Abstract
Background –
Atrial fibrillation (AF) adversely impacts health-related quality of life (hrQoL). While some patients demonstrate improvements in hrQoL, the factors associated with large improvements in hrQoL are not well described.
Methods –
We assessed factors associated with a 1-year increase in AFEQT of 1 standard deviation (>=18 points; 3x clinically important difference), among outpatients in the ORBIT-AF I registry.
Results –
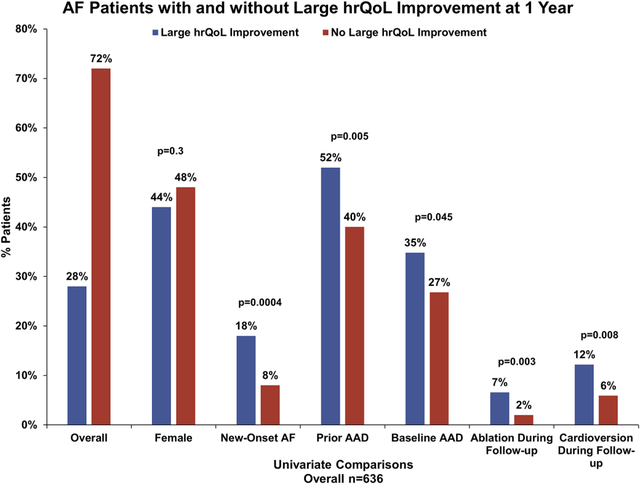
Overall, 28% (181/636) of patients had such a hrQoL improvement. Compared with patients not showing large hrQoL improvement, they were of similar age (median 73 vs. 74, p=0.3), equally likely to be female (44% vs. 48%, p=0.3), but more likely to have newly-diagnosed AF at baseline (18% vs. 8%; p=0.0004), prior antiarrhythmic drug use (52% vs. 40%, 0.005), baseline antiarrhythmic drug use (34.8% vs, 26.8%, p=0.045), and more likely to undergo AF-related procedures during follow-up (AF ablation: 6.6% vs. 2.0%, p=0.003; cardioversion:12.2% vs. 5.9% p=0.008). In multivariable analysis, a history of alcohol abuse (adjusted OR 2.41, p=0.01) and increased baseline diastolic BP (adjusted OR 1.23 per 10-point increase and >65 mm Hg, p=0.04) were associated with large improvements in hrQoL at 1 year, whereas patients with prior stroke/TIA, COPD, and PAD were less likely to improve (p<0.05 for each).
Conclusions –
In this national registry of AF patients, potentially treatable AF risk factors are associated with large hrQoL improvement, whereas less reversible conditions appeared negatively associated with hrQoL improvement. Understanding which patients are most likely to have large hrQoL improvement may facilitate targeting interventions for high-value care that optimizes patient reported outcomes in AF.
Clinical Trial Registration –
clinicaltrials.gov.; Unique Identifier: NCT01165710
Journal Subject Terms: Atrial Fibrillation, Health Services, Quality and Outcomes
Keywords: atrial fibrillation, patient reported outcome, quality of life, AFEQT, response
Graphical Abstract
Introduction
Atrial fibrillation (AF) represents the most common sustained tachyarrhythmia in adults, and accounts for substantial health systems resources.1–3 Additionally, patients with AF demonstrate reduced health-related quality of life (hrQoL), which is similar in magnitude to that experienced by patients who have had a myocardial infarction.4 Certain interventions for AF may improve hrQoL.5–7 However, there can be heterogeneity of responses in hrQoL: some patients may not improve at all while others appear to demonstrate marked improvement in symptom burden over time. Patients with heart rhythm disorders who exhibit large improvement of symptoms and/or hrQoL are often labeled as ‘super-responders’, as is the case for some patients treated with cardiac resynchronization therapy.8
However, the optimal identification of AF patients who are most likely to experience such large improvement in hrQoL, and how to tailor therapy to these patients, remains a challenge.6, 7 Patients’ health status is particularly important for highly symptomatic patients and is not reflected in traditional clinical outcomes of rehospitalization or death. Accordingly, we used data from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry to identify factors associated with large improvements in hrQoL for patients with AF. More specifically, the primary aims of this analysis are: (1) to identify patients who experience large improvements in hrQoL; (2) to understand patient factors associated with large improvements in hrQoL; and (3) to describe interval interventions and outcomes among these patients with large improvements in hrQoL.
Methods
Because of the sensitive nature of the data collected for this study, requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to Dr. Jonathan P. Piccini, at Duke University. The ORBIT-AF registry is a national, US, prospective cohort study of outpatients with AF, enrolled by primary care physicians, cardiologists, and/or electrophysiologists from June 2010 to August 2011. The study was managed and coordinated by the Duke Clinical Research Institute. Sites enrolled consecutive patients with electrocardiographically-documented AF, age 18 years or older. Patients were followed up every 6 months for at least 2 years. Patients were excluded if life expectancy was less than 6 months or AF was felt to be due to a reversible cause.
Data were entered in a web-based case report form, derived primarily from the patient’s medical record. Data elements included demographics, medical history, AF history (including symptoms), medical therapies, vital signs, laboratory and echocardiographic measures, and incident procedures and adverse events. Complete details of the ORBIT-AF design and rationale have been described previously.9
Approximately 20% of all enrolled patients participated in the pre-specified ORBIT-AF hrQoL substudy, as determined by the local site. Among these patients, disease-specific hrQoL was measured using the Atrial Fibrillation Effect on QualiTy-of-Life (AFEQT) Questionnaire.10 This 20-item AF-specific patient-reported outcome (PRO) tool assesses AF-related hrQoL across four domains: symptoms, daily activities, treatment concern, and treatment satisfaction. Each domain is evaluated through 2–4 questions each with a 7-point Likert response ranging from the most severe limitation/symptoms (resulting in a minimum overall score of 0) to no limitation/symptoms (resulting in a maximum overall score of 100). The AFEQT score is calculated based on questions answered, and complete response to all questions is not required to calculate an overall score.
Study Population
The present study included all patients for whom hrQoL improvement could be assessed. This required patients to have baseline and 1-year AFEQT scores, and a baseline AFEQT score low enough to provide an opportunity for significant improvement (<=82). For the purpose of this analysis, we defined large hrQoL improvement as an increase in AFEQT of >=18 points from baseline to one-year follow-up. This was based on one standard deviation above the mean change in AFEQT for this population, and also because it represents a 3-fold greater improvement compared with: (1) the clinically important difference for AFEQT,11, 12 and (2) the improvement in hrQoL observed in recent major clinical trials of AF ablation.6, 7
Statistical Methods
All baseline characteristics and univariate data are presented as frequencies and percentages for categorical variables and medians (25th, 75th percentiles) for continuous variables for those with large hrQoL improvement versus those without. The baseline characteristics were compared using the Chi-square test for categorical variables and the Wilcoxon rank sum test for continuous variables. Events occurring between baseline and the one-year AFEQT measure, by hrQoL improvement status, are described as frequencies and percentages. Statistical comparisons were based on a Chi-square test.
