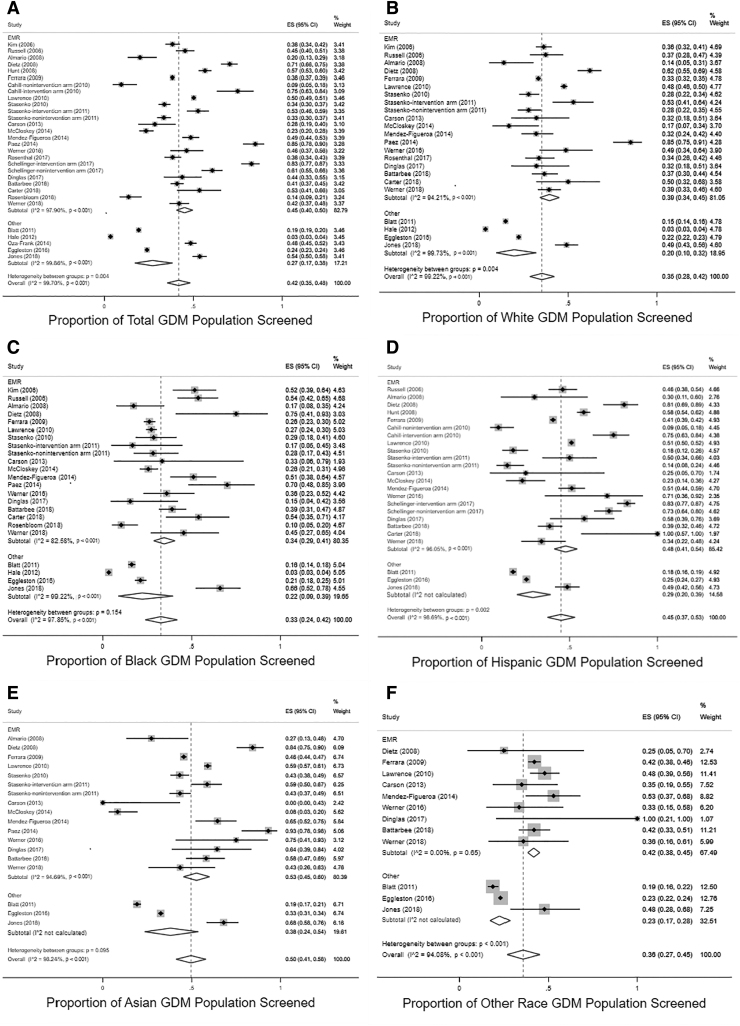
Overall, studies using the EMR as the data source had a pooled proportion of women screened of 45% (95% CI 40%–50%) compared with 27% (95% CI 17%–38%) in studies using other data sources (administrative claims, laboratory data warehouse, survey). As these CIs do not overlap, it can be concluded that this is a statistically significant difference. Significant differences between screening rates by data source were present for white, Hispanic, and other racial and ethnic groups (Fig. 3).
FIG. 3.
Forest plots depicting pooled proportions for postpartum diabetes screening in each racial and ethnic group (A: Total, B: White, C: Black, D: Hispanic, E: Asian, F: Other Race), stratified by data source (EMR vs. other [administrative claims, laboratory data warehouse, or survey data]). EMR, Electronic Medical Record.