To determine which factors were associated with a large hrQoL improvement, backwards selection with an exclusion criterion of 0.05 was used to build a multivariable logistic regression model using the candidate variables in Supplemental Material, Table S1. All continuous variables were evaluated for non-linearity with the outcome, and linear splines were used for variable that did not meet the linear relationship criteria (p < 0.05). Odds ratio (OR) with corresponding 95% confidence interval (CI) and p-value were presented from a multivariable GEE logistic regression model with constant correlation between patients within sites (exchangeable working correlation structure). Missing data was handled with single imputation and imputed values were obtained by Markov chain Monte Carlo (MCMC) or regression methods. Specifically, MCMC was used to create a monotone missing data pattern and then the logistic regression method and regression method were used to imputed binary/ordinal and continuous variables, respectively. Lastly, the discriminate function method was used to impute the remaining nominal variables with missing values. All variables on the candidate variable list had less than 5% missing except: LVEF type (16%), LA diameter type (20%), eGFR (8%) and hematocrit (10%).
Event rates per 100-person years are presented by hrQoL improvement status and compared using Poisson regression. Time zero for event ascertainment was the date of the one-year AFEQT survey date.
All subjects provided written, informed consent, and each site received institutional review board (IRB) approval for this study, according to local regulations. The ORBIT-AF registry is approved by the Duke University IRB. All analyses of the aggregate, de-identified data were performed by the Duke Clinical Research Institute using SAS software (version 9.4, SAS Institute, Cary, North Carolina, USA) and a two-tailed p-value <0.05 was considered significant for all statistical tests.
Sensitivity Analyses
Two sensitivity analyses of the model of large hrQoL improvement were calculated: a) including patients who died from baseline to 1 year as unimproved, and b) in the subset of patients who were diagnosed with AF > 1 year before entry into the study.
Results
Cohort Formation & Patient Characteristics
Among the overall ORBIT-AF population of 10,137 patients from 176 sites, 2,008 subjects participated in the ORBIT-AF hrQoL substudy (from 56% of sites) at baseline. Among these, 94% answered all AFEQT questions at baseline. After excluding patients without 1-year AFEQT assessments (n=661), and those with baseline AFEQT >82 (n=711), this yielded an analysis population of 636 patients. At baseline, these patients had a median overall AFEQT score of 67.6 (IQR 54.6, 75.9). Overall, 181 patients (28%) qualified as having a large improvement in hrQoL with AFEQT improvement of >=18 points from baseline to 1 year (see Figure 1).
Figure 1.
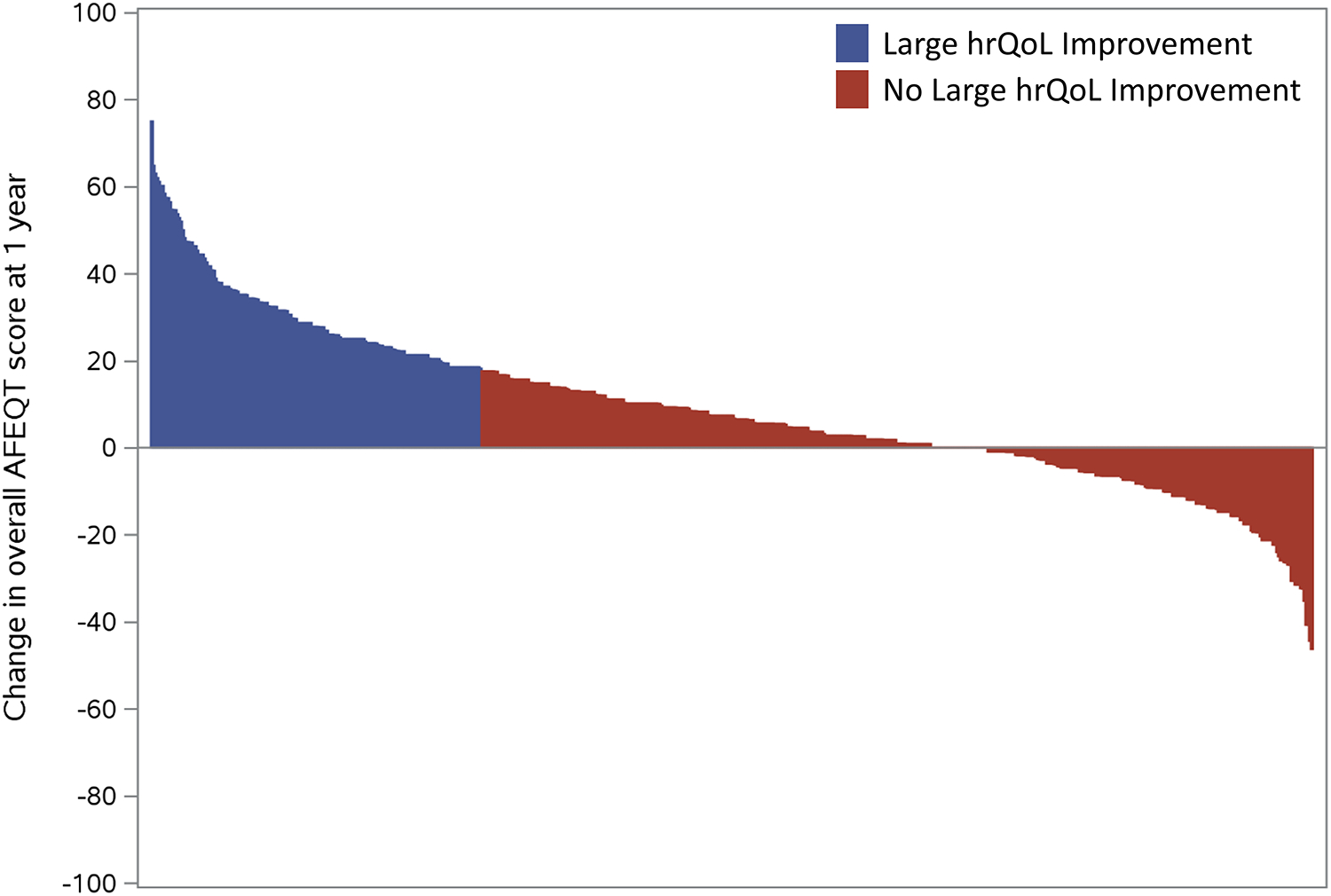
Waterfall plot of all patients in the analytic cohort, by absolute change in overall AFEQT score at 1 year, stratified by presence of large hrQoL improvement during follow-up.
AFEQT: Atrial Fibrillation Effect on QualiTy-of-Life
Baseline characteristics overall and stratified by hrQoL improvement status are shown in Table 1. Compared with those without an improvement in health status, patients experiencing large improvement in AFEQT were of similar age (median 73 vs. 74, p=0.3), and frequency of female sex (44% vs. 48%, p=0.3). However, those with marked improvements in hrQoL were more likely to have newly-diagnosed AF at baseline (18% vs. 8%) and less likely to be persistent/permanent AF (33.7% vs. 46.2%, p=0.0004). Baseline use of rate control medications was not different between the groups, however, those with large hrQoL improvement were more likely to be on antiarrhythmic therapy at baseline (34.8% vs, 26.8%, p=0.045). There was no difference in rates of sinus rhythm on most recent electrocardiogram, between those with or without large hrQoL improvement (32.0% vs. 29.2%, p=0.5). Baseline symptom status, including specific AF symptoms and AFEQT subscales, are shown in the Table 2. Specific symptoms at baseline were generally balanced between the groups, except those with large hrQoL improvement were more likely to experience light-headedness/dizziness (40% vs. 29%, p=0.009) and fatigue (45% vs. 35%, p=0.02). However, patterns of AFEQT domain change at 1 year differed between the groups (Figure 2); patients with large overall improvement in hrQoL at 1 year appeared to demonstrate more improvement in daily activities and treatment concerns.
Table 1.
Baseline characteristics by hrQoL improvement status
| Overall N=636 | No large improvement in hrQoL (<18 point improvement) N=455 | Large improvement in hrQoL (>=18 point improvement) N=181 | P-Value | |
|---|---|---|---|---|
| Age (year) | 74.0 (67.0, 81.0) | 74.0 (67.0, 81.0) | 73.0 (66.0, 80.0) | 0.2966 |
| Female | 300 (47.2%) | 220 (48.4%) | 80 (44.2%) | 0.3442 |
| Race | 0.4318 | |||
| White | 577 (90.7%) | 409 (89.9%) | 168 (92.8%) | |
| Black or African American | 26 (4.1%) | 19 (4.2%) | 7 (3.9%) | |
| Hispanic | 20 (3.1%) | 17 (3.7%) | 3 (1.7%) | |
| Other | 12 (1.9%) | 10 (2.2%) | 2 (1.1%) | |
| Hypertension | 544 (85.5%) | 389 (85.5%) | 155 (85.6%) | 0.9637 |
| Hyperlipidemia | 476 (74.8%) | 349 (76.7%) | 127 (70.2%) | 0.0867 |
| Diabetes | 182 (28.6%) | 137 (30.1%) | 45 (24.9%) | 0.1867 |
| Anemia | 105 (16.5%) | 71 (15.6%) | 34 (18.8%) | 0.3301 |
| Chronic kidney disease (using MDRD) | 236 (37.1%) | 171 (37.6%) | 65 (35.9%) | 0.8588 |
| COPD | 134 (21.1%) | 105 (23.1%) | 29 (16.0%) | 0.0492 |
| Peripheral arterial disease | 87 (13.7%) | 72 (15.8%) | 15 (8.3%) | 0.0126 |
| Obstructive sleep apnea | 160 (25.2%) | 118 (25.9%) | 42 (23.2%) | 0.4744 |
| Frailty | 49 (7.7%) | 38 (8.4%) | 11 (6.1%) | 0.3322 |
| Alcohol abuse | 26 (4.1%) | 15 (3.3%) | 11 (6.1%) | 0.1103 |
| GI bleed | 59 (9.3%) | 46 (10.1%) | 13 (7.2%) | 0.2512 |
| Sinus node dysfunction/sick sinus syndrome | 134 (21.1%) | 109 (24.0%) | 25 (13.8%) | 0.0047 |
| Cardiac implantable device | 207 (32.5%) | 156 (34.3%) | 51 (28.2%) | 0.1382 |
| Congestive heart failure | 198 (31.1%) | 151 (33.2%) | 47 (26.0%) | 0.0762 |
| Ischemic cardiomyopathy etiology | 71 (35.9%) | 54 (35.8%) | 17 (36.2%) | 0.8828 |
| Functional status | 0.9210 | |||
| NYHA Class I | 44 (22.2%) | 34 (22.5%) | 10 (21.3%) | |
| NYHA Class II | 93 (47.0%) | 70 (46.4%) | 23 (48.9%) | |
| NYHA Class III | 55 (27.8%) | 43 (28.5%) | 12 (25.5%) | |
| NYHA Class IV | 6 (3.0%) | 4 (2.6%) | 2 (4.3%) | |
| Prior cerebrovascular events | 95 (14.9%) | 78 (17.1%) | 17 (9.4%) | 0.0134 |
| Significant valvular disease | 173 (27.2%) | 129 (28.4%) | 44 (24.3%) | 0.3017 |
| Moderate/severe mitral stenosis | 11 (1.7%) | 11 (2.4%) | 0 (0.0%) | 0.0350 |
| Prior valve replacement/repair | 55 (8.6%) | 40 (8.8%) | 15 (8.3%) | 0.8385 |
| History of CAD | 202 (31.8%) | 143 (31.4%) | 59 (32.6%) | 0.7754 |
| CHA2DS2VASc score | 4.0 (3.0, 5.0) | 4.0 (3.0, 5.0) | 4.0 (3.0, 4.0) | 0.0039 |
| CHA2DS2VASc score | 0.1942 | |||
| Low: 0 | 11 (1.7%) | 8 (1.8%) | 3 (1.7%) | |
| Medium: 1 | 35 (5.5%) | 21 (4.6%) | 14 (7.7%) | |
| High: 2+ | 590 (92.8%) | 426 (93.6%) | 164 (90.6%) | |
| ORBIT bleeding score | 2.0 (1.0, 3.0) | 2.0 (1.0, 3.0) | 2.0 (1.0, 3.0) | 0.8919 |
| BMI (kg/m2) | 29.7 (25.9, 34.5) | 29.6 (25.8, 34.5) | 30.2 (26.3, 34.4) | 0.3950 |
| Heart rate (bpm) | 70.0 (64.0, 80.0) | 70.0 (64.0, 80.0) | 72.0 (62.0, 80.0) | 0.3851 |
| Systolic blood pressure (mm Hg) | 126.0 (118.0, 138.0) | 126.0 (118.0, 138.0) | 128.0 (120.0, 139.0) | 0.3238 |
| Diastolic blood pressure (mm Hg) | 72.0 (68.0, 80.0) | 72.0 (67.0, 80.0) | 74.0 (70.0, 80.0) | 0.0216 |
| LVEF (%) | 55.0 (50.0, 60.0) | 55.0 (50.0, 60.0) | 55.0 (50.0, 60.0) | 0.7107 |
| Most recent 12-lead electrocardiogram | ||||
| Sinus rhythm | 191 (30.0%) | 133 (29.2%) | 58 (32.0%) | 0.4853 |
| Atrial fibrillation | 347 (54.6%) | 250 (54.9%) | 97 (53.6%) | 0.7572 |