Heterogeneity and publication bias are important considerations when assessing the validity of our findings. The I2 value generated for the total population is quite high at 99.7%, indicating that the variability in results is attributable to variation between studies. The same was true of the I2 for each racial and ethnic group. With stratification by intervention versus nonintervention, heterogeneity was lower in the intervention stratum among racial and ethnic subgroups (31%–88%). Visual inspection of a funnel plot was largely symmetrical implying low risk of publication bias (Supplementary Fig. 1). Begg and Egger's tests also demonstrated no small study effects. In a sensitivity analysis including only studies with 200 or more participants, pooled proportion estimates for screening were not appreciably different overall or in each racial and ethnic group (Table 2).15,27,29–36,38–41,43,44,47,49,51
Discussion
Postpartum screening rates for type 2 diabetes in a population of women with a history of GDM are low among all racial and ethnic groups. Guidelines recommend that women should be screened at multiple intervals after a pregnancy with GDM, and less than half of women in reported studies are being screened. There were also very few studies incorporating black women in interventions intended to improve screening rates. Overall, black women were screened at lower rates than Hispanic and Asian women and similar rates to non-Hispanic white women. This disparity must be addressed in future studies, particularly as black women are at highest risk for progression to type 2 diabetes after a pregnancy with GDM.4,8 In addition, disproportionate maternal mortality and morbidity among black women in the United States is a critical public health problem, highlighting the need for particular focus on care for this population during and after pregnancy.52
Hispanic women also have a high risk for progression to type 2 diabetes after pregnancy with GDM, and more studies have been conducted utilizing interventions to improve screening among Hispanic women than any other racial and ethnic group. These interventions, particularly higher intensity interventions like Centering Pregnancy©, significantly increased screening among Hispanic women and should be implemented and studied at larger scale and among other racial and ethnic groups.32,39,42
To our knowledge, this is the first systematic review to specifically examine racial and ethnic variation in postpartum diabetes screening rates. In addition, our analysis is unique in that metaproportion routines were utilized to estimate pooled proportion estimates. CIs overlapped; however, point estimates for pooled screening rates were at least 10% higher among Asian and Hispanic women than among white and black women. There were a larger number of studies with interventions to improve screening among Hispanic women, and when point estimates for pooled screening proportions were compared among nonintervention groups, these estimates were similar for white, black, and Hispanic women. Nonetheless, even in nonintervention groups, the point estimate for postpartum screening among Asian women was about 10% higher. It is important to recognize both the low overall screening rates and this discrepancy by race and ethnicity as black and Hispanic women are the most likely to progress to type 2 diabetes after GDM.
Our study addresses variation in screening rates reported utilizing different data sources. Studies reporting screening rates from EMR data had pooled proportions for screening that were statistically significantly higher than studies using administrative claims, laboratory data warehouse, or survey data. While this is understandable given the more detailed nature of the EMR and ability to access laboratory results, it is difficult to capture this type of data on the state or national level in the United States as medical record systems vary across clinics and hospital systems. Hence, EMR data reflect screening rates in single, often academic, centers and integrated health systems. With advances in information technology and the establishment of practice-based research networks, future research can focus on connecting EMR systems to capture more accurate screening data across multiple systems in routine care. In addition, future research may focus on development and evaluation of EMR-based interventions aimed at improving diabetes screening rates as well.
This systematic review and meta-analysis has a number of limitations. There was substantial heterogeneity among studies. Sources of heterogeneity may include different study designs and incorporation of interventions to improve screening, study quality, data sources, race/ethnicity classification, time frame, and test used for postpartum screening. We felt it was important to report a pooled screening estimate, despite heterogeneity, to demonstrate in a rigorous way, across many different studies, that screening rates are low across all racial and ethnic groups. Reporting pooled estimates with recognition of heterogeneity among studies can motivate standardization of future randomized studies in this field. Utilizing stratification and metaregression, the heterogeneity was not consistently explained by data source, intervention, use of a recommended test, method for identifying GDM, study quality, study design, or screening time frame.
In addition, a large number of studies were eliminated at the full text review stage because they did not present screening rates stratified by racial and ethnic group. This may have been related to low numbers of underrepresented minorities or inconsistent reporting of screening rates. Some of these excluded studies controlled for race and ethnicity in multivariable regression models assessing associations with screening, but this was typically done using a dichotomous race variable (e.g., white vs. nonwhite). Future research should incorporate screening data by race and ethnicity in an easily accessible manner, particularly as the National Institutes of Health increases the focus on rigor and reproducibility.
Additional challenges were associated with data collection/reporting and definitions of key variables. Studies varied in the way race and ethnicity were defined (self-report, medical record extraction, birth certificate records, and geographic estimates), and some studies did not report how race and ethnicity were collected. There was a lack of consistency among studies in reporting of data for racial/ethnic groups. Some studies reported specific screening on 4–5 groups (white, black, Hispanic, Asian, and other), while other studies incorporated only one group and still others divided data into white and nonwhite populations. For the purposes of reporting pooled proportions, we only reported pooled proportions for the “Other” racial/ethnic category if the study included specific screening rates on white, black, Hispanic, and Asian populations, so as to limit the inadvertent inclusion of these racial and ethnic groups in the “Other” category.
In addition, both GDM diagnosis and outcome definition varied among studies. Addressing the outcome of postpartum screening specifically, studies varied regarding the time frame of postpartum screening as well as the type of testing completed. While a minority of studies had <50% of the eligible population participating, many had >20% loss to follow-up, contributing to the possibility for selection and ascertainment bias. In addition, studies did not report differences in attendance of the postpartum office visit stratified by race/ethnicity so this could contribute to differences in reported screening rates among racial and ethnic groups. However, the overall percent of women attending at least one postpartum visit was much higher than the percent of women with postpartum diabetes screening, so postpartum visit attendance likely explains only a small amount of this variation.