| Atrial Flutter | 11 (1.7%) | 7 (1.5%) | 4 (2.2%) | 0.5581 |
| Paced | 122 (19.2%) | 95 (20.9%) | 27 (14.9%) | 0.0851 |
| Serum creatinine | 1.1 (0.9, 1.3) | 1.1 (0.9, 1.3) | 1.1 (0.8, 1.3) | 0.8398 |
| Estimated creatinine clearance (mL/min)* | 70.7 (50.3, 96.9) | 70.6 (50.7, 96.0) | 71.0 (49.2, 97.1) | 0.7118 |
| Hematocrit (%) | 40.1 (37.1, 42.8) | 40.0 (37.1, 42.9) | 40.4 (37.1, 42.6) | 0.7035 |
| Type of AF | 0.0004 | |||
| First Detected / New Onset | 69 (10.8%) | 37 (8.1%) | 32 (17.7%) | |
| Paroxysmal AF | 296 (46.5%) | 208 (45.7%) | 88 (48.6%) | |
| Persistent/permanent AF | 271 (42.6%) | 210 (46.2%) | 61 (33.7%) | |
| Time to diagnosis from screening (months) | 36.0 (9.0, 79.5) | 40.0 (11.0, 83.0) | 28.0 (3.0, 69.0) | 0.0015 |
| Time from AF diagnosis to enrollment > 12 months | 448 (70.4%) | 335 (73.6%) | 113 (62.4%) | 0.0053 |
| Current AF management strategy | 0.3166 | |||
| Rate Control | 447 (70.3%) | 325 (71.4%) | 122 (67.4%) | |
| Rhythm Control | 189 (29.7%) | 130 (28.6%) | 59 (32.6%) | |
| Prior cardioversions | 174 (27.4%) | 129 (28.4%) | 45 (24.9%) | 0.3734 |
| Treated with antiarrhythmic drug in the past | 275 (43.2%) | 181 (39.8%) | 94 (51.9%) | 0.0053 |
| Prior catheter ablation of AF | 47 (7.4%) | 31 (6.8%) | 16 (8.8%) | 0.3784 |
| Prior atrial flutter ablation | 23 (3.6%) | 11 (2.4%) | 12 (6.6%) | 0.0103 |
| Prior AV Node/HIS bundle ablation | 15 (2.4%) | 11 (2.4%) | 4 (2.2%) | 0.8764 |
| Current Medications at baseline | ||||
| Angiotensin Receptor Blocker | 137 (21.5%) | 98 (21.5%) | 39 (21.5%) | 0.9981 |
| Beta Blockers | 422 (66.4%) | 307 (67.5%) | 115 (63.5%) | 0.3435 |
| Nondihydropyridine CCBs | 116 (18.2%) | 81 (17.8%) | 35 (19.3%) | 0.6513 |
| Digoxin | 167 (26.3%) | 127 (27.9%) | 40 (22.1%) | 0.1331 |
| Statin | 339 (53.3%) | 238 (52.3%) | 101 (55.8%) | 0.4259 |
| Diuretic | 336 (52.8%) | 247 (54.3%) | 89 (49.2%) | 0.2440 |
| Currently on antiarrhythmic drug therapy at baseline | 185 (29.1%) | 122 (26.8%) | 63 (34.8%) | 0.0454 |
| Amiodarone | 48 (7.5%) | 28 (6.2%) | 20 (11.0%) | 0.0351 |
| Dronedarone | 37 (5.8%) | 25 (5.5%) | 12 (6.6%) | 0.5813 |
| Dofetilide | 5 (0.8%) | 3 (0.7%) | 2 (1.1%) | 0.5661 |
| Flecainide | 17 (2.7%) | 9 (2.0%) | 8 (4.4%) | 0.0852 |
| Propafenone | 24 (3.8%) | 17 (3.7%) | 7 (3.9%) | 0.9376 |
| Sotalol | 43 (6.8%) | 32 (7.0%) | 11 (6.1%) | 0.6652 |
| Other antiarrhythmic drug | 10 (1.6%) | 7 (1.5%) | 3 (1.7%) | 0.9134 |
| OAC (warfarin or dabigatran) † | 534 (84.0%) | 379 (83.3%) | 155 (85.6%) | 0.4687 |
As calculated by the Cockcroft-Gault formula.
No other anticoagulants were used in this cohort.
Values are presented as n (%) or median (25th, 75th percentiles), unless noted otherwise.
COPD: chronic obstructive pulmonary disease; BMI: body mass index; OAC: oral anticoagulant.
Table 2.
Baseline symptom status, by hrQoL improvement
| Overall N=636 | No large improvement in hrQoL N=455 | Large improvement in hrQoL N=181 | P-Value | |
|---|---|---|---|---|
| AF Symptoms at Baseline | ||||
| Palpitations | 251 (39.5%) | 173 (38.0%) | 78 (43.1%) | 0.2380 |
| Syncope/fainting | 41 (6.4%) | 28 (6.2%) | 13 (7.2%) | 0.6339 |
| Dyspnea at exertion | 281 (44.2%) | 197 (43.3%) | 84 (46.4%) | 0.4761 |
| Exercise Intolerance | 118 (18.6%) | 80 (17.6%) | 38 (21.0%) | 0.3183 |
| Lightheadedness/dizziness | 204 (32.1%) | 132 (29.0%) | 72 (39.8%) | 0.0087 |
| Dyspnea at Rest | 81 (12.7%) | 52 (11.4%) | 29 (16.0%) | 0.1172 |
| Fatigue | 243 (38.2%) | 161 (35.4%) | 82 (45.3%) | 0.0203 |
| Chest Tightness/discomfort | 90 (14.2%) | 64 (14.1%) | 26 (14.4%) | 0.9224 |
| AFEQT Score - Baseline | ||||
| AFEQT overall score at baseline | 67.6 (54.6, 75.9) | 70.4 (58.3, 76.9) | 57.4 (44.4, 67.6) | <.0001 |
| AFEQT symptoms subscale at baseline | 79.2 (62.5, 91.7) | 83.3 (66.7, 91.7) | 70.8 (54.2, 87.5) | <.0001 |
| AFEQT daily activities subscale at baseline | 54.2 (33.3, 66.7) | 58.3 (37.5, 70.8) | 41.7 (27.1, 58.3) | <.0001 |
| AFEQT treatment concern subscale at baseline | 75.0 (61.1, 86.1) | 77.8 (63.9, 88.9) | 66.7 (50.0, 77.8) | <.0001 |
| AFEQT treatment satisfaction subscale at baseline | 83.3 (66.7, 91.7) | 83.3 (66.7, 91.7) | 83.3 (58.3, 91.7) | 0.0173 |
| AFEQT Score - 1 Year | ||||
| AFEQT overall score at 1 year | 74.1 (60.2, 85.2) | 68.5 (54.8, 78.7) | 90.7 (79.4, 98.1) | <.0001 |
| AFEQT symptoms subscale at 1 year | 87.5 (75.0, 100.0) | 83.3 (70.8, 95.8) | 100.0 (87.5, 100.0) | <.0001 |
| AFEQT daily activities subscale at 1 year | 62.5 (41.7, 83.3) | 54.2 (33.3, 72.9) | 89.6 (66.7, 100.0) | <.0001 |
| AFEQT treatment concern subscale at 1 year | 83.3 (66.7, 94.4) | 77.8 (63.9, 91.7) | 94.4 (83.3, 100.0) | <.0001 |
| AFEQT treatment satisfaction subscale at 1 year | 83.3 (75.0, 100.0) | 83.3 (66.7, 91.7) | 100.0 (83.3, 100.0) | <.0001 |
hrQoL: health-related quality of life; AFEQT: Atrial Fibrillation Effect on QualiTy-of-Life
Figure 2.
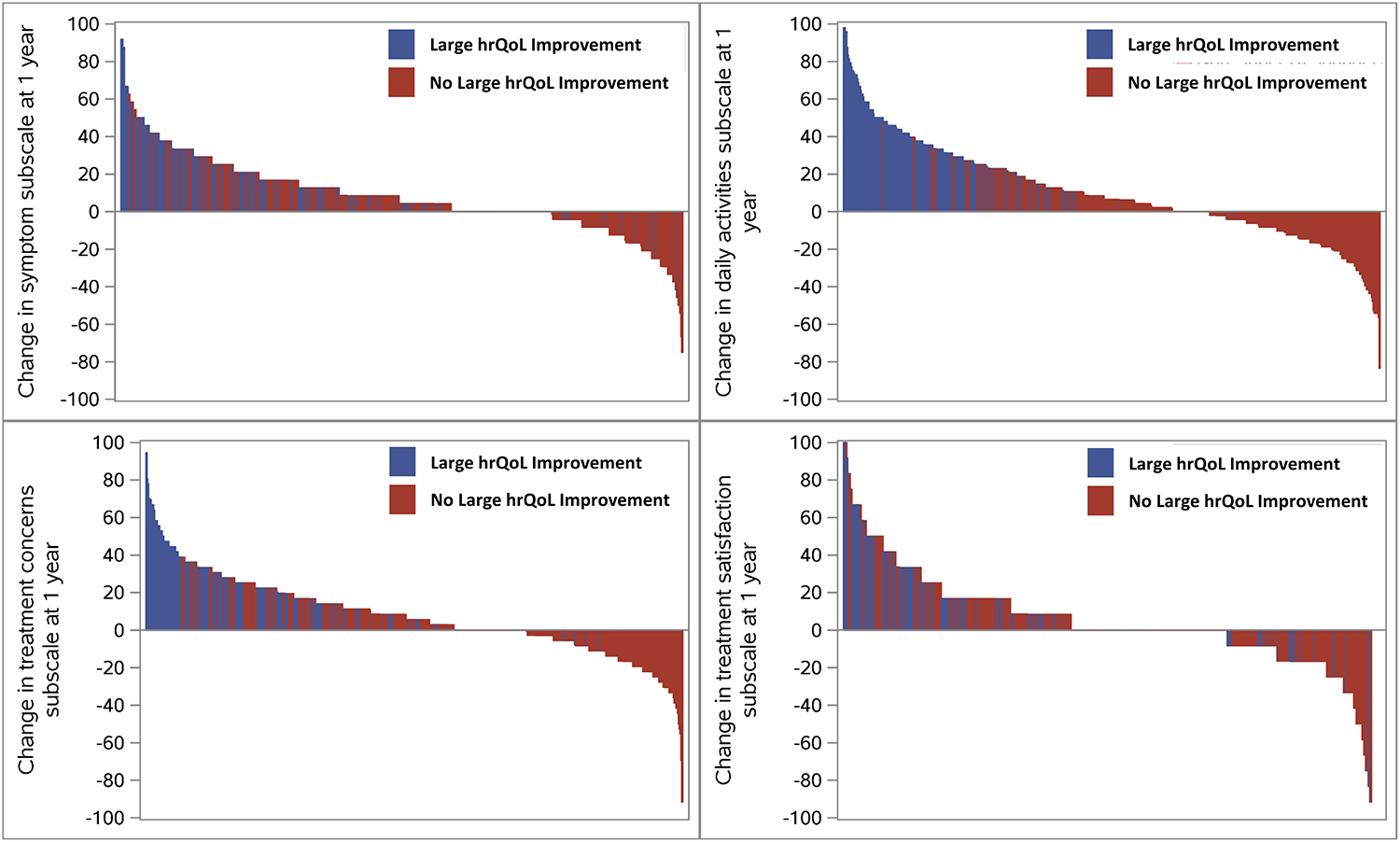
Waterfall plots of AFEQT domain scores, for all analyzed patients, by absolute change in subdomain scores, stratified by presence of large hrQoL improvement at 1 year.
AF: atrial fibrillation; EHRA: European Heart Rhythm Association.
hrQoL Response & Interval Events
Interval interventions and adverse events between baseline and one-year follow-up in these patients are shown in Supplemental Material, Table S2, stratified by hrQoL response status. Overall, patients that experienced large improvement in hrQoL at one year, were more likely to have had interval procedures related to AF, including catheter ablation of AF (6.6% vs. 2.0%, p=0.003), surgical AF ablation (1.1 % vs. 0, p=0.02), and cardioversion (12.2% vs. 5.9%, p=0.008). There were no significant differences in interim adverse clinical events between the two groups, including major bleeding, thromboembolic events, new heart failure, or hospitalization, though event rates were low.
Factors Associated with Large Improvements in hrQoL
Complete results of multivariable analysis to identify factors associated with large improvements in hrQoL are shown in Figure 3. Patients with worse hrQoL at baseline have more opportunity for improvement; in our adjusted analyses, compared to those with baseline score <50, patients with a baseline score >50 had lower odds of AFEQT improvement, and rate of improvement decreased as baseline AFEQT increased (adjusted OR 0.69 per 5-point increase, 95% CI 0.63–0.76; see Supplemental Material, Figure S1). Additionally, patients most likely to experience large hrQoL improvement were those with a history of alcohol abuse (adjusted OR 2.41, 95% CI 1.23–4.70) and patients with elevated diastolic blood pressure at baseline (adjusted OR 1.23 per 10-point increase >65, 95% CI 1.01–1.49). In contrast, patients with prior stroke/TIA (adjusted OR 0.48, 95% CI 0.29–0.8), COPD (adjusted OR 0.67, 95% CI 0.47–0.95), or peripheral arterial disease (PAD; adjusted OR 0.53, 95% CI 0.30–0.93) were less likely to experience a large improvement in hrQoL.
Figure 3.