Despite the challenges and limitations of this current systematic review and meta-analysis, it highlights the need for additional research that can have a meaningful impact. Women often utilize their obstetrician and gynecologist for primary care in the childbearing years, hence, these health care providers are frequently the first-line for postpartum diabetes screening in the first 12 weeks and 1 year postpartum. Our study serves as a call to action for researchers and clinicians to work toward addressing barriers to postpartum diabetes screening among all women.
Conclusion
Our findings are consistent with previous work that has demonstrated low rates of postpartum diabetes screening overall. Greater effort must be directed toward standardizing the measurement, collection, and reporting of racial and ethnic data in screening studies. Given the low screening rates documented among all racial and ethnic groups, and the high burden of subsequent type 2 diabetes and its complications, well-designed studies to examine interventions that comprehensively address the needs of women in the postpartum period are urgently needed. Increasing postpartum screening for diabetes and enhancing prevention of type 2 diabetes among high risk women are essential next steps to achieving health equity.
Supplementary Material
Acknowledgments
The authors acknowledge the Washington University Institute for Clinical and Translational Sciences, Foundation for Barnes Jewish Hospital, NCATS, NCI, and NIDDK at the National Institutes of Health for supporting this research. We also thank Roxann Williams, MPH for assistance with article formatting and preparation.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
C.J.H. was supported for research reported in this publication by Award No. KL2TR002346 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health, and C.J.H. is currently supported by 1K23HD096204-01A1. R.P. was supported by the T32DK007120-44 through NIDDK. K.S. was supported by T32CA190194 through NCI, the Siteman Cancer Center, and the Foundation for Barnes Jewish Hospital. G.A.C. is supported, in part, by the Foundation for Barnes Jewish Hospital and the Washington University Institute for Clinical and Translational Sciences grant UL1TR000448. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding agencies had no role in the design or conduct of this study.
Supplementary Material
References
- 1. DeSisto CL, Kim SY, Sharma AJ. Prevalence Estimates of Gestational Diabetes Mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007–2010. Prev Chronic Dis 2014;11:130415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lavery JA, Friedman AM, Keyes KM, Wright JD, Ananth CV. Gestational diabetes in the United States: Temporal changes in prevalence rates between 1979 and 2010. BJOG 2017;124:804–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. American Diabetes Association. 14. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019;42:S165–S172 [DOI] [PubMed] [Google Scholar]
- 4. Casagrande SS, Linder B, Cowie CC. Prevalence of gestational diabetes and subsequent type 2 diabetes among U.S. women. Diabetes Res Clin Pract 2018;141:200–208 [DOI] [PubMed] [Google Scholar]
- 5. Committee on Practice Bulletin-Obstetrics. ACOG practice bulletin no. 190: Gestational diabetes mellitus. Obstet Gynecol 2018;131:e49–e64 [DOI] [PubMed] [Google Scholar]
- 6. Hunt KJ, Logan SL, Conway DL, Korte JE. Postpartum screening following GDM: How well are we doing? Curr Diab Rep 2010;10:235–241 [DOI] [PubMed] [Google Scholar]
- 7. Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: A systematic review. Diabetes Care 2002;25:1862–1868 [DOI] [PubMed] [Google Scholar]
- 8. Xiang AH, Li BH, Black MH, et al. Racial and ethnic disparities in diabetes risk after gestational diabetes mellitus. Diabetologia 2011;54:3016–3021 [DOI] [PubMed] [Google Scholar]
- 9. Gong Q, Gregg EW, Wang J, et al. Long-term effects of a randomised trial of a 6-year lifestyle intervention in impaired glucose tolerance on diabetes-related microvascular complications: The China Da Qing Diabetes Prevention Outcome Study. Diabetologia 2011;54:300–307 [DOI] [PubMed] [Google Scholar]
- 10. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019;42:S13–S28 [DOI] [PubMed] [Google Scholar]