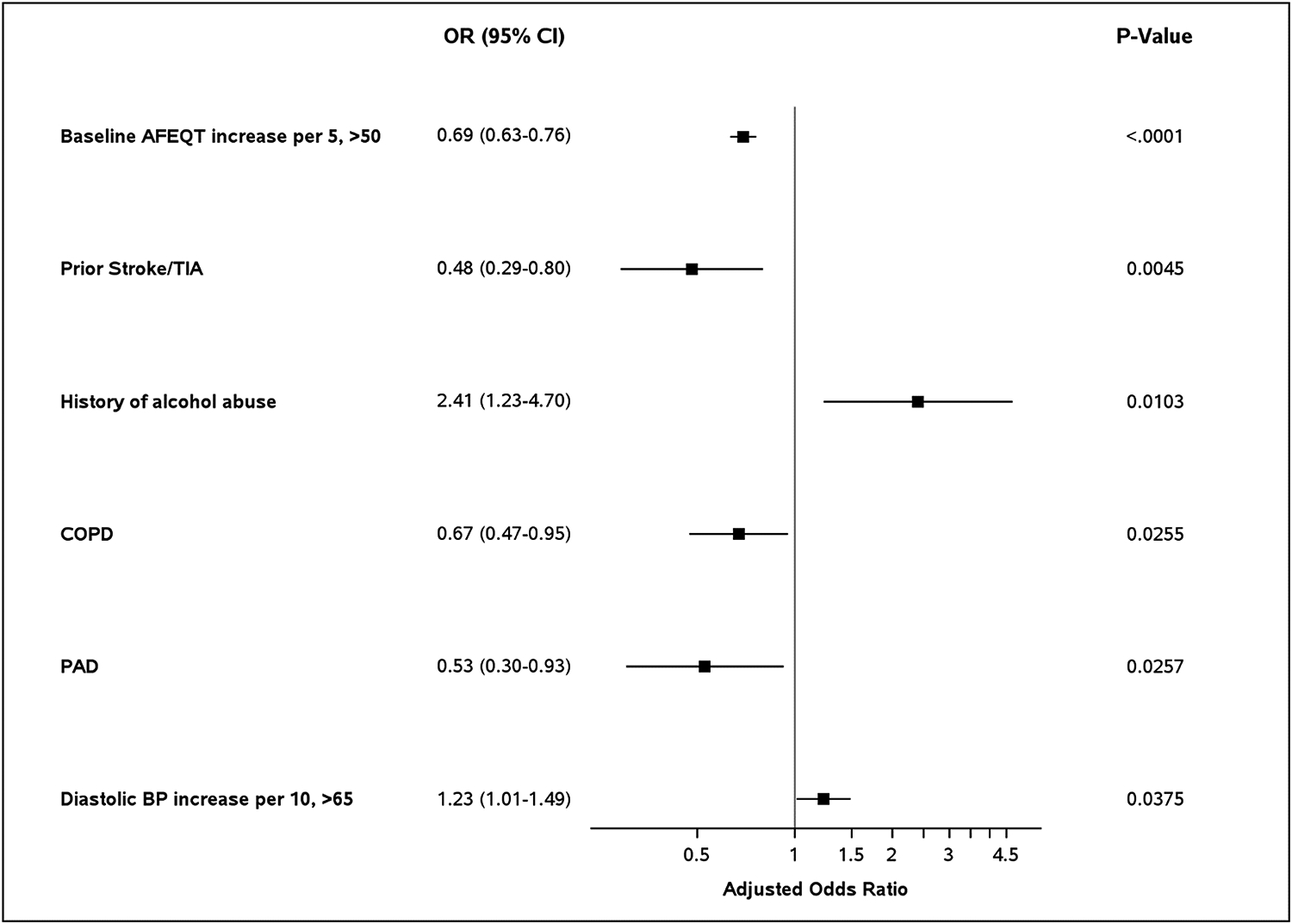
Factors associated with large improvement in hrQoL (n=636) based on the multivariable model.
hrQoL: health-related quality of life; AFEQT: Atrial Fibrillation Effect on QualiTy-of-Life.
Clinical Events & Sensitivity Analyses
During follow-up after 1 year, there was no difference in rates of major bleeding, thromboembolic events, new heart failure, or hospitalization between those with and without significant hrQoL improvement (Supplemental Material, Table S3). In the primary analysis, patients who died during follow-up were excluded as not having the opportunity to improve hrQoL. In a sensitivity analysis including these patients as not improved, multivariate models yielded similar factors associated with large hrQoL improvement (Supplemental Material, Table S4).
After excluding patients with recent AF diagnosis (<1 year of enrollment), the resulting sensitivity analysis population yielded 448 patients of which 113 experienced a large improvement in hrQoL (25%). Baseline characteristics of these subgroups did not differ dramatically from the primary analytic cohort. Multivariate analysis of factors associated with a large improvement in hrQoL in this subset consistently included baseline AFEQT score, prior stroke/TIA, and PAD; instead of COPD, history of anemia was associated with increased odds of experiencing a large improvement in hrQoL (adjusted OR 2.11, 95% CI 1.25–3.55; Supplemental Material, Table S5).
Discussion
In this analysis of symptomatic patients with AF, there are several important conclusions. First, there is a substantial subgroup of AF patients who will demonstrate large improvement in AF hrQoL over one-year follow up, and these changes appear driven by improvements in activities of daily living and treatment concerns. Second, patients who experienced a large improvement in hrQoL at 1 year had higher rates of rhythm control interventions, including baseline antiarrhythmic drug therapy (34.8% vs, 26.8%, p=0.045) and interim AF ablation (6.6% vs. 2.0%, p=0.003). Nevertheless, after adjustment baseline rhythm control therapy (i.e., antiarrhythmic drug use) was not associated with having large hrQoL improvement at one year, whereas potentially modifiable risk factors, including alcohol abuse and elevated diastolic blood pressure, were associated with large symptomatic improvement. Lastly, chronic, difficult-to-treat comorbidities appear to make large hrQoL improvement less likely. These data highlight the potential for significant improvement in symptom status for a condition that is typically associated with poor hrQoL, and further studies are needed to evaluate interventions in the patients most likely to improve.
There are several likely contributors to the symptomatic improvement in these patients with large improvements with hrQoL. Our multivariable model demonstrated both baseline alcohol abuse and elevated diastolic blood pressure were significantly associated with large hrQoL improvement at one year; these are both potentially-modifiable risk factors for AF, which can be effectively treated and may have led to improvement in hrQoL. We acknowledge, however, that we cannot definitely demonstrate a causal relationship between treatment of these conditions and hrQoL response. Additionally, patients with large hrQoL improvement were more likely to have new-onset AF and more recent-diagnosis date – while this may have meant more aggressive rhythm control interventions to improve symptoms, we cannot exclude an element of ‘hedonic adaptation’ leading to improvement in reported hrQoL. The Atrial Fibrillation: Focus on Effective Clinical Treatment Strategies (AFFECTS) registry previously demonstrated differing symptom profiles among patients with paroxysmal versus persistent AF.13 Lastly, while we observed that patients with large hrQoL were more likely to have received medications for rhythm control (prior and baseline antiarrhythmic drug therapy), this was not associated with hrQoL improvement at follow-up.
In contrast, several significant, difficult-to-treat comorbidities were associated with not having significant improvement in hrQoL: COPD, prior stroke/TIA, and PAD. It is well-known that concomitant COPD impacts outcomes, including hrQoL, among AF patients.14 Those with prior cerebrovascular events may have permanent, residual deficits limiting their hrQoL, and patients with PAD may have life-altering symptoms that are difficult to treat, and less likely to be impacted by interventions for AF.
Furthermore, our data on the domains of the AFEQT show more frequent improvement in daily activities and treatment concerns, compared with symptoms and treatment satisfaction – in fact, significant proportions of our patients had no improvement, or worsening, in the later metrics. These findings may underscore the above model, demonstrating that the primary factors associated with large hrQoL improvement (or not) were geared towards risk factors (e.g., hypertension, alcohol) and co-morbidities (e.g., PAD, COPD), and not specific AF interventions. However, the domains also suggest opportunities for further improvement in the hrQoL of these patients – relatively few underwent catheter ablation during follow-up, an intervention proven to improve disease-specific symptoms across AF cohorts.6, 7, 15, 16
These data have important implications for the management of patients with symptomatic AF. Among the primary objectives is to improve hrQoL, and as we have observed, this response is heterogeneous. Understanding factors associated with improvement and decline, and particularly among components of hrQoL, is vital to the appropriate implementation of therapies for both AF and concomitant comorbidities. While rhythm control therapies, primarily antiarrhythmic drugs and catheter ablation, are generally safe and effective, they are not without risk and would ideally target patients most likely to benefit. Ultimately, targeting the appropriate intervention, at the appropriate disease state, and in the appropriate patient, will provide the best opportunities for more consistently, and comprehensively, improving hrQoL in these patients.
Limitations
The data utilized in this analysis are derived from an observational, real-world dataset, and therefore may be subject to sampling bias. Additionally, the analytic cohort was relatively small, with a lower power to detect differences in rarer events between the two groups. There were few events per variable included in the candidate variable list for the factors associated with large improvement in hrQol model, which may have led to overfitting in the model. The assessments of hrQoL were not timed to specific interventions or changes in therapy. Residual measured and unmeasured confounding may account in part for the observed associations, and we cannot ascribe a direct causal effect for factors associated with hrQoL improvement; additionally, there may be some aspect of regression to the mean that contributed to our findings. Lastly, the definition of large improvement in hrQoL was empirically based on both the population distribution and other studies of observed hrQoL changes, and results may vary with different hrQoL change thresholds or in different AF cohorts.
Conclusions
Large improvement in hrQoL occurs in a significant proportion of AF patients at one year, however, we observed heterogeneity in improvement across domains of hrQoL. In addition to lower hrQoL at baseline, additional factors associated with large improvement at one year include potentially reversible risk factors (alcohol, elevated diastolic BP) and less likely among patients with difficult-to-treat comorbidities (prior stroke, COPD, PAD). Understanding the impact of comorbidities and therapeutic interventions on AF hrQoL can facilitate appropriate implementation to optimize these outcomes.
Supplementary Material
Sources of Funding:
The ORBIT-AF registry is sponsored by Janssen Scientific Affairs, LLC, Raritan, NJ. Research reported in this publication was also supported by the National Heart, Lung, And Blood Institute of the NIH under Award Number K23HL143156 (to BAS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures: The following relationships exist related to this presentation: DNH, KP, LT, DES report no relevant disclosures; BAS reports research support from Boston Scientific and Janssen; consulting to Janssen, AltaThera, and Merit Medical; speaking for NACCME (funded by Sanofi). GCF reports consulting for Abbott, Amgen, Bayer, Janssen, Medtronic, Novartis. EH reports modest Speakers Bureau support form Boehringer-Ingelheim and Bayer; Modest Consultant/Advisory Board to Johnson & Johnson, Boehringer-Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Pfizer, and Ortho-McNeil-Janssen. PRK reports modest Consultant/Advisory Board support from Boehringer Ingelheim, Bristol Myers Squibb, Johnson & Johnson, Portola, Merck, Sanofi, and Daiichi Sankyo. KWM financial disclosures prior to August 1, 2013, can be viewed at https://www.dcri.org/about-us/conflict-of-interest/Mahaffey-COI_2011-2013.pdf; disclosures after August 1, 2013, can be viewed at http://med.stanford.edu/profiles/kenneth_mahaffey. EDP reports: significant Research Grant support from Eli Lilly & Company, Janssen Pharmaceuticals, Inc., and the American Heart Association; modest Consultant/Advisory Board support from Boehringer Ingelheim, Bristol-Myers Squibb, Janssen Pharmaceuticals, Inc., Pfizer, and Genentech Inc. JPP receives grants for clinical research from Abbott, American Heart Association, Boston Scientific, Gilead, Janssen Pharmaceuticals, NHLBI, and Philips and serves as a consultant to Abbott, Allergan, ARCA Biopharma, Biotronik, Boston Scientific, Johnson & Johnson, LivaNova, Medtronic, Milestone, Oliver Wyman Health, Sanofi, Philips, and Up-to-Date.