- 11. Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aroda VR, Christophi CA, Edelstein SL, et al. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: The Diabetes Prevention Program Outcomes Study 10 year follow-up. J Clin Endocrinol Metab 2015;100:1646–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin J, O'Connor E, Evans C, Senger C, Rowland M, Groom HC. Behavioral counseling to promote a healthy lifestyle in persons with cardiovascular risk factors: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2014;161:568–578 [DOI] [PubMed] [Google Scholar]
- 14. Tovar A, Chasan-Taber L, Eggleston E, Oken E. Postpartum screening for diabetes among women with a history of gestational diabetes mellitus. Prev Chronic Dis 2011;8:A124. [PMC free article] [PubMed] [Google Scholar]
- 15. Hale NL, Probst JC, Liu J, Martin AB, Bennett KJ, Glover S. Postpartum screening for diabetes among Medicaid-eligible South Carolina women with gestational diabetes. Womens Health Issues 2012;22:e163–e169 [DOI] [PubMed] [Google Scholar]
- 16. Bennett WL, Chang H-Y, Levine DM, et al. Utilization of primary and obstetric care after medically complicated pregnancies: An analysis of medical claims data. J Gen Intern Med 2013;29:636–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herrick CJ, Keller MR, Trolard AM, Cooper BP, Olsen MA, Colditz GA. Postpartum diabetes screening among low income women with gestational diabetes in Missouri 2010–2015. BMC Public Health 2019;19:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009;373:1773–1779 [DOI] [PubMed] [Google Scholar]
- 19. Kim C, Berger DK, Chamany S. Recurrence of gestational diabetes mellitus: A systematic review. Diabetes Care 2007;30:1314–1319 [DOI] [PubMed] [Google Scholar]
- 20. Middleton P, Crowther CA. Reminder systems for women with previous gestational diabetes mellitus to increase uptake of testing for type 2 diabetes or impaired glucose tolerance. Cochrane Database Syst Rev 2014;3:CD009578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jeppesen C, Kristensen JK, Ovesen P, Maindal HT. The forgotten risk? A systematic review of the effect of reminder systems for postpartum screening for type 2 diabetes in women with previous gestational diabetes. BMC Res Notes 2015;8:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Su X, Zhang Z, Qu X, Tian Y, Zhang G. Hemoglobin A1c for diagnosis of postpartum abnormal glucose tolerance among women with gestational diabetes mellitus: Diagnostic meta-analysis. PLoS One 2014;9:e102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bennett WL, Bolen S, Wilson LM, Bass EB, Nicholson WK. Performance characteristics of postpartum screening tests for type 2 diabetes mellitus in women with a history of gestational diabetes mellitus: A systematic review. J Womens Health (Larchmt)) 2009;18:979–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carson MP, Frank MI, Keely E. Original research: Postpartum testing rates among women with a history of gestational diabetes—Systematic review. Prim Care Diabetes 2013;7:177–186 [DOI] [PubMed] [Google Scholar]
- 25. Office of Women's Health Research. Moving into the future with new dimensions and strategies: A vision for 2020 for women's health research. Bethesda, MD: National Institutes of Health, 2010 [Google Scholar]
- 26. Almario CV, Ecker T, Moroz LA, Bucovetsky L, Berghella V, Baxter JK. Obstetricians seldom provide postpartum diabetes screening for women with gestational diabetes. Am J Obstet Gynecol 2008;198:528..e1–528.e5. [DOI] [PubMed] [Google Scholar]
- 27. Blatt AJ, Nakamoto JM, Kaufman HW. Gaps in diabetes screening during pregnancy and postpartum. Obstet Gynecol 2011;117:61–68 [DOI] [PubMed] [Google Scholar]
- 28. Carson MP, Lewis BG, Pagan ER, Evers M. Evaluation of home testing to improve follow up after gestational diabetes (Fingerstick Assessments of sugar two-months postpartum or FAST). Obstet Med 2013;6:120–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dietz PM, Vesco KK, Callaghan WM, et al. Postpartum screening for diabetes after a gestational diabetes mellitus-affected pregnancy. Obstet Gynecol 2008;112:868–874 [DOI] [PubMed] [Google Scholar]
- 30. Eggleston EM, Lecates RF, Zhang F, Wharam JF, Ross-Degnan D, Oken E. Variation in postpartum glycemic screening in women with a history of gestational diabetes mellitus. Obstet Gynecol 2016;128:159–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ferrara A, Peng T, Kim C. Trends in postpartum diabetes screening and subsequent diabetes and impaired fasting glucose among women with histories of gestational diabetes mellitus: A report from the Translating Research Into Action for Diabetes (TRIAD) Study. Diabetes Care 2009;32:269–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hunt KJ, Conway DL. Who returns for postpartum glucose screening following gestational diabetes mellitus? Am J Obstet Gynecol 2008;198:404..e1–404.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim C, Tabaei BP, Burke R, et al. Missed opportunities for type 2 diabetes mellitus screening among women with a history of gestational diabetes mellitus. Am J Public Health 2006;96:1643–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lawrence JM, Black MH, Hsu JW, Chen W, Sacks DA. Prevalence and timing of postpartum glucose testing and sustained glucose dysregulation after gestational diabetes mellitus. Diabetes Care 2010;33:569–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McCloskey L, Bernstein J, Winter M, Iverson R, Lee-Parritz A. Follow-up of gestational diabetes mellitus in an Urban safety net hospital: Missed opportunities to launch preventive care for women. J Womens Health (Larchmt) 2014;23:327–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oza-Frank R. Postpartum diabetes testing among women with recent gestational diabetes mellitus: PRAMS 2009–2010. Matern Child Health J 2014;18:729–736 [DOI] [PubMed] [Google Scholar]
- 37. Paez KA, Eggleston EM, Griffey SJ, et al. Understanding why some women with a history of gestational diabetes do not get tested for diabetes. Womens Health Issues 2014;24:e373–e379 [DOI] [PubMed] [Google Scholar]
- 38. Russell MA, Phipps MG, Olson CL, Welch HG, Carpenter MW. Rates of postpartum glucose testing after gestational diabetes mellitus. Obstet Gynecol 2006;108:1456–1462 [DOI] [PubMed] [Google Scholar]
- 39. Schellinger MM, Abernathy MP, Amerman B, et al. Improved outcomes for Hispanic Women with gestational diabetes using the Centering Pregnancy© Group Prenatal Care Model. Matern Child Health J 2017;21:297–305 [DOI] [PubMed] [Google Scholar]
- 40. Stasenko M, Cheng YW, McLean T, Jelin AC, Rand L, Caughey AB. Postpartum follow-up for women with gestational diabetes mellitus. Am J Perinatol 2010;27:737–742 [DOI] [PubMed] [Google Scholar]
- 41. Stasenko M, Liddell J, Cheng YW, Sparks TN, Killion M, Caughey AB. Patient counseling increases postpartum follow-up in women with gestational diabetes mellitus. Am J Obstet Gynecol 2011;204:522..e1–522.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cahill K, Parietti E, Guttman M. Effective postpartum management of women with gestational diabetes. UPNAAI Nurs J 2010;6:41–44 [Google Scholar]
- 43. Mendez-Figueroa H, Dahlke JD, Daley J, Lopes VV, Coustan DR. Prediction of abnormal postpartum glucose tolerance testing in mild gestational diabetes mellitus. J Reprod Med 2014;59:393–400 [PubMed] [Google Scholar]
- 44. Battarbee AN, Yee LM. Barriers to postpartum follow-up and glucose tolerance testing in women with gestational diabetes mellitus. Am J Perinatol 2018;35:354–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carter EB, Martin S, Temming LA, Colditz GA, Macones GA, Tuuli MG. Early versus 6–12 week postpartum glucose tolerance testing for women with gestational diabetes. J Perinatol 2018;38:118–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dinglas C, Muscat J, Heo H, Islam S, Vintzileos A. Immediate postpartum glucose tolerance testing in women with gestational diabetes: A pilot study. Am J Perinatol 2017;34:1264–1270 [DOI] [PubMed] [Google Scholar]
- 47. Jones KE, Yan Y, Colditz GA, Herrick CJ. Prenatal counseling on type 2 diabetes risk, exercise, and nutrition affects the likelihood of postpartum diabetes screening after gestational diabetes. J Perinatol 2018;38:315–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rosenbloom JI, Blanchard MH. Compliance with postpartum diabetes screening recommendations for patients with gestational diabetes. J Womens Health (Larchmt) 2018;27:498–502 [DOI] [PubMed] [Google Scholar]
- 49. Werner EF, Has P, Kanno L, Sullivan A, Clark MA. Barriers to postpartum glucose testing in women with gestational diabetes mellitus. Am J Perinatol 2019;36:212–218 [DOI] [PubMed] [Google Scholar]
- 50. Werner EF, Has P, Tarabulsi G, Lee J, Satin A. Early postpartum glucose testing in women with gestational diabetes mellitus. Am J Perinatol 2016;33:966–971 [DOI] [PubMed] [Google Scholar]
- 51. Rosenthal EW, Easter SR, Morton-Eggleston E, Dutton C, Zera C. Contraception and postpartum follow-up in patients with gestational diabetes. Contraception 2017;95:431–433 [DOI] [PubMed] [Google Scholar]
- 52. Leonard SA, Main EK, Scott KA, Profit J, Carmichael SL. Racial and ethnic disparities in severe maternal morbidity prevalence and trends. Ann Epidemiol 2019;33:30–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.