Nonstandard Abbreviations and Acronyms:
- ORBIT-AF
Outcomes Registry for Better Informed Treatment of Atrial Fibrillation
- AFEQT
Atrial Fibrillation Effect on QualiTy-of-Life
- AFFECTS
Atrial Fibrillation: Focus on Effective Clinical Treatment Strategies
Appendix:
The Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Investigators and Patients members include the following: R. Mendelson, A. Nahhas, J. Neutel, B. Padanilam, D. Pan, J. Poock, J. Raffetto, R. Greengold, P. Roan, F. Saba, M. Sackett, R. Schneider, Z. Seymour, J. Shanes, J. Shoemaker, V. Simms, N. Smiley, D. Smith, C. Snipes, R. Sotolongo, C. Staniloae, S. Stoltz, D.P. Suresh, T. Tak, A. Tannenbaum, S. Turk, K. Vora, P. Randhawa, J. Zebrack, E. Silva, E. Riley, D. Weinstein, T. Vasiliauskas, S. Goldbarg, D. Hayward, C. Yarlagadda, D. Laurion, A. Osunkoya, R. Burns, T. Castor, D. Spiller, C. Luttman, S. Anton, J. McGarvey, R. Guthrie, G. Deriso, R. Flood, L. Fleischer, J.S. Fierstein, R. Aggarwal, G. Jacobs, N. Adjei, A. Akyea-Djamson, A. Alfieri, J. Bacon, N. Bedwell, P. Berger, J. Berry, R. Bhagwat, S. Bloom, F. Boccalandro, J. Capo, S. Kapadia, R. Casanova, J.E. Morriss III, T. Christensen, J. Elsen, R. Farsad, D. Fox, B. Frandsen, M. Gelernt, S. Gill, S. Grubb, C. Hall, H. Harris, D. Hotchkiss, J. Ip, N. Jaffrani, A. Jones, J. Kazmierski, F. Waxman, G.L. Kneller, A. Labroo, B. Jaffe, M. Lebenthal, D. Lee, M. Lillestol, K. LeClerc, P. Maccaro, N. Mayer, J. Kozlowski, S. Benjamin, R. Detweiler, P. Igic, T. Jackson, J. Pappas, R. Littlefield, A. Frey, R. Vranian, W. Long, P. Grena, A. Arouni, J. Quinn, K. Browne, S. Forman, M. Ebinger, R. Blonder, H. Snyder, S. Slabic, D. Williams, R. Stein, S. Kirkland, K. Cohen, W. Walthall, K. Davis, B. Snoddy, O. Alvarado, C. Leach, S. Rothman, A. Sharma, A. Olatidoye, S. AlMahameed, S. Rosenthal, G. Sutter, W. Reiter, T. Thompson, S. Thew, J. Kobayashi, M. Williams, J. Kramer, S.A. Latif, B. Rhee, A. Adler, D. Ruiz-Serrano, S. Stringam, K. Wolok, A. Focil, S. Butman, H. Ingersoll, R. Borge, Y. Al-Saghir, P. Coats, N. Farris, K. Shore, M.B. Schwartz, C. Gornick, P. Eilat, E. Quinlan, Y. Paliwal, R. Mitra, A. Jingo, A.A. Aslam, L. Allen, R. Watson, S. Voyce, M. Turakhia, D. Goytia-Leos, M. Lurie, G. Mallis, B. Atwater, J. Strobel, J. Murray, D. Fisher, M. Atieh, R. Landes, A. Drabick, E. Harman, B. Ashcraft, M. Krista, A. Videlefsky, E. Rivera Zayas, and A.E. Tan.
References:
- 1.Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104:1534–1539. [DOI] [PubMed] [Google Scholar]
- 2.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. [DOI] [PubMed] [Google Scholar]
- 3.Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–320. [DOI] [PubMed] [Google Scholar]
- 4.Dorian P, Jung W, Newman D, Paquette M, Wood K, Ayers GM, Camm J, Akhtar M, Luderitz B. The impairment of health-related quality of life in patients with intermittent atrial fibrillation: implications for the assessment of investigational therapy. J Am Coll Cardiol. 2000;36:1303–1309. [DOI] [PubMed] [Google Scholar]
- 5.Wokhlu A, Monahan KH, Hodge DO, Asirvatham SJ, Friedman PA, Munger TM, Bradley DJ, Bluhm CM, Haroldson JM, Packer DL. Long-term quality of life after ablation of atrial fibrillation the impact of recurrence, symptom relief, and placebo effect. J Am Coll Cardiol. 2010;55:2308–2316. [DOI] [PubMed] [Google Scholar]
- 6.Mark DB, Anstrom KJ, Sheng S, Piccini JP, Baloch KN, Monahan KH, Daniels MR, Bahnson TD, Poole JE, Rosenberg Y, et al. Effect of Catheter Ablation vs Medical Therapy on Quality of Life Among Patients With Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA. 2019;321:1275–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blomstrom-Lundqvist C, Gizurarson S, Schwieler J, Jensen SM, Bergfeldt L, Kenneback G, Rubulis A, Malmborg H, Raatikainen P, Lonnerholm S, et al. Effect of Catheter Ablation vs Antiarrhythmic Medication on Quality of Life in Patients With Atrial Fibrillation: The CAPTAF Randomized Clinical Trial. JAMA. 2019;321:1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antonio N, Teixeira R, Coelho L, Lourenco C, Monteiro P, Ventura M, Cristovao J, Elvas L, Goncalves L, Providencia LA. Identification of ‘super-responders’ to cardiac resynchronization therapy: the importance of symptom duration and left ventricular geometry. Europace. 2009;11:343–349. [DOI] [PubMed] [Google Scholar]
- 9.Piccini JP, Fraulo ES, Ansell JE, Fonarow GC, Gersh BJ, Go AS, Hylek EM, Kowey PR, Mahaffey KW, Thomas LE, et al. Outcomes registry for better informed treatment of atrial fibrillation: rationale and design of ORBIT-AF. Am Heart J. 2011;162:606–612 e601. [DOI] [PubMed] [Google Scholar]
- 10.Spertus J, Dorian P, Bubien R, Lewis S, Godejohn D, Reynolds MR, Lakkireddy DR, Wimmer AP, Bhandari A, Burk C. Development and validation of the Atrial Fibrillation Effect on QualiTy-of-Life (AFEQT) Questionnaire in patients with atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:15–25. [DOI] [PubMed] [Google Scholar]
- 11.Dorian P, Burk C, Mullin CM, Bubien R, Godejohn D, Reynolds MR, Lakkireddy DR, Wimmer AP, Bhandari A, Spertus J. Interpreting changes in quality of life in atrial fibrillation: How much change is meaningful? Am Heart J. 2013;166:381–387 e388. [DOI] [PubMed] [Google Scholar]
- 12.Holmes DN, Piccini JP, Allen LA, Fonarow GC, Gersh BJ, Kowey PR, O’Brien EC, Reiffel JA, Naccarelli GV, Ezekowitz MD, et al. Defining Clinically Important Difference in the Atrial Fibrillation Effect on Quality-of-Life Score. Circ Cardiovasc Qual Outcomes. 2019;12:e005358. [DOI] [PubMed] [Google Scholar]
- 13.Reiffel JA, Kowey PR, Myerburg R, Naccarelli GV, Packer DL, Pratt CM, Reiter MJ, Waldo AL. Practice patterns among United States cardiologists for managing adults with atrial fibrillation (from the AFFECTS Registry). Am J Cardiol. 2010;105:1122–1129. [DOI] [PubMed] [Google Scholar]
- 14.Durheim MT, Holmes DN, Blanco RG, Allen LA, Chan PS, Freeman JV, Fonarow GC, Go AS, Hylek EM, Mahaffey KW, et al. Characteristics and outcomes of adults with chronic obstructive pulmonary disease and atrial fibrillation. Heart. 2018;104:1850–1858. [DOI] [PubMed] [Google Scholar]
- 15.Wazni OM, Marrouche NF, Martin DO, Verma A, Bhargava M, Saliba W, Bash D, Schweikert R, Brachmann J, Gunther J, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA. 2005;293:2634–2640. [DOI] [PubMed] [Google Scholar]
- 16.Turagam MK, Garg J, Whang W, Sartori S, Koruth JS, Miller MA, Langan N, Sofi A, Gomes A, Choudry S, et al. Catheter Ablation of Atrial Fibrillation in Patients With Heart Failure: A Meta-analysis of Randomized Controlled Trials. Ann Intern Med. 2019;170:41–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


